Recurrence Patterns After Hepatectomy With Very Narrow Resection Margins for Hepatocellular Carcinoma
- 1Division of Liver and Transplantation Surgery, Department of General Surgery, Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Taoyuan, Taiwan
- 2Department of General Surgery, Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Taoyuan, Taiwan
Background: The extent of hepatic resection In HCC depends on the remnant liver reserve or the proximity of the tumor to major vessels. In this study, we evaluated the effects of very close resection margins on postoperative recurrence.
Methods: Consecutive LR for HCC between 2003 and 2009 were studied. Patients were divided into groups with very narrow (≤1 mm) or wider (>1 mm) resection margins. Propensity score matching (PSM) was used to balance demographic, surgical, and pathological factors.
Results: 983 patients were included in the study. After PSM, 173 patients were analyzed in each group. 5-year tumor recurrence and survival rates were comparable. Most recurrences were multiple intrahepatic. Section margin recurrences were similar in both groups. By multivariate analysis, tumor size >5 cm was associated with a very narrow resection margin, whereas low platelet count and tumor macrovascular invasion were significant factors related to tumor recurrence.
Conclusions: Patients with very narrow surgical margins showed outcomes comparable to those with wider surgical margins. Most recurrences were multiple intrahepatic and associated with the degree of portal hypertension and adverse tumor biology. Although wide surgical margins should be aimed whenever possible, a narrow tumor-free margin resection still represents an effective therapeutic strategy.
Introduction
Liver resection (LR) is the mainstay treatment for early hepatocellular carcinoma (HCC) patients. However, even after curative resections, HCC still shows a high recurrence rate (1–3). Among the surgical factors, resection margins have been extensively studied for their effects on postoperative recurrence. During surgery, a wide tumor-free margin is always attempted but the extent of hepatic resection depends on the remnant liver reserve, the depth of the tumor location, and the proximity to major vascular structures (4). Moreover, the resection of excessive liver tissue during surgery may lead to liver dysfunction in patients with liver cirrhosis (5). Liver damage in patients with HCC after resection is also a risk factor associated with recurrence and poor prognosis (6–8).
While a positive surgical margin has a clear impact on oncological outcomes, the significance of close surgical margins remains controversial. Wide margins have been suggested for small (<5 cm) HCCs (9, 10), non-anatomic resections (11), and HCCs with microvascular invasion, without cirrhosis (12), or with high alpha-fetoprotein (AFP) levels (13). However, other investigations demonstrated that margins <1 cm (14) and tumor-negative margins of ≤1 mm had no impact on postoperative recurrence patterns and rates (15, 16).
In this study, we aim to explore the impact of very narrow surgical margins (≤1 mm) on tumor recurrence in patients with HCC who underwent hepatectomy.
Material and Methods
Patients
We retrospectively reviewed the records of patients who underwent LR for HCC at the Chang Gung Memorial Hospital at Linkou, Taiwan, between April 2003 and December 2009. We excluded the patients with intrahospital mortality, mixed type cholangio-hepatocellular carcinoma, fibrolamellar type hepatocellular carcinoma, surgical margin involvement, and post-op follow-up or non-cancer-related survival less than 1 year. The clinical data was obtained from the medical charts and the Taiwan Cancer Registry. The information comprised of the patients' demographics, preoperative laboratory examination, hepatitis serology, surgical features, pathologic features, postoperative complications, tumor staging, tumor recurrence, treatment of tumor recurrence, and the last following-update or date of death.
The study was approved by the institutional review board of the Chang Gung Memorial Hospital (IRB102-4474B).
Hepatectomy
The pre-operative diagnosis of HCC was based on the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver Disease (EASL) guidelines (17, 18).
The criteria for LR and the operative procedures were previously described (2, 5, 19). The extent of liver resection was assessed according to the indocyanine green retention rate at 15 min (ICG R15). ICG R15 was performed by injecting 0.5 mg/kg of ICG into the patients' peripheral vein and drawing a blood sample from another site 15 min later to calculate the retained ratio of ICG. Patients with ICG15 exceeding 20% were carefully selected for major resections, defined as a resection of three or more hepatic segments.
The liver resections were performed using a conventional open approach. Intraoperative ultrasonography was routinely performed in order to confirm resectability and evaluate the relationship between the resection line and major vascular structures. Inflow control with the Pringle maneuver was commonly applied intermittently. Hemivascular control was performed in selected right or left hepatectomies. The liver parenchyma was divided according to the surgeon's preference using a clamp-crushing technique or ultrasonic dissector.
A surgical margin of at least 1 cm was aimed during surgery. However, when the tumor was near major vessels or the patients showed severe comorbidities and liver cirrhosis, a grossly negative macroscopic margin without exposure of the tumor was considered adequate (Supplementary Figure S1). The final resection margin was defined as the shortest microscopic distance from the edge of the tumor to the transection line by histological examination. A wide margin (WM) was defined as a margin >1 mm and a close margin (CM) as a margin ≤ of 1 mm.
Follow up
After surgery, all patients were followed-up every three months. Routine examinations included liver function tests, AFP level, and liver ultrasonography. When ultrasonography revealed a suspicious liver nodule or AFP levels were elevated, tri-phasic computed tomography (CT) or magnetic resonance imaging (MRI) were performed to look for any evidence of tumor recurrence.
The tumor recurrence rate was defined as the interval between the time of liver resection and the detection of recurrence by multiphasic computed tomography, magnetic resonance imaging, and hepatic angiography. The overall survival rate was defined as the interval between the surgery date and the time of death or last follow-up.
Statistical Analysis
Based on an increase in the 5-y tumor recurrence rate of 15% for narrow surgical margin as compared with that for wide surgical margin (14, 20) and assuming an α of 0.05 and a power of 0.80, each treatment group had to include at least 151 patients.
The continuous data were expressed as the median and interquartile ranges. The differences in continuous variables were assessed using Mann-Whitney U tests. The categorical variables were expressed as percentages and analyzed using chi-square tests.
The Kaplan-Meier method with log-rank test was applied to compare survival distributions.
Binary logistic regression was used to examine the variables associated with narrow surgical margins. The Cox proportional hazard regression model was applied to evaluate the risks of tumor recurrence and OS.
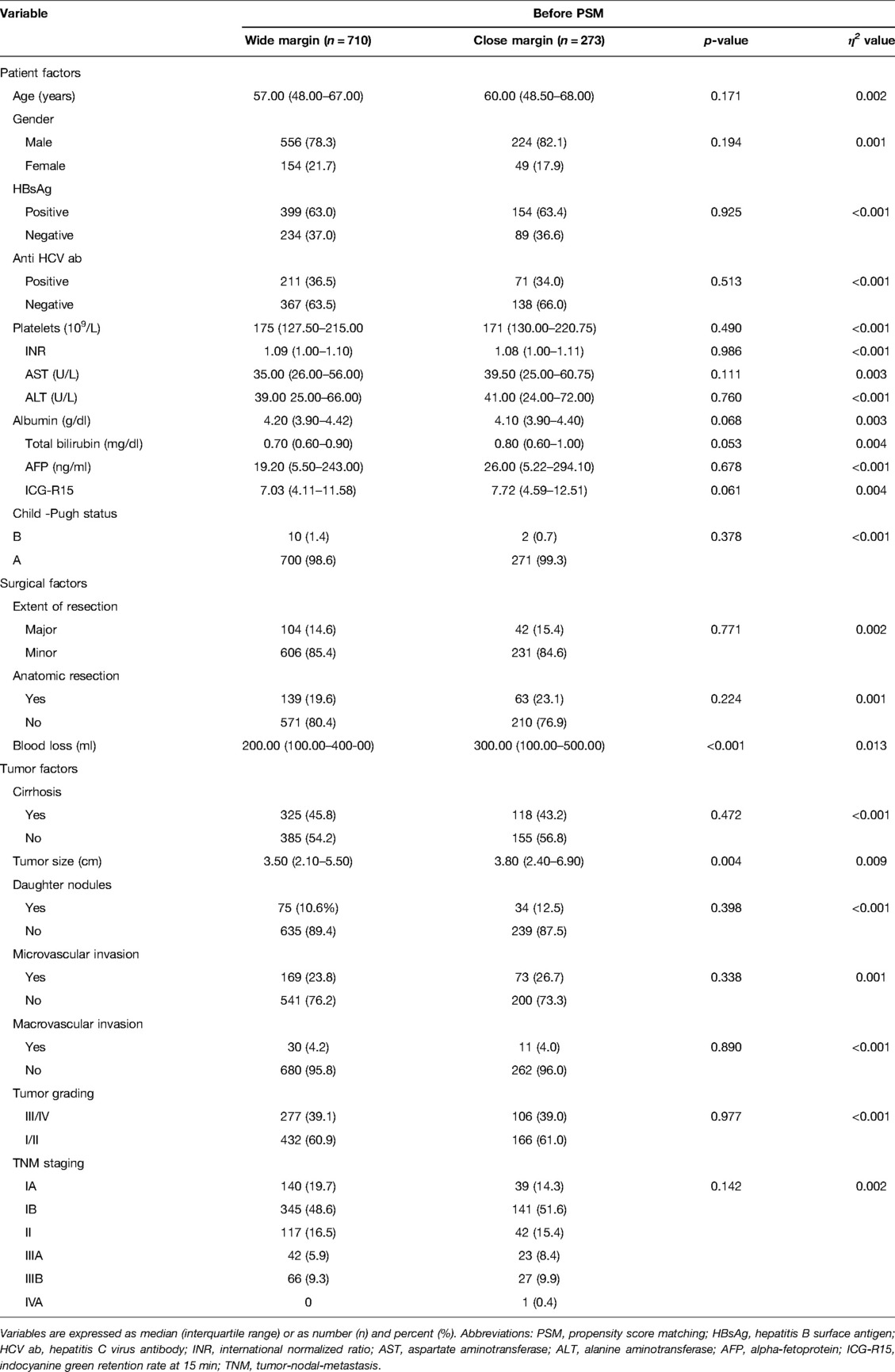
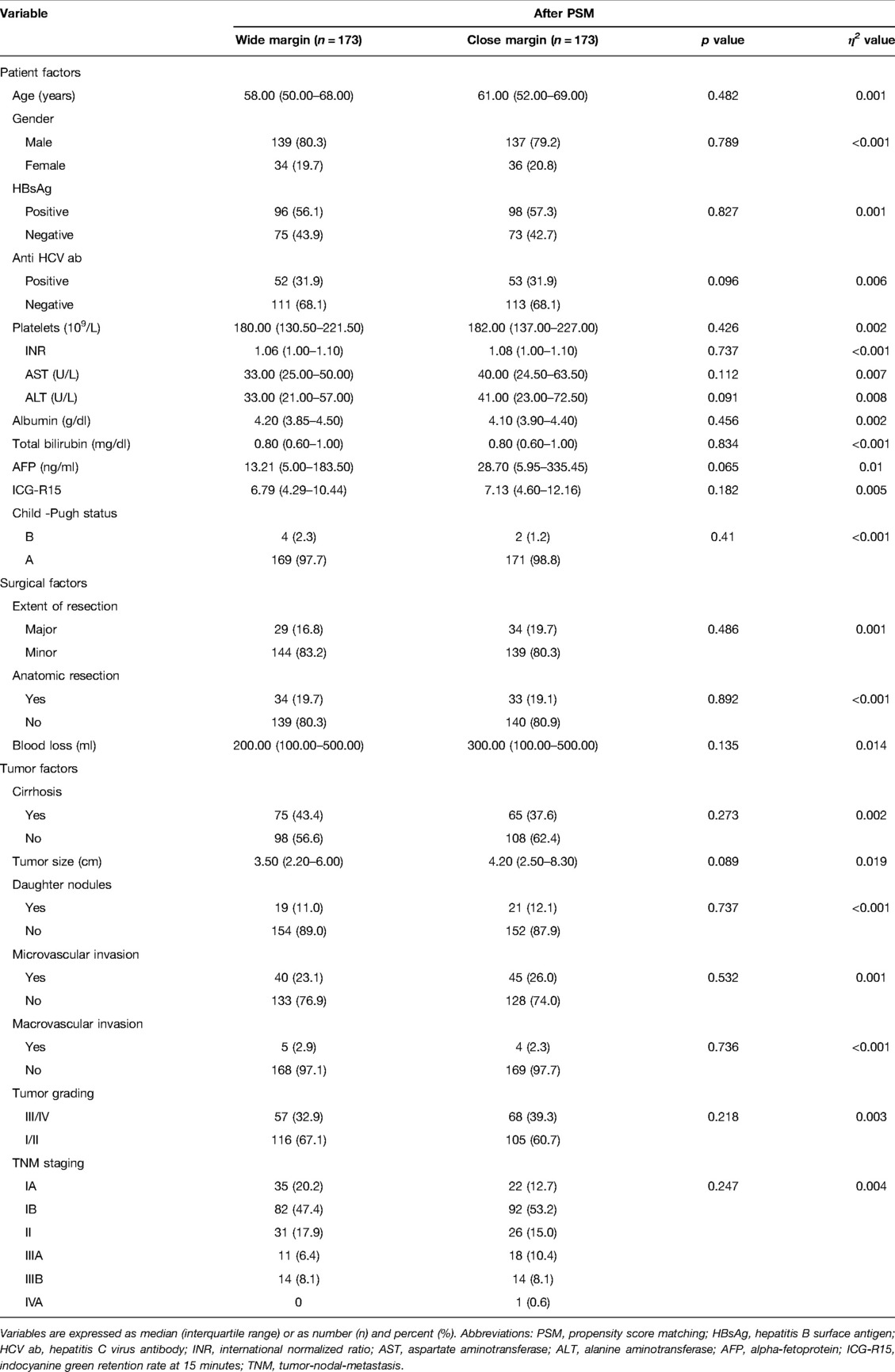
To minimize selection bias, a 1:1 propensity score matching (PSM) was performed using the nearest-neighbor method with a caliper size of 0.05. We included 18 relevant patient, surgical and tumor variables for propensity score generation. These variables included patients age, gender, hepatitis B and C status, platelet counts, albumin levels, Child status, ICG retention rate at 15 min, AFP levels, the extent of LR, intraoperative blood loss, presence of cirrhosis, daughter nodules, microvascular invasion, macrovascular invasion, tumor ruptures, tumor sizes, tumor grading, and tumor/node/metastasis (TNM)/American Joint Committee on Cancer (AJCC) staging system. After propensity score adjustment, both therapy groups were checked again for heterogeneity in covariates with Mann–Whitney U tests. η2 was calculated to confirm the matching balance. p < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS® (SPSS, Chicago, Illinois, USA).
Results
Patients
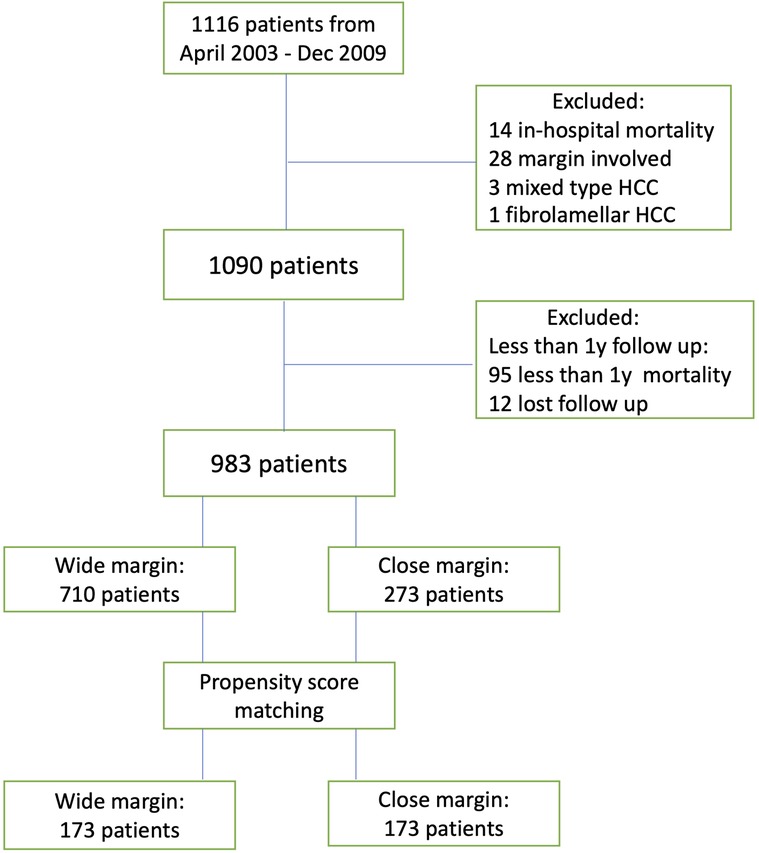
From April 2003 to December 2009, 1,116 patients underwent open liver resection for suspected HCC. After excluding the patients with other diagnoses, in-hospital mortality, surgical margin involvement, and follow-ups of less than 1 year, we included 983 patients in the analysis.
Seven hundred and ten and 273 patients displayed WM and CM, respectively. The median follow-up duration was 85 months (range, 12–196 months) and the last follow-up occurred in September 2019 (Figure 1). After PSM, 173 patients were allocated in each group.
Before PSM, the two groups did not show any differences in the pre-operative factors. The median age of the patients was 58 years old, 79.3% were male, and 63.1% displayed HBV infection. Among the patients, 45.1% had liver cirrhosis, 24.6% tumor microvascular invasion, 4.2% macrovascular invasion, and 11.1% daughter nodules. The median tumor size was 3.5 cm. According to the distribution of TNM staging, we observed that 18.2% of the patients were in stage Ia, 49.4% in stage Ib, 16.2% in stage II, 6.6% in stage IIIa, 9.5% in stage IIIb, and 0.1% in stage IVa. In relation to the surgical and tumor factors, the CM group displayed significantly greater intraoperative blood loss (p < 0.001) and tumor sizes (p = 0.01) (Supplementary Table S1). However, after adjusting for propensity scores, blood loss and tumor sizes were comparable between the two groups (Table 1).
When we analyzed the risk factors associated with CM resections, the univariate and multivariate analysis showed that a tumor size ≥ of 5 cm was the only independent prognostic factor (Table 2).
Tumor Recurrence Rates
Before PSM, the recurrence rates (RR) were significantly higher in the CM group than in the WM group. The median 5-years RR were 63.7% vs. 54.7% and the median 10-years RR were 69% vs. 72.7% (p = 0.014) in the CM and WM groups, respectively (Figure 2A). Following PSM, the RR were not statistically different between the two groups. The median 5-years RR were 54.6% and 63.4% and the median 10-years RR were 69.5% and 72.5% (p = 0.155) in the CM and WM groups, respectively (Figure 2B).
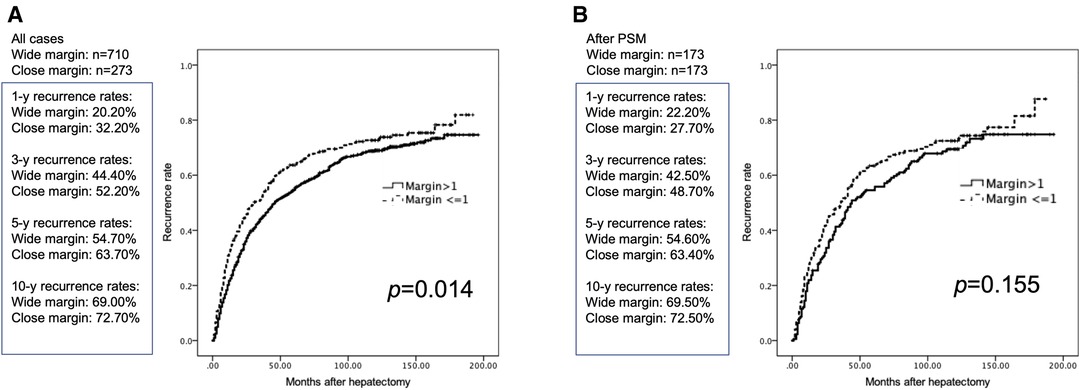
Figure 2. Cumulative postoperative tumor recurrence rates in patients with margin >1 and ≤1 mm. (A) Before PSM. (B) After PSM.
Risks Factor for Tumor Recurrence
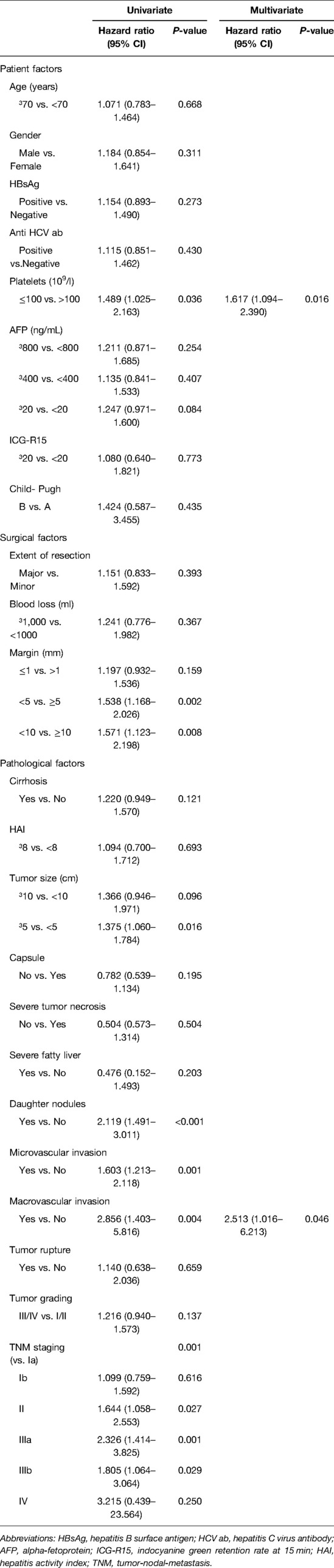
After PSM, by univariate analysis, we observed that a low platelet count (platelet count ≤100,000/μl), a tumor size ≥ of 5 cm, the presence of daughter nodules, tumor macrovascular invasion, and tumor TNM staging were significant prognostic factors for tumor recurrence. The multivariate analysis showed that a low platelet count and the presence of tumor macrovascular invasion were the only significant factors for tumor recurrence (Table 3).
Patterns of Tumor Recurrence and Patient Survival
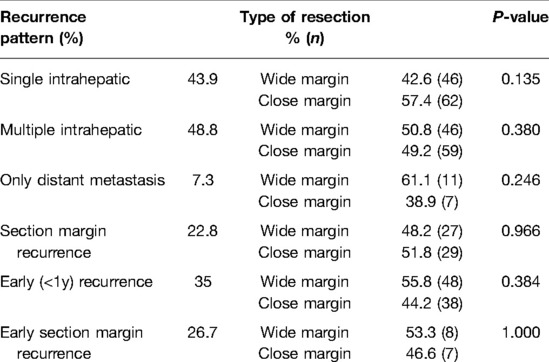
We observed that 43.9% of the patients developed single intrahepatic recurrence, 48.8% experienced multiple intrahepatic recurrences, and 7.3% displayed distant metastasis without intrahepatic recurrence. The patterns of tumor recurrence were not significantly different between the CM and WM patients. When we further evaluated the impact of close resection margins on section margin recurrence (recurrence at ≤1 cm from the resection margin regardless of whether there was any simultaneous intra- or extrahepatic recurrence) or early recurrence (recurrence ≤1 year), we also did not find any significant difference between the two groups (Table 4). Finally, the long-term OS was similar between the two groups. The 5-year and 10-year survival rates were 67.5% and 52.2% in the CM group and 74.3% and 54.9% in the WM group (p = 0.160), respectively (Supplementary Figure S2). For the patients with tumor microvascular invasion, satellite nodules or tumor macrovascular invasion, there were also no significant differences in recurrence and survival rates between the two groups (Supplementary Figure S3).
Discussion
A wide tumor-free margin is paramount during oncological resections to avoid residual tumors at the resection site to promote tumor recurrence. However, during liver resections for HCC, wide surgical margins are limited by the presence of portal hypertension or liver cirrhosis. Additionally, major hepatic resections are associated with increased morbidity. Likewise, the tumors close to major hepatic veins or branches of the Glisson's pedicles are detached from the vessels with CUSA® and a sufficient surgical margin is sacrificed to preserve more liver parenchyma. Therefore, we conducted the present study to investigate the impact of very close margins on the outcome of patients with HCC undergoing resection.
Patients with HCC differ considerably in their baseline liver function and tumor characteristics. To reduce confounding variables and equate the treatment groups, we conducted PSM in 983 patients with long-term follow up. After a median follow-up of 85 months, our study did not show any statistical differences in tumor recurrence rates in the close and wide resection groups. By multivariate analysis, we also found that a low platelet count and the presence of tumor macrovascular invasion were the only independent prognostic factors, which underscores the impact of patients' characteristics and tumor biology on surgical factors.
The prognostic significance of wide surgical margins has been addressed in many previous studies with different cutoff values ranging from 2 cm to no margin. Poon et al. analyzed patients with <1 cm and ≥1 cm resection margins and the two groups showed comparable recurrence rates (14). On multivariate analysis, the authors found that only a pTNM stage of III/IV and perioperative transfusions were significant risk factors of tumor recurrence. Oguro et al. did not show any difference in the recurrence-free and overall survival of HCC patients undergoing macroscopic no-margin hepatectomy. Although a microscopically positive surgical margin was more frequent in the no-margin hepatectomy group than the control group, a microscopically positive margin was not associated with a higher incidence of recurrence in the remnant liver (15). Similarly, in another study investigating HCC patients who underwent resection with exposure of the tumor surface, the authors did not show any significant differences in the recurrence and survival rates between the tumor exposure group and the non-exposure group (21). Notably, the influence of tumor encapsulation was not observed in close marginal resections. In our study, we also did not find any correlation between the presence of tumor capsule and postoperative outcomes.
Several reports showed that the width of the resection margins had no impact on tumor recurrence or patient survival. However, several other studies also associated improved outcomes with wider surgical margins (12, 22, 23). Nara et al. categorized the patients according to the macroscopic appearance of HCC and reported that a wide resection resulted in better recurrence-free survival in patients with non-simple nodular type tumors without cirrhosis (20). However, the recurrence-free survival rates were not affected by the type of resection in patients with cirrhosis. The only prospective randomized trial that stratified the patients according to surgical margins (1 or 2 cm) advocated that wider margins gave a survival advantage only to the patients with HCC ≤2 cm (10). However, the group of patients with the survival advantage was very small (i.e., wide margins 12 patients, narrow margins 10 patients) and the margins width did not show any significant impact on tumor recurrence, regardless of HCC size.
Recurrences after surgery are mostly intrahepatic (24–26) and wide resections are aimed to avoid recurrences at the resection site. A previous study examined the patterns of intrahepatic micrometastases using large pathologic sections on liver specimens with ample resection margins and reported that the spread of micrometastases ranged from 0.05 to 6.1 cm (27). This supports the concept that extensive anatomic resections can achieve better tumor clearance by removing tumor-bearing portal territories. However, tumors can propagate proximally and distally after microscopic portal vein invasion (28) and the tumor dissemination can involve nonadjacent hepatic segments (29, 30). Additionally, tumor recurrence may also result from metachronous tumors that arise in the oncogenic cirrhotic liver (31–33). Therefore, even with extensive resections, it is difficult to eliminate the disease in every patient. In this study, most recurrences occurred in distal liver segments or multiple segments regardless of the margin width. This suggests that most recurrences were due to intrahepatic distant metastasis or multicentric carcinogenesis. In our study, overall 48.8% of the tumor recurrences were multiple intrahepatic recurrences and close resection margins were not associated with increased recurrences. In addition, early tumor recurrence is another concern after surgery for HCC, and is a leading cause of death within 2 years (34). Previously reported risk factors of early tumor recurrence included vascular invasion and positive margins (34) but the extension and the type of resection did not show any correlation with the risk of recurrence provided that the surgery was radical (3). In this study, the rates of early tumor recurrence were similar between the two section margin groups and even the subgroup of patients with section margin recurrences.
Our study has several limitations. As a retrospective study, confounding variables and selection bias cannot be fully eliminated even after PSM. Furthermore, to achieve a sufficiently long follow-up, we included patients who underwent liver resections before 2009. As a result, we did not include patients who underwent laparoscopic resections because this approach was not widely performed before 2009. Additionally, the histological and genetic features of the recurrent tumors were not analyzed or compared with the primary tumors, so as to classify as intrahepatic metastases or de novo hepatocarcinogenesis, which would provide a better insight into real tumor-free resection margins.
In conclusion, the patients with very narrow surgical margins showed outcomes comparable to those with wider margins. Most recurrences were multiple intrahepatic recurrences related to the degree of portal hypertension and adverse tumor biology. Although wide surgical margins should be whenever possible, a narrow tumor-free margin resections still represent an effective therapeutic strategy.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by The study was approved by the institutional review board of the Chang Gung Memorial Hospital (IRB102-4474B). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
CHC: drafting the manuscript and data collection. CHC, YL, HCH, JCL, YCW, THW, CFL: data collection, TJW, HSC, KMC and WCL designing and revising the manuscript. All authors contributed to the article and approved the submitted version.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.926728/full#supplementary-material.
Supplementary Figure 1 | Abdominal computed tomography and intraoperative images of tumors overriding the middle hepatic vein (A) or the porta hepatis (B). The tumors were detached from the major vessels without a macroscopic margin.
Supplementary Figure 2 | Cumulative postoperative patient survival rates in patients with margin >1 and ≤1 mm after PSM.
Supplementary Figure 3 | Cumulative postoperative tumor recurrence and patient survival rates in patients with tumor microvascular invasion (A), satellite nodules (B) and tumor macrovascular invasion (C) according to margin >1 and ≤1 mm.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Shimada M, Takenaka K, Gion T, Fujiwara Y, Kajiyama K, Maeda T, et al. Prognosis of recurrent hepatocellular carcinoma: a 10-year surgical experience in Japan. Gastroenterology. (1996) 111(3):720–6. doi: 10.1053/gast.1996.v111.pm8780578
2. Yeh CN, Lee WC, Chen MF, Tsay PK. Predictors of long-term disease-free survival after resection of hepatocellular carcinoma: two decades of experience at Chang Gung Memorial Hospital. Ann Surg Oncol. (2003) 10(8):916–21. doi: 10.1245/aso.2003.09.012
3. Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GA, et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. (2006) 243(2):229–35. doi: 10.1097/01.sla.0000197706.21803.a1
4. Cheng CH, Yu MC, Wu TH, Lee CF, Chan KM, Chou HS, et al. Surgical resection of centrally located large hepatocellular carcinoma. Chang Gung Med J. (2012) 35(2):178–91. doi: 10.4103/2319-4170.106153
5. Dahiya D, Wu TJ, Lee CF, Chan KM, Lee WC, Chen MF. Minor versus major hepatic resection for small hepatocellular carcinoma (HCC) in cirrhotic patients: a 20-year experience. Surgery. (2010) 147(5):676–85. doi: 10.1016/j.surg.2009.10.043
6. Tarao K, Rino Y, Ohkawa S, Shimizu A, Tamai S, Miyakawa K, et al. Association between high serum alanine aminotransferase levels and more rapid development and higher rate of incidence of hepatocellular carcinoma in patients with hepatitis C virus-associated cirrhosis. Cancer. (1999) 86(4):589–95. doi: 10.1002/(sici)1097-0142(19990815)86:4<589::aid-cncr7>3.0.co
7. Hanazaki K, Wakabayashi M, Sodeyama H, Kajikawa S, Amano J. Hepatic function immediately after hepatectomy as a significant risk factor for early recurrence in hepatocellular carcinoma. Hepatogastroenterology. (1999) 46(30):3201–7.
8. Cho WR, Hung CH, Chen CH, Lin CC, Wang CC, Liu YW, et al. Ability of the post-operative ALBI grade to predict the outcomes of hepatocellular carcinoma after curative surgery. Sci Rep. (2020) 10(1):7290. doi: 10.1038/s41598-020-64354-0
9. Tsilimigras DI, Sahara K, Moris D, Hyer JM, Paredes AZ, Bagante F, et al. Effect of surgical margin width on patterns of recurrence among patients undergoing R0 hepatectomy for T1 hepatocellular carcinoma: an international multi-institutional analysis. J Gastrointest Surg. (2020) 24(7):1552–60. doi: 10.1007/s11605-019-04275-0
10. Shi M, Guo RP, Lin XJ, Zhang YQ, Chen MS, Zhang CQ, et al. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Ann Surg. (2007) 245(1):36–43. doi: 10.1097/01.sla.0000231758.07868.71
11. Dong S, Wang Z, Wu L, Qu Z. Effect of surgical margin in R0 hepatectomy on recurrence-free survival of patients with solitary hepatocellular carcinomas without macroscopic vascular invasion. Medicine (Baltimore). (2016) 95(44):e5251. doi: 10.1097/md.0000000000005251
12. Wang H, Yu H, Qian YW, Cao ZY, Wu MC, Cong WM. Impact of surgical margin on the prognosis of early hepatocellular carcinoma (≤5 cm): a propensity score matching analysis. Front Med (Lausanne). (2020) 7:139. doi: 10.3389/fmed.2020.00139
13. Lee JC, Cheng CH, Wang YC, Wu TH, Lee CF, Wu TJ, et al. Clinical relevance of alpha-fetoprotein in determining resection margin for hepatocellular carcinoma. Medicine (Baltimore). (2019) 98(11):e14827. doi: 10.1097/md.0000000000014827
14. Poon RT, Fan ST, Ng IO, Wong J. Significance of resection margin in hepatectomy for hepatocellular carcinoma: a critical reappraisal. Ann Surg. (2000) 231(4):544–51. doi: 10.1097/00000658-200004000-00014
15. Oguro S, Yoshimoto J, Imamura H, Ishizaki Y, Kawasaki S. Clinical significance of macroscopic no-margin hepatectomy for hepatocellular carcinoma. HPB (Oxford). (2018) 20(9):872–80. doi: 10.1016/j.hpb.2018.03.012
16. Lee JW, Lee YJ, Park KM, Hwang DW, Lee JH, Song KB. Anatomical resection but not surgical margin width influence survival following resection for HCC, a propensity score analysis. World J Surg. (2016) 40(6):1429–39. doi: 10.1007/s00268-016-3421-5
17. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD Guidelines for the treatment of hepatocellular carcinoma. Hepatology. (2018) 67(1):358–80. doi: 10.1002/hep.29086
18. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. (2018) 69(1):182–236. doi: 10.1016/j.jhep.2018.03.019.
19. Cheng CH, Lee CF, Wu TH, Chan KM, Chou HS, Wu TJ, et al. Evaluation of the new AJCC staging system for resectable hepatocellular carcinoma. World J Surg Oncol. (2011) 9:114. doi: 10.1186/1477-7819-9-114
20. Nara S, Shimada K, Sakamoto Y, Esaki M, Kishi Y, Kosuge T, et al. Prognostic impact of marginal resection for patients with solitary hepatocellular carcinoma: evidence from 570 hepatectomies. Surgery. (2012) 151(4):526–36. doi: 10.1016/j.surg.2011.12.002
21. Matsui Y, Terakawa N, Satoi S, Kaibori M, Kitade H, Takai S, et al. Postoperative outcomes in patients with hepatocellular carcinomas resected with exposure of the tumor surface: clinical role of the no-margin resection. Arch Surg. (2007) 142(7):596–602. doi: 10.1001/archsurg.142.7.596
22. Liu L, Shui Y, Yu Q, Guo Y, Zhang L, Zhou X, et al. Narrow-margin hepatectomy resulted in higher recurrence and lower overall survival for R0 resection hepatocellular carcinoma. Front Oncol. (2020) 10:610636. doi: 10.3389/fonc.2020.610636
23. Zhong FP, Zhang YJ, Liu Y, Zou SB. Prognostic impact of surgical margin in patients with hepatocellular carcinoma: a meta-analysis. Medicine (Baltimore). (2017) 96(37):e8043. doi: 10.1097/md.0000000000008043
24. Belghiti J, Panis Y, Farges O, Benhamou JP, Fekete F. Intrahepatic recurrence after resection of hepatocellular carcinoma complicating cirrhosis. Ann Surg. (1991) 214(2):114–7. doi: 10.1097/00000658-199108000-00004
25. Chan KM, Lee WC, Hung CF, Yu MC, Jan YY, Chen MF. Aggressive multimodality treatment for intra-hepatic recurrence of hepatocellular carcinoma following hepatic resection. Chang Gung Med J. (2005) 28(8):543–50.
26. Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg. (1999) 229(2):216–22. doi: 10.1097/00000658-199902000-00009
27. Shi M, Zhang CQ, Zhang YQ, Liang XM, Li JQ. Micrometastases of solitary hepatocellular carcinoma and appropriate resection margin. World J Surg. (2004) 28(4):376–81. doi: 10.1007/s00268-003-7308-x
28. Mitsunobu M, Toyosaka A, Oriyama T, Okamoto E, Nakao N. Intrahepatic metastases in hepatocellular carcinoma: the role of the portal vein as an efferent vessel. Clin Exp Metastasis. (1996) 14(6):520–9. doi: 10.1007/bf00115112
29. Okusaka T, Okada S, Ueno H, Ikeda M, Shimada K, Yamamoto J, et al. Satellite lesions in patients with small hepatocellular carcinoma with reference to clinicopathologic features. Cancer. (2002) 95(9):1931–7. doi: 10.1002/cncr.10892
30. Nakashima Y, Nakashima O, Tanaka M, Okuda K, Nakashima M, Kojiro M. Portal vein invasion and intrahepatic micrometastasis in small hepatocellular carcinoma by gross type. Hepatol Res. (2003) 26(2):142–7. doi: 10.1016/s1386-6346(03)00007-x
31. Hoshida Y. Risk of recurrence in hepatitis B-related hepatocellular carcinoma: impact of viral load in late recurrence. J Hepatol. (2009) 51(5):842–4. doi: 10.1016/j.jhep.2009.08.003
32. Kubo S, Kinoshita H, Hirohashi K, Tanaka H, Tsukamoto T, Hamba H, et al. Patterns of and risk factors for recurrence after liver resection for well-differentiated hepatocellular carcinoma: a special reference to multicentric carcinogenesis after operation. Hepatogastroenterology. (1999) 46(30):3212–5.
33. Matsumoto Y, Fujii H, Matsuda M, Kono H. Multicentric occurrence of hepatocellular carcinoma: diagnosis and clinical significance. J Hepatobiliary Pancreat Surg. (2001) 8(5):435–40. doi: 10.1007/s005340100006
Keywords: hepatocellu, hepatectomy, margin, recurrence pattern, recurrence factors
Citation: Cheng C, Lai Y, Hung H, Lee J, Wang Y, Wu T, Lee C, Wu T, Chou H, Chan K and Lee W (2022) Recurrence Patterns After Hepatectomy With Very Narrow Resection Margins for Hepatocellular Carcinoma. Front. Surg. 9:926728. doi: 10.3389/fsurg.2022.926728
Received: 23 April 2022; Accepted: 20 June 2022;
Published: 12 July 2022.
Edited by:
Alessandro Vitale, University Hospital of Padua, ItalyReviewed by:
Liu Weiren, Fudan University, ChinaWeixing Guo, Eastern Hepatobiliary Surgery Hospital, China
Copyright © 2022 Cheng, Lai, Hung, Lee, Wang, Wu, Lee, Wu, Chou, Chan and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei-Chen Lee weichen@cgmh.org.tw
Specialty section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Chih-Hsien Cheng
Chih-Hsien Cheng Yin Lai
Yin Lai Hao-Chien Hung1
Hao-Chien Hung1  Kun-Ming Chan
Kun-Ming Chan Wei-Chen Lee
Wei-Chen Lee