Subcutaneous Vulvar Flap Viability Evaluation With Near-Infrared Probe and Indocyanine Green for Vulvar Cancer Reconstructive Surgery: A Feasible Technique
- 1Department of Medicine and Surgery, University of Parma, Parma, Italy
- 2Department of Gynecologic Oncology, University of Palermo, Palermo, Italy
- 3Department of Women's and Children's Health, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy
- 4Department of Medicine and Health Science “V.Tiberio”, Università degli Studi del Molise, Campobasso, Italy
- 5Department of Human Pathology of the Adult and of the Childhood “Gaetano Barresi”, Università di Messina, Messina, Italy
Introduction: Vulvar cancer is a rare condition affecting older women and accounts for 3–5% of all gynecological cancers. Primary surgical treatment involves the removal of a large amount of tissue for which reconstructive surgery is often necessary with a high rate of postoperative complications. Despite several techniques for the evaluation of vulvar flap viability have been proposed, many methods cannot be performed during surgery and require expensive devices often missing in a gynecological clinic. This study aims to verify the feasibility and the safety of the vulvar flap viability evaluation through a near-infrared endoscopic probe and Indocyanine green (ICG) tracer in a small group of patients and to evaluate long-term vulvar flap outcomes.
Methods: Patients with primary vulvar cancer who required surgical treatment and subsequent vulvar flap reconstructive surgery were prospectively included in the study. A 25 mg ICG vial diluted in 20 ml of saline solution was intravenously infused before closing the skin edges of the flaps. All patients were given 0.2 mg/kg body weight of intravenous ICG. After 10–15 min, a near-infrared endoscopic probe was used to evaluate the vulvar flap viability.
Results: Of the 18 patients who underwent radical vulvectomy for vulvar cancer during the study period, 15 were included in the analysis. All packaged surgical flaps showed tracer uptake on the surgical margin. No intro-operative complications were recorded neither surgery-related nor to dye infusion. No surgical infection, dehiscence, or necrosis was recorded.
Conclusions: Vulvar flap viability assessment using Indocyanine green and a laparoscopic infrared probe is a feasible method. All cases included in the analysis showed a dye uptake on the surgical edge of the flap. Further, prospective studies are needed to confirm the method in clinical practice and to evaluate its superiority over simple subjective clinical evaluation.
Introduction
Vulvar cancer is a rare condition affecting older women and accounts for 3–5% of all gynecological cancers (1). Primary surgical treatment is radical vulvectomy with inguinal nodal staging (2). A disease-free margin of 8 mm by the primary lesion should be ensured, and this could lead to extensive demolition surgery which may require a plastic reconstructive surgical time (3).
Reconstructive surgery should replace the removed tissue with a healthy donor area through tension-free skin closure. Although, multiple reconstructive surgical techniques have been proposed over the years, the rate of postoperative complications remains high (4, 5). In addition, surgical complications for malignant gynecological pathology are affected by a high complication rate (6). In particular, wound dehiscences, low genital tract infections, vaginal and anovaginal shrinkage, urethral damage, and anti-aesthetic reconstructions may worsen the patient's quality of life and could require reintervention (7–11).
The fasciocutaneous advancement flaps are the most used by the oncologist gynecologist for their easier feasibility with satisfactory surgical outcomes (12). Hand et al. reported 33 and 15% of surgical dehiscence and infectious complications, respectively with V-Y fasciocutaneous flap reconstruction (13).
A key point for the vulvar flap success is the evaluation of the recovered tissue viability after its displacement. Despite several techniques for the evaluation of vulvar flap viability have been proposed, many methods cannot be performed during surgery and require expensive devices often missing in a gynecological clinic (14–16). Angiography with Indocyanine green (ICG) shows a 90% sensitivity in the flap vitality assessment but the clinical evaluation of color, turgor, and bleeding of the flap still represent a crucial aspect that should always be evaluated (17). Furthermore, a unanimous consensus on which is the best method to use has not been reached worldwide (2, 18).
We previously proposed for the first time the vulvar flap viability evaluation using a near-infrared endoscopic probe and ICG tracer (19). The method, applied on a single patient, proved to be safe, easy to perform and did not require missing devices in common gynecological clinics.
This study aims to verify the feasibility and the safety of the vulvar flap viability evaluation through a near-infrared endoscopic probe and ICG tracer in a small group of patients and to evaluate long-term vulvar flap outcomes.
Methods
Patients with primary vulvar cancer who required surgical treatment and subsequent vulvar flap reconstructive surgery from January to November 2019 accessing the department of Gynecology and Obstetrics of the University of Parma were prospectively included in the pilot study.
According to the National Comprehensive Cancer Network (NCCN) guidelines, all patients underwent modulated surgery and subsequent adjuvant therapy related to the site and size of the primary lesion (2). The vulvar flap used was chosen concerning the site and the amount of tissue to be replaced (18).
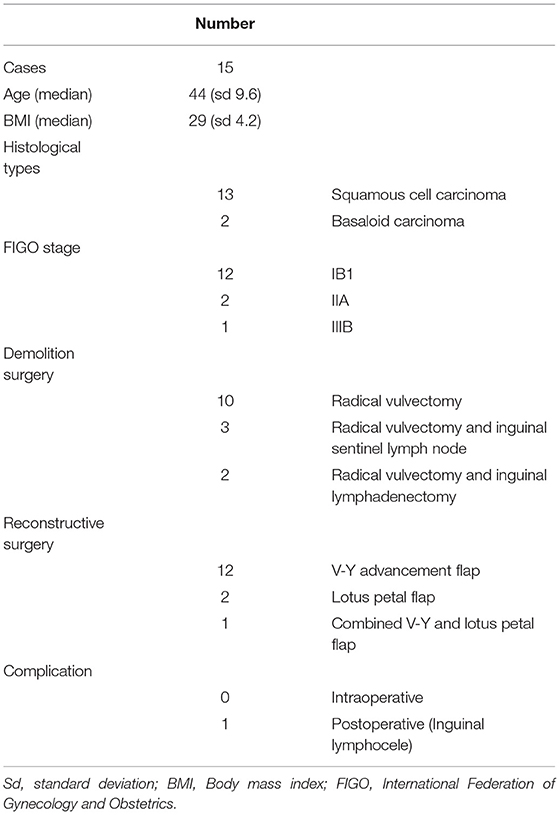
A 25 mg ICG vial diluted in 20 ml of saline solution was intravenously infused before closing the skin edges of the flaps. After 10–15 min, a near-infrared endoscopic probe (Storz Endoscopic IMAGE 1SH3-link TC300 and Light source Storz D-light P 20133720) was used to evaluate the vulvar flap viability. A subsequent flap evaluation was performed after closing the skin edges of the flaps, 30 min after the ICG infusion. A double-check by two different operators to verify the flap viability was performed.
Anamnestic data, BMI, age at the time of surgery, type of surgery, type of reconstructive flap, International Federation of Gynecology and Obstetrics (FIGO) stage, intraoperative, and postoperative complications were collected for each patient.
All patients underwent clinical follow-up at our department every 3 months. All patients who did not perform reconstructive vulval flap surgery, patients who did not provide their informed consent, patients who did not perform a clinical follow-up for at least 6 months, or with known allergy to the contrast agents were excluded from the study. Furthermore, patients with multiple comorbidities, or with American Society of Anesthesiologists (ASA) status 3 were excluded from the analysis.
All surgical procedures, both in the demolition and reconstructive phase, were performed by gynecologists oncologists dedicated to vulvar cancer.
All patients provided their informed consent for the writing of the article and the publication of the images and videos in an anonymous format. The study was approved by the ethics committee of the University of Parma, with protocol number: 0001123.
Results
Of the 18 patients who underwent radical vulvectomy for vulvar cancer in our department during the study period, 15 were included in the analysis. Two patients did not provide informed consent for study participation and one did not perform post-operative follow-up.
Median age was 74 years with standard deviation (sd) 9.6, median body mass index (BMI) was 29 (sd 4.2), 13 patients had squamous cancer, and two basaloid histology. Twelve patients were FIGO stage IB1, one IIIB, and two cases showed FIGO stage IIA. The median lesion diameter was 20 mm. All patients underwent radical vulvectomy, the sentinel lymph node was removed bilaterally in 3 patients, and bilateral inguinal lymphadenectomy was performed in two patients. All patients underwent reconstructive surgery after vulvectomy, 12 patients with a V-Y advancement flap, two with a lotus petal flap, and one with a combined V-Y flap and lotus petal flap for hemivulva. Patient characteristics are shown in Table 1. All packaged surgical flaps showed tracer uptake on the surgical margin. No intro-operative complications were recorded neither surgery-related nor to dye infusion. No surgical infection, dehiscence, or necrosis was recorded. One patient showed a lymphocele at the site of inguinal lymphadenectomy that was subsequently surgically drained.
Figures 1–3 show the preoperative, intraoperative, and 3-month follow-up cases.

Figure 1. Fasciocutaneous advancement V-Y flap (preoperative, postoperative, and three-month follow-up).
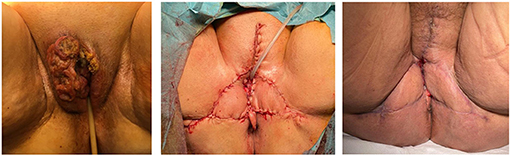
Figure 2. Bilateral fasciocutaneous advancement V-Y flap (preoperative, postoperative, and three-month follow-up).
Discussion
The present case series has confirmed the feasibility of the intraoperative technique of vulvar flap vitality assessment using indocyanine green and laparoscopic probe. No intra and postoperative procedure-related complications were reported.
In 1980, Magrina et al. first described how intravenous fluorescein with ultraviolet light allowed a good identification of poorly vascularized areas following radical vulvectomy (20). However, at the time, a single skin incision was performed for inguinal lymphadenectomy and radical vulvectomy significantly increasing the rate of postoperative complications compared to the double inguinal-vulvar incisions (2). Over the years, different methods have been described for flap vitality evaluation both in gynecologic and plastic surgery. Oliver et al. (21) with orthogonal polarization imaging, Furukawa et al. (22) with indocyanine-green fluorescence angiography, and Zhu et al. (23) via diffuse reflectance spectroscopy reported the reproducibility of different techniques to assess skin flap vascularity. Unfortunately, all of these methods require expensive machinery, often missing in common gynecological clinics.
To overcome this problem, we experimented with an evaluation of the vulvar flap viability using devices normally used in gynecological surgery for sentinel lymph node identification in endometrial, cervical, or ovarian cancer (24–29). Through the laparoscopic infrared probe and ICG dye was possible to evaluate the vascularization of the vulvar flap surgical margins. The dye administered intravenously will reach the margins of the surgical wound showing, where present, a good vascularization of the flap. In non-uptake of the dye cases, the flap is probably not adequately vascularized and could result in postoperative surgical dehiscence. Furthermore, in these cases, a resection of the surgical margins or the packaging of a new vulvar flap may be necessary.
We know that this method has limitations. The major limit is the subjective assessment of tracer uptake. Unlike angiography which shows an objective assessment of the degree of flap vascularization, with our method, the evaluation should be limited to the uptake vs. non-uptake. However, in line with our method and as reported by several authors, a standardized method for vulvar flap viability evaluation is still absent, and subjective clinical evaluation still represents one of the best methods for assessing vulvar flap vitality (30–32). Furthermore, it would be useful to prospectively evaluate the flap viability using ICG and infrared probes with only clinical evaluation to assess whether this new method allows better surgical outcomes. Furthermore, both the complication and the reoperation that could be avoided with this method would certainly improve the patients' quality of life, as reported for other surgeries (33–37).
To conclude, recent studies are successfully investigating the use of the combined ICG and technetium 99 m Nanocolloid for sentinel lymph node identification in vulvar cancer patients (38, 39). In line with these authors, we believe that the ICG is a safe method, in which the oncologist gynecologist is confident, and that could shortly find different uses in vulvar cancer patients. To date, a prospective multicenter study with a control group is planned to confirm the results of the present pilot study.
Conclusions
Vulvar flap viability assessment using Indocyanine green and a laparoscopic infrared probe is a feasible method. All cases included in the analysis showed a dye uptake on the surgical edge of the flap. Further, prospective studies are needed to confirm the method in clinical practice and to evaluate its superiority over simple subjective clinical evaluation.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by University of Parma. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
VC and RB: project development, data collection, manuscript writing, and editing. LM, GA, GS, and AR: data collection and manuscript writing. SG, FC, AE, and SC: protocol/project development and manuscript writing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer [ET] declared a shared affiliation, with no collaboration, with the authors [AR and SG] to the handling editor.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rogers LJ, Cuello MA. Cancer of the vulva. Int J Gynaecol Obs. (2018) 143(Suppl. 2):4–13. doi: 10.1002/ijgo.12609
2. National Comprehensive Cancer Network. Vulvar Cancer (Squamous Cell Carcinoma) Version 1.2020. Available online at: https://www.nccn.org/professionals/physician_gls/pdf/vulvar.pdf (accessed April 18, 2020).
3. Woelber L, Choschzick M, Eulenburg C, Hager M, Jaenicke F, Gieseking F, et al. Prognostic value of pathological resection margin distance in squamous cell cancer of the vulva. Ann Surg Oncol. (2011) 18:3811–8. doi: 10.1245/s10434-011-1778-0
4. Wills A, Obermair A. A review of complications associated with the surgical treatment of vulvar cancer. Gynecol Oncol. (2013) 131:467–79. doi: 10.1016/j.ygyno.2013.07.082
5. Salgarello M, Farallo E, Barone-Adesi L, Cervelli D, Scambia G, Salerno G, et al. Flap algorithm in vulvar reconstruction after radical, extensive vulvectomy. Ann Plast Surg. (2005) 54:184–90. doi: 10.1097/01.sap.0000141381.77762.07
6. Bizzarri N, du Bois A, Fruscio R, De Felice F, De Iaco P, Casarin J, et al. Is there any therapeutic role of pelvic and para-aortic lymphadenectomy in apparent early stage epithelial ovarian cancer? Gynecol Oncol. (2021) 160:56–63. doi: 10.1016/j.ygyno.2020.10.028
7. Hellinga J, Te Grootenhuis NC, Werker PMN, de Bock GH, van der Zee AGJ, Oonk MHM, et al. Quality of life and sexual functioning after vulvar reconstruction with the lotus petal flap. Int J Gynecol Cancer. (2018) 28:1728–36. doi: 10.1097/IGC.0000000000001340
8. Capozzi VA, Riemma G, Rosati A, Vargiu V, Granese R, Ercoli A, et al. Surgical complications occurring during minimally invasive sentinel lymph node detection in endometrial cancer patients. A systematic review of the literature and metanalysis. Eur J Surg Oncol. (2021). doi: 10.1016/j.ejso.2021.03.253
9. Cianci S, Rosati A, Capozzi VA, Tarascio M, Uccella S, Palumbo M, et al. Quality of life and sexual functioning of patient affected by endometrial cancer. Minerva Med. (2020) 112:81–95. doi: 10.23736/S0026-4806.20.07081-0
10. Gueli Alletti S, Perrone E, Cretì A, Cianci S, Uccella S, Fedele C, et al. Feasibility and perioperative outcomes of percutaneous-assisted laparoscopic hysterectomy: a multicentric Italian experience. Eur J Obstet Gynecol Reprod Biol. (2020) 245:181–5. doi: 10.1016/j.ejogrb.2019.12.020
11. Scaletta G, Quagliozzi L, Cianci S, Vargiu V, Mele MC, Scambia G, et al. Management of postoperative chylous ascites after surgery for ovarian cancer: a single-institution experience. Updates Surg. (2019) 71:729–34. doi: 10.1007/s13304-019-00656-x
12. Hand LC, Maas TM, Baka N, Mercier RJ, Greaney PJ, Rosenblum NG, et al. Utilizing V-Y fasciocutaneous advancement flaps for vulvar reconstruction. Gynecol Oncol Rep. (2018) 26:24–8. doi: 10.1016/j.gore.2018.08.007
13. Kim SI, Lee M, Kim HS, Chung HH, Kim JW, Park NH, et al. Effect of BRCA mutational status on survival outcome in advanced-stage high-grade serous ovarian cancer. J Ovarian Res. (2019) 12:40. doi: 10.1186/s13048-019-0511-7
14. Holm C, Mayr M, Becker A, Pfeiffer UJ, Miihlbauer W. Intraoperative evaluation of skin-flap viability using laser-induced fluorescence of indocyanine green. Br J Plast Surg. (2002) 55:635–44. doi: 10.1054/bjps.2002.3969
15. Krishnan KG, Schackert G, Steinmeier R. The role of near-infrared angiography in the assessment of post-operative venous congestion in random pattern, pedicled island and free flaps. Br J Plast Surg. (2005) 58:330–8. doi: 10.1016/j.bjps.2004.10.003
16. Yeoh MS, Kim DD, Ghali GE. Fluorescence angiography in the assessment of flap perfusion and vitality. Oral Maxillofac Surg Clin North Am. (2013) 25:61–6, vi. doi: 10.1016/j.coms.2012.11.004
17. Sloan GM, Sasaki GH. Non-invasive monitoring of tissue viability. Clin Plast Surg. (1985) 12:185–95. doi: 10.1016/S0094-1298(20)31689-8
18. Royal College of Obstetricians & Gynaecoloists. Guidelines for the Diagnosis and Management of Vulval Carcinoma. Version May.2014. Available online at: https://www.rcog.org.uk/globalassets/documents/guidelines/vulvalcancerguideline.pdf (accessed April 18, 2020).
19. Capozzi VA, Ceni V, Sozzi G, Cianciolo A, Gambino G, Pugliese M, et al. Endoscopic near infrared and Indocyanine green to verify the viability of the subcutaneous flap for vulvar cancer. Gynecol Oncol. (2019) 154:653–4 doi: 10.1016/j.ygyno.2019.06.018
20. Magrina JF, Masterson BJ. Evaluation of groin and vulvar flap viability by the use of intravenous fluorescein. Gynecol Oncol. (1981) 11:96–101. doi: 10.1016/0090-8258(81)90014-7
21. Olivier WA, Hazen A, Levine JP, Soltanian H, Chung S, Gurtner GC. Reliable assessment of skin flap viability using orthogonal polarization imaging. Plast Reconstr Surg. (2003) 112:547–55. doi: 10.1097/01.PRS.0000070968.42857.43
22. Furukawa H, Hayashi T, Oyama A, Funayama E, Murao N, Yamao T, et al. Effectiveness of intraoperative indocyanine-green fluorescence angiography during inguinal lymph node dissection for skin cancer to prevent postoperative wound dehiscence. Surg Today. (2015) 45:973–8. doi: 10.1007/s00595-014-0996-z
23. Zhu C, Chen S, Chui CH, Tan BK, Liu Q. Early prediction of skin viability using visible diffuse reflectance spectroscopy and autofluorescence spectroscopy. Plast Reconstr Surg. (2014) 134:240e−7e. doi: 10.1097/PRS.0000000000000399
24. Cianci S, Gueli Alletti S, Rumolo V, Rosati A, Rossitto C, Cosentino F, et al. Total laparoscopic hysterectomy for enlarged uteri: factors associated with the rate of conversion to open surgery. J Obstet Gynaecol. (2019) 39:805–10. doi: 10.1080/01443615.2019.1575342
25. Gueli Alletti S, Cianci S, Perrone E, Fanfani F, Vascone C, Uccella S, et al. Technological innovation and personalized surgical treatment for early-stage endometrial cancer patients: a prospective multicenter Italian experience to evaluate the novel percutaneous approach. Eur J Obstet Gynecol Reprod Biol. (2019) 234:218–22. doi: 10.1016/j.ejogrb.2019.01.024
26. Cosentino F, Vizzielli G, Turco LC, Fagotti A, Cianci S, Vargiu V, et al. Near-infrared imaging with indocyanine green for detection of endometriosis lesions (Gre-Endo Trial): a pilot study. J Minim Invasive Gynecol. (2018) 25:1249–54. doi: 10.1016/j.jmig.2018.02.023
27. Uccella S, Zorzato PC, Lanzo G, Fagotti A, Cianci S, Gallina D, et al. The role of sentinel node in early ovarian cancer: a systematic review. Minerva Med. (2019) 110:358–66. doi: 10.23736/S0026-4806.19.06145-7
28. Zorzato PC, Bosco M, Franchi MP, Mariani A, Cianci S, Garzon S, et al. Sentinel lymph-node for endometrial cancer treatment: review of literature. Minerva Med. (2020) 112:70–80. doi: 10.23736/S0026-4806.20.07117-7
29. Vizzielli G, Cosentino F, Raimondo D, Turco LC, Vargiu V, Iodice R, et al. Real three-dimensional approach vs two-dimensional camera with and without real-time near-infrared imaging with indocyanine green for detection of endometriosis: a case-control study. Acta Obstet Gynecol Scand. (2020) 99:1330–8. doi: 10.1111/aogs.13866
30. Benedetti Panici P, Di Donato V, Bracchi C, Marchetti C, Tomao F, Palaia I, et al. Modified gluteal fold advancement V-Y flap for vulvar reconstruction after surgery for vulvar malignancies. Gynecol Oncol. (2014) 132:125–9. doi: 10.1016/j.ygyno.2013.10.037
31. Tim CR, Martignago CCS, da Silva VR, Dos Santos ECB, Vieira FN, Parizotto NA, et al. A comparison of three methods for the analysis of skin flap viability: reliability and validity. Adv Wound Care. (2018) 7:157–63. doi: 10.1089/wound.2017.0758
32. Lee PK, Choi MS, Ahn ST, Oh DY, Rhie JW, Han KT. Gluteal fold V-Y advancement flap for vulvar and vaginal reconstruction: a new flap. Plast Reconstr Surg. (2006) 118:401–6. doi: 10.1097/01.prs.0000227683.47836.28
33. Turco LC, Scaldaferri F, Chiantera V, Cianci S, Ercoli A, Fagotti A, et al. Long-term evaluation of quality of life and gastrointestinal well-being after segmental colo-rectal resection for deep infiltrating endometriosis (ENDO-RESECT QoL). Arch Gynecol Obstet. (2020) 301:217–28 doi: 10.1007/s00404-019-05382-8
34. Cianci S, Rosati A, Rumolo V, Gueli Alletti S, Gallotta V, Turco LC, et al. Robotic single-port platform in general, urologic, and gynecologic surgeries: a systematic review of the literature and meta-analysis. World J Surg. (2019) 43:2401–19. doi: 10.1007/s00268-019-05049-0
35. Rosati A, Gueli Alletti S, Capozzi VA, Mirandola M, Vargiu V, Fedele C, et al. Role of ultrasound in the detection of recurrent ovarian cancer: a review of the literature. Gland Surg. (2020) 9:1092–101. doi: 10.21037/gs-20-357
36. Uccella S, Capozzi VA, Ricco' M, Perrone E, Zanello M, Ferrari S, et al. Sexual function following laparoscopic versus transvaginal closure of the vaginal vault after laparoscopic hysterectomy: secondary analysis of a randomized trial by the italian society of gynecological endoscopy using a validated questionnaire. J Minim Invasive Gynecol. (2020) 27:186–94. doi: 10.1016/j.jmig.2019.03.018
37. Scaletta G, Dinoi G, Capozzi V, Cianci S, Pelligra S, Ergasti R, et al. Comparison of minimally invasive surgery with laparotomic approach in the treatment of high risk endometrial cancer: a systematic review. Eur J Surg Oncol. (2020) 46:782–8. doi: 10.1016/j.ejso.2019.11.519
38. Soergel P, Hertel H, Nacke AK, Klapdor R, Derlin T, Hillemanns P. Sentinel lymphadenectomy in vulvar cancer using near-infrared fluorescence from indocyanine green compared with technetium 99 m nanocolloid. Int J Gynecol Cancer. (2017) 27:805–12. doi: 10.1097/IGC.0000000000000996
Keywords: vulvar cancer, indocyanine green, laparoscopic near-infrared probe, flap viability, vulvar flap
Citation: Capozzi VA, Monfardini L, Sozzi G, Armano G, Rosati A, Gueli Alletti S, Cosentino F, Ercoli A, Cianci S and Berretta R (2021) Subcutaneous Vulvar Flap Viability Evaluation With Near-Infrared Probe and Indocyanine Green for Vulvar Cancer Reconstructive Surgery: A Feasible Technique. Front. Surg. 8:721770. doi: 10.3389/fsurg.2021.721770
Received: 07 June 2021; Accepted: 05 July 2021;
Published: 09 August 2021.
Edited by:
Giuseppe Vizzielli, Azienda Sanitaria Universitaria Integrata di Udine, ItalyReviewed by:
Silvia Zermano, University of Udine, ItalyElena Teodorico, Agostino Gemelli University Polyclinic, Italy
Francesca Previtera, University of Udine, Italy
Copyright © 2021 Capozzi, Monfardini, Sozzi, Armano, Rosati, Gueli Alletti, Cosentino, Ercoli, Cianci and Berretta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesco Cosentino, cosentino.francesco@libero.it
 Vito Andrea Capozzi
Vito Andrea Capozzi Luciano Monfardini
Luciano Monfardini Giulio Sozzi
Giulio Sozzi Giulia Armano
Giulia Armano Andrea Rosati
Andrea Rosati Salvatore Gueli Alletti
Salvatore Gueli Alletti Francesco Cosentino
Francesco Cosentino Alfredo Ercoli5
Alfredo Ercoli5  Stefano Cianci
Stefano Cianci