Organic Selenium Ameliorates Staphylococcus aureus-Induced Mastitis in Rats by Inhibiting the Activation of NF-κB and MAPK Signaling Pathways
- 1College of Veterinary Medicine, Yangzhou University, Yangzhou, China
- 2Jiangsu Co-innovation Center for Prevention and Control of Important Animal Infectious Diseases and Zoonoses, Yangzhou, China
Mastitis is an economically important disease in dairy cows, which is often caused by Staphylococcus aureus (S. aureus). Selenium is an indispensable element for physiological function and contributes to reduce injury of the mammary glands in mastitis. However, adequate sources of selenium have always been an important consideration for livestock. Therefore, the study aimed to explore the protective effect and mechanism of Selenohomolanthionine (SeHLan) on mastitis induced by S. aureus. The S. aureus-induced rat model was established and three doses (0.2, 2, 20 μg/kg body weight/day) of dietary OS were supplemented. The bacterial load, histopathology, and myeloperoxidase (MPO) of the mammary glands were performed and determined. Cytokines, including interleukin (IL)-1β, TNF-α, and IL-6, were detected using qRT-PCR. The key proteins of NF-κB and MAPK signaling pathways were analyzed by Western blot. The results revealed that OS supplementation could reduce the recruitment of neutrophils and macrophages in mammary tissues, but did not decrease S. aureus load in the tissues. The overexpression levels of IL-1β, TNF-α, and IL-6 induced by S. aureus were inhibited after OS treatment. Furthermore, the increased phosphorylation of NF-κB and MAPKs proteins were also suppressed. The results suggest that dietary supplementation with adequate OS during pregnancy contributes to protect the mammary glands from injury caused by S. aureus and alleviate the inflammatory response.
Introduction
Mastitis, inflammation of the mammary glands, remains a significant health problem in both human beings and animals (1, 2). In cattle, mastitis is recognized as one of the most costly inflammatory diseases, which compromises the cow welfare and food safety (3). Bacterial infection is the main etiology in bovine mastitis and a wide variety of pathogens have been reported to cause the disease (4). S. aureus is one of the most prevailing pathogenic bacteria, which causes clinical and subclinical mastitis in dairy herds (5, 6). It was characterized by chronic, recurrent and lower cure rate in mastitis induced by S. aureus (7, 8). Although vaccines have been used to prevent mastitis caused by S. aureus, bovine mastitis mainly depends on antibiotic treatment. However, neither is effective in preventing S. aureus mastitis (9). Furthermore, ineffective treatment can also induce antimicrobial resistance in bacteria and drug residue in the products (10). So, it is necessary to develop alternative or supportive approaches to reduce the antibiotic usage in dairy cows.
There were extensive investigations in the defense mechanisms of the mammary glands. The successful triggering of pattern recognition receptors (PRRs) in both immune and non-immune cells by their ligands activates the downstream of nuclear transcription factor-kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways, which initiate the inflammatory cascade by promoting the production of cytokines (11). Cytokines facilitate the recruitment of leukocytes at the infectious sites, which play an essential role in the early stage of S. aureus infection (12). Neutrophils are the main defensive cells during the early stage of the infection (13). The process of mastitis can be influenced not only by bacterial virulence factors but also by host-related factors, including the nutrition conditions from involution to lactation (12).
Selenium is necessary to maintain excellent reproductive performances in dairy cattle due to its beneficial biochemical and pharmacological properties (14, 15). And Se supplementation showed protective effects on the mammary gland health, which decreased the somatic cells count and incidence rate of the mastitis (16). SeHLan is a new selenoamino acid discovered in selenized Japanese pungent radish (17). Organic selenium supplementations, also including selenomethionine (SeMet), are mainly derived from Se-rich yeast Saccharomyces cerevisiae. However, SeHLan is obtained from Candida utilis (18). The metabolic pathway of SeHLan is simpler than SeMet. There are three metabolic pathways of SeMet, including trans-selenation for selenoprotein synthesis, replacing methionine for peptide synthesis, and methylation to form selenide or dimethylselenide. In contrast, SeHLan is only involved in the trans-selenation pathway, so SeHLan is affecting the metabolic pathway of methionine less and is more effective for production of selenoprotein (19–21). A previous study reported that SeHLan showed different tissue accumulation from SeMet in rats. SeMet was more easily distributed in the pancreas and caused pancreas damage, whereas SeHLan was preferably distributed in kidney and caused kidney damage. But the kidney damage can be avoided when SeHLan was administered at a lower dose than 1.0 mg Se/Kg body weight (19). It has been reported that SeHLan increased the survival rate of septic mice from 0.1 to 0.5 within 72 h after intraperitoneal injection of LPS, which was more effective than SeMet and selenite (22). So SeHLan is expected to be a potential Se source for livestock. As a new Se source, SeHLan has been given in dairy cows. However, the protective effects of SeHLan on the mammary glands during mastitis are unclear. So, the study was conducted to investigate what to the functions SeHLan played during mastitis induced by S. aureus and explore the potential mechanism using a rat mastitis model.
Materials and Methods
Materials
Organic selenium (purity ≥ 98%) was supplied by ABNA Trading Co., Ltd. (Shanghai, China). The organic selenium was derived from Candida utilis with analysis of 4,000 mg/kg of total Se and the main form of organic selenium is SeHLan (not <75%). The primary antibodies of IκB-α (#4812), p-IκB-α (#2859), p65 (#8242), p-p65 (#3033), JNK (#9258), p-JNK (#4668), ERK (#4695), p-ERK (#4370), p38 (#8690), p-p38 (#4511), β-actin (#4970) were provided by Cell Signaling Technology company (Boston, MA, USA). The myeloperoxidase assay kits were obtained from Nanjing Jiancheng Technology Co., Ltd. (Nanjing, China).
Animals and Experimental Groups
Thirty pregnant Wistar rats of 8–10 weeks old (weight: 180–220 g) were obtained from the Laboratory Animal Center of Yangzhou University (Yangzhou, China). They were raised in standard plastic cages under a 12 h light/dark cycle. The room temperature was kept at 23 ± 2°C with a relative humidity of 55 ± 5%. A basal diet and distilled water were provided ad libitum. The rats were randomly divided into 5 groups (n = 6) as following: (a) control group, (b) S. aureus group, (c–e) S. aureus + OS-supplemented groups (0.2, 2, 20 μg/kg body weight/day, dissolved in distilled water). From the 1st day of pregnancy, rats in the groups(c-e) were administrated with OS orally until the end of the experiment. On the 4th day after parturition, the rats were anesthetized with isoflurane. L4 and R4 mammary glands respectively in the groups (b–e) were infused with 100 μL S. aureus suspension (2 × 107 CFU/ mL) via the teat duct. The rats in the control group were infused with equal volumes of saline. Finally, all the rats were anesthetized with isoflurane at 12 h post infection. Part of the mammary tissue samples were collected aseptically for bacterial load. Some mammary tissue samples were prepared for histological studies. Other mammary tissues were immediately stored at −80°C.
Determination of the Bacterial Load in the Mammary Glands
The S. aureus load in the mammary glands were detected as described previously (23). Briefly, the mammary tissues were homogenized in 1 mL phosphate buffered saline using a tissue grinder. The gland homogenates with 10-fold serial dilution method were plated on LB agar plates, incubated at 37°C. Then S. aureus colonies were counted.
Histopathological Analysis
The mammary tissues were trimmed and fixed in 10% formalin. Then the tissues were embedded with paraffin wax and dehydrated in the graded alcohols. The sections were obtained and stained with hematoxylin and eosin (H&E). Histopathological changes were discovered and pictures were taken under a light microscope (Olympus, Japan).
Determination of Myeloperoxidase Activity
Myeloperoxidase (MPO) activity in the mammary tissues were detected using the commercial kits according to the manufacturer's instructions. Briefly, the mammary gland tissues were homogenized with the extraction buffer (1:19, w/v). Then the homogenate was mixed with reaction buffer (9:1, v/v), and cultured at 37°C for 15 min. The enzymatic activity was calculated according to the absorbance at 460 nm.
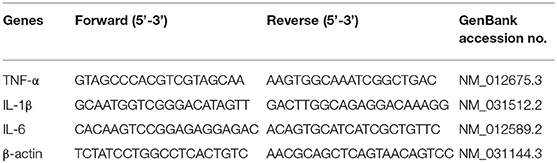
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
Trizol reagent was used to extract total RNA from the mammary tissues (Vazyme Biotech Co., Ltd, China). The concentration and purity of RNA were measured using NanoDrop 2000 spectrophotometer (Thermo Scientific, USA) as described previously (24). The RNA samples of A260/280 ratio ranging from 1.90 to 2.0 were selected for cDNA synthesis using HiScript® QRT SuperMix (Vazyme Biotech Co., Ltd, China). The specific primers used in this study were listed in Table 1, according to literature reports (25). The qRT-PCR was conducted using the CFX ConnectTM Real-Time System (Bio-Rad Instruments, Hercules, CA, USA) and ChanQTM SYBR® Qpcr Master Mix in a 20 μL reaction systems according to the recommended conditions (Vazyme Biotech Co., Ltd, China). The results were analyzed using the 2−ΔΔCt method as previous description (26).
Western Blot Analysis
The mammary gland tissues were homogenized with the RIPA lysis buffer containing protease and phosphatase inhibitors, and then centrifuged at 12,000 r/min for 15 min at 4°C. The protein concentration was determined using a BCA protein assay kit (Beyotime Biotechnology Co. Ltd, Shanghai, China). Equal amounts of protein were separated in 10% SDS-polyacrylamide gels and transferred to polyvinylidene difluoride membrane (Millipore, Germany). Subsequently, Tris-buffered saline containing 0.05% Tween 20 (TBST) and 5% skimmed milk were used to block the membranes at room temperature for 1 h. The membranes were incubated with primary antibodies against p65, p-p65, IκB-α, p-IκB-α, JNK, p-JNK, ERK, p-ERK, p38, p-p38, and β-actin (all at 1:1000 dilution in 5%BSA) at 4°C overnight, followed by incubation with secondary antibodies, conjugated with horseradish peroxidase, at room temperature for 1 h. Finally, the blots were visualized using enhanced chemiluminescence (ECL) assay according to the manufacturer's instructions. Bands were scanned and analyzed using Image J. β-actin antibody was used to prove equal loading of blots.
Statistical Analysis
Data were expressed as the mean ± standard error of the mean (SEM) from at least three independent experiments. The obtained data were analyzed by SPSS Statistics 22.0 software (IBM, USA). Statistical significance was analyzed using one-way factorial analysis of variance (ANOVA) and evaluated using Tukey's multiple-comparisons test. P < 0.05 was considered statistically significant.
Results
Effects of OS on Bacterial Load of the Mammary Glands
In order to verify whether OS contributes to the mammary gland against S. aureus infection, S. aureus load of the mammary glands were detected at 12 h after infection. No bacterial colonization was observed in the control group. Compared with the S. aureus group, low-dose OS supplementation reduced S. aureus load in the mammary glands slightly, but there was no significant difference. In middle- and high-dose OS-supplemented groups, there were no significant decrease in S. aureus load of the mammary glands compared to S. aureus group (Figure 1).
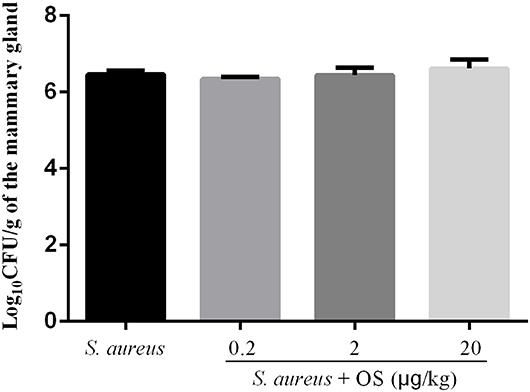
Figure 1. S. aureus load in the mammary glands. Values are presented as the mean Log10CFU/g of the mammary gland ± SEM (n = 5).
Histopathological Changes
The histopathological changes of the mammary tissues were evaluated as shown in Figure 2. There were no histopathological changes in the control group (Figure 2A). However, obvious inflammatory changes were observed in mammary tissues of S. aureus group, which were characterized by neutrophils and macrophages infiltrating in the alveoli lumens, ducts, perivascular, and connective tissues (Figure 2B). While, OS reduced the infiltration of immune cells and alleviated damage of the mammary glands (Figures 2C–E).
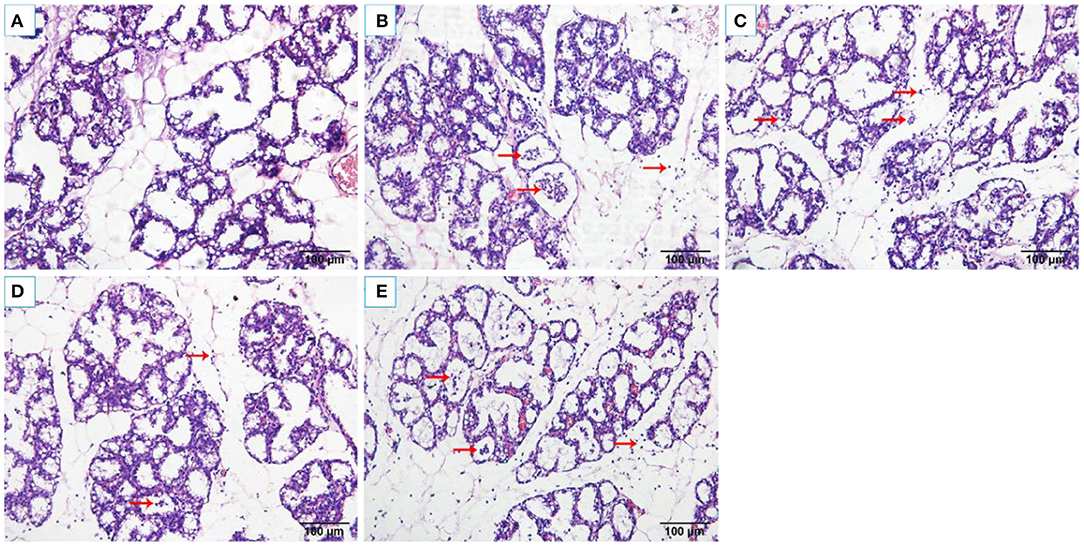
Figure 2. Histological changes of the rat mammary tissues (H&E 200×). (A) control group. (B) S. aureus group. (C–E) S. aureus + OS-supplemented groups (0.2, 2, 20 μg/kg body weight/day, respectively). The red arrows indicate the inflammatory cells infiltration and some debris of the mammary epithelial cells in mammary tissues.
MPO Activity
MPO activity was used as a biomarker for the infiltration of neutrophils and macrophages, which plays important roles in evaluating the development of mastitis (27). The activity of MPO was detected in mammary tissues (Figure 3). Compared with the control group, the activity of MPO increased significantly after S. aureus infection (p < 0.01). OS supplementation effectively depressed the activity of MPO induced by S. aureus (p < 0.05). There was no significant difference between different OS-supplemented groups, although 2 μg/kg OS treatment decreased the most in the MPO activity.
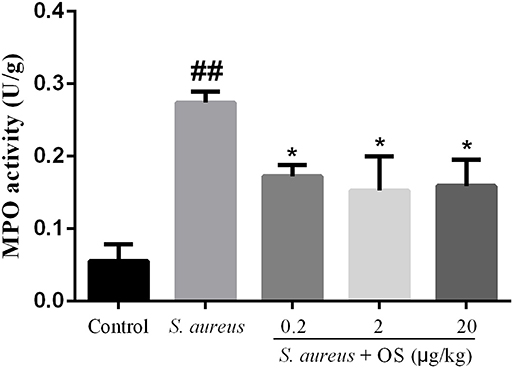
Figure 3. MPO activity in mammary tissues. Data are mean ± SEM (n = 5), ##p < 0.01 vs. control. *p < 0.05 vs. S. aureus.
Inflammatory Cytokines mRNA Expression Analysis
The effects of OS on the mRNA expression levels of TNF-α, IL-1β, and IL-6 induced by S. aureus were measured by qRT-PCR. As shown in Figure 4, the mRNA expression levels of TNF-α, IL-1β, and IL-6 in the S. aureus group increased significantly, relative to the control group (p < 0.01). Compared to the S. aureus group, OS significantly inhibited the mRNA expression levels of TNF-α, IL-1β, and IL-6 after S. aureus infection (p < 0.01). Similarly, the mRNA expression levels decreased most in the 2 μg/kg OS treatment group, but there was no significant difference relative to the other two OS-supplemented groups.
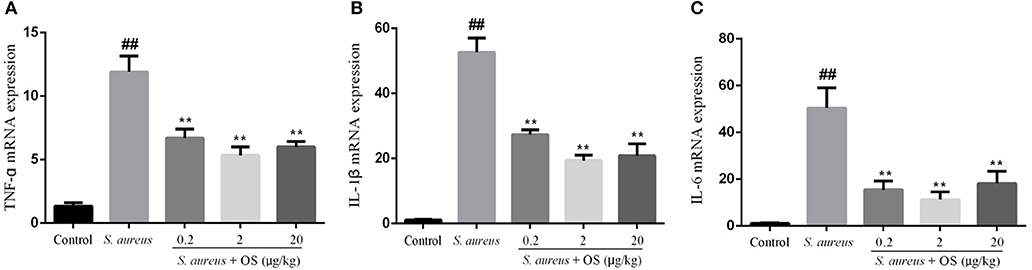
Figure 4. The effects of OS on the mRNA expression levels of TNF-α (A), IL-1β (B), and IL-6 (C). Data are mean ± SEM, ##p < 0.01 vs. control. **p < 0.01 vs. S. aureus.
OS Inhibits NF-κB Signaling Pathway Activation
To further determine the anti-inflammatory mechanism of OS, we evaluated the effects of OS on the phosphorylation levels of p65 and IκB-α proteins with western blot analysis. Phosphorylation of p65 and IκB-α proteins increased markedly after S. aureus infection, compared with the control group (p < 0.01). The phosphorylation of p65 protein was inhibited by OS supplementation with different concentrations (p < 0.01) when the mammary glands were infected by S. aureus (Figure 5). The middle-dose OS decreased the most in the phosphorylation of p65 protein, but no significant difference was observed compared to the low- and high-dose OS treatment. The phosphorylation level of IκB-α protein decreased significantly with the increase of OS concentration (p < 0.05, p < 0.01), compared with the S. aureus group. Therefore, OS could regulate the inflammatory response to some extent by inhibiting NF-κB signaling pathway activation.
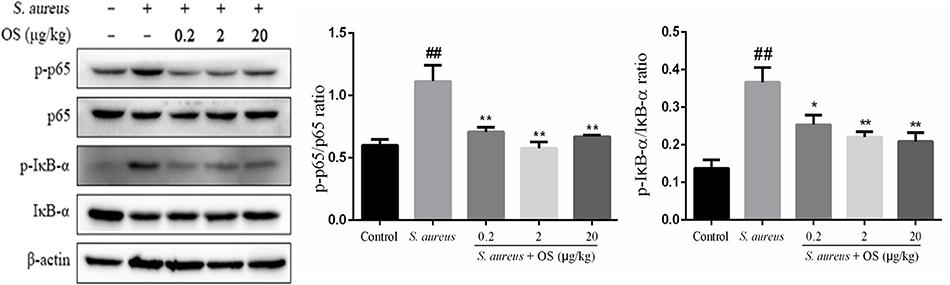
Figure 5. Effects of OS on S. aureus-induced NF-κB signaling pathway in the mammary glands. Western blot was used to detected the phosphorylation levels of p65 and IκB-α proteins. Data are represented as the mean ± SEM (n = 3). ##p < 0.01 vs. control. *p < 0.05 vs. S. aureus. **p < 0.01 vs. S. aureus.
OS Inhibits MAPK Signaling Pathway Activation
In this study, the phosphorylation of p38, JNK, and ERK proteins were significantly upregulated after S. aureus infection, compared to the control group (p < 0.01). However, OS could inhibit S. aureus-induced phosphorylation of p38 and JNK proteins in different OS-supplemented groups (p < 0.01). And the phosphorylation of ERK protein was suppressed in middle- and high-dose OS-supplemented groups (p < 0.01) (Figure 6).
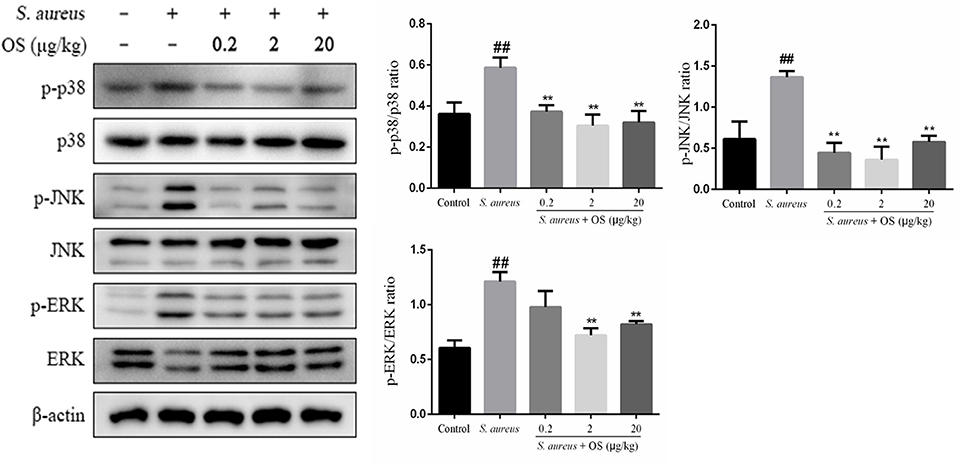
Figure 6. The effects of OS on MAPKs signaling pathway in the mammary glands. Western blot was used to assay the phosphorylation levels of p38, JNK, and ERK proteins. Data are mean ± SEM (n ≥ 3). ##p < 0.01 vs. control. **p < 0.01 vs. S. aureus.
Discussion
Mastitis is a common disease in dairy cattle negatively affecting economic return and animal health, which seriously hampers the development of dairy industries (28). S. aureus is one of the main pathogens involved in bovine intramammary infection (4). The infection not only affects the milk quality, but also poses a huge threat to food safety due to bacterial toxins and antibiotic residues (29, 30). Selenium was widely applied in dietary supplements for its antioxidative and anti-inflammatory effects (24, 31). In this study, the anti-inflammatory functions of OS were evaluated using a rat mastitis model induced by S. aureus.
It is essential to eliminate the pathogens to control the infection. It has been found that the bactericidal activity could be improved in cow milk whey with Se supplementation (32). It is unclear whether Se can reduce the bacterial load of the mammary glands after S. aureus infection. In this study, OS supplementation has no significant effect on reducing S. aureus load in the mammary glands. Histopathological examination showed that a large number of neutrophils and macrophages infiltrated mammary tissues after S. aureus infection, which were similar to previous studies (25, 33). But OS supplementation can reduce the infiltration of inflammatory cells and alleviate the tissue damage. The MPO activity was a typical marker of inflammatory cell infiltration (34). In line with histopathological observations, MPO activity increased significantly after S. aureus infection, but the increased MPO activity was suppressed in the OS supplemented groups. Therefore, OS supplementation alleviated the inflammatory response of mastitis induced by S. aureus, which may support the prevention and treatment of mastitis.
Inflammatory cytokines are essential for the initiation and development of mastitis (12). A previous study demonstrated that S. aureus was able to cause inflammatory responses by inducing the production of TNF-α, IL-1β, and IL-6 in the mammary glands of mice (24). In this study, the increased gene expression of TNF-α, IL-1β, and IL-6 were observed in the mammary glands of postpartum rats infected with S. aureus. TNF-α plays a key role in the initiation and orchestration of inflammation and immunity (32). In the earliest stage of inflammation, IL-1β acts as a trigger for a subsequent cascade of inflammatory cytokines (35). IL-6 increases quickly in acute inflammation, which is often associated with injury, infections, and other stress (36). The adequate production of cytokines is very important for immune response and host defense. However, persistent production of the inflammatory-related cytokines could result in swelling and rupture of the mammary cells, eventually causing tissue damage (37). The anti-inflammatory effects of Se compounds have been reported. Vunta et al. (38) reported that sodium selenite significantly decreased LPS-induced expression of TNF-α in bone marrow-derived macrophages. Ma et al. (24) found that Se-(methyl) selenocysteine hydrochloride had anti-inflammatory effects in S. aureus-induced mice mastitis via decreasing the gene expression levels of TNF-α, IL-1β, and increasing the gene expression level of IL-10. In our results, the overexpression of TNF-α, IL-1β, and IL-6 induced by S. aureus in the mammary glands of postpartum rats were downregulated after OS treatment across the given dose range. The results indicate that OS could alleviate the inflammatory response and the tissue damage by inhibiting the gene transcription of pro-inflammatory cytokines.
NF-κB is critical for the transcription of pro-inflammatory genes, which play a vital role in the amplification of inflammation (39, 40). The activation of NF-κB typically involves the degradation of IκB-α, followed by nuclear translocation of NF-κB p65 subunit (41). NF-κB dimers remain inactive by IκB proteins in the cytoplasm. When inflammation occurs, the IκB kinase triggers IκB-α ubiquitination and proteasome degradation via phosphorylation, which allows NF-κB p65 to accumulate in the nucleus to elicit the production of cytokines, such as TNF-α, IL-1β, and IL-6 (42). Previous studies have shown that S. aureus infection can increase the phosphorylation of IκB-α and p65 proteins, and activate NF-κB signaling pathway in lung and uterine tissues (43, 44). In this study, the phosphorylation of IκB-α and p65 proteins in mammary tissues were increased significantly after S. aureus infection, which indicated that NF-κB was involved in the inflammatory response. It has been demonstrated that SeMet was able to alleviate LPS-induced inflammatory response through inhibiting NF-κB activation in the chicken trachea (45). Wang et al. (46) observed Na2SeO3 suppressed S. aureus-induced NF-κB activation in bovine mammary epithelial cells. In this study, the increased phosphorylation of IκB-α and p65 proteins in the rat mammary glands infected with S. aureus were suppressed after OS supplementation. Combining with the inhibition of OS on inflammatory cytokines mRNA expression, our results indicated that the NF-κB signaling pathway was negatively regulated by OS in S. aureus-induced mastitis, at least partly, downregulation of the inflammatory genes expression and ultimately reducing the inflammatory response.
MAPK signaling pathway also plays a critical role in regulation of inflammatory response and the MAPKs p38, ERK, and JNK are the best-studied major MAP kinases (47). Upon pathogen challenge, p38 acts as a pivotal kinase involved in controlling the production of cytokines, such as TNFα, IL-1β, and IL-6 (48). JNK is also associated with regulating the expression and activation of inflammatory mediators (49). It has been revealed that ERK facilitates the development of inflammation-associated cancer by regulating the expression of inflammatory cytokines (50). Previous studies reported that the increased phosphorylation of the p38, ERK, and JNK proteins were involved in regulating the inflammatory response caused by S. aureus in mice mastitis (26) and pneumonia (51). In this study, the phosphorylation of p38, ERK, and JNK proteins were upregulated after S. aureus infection, which were similar to previous studies. It has been suggested that Se can reduce the inflammatory response caused by infection through regulating the MAPK signaling pathway. Liu et al. (52) reported that Se alleviated the inflammation induced by LPS via p38 MAPK in chicken myocardial. In RAW264.7 macrophages, MAPKs (p38, ERK, and JNK) took part in the inhibitory effects of Na2SeO3 on increased gene expression of cytokines induced by S. aureus (53). In this study, the elevated phosphorylation of MAPKs proteins induced by S. aureus were reversed by OS, indicating the involvement of MAPKs in OS-modulated anti-inflammatory effects in rat mastitis.
In conclusion, OS supplementation can suppress MPO activity and reduce the infiltration of inflammatory cells, thus protect mammary tissues from damage caused by excessive inflammatory response. The anti-inflammatory effects of OS on S. aureus-induced mastitis may be related to the process of suppressing the expression of inflammatory cytokine genes via inhibiting the activation of NF-κB and MAPK signaling pathways. The findings suggest that OS supplementation could be used as a potential treatment for mastitis.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
This study was approved by the Institutional Animal Care and Use Committee of Yangzhou University (No: YZUDWSY2017-0029).
Author Contributions
HW and JiL conceived and designed the study. KL, TD, LF, LC, and JuL performed the experiments. KL, LC, and XM analyzed the data. KL wrote the manuscript. HW, JiL, GZ, and CQ revised it critically for important content. All authors read and approved the final version of the manuscript.
Funding
This work was supported by the Earmarked fund for Jiangsu Agricultural Industry Technology System (No. JATS [2019]461), Natural Science Foundation of Jiangsu Province (No. BK20160062), Outstanding Young Backbone Teacher Foundation of Yangzhou University, Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and Top-notch Academic Programs Project of Jiangsu Higher Education Institutions (TAPP).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Marin M, Arroyo R, Espinosa-Martos I, Fernandez L, Rodriguez JM. Identification of emerging human mastitis pathogens by MALDI-TOF and assessment of their antibiotic resistance patterns. Front Microbiol. (2017) 8:1258–71. doi: 10.3389/fmicb.2017.01258
2. Xu T, Deng R, Li X, Zhang Y, Gao MQ. RNA-seq analysis of different inflammatory reactions induced by lipopolysaccharide and lipoteichoic acid in bovine mammary epithelial cells. Microb Pathog. (2019) 130:169–77. doi: 10.1016/j.micpath.2019.03.015
3. Fogsgaard KK, Bennedsgaard TW, Herskin MS. Behavioral changes in freestall-housed dairy cows with naturally occurring clinical mastitis. J Dairy Sci. (2015) 98:1730–38. doi: 10.3168/jds.2014-8347
4. Murphy MP, Niedziela DA, Leonard FC, Keane OM. The in vitro host cell immune response to bovine-adapted Staphylococcus aureus varies according to bacterial lineage. Sci Rep. (2019) 9:6134–47. doi: 10.1038/s41598-019-42424-2
5. Grispoldi L, Massetti L, Sechi P, Iulietto MF, Ceccarelli M, Karama M, et al. Short communication: characterization of enterotoxin-producing Staphylococcus aureus isolated from mastitic cows. J Dairy Sci. (2019) 102:1059–65. doi: 10.3168/jds.2018-15373
6. Yu GM, Tan W. Melatonin inhibits lipopolysaccharide-induced inflammation and oxidative stress in cultured mouse mammary tissue. Mediators Inflamm. (2019) 2019:1–10. doi: 10.1155/2019/8597159
7. Szweda P, Schielmann M, Frankowska A, Kot B, Zalewska M. Antibiotic resistance in Staphylococcus aureus strains isolated from cows with mastitis in eastern poland and analysis of susceptibility of resistant strains to alternative nonantibiotic agents: lysostaphin, nisin and polymyxin B. J Vet Med Sci. (2014) 76:355–62. doi: 10.1292/jvms.13-0177
8. Schmidt T, Kock MM, Ehlers MM. Diversity and antimicrobial susceptibility profiling of staphylococci isolated from bovine mastitis cases and close human contacts. J Dairy Sci. (2015) 98:6256–69. doi: 10.3168/jds.2015-9715
9. Furukawa M, Yoneyama H, Hata E, Iwano H, Higuchi H, Ando T, et al. Identification of a novel mechanism of action of bovine IgG antibodies specific for Staphylococcus aureus. Vet Res. (2018) 49:22–34. doi: 10.1186/s13567-018-0517-y
10. Krömker V, Leimbach S. Mastitis treatment-Reduction in antibiotic usage in dairy cows. Reprod Domest Anim. (2017) 52:21–9. doi: 10.1111/rda.13032
11. Oviedo-Boyso J, Bravo-Patino A, Baizabal-Aguirre VM. Collaborative action of Toll-like and NOD-like receptors as modulators of the inflammatory response to pathogenic bacteria. Mediators Inflamm. (2014) 2014:1–16. doi: 10.1155/2014/432785
12. Aitken SL, Corl CM, Sordillo LM. Immunopathology of mastitis: insights into disease recognition and resolution. J Mammary Gland Biol. (2011) 16:291–304. doi: 10.1007/s10911-011-9230-4
13. Lahouassa H, Moussay E, Rainard P, Riollet C. Differential cytokine and chemokine responses of bovine mammary epithelial cells to Staphylococcus aureus and Escherichia coli. Cytokine. (2007) 38:12–21. doi: 10.1016/j.cyto.2007.04.006
14. Hosnedlova B, Kepinska M, Skalickova S, Fernandez C, Ruttkay-Nedecky B, Malevu TD, et al. A summary of new findings on the biological effects of selenium in selected animal species-a critical review. Int J Mol Sci. (2017) 18:2209–56. doi: 10.3390/ijms18102209
15. Salman S, Khol-Parisini A, Schafft H, Lahrssen-Wiederholt M, Hulan HW, Dinse D, et al. The role of dietary selenium in bovine mammary gland health and immune function. Anim Health Res Rev. (2009) 10:21–34. doi: 10.1017/S1466252308001588
16. Misra N, Wines TF, Knopp CL, Hermann R, Bond L, Mitchell B, et al. Immunogenicity of a Staphylococcus aureus-cholera toxin A2/B vaccine for bovine mastitis. Vaccine. (2018) 36:3513–21. doi: 10.1016/j.vaccine.2018.04.067
17. Ogra Y, Kitaguchi T, Ishiwata K, Suzuki N, Iwashita Y, Suzuki KT. Identification of selenohomolanthionine in selenium-enriched Japanese pungent radish. J Anal Atom Spectr. (2007) 22:1390–6. doi: 10.1039/b707348h
18. Bierla K, Suzuki N, Ogra Y, Szpunar J, Lobinski R. Identification and determination of selenohomolanthionine - the major selenium compound in torula yeast. Food Chem. (2017) 237:1196–201. doi: 10.1016/j.foodchem.2017.06.042
19. Tsuji Y, Mikami T, Anan Y, Ogra Y. Comparison of selenohomolanthionine and selenomethionine in terms of selenium distribution and toxicity in rats by bolus administration. Metallomics. (2010) 2:412–9. doi: 10.1039/c004026f
20. Ogra Y, Kitaguchi T, Ishiwata K, Suzuki N, Toida T, Suzuki KT. Speciation of selenomethionine metabolites in wheat germ extract. Metallomics. (2009) 1:78–86. doi: 10.1039/b813118j
21. Ohta Y, Suzuki KT. Methylation and demethylation of intermediates selenide and methylselenol in the metabolism of selenium. Toxicol Appl Pharm. (2008) 226:169–77. doi: 10.1016/j.taap.2007.09.011
22. Anan Y, Ogra Y. Toxicological and pharmacological analysis of selenohomolanthionine in mice. Toxicol Res. (2013) 2:115–22. doi: 10.1039/c2tx20050c
23. Pereyra EAL, Sacco SC, Dure A, Baravalle C, Renna MS, Andreotti CS, et al. Immune response of Staphylococcus aureus strains in a mouse mastitis model is linked to adaptive capacity and genotypic profiles. Vet Microbiol. (2017) 204:64–76. doi: 10.1016/j.vetmic.2017.04.009
24. Ma J, Zhu S, Guo Y, Hao M, Chen Y, Wang Y, et al. Selenium attenuates Staphylococcus aureus mastitis in mice by inhibiting the activation of the NALP3 inflammasome and NF-κB/MAPK pathway. Biol Trace Elem Res. (2018) 191:159–66. doi: 10.1007/s12011-018-1591-8
25. Wang H, Yu G, Yu H, Gu M, Zhang J, Meng X, et al. Characterization of TLR2, NOD2, and related cytokines in mammary glands infected by Staphylococcus aureus in a rat model. Acta Vet Scand. (2015) 57:25–31. doi: 10.1186/s13028-015-0116-0
26. Zhang ZB, Guo YF, Li CY, Qiu CW, Guo MY. Selenium influences mmu-miR-155 to inhibit inflammation in Staphylococcus aureus-induced mastitis in mice. Food Funct. (2019) 10:6543–55. doi: 10.1039/c9fo01488h
27. Depreester E, Meyer E, Demeyere K, Van Eetvelde M, Hostens M, Opsomer G. Flow cytometric assessment of myeloperoxidase in bovine blood neutrophils and monocytes. J Dairy Sci. (2017) 100:7638–47. doi: 10.3168/jds.2016-12186
28. Wang D, Zhang L, Zhou X, He Y, Yong C, Shen M, et al. Antimicrobial susceptibility, virulence genes, and randomly amplified polymorphic DNA analysis of Staphylococcus aureus recovered from bovine mastitis in Ningxia, China. J Dairy Sci. (2016) 99:9560–69. doi: 10.3168/jds.2016-11625
29. Schmidt T, Kock MM, Ehlers MM. Molecular characterization of Staphylococcus aureus isolated from bovine mastitis and close human contacts in south african dairy herds: genetic diversity and inter-species host transmission. Front Microbiol. (2017) 8:511–29. doi: 10.3389/fmicb.2017.00511
30. Asli A, Brouillette E, Ster C, Ghinet MG, Brzezinski R, Lacasse P, et al. Antibiofilm and antibacterial effects of specific chitosan molecules on Staphylococcus aureus isolates associated with bovine mastitis. PLoS ONE. (2017) 12:6988–7011. doi: 10.1371/journal.pone.0176988
31. Zhang W, Zhang R, Wang T, Jiang H, Guo M, Zhou E, et al. Selenium inhibits LPS-induced pro-inflammatory gene expression by modulating MAPK and NF-κB signaling pathways in mouse mammary epithelial cells in primary culture. Inflammation. (2014) 37:478–85. doi: 10.1007/s10753-013-9761-5
32. Fischer R, Maier O. Interrelation of oxidative stress and inflammation in neurodegenerative disease: role of TNF. Oxid Med Cell Long. (2015) 2015:1–18. doi: 10.1155/2015/610813
33. Jiang KF, Zhao G, Deng GZ, Wu HC, Yin NN, Chen XY, et al. Polydatin ameliorates Staphylococcus aureus-induced mastitis in mice via inhibiting TLR2-mediated activation of the p38 MAPK/NF-κB pathway. Acta Pharmacol Sin. (2017) 38:211–22. doi: 10.1038/aps.2016.123
34. Suresh S, Sankar P, Telang AG, Kesavan M, Sarkar SN. Nanocurcumin ameliorates Staphylococcus aureus-induced mastitis in mouse by suppressing NFκB signaling and inflammation. Int Immunopharmacol. (2018) 65:408–12. doi: 10.1016/j.intimp.2018.10.034
35. Krishnan SM, Sobey CG, Latz E, Mansell A, Drummond GR. IL-1β and IL-18: inflammatory markers or mediators of hypertension? Br J Pharmacol. (2014) 171:5589–602. doi: 10.1111/bph.12876
36. Shaukat A, Guo YF, Jiang K, Zhao G, Wu H, Zhang T, et al. Ginsenoside Rb1 ameliorates Staphylococcus aureus-induced acute lung injury through attenuating nf-κb and MAPK activation. Microb Pathog. (2019) 132:302–12. doi: 10.1016/j.micpath.2019.05.003
37. He C, Zhao Y, Jiang X, Liang X, Yin L, Yin Z, et al. Protective effect of ketone musk on LPS/ATP-induced pyroptosis in J774A.1 cells through suppressing NLRP3/GSDMD pathway. Int Immunopharmacol. (2019) 71:328–35. doi: 10.1016/j.intimp.2019.03.054
38. Vunta H, Belda BJ, Arner RJ, Channa Reddy C, Vanden Heuvel JP, Sandeep Prabhu K. Selenium attenuates pro-inflammatory gene expression in macrophages. Mol Nutr Food Res. (2008) 52:1316–23. doi: 10.1002/mnfr.200700346
39. Iwai K. Diverse roles of the ubiquitin system in NF-κB activation. Biochim Biophys Acta. (2014) 1843:129–36. doi: 10.1016/j.bbamcr.2013.03.011
40. Sun S, Wang J, Wang J, Wang F, Yao S, Xia H. Maresin 1 mitigates sepsis-associated acute kidney injury in mice via inhibition of the NF-κB/STAT3/MAPK pathways. Front Pharmacol. (2019) 10:1323–35. doi: 10.3389/fphar.2019.01323
41. Lo JY, Kamarudin MNA, Hamdi OAA, Khalijah A, Habsah AK. Curcumenol isolated from curcuma zedoaria suppresses Akt-mediated NF-κB activation and p38 MAPK signaling pathway in LPS-stimulated BV-2 microglial cells. Food Funct. (2015) 6:3550–59. doi: 10.1039/x0xx00000x
42. Wullaert A, Bonnet MC, Pasparakis M. NF-κB in the regulation of epithelial homeostasis and inflammation. Cell Res. (2011) 21:146–58. doi: 10.1038/cr.2010.175
43. Yu B, Shen Y, Qiao J, Cui Q. Geniposide attenuates Staphylococcus aureus-induced pneumonia in mice by inhibiting NF-κB activation. Microb Pathog. (2017) 112:117–21. doi: 10.1016/j.micpath.2017.09.050
44. Hu X, Guo J, Xu M, Jiang P, Yuan X, Zhao C, et al. Clostridium tyrobutyricum alleviates Staphylococcus aureus-induced endometritis in mice by inhibiting endometrial barrier disruption and inflammatory response. Food Funct. (2019) 10:6699–710. doi: 10.1039/c9fo00654k
45. Shi X, Wang W, Zheng S, Zhang Q, Xu S. Selenomethionine relieves inflammation in the chicken trachea caused by LPS though inhibiting the NF-κB pathway. Biol Trace Elem Res. (2019) 194:525–35. doi: 10.1007/s12011-019-01789-1
46. Wang H, Bi C, Wang Y, Sun J, Meng X, Li J. Selenium ameliorates staphylococcus aureus-induced inflammation in bovine mammary epithelial cells by inhibiting activation of TLR2, NF-κB and MAPK signaling pathways. BMC Vet Res. (2018) 14:197–205. doi: 10.1186/s12917-018-1508-y
47. Santulli P, Marcellin L, Tosti C, Chouzenoux S, Cerles O, Borghese B, et al. MAP kinases and the inflammatory signaling cascade as targets for the treatment of endometriosis? Expert Opin Ther Targets. (2015) 19:1465–83. doi: 10.1517/14728222.2015.1090974
48. Jiang F, Guan H, Liu D, Wu X, Fan M, Han J. Flavonoids from sea buckthorn inhibit the lipopolysaccharide-induced inflammatory response in RAW264.7 macrophages through the MAPK and NF-κB pathways. Food Funct. (2017) 8:1313–22. doi: 10.1039/c6fo01873d
49. Huang P, Han J, Hui L. MAPK signaling in inflammation-associated cancer development. Protein Cell. (2010) 1:218–26. doi: 10.1007/s13238-010-0019-9
50. Guerra C, Schuhmacher AJ, Canamero M, Grippo PJ, Verdaguer L, Perez-Gallego L, et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. (2007) 11:291–302. doi: 10.1016/j.ccr.2007.01.012
51. An Y, Wang Y, Zhan J, Tang X, Shen K, Shen F, et al. Fosfomycin protects mice from Staphylococcus aureus pneumonia caused by α-hemolysin in extracellular vesicles by inhibiting MAPK-regulated NLRP3 inflammasomes. Front Cell Infect Microbiol. (2019) 9:253–71. doi: 10.3389/fcimb.2019.00253
52. Liu J, Wang S, Zhang Q, Li X, Xu S. Selenomethionine alleviates LPS-induced chicken myocardial inflammation by regulating the miR-128-3p-p38 MAPK axis and oxidative stress. Metallomics. (2020) 12:54–64. doi: 10.1039/c9mt00216b
Keywords: organic selenium, selenohomolanthionine, mastitis, Staphylococcus aureus, NF-κB, MAPK
Citation: Liu K, Ding T, Fang L, Cui L, Li J, Meng X, Zhu G, Qian C, Wang H and Li J (2020) Organic Selenium Ameliorates Staphylococcus aureus-Induced Mastitis in Rats by Inhibiting the Activation of NF-κB and MAPK Signaling Pathways. Front. Vet. Sci. 7:443. doi: 10.3389/fvets.2020.00443
Received: 05 March 2020; Accepted: 19 June 2020;
Published: 30 July 2020.
Edited by:
Natali Krekeler, The University of Melbourne, AustraliaReviewed by:
Yunhe Fu, Jilin University, ChinaMengyao Guo, Huazhong Agricultural University, China
Yongguo Cao, Jilin University, China
Copyright © 2020 Liu, Ding, Fang, Cui, Li, Meng, Zhu, Qian, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Heng Wang, sdaulellow@163.com; Jianji Li, yzjjli@163.com
 Kangjun Liu
Kangjun Liu Tao Ding
Tao Ding Li Fang
Li Fang Luying Cui
Luying Cui Jun Li1,2
Jun Li1,2  Xia Meng
Xia Meng Guoqiang Zhu
Guoqiang Zhu Chen Qian
Chen Qian Heng Wang
Heng Wang Jianji Li
Jianji Li