Implementation of streamlining measures in selecting and prioritising complex cases for the cancer multidisciplinary team meeting: a mini review of the recent developments
- 1Department of Urology, University College London Hospitals NHS Foundation Trust, London, United Kingdom
- 2Department of Urology, The Specialty Hospital, Amman, Jordan
- 3Department of Surgery and Cancer, Imperial College London, London, United Kingdom
- 4Barts Cancer Institute, Queen Mary University of London, London, United Kingdom
- 5Department of Urology, Barts Health NHS Trust, London, United Kingdom
In January 2020, NHS England and NHS Improvement, in the United Kingdom, issued a permissive framework for streamlining cancer multidisciplinary (MDT) meetings. Streamlining is defined as a process whereby complex cases are prioritized for full discussion by an MDT in an MDT meeting (MDM), while the management of straightforward cases is expedited using Standards of Care (SoC). SoC are points in the pathway of patient management where there are recognized guidelines and clear clinical consensus on the options for management and should be regionally agreed and uniformly applied by regional Cancer Alliances. While this report marks the first major change in cancer MDT management since the Calman-Hine report in 1995, its implementation, nationally, has been slow with now nearly four years since its publication. It is argued however that streamlining is a necessary step in ensuring the viability of MDT processes, and therefore maintaining patient care in the current socioeconomic context of rising workload and cancer incidence, financial pressures, and workforce shortages. In this mini review, we offer a succinct summary of the recent developments around the implementation of the 2020 streamlining framework, including challenges and barriers to its implementation, and the potential future directions in this field, which we propose should increase utilisation of implementation science. We conclude that ensuring successful implementation of the framework and the SOC requires securing a buy-in from key stakeholders, including MDTs and hospital management teams, with clearly defined (a) management approaches that include triage (e.g. through a mini MDT meeting), (b) assessment of case complexity (something that directly feeds into the SOC), and (c) roles of the MDT lead and the members, while acknowledging that the SOC cannot be universally applied without the consideration of individual variations across teams and hospital Trusts.
1 Introduction
Multidisciplinary team (MDT) meetings are an essential part of cancer care, bringing together healthcare professionals from different disciplines (e.g., oncologists, surgeons, cancer nurse specialists, radiologists, pathologists, physicians, and in some cancers allied health professionals) to discuss patient cases, review diagnostics and develop treatment recommendations (1). However, MDT meetings can be time-consuming and resource-intensive, particularly when discussing straightforward cases that do not require true multidisciplinary input (2). This is further compounded by rising cancer incidents, staff shortages and financial pressures on healthcare (3, 4). Accordingly, there is a need to prioritize complex cases, which are those known to benefit most from a multidisciplinary approach. Indeed, focusing on complex cases has been recommended in several UK national policy documents in recent years (1, 5).
Streamlining is a process whereby complex cases are prioritized for full discussion, while the management of straightforward cases is expedited using Standards of Care (SoC; 4). SoC are points in the pathway of patient management where there are recognised guidelines and clear clinical consensus on the options for management and should be regionally agreed and uniformly applied by regional Cancer Alliances (6). By streamlining cases listed for cancer MDT review, healthcare professionals can work towards improving the efficiency and effectiveness of MDT meetings while still providing high-quality care for cancer patients (3, 7). This review will explore the implementation of the 2020 streamlining framework, emphasizing the vital role of implementation science and the importance of securing buy-in from key stakeholders.
2 The 2020 streamlining framework and its implementation
The guidance from NHS England (5) marks a departure from the NHS directives of the past 20-years (8). It suggests that not all cases require discussion and that the focus should be on complex cases. This provides cancer MDTs with a clear mandate to implement changes. The question of what constitutes a complex case, is however not so readily answered in the guidance. This question is an important one, as failure to streamline MDT processes using existing best evidence means that while the team's caseload may become more manageable, the care quality could be compromised by returning to the unwarranted variation in care that was evident before the introduction of MDTs (9, 10).
What constitutes the complexity of a cancer case for MDT discussion has been addressed scientifically and concurrently with the NHS England guidance by Soukup and colleagues, who spent 2-years undertaking an NIHR-funded mixed methods study, with input from hundreds of cancer experts, and data from hundreds of cancer MDT case discussions across the UK (11). The researchers found that each professional group within an MDT holds a unique perspective on the question of what constitutes a complex case. Their algorithm, Measure of Case-Discussion Complexity: the MeDiC tool, includes 26 psychometrically validated indicators of complexity that represent the perspectives of all professional groups that make up an MDT. This tool allows MDTs to scientifically measure case complexity and apply this to streamlining and the selection of cases for the SoC. Subsequent research demonstrated feasibility and utility of the MeDiC tool in urology MDTs (12).
Implementing the streamlining guidance, along with structuring and organizing the MDT meeting, will require time and effort, especially if using scientific tools such as MeDiC. What resource is required, and how this is best utilized is open to debate, and the optimal strategy will likely vary from one team to another. It is hoped however, that this investment will pay dividends by allowing better utilization of these resources by focusing on complex cases, and reducing unnecessary delay for cases that meet SoC (7).
In addition, several models have been proposed to facilitate triage of cases referred to the MDT meeting, and efficient decision-making and patient management (9, 13).
The first model, referred to as the Mini MDT, constitutes a core team comprising the MDT Coordinator, MDT lead (or deputy), radiologist, and pathologist. Within this framework, all cases are subject to discussion within the Mini MDT (14). The Mini MDT collectively evaluates the results of investigations and decides whether a case should be referred for SoC management or necessitates full MDT discussion. Cases designated for SoC management have their management recommendations meticulously documented, while MDT cases undergo comprehensive deliberation during the subsequent full MDT meeting, involving the complete team.
The Pre-MDT triage model (15), on the other hand, adopts a different approach. It involves a smaller triage team, consisting of the MDT Lead (or deputy) and the MDT Coordinator. For a case to be considered under this model, the radiology and pathology reports must have been reviewed and reported by a core MDT member. The pre-MDT triage team systematically reviews all cases and makes determinations regarding their categorization, either for SoC management or full MDT discussion. As with the previous model, SoC cases have their management recommendations meticulously documented and integrated into the MDT minutes, while MDT cases are subsequently subjected to comprehensive examination during the full MDT meeting, where the entire team contributes to the discussion.
A third suggested model places emphasis on active engagement from all clinicians involved in the MDT (13). The prerequisite, once again, is that radiology and pathology reports must have been reported by a core MDT member. Under this model, referring clinicians take on the responsibility of assessing whether a case warrants SoC management or necessitates full MDT discussion. The referring clinician then documents the management recommendation accordingly. Subsequently, the MDT lead reviews both the SoC and MDT lists to ensure the appropriate categorization. Cases designated for full MDT discussion are deliberated upon in detail during the MDT meeting, involving the complete team.
These three models for MDT triage enable the allocation of appropriate resources and the identification of cases that necessitate comprehensive MDT discussion. By adhering to specific criteria and actively involving relevant team members, these models facilitate streamlined decision-making processes, ultimately ensuring the delivery of optimal patient care outcomes (16).
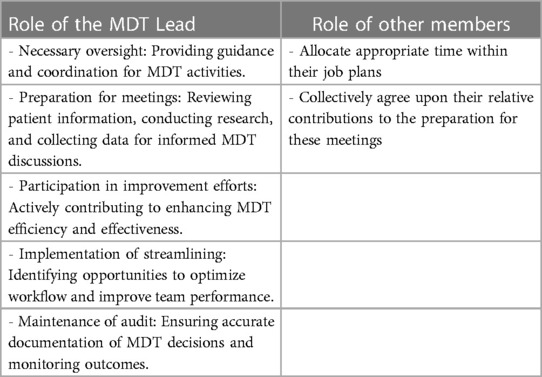
In this process, the role of the MDT lead holds significant importance. To ensure that the individual appointed for this role possesses the requisite experience, interest, and credibility, a competitive interview process should be considered (17). This interview process serves to assess the candidate's qualifications and suitability for the position. Furthermore, it is imperative that the role of the MDT lead is clearly defined, with a well-defined description outlining the responsibilities and expectations associated with the position (13). To ensure effective execution of the MDT, members should cover various essential activities listed in Table 1.
The roles within MDTs, as detailed in Table 1, are pivotal to the streamlining and efficiency of cancer care, yet their effective implementation necessitates adaptability to the unique environments of various teams and hospital trusts. The MDT Lead plays a crucial role in providing necessary oversight, preparation for meetings, participation in improvement efforts, implementation of streamlining strategies, and maintaining audits. This role, however, must be flexible enough to accommodate the diverse challenges and resources of different healthcare settings. For instance, in smaller trusts, the MDT Lead might engage more directly in the preparation and review of patient information, while in larger settings, their focus might shift towards strategic coordination and oversight of streamlining efforts.
Similarly, the roles of other team members, who contribute to meeting preparation and actively participate in streamlining and improvement initiatives, must be attuned to the specific dynamics of their team. In some settings, team members might allocate significant time to the detailed preparation of cases due to the complexity or volume of patients, while in others, their focus might be on collective efficiency and streamlined decision-making processes. Adaptability in these roles is essential to cater to varying patient loads, resource availability, and organizational structures across different hospital trusts.
By recognizing and adapting to this variability, MDTs can ensure that their roles are not only clearly defined but also flexible and responsive to their specific healthcare environment. This adaptability is key to maintaining high standards of patient care, irrespective of the differing contexts and challenges presented by each trust.
3 Challenges, barriers, and the role of implementation science
While efforts to streamline MDT meetings are crucial, the potential disadvantages of implementing streamlining in MDT meetings needs to be considered. The specific disadvantages may vary depending on the context and implementation approach, and some to consider are listed in Table 2. It is important to carefully consider these and design streamlining strategies that strike a balance between efficiency and comprehensive patient care (18). Engaging MDT members, maintaining open lines of communication, and regularly evaluating the impact of streamlining efforts can help address these disadvantages and optimize the benefits of streamlining in cancer MDT meetings (19).
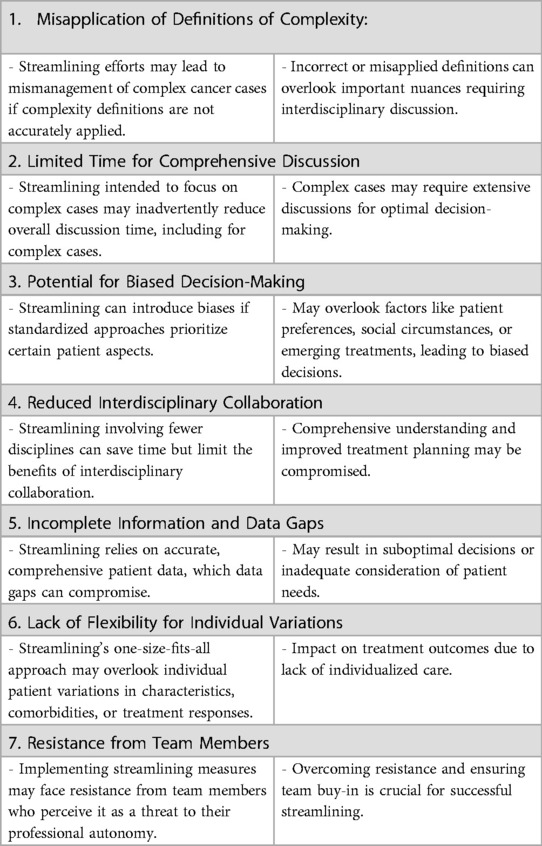
Table 2. The complexities and potential drawbacks of streamlining in multidisciplinary team (MDT) meetings.
It is also important to anticipate different barriers to implementation of streamlining, such as for example challenges in selecting and prioritising complex cases (20, 21). Resistance to change is a common hurdle, as implementing streamlining measures often disrupt established routines and roles within the MDT. Additionally, necessary resources are often found to be lacking, including dedicated personnel and technology upgrades. This is improved by engaging various stakeholders, from clinicians to administrators, as disengagement from specific groups can hamper progress (22).
Challenges in the form of communication and coordination within the team can further complicate the process. Given that MDT meetings are already time-limited, time constraints can lead to rushed decision-making when introducing streamlining measures. Furthermore, an efficient streamlining process relies on access to accurate patient data, test results, and treatment guidelines (20). Barriers related to data availability, privacy concerns, or incomplete information can hinder the streamlining process. Finally, institutional culture, existing policies, and governance structures may either facilitate or hinder streamlining efforts, and legal and regulatory considerations must be navigated carefully.
4 Strategies for successful implementation
Mitigating the barriers necessitates the adoption of comprehensive strategies (21). It involves effective change-management strategies, adequate resource allocation, stakeholder engagement, and clear communication regarding the advantages of streamlining. Collaboration among MDT members, along with leadership support and commitment to adapt and learn from the implementation process, is key to overcoming these challenges and successfully streamlining cancer MDT meetings.
It is therefore important to consider optimising existing processes in MDT meetings before embarking on significant changes to the standard operating procedures (9, 23). First, a comprehensive assessment and audit of the current local circumstances is imperative, encompassing a meticulous evaluation of case volume, temporal allocation, personnel availability, and their respective contributions (24). Secondly, a revision of the clinical data available for decision-making should encompass comorbidities, social determinants, performance metrics, radiological findings, pathology reports, and patient perspectives where accessible (25). Furthermore, a judicious approach to measuring case complexity should be adopted, and the employment of a structured template or proforma is recommended (26). The team should refrain from discussing cases that could be appropriately managed elsewhere, focusing solely on cancer cases during the MDT meeting.
To facilitate a comprehensive, holistic, and patient-centred care, the recognition and cultivation of good team dynamics, effective meeting management, and the mitigation of disruptive behaviours and distractions are important. Avoiding excessively lengthy meetings and incorporating breaks to refresh participants are also important considerations (27). Finally maintaining representative and transparent record-keeping is crucial.
Lastly, establishing SoC is critical as it requires a clear consensus regarding the most effective care management for patients (28). These standards hinge on several key factors: firstly, the condition must be categorized within the criteria for low complexity, furthermore, patients must meet the eligibility criteria with minimal comorbidities, indicating lower chances of adverse outcomes. Secondly, a robust consensus should exist regarding the optimal management strategies for the specific condition in question (22), while patients' willingness and ability to adhere to the recommended approach is considered. This approach could ensure consistent, safe, and effective management practices for low-risk conditions, ultimately optimizing patient outcomes and judiciously allocating resources.
5 Future directions and role of implementation science
Implementation science plays a pivotal role in the streamlining of MDT meetings in cancer care, bridging the gap between established guidelines and their practical application. With its focus on the methods to promote the uptake of research findings into routine healthcare practices, it offers invaluable insights for enhancing MDT meeting efficiency and effectiveness. It provides a structured framework for identifying, analyzing, and overcoming the barriers to successful implementation. By employing implementation science principles (e.g., 29–33), MDT meetings can adopt a more systematic and evidence-based approach to prioritize complex cases, optimize decision-making processes, and adapt to the unique challenges of different healthcare settings. As outlined in the recent paper (7), it equips MDMs with the necessary tools to evolve from traditional, all-encompassing discussions to a more focused and strategic model of patient case review, which is crucial in the current landscape of increasing cancer incidence and resource constraints (7).
Securing stakeholder buy-in is also a critical component in the successful implementation of streamlined MDT meeting processes. Implementation science emphasizes the need for engaging all key stakeholders—from managers to oncologists and pathologists to nurses and administrative staff—ensuring that each voice is heard in shaping the implementation. This inclusive approach not only fosters a sense of ownership among MDT members but also facilitates the identification of team-centred solutions that are sensitive to the unique dynamics and needs of each team. Moreover, implementation science provides the tools and methodologies (e.g., 33) to tailor the streamlining strategies to fit diverse settings, acknowledging that a one-size-fits-all approach is rarely effective. By leveraging these principles, healthcare organizations can develop and implement streamlining strategies that are both effective and sustainable. These tailored strategies not only streamline the decision-making process but also enhance collaboration and communication within teams, ultimately leading to a more agile and responsive cancer care system. Through this lens, implementation science is not just a facilitator for change; it is a catalyst for creating a more dynamic, efficient, and patient-centered MDT model (7).
In addition to these direct applications, a critical aspect of implementation science in streamlining MDT meetings lies in its contribution to building and expanding a knowledge base. As MDTs adopt these streamlined approaches, the collection, analysis, and dissemination of data on their implementation and clinical effectiveness will become invaluable. This ongoing process of knowledge creation not only informs the refinement of current practices but also serves as a rich resource for other teams embarking on similar streamlining journeys. By systematically documenting successes, challenges, and lessons learned, a robust body of evidence can be generated. This evidence base is essential not only for continuous improvement within individual teams but also for advancing the overall practice of cancer care. It supports the development of best practices that can be shared and adapted across different contexts, further enhancing the capability of MDTs to provide high-quality, efficient, and patient-focused care in an ever-evolving healthcare landscape (7, 29–33).
6 Conclusion
The workload of MDTs is on the rise, while the effectiveness of MDT processes exhibits variability. To enhance effectiveness and efficiency, streamlining measures need to be implemented. It is crucial to concentrate the MDT meetings on complex cases, as these often require comprehensive interdisciplinary collaboration. Successfully implementing SoC necessitates directing attention towards several key factors, including areas of consensus, complexity of cases, local agreement, the operational model, and acknowledging that SoC cannot be universally applied without consideration of individual variations. Looking ahead, the integration of implementation science principles will be crucial in adapting and evolving the streamlining practices to meet the diverse needs of cancer care.
Author contributions
TA: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing. RA-M: Writing – original draft, Writing – review & editing, Visualization. BL: Conceptualization, Supervision, Validation, Writing – original draft, Writing – review & editing. TS: Conceptualization, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
The Specialty Hospital, Amman, Jordan, kindly supported the publication fees for this article. Infrastructure support for this research for TS was provided by the NIHR London Medtech and In vitro diagnostic Co-operative.
Conflict of interest
BL and TS received funding from Cancer Alliances and NHS England for training MDTs in assessment and quality improvement methods in the United Kingdom; and honoraria for public speaking from Parsek. TS received consultancy fees from Roche Diagnostics, Parsek and Salutare. BL received consultancy fees from Digital Surgery Ltd, MDOUTLOOK; and honoraria from Astra Zeneca and Astellas.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. NHS England. Streamlining Multi-Disciplinary Team Meetings—Guidance for Cancer Alliances. Available online at: https://www.england.nhs.uk/publication/streamlining-mdt-meetings-guidance-cancer-alliances/ (accessed September 4, 2023).
2. Blazeby JM, Wilson L, Metcalfe C, Nicklin J, English R, Donovan JL. Analysis of clinical decision-making in multi-disciplinary cancer teams. Ann Oncol. (2006) 17(3):457–60. doi: 10.1093/annonc/mdj102
3. Robertson R, Wenzel L, Thompson J, Charles A. Understanding NHS Financial Pressures. How Are They Affecting Patient Care? UK: The King’s Fund (2017). p. 124.
4. NHS England. Achieving World-Class Cancer Outcomes: One Year On 2015–16. Available online at: https://www.england.nhs.uk/cancer/strategy/cancer-one-year-on-2/ (Accessed September 4, 2023).
5. Cancer Research UK. Improving the effectiveness of multidisciplinary team meetings in cancer services. Available online at: https://www.cancerresearchuk.org/about-us/we-develop-policy/our-policy-on-cancer-services/improving-the-effectiveness-of-mdts-in-cancer-services (accessed September 4, 2023).
6. Vinod SK, Sidhom MA, Delaney GP. Do multidisciplinary meetings follow guideline-based care? J Oncol Pract. (2010) 6(6):276–81. doi: 10.1200/JOP.2010.000019
7. Soukup T, Stewart GD, Lamb BW. Defining an evidence-based strategy for streamlining cancer multidisciplinary team meetings. Lancet Oncol. (2023) 24(10):1061–3. doi: 10.1016/S1470-2045(23)00440-0
8. Calman K, Hine D. A Policy Framework for Commissioning Cancer Services. A Report by the Expert Advisory Group on Cancer to Chief Medical Officers of England and Wales. London, UK: Department of Health and Social Care (1995). 34.
9. Soukup T, Lamb BW, Sevdalis N, Green JS. Streamlining cancer multidisciplinary team meetings: challenges and solutions. Br J Hosp Med. (2020) 81(3):1–6. doi: 10.12968/hmed.2020.0024
10. Raine R, Xanthopoulou P, Wallace I, Nic A’ Bháird C, Lanceley A, Clarke A, et al. Determinants of treatment plan implementation in multidisciplinary team meetings for patients with chronic diseases: a mixed-methods study. BMJ Qual Saf. (2014) 23(10):867–76. doi: 10.1136/bmjqs-2014-002818
11. Soukup T, Morbi A, Lamb BW, Gandamihardja TAK, Hogben K, Noyes K, et al. A measure of case complexity for streamlining workflow in multidisciplinary tumor boards: mixed methods development and early validation of the MeDiC tool. Cancer Med. (2020) 9(14):5143–54. doi: 10.1002/cam4.3026
12. Wihl J, Falini V, Borg S, Stahl O, Jiborn T, Ohlsson B, et al. Implementation of the measure of case discussion complexity to guide selection of prostate cancer patients for multidisciplinary team meetings. Cancer Med. (2023) 12(14):15149–58. doi: 10.1002/cam4.6189
13. Lamb BW, Brown KF, Nagpal K, Vincent C, Green JS, Sevdalis N. Quality of care management decisions by multidisciplinary cancer teams: a systematic review. Ann Surg Oncol. (2011) 18(8):2116–25. doi: 10.1245/s10434-011-1675-6
14. Memon AA, Godbole C, Tzivanakis A, Mohamed F, Dayal S, Cecil T, et al. Cancer MDT’s oversubscribed and need to change: a novel clinical radiological assessment meeting (CRAM) as a “mini-MDT” reduces referral response times and MDT workload. Eur J Surg Oncol. (2023) 49(2):e196–7. doi: 10.1016/j.ejso.2022.11.631
15. Merker L, Conroy S, El-Wakeel H, Laurence N. Streamlining the multi-disciplinary team meeting: the introduction of robust pre-preparation methods and its effect on the length of case discussions. J Multidiscip Healthc. (2023) 16:613–22. doi: 10.2147/JMDH.S387174
16. Lumenta DB, Sendlhofer G, Pregartner G, Hart M, Tiefenbacher P, Kamolz LP, et al. Quality of teamwork in multidisciplinary cancer team meetings: a feasibility study. PLoS One. (2019) 14(2):e0212556. doi: 10.1371/journal.pone.0212556
17. Fleissig A, Jenkins V, Catt S, Fallowfield L. Multidisciplinary teams in cancer care: are they effective in the UK? Lancet Oncol. (2006) 7(11):935–43. doi: 10.1016/S1470-2045(06)70940-8
18. Bohmeier B, Schellenberger B, Diekmann A, Ernstmann N, Ansmann L, Heuser C. Opportunities and limitations of shared decision making in multidisciplinary tumor conferences with patient participation—a qualitative interview study with providers. Patient Educ Couns. (2021) 104(4):792–9. doi: 10.1016/j.pec.2020.09.007
19. Walraven JEW, Verhoeven RHA, Meulen RV, Hoeven JJMV, Lemmens VEPP, Hesselink G, et al. Facilitators and barriers to conducting an efficient, competent and high-quality oncological multidisciplinary team meeting. BMJ Open Qual. (2023) 12(1):e002130. doi: 10.1136/bmjoq-2022-002130
20. Wright FC, Lookhong N, Urbach D, Davis D, McLeod RS, Gagliardi AR. Multidisciplinary cancer conferences: identifying opportunities to promote implementation. Ann Surg Oncol. (2009) 16(10):2731–7. doi: 10.1245/s10434-009-0639-6
21. Taylor C, Harris J, Stenner K, Sevdalis N, Green SAJ. A multi-method evaluation of the implementation of a cancer teamwork assessment and feedback improvement programme (MDT-FIT) across a large integrated cancer system. Cancer Med. (2021) 10(4):1240–52. doi: 10.1002/cam4.3719
22. Jalil R, Ahmed M, Green JS, Sevdalis N. Factors that can make an impact on decision-making and decision implementation in cancer multidisciplinary teams: an interview study of the provider perspective. Int J Surg. (2013) 11(5):389–94. doi: 10.1016/j.ijsu.2013.02.026
23. Winters DA, Soukup T, Sevdalis N, Green JSA, Lamb BW. The cancer multidisciplinary team meeting: in need of change? History, challenges and future perspectives. BJU Int. (2021) 128(3):271–9. doi: 10.1111/bju.15495
24. Taylor C, Brown K, Lamb B, Harris J, Sevdalis N, Green JS. Developing and testing TEAM (team evaluation and assessment measure), a self-assessment tool to improve cancer multidisciplinary teamwork. Ann Surg Oncol. (2012) 19(13):4019–27. doi: 10.1245/s10434-012-2493-1
25. Stairmand J, Signal L, Sarfati D, Jackson C, Batten L, Holdaway M, et al. Consideration of comorbidity in treatment decision making in multidisciplinary cancer team meetings: a systematic review. Ann Oncol. (2015) 26(7):1325–32. doi: 10.1093/annonc/mdv025
26. Guirado M, Sanchez-Hernandez A, Pijuan L, Teixido C, Gómez-Caamaño A, Cilleruelo-Ramos Á. Quality indicators and excellence requirements for a multidisciplinary lung cancer tumor board by the spanish lung cancer group. Clin Transl Oncol. (2022) 24(3):446–59. doi: 10.1007/s12094-021-02712-8
27. Walraven JEW, van der Hel OL, van der Hoeven JJM, Lemmens VEPP, Verhoeven RHA, Desar IME. Factors influencing the quality and functioning of oncological multidisciplinary team meetings: results of a systematic review. BMC Health Serv Res. (2022) 22(1):829. doi: 10.1186/s12913-022-08112-0
28. Lamb BW, Linton KD, Narahari K. BAUS Oncology guidance for implementing streamlining in cancer MDT meetings: selecting standards of care and operational considerations. J Clin Urol. (2023). doi: 10.1177/20514158231168463
29. The Medical Research Council. Developing and evaluating complex interventions: new guidance. Available online at: https://www.mrc.ac.uk/documents/pdf/complex-interventions-guidance/ (accessed August 14, 2023).
30. Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new medical research council guidance. Int J Nurs Stud. (2013) 50:587–92. doi: 10.1016/j.ijnurstu.2012.09.010
31. Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. (2011) 38:65–76. doi: 10.1007/s10488-010-0319-7
32. Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. (2012) 50:217–26. doi: 10.1097/MLR.0b013e3182408812
33. Hull L, Goulding L, Khadjesari Z, Davis R, Healey A, Bakolis I, et al. Designing high-quality implementation research: development, application, feasibility and preliminary evaluation of the implementation science research development (ImpRes) tool and guide. Implement Sci. (2019) 14:80. doi: 10.1186/s13012-019-0897-z
Keywords: cancer treatment, health care quality, standard of care, outcome assessment, shared decision making, multidisciplinary team, multidisciplinary team meeting, tumor board
Citation: Al-Hammouri T, Almeida-Magana R, Soukup T and Lamb B (2024) Implementation of streamlining measures in selecting and prioritising complex cases for the cancer multidisciplinary team meeting: a mini review of the recent developments. Front. Health Serv. 4:1340320. doi: 10.3389/frhs.2024.1340320
Received: 17 November 2023; Accepted: 29 January 2024;
Published: 12 March 2024.
Edited by:
Xiaolin Wei, University of Toronto, CanadaReviewed by:
Anthony Ta, University of Melbourne, Australia© 2024 Al-Hammouri, Almeida-Magana, Soukup and Lamb. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tayana Soukup t.soukup@ic.ac.uk
†These authors have contributed equally to this work and share senior authorship
 Tarek Al-Hammouri1,2
Tarek Al-Hammouri1,2  Tayana Soukup
Tayana Soukup Benjamin Lamb
Benjamin Lamb