Curative efficacy of entomopathogenic nematodes against white grubs in honeysuckle fields
- 1Shandong Key Laboratory of Plant Virology, Institute of Plant Protection, Shandong Academy of Agricultural Sciences, Jinan, China
- 2State Key Laboratory of Crop Biology, College of Agronomy, Shandong Agricultural University, Tai'an, China
- 3Shandong Key Laboratory of Crop Biology, College of Agronomy, Shandong Agricultural University, Tai'an, China
Root-feeding white grubs are one of the most serious pests of honeysuckle trees (Lonicera japonica) in China, directly damaging their roots and facilitating infection by soil pathogens. Entomopathogenic nematodes (EPNs) are considered as potential control agents against soil-dwelling insect pests. This study aimed to identify effective EPN species against white grubs through bioassay and field experiments. Among the EPN species screened against Holotrichia oblita under laboratory conditions, Steinernema feltiae and Heterorhabditis indica had low virulence, while S. longicaudum, S. glaseri, and H. bacteriophora applied at a rate of 400 IJs/larva caused a higher corrected mortality (80.00 ± 5.77%), which screened them as good candidates for future applications. The field experiments showed that both S. longicaudum and H. bacteriophora were approximately as effective in reducing white grubs as the insecticide phoxim, whereas S. glaseri caused a significantly lower reduction compared with these two EPNs and phoxim. Plant mortalities obtained from S. longicaudum, H. bacteriophora and the insecticide treatment plots were significantly lower than those observed in the water-treated control plots. All EPNs examined could establish well in the treated honeysuckle fields for 42 d, confirmed by Tenebrio molitar larvae baiting technique. Our findings suggest that EPNs could provide curative efficacy against white grubs and significantly reduce plant death in honeysuckle fields.
1. Introduction
Honeysuckle, Lonicera japonica Thunb, is a Chinese medicinal plant native to East Asia and can be easily grown all over the world. It is renowned for its active compounds and widespread pharmacological effects on heat-evil, dysentery and swellings, body protection and lifespan extension as recorded in the famous classical book of Chinese material media “Ben Cao Gang Mu” (Shang et al., 2011). So many beneficial effects including anti-viral (Ding et al., 2017), anti-bacterial (Rahman and Sun, 2009), anti-oxidant (Kong et al., 2017), anti-inflammatory (Tang et al., 2016), anti-diabetic (Han et al., 2015) and neuroprotective (Wang et al., 2014) have been demonstrated for this plant. Moreover, it is also used as cosmetics, food products, and healthy beverages worldwide (Wang, 2010; Fang et al., 2020). Along with the great changes in the environment, food consumption, and lifestyle observed in the modern society, honeysuckle is playing an increasingly important role in our daily life (Yang et al., 2018). Honeysuckle cultivation has been expanded as the demand increases (Hu et al., 2022), while the problem of white grubs is becoming more and more serious (Xin, 2017; Li, 2022).
White grubs, which are the root-feeding larvae of scarab beetles, are one of the most severe soil-dwelling pests and are increasingly damaging honeysuckle cultivation (Xu and Wei, 2021). Holotrichia oblita Faldermann is one of the dominant species found in honeysuckle fields and it generally co-occurs with other white grub species, such as Brahmina faldermanni Kraatz and Maladera orientalis Motschulsky (Li, 2022). These larvae feed on honeysuckle roots, facilitating their infection by other soil pathogens and subsequent decay (Gao et al., 2020). The damages caused to the roots affect the entire plant, with serious impacts on tree growth and flowering, eventually leading to the plant's death (Liu et al., 2017; Gao et al., 2020).
For many years, white grubs in honeysuckle fields have been mainly controlled using chemical insecticides, such as phoxim and chlorpyrifos (Liu et al., 2017). However, the efficacy of these products is not always satisfactory as white grubs live concealed in the soil and in addition to the development of insecticide resistance (Gao et al., 2020). Therefore, in light of the increasing environmental and human safety concerns, and of the importance of honeysuckle flowers for medical purposes, alternative biological strategies are urgently needed to control white grubs in honeysuckle fields.
Entomopathogenic nematodes (EPNs) are known as potential biological control agents and have been used to control a variety of soil-dwelling insects due to their superior ability to actively search for hosts (Grewal et al., 2005; Georgis et al., 2006). Some EPN species have been shown to be potentially highly efficient against different white grub species in turf grass or peanut fields, such as Steinernema scarabaei, S. longicaudum, S. glaseri, Heterorhabditis bacteriophora, and H. zealandica (Tamson and Alm, 1995; Koppenhöfer et al., 2000, 2002; Koppenhöfer and Fuzy, 2003a,b; Grewal et al., 2004; Du et al., 2009; Guo et al., 2015). However, knowledge of EPNs application to control white grubs in honeysuckle fields is still limited.
The successful application of EPNs strictly depends on environmental factors, such as soil texture, moisture, and temperature (Shapiro-Ilan et al., 2012a; Guo et al., 2015). Honeysuckle cultivation needs to pay more attention to the geo-herbalism (Zhang et al., 2003; Duan et al., 2019), for the soil characteristics is of great importance to the content of active compounds in honeysuckle flowers (Chen et al., 2021). Yimeng mountain area is the natural planting area for honeysuckle (Liu et al., 2008). Pingyi county, which is located in the Yimeng Mountains, is the largest honeysuckle production area in China (Zhang, 2021). The soil here is sandy and arid, which favors the accumulation of the plants' active compounds (Chen et al., 2021). Whether these soil conditions are also suitable for the successful application of EPNs needs to be explored.
More importantly, it is necessary to choose the appropriate EPN species to control the target pest by considering their virulence, environmental tolerance, and even persistence (Shapiro-Ilan et al., 2002, 2006a,b). The virulence of EPNs to white grubs varies with EPN and white grub species (Koppenhöfer and Fuzy, 2003a,b; Grewal et al., 2004). Although there are some differences in the virulence of each EPN to different white grub species, certain EPNs were shown to be pathogenic to several white grubs, as observed for H. bacteriophora against Popilia japonica Newman (Selvan et al., 1994), Maladera matrida Argaman (Glazer and Gol'Berg, 1993), H. parallela Motschulsky (Guo et al., 2013), and H. oblita (Guo et al., 2015). Little is known on the efficacy of certain EPNs against white grubs in the honeysuckle fields. More EPN species are needed to be screened for providing more alternatives to effectively control white grubs that always co-occur in the same honeysuckle fields.
Therefore, five EPN species, i.e., S. longicaudum X-7, S. glaseri KG, S. feltiae SN, H. bacteriophora H06, and H. indica LN2, reported with high virulence or good performance in the fields against several pests, for example, fungus gnats, Lepidopterous pests and white grubs (Yan et al., 2014; Wang et al., 2021), were chosen for bioassay screening against H. oblita, one of the dominant white grub species in honeysuckle fields. Subsequently, the control efficacy of high virulent EPN species screened in the bioassay was evaluated in the honeysuckle fields in the present study.
2. Materials and methods
2.1. EPNs
The Steinernema longicaudum X-7, S. glaseri KG, S. feltiae SN, H. bacteriophora H06, and H. indica LN2 species used in this study were provided by Weifang Hongrun Agriculture Science and Technology Co., LTD, China. Infective juveniles (IJs) were cultured in vitro in solid sponge media using the method described in Bedding (1981) with modifications (Han, 1996) and were formulated with vermiculite (200 mesh). IJ suspensions were used for experiments if more than 95% of IJs were alive, which was assessed using a microscope before the experiments (Yan et al., 2013).
2.2. Insects
The second instar larvae of H. oblita used for the bioassays were provided by the Cangzhou Academy of Agriculture and Forestry Science, China. The white grubs were reared and fed on dry potato pieces (0.5 × 0.5 × 0.5 cm). The size and weight of each instar larva were consistent with the measurements reported in Guo et al. (2015). The larvae were individually kept in plastic cups (with a diameter of 4.3, height of 7 cm, and a 2-mm-diameter hole in the lid) filled with 50 g of sandy soil (10% w/w soil moisture) at 25 ± 2°C and 50% relative humidity (RH). Six wheat seeds were added to each cup as food. The cups were kept at 25°C for 24 h and only grubs that showed signs of activity were selected for the bioassays (Koppenhöfer and Fuzy, 2008).
Yellow mealworms, Tenebrio molitor L., were purchased from Shandong Taian Wuma market. The mealworms were reared in a controlled room with 25 ± 2°C and 50% RH, and fed on wheat bran and fresh vegetable leaves. Similarly sized 9th- to 11th-instar larvae were chosen to evaluate nematode persistence by burying them in soil samples collected from the field experiments (Guo et al., 2015).
2.3. Bioassays
2.3.1. Virulence of different EPN species to second instar H. oblita larvae
One mL of IJ suspensions containing 200 IJs of S. longicaudum, S. glaseri, S. feltiae, H. bacteriophora, or H. indica was applied into each cup containing the H. oblita larvae (equal to 1.5 × 109 IJs ha−1). A similar volume of water without nematodes was added to the soil of control treatment. Three replicates were set for each treatment or control and each replicate tested 10 individual larvae. After the treatment, the cups were placed in the dark at 25 ± 2°C and 75 ± 5% RH. White grub mortality was assessed after 4, 7, and 14 d; the cadavers were placed onto moist filter paper and were dissected 3 d later to evaluate IJ invasion (Yan et al., 2013).
2.3.2. Effect of highly virulent EPN species applied at different rates
The bioassay was performed in plastic cups, as described above. IJ suspensions of the most effective EPN species screened in the first step, i.e., S. longicaudum, S. glaseri, or H. bacteriophora, were prepared. One mL of the nematode suspension containing 400, 200, 100, or 50 IJs was applied into each cup with one grub (equal to 3.0 × 109, 1.5 × 109, 7.5 × 108 or 3.75 × 108 IJs ha−1). Water without IJs was used as control. Ten cups with 10 individual larvae were set as one replicate and three replicates were set for each treatment or control. All cups were placed in the dark at 25 ± 2°C and 75 ± 5% RH. Grub mortality was assessed as described in the previous section.
2.3.3. Field experiments
Two field trials were conducted in different honeysuckle fields in the Pingyi area, China. Before the treatments, the presence of native EPN populations in the fields was assessed by baiting soil samples with yellow mealworms as described in Liu et al. (2009). No EPN populations were detected in the experimental fields. Grub population was estimated based on Du et al. (2009) and species were identified following the guidelines reported in Wei et al. (1989) and Cao and Li (2017). In brief, 30 honeysuckle plants were randomly selected and the soil around their roots (diameter = 40 cm, depth = 20 cm) was removed to identify larval species and calculate population abundance.
The first experiment was performed in a honeysuckle field in Fumin village (N35°15′23′′, E117°40′54′′) on August 26, 2020, at 1:30 pm, to determine which EPN species to apply against white grubs and at which rates. The sandy soil in the field had a water content of 8.09 ± 0.53%. The day was sunny, air temperature was 29°C and soil temperature was 27°C at a depth of 5 cm. The honeysuckle plants in the experiment field were 3 years old. Each plant covered an area of ~1.15 m2 (diameter = 1.21 m). Each experimental plot had an area of 48 m2 (15 m × 3.2 m) with a 1.6-m buffer space set between plots and containing 36 honeysuckle plants spaced 1.6-m apart within a row. The white grub species present in the experiment field were H. oblita, Brahmina faldermanni, and Serica orientalis with a ratio of 5: 4: 6. The population density was 5.67 ± 0.61 larvae per plant, and the larvae were mainly in the first, second, and third instar with a ratio of 1: 7: 2. S. longicaudum, S. glaseri, and H. bacteriophora treatments were applied at 3.0 × 109, 1.5 × 109, and 7.5 × 108 IJs/ha, respectively.
The second experiment was conducted in Nanwan village (N35°16′56″, E117second) on August 18, 2021, at 4:30 pm, to assess the efficacy of EPNs against white grubs at the selected application rate and the protection provided to honeysuckle plants. The sandy soil in the fieldhad a water content of 6.22 ± 0.34%; the day was sunny with an air temperature of 27°C and soil temperature of 26°C at a depth of 5 cm. The honeysuckle plants were 3 years old. Each plant covered an area of ~1.04 m2 (diameter = 1.15 m). Each experimental plot had an area of ~100 m2 (33.3 m × 3.0 m) with a 1.5-m buffer space set between plots and contained 75 honeysuckle plants spaced 1.6 m apart within a row. The white grub species present in this experiment field were H. oblita, S. orientalis, and Hoplosternus incanus with a ratio of 8: 9: 2. The population density was 3.20 ± 0.05 larvae per plant and the larvae were mainly in the first, second, and third instar with a ratio of 1: 8: 1. S. longicaudum, S. glaseri, and H. bacteriophora treatments were applied at 1.5 × 109 IJs/ha.
In both experiments, phoxim (EC 48%, Shandong United Pesticide Industry Co. Ltd, Jinan, China) at a dosage of 4,500 mL/ha was used as a positive control. Water without IJs or insecticide was set as a negative control. In the first and second experiments, 15 L and 30 L of water, respectively, containing different concentrations of IJs or phoxim were sprayed on the soil around each plant root. A similar volume of only water was used for the control experiment. No additional irrigation or other insecticides were supplied. Each treatment was conducted in four replicates (plots) and all the plots were arranged in a randomized complete block design. Throughout both experiments, soil temperature at a depth of 5 cm ranged from 16 to 25°C.
White grub populations were monitored 7, 21, and 42 d after treatment (DAT) in both experiments. In the second experiment, plants selected for the larval abundance were excluded; the number of dead plants and total plants in each plot were determined on May 15, 2022, to calculate plant mortality.
EPN persistence in the soil was evaluated by assessing the mortality of yellow mealworm larvae buried in the soil samples 7, 14, 21, 28, 42 d after EPN application in both experiments. Soil sample (10 cm × 10 cm × 10 cm) around each plant roots was taken and five soil samples were taken from each plot. Then, 10 mealworm larvae were put in each soil sample and mortality was assessed 4 d later. Dead larvae were incubated in petri dishes with moist filter paper and were dissected 3 d later to estimate IJ invasion (Yan et al., 2013).
2.4. Statistical analysis
The H. oblita and T. molitor bioassay data were corrected for control mortality using Abbott's formula (Abbott, 1925). The percentage reductions in white grubs in the field experiments were calculated based on Liu et al. (2007) and Guo et al. (2013, 2015). Plant mortality was calculated using the following equation:
where Pd is the percentage of dead plants in each plot, and Nd and Na indicate the number of dead plants and the total number of plants in each plot, respectively.
Arcsine square root transformation was applied to the percentage data before statistical analysis in SPSS 16.0 (SPSS Inc., Chicago, IL). Means were separated using Tukey's test and differences among means were considered significant at P < 0.05.
3. Results
3.1. Virulence of different EPN species against H. oblita
A significant difference was observed between white grub mortalities (hereafter referred to as “mortalities”) caused by different EPN species (Figure 1). At 4 DAT, S. longicaudum and H. bacteriophora caused higher mortalities than S. feltiae and H. indica, while their values were not significantly different from the mortalities associated with S. glaseri (F = 8.401; df = 4, 10; P = 0.003). With time, an increase in mortality was observed in all treatments. Until 7 DAT, S. longicaudum, S. glaseri, and H. bacteriophora, caused significantly higher mortalities than S. feltiae and H. indica (F = 6.110; df = 4, 10; P = 0.009). At 14 DAT, the mortalities (63.33 ± 3.33% to 66.67 ± 3.33%) observed in the treatments with S. longicaudum, S. glaseri, and H. bacteriophora, were significantly higher than that by H. indica (F = 9.357; df = 4, 10; P = 0.002).
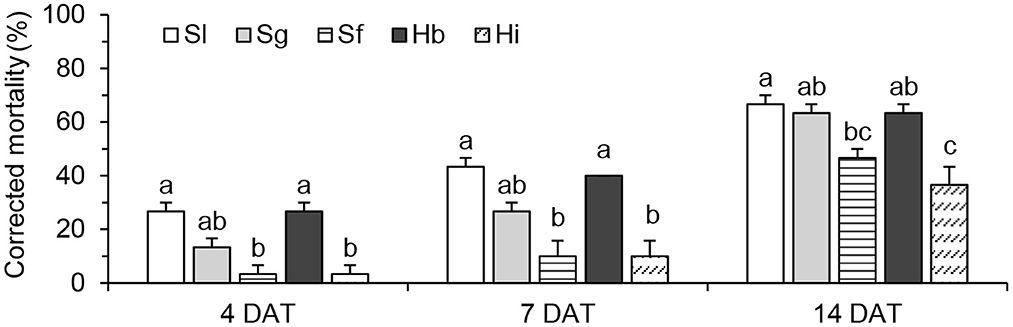
Figure 1. Corrected mortality of Holotrichia oblita Faldermann treated with Steiernema longicaudum X-7 (Sl), S. glaseri KG (Sg), S. feltiae SN (Sf), Heterorhabditis bacteriophora H06 (Hb), and H. indica LN2 (Hi) evaluated at 4, 7 and 14 DAT. Different letter(s) on the bars represent significant differences among treatments on the same day (P < 0.05, Tukey's test).
3.2. Effects of application rates on the virulence of superior EPNs
White grub mortalities varied with EPN application rates (Figure 2). Generally, the higher application rates were, the higher mortalities were obtained. White grub mortalities kept increasing with time. At 4 DAT, the mortalities caused by S. longicaudum at 400 IJs/larva and H. bacteriophora at 400 and 200 IJs/larva were significantly higher than that incurred by the same EPN species applied at lower rates (F = 5.020; df = 11, 24; P < 0.001). No significant difference was observed among the three application rates used for S. glaseri. At 14 DAT, the highest mortalities, ranging from 70.00 ± 5.77% to 80.00 ± 5.77%, were caused by S. longicaudum, S. glaseri, and H. bacteriophora at 400 IJs/larva (F = 4.478; df = 11, 24; P = 0.001).
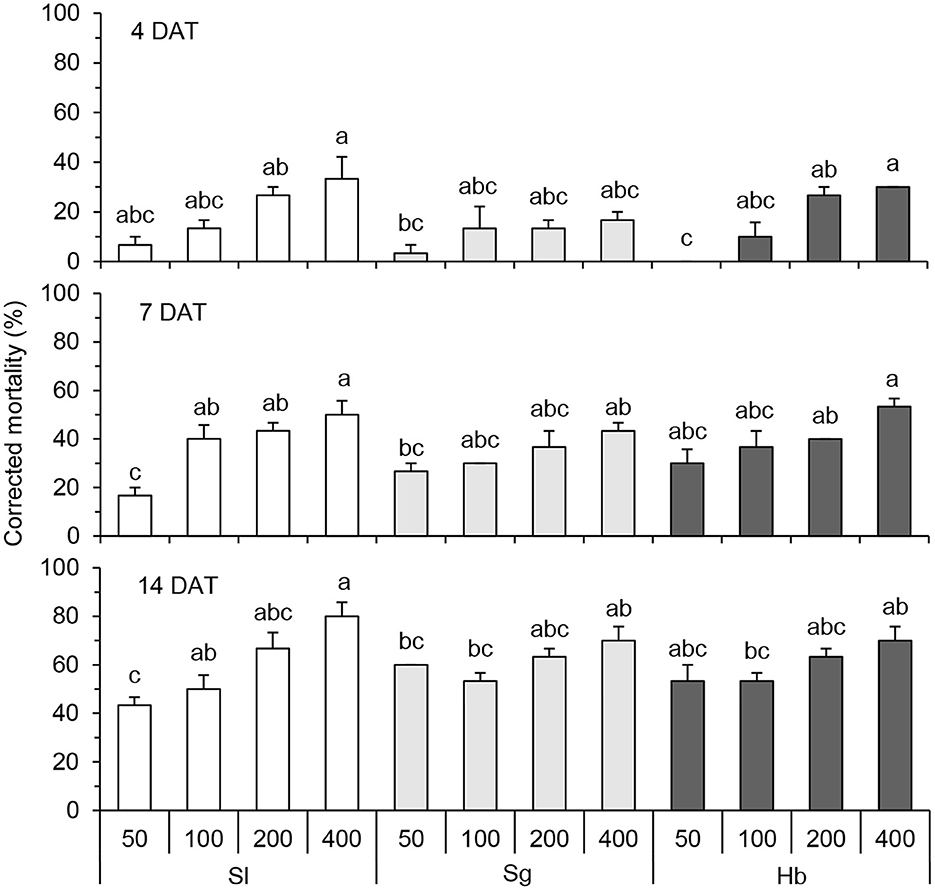
Figure 2. Corrected mortality of Holotrichia oblita Faldermann treated with Steinernema longicaudum X-7 (Sl), S. glaseri KG (Sg), and Heterorhabditis bacteriophora H06 (Hb) at rates of 50, 100, 200, and 400 IJs per larva evaluated at 4, 7, and 14 DAT. Different letter(s) on the bars represent significant differences among treatments on the same day (P < 0.05, Tukey's test).
3.3. Effects of EPN application in honeysuckle fields
The reduction in white grub population (hereafter referred to as “grub reduction”) in the EPN- and phoxim-treated plots at different sampling times in the two experiments were shown in Table 1.
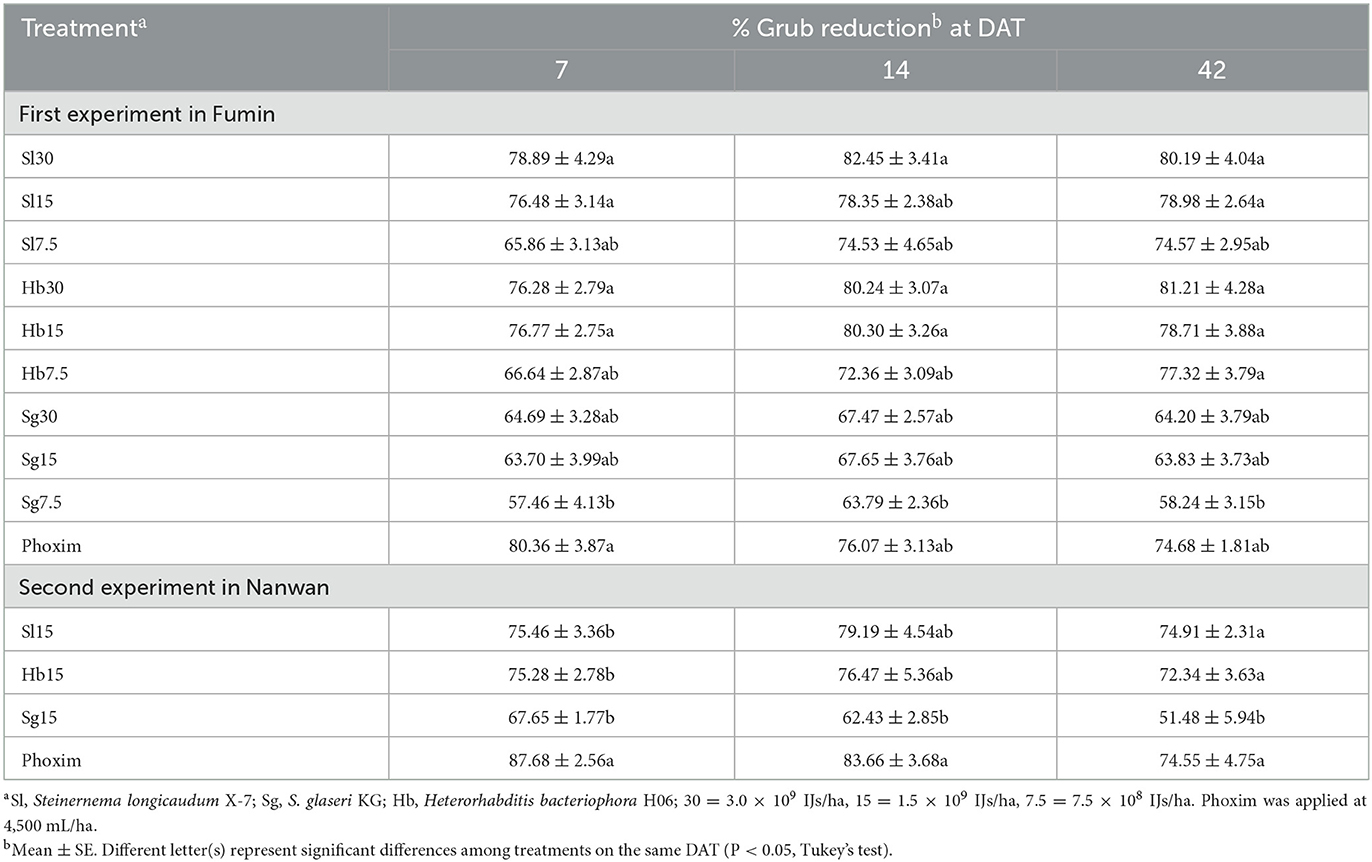
Table 1. Percentage reduction in white grubs obtained from different treatments at 7, 21, and 42 days after treatment (DAT) in honeysuckle fields in Fumin (first experiment) and Nanwan (second experiment), Shandong, China.
In the first experiment, the treatments with S. longicaudum and H. bacteriophora at all application rates showed high efficacy against white grubs. At 7 DAT, when compared insecticide phoxim, S. longicaudum and H. bacteriophora at all application rates caused similar grub reduction, while S. glaseri at 7.5 × 108 IJs/ha caused a significantly lower grub reduction (F = 4.754, df = 9, 30; P = 0.001). No significant difference was observed among the grub reduction from the treatments with the same EPNs at different application rates. However, the application of S. longicaudum and H. bacteriophora at the high rates of 3.0 × 109 and 1.5 × 109 IJs/ha and low rate of 7.5 × 108 IJs/ha caused > 76% and ≈ 65% grub reduction, respectively. However, grub reduction caused by both EPN species applied at 7.5 × 108 IJs/ha significantly increased over time, reaching 77.32 ± 3.79%. All rates of S. longicaudum and H. bacteriophora caused grub reductions ranging from 74.57 ± 4.65% to 81.21 ± 4.30% after 42 DAT, which were not significantly different from those obtained using phoxim, but significantly higher than the reductions obtained using S. glaseri at 7.5 × 108 IJs/ha (F = 4.590, df = 9, 30; P = 0.001).
In the second experiment, S. longicaudum and H. bacteriophora were approximately as efficient as phoxim in reducing white grub population. At 7 DAT, phoxim was more effective against white grubs when compared with the EPNs. The grub reductions caused by S. longicaudum, S. glaseri, and H. bacteriophora at 1.5 × 109 IJs/ha were 75.46 ± 3.36%, 67.65 ± 1.77%, and 75.28 ± 2.78%, respectively, significantly lower than that caused by phoxim (F = 9.687; df = 3, 12; P = 0.002). However, with time, the differencesbetween phoxim and the EPNs S. longicaudum and H. bacteriophora, were reduced to zero. Until 42 DAT, these two species and phoxim had the same efficacy, achieving grub reductions that were significantly higher than that caused by S. glaseri KG (F = 6.292; df = 3, 12; P = 0.008).
3.4. Effects of EPN application on plant mortality
Plant mortality in different treatment plots was shown in Figure 3. Plant death from plots treated with S. longicaudum, S. glaseri, H. bacteriophora, and phoxim were 0.84 ± 0.48%, 2.09 ± 0.41%, 0.83 ± 0.41%, and 0.85 ± 0.49%, respectively. No significant difference was observed among the treatments with the EPNs and phoxim. While the mortalities observed in plots treated with S. longicaudum, H. bacteriophora, and phoxim were all significantly lower than those in the control plots treated with water (4.19 ± 0.49%) (F = 4.442; df = 4, 15 P = 0.014).
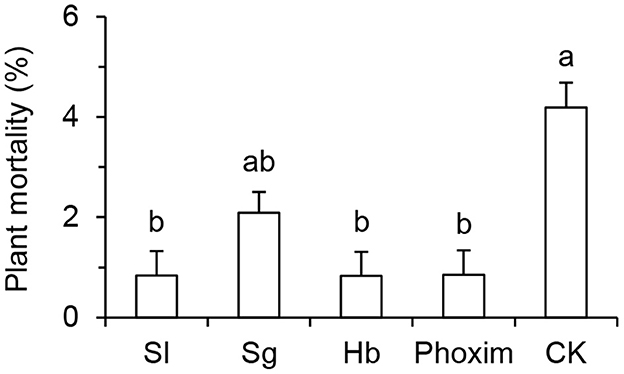
Figure 3. Plant mortality observed in different plots treated with Steinernema longicaudum X-7 (Sl), S. glaseri KG (Sg), and Heterorhabditis bacteriophora H06 (Hb) at 1.5 × 109 IJs/ha, and phoxim at 4,500 mL/ha evaluated on May 13, 2022. Different letter(s) on the bars represent significant differences among treatments and the water-based control (CK) (P < 0.05, Tukey's test).
3.5. EPN persistence
The mortalities of baited yellow mealworm larvae were calculated. S. longicaudum, S. glaseri, and H. bacteriophora were able to persist in the soil for 42 d after application (Table 2). Yellow mealworm mortalities ranged from 20.00 ± 4.08% to 45.00 ± 2.89% in the first field trail and from 27.50 ± 4.79% to 45.00 ± 2.89% in the second one. No significant difference was observed among treatments on the same sampling day (experiment 1: F ≤ 1.586; df = 8, 27; P ≥ 0.176; experiment 2: F ≤ 0.984; df = 2, 9; P ≥ 0.411).
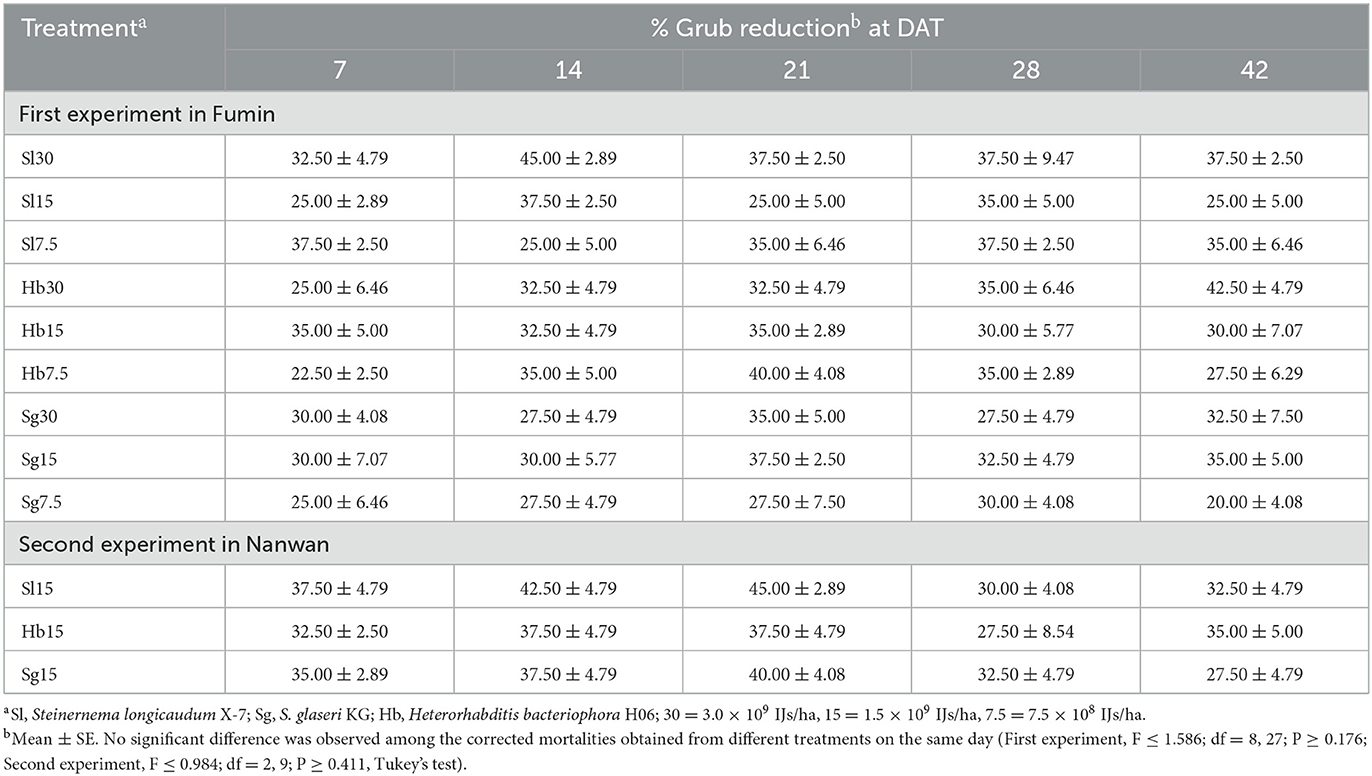
Table 2. Corrected mortality of Tenebrio molitor bait larvae in soil samples collected from the first (Fumin) and second (Nanwan) experiments conducted in Shandong, China at 7, 14, 21, 28, and 42 DAT.
4. Discussion
The screening of EPN species is critical to achieve a successful biocontrol of pests. Foremost, suitable EPN species must be matched with the target pest (Lacey and Georgis, 2012; Shapiro-Ilan and Dolinski, 2015). Among the EPN species tested via bioassay in this study, S. longicaudum, S. glaseri, and H. bacteriophora showed high virulence to H. oblita, which was congruent with previous studies that S. longicaudum (Li et al., 2007; Du et al., 2009; Guo et al., 2013), S. glaseri, and H. bacteriophora were highly pathogenic to a variety of scarab larvae (Grewal et al., 2005; Koppenhöfer and Fuzy, 2006). In contrast, our results showed that S. feltiae and H. indica were slightly virulent to grubs. The virulence of EPNs varies with different EPN species and target pests (Lacey et al., 2015) and little is known about that caused by S. feltiae and H. indica to scarab larvae. These two species have been reported to have a wide host range, with high virulence to fungus gnats (Zhao, 2013; Yan et al., 2019) and Lepidopterous pests (Lacey et al., 2015; Wang et al., 2021). Their lack of virulence to scarab larvae maybe partly due to their failure in overcoming host defenses (Wang et al., 1995; Lara-Reyes et al., 2021).
Although we firstly screened EPN species through laboratory bioassays to narrow down the candidates, the importance of confirming the virulence determined via bioassay by conducting subsequent filed trials cannot be overemphasized (Shapiro-Ilan et al., 2012b). In the honeysuckle fields treated in this study, the efficacy of S. glaseri against white grubs was not satisfactory. Although this was the first EPN species used to control white grubs at large scales (Gaugler et al., 1992), studies have shown that its field efficacy has deteriorated (Selvan et al., 1994; Converse and Grewal, 1998). Long-term laboratory culture may be one of the main factors responsible for its reduced performance (Converse and Grewal, 1998; Lee et al., 2002). Moreover, the potential virulence of S. glaseri against other white grub species co-occurring in same field may be another factor affecting its field efficacy. This virulence remains to be determined, as in this study we only assessed the virulence against the larvae of H. oblita.
Unlike S. glaseri KG, both S. longicaudum and H. bacteriophora achieved an acceptable level of grub control in the treated honeysuckle fields, where multiple species of white grubs co-occurred. To ensure a successful control, it is important that EPN species are highly pathogenic to several grub species, as these have overlapping geographic ranges and may often co-occur in the same fields (Grewal et al., 2004). Steinernema longicaudum and H. bacteriophora have been shown to perform well against different white grub species; for example, S. longicaudum proved to be effective against Polyphylla gracilicornis (Fan, 2015) and Holotrichia ovata (Zhang et al., 2006) in bioassays, and against Exomala orientalis in turf grass (Lee et al., 2002) and H. parallela in peanut fields (Guo et al., 2013), while H. bacteriophora performed well against Popillia japonica in turf grass (Koppenhöfer and Fuzy, 2003a,b; Grewal et al., 2004; Torrini et al., 2020), H. parallela (Guo et al., 2013), and H. oblita (Guo et al., 2015) in peanut fields. Although we did not test the virulence of either EPN to the white grub species mentioned above via bioassay, we believe that both are suitable to control them based on the results of the present study and the good field performance reported in previous studies.
In addition to the suitability of EPN species, adequate environmental conditions, especially in terms of soil moisture, are considered as another important factor in EPN application (Kaya, 1990; Shapiro-Ilan et al., 2006a). The honeysuckle trees in this study were planted in hill fields with sandy soil characterized by poor water retention. However, according to our data, the soil moisture detected during the experimental period ranged from 8.07 to 16.33%, and EPNs could establish well in this soil. The honeysuckle trees in the experiment fields were 3-years-old, with lush vines covering the ground. We speculated that the good level of shade and frequent rainfall in summer and autumn contributed to the adequate soil moisture. Generally, white grub outbreaks in honeysuckle are persistent (Li, 2022). In this study, EPNs could reduce white grub populations in the long term in the fields, which indicated that soil conditions, including soil moisture, texture, and temperature (16–25°C), favored EPN establishment, dispersal, and contact with hosts (Guo et al., 2015).
In the present study, plant mortalities in the plots treated with S. longicaudum, H. bacteriophora, and phoxim were significantly reduced by a rate of ≈80% compared with the values observed in the control plots treated with water. This indicated that the reduction in white grubs obtained through the above-mentioned treatments could lower plant mortality. To improve the curative qualities of honeysuckle flowers, more attention should also be paid to ecological planting. In particular, the application of EPNs to control pests dwelling below the ground is of great significance for ecological planting, not only for biological control purposes, but also to enhance plant defenses (Helms et al., 2019). Further studies should focus on the effects of EPNs on the soil system and the quality of honeysuckle flowers after EPN application.
In our study, both S. longicaudum and H. bacteriophora treatments performed well against white grubs in honeysuckle fields. However, H. bacteriophora may be considered as a more promising agent due to its relatively lower production cost (Guo et al., 2013) and higher stability under unfavorable conditions (Yan et al., 2010) compared with S. longicaudum. The EPN application rate is of paramount importance, varying across target pests and environmental settings (Shapiro-Ilan and Dolinski, 2015). Generally, higher application rates could enhance the efficacy of the treatments to some degree, achieving results within a shorter period of time (Guo et al., 2015). This would also entail an increase in costs; however, applying lower EPN rates will increase the risk of low efficacy against white grubs (Shapiro-Ilan et al., 2006a), as suggested by the data in our first experiment. Our results showed that 1.5 × 109 IJs/ha would be an optimal application rate for honeysuckle fields, considering that higher rates did not determine a greater reduction in white grubs at all.
In summary, the present study highlighted the potential of using EPNs against white grubs in honeysuckle fields. Additional studies are needed on how to accelerate the effects of EPN treatments through the joint application of EPNs and other entomopathogenic agents, such as Metarhizium anisopliae and Beauveria bassiana, among others, which will improve efficacy.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
XL, WG, and YY conceived and designed the research. XL, SL, LL, HC, and YS conducted experiments. XL analyzed the data and produced a draft of the manuscript. XM, WG, YY, JW, XF, and ZS provided comments on various drafts. All authors read and approved the final manuscript.
Funding
This study was supported by the earmarked fund for CARS (CARS-21), Shandong Provincial Natural Science Foundation (ZR2019BC113), and Shandong Modern Agricultural Industry Technical System Project of China (SDAIT- 20-04).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbott, W. S. (1925). A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 18, 265–267. doi: 10.1093/jee/18.2.265a
Bedding, R. A. (1981). Low cost in vitro mass production of Neoaplectana and Heterorhabditis species (Nematoda) for field control of insect pests. Nematologica 27, 109–114. doi: 10.1163/187529281X00115
Cao, Y. Z., and Li, K. B. (2017). Map of Common Underground Pests in China. Beijing: China Agricultural Science and Technology Press.
Chen, Q. Q., Weng, S. Q., Cheng, L., Chen, X. Q., Lu, D. J., Zhou, J. M., et al. (2021). Relationship between genuine honeysuckle quality and soil fertility. Soils 53, 732–738.
Converse, V., and Grewal, P. S. (1998). Virulence of entomopathogenic nematodes to the western masked chafer Cyclocephala hirta (Coleoptera: Scarabaeidae). J. Econ. Entomol. 91, 428–432. doi: 10.1093/jee/91.2.428
Ding, Y., Cao, Z., Cao, L., Ding, G., Wang, Z., and Xiao, W. (2017). Antiviral activity of chlorogenic acid against influenza A (H1N1/H3N2) virus and its inhibition of neuraminidase. Sci. Rep.7, 45723.
Du, X., Liu, Q., Zhang, L., Liang, L., Xie, N., and Zhang, S. (2009). Application technology of Steinernema longicaudum BPS strain in peanut fields for chafer grub control. Agrochemica 48, 379–388.
Duan, H. F., Wu, Q. N., Zhu, Y. Y., Liu, C. C., Huang, Z. H., Qian, D. W., et al. (2019). UPLC was used to determine 10 components in honeysuckle from different areas. Chin. Trad. Herb. Drugs 50, 5858–5864.
Fan, F. F. (2015). Infectivity of Entomopathogenic Nematodes to Grassland Soil Pest White Grubs on Alpine Areas (Master's thesis). Xining: Qinghai University.
Fang, H., Qi, X., Li, Y., Yu, X., Xu, D., Liang, C., et al. (2020). De novo transcriptomic analysis of light-induced flavonoid pathway, transcription factors in the flower buds of Lonicera japonica. Trees 34, 267–283. doi: 10.1007/s00468-019-01916-4
Gao, L. F., Zhou, X. J., Shen, J. D., and Zhao, Q. (2020). Investigation and occurrence regularity of main insect pests of honeysuckle in Nanyang city. Bull. Agric. Sci. Technol. 211–213.
Gaugler, R., Campbell, J. F., Selvan, S., and Lewis, E. E. (1992). Largescale inoculative releases of the entomopathogenic nematode Steinernema glaseri: assessment 50 years later. Biol. Control. 2, 181–187. doi: 10.1016/1049-9644(92)90057-K
Georgis, R., Koppenhöfer, A. M., Lacey, L. A., Be'lair, G., Duncan, L. W., Grewal, P. S., et al. (2006). Successes and failures in the use of parasitic nematodes for pest control. Biol. Control 38, 103–123. doi: 10.1016/j.biocontrol.2005.11.005
Glazer, I., and Gol'Berg, A. (1993). Field efficacy of entomopathogenic nematodes against the bettie Maladera malrida (Coleoptera: Scarabaeidae). Bioconlr. Sci. Technol. 3, 367–376. doi: 10.1080/09583159309355291
Grewal, P. S., Koppenhöfer, A. M., and Choo, H. Y. (2005). “Lawn, turfgrass and pasture applications,” in Nematodes as Biocontrol Agents, eds P. S. Grewal, R. U. Ehlers, and D. I. Shapiro-Ilan (Wallingford, CABI Publishing), 115–146. doi: 10.1079/9780851990170.0115
Grewal, P. S., Power, K. T., Grewal, S. K., Suggars, A., and Haupricht, S. (2004). Enhanced consistency in biological control of white grubs (Coleoptera: Scarabaeidae) with new strains of entomopathogenic nematodes. Biol. Control 30, 73–82. doi: 10.1016/j.biocontrol.2003.09.016
Guo, W. X., Yan, X., Zhao, G. Y., and Han, R. C. (2013). Efficacy of entomopathogenic Steinernema and Heterorhabditis nematodes against white grubs (Coleoptera: Scarabaeidae) in peanut fields. J. Econ. Entomol. 106, 1112–1117. doi: 10.1603/EC12477
Guo, W. X., Yan, X., Zhao, G. Y., and Han, R. C. (2015). Efficacy of entomopathogenic Steinernema and Heterorhabditis nematodes against Holotrichia oblita. J. Pest. Sci. 88, 359–368. doi: 10.1007/s10340-014-0626-y
Han, J. M., Kim, M. H., Choi, Y. Y., Lee, H., Hong, J., and Yang, W. M. (2015). Effects of Lonicera japonica thunb. on type 2 diabetes via ppar- γ activation in rats. Phytother Res. 29, 1616–1621. doi: 10.1002/ptr.5413
Han, R. C. (1996). The effects of inoculum size on yield of Steinernema carpocapsae and Heterorhabditis bacteriophora in liquid culture. Nematologica 42, 546–553. doi: 10.1163/004625996X00045
Helms, A. M., Ray, S., Matulis, N. L., Kuzemchak, M. C., Grisales, W., Tooker, J. F., et al. (2019). Chemical cues linked to risk: cues from below-ground natural enemies enhance plant defences and influence herbivore behaviour and performance. Funct. Ecol. 33, 1–11. doi: 10.1111/1365-2435.13297
Hu, K. J., Wang, H. N., and Yuan, H. F. (2022). Analysis on the progress of Chinese honeysuckle research. J. Hebei North Univ. 7, 38–42.
Kaya, H. K. (1990). “Soil ecology,” in Entomopathogenic Nematodes in Biological Control, eds R. Gaugler, and H. K. Kaya (Boca Raton, FL: CRC Press), 93–116.
Kong, D., Li, Y., Bai, M., Deng, Y., Liang, G., and Wu, H. (2017). A comparative study of the dynamic accumulation of polyphenol components and the changes in their antioxidant activities in diploid and tetraploid Lonicera japonica. Plant Physiol. Biochem. 112, 87–96. doi: 10.1016/j.plaphy.2016.12.027
Koppenhöfer, A. M., Brown, I. M., Gaugler, R., Grewal, P. S., Kaya, H. K., and Klein, M. G. (2000). Synergism of entomopathogenic nematodes and imidacloprid against white grubs: greenhouse and field evaluation. Biol. Control 19, 245–252. doi: 10.1006/bcon.2000.0863
Koppenhöfer, A. M., Cowles, R. S., Cowles, E. A., Fuzy, E. M., and Baumgartner, L. (2002). Comparison of neonicotinoid insecticides as synergists for entomopathogenic nematodes. Biol. Control 24, 90–97. doi: 10.1016/S1049-9644(02)00008-7
Koppenhöfer, A. M., and Fuzy, E. M. (2003a). Steinernema scarabaei for the control of white grubs. Biol. Control 28, 47–59. doi: 10.1016/S1049-9644(03)00048-3
Koppenhöfer, A. M., and Fuzy, E. M. (2003b). Effects of turfgrass endophytes (Clavicipitaceae: Ascomycetes) on white grub (Coleoptera: Scarabaeidae) control by the entomopathogenic nematode Heterorhabditis bacteriophora (Rhabditida: Heterorhabditidae). Environ. Entomol. 32, 392–396. doi: 10.1603/0046-225X-32.2.392
Koppenhöfer, A. M., and Fuzy, E. M. (2006). Effect of soil type on infectivity and persistence of the entomopathogenic nematodes Steinernema scarabaei, Steinernema glaseri, Heterorhabditis zealandica, and Heterorhabditis bacteriophora. J. Invertebr. Pathol. 92, 11–22. doi: 10.1016/j.jip.2006.02.003
Koppenhöfer, A. M., and Fuzy, E. M. (2008). Early timing and new combinations to increase the efficacy of neonicotinoid–entomopathogenic nematode (Rhabditida: Heterorhabditidae) combinations against white grubs (Coleoptera: Scarabaeidae). Pest Manag. Sci. 64, 725–735. doi: 10.1002/ps.1550
Lacey, L. A., and Georgis, R. (2012). Entomopathogenic nematodes for control of insect pests above and below ground with comments on commercial production. J. Nematol. 44, 218–225.
Lacey, L. A., Grzywacz, D., Shapiro-Ilan, D. I., Frutos, R., Brownbridge, M., and Goettel, M. S. (2015). Insect pathogens as biological control agents: back to the future. J. Invertebr. Pathol. 132, 1–41. doi: 10.1016/j.jip.2015.07.009
Lara-Reyes, N., Jiménez-Cortés, J. G., Canales-Lazcano, J., Franco, B., Krams, I., and Contreras-Garduño, J. (2021). Insect immune evasion by dauer and nondauer entomopathogenic nematodes. J. Parasitol. 107, 115–124. doi: 10.1645/20-61
Lee, D. W., Choo, H. Y., Kaya, H. K., Lee, S. M., Smitley, D. R., Shin, H. K., et al. (2002). Laboratory and field evaluation of Korean entomopathogenic nematodes isolates against the oriental beetle Exomala orientalis (Coleoptera: Scarabaeidae). J. Econ. Entomol. 95, 918–926. doi: 10.1603/0022-0493-95.5.918
Li, J., Sun, C., Kang, Y., and Ma, J. (2007). Control effect of entomopathogenic nematodes against grubs in peanut field. Agrochemica 46, 62–63.
Li, X. (2022). Effects of Entomopathogenic Nematodes for the Control of White Grubs in Honeysuckle Fields (master's thesis). Tai'an: Shandong Agricultural University.
Liu, N., Yang, X. H., Chen, Z. G., Zhao, J., Xu, J., and Sun, T. (2008). Present situation of honeysuckle variety resources and cultivation and utilization in Yimeng Mountain area. China Seed Ind. 4, 57–58. doi: 10.19462/j.cnki.1671895x.2008.04.035
Liu, Q. Z., Li, J. X., Xu, X. J., Sun, C. M., Kang, Y. J., Zhou, H. Y., et al. (2007). The preliminary study on grub control with Rhabditis (Oscheius) spp in peanut fields. Acta Agric. Boreali-Sin 22, 250–253.
Liu, S. S., Ke-Bin, L. I., Liu, C. Q., Wang, Q. L., Yin, J., and Cao, Y. Z. (2009). Identification of a strain of Heterorhabditis (Nematoda:Heterorhabditidae) from Hebei and its virulence to white grubs. Acta Entomol. Sin. 52, 959–966.
Liu, Y. H., Lu, X., Jia, H. M., Xin, Z. S., and He, Y. Z. (2017). Study on efficacy of insecticides against Serica orientalis in honeysuckle field. China Plant Prot. 37, 66–69.
Rahman, A., and Sun, C. K. (2009). In vitro control of food-borne and food spoilage bacteria by essential oil and ethanol extracts of Lonicera japonica thunb. Food Chem. 116, 670–675. doi: 10.1016/j.foodchem.2009.03.014
Selvan, S., Grewal, P. S., Gaugler, R., and Tomalak, M. (1994). Evaluation of Steinernematid nematodes against Popillia japonica (Coleoptera: Scarabaeidae) larvae: species, strains, and rinse after application. J. Econ. Enlomol. 87, 605–609. doi: 10.1093/jee/87.3.605
Shang, X., Pan, H., Li, M., Miao, X., and Ding, H. (2011). Lonicera japonica thunb.: ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Econ. Enlomol. 138, 1–21. doi: 10.1016/j.jep.2011.08.016
Shapiro-Ilan, D. I., Bruck, D. J., and Lacey, L. A. (2012b). “Principles of epizootiology and microbial control,” in Insect Pathology, eds F. E. Vega, and H. K. Kaya (Amsterdam: Elsevier), 29–72. doi: 10.1016/B978-0-12-384984-7.00003-8
Shapiro-Ilan, D. I., and Dolinski, C. (2015). “Entomopathogenic nematode application technology,” in Nematode Pathogenesis of Insects and Other Pests, Sustainability in Plant and Crop Protection, ed R. Campos-Herrera (Switzerland, Springer), 231–254. doi: 10.1007/978-3-319-18266-7_9
Shapiro-Ilan, D. I., Gouge, D. H., and Koppenhöfer, A. M. (2002). “Factors affecting commercial success: case studies in cotton, turf and citrus,” in Entomopathogenic Nematology, ed R. Gaugler (Walingford, CABI Publishing), 333–355. doi: 10.1079/9780851995670.0333
Shapiro-Ilan, D. I., Gouge, D. H., Piggott, S. J., and Fife, J. P. (2006a). Application technology and environmental considerations for use of entomopathogenic nematodes in biological control. Biol. Control 38, 124–133. doi: 10.1016/j.biocontrol.2005.09.005
Shapiro-Ilan, D. I., Han, R., and Dolinski, C. (2012a). Entomopathogenic nematode production and application technology. J. Nematol. 44, 206–217.
Shapiro-Ilan, D. I., Stuart, R. J., and McCoy, C. W. (2006b). A comparison of entomopathogenic nematode longevity in soil under laboratory conditions. J. Nematol. 38, 119–129.
Tamson, Y., and Alm, S. R. (1995). Evaluation of Steinernema glaseri (Nematoda: Steinernematidae) for biological control of Japanese and oriental beetles (Coleoptera: Scarabaeidae). J. Econ. Enlomol. 88, 1251–1255. doi: 10.1093/jee/88.5.1251
Tang, Y. L., Yin, L., Zhang, Y. D., Huang, X., Zhao, F. L., Cui, X. B., et al. (2016). Study on anti-inflammatory efficacy and correlative ingredients with pharmacodynamics detected in acute inflammation rat model serum from caulis Lonicerae japonicae. Phytomedicine 23, 597–610. doi: 10.1016/j.phymed.2016.01.016
Torrini, G., Paoli, F., Mazza, G., Simoncini, S., Benvenuti, C., Strangi, A., et al. (2020). Evaluation of indigenous entomopathogenic nematodes as potential biocontrol agents against Popillia japonica (Coleoptera: Scarabaeidae) in northern Italy. Insects 11, 804. doi: 10.3390/insects11110804
Wang, J., Dai, K., Kong, X. X., Cao, L., Qu, L., Jin, Y. L., et al. (2021). Research progress and perspective on entomopathogenic nematodes. J. Environ. Entomol. 43, 811–839.
Wang, P., Liao, W., Fang, J., Liu, Q., Yao, J., Hu, M., et al. (2014). A glucan isolated from flowers of Lonicera japonica thunb. inhibits aggregation and neurotoxicity of aβ42. Carbohydr. Polym. 110, 142–147. doi: 10.1016/j.carbpol.2014.03.060
Wang, Y., Campbell, J. F., and Gaugler, R. (1995). Infection of entomopathogenic nematodes Steinernema glaseri and Heterorhabditis bacteriophora against Popillia japonica (Coleoptera: Scarabaeidae) larvae. J. Invertebr. Pathol. 66, 178–184. doi: 10.1006/jipa.1995.1081
Wei, H. J., Zhang, Z. L., and Wang, M. Z. (1989). Underground Pests in China. Shanghai: Shanghai Science and Technology Press.
Xin, Z. S. (2017). Occurrence and biocontrol technology of scarab in honeysuckle field, Julu county. Modern Rural Sci. Technol. 5, 39.
Xu, X. Y., and Wei, K. (2021). Activity regularity and comprehensive control measures of white grubs from honeysuckle field. Agric. Knowl. 19, 46–48.
Yan, X., Guo, W. X., Zhao, G. Y., and Han, R. C. (2014). Research advances in subterranean pest control by entomopathogenic nematodes. J. Environ. Entomol. 36, 1018-1024.
Yan, X., Han, R., Moens, M., Chen, S., and Clercq, P. (2013). Field evaluation of entomopathogenic nematodes for biological control of striped flea beetle, Phyllotreta striolata (Coleoptera: Chrysomelidae). Biocontrol 58, 247–256. doi: 10.1007/s10526-012-9482-y
Yan, X., Liu, X. J., Han, R. C., Chen, S. L., Clercq, P. D., and Moens, M. (2010). Osmotic induction of anhydrobiosis in entomopathogenic nematodes of the genera Heterorhabditis and Steinernema. Biol. Control 53, 325–330. doi: 10.1016/j.biocontrol.2010.01.009
Yan, X., Zhao, G., and Han, R. (2019). Integrated management of chive gnats (Bradysia odoriphaga Yang and Zhang) in chives using entomopathogenic nematodes and low-toxicity insecticides. Insects 10, 161. doi: 10.3390/insects10060161
Yang, Z. Z., Yu, Y. T., Lin, H. R., Liao, D. C., Cui, X. H., and Wang, H. B. (2018). Lonicera japonica extends lifespan and healthspan in Caenorhabditis elegans. Free Radic. Biol. Med. 129, 310–322. doi: 10.1016/j.freeradbiomed.2018.09.035
Zhang, A. J. (2021). Development countermeasures of Lonicer japonicaindustry in Pingyi under the strategy of rural revitalization. Chin. Wild Plant Resour. 40, 81–85.
Zhang, C. Y., Li, P., Qi, H., and Li, J. (2003). Analysis on the geologic background and physicochemical properties of cultivated soil of flos lonicerae in the geo-authentic and non-authentic producing areas. China J. Chin. Mater. Med. 28, 114–117.
Zhang, Z. R., Cao, L., Liu, X. L., Wang, G. H., Xu, Z. F., and Han, R. C. (2006). Screening of the synergistic agents of Steinernema longicaudum X-7 in entomopathogenic nematodes. Insect Knowl. 68–73.
Keywords: white grubs, entomopathogenic nematode, honeysuckle, biological control, field efficacy, ecological planting
Citation: Li X, Men X, Wang J, Lv S, Li L, Cui H, Song Y, Fang X, Song Z, Guo W and Yu Y (2023) Curative efficacy of entomopathogenic nematodes against white grubs in honeysuckle fields. Front. Sustain. Food Syst. 7:1155133. doi: 10.3389/fsufs.2023.1155133
Received: 31 January 2023; Accepted: 29 March 2023;
Published: 17 April 2023.
Edited by:
Javad Karimi, Ferdowsi University of Mashhad, IranReviewed by:
Muhammad Hafeez, Zhejiang University, ChinaHana Haji Allahverdipour, Iranian Research Institute of Plant Protection (IRIPP), Iran
Copyright © 2023 Li, Men, Wang, Lv, Li, Cui, Song, Fang, Song, Guo and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenxiu Guo, wenxiu.guo@163.com; Yi Yu, robertyuyi@163.com
 Xia Li1,2,3
Xia Li1,2,3  Xingyuan Men
Xingyuan Men Jianhua Wang
Jianhua Wang Lili Li
Lili Li Hongying Cui
Hongying Cui Yingying Song
Yingying Song Zhenqiao Song
Zhenqiao Song Wenxiu Guo
Wenxiu Guo