- 1Warwickshire Institute for the Study of Diabetes, Endocrinology and Metabolism (WISDEM), University Hospitals Coventry and Warwickshire NHS Trust, Coventry, United Kingdom
- 2Warwick Medical School, University of Warwick, Coventry, United Kingdom
- 3School of Health and Society, Faculty of Education, Health and Wellbeing, University of Wolverhampton, Wolverhampton, United Kingdom
- 4Centre for Sport, Exercise and Life Sciences, Research Institute for Health and Wellbeing, Coventry University, Coventry, United Kingdom
- 5Chester Medical School, University of Chester, Shrewsbury, United Kingdom
- 6Clinical Evidence-Based Information Service (CEBIS), University Hospitals Coventry and Warwickshire NHS Trust, Coventry, United Kingdom
- 7Department of Biochemistry and Immunology, University Hospitals Coventry and Warwickshire NHS Trust, Coventry, United Kingdom
- 8Institute for Cardiometabolic Medicine, University Hospitals Coventry and Warwickshire NHS Trust, Coventry, United Kingdom
- 9Aston Medical School, College of Health and Life Sciences, Aston University, Birmingham, United Kingdom
- 10College of Health, Psychology and Social Care, University of Derby, Derby, United Kingdom
- 11Laboratory of Dietetics and Quality of Life, Department of Food Science and Human Nutrition, School of Food and Nutritional Sciences, Agricultural University of Athens, Athens, Greece
Background: Gestational diabetes mellitus (GDM) is a prevalent condition where diabetes is diagnosed during pregnancy, affecting both maternal and fetal outcomes. Retinol-binding protein 4 (RBP4) is a circulating adipokine which belongs to the lipocalin family and acts as a specific carrier protein that delivers retinol (vitamin A) from the liver to the peripheral tissues. Growing data indicate that circulating RBP4 levels may positively correlate with GDM. Thus, this systematic review and meta-analysis aimed to investigate the potential relationship between circulating RBP4 levels and GDM when measured at various stages of pregnancy.
Methods: MEDLINE, CINAHL, EMCARE, EMBASE, Scopus, and Web of Science databases were searched to identify studies comparing pregnant women with and without GDM, whose circulating RBP4 levels were measured in at least one pregnancy trimester. Findings were reported using standardized mean difference (SMD) and random-effects models were used to account for variability among studies. Furthermore, the risk of bias was assessed using the RoBANS tool.
Results: Out of the 34 studies identified, 32 were included in the meta-analysis (seven with circulating RBP4 levels measured in the first trimester, 19 at 24–28 weeks, and 14 at >28 weeks of pregnancy). RBP4 levels were statistically higher in the GDM group than in controls when measured during all these pregnancy stages, with the noted RBP4 SMD being 0.322 in the first trimester (95% CI: 0.126–0.517; p < 0.001; 946 GDM cases vs. 1701 non-GDM controls); 0.628 at 24–28 weeks of gestation (95% CI: 0.290–0.966; p < 0.001; 1776 GDM cases vs. 1942 controls); and 0.875 at >28 weeks of gestation (95% CI: 0.252–1.498; p = 0.006; 870 GDM cases vs. 1942 non-GDM controls). Significant study heterogeneity was noted for all three pregnancy timepoints.
Conclusion: The present findings indicate consistently higher circulating RBP4 levels in GDM cases compared to non-GDM controls, suggesting the potential relevance of RBP4 as a biomarker for GDM. However, the documented substantial study heterogeneity, alongside imprecision in effect estimates, underscores the need for further research and standardization of measurement methods to elucidate whether RBP4 can be utilized in clinical practice as a potential GDM biomarker.
Systematic review registration: PROSPERO (CRD42022340097: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022340097).
1 Introduction
Diabetes diagnosed during pregnancy, i.e., gestational diabetes mellitus (GDM), is a highly prevalent condition that is typically characterized by hyperglycemia, glucose intolerance, and insulin resistance, potentially resulting in adverse effects for both the mother and the fetus (1). The reported GDM prevalence rates range from 1 to 14% depending on the studied population, with Asia, Latin America, and the Middle East regions exhibiting higher prevalence rates, whilst inconsistencies in the testing protocols and diagnostic criteria further contribute to the varying GDM prevalence rates reported worldwide (2). In the United Kingdom, approximately 1 in 23 pregnancies is affected by GDM (3). GDM frequently resolves soon after delivery, but these women are more likely to experience GDM in subsequent pregnancies and have an increased risk of later developing type 2 diabetes (4, 5).
Several factors contribute to a higher risk of developing GDM, including an increased body mass index (BMI) at overweight or obesity levels, excessive weight gain during pregnancy, specific ethnic backgrounds (e.g., women from South Asia), genetic factors, a personal or family history of GDM, and polycystic ovary syndrome (PCOS) (6–8). Currently, to diagnose GDM, most pregnant women are offered an oral glucose tolerance test (OGTT) between 24 and 28 weeks of gestation or earlier for those considered at high risk (9). However, using pre-diagnostic risk factor screening alone is not always an effective method of identifying women at risk of GDM, as shown by meta-analysis data (9). This highlights that there is still a need for novel biomarkers to more accurately identify women at high GDM risk. As such, recent research in the field of GDM has focused on studying an array of biomarkers which can be measured in the circulation of pregnant women and are linked to the complex pathophysiology of the condition, such as biomarkers associated with obesity-related inflammation, insulin resistance, and those derived from the adipose tissue (i.e., adipokines) or the placenta.
Retinol-binding protein 4 (RBP4) is a 21-kDa protein (10), which is secreted mainly by the liver and adipose tissue, and was initially identified as a transport protein for retinol (vitamin A) and other retinoid derivatives in the bloodstream (11). A 2005 study showed for the first time the potential involvement of RBP4 in the pathogenesis of type 2 diabetes (11), with the expression of RBP4 playing a regulatory role in glucose metabolism in both the liver and skeletal muscle. Indeed, the decreased expression of glucose transporter-4 (GLUT4) is linked to increased RBP4 secretion from the adipose tissue, which leads to increased hepatic gluconeogenesis and reduced glucose uptake in the muscle, ultimately resulting in increased blood glucose levels, impaired glucose tolerance, and diabetes (12). Furthermore, recent studies have also revealed close associations between RBP4 and cardiovascular disease (CVD) and related risk factors, such as obesity, hypertension, dyslipidemia, heart failure, and coronary heart disease (10).
In this context, there has been increasing interest in investigating the potential role of RBP4 as a novel biomarker for GDM. However, the reported results have been inconsistent, with previous meta-analyses suggesting that serum RBP4 levels in early pregnancy show an independent positive association with GDM risk (13), and that Asian women with GDM had increased circulating RBP4 levels during the second/third pregnancy trimester (14). Although such data support the hypothesis that circulating RBP4 may be linked to GDM (15), there is still a need for a comprehensive systematic analysis and an updated meta-analysis of the relevant published studies examining the association between GDM and circulating RBP4 levels measured during all pregnancy stages/trimesters. Therefore, the present systematic review and meta-analysis aimed to explore this potential relationship across the pregnancy duration, providing an up-to-date critical synthesis of the relevant available data.
2 Materials and methods
The present systematic review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) (16) guidelines (Supplementary Table S1.1), and was prospectively registered on PROSPERO (International Prospective Register of Systematic Reviews – University of York), with the registration number CRD42022340097.
2.1 Search strategy and data sources
A search was conducted based on a predefined search strategy and was adapted to the syntax and appropriate subject headings of the following databases: MEDLINE, CINAHL, EMCARE, EMBASE via Ovid, Scopus, and Web of Science. Reference lists were also browsed to ensure literature saturation. Final searches were completed in June 2023, and the main search strategy for MEDLINE is presented in Table 1, whilst all other search strategies are detailed in Supplementary data and Supplementary material 1.2.
2.2 Eligibility criteria
Eligible articles included those conducted in adult (age > 18 years old) pregnant women with and without GDM, whose circulating levels of RBP4 were measured during at least one pregnancy trimester. No restrictions were imposed regarding the year of publication, type of setting, language, or timing of RBP4 measurement during the pregnancy. All observational study designs were included, while single case reports, expert opinion manuscripts, commentaries, animal studies, and review articles were excluded.
2.3 Study selection and data extraction
The study selection and data extraction processes were conducted independently by two reviewers (BML and LL), and any discrepancies or disagreements were resolved through consultation with a third reviewer (CK).
The initial selection of potentially eligible studies was based on title and abstract screening and was performed using the Rayyan software (17), following a predefined protocol. Papers considered eligible progressed to a full-text review.
A standardized data extraction form was developed to extract relevant information from the included eligible studies. The extracted data included country of origin, study design, patient demographics, number of participants, and relevant study outcomes/findings (e.g., circulating RBP4 levels). In addition, attempts were made to contact the corresponding study investigators in cases where relevant data on circulating RBP4 levels were missing or reported as median and interquartile range (IQR). Where relevant responses were not received (18–25), median and IQR data were transformed using the formulas provided by Luo et al. (26) and Wan et al. (27). Furthermore, for one study (28) these values were extracted from figures using a plot digitizer,1 as previously reported (29).
Herein, data on circulating RBP4 levels are reported as mean and standard deviations (SDs) (30). For certain included studies (25, 31–33), it was necessary to combine study groups; this was done using recommended formulae (34).
When multiple methods were used to measure circulating RBP4 levels (23, 24), the enzyme linked immunosorbent assay (ELISA) result was chosen as the most commonly utilized method. Additionally, for Tepper et al. (35), a sensitivity analysis was conducted by switching the data to Western Blot due to the differences observed between measurements.
2.4 Quality assessment
The risk of bias for each included study was assessed independently by two reviewers (BML and LL) using the Risk of Bias Assessment Tool for Nonrandomized Studies (RoBANS) (36), which covers six domains, namely: selection of participants, confounding variables, exposure measurement, blinding of outcome assessment, incomplete outcome data, and selective outcome reporting. For each domain, the risk of bias was assessed as low, high, or unclear. Any disagreements were resolved through discussion between reviewers and if needed, consultation with a third reviewer (CK).
2.5 Statistical analysis
The statistical analysis was performed using Comprehensive Meta-Analysis Version 4.0 (37). The results were reported using the standardized mean difference (SMD) to quantify the magnitude of the effect and 95% confidence intervals (CI) as a measure of precision around effect estimates. The effect size represents the SMD between circulating RBP4 levels in the GDM group and the pregnant control group at different timepoints (i.e., at the first trimester, 24–28 weeks of gestation, and > 28 weeks of gestation).
A random-effects model was used for the performed meta-analysis, and the effect size for each timepoint was calculated. Heterogeneity among studies was assessed using Cochran’s Q and I2 statistics, and was considered significant if p < 0.1 in the Q-test whilst for the I2: 0–40% heterogeneity might not be important; 30–60% may represent moderate heterogeneity; 50–90% may represent substantial heterogeneity; and 75–100% represents considerable heterogeneity (30).
To investigate heterogeneity, we sub-grouped studies based upon the country in which they were conducted, the diagnostic criteria used to identify GDM cases, and the RBP4 measurement method/assay. It was not possible to sub-group based upon any other variable due to the incompleteness of reporting. Supplementary Table S2.1 presents the summary of effect estimates and heterogeneity for the sub-groups at each pregnancy stage.
For the studies where mean and SDs were calculated (18–25), sensitivity analysis was performed, removing studies that contained data significantly skewed away from the normal distribution (19, 21, 22, 24).
Where analyses included ten or more studies (30), publication bias was assessed using the Egger’s test and regression intercept. Additionally, a Duval and Tweedie’s trim-and-fill analysis was conducted to obtain an adjusted summary effect that accounts for publication bias.
3 Results
3.1 Study selection
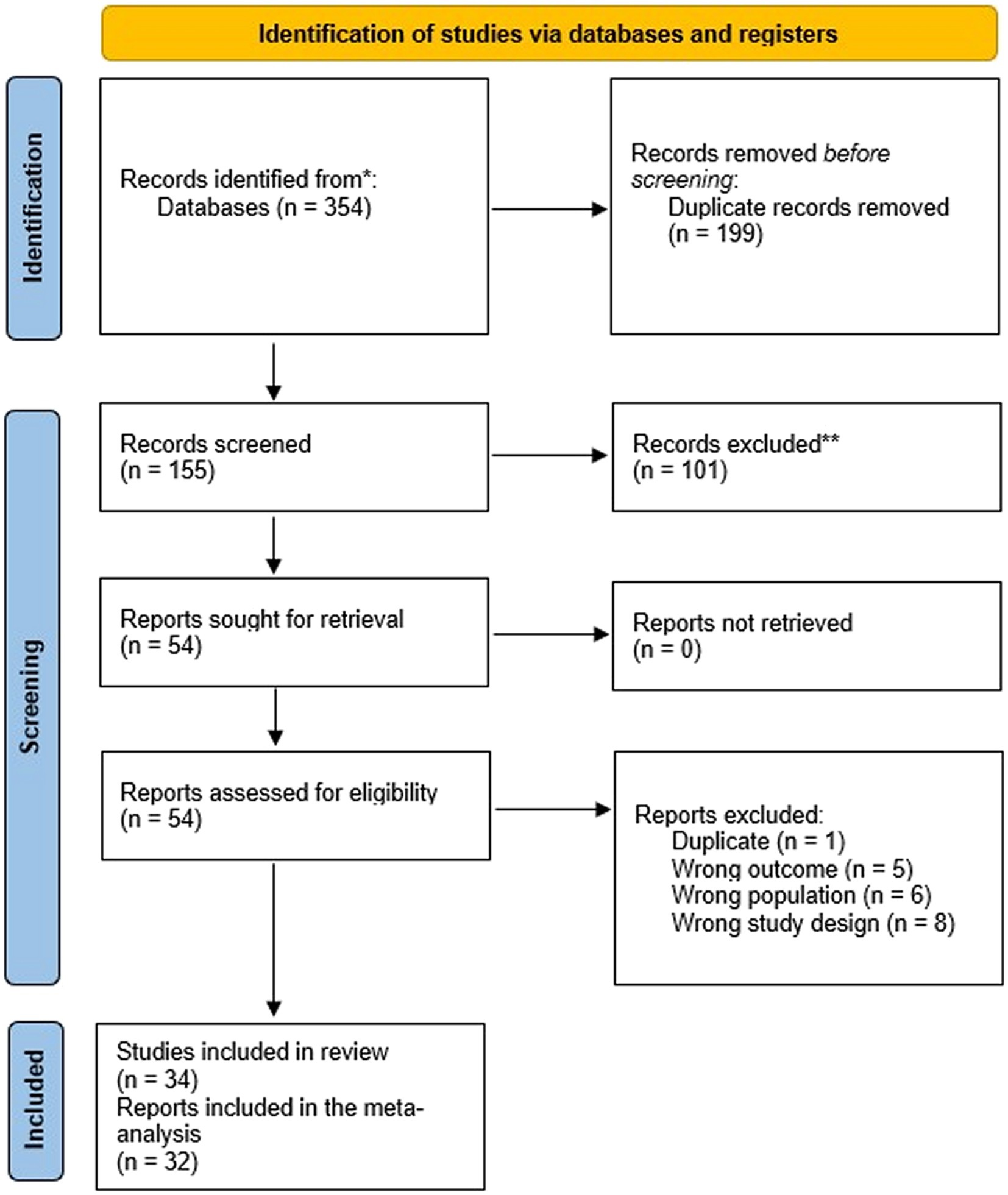
A total of 354 articles were initially identified from the searched databases. Following deduplication in RefWorks, this number was refined to 155 unique records that required screening. Out of these, 101 records were excluded during the title and abstract screening process. The remaining 54 were successfully retrieved and the full texts were assessed for eligibility, resulting in the exclusion of 20 reports for various reasons, i.e., one was a duplicate, five had the wrong outcome, six involved the wrong population, and eight had the wrong study design (Figure 1). Furthermore, two studies (38, 39) were included in the review, but excluded from the meta-analysis because the reported data on RBP4 levels could not be extracted/converted for meta-analysis and repeated attempts to contact the authors were unsuccessful.
3.2 Risk of bias assessment
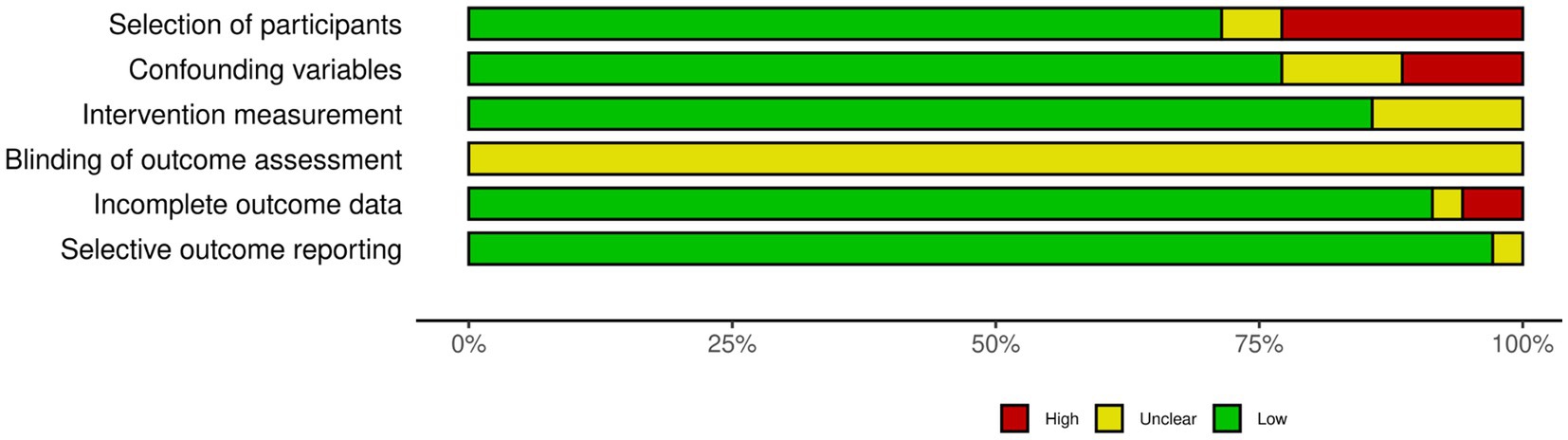
The risk of bias assessment of the included studies is presented in Figure 2 and in Supplementary Figure S2.2. Most studies (n = 24; 70.5%) had a low risk of bias in participant selection, although some lacked clarity in their selection methods (eight studies with high risk of bias, and two with unclear; Supplementary Figure S2.2). When it came to controlling for confounding variables, 27 studies (77%) were rated as having a low risk of bias, with four having an unclear risk, and three having a high risk in this regard. When assessing the exposure measurement, in five studies the exact criteria used to diagnose GDM were unclear, while the rest of the studies were classified as having a low risk of bias (87.1%). In terms of utilizing a valid measurement method for RBP4, 32 studies (94.1%) had a low risk of bias, but two had unclear measurement methods. Given that none of the studies were interventional, and therefore did not report on assessor blinding, all had an unclear risk of bias in blinding the outcome assessment. Concerning handling incomplete outcome data, one study was at a high risk of bias, while one other had an unclear risk in this category; the remaining studies (n = 32; 91%) were judged to have a low risk of bias. In the selective outcome reporting domain, all studies apart from one had a low risk of bias (13, 18–25, 28, 31–33, 35, 39–57); Zhu et al. (58) was judged to have an unclear risk.
3.3 Main characteristics of the included studies
The main characteristics of the included studies are presented in Table 2, and reported circulating RBP4 levels are presented in Supplementary Table S1.3. Of the 34 eligible studies, nine measured circulating RBP4 levels in the first trimester, 21 at 24–28 weeks, and 14 at >28 weeks of gestation. However, two studies did not report the measured RBP4 levels in a way that could be extracted (38, 39), so were not included in the meta-analysis. When sensitivity analyses were conducted by removing the studies with skewed data, the effect on estimates was negligible, therefore they were included in the analysis. The final selected studies included a total of 3,595 GDM cases and 4,544 non-GDM controls.
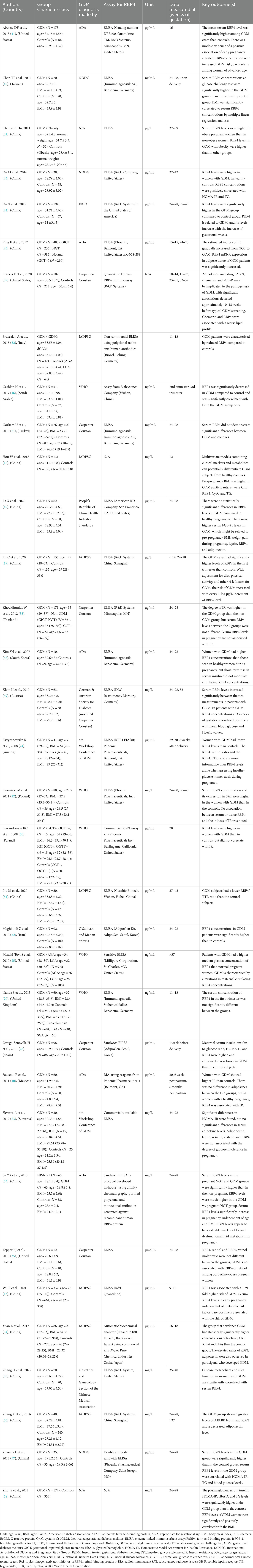
Table 2. General characteristics of the eligible studies included in the present systematic review and meta-analysis.
3.4 Circulating RBP4 levels in the first trimester of pregnancy
From the nine studies that examined the relationship between circulating RBP4 levels during the first pregnancy trimester and GDM, seven were meta-analyzed (13, 18–20, 32, 41, 54) (946 GDM vs. 1701 non-GDM controls). Based on these, circulating RBP4 levels were statistically higher in pregnant women with GDM compared to pregnant controls (SMD: 0.322; 95% CI: 0.126 to 0.517; p < 0.001) (Figure 3). Moreover, there was substantial heterogeneity among these studies (I2 = 80%), although it is essential to acknowledge that the low number of eligible studies may limit the reliability of heterogeneity estimates (30). Additionally, removal of the study with skewed data in a sensitivity analysis (19) slightly reduced the SMD (0.309, 95% CI: 0.078–0.539; p = 0.009) (Supplementary Table S2.3).
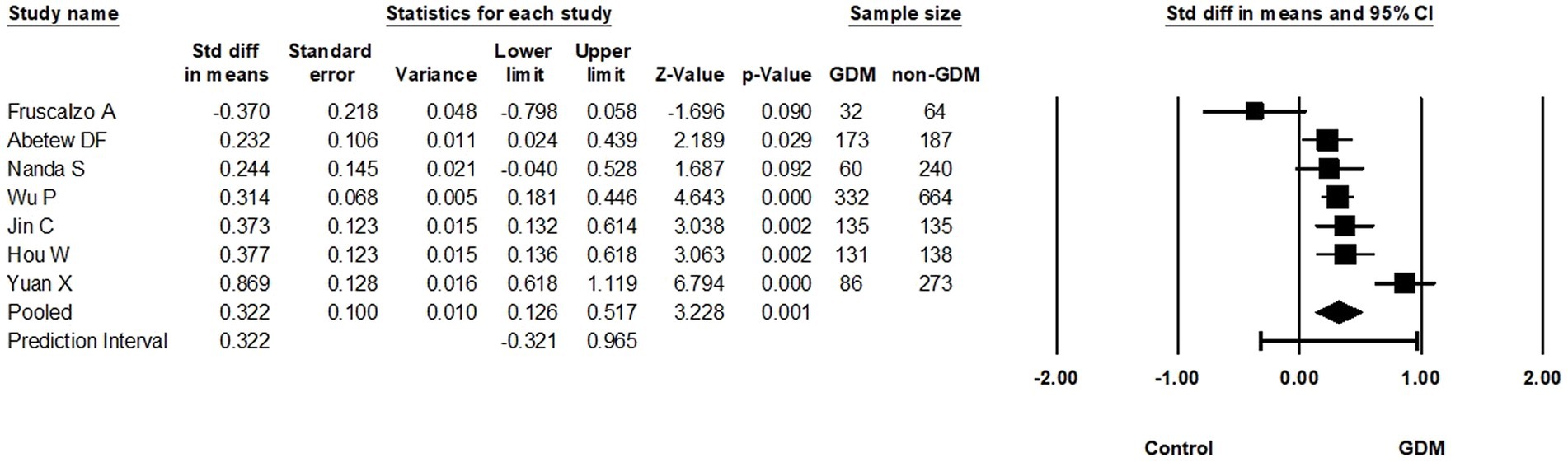
Figure 3. Forest plot of circulating RBP4 levels: gestational diabetes mellitus (GDM) compared to control in the first trimester of pregnancy. Std diff in means: standardized mean difference; CI: Confidence intervals.
3.5 Circulating RBP4 levels at 24–28 weeks of gestation
A total of 19 studies investigated the relationship between circulating RBP4 and GDM at 24–28 weeks of gestation and reported the corresponding RBP4 levels, with 1776 GDM cases and 1942 non-GDM controls in the performed meta-analysis. When compared to controls, circulating RBP4 levels at 24–28 weeks of gestation were significantly higher in women with GDM (SMD: 0.628; 95% CI: 0.290–0.966; p < 0.001) (Figure 4). Heterogeneity among these studies was considerable (I2 = 95%). Furthermore, when switching the reported RBP4 data from the Tepper et al. study (35) to their Western Blot reported data, the effect estimate remained similar at 0.620 (95% CI: 0.282–0.959; p < 0.001). Additionally, removal of the skewed studies (19, 21, 22) increased the effect size (SMD: 0.702; 95% CI: 0.289–1.115; p = 0.001). A one-study-removed analysis was also performed, as presented in Supplementary Figure S2.4.
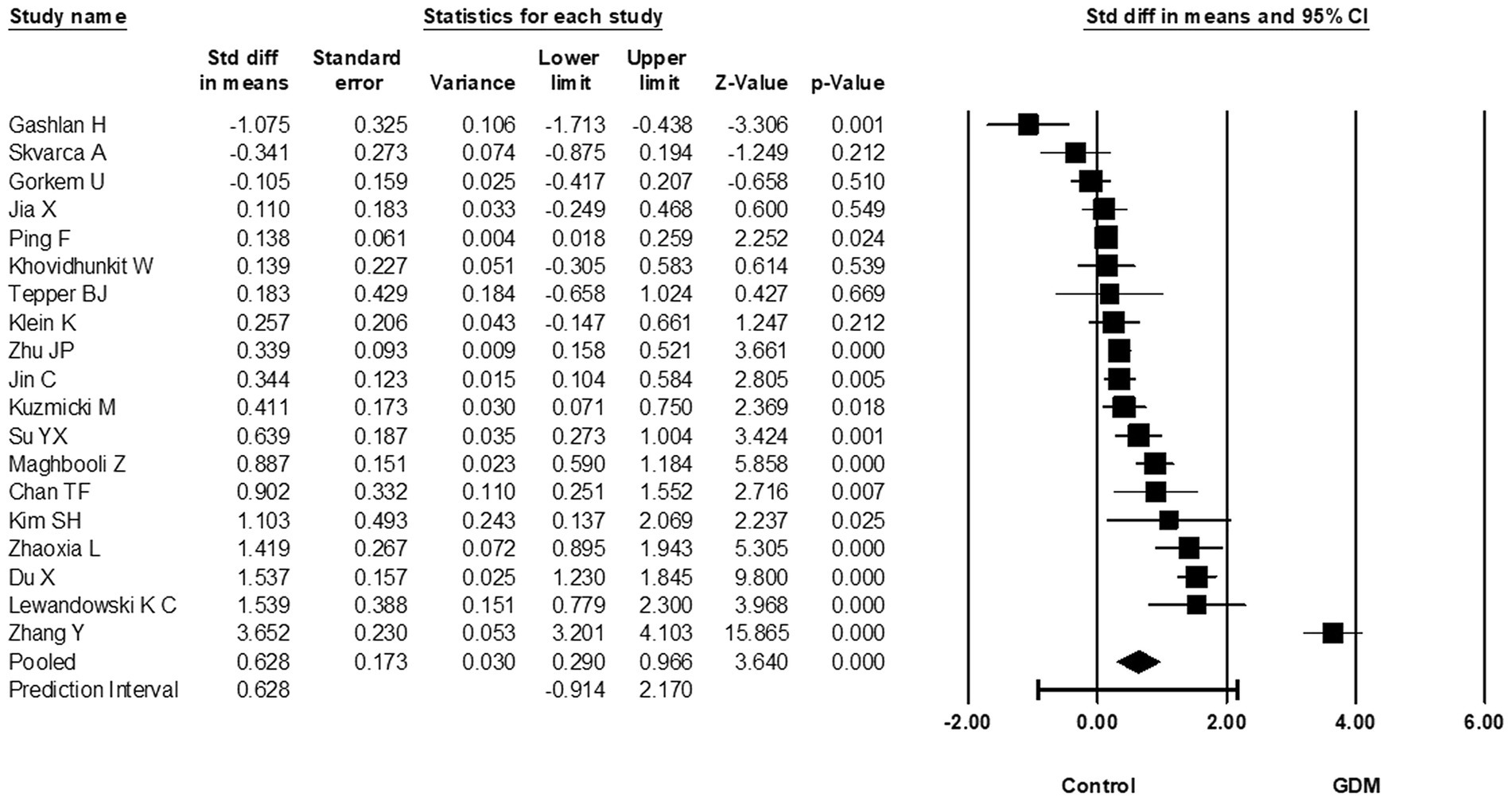
Figure 4. Forest plot of circulating RBP4 levels: gestational diabetes mellitus (GDM) compared to control at 24-28 weeks of gestation. Std diff in means: standardized mean difference; CI: Confidence intervals.
3.6 Circulating RBP4 levels at more than 28 weeks of gestation
In total, 14 eligible studies compared circulating RBP4 at >28 weeks of gestation and reported the corresponding RBP4 levels (870 GDM cases vs. 901 non-GMD controls). Based on these, circulating RBP4 levels at >28 weeks of pregnancy were statistically higher in women with GDM compared to non-GDM controls (SMD: 0.875; 95% CI: 0.252–1.498; p = 0.006) (Figure 5). Considerable heterogeneity was noted among these studies (I2 = 97%), suggesting potential differences in the true effect sizes among the populations under investigation. Furthermore, removal of the study with skewed data (24) slightly increased the SMD to 0.984 (95% CI: 0.348–1.620). A one-study-removed analysis was also performed, as presented in Supplementary Figure S2.5.
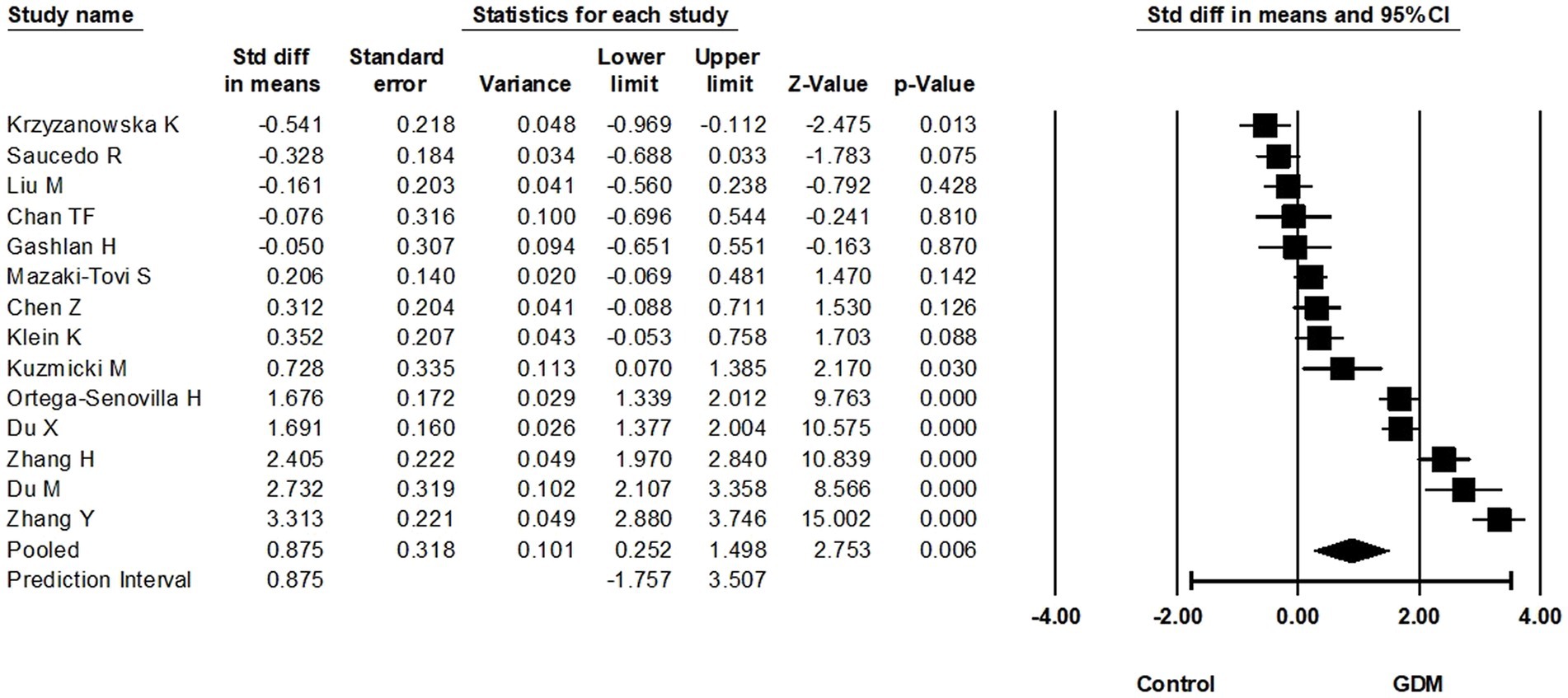
Figure 5. Forest plot of circulating RBP4 levels: gestational diabetes mellitus (GDM) compared to control at more than 28 weeks of gestation. Std diff in means: standardized mean difference; CI: Confidence intervals.
3.7 Sub-group analysis
During the first trimester, sub-group analyses were completed for GDM diagnosis, RBP4 measurement method/assay, and country of study (Supplementary Table S2.1). Regarding the applied GDM diagnostic criteria, only one sub-group (IADPSG criteria) had more than one study in; in this group, the effect estimate was increased (SMD: 0.347, 95% CI: 0.073–0.621), but so too was the degree of heterogeneity (I2 = 85.4%). For RBP4 measurement, there was also only one subgroup with more than one study included. Three studies used an R&D Systems ELISA (SMD: 0.305, 95% CI 0.203–0.406) which reduced the I2 in that sub-group to 0%. Similarly for country in which included studies were conducted, it was only studies from China that constituted a group including multiple studies; the effect estimate was larger (SMD: 0.473, 95% CI: 0.237–0.708) in this sub-group, but the I2 was practically unchanged.
For 24–28 weeks of gestation, the sub-group analysis based upon the applied GDM diagnostic criteria identified four sub-groups that contained more than one study (Supplementary Table S2.1). As such, studies using the ADA, or Carpenter-Coustan, or 4th Workshop conference criteria were grouped together based on the applied GDM diagnostic cut-offs/criteria specified in the corresponding papers (7 studies, 1894 participants). For these, the statistical effect was lost, whilst the I2 was reduced to 63.5%. For the sub-group of studies using the IADPSG, or FIGO, or People’s Republic of China Health Industry Standards criteria (4 studies, 931 participants), the SMD increased to 1.402 (95% CI: 0.084 to 2.721) and so too did the I2 (98.5%). When the studies using the WHO criteria were grouped there was still considerable heterogeneity (93.0%), and the statistical effect was lost. Finally, for the two studies using the NDDG GDM criteria, the effect estimate retained statistical significance (SMD: 1.198, 95% CI: 0.696–1.699), whilst heterogeneity was reduced to an amount that may not be important (I2 = 32.1%). When RBP4 measurement method/assay was sub-grouped, three sub-groups were created (Supplementary Table S2.1). The sub-groups which used either an R&D Systems (five studies; SMD: 1.151, 95% CI: 0.042–2.260) or a Phoenix Pharmaceuticals (five studies; SMD: 0.699, 95% CI: 0.167–1.232) ELISA retained statistical effects, but with considerable heterogeneity (I2 = 98.1 and 88.3%, respectively). For the third sub-group which used an Immundiagnostik AG ELISA, the statistical effect estimate was lost, whilst there was still evidence of substantial heterogeneity (I2 = 78.8%). Finally, for sub-group analysis based upon country in which included studies were conducted, only two countries had more than one study, namely China (8 studies, SMD: 1.001; I2 = 97.6%) and Poland (2 studies, SMD: 0.922; I2 = 85.8%). A considerable degree of heterogeneity was apparent in both these sub-groups, while a statistical effect was retained for the studies from China only (Supplementary Table S2.1).
When sub-group analysis was completed based upon the applied GDM diagnostic criteria for the >28 weeks of gestation timepoint (Supplementary Table S2.1), a statistical effect was not retained for any of these sub-groups. The heterogeneity remained at a considerable level (I2 > 95%) for all but one of these sub-groups, namely the sub-group of studies that applied the WHO criteria for which the heterogeneity was reduced to a level that may not be important (3 studies; I2 = 34.7%). When RBP4 measurement methods/assays were sub-grouped, only two sub-groups were formed. For the four studies which used the R&D Systems ELISA (SMD: 2.337, 95% CI: 2.130–2.544) a statistical effect was retained, whereas for the two studies which used the Phoenix Pharmaceuticals ELISA the statistical effect was lost. Both these sub-groups demonstrated a considerable amount of heterogeneity (I2 ≥ 90%). Finally, based upon country in which included studies were conducted, sub-group analysis was possible only for China (six studies) and Austria (two studies). A statistical effect was retained for the studies from China (SMD: 1.708, 95% CI: 0.634–2.782), but not for the studies from Austria. Both these sub-groups had a considerable degree of heterogeneity (I2 > 88%).
3.8 Publication bias
Egger’s regression intercept test indicated that publication was not present at 24–28 weeks of gestation (t = 1.3, p = 0.2) (Supplementary Figure S2.6) nor at >28 weeks of gestation (t = 0.2, p = 0.8) (Supplementary Figure S2.7).
4 Discussion
The pathogenesis of GDM remains a subject of intense research interest due to the increasing GDM prevalence and the potential significant health implications for both mothers and offspring. In this context, recent research has further focused on novel factors (e.g., circulating adipokines such as RBP4) which appear implicated in the pathogenesis of GDM and may be utilized as GDM biomarkers (59). Therefore, this systematic review and meta-analysis aimed to offer up-to-date, comprehensive evidence on the relationship between circulating RBP4 levels and GDM at various timepoints across the pregnancy. The present meta-analyses included data from seven eligible studies examining circulating RBP4 levels in the first trimester, 19 studies at 24–28 weeks, and 13 studies at >28 weeks of pregnancy. Overall, the results showed statistically higher RBP4 levels in women with GDM compared to non-GDM controls at these different pregnancy timepoints.
Indeed, such a statistical difference in the circulating RBP4 levels was evident during the first trimester when women with and without GDM were compared. This finding suggests that circulating RBP4 levels in early pregnancy may be an early biomarker for GDM; although, the limited number of eligible existing studies for this early pregnancy timepoint warrants caution in interpreting this finding. Nevertheless, this is in accord to that from a previous meta-analysis from Wu et al. (13) on the association between RBP4 levels in early pregnancy and GDM risk. However, the paucity of relevant data for this pregnancy trimester/timepoint was also noted in this previous meta-analysis, together with potential ethnic-related differences; hence, further research is clearly required to determine if circulating RBP4 has potential as a GDM-related biomarker during the first trimester.
The present meta-analysis also revealed statistically higher circulating RBP4 levels in GDM cases compared to non-GDM controls at 24–28 weeks of gestation. The noted moderate effect size during this pregnancy period suggests that such elevated circulating RBP4 levels may be associated to GDM. This finding is in accord with previous research indicating the potential role of RBP4 in insulin resistance and glucose metabolism regulation after the first trimester of pregnancy (13–15). Thus, monitoring circulating RBP4 levels in pregnant women during the second trimester could be further explored as a potential GDM biomarker.
Finally, at >28 weeks of pregnancy, our meta-analysis also revealed higher circulating RBP4 levels in patients with GDM compared to non-GDM controls. The relatively large effect size noted for this gestation period indicates a potential relationship between these RBP4 levels in late pregnancy and GDM. Indeed, it is plausible that elevated circulating RBP4 levels at this stage may reflect an intensified insulin-resistant state, a hallmark of GDM, although further research is also required to establish this link.
Collectively, the findings of the present systematic review and meta-analysis offer updated evidence, which is also in line with Huang et al. (14) who conducted the first reported meta-analysis of observational studies aiming to investigate the relationship between circulating RBP4 levels and GDM. Indeed, their data included a total of 14 studies with 884 women with GDM and 1,251 normoglycemic pregnant women. Similar to the present meta-analysis, their overall results showed that circulating RBP4 levels were significantly higher in women with GDM compared to the studied controls. However, their stratified results indicated that this significant difference was observed only in the second/third trimester and was limited to Asian populations. This may be, at least in part, attributed to the lower number of eligible studies analyzed by Huang et al. (14), whilst potential ethnic differences in circulating RBP4 levels in pregnancy and GDM merits further targeted research. Another meta-analysis by Hu et al. (15) also included 14 case–control studies on serum RBP4 levels and GDM risk, involving a total of 647 GDM cases and 620 controls. This showed that high serum RBP4 levels represent a risk factor for GDM, with a pooled SMD of 0.758 (95% CI: 0.387–1.128). Their subgroup analyses based on gestational age at blood sampling and diagnostic criteria were consistent with the overall results, supporting the hypothesis that elevated RBP4 is a modest independent risk factor for GDM. However, in contrast with our present findings, no association was found by Hu et al. between circulating RBP4 levels and GDM in the first trimester. This may be partly due to changing insulin resistance levels during pregnancy; however, it should be noted that our meta-analysis included seven studies which assessed circulating RBP4 levels during the first trimester, while only one such study was included in the analyses by Hu et al. (15), potentially reducing the reliability of their stratified analysis on this point. Finally, another meta-analysis (60) that focused on the association of leptin and RBP4 with GDM risk included six studies with a total of 2,715 participants and 841 cases of GDM. In that meta-analysis, serum RBP4 levels also showed a significant positive association with the overall GDM risk, and pregnant women with the highest serum RBP4 levels were 2.04-fold more prone to GDM than those with the lowest levels. However, as with our findings, significant heterogeneity of the included studies was also noted (60). Overall, the exiting evidence supports the association of higher circulating RBP4 levels during pregnancy in patients with GDM, whilst this association appears to be more consistent in later pregnancy stages (second/third trimester), as was also documented in the aforementioned previous meta-analyses (14, 15). While this growing evidence is promising, further research is still required to advance our understanding, validate previous findings, and better explore the clinical implications of circulating RBP4 in the context of GDM.
The present meta-analysis has several strengths, including a comprehensive study selection process, thorough risk of bias assessment, and a relatively large sample size of 32 included studies with 3,595 GDM cases and 4,544 non-GDM controls, which is larger than previous meta-analyses on this topic. Indeed, by including detailed temporal analyses at different (early, mid, and late) pregnancy stages, the present work adds to the understanding of the potential association between circulating RBP4 levels and GDM. Moreover, the performed sensitivity analyses, addressing skewed data and the impact of specific studies, enhance the robustness of the present findings. Finally, our systematic review and meta-analysis addresses and evaluates potential publication bias, contributing to the overall reliability of the reported results.
However, certain limitations of the present work should also be acknowledged. Firstly, the total number of existing eligible studies included in some analyses was limited, which may have affected the robustness of the results. In addition, the study designs, participant characteristics, and laboratory methods for measuring RBP4 varied among the included studies, contributing to the observed high heterogeneity which may affect the reliability of the meta-analysis results, whilst inconsistencies in how relevant data are reported across the included studies might affect the accuracy of the present meta-analysis. A meta-regression would have been useful to help explain the high degree of heterogeneity in the analyses, but this was not performed due to inconsistencies with how continuous variables were reported across the identified studies (i.e., not all studies reported all variables). Moreover, variation in the methods used to measure circulating RBP4 levels across the identified studies could impact on the comparability of the results. Notably, most of the included studies were retrospective case–control studies, thus causality could not be established, whilst this may also introduce bias. The generalizability of the findings may be also limited by the small sample sizes of some of the included eligible studies. Furthermore, the identified statistically significant differences between GDM and non-GMD pregnancies cannot be necessarily considered as clinically significant, particularly given the proximity of the lower bound CI to zero. As is also common in systematic reviews, the possibility of publication bias, where studies with significant findings are more likely to be published, may have introduced a bias regarding the eligible studies which are published and are subsequently included in the searched databases. Finally, although multiple established biomedical databases were searched, the present systematic review identified only articles with English-language abstracts and main text written in either English or Chinese, which may have introduced a potential language bias.
5 Conclusion
The present systematic review and meta-analysis offers updated and comprehensive data which suggest that circulating RBP4 levels measured at different pregnancy timepoints/stages are higher in patients with GDM compared to non-GDM controls. Taken together with previous findings, this suggests that circulating RBP4 could be considered as a potential biomarker associated with GDM. Given that circulating levels of RBP4 are not routinely measured in the clinical practice, it is plausible that standardizing the method/assay for measuring circulating RBP4 in routine practice and adding this measurement as part of the GDM risk assessment visit/protocol in the context of antenatal care may be helpful to promptly identify those at high risk. However, the scarcity of relevant data particularly for early pregnancy and the noted high study heterogeneity, as well as factors relating to variability in RBP4 measurement methods and GDM diagnostic criteria/protocols, highlight the need for additional research in this field. Particularly, prospective (including the first trimester) and large-scale cohort studies across diverse populations and with standardized measurements of circulating RBP4 are needed to validate the present findings and confirm the generalizability of existing evidence. Future studies should also explore the potential underlying biological mechanisms which may link RBP4 to GDM, considering key pathophysiologic factors, such as insulin resistance and obesity-related inflammation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
BL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. CK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. LL: Data curation, Formal analysis, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. ADav: Data curation, Writing – original draft, Writing – review & editing. ADal: Data curation, Formal analysis, Software, Writing – original draft, Writing – review & editing. KC: Data curation, Writing – original draft, Writing – review & editing. HR: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. IK: Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Investigation.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to thank the University Hospitals Coventry and Warwickshire (UHCW) NHS Trust for the ongoing support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1348970/full#supplementary-material
Footnotes
References
1. Plows, JF , Stanley, J , Baker, P , Reynolds, C , and Vickers, M . The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. (2018) 19:3342. doi: 10.3390/ijms19113342
2. Khambule, L , and George, JA . The role of inflammation in the development of GDM and the use of markers of inflammation in GDM screening. Adv Exp Med Biol. (2019) 1134:217–42. doi: 10.1007/978-3-030-12668-1_12
3. National Institute for Health and Care Excellence (NICE) . Diabetes in Pregnancy: Management from Preconception to the Postnatal Period. London: National Institute for Health and Care Excellence (NICE) (2020).
4. Getahun, D , Fassett, MJ , and Jacobsen, SJ . Gestational diabetes: risk of recurrence in subsequent pregnancies. Am J Obstet Gynecol. (2010) 203:467.e1–6. doi: 10.1016/j.ajog.2010.05.032
5. Noctor, E , and Dunne, FP . Type 2 diabetes after gestational diabetes: the influence of changing diagnostic criteria. World J Diabetes. (2015) 6:234–44. doi: 10.4239/wjd.v6.i2.234
6. Durnwald, C . Gestational diabetes: linking epidemiology, excessive gestational weight gain, adverse pregnancy outcomes, and future metabolic syndrome. Semin Perinatol. (2015) 39:254–8. doi: 10.1053/j.semperi.2015.05.002
7. Jenum, AK , Mørkrid, K , Sletner, L , Vange, S , Torper, JL , Nakstad, B, et al. Impact of ethnicity on gestational diabetes identified with the WHO and the modified International Association of Diabetes and Pregnancy Study Groups criteria: a population-based cohort study. Eur J Endocrinol. (2012) 166:317–24. doi: 10.1530/EJE-11-0866
8. Anghebem-Oliveira, MI , Martins, BR , Alberton, D , Ramos, EAS , Picheth, G , and Rego, FGM . Type 2 diabetes-associated genetic variants of FTO, LEPR, PPARg, and TCF7L2 in gestational diabetes in a Brazilian population. Arch Endocrinol Metab. (2017) 61:238–48. doi: 10.1590/2359-3997000000258
9. Farrar, D , Simmonds, M , Bryant, M , Lawlor, DA , Dunne, F , Tuffnell, D, et al. Risk factor screening to identify women requiring oral glucose tolerance testing to diagnose gestational diabetes: a systematic review and meta-analysis and analysis of two pregnancy cohorts. PLoS One. (2017) 12:e0175288. doi: 10.1371/journal.pone.0175288
10. Ji, Y , Song, J , Su, T , and Gu, X . Adipokine retinol binding protein 4 and cardiovascular diseases. Front Physiol. (2022) 13:856298. doi: 10.3389/fphys.2022.856298
11. Yang, Q , Graham, TE , Mody, N , Preitner, F , Peroni, OD , Zabolotny, JM, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. (2005) 436:356–62. doi: 10.1038/nature03711
12. Saucedo, R , Valencia, J , Basurto, L , Hernandez, M , Puello, E , Zarate, A, et al. The retinol binding Protein-4 (RBP4) gene and Gestational diabetes In: R Rajendram, VR Preedy, and VB Patel, editors. Nutrition and Diet in Maternal Diabetes: An Evidence-Based Approach. Cham: Springer International Publishing (2018). 135–45.
13. Wu, P , Wang, Y , Ye, Y , Yang, X , Lu, Q , Liu, Y, et al. Serum retinol-binding protein 4 levels and risk of gestational diabetes mellitus: a nested case-control study in Chinese women and an updated meta-analysis. Diabetes Metab Res Rev. (2022) 38:e3496. doi: 10.1002/dmrr.3496
14. Huang, QT , Huang, Q , Luo, W , Li, F , Hang, LL , Yu, YH, et al. Circulating retinol-binding protein 4 levels in gestational diabetes mellitus: a meta-analysis of observational studies. Gynecol Endocrinol. (2015) 31:337–44. doi: 10.3109/09513590.2015.1005594
15. Hu, S , Liu, Q , Huang, X , and Tan, H . Serum level and polymorphisms of retinol-binding protein-4 and risk for gestational diabetes mellitus: a meta-analysis. BMC Pregnancy Childbirth. (2016) 16:52. doi: 10.1186/s12884-016-0838-7
16. Page, MJ , McKenzie, JE , Bossuyt, PM , Boutron, I , Hoffmann, TC , Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
17. Ouzzani, M , Hammady, H , Fedorowicz, Z , and Elmagarmid, A . Rayyan-a web and mobile app for systematic reviews. Syst Rev. (2016) 5:210. doi: 10.1186/s13643-016-0384-4
18. Hou, W , Meng, X , Zhao, A , Zhao, W , Pan, J , Tang, J, et al. Development of multimarker diagnostic models from metabolomics analysis for gestational diabetes mellitus (GDM). Mol Cell Proteomics. (2018) 17:431–41. doi: 10.1074/mcp.RA117.000121
19. Jin, C , Lin, L , Han, N , Zhao, Z , Liu, Z , Luo, S, et al. Plasma retinol-binding protein 4 in the first and second trimester and risk of gestational diabetes mellitus in Chinese women: a nested case-control study. Nutr Metabolism. (2020) 17:1–7. doi: 10.1186/s12986-019-0425-9
20. Nanda, S , Nikoletakis, G , Markova, D , Poon, LCY , and Nicolaides, KH . Maternal serum retinol-binding protein-4 at 11-13 weeks' gestation in normal and pathological pregnancies. Metab Clin Exp. (2013) 62:814–9. doi: 10.1016/j.metabol.2012.12.011
21. Gorkem, U , Kucukler, FK , Togrul, C , and Gungor, T . Are adipokines associated with gestational diabetes mellitus? J Turk German Gynecol Assoc. (2016) 17:186–90. doi: 10.5152/jtgga.2016.16112
22. Kuzmicki, M , Telejko, B , Wawrusiewicz-kurylonek, N , Nikolajuk, A , Zwierz-gugala, D , Jelski, W, et al. Retinol-binding protein 4 in adipose and placental tissue of women with gestational diabetes. Gynecol Endocrinol. (2011) 27:1065–9. doi: 10.3109/09513590.2011.579651
23. Skvarca, A , Tomazic, M , Krhin, B , Blagus, R , and Janez, A . Adipocytokines and insulin resistance across various degrees of glucose tolerance in pregnancy. J Int Med Res. (2012) 40:583–9. doi: 10.1177/147323001204000220
24. Krzyzanowska, K , Zemany, L , Krugluger, W , Schernthaner, GH , Mittermayer, F , Schnack, C, et al. Serum concentrations of retinol-binding protein 4 in women with and without gestational diabetes. Diabetologia. (2008) 51:1115–22. doi: 10.1007/s00125-008-1009-9
25. Mazaki-Tovi, S , Romero, R , Vaisbuch, E , Kusanovic, JP , Chaiworapongsa, T , Kim, SK, et al. Retinol-binding protein 4: a novel adipokine implicated in the genesis of LGA in the absence of gestational diabetes mellitus. J Perinat Med. (2010) 38:147–55. doi: 10.1515/jpm.2010.044
26. Luo, D , Wan, X , Liu, J , and Tong, T . Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. (2018) 27:1785–805. doi: 10.1177/0962280216669183
27. Wan, X , Wang, W , Liu, J , and Tong, T . Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
28. Ortega-Senovilla, H , Schaefer-Graf, U , Meitzner, K , Abou-Dakn, M , Graf, K , Kintscher, U, et al. Gestational diabetes mellitus causes changes in the concentrations of adipocyte fatty acid-binding protein and other adipocytokines in cord blood. Diabetes Care. (2011) 34:2061–6. doi: 10.2337/dc11-0715
29. Jelicic Kadic, A , Vucic, K , Dosenovic, S , Sapunar, D , and Puljak, L . Extracting data from figures with software was faster, with higher interrater reliability than manual extraction. J Clin Epidemiol. (2016) 74:119–23. doi: 10.1016/j.jclinepi.2016.01.002
30. Higgins, JPT, Thomas, J, Chandler, J, Cumpston, M, Li, T, Page, MJ, et al. eds. Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 (Updated August 2023). Chichester, United Kingdom: Cochrane. (2023).
31. Chen, Zy , and Du, J . Change of Serum Retinol-Binding Protein-4 Level in Pregnancy Obese Subjects and GDM and Related Factors Journal of China Medical University (2011). 405:465–468.
32. Fruscalzo, A , Londero, AP , Driul, L , Henze, A , Tonutti, L , Ceraudo, M, et al. First trimester concentrations of the TTR-RBP4-retinol complex components as early markers of insulin-treated gestational diabetes mellitus. Clin Chem Lab Med. (2015) 53:1643–51. doi: 10.1515/cclm-2014-0929
33. Khovidhunkit, W , Pruksakorn, P , Plengpanich, W , and Tharavanij, T . Retinol-binding protein 4 is not associated with insulin resistance in pregnancy. Metab Clin Exp. (2012) 61:65–9. doi: 10.1016/j.metabol.2011.05.019
34. Liengme, B , and Hekman, K . Liengme's Guide to Excel 2016 for Scientists and Engineers:(Windows and Mac). London, United Kingdom: Academic Press (2019).
35. Tepper, BJ , Kim, YK , Shete, V , Shabrova, E , and Quadro, L . Serum retinol-binding protein 4 (RBP4) and retinol in a cohort of borderline obese women with and without gestational diabetes. Clin Biochem. (2010) 43:320–3. doi: 10.1016/j.clinbiochem.2009.10.013
36. Kim, SY , Park, JE , Lee, YJ , Seo, HJ , Sheen, SS , Hahn, S, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. (2013) 66:408–14. doi: 10.1016/j.jclinepi.2012.09.016
37. Borenstein, M . Comprehensive meta-analysis software In: JPT Higgins, M Egger, and GD Smith, editors. Systematic Reviews in Health Research: Meta-Analysis in Context. 3rd ed (2022). Chichester, United Kingdom: BMJ Books, Wiley Blackwell, John Wiley & Sons Ltd.
38. Du, C , and Kong, F . A prospective study of maternal plasma concentrations of retinol-binding protein 4 and risk of gestational diabetes mellitus. Ann Nutr Metab. (2019) 74:1–8. doi: 10.1159/000494888
39. Francis, EC , Li, M , Hinkle, SN , Cao, Y , Chen, J , Wu, J, et al. Adipokines in early and mid-pregnancy and subsequent risk of gestational diabetes: a longitudinal study in a multiracial cohort. BMJ Open Diabetes Res Care. (2020) 8:8. doi: 10.1136/bmjdrc-2020-001333
40. Saucedo, R , Zarate, A , Basurto, L , Hernandez, M , Puello, E , Galvan, R, et al. Relationship between circulating adipokines and insulin resistance during pregnancy and postpartum in women with gestational diabetes. Arch Med Res. (2011) 42:318–23. doi: 10.1016/j.arcmed.2011.06.009
41. Abetew, DF , Qiu, C , Fida, NG , Dishi, M , Hevner, K , Williams, MA, et al. Association of retinol binding protein 4 with risk of gestational diabetes. Diabetes Res Clin Pract. (2013) 99:48–53. doi: 10.1016/j.diabres.2012.10.023
42. Chan, TF , Chen, HS , Chen, YC , Lee, CH , Chou, FH , Chen, IJ, et al. Increased serum retinol-binding protein 4 concentrations in women with gestational diabetes mellitus. Reprod Sci. (2007) 14:169–74. doi: 10.1177/1933719106298407
43. du, M , Wang, B , Liang, Z , Dong, M , and Chen, D . The relationship between retinol-binding protein 4 concentrations and gestational diabetes mellitus in Chinese women. Gynecol Obstet Investig. (2016) 81:174–80. doi: 10.1159/000398794
44. du, X , Dong, Y , Xiao, L , Liu, GH , Qin, W , and Yu, H . Association between retinol-binding protein 4 concentrations and gestational diabetes mellitus (A1GDM and A2GDM) in different pregnancy and postpartum periods. Ann Transl Med. (2019) 7:479. doi: 10.21037/atm.2019.08.45
45. Ping, F , Xiang, HD , Li, M , Li, W , Liu, JT , Nie, M, et al. Effects of variation in retinol binding protein 4 gene and adipose specific expression of gestational diabetes in Beijing, China. Diabetes Res Clin Pract. (2012) 97:283–9. doi: 10.1016/j.diabres.2012.02.017
46. Gashlan, HM . Relationship between levels of retinol binding protein 4, Vaspin and Chemerin and insulin resistance in gestational diabetes mellitus. Int J Pharm Res Allied Sci. (2017) 6:236–50.
47. Jia, X , Bai, L , Ma, N , and Lu, Q . The relationship between serum adipokine fibroblast growth factor-21 and gestational diabetes mellitus. J Diabetes Invest. (2022) 13:2047–53. doi: 10.1111/jdi.13889
48. Kim, SH , Choi, HJ , and Im, JA . Retinol-binding protein 4 responses during an oral glucose tolerance testing in women with gestational diabetes mellitus. Clin Chim Acta. (2008) 391:123–5. doi: 10.1016/j.cca.2008.01.030
49. Klein, K , Bancher-Todesca, D , Leipold, H , Knöfler, M , Haslinger, P , Handisurya, A, et al. Retinol-binding protein 4 in patients with gestational diabetes mellitus. J Women’s Health. (2010) 19:517–21. doi: 10.1089/jwh.2009.1615
50. Lewandowski, KC , Stojanovic, N , Bienkiewicz, M , Tan, BK , Prelevic, GM , Press, M, et al. Elevated concentrations of retinol-binding protein-4 (RBP-4) in gestational diabetes mellitus: negative correlation with soluble vascular cell adhesion molecule-1 (sVCAM-1). Gynecol Endocrinol. (2008) 24:300–5. doi: 10.1080/09513590802141052
51. Liu, MT , Chen, YM , and Chen, DQ . Association between transthyretin concentrations and gestational diabetes mellitus in Chinese women. Arch Gynecol Obstet. (2020) 302:329–35. doi: 10.1007/s00404-020-05599-y
52. Maghbooli, Z , Hossein-nezhad, A , Mirzaei, K , Karimi, F , Besharati, A , Omidfar, K, et al. Association between retinol-binding protein 4 concentrations and gestational diabetes mellitus and risk of developing metabolic syndrome after pregnancy. Reprod Sci. (2010) 17:196–201. doi: 10.1177/1933719109351097
53. Su, YX , Hong, J , Yan, Q , Xu, C , Gu, WQ , Zhang, YF, et al. Increased serum retinol-binding protein-4 levels in pregnant women with and without gestational diabetes mellitus. Diabetes Metab. (2010) 36:470–5. doi: 10.1016/j.diabet.2010.06.006
54. Yuan, XS , Shi, H , Wang, HY , Yu, B , and Jiang, J . Ficolin-3/adiponectin ratio for the prediction of gestational diabetes mellitus in pregnant women. J Diabetes Invest. (2018) 9:403–10. doi: 10.1111/jdi.12688
55. Zhang, H , and Sun, T . Correlation of blood glucose and pancreatic islet function with serum retinol-binding protein 4, serum cystatin C, and human new satiety molecule Protein-1 in pregnant women with gestational diabetes mellitus. Evid Based Complement Alternat Med. (2022) 2022:4247412. doi: 10.1155/2022/4247412
56. Zhang, Y , Zhang, HH , Lu, JH , zheng, SY , Long, T , Li, YT, et al. Changes in serum adipocyte fatty acid-binding protein in women with gestational diabetes mellitus and normal pregnant women during mid- and late pregnancy. J Diabetes Invest. (2016) 7:797–804. doi: 10.1111/jdi.12484
57. Zhaoxia, L , Mengkai, D , Qin, F , and Danqing, C . Significance of RBP4 in patients with gestational diabetes mellitus: a case-control study of Han Chinese women. Gynecol Endocrinol. (2014) 30:161–4. doi: 10.3109/09513590.2013.871515
58. Zhu, JP , Ji, Y , Tan, M , and Teng, Y . Variations of retinol-binding protein 4 level of patients with non-obese gestational diabetes mellitus and the influence factors. J Shanghai Jiaotong Univ (Med Sci). (2014) 34:1506–10. doi: 10.3969/j.issn.1674-8115.2014.10.018
59. Valencia-Ortega, J , González-Reynoso, R , Ramos-Martínez, EG , Ferreira-Hermosillo, A , Peña-Cano, MI , Morales-Ávila, E, et al. New insights into Adipokines in gestational diabetes mellitus. Int J Mol Sci. (2022) 23:6279. doi: 10.3390/ijms23116279
60. Mousavi, SN , Bahramfard, T , Rad, EY , Hosseinikia, M , and Saboori, S . Association of Leptin and Retinol Binding Protein 4 with the risk of gestational diabetes: a systematic review and Meta-analysis of observational studies. Indian J Endocrinol Metab. (2023) 27:96–104. doi: 10.4103/ijem.ijem_385_22
Keywords: retinol-binding protein 4, RBP4, gestational diabetes mellitus, GDM, pregnancy, systematic review, meta-analysis
Citation: Leca BM, Kite C, Lagojda L, Davasgaium A, Dallaway A, Chatha KK, Randeva HS and Kyrou I (2024) Retinol-binding protein 4 (RBP4) circulating levels and gestational diabetes mellitus: a systematic review and meta-analysis. Front. Public Health. 12:1348970. doi: 10.3389/fpubh.2024.1348970
Edited by:
Yanhui Lu, Food and Drug Administration, United StatesReviewed by:
Yun Shen, Pennington Biomedical Research Center, United StatesJose Rafael Villafan Bernal, National Institute of Genomic Medicine (INMEGEN), Mexico
Copyright © 2024 Leca, Kite, Lagojda, Davasgaium, Dallaway, Chatha, Randeva and Kyrou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bianca M. Leca, bianca.leca@uhcw.nhs.uk
†These authors share senior authorship
 Bianca M. Leca
Bianca M. Leca Chris Kite1,3,4,5
Chris Kite1,3,4,5 Harpal S. Randeva
Harpal S. Randeva Ioannis Kyrou
Ioannis Kyrou