Management of borderline ovarian tumors: A tertiary referral center experience in Egypt
- 1Department of Surgical Oncology, Oncology Center, Mansoura University, Mansoura, Egypt
- 2Ministry of Health, Consultant of Obstetrics and Gynecology, Mansoura, Egypt
Background: In this retrospective study, we discuss our experience as a large tertiary referral center in Egypt in the management and follow-up of borderline tumors
Patients and methods: This is a retrospective cohort study where all patients diagnosed with a borderline ovarian tumor at Oncology Center Mansoura University from November 2014 to June 2020 were included. Demographics, preoperative, operative, postoperative, pathologic, and oncologic follow-up data were retrieved from a prospectively maintained electronic database. The included patients were followed until April 2022.
Results: We included 27 patients with borderline ovarian tumors. The mean age of the study patients was 47.67 ± 16.39 years. The median CA 125 was 33 (6–304 U/ml). Frozen section examination was utilized in 13 patients (48.14%), where a diagnosis of borderline ovarian tumors was revealed in 8 patients. Recurrence was reported in one patient with serous type after approximately 26 months. The most common pathological type in our cohort was the mucinous borderline type reported in 14 patients (51.9%), followed by the serous type reported in 11 patients (40.7%), and the seromucinous type in 1 patient only. Patients with mucinous borderline type were significantly younger (40.083 ± 18.47 vs. 53.73 ± 11.91 years, p = 0.028). Interestingly, Cancer Antigen 125 levels were significantly higher in mucinous than serous and seromucinous types [67(16–304) vs. 20(6–294.6) U/ml, p = 0.027]. On the other hand, the radiological tumor size of serous and seromucinous types was larger than that of the mucinous type [23(19–31) cm vs. 8(5–20) cm, p = 0.001]. Over a median follow-up period of 58.66 (54.16–63.16) months, only one postoperative mortality was reported, while only one recurrence was reported.
Conclusion: Borderline ovarian tumors still represent a dilemma either in diagnosis or management. A frozen section examination could help to reach a preliminary diagnosis. Total abdominal hysterectomy and bilateral salpingo-oophorectomy are the cornerstone of surgical management; however, fertility-sparing surgery could be a valid option for women desiring fertility.
Introduction
Borderline ovarian tumors (BOTs) are known to be a certain type of tumor that has a higher mitotic activity than the benign tumors but with no stromal invasion like other malignant epithelial ovarian tumors (1). Borderline ovarian tumors were first described in the literature about 100 years ago, and they nearly account for 10%–15% of all ovarian tumors (2, 3). Serous borderline tumors account for the majority of BOT with an incidence of about 50%, followed by mucinous type, which accounts for about 40% of diagnosed BOTs (4). BOTs are usually diagnosed during the reproductive age, with nearly 30% of cases diagnosed before 40 years (5, 6), and luckily, most of these tumors are diagnosed at an early stage while still confined to the ovaries with a 5-year survival rate approaching 95% (7). Radical surgery including hysterectomy and salpingo-oophorectomy had been the gold standard for the management of these tumors for decades; however, fertility-sparing surgery with preservation of the uterus and at least one ovary has been widely adopted recently as a better choice in the management of these tumors without compromising the oncological outcome, especially in the young age group of patients (8–11). Unilateral cystectomy also had been offered as a treatment option in the case of unilateral tumors; however, several studies have reported a higher recurrence rate despite not affecting the overall survival (8–11). The use of frozen sections in ovarian tumors has been increasing recently in several institutes worldwide; however, the diagnosis of BOT, particularly by frozen sections, is a real challenge with lower sensitivity and specificity rates than benign and malignant tumors (12–14).
Herein, we share and discuss our experience as a large tertiary referral center in Egypt in the management and follow-up of borderline tumors.
Patients and methods
This is a retrospective cohort study where all patients diagnosed with a borderline ovarian tumor at Oncology Center Mansoura University from November 2014 to June 2020 were included. We included all patients who had a pathological diagnosis of borderline ovarian neoplasm, either the serous, mucinous, or seromucinous type. Patients who were operated by either laparotomy or laparoscopy, fertility-preserving surgery, or total abdominal hysterectomy and bilateral salpingoophrectomy were included. Patients diagnosed with invasive epithelial carcinoma or those with missing data were excluded. Demographics, preoperative, operative, postoperative, pathologic, and oncologic follow-up data were retrieved from a prospectively maintained electronic database. The included patients were followed until April 2022.
Data were fed to the computer and analyzed using IBM Corp. Released 2013 (IBM SPSS Statistics for Windows, Version 22.0; IBM Corp., Armonk, NY). Qualitative data were described using numbers and percentages. Quantitative data were described using median (minimum and maximum) and mean ± standard deviation for parametric data after testing normality using the Kolmogorov–Smirnov test. The significance of the obtained results was judged at the 0.05 level. The chi-square test, Fischer exact test, and Monte Carlo test for comparison of two or more groups as appropriate were used for qualitative variables. Student’s t-test and the Mann–Whitney U test were used to compare two independent groups of normally and non-normally distributed data.
Results
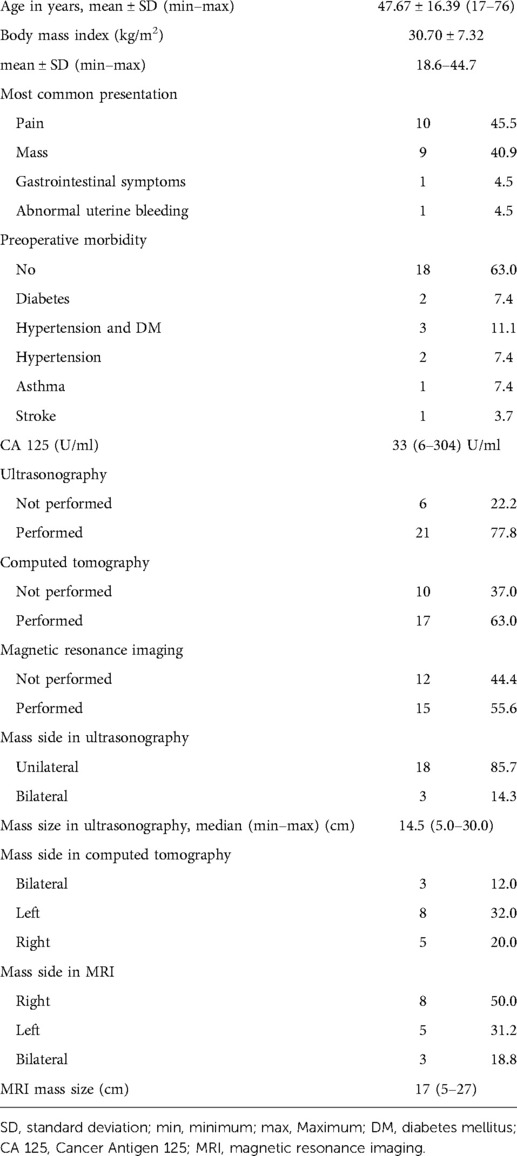
We included 27 patients with borderline ovarian tumors. The mean age of the study patients was (mean ± standard deviation 47.67 ± 16.39 years). Most of the patients (86.4%) presented with vague abdominal symptoms and abdominal mass. The median CA 125 was 33 (6–304 U/ml). The remaining demographic and preoperative criteria are listed in Table 1.
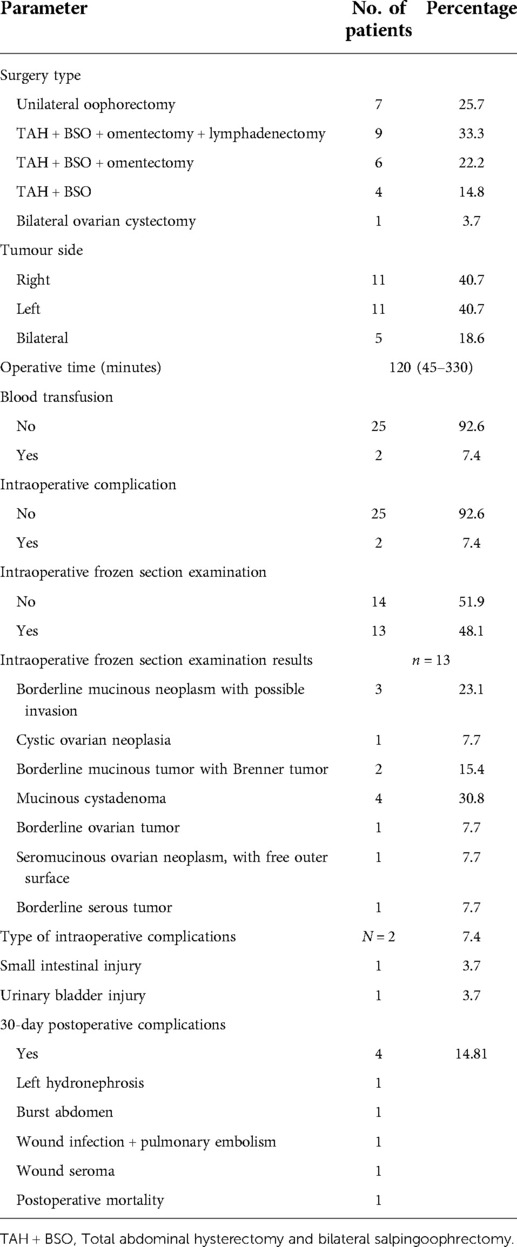
The tumor affected one ovary in 22 patients (81.4%), while it was bilateral in 5 patients (18.6%). Total abdominal hysterectomy and bilateral salpingo-oophorectomy were performed in 19 patients (70.3%), while unilateral oophorectomy was performed in 7 patients (25.7%). One patient had bilateral seromucinous borderline tumor and was managed by bilateral cystectomy only as fertility-preserving surgery after a multidisciplinary decision. The infracolic omentum was resected in 16 patients (59.25%) and was proved to be free from deposits after pathological evaluation. Lymphadenectomy was done in eight patients (32%), whereas pelvic lymphadenectomy was done in five patients, paraortic in one patient, and both lymph node groups in two patients. All dissected lymph nodes were free from tumor tissue. It should be noted that there was a clinical suspicion of ovarian cancer preoperatively in those patients.
The frozen section examination was utilized in 13 patients (48.14%), where a diagnosis of borderline ovarian tumors was revealed in 8 patients. The laparoscopic approach was used in two patients where conversion to open was performed in one of them due to adhesions. Table 2 illustrates the operative and postoperative parameters of the studied patients.
Intraoperative complication was reported in two patients (7.4%), whereas small intestinal and urinary bladder injury was reported equally in one patient. The 30-day postoperative complications were reported in four cases. The most common was wound-related, including either seroma, wound infection, or burst abdomen. Recurrence was reported in one patient with serous type after approximately 26 months. Resection of the recurrent mass was performed where pathological assessment revealed a recurrent ovarian borderline serous tumor. One patient succumbed to pulmonary embolism in the first month after her surgery.
The most common pathological type in our cohort was the mucinous borderline type reported in 14 patients (51.9%), followed by the serous type reported in 11 patients (40.7%) and the seromucinous type in 1 patient only. The microinvasive component was reported in six patients (22.2%); one of these patients had bilateral synchronous borderline line serous tumor on one side and low-grade serous carcinoma on top of borderline serous tumor on the contralateral side.
We compared the parameters of patients diagnosed with mucinous borderline type with those of serous and seromucinous types (Table 3). Patients with the mucinous borderline type were significantly younger (40.083 ± 18.47 vs. 53.73 ± 11.91 years, p = 0.028). Patients with serous and seromucinous types presented more with pelviabdominal mass than those with the mucinous type who presented with vague abdominal pain. Interestingly, Cancer Antigen 125 levels were significantly higher in mucinous than serous and seromucinous types [67(16–304) vs. 20(6–294.6) U/ml, p = 0.027]. On the other hand, the radiological tumor size of serous and seromucinous types was larger than that of the mucinous type [23(19–31) cm vs. 8(5–20) cm, p = 0.001]. Over a median follow-up period of 58.66 (54.16–63.16) months, only one postoperative mortality was reported, while no recurrence events were reported.
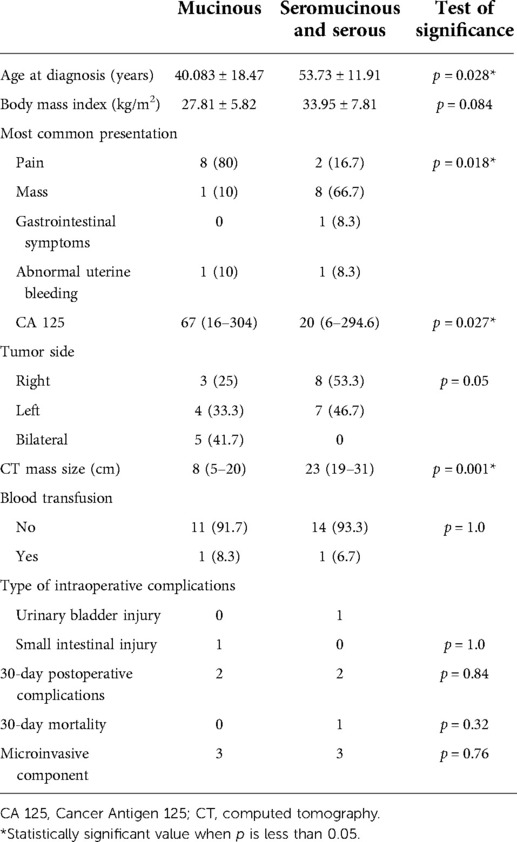
Table 3. Sociodemographic and clinicopathological characteristic distribution according to the pathology of the studied patients.
Discussion
BOTs represent a dilemma whether in preoperative diagnosis based on radiological and clinical signs and symptoms or in frozen section diagnosis during the operation. We discuss our experience as a major oncology hospital in Egypt in managing such cases.
BOTs are reported to be diagnosed usually during the women's reproductive age, with about 30% of cases diagnosed before the age of 40 years (5, 6). The mean age of patients in our study group was 47.67 ± 16.39 years, and the patients with borderline mucinous type were significantly younger than patients diagnosed with borderline serous and seromucinous types (40.083 ± 18.47 vs. 53.73 ± 11.91 years, p = 0.028).
Most of our patients (86.4%) were diagnosed with vague abdominal symptoms, mainly abdominal pain and mass. Several authors reported the same findings in diagnosing BOTs (15, 16). A study conducted in Norway revealed that nearly 75% of patients with BOTs had at least a single symptom at the time of diagnosis. Others reported that more than 80% of women with BOTs have variable abdominal symptoms whether abdominal pain, discomfort, distention, or other gynecological and urological symptoms (17–20).
CA-125 had been widely used in the preoperative assessment of ovarian tumors especially in ovarian cancer as a marker that aids diagnosis and gives an indicator of prognosis and response to chemotherapy (21). The use of CA 125 in BOTs is controversial, and several studies reported that it should not be used as a diagnostic tool in BOTs (22, 23). In our study, we reported a median CA 125 level of 33 U/ml, and it was not significantly elevated in most of our patients. Lenhard et al. also reported a median CA 125 level of 34.7 U/ml in a single-center retrospective study, which is nearly the same value reported in our study (24). Another study reported relatively lower CA 125 values in BOT compared to ovarian cancer (25). While few studies reported relatively higher CA 125 levels in borderline serous than in mucinous subtypes (26, 27), we reported significantly higher CA 125 levels in borderline mucinous than serous and seromucinous types.
BOTs, as reported in the literature, are usually diagnosed in an early stage (7). Twenty-two patients in our study, representing 81.4%, were diagnosed with unilateral tumors, while only 5 patients had a bilateral tumor at the time of the diagnosis. Unilateral cystectomy or salpingo-oophorectomy with fertility preservation is now considered the ideal line of treatment in such cases; however, a higher recurrence rate was documented with unilateral cystectomy without affecting the oncological outcome (8–11). In our study, there is heterogeneity in the surgical management of such cases, and this may be attributed to some factors.
The first factor is that, in Egypt, we have a low median age of first marriage of 20.8 years, as reported by UNICEF, compared to western countries, so most of our patients were already married with offspring at the time of surgery, so fertility-sparing surgery was not the choice in such patients. Nineteen patients, representing 70.3% of our study group, had a total abdominal hysterectomy and bilateral salpingo-oophorectomy.
The second factor is that lymphadenectomy was done in eight patients based on clinical and radiological suspicion of ovarian carcinoma instead of BOTs.
The third factor is that we did not use the frozen section examination in all of our patients, and also the frozen section examination was not conclusive in all cases as 8 patients out of 13 (61.5%) were found to be the same diagnosis in paraffin sections as the frozen sections.
According to the WHO classification, the serous borderline tumor represents about 50% of diagnosed cases, followed by mucinous type, which represents about 40% of diagnosed cases. At the final pathology in our study, the most common type was the mucinous borderline (51.9%), followed by the serous type (40.7%), while the seromucinous type was only recorded in one patient. We also reported microinvasion in six patients (22%) in our study. Although the presence of a microinvasive component was reported to be an indicator of a higher recurrence rate (28), other studies denied the association between the presence of microinvasion and the recurrence or survival rate (29, 30). We did not report any recurrences associated with the presence of microinvasion in our study.
Interestingly the radiological tumor size whether by ultrasonography or MRI was significantly larger in serous and seromucinous types than mucinous type in our study 23(19–31) cm vs. 8(5–20) cm, p = 0.001), although it is reported in several studies that the mucinous tumors usually tend to be larger in comparison to serous tumors (31–33).
The recurrence rate in BOTs is relatively low and ranges from 0% to 25%, with a higher risk in patients who underwent a unilateral or bilateral cystectomy (3, 8, 34–36). In our study, we did not report any recurrence in a median follow-up period of 58.66 months, but most of our patients were not treated with fertility-preserving surgery as in other studies, and also the relatively small sample size might have an effect on the recurrence rates.
Surgery is considered the main line of treatment for borderline ovarian tumors. Treatment guidelines recommend tailoring the surgical decision according to the histologic and clinical features of the tumor and the age of the patient. Fertility-sparing surgery is a valid option for young females with BOTs, while total hysterectomy and bilateral salpingoophrectomy are reserved for menopausal females. The most common risk factors for relapse or recurrence are ovarian cystectomy and incomplete staging. According to the NCCN guidelines, patients who had incomplete staging or surgery should be managed depending on fertility desire and the presence of invasive implants, where patients who desire to preserve their fertility could be treated by fertility-preserving surgery and resection of any residual disease. The NCCN guideline recommends tailoring the decision of lymphadenectomy on a case-by-case basis in view of the available evidence from the literature about no improved survival after lymphadenectomy and omentectomy for BOTs. In the present study, conservative surgery was performed in 29.4% of the patients, while more aggressive approaches including omentectomy and/or lymphadenectomy were performed in 55.5% of them.
The present study highlighted the management of borderline ovarian cancer in a tertiary center in a developing country. We believe that this study is crucial to arouse concerns about the urgent need to unify the management of this type of tumor among the gyneoncologists in developing countries. However, it is worth mentioning that this study had some limitations, including the retrospective design, the relatively small sample size, and the heterogeneity of the surgical management as not all the patients were operated by gynecologic oncologists and most of our patients did not have a fertility-sparing surgery.
Although most guidelines consider complete surgical staging as the standard of care in the management of BOTs, a large proportion of the patients is incompletely staged (37). The main concern about incomplete staging is that it could hinder the detection of advanced diseases that could be surgically resected or confirm the need for adjuvant therapy. In their series, Romeo et al. reported that 10.9% of relapse in all of them was staged as FIGO stage I after incomplete staging (37). In the present study, no recurrence events were reported in patients who underwent conservative surgery.
Interestingly, the benefit of restaging surgery after the incomplete staging is still debatable. In their study, Lecointre et al. highlighted the main indications of restaging surgery as cystectomy in mucinous tumor or serous tumor with a micropapillary component, incomplete peritoneal exploration, a defective surgical technique that led to seeding, and peritoneal lesions after the primary surgery in the case of residual gross (38).
Borderline ovarian tumors still represent a dilemma either in diagnosis or management. A frozen section examination could help to reach a preliminary diagnosis. Total abdominal hysterectomy and bilateral salpingo-oophorectomy are the cornerstone of surgical management. However, fertility-sparing surgery could be a valid option for women desiring fertility. Future studies are awaited to address the incidence of recurrence and its risk factors to guide the best management protocol.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Research Board (IRB), Faculty of Medicine, Mansoura University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
KG: writing. BR, MA, AF, ME: statistics and data collection. MZ: writing. All authors contributed to the article and approved the submitted version.
Funding
This is a retrospective study conducted at Oncology Center Mansoura University and has not received funding from any sources.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ovarian Tumour Panel of the Royal College of Obstetricians and Gynaecologists. Ovarian epithelial tumours of borderline malignancy: pathological features and current status. Br J Obstet Gynaecol. (1983) 90(8):743–50. doi: 10.1111/j.1471-0528.1983.tb09305.x
2. Taylor HC. Malignant and semi-malignant tumours of the ovary. Surg Gynecol Obs. (1929) 48:204–30.
3. Tropé CG, Kaern J, Davidson B. Borderline ovarian tumours. Best Pract Res Clin Obstet Gynaecol. (2012) 26(3):325–36. doi: 10.1016/j.bpobgyn.2011.12.006
4. Hauptmann S, Friedrich K, Redline R, Avril S. Ovarian borderline tumors in the 2014 WHO classification: evolving concepts and diagnostic criteria. Virchows Arch. (2017) 470(2):125–42. doi: 10.1007/s00428-016-2040-8
5. Zanetta G, Rota S, Lissoni A, Meni A, Brancatelli G, Buda A. Ultrasound, physical examination, and CA 125 measurement for the detection of recurrence after conservative surgery for early borderline ovarian tumors. Gynecol Oncol. (2001) 81(1):63–6. doi: 10.1006/gyno.2000.6099
6. Harter P, Gershenson D, Lhomme C, Lecuru F, Ledermann J, Provencher DM, et al. Gynecologic Cancer InterGroup (GCIG) consensus review for ovarian tumors of low malignant potential (borderline ovarian tumors). Int J Gynecol Cancer Off J Int Gynecol Cancer Soc. (2014) 24(9 Suppl 3):S5–8. doi: 10.1097/IGC.0000000000000282
7. Bourdel N, Huchon C, Abdel Wahab C, Azaïs H, Bendifallah S, Bolze PA, et al. Borderline ovarian tumors: French guidelines from the CNGOF. Part 2. Surgical management, follow-up, hormone replacement therapy, fertility management and preservation. J Gynecol Obstet Hum Reprod. (2021) 50(1):101966. doi: 10.1016/j.jogoh.2020.101966
8. Li N, Ming X, Li Z. Unilateral cystectomy and serous histology are associated with relapse in borderline ovarian tumor patients with fertility-sparing surgery: a systematic review and meta-analysis. Arch Gynecol Obstet. (2020) 302(5):1063–74. doi: 10.1007/s00404-020-05716-x
9. Vancraeynest E, Moerman P, Leunen K, Amant F, Neven P, Vergote I. Fertility preservation is safe for serous borderline ovarian tumors. Int J Gynecol Cancer. (2016) 26(8):1399 LP–1406. doi: 10.1097/IGC.0000000000000782
10. Helpman L, Yaniv A, Beiner ME, Aviel-Ronen S, Perri T, Ben-Baruch G, et al. Fertility preservation in women with borderline ovarian tumors—how does it impact disease outcome? A cohort study. Acta Obstet Gynecol Scand. (2017) 96(11):1300–6. doi: 10.1111/aogs.13203
11. Karlsen NMS, Karlsen MA, Høgdall E, Nedergaard L, Christensen IJ, Høgdall C. Relapse and disease specific survival in 1143 Danish women diagnosed with borderline ovarian tumours (BOT). Gynecol Oncol. (2016) 142(1):50–3. doi: 10.1016/j.ygyno.2016.05.005
12. Ratnavelu ND, Brown AP, Mallett S, Scholten RJ, Patel A, Founta C, et al. Intraoperative frozen section analysis for the diagnosis of early stage ovarian cancer in suspicious pelvic masses. Cochrane Database Syst Rev. (2016) 3(3):CD010360. doi: 10.1002/14651858.CD010360.pub2
13. Palakkan S, Augestine T, Valsan MK, Vahab KPA, Nair LK. Role of frozen section in surgical management of ovarian neoplasm. Gynecol Minim Invasive Ther. (2020) 9(1):13–7. doi: 10.4103/GMIT.GMIT_2_19
14. Md Arshad NZ, Ng BK, Md Paiman NA, Abdullah Mahdy Z, Mohd Noor R. Intra-operative frozen sections for ovarian tumors – a tertiary center experience. Asian Pac J Cancer Prev. (2018) 19(1):213–8. doi: 10.22034/APJCP.2018.19.1.213
15. Hamilton W, Peters TJ, Bankhead C, Sharp D. Risk of ovarian cancer in women with symptoms in primary care: population based case-control study. Br Med J. (2009) 339:b2998. doi: 10.1136/bmj.b2998
16. Carbonnel M, Layoun L, Poulain M, Tourne M, Murtada R, Grynberg M, et al. Serous borderline ovarian tumor diagnosis, management and fertility preservation in young women. J Clin Med. (2021) 10(18):4233. doi: 10.3390/jcm10184233
17. Vine MF, Ness RB, Calingaert B, Schildkraut JM, Berchuck A. Types and duration of symptoms prior to diagnosis of invasive or borderline ovarian tumor. Gynecol Oncol. (2001) 83(3):466–71. doi: 10.1006/gyno.2001.6411
18. Webb PM, Purdie DM, Grover S, Jordan S, Dick M-L, Green AC. Symptoms and diagnosis of borderline, early and advanced epithelial ovarian cancer. Gynecol Oncol. (2004) 92(1):232–9. doi: 10.1016/j.ygyno.2003.09.005
19. Olsen CM, Cnossen J, Green AC, Webb PM. Comparison of symptoms and presentation of women with benign, low malignant potential and invasive ovarian tumors. Eur J Gynaecol Oncol. (2007) 28(5):376–80.17966216
20. Paulsen T, Kaern J, Kjaerheim K, Tropé C, Tretli S. Symptoms and referral of women with epithelial ovarian tumors. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. (2005) 88(1):31–7. doi: 10.1016/j.ijgo.2004.09.008
21. Zhang M, Cheng S, Jin Y, Zhao Y, Wang Y. Roles of CA125 in diagnosis, prediction, and oncogenesis of ovarian cancer. Biochim Biophys Acta Rev Cancer. (2021) 1875(2):188503. doi: 10.1016/j.bbcan.2021.188503
22. Eymerit-Morin C, Brun JL, Vabret O, Devouassoux-Shisheboran M. Borderline ovarian tumours: CNGOF guidelines for clinical practice—biopathology of ovarian borderline tumors. Gynecol Obstet Fertil Senol. (2020) 48(9):629–45. doi: 10.1016/j.gofs.2020.05.007
23. Denewar FA, Takeuchi M, Urano M, Kamishima Y, Kawai T, Takahashi N, et al. Multiparametric MRI for differentiation of borderline ovarian tumors from stage I malignant epithelial ovarian tumors using multivariate logistic regression analysis. Eur J Radiol. (2017) 91:116–23. doi: 10.1016/j.ejrad.2017.04.001
24. Lenhard MS, Nehring S, Nagel D, Mayr D, Kirschenhofer A, Hertlein L, et al. Predictive value of CA 125 and CA 72-4 in ovarian borderline tumors. Clin Chem Lab Med. (2009) 47(5):537–42. doi: 10.1515/CCLM.2009.134
25. Eltabbakh GH, Yadav PR, Morgan A. Clinical picture of women with early stage ovarian cancer. Gynecol Oncol. (1999) 75(3):476–9. doi: 10.1006/gyno.1999.5621
26. Kolwijck E, Thomas CMG, Bulten J, Massuger LFAG. Preoperative CA-125 levels in 123 patients with borderline ovarian tumors: a retrospective analysis and review of the literature. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc. (2009) 19(8):1335–8. doi: 10.1111/IGC.0b013e3181a83e04
27. Gotlieb WH, Soriano D, Achiron R, Zalel Y, Davidson B, Kopolovic J, et al. CA 125 Measurement and ultrasonography in borderline tumors of the ovary. Am J Obstet Gynecol. (2000) 183(3):541–6. doi: 10.1067/mob.2000.105940
28. Bois A, Ewald-Riegler N, Bois O, Harter P. Borderline-Tumoren des Ovars – eine systematische Übersicht. Geburtshilfe Frauenheilkd. (2009) 69:807–33. doi: 10.1055/s-0029-1186007
29. Prat J, De Nictolis M. Serous borderline tumors of the ovary: a long-term follow-up study of 137 cases, including 18 with a micropapillary pattern and 20 with microinvasion. Am J Surg Pathol. (2002) 26(9):1111–28. doi: 10.1097/00000478-200209000-00002
30. Ronnett BM, Kajdacsy-Balla A, Gilks CB, Merino MJ, Silva E, Werness BA, et al. Mucinous borderline ovarian tumors: points of general agreement and persistent controversies regarding nomenclature, diagnostic criteria, and behavior. Hum Pathol. (2004) 35(8):949–60. doi: 10.1016/j.humpath.2004.03.006
31. Laurent P-E, Thomassin-Piana J, Jalaguier-Coudray A. Mucin-producing tumors of the ovary: MR imaging appearance. Diagn Interv Imaging. (2015) 96(11):1125–32. doi: 10.1016/j.diii.2014.11.034
32. Seidman JD, Kurman RJ, Ronnett BM. Primary and metastatic mucinous adenocarcinomas in the ovaries: incidence in routine practice with a new approach to improve intraoperative diagnosis. Am J Surg Pathol. (2003) 27(7):985–93. doi: 10.1097/00000478-200307000-00014
33. Borrelli GM, de Mattos LA, Andres MdeP, Gonçalves MO, Kho RM, Abrão MS. Role of imaging tools for the diagnosis of borderline ovarian tumors: a systematic review and meta-analysis. J Minim Invasive Gynecol. (2017) 24(3):353–63. doi: 10.1016/j.jmig.2016.12.012
34. Anfinan N, Sait K, Ghatage P, Nation J, Chu P. Ten years experience in the management of borderline ovarian tumors at Tom Baker Cancer Centre. Arch Gynecol Obstet. (2011) 284(3):731–5. doi: 10.1007/s00404-010-1713-9
35. Silva EG, Gershenson DM, Malpica A, Deavers M. The recurrence and the overall survival rates of ovarian serous borderline neoplasms with noninvasive implants is time dependent. Am J Surg Pathol. (2006) 30(11):1367–71. doi: 10.1097/01.pas.0000213294.81154.95
36. Uzan C, Muller E, Kane A, Rey A, Gouy S, Bendiffallah S, et al. Prognostic factors for recurrence after conservative treatment in a series of 119 patients with stage I serous borderline tumors of the ovary. Ann Oncol Off J Eur Soc Med Oncol. (2014) 25(1):166–71. doi: 10.1093/annonc/mdt430
37. Romeo M, Pons F, Barretina P, Radua J. Incomplete staging surgery as a major predictor of relapse of borderline ovarian tumor. World J Surg Oncol. (2013) 11:13. doi: 10.1186/1477-7819-11-13
Keywords: borderline, ovarian borderline tumor (BOT), ovarian borderline malignancy, ovarian cancer, borderline (»proliferating«) mucinous tumor
Citation: Gaballa K, Abdelkhalek M, Fathi A, Refky B, Belal K, elaraby M and Zuhdy M (2022) Management of borderline ovarian tumors: A tertiary referral center experience in Egypt. Front. Surg. 9:962820. doi: 10.3389/fsurg.2022.962820
Received: 6 June 2022; Accepted: 1 August 2022;
Published: 2 September 2022.
Edited by:
Carmine Conte, Agostino Gemelli University Polyclinic (IRCCS), ItalyReviewed by:
Carlo Ronsini, Università degli Studi della Campania “Luigi Vanvitelli”, ItalyGuido Lancellotti, Agostino Gemelli University Polyclinic (IRCCS), Italy
© 2022 Gaballa, Abdelkhalek, Fathi, Refky, Belal, elaraby and Zuhdy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khaled Gaballa Khaledgaballah85@gmail.com
Specialty Section: This article was submitted to Obstetrics and Gynecological Surgery, a section of the journal Frontiers in Surgery
 Khaled Gaballa
Khaled Gaballa Mohamed Abdelkhalek1
Mohamed Abdelkhalek1  Basel Refky
Basel Refky Mohammad Zuhdy
Mohammad Zuhdy