Comparative Effects of L. plantarum CGMCC 1258 and L. reuteri LR1 on Growth Performance, Antioxidant Function, and Intestinal Immunity in Weaned Pigs
- 1State Key Laboratory of Livestock and Poultry Breeding, Ministry of Agriculture Key Laboratory of Animal Nutrition and Feed Science in South China, Guangdong Key Laboratory of Animal Breeding and Nutrition, Maoming Branch, Guangdong Laboratory for Lingnan Modern Agriculture, Institute of Animal Science, Guangdong Academy of Agricultural Sciences, Guangzhou, China
- 2College of Animal Science, Institute of Animal Nutrition and Feed Science, Guizhou University, Guiyang, China
Lactobacillus plantarum CGMCC 1258 and Lactobacillus reuteri LR1 are two important strains of probiotics. However, their different advantages in the probiotic effect of weaned pigs are still poorly understood. Therefore, the study was to investigate the comparative effects of dietary supplementation of L. plantarum CGMCC 1258 and L. reuteri LR1 on growth performance, antioxidant function, and intestinal immunity in weaned pigs. Ninety barrows [initial body weight (BW) = 6.10 ± 0.1 kg] 21 days old were randomly divided into 3 treatments with 5 replicates, each replicate containing 6 pigs. Pigs in control (CON) were fed a basal diet, and the basal diets supplemented with 5 × 1010 CFU/kg L. plantarum CGMCC 1258 (LP) or L. reuteri LR1 (LR) for 42 days, respectively. The results showed that LP increased (p < 0.05) serum superoxide dismutase (SOD), and decreased (p < 0.05) serum malondialdehyde (MDA) and the expression and secretion of interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ) in intestinal mucosa, but has no significant effect on growth performance and diarrheal incidence. However, LR increased (p < 0.05) final BW and average daily gain (ADG), reduced (p < 0.05) 29–42-day diarrheal incidence, decreased (p < 0.05) the expression and secretion of IL-1β, IL-6, TNF-α, and IFN-γ, and increased (p < 0.05) the expression of transforming growth factor-β (TGF-β) in intestinal mucosa. In addition, the serum glutathione peroxidase (GSH-PX), mRNA relative expression of Na+-K+-2Cl– co-transporter 1 (NKCC1) and cystic fibrosis transmembrane conductance regulator (CFTR) and the content of toll-like relative (TLR2) and TLR4 in the jejunum, and secretory immunoglobulin (sIgA) content of ileal mucosa were higher (p < 0.05) than LP. Collectively, dietary L. plantarum CGMCC 1258 improved intestinal morphology, intestinal permeability, intestinal immunity, and antioxidant function in weaned pigs. Dietary L. reuteri LR1 showed better growth performance, a lower incidence of diarrhea, better intestinal morphology, and a higher extent of immune activation in weaned pigs.
Introduction
Early weaning is often associated with a range of disorders in pigs including digestive upset, low feed intake, poor immunocompetence, diarrhea, and reduced growth performance (1, 2). After the widespread restriction of the use of growth-promoting antibiotics, probiotic additives have played an important role in improving immune response, intestinal microbial balance, and the pH of the gastrointestinal tract of weaned pigs (3). Lactobacillus is a widely used probiotic agent. Lactobacillus plantarum and Lactobacillus reuteri have been used in vertebrates such as pigs, chickens, and humans (4). L. plantarum and L. reuteri improve intestinal health by producing exopolysaccharides to increase intestinal adhesion and colonization of probiotics. Currently, L. plantarum and L. reuteri may promote host immunity and intestinal physiological functions by coregulating pro-inflammatory and anti-inflammatory cytokines that has been proven in many ways (5, 6). In addition, previous studies have shown that L. plantarum CJLP243 (1 × 1010 CFU/kg) or L. plantarum CGMCC 1258 (5 × 1010 CFU/kg) can improve growth performance and enhance the defense of intestinal epithelial barrier in weaned pigs challenged by Escherichia coli (7, 8). L. plantarum ZJ316 also improved the growth performance of weaned pigs under normal feeding conditions (9). For L. reuteri, L. reuteri D8 promotes the development of intestine mucosal system and maintains intestinal mucosal barrier (10). Previous studies in this laboratory have showed that a strain of L. reuteri LR1 isolated from the feces of healthy piglets showed bile resistance and notable acid (11). Dietary L. reuteri LR1 supplemented at 5 × 1010 CFU/kg improved growth performance, epithelial barrier function, and enhanced amino acid metabolism in weaned pigs (12, 13). However, under the premise that the two strains of L. plantarum CGMCC 1258 and L. reuteri LR1 are known to have good probiotic effects on weaned pigs, their different advantages in the probiotic effect on weaned pigs are still lacking. Hence, the present study was conducted to investigate the differential effects of L. plantarum CGMCC 1258 and L. reuteri LR1 on the growth performance, antioxidant function, and intestinal immunity in weaned pigs.
Materials and Methods
These experiments were conducted in accordance with Chinese guidelines for animal welfare and experimental protocols, and all animal procedures were approved by the Animal Care and Use Committee of Guangdong Academy of Agricultural Sciences (Permit Number: GAASIAS-2015-012). The L. plantarum CGMCC 1258 strain was provided by Dr. Hang Xiaomin (Institute of Science Life of Onlly, Shanghai Jiao Tong University, Shanghai, China), and the strain was originally isolated from the feces of healthy infants (14). The L. reuteri LR1 strain was originally isolated from the feces of healthy 35-day-old weaned pigs in our laboratory (11).
Animals and Diets
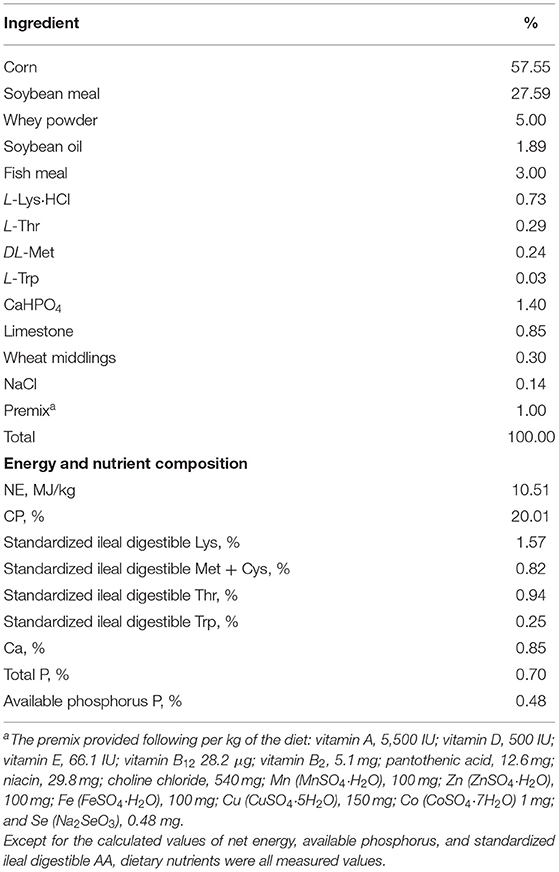
A total of 90 barrows [Duroc × (Landrace × Yorkshire), 21 d of age, body weight (BW) = 6.10 ± 0.1 kg] were randomly allocated to three groups (five replicates per group and six pigs per replicate). Control group (CON) were fed a corn–soybean meal basal diet, L. plantarum CGMCC 1258 group (LP) were fed the basal diet supplemented with 5 × 1010 CFU/kg L. plantarum CGMCC 1258, and L. reuteri LR1 group (LR) were fed the basal diet supplemented with 5 × 1010 CFU/kg L. reuteri LR1 for 42 days. Experimental diets were formulated to meet the nutrient requirements for pigs proposed by the National Research Council (15), and the ingredient compositions and nutrient levels of the basal diets are listed in Table 1. The measurements of crude protein (CP), Ca, and P refer to GB/T 6432-2018 (China), GB/T 6436-2018 (China), and GB/T 6437-2018 (China), respectively. The diets used in the experiment were all mash feed. The pigs were provided feed and water ad libitum throughout the experiment.
Sample Collection
For each pen, two pigs were randomly selected for blood collection and slaughter sampling. Blood samples and tissue sampling from all weaned pigs were completed on day 43. Blood samples were collected intravenously into 10-ml vacuum tubes without anticoagulant, centrifuged at 3,000 × g at 4°C for 15 min to obtain serum, and stored at −80°C until further assay. After blood collection, one pig per pen was anesthetized by intravenous injection of pentobarbital sodium (30 mg/kg BW) and killed by bloodletting. Approximately 1-cm lengths of middle duodenum, middle jejunum, and distal ileum specimens were collected without rinsing and fixed in 4% paraformaldehyde. Approximately 10-cm lengths of jejunum and ileum were cut open to expose the intestinal lumen, rinsed with phosphate buffered saline, and the mucosa were scraped by sterile glass microscope slide, and then the samples were quickly frozen in liquid nitrogen and stored at −80°C until analyses.
Performance and Diarrhea Measurements
Feed intake was measured every day during the entire experiment, and pigs were weighed on days 0 and 42 to calculate average daily gain (ADG), average daily feed intake (ADFI), and gain:feed ratio (G/F). In addition, the diarrhea was observed and recorded in each pen at 9:00 and 16:00 every day. Diarrhea is evaluated according to the shape of the stool; strips or pellets are normal stools, while flat or liquid stools are diarrhea stools. Diarrhea incidence was calculated at the end of the experiment for each enclosure from 1 to 14 days, 15 to 28 days, 28 to 42 days, and 1 to 42 days. Diarrhea incidence was calculated according to the formula: diarrheal incidence (%) = [total number of pigs with diarrhea in each pen × diarrhea days/(6 pigs × the number of days)] × 100.
Analysis of Intestinal Morphology
The collected fixed samples ileum, jejunum, and duodenum were dehydrated and embedded in paraffin. Sections of 5 μm thickness were stained coated with H&E. Nine well-oriented and intact villi and adjacent crypts were measured each section using Image-Pro software (Media Cybernetics, Rockville, MD), and the villus height to crypt depth ratio (V/C) was calculated. The images were obtained by an Axio Scope A1 microscope (Zeiss, Germany).
Analysis of Antioxidant Function and Intestinal Cytokines
Lactate dehydrogenase (LDH, A020-2-2), the activities of superoxide dismutase (SOD, A001-3-2), malondialdehyde (MDA, A003-1-2), glutathione peroxidase (GSH-PX, A005-1-2), urea nitrogen (SUN, C013-2-1), and glucose (GLU, F006-1-1) in serum were estimated using a commercial kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The contents of immunoglobulin G (IgG, FU-Z076), lipopolysaccharide (LPS, YS04547B), insulin-like growth factor 1 (IGF-1, FU-Z135), and diamine oxidase (DAO, FU-Z050) in serum were estimated using ELISA kits (Beijing FangCheng Bioengineering Institute, Beijing, China). To obtain a 10% intestinal mucosa supernatant, 0.4 g of intestinal mucosa was added to 3.6 ml of 0.86% normal saline, homogenized in ice water with a tissue homogenizer, and centrifuged at 3,000 × g at 4°C for 10 min. The content of total protein in the supernatant was determined by BCA protein analysis kit (Thermo Fisher Scientific, Waltham, MA, 23227). The levels of interleukin-1β (IL-1β, H002), IL-6, tumor necrosis factor-α (TNF-α, FU-Z149), transforming growth factor-β (TGF-β, FU- FU-Z014), interferon-γ (IFN-γ, FU-Z054), secretory immunoglobulin (sIgA, H108-2) (Beijing FangCheng Bioengineering Institute), and TLR2 (ml027585) and TLR4 (ml027583) in intestinal mucosa were estimated using ELISA kits (Mlbio Bioengineering, Shanghai, China).
Real-Time PCR for Relative Measurement of Intestinal Diarrhea-Related Ion Channel Genes and Cytokines
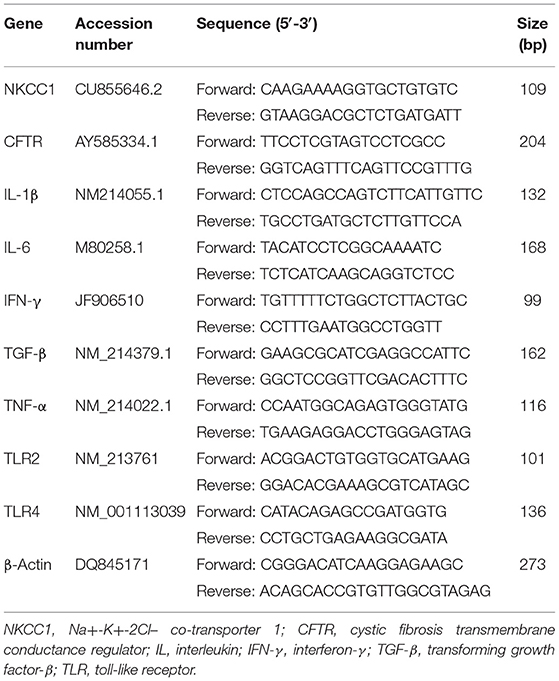
Total RNA of the mucosa of jejunum and ileum was extracted following the Trizol Reagent Instructions (Invitrogen, Carlsbad, CA). The total RNA was quantified using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA). RNA purity was assessed by determining the ratio of absorbance at 260 nm to that at 280 nm, and RNA (2 μg) was used to generate cDNA in a volume of 20 μl using a PrimeScript II 1st Strand cDNA Synthesis Kit (Takara, Tokyo, Japan). PCR amplification was performed in a total volume of 20 μl containing 10 μl of master mix (SYBR PCR Master Mix; Applied Biosystems), 1.0 μl of gene-specific primers (Table 2), 6.0 μl of RNAse-free water, and 2 μl 10-fold diluted cDNA. The thermocycler protocol consisted of 1 min at 95°C followed by 39 cycles of 10 s at 95°C, 30 s at 60°C, and 30 s at 72°C. β-Actin was used as a housekeeping gene. The fold changes were calculated for each sample using the 2−ΔΔCt method, and data for each target transcript were normalized to control pigs; ΔΔCT = (CT,Target – CT,β−actin)Treatment – (CT,Target – CT,β−actin)Control.
Statistical Analyses
The pen was the experimental unit. Statistical significance analysis was determined by one-way ANOVA with Tukey's test using SPSS 19.0 software (SPSS Inc., Chicago, IL). All data were expressed as the means ± SEM. The differences were significant at p < 0.05.
Results
Effects of L. plantarum CGMCC 1258 and L. reuteri LR1 on Growth Performance and Diarrhea in Weaned Pigs
LR but not LP increased final BW (p = 0.013) and ADG (p = 0.013), and reduced (p = 0.025) 29–42-day diarrheal incidence compared with CON (Table 3). However, no significant differences were observed on ADFI and neither on 1–42-day diarrheal incidence between treatments (p = 0.154).
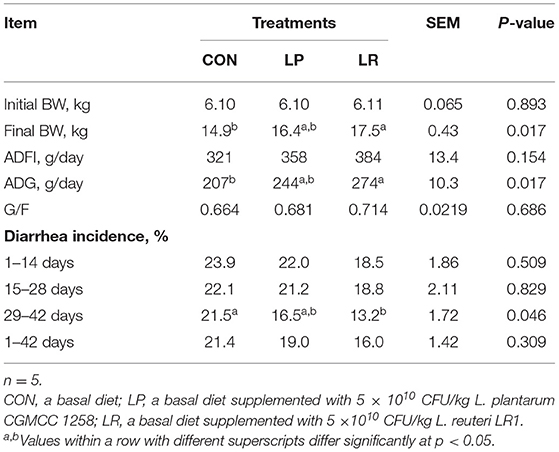
Table 3. Effects of L. plantarum CGMCC 1258 and L. reuteri LR1 on growth performance and diarrhea of weaned pigs.
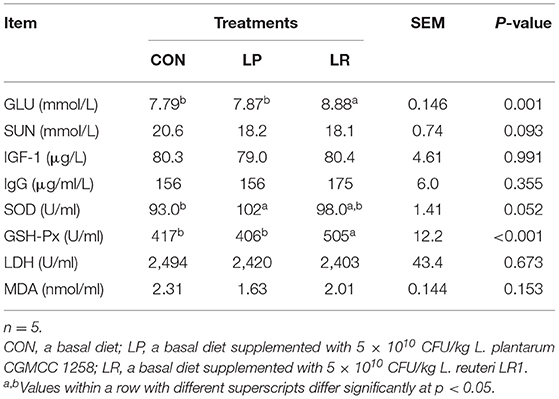
Effects of L. plantarum CGMCC 1258 and L. reuteri LR1 on Antioxidant Function in Weaned Pigs
LR increased serum GLU (p = 0.007) compared with CON and LP (Table 4). In addition, the LP increased (p = 0.043) serum SOD compared with CON. LR increased serum GSH-Px compared with both CON (p = 0.002) and LP (p = 0.001).
Effects of L. plantarum CGMCC 1258 and L. reuteri LR1 on Intestinal Morphology in Weaned Pigs
LP increased duodenal villus height compared with CON (p = 0.0003) and LR (p = 0.018), and the jejunal villus height of LR was higher (p = 0.041) than that of CON (Table 5). In addition, LP increased (p = 0.001) the V/C of duodenum compared with CON. LR increased the V/C of jejunum (p = 0.011) and ileum (p = 0.018) compared with CON.
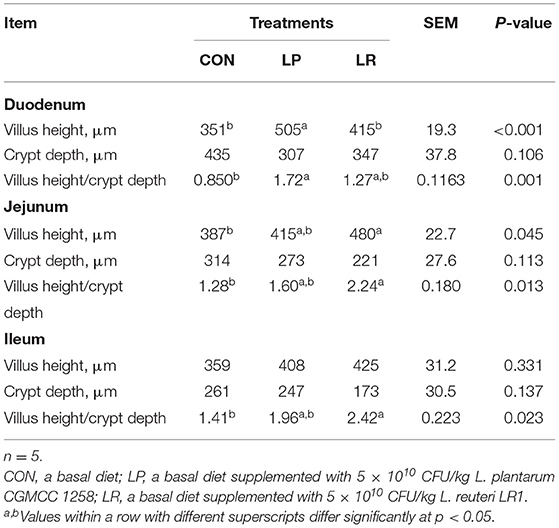
Table 5. Effects of L. plantarum CGMCC 1258 and L. reuteri LR1 on intestinal morphology in weaned pigs.
Effects of L. plantarum CGMCC 1258 and L. reuteri LR1 on Intestinal Permeability in Weaned Pigs
The expression of Na+-K+-2Cl– co-transporter 1 (NKCC1) in jejunal mucosa was decreased by LR compared with CON (p = 0.025) and LP (p = 0.029) (Figure 1). Meanwhile, LR decreased the expression of and cystic fibrosis transmembrane conductance regulator (CFTR) in jejunal mucosa compared with CON (p = 0.036) and LP (p = 0.038). In addition, both LP (p = 0.001) and LR (p = 0.002) decreased serum DAO above CON, similar to the effect of LP (p = 0.0002) and LR (p = 0.0004) on serum LPS compared with CON.
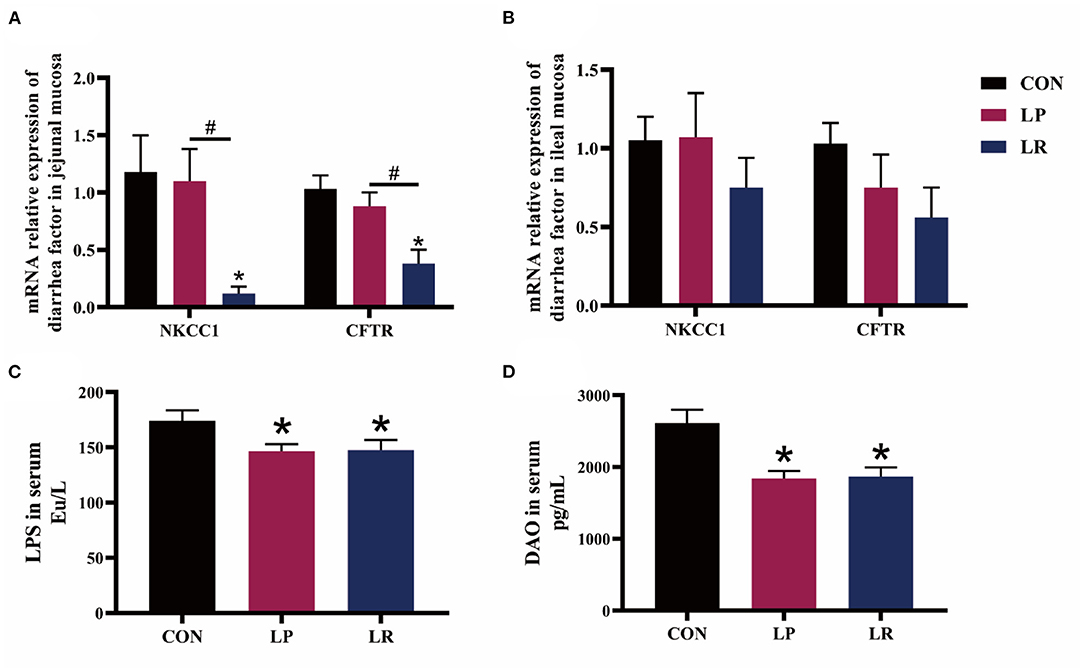
Figure 1. Effects of L. plantarum CGMCC 1258 and L. reuteri LR1 on diarrhea-related ion channel genes and intestinal permeability in weaned pigs. The relative mRNA expression levels of NKCC1 and CFTR in jejunal mucosa (A) and ileal mucosa (B) were determined via real-time PCR. Levels of LPS (C) and DAO (D) in serum determined by ELISA. NKCC1, Na+-K+-2Cl– co-transporter 1; CFTR, cystic fibrosis transmembrane conductance regulator; LPS, lipopolysaccharide; DAO, diamine oxidase. All data are expressed as the mean ± SEM (n = 5). Differences were determined by one-way ANOVA followed by Tukey test. *p < 0.05 compared with CON, #p < 0.05 compared with LP.
Effects of L. plantarum CGMCC 1258 and L. reuteri LR1 on Intestinal Cytokines in Weaned Pigs
Figure 2, Table 6 show that both LP (p = 0.029) and LR (p = 0.025) decreased IL-1β transcripts in jejunal mucosa and LP decreased (p = 0.004) ileal mucosa content of IL-1β compared with CON. LR decreased jejunal mucosa (p = 0.041) and ileal mucosa (p = 0.004) content of IL-6 compared with CON. The relative mRNA expression of TNF-α in jejunal mucosa was decreased by LR compared with CON (p = 0.046) and LP (p = 0.049). Both LP (p = 0.017) and LR (p = 0.011) decreased the TNF-α content of jejunal mucosa compared with CON. LR decreased (p = 0.039) the mRNA expression of IFN-γ in ileum mucosa compared with CON. LR increased (p < 0.05) the mRNA expression of TGF-β in jejunal mucosa compared with CON, and the TGF-β content in jejunal mucosa was increased in pigs fed LR compared with CON (p = 0.001) and LP (p = 0.043). In addition, LP (p = 0.0002) and LR (p = 0.0003) increased the sIgA content of the jejunal mucosa compared with CON, and concentrations of sIgA in ileal mucosa in LR was higher than in CON (p = 0.018) and LP (p = 0.009).
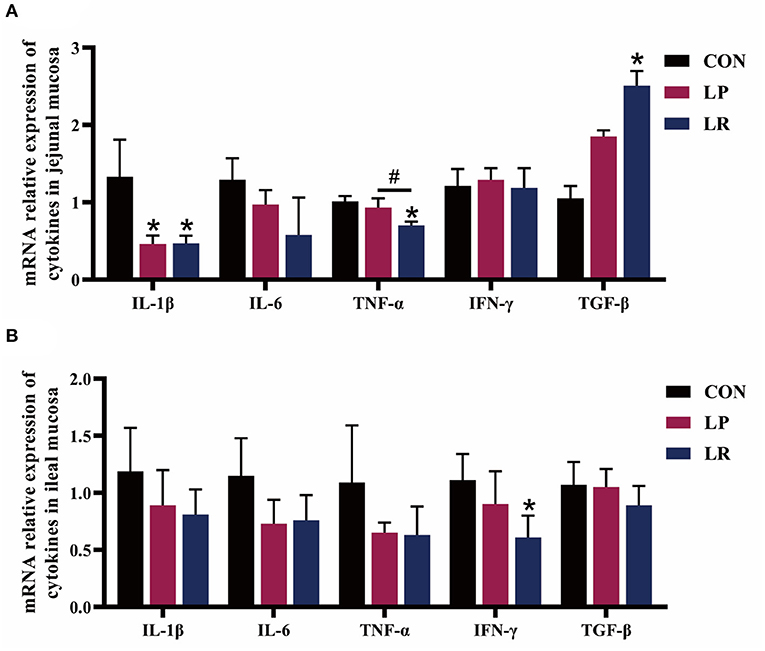
Figure 2. Effect of L. plantarum CGMCC 1258 and L. reuteri LR1 on the expression of cytokines in intestinal in weaned pigs. The relative mRNA expression levels of IL-1β, IL-6, TNF-α, IFN-γ, and TGF-β in the jejunal mucosa (A) and ileal mucosa (B) were determined via real-time PCR. All data are expressed as the mean ± SEM (n = 5). Differences were determined by one-way ANOVA followed by Tukey test. *p < 0.05 compared with CON, #p < 0.05 compared with LP.
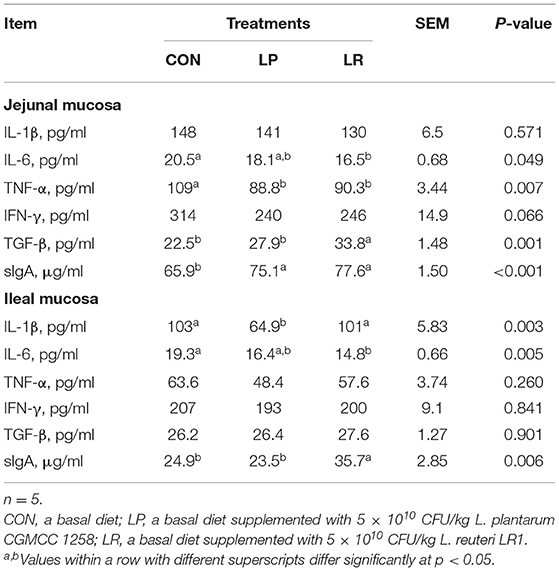
Table 6. Effects of L. plantarum CGMCC 1258 and L. reuteri LR1 on intestinal mucosal cytokines and sIgA concentrations of weaned pigs.
Effects of L. plantarum CGMCC 1258 and L. reuteri LR1 on TLRs in the Intestinal Mucosa in Weaned Pigs
LR increased (p = 0.003) content of TLR2 in the ileal mucosa compared with CON, and the content of TLR2 in the jejunal mucosa of LR is higher (p = 0.015) than that of LP (Figure 3). Both LP (p = 0.001) and LR (p = 0.003) increased content of TLR4 in ileal mucosa compared with CON, and LR increased content of TLR4 in jejunal mucosa compared with CON (p = 0.012) and LP (p = 0.003).
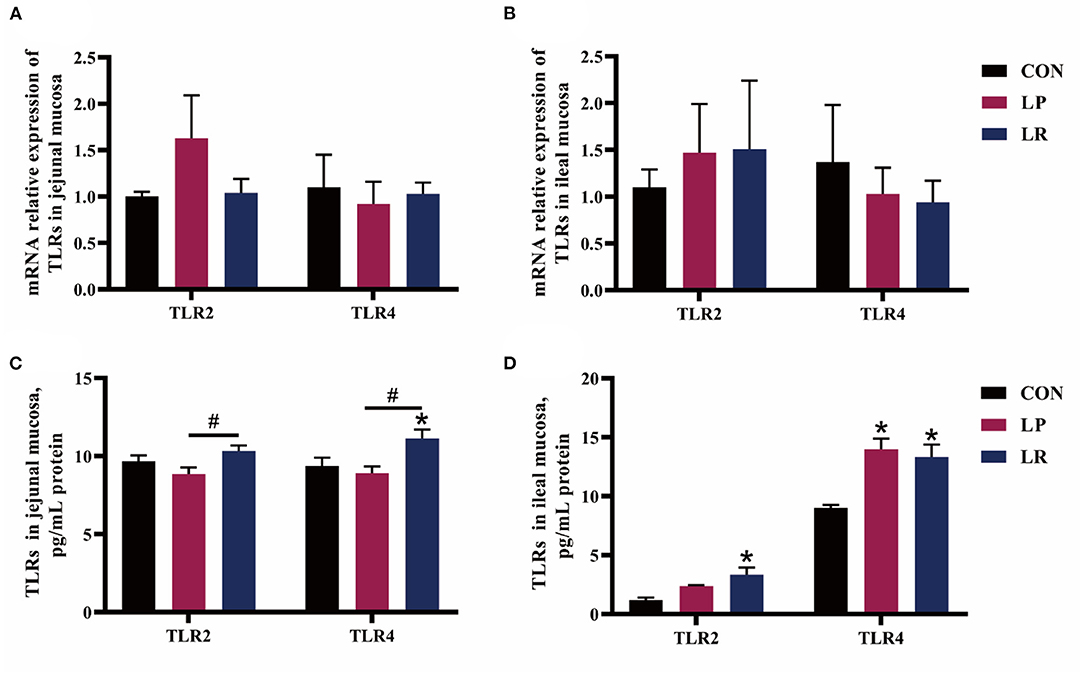
Figure 3. Effects of L. plantarum CGMCC 1258 and L. reuteri LR1 on TLRs in intestinal mucosa in weaned pigs. The relative mRNA expression levels of TLRs in the jejunal mucosa (A) and ileal mucosa (B) were determined via real-time PCR. Levels of TLRs in the jejunal mucosa (C) and ileal mucosa (D) determined by ELISA. All data are expressed as the mean ± SEM (n = 5). Differences were determined by one-way ANOVA followed by Tukey test. *p < 0.05 compared with CON, #p < 0.05 compared with LP.
Discussion
In the present study, we compared the effects of L. plantarum CGMCC 1258 and L. reuteri LR1 on the growth performance, antioxidant function, and intestinal immune function of weaned pigs. The results showed that both dietary L. plantarum CGMCC 1258 and L. reuteri LR1 supplementation at 5 × 1010 CFU/kg improved the antioxidant function, intestinal morphology, and intestinal immunity of weaned pigs, and they are consistent in improving intestinal permeability. However, L. plantarum CGMCC 1258 has a better effect on SOD and L. reuteri LR1 has better effects on ADG, diarrheal incidence, GLU, GSH-Px, intestinal morphology, and intestinal immunity.
Generally, the level of GLU is positively correlated with the digestion and absorption of carbohydrates in the intestine of pigs. In this study, L. reuteri LR1 but not L. plantarum CGMCC 1258 significantly increased the GLU content in the serum. The results suggest that L. reuteri LR1 improves serum GLU of weaned pigs, which was more advantageous than L. plantarum CGMCC 1258.
Weaning often induces a large number of reactive oxygen radicals in the piglets, causing oxidative stress and resulting in reduced piglet production performance and immune function (16). SOD and GSH-Px can scavenge reactive oxygen radicals in the body and are the main antioxidant enzymes in the body. MDA is a small molecule product produced at the termination stage of lipid peroxidation reaction, and its content can reflect the degree of lipid oxidation caused by reactive oxygen species in the organism (17). The strain of L. plantarum 423, L. plantarum 200655, and L. plantarum RG14 showed strong free radical–scavenging activity, and exert strong antioxidant capacity of human umbilical vein endothelial cells, human colon adenocarcinoma cell line, and post-weaning lambs, respectively (18–20). According to the mechanisms related to probiotic effects, L. plantarum and L. reuteri have been reported to limit excessive amounts of reactive radicals against oxidative stress in vivo (21, 22). Another study showed that dietary supplementation of L. plantarum ZLP001 increased the activity of serum SOD and GSH-Px in weaned pigs and reduced MDA content (23). The feeding with L. reuteri KT260178 increased the plasma total antioxidant capacity (T-AOC), SOD, and GSH-Px, which did not increase MDA in suckling piglets (24). However, the antioxidant function of L. plantarum CGMCC 1258 and L. reuteri LR1 has not been reported. In this study, L. plantarum CGMCC1258 increased serum SOD enzyme activity of weaned pigs, while L. reuteri LR1 increased the enzyme activity of GSH-Px. Taken together, L. reuteri LR1 improved the antioxidant function mainly by regulating GSH-Px, while L. plantarum CGMCC 1258 mainly affects SOD.
Probiotics are often used to improve the performance and intestinal health of pigs. Our data showed that the L. reuteri LR1 increased the ADG, but L. plantarum CGMCC 1258 has no significant effects on the growth performance of weaned pigs. The results of L. plantarum CGMCC 1258 in this experiment was different from a previous study, but the results of L. reuteri LR1 are consistent. Other strains of L. plantarum (such as CJLP243) and the our previous researched on L. plantarum CGMCC 1258 strain can improve the growth performance of weaned pigs by Escherichia coli challenge (7, 8), and L. plantarum ZJ316 and L. reuteri LR1 also improved the growth performance of weaned pigs under normal feeding conditions (9, 12, 25). We have also previously study that the L. reuteri LR1 strain improved the growth performance of weaned pigs under normal feeding conditions (12). Under normal feeding conditions of this experiment, there was no significant effect of L. plantarum CGMCC 1258 on growth performance of weaned pigs, which is quite different from the past, which may be related to the isolation of L. plantarum CGMCC 1258 strain from infant feces and the different experimental conditions. However, its influence mechanism needs more in-depth study.
In the present study, L. reuteri LR1 but not L. plantarum CGMCC1258 reduced the incidence of diarrhea in weaned pigs during the period of 29–42 days. Lee et al. (7), Yang et al. (8), and Suo et al. (9) showed that L. plantarum ZJ316 was effective in reducing diarrhea incidence in weaned pigs, but the reductions in diarrhea incidence with L. plantarum CGMCC1258 were all in the Escherichia coli challenge feeding mode (7–9). Probiotics play a detoxification role by inhibiting the reproduction of pathogenic bacteria, removing intestinal metabolites and bacteriocins (26). In the absence of E. coli challenge and under conditions of good intestinal health, L. plantarum CGMCC1258 was unable to exert significant antimicrobial and detoxification abilities in the intestine of weaned pigs. This may be the reason why L. plantarum CGMCC1258 is different from previous studies. In addition, stimulants such as enterotoxin and inflammatory mediators stimulate intestinal mucosal cells, and activate CFTR at the top of intestinal mucosal cells through G protein-coupled signaling pathways and phosphorylation, leading to a large amount of intracellular Cl– and water secretion, causing watery diarrhea (27). The activities of basolateral transport proteins NKCC1 are the rate-limiting steps of ion and fluid secretions in Cl-secreting epithelia (28, 29). For diarrhea-related ion channel genes, we found that L. reuteri LR1 reduced the expression of NKCC1 and CFTR in the intestine of piglets, which may be one of the reasons for its reduction of diarrhea. Taken together, L. reuteri LR1 showed better effects on reduced diarrhea of weaned pigs than L. plantarum CGMCC 1258.
The intestinal barrier plays an important role in resisting the invasion of intestinal bacteria and pathogenic allergens into the mucosa (30). When the intestinal mucosa is damaged, the increase in intestinal permeability leads to more DAO and LPS from the tissues into the peripheral blood circulation (31, 32). Pan et al. (33) have found that the addition of probiotics (mainly Bacillus licheniformis and Saccharomyces cerevisiae) could reduce the intestinal damage caused by the enterotoxigenic Escherichia coli K88 challenge through reducing the serum DAO content of weaned pigs (33). In this study, both L. plantarum CGMCC1258 and L. reuteri LR1 reduced the content of DAO and LPS in the serum in weaned pigs. The formation of villi and crypt in the intestine enlarged the surface area of the intestinal mucosa, and not only promoted the efficient absorption of nutrients but also generated a protected stem cell niche (10). We found that L. plantarum CGMCC1258 improved the intestinal morphology of the duodenum, while L. reuteri LR1 improved the intestinal morphology of the jejunum and ileum. This result showed that L. plantarum CGMCC1258 and L. reuteri LR1 enhanced the intestinal barrier function, and L. reuteri LR1 can better improve the intestinal morphology of weaned pigs.
The increased expression and secretion of pro-inflammatory factors IL-1β, IL-6, TNF-α, and IFN-γ are stimulated by weaning stress or pathogenic invasion (34, 35). The strain of L. plantarum CGMCC1258 and L. plantarum ACTT 8014 could effectively increase the protein levels of the natural cytotoxic receptor family of natural killer cells, and alleviates the pathological changes of intestinal tissues of animal intestinal inflammation models (36, 37). The L. plantarum 299v facilitates the gut health of suckling piglets by improved the intestinal morphology and intestinal barrier function and microflora (38). In this study, L. plantarum CGMCC1258 reduced the gene expression of IL-1β and the content of IL-1β and TNF-α in the intestinal mucosa, while L. reuteri LR1 reduced the gene expression of IL-1β, TNF-α, and IFN-γ and the content of IL-6 and TNF-α, and increased the TGF-β expression. Yi et al. (12) found that L. reuteri LR1 increased the content of TGF-β in the ileum and improve the intestinal immunity of weaned pigs (12). Collectively, these two strains of Lactobacillus have great differences in regulating the expression of IL-1β, IL-6, TNF-α, and TGF-β in the intestinal mucosa, and L. reuteri LR1 showed better anti-inflammatory ability than L. plantarum CGMCC 1258. The different effects of L. reuteri LR1 and L. plantarum CGMCC 1258 on intestinal immunity in weaned pigs may be related to the different hosts from which the strains originate, with L. reuteri LR1 from piglet feces readily attaching to the gastrointestinal tract and acting, while L. plantarum CGMCC 1258 from infants has lower effects because the piglet probably has not evolved to specifically recognize this strain.
The sIgA has been proven as the first line of defense in intestinal mucosa, effectively preventing the adhesion and penetration of pathogen in intestinal epithelial cells (39). The TLRs play an important role in recognizing bacterial signals and initiating intestinal immune responses. The TLR2 detects lipoprotein and peptidoglycans of gram-positive bacteria and gram-negative bacteria, and TLR4 can recognize LPS of gram-negative bacteria (40). The activation of TLR2 enhances the expression of antimicrobial peptides and tight junction proteins (41, 42). The L. plantarum 299v or L. plantarum CGMCC 1258 increased the expression of tight junction proteins, which was related to the expression of TLR2 in the pig intestine (8, 38). Another study showed that L. reuteri LR1 increased the expression of intestinal antimicrobial peptides, tight junction protein, and sIgA secretion, which was related to the increase of TLR2 and TLR4 expression (12). In the present study, L. plantarum CGMCC 1258 increased TLR4 levels only in ileum, and L. reuteri LR1 increased TLR2 and TLR4 levels in jejunum and ileum. The probiotic preparations containing L. plantarum CGMCC 1258 can significantly increase the sIgA content of the ileal mucosa of pigs 21 days after weaning, and also increased the sIgA content of jejunal mucosa (43). We found that dietary supplement L. plantarum CGMCC 1258 and L. reuteri LR1 increased the sIgA content in the jejunal mucosa of pigs, and L. reuteri LR1 can increase the sIgA content in the ileal mucosa. Collectively, these data suggest that L. plantarum CGMCC 1258 and L. reuteri LR1 may mediate TLRs-related pathways to regulate the secretion of sIgA and improved the intestinal immune response, but LR has a stronger influence.
Conclusions
In conclusion, dietary L. plantarum CGMCC 1258 supplementation at 5 × 1010 CFU/kg improved intestinal morphology, intestinal permeability, intestinal immunity, and antioxidant function in weaned pigs. However, dietary L. reuteri LR1 supplementation at 5 × 1010 CFU/kg showed higher improvements in growth performance, incidence of diarrhea, intestinal morphology, and a higher extent of immune activation in weaned pigs.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
These experiments were conducted in accordance with Chinese guidelines for animal welfare and experimental protocols, and all animal procedures were approved by the Animal Care and Use Committee of Guangdong Academy of Agricultural Sciences (Permit Number: GAASIAS-2015-012).
Author Contributions
QT, HY, and LW: conceptualization and investigation. QW and XY: methodology. SH, WH, and YX: data curation, formal analysis, and software. HY, QW, and YX: validation and visualization. QT and HY: writing—original draft preparation. LW and ZJ: writing—review and editing, funding acquisition, project administration, and resources. All authors have read and agreed to the published version of the article.
Funding
This study was funded by the National Key Research and Development Program of China (2018YFD0501101), China Agriculture Research System of MOF and MARA, the Science and Technology Program of Guangdong Academy of Agricultural Sciences (R2020PY-JG009), and Special fund for scientific innovation strategy-construction of high-level Academy of Agriculture Science (R2016YJ-YB2003, R2019PY-QF005, R2018QD-068).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the staff and postgraduate students of Institute of Animal Science of Guangdong Academy of Agricultural Sciences for providing technical assistance.
References
1. Che L, Zhan L, Fang Z, Lin Y, Yan T, Wu D. Effects of dietary protein sources on growth performance and immune response of weanling pigs. Livest Sci. (2012) 148:1–9. doi: 10.1016/j.livsci.2012.04.019
2. Pu J, Chen D, Tian G, He J, Zheng P, Mao X, et al. Effects of benzoic acid, bacillus coagulans and oregano oil combined supplementation on growth performance, immune status and intestinal barrier integrity of weaned piglets. Anim Nutr. (2020) 6:152–9. doi: 10.1016/j.aninu.2020.02.004
3. Hu S, Wang L, Jiang Z. Dietary additive probiotics modulation of the intestinal microbiota. Protein Peptide Lett. (2017) 24:382–7. doi: 10.2174/0929866524666170223143615
4. Shin D, Chang SY, Bogere P, Won K, Choi J, Choi Y, et al. Beneficial roles of probiotics on the modulation of gut microbiota and immune response in pigs. PLoS ONE. (2019) 14:e220843. doi: 10.1371/journal.pone.0220843
5. Hou C, Zeng X, Yang F, Liu H, Qiao S. Study and use of the probiotic Lactobacillus reuteri in pigs: a review. J Anim Sci Biotechnol. (2015) 6:14. doi: 10.1186/s40104-015-0014-3
6. Zhao W, Peng C, Sakandar HA, Kwok L, Zhang W. Meta-analysis: randomized trials of Lactobacillus plantarum on immune regulation over the last decades. Front Immunol. (2021) 12:643420. doi: 10.3389/fimmu.2021.643420
7. Lee JS, Awji EG, Lee SJ, Tassew DD, Park YB, Park KS, et al. Effect of Lactobacillus plantarum CJLP243 on the growth performance and cytokine response of weaning pigs challenged with enterotoxigenic Escherichia coli. J Anim. Sci. (2012) 90:3709–17. doi: 10.2527/jas.2011-4434
8. Yang KM, Jiang ZY, Zheng CT, Wang L, Yang XF. Effect of Lactobacillus plantarum on diarrhea and intestinal barrier function of young piglets challenged with enterotoxigenic Escherichia coli K88. J Anim Sci. (2014) 92:1496–503. doi: 10.2527/jas.2013-6619
9. Suo C, Yin Y, Wang X, Lou X, Song D, Wang X, et al. Effects of Lactobacillus plantarum ZJ316 on pig growth and pork quality. BMC Vet Res. (2012) 8:89. doi: 10.1186/1746-6148-8-89
10. Wang M, Wu H, Lu L, Jiang L, Yu Q. Lactobacillus reuteri promotes intestinal development and regulates mucosal immune function in newborn piglets. Front Vet Sci. (2020) 7:42. doi: 10.3389/fvets.2020.00042
11. Wang Z, Wang L, Chen Z, Ma X, Yang X, Zhang J, et al. In vitro evaluation of swine-derived Lactobacillus reuteri: probiotic properties and effects on intestinal porcine epithelial cells challenged with enterotoxigenic Escherichia coli K88. J Microbiol Biotechn. (2016) 26:1018–25. doi: 10.4014/jmb.1510.10089
12. Yi H, Wang L, Xiong Y, Wen X, Wang Z, Yang X, et al. Effects of Lactobacillus reuteri LR1 on the growth performance, intestinal morphology, and intestinal barrier function in weaned pigs. J Anim Sci. (2018) 96:2342–51. doi: 10.1093/jas/sky129
13. Yi H, Yang G, Xiong Y, Wu Q, Xiao H, Wen X, et al. Integrated metabolomic and proteomics profiling reveals the promotion of Lactobacillus reuteri LR1 on amino acid metabolism in the gut-liver axis of weaned pigs. Food Funct. (2019) 1:7387–96. doi: 10.1039/C9FO01781J
14. Xia Y, Chen H, Zhang M, Jiang Y, Hang X, Qin H. Effect of Lactobacillus plantarum LP-Onlly on gut flora and colitis in interleukin-10 knockout mice. J Gastroen Hepatol. (2011) 26:405–11. doi: 10.1111/j.1440-1746.2010.06498.x
15. NRC. Nutrient Requirements of Swine. 11th revised ed. Washington, DC: The National Academies Press (2012).
16. Atkins HL, Geier MS, Prisciandaro LD, Pattanaik AK, Forder REA, Turner MS, et al. Effects of a Lactobacillus reuteri BR11 mutant deficient in the cystine-transport system in a rat model of inflammatory bowel disease. Digest Dis Sci. (2012) 57:713–9. doi: 10.1007/s10620-011-1943-0
17. Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Anal Biochem. (2017) 524:13–30. doi: 10.1016/j.ab.2016.10.021
18. Wang M, Lei M, Samina N, Chen L, Liu C, Yin T, et al. Impact of Lactobacillus plantarum 423 fermentation on the antioxidant activity and flavor properties of rice bran and wheat bran. Food Chem. (2020) 330:127156. doi: 10.1016/j.foodchem.2020.127156
19. Yang S, Lee J, Lim S, Kim Y, Lee N, Paik H. Antioxidant and immune-enhancing effects of probiotic Lactobacillus plantarum 200655 isolated from kimchi. Food Sci Biotechnol. (2019) 28:491–9. doi: 10.1007/s10068-018-0473-3
20. Izuddin WI, Humam AM, Loh TC, Foo HL, Samsudin AA. Dietary postbiotic Lactobacillus plantarum improves serum and ruminal antioxidant activity and upregulates hepatic antioxidant enzymes and ruminal barrier function in post-weaning lambs. Antioxidants. (2020) 9:250. doi: 10.3390/antiox9030250
21. DÜz M, DoGan YN, DoGan I. Antioxidant activity of Lactobacillus plantarum, Lactobacillus sake and Lactobacillus curvatus strains isolated from fermented Turkish Sucuk. An Acad Bras Cienc. (2020) 92:e20200105. doi: 10.1590/0001-3765202020200105
22. Amaretti A, di Nunzio M, Pompei A, Raimondi S, Rossi M, Bordoni A. Antioxidant properties of potentially probiotic bacteria: in vitro and in vivo activities. Appl Microbiol Biot. (2013) 97:809–17. doi: 10.1007/s00253-012-4241-7
23. Wang J, Ji HF, Wang SX, Zhang DY, Liu H, Shan DC, et al. Lactobacillus plantarum ZLP001: in vitro assessment of antioxidant capacity and effect on growth performance and antioxidant status in weaning piglets. Asian Austral J Anim. (2012) 25:1153–8. doi: 10.5713/ajas.2012.12079
24. Yang J, Wang C, Liu L, Zhang M. Lactobacillus reuteri KT260178 supplementation reduced morbidity of piglets through its targeted colonization, improvement of cecal microbiota profile, and immune functions. Probiotics Antimicrob Proteins. (2020) 12:194–203. doi: 10.1007/s12602-019-9514-3
25. Tian Z, Cui Y, Lu H, Ma X. Effects of long-term feeding diets supplemented with Lactobacillus reuteri LR1 on growth performance, digestive and absorptive function of the small intestine in pigs. J Funct Foods. (2020) 71:104010. doi: 10.1016/j.jff.2020.104010
26. Suez J, Zmora N, Segal E, Elinav E. The pros, cons, and many unknowns of probiotics. Nat Med. (2019) 25:716–29. doi: 10.1038/s41591-019-0439-x
27. Viswanathan VK, Hodges K, Hecht G. Enteric infection meets intestinal function: how bacterial pathogens cause diarrhoea. Nat Rev Microbiol. (2009) 7:110–9. doi: 10.1038/nrmicro2053
28. Hoque KM, Chakraborty S, Sheikh IA, Woodward OM. New advances in the pathophysiology of intestinal ion transport and barrier function in diarrhea and the impact on therapy. Expert Rev Anti-Infe. (2012) 10:687–99. doi: 10.1586/eri.12.47
29. Reynolds A, Parris A, Evans LA, Lindqvist S, Sharp P, Lewis M, et al. Dynamic and differential regulation of NKCC1 by calcium and cAMP in the native human colonic epithelium. J Physiol. (2007) 582:507–24. doi: 10.1113/jphysiol.2007.129718
30. Camilleri M, Madsen K, Spiller R, Van Meerveld BG, Verne GN. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil. (2012) 24:503–12. doi: 10.1111/j.1365-2982.2012.01921.x
31. Fukudome I, Kobayashi M, Dabanaka K, Maeda H, Okamoto K, Okabayashi T, et al. Diamine oxidase as a marker of intestinal mucosal injury and the effect of soluble dietary fiber on gastrointestinal tract toxicity after intravenous 5-fluorouracil treatment in rats. Med Mol Morphol. (2014) 47:100–7. doi: 10.1007/s00795-013-0055-7
32. Nielsen C, Lindholt JS, Erlandsen EJ, Mortensen FV. D-lactate as a marker of venous-induced intestinal ischemia: an experimental study in pigs. Int J Surg. (2011) 9:428–32. doi: 10.1016/j.ijsu.2011.04.004
33. Pan L, Zhao PF, Ma XK, Shang QH, Xu YT, Long SF, et al. Probiotic supplementation protects weaned pigs against enterotoxigenic Escherichia coli K88 challenge and improves performance similar to antibiotics. J Anim Sci. (2017) 95:2627–39. doi: 10.2527/jas.2016.1243
34. Yang F, Wang A, Zeng X, Hou C, Liu H, Qiao S. Lactobacillus reuteri I5007 modulates tight junction protein expression in IPEC-J2 cells with LPS stimulation and in newborn piglets under normal conditions. BMC Microbiol. (2015) 15:32. doi: 10.1186/s12866-015-0372-1
35. Zanello G, Berri M, Dupont J, Sizaret P, D'Inca R, Salmon H, et al. Saccharomyces cerevisiae modulates immune gene expressions and inhibits ETEC-mediated ERK1/2 and p38 signaling pathways in intestinal epithelial cells. PLoS ONE. (2011) 6:e18573. doi: 10.1371/journal.pone.0018573
36. Qiu Y, Jiang Z, Hu S, Wang L, Ma X, Yang X. Lactobacillus plantarum enhanced il-22 production in natural killer (nk) cells that protect the integrity of intestinal epithelial cell barrier damaged by enterotoxigenic Escherichia coli. Int J Mol Sci. (2017) 18:2409. doi: 10.3390/ijms18112409
37. Ciobanu L, Tefas C, Oancea DM, Berce C, Vodnar D, Mester A, et al. Effect of Lactobacillus plantarum ACTT 8014 on 5-fluorouracil induced intestinal mucositis in Wistar rats. Exp Ther Med. (2020) 20:209. doi: 10.3892/etm.2020.9339
38. Wang Q, Sun Q, Qi R, Wang J, Qiu X, Liu Z, et al. Effects of Lactobacillus plantarum on the intestinal morphology, intestinal barrier function and microbiota composition of suckling piglets. J Anim Physiol An N. (2019) 103:1908–18. doi: 10.1111/jpn.13198
39. Russell MW, Kilian M. Biological activities of IgA. Mucosal Immunol. (2005) 14:267–89. doi: 10.1016/B978-012491543-5/50018-8
40. Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. (2011) 34:637–50. doi: 10.1016/j.immuni.2011.05.006
41. Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology. (2004) 127:224–38. doi: 10.1053/j.gastro.2004.04.015
42. Lai Y, Cogen AL, Radek KA, Park HJ, Macleod DT, Leichtle A, et al. Activation of TLR2 by a small molecule produced by Staphylococcus epidermidis increases antimicrobial defense against bacterial skin infections. J Invest Dermatol. (2010) 130:2211–21. doi: 10.1038/jid.2010.123
Keywords: Lactobacillus plantarum, Lactobacillus reuteri, antioxidant function, intestinal immunity, weaned pigs
Citation: Tang Q, Yi H, Hong W, Wu Q, Yang X, Hu S, Xiong Y, Wang L and Jiang Z (2021) Comparative Effects of L. plantarum CGMCC 1258 and L. reuteri LR1 on Growth Performance, Antioxidant Function, and Intestinal Immunity in Weaned Pigs. Front. Vet. Sci. 8:728849. doi: 10.3389/fvets.2021.728849
Received: 22 June 2021; Accepted: 12 October 2021;
Published: 11 November 2021.
Edited by:
Rita Payan Carreira, University of Evora, PortugalReviewed by:
Richard Faris, Cargill, United StatesSusana María Martín-Orúe, Universitat Autònoma de Barcelona, Spain
Copyright © 2021 Tang, Yi, Hong, Wu, Yang, Hu, Xiong, Wang and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Wang, wangli1@gdaas.cn; Zongyong Jiang, jiangzy@gdaas.cn
†These authors have contributed equally to this work
 Qingsong Tang
Qingsong Tang Hongbo Yi1†
Hongbo Yi1†  Shenglan Hu
Shenglan Hu Yunxia Xiong
Yunxia Xiong