Middle cerebral artery infarction, A rare complication of intracranial cryptococcoma in an immunocompetent patient: A case report and literature review
- Department of Neurosurgery, Chang Gung Memorial Hospital, Linkou Branch, Taoyuan, Taiwan
Background: This report presents the first case of intracranial cryptococcoma arising from the right frontal lobe causing right middle cerebral artery infarction. Intracranial cryptococcomas usually occur in the cerebral parenchyma, basal ganglia, cerebellum, pons, thalamus, and choroid plexus; they may mimic intracranial tumors, but seldom cause infarction. Of the 15 cases of pathology-confirmed intracranial cryptococcomas in the literature, no case has been complicated by middle cerebral artery (MCA) infarction. Here, we discuss a case of intracranial cryptococcoma with an ipsilateral middle cerebral artery infarction.
Case Description: A 40-year-old man was referred to our emergency room due to progressive headaches and acute left hemiplegia. The patient was a construction worker with no history of avian contact, recent travel, or human immunodeficiency virus (HIV) infection. Brain computed tomography (CT) showed an intra-axial mass, and subsequent magnetic resonance imaging (MRI) delineated a large mass of 53 mm in the right middle frontal lobe and a small lesion of 18 mm in the right caudate head, with marginal enhancement and central necrosis. A neurosurgeon was consulted in view of the intracranial lesion, and the patient underwent en-bloc excision of the solid mass. The pathology report later identified a Cryptococcus infection rather than malignancy. The patient underwent 4 weeks of postoperative treatment with amphotericin B plus flucytosine; he then received subsequent oral antifungal treatment for 6 months, and had neurologic sequelae that manifested as left side hemiplegia.
Conclusion: Diagnosis of fungal infections in the CNS remains challenging. This is especially true of Cryptococcus CNS infections that present as a space-occupying lesion in an immunocompetent patient. A Cryptococcus infection should be considered in the differential diagnoses in patients with brain mass lesions, as this infection can be misdiagnosed as a brain tumor.
Introduction
Diagnosis and treatment of central nervous system (CNS) cryptococcoma is more complicated than of meningitis. First, cryptococcomas are radiologically non-specific, and may be mistaken for malignant lesions; diagnosis may therefore only be made on histologic examination after resection. Second, the symptoms are unspecific. Common symptoms identified in our literature review, which included headaches, seizures and fever, are often seen in other CNS diseases as well. The common initial signs of cryptococcomas are an increasing intracranial pressure (IICP) and neurological focal signs. There has been no case in which hemiplegia was present according to our literature review. Of the 15 cases of pathology-confirmed intracranial cryptococcoma in the literature, there was no case complicated by middle cerebral artery (MCA) infarction. Here, we discuss a case of intracranial cryptococcoma with an ipsilateral MCA infarction.
Clinical presentation
A 40-year-old male patient presenting with progressive headaches and sudden-onset left-sided weakness was referred to our emergency room. The patient did not have a prior history of any systemic diseases and was otherwise healthy. He worked as a construction worker and denied having any contact with pigeons. At presentation, he was lethargic and febrile, with left-side weakness. Neurologic examination revealed a Glasgow coma scale (GCS) of E3V5M6 with left-sided hemiplegia (muscle power: grade 0), normal cranial nerves, and a positive Babinski sign. Emergency brain computed tomography (CT) was arranged for highly-suspected MCA infarction, and showed an intra-axial mass lesion at the right frontal lobe with severe perifocal edema. A marked midline shift to the left was also noted. A blood test revealed leukocytosis and an elevated C-reactive protein (CRP) level, while chest x-ray showed left lower-lobe consolidation with left paratracheal lymph node enlargement and pleural effusion. Subsequent magnetic resonance imaging (MRI) further delineated a large mass of 53 mm in the right middle frontal lobe with an avid marginal enhancement and central necrosis (Figure 1). Another small lesion of 18 mm in the right caudate head with similar enhancement plus hemorrhaging and diffuse swelling of the right frontal and temporal-occipital lobes was also detected (Figure 2). Right MCA infarction was also apparent on the MRI images (Figure 3). The initial differential diagnoses included a brain abscess, glioma and metastasis. An emergency consultation with a neurosurgeon was conducted under suspicion of a high-grade brain tumor causing cerebral infarct. The patient underwent an emergency craniotomy with en-bloc excision of an elastic solid mass (Figure 4).
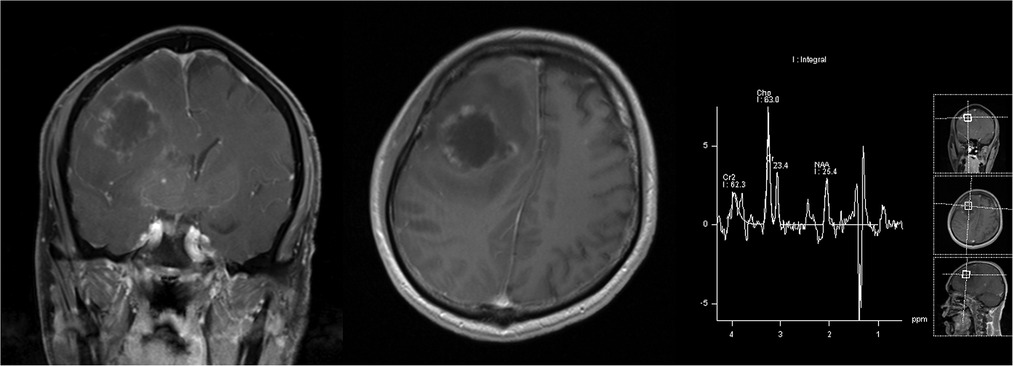
Figure 1. Post-contrast T1-weighted images showed a right frontal mass with a rugged marginal enhancement and possible extensive interior necrotic change; this was also suggested by the presence of a lactic acid peak on MR spectroscopy.
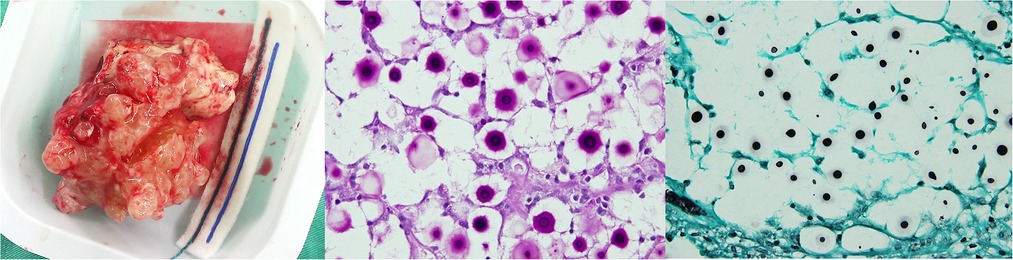
Figure 2. Diffusion-weighted images showed a right frontal mass without water restriction that differed from the abscess (white arrowhead), and a large area of water restriction in the right frontotemporal lobe, suggesting a massive arterial infarction in the MCA vascular region (black arrowhead).
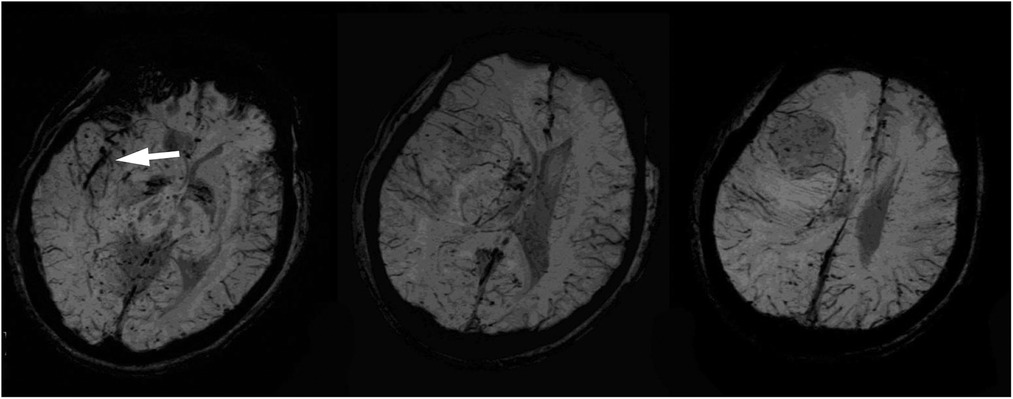
Figure 3. Susceptibility-weighted images showed a mildly increased vascularity in the right frontal mass and a curvilinear dark signal in the right sylvian fissure cistern near the thrombosis of the middle cerebral artery (white arrowhead).
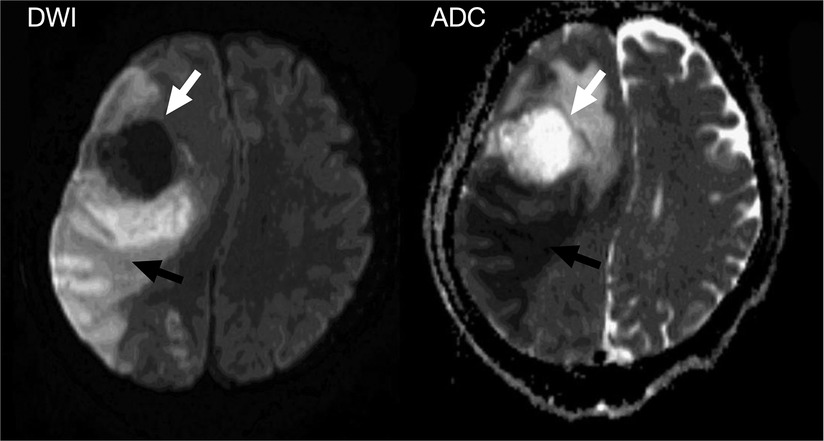
Figure 4. Gross appearance of the resected infectious tumor; microscopic section with PAS; and GMS stain showing hyphal formation.
After this initial operation, the patient's consciousness became clearer (GCS: E4V5M6); however, the left hemiparesis remained (muscle power grade 1–2). On the 4th day after tumor excision, disturbance of consciousness was again noted, with a GCS of E1V1M5. Emergency brain CT showed deteriorating right hemisphere swelling, a leftward midline shift, obstructive hydrocephalus, and brainstem compression. A frontal lobectomy was performed 6 cm from the anterior frontal tip for decompression. Postoperative recovery was uneventful, except for intermittent episodes of fever that were difficult to control. The pathology report later identified a Cryptococcus infection (Figure 4). An infectious disease doctor was consulted, and the patient was started on antifungal treatment with amphotericin B plus flucytosine as per recommendations. After 4 weeks of treatment, the patient recovered well but with continued left hemiparesis. The patient received consolidation therapy with fluconazole (800 mg) for 8 weeks, followed by maintenance therapy with fluconazole (200 mg) for 9 months after discharge. Currently, no recurrence has been noted since completion of the antifungal therapy.
Discussion
The current case was an intracranial cryptococcoma with ipsilateral MCA infarction in an immunocompetent patient. Due to the uncommon nature of this case, a comprehensive literature review to examine the incidence, clinical manifestations and complications of cryptococcoma was conducted.
Cryptococcus meningitis is known to be one of the most common opportunistic infections of the CNS. A review of the literature showed that the incidence of Cryptococcus meningitis is approximately one-million people per year, with at least 600,000 mortalities worldwide. Infections most commonly occur in immunosuppressed hosts (1). Another global review study indicated a significant difference in incidence between high-income and middle to low income countries. The 90-day case-fatality rate from HIV-associated Cryptococcus meningitis in East Asia, Oceania, Western Europe, and the US is 9%, as compared with 55% in other parts of Asia and South America, and 70% in sub-Saharan Africa. This disparity is due to a combination of earlier access to antiretroviral therapy and the availability of fungicidal drugs in high-income countries (2).
The overall CNS involvement in cryptococcosis was estimated to be 42% in Taiwan (3). The incidence of Cryptococcus meningitis ranges from 4.0 to 5.5 per million people per year, with an average incidence of 4.7 per million people per year. A national multicenter epidemiology study in Taiwan from 1997 to 2010 revealed a higher prevalence of cryptococcosis in HIV-negative patients (73%). In addition, 15% of patients had no major comorbidity factors, such as chronic hepatitis or diabetes mellitus. Meningoencephalitis was the most common presentation of cryptococcosis (58.9%) (4).
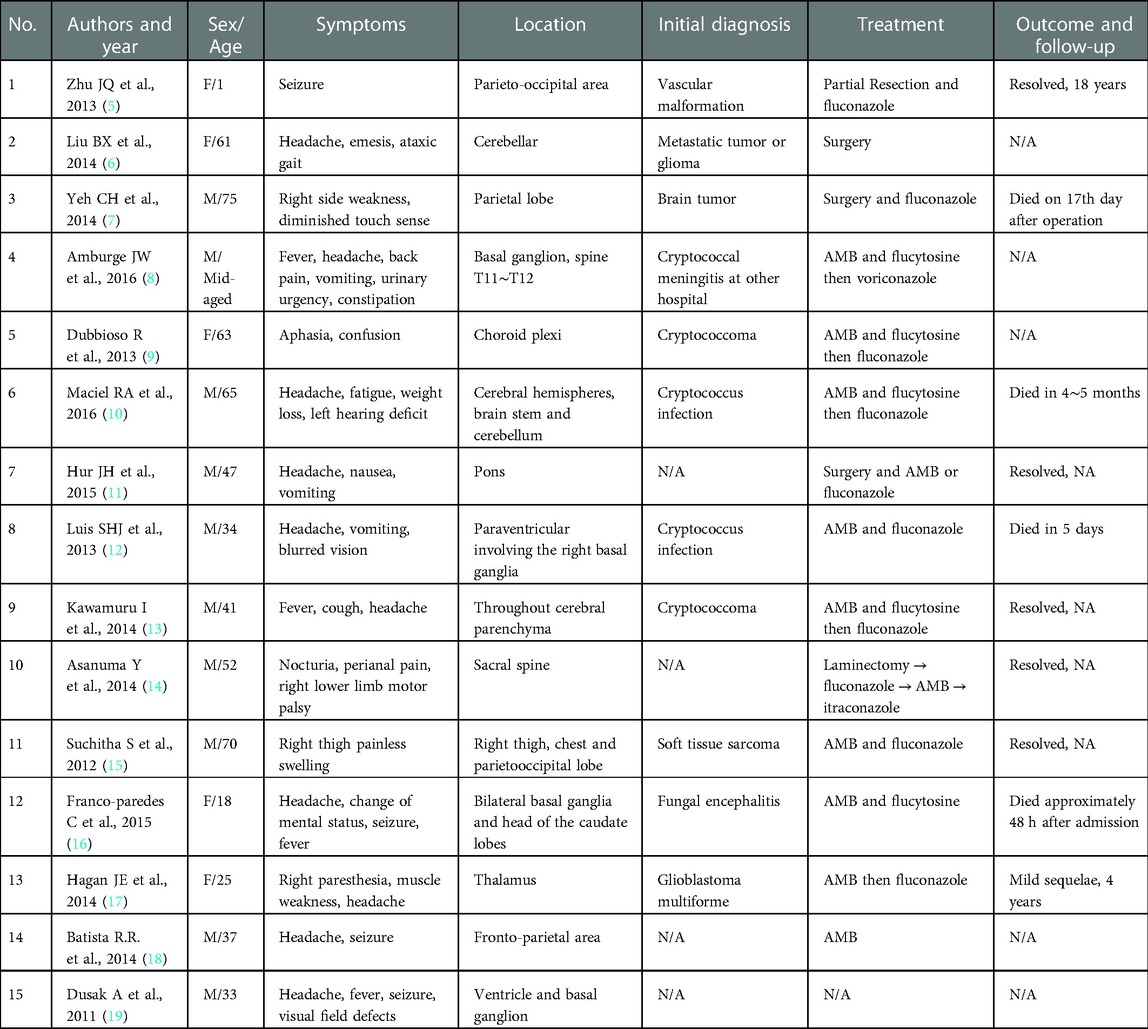
Compared with these review studies, our case study involved a relatively young male with an HIV-negative status and no underlying conditions such as liver disease, diabetes mellitus, malignancy, kidney disease, or solid organ transplantation. To date, there have only been case reports of cryptococcoma in immunocompetent patients, and no large series studies; therefore, we reviewed the literature to identify these case reports. Table 1 summarizes the case reports from 2013/1/1 to 2016/12/31. To the best of our knowledge, only 15 case reports exist. Headaches, which were the most common symptom, occurred in 10 cases (67%); five patients presented with seizures (33%), four with vomiting (27%), four with a fever (27%), and one with a change in mental status (7%). Muscle weakness was noted in three patients (20%).
It was generally known that cryptococcomas can present as a single lesion or multiple lesions and occur in the cerebral parenchyma, basal gangalion, cerebellum, pons, and choroid plexus. In our literature review, there were 6 cases of cryptococcoma occurring in the cerebral parenchyma (40%) and 3 cases in the basal ganglion (20%) (Table 1). However, no case presented with MCA infarction according to the review, which implies that the current case had a particularly rare presentation with an unusual large MCA infarction.
In CNS fungal infections, acute cerebrovascular events take the form of either ischemic (commonly) or hemorrhagic (uncommonly) strokes. Aspergillus, Zygomycetes, Candida, Coccidioides, Histoplasma, Cryptococcus, Penicillium, etc. are all known fungal infections rarely presenting with acute cerebrovascular events (20). R. Raman et al. stated the hypothesis that gradual contiguous involvement of the skull base structures in cases of prolonged paranasal fungal sinusitis (commonly aspergillosis, zygomycosis, cladosporiosis, etc.) leads to angio-invasion, which in turn results in fungal vasculitis, and thereafter thrombotic occlusions occur in the major branches of the cerebral vasculature at the skull base: internal carotid arteries and/or vertebro-basilar system (20). In the current case, our hypothesis was that hyphae invaded the vessel walls of major vasculature at the skull base and caused cerebral arterial thrombosis, cerebral infarction and cerebritis. Sharma et.al stated that in a series of 170 patients, there were 45 cases of major cerebral artery thrombosis, especially either in the ICA or the basilar artery. Ischemic cerebral strokes also result due to cardiac emboli in patients with fungal endocarditis.
Diagnosis of CNS cryptococcoma remains difficult. According to our review, 4/15 (27%) patients were misdiagnosed and treated for brain tumors or a vascular malformation until pathologic examination confirmed a Cryptococcus infection. In the review of Li, Q et al., the rate of misdiagnosis was 9/17 (53%) (21). The reasons for frequent misdiagnosis are described below.
First, symptoms are nonspecific. The common symptoms identified in our review, which included headaches, seizures and fever, are often seen in other CNS diseases. The common initial signs of cryptococcomas are an increasing intracranial pressure (IICP) and neurological focal signs (6). These symptoms are also present in brain tumors or abscesses. The IICP sign leads to a poor prognosis and makes diagnosis more difficult. Typically, Cryptococcus infections can be detected by CSF antigen titer and CSF analysis via lumbar puncture, which can be less invasive than a surgical biopsy. In our case, lumbar puncture was not possible due to the contraindication of IICP.
Second, it is difficult to diagnose cryptococcoma on the basis of imaging. However, according to some of the reports in the literature, the imaging characteristics of CNS cryptococcoma could also be seen in granulomatous tumors and abscesses (6). The common findings in images of brain cryptococcoma were multifocal basal ganglia and midbrain T2 hyperintensities due to gelatinous pseudocysts (cryptococcomas) and dilated Virchow-Robin spaces (22, 23). In our case, the small lesion in the caudate head also represented a typical image-based finding. Brain infarction complicated by infection is commonly seen in aspergillosis, but not in cryptococcosis (24, 25). Multiple lesions with infarctions or hemorrhaging are of a random distribution due to the angio-invasive nature. Hemorrhaging occurs in approximately 25% of lesions (25).
Third, brain abscesses arising from Cryptococcus infections are considered rare in immunocompetent patients. The incidence of other intracranial lesions is higher than those arising from Cryptococcus infection. However, a review of local studies revealed that Cryptococcus meningitis in HIV-negative patients is not uncommon in Taiwan (73% without HIV infection) (4), and that Cryptococcus neoformans is the most frequent species in CNS cryptococcosis (1). A genotype study by Tseng, H.K., et al. showed that C. neoformans is the most prevalent species of Cryptococcus in Taiwan (4). In addition, the variant Cryptococcus gattii often infects immunocompetent patients and grows mainly in the soil under Eucalyptus trees; it is not present in avian feces (16).
In our case, the patient was a non-HIV-infected, non-transplant host, but presented with cryptococcoma and not meningoencephalitis. For the treatment of a non-HIV-infected, non-transplant host with complications, we followed the practical guidelines of the Infectious Disease Society of America (26): induction therapy administered as amphotericin B (0.7–1 mg/kg per day I.V.), liposomal amphotericin B (3–4 mg/kg per day I.V.), or amphotericin B lipid complex (5 mg/kg per day I.V.) plus flucytosine (100 mg/kg per day orally in 4 divided doses) for at least 6 weeks. Consolidation and maintenance therapy was completed with fluconazole (400–800 mg per day orally) for 6–18 months. The guidelines also suggest surgery for large (≥3-cm) or accessible lesions with mass effects. In our case, the large lesion measured 5.3 cm with a mass effect; thus, surgical intervention was recommended.
Conclusion
Diagnosing fungal infections, especially Cryptococcus CNS infections, that present as a space-occupying lesion in an immunocompetent patient remains challenging according to the unspecific symptoms. Intracranial cryptococcoma can lead to ischemic cerebral stroke, and can mimic a brain tumor, and therefore Cryptococcus infection should always be considered in patients with brain mass lesions with abnormal lab data.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by IRB of Chang Gung Memorial Hospital . The patients/participants provided their written informed consent to participate in this study.
Author contributions
LYC, CSC, TCC and CTW completed the manuscript. JSM, HSH, LZH were responsible for pathologic diagnosis. CSC, CCT, TPH, were responsible for the imaging interpretation. HYC is the corresponding author, who supervised the completion of this manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgment
We acknowledge the assistance from the IRB of Chang Gung Memorial Hospital.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. (2009) 23:525–30. doi: 10.1097/QAD.0b013e328322ffac
2. Sloan DJ, Parris V. Cryptococcal meningitis: epidemiology and therapeutic options. Clin Epidemiol. (2014) 6:169–82. doi: 10.2147/CLEP.S38850
3. Chen YY, Lai CH. Nationwide population-based epidemiologic study of cryptococcal meningitis in Taiwan. Neuroepidemiology. (2011) 36:79–84. doi: 10.1159/000323390
4. Tseng HK, Liu CP, Ho MW, Lu PL, Lo HJ, Lin YH, et al. Taiwan infectious diseases study network for C: microbiological, epidemiological, and clinical characteristics and outcomes of patients with cryptococcosis in Taiwan, 1997–2010. PLoS One. (2013) 8:e61921. doi: 10.1371/journal.pone.0061921
5. Zhu JQ, Tao XF, Bao WQ, Hao NX, Wu XR. Calcified cerebral cryptococcal granuloma. Indian J Pediatr. (2013) 80:345–8. doi: 10.1007/s12098-012-0758-0
6. Liu BX, Dai XJ, Liu H, Gong HH, Wang YX, Zhang LL. Cerebellar cryptococcosis characterized by a space-occupying lesion in an immunocompetent non-HIV patient. Neuropsychiatr Dis Treat. (2015) 11:21–4. doi: 10.2147/NDT.S75432
7. Yeh CH, Lin SF, Chiu MC, Kuo CL, Huang HT, Shoung HM. Cerebral cryptococcoma in an HIV-negative patient: experience learned from a case. J Neuropsychiatry Clin Neurosci. (2014) 26:E34–35. doi: 10.1176/appi.neuropsych.13070161
8. Amburgy JW, Miller JH, Ditty BJ, Vande Lune P, Muhammad S, Fisher WS 3rd. Cryptococcus gattii in an immunocompetent patient in the southeastern United States. Case Rep Infect Dis. (2016) 2016:8280915. doi: 10.1155/2016/8280915
9. Dubbioso R, Pappata S, Quarantelli M, D'Arco F, Manganelli F, Esposito M, et al. Atypical clinical and radiological presentation of cryptococcal choroid plexitis in an immunocompetent woman. J Neurol Sci. (2013) 334:180–2. doi: 10.1016/j.jns.2013.08.010
10. Maciel RA, Ferreira LS, Wirth F, Rosa PD, Aves M, Turra E, et al. Corticosteroids for the management of severe intracranial hypertension in meningoencephalitis caused by cryptococcus gattii: a case report and review. J Mycol Med. (2017) 27:109–12. doi: 10.1016/j.mycmed.2016.09.003
11. Hur JH, Kim JH, Park SW, Cho KG. Cryptococcal brainstem abscess mimicking brain tumors in an immunocompetent patient. J Korean Neurosurg Soc. (2015) 57:50–3. doi: 10.3340/jkns.2015.57.1.50
12. Hernandez YU, Monzon JF, Delgado-Hernandez R, Soto-Hernandez JL, Cardenas G. Cryptococcoma of the brain in an immunocompetent man. Natl Med J India. (2013) 26:216–7.24758445
13. Kawamura I, Kamei K, Yarita K, Ohkusu M, Ito K, Tsukahara M, et al. Cryptococcus gattii genotype VGIIb infection in Japan. Med Mycol J. (2014) 55:E51–54. doi: 10.3314/mmj.55.E51
14. Asanuma Y, Fujimoto H, Nakabayashi H, Akeda K, Asanuma K, Tanaka M, et al. Extradural cryptococcoma at the sacral spine without bone involvement in an immunocompetent patient. J Orthop Sci. (2014) 19:1040–5. doi: 10.1007/s00776-013-0413-2
15. Suchitha S, Sheeladevi CS, Sunila R, Manjunath GV. Disseminated cryptococcosis in an immunocompetent patient: a case report. Case Rep Pathol. (2012) 2012:652351. doi: 10.1155/2012/652351
16. Franco-Paredes C, Womack T, Bohlmeyer T, Sellers B, Hays A, Patel K, et al. Marr KA: management of cryptococcus gattii meningoencephalitis. Lancet Infect Dis. (2015) 15:348–55. doi: 10.1016/S1473-3099(14)70945-4
17. Hagan JE, Dias JS, Villasboas-Bisneto JC, Falcao MB, Ko AI, Ribeiro GS. Puerperal brain cryptococcoma in an HIV-negative woman successfully treated with fluconazole: a case report. Rev Soc Bras Med Trop. (2014) 47:254–6. doi: 10.1590/0037-8682-0215-2013
18. Batista RR, Gasparetto EL. Uncommon presentation of intracranial cryptococcoma in an immunocompetent patient. AJNR Am J Neuroradiol. (2012) 33:E26. author reply E27. doi: 10.3174/ajnr.A2988
19. Dusak A, Hakyemez B, Kocaeli H, Bekar A. Magnetic resonance spectroscopy findings of pyogenic, tuberculous, and cryptococcus intracranial abscesses. Neurochem Res. (2012) 37:233–7. doi: 10.1007/s11064-011-0622-z
20. Sharma RR. Fungal infections of the nervous system: current perspective and controversies in management. Int J Surg. (2010) 8:591–601. doi: 10.1016/j.ijsu.2010.07.293
21. Li Q, You C, Liu Q, Liu Y. Central nervous system cryptococcoma in immunocompetent patients: a short review illustrated by a new case. Acta Neurochir (Wien). (2010) 152:129–36. doi: 10.1007/s00701-009-0311-8
22. Jain KK, Mittal SK, Kumar S, Gupta RK. Imaging features of central nervous system fungal infections. Neurol India. (2007) 55:241–50. doi: 10.4103/0028-3886.35685
23. Saigal G, Post MJ, Lolayekar S, Murtaza A. Unusual presentation of central nervous system cryptococcal infection in an immunocompetent patient. AJNR Am J Neuroradiol. (2005) 26:2522–6.16286394
24. Sparano JA, Gucalp R, Llena JF, Moser FG, Wiernik PH. Cerebral infection complicating systemic aspergillosis in acute leukemia: clinical and radiographic presentation. J Neurooncol. (1992) 13:91–100. doi: 10.1007/BF00172950
25. Tempkin AD, Sobonya RE, Seeger JF, Oh ES. Cerebral aspergillosis: radiologic and pathologic findings. Radiographics. (2006) 26:1239–42. doi: 10.1148/rg.264055152
Keywords: MCA infarction, cryptococcomas, rare complication, intracranial infection, immunocompetent adult infection
Citation: Li Y, Tseng C, Chien S, Huang S, Chang T, Chen C, Tu P, Liu Z and Huang Y (2023) Middle cerebral artery infarction, A rare complication of intracranial cryptococcoma in an immunocompetent patient: A case report and literature review. Front. Surg. 10:1083833. doi: 10.3389/fsurg.2023.1083833
Received: 29 October 2022; Accepted: 12 January 2023;
Published: 15 February 2023.
Edited by:
Jian Wu, Capital Medical University, ChinaReviewed by:
Lars-Peder Pallesen, University Hospital Carl Gustav Carus, GermanyMajed Katati, University of Granada, Spain
© 2023 Li, Tseng, Chien, Huang, Chang, Chen, Tu, Liu and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yin-Cheng Huang ns3068@gmail.com
Specialty Section: This article was submitted to Neurosurgery, a section of the journal Frontiers in Surgery
Abbreviations CNS, central nervous system; HIV, human immunodeficiency virus; GCS, Glasgow coma scale.
 Ying-Ching Li
Ying-Ching Li Chun-Chia Tseng
Chun-Chia Tseng  Sheng-Han Huang
Sheng-Han Huang Po-Hsun Tu
Po-Hsun Tu Zhuo-Hao Liu
Zhuo-Hao Liu