Risks of metabolic syndrome in the ADVANCE and NAMSAL trials
- 1TransVIHMI, University of Montpellier, IRD, INSERMI, Montpellier, France
- 2Infectious Diseases, Regional Military Hospital Number 1, Yaoundé, Cameroon
- 3Faculty of Medicine and Pharmaceutical Sciences, University of Dschang, Dschang, Cameroon
- 4Day Stay Hospital, Central Hospital of Yaoundé, Henri-Dunant, Yaoundé, Cameroon
- 5ANRS Cameroon Site, Central Hospital of Yaoundé, Henri-Dunant, Yaoundé, Cameroon
- 6Division of Infectious Diseases, HIV-AIDS Unit, Genva University Hospitals, Geneva, Switzerland
- 7Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
- 8School of Medicine and Veterinary Medicine, University of Edinburgh, Edinburgh, United Kingdom
- 9London School of Economics and Political Science, LSE Health, London, United Kingdom
- 10Unitaid, Global Health Campus, Le Grand-Saconnex, Switzerland
- 11Faculty of Medicine, Imperial College London, London, United Kingdom
- 12Department of Pharmacology and Therapeutics, University of Liverpool, Liverpool, United Kingdom
Introduction: The ADVANCE and NAMSAL trials evaluating antiretroviral drugs have both reported substantial levels of clinical obesity in participants. As one of the main risk factors for metabolic syndrome, growing rates of obesity may drive metabolic syndrome development. This study aims to evaluate the risk of metabolic syndrome in the ADVANCE and NAMSAL trials.
Methods: The number of participants with metabolic syndrome was calculated at baseline and week 192 as central obesity and any of the following two factors: raised triglycerides, reduced HDL-cholesterol, raised blood pressure and raised fasting glucose. Differences between the treatment arms were calculated using the χ2 test.
Results: Across all visits to week 192, treatment-emergent metabolic syndrome was 15% (TAF/FTC + DTG), 10% (TDF/FTC + DTG) and 7% (TDF/FTC/EFV) in ADVANCE. The results were significantly higher in the TAF/FTC + DTG arm compared to the TDF/FTC/EFV arm (p < 0.001), and the TDF/FTC + DTG vs. the TDF/FTC/EFV arms (p < 0.05) in all patients, and in females. In NAMSAL, the incidence of treatment-emergent metabolic syndrome at any time point was 14% (TDF/3TC + DTG) and 5% (TDF/3TC + EFV) (p < 0.001). This incidence was significantly greater in the TDF/3TC/DTG arm compared to the TDF/3TC/EFV arm in all patients (p < 0.001), and in males (p < 0.001)
Conclusion: In this analysis, we highlight treatment-emergent metabolic syndrome associated with dolutegravir, likely driven by obesity. Clinicians initiating or monitoring patients on INSTI-based ART must counsel for lifestyle optimisation to prevent these effects.
Introduction
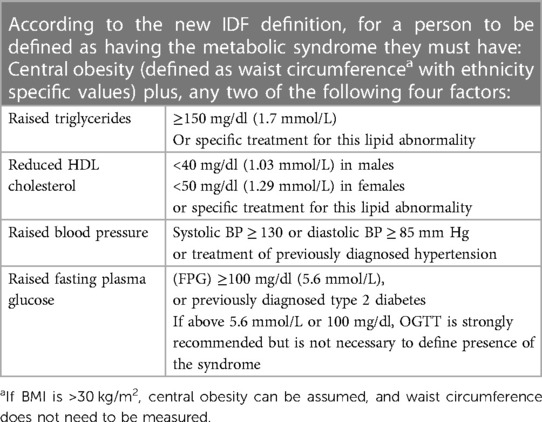
Metabolic syndrome is a cluster of clinical features comprising obesity, dyslipidaemia, hypertension, and impaired glucose tolerance. It has been defined by the International Diabetes Federation as having central obesity plus any two of the following: (1) raised triglycerides, (2) reduced high-density lipoprotein (HDL) cholesterol or on lipid lowering therapy, (3) hypertension, and (4) raised fasting plasma glucose or diagnosis of type 2 diabetes (1) (See Table 1). The mechanism of development is not fully understood, but has been linked to genetic predisposition, chronic inflammation, neurohormonal activation, and insulin resistance (2, 3). Visceral adiposity has been shown to be a significant trigger to activate pathways of metabolic syndrome development (2, 4).
There has been a suggestion that metabolic syndrome may be more prevalent in people living with HIV (PLWH) in a meta-analysis conducted in Sub Saharan Africa, with a prevalence of 21.5% in PLWH compared to 12% in uninfected populations (5). The global prevalence has been estimated to be between 16.7% and 31.3% among PLWH in another study (6).
It has been widely reported that metabolic syndrome predisposes to development of cardiovascular disease and type II diabetes, with an estimated two and five-fold increased risk respectively, compared to those without the syndrome (1). It has also been linked to co-morbidities including cancer development, polycystic ovarian syndrome (7), obstructive sleep apnoea (8), chronic kidney disease (9), and non-alcoholic fatty liver disease (10, 11).
Previously, protease inhibitors used to treat HIV were associated with metabolic syndrome development (12). However, there is now a significant body of evidence associating weight gain and obesity with integrase inhibitors (INSTI) (13, 14), which are recommended as the preferred first line antiretroviral in the WHO guidelines for treatment of HIV-1 infection (15). Tenofovir alafenamide, often used in combination with INSTIs, has also been implicated in weight gain and obesity (13, 16). Weight gain associated with both INSTIs +/− TAF, has been greater in Black ethnicity, and females (13, 16). As one of the main risk factors for metabolic syndrome, growing rates of obesity within this patient population may drive metabolic syndrome development.
The NAMSAL-ANRS-12313 (NAMSAL) trial is a phase 3 open-label randomised trial which compared the efficacy of TDF/3TC + DTG to TDF/3TC + EFV (17). The trial recruited adults with HIV-1 infection who had not received antiretroviral therapy from three hospitals in Cameroon. The ADVANCE trial is a phase 3 randomised controlled open-label trial which evaluated the efficacy and safety of TAF/FTC + DTG and TDF/FTC + DTG, as compared with TDF/FTC/EFV (18). The trial recruited participants from routine HIV testing sites, based in Johannesburg, South Africa.
The ADVANCE and NAMSAL trials have both reported substantial levels of clinical obesity (17, 18). Weight gain has been more pronounced on the DTG arms of both trials, disproportionally affecting females, and particularly those on the TAF/FTC backbone on the ADVANCE trial (17, 18). This study aims to evaluate the risk of metabolic syndrome in the ADVANCE and NAMSAL trials.
Methods
Study design
The ADVANCE and NAMSAL trials were the first open-label, non-inferiority phase 3 randomised controlled trials of DTG with treatment-naïve participants in low- to middle-income country settings (LMIC), with recruitment of >99% Black Africans in both studies (17, 18). Ethics approval and written, informed consent for all participants were obtained and further details on these can be found in the original papers (17, 18). Study visits were planned at baseline, weeks 4, 12 and every 12 weeks thereafter until week 192. At each visit, data was recorded on parameters including HIV RNA, weight, systolic/diastolic blood pressure, fasting glucose, cholesterol, HDL, LDL and triglycerides. Both ADVANCE and NAMSAL ended at Week 192.
Statistical analyses
Baseline characteristics including age, sex, weight, HIV RNA, CD4 count, and the metabolic parameter levels were calculated for each treatment arm in both the ADVANCE and NAMSAL trials. Count outcomes are summarised as number (%) and continuous outcomes as median (IQR). We also evaluated how many participants had abnormalities in any of the metabolic parameters (BMI, blood pressure, fasting glucose, triglycerides, HDL) at baseline and week 192. Results are displayed as number (%).
Metabolic syndrome at any time up to week 192 was calculated in both trials as central obesity (BMI > 30 kg/m2) and any of the following two factors: raised triglycerides (≥1.7 mmol/L), reduced HDL-cholesterol (<1.29 mmol/L), raised systolic or diastolic blood pressure (SBP ≥ 130 mm Hg or DBP ≥ 85 mm Hg) and raised fasting glucose (≥5.6 mmol/L), as per the definition set by the International Diabetes Federation (1) (Table 1). Participants who already had metabolic syndrome at baseline were excluded from the analysis. Results are displayed as number (%) of participants with metabolic syndrome.
Statistical analysis was conducted using STATA/IC version 16 (StataCorp LLC, College Station, TX, USA). Differences between the treatment arms were calculated using the χ2 test. The significance threshold was set at 5% (two-sided).
Results
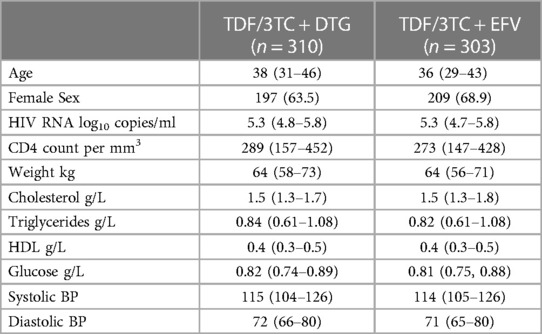
In the ADVANCE trial there were a total of 1,053 randomised participants (TAF/FTC + DTG = 351, TDF/FTC + DTG = 351, TDF/FTC + EFV = 351). The median age of participants was 32 in the TAF/FTC + DTG and TDF/FTC + DTG arms and 33 in the TDF/FTC + EFV arm. Females comprised 61% of participants in the TAF/FTC + DTG arm, 59% in the TDF/FTC + DTG arm and 57% in the TDF/FTC + EFV arm. The median weight of participants was 66.4 kg, 66.3 kg and 66.4 kg in the TAF/FTC + DTG, TDF/FTC + DTG and TDF/FTC + EFV arms respectively. In the NAMSAL trial there were a total of 616 randomised participants (TDF/3TC + DTG = 310, TDF/3TC + EFV = 303). The median age of participants was 38 in the TDF/3TC + DTG arm and 36 in the TDF/3TC + EFV arm. 63.5% of participants in the TDF/3TC + DTG arm and 68.3% in the TDF/3TC + EFV arm were females. The median weight of participants was 64 kg in both arms. Further details on baseline characteristics of participants in ADVANCE and NAMSAL are presented in Tables 2A,B.
Advance
Weight
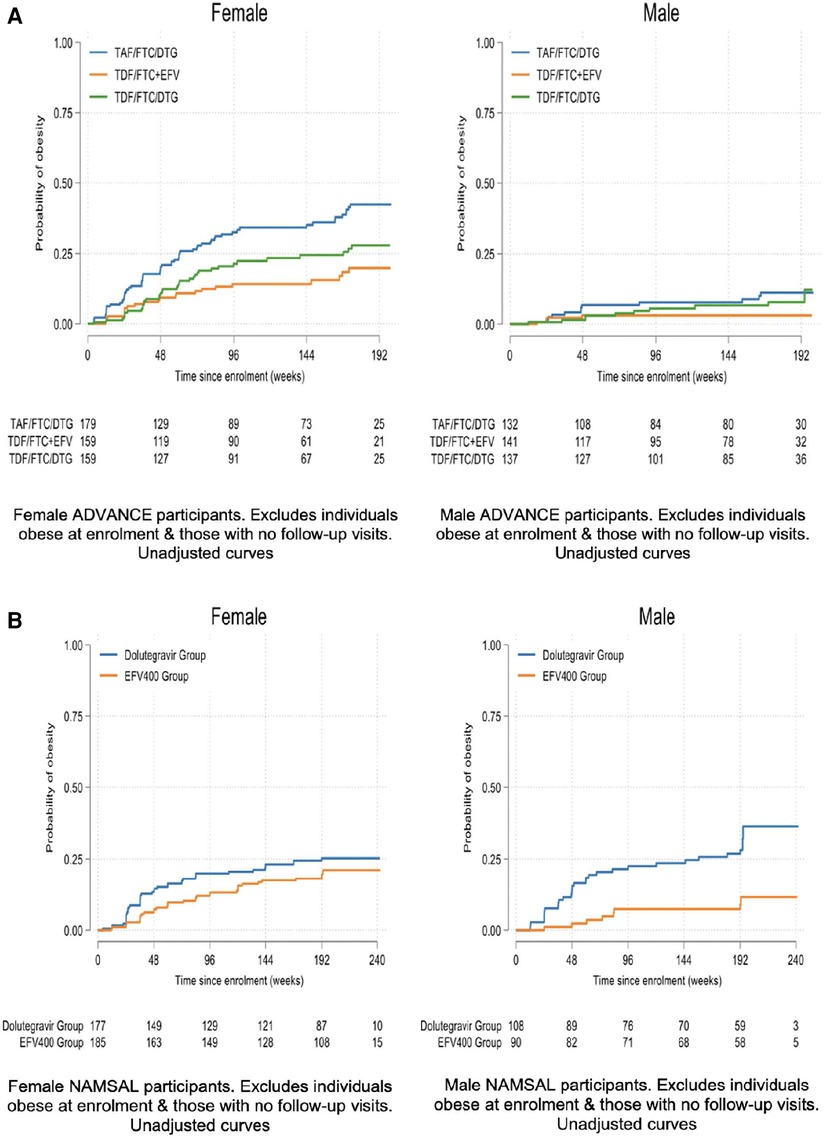
At week 192, mean weight change across the TAF/FTC + DTG, TDF/FTC + DTG and TDF/FTC/EFV arms were 9.93 kg, 6.65 kg and 5.01 kg in females and 7.18 kg, 4.87 kg and 1.29 kg in males Figure 1A displays the time to clinical obesity across the 3 arms.
Changes in metabolic parameters
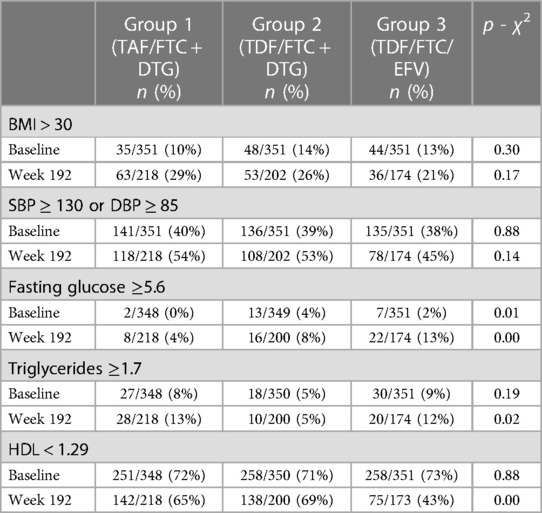
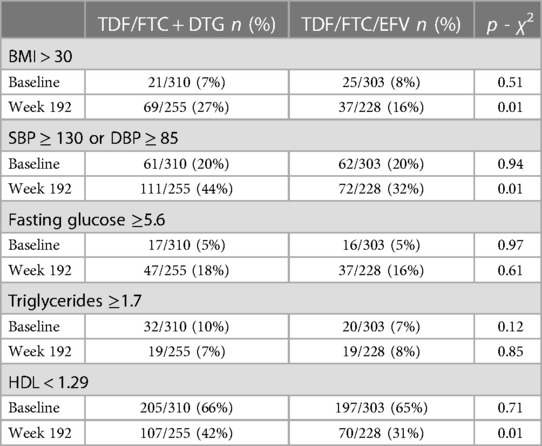
In the ADVANCE trial, the only significant difference in metabolic abnormalities between treatment arms at baseline was for fasting glucose (p = 0.014). At week 192 there were significant differences between treatment arms for fasting glucose (p = 0.004), triglycerides (p = 0.017) and HDL (p < 0.00001). Table 3A shows the number (%) of participants with abnormalities in metabolic parameters stratified by treatment arm at baseline and week 192.
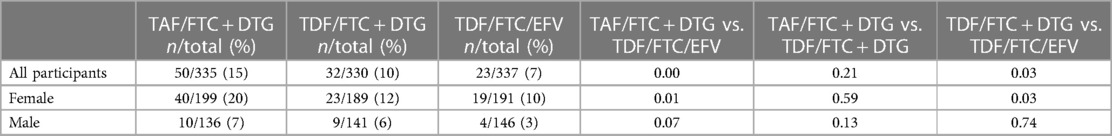
Metabolic syndrome
51 (5%) participants had metabolic syndrome at baseline in the ADVANCE trial. There were no differences between the treatment arms. Across all study visits to week 192, treatment-emergent metabolic syndrome was 15% (TAF/FTC + DTG), 10% (TDF/FTC + DTG) and 7% (TDF/FTC/EFV) (Table 4A). The results were significantly higher in the TAF/FTC + DTG compared to the TDF/FTC/EFV arm (p < 0.001), and the TDF/FTC + DTG vs. the TDF/FTC/EFV arms (p < 0.05) in all patients, and in females. The results in males were non-significant. Table 4A shows the results stratified by study visit and sex. NAMSAL.
Weight
At week 192, mean weight change across the TDF/3TC + DTG and TDF/3TC + EFV arms were 6.37 kg and 5.38 kg in females and 5.13 kg and 3.51 kg in males. Figure 1B displays the time to clinical obesity across the 3 arms.
Changes in metabolic parameters
In the NAMSAL trial, there was no significant difference in metabolic abnormalities between treatment arms at baseline (Table 3B). At week 192 there was a significant difference between treatment arms for BMI (p < 0.01), raised blood pressure (p < 0.01), and HDL (p < 0.05). Table 3B shows the number (%) of participants with abnormalities in metabolic parameters stratified by treatment arm at baseline and week 192.
Metabolic syndrome
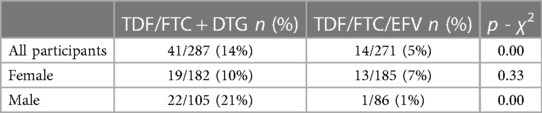
In the NAMSAL trial, at baseline 18 (3%) participants had metabolic syndrome with no difference between the two treatment arms. Across all participants, the incidence of treatment-emergent metabolic syndrome was 14% (TDF/3TC + DTG) and 5% (TDF/3TC + EFV) at any time up to week 192 (p < 0.01) (Table 4B). The incidence of treatment-emergent metabolic syndrome was significantly greater in the TDF/3TC/DTG arm compared to the TDF/3TC/EFV arm in all patients (p < 0.001), and in males (p = 0.002) up to week 192 of the trial. The differences were not significant in females.
Discussion
In ADVANCE, across all study visits to week 192, treatment-emergent metabolic syndrome was present in 15% (TAF/FTC + DTG), 10% (TDF/FTC + DTG) and 7% (TDF/FTC/EFV) of participants. In NAMSAL the incidence of treatment-emergent metabolic syndrome was 14% (TDF/3TC + DTG) and 5% (TDF/3TC + EFV) at any time up to week 192. We highlight treatment-emergent metabolic syndrome associated with dolutegravir, likely driven by obesity. There is some evidence of metabolic syndrome associated with INSTI use in other trials. However, there is limited data coming from other randomised trials.
In a Zambian cross-sectional study, a DTG-based regimen was associated with metabolic syndrome (OR: 2.10, 95% CI: 1.05–4.20) (19). The ACTG A5001 and A5322 trials observed changes in weight gain to correspond with lower levels of HDL, and higher levels of LDL cholesterol, total cholesterol, triglyceride levels and fasting glucose (20). Similarly, 10% weight gain in NAMSAL was associated with a rise in cholesterol levels (21).
By contrast, in the REPRIEVE cohort study, despite being associated with a significant higher odds of developing obesity, INSTI use was not associated with metabolic syndrome, or differences in glucose, LDL, or hypertension (22). A randomised switch study from TDF/FTC/NNRTI to ABC/3TC/DTG saw significant weight gain in the DTG arm, but no associated reduced insulin sensitivity or treatment-emergent metabolic syndrome (23). Similarly, in the ATHENA cohort, weight gain of ≥10% was observed following switch to INSTI and/or TAF, without significant changes in metabolic parameters (24). In the TANGO trial, switching from a 3-/4-drug TAF based regimen to DTG/3TC, resulted in improvements in metabolic parameters (25). No significant differences in odds of metabolic syndrome were seen between the treatment groups in this trial (25).
Case reports have described incidences of hyperglycaemia associated with INSTI use (26, 27, 28, 29). In some of these cases, the hyperglycaemia was independent of weight gain, and associated with ketoacidosis, suggestive that the mechanism of dysglycaemia may not be associated with obesity. Another study showed cases of hyperglycaemia in pre-treated patients independent of weight gain and associated with ketoacidosis (30). There is mixed evidence relating INSTIs to insulin resistance. Some studies have shown association with insulin resistance (31, 32) and small rises in glycated haemoglobin (33, 34). A recent analysis of the FDA Adverse Event Reporting System linked INSTIs to greater odds of hyperglycaemia or new onset diabetes (ROR: 2.16, CI: 1.96–2.38) (35). However, there is also evidence opposing the association of INSTIs to insulin resistance and diabetes (36, 37).
Previous analyses of the ADVANCE trial looking at 10-year risk of developing diabetes using the QDiabetes equation, a risk equation validated in populations of Black ethnicity, have shown that TAF/FTC + DTG was associated with greater risk of developing diabetes (38).
INSTIs have been associated with increases in fat gain and body circumference (39, 40, 41, 42, 43). In the ADVANCE trial, DTG, combined with TAF was associated with increases in both mass and volume of VAT compared with the TDF/FTC/DTG and the TDF/FTC + EFV arms (16). They have generally been found to be lipid neutral (44, 45, 46, 47).
In two observational studies, dolutegravir was not associated with a significant change in glucose metabolism (48, 49). However, both studies were based on follow-up to 48 weeks. Such short-term studies may not be able to demonstrate metabolic syndrome.
Strengths and limitations
A strength of this analysis is that both NAMSAL and ADVANCE were randomised controlled trials which included follow up data until week 192. Participants were recruited across different regions from HIV testing sites and hospitals which increases the generalisability of the analysis. A limitation is that in both trials, participants were lost to follow up to week 192.
Implications for the future
This paper highlights new evidence of metabolic syndrome, primarily driven by weight gain and obesity, associated with the use of INSTIs. There is currently limited data from other randomised trials, particularly with INSTI use. Metabolic syndrome has a multitude of adverse health consequences which must be monitored for. Clinicians initiating or monitoring patients on INSTI-based ART must counsel for lifestyle optimisation to prevent these effects. Close monitoring of glucose, blood pressure and lipids is essential, with prompt initiation of anti-diabetic medications, anti-hypertensives, and anti-cholesterol agents where required. For those who have developed clinical obesity, anti-obesity drugs should also be considered to enhance weight loss. These can be cheaply manufactured and serve as an alternative to bariatric surgery which may not be a feasible option in low- to middle-income countries.
Data availability statement
Data can be made available if needed. Requests to access these datasets should be directed to Manya Mirchandani manya.6269@gmail.com.
Ethics statement
The trial was approved by an institutional review board (the human research ethics committee at the University of the Witwatersrand) and received local regulatory approval. ADVANCE: https://www.nejm.org/doi/full/10.1056/NEJMoa1902824 Approval from the Cameroon National Ethics Committee was obtained in November 2015. NAMSAL: https://www.nejm.org/doi/full/10.1056/NEJMoa1904340. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ED, TT, CK, MM-E and AC contributed data from NAMSAL. FV, SS, GA and BB contributed data from ADVANCE. TP, BS, KM, CC, AH and MM performed the data analysis, writing and critiquing of the paper. All authors contributed to the article and approved the submitted version.
Funding
UNITAID.
Conflict of interest
FV reports receiving lecture fees and travel support from Roche, grant support, advisory board fees, and provision of drugs from Gilead Sciences, advisory board fees from ViiV Healthcare, lecture fees from Merck and Adcock Ingram, and lecture fees and advisory board fees from Johnson & Johnson and Mylan.
The reviewer NC declared a shared affiliation with the author(s) FV, SS, BB, GA, AT to the handling editor at the time of review.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Alberti G, Zimmet P, Shaw J, Grundy SM. The IDF consensus worldwide definition of the metabolic syndrome. Belgium: International Diabetes Federation (2006).
2. Fahed G, Aoun L, Bou Zerdan M, Allam S, Bou Zerdan M, Bouferraa Y, et al. Metabolic syndrome: updates on pathophysiology and management in 2021. Int J Mol Sci. (2022) 23(2):786. doi: 10.3390/ijms23020786
3. Dizaji BF. The investigations of genetic determinants of the metabolic syndrome. Diabetes Metab Syndr. (2018) 12(5):783–9. doi: 10.1016/j.dsx.2018.04.009
4. Matsuzawa Y, Funahashi T, Nakamura T. The concept of metabolic syndrome: contribution of visceral fat accumulation and its molecular mechanism. J Atheroscler Thromb. (2011) 18(8):629–39. doi: 10.5551/jat.7922
5. Todowede OO, Mianda SZ, Sartorius B. Prevalence of metabolic syndrome among HIV-positive and HIV-negative populations in sub-Saharan Africa—a systematic review and meta-analysis. Syst Rev. (2019) 8(1):4. doi: 10.1186/s13643-018-0927-y
6. Nguyen KA, Peer N, Mills EJ, Kengne AP. A meta-analysis of the metabolic syndrome prevalence in the global HIV-infected population. PLoS One. (2016) 11(3):e0150970. doi: 10.1371/journal.pone.0150970
7. Abraham Gnanadass S, Divakar Prabhu Y, Valsala Gopalakrishnan A. Association of metabolic and inflammatory markers with polycystic ovarian syndrome (PCOS): an update. Arch Gynecol Obstet. (2021) 303(3):631–43. doi: 10.1007/s00404-020-05951-2
8. Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol. (2013) 62(7):569–76. doi: 10.1016/j.jacc.2013.05.045
9. Prasad GVR. Metabolic syndrome and chronic kidney disease: current status and future directions. World J Nephrol. (2014) 3(4):210–9. doi: 10.5527/wjn.v3.i4.210
10. Dietrich P, Hellerbrand C. Non-alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Pract Res Clin Gastroenterol. (2014) 28(4):637–53. doi: 10.1016/j.bpg.2014.07.008
11. Swarup S, Goyal A, Grigorova Y, Zeltser R. Metabolic syndrome. In: Statpearls. Treasure Island, FL, United States: StatPearls Publishing (2022). Available from: http://www.ncbi.nlm.nih.gov/books/NBK459248/ (cited August 23, 2022)
12. Jericó C, Knobel H, Montero M, Ordoñez-Llanos J, Guelar A, Gimeno JL, et al. Metabolic syndrome among HIV-infected patients: prevalence, characteristics, and related factors. Diabetes Care. (2005) 28(1):132–7. doi: 10.2337/diacare.28.1.132
13. Sax PE, Erlandson KM, Lake JE, McComsey GA, Orkin C, Esser S, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis Off Publ Infect Dis Soc Am. (2020) 71(6):1379–89. doi: 10.1093/cid/ciz999
14. Shah S, Hindley L, Hill A. Are new antiretroviral treatments increasing the risk of weight gain? Drugs. (2021) 81(3):299–315. doi: 10.1007/s40265-020-01457-y
15. World Health Organisation. Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: Recommendations for a public health approach. Geneva: World Health Organisation (2021).
16. Venter WDF, Sokhela S, Simmons B, Moorhouse M, Fairlie L, Mashabane N, et al. Dolutegravir with emtricitabine and tenofovir alafenamide or tenofovir disoproxil fumarate versus efavirenz, emtricitabine, and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection (ADVANCE): week 96 results from a randomised, phase 3, non-inferiority trial. Lancet HIV. (2020) 7(10):E666–76. doi: 10.1016/S2352-3018(20)30241-1
17. NAMSAL ANRS 12313 Study Group, Kouanfack C, Mpoudi-Etame M, Omgba Bassega P, Eymard-Duvernay S, Leroy S, et al. Dolutegravir-based or low-dose efavirenz-based regimen for the treatment of HIV-1. N Engl J Med. (2019) 381(9):816–26. doi: 10.1056/NEJMoa1904340
18. Venter WDF, Moorhouse M, Sokhela S, Fairlie L, Mashabane N, Masenya M, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med. (2019) 381(9):803–15. doi: 10.1056/NEJMoa1902824
19. Hamooya BM, Mulenga LB, Masenga SK, Fwemba I, Chirwa L, Siwingwa M, et al. Metabolic syndrome in Zambian adults with human immunodeficiency virus on antiretroviral therapy. Medicine. (2021) 100(14):e25236. doi: 10.1097/MD.0000000000025236
20. Lake JE, Wu K, Bares SH, Debroy P, Godfrey C, Koethe JR, et al. Risk factors for weight gain following switch to integrase inhibitor-based antiretroviral therapy. Clin Infect Dis Off Publ Infect Dis Soc Am. (2020) 71(9):ciaa177. doi: 10.1093/cid/ciaa177
21. Calmy A, Tovar-Sanchez T, Kouanfack C, Mpoudi-Etame M, Leroy S, Perrineau S, et al. Dolutegravir-based and low-dose efavirenz-based regimen for the initial treatment of HIV-1 infection (NAMSAL): week 96 results from a two-group, multicentre, randomised, open label, phase 3 non-inferiority trial in Cameroon. Lancet HIV. (2020) 7(10):e677–87. doi: 10.1016/S2352-3018(20)30238-1
22. Kileel EM, Lo J, Malvestutto C, Fitch KV, Zanni MV, Fichtenbaum CJ, et al. Assessment of obesity and cardiometabolic status by integrase inhibitor use in REPRIEVE: a propensity-weighted analysis of a multinational primary cardiovascular prevention cohort of people with human immunodeficiency virus. Open Forum Infect Dis. (2021) 8(12):ofab537. doi: 10.1093/ofid/ofab537
23. Ibrahim F, Samarawickrama A, Hamzah L, Vincent R, Gilleece Y, Waters L, et al. Bone mineral density, kidney function, weight gain and insulin resistance in women who switch from TDF/FTC/NNRTI to ABC/3TC/DTG. HIV Med. (2021) 22(2):83–91. doi: 10.1111/hiv.12961
24. Verburgh ML, Wit FWNM, Boyd A, Verboeket SO, Reiss P, van der Valk M. One in 10 virally suppressed persons with HIV in The Netherlands experiences ≥10% weight gain after switching to tenofovir alafenamide and/or integrase strand transfer inhibitor. Open Forum Infect Dis. (2022) 9(7):ofac291. doi: 10.1093/ofid/ofac291
25. van Wyk J, Ait-Khaled M, Santos J, Scholten S, Wohlfeiler M, Ajana F, et al. Brief report: improvement in metabolic health parameters at week 48 after switching from a tenofovir alafenamide-based 3- or 4-drug regimen to the 2-drug regimen of dolutegravir/lamivudine: the TANGO study. J Acquir Immune Defic Syndr 1999. (2021) 87(2):794–800. doi: 10.1097/QAI.0000000000002655
26. Nolan NS, Adamson S, Reeds D, O’Halloran JA. Bictegravir-based antiretroviral therapy-associated accelerated hyperglycemia and diabetes mellitus. Open Forum Infect Dis. (2021) 8(5):ofab077. doi: 10.1093/ofid/ofab077
27. McLaughlin M, Walsh S, Galvin S. Dolutegravir-induced hyperglycaemia in a patient living with HIV. J Antimicrob Chemother. (2018) 73(1):258–60. doi: 10.1093/jac/dkx365
28. Rebeiro PF, Rebeiro PF, Jenkins C, Bian A, Lake J, Lake J, et al. LB9. The effect of initiating integrase inhibitor-based vs. non-nucleoside reverse transcriptase inhibitor-based antiretroviral therapy on progression to diabetes among North American persons in HIV care. Open Forum Infect Dis. (2019) 6(Suppl 2):S996–7. doi: 10.1093/ofid/ofz415.2492
29. Fong PS, Flynn DM, Evans CD, Korthuis PT. Integrase strand transfer inhibitor-associated diabetes mellitus: a case report. Int J STD AIDS. (2017) 28(6):626–8. doi: 10.1177/0956462416675107
30. Lamorde M, Atwiine M, Owarwo NC, Ddungu A, Laker EO, Mubiru F, et al. Dolutegravir-associated hyperglycaemia in patients with HIV. Lancet HIV. (2020) 7(7):E461–2. doi: 10.1016/S2352-3018(20)30042-4
31. Dirajlal-Fargo S, Moser C, Brown TT, Kelesidis T, Dube MP, Stein JH, et al. Changes in insulin resistance after initiation of raltegravir or protease inhibitors with tenofovir-emtricitabine: AIDS clinical trials group A5260s. Open Forum Infect Dis. (2016) 3(3):ofw174. doi: 10.1093/ofid/ofw174
32. Gianotti N, Muccini C, Galli L, Poli A, Spagnuolo V, Andolina A, et al. Homeostatic model assessment for insulin resistance index trajectories in HIV-infected patients treated with different first-line antiretroviral regimens. J Med Virol. (2019) 91(11):1937–43. doi: 10.1002/jmv.25541
33. Aldredge A, Lahiri CD, Summers NA, Mehta CC, Angert CD, Kerchberger AM, et al. 980. Effects of integrase strand-transfer inhibitor use on lipids, glycemic control, and insulin resistance in the women’s interagency HIV study (WIHS). Open Forum Infect Dis. (2019) 6(Supplement_2):S38. doi: 10.1093/ofid/ofz359.082
34. Summers NA, Lahiri CD, Angert CD, Aldredge A, Mehta CC, Ofotokun I, et al. Metabolic changes associated with the use of integrase strand transfer inhibitors among virally-controlled women. J Acquir Immune Defic Syndr 1999. (2020) 85(3):355–62. doi: 10.1097/QAI.0000000000002447
35. Murray MM, Harpe SE. 106. META-INSTI: metabolic adverse events following integrase strand transfer inhibitor administration in spontaneous adverse event reports. Open Forum Infect Dis. (2020) 7(Suppl 1):S182. doi: 10.1093/ofid/ofaa439.416
36. Calza L, Colangeli V, Borderi M, Coladonato S, Tazza B, Bon I, et al. Improvement in insulin sensitivity and serum leptin concentration after the switch from a ritonavir-boosted PI to raltegravir or dolutegravir in non-diabetic HIV-infected patients. J Antimicrob Chemother. (2019) 74(3):731–8. doi: 10.1093/jac/dky507
37. Lo J, Oyee J, Crawford M. Dolutegravir and insulin resistance. Conference on retroviruses and opportunistic infections; Seattle, USA (2019).
38. McCann K, Shah S, Hindley L, Hill A, Qavi A, Simmons B, et al. Implications of weight gain with newer antiretrovirals: 10-year predictions of cardiovascular disease and diabetes. AIDS Lond Engl. (2021) 35(10):1657–65. doi: 10.1097/QAD.0000000000002930
39. Lennox JL, Dejesus E, Berger DS, Lazzarin A, Pollard RB, Ramalho Madruga JV, et al. Raltegravir versus efavirenz regimens in treatment-naive HIV-1-infected patients: 96-week efficacy, durability, subgroup, safety, and metabolic analyses. JAIDS J Acquir Immune Defic Syndr. (2010) 55(1):39–48. doi: 10.1097/QAI.0b013e3181da1287
40. Reynes J, Trinh R, Pulido F, Soto-Malave R, Gathe J, Qaqish R, et al. Lopinavir/ritonavir combined with raltegravir or tenofovir/emtricitabine in antiretroviral-naive subjects: 96-week results of the PROGRESS study. AIDS Res Hum Retroviruses. (2013) 29(2):256–65. doi: 10.1089/aid.2011.0275
41. McComsey GA, Moser C, Currier J, Ribaudo HJ, Paczuski P, Dubé MP, et al. Body composition changes after initiation of raltegravir or protease inhibitors: ACTG A5260s. Clin Infect Dis Off Publ Infect Dis Soc Am. (2016) 62(7):853–62. doi: 10.1093/cid/ciw017
42. Vizcarra P, Vivancos MJ, Pérez-Elías MJ, Moreno A, Casado JL. Weight gain in people living with HIV switched to dual therapy: changes in body fat mass. AIDS. (2020) 34(1):155–7. doi: 10.1097/QAD.0000000000002421
43. Debroy P, Sim M, Erlandson KM, Falutz J, Prado CM, Brown TT, et al. Progressive increases in fat mass occur in adults living with HIV on antiretroviral therapy, but patterns differ by sex and anatomic depot. J Antimicrob Chemother. (2019) 74(4):1028–34. doi: 10.1093/jac/dky551
44. Giacomet V, Lazzarin A, Manzo A, Longoni E, Maruca K. Body fat and lipid profile changes in HIV-infected youths switched to dolutegravir. In (2020).
45. Taramasso L, Bonfanti P, Ricci E, Orofino G, Squillace N, Menzaghi B, et al. Factors associated with weight gain in people treated with dolutegravir. Open Forum Infect Dis. (2020) 7(6):ofaa195. doi: 10.1093/ofid/ofaa195
46. Zimmerman M, DeSimone J, Schafer JJ. 332. Exploring the prevalence and characteristics of weight gain and other metabolic changes in patients with HIV infection switching to integrase inhibitor containing ART. Open Forum Infect Dis. (2019) 6(Supplement_2):S176–7. doi: 10.1093/ofid/ofz360.405
47. Ramgopal M, Maggiolo F, Ward D, Lebouche B, Rizzardini G, Molina JM, et al. Pooled analysis of 4 international trials of bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF) in adults aged >65 or older demonstrating safety and efficacy: week 48 results. In San Francisco, USA, abstract OAB0403, (2020).
48. Mulindwa F, Castelnuovo B, Brusselaers N, Nabwana M, Bollinger R, Laker E, et al. Dolutegravir use over 48 weeks is not associated with worsening insulin resistance and pancreatic beta cell function in a cohort of HIV-infected Ugandan adults. (2023).
Keywords: metabolic syndrome, cardiovascular, antriretroviral medication, tenoforvir disoproxil fumarate, tenofovir alafenamide
Citation: Tovar Sanchez T, Mpoudi-Etame M, Kouanfack C, Delaporte E, Calmy A, Venter F, Sokhela S, Bosch B, Akpomiemie G, Tembo A, Pepperrell T, Simmons B, Casas CP, McCann K, Mirchandani M and Hill A (2023) Risks of metabolic syndrome in the ADVANCE and NAMSAL trials. Front. Reprod. Health 5:1133556. doi: 10.3389/frph.2023.1133556
Received: 29 December 2022; Accepted: 1 September 2023;
Published: 18 September 2023.
Edited by:
Mohammed Lamorde, Makerere University, UgandaReviewed by:
Christina Bothou, University Hospital Zurich, SwitzerlandNomathemba Chandiwana, University of the Witwatersrand, South Africa
Barbara Castelnuovo, Makerere University, Uganda
© 2023 Tovar Sanchez, Mpoudi-Etame, Kouanfack, Delaporte, Calmy, Venter, Sokhela, Bosch, Akpomiemie, Tembo, Pepperrell, Simmons, Casas, Mccann, Mirchandani and Hill. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrew Hill microhaart@aol.com
 Tamara Tovar Sanchez1
Tamara Tovar Sanchez1  Mireille Mpoudi-Etame
Mireille Mpoudi-Etame Eric Delaporte
Eric Delaporte Angela Tembo
Angela Tembo Manya Mirchandani
Manya Mirchandani