Potentials of underutilized legumes in food security
- 1Food Security and Safety Focus Area, Faculty of Natural and Agricultural Sciences, North-West University, Mmabatho, South Africa
- 2Genetic Resources Centre, International Institute of Tropical Agriculture, Ibadan, Nigeria
- 3Department of Microbiology, Obafemi Awolowo University, Ile-Ife, Nigeria
Adopting underutilized legumes in tackling food security is essential, especially in this era of climate change. Underutilized legumes are embedded with inherent potentials such as the ability to survive in extreme conditions (such as temperature, drought, pH, saline, etc.), high nitrogen-fixing potential, weed and disease control ability, and high nutrient status. Underutilized legumes can improve the yield of companion crops in an intercropping system and as a subsequent crop (due to their residual effects). They possess symbiotic and non-symbiotic organisms in their nodules, and also have different organisms in their bulk soil, rhizoplanes and rhizosphere, which can fix nitrogen, solubilize phosphorus or produce exudates which help in improving plant growth. Also, they contain some phytochemicals, including alkaloids, saponin, amino acids, organic and inorganic minerals, and compounds that help improve human health and prevent diseases. Hence, this review discusses the current status, role, challenges and the prospects of underutilized legumes in food security.
Introduction
Food security is a global challenge in recent years, especially with the ever-growing population and crop yield reduction, making it difficult to feed the increasing populace. Food security is when there is physical, economic, and social accessibility of sufficient and nutritious food for everyone (1, 2). About 850 million of the world population are extremely hungry, leading to economic challenges and difficulties in the achievement of the sustainable development goals (SDG) (3). Food security is affected by factors such as economy, climate change, environment, lack of storage and processing facilities (leading to postharvest loss), underutilization of some crops with essential values, seed quality, and soil nutrient status (4–12). The utilization of underutilized crops (orphan crops), especially legumes, will go a long way in alleviating food insecurity; this is because of their inherent properties, such as the ability to survive drought, their high nutrient status, and most especially, their high nitrogen-fixing potentials which consequently increase plant growth, crop yield and food production (13, 14). Underutilized legumes are crops that are well known in their countries of origin owing to their economic, cultural, or agronomic values but are generally abandoned by plant breeders, policymakers, consumers, agricultural researchers, donors, extension services, and technology providers (15, 16). This reduces their general acceptability and information available on them and makes it impossible to unleash the potential embedded in them. Nitrogen is the most crucial nutrient in the soil, and its presence enhances crop yield (17); it accounts for 78-79% of the total gas in nature in an inert state and unabsorbable by plants. Plants use ammonium and nitrate forms of nitrogen which are easily lost from the soil through denitrification, crop harvesting, and leaching (17). Legumes are nitrogen-fixing plants from the family Leguminosae that form a symbiotic relationship with a group of bacteria collectively called rhizobia. Rhizobia are concealed in a specialized structure on the root of legumes (nodules), and they fix air nitrogen into a plant usable form (ammonia) using nitrogenase complex enzyme (18, 19). Therefore, the intervention of legumes capable of fixing nitrogen will help to significantly improve food security (20, 21). Thus, it is important to create awareness and understand the mechanism adopted by underutilized legumes in ensuring food security in a bid to ensure the maximum utilization of their potentials; hence this review.
Mechanism used by underutilized legumes to boost food security
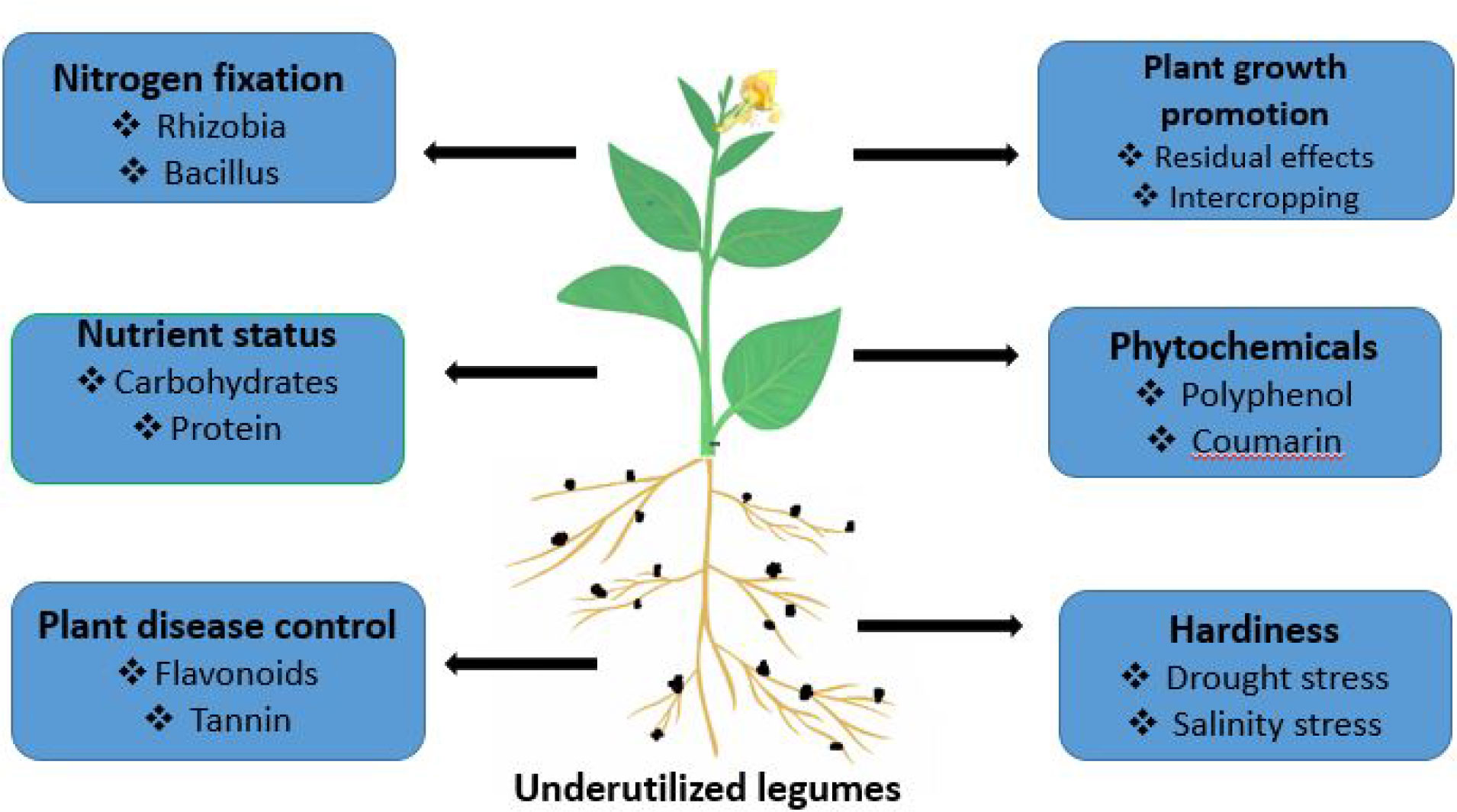
Underutilized legumes can improve food security due to their high nutrient status, ability to improve soil nutrient, potentials to alleviate climate change effects, resilience to adverse effects of climate change such as (erosion, disease emergence, etc.), plant disease control potential, and weed control (13, 19, 22) (Figure 1).
Nutritional status of underutilized legumes
Malnutrition (including protein and micronutrient malnutrition) has been reported to be the leading cause of stunted growth (especially in children below 5 years) as well as child and infant death globally (23). Malnutrition which could be in form of undernutrition (leading to stunted growth, underweight, mineral and vitamin deficiency) or over nutrition (resulting in cancer, diabetes mellitus, stroke, and heart disease) leads to poverty and a reduction in economic growth and productivity (24). According to WHO, around 115 million children below five years of age are stunted, 462 million adults are underweight, 41 million children are overweight and obese, and 1.9 billion adults are overweight and obese, hence, a reduction in malnutrition would help to decrease diseases globally by 32% (24). Malnutrition, especially protein deficiency has been reported to impede fetal brain development, resulting to an abnormal brain physiology and anatomy (25). Malnutrition leads to a high mortality rate, increased cost for diseases treatment and a low rate of recovery from sickness (26–29). Worldwide, malnutrition is responsible for 45% of death in children (30).
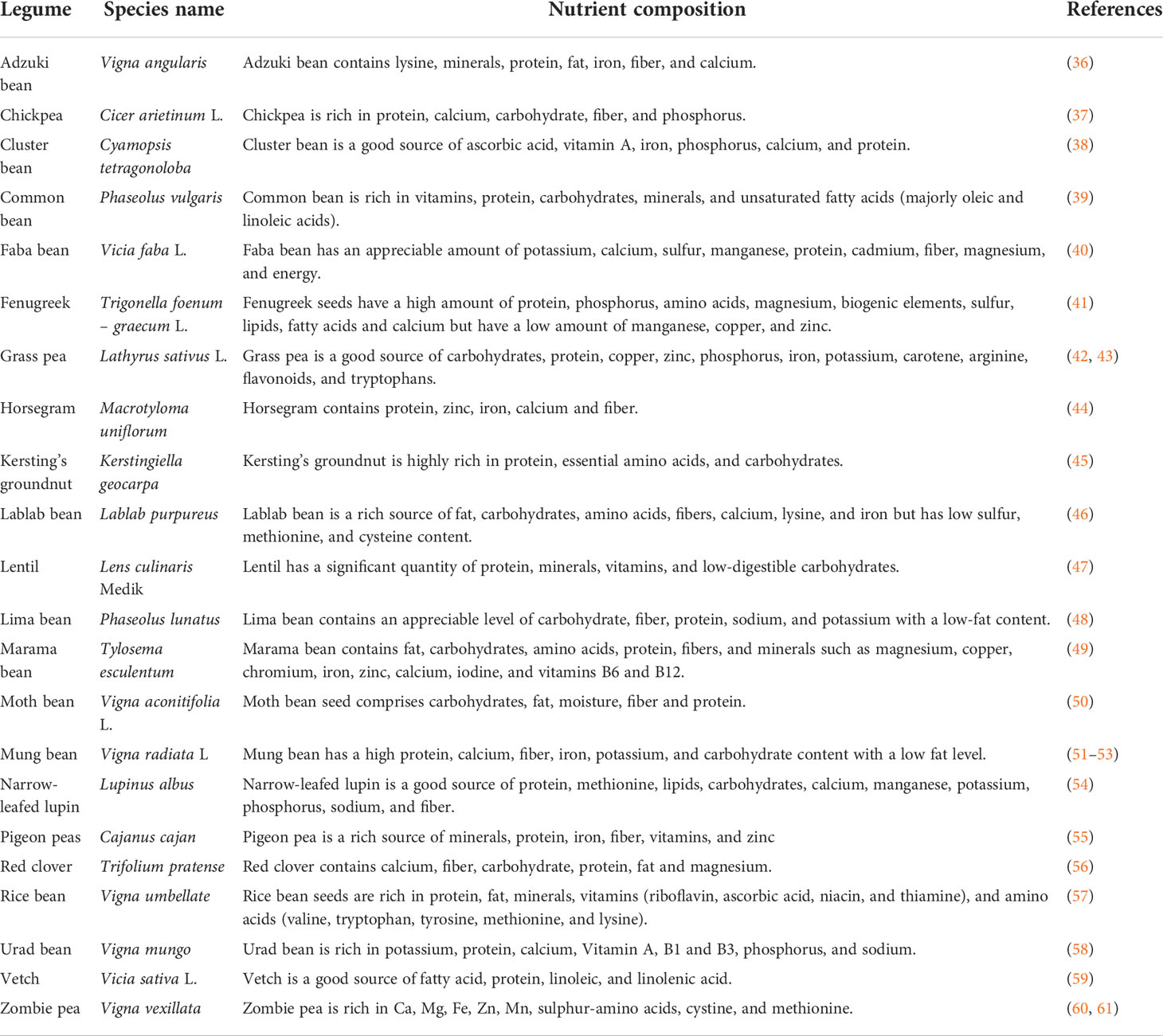
Many underutilized legumes are very high in nutrients and can promote good health and ensure food security (31). They are very affordable and rich in protein, one of the most essential nutrients needed for good living, especially in this era where animal protein sources pose a health threat to humans and are being discouraged. This has led to the promotion and recommendation of plant-based protein to fulfill the nutritional needs of the ever-growing population (32). Soetan and Adeola (33) researched to reveal the nutritional properties of different underutilized legumes which include Bambara groundnut (Vigna subterranean), Jack bean (Canavalia ensiformis), Lima bean (Phaseolus lunatus) and sword bean (Canavalia gladiata). These researchers observed that these plants are rich in protein, fiber, fat, calcium, phosphorus, sodium, potassium, iron and magnesium. Cassia hirsuta L. has equally been reported to contain high protein, lipid, potassium, fiber, carbohydrates, and energy contents (34), while velvet bean (Mucuna pruriens) has been reported to have optimum crude protein, lipid, fiber, carbohydrate, energy, calcium, potassium, phosphorus, zinc, manganese and magnesium content which is capable of supplying human nutritional needs (35). Examples of other underutilized legumes and nutrient composition are listed in Table 1 below:
Some underutilized legumes have multiple edible parts; for instance, different parts of winged bean which include the flower, seeds, pod case, immature pods, tuberous roots, and leaves (62), similarly, the seeds and leaves of fenugreek are edible (63), also, marama bean produces edible grains and tubers beneath their root (49), zombie pie produces edible seeds and tubers (16) and African yam bean produces edible tubers and seeds (64). The edibility of multiple parts of underutilized legumes gives a chance for the human nutritional requirements to be satisfied through different diets, especially for picky eaters, making it possible for the same or similar nutrients to be supplied from different parts of the same plant.
Phytochemicals and compounds in underutilized legumes
The concept of food security involves the existence of safe and nutritious food for all people to meet their dietary needs and promote healthy life (65). Hence, other medicinal or health benefits which can be provided by food is essential to promote food security. Underutilized legumes contain some phytochemicals which can help to prevent diseases in humans; example of such include winged bean, sword bean, velvet bean, jack bean, and scarlet bean, which are rich in antioxidants and phenols and are capable of promoting health status in humans (66).
Coumarin, polyphenol, and flavonoid are available in abundant quantity in fenugreek and have been reported to cure cardiovascular and other chronic diseases (67). Common bean, chickpea, lablab bean, winged bean, faba bean, and pigeon peas contain saponin that have antioxidative, antidiabetic, antitumor, hyperlipidemia, hepatoprotective, anticarcinogenic, antiviral, hypocholesterolemic, and antihepatic effects on humans (68). Common bean contain some level of lectin and lectin-related compounds (69). African yam bean has polypeptides embedded in its albumin fraction with a size not less than 26 kDa sizes (70). Bambara groundnut contains flavonoids, steroids, and saponins which were reported to be responsible for its anti-tubercular nature (71, 72). Amino acids are found in almost all underutilized legumes, and are very helpful in gene expression, treating genetic disorders, boosting the immune system (by producing cytokinins, immunoglobulins, etc.), and regulating the metabolic activities in the body; for instance, arginine prevents oxidation stress, enhances glucose and fatty acid metabolism and prevents non-communicable diseases (47). Similarly, amino acids such as valine, alanine, isoleucine, leucine, serine, threonine, proline, etc., produce signal molecules or hormones that regulate lymphocyte multiplication (lymphocytes carry the memory for invading pathogens and reduce pathogen infiltration) (47). Branched chained amino acids (valine, leucine, and isoleucine) also help to maintain blood sugars and promote reproduction in women by normalizing the embryo implantation, blastocyst development, and fetal development through the production of hormones; it as well enhances the function of the mammary gland (73). In addition, amino acid oxidases from L-isomers of leucine, tyrosine, phenylalanine and tryptophan have antimicrobial properties, which helps to boost the human immune system and fight pathogens (74). Further research should be carried out to test plant extracts from the leaves, flower, roots, and stem bark of underutilized legume in the laboratory for antimicrobial and diseases (e.g. cancer) control potentials, and if successful, they should be further developed into orthodox medicine for human use.
Nitrogen fixation by underutilized legumes
Nitrogen is the most crucial nutrient in the soil, and its presence enhances crop yield (17); it accounts for 78-79% of the total gas in nature, which is in an inert state and unabsorbable by plants. Plants use ammonium and nitrate forms of nitrogen are lost from the soil through denitrification, crop harvesting, and leaching (17). The intervention of legumes capable of fixing nitrogen will help in significantly in improving food security (20, 21). Legumes are nitrogen-fixing plants that form a symbiotic relationship with a group of bacteria called rhizobia. These rhizobia are concealed in a specialized structure on the root of legumes (nodules), and they fix air nitrogen into a plant usable form (ammonia) using nitrogenase complex enzyme contained in them (18). Rhizobium are gram-negative, aerobic, motile, rod-shaped, non-sporing bacteria; they live in water, soil, and plants, and they multiply through cell division using acids, alcohol, and sugar as their energy source (75). Nodulation is the development of nodules on the root of leguminous plants. Firstly, flavonoid (a plant metabolite) is dispersed into the root zone of legumes (76). This attracts infective rhizobium cells to the root of a susceptible legume seedling; the legume provides them with food and shelter, leading to an increase in rhizobial population and plant’s root hair colonization. When the root hair curls, a rhizobium enters, multiplies and forms an infection thread, the infection thread enters the root cortex, where other root cell infection takes place; thereafter, cell division increases, leading to the formation of an embryonic nodule (77). Variations occur in the shape, size, texture, location, and color of nodules and the effectiveness of the nodules is essential to assess the outcome of the legume-rhizobium symbiotic relationship. Nodule color reflects the nitrogen-fixing potentials of nodules; effective nodules appear deep red or pink in color arising from the leghemoglobin pigment (78). Hassen et al. (79) reported the production of effective nodules isolated from Bambara groundnut landraces which are pink in color. When legumes are fertilized using synthetic chemicals, the effective rhizobia strain produces small nodules which remain passive until the synthetic nitrogen are exhausted (77). Effective nodules are large and clustered on the plant’s primary and upper lateral roots and usually evaluated by weight or volume during the late plant flowering stage, while ineffective nodules are usually tiny, many and widely distributed on leguminous roots.
Nitrogen fixation potentials has been reported in underutilized legumes such as Bambara groundnut, Kersting’s groundnut, African yam bean and winged bean (80). Rhizobium belonging to the strain KUL-Z3, KUL-GP, KUL-JN and KUL-BH were isolated from winged bean (81). Equally, Ribeiro et al. (82) discovered novel strains of rhizobium lineage in common bean, the strains discovered were majorly Rhizobium leguminosarum, Rhizobium etli and Rhizobium phaseoli. If well investigated, these rhizobia could probably be capable of promoting food security better than others that have been previously identified from well-known legumes. For instance, they could have the ability to produce antibiotics or other metabolites which can promote the growth of plants by enhancing the release of nutrients or by controlling or preventing plant diseases.
Nitrogen fixation has also been demonstrated in Kersting’s groundnut, where high nitrogen-fixing ability was expressed by Bradyrhizobium kavangense 14-3T, Bradyrhizobium elkanii, Bradyrhizobium pachyrhizi PAC48T, Bradyrhizobium vignae 7-2T, and Bradyrhizobium subterraneum 58-2-1T. These microsymbionts enhanced plant growth promotions via increased leaf chlorophyll, nodulation and photosynthesis, which all helps to increase plant yield (83). Likewise, the lablab bean’s ability to associate with nitrogen-fixing rhizobia such as Mesorhizobium ensifer and Bradyrhizobium species has been reported (84). However, there could be variability in the nitrogen-fixing potentials of different rhizobia, for instance, Hailu Gunnabo et al. (85) carried out research that revealed that of all the Rhizobium species isolated from the common bean in Ethiopia, only Rhizobium phaseoli and Rhizobium etli were predominant. This could be due to the ability to out-compete other organisms.
Non-symbiotic bacteria and cyanobacteria residing in the bulk soil, rhizosphere or rhizoplane of plants can fix nitrogen by using nitrogenase enzymes at normal temperature and pressure (86). Free-living bacteria utilize soil nitrogen for their metabolic activities and are active only under specific conditions; they use high molecular weight nitrogen by releasing proteases and chitinases enzymes which break down the complex nitrogen for plant use (87). Examples of free-living nitrogen-fixing bacteria include Azotobacter species, Bacillus species, and Azospirillum species, with Azospirillum sp. being one of the most efficient free-living nitrogen-fixing diazotrophs (88, 89). Zoundji et al. (90) reported that Rhizobium species was able to increase the dry shoot matter of Bambara groundnut. Similarly, Rhizobium species and Pseudomonas species were able to increase the biomass and yield of common bean by 25% and 15% respectively (91). Aspergillus species and Penicillium species significantly increased the root length, shoot dry weight, height, nodule number, pod number, 50 seed weight, nodule dry weight and phosphorus content of Phaseolus vulgaris L. (92). The two fungal species (Aspergillus species and Penicillium species) reported by Elias et al. (92) were able to increase the yield and yield parameters of Phaseolus vulgaris because they have phosphorus solubilizing ability. Few studies have been carried out on the endophytes living in different organs other than the roots of underutilized legumes such as the flower, seeds, root, and stem. Endophytes are organisms that help plants to store nutrients, recycle nutrients, and in soil bioremediation (93). Chimwamurombe et al. (94) reported the presence of endophytes belonging to the species Chitinophaga sp., Rhizobium sp., Massilia species, Microbacterium species, Burkholderia species, Kosakonia species, Mucilaginibacter species, Curtobacterium species, Bacillus species, Pseudorhodoferax species, Methylobacterium species, Caulobacter species, Sphingomonas, species, and Pantoea species. in the seed of marama bean. These researchers reported the presence of exudates such as protease, siderophores, indole acetic acid (IAA), endoglucanase, and 1-aminocyclopropane-1-carboxylic acid (ACC deaminase), which enhanced the nitrogen-fixing and phosphorus solubilizing abilities of the endophytes (94). Parsa et al. (95) also assessed common beans for fungal endophytes and realized that the prevalent species were Fusarium oxysporum, Cladosporium cladosporioides and Xylaria species. Some of the microbes mentioned above can be used in bioremediation process, which can rejuvenate the soil and make it available for plant growth and for other purposes.
There are records of free-living microbes from the rhizosphere and bulk soil of underutilized legumes. Some of them are embedded with other attributes aside from nitrogen fixation. For example, the rhizosphere and bulk soil of marama bean has been found to contain bacteria from the species Klebsiella, Bacillus, Acinetobacter, Kosakonia, Raoultella, Arthrobacter, Burkholderia, these organisms are embedded with the ability to solubilize phosphorus, and to produce catalase, ammonia, hydrogen cyanide, protease as well as ACC deaminase activity (96). In another research which involved lima bean, the presence of a high population of microbes was reported in the rhizosphere, and they belong majorly to the species Gaiella, Streptomyces, Nitrososphaeraceae, Acidobacteria, Rhizobium, Conexibacter, Bacillus, Burkholderiaceae, Novosphingobium and Synechococcus (Cyanobacteria) (97). However, there is a research gap in literature regarding the quantification of nitrogen fixed and the existence of non-symbiotic nitrogen-fixing bacteria inhabiting the nodules, rhizosphere, bulk soil and rhizoplane of many underutilized legumes; hence, further researches should be carried out to unravel these information. If the amount of nitrogen fixed by specific underutilized legumes in intercrops and as subsequent crops are confirmed, the deficient nitrogen needed by crops can be complimented using synthetic nitrogen source; this will reduce the application of synthetic fertilizer and the greenhouse gases emission associated with them.
Ability to survive extreme conditions
Underutilized legumes can survive in extreme conditions due to the presence of soil microflora in their rhizosphere and nodules and some inherent traits they possess, a term referred to as hardiness (Figure 1). Underutilized legumes have peculiar physiological makeup and harbor microbes that can survive and be active in extreme conditions such as saline, drought, extreme pH, temperature, etc. (16, 98, 99). Degefu et al. (100) carried out an experiment on pigeon pea and revealed that Bradyrhizobium elkanii and Bradyrhizobium japonicum survive in saline and drought soils in Ethiopia, where they help the plant to alleviate the stress conditions. In mung beans, strains of Pseudomonas and Rhizobium which produces auxin and ACC deaminase helped the plant to survive in saline environment by inducing salinity tolerance (101). Common bean was also evaluated for the ability to survive in a saline condition and it was detected that the presence of different Rhizobium species designated as PvMb1, ISRA352, PvNk7, and PvNk8 helped it to alleviate salinity stress and enhanced plant growth and osmolyte content (glycine, betaine and proline) (102). Similarly, Rhizobium radiobacter from mung beans produced extracellular polymeric substances which was used to bioremediate arsenic and increased the survival and stress tolerance of the plant to arsenic pollutant (103).
In addition, salt and pH tolerance was recorded in common bean which accommodates Rhizobium species. in their nodules, the effective isolates recovered were HUCRM3B, HUCRM2D, HUCRM5C and HUCRM9C, the species helped common bean to alleviate pH and salt stress (104). The presence of microflora in soil with high salinity content and soil with extreme pH indicates that the microbes utilizes the salt and pH for their growth and can thus be developed into commercially available products which can be used in bioremediation; hence, helping to put to use land that are neglected because of their natural extreme chemical properties or high chemical properties arising from human activities and pollutions.
Plant disease control
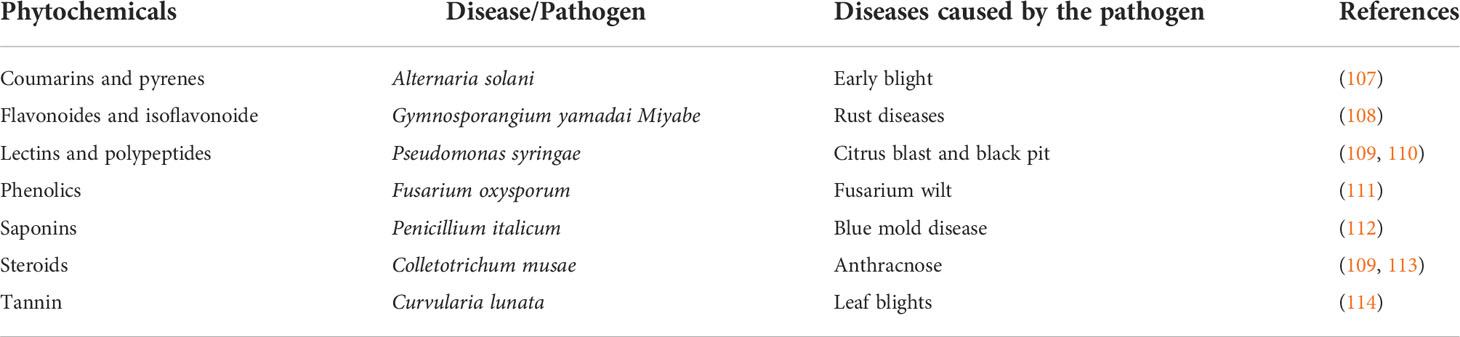
The use of plant extract from underutilized legumes to control plant diseases has not been extensively explored. Some phytochemicals are found in underutilized legumes; these chemicals, which are also present in some other plants, have been reported to be capable of controlling plant diseases (Figure 1). African yam bean contains tannin (16); although tannin is an antinutrient which prevent the absorption of nutrient during consumption, on the other side its potential to control plant disease could be an advantage. Equally, Adegboyega et al. (105) reported the presence of tannin and phytate in the seeds and tubers of winged beans. Flavonoids, tannin, and phenols were also reported to be present in common beans with an increased concentration in a water-stressed condition (67). Fenugreek contains coumarin, polyphenol, and flavonoid in abundant quantity (67), and saponin in common bean, chickpea, lablab bean, winged bean, faba bean, and pigeon peas has also been established (68). Sparvoli et al. (69) and Ajibola et al. (106) has equally reported the presence of lectin in common bean and the presence of polypeptides in African yam bean, respectively. Bambara groundnut also contains falvonoids, steroids, and saponins which were reported to be responsible for its anti-tubercular nature (71, 72). Many of the phytochemicals that are produced by the aforementioned underutilized legumes have been reported to be used in curing some plant diseases. The causative agent of diseases they cure and the mechanism of action of the plant diseases they control are detailed in Table 2 below. Research should be intensified to unravel some other existing and novel phytochemicals in underutilized legumes which can be useful in the control of plant diseases. The presence of all these aforementioned phytochemicals from other plants has been reported by some other researchers to be capable of controlling plant diseases (Table 2). The ability of these chemicals from underutilized legumes to control plant diseases should as well be tested and the mechanisms underlying their production in underutilized legumes should be properly understood; perhaps it could be used to increase the level of phytochemical production by underutilized legumes for plant disease control.
Promotion of companion/subsequent plant growth
Legumes are capable of fixing nitrogen for themselves; however, other plants can as well benefit from this fixation (115). A neighboring plant in an intercropping system (116) or a succeeding plant (117) can utilize the nitrogen fixed biologically by legumes. When legumes are planted in a monocropping system and harvested, only the seeds are harvested. The other parts, e.g., the roots, stems, and other non-edible portions, are left behind, and they decompose to release nutrients. In an intercropping system, the roots of the plants grow and die several times during the life cycle of the plants and lead to the release of nitrogen by mineralization, nitrogen is also made available by mycorrhizae fungi and the root exudates released during intercropping (118). Mycorrhizae network between roots of legumes and companion crops also meditates nitrogen transfer and uptake respectively (119). More research is needed on the mechanism of nutrient transfer between nodulating underutilized legumes and non-nodulating plants, as this would enhance tapping into the potentials of underutilized legumes for improved food security. In a study by Giller et al. (120), the transfer of nitrogen from common bean to maize which was the companion crop during intercropping was recorded, though the quantity transferred was not too significant.
In contrast, in a previous research carried out by Egbe et al. (121), it was reported that the weight and nodule number of Bambara groundnut, when intercropped with maize decreased, while the nodules numbers increased and the weight was indifferent when Bambara groundnut was intercropped with cowpea. The researchers as well reported that the nitrogen-fixing potentials decreased with a lower planting density of Bambara groundnut when it was intercropped with cowpea and maize. Also, nitrogen fixation (using root yield nitrogen) measured was not different when Bambara groundnut was intercropped with both cowpea and maize (121). Owing to the different reports on nutrient transfer during intercropping, it is necessary to understand the principles underlying the transfer of nutrients during intercropping as this will help to maximally tap into the potentials of increasing nitrogen transfer from legumes to cereals.
Asides intercropping, underutilized legumes could make nitrogen available for subsequent crops through decomposition, a process referred to as residual effect (Figure 1). A study by Uher et al. (122) revealed that the forage quality from maize intercropped with common bean led to a high crude protein as well as a reduced amount of neutral and acid detergent fiber, consequently a higher nutrient digestibility, even though different densities of intercropping was used. Gebremichael et al. (123) also noted that the yield of sorghum planted in an intercropping system and as a subsequent crop using pigeon peas had an increased grain yield. This indicates that intercropping using underutilized legumes have a positive effect on the nitrogen-fixing potentials, nutritional outputs, forage, and grain yield which all have an effect on food security. It is therefore very vital to carry out more studies to determine the compatibility of different accessions and species of underutilized crops as intercrops and preceding crops, as this will help farmers to choose the best option to realize their desired output (i.e. forage, grain yield, etc.).
Factors impeding the usage of underutilized legumes for food security
The utilization of underutilized legumes in promoting food security are hindered by different factors, which include the presence of anti-nutrients, long cooking duration, low demand by the populace, shattering of the pod, high cost of production and poor digestibility (16, 37). Some climbing underutilized climbing legumes require staking, which incurs additional costs for farmers; for instance, African yam bean requires staking, which may be too expensive for farmers (124). The production of some other underutilized legumes is also laborious; for example, bambara groundnut is a shrub and the manual weeding, milling and harvesting could be stressful or expensive if the farmer employs people to carry out the maintenance operations (125). Long cooking duration is another problem associated with underutilized legumes (126). Most of the underutilized legumes take several hours to cook, making it impossible for them to serve immediate hunger needs. In a study by Owusu et al. (126), the long cooking duration of lima bean was reported. However, some methods have been used to hasten the cooking time of underutilized legumes and these include presoaking, dehulling, and molecular techniques such as Genome-Wide Association Study and Quantitative Trait Loci, which can be used to identify the loci which controls the cooking duration and can be altered to reduce the cooking duration (127). Equally, novel breeding techniques such as transcription activator-like effector nucleases (TALENS), clustered regularly interspaced short palindromic repeats (CRISPR/Cas9) and zinc finger nucleases (ZFNs) are available to insert the desired traits into plants (127). The use of organic tenderizers can also be experimented as organic tenderizers are natural and not associated with harmful side effects compared to their chemical counterparts; such tenderizers include unripe pawpaw, kiwi, etc. Enhancing the cooking time of underutilized legumes will go a long way in promoting their acceptability and utilization.
The shattering of the pods of underutilized legumes is another challenge that impedes their production and reduces their yield (127). The presence of anti-nutrients and properties such as hydrogen cyanides, saponins, oxalates, phytates, alkaloids, flatulence factors, tannins and heamagglutinins in underutilized legumes reduce their acceptability (128). However, many solutions have been prescribed to reduce the anti-nutrient factors in legumes, and these include autoclaving, parching in hot sand, soaking in water, microwave treatment, boiling in water, fermentation, dehulling, soaking in acidic or alkaline solution, roasting, simmering, pounding, baking, air-drying, steaming, and soaking in lime solutions (129). The purpose or proposed use of underutilized legumes could be considered before deciding to reduce their anti-nutrients. For instance the anti-nutrients are chemicals of interest for the pharmaceutical industries; hence, if the crops are meant to be used for drug production, antinutrients should be enhanced and not reduced but for consumption purposes, the reduction of antinutrients is very important.
Prospects, recommendations and research gaps in underutilized legumes
A lot of microorganisms are associated with the nodules, rhizosphere, rhizoplane, and bulk soil of legumes generally, with few studies reporting these in underutilized legumes. Since underutilized legumes have the potentials to host novel microbes with desirable characteristics and traits to improve crop yield (such as the ability to survive in extreme temperature, pH, salinity and water stressed situation, their insecticidal and pesticidal ability and the potential to fix high nitrogen), such microbes should be developed into products such as inoculum, biofertilizers, biochar, biopesticides, bioinsecticides, etc. to increase the growth and yield of both leguminous and non-leguminous plants. Plant extracts can also be produced using different parts of underutilized legumes such as the roots, stems, pods, leaves, etc., and tested for their ability to treat plant diseases and drive away pests and insects.
Underutilized legumes should be incorporated into different planting system so as to determine the best planting system that suits them and fully unleash their potentials to improve food security. Furthermore, the compatibility of underutilized crops with other crops, the principles underlying the transfer of nitrogen from nitrogen-fixing underutilized legumes to companion crops and the yield outcome during intercropping should be well understood in order to maximize the advantages associated with their intercropping.
In addition, research should be intensified on the application of legumes to adapt and mitigate climate change effects which include pests, erosion, drought, salinity and diseases; this will help to maximize land resources. Also, organic tenderizers such as unripe pawpaw and kiwi which are being used locally as tenderizers to soften beefs while cooking, should be tested for their ability to reduce the cooking time of underutilized legumes by softening them, as organic tenderizers are natural and not associated with harmful side effects compared to their chemical counterparts.
Due to the high nutritional and medicinal properties of many underutilized legumes, essential nutrients can be extracted from them and made into nutritional supplements or tablets for the treatment and prevention of ailments. Other edible products such as cooking oil can be extracted from underutilized legumes since similar legumes (e.g., soybean are processed into oil) are used to produce cooking oil and some underutilized legumes have high lipid contents. The processing of underutilized legumes into flour will prolong their shelf life and prevent spoilage; the flowers can therefore be incorporated in baking of breads and other pastries.
Finally, creating awareness of underutilized legumes will help to reduce the problem of low demand. If well publicized, many people, even if they do not like the taste of underutilized legumes, can feed on them for their health benefits. If underutilized legumes are used as raw materials in industries (e.g. for oil and medications), demand from such will help to increase the production and encourage more farmers to produce them; hence, prevent them from going into extinction and promoting food security.
Conclusion
The intervention of underutilized legumes in tackling food insecurity should be emphasized owing to their important characteristics. Many research has been carried out on the nitrogen-fixing potentials of well-known legumes. Hence, adequate awareness and information from researchers on the nitrogen-fixing potentials of underutilized legumes will remarkably enhance their utilization; perhaps there may be some of them that fix more nitrogen than many well-known legumes, which will promote their usage as preceding crop or in an intercrop in a view to improving food security. This will reduce the application of fertilizers by farmers, consequently reducing the cost of farmer’s production and alleviating the effects of climate change since a reduction in fertilizer application will lead to the reduction in the release of greenhouse gases. In addition, underutilized legumes can bioremediate the soil and survive in extreme environments owing to the presence of rhizobia and other plant growth promoting microbes in them, which produces or enhances the production of metabolites which makes the environment more conducive for the plants. Equally, underutilized legumes are very high in nutrients, making them a good source of satisfying human nutritional needs. However, underutilized legumes are faced with challenges such as long cooking duration, low demand, anti-nutrient factors etc. Some methods have been reported to tackle these challenges, for instance, organic tenderizers and the use of molecular techniques such as TALENS, ZFNs, etc. has been recommended to reduce the cooking time, antinutrient factors are also reduced through different processing such as dehulling, soaking, etc., also the knowledge of the underutilized legume’s health benefit will help to promote their demand. Hence, it is very necessary to carry out more research to unravel the potentials embedded in underutilized legumes and promote their use to improve food security.
Author contributions
MSA managed the literature searches and wrote the manuscript’s first draft. OAO, MA, OB, and OO were involved in the conceptualization, reviewing of the drafts and proofreading of the manuscripts. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Global Crop Diversity Trust through Genetic Resources Center, International Institute of Tropical Agriculture, Ibadan, Nigeria.
Acknowledgments
The authors appreciate the International Institute of Tropical Agriculture for the Graduate Research Fellowship award to MSA. Also, the authors sincerely appreciate the North-West University, South Africa, for the Institutional bursary awarded to the first author.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Liese A. Food security. In: The Oxford handbook of governance limited statehood Oxford, England, United Kingdom: Oxford University Press (2018). p. 459–78.
2. Wani SP, Sawargaonkar GL. Future smart crops for paddy fallow agri-food systems in southeast Asia. In: Future smart food-rediscovering hidden treasures of neglected underutilized species for zero hunger in Asia. Bangkok: FAO (2018). p. 61–78.
3. Kousar S, Ahmed F, Pervaiz A, Bojnec S. Food insecurity, population growth, urbanization and water availability: The role of government stability. Sustainability (2021) 13(22):12336. doi: 10.3390/su132212336
4. Wang Z-g, Bao X-g, Li X-f, Jin X, Zhao J-h, Sun J-h, et al. Intercropping maintains soil fertility in terms of chemical properties and enzyme activities on a timescale of one decade. Plant Soil (2015) 391(1):265–82. doi: 10.1007/s11104-015-2428-2
5. Szabo S. Urbanisation and food insecurity risks: Assessing the role of human development. Oxford Dev Stud (2016) 44(1):28–48. doi: 10.1080/13600818.2015.1067292
6. Weih M, Westerbergh A, Lundquist P-O. “Role of nutrient-efficient plants for improving crop yields: bridging plant ecology, physiology, and molecular biology,”. In: Plant macronutrient use efficiency. Cambridge, Massachusetts: Academic press (2017). p. 31–44.
7. Abiad MG, Meho LI. Food loss and food waste research in the Arab world: A systematic review. Food Secur (2018) 10(2):311–22. doi: 10.1007/s12571-018-0782-7
8. Chauhan N, Vaidya D, Pandit A. Underutilized grains of Himalayan region: A mini review. J J Pharmacognosy Phytochem (2018) 7(1):1044–7. https://www.phytojournal.com/archives/2018/vol7issue1/PartN/6-4-484-543.pdf
9. Richardson KJ, Lewis KH, Krishnamurthy PK, Kent C, Wiltshire AJ, Hanlon HM. Food security outcomes under a changing climate: impacts of mitigation and adaptation on vulnerability to food insecurity. Climatic Change (2018) 147(1):327–41. doi: 10.1007/s10584-018-2137-y
10. Begna T. Role and economic importance of crop genetic diversity in food security. Int J Agric Sci Food Technol (2021) 7(1):164–9. doi: 10.17352/2455-815X.000104
11. Rahman MS, Toiba H, Huang W-C. The impact of climate change adaptation strategies on income and food security: Empirical evidence from small-scale fishers in Indonesia. Sustainability (2021) 13(14):7905. doi: 10.3390/su13147905
12. Setsoafia ED, Ma W, Renwick A. Effects of sustainable agricultural practices on farm income and food security in northern Ghana. Agric Food Economics (2022) 10(1):1–15. doi: 10.1186/s40100-022-00216-9
13. Bano SA, Iqbal SM. Biological nitrogen fixation to improve plant growth and productivity. J Int J Agric Innovat. Res (2016) 4:596–9. https://ijair.org/administrator/components/com_jresearch/files/publications/1_IJAIR_1732_Final.pdf
14. Olanrewaju OS, Oyatomi O, Babalola OO, Abberton M. Breeding potentials of bambara groundnut for food and nutrition security in the face of climate change. Front Plant Sci (2021) 12:1–14. doi: 10.3389/fpls.2021.798993
15. Mabhaudhi T, Chimonyo VG, Chibarabada TP, Modi AT. Developing a roadmap for improving neglected and underutilized crops: A case study of south Africa. Front Plant Sci (2017) 8:2143. doi: 10.3389/fpls.2017.02143
16. Popoola J, Ojuederie O, Omonhinmin C, Adegbite A. "Neglected and underutilized legume crops: Improvement and future prospects,". In: Recent advances in grain crops research. London, UK: IntechOpen (2019).
17. Leghari SJ, Wahocho NA, Laghari GM, HafeezLaghari A, MustafaBhabhan G, HussainTalpur K, et al. Role of nitrogen for plant growth and development: A review. J Adv Environ Biol (2016) 10(9):209–19. https://link.gale.com/apps/doc/A472372583/AONE?u=anon~a3780ecd&sid=googleScholar&xid=6d264c51
18. Liu A, Contador CA, Fan K, Lam H-M. Interaction and regulation of carbon, nitrogen, and phosphorus metabolisms in root nodules of legumes. J Front Plant Sci (2018) 9:1860. doi: 10.3389/fpls.2018.01860
19. Ajilogba CF, Olanrewaju OS, Babalola OO. Improving bambara groundnut production: Insight into the role of omics and beneficial bacteria. Front Plant Sci (2022) 13. doi: 10.3389/fpls.2022.836133
20. Rosegrant MW, Cline SA. Global food security: challenges and policies. J Sci (2003) 302(5652):1917–9. doi: 10.1126/science.1092958
21. Nnamani CV, Ajayi SA, Oselebe HO, Atkinson CJ, Igboabuchi AN, Ezigbo EC. Sphenostylis stenocarpa (ex. a. rich.) harms., a fading genetic resource in a changing climate: prerequisite for conservation and sustainability. Plants (2017) 6(3):30. doi: 10.3390/plants6030030
22. Bitire TD, Abberton M, Oyatomi O, Babalola OO. Effect of bradyrhizobium japonicum strains and inorganic nitrogen fertilizer on the growth and yield of bambara groundnut (Vigna subterranea (L.) verdc) accessions. Front Sustain Food Syst (2022) 231:1–12. doi: 10.3389/fsufs.2022.913239
23. WHO. World health organization;. the state of food security and nutrition in the world 2018. In: Building climate resilience for food security and nutrition. Rome, Italy: Food and Agriculture Organization (2018).
24. Dukhi N. Global prevalence of malnutrition: evidence from literature. Malnutrition (2020) 1:1–16. doi: 10.5772/intechopen.92006
25. Rushmore R, McGaughy J, Mokler D, Rosene D. The enduring effect of prenatal protein malnutrition on brain anatomy, physiology and behavior. Nutr Neurosci (2022) 25(7):1392–9. doi: 10.1080/1028415X.2020.1859730
26. Norman K, Pichard C, Lochs H, Pirlich M. Prognostic impact of disease-related malnutrition. J.C.n. (2008) 27(1):5–15. doi: 10.1016/j.clnu.2007.10.007
27. Goates S, Du K, Braunschweig CA, Arensberg MB. Economic burden of disease-associated malnutrition at the state level. PloS One (2016) 11(9):e0161833. doi: 10.1371/journal.pone.0161833
28. Lanctin DP, Merced-Nieves F, Mallett RM, Arensberg MB, Guenter P, Sulo S, et al. Prevalence and economic burden of malnutrition diagnosis among patients presenting to united states emergency departments. Acad Emergency Med (2021) 28(3):325–35. doi: 10.1111/acem.13887
29. Correia MIT, Tappenden KA, Malone A, Prado CM, Evans DC, Sauer AC, et al. Utilization and validation of the global leadership initiative on malnutrition (GLIM): A scoping review. Clin Nutr (2022) 41(2022):687–97. doi: 10.1016/j.clnu.2022.01.018
30. Ntaganda J. Analysis of risk factors that influence stunting among Rwandan children under the age of five. Afr J Food Agriculture Nutr Dev (2022) 22(5):1–10. doi: 10.18697/ajfand.110.21125
31. Cheng A. Shaping a sustainable food future by rediscovering long-forgotten ancient grains. Plant Sci (2018) 269:136–42. doi: 10.1016/j.plantsci.2018.01.018
32. Cheng A, Raai MN, Zain NAM, Massawe F, Singh A, Wan W.A.A.Q.I. In search of alternative proteins: unlocking the potential of underutilized tropical legumes. Food Secur (2019) 11(6):1205–15. doi: 10.1007/s12571-019-00977-0
33. Soetan K, Adeola A. Comparative nutritional and functional properties of selected underutilized legumes. Nigerian J Anim Production (2018) 45(3):96–106-196–106. doi: 10.51791/njap.v45i3.441
34. Vadivel V, Janardhanan K. Chemical composition of the underutilized legume Cassia hirsuta L. Plant Foods Human Nutr (2000) 55(4):369–81. doi: 10.1023/A:1008117010991
35. Janardhanan VVK Nutritional and anti-nutritional composition of velvet bean: an under-utilized food legume in South India. Inter J Food Sci Nutr (2000) 51(4):279–87.
36. Agarwal S, Chauhan ES. Adzuki beans-physical and nutritional characteristics of beans and its health benefits. J Food Sci Nutr J (2019) 9(4):304–10. https://www.ijhsr.org/IJHSR_Vol.9_Issue.4_April2019/IJHSR_Abstract.043.html
37. Yegrem L. Nutritional composition, antinutritional factors, and utilization trends of ethiopian chickpea (Cicer arietinum l.). Int J Food Sci Nutr (2021) 2021:10. doi: 10.1155/2021/5570753
38. Ramanjaneyulu A, Madhavi A, Neelima T, Naresh P, Reddy KI, Srinivas A. Effect of row spacing and sowing time on seed yield, quality parameters and nutrient uptake of guar [Cyamopsis tetragonoloba (L.) taub] in semi arid climate of southern telanagana, India. Legume Research-An Int J (2018) 41(2):287–92. doi: 10.1155/2021/5570753
39. Celmeli T, Sari H, Canci H, Sari D, Adak A, Eker T, et al. The nutritional content of common bean (Phaseolus vulgaris l.) landraces in comparison to modern varieties. Agronomy (2018) 8(9):166. doi: 10.3390/agronomy8090166
40. Khazaei H, Vandenberg A. Seed mineral composition and protein content of faba beans (Vicia faba l.) with contrasting tannin contents. Agronomy (2020) 10(4):511. doi: 10.3390/agronomy10040511
41. Zuk-Gołaszewska K, Wierzbowska J. Fenugreek: productivity, nutritional value and uses. J Elementol (2017) 22(3):1067–80. doi: 10.5601/jelem.2017.22.1.1396
42. Kosev V, Vasileva V. Biochemical assessment of grass pea (Lathyrus sativus l.) varieties. J Global Innov Agric Soc Sci (2018) 6(1):23–7. https://www.researchgate.net/publication/328391692_KOSEVVASILEVA_JGIASS_2018
43. Angelova VR. Assessment of soil contamination on the content of macro and microelements and the quality of grass pea seeds (Lathyrus sativus l.). Int J Environ Ecol Eng (2019) 13(11):651–8. doi: 10.5281/zenodo.3593104
44. Herath HT, Samaranayake M, Liyanage S, Abeysekera W. Horse gram: an incredible food grain as a potential source of functional and nutritional food ingredient. Int J Food Sci Nutr (2020) 5:93–101. http://www.foodsciencejournal.com/archives/2020/vol5/issue4/5-2-16
45. Jaiswal SK, Mohammed M, Dakora FD. Microbial community structure in the rhizosphere of the orphan legume kersting’s groundnut [Macrotyloma geocarpum (Harms) marechal & baudet]. Mol Biol Rep (2019) 46(4):4471–81. doi: 10.1007/s11033-019-04902-8
46. Tjandra Nugraha D, Bata FS. Evaluation of lablab bean (Lablab purpureus (L.) sweet) sprout milk fortificated with eggshell extracted calcium. Prog Agric Eng Sci (2021) 16(S2):9–18. doi: 10.1007/s11033-019-04902-8
47. Salaria S, Boatwright JL, Thavarajah P, Kumar S, Thavarajah D. Protein biofortification in lentils (Lens culinaris medik.) toward human health. Front Plant Sci (2022) 934. doi: 10.3389/fpls.2022.869713
48. Farinde EO, Olanipekun OT, Olasupo RB. Nutritional composition and antinutrients content of raw and processed lima bean (Phaseolus lunatus). Ann Food Sci Technol (2018) 19:250–64. https://www.semanticscholar.org/paper/NUTRITIONAL-COMPOSITION-AND-ANTINUTRIENTS-CONTENT-Farinde-Olanipekun/3eade01302d84908b600536951a2dd04cbe0e3c4
49. Omotayo AO, Aremu AO. Marama bean [Tylosema esculentum (Burch.) a. schreib.]: an indigenous plant with potential for food, nutrition, and economic sustainability. Food Funct (2021) 12(6):2389–403. doi: 10.1039/D0FO01937B
50. Deshmukh B, Pawar V. Effects of different pretreatments on physicochemical and anti nutritional quality of moth bean. J Pharmacognosy Phytochem (2020) 9(1):1965–8. https://www.phytojournal.com/archives/2020/vol9issue1/PartAG/9-1-457-658.pdf
51. Dahiya P, Linnemann A, Van Boekel M, Khetarpaul N, Grewal R, Nout M. Mung bean: Technological and nutritional potential. Crit Rev Food Sci Nutr J (2015) 55(5):670–88. doi: 10.1080/10408398.2012.671202
52. Skylas DJ, Blanchard CL, Quail K. Variation in nutritional composition of australian mungbean varieties. J Agric Sci (2017) 9(5):45–53. doi: 10.1080/10408398.2012.671202
53. Johnson J, Collins T, Power A, Chandra S, Skylas D, Portman D, et al. Antioxidative properties and macrochemical composition of five commercial mungbean varieties in Australia. Legume Sci (2020) 2(1):e27. doi: 10.5539/jas.v9n5p45
54. Arnoldi A, Boschin G, Zanoni C, Lammi C. The health benefits of sweet lupin seed flours and isolated proteins. J Funct Foods (2015) 18:550–63. doi: 10.1002/leg3.27
55. Anaemene D. A comparative evaluation of the nutrient and anti-nutrient compositions of four pigeon pea (Cajanus cajan) varieties. Anchor Univ J Sci Technol (2020) 1(1):102–9. doi: 10.1016/j.jff.2015.08.012
56. Kumar R, Joshi R, Kumar R, Srivatsan V, Chawla A, Patial V, et al. Nutritional quality evaluation and proteome profile of forage species of Western himalaya. Grassland Science (2022) 68:214–25. doi: 10.1111/grs.12357
57. Dhillon PK, Tanwar B. Rice bean: A healthy and cost-effective alternative for crop and food diversity. Food Secur (2018) 10(3):525–35. doi: 10.1111/grs.12357
58. Dineshkumar R, Subramanian J, Sampathkumar P. Prospective of chlorella vulgaris to augment growth and yield parameters along with superior seed qualities in black gram, vigna mungo (L.). Waste Biomass Valorization (2020) 11(4):1279–87. doi: 10.1007/s12571-018-0803-6
59. Grela ER, Samolińska W, Rybiński W, Kiczorowska B, Kowalczuk-Vasilev E, Matras J, et al. Nutritional and anti-nutritional factors in vicia sativa l. seeds and the variability of phenotypic and morphological characteristics of some vetch accessions cultivated in European countries. Animals (2020) 11(1):44. doi: 10.1007/s12649-018-0465-9
60. Siddhuraju P, Vijayakumari K, Janardhanan K. Chemical analysis and nutritional assessment of the less known pulses, vigna aconitifolia (Jacq.) marechal andVigna vexillata (L.) a. rich. Plant Foods Hum Nutr (1994) 45(2):103–11. doi: 10.1007/BF01088467
61. Marconi E, Ruggeri S, Carnovale E. Chemical evaluation of wild under-exploited vigna spp. seeds. Food Chem (1997) 59(2):203–12. doi: 10.1007/BF01088467
62. Mohanty CS, Singh V, Chapman MA. Winged bean: An underutilized tropical legume on the path of improvement, to help mitigate food and nutrition security. Scientia Hortic (2020) 260:108789. doi: 10.1016/S0308-8146(96)00172-0
63. Aasim M, Baloch F, Nadeem M, Bakhsh A, Sameeullah M, Day S. "Fenugreek (Trigonella foenum-graecum l.): An underutilized edible plant of modern world,". In: Global perspectives on underutilized crops. Springer: Cham, Switzerland (2018). p. 381–408.
64. Ibirinde DO, Aremu CO, Balogun K, Oladokun L. Assessment of seed and tuber production potential in varieties of sphenostylis stenocarpa (Africa yam bean). Agric Sci (2019) 10:870–81. doi: 10.4236/as.2019.107066
65. Mohamed AA. Food security situation in Ethiopia: a review study. Int J Health Economics Policy (2017) 2(3):86–96. doi: 10.11648/j.hep.20170203.11
66. Koley TK, Maurya A, Tripathi A, Singh B, Singh M, Bhutia T, et al. Antioxidant potential of commonly consumed underutilized leguminous vegetables. Int J Vegetable Sci (2019) 25(4):362–72. doi: 10.1080/19315260.2018.1519866
67. Gaafar AA, Ali SI, El-Shawadfy MA, Salama ZA, Sękara A, Ulrichs C, et al. Ascorbic acid induces the increase of secondary metabolites, antioxidant activity, growth, and productivity of the common bean under water stress conditions. Plants (2020) 9(5):627. doi: 10.1080/19315260.2018.1519866
68. Mohan V, Tresina P, Daffodil E. Antinutritional factors in legume seeds: characteristics and determination. In: Encyclopedia of food and health. Amsterdam, Netherlands: Elsevier Science (2016). p. 211–20.
69. Sparvoli F, Lanave C, Santucci A, Bollini R, Lioi L. Lectin and lectin-related proteins in Lima bean (Phaseolus lunatus l.) seeds: biochemical and evolutionary studies. Plant Mol Biol (2001) 45(5):587–97. doi: 10.1023/A:1010647310311
70. Ajibola CF, Malomo SA, Fagbemi TN, Aluko RE. Polypeptide composition and functional properties of African yam bean seed (Sphenostylis stenocarpa) albumin, globulin and protein concentrate. Food Hydrocolloids (2016) 56:189–200. doi: 10.1016/j.foodhyd.2015.12.013
71. Kumar JK, Prasad AD, Chaturvedi V. Phytochemical screening of five medicinal legumes and their evaluation for in vitro anti-tubercular activity. Ayu (2014) 35(1):98. doi: 10.1016/j.foodhyd.2015.12.013
72. Udeh EL, Nyila MA, Kanu SA. Nutraceutical and antimicrobial potentials of bambara groundnut (Vigna subterranean): A review. Heliyon (2020) 6(10):e05205. doi: 10.4103/0974-8520.141897
73. Zhang X, Wang W, Guo N, Zhang Y, Bu Y, Zhao J, et al. Combining QTL-seq and linkage mapping to fine map a wild soybean allele characteristic of greater plant height. BMC Genomics (2018) 19(1):1–12. doi: 10.1016/j.heliyon.2020.e05205
74. Phua C, Vejayan J, Ambu S, Ponnudurai G, Gorajana A. Purification and antibacterial activities of an l-amino acid oxidase from king cobra (Ophiophagus hannah) venom. J Venomous Anim Toxins including Trop Dis (2012) 18(2):198–207. doi: 10.1186/s12864-018-4582-4
75. Wisplinghoff H. "Pseudomonas spp., acinetobacter spp. and miscellaneous gram-negative bacilli,". In: Infectious diseases, vol. 1579-1599. Elsevier: Amsterdam, The Netherlands (2017).
76. Adeleke BS, Babalola OO, Glick BR. Plant growth-promoting root-colonizing bacterial endophytes. Rhizosphere (2021) 20:100433. doi: 10.1016/j.rhisph.2021.100433
77. Venado RE, Liang J, Marín M. Rhizobia infection, a journey to the inside of plant cells. Adv Botanical Res (2020) 94:97–118. doi: 10.1016/j.rhisph.2021.100433
78. Yadav N, Yadav S, Yadav M, Kumar R, Yadav L, Yadav N, et al. Growth and productivity of groundnut (Arachis hypogaea l.) under varying levels and sources of sulphur in semi-arid conditions. Legume Res (2017) 41:1–7. doi: 10.1016/bs.abr.2019.09.007
79. Hassen AII, van Vuuren A, Bopape FL, Gerrano AS. Nodulation compatibility and symbiotic performance of rhizobia spp. with different landraces of bambara groundnut (Vigna subterranea (L.) verdc.) collections. Res Square (2022) 1:1–24. doi: 10.21203/rs.3.rs-1233082/v1
80. Paliwal R, Abberton M, Faloye B, Olaniyi O. Developing the role of legumes in West Africa under climate change. Curr Opin Plant Biol (2020) 56:242–58. doi: 10.1016/j.pbi.2020.05.002
81. Iruthayathas E, Vlassak K. Competition between winged bean psophocarpus tetragonolobus (L) DC rhizobium strains for nodulation. Z für Pflanzenernährung und Bodenkunde (1985) 148(5):536–43. doi: 10.1016/j.pbi.2020.05.002
82. Ribeiro RA, Ormeno-Orrillo E, Dall'Agnol RF, Graham PH, Martinez-Romero E, Hungria M. Novel rhizobium lineages isolated from root nodules of the common bean (Phaseolus vulgaris l.) in Andean and mesoamerican areas. Res Microbiol (2013) 164(7):740–8. doi: 10.1002/jpln.19851480510
83. Mohammed M, Jaiswal SK, Dakora FD. Insights into the phylogeny, nodule function, and biogeographic distribution of microsymbionts nodulating the orphan kersting’s groundnut [Macrotyloma geocarpum (Harms) marechal & baudet] in African soils. Appl Environ Microbiol (2019) 85(11):e00342–00319. doi: 10.1128/AEM.00342-19
84. Chang YL, Wang ET, Sui XH, Zhang XX, Chen WX. Molecular diversity and phylogeny of rhizobia associated with lablab purpureus (Linn.) grown in southern China. Systematic Appl Microbiol Biotechnol (2011) 34(4):276–84. doi: 10.1016/j.syapm.2008.04.004
85. Hailu Gunnabo A, Geurts R, Wolde-Meskel E, Degefu T, E. Giller K, van Heerwaarden J. Phylogeographic distribution of rhizobia nodulating common bean (Phaseolus vulgaris l.) in Ethiopia. FEMS Microbiol Ecol (2021) 97(4):fiab046. doi: 10.1093/femsec/fiab046
86. Liu D, Liberton M, Yu J, Pakrasi HB, Bhattacharyya-Pakrasi M. Engineering nitrogen fixation activity in an oxygenic phototroph. J MBio (2018) 9(3):e01029–01018. doi: 10.1128/mBio.01029-18
87. Norman JS, Friesen ML. Complex n acquisition by soil diazotrophs: how the ability to release exoenzymes affects n fixation by terrestrial free-living diazotrophs. ISME J (2017) 11(2):315–26. doi: 10.1128/mBio.01029-18
88. Suhag M. Potential of biofertilizers to replace chemical fertilizers. Int Adv Res J Sci Eng. Technol (2016) 3(5):163–7. doi: 10.1038/ismej.2016.127
89. Talabani S, Fattah O, Khider A. Classical and molecular approaches for identification of rhizobium leguminosarium, azotobacter chroococcum and bacillus megaterium. Appl Ecol Environ Res (2019) 17(5):12491–506. doi: 10.15666/aeer/1705_1249112506
90. Zoundji MCC, Ahoglé AMA, Akplo TM, Gangnon SO, Montéiro D, Zanvo Y, et al. Resistance to abiotic stress and effectiveness of native rhizobia on bambara groundnut [Vigna subterranea (L.) verdc.] in Benin. Open J Soil Sci (2022) 12(6):193–215. doi: 10.15666/aeer/1705_1249112506
91. Pastor-Bueis R, Jimenez-Gomez A, Barquero M, Mateos PF, González-Andrés F. Yield response of common bean to co-inoculation with rhizobium and pseudomonas endophytes and microscopic evidence of different colonised spaces inside the nodule. Eur J Agron (2021) 122:126187. doi: 10.4236/ojss.2022.126008
92. Elias F, Muleta D, Woyessa D. Effects of phosphate solubilizing fungi on growth and yield of haricot bean (Phaseolus vulgaris l.) plants. J Agric Sci (2016) 8(10):204–18. doi: 10.1016/j.eja.2020.126187
93. Adeleke BS, Fadiji AE, Ayilara MS, Igiehon ON, Nwachukwu BC, Babalola OO. Strategies to enhance the use of endophytes as bioinoculants in agriculture. Horticulturae (2022) 8(6):498. doi: 10.5539/jas.v8n10p204
94. Chimwamurombe PM, Grönemeyer JL, Reinhold-Hurek B. Isolation and characterization of culturable seed-associated bacterial endophytes from gnotobiotically grown marama bean seedlings. FEMS Microbiol Ecol (2016) 92(6):fiw083. doi: 10.3390/horticulturae8060498
95. Parsa S, García-Lemos AM, Castillo K, Ortiz V, López-Lavalle LAB, Braun J, et al. Fungal endophytes in germinated seeds of the common bean, phaseolus vulgaris. Fungal Biol (2016) 120(5):783–90. doi: 10.1093/femsec/fiw083
96. Olaf SK, Jean-Damascene U, Percy MC. Isolation and characterization of culturable bacteria from bulk soil samples and the rhizosphere of arid-adapted tylosema esculentum (Burchell). a. schreiber (Marama bean) in Namibia. Afr J Biotechnol (2015) 14(11):944–52. doi: 10.1016/j.funbio.2016.01.017
97. Da Silva JL, Mendes LW, Rocha SMB, Antunes JEL, Oliveira L, Melo VMM, et al. Domestication of Lima bean (Phaseolus lunatus) changes the microbial communities in the rhizosphere. Microbial Ecol (2022) 2022:1–11. doi: 10.1007/s00248-022-02028-2
98. Cullis C, Kunert KJ. Unlocking the potential of orphan legumes. J Exp Bot (2017) 68(8):1895–903. doi: 10.1007/s00248-022-02028-2
99. Ibny FY, Jaiswal SK, Mohammed M, Dakora FD. Symbiotic effectiveness and ecologically adaptive traits of native rhizobial symbionts of bambara groundnut (Vigna subterranea l. verdc.) in Africa and their relationship with phylogeny. Sci Rep (2019) 9(1):1–17. doi: 10.1038/s41598-019-48944-1
100. Degefu T, Wolde-meskel E, Adem M, Fikre A, Amede T, Ojiewo C. Morphophysiological diversity of rhizobia nodulating pigeon pea (Cajanus cajan l. millsp.) growing in Ethiopia. Afr J Biotechnol (2018) 17(6):167–77. doi: 10.5897/AJB2017.16338
101. Ahmad M, Zahir ZA, Nazli F, Akram F, Arshad M, Khalid M. Effectiveness of halo-tolerant, auxin producing pseudomonas and rhizobium strains to improve osmotic stress tolerance in mung bean (Vigna radiata l.). Braz J Microbiol (2013) 44:1341–8. doi: 10.1590/S1517-83822013000400045
102. Laurette NN, Stephane YGH, Firmin SL, Laurence TSA, Lambert DKJ, Dieudonne N. Salt-tolerant rhizobia for enhancing common bean (Phaseolus vulgaris l.) productivity under salt stress. Rev Plant Stud (2022) 9(1):1–11. doi: 10.1590/S1517-83822013000400045
103. Deepika K, Raghuram M, Kariali E, Bramhachari P. Biological responses of symbiotic rhizobium radiobacter strain VBCK1062 to the arsenic contaminated rhizosphere soils of mung bean. Ecotoxicology Environ Saf (2016) 134:1–10. doi: 10.1016/j.ecoenv.2016.08.008
104. Mekonnen M, Kebede A. Improvement of tolerance to high salinity and extreme ph conditions in common bean (Phaseolus vulgaris l.) nodulating Rhizobial isolates from hararghe lowlands and mid altitudes, Eastern Ethiopia, through physical and chemical mutagenesis. Int J Innovative Pharm Sci (2021) 9(05):1–31. doi: 10.1016/j.ecoenv.2016.08.008
105. Adegboyega TT, Abberton MT, AbdelGadir AH, Dianda M, Maziya-Dixon B, Oyatomi OA, et al. Nutrient and antinutrient composition of winged bean (Psophocarpus tetragonolobus (L.) DC.) seeds and tubers. J Food Qual (2019) 2019:8. doi: 10.1155/2019/3075208
106. Ajibola CF, Malomo SA, Fagbemi TN, Aluko RE. Polypeptide composition and functional properties of African yam bean seed (Sphenostylis stenocarpa) albumin, globulin and protein concentrate. J Food Hydrocolloids (2016) 56:189–200. doi: 10.1016/j.foodhyd.2015.12.013
107. Nahunnaro H, Bayaso I. Inhibitory activity of plant extracts on the early blight pathogen alternaria solani. Global J Agric Sci (2012) 11(1):57–62. doi: 10.1016/j.foodhyd.2015.12.013
108. Lu Y, Chen Q, Bu Y, Luo R, Hao S, Zhang J, et al. Flavonoid accumulation plays an important role in the rust resistance of malus plant leaves. Front Plant Sci (2017) 8:1286. doi: 10.4314/gjass.v11i1.10
109. Rana S, Pal R, Vaiphei K, Sharma SK, Ola R. Garlic in health and disease. Nutr Res Rev (2011) 24(1):60–71. doi: 10.3389/fpls.2017.01286
110. Mougou I, Boughalleb-M’hamdi N. Biocontrol of pseudomonas syringae pv. syringae affecting citrus orchards in Tunisia by using indigenous bacillus spp. and garlic extract. Egyptian J Biol Pest Control (2018) 28(1):1–11. doi: 10.1017/S0954422410000338
111. Were E, Schöne J, Viljoen A, Rasche F. Phenolics mediate suppression of fusarium oxysporum f. sp. cubense TR4 by legume root exudates. Rhizosphere (2022) 21:100459. doi: 10.1016/j.rhisph.2021.100459
112. Sadeghi M, Zolfaghari B, Senatore M, Lanzotti V. Spirostane, furostane and cholestane saponins from Persian leek with antifungal activity. Food Chem (2013) 141(2):1512–21. doi: 10.1016/j.rhisph.2021.100459
113. Khaliq G, Abbas HT, Ali I, Waseem M. Aloe vera gel enriched with garlic essential oil effectively controls anthracnose disease and maintains postharvest quality of banana fruit during storage. Horticulture Environ Biotechnol (2019) 60(5):659–69. doi: 10.1016/j.foodchem.2013.04.009
114. Bhagat S, Dutta U, Mahajan T. Antifungal activity of important botanicals against plant pathogens. Int J Curr Microbiol App. Sci (2019) 8(10):531–45. doi: 10.1007/s13580-019-00159-z
115. Carranca C, Torres MO, Madeira M. Underestimated role of legume roots for soil n fertility. Agron Sustain Dev (2015) 35(3):1095–102. doi: 10.20546/ijcmas.2019.810.058
116. Zhang H, Zeng F, Zou Z, Zhang Z, Li Y. Nitrogen uptake and transfer in a soybean/maize intercropping system in the karst region of southwest China. Ecol Evol (2017) 7(20):8419–26. doi: 10.1007/s13593-015-0297-y
117. Rahman MM, Islam AM, Azirun SM, Boyce AN. Tropical legume crop rotation and nitrogen fertilizer effects on agronomic and nitrogen efficiency of rice. Sci World J (2014) 2014:490841. doi: 10.1155/2014/490841
118. Thilakarathna MS, McElroy MS, Chapagain T, Papadopoulos YA, Raizada MN. Belowground nitrogen transfer from legumes to non-legumes under managed herbaceous cropping systems. a review. Agron Sustain Dev (2016) 36(4):1–16. doi: 10.1155/2014/490841
119. Thilakarathna MS, Chapagain T, Ghimire B, Pudasaini R, Tamang BB, Gurung K, et al. Evaluating the effectiveness of rhizobium inoculants and micronutrients as technologies for Nepalese common bean smallholder farmers in the real-world context of highly variable hillside environments and indigenous farming practices. Agriculture (2019) 9(1):20. doi: 10.3390/agriculture9010020
120. Giller KE, Ormesher J, Awah FM. Nitrogen transfer from phaseolus bean to intercropped maize measured using 15N-enrichment and 15N-isotope dilution methods. Soil Biol Biochem (1991) 23(4):339–46. doi: 10.3390/agriculture9010020
121. Egbe MO, Alhassan GA, Ijoyah M. Nodulation, nitrogen yield and fixation by bambara groundnut (Vigna subterranea (L.) verdc.) landraces intercropped with cowpea and maize in southern Guinea savanna of Nigeria. Agric Sci (2013) 1(4):15–28. doi: 10.1016/0038-0717(91)90189-Q
122. Uher D, Svečnjak Z, Svečnjak Z, Dujmovic-Purgar D, Jareš D, Horvatic I. Influence of intercropping maize with climbing bean on forage yield and quality. Agron Int J (2019) 4:60–7. doi: 10.7251/AGRENG1903060U
123. Gebremichael A, Bekele B, Tadesse B. Evaluation of the effect of sorghum-legume intercropping and its residual effect on yield of sorghum in yeki woreda, sheka zone, Ethiopia. Int J Agric Research Innovation Technol (2019) 9(2355-2020-1128):62–6. doi: 10.7251/AGRENG1903060U
124. Oluwole OO, Aworunse OS, Aina AI, Oyesola OL, Popoola JO, Oyatomi OA, et al. A review of biotechnological approaches towards crop improvement in African yam bean (Sphenostylis stenocarpa hochst. ex a. rich.). Heliyon (2021) 7(11):e08481. doi: 10.1016/j.heliyon.2021.e08481
125. Lin Tan X, Azam-Ali S, Goh EV, Mustafa MA, Chai HH, Kuan Ho W, et al. Bambara groundnut: An underutilized leguminous crop for global food security and nutrition. Front Nutr (2020) 7:276. doi: 10.1016/j.heliyon.2021.e08481
126. Owusu KD, Newman CF, Gabriel A-G. Evaluation of physical and cooking characteristics of five improved Lima beans. World (2018) 2(2):38–43. doi: 10.3389/fnut.2020.601496
127. Shitta NS, Edemodu AC, Abtew WG, Tesfaye AA. A review on the cooking attributes of African yam bean (Sphenostylis stenocarpa). London, UK: IntechOpen (2021).
128. Soetan K. Antinutritional factors of five selected underutilized legumes. Food Sci Qual Manage (2018) 76:92–100. https://core.ac.uk/download/pdf/234684765.pdf
Keywords: orphan crops, nitrogen fixation, phytochemicals, rhizobium, anti-nutrients
Citation: Ayilara MS, Abberton M, Oyatomi OA, Odeyemi O and Babalola OO (2022) Potentials of underutilized legumes in food security. Front. Soil Sci. 2:1020193. doi: 10.3389/fsoil.2022.1020193
Received: 15 August 2022; Accepted: 12 September 2022;
Published: 30 September 2022.
Edited by:
Benedicta Essel Ayamba, Council for Scientific and Industrial Research (CSIR), GhanaReviewed by:
Mustapha Mohammed, University for Development Studies, GhanaDebasis Mitra, National Rice Research Institute, (ICAR), India
Copyright © 2022 Ayilara, Abberton, Oyatomi, Odeyemi and Babalola. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Abberton, m.abberton@cgiar.org
 Modupe S. Ayilara
Modupe S. Ayilara Michael Abberton2*
Michael Abberton2*  Olaniyi A. Oyatomi
Olaniyi A. Oyatomi Olubukola O. Babalola
Olubukola O. Babalola