The effect of unpredictability on the perception of breathlessness: a narrative review
- Research Group Health Psychology, Department of Psychology and Educational Sciences, KU Leuven, Leuven, Belgium
Breathlessness is an aversive bodily sensation impacting millions of people worldwide. It is often highly detrimental for patients and can lead to profound distress and suffering. Notably, unpredictable breathlessness episodes are often reported as being more severe and unpleasant than predictable episodes, but the underlying reasons have not yet been firmly established in experimental studies. This review aimed to summarize the available empirical evidence about the perception of unpredictable breathlessness in the adult population. Specifically, we examined: (1) effects of unpredictable relative to predictable episodes of breathlessness on their perceived intensity and unpleasantness, (2) potentially associated neural and psychophysiological correlates, (3) potentially related factors such as state and trait negative affectivity. Nine studies were identified and integrated in this review, all of them conducted in healthy adult participants. The main finding across studies suggested that unpredictable compared to predictable, breathlessness elicits more frequently states of high fear and distress, which may contribute to amplify the perception of unpredictable breathlessness, especially its unpleasantness. Trait negative affectivity did not seem to directly affect the perception of unpredictable breathlessness. However, it seemed to reinforce state fear and anxiety, hence possible indirect modulatory pathways through these affective states. Studies investigating neural correlates of breathlessness perception and psychophysiological measures did not show clear associations with unpredictability. We discuss the implication of these results for future research and clinical applications, which necessitate further investigations, especially in clinical samples suffering from breathlessness.
1 Introduction
Breathlessness, defined as a subjective experience of breathing discomfort which may vary in quality and intensity (1, 2), is a prevalent condition affecting approximately one-fourth of the European adult population (3). Its prevalence increases with aging (3–5), but is not limited to a specific age group as it represents a significant symptom in various respiratory (e.g., asthma, chronic obstructive pulmonary disease) and non-respiratory diseases and disorders (lung cancer, cardiovascular diseases, anxiety, obesity) (5). Its consequences are often highly detrimental for the quality of life of individuals, including difficulties to perform daily tasks, to engage in social activities and to continue their careers and normal lives. Breathlessness is typically experienced as highly aversive and can cause panic and fear of dying which both profoundly undermine well-being (6). Affected individuals are at increased risk of becoming socially isolated (7–9) or to develop affective disorders such as depression and anxiety, which are often associated with the severity of breathlessness (5, 10–14). Moreover, the loss of autonomy of patients and their distress is also demanding for their relatives and caregivers, who frequently develop worries and concerns resulting in reductions in their quality of life (8, 9, 15).
Qualitative studies looking at patient testimonials have proposed to distinguish between continuous and episodic breathlessness (16, 17), the latter referring to a transient exacerbation of the symptom. Episodic breathlessness can manifest predictably, triggered by specific factors known to the patient such as emotions (e.g., panic), physical activities, comorbid diseases (e.g., infections) or environmental circumstances like dust or heat (17–19). It can also occur unpredictably, either because of a lack of discernible triggers or because it emerges at a sudden unusual threshold such as after a faint instead of a strong emotion (16, 18). Unpredictable breathlessness tends to occur less frequently than predictable episodes (18). Yet, unpredictability has been reported by patients to be particularly distressing because (1) it impairs their coping abilities and leads to feelings of loss of control and frustration (9, 18), (2) unpredictable breathlessness is often perceived as more severe and unpleasant (18), and (3) (as a possible consequence of the second point) catastrophizing thoughts and fear of suffocation may trigger panic (6, 18, 19) and initiate a vicious circle as panic contributes to the further aggravation and persistence of breathlessness (14, 16, 20). However, all these observations have been made in clinical contexts and need supporting evidence from controlled experimental contexts to better understand the dynamics and mechanisms influencing the perception of unpredictable breathlessness.
Moreover, an increasing number of studies have investigated the neural mechanisms underlying the perception of breathlessness using different neuroimaging techniques such as functional magnetic resonance imaging (21–24) and electroencephalography (EEG) (25–32). These studies commonly reported activations during experimentally induced breathlessness in affect-related limbic brain areas (22–24) and respective associations with state and trait negative affect (24, 33). Similarly, attentional and affective states were shown to modulate the neural processing of respiratory stimuli (28, 30–32, 34, 35). However, respective interactions with the unpredictability of breathlessness remain poorly understood.
In the present manuscript, we review the currently available experimental evidence about the effect of unpredictability on the perception of breathlessness in the adult population. In addition, we explore potential mechanisms underlying the effect of breathlessness unpredictability such as state and trait fear/anxiety, fear of suffocation and potential psychophysiological and neural correlates (Figure 1). We also briefly discuss other clinically relevant concepts such as controllability and expectations, and make relevant parallels with the literature on unpredictable pain perception. We conclude with a brief overview of the implications for future research and clinical applications.
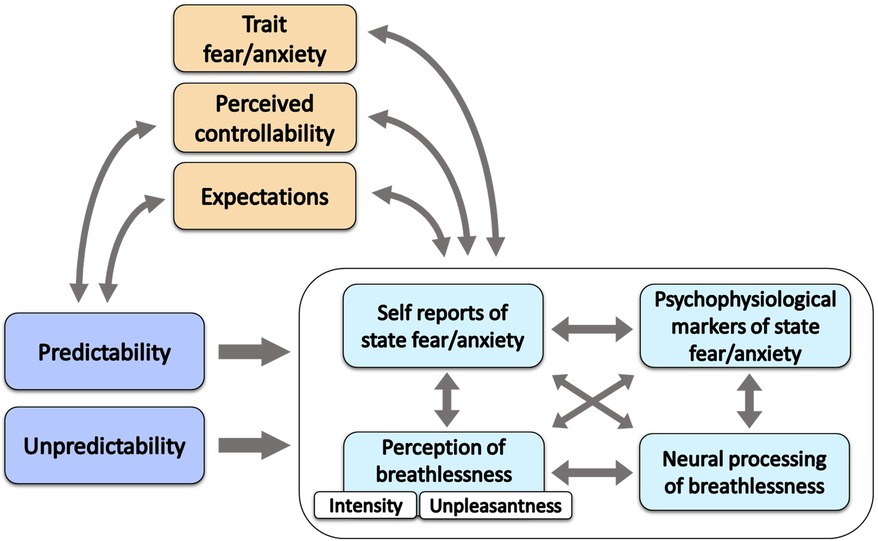
Figure 1. Schematic representation of the reviewed dynamics related to (un)predictability of breathlessness. The arrows in the figure represent possible dynamics. (Un)predictability (darker blue) may influence the components of breathlessness experience, i.e., the perception of breathlessness, the elicited affective states and their psychophysiological and neural markers (light blue). It can also be assumed that these components are interdependent. Trait fear/anxiety, perceived controllability and expectations (orange) can be associated with (un)predictability. For instance, different (un)predictable contexts may elicit different expectations, and less precise expectations can be considered more unpredictable. Moreover, unpredictability and uncontrollability are entangled as knowledge is often required to exert control and to have feelings of control. Trait fear/anxiety, perceived controllability and expectations (orange) can also modulate future breathlessness experiences (light blue) and be modulated by previous breathlessness experiences.
2 Method
We adopted a structured, but not systematic, search strategy that we present hereafter as a good practice statement and to clarify the scope of this narrative review. We searched the PubMed database for relevant articles in English language up to June 2023. The references of the included articles were also screened for other relevant studies. We accepted experimental studies measuring either of the following outcomes: self-reported breathlessness intensity, self-reported breathlessness unpleasantness, and fear-or-anxiety-related psychophysiological and electrophysiological measurements obtained in (un)predictable breathlessness contexts [e.g., electromyographic (EMG) startle response, skin conductance response (SCR), respiratory-related evoked potentials (RREP)]. Studies were valid for inclusion when one condition used more predictable breathlessness stimuli than another condition which was by consequence more unpredictable. Moreover, breathlessness stimuli had to be comparable in terms of type of breathlessness manipulation (e.g., inspiratory resistive loads, CO2 enriched-air, complete breathing obstruction…), intensity and duration between the (more) predictable and the (more) unpredictable conditions.
3 Results
3.1 Identified studies
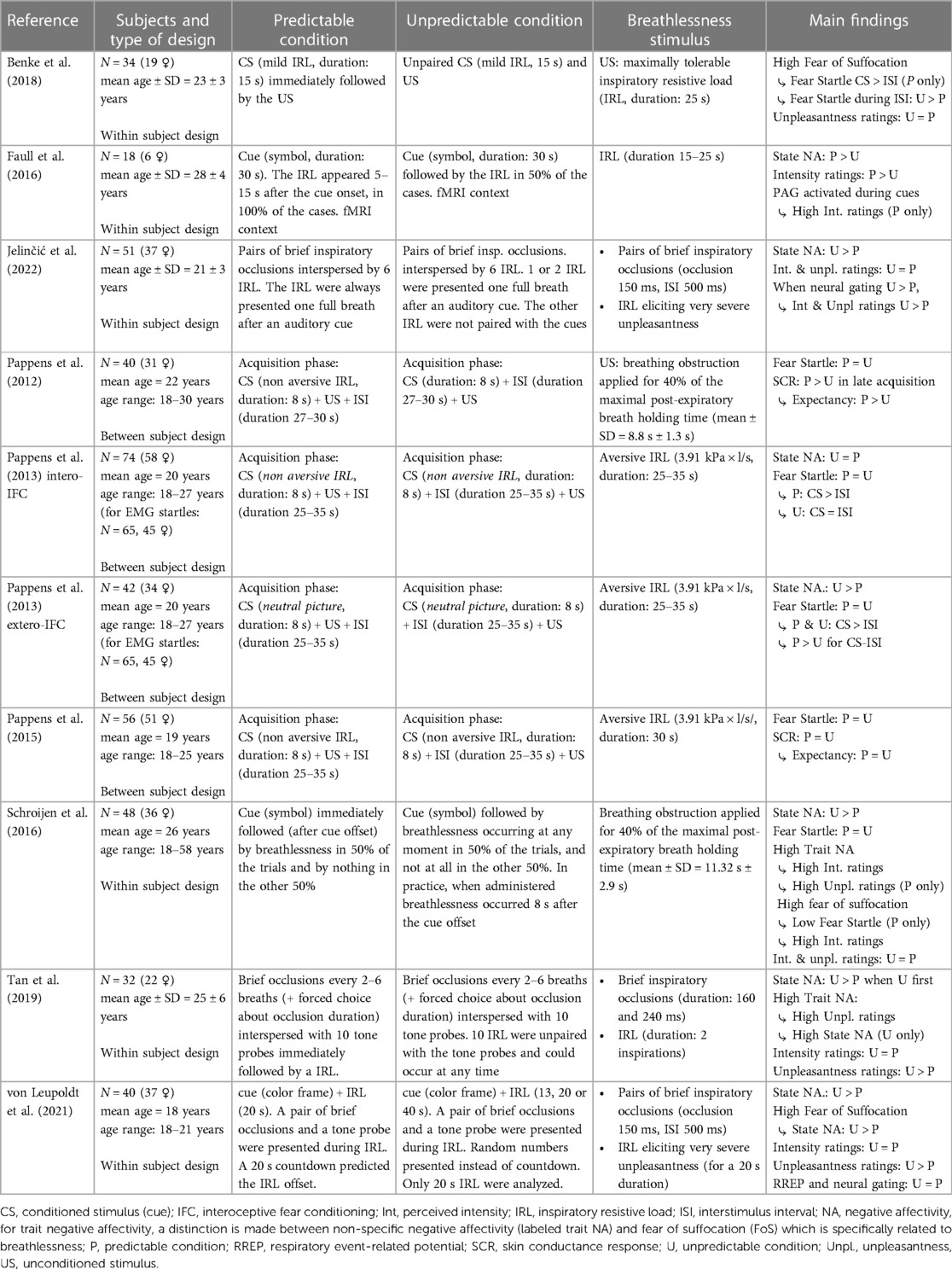
Nine relevant studies were included in this review (see summary descriptions in Table 1), with a total number of 435 participants (343 females; 92 males, mean age: 21.7 years). The experimental manipulations of breathlessness ranged from inspiratory resistive loads (inspiration difficult but possible) to inspiratory occlusions (inspiration briefly interrupted), to full breathing occlusions (inspiration and exhalation impossible). In some cases, inspiratory resistive loads and inspiratory occlusions were delivered simultaneously whereas in other cases, they were delivered alternately. All studies included self-reports and/or psychophysiological measures (e.g., SCR or EMG startle response) of state fear and anxiety. Five of these studies included measures of trait fear and anxiety (25, 36–38) or trait negative affectivity (26). Lastly, 3 studies included measures of neural activity such as functional magnetic resonance imaging (fMRI) (21) and RREPs (25, 26). Two articles were identified as relevant, but later excluded because of an absence of time interval between the conditioned stimulus CS (CO2-enriched air for 5 s) and the unconditioned stimulus US (CO2-enriched air for 15 s) in the predictable condition, and because the CS and US were not distinguishable from one another in terms of intensity, thus making the dyspneic CO2 stimulation period appear longer in the predictable than in the unpredictable condition (39, 40). Below, we will present the main results of the included studies grouped by outcome domain.
3.2 State negative affectivity
Across the reviewed studies, results mainly showed that unpredictable breathlessness induces more anxiety and fear than predictable breathlessness (25, 26, 37, 38, 41). For instance, Schroijen et al. (37) used cues to manipulate the predictability and anticipation of breathing occlusions. They found significantly higher anxiety ratings in the condition with unpredictable delayed breathing occlusions compared to the predictable condition with non-delayed breathing occlusions. Specifically, unpredictability and anticipation seems to have reduced the distinction between the threatening phases (during the cue) and the safe phases (interstimulus interval before the cue), which resulted in an increase in fear and anxiety during the safe phases of the unpredictable condition relative to the predictable condition. Similar trends towards higher fear in the condition with unpredictable and delayed inspiratory resistive loads have also been observed by Pappens et al. (41). In the same vein, further manipulations of the (un)predictability of inspiratory resistive loads by von Leupoldt et al. (25) and Jelinčić et al. (26) showed that fear of breathlessness was exacerbated in unpredictable conditions for both onset and duration types of unpredictability. The effect of onset unpredictability can however be mitigated by Tan's et al. results (38) showing that it concerns only the participants who started with the unpredictable condition, and not those who started with the predictable condition. These specific findings may also suggest that experiencing first a predictable context could decrease the threatening aspect of the following unpredictable context, hence reducing unpredictability-elicited fear. Importantly, a study of Faull et al. (21) showed results contrasting with the other studies, with higher anxiety scores in the predictable compared to the unpredictable condition. This may possibly be related to design specificities such as the fact that some elements of unpredictability were also included in the (more) predictable condition. The delivered breathlessness stimuli were induced by inspiratory resistive loads, with a 100% cue contingency in the predictable condition and a 50% cue-contingency in the unpredictable condition. However, both predictable and unpredictable conditions contained variations of anticipation (5–15 s) and stimulus duration (15–25 s) which could have dampened the distinction between predictability and unpredictability. Moreover, this study was conducted in an fMRI scanner, an environment possibly more aversive than usual laboratory settings due to supine position and/or space restriction.
Instead of using (only) subjective reports of fear and anxiety, several studies by Schroijen et al. (37) and Pappens et al. (41–43) included electromyographic measurements of the fear-potentiated startle response [a startle reflex potentiated by fear and anxiety (44)] as an indicator of the successful learning of cue-breathlessness contingencies in fear conditioning designs1. None of the breathlessness fear-conditioning studies identified for this review found overall significant differences in the magnitude of the startle responses between the predictable and unpredictable conditions. However, the authors reported that, within the predictable condition, the startle responses were more pronounced during the presentations of the conditioned stimulus1 (CS, threatening phase) than during inter-stimulus interval (safe phase). This suggests that the anticipation of an upcoming breathlessness episode during a predictable CS induces a state of fear and vigilance, which disappears during the safe interstimulus intervals (ISI). The self-reports showing an increase in fear during the late as compared to the early phase of the predictable CS presentations further support this idea of an alternance of fear and safety phases in the predictable condition (41). On the contrary, the absence of such a difference in fear between the CS and the interstimulus interval in the unpredictable conditions seems to indicate that fear is maintained during the entire unpredictable condition.
In addition to the fear-potentiated startle responses, Pappens et al. (42) measured skin conductance responses (SCR) as a fear index during the presentation of the CS. They found that the CS elicited higher SCR in the predictable than in the unpredictable condition, especially in the 2nd and 3rd blocks of the acquisition phase (i.e., in the last two blocks). The self-reports of breathlessness expectancy showed that the CS-US contingencies were learned from the 2nd block onward in the predictable condition but not in the unpredictable condition. Therefore, it can be inferred that the more elevated SCR during the predictable CS most likely represented an elevated arousal caused by the learned fear of imminent breathlessness. In a later experiment, Pappens et al. (43), did not observe any significant difference in SCR between the predictable and unpredictable conditions when differences in expectancy were also non-significant.
In short, except for one study (21) the reviewed studies consistently highlight more self-reported fear and anxiety in unpredictable breathlessness contexts. This is especially the case during safe phases (i.e., ISI) rendered less distinguishable from threatening phases by unpredictability. Physiological measures such as SCR have also suggested that the threatening phases (i.e., CS) could sometimes elicit more fear in predictable than unpredictable contexts. This effect seems likely due to fear learning which is facilitated in predictable conditions by the clear cue-breathlessness contingencies.
3.3 Trait negative affectivity
A large body of literature has shown associations between trait negative affectivity (especially fear/anxiety and depression) and increased breathlessness severity (5, 10–13, 45–48). Unsurprisingly, similar effects have also been found in the reviewed studies. For instance, Jelinčić et al. (26) measured trait negative affectivity with the Positive And Negative Affect Schedule [PANAS, (49)] and observed that higher scores were associated with higher unpleasantness ratings to the brief inspiratory occlusions. However, they did not find any interaction between trait negative affectivity and unpredictability. Likewise, Schroijen et al. (37) and Tan et al. (38) reported associations between higher anxiety sensitivity and higher breathlessness intensity (37) and breathlessness unpleasantness (38), this independently of the predictability of the stimulus. However, it can be noted that a predictability-related association between anxiety sensitivity and breathlessness unpleasantness was found by Schroijen et al. (37). No such association between anxiety sensitivity and breathlessness unpleasantness was found in the unpredictable condition. Regarding the relationship between trait and state fear/anxiety, Tan et al. (38) observed that higher anxiety sensitivity related to increased ratings of state anxiety in the unpredictable condition, but not in the predictable condition. No association between trait anxiety and state fear/anxiety was found by Schroijen et al. (37).
Fear of suffocation is another affective personality trait to consider when investigating breathlessness perception. Research has demonstrated that fear of suffocation is a more reliable predictor of state fear/anxiety than anxiety sensitivity (50). In the reviewed studies, fear of suffocation has been associated with the amplitude of the fear-potentiated startle response (36), an indicator of state fear and anxiety. In particular, the affective distress in the unpredictable breathlessness condition appeared to be accentuated by high fear of suffocation. Von Leupoldt et al. (25) observed that the participants with high relative to low fear of suffocation showed overall higher state fear and SCR during the breathlessness experiment and showed specifically higher fear reports for unpredictable than predictable breathlessness. In the same vein, Benke et al. (36) found that the participants with high fear of suffocation (compared to those with low fear of suffocation) exhibited higher startle responses during the safe phase (ISI) of the unpredictable compared to the predictable conditions. Additionally, high fear of suffocation has been shown to relate to increased state fear caused by the predictable imminence of breathlessness. In particular, Benke et al. (36) observed in participants with high fear of suffocation higher startle responses during the predictable threatening phase (CS) than during the predictable safe phase (ISI). Together, these results suggest that high fear of suffocation increases state fear and contributes to the maintenance of high levels of fear in unpredictable contexts, whereas in a predictable context, high fear of suffocation transiently increases fear only when breathlessness is imminent. However, it has to be noted that not all research findings are univocal. For example, Schroijen et al. (37) observed a contradictory negative correlation between fear of suffocation and the fear-potentiated startle in the predictable condition. Moreover, they did not find any association between fear of suffocation and self-reported state fear/anxiety. Regarding breathlessness intensity and unpleasantness, von Leupoldt et al. (36) did not find any association with fear of suffocation whereas Schroijen et al. (36) found a significant medium correlation (r = 0.38) between fear of suffocation and breathlessness intensity.
In summary, the existing literature on breathlessness unpredictability points at a potential modulatory effect of trait negative affectivity, especially fear of suffocation, on state negative affectivity. Differential modulatory effects in predictable and unpredictable contexts appear often contradictory and require more comprehensive investigations.
3.4 Perceived intensity and unpleasantness
Five studies measured the perceived intensity (21, 25, 26, 37, 38) and unpleasantness (25, 26, 36–38) of experimentally-induced predictable and unpredictable breathlessness episodes.
For breathlessness intensity, only one study by Faull et al. (21) showed greater ratings for inspiratory resistive loads in the predictable compared to the unpredictable condition. As previously stated, this study denoted from the other studies by the presence of several unpredictability components within the more predictable condition, and by its fMRI scanner environment which may have been somewhat more aversive than usual laboratory settings due to supine position or space restrictions. These potential confounds may possibly explain the heightened perceived breathlessness intensity in the predictable condition in this study, whereas the other studies did not show any significant influences of (un)predictability on breathlessness intensity perception (25, 26, 37, 38).
For breathlessness unpleasantness, the studies of Tan et al. (38), and von Leupoldt et al. (25) manipulated respectively the predictability of the onset and offset (= duration) of short episodes of breathlessness induced by inspiratory resistive loads. In both cases, more unpleasantness was found in the unpredictable conditions. Three other experiments measured unpleasantness [valence of the condition in Benke et al. (36); breathlessness unpleasantness in Jelinčić et al. (26) and Schroijen et al. (37)], but they did not find any significant modulation by (un)predictability. Interestingly, the experimental manipulation in the study by Jelinčić et al. (26) may have possibly affected breathlessness ratings. Each participant was administered two types of stimuli: electrocutaneous stimulations and resistive-load-induced breathlessness, in different blocks. These stimuli were initially calibrated to elicit the same level of unpleasantness. However, breathlessness turned out to be rated as less unpleasant (and intense) than the electrocutaneous stimulation during the experimental task. Previous work has shown that a perceptual anchor can create a floor effect in the reported perception of distant items (51). Therefore, it may be questioned whether the electrocutaneous stimulations (possible anchor) hampered unpredictability-driven modulations of breathlessness unpleasantness (distant items).
Overall, enhancing effects of unpredictability on breathlessness perception appear inconsistently and only for the unpleasantness dimension. None of the identified studies report significantly higher perception of breathlessness intensity in unpredictable as compared to predictable conditions. One studies even shows higher breathlessness intensity ratings in the predictable condition (21), but this effect could be caused by potential confounders.
3.5 Neural correlates
As compared to self-reports and psychophysiological measurements, recordings of brain activity offer a different perspective on the perception of breathlessness with more emphasis on underlying (cognitive) processes. Only a few studies have investigated the effects of unpredictability on neural correlates of breathlessness perception. One of them, conducted by Faull et al. (21), consisted of an fMRI study focusing on the activation of the periaqueductal grey (PAG) in response to predictable and unpredictable inspiratory resistive loads. The PAG is a nucleus in the midbrain which has been associated with defensive behaviors (52), with fear-anxiety (52, 53) and with pain modulation (52, 53). The PAG has also been shown to be involved in the brain processing of respiration and respiratory sensations (52, 54, 55). Faull et al. (21), showed that some areas of the PAG were activated during predictable breathlessness stimuli (lateral and ventrolateral areas) as well as during their anticipation (ventrolateral areas), but these activations were not significantly different from those in the unpredictable condition. They also report that the activation in the lateral PAG during the anticipation of predictable breathlessness stimuli was correlated with perceived breathlessness intensity, but no such effect could be found for anxiety nor for the activation of the PAG during the inspiratory resistive loads (21). Based on these results, Faull et al. (21) proposed that the PAG could play a role in the modulation of perceived breathlessness, as it does for pain (52, 53). However, they could not find any clear association between activations in the PAG and unpredictability.
The respiratory-related-evoked-potentials (RREP) are another cortical response to breathlessness, measured with an electroencephalogram (EEG). Some evidence showed that increased amplitudes of the RREP are associated with increased breathlessness perception (27, 28, 56). The RREP can also be used to assess the capacity of the brain to filter out redundant and irrelevant information related to respiration, such as the second brief inspiratory occlusion in a pair of occlusions presented within one inspiration (25, 26), a process called neural gating (25, 26, 29, 57). In other words, a redundant breathlessness stimulation would be less deeply processed by the brain, resulting in decreased RREP amplitudes and decreased breathlessness perception (27, 28, 30, 56). In a first study, von Leupoldt et al. (25) used pairs of brief inspiratory occlusions administered during breathing through inspiratory resistive loads of either predictable or unpredictable duration. In spite of having found higher breathlessness unpleasantness in the unpredictable condition, this effect did not translate into higher RREP amplitudes. Moreover, neural gating did not seem to be affected by (un)predictability. However, the methodological choice of administering the pairs of brief occlusions during inspiratory resistive loads may have contributed to shift participants' attention from the occlusions to the loads, resulting in a possible mitigation of subtle effects of unpredictability (25) on RREPs and neural gating. In a second study by Jelinčić et al. (26), the authors avoided this possible incidental attentional capture by administering the paired inspiratory occlusions alternately with (un)predictable inspiratory resistive loads. Although the authors found no main effect of unpredictability on neural gating, they found additional interesting associations between the neural gating and the perception of the brief inspiratory occlusions. Notably, they report that higher neural gating (higher RREP reduction) in the unpredictable condition was associated with higher perceived breathlessness unpleasantness and intensity of the inspiratory occlusions. These results appear counterintuitive since smaller RREPs and higher neural gating are usually associated with reduced breathlessness perception (27, 28, 30, 34, 56). In a previous study with similar counterintuitive findings (58), the authors hypothesized that, in other sensory modalities, reduced amplitudes of event-related potentials for redundant stimulations, as observed with neural gating, relate more to a decrease in saliency than to a reduction of perceived intensity (59–61). Therefore, it may be argued that the neural gating of respiratory sensations is also impacted by saliency, and perhaps only influenced by breathlessness perception in specific circumstances which remain to be identified.
Overall, the few studies exploring the neural correlates of breathlessness perception, did not find clear cut results regarding the unpredictability of breathlessness. It appears that some measures of brain activity, such as the amplitudes of event-related potentials (RREP) and the activation of the PAG, may be related to breathlessness perception, as is the case for another aversive sensation: pain. However, results for breathlessness unpredictability are scarce and sometimes surprisingly contradictory, thus pointing at the complex interplay between different cognitive processes (e.g., attention, saliency, perception) that need to be further disentangled to better understand neural correlates of breathlessness unpredictability.
4 Discussion
4.1 Summary of main findings
The most consistent finding across studies was that states of increased fear/anxiety were more frequently observed in unpredictable breathlessness conditions. The psychophysiological markers suggested that fear/anxiety is sustained during entire unpredictable conditions, but that it alternates between lower and higher states in predictable conditions depending on whether the participants experience a safe phase or a cue predicting imminent breathlessness. More constant fear/anxiety without clear safe phases may possibly explain why unpredictable breathlessness is often perceived as more unpleasant or intense in clinical (18) than non-clinical samples (25, 38).
Trait anxiety has often been associated with increased state fear/anxiety as well as with overall increased breathlessness perception. However, it does not seem to directly influence in a differential manner predictable and unpredictable episodes of breathlessness. Similarly, the results regarding fear of suffocation are not clear-cut, but suggest that it can increase state fear in both the predictable and unpredictable conditions. In other words, indirect modulatory pathways of trait negative affectivity via short-lasting affective states may exist, but require further investigations.
The reviewed experimental studies suggest that unpredictability impacts more the affective than the sensory dimension of breathlessness. Specifically, higher ratings in the unpredictable than predictable condition were more frequently reported for breathlessness unpleasantness than breathlessness intensity. However, several studies were not able to show such findings, in some cases perhaps because of differences in the employed designs and stimuli, requiring further research efforts. Moreover, observed effects of breathlessness unpredictability have not yet been clearly found to relate to neural processing patterns in specific brain areas nor to the neural gating of respiratory sensations. Neural gating may relate mainly to saliency processes (58), with effects on breathlessness perception only in some specific cases, but these hypotheses remain to be empirically confirmed.
4.2 Implication for future research and clinical practice
Given the sometimes contrasting or null findings observed in the reviewed studies, future research on the effects of unpredictability on the perception of breathlessness is needed. For example, future studies should further examine potential brain mechanisms involved with unpredictability and clarify the potential moderating role of fear of suffocation. Moreover, direct comparisons between different qualities of breathlessness stimuli (e.g., exercise-induced, CO2-induced) may potentially reveal different effects of unpredictability. Importantly, the reviewed studies included exclusively healthy volunteers whose perception of breathlessness may not fully reflect the experience of individuals afflicted with breathlessness. Thus, studies in different clinical samples suffering from breathlessness are required, especially studies in clinical settings as they would be informative to explore effects of (un)predictability during treatments of breathlessness, for example during exercise in rehabilitation contexts.
For pain, another aversive bodily sensation, evidence has shown that unpredictability does not always directly enhance pain perception (62–64). Instead, several variables including pain expectations can partially explain the relationship between unpredictability and pain perception (62–64). Such effects have not yet been systematically confirmed for breathlessness, although research has already repeatedly suggested comparable influences of expectations on breathlessness perception in both clinical and non-clinical samples (22, 65–70). Negative expectations are also an important element of catastrophizing, itself associated with worse quality of life (71). This highlights the need for future investigations into the effects of expectations on breathlessness.
In the reviewed studies, responses to unpredictable breathlessness did not seem to differ with respect to the type of unpredictability (onset, offset). However, effects of unpredictable intensities of breathlessness have not yet been investigated. According to research with other aversive bodily sensations such as pain (63, 64), this type of manipulation of unpredictability is more likely to create differences in pain perception between the predictable and unpredictable conditions because of underlying differences in expected pain intensities. In the absence of clear differences in expectations, a recent meta-analysis revealed that unpredictability did not significantly influence pain perception, and that for all types of unpredictability (onset, duration, intensity, location) (72). Whether similar effects would hold for breathlessness is currently unknown, warranting future studies. The aforementioned meta-analyses (72) also presented significant moderating effects of state negative affectivity on unpredictable pain perception echoing the present findings for breathlessness.
Controllability is entangled with predictability (73). Not all predictable events would be controllable, but controllability would require some minimal knowledge about the event (e.g., onset, offset, intensity…), therefore some predictability (73). The same reasoning implies that a completely unpredictable event is also uncontrollable. This question about the contingency between uncontrollability and unpredictability is of major importance since higher perceived control over the course of a respiratory disease has been regularly associated with lower symptom severity, lower depression and anxiety and a better quality of life (74–78). The beliefs about the lack of control on breathlessness may also cause panic (19, 79), a highly aversive state which can further increase or maintain breathlessness. Another implication could be that the detrimental effects attributed to unpredictability instead originate from uncontrollability. Therefore, careful considerations about potentially confounding effects of uncontrollability should be an integral part of future research on unpredictability, also in the field of breathlessness.
Research on unpredictability is particularly relevant to reduce the burden associated with breathlessness and to improve the quality of life of patients, two key objectives of pulmonary rehabilitation. Reducing the perceived unpredictability of breathlessness may also potentially decrease negative affect and, through the latter, breathlessness experiences (5, 6, 14, 16, 18–20). Moreover, this may encourage individuals to be more physically active and to re-engage into social activities (7–9, 18). The present review notably suggests that the absence of clear safe phases in unpredictable contexts is likely responsible for the maintenance of heightened negative affective states, hence a possible treatment target. Controllability, because of its entanglement with unpredictability, offers another possible treatment target. Restoring perceived control over breathlessness [e.g., control over catastrophizing thoughts, emotions, coping strategies… (19, 80),], as already assessed in some pulmonary rehabilitation programs (81), may possibly reduce perceived unpredictability. Yet, it has to be noted that controllability might not be beneficial to all individuals, with sometimes positive effects only for male (82) or less fearful patients (83). All these findings emphasize the need for more research on treatments, including unpredictability management.
5 Conclusion
Overall, available experimental studies about breathlessness and unpredictability are still rare and results are often not uniform. The current review provides some preliminary answers to understand why unpredictable breathlessness episodes may be particularly distressing for patients. A constant fear and unrest in unpredictable conditions seems associated with the subjective exacerbation of breathlessness, especially its unpleasantness. The known effects of trait negative affectivity on breathlessness do not appear to depend on (un)predictability. However, they suggest possible indirect effects on the perception of unpredictable breathlessness through reinforcements of state fear and anxiety. Taken together, the present observations on the unpredictability of breathlessness remain to be confirmed and extended by further investigations on different types of unpredictability and on its associations with expectations and uncontrollability, especially in clinical samples and treatment contexts. These investigations should also include studies on neural and psychophysiological correlates of unpredictable breathlessness.
Author contributions
FP: Writing – original draft. DT: Funding acquisition, Supervision, Writing – review & editing. AL: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This research was supported by an infrastructure grant from the Research Foundation Flanders (FWO) and the Research Fund KU Leuven, Belgium (AKUL/19/06; I011320N), by the “Asthenes” long-term structural funding Methusalem grant (METH/15/011) from the Flemish Government, Belgium, by a project grant from the FWO, Belgium (G0C1921N) and by a KU Leuven Starting Grant (STG/19/025) to Diana Torta.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnote
1In conditioning studies, a neutral cue (conditioned stimulus CS) is repeatedly presented with an aversive stimulus [unconditioned stimulus US, here inspiratory resistive loads (46, 48) or breathing obstructions (23, 47)] causing fear and anxiety. After an acquisition phase, the neutral cue becomes aversive and able to elicit fear and anxiety, even when the breathlessness stimulus is not jointly presented.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. American Thoracic Society. Dyspnea: mechanisms, assessment, and management, a consensus statement. Am J Respir Crit Care Med. (1999) 159:321–40. doi: 10.1164/ajrccm.159.1.ats898
2. Parshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL, Bourbeau J, et al. An official American thoracic society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. (2012) 185:435–52. doi: 10.1164/rccm.201111-2042ST
3. Grønseth R, Vollmer WM, Hardie JA, Ólafsdóttir IS, Lamprecht B, Buist AS, et al. Predictors of dyspnoea and prevalence: results from the BOLD study. Eur Respir J. (2013) 43:1610–20. doi: 10.1183/09031936.00036813
4. Bowden JA, To THM, Abernethy AP, Currow DC. Predictors of chronic breathlessness: a large population study. BMC Public Health. (2011) 11, Available online at: http://www.biomedcentral.com/1471-2458/11/33. doi: 10.1186/1471-2458-11-33
5. Sandberg J, Olsson M, Ekström M. Underlying conditions contributing to breathlessness in the population. Curr Opin Support Palliat Care. (2021) 15:219–55. doi: 10.1097/SPC.0000000000000568
6. Banzett RBB, Sheridan AR, Baker KM, Lansing RW, Stevens JP. “Scared to death” dyspnea from the hospitalized patient’s perspective. BMJ Open Respir Res. (2020) 7. doi: 10.1136/bmjresp-2019-000493
7. Brighton LJ, Chilcot J, Maddocks M. Social dimensions of chronic respiratory disease: stigma, isolation, and loneliness. Curr Opin Support Palliat Care. (2022) 16:195–202. doi: 10.1097/SPC.0000000000000616
8. Hutchinson A, Barclay-Klingle N, Galvin K, Johnson MJ. Living with breathlessness: a systematic literature review and qualitative synthesis. Eur Respir J. (2018) 51. doi: 10.1183/13993003.01477-2017
9. Lovell N, Etkind SN, Bajwah S, Maddocks M, Higginson IJ. Control and context are central for people with advanced illness experiencing breathlessness: a systematic review and thematic synthesis. J Pain Symptom Manag. (2018) 57:140–55. doi: 10.1016/j.jpainsymman.2018.09.021
10. McKenzie E, Zhang L, Chan S, Zaki P, Razvi Y, Tsao M, et al. Symptom correlates of dyspnea in advanced cancer patients using the Edmonton symptom assessment system. Support Care Cancer. (2019) 28:87–98. doi: 10.1007/s00520-019-04787-0
11. Trevisan C, Vianello A, Zanforlini BM, Curreri C, Maggi S, Noale M, et al. The mutual association between dyspnea and depressive symptoms in older adults: a 4-year prospective study. Aging Ment Health. (2019) 24:993–1000. doi: 10.1080/13607863.2019.1582005
12. von Siemens SM, Jorres RA, Behr J, Alter P, Lutter J, Lucke T, et al. Effect of COPD severity and comorbidities on the results of the PHQ-9 tool for the diagnosis of depression: results from the COSYCONET cohort study. Respir Res. (2019) 20:30–40. doi: 10.1186/s12931-019-0997-y
13. Yohannes AM, Dryden S, Hanania NA. Validity and responsiveness of the depression-anxiety stress scales-21 (DASS-21) in COPD. Chest. (2018) 155:1166–77. doi: 10.1016/j.chest.2018.12.010
14. von Leupoldt A, Denutte Y. Affective traits, states and breathlessness. Curr Opin Support Palliat Care. (2020) 14:182–9. doi: 10.1097/SPC.0000000000000506
15. Reitzel T, Bergmann A, Schloesser K, Pauli B, Eisenmann Y, Randerath W, et al. The experience of episodic breathlessness from the perspective of informal caregivers: a qualitative interview study. Ann Palliat Med. (2022) 11:2225–34. doi: 10.21037/apm-21-336
16. Simon TS, Higginson IJ, Benalia H, Gysels M, Murtagh FEM, Spicer J, et al. Episodes of breathlessness: types and patterns – a qualitative study exploring experience of patients with advanced diseases. Palliat Med. (2013) 27:524–32. doi: 10.1177/0269216313480255
17. Simon ST, Weingärtner V, Econ H, Higginson IJ, Raymond V, Bausewein C. Definition, categorization, and terminology of episodic breathlessness: consensus by an international delphi survey. J Pain Symptom Manag. (2014) 47:828–38. doi: 10.1016/j.jpainsymman.2013.06.013
18. Linde P, Hanke G, Voltz R, Simon ST. Unpredictable episodic breathlessness in patients with advanced chronic obstructive pulmonary disease and lung cancer: a qualitative study. Support Care Cancer. (2017) 26:1097–104. doi: 10.1007/s00520-017-3928-9
19. Schloesser K, Bergmann A, Eisenmannn Y, Pauli B, Pralong A, Hellmich M, et al. Interaction of panic and episodic breathlessness among patients with life-limiting diseases: a cross-sectional study. Ann Palliat Med. (2023) 12. doi: 10.21037/apm-22-1304
20. Van Diest I. Interoception, conditioning, and fear: the panic threesome. Psychophysiology. (2023) 56. doi: 10.1111/psyp.13421
21. Faull OK, Jenkinson M, Ezra M, Pattinson KTS. Conditioned respiratory threat in the subdivisions of the human periaqueductal gray. eLife. (2016) 5. doi: 10.7554/eLife.12047
22. Marlow LL, Faull OK, Finnegan SL, Pattinson KTS. Breathlessness and the brain: the role of expectation. Curr Opin Support Palliat Care. (2019) 13:200–10. doi: 10.1097/SPC.0000000000000441
23. Pattinson KTS, Johnson MJ. Neuroimaging of central breathlessness mechanisms. Curr Opin Support Palliat Care. (2014) 8:225–33. doi: 10.1097/SPC.0000000000000069
24. Stoeckel MC, Esser RW, Gamer M, Büchel C, von Leupoldt A. Dyspnea catastrophizing and neural activations during the anticipation and perception of dyspnea. Psychophysiology. (2017) 55:1–10. doi: 10.1111/psyp.13004
25. von Leupoldt A, Ashoori M, Jelinčić V, Herzog M, Van Diest I. The impact of unpredictability of dyspnea offset on dyspnea perception, fear, and respiratory neural gating. Psychophysiology. (2021) 58. doi: 10.1111/psyp.13807
26. Jelinčić V, Torta DM, Van Diest I, von Leupoldt A. The effects of unpredictability and negative affect on perception and neural gating in different interoceptive modalities. Biol Psychol. (2022) 169. doi: 10.1016/j.biopsycho.2022.108267
27. von Leupoldt A, Bradley MM, Lang PJ, Davenport PW. Neural processing of respiratory sensations when breathing becomes more difficult and unpleasant. Front Physiol. (2010) 1. doi: 10.3389/fphys.2010.00144
28. Reijnders T, Troosters T, Janssens W, Gosselink R, Langer D, Davenport PW, et al. Brain activations to dyspnea in patients with COPD. Front Physiol. (2020) 11. doi: 10.3389/fphys.2020.00007
29. Chan P-YS, Davenport PW. Respiratory-related evoked potential measures of respiratory sensory gating. J Appl Physiol. (2008) 105:1106–13. doi: 10.1152/japplphysiol.90722.2008
30. Herzog M, Sucec J, Van Diest I, Van den Bergh O, Chan P-YS, Davenport P, et al. Reduced neural gating of respiratory sensations is associated with increased dyspnoea perception. Eur Respir J. (2018a) 52. doi: 10.1183/13993003.00559-2018
31. Herzog M, Sucec J, Van Diest I, Van den Bergh O, Chenivesse C, Davenport P, et al. Observing dyspnoea in others elicits dyspnoea, negative affect and brain responses. Eur Respir J. (2018) 51. doi: 10.1183/13993003.02682-2017
32. von Leupoldt A, Chan P-YS, Bradley MM, Lang PJ, Davenport PW. The impact of anxiety on the neural processing of respiratory sensation. NeuroImage. (2011) 55:247–52. doi: 10.1016/j.neuroimage.2010.11.050
33. Chan P-YS, von Leupoldt A, Liu C-Y, Hsu S-C. Respiratory perception measured by cortical neural activations in individuals with generalized anxiety disorders. Respir Physiol Neurobiol. (2014) 204:36–40. doi: 10.1016/j.resp.2014.09.009
34. Chan P-Y, Cheng C-H, Jhu Y-J, Chen C-L, von Leupoldt A. Being anxious, thinking positively: the effect of emotional context on respiratory sensory gating. Front Psychol. (2016) 7. doi: 10.3389/fphys.2016.00019
35. von Leupoldt A, Chan P-YS, Esser R, Davenport P. Emotions and neural processing of respiratory sensations investigated with respiratory-related evoked potentials. Psychosom Med. (2013) 73:244–52. doi: 10.1097/PSY.0b013e31828251cf
36. Benke C, Alius MG, Hamm AO, Pané-Farré CA. Cue and context conditioning to respiratory threat: effects of suffocation fear and implications for the etiology of panic disorder. Int J Psychophysiol. (2020) 124:33–42. doi: 10.1016/j.ijpsycho.2018.01.002
37. Schroijen M, Fantoni S, Rivera C, Vervliet B, Schruers K, Van den Bergh O, et al. Defensive activation to (un)predictable interoceptive threat: the NPU respiratory threat test (NPUr). Psychophysiology. (2016) 53:905–13. doi: 10.1111/psyp.12621
38. Tan Y, Van den Bergh O, Qiu J, von Leupoldt A. The impact of unpredictability on dyspnea perception, anxiety and interoceptive error processing. Front Physiol. (2019) 10. doi: 10.3389/fphys.2019.00535
39. Acheson DT, Forsyth JP, Prenoveau JM, Bouton ME. Interoceptive fear conditioning as a learning model of panic disorder: an experimental evaluation using 20% CO2-enriched air in a non-clinical sample. Behav Res Ther. (2007) 45:2280–94. doi: 10.1016/j.brat.2007.04.008
40. Acheson DT, Forsyth JP, Moses E. Interoceptive fear conditioning and panic disorder: the role of conditioned stimulus-unconditioned stimulus predictability. Behav Ther. (2012) 43:174–89. doi: 10.1016/j.beth.2011.06.001
41. Pappens M, Van den Bergh O, Vansteenwegen D, Ceunen E, De Peuter S, Van Diest I. Learning to fear obstructed breathing: comparing interoceptive and exteroceptive cues. Biol Psychol. (2013) 92:36–42. doi: 10.1016/j.biopsycho.2011.05.004
42. Pappens M, Smets E, Vansteenwegen D, Van den Bergh O, Van Diest I. Learning to fear of suffocation: a new paradigm for interoceptive fear conditioning. Psychophysiology. (2012) 49:821–8. doi: 10.1111/j.1469-8986.2012.01357.x
43. Pappens M, Vandenbossche E, Van den Bergh O, Van Diest I. Interoceptive fear learning to mild breathlessness as a laboratory model for unexpected panic attacks. Front Psychol. (2015) 6. doi: 10.3389/fpsyg.2015.01150
44. Grillon C, Ameli R, Foot M, Davis M. Fear-potentiated startle: relationship to the level of state/trait anxiety in healthy subjects. Biol Psychiatry. (1993) 33:566–74. doi: 10.1016/0006-3223(93)90094-T
45. Janssens T, Van de Moortel Z, Geidl W, Carl J, Pfeifer K, Lehbert N, et al. Impact of disease-specific fears on pulmonary rehabilitation trajectories in patients with COPD. J Clin Med. (2019) 8. doi: 10.3390/jcm8091460
46. Put C, Van den Bergh O, Van Ongeval E, De Peuter S, Demedts M, Verleden G. Negative affectivity and the influence of suggestion on asthma symptoms. J Psychosom Res. (2004) 57:249–55. doi: 10.1016/S0022-3999(03)00541-5
47. Reijnders T, Schuler M, Wittman M, Jelusic D, Troosters T, Janssens W, et al. The impact of disease-specific fears on outcome measures of pulmonary rehabilitation in patients with COPD. Respir Med. (2019) 146:87–95. doi: 10.1016/j.rmed.2018.12.004
48. von Leupoldt A, Taube K, Lehmann K, Fritzsche A, Magnussen H. The impact of anxiety and depression on outcomes of pulmonary rehabilitation in patients with COPD. Chest. (2011) 140:730–6. doi: 10.1378/chest.10-2917
49. Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. (1988) 54:1063–70. doi: 10.1037//0022-3514.54.6.1063
50. McNally RJ, Eke M. Anxiety sensitivity, suffocation fear, and breath-holding duration as predictors of response to carbon dioxide challenge. J Abnorm Psychol. (1996) 105:146–9. doi: 10.1037/0021-843X.105.1.146
51. Sherif M, Taub D, Hovland CI. Assimilation and contrast effects of anchoring stimuli on judgments. J Exp Psychol. (1958) 55:150–5. doi: 10.1037/h0048784
52. Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol. (1995) 46:575–605. doi: 10.1016/0301-0082(95)00009-K
53. Linnman C, Moulton EA, Barmettler G, Becerra L, Borsook D. Neuroimaging of the periaqueductal gray: state of the field. NeuroImage. (2012) 60:505–22. doi: 10.1016/j.neuroimage.2011.11.095
54. von Leupoldt A, Sommer T, Kegat S, Eippert F, Baumann HJ, Klose H, et al. Down-regulation of insular cortex responses to dyspnea and pain in asthma. Am J Respir Crit Care Med. (2009) 180:200–6. doi: 10.1164/rccm.200902-0300OC
55. von Leupoldt A, Brassen S, Baumann HJ, Klose H, Büchel C. Structural brain changes related to disease duration in patients with asthma. PLoS One. (2011) 6. doi: 10.1371/journal.pone.0023739
56. von Leupoldt A, Vovk A, Bradley MM, Lang PJ, Davenport PW. Habituation in neural processing and subjective perception of respiratory sensations. Psychophysiology. (2011) 48:808–12. doi: 10.1111/j.1469-8986.2010.01141.x
57. Chan P-YS, Chang W-P, Cheng C-H, Liu C-Y, von Leupoldt A, Hsu A-L, et al. The impact of emotional context on neural substrates of respiratory sensory gating. Front Neurosci. (2022) 16. doi: 10.3389/fnins.2022.1004271
58. Jelinčić V, Torta DM, Van Diest I, von Leupoldt A. Cross-modal relationships of neural gating with the subjective perception of respiratory and somatosensory sensations. Psychophysiology. (2020) 58. doi: 10.1111/psyp.13710
59. Iannetti GD, Hughes NP, Lee MC, Mouraux A. Determinants of laser-evoked EEG responses: pain perception or stimulus saliency? J Neurophysiol. (2008) 100:815–28. doi: 10.1152/jn00097.2008
60. Wang AL, Mouraux A, Liang M, Iannetti GD. Stimulus novelty, and not neural refractoriness, explains the repetition suppression of laser-evoked potentials. J Neurophysiol. (2010) 104:2116–24. doi: 10.1152/jn.01088.2009
61. Van den Broeke E, Van Rijn CM, Biurrun Manresa JA, Andersen OK, Arendt-Nielsen L, Wilder-Smith OHG. Neurophysiological correlates of nociceptive heterosynaptic long-term potentiation in humans. J Neurophysiol. (2010) 103:2107–13. doi: 10.1152/jn.00979.2009
62. Zaman J, Van Oudenhove L, Vlaeyen JWS. Uncertainty in a context of pain: disliked but also more painful? Pain. (2020) 162:995–8. doi: 10.1097/j.pain.0000000000002106
63. Brown CA, Seymour B, Boyle Y, El-Deredy W, Jones AKP. Modulation of pain ratings by expectation and uncertainty: behavioral characteristics and anticipatory neural correlates. Pain. (2008) 135:240–50. doi: 10.1016/j.pain.2007.05.022
64. Pavy F, Zaman J, von Leupoldt A, Torta D. Expectations underlie the effects of unpredictable pain: a behavioral and EEG study. Pain. (2023). doi: 10.1097/j.pain.0000000000003046
65. De Peuter S, Van Diest I, Lemaigre V, Li W, Verleden G, Demedts M, et al. Can subjective asthma symptoms be learned? Psychosom Med. (2005) 67:454–61. doi: 10.1097/01.psy.0000160470.43167.e2
66. Finnegan SL, Dearlove DJ, Morris P, Freeman D, Sergeant M, Taylor S, et al. Breathlessness in a virtual world: an experimental paradigm testing how discrepancy between VR visual gradients and pedal resistance during stationary cycling affects breathlessness perception. PLoS One. (2023) 18. doi: 10.1371/journal.pone.0270721
67. Jaén C, Dalton P. Asthma and odors: the role of risk perception in asthma exacerbation. J Psychosom Res. (2014) 77:302–8. doi: 10.1016/j.jpsychores.2014.07.002
68. Vleminckx E, Sprenger C, Büchel C. Expectation and dyspnoea: the neurobiological basis of respiratory nocebo effects. Eur Respir J. (2021) 58. doi: 10.1183/13993003.03008-2020
69. Van den Bergh O, Stegen K, Van Diest I, Raes C, Stulens P, Eelen P, et al. Acquisition and extinction of somatic symptoms in response to odours: a pavlovian paradigm relevant to multiple chemical sensitivity. Occup Environ Med. (1999) 56:295–301. doi: 10.1136/oem.56.5.295
70. Vinckier F, Betka S, Nion N, Serresse L, Similowski T. Harnessing the power of anticipation to manage respiratory-related brain suffering and ensuing dyspnoea: insights from the neurobiology of the respiratory nocebo effect. Eur Respir J. (2021) 58. doi: 10.1183/13993003.01876-2021
71. Maras D, Balfour L, Tasca GA, Gaudet E, Aaron SD, Cameron WD, et al. Breathlessness catastrophizing relates to poorer quality of life in adults with cystic fibrosis. J Cyst Fibros. (2019) 18:150–7. doi: 10.1016/j.jcf.2018.08.008
72. Pavy F, Zaman J, Van den Noortgate W, Scarpa A, von Leupoldt A, Torta D. The effect of unpredictability on the perception of pain: a systematic review and meta-analysis. PsyarXiv. (2023). doi: 10.31234/osf.io/4wez7
73. Mineka S, Kihlstrom JF. Unpredictable and uncontrollable events: a new perspective on experimental neurosis. J Abnorm Psychol. (1978) 2:256–71. doi: 10.1037/0021-843X.87.2.256
74. Borges-Santos E, Wada JT, da Silva CM, Silva RA, Stelmach R, Carvalho CR, et al. Anxiety and depression are related to dyspnea and clinical control but not with thoracoabdominal mechanics in patients with COPD. Respir Physiol Neurobiol. (2015) 210:1–6. doi: 10.1016/j.resp.2015.01.011
75. Calfee C, Katz P, Yelin E, Iribarren C, Eisner M. The influence of perceived control of asthma on health outcomes. Chest. (2006) 130:1312–8. doi: 10.1378/chest.130.5.1312
76. Katz PP, Yelin EH, Eisner MD, Blanc PD. Perceived control of asthma and quality of life among adults with asthma. Ann Allergy Asthma Immunol. (2002) 89:251–8. doi: 10.1016/S1081-1206(10)61951-5
77. Simpson E, Jones MC. An exploration of self-efficacy and self-management in COPD patients. Br J Nurs. (2013) 22:1105–9. doi: 10.12968/bjon.2013.22.19.1105
78. Stubs MA, Clark VL, Gibson PG, Yorke J, McDonald VM. Associations of symptoms of anxiety and depression with health-status, asthma control, dyspnoea, dysfunction breathing and obesity in people with severe asthma. Respir Res. (2022) 23:341–53. doi: 10.1186/s12931-022-02266-5
79. Hallas CN, Howard C, Theadom A, Wray J. Negative beliefs about breathlessness increases panic for patients with chronic respiratory disease. Psychol Health Med. (2012) 17:467–77. doi: 10.1080/13548506.2011.626434
80. Booth S, Johnson MJ. Improving the quality of life of people with advanced respiratory disease and severe breathlessness. Breathe. (2019) 15:198–215. doi: 10.1183/20734735.0200-2019
81. Fischer M, Scharloo M, Abbink J, Van’t Hul A, Van Ranst D, Rudolphus A, et al. The dynamics of illness perceptions: testing assumptions of Leventhal’s common-sense model in a pulmonary rehabilitation setting. Br J Health Psychol. (2010) 15:887–903. doi: 10.1348/135910710X492693
82. Brown JC, Boat R, Williams NC, Johnson MA, Sharpe GR. The effect of trait self-control on dyspnoea and tolerance to a CO2 rebreathing challenge in healthy males and females. Physiol Behav. (2022) 255. doi: 10.1016/j.physbeh.2022.113944
Keywords: breathlessness, dyspnea, respiration, unpredictability, uncertainty, fear, anxiety, neural processing
Citation: Pavy F, Torta DM and von Leupoldt A (2024) The effect of unpredictability on the perception of breathlessness: a narrative review. Front. Rehabil. Sci. 4:1339072. doi: 10.3389/fresc.2023.1339072
Received: 15 November 2023; Accepted: 21 December 2023;
Published: 9 January 2024.
Edited by:
Eleonora Volpato, Fondazione Don Carlo Gnocchi Onlus (IRCCS), ItalyReviewed by:
Olivia Kate Harrison, University of Otago, New ZealandCátia Paixão, University of Aveiro, Portugal
© 2024 Pavy, Torta and von Leupoldt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fabien Pavy fabien.pavy@kuleuven.be
 Fabien Pavy
Fabien Pavy Diana M. Torta
Diana M. Torta Andreas von Leupoldt
Andreas von Leupoldt