Oral Chronic Graft-Versus-Host Disease
- 1Department of Oral Medicine, University of Washington/Seattle Cancer Care Alliance, Seattle, WA, United States
- 2Division of Oral Medicine and Dentistry, Brigham and Women's Hospital and Dana Farber Cancer Institute Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, United States
Chronic oral graft-versus-host disease (cGVHD) is a complex, frequent, and highly impactful complication of allogeneic hematopoietic cell transplantation (alloHCT). It represents the leading cause of morbidity and mortality in long-term alloHCT survivors. cGVHD can affect almost any visceral organ system and commonly affects the skin, eyes and mouth, manifesting with signs and symptoms similar to other known immune-mediated and autoimmune diseases. Oral manifestations of GVHD include inflammation, thinning, and ulceration of oral mucosal tissues (similar to lichen planus), lymphocyte-mediated salivary gland dysfunction (similar to Sjögren/Sicca Syndrome), and decreased oral opening (trismus) secondary to sclerosis of oral and perioral tissues (analogous to limitation in scleroderma). Potential sequelae include severe mucosal pain, compromised nutrition, weight loss, limitation in opening, and sometimes irreversible fibrosis of the salivary glands. While some cases can be managed with topical therapies, management may also require long-term targeted immunosuppressive and/or corticosteroid therapy with associated risk of local and systemic infection, hyperglycemia, kidney dysfunction, osteopenia/osteoporosis, and possibly secondary malignancies. The aim of this mini-review is to provide an up-to-date review of literature related to the diagnosis and management of oral cGVHD to aid dental and medical clinicians in optimizing oral cGVHD therapy while minimizing potential adverse effects.
Introduction
Chronic graft-versus-host disease (cGVHD) is a common, pleotropic disorder with distinct manifestations throughout the body. cGVHD is diagnosed in 30–50% of allogeneic hematopoietic cell transplantation (alloHCT) recipients with more than 90% diagnosed within 12 months [1–3]. Incidence is increasing due to greater frequency of alloHCT, improved survivorship, and trends in donor selection, graft source, and other factors [2, 4]. It represents the leading cause of morbidity and mortality in long-term survivors otherwise in remission from their hematological disease [5–11].
Oral cGVHD
Oral cGVHD is characterized by lichenoid mucositis, immune-mediate salivary dysfunction, and tissue sclerosis. Recent studies suggest that each represents a discrete clinical entity with little interrelationship [12, 13]. Though oral cGVHD is not independently associated with mortality, it may cause significant morbidity, making oral therapy an important component in comprehensive management [13–18].
Symptoms in mucosal cGVHD range from asymptomatic lichenoid changes to severely painful ulcerations which can be disabling (Figures 1A,B). Often minimal pain is reported at rest, though thinning and ulceration of the oral mucosa regularly cause sensitivity to previously tolerated stimuli [19, 20]. Common triggers include acidic, spicy or highly seasoned foods, carbonated beverages, alcohol/alcohol-based products, and flavoring agents such as mint in toothpaste [14, 19–22]. Tissue irritation may compromise nutrition, mastication, speech, swallowing, social interactions, and ability to perform effective oral hygiene, particularly when salivary dysfunction is also present [13, 23]. Mucosal ulceration compromises barrier function increasing risk for oral-sourced bacteremia [24].
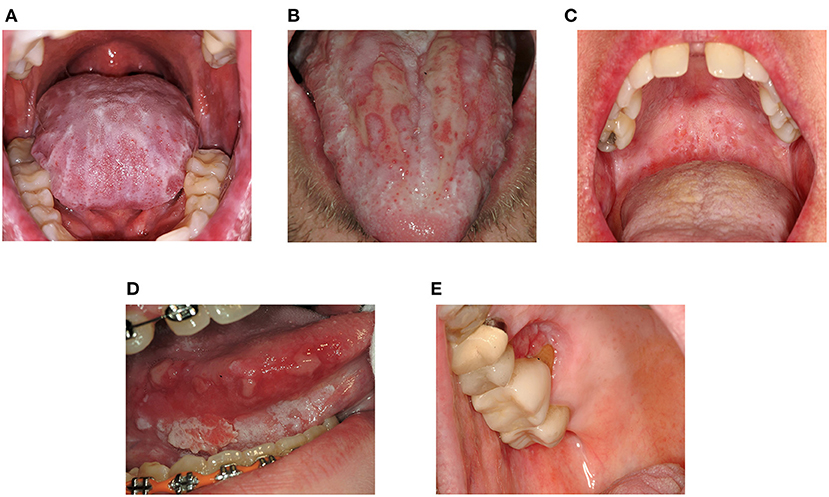
Figure 1. Clinical features of oral mucosal cGVHD and Oral Squamous Cell Carcinoma. (A) Dense lichenoid reticulations involving the dorsal tongue (B) Pseudomembrane-covered ulcerations of the dorsal tongue surrounded by lichen-like changes (lichenoid hyperkeratosis) (C) Superficial mucoceles of the left soft palate. Note the prominent minor salivary glands and thin lichenoid striations affecting the hard and soft palates (D) Squamous cell carcinoma of the right ventrolateral tongue in a patient with longstanding oral cGVHD (E) Squamous cell carcinoma of the right hard palate at a site of persistent cGVHD involvement.
Oral health and health-related quality of life are further impaired by immune-mediated salivary dysfunction which is especially impactful [12, 13]. Dysfunction is associated with xerostomia (the subjective impression of dry mouth), increased adherence of bacterial plaque and debris, difficulty swallowing, and decreased ability to clear viscous secretions. Qualitative and quantitative changes in saliva increase susceptibility to dental caries, oral candidiasis, and mucosal breakdown [25–28]. Dry mouth exacerbates mucosal symptoms while independently decreasing quality of life [13]. Mucoceles, dome-shaped fluid-filled “blisters” arising from minor salivary glands, are also common in cGVHD, though not specific to the disease [19, 22] (Figure 1C).
Function may also be limited by trismus resulting from oral sclerosis which has been described as a late effect of oral cGVHD. Pathophysiology is not well-understood [14, 20, 29]. Recent work found association between limited mouth opening and skin sclerosis, but not lichenoid mucositis suggesting a cutaneous rather than mucosal process [12]; however, limitation may be multifactorial as chronic inflammation can cause mucosal scarring [19, 22]. Trismus can impact nutrition, oral hygiene, and ability to comfortably complete dental procedures [22].
Taste-alterations have been described [30–32].
Diagnosis and Staging of cGVHD
In 2014 the NIH Diagnosis and Staging Working Group revised standards established in 2005 [33] to clarify enrollment criteria for clinical trials, align disease staging with treatment prognosis, and aid in treatment selection [34]. Diagnostic criteria were updated for the skin, mouth, lungs, and genitalia and organ severity scores revised in eight organs to improve global severity scoring. Diagnostic and distinctive features are defined for each system with diagnosis confirmed by the presence of one diagnostic feature, or one distinctive feature supported by a confirmatory test (e.g., biopsy). Conversely, acute GVHD, which was initially defined by time of occurrence (<100 days post-alloHCT), is now diagnosed and staged based on rash, total bilirubin elevation, and diarrhea. Overlap of acute and chronic GVHD can be seen and may relate to worse clinical outcome [3]. Though diagnostic criteria are foundational, some patients with equivocal diagnoses may also require therapy to minimize adverse effects of alloimmunity [3].
Diagnosis of Oral cGVHD
The mouth is commonly affected by cGVHD with up to 83% of cGVHD patients meeting diagnostic criteria [35, 36]. Oral cGVHD may co-occur with disease in other visceral organs or present as the initial or only site of involvement [14, 16, 22, 37]. The high incidence and ease of oral examination may aid in diagnosis of emerging disease [37]. Diagnosis is based on visual examination supplemented by history and global health status [14, 19]. Pathophysiology of oral cGVHD is not fully characterized, though findings of inflammation and fibrosis mirror other systems [3, 14]. Lichen planus-like changes are diagnostic and do not require confirmational biopsy. They may present as Wickham striae, lichenoid patches, or plaques. The 2014 NIH criteria replaced the term “hyperkeratotic plaques” [33] with “lichen-like changes” to differentiate from other causes of oral hyperkeratosis which may be reactive (frictional or chemical induced), infectious (pseudomembranous and hyperplastic candidiasis), or potentially malignant (idiopathic leukoplakia) [34]. Distinguishing cGVHD from idiopathic leukoplakia is especially important given increased risk of oral malignancy after alloHCT [38–43].
Distinctive features include mucosal atrophy, pseudomembranes, ulcers, mucoceles, and xerostomia. Distinctive features must be differentiated from other conditions common in the AlloHCT population including candidiasis, recurrent herpetic infection, drug reaction, mucosal trauma, recurrent or primary malignancy, and salivary dysfunction secondary to xerogenic medications and/or polypharmacy [19, 29, 34]. Biopsy, culture, viral PCR, and sialometry may be valuable in confirming diagnosis [32]. When cGVHD is suspected, biopsy should be obtained from non-ulcerated tissue and reviewed by an experienced pathologist [22, 44].
Severity Scoring and Response Criteria
Severity scores are used to quantify organs affected by cGVHD and resulting level of functional impairment [34]. Global and organ-specific scores are used in treatment planning which is strongly influenced by extent and severity of disease [34, 45, 46]. Topical and local therapies are favored in mild cases (confined to ≤ 2 non-respiratory organs each with a maximum score of 1) [3, 45] while systemic therapy is often required in moderate to severe disease. The NIH Global Severity Score is a simple instrument that can be used by non-specialists to assess functional impact of cGVHD [34]. Eight organs are scored from 0 to 3 with higher scores indicating greater disability [34, 36]. An oral score of 1 reflects disease that is not significantly impacting nutritional intake while a 3 indicates major dietary limitations caused by oral symptoms [34]. Asymptomatic oral lichenoid changes do not impact global scoring as they do not affect nutrition [34]. Patients should also rate their peak sensitivity (i.e., irritation from normally tolerated stimuli) over the past week using a 0 to 10 scale either alone or in combination with the oral questions on the Lee cGVHD symptom scale [46, 47].
Organ-specific response criteria are intended for use by specialists to capture higher level detail [46]. The preferred oral instrument is the NIH Modified Oral Mucosa Score (OMRS) which assigns scores for mucosal erythema (0–3), lichen-like changes (0–3), and tissue ulceration [0–6] based on severity and surface area affected. Final scores range from 0 to 12 with scores of ≥2 representing clinically significant disease. Score change of ≥2 indicates disease progression (if increasing) or response to therapy (if decreasing) [46, 48, 49]. Mucoceles have been removed from the scale due to challenges in reporting and lack of correlation with clinical outcomes [18, 46, 50, 51]. Lichenoid changes, erythema, and symptoms scores are most strongly associated with perceived change in disease status [37].
Prophylaxis and Management
GVHD prophylaxis and treatment are complex and determined by extent of disease, co-occurrence of acute and chronic GVHD, degree of functional impairment, and patient specific factors including likelihood of end organ damage [45, 46, 52]. Therapy must balance GVHD-associated morbidity against the benefit of the graft-versus-leukemia (GVL) effect which decreases risk for relapse [53–55]. Standard prophylaxis includes a calcineurin inhibitor and an antimetabolite (most commonly methotrexate) [45]. Systemic corticosteroids, specifically prednisone 1 mg/kg, is well-established as first-line therapy [3, 45], though mild localized disease may be limited to topical or local corticosteroids. Addition of a non-steroidal agent in initial therapy has not shown additional benefit in patients with standard risk GVHD [56–58]. Non-steroidal immunomodulatory medication should be added in in severe GVHD to limit end organ damage and adverse effects associated with extended systemic corticosteroid use [45, 59]. Second-line therapy varies widely. Choice is often center-specific as studies comparing relative effectiveness are lacking [45]. Sirolimus, tacrolimus, cyclosporine, mycophenolate mofetil, pentostatin, and extracorporeal photopheresis are commonly used off-label [52]. The FDA has only been recently approved ibrutinib (2017) [60], ruxolitinib (2021) [61] and belumosudil (2021) [62] in the treatment of cGVHD.
While systemic immunomodulating medications are essential in treatment, they are associated with a variety of adverse effects including impaired immune function, decreased bone density, diabetes, renal dysfunction, neurologic side effects, and in some cases secondary malignancy [32, 63]. Risk mitigation is critical as most patients require systemic therapy beyond 2 years with up to 15% extending past 7 years [14, 56, 64]. Optimizing non-systemic therapies may help to limit prolonged use, particularly when the oral cavity is the primary site of involvement [16, 23, 32, 65, 66].
Oral Mucosal cGVHD
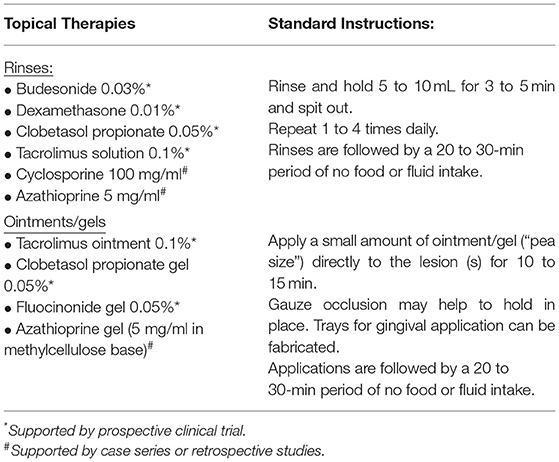
Topical therapies are the cornerstone of oral cGVHD management and are valuable even when systemic treatment is required as combined therapy has greater effect than systemic alone [32, 65–68] (Table 1). Furthermore, the mouth is one of few organs in which aggressive topical therapy may successfully manage moderate-to-severe disease [65]. Oral cGVHD should be treated when there is loss of barrier function and/or when oral sensitivity is negatively affecting quality of life [14, 20, 32, 67]. Asymptomatic lichenoid changes do not require therapy as treating to “disease resolution” has limited clinical benefit. This practice is consistent with treatment approaches in other organs [69]. Data from cGVHD Consortium sites has confirmed that treatment behavior follows these recommendations with topical therapies more likely to be used in patients reporting pain and decreased oral function [70]. Follow-up is recommended to confirm symptom control and restoration of mucosal integrity. Therapy should be tapered over time to the lowest frequency (and potency) required to maintain effectiveness in symptomatic mitigation [16, 23].
Medication selection is based on the extent of oral lesions, medication potency, cost, availability, and patient preference [19, 23]. There are currently no FDA-approved topical therapies for oral cGVHD, though corticosteroids and calcineurin-inhibitors are regularly used in practice. Rinses are recommended when lesions are widespread to facilitate application to all sites. Localized lesions may be treated with higher potency gels or ointments which can be applied under gauze occlusion to maximize local effect. Numerous topical steroids, topical non-steroidal agents (e.g., tacrolimus [71–75], cyclosporine [76], sirolimus [77], azathioprine [78, 79], thalidomide [80]), and phototherapies (Photobiomodulation [81], PUVA [82, 83], UVB [84]) have been used in clinical practice and excellent evidence summaries have been previously published in national and international consensus documents [32, 65, 85, 86] and comprehensive reviews [14, 22, 66]. Surveys indicates that over 90% of specialists initially favor topical steroids with tacrolimus the preferred second-line alternative [87]. Among topical therapies only clobetasol, dexamethasone, tacrolimus solution and budesonide effervescence tablets have been analyzed in randomized trials [68, 72, 88]. Evidence-based practice is hindered by availability as only dexamethasone and prednisolone solutions are commercially manufactured in the United States [23, 65]. Other agents may be compounded; however, this increases cost to the patient as compounded medications are unlikely to be covered by insurance [23].
Topical steroids are generally well-tolerated, but patients must be monitored for potential adverse effects. Secondary candidiasis with topical steroids is not uncommon and [63, 88, 89] and prophylactic antifungal coverage is often used in clinical practice [16, 65, 87]. Risk factors for oral candidiasis, including immunosuppression, altered quantity and composition of saliva, and the use of medications that alter the normal oral flora (e.g., antibiotics, steroid inhalers), are common in people living with cGVHD. Candidiasis should therefore be considered when presumed oral cGVHD is not responsive to topical steroids. Whereas systemic uptake of tacrolimus has been described in case series and blood levels should be periodically monitored to rule it out [75]. Limited data is available for topical steroid absorption in the cGVHD population. Nonetheless patients should be monitored for cushingoid features or other signs of adrenal suppression [22]. The best available evidence is a study of 62 patients with severe erosive lichen planus treated with clobetasol propionate 0.05% rinse (10 mL for 5 min TID for 2 to 6 weeks based on response). Plasma cortisol levels showed signs of suppression in 85.5% of patients during initial therapy with only 4% of patients effected in the maintenance phase (suggesting lower systemic absorption after mucosal integrity is reestablished [90]. There were no major adverse events and dose reduction was effective in reversing cushingoid features and capillary fragility. Topical budesonide has been proposed as a preferred alternative for extended use due to low transmucosal absorption and poor systemic bioavailability [68].
Topical therapies may also be useful adjuncts in lesion assessment, though biopsy is required for definitive diagnosis. For example, short (e.g., 2 week) therapeutic trials of high potency topical steroids have been recommended in differentiation of lichenoid hyperkeratosis from leukoplakia. Immune-mediated lesions, such as cGVHD, are likely to respond to topical therapy while oral potentially malignant lesions will not. Non-responsive lesions should be biopsied to rule out epithelial dysplasia and/or squamous cell carcinoma [32, 65]. Intralesional injections with triamcinolone acetonide (40 mg/mL) have also shown value in treatment of persistent oral ulcerations which must also be differentiated from oral malignancy [91].
Salivary Dysfunction
Treatment of salivary dysfunction focuses on reestablishing oral lubrication to improve comfort and function while simultaneously minimizing risk of dental sequelae related to hyposalivation. Patients are encouraged to take frequent sips of water to moisten the mouth and maintain hydration. Liquid intake during mealtime, or when swallowing medications, can help to limit dysphagia if swallowing function is otherwise normal. Sugar-free candy, mints, and chewing gum can provide gustatory and masticatory stimulation to glands to increase salivary flow during the day [92]. All are inexpensive and widely available without a prescription. Normal saline rinses, over-the-counter coating agents, in the form of rinses, sprays, and gels, and artificial saliva may temporarily reduce xerostomia, but must be regularly reapplied [16, 32, 65]. Adhesive tablets, such as XyliMelts®, slowly dissolve over time while simultaneously stimulating flow. They may be especially useful during sleep along with other longer lasting agents (e.g., gels, oil-based products).
Systemic sialagogues are commonly used off-label in the treatment of severe cGVHD-related salivary dysfunction. Pilocarpine is FDA-approved for the treatment of radiation-induced dry mouth in head and neck cancer patients and the treatment of dry mouth and dry eyes in Sjögren syndrome, while Cevimeline is approved in Sjögren syndrome only. Data in the cGVHD population is limited [93, 94]. Daily use of pilocarpine has been associated with increased salivary output, improved oral function, and restoration of normal sialometric properties, though data is limited to one randomized trial and several open enrollment studies [95–98]. Cevimeline has shown similar safety and efficacy in other populations [99, 100], and case series suggest it is an effective alternative in cGVHD [101]. A survey of practitioners in specialty health centers confirmed pilocarpine as the most common first-line therapy for salivary dysfunction in cGVHD (41.7%) with saliva substitutes favored as first-line palliative therapy [102]. The FDA cites hypersensitivity, uncontrolled asthma, acute iritis, and narrow angle glaucoma as contraindications [103] while consensus guidelines in cGVHD also advise against use in patients with cardiac disease and obstructive pulmonary disease (including pulmonary GVHD) [65]. Preexisting pulmonary and gastrointestinal GVHD may be exacerbated due to increase in bronchial and gastric secretions [16, 19, 87]. Sweating and flushing, commonly reported in other populations, is reported to be uncommon in the cGVHD population [19]. Titration of the medication over 2 weeks can be helpful in mitigating this effect when present. Side effect profiles are similar, and choice may be based on relative out of pocket cost [19].
Oral Sclerosis
Stretching and physical therapy are the most common therapies for oral and perioral sclerosis. Use of long-term, sequential intralesional steroid injections have also been described [65].
Palliative and Ancillary Therapy
During active therapy, palliative and ancillary therapies can be helpful to decrease pain, mucosal trauma, secondary infection, and dental sequelae. Dietary modifications, favoring bland and soft foods, and adjustments to oral hygiene practices (e.g., soft bristle toothbrush, non-mint and sodium lauryl sulfate-free toothpaste, non-alcohol-based rinses) can help to decrease mucosal sensitivity [19, 20]. Topical adhesive agents, such as ZilactinB® or Orabase®, can be applied to localized ulcerations to decrease pain and recurrent trauma while occlusal guards or Essix retainers can minimize frictional irritation from sharp or malpositioned teeth. Effective plaque control and judicious use of topical antimicrobials, such as chlorhexidine gluconate, can decrease gingivitis improving gingival comfort and decreasing risk for secondary infection [20].
Topical anesthetics, such as lidocaine or benzocaine, are recommended if soft tissue pain is limiting ability to eat or perform effective oral hygiene. Numerous preparations are available, though 2% viscous lidocaine is favored in consensus documents [32, 65, 67]. Care should be taken to avoid trauma after application, particularly in pediatric patients [65]. Gargling and swallowing should be avoided to decrease risk for aspiration [65]. Studies in oral mucositis patients have shown low systemic absorption and risk is low with oral application when used as directed [104]. Methemoglobinemia has been described after application to large mucosal surfaces (e.g., bronchoscopy) and with excessive oral use [105–108].
Late Oral Complications
Dental Caries
Patients with salivary cGVHD are at high risk for dental caries. Extensive caries has been described within 2 years of diagnosis [27]. Prevention is paramount as progressive caries increases risk for oral-source infection, the need for invasive dental procedures, and overall cost of care. Dietary counseling is recommended to limit refined sugar and other fermentable carbohydrates [109, 110]. Daily application of prescription fluoride gel is recommended and may be supplemented by professional fluoride application and shorter recall intervals in cases of severe hyposalivation [20, 32, 65, 87].
Second Malignancy
NIH consensus recommendations call for annual oral examinations in AlloHCT patients surviving beyond 1 year [111] due to increased risk for oral cancer [39–43, 112] (Figures 1D,E). Elevated risk for oral squamous cell carcinoma in oral cGVHD patients has prompted other groups to recommend more frequent assessment [87] analogous to other oral premalignant disorders [113]. Long-term follow-up is essential as the risk increases over time [114–117].
Summary
Oral cGVHD is a common complication of AlloHCT associated with decreased quality of life. Oral cGVHD includes three distinct manifestations: lichenoid mucositis, salivary gland dysfunction, and tissue sclerosis resulting in trismus. Complications include tissue sensitivity, loss of mucosal integrity, infection risk, xerostomia, and compromised oral function. Topical, intralesional, palliative, and ancillary interventions are essential in managing these complications and may help to limit prolonged use of systemic immunomodulatory agents. Long-term follow-up is essential due to elevated risk for oral cancer which increases with time.
Author Contributions
DD drafted and critically revised the manuscript. HS critically revised the manuscript. All authors give final approval and agree to be accountable for all aspects of the work.
Funding
Research reported in this publication was supported by the National Institute of Dental and Craniofacial Research of the National Institutes of Health under award number R01DE028336 which funded the entirety of the work. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lee SJ, Flowers M. Recognizing and managing chronic graft-versus-host disease hematology. Am Soc Hematol Educ Program. (2008) 2008:134–41. doi: 10.1182/asheducation-2008.1.134
2. Arora M, Cutler CS, Jagasia MH, Pidala J, Chai X, Martin PJ, et al. Late acute and chronic graft-versus-Host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. (2016) 22:449–55. doi: 10.1016/j.bbmt.2015.10.018
3. Lee SJ. Classification systems for chronic graft-versus-host disease. Blood. (2017) 129:30–7. doi: 10.1182/blood-2016-07-686642
4. Arai S, Arora M, Wang T, Spellman SR, He W, Couriel DR, et al. Increasing incidence of chronic graft-versus-host disease in allogeneic transplantation: a report from the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. (2015) 21:266–74. doi: 10.1016/j.bbmt.2014.10.021
5. Fraser CJ, Bhatia S, Ness K, Carter A, Francisco L, Arora M, et al. Impact of chronic graft-versus-host disease on the health status of hematopoietic cell transplantation survivors: a report from the bone marrow transplant survivor study. Blood. (2006) 108:2867–73. doi: 10.1182/blood-2006-02-003954
6. Pidala J, Kurland B, Chai X, Majhail N, Weisdorf DJ, Pavletic S, et al. Patient–reported quality of life is associated with severity of chronic graft-versus-host disease as measured by NIH criteria: report on baseline data from the chronic GVHD consortium. Blood. (2011) 117:4651–7. doi: 10.1182/blood-2010-11-319509
7. Bhatia S, Dai C, Landier W, Hageman L, Wu J, Schlichting E, et al. Trends in late mortality and life expectancy after allogeneic blood or marrow transplantation over 4 decades: a Blood or marrow transplant survivor study report. JAMA Oncol. (2021) 7:1626–34. doi: 10.1001/jamaoncol.2021.3676
8. Socie G, Stone JV, Wingard JR, Weisdorf D, Henslee-Downey PJ, Bredeson C, et al. Long-term survival and late deaths after allogeneic bone marrow transplantation. late effects working committee of the international bone marrow transplant registry. N Engl J Med. (1999) 341:14–21. doi: 10.1056/NEJM199907013410103
9. Wingard JR, Majhail NS, Brazauskas R, Wang Z, Sobocinski KA, Jacobsohn D, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. (2011) 29:2230–9. doi: 10.1200/JCO.2010.33.7212
10. Goldman JM, Majhail NS, Klein JP, Wang Z, Sobocinski KA, Arora M, et al. Relapse and late mortality in 5–year survivors of myeloablative allogeneic hematopoietic cell transplantation for chronic myeloid leukemia in first chronic phase. J Clin Oncol. (2010) 28:1888–95. doi: 10.1200/JCO.2009.26.7757
11. Martin PJ, Counts GW, Jr Appelbaum FR, Lee SJ, Sanders JE, Deeg HJ, et al. Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. J Clin Oncol. (2010) 28:1011–6. doi: 10.1200/JCO.2009.25.6693
12. Bassim CW, Fassil H, Mays JW, Edwards D, Baird K, Steinberg SM, et al. Oral disease profiles in chronic graft versus host disease. J Dent Res. (2015) 94:547–54. doi: 10.1177/0022034515570942
13. Imanguli MM, Atkinson JC, Mitchell SA, Avila DN, Bishop RJ, Cowen EW, et al. Salivary gland involvement in chronic graft-versus-host disease: prevalence, clinical significance, and recommendations for evaluation. Biol Blood Marrow Transplant. (2010) 16:1362–9. doi: 10.1016/j.bbmt.2010.03.023
14. Fall–Dickson JM, Pavletic SZ, Mays JW, Schubert MM. Oral complications of chronic graft-versus-Host disease. J Natl Cancer Inst Monogr. (2019) 2019:53. doi: 10.1093/jncimonographs/lgz007
15. Lynch Kelly D, Lyon DE, Ameringer SA, Elswick R, Symptoms K. cytokines and quality of life in patients diagnosed with chronic graft-versus-host disease following allogeneic hematopoietic stem cell transplantation. Oncol Nurs Forum. (2015) 42:265–75. doi: 10.1188/15.ONF.265-275
16. Hull K, Kerridge I, Avery S, McCullough M, Ritchie D, Szer J. Oral chronic graft-versus-host disease in Australia: clinical features challenges in management. Intern Med J. (2015)45:702–10. doi: 10.1111/imj.12812
17. Fall–Dickson JM, Mitchell SA, Marden S, Ramsay ES, Guadagnini JP, Wu T, et al. Oral symptom intensity, health-related quality of life, and correlative salivary cytokines in adult survivors of hematopoietic stem cell transplantation with oral chronic graft-versus-host disease. Biol Blood Marrow Transplant. (2010) 16:948–56. doi: 10.1016/j.bbmt.2010.01.017
18. Bassim CW, Fassil H, Mays JW, Edwards D, Baird K, Steinberg SM, et al. Validation of the national institutes of health chronic GVHD oral mucosal score using component–specific measures. Bone Marrow Transplant. (2014) 49:116–21. doi: 10.1038/bmt.2013.137
19. Treister N, Duncan C, Cutler C, Lehmann L. How we treat oral chronic graft-versus-host disease. Blood. (2012) 120:3407–18. doi: 10.1182/blood-2012-05-393389
20. Schubert MM, Correa ME. Oral graft-versus-host disease. Dent Clin North Am. (2008) 52:79–109. doi: 10.1016/j.cden.2007.10.004
21. Treister NS, Woo SB, O'Holleran EW, Lehmann LE, Parsons SK, Guinan EC. Oral chronic graft-versus-host disease in pediatric patients after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. (2005) 11:721–31. doi: 10.1016/j.bbmt.2005.06.002
22. Mays JW, Fassil H, Edwards DA, Pavletic SZ, Bassim CW. Oral chronic graft-versus-host disease: current pathogenesis, therapy, and research. Oral Dis. (2013) 19:327–46. doi: 10.1111/odi.12028
23. Elad S, Zinchuk K, Li S, Cutler C, Liesveld J, Treister N. Economic and practical considerations in the treatment of oral mucosal chronic graft-versus-host disease. Biol Blood Marrow Transplant. (2018) 24:1748–53. doi: 10.1016/j.bbmt.2018.02.022
24. Laheij AM, de Soet JJ, von dem Borne PA, Kuijper EJ, Kraneveld EA, van Loveren C, et al. Oral bacteria and yeasts in relationship to oral ulcerations in hematopoietic stem cell transplant recipients. Support Care Cancer. (2012) 20:3231–40. doi: 10.1007/s00520-012-1463-2
25. Nagler RM, Nagler A. Salivary gland involvement in graft-versus-host disease: the underlying mechanism implicated treatment. Isr Med Assoc J. (2004)6:167–72.
26. Nagler RM, Nagler A. Major salivary gland involvement in graft-versus-host disease: considerations related to pathogenesis, the role of cytokines and therapy. Cytokines Cell Mol Ther. (1999) 5:227–32.
27. Castellarin P, Stevenson K, Biasotto M, Yuan A, Woo SB, Treister NS. Extensive dental caries in patients with oral chronic graft-versus-host disease. Biol Blood Marrow Transplant. (2012) 18:1573–9. doi: 10.1016/j.bbmt.2012.04.009
28. Piccin A, Tagnin M, Vecchiato C, Al-Khaffaf A, Beqiri L, Kaiser C, et al. Graft-versus-host disease (GVHD) of the tongue and of the oral cavity: a large retrospective study. Int J Hematol. (2018) 108:615–21. doi: 10.1007/s12185-018-2520-5
29. Woo SB, Lee SJ, Schubert MM. Graft-vs-host disease. Crit Rev Oral Biol Med. (1997) 8:201–16. doi: 10.1177/10454411970080020701
30. Boer CC, Correa ME, Miranda EC, de Souza CA. Taste disorders and oral evaluation in patients undergoing allogeneic hematopoietic sCT. Bone Marrow Transplant. (2010) 45:705–11. doi: 10.1038/bmt.2009.237
31. Scordo M, Shah GL, Peled JU, Preston EV, Buchan ML, Epstein JB, et al. Unlocking the complex flavors of dysgeusia after hematopoietic cell transplantation. Biol Blood Marrow Transplant. (2018) 24:425–32. doi: 10.1016/j.bbmt.2017.10.022
32. Meier JK, Wolff D, Pavletic S, Greinix H, Gosau M, Bertz H, et al. Oral chronic graft-versus-host disease: report from the international consensus conference on clinical practice in cGVHD. Clin Oral Investig. (2011) 15:127–39. doi: 10.1007/s00784-010-0450-6
33. Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: diagnosis i, and staging working group report. Biol Blood Marrow Transplant. (2005) 11:945–56. doi: 10.1016/j.bbmt.2005.09.004
34. Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: i. the2014 diagnosis and staging working group report. Biol Blood Marrow Transplant. (2015) 21:389–401 e1. doi: 10.1016/j.bbmt.2014.12.001
35. Flowers ME, Parker PM, Johnston LJ, Matos AV, Storer B, Bensinger WI, et al. Comparison of chronic graft-versus-host disease after transplantation of peripheral blood stem cells versus bone marrow in allogeneic recipients: Long-term follow-up of a randomized trial. Blood. (2002) 100:415–9. doi: 10.1182/blood-2002-01-0011
36. Arai S, Jagasia M, Storer B, Chai X, Pidala J, Cutler C, et al. Global and organ-specific chronic graft-versus-host disease severity according to the (2005) NIH consensus criteria. Blood. (2011) 118:4242–9. doi: 10.1182/blood-2011-03-344390
37. Treister N, Chai X, Kurland B, Pavletic S, Weisdorf D, Pidala J, et al. Measurement of oral chronic GVHD: results from the chronic GVHD consortium. Bone Marrow Transplant. (2013) 48:1123–8. doi: 10.1038/bmt.2012.285
38. Mawardi H, Elad S, Correa ME, Stevenson K, Woo SB, Almazrooa S, et al. Oral epithelial dysplasia and squamous cell carcinoma following allogeneic hematopoietic stem cell transplantation: clinical presentation and treatment outcomes. Bone Marrow Transplant. (2011) 46:884–91. doi: 10.1038/bmt.2011.77
39. Baker KS, Leisenring WM, Goodman PJ, Ermoian RP, Flowers ME, Schoch G, et al. Total body irradiation dose and risk of subsequent neoplasms following allogeneic hematopoietic cell transplantation. Blood. (2019) 133:2790–9. doi: 10.1182/blood.2018874115
40. Chen MH, Chang PM, Li WY, Hsiao LT, Hong YC, Liu CY, et al. High incidence of oral squamous cell carcinoma independent of HPV infection after allogeneic hematopoietic SCT in taiwan. Bone Marrow Transplant. (2011) 46:567–72. doi: 10.1038/bmt.2010.163
41. Curtis RE, Metayer C, Rizzo JD, Socie G, Sobocinski KA, Flowers ME, et al. Impact of chronic GVHD therapy on the development of squamous-cell cancers after hematopoietic stem-cell transplantation: an international case-control study. Blood. (2005) 105:3802–11. doi: 10.1182/blood-2004-09-3411
42. Demarosi F, Soligo D, Lodi G, Moneghini L, Sardella A, Carrassi A. Squamous cell carcinoma of the oral cavity associated with graft versus host disease: report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (2005) 100:63–9. doi: 10.1016/j.tripleo.2004.12.008
43. Kruse AL, Gratz KW. Oral carcinoma after hematopoietic stem cell transplantation-a new classification based on a literature review over 30 years. Head Neck Oncol. (2009) 1:29. doi: 10.1186/1758-3284-1-29
44. Pavletic SZ, Martin P, Lee SJ, Mitchell S, Jacobsohn D, Cowen EW, et al. Measuring therapeutic response in chronic graft-versus-host disease: national institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: iv. response criteria working group report. Biol Blood Marrow Transplant. (2006) 12:252–66. doi: 10.1016/j.bbmt.2006.01.008
45. Penack O, Marchetti M, Ruutu T, Aljurf M, Bacigalupo A, Bonifazi F, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the european society for blood and marrow transplantation. Lancet Haematol. (2020) 7:e157–67. doi: 10.1016/S2352-3026(19)30256-X
46. Lee SJ, Wolff D, Kitko C, Koreth J, Inamoto Y, Jagasia M, et al. Measuring therapeutic response in chronic graft-versus-host disease. national institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: iv. the 2014 response criteria working group report. Biol Blood Marrow Transplant. (2015) 21:984–99. doi: 10.1016/j.bbmt.2015.02.025
47. Lee S, Cook EF, Soiffer R, Antin JDevelopment H. and validation of a scale to measure symptoms of chronic graft-versus-host disease. Biol Blood Marrow Transplant. (2002) 8:444–52. doi: 10.1053/bbmt.2002.v8.pm12234170
48. Fassil H, Bassim CW, Mays J, Edwards D, Baird K, Steinberg SM, et al. Oral chronic graft-vs-host disease characterization using the NIH scale. J Dent Res. (2012) 91(7 Suppl):45S−51S. doi: 10.1177/0022034512450881
49. Mitchell SA, Jacobsohn D, Thormann Powers KE, Carpenter PA, Flowers ME, Cowen EW, et al. A multicenter pilot evaluation of the national institutes of health chronic graft-versus-host disease (cGVHD) therapeutic response measures: feasibility, interrater reliability, and minimum detectable change. Biol Blood Marrow Transplant. (2011) 17:1619–29. doi: 10.1016/j.bbmt.2011.04.002
50. Treister NS, Stevenson K, Kim H, Woo SB, Soiffer R, Cutler C. Oral chronic graft-versus-host disease scoring using the NIH consensus criteria. Biol Blood Marrow Transplant. (2010) 16:108–14. doi: 10.1016/j.bbmt.2009.09.010
51. Elad S, Zeevi I, Or R, Resnick IB, Dray L, Shapira MY. Validation of the national institutes of health (NIH) scale for oral chronic graft-versus-host disease (cGVHD). Biol Blood Marrow Transplant. (2010) 16:62–9. doi: 10.1016/j.bbmt.2009.08.018
52. Garnett C, Apperley JF, Pavlu Treatment J. and management of graft-versus-host disease: improving response and survival. Ther Adv Hematol. (2013) 4:366–78. doi: 10.1177/2040620713489842
53. Blazar BR, Hill GR, Murphy WJ. Dissecting the biology of allogeneic hSCT to enhance the gvT effect whilst minimizing GVHD. Nat Rev Clin Oncol. (2020) 17:475–92. doi: 10.1038/s41571-020-0356-4
54. Weiden PL, Flournoy N, Sanders JE, Sullivan KM, Thomas ED. Antileukemic effect of graft-versus-host disease contributes to improved survival after allogeneic marrow transplantation. Transplant Proc. (1981) 13:248–51.
55. Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. (1990) 75:555–62. doi: 10.1182/blood.V75.3.555.555
56. Koc S, Leisenring W, Flowers ME, Anasetti C, Deeg HJ, Nash RA, et al. Therapy for chronic graft-versus-host disease: a randomized trial comparing cyclosporine plus prednisone versus prednisone alone. Blood. (2002) 100:48–51. doi: 10.1182/blood.V100.1.48
57. Sullivan KM, Witherspoon RP, Storb R, Weiden P, Flournoy N, Dahlberg S, et al. Prednisone and azathioprine compared with prednisone and placebo for treatment of chronic graft-vs-host disease: prognostic influence of prolonged thrombocytopenia after allogeneic marrow transplantation. Blood. (1988) 72:546–54. doi: 10.1182/blood.V72.2.546.bloodjournal722546
58. Arora M, Wagner JE, Davies SM, Blazar BR, Defor T, Enright H, et al. Randomized clinical trial of thalidomide, cyclosporine, and prednisone versus cyclosporine and prednisone as initial therapy for chronic graft-versus-host disease. Biol Blood Marrow Transplant. (2001) 7:265–73. doi: 10.1053/bbmt.2001.v7.pm11400948
59. Flowers ME, Martin PJ. How we treat chronic graft-versus-host disease. Blood. (2015) 125:606–15. doi: 10.1182/blood-2014-08-551994
60. Center for Drug Evaluation and Research. FDA expands ibrutinib indications to chronic GVHD. Food US, Drug Administration. FDA. Available online at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-expands-ibrutinib-indications-chronic-gvhd (accessed Feb 2, 2022).
61. Center for Drug Evaluation and Research. FDA approves ruxolitinib for chronic graft-versus-host disease. Food US, Drug Administration. FDA. Available online at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ruxolitinib-chronic-graft-versus-host-disease (accessed Feb 2, 2022).
62. Center for Drug Evaluation and Research. FDA approves Belumosudil for chronic graft-versus-host disease. Food US, Drug Administration. FDA. Available online at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-belumosudil-chronic-graft-versus-host-disease (accessed Feb 2, 2022).
63. Sari I, Altuntas F, Kocyigit I, Sisman Y, Eser B, Unal A, et al. The effect of budesonide mouthwash on oral chronic graft versus host disease. Am J Hematol. (2007) 82:349–56. doi: 10.1002/ajh.20814
64. Stewart BL, Storer B, Storek J, Deeg HJ, Storb R, Hansen JA, et al. Duration of immunosuppressive treatment for chronic graft-versus-host disease. Blood. (2004) 104:3501–6. doi: 10.1182/blood-2004-01-0200
65. Carpenter PA, Kitko CL, Elad S, Flowers ME, Gea-Banacloche JC, Halter JP, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: the 2014 ancillary therapy and supportive care working group report. Biol Blood Marrow Transplant. (2015) 21:1167–87. doi: 10.1016/j.bbmt.2015.03.024
66. Mawardi H, Hashmi SK, Elad S, Aljurf M, Treister N. Chronic graft-versus-host disease: current management paradigm and future perspectives. Oral Dis. (2019) 25:931–48. doi: 10.1111/odi.12936
67. Couriel D, Carpenter PA, Cutler C, Bolanos-Meade J, Treister NS, Gea-Banacloche J, et al. Ancillary therapy and supportive care of chronic graft-versus-host disease: national institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: v. ancillary therapy and supportive care working group report. Biol Blood Marrow Transplant. (2006) 12:375–96. doi: 10.1016/j.bbmt.2006.02.003
68. Elad S, Zeevi I, Finke J, Koldehoff M, Schwerdtfeger R, Wolff D, et al. Improvement in oral chronic graft-versus-host disease with the administration of effervescent tablets of topical budesonide-an open, randomized, multicenter study. Biol Blood Marrow Transplant. (2012) 18:134–40. doi: 10.1016/j.bbmt.2011.06.001
69. Inamoto Y, Martin PJ, Chai X, Jagasia M, Palmer J, Pidala J, et al. Clinical benefit of response in chronic graft-versus-host disease. Biol Blood Marrow Transplant. (2012) 18:1517–24. doi: 10.1016/j.bbmt.2012.05.016
70. Yuan A, Chai X, Martins F, Arai S, Arora M, Correa ME, et al. Oral chronic GVHD outcomes and resource utilization: a subanalysis from the chronic GVHD consortium. Oral Dis. (2016) 22:235–40. doi: 10.1111/odi.12429
71. Mawardi H, Stevenson K, Gokani B, Soiffer R, Treister N. Combined topical dexamethasone/tacrolimus therapy for management of oral chronic GVHD. Bone Marrow Transplant. (2010) 45:1062–7. doi: 10.1038/bmt.2009.301
72. Treister N, Li S, Kim H, Lerman M, Sultan A, Alyea EP, et al. An open-Label phase iI randomized trial of topical dexamethasone and tacrolimus solutions for the treatment of oral chronic graft-versus-Host disease. Biol Blood Marrow Transplant. (2016) 22:2084–91. doi: 10.1016/j.bbmt.2016.08.020
73. Eckardt A, Starke O, Stadler M, Reuter C, Hertenstein B. Severe oral chronic graft-versus-host disease following allogeneic bone marrow transplantation: highly effective treatment with topical tacrolimus. Oral Oncol. (2004) 40:811–4. doi: 10.1016/j.oraloncology.2004.02.003
74. Sanchez AR, Sheridan PJ, Rogers RS. Successful treatment of oral lichen planus-like chronic graft-versus-host disease with topical tacrolimus: a case report. J Periodontol. (2004) 75:613–9. doi: 10.1902/jop.2004.75.4.613
75. Albert MH, Becker B, Schuster FR, Klein B, Binder V, Adam K, et al. Oral graft vs. host disease in children-treatment with topical tacrolimus ointment. Pediatr Transplant. (2007) 11:306–11. doi: 10.1111/j.1399-3046.2006.00666.x
76. Epstein JB, Truelove EL. Topical cyclosporine in a bioadhesive for treatment of oral lichenoid mucosal reactions: an open label clinical trial. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (1996) 82:532–6. doi: 10.1016/S1079-2104(96)80198-3
77. Treister N, Li S, Soiffer R, Cutler C. Topical sirolimus for management of refractory oral chronic graft-versus-host disease. Oral Dis. (2021) 27:1451–4. doi: 10.1111/odi.13676
78. Epstein JB, Nantel S, Sheoltch SM. Topical azathioprine in the combined treatment of chronic oral graft-versus-host disease. Bone Marrow Transplant. (2000) 25:683–7. doi: 10.1038/sj.bmt.1702192
79. Epstein JB, Gorsky M, Epstein MS, Nantel S. Topical azathioprine in the treatment of immune–mediated chronic oral inflammatory conditions: a series of cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (2001) 91:56–61. doi: 10.1067/moe.2001.111130
80. St John L, Gordon SM, Childs R, Marquesen M, Pavletic SZ, Wu TX, et al. Topical thalidomide gel in oral chronic GVHD and role of in situ cytokine expression in monitoring biological activity. Bone Marrow Transplant. (2013) 48:610–1. doi: 10.1038/bmt.2012.178
81. Epstein JB, Raber–Durlacher JE, Epstein GL, Hazenberg MD, Tzachanis D, Spielberger RT. Chronic oral graft-versus-host disease: induction and maintenance therapy with photobiomodulation therapy. Support Care Cancer. (2021) 29:1387–94. doi: 10.1007/s00520-020-05626-3
82. Wolff D, Anders V, Corio R, Horn T, Morison WL, Farmer E, et al. Oral pUVA and topical steroids for treatment of oral manifestations of chronic graft-vs-host disease. Photodermatol Photoimmunol Photomed. (2004) 20:184–90. doi: 10.1111/j.1600-0781.2004.00102.x
83. Redding SW, Callander NS, Haveman CW, Leonard DL. Treatment of oral chronic graft-versus-host disease with pUVA therapy: case report and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (1998) 86:183–7. doi: 10.1016/S1079-2104(98)90123-8
84. Elad S, Garfunkel AA, Enk CD, Galili D, Or R. Ultraviolet b irradiation: a new therapeutic concept for the management of oral manifestations of graft-versus-host disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (1999) 88:444–50. doi: 10.1016/S1079-2104(99)70059-4
85. Wolff D, Gerbitz A, Ayuk F, Kiani A, Hildebrandt GC, Vogelsang GB, et al. Consensus conference on clinical practice in chronic graft-versus-host disease (GVHD): first-line and topical treatment of chronic GVHD. Biol Blood Marrow Transplant. (2010) 16:1611–28. doi: 10.1016/j.bbmt.2010.06.015
86. Wolff D, Schleuning M, von Harsdorf S, Bacher U, Gerbitz A, Stadler M, et al. Consensus conference on clinical practice in chronic GVHD: second-Line treatment of chronic graft-versus-Host disease. Biol Blood Marrow Transplant. (2011) 17:1–17. doi: 10.1016/j.bbmt.2010.05.011
87. Elad S, Jensen SB, Raber–Durlacher JE, Mouradian N, Correa EM, Schubert MM, et al. Clinical approach in the management of oral chronic graft-versus-host disease (cGVHD) in a series of specialized medical centers. Support Care Cancer. (2015) 23:1615–22. doi: 10.1007/s00520-014-2503-x
88. Noce CW, Gomes A, Shcaira V, Correa ME, Moreira MC, Silva Junior A, et al. Randomized double-blind clinical trial comparing clobetasol and dexamethasone for the topical treatment of symptomatic oral chronic graft-versus-host disease. Biol Blood Marrow Transplant. (2014) 20:1163–8. doi: 10.1016/j.bbmt.2014.04.009
89. Park AR, La HO, Cho BS, Kim SJ, Lee BK, Rhie JY, et al. Comparison of budesonide and dexamethasone for local treatment of oral chronic graft-versus-host disease. Am J Health Syst Pharm. (2013) 70:1383–91. doi: 10.2146/ajhp120567
90. Gonzalez-Moles MA, Scully C. HPA-suppressive effects of aqueous clobetasol propionate in the treatment of patients with oral lichen planus. J Eur Acad Dermatol Venereol. (2010) 24:1055–9. doi: 10.1111/j.1468-3083.2010.03591.x
91. Fantozzi PJ, Treister N, Shekar R, Woo SB, Villa A. Intralesional triamcinolone acetonide therapy for inflammatory oral ulcers. Oral Surg Oral Med Oral Pathol Oral Radiol. (2019) 128:485–90. doi: 10.1016/j.oooo.2019.07.024
93. Imanguli MM, Alevizos I, Brown R, Pavletic SZ, Atkinson JC. Oral graft-versus-host disease. Oral Dis. (2008) 14:396–412. doi: 10.1111/j.1601-0825.2008.01448.x
94. Mousavian M, Sroussi H, Villa A, Cutler C, Treister N. Use of prescription sialagogues for management of xerostomia in chronic graft-versus-host-disease. Transplant Cell Ther. (2021) 27:480 e1– e5. doi: 10.1016/j.jtct.2021.02.020
95. Singhal S, Powles R, Treleaven J, Rattenbury H, Mehta J. Pilocarpine hydrochloride for symptomatic relief of xerostomia due to chronic graft-versus-host disease or total–body irradiation after bone-marrow transplantation for hematologic malignancies. Leuk Lymphoma. (1997) 24:539–43. doi: 10.3109/10428199709055591
96. Nagler RM, Nagler A. Pilocarpine hydrochloride relieves xerostomia in chronic graft-versus-host disease: a sialometrical study. Bone Marrow Transplant. (1999) 23:1007–11. doi: 10.1038/sj.bmt.1701752
97. Nagler RM, Nagler A. The effect of pilocarpine on salivary constituents in patients with chronic graft-versus-host disease. Arch Oral Biol. (2001) 46:689–95. doi: 10.1016/S0003-9969(01)00035-8
98. Agha-Hosseini F, Mirzaii-Dizgah I, Ghavamzadeh L, Ghavamzadeh A, Tohidast-Acrad Z. Effect of pilocarpine hydrochloride on unstimulated whole saliva flow rate and composition in patients with chronic graft-versus-host disease (cGVHD). Bone Marrow Transplant. (2007) 39:431–4. doi: 10.1038/sj.bmt.1705621
99. Brimhall J, Jhaveri MA, Yepes JF. Efficacy of cevimeline vs. pilocarpine in the secretion of saliva: a pilot study. Spec Care Dentist. (2013) 33:123–7. doi: 10.1111/scd.12010
100. Fife RS, Chase WF, Dore RK, Wiesenhutter CW, Lockhart PB, Tindall E, et al. Cevimeline for the treatment of xerostomia in patients with sjogren syndrome: a randomized trial. Arch Intern Med. (2002) 162:1293–300. doi: 10.1001/archinte.162.11.1293
101. Carpenter PA, Schubert MM, Flowers ME. Cevimeline reduced mouth dryness and increased salivary flow in patients with xerostomia complicating chronic graft-versus-host disease. Biol Blood Marrow Transplant. (2006) 12:792–4. doi: 10.1016/j.bbmt.2006.03.010
102. Elad S, Raber-Durlacher JE, Brennan MT, Saunders DP, Mank AP, Zadik Y, et al. Basic oral care for hematology-oncology patients and hematopoietic stem cell transplantation recipients: a position paper from the joint task force of the multinational association of supportive care in cancer/International society of oral oncology (MASCC/ISOO) and the european society for blood and marrow transplantation (EBMT). Support Care Cancer. (2015) 23:223–36. doi: 10.1007/s00520-014-2378-x
103. Salagen (pilocarpine hydrochloride) DRUGS@FDA: FDA-approved drugs. Access Data.fda.gov. Available online at: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=020237 (accessed Feb 2, 2022).
104. Elad S, Cohen G, Zylber-Katz E, Findler M, Galili D, Garfunkel AA, et al. Systemic absorption of lidocaine after topical application for the treatment of oral mucositis in bone marrow transplantation patients. J Oral Pathol Med. (1999) 28:170–2. doi: 10.1111/j.1600-0714.1999.tb02018.x
105. Yamashita S, Sato S, Kakiuchi Y, Miyabe M, Yamaguchi H. Lidocaine toxicity during frequent viscous lidocaine use for painful tongue ulcer. J Pain Symptom Manage. (2002) 24:543–5. doi: 10.1016/S0885-3924(02)00498-0
106. Kwok S, Fischer JL, Rogers J, Benzocaine D. Lidocaine induced methemoglobinemia after bronchoscopy: a case report. J Med Case Rep. (2008) 2:16. doi: 10.1186/1752-1947-2-16
107. Kotler RL, Hansen-Flaschen J, Casey MP. Severe methaemoglobinaemia after flexible fibreoptic bronchoscopy. Thorax. (1989) 44:234–5. doi: 10.1136/thx.44.3.234
108. Nappe TM, Pacelli AM, Katz K. An atypical case of methemoglobinemia due to self-administered benzocaine. Case Rep Emerg Med. (2015) 2015:670979. doi: 10.1155/2015/670979
109. Sheiham A, James WP. A reappraisal of the quantitative relationship between sugar intake and dental caries: the need for new criteria for developing goals for sugar intake. BMC Public Health. (2014) 14:863. doi: 10.1186/1471-2458-14-863
110. Moynihan PJ, Kelly SA. Effect on caries of restricting sugars intake: systematic review to inform WHO guidelines. J Dent Res. (2014) 93:8–18. doi: 10.1177/0022034513508954
111. Majhail NS, Rizzo JD, Lee SJ, Aljurf M, Atsuta Y, Bonfim C, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Bone Marrow Transplant. (2012) 47:337–41. doi: 10.1038/bmt.2012.5
112. Atsuta Y, Suzuki R, Yamashita T, Fukuda T, Miyamura K, Taniguchi S, et al. Continuing increased risk of oral/esophageal cancer after allogeneic hematopoietic stem cell transplantation in adults in association with chronic graft-versus-host disease. Ann Oncol. (2014) 25:435–41. doi: 10.1093/annonc/mdt558
113. Epstein JB, Gorsky M, Fischer D, Gupta A, Epstein M, Elad S. A survey of the current approaches to diagnosis and management of oral premalignant lesions. J Am Dent Assoc. (2007) 138:1555–62; quiz 614. doi: 10.14219/jada.archive.2007.0104
114. Rizzo JD, Curtis RE, Socie G, Sobocinski KA, Gilbert E, Landgren O, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. (2009) 113:1175–83. doi: 10.1182/blood-2008-05-158782
115. Inamoto Y, Shah NN, Savani BN, Shaw BE, Abraham AA, Ahmed IA, et al. Secondary solid cancer screening following hematopoietic cell transplantation. Bone Marrow Transplant. (2015) 50:1013–23. doi: 10.1038/bmt.2015.63
116. Elad S, Zadik Y, Zeevi I, Miyazaki A, de Figueiredo MA, Or R. Oral cancer in patients after hematopoietic stem-cell transplantation: Long-term follow-up suggests an increased risk for recurrence. Transplantation. (2010) 90:1243–4. doi: 10.1097/TP.0b013e3181f9caaa
Keywords: chronic GVHD, hematopoietic cell transplantation, oral medicine, dental, supportive care
Citation: Dean D and Sroussi H (2022) Oral Chronic Graft-Versus-Host Disease. Front. Oral. Health 3:903154. doi: 10.3389/froh.2022.903154
Received: 24 March 2022; Accepted: 18 April 2022;
Published: 20 May 2022.
Edited by:
Omar Kujan, University of Western Australia, AustraliaReviewed by:
Sven E. Niklander, Universidad Andres Bello, ChileLalima Tiwari, University of Western Australia, Australia
Copyright © 2022 Dean and Sroussi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David Dean, ddean2@uw.edu
 David Dean
David Dean Herve Sroussi
Herve Sroussi