Neoadjuvant Systemic Therapy in Localized and Locally Advanced Renal Cell Carcinoma
- 1Urology Service, Memorial Sloan Kettering Cancer Center, New York, NY, United States
- 2Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, United States
While the majority of renal cell carcinoma (RCC) cases present at an early stage, a significant number of patients are diagnosed with either locally advanced or metastatic disease. While surgical resection remains the definitive curative management in the localized setting, many patients experience disease relapse and the 5-year recurrence rate following nephrectomy nears 60% for patients with high-risk localized disease. As systemic therapies including anti-angiogenesis, immune checkpoint blockade, and combinations thereof have evolved with dramatic improvements in survival outcomes for patients with metastatic RCC, there is a renewed interest in exploring the utility of these agents in the upfront neoadjuvant and adjuvant setting. Neoadjuvant therapy, administered prior to definitive surgery, aims to eradicate micro-metastatic disease early on and reduce surgical complexity with the overall goals of lowering perioperative morbidity and increasing post-operative recurrence-free and progression-free survival. In this chapter, we present an overview of previously completed and ongoing neoadjuvant systemic therapy clinical trials for patients with localized and locally advanced RCC and discuss potential considerations regarding the utility and future study of neoadjuvant therapy for the optimal management of localized RCC.
1 Introduction
While renal cell carcinoma (RCC) accounts only for 2-3% of all adult malignant neoplasms, it is considered highly lethal, with a 16.9% five-year mortality rate (1). One cause for this high mortality is the significant proportion of patients who present with localized stage III and/or advanced stage IV disease (13.9% and 18.7%, respectively) (2). Another cause for the persistent high mortality rate of RCC is the relatively high rate of disease recurrence and metastases following surgical resection for high-risk localized disease patients [such as T3 stage, Fuhrman grade ≥ 2, sarcomatoid differentiation, and nodal involvement (3, 4)], with 5-year recurrence rates near 60% (3, 5, 6), and corresponding 5-year survival rates of 63% and 53% for stage II and stage III RCC (7).
The management of advanced RCC has undergone many advancements in the past 2 decades, with the introduction of targeted therapy agents – particularly vascular endothelial growth factor inhibitor (VEGFi), and immune checkpoint inhibitor (IO) agents, and IO/IO or IO/VEGFi combination therapies. As these agents have become standard agents for disease control for patients with advance disease (8, 9), their use in the adjuvant setting has been explored (8, 10–14). However, only two completed prospective studies have noted significant improvement in disease-free survival (DFS). S-TRAC, which studied adjuvant sunitinib for 1 year, showed an improvement in DFS [HR 0.76; 95% CI, 0.59 to 0.98, p = 0.03 (10)] but no significant improvement in overall survival (OS). Recently, KeyNote-564, a phase III study investigating adjuvant pembrolizumab for 1 year for high risk disease, noted a significant improvement in DFS [HR 0.54; 95% CI, 0.30 to 0.96 (15)] while benefit to OS, if any, has not yet been established pending maturation of data (15). This introduction of IO therapy in the adjuvant setting has expanded the options for patients with high-risk disease, and its impact on the natural history of disease will require further study.
As with most treatments in cancer therapy, the above targeted therapy agents were first investigated in treatment-refractory advanced RCC, then as first-line therapy for advanced RCC, followed by evaluation in the adjuvant and, finally, in the setting of neoadjuvant systemic therapy (NA-ST), as it offers several theoretical benefits over adjuvant therapy. These advantages are broadly classified into (1) perioperative benefits, including downsizing or downstaging of a surgically difficult or otherwise unresectable tumors, reducing surgical morbidity by reduction of tumor complexity, and allowing for an organ sparing approach in patients with limited baseline renal function; and (2) early and prompt oncologic control, reducing post-operative recurrence risk and eradication of micrometastatic disease (16–18).
Here, we provide an overview of current literature and ongoing trials of NA-ST in localized and locally advanced RCC. Of note, our review focuses on the implementation of NA-ST in RCC patients with no evidence of metastatic disease (M0) at the time of surgery, which we and others define as the target for true neoadjuvant ST (18, 19), in contrast to trials of presurgical systemic therapy followed by consolidative or cytoreductive surgery for patients with limited M1 disease (20–23), a topic that is outside the scope of this review. We will review published trials of NA-ST, studying their outcomes and adverse effects of their therapeutic agents, followed by a review of ongoing trials and future directions in this field, and discuss the current state and limitations of NA-ST for RCC as the current treatment landscape evolves.
2 Materials and Methods
We queried PubMed, Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov using the keywords (“neoadjuvant” and “renal cell”) to identify candidate articles, including published and ongoing trials. Inclusion criteria for ST trials and retrospective series reviewed in this chapter were: use of neoadjuvant targeted ST (VEGFi and/or IO), treatment of localized or locally advanced M0 RCC, and having a publication or full-article translation in the English language. Articles with no corresponding English language publication, editorials, case reports, and studies of non-targeted therapy (such as IL-2, IFN-gamma) were excluded, as well as trials that included both M0 and M1 patients, as we believe such studies to be of presurgical systemic therapy followed by cytoreductive nephrectomy, a currently debated topic that is outside the scope of this chapter. Based on this distinction, we excluded 2 published and one ongoing prospective trial that enrolled both M0 and M1 cases (20, 21, 24), and a retrospective series of presurgical sunitinib for tumor downsizing prior to partial nephrectomy in a select group of M0 and M1 patients (25).
Adverse events (AEs) including toxicity profile of implemented systemic therapies and post-operative complications in included trials were also summarized using CTCAE (Common Terminology Criteria for Adverse Events) grading, focusing on grade 3-5 events (26).
3 Results
3.1 Overview of Literature Search Results
We identified 4 published prospective trials, and 13 ongoing and/or recently completed trials in NA-ST of localized or locally advanced M0 RCC. We also identified two unpublished studies that were terminated early due to poor accrual (phase I pembrolizumab study (27), phase II sunitinib study (28)). In terms of ST agents, all published trials utilized VEGFi monotherapy agents, while most ongoing trials have shifted to IO-based therapy. Only 1/4 published trials evaluated preoperative objective response rate (ORR) as a primary outcome, while all (10/10) of the ongoing phase II trials list ORR or pathologic response rates as their primary outcomes of interest.
3.2 Summary of Published Clinical Trials
We identified 4 published and completed trials of NA-ST for localized RCC. All studies utilized neoadjuvant VEGFi tyrosine kinase inhibitor (TKI) monotherapy (pazopanib, axitinib, sorafenib), with objective response rate (ORR) as a primary or secondary outcome. The results of published prospective clinical trials are discussed below by the implemented ST agent, with a summary in Table 1. Incidence and nature of AEs for the agents utilized in these trials are summarized in Table 2.
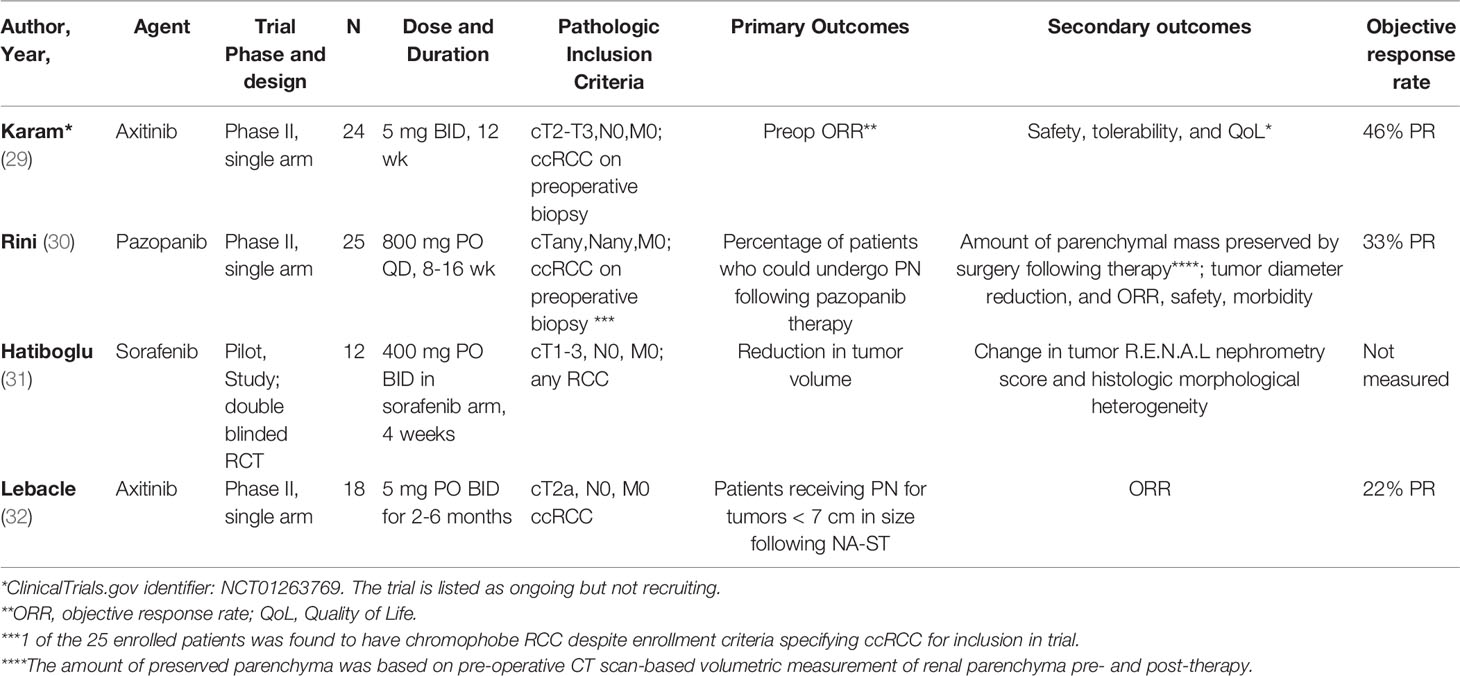
Table 1 Published Trials in Neoadjuvant Therapy in Locally Advanced, Non-Metastatic Renal Cell Carcinoma.
3.2.1 Axitinib
Axitinib is a potent oral TKI used for treatment of advanced RCC as a monotherapy and, more recently, in combination with IO agents in the first-line setting (33, 34). Unlike multi-targeting earlier TKIs (e.g., sorafenib, sunitinib, pazopanib), axitinib is more selective for VEGFR, with a shorter plasma half-life and less need for dose titration (35). Axitinib was first evaluated as NA-ST in a single arm, single center, phase 2 trial by Karam et al. (2014; NCT01263769) (29). A course of pre-operative axitinib 5 mg twice daily for up to 12 weeks was investigated in 24 patients with surgically resectable cT2-T3b disease and biopsy-confirmed clear cell carcinoma (ccRCC), followed by partial or radical nephrectomy. The primary outcome was objective response rate (ORR) by RECIST v1.1 criteria (36). Most (22/24) patients completed their 12-week axitinib regimen without requiring dose modification; one patient completed only 11 weeks due to transient grade 3 elevation of liver enzymes and thrombocytopenia, and another stopped treatment at 7 weeks due to development of AKI, with later recovery, then was taken to surgery earlier than scheduled. The authors noted a partial response (PR) in 11/24 (46%) patients, and no disease progression on pre-operative CT scans taken following completion of axitinib regimen, with a corresponding reduction in median tumor size of 28.3%.
In a more recent trial by Lebacle et al. (2018) (32), axitinib was investigated for downstaging of cT2aN0-NxM0 ccRCC patients who were deemed not suitable for partial nephrectomy (PN). The primary outcome was the number of patients receiving PN for tumors < 7 cm in size following NA-ST, a decision determined by the surgeon based on the preceding pre-operative CT scan. Axitinib 5 mg twice daily was given for 2-6 months pre-operatively, depending on radiologic response – patients received radical nephrectomy (RN) if the tumor continued to enlarge and continued therapy stable per investigator review. Patients who tolerated axitinib with AEs > grade 2 during a 2-week period had their dose gradually up-titrated to a maximum of 10 mg twice daily per FDA label. The study enrolled 18 patients, with most (12) receiving 2 months of axitinib 5 mg BID; 3 and 3 patients received 4 and 6 months of axitinib pre-operatively, and only one patient required dose reduction to 3 mg for unclear reasons. At the end of the study, the primary outcome was considered reached in 12 of the 18 enrolled patients, with 16 undergoing PN. ORR was the secondary outcome, with partial response and stable disease in 3 and 14 patients, respectively. Median reduction in tumor diameter and R.E.N.A.L nephrometry score (37) were 12 mm and 1, respectively. The study reported the incidence of local recurrence and metastatic disease at 2-year follow-up (2 and 6 patients, respectively), which was attributed to the upstaging of 41% (7) tumors from cT2 to pT3a on final pathology, and the 11% rate of positive margins. The authors ultimately concluded that axitinib was a feasible neoadjuvant ST that produced a modest decrease in size and complexity of cT2 tumors, and may in turn make tumors more amenable to PN over RN.
3.2.2 Pazopanib
Pazopanib is an oral, multi-targeting TKI agent that inhibits tyrosine kinases associated with VEGFR, platelet-derived growth factor (PDGF) receptor and Kit receptor (38). In addition to being evaluated in the management of metastatic RCC (39), pazopanib was also evaluated in the adjuvant setting in the phase III PROTECT trial (1 year of pazopanib 800 mg daily, later dose reduced to 600 mg daily due to due high attrition rates attributed to drug toxicity), but did not demonstrate a recurrence-free survival (RFS) or OS benefit compared to placebo (40). Similar to the aforementioned axinitib trial by Lebacle et al, neoadjuvant pazopanib was evaluated for improving the number of patients eligible for PN in a phase II trial of localized ccRCC by Rini et al (30). Specifically, patients enrolled in the trial had to meet at least one preoperative criteria: (1) their PN or RN was likely to yield a glomerular filtration rate of less than 30 ml/minute/1.73 m2, or (2) their planned PN was deemed high risk due to high complexity, defined as either R.E.N.A.L. nephrometry score of 10-12 and/or tumor location being adjacent to hilar vessels. The primary endpoint was the percentage of patients who could undergo PN after pazopanib therapy, while secondary endpoints included estimated preserved functional renal parenchyma, based on CT scan-based volumetric measurement of renal parenchyma pre- and post-ST, along with reduction in tumor volume, and ORR. The trial enrolled 25 patients who received pazopanib 800 mg PO daily for up to 16 weeks. Of these, 13 patients were deemed ineligible for PN based on surgeon assessment pre-therapy, with 6/13 (46%) patients developing a sufficient response to be deemed PN-eligible post-therapy, along with an estimated improvement of preserved functional parenchyma from 107 cc pre-therapy to 173 cc post-therapy (p = 0.0015) and median tumor diameter reduction from 7.3 cm to 5.5 cm following therapy (p < 0.0001). However, overall ORR was only 33% for ccRCC patients, with 16 (64%) of patients developing grade 3 AEs.
3.2.3 Sorafenib
Sorafenib an oral multi-targeting TKI including VEGFR2, FLT3, PDGF receptor, and fibroblast growth factor receptor-1 (FGFR1). In addition to its proximal signaling effects, this molecule also inhibits downstream Raf kinases which serve as important mediators of the Ras/Raf/MEK pathway (41). Adjuvant sorafenib was evaluated in a phase 3 trial by Eisen et al (12), noting no improvement in DFS or OS compared to placebo. It was also studied as potential NA-ST agent for localized RCC in a randomized, placebo-controlled trial by Hatiboglu et al (31) in patients with clinical stage I-III RCC and cN0/M0 disease. Patients were enrolled into either sorafenib or placebo arms (allocation ratio 3:1, respectively; sorafenib 400 mg PO BID for 4 weeks). The primary outcome was reduction in tumor volume, along with assessment of R.E.N.A.L scores. However, despite enrolling 20 patients, only 12 proceeded with therapy followed by surgery (9 sorafenib, 3 placebo), as 3 had to be excluded for not meeting inclusion criteria following further investigation, and 5 withdrew due to concerns regarding side effects of surgery and/or delaying surgery. Of the 12 patients who proceeded with the trial, only 3 of the 9 patients in the sorafenib arm completed the planned course of sorafenib, while 4 patients underwent dose modification to 200 mg BID due to grade 3 toxicity, and 1 patient discontinued it completely due to serious AEs on day 5 of treatment, with recurrence of these AEs on resuming treatment at 100 mg BID. At the conclusion of the study, median reduction of tumor in the sorafenib arm was 29% with tumor shrinkage in 8/9 patients (range -4% to 61.1%), versus no change in the placebo arm, but with no statistically significant change in R.E.N.A.L scores compared to pre-treatment in either arm.
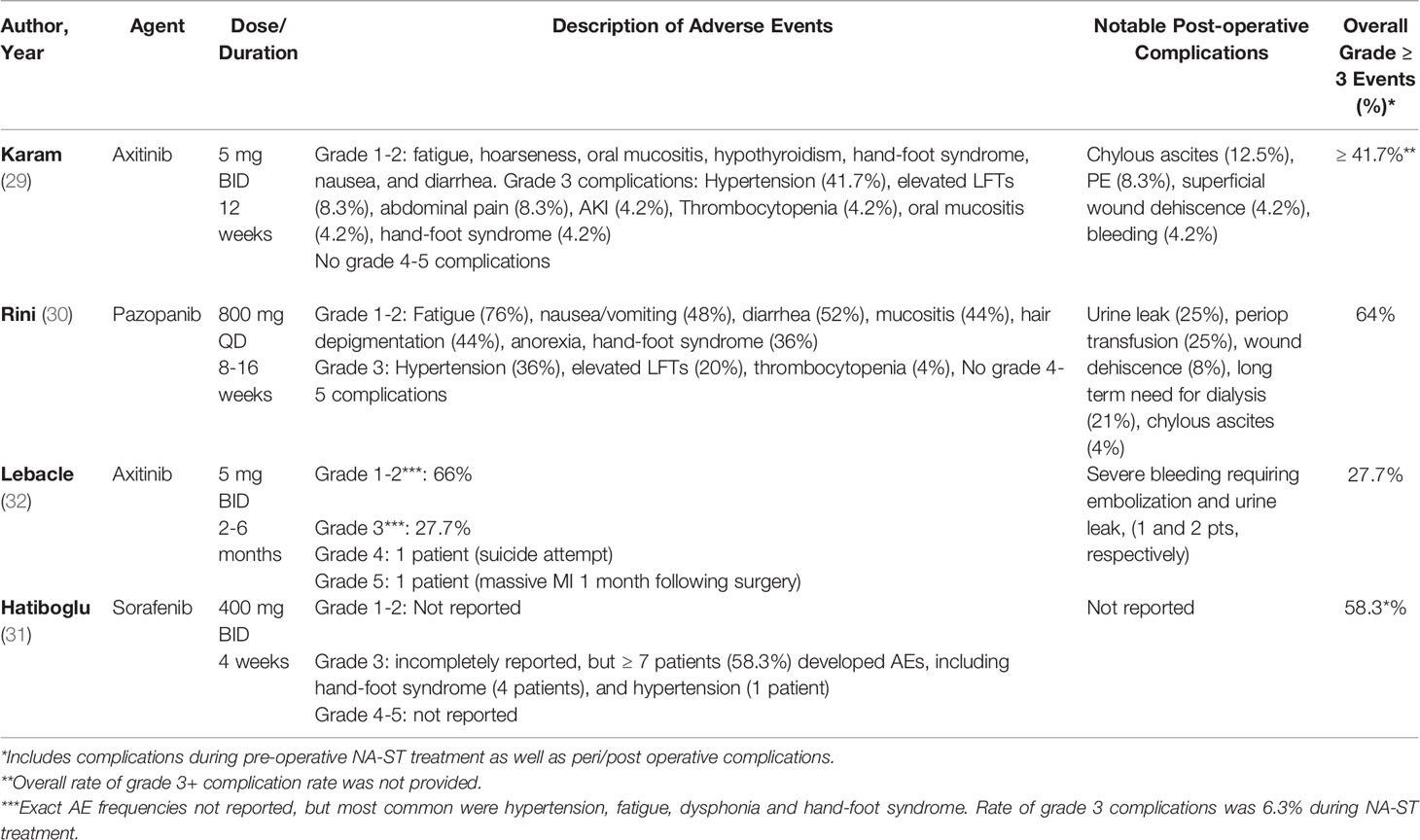
3.2.4 Adverse Effects of NA-ST in Published Trials
Adverse effects of treatment and post-operative complications for published trials are summarized in Table 3. While no CTCAE grade 4-5 complications related to treatment were noted with NA-ST, 27.7-64% of patients experienced grade 3 complications overall, which were predominantly hypertension, elevated liver AST/ALT, abdominal pain, and gastrointestinal side effects (ileus, nausea, vomiting, poor appetite). TKI-specific side effects including oral mucositis and hand-foot syndrome were mostly grade 1-2, and rarely required dose adjustment (Table 2). However, only a minority of patients required early discontinuation of treatment (2 in axitinib trial by Karam et al, 1 in sorafenib trial by Hatiboglu et al), and there were no reported treatment-related deaths.
While 3 of the 4 published studies reported post-operative complications, noted for chylous ascites and superficial wound dehiscence (12.5% and 8.3% with axitinib by Karam et al (29); 4% and 8% with pazopanib by Rini et al (30), respectively), it is difficult to determine if these complications were related to NA-ST or not without a control arm, particularly given the small cohort sizes and the preference for partial over radical nephrectomy in two of the trials due to their outcome of interest being the facilitation of performing this procedure.
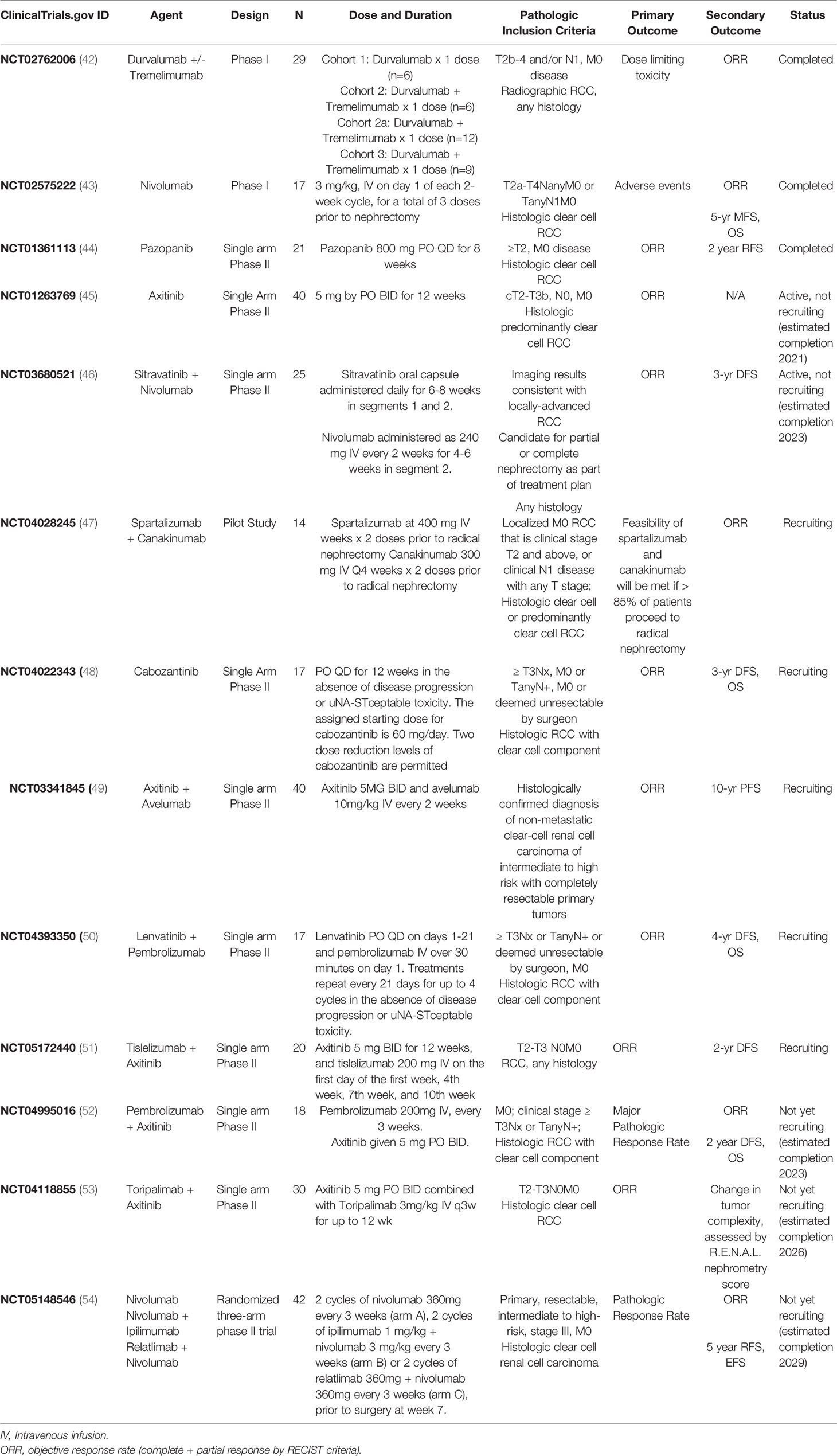
3.3 Summary of Ongoing and Recently Completed Trials
We identified 13 ongoing and/or recently completed trials in NA-ST of localized or locally advanced M0 RCC: 1 pilot study, 2 phase I studies, and 10 phase II studies, summarized in Table 3. While only 1 of the published studies reported recurrence-free survival outcomes, most (9/13) of the ongoing trials plan to report disease-free, recurrence-free, or progression free survival (DFS, RFS, PFS) as secondary outcomes, and only 1 has OS as a secondary endpoint.
As with published studies, most (11/13) of the ongoing studies are focused on patients with a clear cell component. Not surprisingly, most of these trials have shifted from VEGFi monotherapy to investigating the use of IO agents as monotherapy or in combination with other IO or VEGFi agents in the neoadjuvant setting; only 2 ongoing studies utilize VEGFi monotherapy: NCT01263769 (aforementioned trial of axitinib by Karam et al (29); no longer recruiting), and NCT04022343 (cabozantinib, actively recruiting), while the remaining 11 studies utilize IO monotherapy (2 studies) or IO-based combination therapy (9 studies). This momentum in prospective trials on investigating the role of peri-operative immunotherapy reflects a significant paradigm shift in clinical oncology in the acceptance of this modality as an additional pillar in the treatment of localized disease. While outside the scope of this review, the incorporation of immune checkpoint inhibitors as adjuvant therapy is being actively pursued in parallel with many of the studies in the neoadjuvant space (55, 56).
The rationale for utilizing immunotherapy earlier in the course of the disease centers on the ability of immunotherapy to augment anti-tumor immune surveillance which may ultimately confer effective treatment of micro-metastatic disease compared with targeted therapies. NCT02575222 (43) was a recently completed phase I trial that accrued 17 patients with non-metastatic high-risk clear cell RCC to investigate the role of nivolumab monotherapy, an anti-PD-1 monoclonal antibody that is also currently under investigation in the adjuvant setting (55). Results from this study are pending; the primary outcome is safety as assessed by the number of participants experiencing AEs, with ORR and survival data as secondary outcomes. NCT02595918 (57) was another pilot phase I trial investigating nivolumab monotherapy in the pre-operative setting. The original inclusion criteria included a goal of 29 patients with localized RCC or low-volume metastatic disease. This study was ultimately terminated in August 2020 due to low accrual. Study outcomes, which include safety and feasibility (primary objective) as well as overall ORR and RFS (secondary objectives), are pending report. Notably, the PROSPER trial [NCT03055013 (58, 59)] is an active, multicenter randomized Phase III study with planned enrollment of 766 patients and seeks to investigate nivolumab in the neoadjuvant setting. While the inclusion criteria allow for M1 disease, the presumed M1 site must be rendered “no evidence of disease” by metastasectomy, thermal ablation or stereotactic radiation within 12 weeks of the initial procedure. This study has completed recruitment with estimated study completion in late 2023 and will report event-free survival as a primary outcome, with overall survival, RFS, and incidence of toxicity as secondary outcomes.
Another treatment paradigm under active investigation in the neoadjuvant setting is that of combination VEGFi plus IO therapy agents. Recent pre-clinical research on intratumoral immune components after pretreatment of RCC suggest a potential synergism for TKI with anti-PD-1/L1 therapy (60–62), and the significant improvement in metastatic disease control demonstrated by trials of VEGFi/IO combination therapies (33, 34, 63–65), have in turn raised interest in neoadjuvant TKI/IO combination therapies. Six ongoing trials, NCT04118855 (53), NCT04995016 (52), NCT03680521 (46), NCT03341845 (49), NCT04393350 (50), and NCT05172440 (51) are currently investigating this strategy in the neoadjuvant setting. Several of the studies will include additional correlative studies to monitor the true effect on the tumor microenvironment and seek to define molecular biomarkers to associated with treatment response as secondary goals.
4 Discussion
4.1 Current Evidence for NA-ST in RCC
In this chapter, we reviewed the current state of literature on NA-ST in RCC, focusing on published prospective trials of localized or locally advanced RCC, which we considered as “true” NA-ST, compared to presurgical systemic therapy for known metastatic or otherwise unresectable disease. Despite the theoretical benefits and advantages for NA-ST, we found the evidence for benefit of NA-ST in RCC to be quite limited, and not sufficient to support it as a treatment approach outside of a clinical trial.
The application of systemic therapies in the perioperative setting leverages the successful use of these agents in metastatic disease. Subsequently, trials investigating adjuvant VEGF inhibition in advanced localized disease have demonstrated mixed results especially in light of the significant side effects associated with its use. Pooled analyses in recent systematic reviews have demonstrated no significant improvement in disease-specific or overall survival (66, 67).
In the neoadjuvant setting, published studies on VEGFi TKIs have demonstrated modest responses. However, all 4 published prospective NA-ST in RCC trials were small scale pilot or phase II trials of VEGFi monotherapy for mostly clear cell carcinoma patients, with significant heterogeneity in the primary cohort and outcome of interest, therapeutic agent, and design. The primary outcome of interest for most (3/4) published trials was reduction of tumor volume and/or surgical complexity, which in turn was to allow for PN (2/4 trials) in patients at risk for significant decline in renal function with RN who were deemed to be difficult candidates for PN by the recruiting surgeon. The duration of NA-ST varied significantly, from only 4 weeks [sorafenib, Hatiboglu et al. (31)] to 6 months [pazopanib, Rini et al. (30)]. Similarly, the wait period between completion of systemic therapy and surgical intervention varied by study, from only 36 hours pre-operatively [axitinib, Karam et al. (29)], to ≥ 7 days pre-operatively [pazopanib, Rini et al. (30)], or was left to the discretion of the medical oncologist and surgeon [sorafenib, Hatibglou et al. (31); axitinib, Lebacle et al. (32)]. ORR was assessed in 3/4 of the published prospective trials, with a limited overall partial response rate (22-46%). Overall, it is difficult to generalize the results of these studies for clinical practice given the small study size, the use of different agents and regimens in each study with no control arm (except for one study), and the inherent selection bias in the 2 studies where patient enrollment was predicated on being deemed poor candidates for PN based on the recruiting surgeon’s assessment. Of the 4 studies, only one study [Lebacle et al. (32)] attempted to evaluate oncologic outcomes by providing 2-year follow-up results, while the remaining studies focused on tumor response rates and feasibility of preforming PN following NA-ST.
Interestingly, despite being the only FDA-approved VEGFi TKI agent for high risk localized disease (68), neoadjuvant sunitinib was evaluated in only two clinical trials: a phase II, 20-patient trial by Hellenthal et al. (2010) (20) (excluded from our main analysis due to inclusion of M1 patients), and a single phase II trial (NCT00480935) which was terminated due to poor accrual (28). In the Hellenthal et al. series, the primary objective was assessing the safety of sunitinib (37.5 mg daily for 3 months) as NA-ST and of surgery following this NA-ST regimen. ORR was a secondary outcome for this study, with only one patient achieving formal partial response and the remainder deemed to have stable disease per RECIST criteria. However, 17/20 (85%) patients exhibited decrease in tumor size on two-month follow-up, with a median change in tumor diameter of -11.8% (range -27 to 11%).
In addition to the absence of strong evidence to support reliable oncologic benefits or improved survival outcomes in the discussed trials, further concerns with NA-ST include its potential effects on post-operative recovery and post-operative complications, and downstream effects of delay of surgery, particularly for non-responders to these agents. While post-operative complications were reported in 3/4 published trials, they were felt to be within expected complication rates by their respective authors and whether NA-ST had an effect on the incidence of these complications was difficult to discern due to the absence of a control arm. Finally, the relevance of these published studies to current and future management of RCC is significantly limited by their reliance on VEGFi monotherapy, while combination IO/TKI or IO/IO agents have become the new frontline therapy for advanced RCC.
Fortunately, ongoing and future trials of NA-ST in RCC address several of the above limitations, starting with the utilization of IO-based therapies, which have proven to be more effective and, in some studies, less likely to result in cumulative and significant AEs compared to VEGFi agents. In addition to the change of utilized ST agents, these trials have notably shifted their focus from perioperative outcomes, such as facilitation of partial nephrectomy, towards improving oncologic outcomes (DFS, RFS, PFS, and in one study, OS), as well as more immediate outcomes, such as radiologic or pathologic response. Inclusion criteria for these studies generally require patients with higher risk for disease recurrence (≥ cT2) and, as most of the evidence for newer ST agents comes from trials in ccRCC patients, most trials require tumors to be biopsy-proven, predominantly clear cell RCC. Treatment regimens for these trials mirror those used in metastatic RCC, but with fewer cycles, and overall treatment periods of 4-12 weeks.
4.2 Future Directions
The management of advanced RCC has undergone many advancements in the past 2 decades, with the introduction of targeted therapy agents – VEGFi, mammalian target of rapamycin (mTOR), IO agents; and IO/IO or IO/TKI combination therapies. As with most treatments in cancer therapy, these agents were first investigated in treatment-refractory advanced RCC, then as first-line therapy for advanced RCC, followed by evaluation in the adjuvant and, finally, the neoadjuvant setting. This natural evolution of systemic therapies is reflected in the gradual progression of NA-ST in RCC from TKI monotherapies to IO monotherapies, and more recently, IO/TKI and IO/IO combination therapies.
In addition to IO/TKI and IO/IO combination therapies, recent studies have shown promise with targeting of hypoxia-inducible factor 2-α (HIF-2α), a transcription factor that is constitutively activated upon mutation of VHL gene, leading to induction of several oncogenic pathways involved in the pathogenesis of several benign and malignant neoplasms, including ccRCC (69), particularly in patients with von Hippel-Lindau disease (70, 71). In this regard, Belzutifan (MK-6482) is a potent small molecule inhibitor of HIF-2α that has shown impressive activity in neoplasms associated with VHL disease, with a recent phase 2 trial of this agent in patients with renal cell carcinoma associated with VHL disease, noting an ORR of 49% in patients with RCC kidney tumors (95% CI: 36-62) (72). As new agents like this are investigated in the advanced ccRCC (73, 74) space, their utility in the adjuvant and neoadjuvant setting may soon be explored.
As with ST in the metastatic setting, the potential benefits of NA-ST in RCC patients may be improved by the identification of candidates who are more likely to respond to NA-ST through biomarkers predictive of therapeutic response, thereby reducing concerns for unnecessary toxicity and delayed treatment in non-responders to NA-ST. While no such biomarkers have been investigated for NA-ST in RCC, genomic markers including mutational, transcriptomic, and epigenetic markers have been investigated in advanced RCC. Examples of such markers include expression of IO-targets (PD1, PDL1, CTLA-4), as well as composite gene expression signatures predictive of response to TKI monotherapy (75, 76) and IO-based therapies (75–78) in recent trials of IO-based agents in advanced RCC. However, none of the current markers for IO-based therapy and VEGFi-therapy have been externally validated or approved for clinical use for advanced RCC, and their applicability to predicting response to NA-ST in localized or locally-advanced RCC is unclear.
While radiation therapy has long been considered an ineffective modality to treat localized RCC due to the associated adverse effects of radiation on healthy tissue, as well as the documented radioresistance of RCC cells, newer radiation techniques have demonstrated the ability to overcome these limitations. New advances in radiotherapy recognized that while conventionally fractionated therapy (eg, 1.8 – 3.0 Gy) likely fails to generate the associated endothelial apoptotic response necessary for tumor death, high-dose, hypo-fractionated stereotactic ablative radiotherapy (SAbR) is a strategy that has demonstrated success in patients with extracranial metastases in several trials (79, 80).
Given this early success, Margulis et al. reported a single arm phase 1/2 prospective trial investigating the use of 40 Gy in 5 fractions to patients with RCC associated with IVC tumor thrombus. A total of 6 patients were included in the final analysis, with 3 patients who had M1 disease (81). These authors reported minimal treatment-associated adverse events and no intraoperative complications or technical difficulties (81). While the small number of this cohort limits conclusions in terms of oncologic outcomes, it highlights that Neo-SAbR is feasible and safe for evaluation in phase II setting which remains ongoing [NCT02473536 (82)].
Finally, multimodal neoadjuvant therapy approaches utilizing both systemic and radiation therapies are being investigated; NCT05024318 (24) will seek to assess the efficacy of stereotactic radiotherapy prior to nephrectomy in combination with neoadjuvant pembrolizumab versus SABR alone plus nephrectomy. The study plans to enroll 26 patients with locally advanced disease, but allows for low-volume metastatic disease in patients who are candidates for cytoreductive nephrectomy (24).
4.3 Summary
Currently, there is limited evidence for the use of NA-ST to improve oncologic outcomes in RCC, such as recurrence-free survival, metastasis-free survival, or cancer-specific survival, as well as perioperative outcomes – to facilitate surgery of potentially unresectable tumors, or cases where nephron sparing surgery is preferred. This is partly due to the heterogeneity within published studies in terms of patient selection criteria, differences in types and intensity of treatment regimens, and trial design endpoints. As the field looks forward to novel prospective neoadjuvant trials, it will be critical to incorporate both surgical outcomes, including pre-surgical complexity and morbidity along, with clinical outcomes like pathologic downstaging and RFS to ensure robust evaluation of the impact of NA-ST. Further, neoadjuvant studies offer the unique advantage of potentially pre- and post-treatment correlative tissue analyses, and distinctively present a window of opportunity to uncover novel biomarkers associated with the evolving effects of systemic therapy. With the rapidly changing landscape of IO and IO/VEGFi combinations in the adjuvant and metastatic setting, the neoadjuvant space is readily poised to integrate and build upon these efforts for future study.
Author Contributions
SK and SJ have contributed equally to this work (manuscript writing, data collection, critical analysis) and share first authorship. AH and RK have contributed equally to this work and share last authorship (manuscript writing, critical analysis, and supervision of first authors). All authors contributed to the article and approved the submitted version.
Funding
SK and SJ are supported by the following grants: Cancer Center Support Grant (NIH, NCI; 2P30CA008748-48). RK is supported by the following grants: Supported (in part) by the Academy of Kidney Cancer Investigators of the CDMRP/DOD (KC200127). AH is funded by MSKCC’s institutional NCI P30 Cancer Center Support Grant (CCSG) (P30 CA008748).
Conflict of Interest
AH reports advisory board consultation for Merck. RK reports advisory board consultation for Eisai and reports receiving institutional research funding from Pfizer and Takeda.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2022. CA Cancer J Clin (2022) 72:7–33. doi: 10.3322/caac.21708
2. Kane CJ, Mallin K, Ritchey J, Cooperberg MR, Carroll PR. Renal Cell Cancer Stage Migration: Analysis of the National Cancer Data Base. Cancer (2008) 113:78–83. doi: 10.1002/cncr.23518
3. Lam JS, Shvarts O, Leppert JT, Pantuck AJ, Figlin RA, Belldegrun AS. Postoperative Surveillance Protocol for Patients With Localized and Locally Advanced Renal Cell Carcinoma Based on a Validated Prognostic Nomogram and Risk Group Stratification System. J Urol (2005) 174:466–72. doi: 10.1097/01.ju.0000165572.38887.da
4. Leibovich BC, Lohse CM, Cheville JC, Zaid HB, Boorjian SA, Frank I, et al. Predicting Oncologic Outcomes in Renal Cell Carcinoma After Surgery. Eur Urol (2018) 73:772–80. doi: 10.1016/j.eururo.2018.01.005
5. Figlin RA, Leibovich BC, Stewart GD, Negrier S. Adjuvant Therapy in Renal Cell Carcinoma: Does Higher Risk for Recurrence Improve the Chance for Success? Ann Oncol (2018) 29:324–31. doi: 10.1093/annonc/mdx743
6. Dabestani S, Thorstenson A, Lindblad P, Harmenberg U, Ljungberg B, Lundstam S. Renal Cell Carcinoma Recurrences and Metastases in Primary non-Metastatic Patients: A Population-Based Study. World J Urol (2016) 34:1081–6. doi: 10.1007/s00345-016-1773-y
7. Cancer RPSEER Stat Fact Sheets: Kidney and Renal Pelvis Cancer (2021). SEER Natl. Cancer Inst. Available at: https://seer.cancer.gov/statfacts/html/kidrp.html (Accessed November 28, 2021).
8. Gulati S, Vaishampayan U. Current State of Systemic Therapies for Advanced Renal Cell Carcinoma. Curr Oncol Rep (2020) 22:26. doi: 10.1007/s11912-020-0892-1
9. Rappold PM, Silagy AW, Kotecha RR, Hakimi AA. Immune Checkpoint Blockade in Renal Cell Carcinoma. J Surg Oncol (2021) 123:739–50. doi: 10.1002/jso.26339
10. Ravaud A, Motzer RJ, Pandha HS, George DJ, Pantuck AJ, Patel A, et al. Adjuvant Sunitinib in High-Risk Renal-Cell Carcinoma After Nephrectomy. N Engl J Med (2016) 375:2246–54. doi: 10.1056/NEJMoa1611406
11. Haas NB, Manola J, Uzzo RG, Flaherty KT, Wood CG, Kane C, et al. Adjuvant Sunitinib or Sorafenib for High-Risk, Non-Metastatic Renal-Cell Carcinoma (ECOG-ACRIN E2805): A Double-Blind, Placebo-Controlled, Randomised, Phase 3 Trial. Lancet (2016) 387:2008–16. doi: 10.1016/S0140-6736(16)00559-6
12. Eisen T, Frangou E, Oza B, Ritchie AWS, Smith B, Kaplan R, et al. Adjuvant Sorafenib for Renal Cell Carcinoma at Intermediate or High Risk of Relapse: Results From the SORCE Randomized Phase III Intergroup Trial. J Clin Oncol (2020) 38:4064–75. doi: 10.1200/JCO.20.01800
13. Motzer RJ, Russo P, Haas N, Doehn C, Donskov F, Gross-Goupil M, et al. Adjuvant Pazopanib Versus Placebo After Nephrectomy in Patients With Localized or Locally Advanced Renal Cell Carcinoma: Final Overall Survival Analysis of the Phase 3 PROTECT Trial. Eur Urol (2021) 79:334–8. doi: 10.1016/j.eururo.2020.12.029
14. ClinicalTrials.gov.: S0931. Everolimus in Treating Patients With Kidney Cancer Who Have Undergone Surgery - Full Text View - ClinicalTrials.Gov. Available at: https://clinicaltrials.gov/ct2/show/NCT01120249 (Accessed January 6, 2022).
15. Choueiri TK, Tomczak P, Park SH, Venugopal B, Ferguson T, Chang Y-H, et al. Adjuvant Pembrolizumab After Nephrectomy in Renal-Cell Carcinoma. N Engl J Med (2021) 385:683–94. doi: 10.1056/NEJMoa2106391
16. Thillai K, Allan S, Powles T, Rudman S, Chowdhury S. Neoadjuvant and Adjuvant Treatment of Renal Cell Carcinoma. Expert Rev Anticancer Ther (2012) 12:765–76. doi: 10.1586/era.12.56
17. Timsit M-O, Albiges L, Méjean A, Escudier B. Neoadjuvant Treatment in Advanced Renal Cell Carcinoma: Current Situation and Future Perspectives. Expert Rev Anticancer Ther (2012) 12:1559–69. doi: 10.1586/era.12.142
18. Westerman ME, Shapiro DD, Wood CG, Karam JA. Neoadjuvant Therapy for Locally Advanced Renal Cell Carcinoma. Urol Clin NA (2020) 47:329–43. doi: 10.1016/j.ucl.2020.04.010
19. Bex A, Powles T, Karam JA. Role of Targeted Therapy in Combination With Surgery in Renal Cell Carcinoma. Int J Urol (2016) 23:5–12. doi: 10.1111/iju.12891
20. Hellenthal NJ, Underwood W, Penetrante R, Litwin A, Zhang S, Wilding GE, et al. Prospective Clinical Trial of Preoperative Sunitinib in Patients With Renal Cell Carcinoma. J Urol (2010) 184:859–64. doi: 10.1016/j.juro.2010.05.041
21. Cowey CL, Amin C, Pruthi RS, Wallen EM, Nielsen ME, Grigson G, et al. Neoadjuvant Clinical Trial With Sorafenib for Patients With Stage II or Higher Renal Cell Carcinoma. J Clin Oncol (2010) 28:1502–7. doi: 10.1200/JCO.2009.24.7759
22. Silberstein JL, Millard F, Mehrazin R, Kopp R, Bazzi W, DiBlasio CJ, et al. Feasibility and Efficacy of Neoadjuvant Sunitinib Before Nephron-Sparing Surgery. BJU Int (2010) 106:1270–6. doi: 10.1111/j.1464-410X.2010.09357.x
23. Rini BI, Garcia J, Elson P, Wood L, Shah S, Stephenson A, et al. The Effect of Sunitinib on Primary Renal Cell Carcinoma and Facilitation of Subsequent Surgery. J Urol (2012) 187:1548–54. doi: 10.1016/j.juro.2011.12.075
24. ClinicalTrials.gov: NCT05024318:. NeoAdjuvant Pembrolizumab and STEreotactic Radiotherapy Prior to Nephrectomy for Renal Cell Carcinoma - Full Text View - ClinicalTrials.Gov (2021). Available at: https://clinicaltrials.gov/ct2/show/NCT05024318 (Accessed January 12, 2022).
25. Lane BR, Derweesh IH, Kim HL, O'Malley R, Klink J, Ercole CE, et al. Presurgical Sunitinib Reduces Tumor Size and may Facilitate Partial Nephrectomy in Patients With Renal Cell Carcinoma. Urol Oncol Semin Orig Investig (2015) 33:112. doi: 10.1016/j.urolonc.2014.11.009
26. Dindo D, Demartines N, Clavien P-A. Classification of Surgical Complications: A New Proposal With Evaluation in A Cohort of 6336 Patients and Results of a Survey. Ann Surg (2004) 240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae
27. ClinialTrials.gov: NCT02212730:. A Study Evaluating the Effect of Pembrolizumab (MK-3475) in Participants With Renal Cell Cancer (MK-3475-031) - Full Text View - ClinicalTrials.Gov (2014). Available at: https://clinicaltrials.gov/ct2/show/NCT02212730 (Accessed January 12, 2022).
28. ClinicalTrials.gov: NCT00480935:. A Study of Neoadjuvant Sutent for Patients With Renal Cell Carcinoma (2007). Available at: https://clinicaltrials.gov/ct2/show/NCT00480935 (Accessed January 11, 2022).
29. Karam JA, Devine CE, Urbauer DL, Lozano M, Maity T, Ahrar K, et al. Phase 2 Trial of Neoadjuvant Axitinib in Patients With Locally Advanced Nonmetastatic Clear Cell Renal Cell Carcinoma. Eur Urol (2014) 66:874–80. doi: 10.1016/j.eururo.2014.01.035
30. Rini BI, Plimack ER, Takagi T, Elson P, Wood LS, Dreicer R, et al. A Phase II Study of Pazopanib in Patients With Localized Renal Cell Carcinoma to Optimize Preservation of Renal Parenchyma. J Urol (2015) 194:297–303. doi: 10.1016/j.juro.2015.03.096
31. Hatiboglu G, Hohenfellner M, Arslan A, Hadaschik B, Teber D, Radtke JP, et al. Effective Downsizing But Enhanced Intratumoral Heterogeneity Following Neoadjuvant Sorafenib in Patients With non-Metastatic Renal Cell Carcinoma. Langenbeck’s Arch Surg (2017) 402:637–44. doi: 10.1007/s00423-016-1543-8
32. Lebacle C, Bensalah K, Bernhard J-C, Albiges L, Laguerre B, Gross-Goupil M, et al. Evaluation of Axitinib to Downstage Ct2a Renal Tumours and Allow Partial Nephrectomy: A Phase II Study. BJU Int (2019) 123:804–10. doi: 10.1111/bju.14581
33. Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab Plus Axitinib Versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med (2019) 380:1103–15. doi: 10.1056/NEJMoa1816047
34. Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab Plus Axitinib Versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med (2019) 380:1116–27. doi: 10.1056/NEJMoa1816714
35. Chen Y, Tortorici MA, Garrett M, Hee B, Klamerus KJ, Pithavala YK, et al. Clinical Pharmacology of Axitinib. Clinical Pharmacokinetics (2013) 52:713–25. doi: 10.1007/s40262-013-0068-3
36. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur J Cancer (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
37. Kutikov A, Uzzo RG. The R.E.N.A.L. Nephrometry Score: A Comprehensive Standardized System for Quantitating Renal Tumor Size, Location and Depth. J Urol (2009) 182:844–53. doi: 10.1016/j.juro.2009.05.035
38. Sonpavde G, Hutson TE. Pazopanib: A Novel Multitargeted Tyrosine Kinase Inhibitor. Curr Oncol Rep (2007) 9:115–9. doi: 10.1007/s11912-007-0007-2
39. Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, et al. Pazopanib Versus Sunitinib in Metastatic Renal-Cell Carcinoma. N Engl J Med (2013) 369:722–31. doi: 10.1056/NEJMoa1303989
40. Motzer RJ, Haas NB, Donskov F, Gross-Goupil M, Varlamov S, Kopyltsov E, et al. Randomized Phase III Trial of Adjuvant Pazopanib Versus Placebo After Nephrectomy in Patients With Localized or Locally Advanced Renal Cell Carcinoma. J Clin Oncol (2017) 35:3916–23. doi: 10.1200/JCO.2017.73.5324
42. ClinicalTrials.gov: NCT02762006:. Neoadjuvant MEDI 4736 +/- Tremelimumab in Locally Advanced Renal Cell Carcinoma - Full Text View - ClinicalTrials.Gov (2016). Available at: https://clinicaltrials.gov/ct2/show/NCT02762006 (Accessed January 18, 2022).
43. ClinicalTrials.gov: NCT02575222:. Study of Neoadjuvant Nivolumab in Patients With Non-Metastatic Stage II-IV Clear Cell Renal Cell Carcinoma - Full Text View - ClinicalTrials.Gov (2016). Available at: https://www.clinicaltrials.gov/ct2/show/NCT02575222 (Accessed January 18, 2022).
44. ClinicalTrials.gov: NCT01361113:. Neoadjuvant Pazopanib in Renal Cell Carcinoma - Full Text View - ClinicalTrials.Gov. Available at: https://clinicaltrials.gov/ct2/show/NCT01361113 (Accessed January 18, 2022).
45. ClincalTrials.gov: NCT01263769:. Phase 2 Trial of Neoadjuvant Axitinib in Patients With Locally Advanced Nonmetastatic Clear Cell Renal Cell Carcinoma. 2011. Available at: https://linkinghub.elsevier.com/retrieve/pii/S0302283814001146.
46. ClinicalTrials.gov: NCT03680521:. Neoadjuvant Sitravatinib in Combination With Nivolumab in Patients With Clear Cell Renal Cell Carcinoma - Full Text View - ClinicalTrials.Gov (2018). Available at: https://clinicaltrials.gov/ct2/show/NCT03680521 (Accessed January 12, 2022).
47. ClinicalTrials.gov: NCT04028245:. A Study of Combination Spartalizumab and Canakinumab in Patients With Localized Clear Cell Renal Cell Carcinoma - Full Text View - ClinicalTrials.Gov (2019). Available at: https://clinicaltrials.gov/ct2/show/NCT04028245 (Accessed January 18, 2022).
48. Anon: NCT04022343:. Neoadjuvant Cabozantinib in Treating Patients With Locally Advanced Kidney Cancer - Full Text View - ClinicalTrials.Gov (2019). Available at: https://clinicaltrials.gov/ct2/show/NCT04022343 (Accessed January 18, 2022).
49. ClinicalTrials.gov: NCT03341845:. Neoadjuvant Axitinib Plus Avelumab in Patients With Localized Renal Cell Carcinoma (2018). Available at: https://clinicaltrials.gov/ct2/show/NCT03341845.
50. ClinicalTrials.gov: NCT04393350:. Lenvatinib and Pembrolizumab Before Surgery for the Treatment of Locally Advanced Non-Metastatic Kidney Cancer - Full Text View - ClinicalTrials.Gov (2020). Available at: https://clinicaltrials.gov/ct2/show/NCT04393350 (Accessed January 18, 2022).
51. ClinicalTrials.gov: NCT05172440:. A Study on the Safety and Effectiveness of Tislelizumab Combined With Axitinib for Neoadjuvant Treatment of ccRCC - Full Text View - ClinicalTrials.Gov (2021). Available at: https://clinicaltrials.gov/ct2/show/NCT05172440?cond=Renal+Cell+Carcinoma&intr=Tislelizumab&draw=2&rank=1 (Accessed January 18, 2022).
52. ClinicalTrials.gov: NCT04995016:. Pembrolizumab and Axitinib as Neoadjuvant Therapy for Locally Advanced Non-Metastatic Clear Cell Renal Cell Carcinoma - Full Text View - ClinicalTrials.Gov (2021). Available at: https://clinicaltrials.gov/ct2/show/NCT04995016 (Accessed January 18, 2022).
53. ClinicalTrials.gov: NCT04118855:. Toripalimab Combined With Axitinib as Neoadjuvant Therapy in Patients With Non-Metastatic Locally Advanced Nonmetastatic Clear Cell Renal Cell Carcinoma - Full Text View - ClinicalTrials.Gov (2020). Available at: https://clinicaltrials.gov/ct2/show/NCT04118855 (Accessed January 18, 2022).
54. ClinicalTrials.gov: NCT05148546:. Neoadjuvant Study With Combination Immuno-Oncology for Primary Clear Cell Renal Cell Cancer - Full Text View - ClinicalTrials.Gov (2021). Available at: https://clinicaltrials.gov/ct2/show/NCT05148546 (Accessed January 18, 2022).
55. Bex A, Russo P, Tomita Y, Grünwald V, Ramirez L-M, McHenry BM, et al. A Phase III, Randomized, Placebo-Controlled Trial of Nivolumab or Nivolumab Plus Ipilimumab in Patients With Localized Renal Cell Carcinoma at High-Risk of Relapse After Radical or Partial Nephrectomy (CheckMate 914). J Clin Oncol (2020) 38:TPS5099–TPS5099. doi: 10.1200/JCO.2020.38.15_suppl.TPS5099
56. Oza B, Frangou E, Smith B, Bryant H, Kaplan R, Choodari-Oskooei B, et al. RAMPART: A Phase III Multi-Arm Multi-Stage Trial of Adjuvant Checkpoint Inhibitors in Patients With Resected Primary Renal Cell Carcinoma (RCC) at High or Intermediate Risk of Relapse. Contemp Clin Trials (2021) 108:106482. doi: 10.1016/j.cct.2021.106482
57. ClinicalTrials.gov: NCT02595918:. Nivolumab in Treating Patients With High-Risk Kidney Cancer Before Surgery - Full Text View - ClinicalTrials.Gov (2016). Available at: https://clinicaltrials.gov/ct2/show/NCT02595918 (Accessed January 27, 2022).
58. ClinicalTrials.gov: NCT03055013:. Nivolumab in Treating Patients With Localized Kidney Cancer Undergoing Nephrectomy - Full Text View - ClinicalTrials.Gov (2017). Available at: https://clinicaltrials.gov/ct2/show/NCT03055013 (Accessed January 27, 2022).
59. Harshman LC, Puligandla M, Haas NB, Allaf M, Drake CG, McDermott DF, et al. PROSPER: A Phase III Randomized Study Comparing Perioperative Nivolumab (Nivo) Versus Observation in Patients With Localized Renal Cell Carcinoma (RCC) Undergoing Nephrectomy (ECOG-ACRIN 8143). J Clin Oncol (2019) 37:TPS684–4. doi: 10.1200/JCO.2019.37.7_suppl.TPS684
60. Yi M, Jiao D, Qin S, Chu Q, Wu K, Li A. Synergistic Effect of Immune Checkpoint Blockade and Anti-Angiogenesis in Cancer Treatment. Mol Cancer (2019) 18:60. doi: 10.1186/s12943-019-0974-6
61. Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, Pointet A-L, et al. VEGF-A Modulates Expression of Inhibitory Checkpoints on CD8+ T Cells in Tumors. J Exp Med (2015) 212:139–48. doi: 10.1084/jem.20140559
62. Allen E, Jabouille A, Rivera LB, Lodewijckx I, Missiaen R, Steri V, et al. Combined Antiangiogenic and Anti–PD-L1 Therapy Stimulates Tumor Immunity Through HEV Formation. Sci Transl Med (2017) 9. doi: 10.1126/scitranslmed.aak9679
63. Amin A, Plimack ER, Ernstoff MS, Lewis LD, Bauer TM, McDermott DF, et al. Safety and Efficacy of Nivolumab in Combination With Sunitinib or Pazopanib in Advanced or Metastatic Renal Cell Carcinoma: The CheckMate 016 Study. J Immunother Cancer (2018) 6:109. doi: 10.1186/s40425-018-0420-0
64. Motzer RJ, Powles T, Atkins MB, Escudier B, McDermott DF, Suarez C, et al. IMmotion151: A Randomized Phase III Study of Atezolizumab Plus Bevacizumab vs Sunitinib in Untreated Metastatic Renal Cell Carcinoma (mRCC). J Clin Oncol (2018) 36:578–8. doi: 10.1200/JCO.2018.36.6_suppl.578
65. Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, et al. Nivolumab Plus Cabozantinib Versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med (2021) 384:829–41. doi: 10.1056/NEJMoa2026982
66. Siddiqi R, Bin RI, Islam M, Sipra QUAR, Ryu AJ, Raina A, et al. Adjuvant Therapy in High-Risk Renal Cell Cancer: A Systematic Review and Cumulative Meta-Analysis. J Clin Oncol (2020) 38. doi: 10.1200/JCO.2020.38.6_suppl.708
67. Botrel TEA, Clark O, Bretas FFH, Sadi MV, Ferreira U, Paladini L, et al. Efficacy of Adjuvant Vascular Endothelial Growth Factor Receptor (VEGFR) Tyrosine Kinase Inhibitors (VEGFRi) in Renal Cell Carcinoma (RCC): A Systematic Review and Meta-Analysis. J Clin Oncol (2020) 38. doi: 10.1200/JCO.2020.38.6_suppl.680
68. Martinez Chanza N, Tripathi A, Harshman LC. Adjuvant Therapy Options in Renal Cell Carcinoma: Where Do We Stand? Curr Treat Options Oncol (2019) 20:44. doi: 10.1007/s11864-019-0639-0
69. Choueiri TK, Kaelin WG. Targeting the HIF2–VEGF Axis in Renal Cell Carcinoma. Nat Med (2020) 26:1519–30. doi: 10.1038/s41591-020-1093-z
70. Couch V, Lindor NM, Karnes PS, Michels VV. Von Hippel-Lindau Disease. Mayo Clin Proc (2000) 75:265–72. doi: 10.1016/S0025-6196(11)65031-3
71. Turner KJ, Moore JW, Jones A, Taylor CF, Cuthbert-Heavens D, Han C, et al. Expression of Hypoxia-Inducible Factors in Human Renal Cancer: Relationship to Angiogenesis and to the Von Hippel-Lindau Gene Mutation. Cancer Res (2002) 62.
72. Jonasch E, Donskov F, Iliopoulos O, Rathmell WK, Narayan VK, Maughan BL, et al. Belzutifan for Renal Cell Carcinoma in Von Hippel–Lindau Disease. N Engl J Med (2021) 385:2036–46. doi: 10.1056/NEJMoa2103425
73. Choueiri TK, Bauer TM, Papadopoulos KP, Plimack ER, Merchan JR, McDermott DF, et al. Inhibition of Hypoxia-Inducible Factor-2α in Renal Cell Carcinoma With Belzutifan: A Phase 1 Trial and Biomarker Analysis. Nat Med (2021) 27:802–5. doi: 10.1038/s41591-021-01324-7
74. ClinicalTrials.gov: MK-6482:. A Study of Pembrolizumab (MK-3475) in Combination With Belzutifan (MK-6482) and Lenvatinib (MK-7902), or Pembrolizumab/Quavonlimab (MK-1308A) in Combination With Lenvatinib, Versus Pembrolizumab and Lenvatinib, for Treatment of Advanced Clear Cell . Available at: https://clinicaltrials.gov/ct2/show/NCT04736706 (Accessed January 27, 2022).
75. McDermott DF, Huseni MA, Atkins MB, Motzer RJ, Rini BI, Escudier B, et al. Clinical Activity and Molecular Correlates of Response to Atezolizumab Alone or in Combination With Bevacizumab Versus Sunitinib in Renal Cell Carcinoma. Nat Med (2018) 24:749–57. doi: 10.1038/s41591-018-0053-3
76. Motzer RJ, Robbins PB, Powles T, Albiges L, Haanen JB, Larkin J, et al. Avelumab Plus Axitinib Versus Sunitinib in Advanced Renal Cell Carcinoma: Biomarker Analysis of the Phase 3 JAVELIN Renal 101 Trial. Nat Med (2020) 26:1733–41. doi: 10.1038/s41591-020-1044-8
77. Şenbabaoğlu Y, Gejman RS, Winer AG, Liu M, Van Allen EM, de Velasco G, et al. Tumor Immune Microenvironment Characterization in Clear Cell Renal Cell Carcinoma Identifies Prognostic and Immunotherapeutically Relevant Messenger RNA Signatures. Genome Biol (2016) 17:231. doi: 10.1186/s13059-016-1092-z
78. Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, et al. IFN-γ-Related mRNA Profile Predicts Clinical Response to PD-1 Blockade. J Clin Invest (2017) 127:2930–40. doi: 10.1172/JCI91190
79. Zaorsky NG, Lehrer EJ, Kothari G, Louie AV, Siva S. Stereotactic Ablative Radiation Therapy for Oligometastatic Renal Cell Carcinoma (SABR ORCA): A Meta-Analysis of 28 Studies. Eur Urol Oncol (2019) 2:515–23. doi: 10.1016/j.euo.2019.05.007
80. Tang C, Msaouel P, Hara K, Choi H, Le V, Shah AY, et al. Definitive Radiotherapy in Lieu of Systemic Therapy for Oligometastatic Renal Cell Carcinoma: A Single-Arm, Single-Centre, Feasibility, Phase 2 Trial. Lancet Oncol (2021) 22:1732–9. doi: 10.1016/S1470-2045(21)00528-3
81. Margulis V, Freifeld Y, Pop LM, Manna S, Kapur P, Pedrosa I, et al. Neoadjuvant SABR for Renal Cell Carcinoma Inferior Vena Cava Tumor Thrombus—Safety Lead-In Results of a Phase 2 Trial. Int J Radiat Oncol (2021) 110:1135–42. doi: 10.1016/j.ijrobp.2021.01.054
82. Hannan R, : NCT02473536:. Neo-Adjuvant SABR for IVC Tumor Thrombus in Newly Diagnosed RCC. ClinicalTrials.Gov. Available at: https://clinicaltrials.gov/ct2/show/NCT02473536 (Accessed January 4, 2022).
Keywords: neoadjuvant, targeted therapy, systemic therapy, renal cell carcinoma, clear cell
Citation: Khaleel S, Jiang S, Kotecha RR and Hakimi AA (2022) Neoadjuvant Systemic Therapy in Localized and Locally Advanced Renal Cell Carcinoma. Front. Urol. 2:864778. doi: 10.3389/fruro.2022.864778
Received: 28 January 2022; Accepted: 25 March 2022;
Published: 29 April 2022.
Edited by:
Juan Gomez Rivas, Hospital Clínico San Carlo, SpainReviewed by:
Alvaro Pinto, University Hospital La Paz, SpainRiccardo Campi, Careggi Hospital, Italy
Ignacio Puche-Sanz, Virgen de las Nievas University Hospital, Spain
Alvaro Juarez, University Hospital of Jerez de la Frontera, Spain
Copyright © 2022 Khaleel, Jiang, Kotecha and Hakimi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: A. Ari Hakimi, hakimia@mskcc.org; Ritesh R. Kotecha, kotechar@mskcc.org
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
 Sari Khaleel
Sari Khaleel Song Jiang
Song Jiang Ritesh R. Kotecha
Ritesh R. Kotecha A. Ari Hakimi1*‡
A. Ari Hakimi1*‡