- 1Institute of the History, Philosophy and Ethics of Medicine, Ulm University, Ulm, Germany
- 2Ethics of Medicine, Medical Faculty, University of Augsburg, Augsburg, Germany
Introduction: There is currently no binding, internationally accepted and successful approach to ensure global equitable access to healthcare during a pandemic. The aim of this ethical analysis is to bring into the discussion a legally regulated vaccine allocation as a possible strategy for equitable global access to vaccines. We focus our analysis on COVAX (COVID-19 Vaccines Global Access) and an existing EU regulation that, after adjustment, could promote global vaccine allocation.
Methods: The main documents discussing the two strategies are examined with a qualitative content analysis. The ethical values reasonableness, openness and transparency, inclusiveness, responsiveness and accountability serve as categories for our ethical analysis.
Results: We observed that the decision-making processes in a legal solution to expand access to vaccines would be more transparent than in COVAX initiative, would be more inclusive, especially of nation states, and the values responsiveness and accountability could be easily incorporated in the development of a new regulation.
Discussion: A legal strategy that offers incentives to the pharmaceutical industry in return for global distribution of vaccines according to the Fair Priority Model is an innovative way to achieve global and equitable access to vaccines. However, in the long term, achieving the Sustainable Development Goals will require from all nations to work in solidarity to find durable solutions for global vaccine research and development. Interim solutions, such as our proposed legal strategy for equitable access to vaccines, and efforts to find long-term solutions must be advanced in parallel.
1. Background
The COVID-19 pandemic once again shows that the global allocation of medical goods—such as vaccines—is far from being fair and conducive to common public health goals. While rich countries have offered access to vaccines to all their citizens (1), least developed countries have not been able to guarantee adequate access even to top priority groups, such as health professionals (2, 3). Since national borders cannot contain a pandemic and other infectious diseases will emerge after COVID-19 (4), long-term strategies for global vaccine distribution are needed.
An available tool to improve access to COVID-19 vaccines in low-income countries is to issue compulsory licenses, as foreseen in the Trade-Related Aspects of Intellectual Property Rights (TRIPS) Agreement. The TRIPS Agreement of the World Trade Organization contains specified exceptions to make the protection of intellectual property compatible with responsibilities to address public health emergencies (5). Critics state that compulsory licenses are insufficient to expand access to medical goods (6, 7).
As a response to the shortcomings of compulsory licensing, the COVID-19 Vaccines Global Access Facility (COVAX) initiative has been started. COVAX, which is co-led by The Vaccine Alliance (Gavi), the Coalition for Epidemic Preparedness Innovations (CEPI), and the World Health Organization (WHO) aims to guarantee fair and equitable access to COVID-19 vaccines in each of the more than 190 voluntarily participating countries around the world, regardless of their financial resources. COVAX intended to deliver two billion doses of vaccine by the end of 2021. Initially 3% and later 20% of the population of the participating countries were to be supplied, with priority being given to healthcare workers and high-risk groups. Following, the population-based allocation criteria were to be replaced by a weighted allocation based on the countries' risk assessment (8). COVAX contributes to a more equitable global distribution of vaccines, but consistent implementation is lacking. The aim for vaccine doses delivered in 2021 was not achieved and huge inequalities remain between high-income countries and countries in the Global South (2, 9).
Neither the voluntary nature of COVAX nor the TRIPS flexibilities for public health emergencies currently lead to a fair global distribution of COVID-19 vaccines. It is therefore necessary to think about other strategies, as we are likely to face similar events in the future.
There are however legal instruments that remain underused to regulate a fair distribution of COVID-19 vaccines. The European Union (EU) has already implemented legal incentives in the past to motivate pharmaceutical companies to develop drugs for economically less profitable population groups (10, 11). Comparable regulations also exist in the United States, Japan, Australia and Singapore (12). At the EU level, improving access is possible through an ordinary legislative procedure in accordance with Article 294 Treaty on the Functioning of the EU (TFEU) (13). The enactment of an EU regulation is decided by representatives of the EU member states. The EU could create incentives for pharmaceutical companies to ensure fair global allocation through the Fair Priority Model while still serving markets in high-income countries.
The Fair Priority Model is a proposal for fair global vaccine allocation. It was developed by a group of international ethicists during the COVID-19 pandemic. Given the initial shortage of vaccine supplies, distribution follows three phases: In phase 1, the focus is on preventing deaths. A commonly used global health metric, the “Standard Expected Years of Life Lost” (SEYLL) is used here for the calculation. Phase 2 should focus on the overall economic and social impact of the pandemic. Here, the researchers propose two additional metrics in addition to SEYLL: The extent of poverty avoidance (“poverty gap”) and declines in gross national income (GNI) due to vaccine administration. In phase 3, returning to full functioning is the goal. A ranking of transmission rates is to serve as a scale here (14).
While the TRIPS waiver has already been discussed in detail in the literature (15, 16), alternative allocation approaches such as voluntariness of the COVAX model or governance through legislation have hardly been analyzed from an ethical perspective so far. We focus specifically on the decision-making processes on fair access to vaccines. The aim of this paper is to offer guidance on the different ethical values that need to be addressed while deciding over the equitable distribution of vaccines.
The following research question should be answered: To what extent do two different allocation strategies to improve access to COVID-19 vaccines meet the values of an ethical decision-making process?
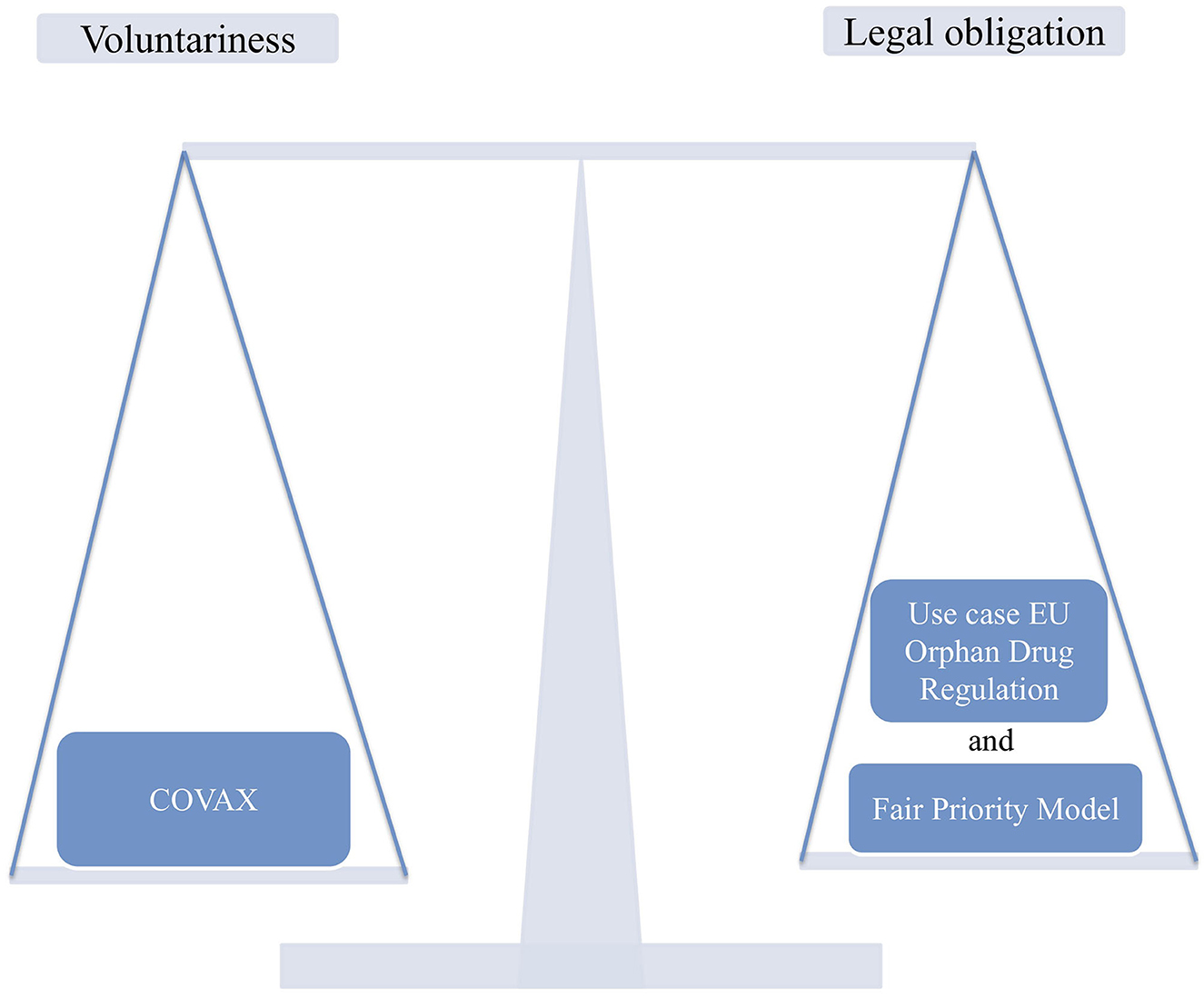
We compare a voluntary approach to global vaccine allocation, as is currently done under the COVAX program, with an existing EU regulation that, after adjustment, could promote global vaccine allocation. Therefore, we use the Regulation (EC) No. 141/2000 on orphan medicinal products (Orphan Drug Regulation), which grants incentives for pharmaceutical companies to encourage drug development and research, as a reference. In our case, incentives should be set for pharmaceutical companies that allocate vaccines globally according to the Fair Priority Model of Emanuel et al. (14) (Figure 1). Similar tools could be implemented to allocate vaccines worldwide.
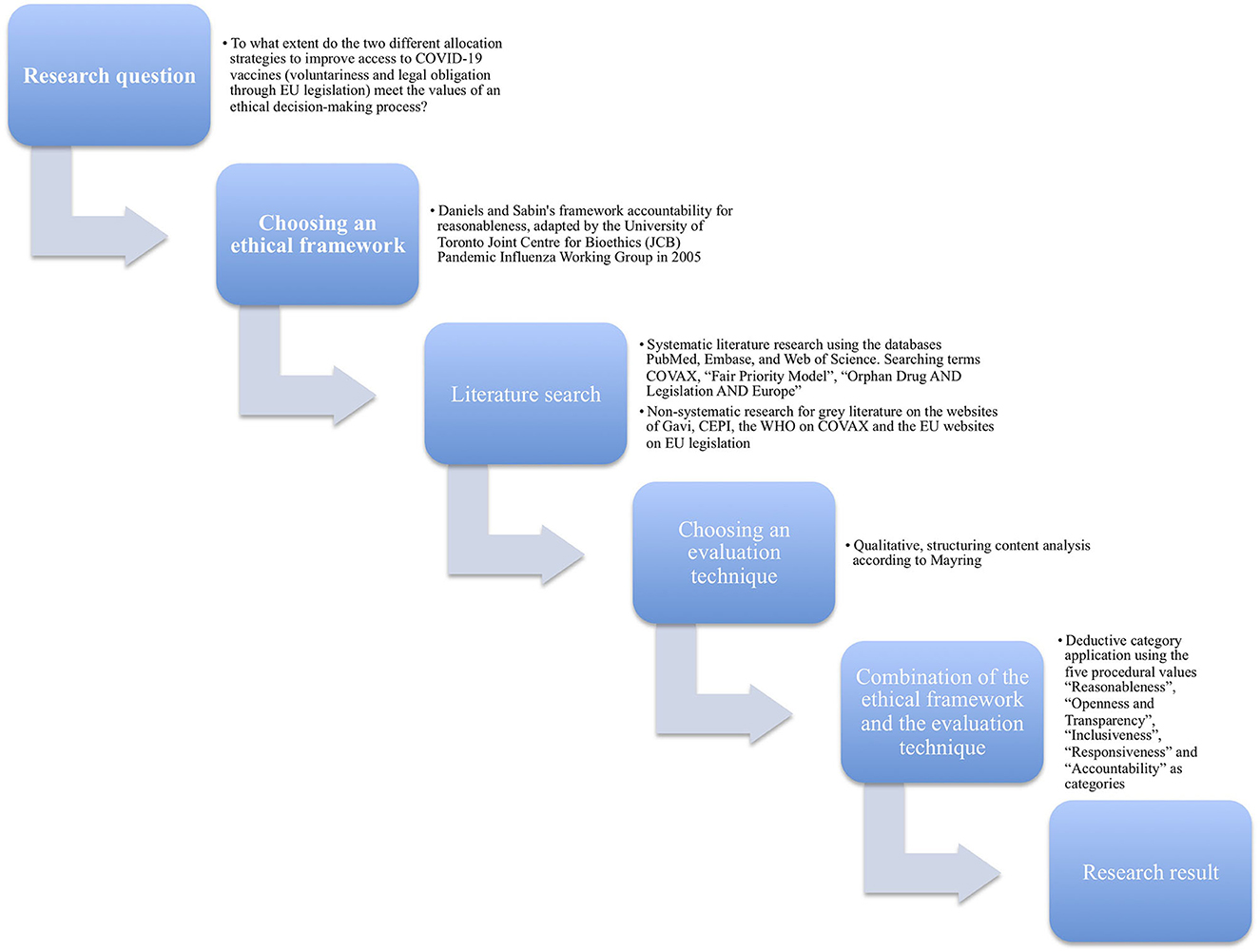
To answer the research question a qualitative, structuring content analysis was performed. Our main findings are discussed in relation to global access to vaccines. The lessons learned can help to improve decision-making about fair access to vaccines or other life-saving drugs in comparable public health emergencies in the future.
2. Methods
This is a comparative ethical analysis of two different strategies of COVID-19 vaccine allocation. The ethical values reasonableness, openness and transparency, inclusiveness, responsiveness and accountability serve as categories for our ethical analysis. The main documents discussing the two strategies are analyzed using a qualitative content analysis (Figure 2).
2.1. Framework
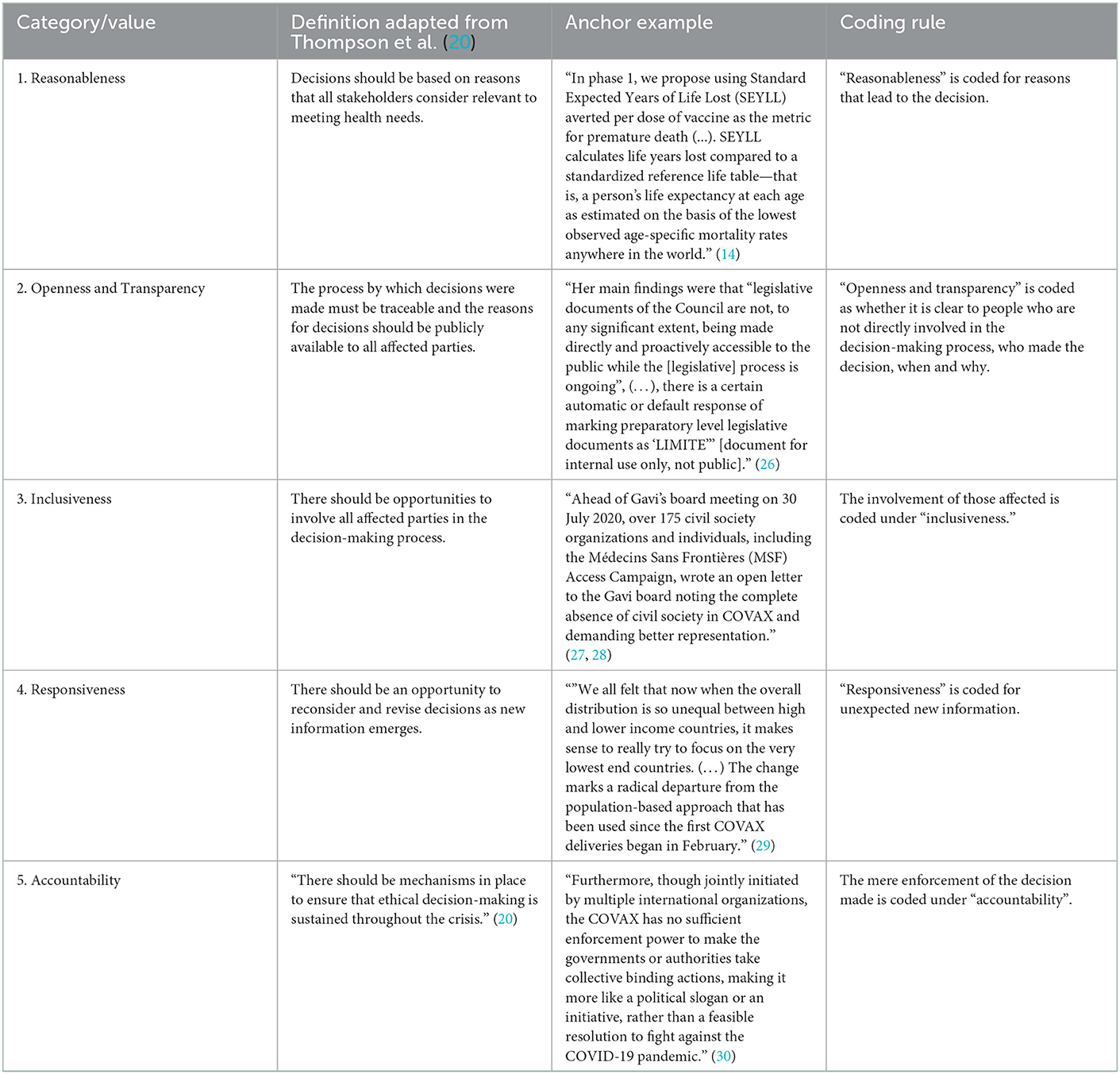
The analysis is based on the Daniels and Sabin's framework accountability for reasonableness (17), in its adaptation by the University of Toronto Joint Center for Bioethics (JCB) Pandemic Influenza Working Group in 2005 (18). The background to this approach is that allocation decisions must be made even when there is disagreement among reasonable people about what kind of allocation strategy is ethically preferable. This framework is a proposal for a fair process for priority setting in healthcare resource allocation with far-reaching influence worldwide (19–21). By using it, it is possible to analyze the extent to which different allocation decisions regarding COVID-19 vaccines meet the ethical values of a good-decision-making process. The Canadian adaptation of the framework is based on the experiences of the Severe Acute Respiratory Syndrome (SARS) outbreak in 2002/2003 in Canada and is therefore well suited for comparable challenges in the COVID-19 pandemic. According to the JCB Working Group, for a fair decision-making process with limited resources, the following five procedural values should be met (18, 20):
• Reasonableness
• Openness and transparency
• Inclusiveness
• Responsiveness
• Accountability
We deductively analyzed these five categories to explore the extent to which the two strategies meet the values of an ethical decision-making process. The use of these original categories has been used successfully in previous research (22–24) and are considered well-established values for an ethical decision-making process. These five categories have the advantage of allowing to assess how five different values are gradually being met, permitting a full ethical analysis instead of merely constituting an ethics “tick-box” exercise. These values influence the public perception and expectations of how decisions on vaccines accessibility are done according to ethical standards.
2.2. Literature search
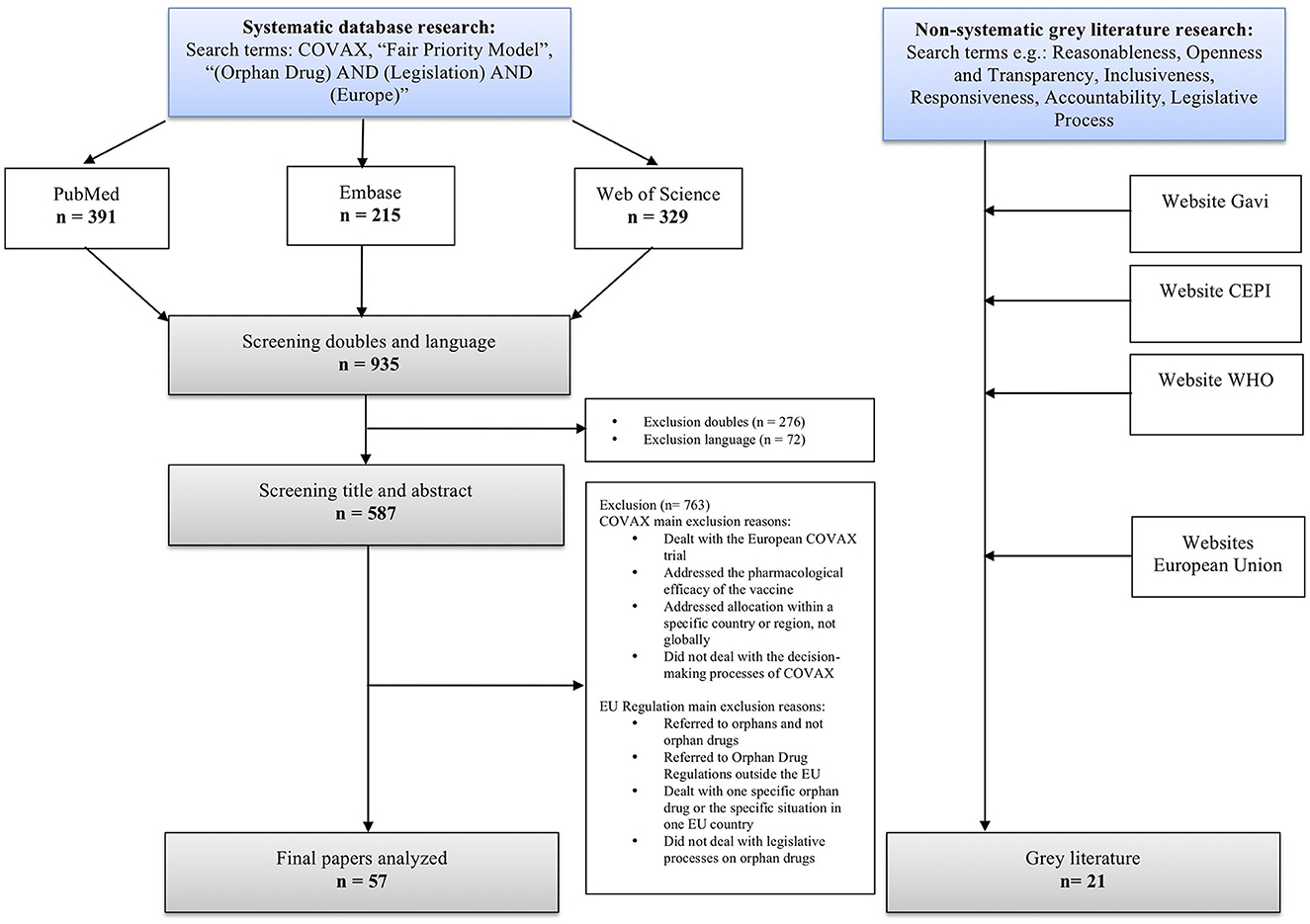
A non-standardized systematic literature search was performed on May 15, 2022, using standard settings in three of the databases: PubMed, Embase, and Web of Science. We used COVAX, “Fair Priority Model”, “Orphan Drug AND Legislation AND Europe” as searching terms. In addition, we conducted a non-systematic research for gray literature, focusing on policy reports by e.g., CEPI, Gavi, and the WHO on COVAX and the EU websites on the legislative process. The systematic literature search yielded 935 results. After removing duplicates and publications that did not meet inclusion criteria, 57 publications remained. To complement these sources, additional 21 articles from gray literature supplemented them to diversify sources (Figure 3).
2.3. Content analysis
A qualitative, structuring content analysis according to Mayring (25) was carried out. The five values of the adapted accountability for reasonableness approach (20) were selected as deductive categories for the ethical analysis. Associated definitions were supplemented with anchor examples from the reviewed literature and compiled in a coding guide as proposed by Mayring (25). In the event that there were delimitation problems between categories, rules were formulated in order to enable an unambiguous assignment. The coding rules were created based on the definitions of the categories and, if necessary, specified. For example, the value openness and transparency was coded with a focus on those not involved in the decision-making process and inclusiveness on those involved (Table 1). The full texts were analyzed and applicable text passages were tagged with a number that stood for one of the five ethical values. In case a statement could be classified under more than one value we opted for the most accentuated value. Disagreements in the assignment to the five values were discussed in the multi-professional team of authors consisting of experts in philosophy (C.T., F.S.), political science (M.O.), history and ethics of medicine (F.S.), and drug law and human geography (K.V.).
3. Results
In this section, we report the results of our qualitative content analysis using five deductively formed categories (Table 2).
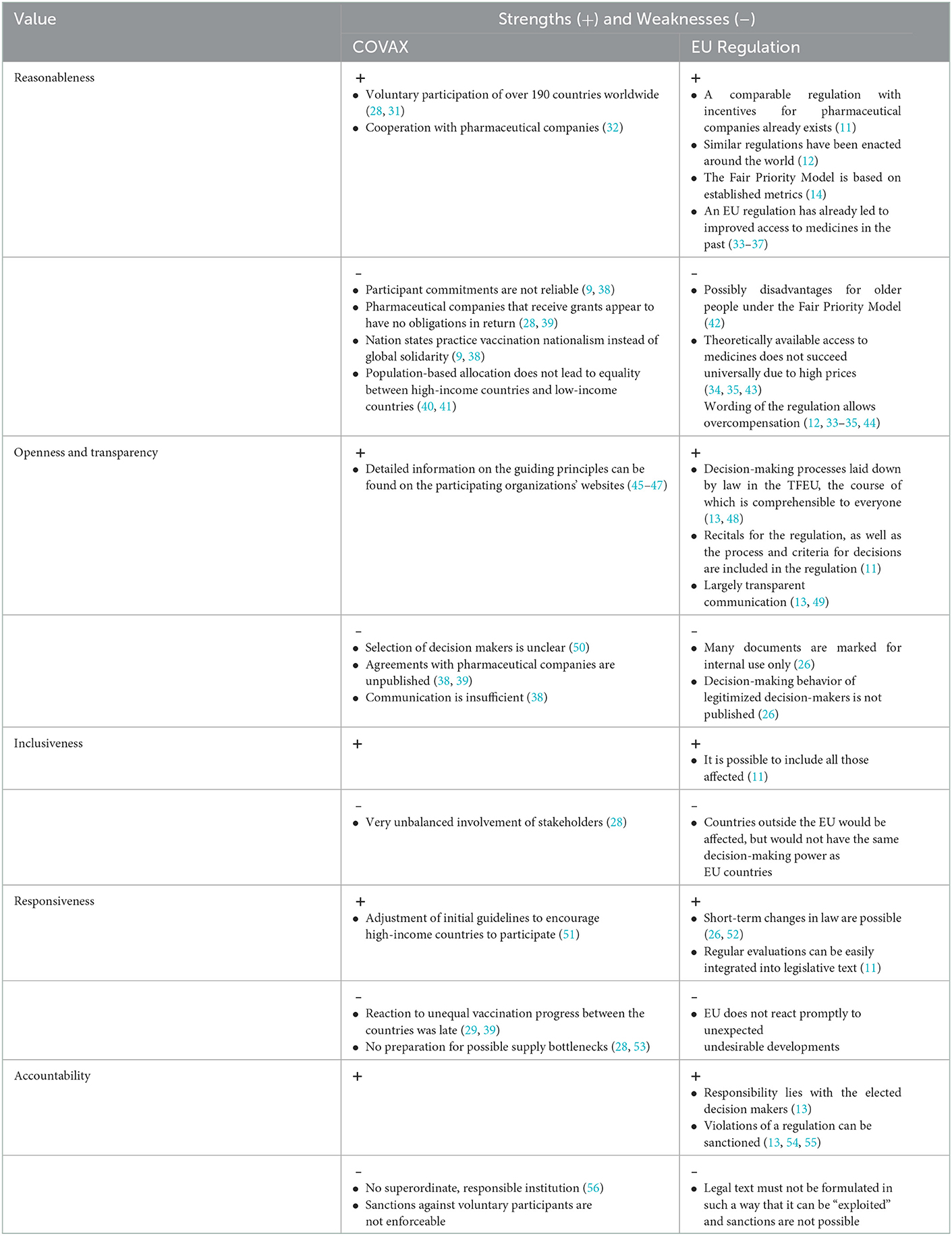
Table 2. Summary strengths and weaknesses of the decision-making process at COVAX and a legislative strategy.
3.1. Reasonableness
According to the ethical value of reasonableness, decisions should be based on reasons that can be considered intelligible by all stakeholders (20). These reasons need to be evidence-based to effectively meet health needs during a pandemic and compatible with shared values, such as human rights, solidarity and international cooperation, and non-discrimination (18, 57, 58).
Countries with delayed access to vaccines have greater potential to develop new viral variants, while coordinated vaccine sharing can reduce the risk of infection, deaths, and the financial burden of a pandemic worldwide (14, 59, 60). Because certain groups are at higher risk during a pandemic, such as the elderly, people with disabilities, the chronically ill, refugees, prison inmates, people in poor health, and healthcare professionals, these groups should have a preferential access to vaccines (21).
3.1.1. COVAX and the value of reasonableness
The voluntary participation of more than 190 countries in COVAX shows that they agree in principle with the idea of a global initiative to increase access to vaccines (28, 31). Bilateral treaties and lack of compliance with COVAX principles, however, are seen as a sign that improving reputation and self-image are greater concerns than global solidarity (9, 38). Vaccine manufacturers have also announced their support for fair global vaccine distribution (61–63). However, the analyzed literature criticizes that they do not act accordingly and do not reliably support COVAX (28, 39).
It is argued that pharmaceutical companies generally lack financial incentives to develop vaccines for infectious diseases and it takes too long to develop new vaccines when relying on the market alone (64, 65). To reduce investment risk for pharmaceutical companies, COVAX, and with it public funding, has committed to purchase large quantities of vaccine upon successful development and incentivized high-risk investments in global vaccine production capacity (59, 66). Criticized are lacking mechanism to ensure sharing of knowledge and data with other companies, and impediment of vaccine donation and resale (28, 67, 68).
In practice COVAX makes a clear distinction between economically strong and weak countries (32, 66). There are not only special incentives and concessions for high-income countries, but also additional requirements for low-income countries (28, 51, 64, 69). Population-based proportional allocation as well as prioritizing healthcare workers and the elderly is criticized for giving higher shares of vaccines to countries with many health care workers and an elderly population, at the cost of countries with weak health systems and young populations (40, 41).
3.1.2. Legislation and the value of reasonableness
The idea behind the orphan drugs legislation is that the pharmaceutical industry is not developing enough drugs for rare diseases under normal market conditions and therefore political intervention is required (11, 64, 70). The ten-year market exclusivity included in the regulation, which is judged to be more beneficial than patent protection, is one of several important incentives for engagement of pharmaceutical companies (33, 34, 71). The Orphan Drug Regulation is seen as positive for research and development of orphan drugs (33–37). Nevertheless, this does not result in all people within the EU having equal access to EU-approved orphan drugs; one of the reasons is the high price of many of these products (34, 35, 43). Authorities have limited negotiating power, as they do not have verifiable information on the actual development and manufacturing costs and are under pressure from patient associations and the media to approve the drugs (12).
The principle of equity and solidarity was decisive for the Orphan Drug Regulation in the EU, but also worldwide (12, 72). Patients suffering from rare diseases should have the right to the same quality of care as other patients (11). This is reflected in the Fair Priority Model: all people worldwide should have equitable access to vaccines. The strategy, which is guided by the values of benefit to the individual and limitation of harm, priority for the disadvantaged, and global equity, and aims to allocate vaccine globally using metrics such as GNI and SEYLL, was deemed effective by the model's developers (14). A critic of the Fair Priority Model points out that using SEYLL as a metric leads to a disadvantage for older people and contradicts the equal treatment of all lives (42).
The Orphan Drug Regulation contains two criteria for a designation as an orphan drug and obtain funding: Either no more than five out of ten thousand people in the EU are affected (prevalence route), or that without incentives, the drug would probably not generate sufficient profit to justify the necessary investment (return on investment route) [Article 3(1) Orphan Drug Regulation] (11). An evaluation after 20 years of Orphan Drug Regulation in Europe shows that not a single drug was brought onto the market that received support because it would otherwise not have generated sufficient profit; all benefited from the low prevalence of the diseases (44). In the opinion of many authors, the Orphan Drug Regulation leads to market failure and overcompensation, since, for example, even ibuprofen received orphan drug status and was able to benefit from privileges (12, 33–35, 44).
3.2. Openness and transparency
The ethical value of openness and transparency are met when decision-making processes, including arguments brought up in the deliberative processes, are disclosed. Moreover, a communication plan should be established and followed to adequately inform the participating parties about the main factors affecting the decisions (18). This increases trust toward decision-makers, decisions are more likely to be perceived as fair and it promotes solidarity (18, 21).
3.2.1. COVAX in relation to openness and transparency
The analyzed literature provides detailed information on the working structure and guiding principles of the organizations involved in COVAX, as well as the rationale for global vaccine distribution (45–47, 50, 73, 74). However, the roles of the various organizations and bodies in decision-making remain unclear. It is not comprehensible for the public to identify who is responsible to whom (75). For example, there is no explanation for the selection process of committee members; unclear is also which organization or company they represent (50). The late inclusion of civil society and the participation of numerous decision-makers linked to the pharmaceutical industry (28) are not justified in detail.
The contracts with pharmaceutical companies are mostly not public, which leads to major transparency gaps (38, 39). It remains unclear according to which criteria the selection of vaccines receiving funding was made (32, 68, 69). Since the development costs are not known, it is not possible to verify whether the financial support is appropriate in relation to the incurred development costs (76). Although there is a public accountability requirement for research institutions that receive government funding, COVAX lacks transparency here (77). Furthermore, due to inclusion of vague terms in the agreements between COVAX and manufacturers, such as “statement of intent”, there are doubts about their binding nature (76). It is unclear what the consequences are for manufacturers if supply agreements are not met (68).
We found little evidence of a communication plan regarding the global distribution of vaccines (50). Minutes of the COVAX Coordination Meetings are not publicly accessible (38). Interview requests from researchers or inquiries about specific decisions at COVAX were not answered (38).
3.2.2. Legislation in relation to openness and transparency
The legislative procedure for EU regulations, the legitimacy of the decision-makers and also the voting modalities are laid down by law and are thus easily comprehensible to the public [Article 294 TFEU, Articles 14 and 16 Treaty on European Union (TEU)] (13, 48). However, an evaluation in this regard revealed deficits. In particular, it was criticized that documents during the legislative process were not made sufficiently accessible to the public. It had also not been systematically recorded which positions were held by the individual member states (26). The EU citizens are therefore unable to understand the work of their elected representatives in the European Parliament (78) and influence them when necessary.
The recitals that led to the regulations are disclosed in the preamble of each EU regulation (79). In the subsequent normative part, for example, in the case of the Orphan Drug Regulation, the incentives for pharmaceutical manufacturers (Articles 7 to 9 Orphan Drug Regulation), the criteria for the designation of a drug as an orphan drug (Article 3 Orphan Drug Regulation) and who decides on this (Article 4) are regulated (11). If a new EU vaccine regulation were to be implemented, comparable standards would have to be drawn up, but in addition, the mandatory global distribution according to the Fair Priority Model would have to be made a condition. The latter is based, among other things, on the well-known parameters SEYLL and GNI (14), the calculation of which is verifiable for the public.
On the EU side, the European Commission's Directorate-General for Communication is responsible for public information (49). In addition, EU regulations are published in the Official Journal of the European Union and are accessible to everyone (Article 297(3) TFEU) (13).
3.3. Inclusiveness
Inclusiveness demands opportunities for all affected parties to voice their concerns and ideas in decision-making processes (20). It is intended to ensure that the decision-making process is based on ethical values shared by all those affected. Ideally, expanding participation in decision-making processes helps to balance power between different interest groups, facilitate a review by people with diverse expertise, and increase the acceptance of a decision among those who do not agree with it (20, 21).
3.3.1. COVAX and the value of inclusiveness
COVAX has four groups of stakeholders: Self-funded countries, subventioned countries, vaccine manufacturers, and global health institutions (31). This leads to a mix of public and private organizations, as well as individuals without a common mission or shared values (75, 80). Key decisions are made by the “COVAX Coordination Meeting,” which includes as permanent participants the two CEOs of CEPI and Gavi, other CEPI, Gavi and WHO leaders, two representatives of the pharmaceutical industry, a member of UNICEF (United Nations International Children's Emergency Fund) and a representative of civil society (50). Of the government representatives involved in some way in COVAX's governance structure, 81% are from self-funded countries (28). The dominant role of pharmaceutical companies in the COVAX decision-making process, as well as their impact on global vaccine allocation, is frequently affirmed (28, 39, 51, 76). Civil society organizations were included in the COVAX decision-making process only after a letter of complaint was made public (28).
Although the WHO has expertise, credibility, and experience in promoting equitable access to health care (81) and acts on behalf of its members, it does not have an overarching role in COVAX (75). Nor do the states themselves have any way of influencing the contracts that COVAX signs (82). There is wide consensus on COVAX not responding sufficiently to the concerns of nation states (51, 76, 83).
3.3.2. Legislation and the value of inclusiveness
Since legislation at the EU level is made jointly by the European Parliament (elected representatives of the citizens of the Union) and the European Council (representatives of the Member States at ministerial level) (Article 294 TFEU, Articles 14 and 16 TEU) (13, 48), the interests of the states are discussed in the legislative process. Further involvement of others can be defined in the legal text. For example, the Orphan Drug Regulation stipulates that a Committee for Orphan Medicinal Products (COMP) examines the extent to which medicinal products can receive orphan drug status and thus funding [Article 4(2) Orphan Drug Regulation]. Patient organizations are represented in the COMP and there is the possibility to consult experts [Article 4(3) Orphan Drug Regulation] (11).
Since the Fair Priority Model envisages global vaccine distribution (14), a solution will have to be found to include countries outside Europe in the decision-making process of an EU regulation. Comparable to COMP, a committee could be established consisting of representatives from non-EU countries. Experts from pharmaceutical companies would also need to be involved to ensure their participation and reduce opposition.
3.4. Responsiveness
Decision-making mechanisms can be considered to meet the value of responsiveness, when continuous review processes are implemented and decisions can be timely revised as new information emerges (18).
3.4.1. COVAX and the value of responsiveness
Although it is acknowledged that global cooperation is necessary to end a pandemic, participation in COVAX was initially not attractive for many countries (51, 76). Both high- and low-income countries rather concluded bilateral agreements with pharmaceutical manufacturers (39). It required concessions on part of COVAX to get states to participate (51). For example, contrary to the original idea, signing bilateral contracts stopped being an impediment for joining COVAX (31). In addition, self-funded countries were allowed to choose a specific vaccine, although product neutrality was originally intended, and, unlike assisted countries, they were allowed to purchase vaccines for up to 50% of the population through COVAX instead of the 20% originally envisioned (51, 83).
The distribution of vaccine to all countries in proportion to population was also criticized. No distinction was made with regard to different levels of pandemic impact or existing vaccine availability. Countries with extremely low mortality rates and those who already had an adequately supply of vaccines also received their share (51, 69). Despite a shortage of available vaccine, COVAX did not initially deviate from proportional distribution. Only in mid-2021, when it became clear that < 3% of people in low-income countries received their first dose, compared with more than 60% in high-income countries, and vaccine manufacturers were prioritizing commitments to bilateral contracts before COVAX, COVAX changed its mode of distribution (29, 39). Instead of treating all countries equally and distributing vaccine proportionally to population, COVAX now focuses on countries where COVAX is the primary source—a complete departure from the original population-proportional allocation (29, 45).
Systematic preparation for possible developments is lacking: COVAX was neither prepared for rich countries buying away most of the vaccines available on the market, nor for planned deliveries not to take place (28, 53).
3.4.2. Legislation and the value of responsiveness
The application of the Orphan Drug Regulation in practice revealed some unforeseen effects that need to be adjusted. It is undisputed that orphan drugs sometimes have high prices and generate very high profits (33–35, 44, 70). However, it is not possible to assess whether the prices are exaggerated because, on the one hand, data such as development costs do not have to be disclosed and, on the other hand, there is a lack of a benchmark to assess this (12, 35). In addition, the analyzed publications frequently address an unbalanced research activity with a very high concentration on oncological diseases (33, 35, 44, 72, 84). Although these aspects have been known for years, the Orphan Drug Regulation has not been adapted in this respect.
The EU needs an average of 19 months for a legislative procedure (52), in principle, however, it could adjust regulations at any time. This was proven during the COVID-19 pandemic: the parliament accepted urgent proposals within a period of 1 to 3 months, made exceptions to deadlines and, for reasons of urgency, allowed regulations to come into force on the day of their publication (26, 85).
Law can also stipulate a systematic consideration of new findings. The Orphan Drug Regulation foresees a report on its evaluation (Article 10 Orphan Drug Regulation) (11). In addition, the European Medicines Agency (EMA) regularly reports on the work of the Committee for Orphan Medicinal Products (COMP) (86). With Article 8(2) and (3), the Orphan Drug Regulation has a mechanism to ensure that it is possible to respond to changing information (11). Under certain conditions, market exclusivity can thus be withdrawn or challenged (35, 37).
3.5. Accountability
The value accountability is addressed when mechanism are in place to implement and enforce decisions, responsibilities are clearly defined, and decision-makers can be held accountable for their actions and omissions (18, 20, 21).
3.5.1. COVAX and the value of accountability
COVAX deviates significantly in terms of accountability from the ideal. The chairs of the COVAX Coordination Meeting, the most powerful decision-making body at COVAX, are accountable only to their own organizations, CEPI and Gavi (28). The mixture of public and private organizations without legitimacy and clear responsibilities in the decision-making process jeopardizes public interests (75, 87). It is consistently criticized that there is neither a responsible higher-level organization nor an enforcement mechanism that takes effect if promises made to COVAX are not kept, so that COVAX is completely dependent on goodwill (28, 30, 38, 56, 88–90). Of the 945 million doses of vaccine pledged by high-income countries by October 2021, only 13% were delivered to COVAX (29). Calls by WHO and NGOs to wait until 10% of the world's population is vaccinated before starting with booster doses have been ineffective (59). Contradictions and violations of declarations of intent by pharmaceutical companies who announced that they would only make very small profits, would not charge excessive prices, would work for fair vaccine distribution and would supply agreed quantities cannot be sanctioned by COVAX (28, 38, 61–63, 83).
3.5.2. Legislation and the value of accountability
In the case of an EU regulation, the responsibilities in the decision-making process, are clearly regulated in the TFEU, in particular in Article 294 TFEU (13). The decision-makers are legitimized by regular elections in the EU member states. However, it is questioned whether the current legislation is sufficient to effectively prevent abuse (44).
If pharmaceutical companies that receive incentives based on EU regulation violate the allocation of a vaccine according to the Fair Priority Model, this could be sanctioned. Since the primary responsibility for the application of a regulation lies with the authorities of the individual Member States (54), supplementary laws with provisions on penalties and fines may be required at national level. If an EU member state violates an EU regulation, formal infringement proceedings are initiated, which are regulated in Article 258 et seq. TFEU (13, 55).
4. Discussion
A legal strategy that offers incentives to the pharmaceutical industry in return for global distribution of vaccines according to the Fair Priority Model is an innovative way to achieve global and equitable access to vaccines. In our analysis of the decision-making processes of COVAX and an EU legal strategy, COVAX deviated significantly from ideal ethical decision-making processes compared to the examined legal strategy. We found significant deficits in COVAX, especially with regard to the ethical values of transparency, inclusiveness and accountability. The decision-making process in a legal regulation is more transparent than COVAX, has broader participation, especially of nation states, and the ethical values of responsiveness and accountability can easily be considered in the development of a new regulation. Therefore, we propose to use the advantages of this strategy and complement it with the positive aspects of COVAX. We continue with a discussion of the key challenges, using the five ethical values, when facilitating global access to vaccines through a legislative strategy.
Both COVAX and the Orphan Drug Regulation have strengths and weaknesses in terms of reasonableness. COVAX already showed that, in principle, there is worldwide agreement on fairer vaccine allocation. While COVAX is based on population-proportional allocation, the proposed legislative strategy relies on vaccine allocation according to the Fair Priority Model, which uses established measurements such as SEYLL and GNI. In a paper published in late 2020, scientists argued that COVAX better succeeds in uniting national and international interests (88). From today's perspective, it is obvious that COVAX has not succeeded in doing so. Instead of supporting global solidarity, nation states engaged in vaccine nationalism and “vaccine diplomacy” (38). A worldwide strategy, similar to EU regulation could be more binding when it comes to achieving the goal of a fair global distribution of vaccines according to the Fair Priority Model. Since the EU has managed to ensure equitable access to vaccines within Europe (82, 91), a similar model could be applied globally. But why should the EU take the first step? Global health is geopolitics (92). The EU claims a leadership role in the world and is committed to fair access to vaccines worldwide (93). In April 2022 the EU reconfirmed that it would also make a contribution to global solidarity in the coming pandemic phase (94). In the context of COVAX, it operated at the beginning as a mediator between international actors such as WHO, Gavi and the Gates Foundation (91). However, the EU fell behind the US and China in vaccine donations during the pandemic (38, 91, 95). If the EU took the first step in a legal strategy for global access to vaccines, it could regain diplomatic recognition and influence.
To achieve the goal of global access to vaccines, cooperation with the pharmaceutical industry is essential. The comparison with the Orphan Drug Regulation as a use case is adequate, as especially the aspect of cooperation between governments and pharmaceutical companies to improve access to medical goods can be easily transferred from orphan drugs to vaccines. From an ethical perspective, pharmaceutical companies should help optimize vaccine production, fair distribution, sustainability, and accountability in the context of COVID-19 vaccines (90). Incentives such as extended market exclusivity in a legal strategy could increase their engagement. Linking incentives to mandatory distribution of vaccines under the Fair Priority Model could simultaneously prevent pharmaceutical companies from being disproportionately favored and ensure that there is a balance between sufficient incentives and their social contribution. Undesirable overcompensation as in the Orphan Drug Regulation must be avoided. Strict attention must be paid to ensuring that vaccines are not only theoretically available worldwide, but are also financially affordable.
The challenge of disproportionately disregarding older people due to the application of SEYLL in the Fair Priority Model (42) must not be neglected. However, generally accepted criteria of equity for the distribution of limited vaccines do not exist.
The lack of openness and transparency at COVAX has led to a great loss of trust in COVAX, high-income countries and pharmaceutical companies. On the other side, although the EU legislative process is already considered as transparent, there is still room for improvement. In order to promote trust, the proposed legislative strategy should take into account the criticism of transparency mentioned in the “Activity Report: Development and Trends of the Ordinary Legislative Procedure” from 2019 (26) and initiate appropriate measures. It should ensure that fewer documents are marked “for internal use only” during the legislative process and that the public can comprehend the voting behavior of their elected representatives.
In both COVAX and a legal strategy, inclusiveness proved to be a significant ethical value. Countries worldwide would benefit from a regulation and receive a fair share of vaccine through a legislative strategy similar to the analyzed EU regulation. They could distribute it within their country according to their needs. The Fair Priority Model affects their sovereignty only in extreme cases (14). Solidarity as a justification for exclusionary decision-making processes is insufficient as a rationale. It must be ensured that the views of all recipient countries are not neglected. At the same time, this is where the greatest weakness of our regulatory approach is revealed: it prevents decolonization of global health and instead supports further dependence of low-income countries on high-income countries and so-called “vaccine apartheid” (9). Instead of promoting research and development in developing countries, as required by Goal 9 of the Sustainable Development Goals, the proposed legislative strategy, like COVAX, continues a traditional aid and charity model (38, 83, 96). Thus, this strategy can only be an interim solution on the path to the ideal: globally equitable access to vaccines achieved through global vaccine research and development (97). In the short term, COVAX was not a perfect solution, but it was a quick fix; in the medium term, a legislative strategy could bridge the gap to the long-term goal of global vaccine research and development.
A worldwide legal strategy including an allocation of vaccines according to the Fair Priority Model would meet the demands of the ethical value responsiveness well, since due to the metrics used, it is possible to react flexibly to new information or unforeseen circumstances. It is also assumed that high-income countries prefer an allocation according to the Fair Priority Model to a population-based proportional allocation used by COVAX. In the event of a major outbreak in a high-income country, the Fair Priority Model with its flexible metric SEYLL would mean preferential adjusted vaccine supply rather than suffering the disadvantages of rigid, population-based proportional allocation (98).
Furthermore, a worldwide legal strategy should include a mechanism that takes effect when it becomes apparent that access to vaccines is not possible as intended despite the regulation or unforeseen loopholes in the regulation become apparent. It seems to make sense to build standards directly into the regulation that specify a time window that must lead to an adaptation of the regulation if deficits in the regulation come to light. For example, a regular evaluation would be conceivable; in the case of the Orphan Drug Regulation, this was planned in accordance with Article 10 in 2006, 6 years after the regulation came into force (11). We think this interval is too long. Especially during a pandemic, rapid action is necessary and crucial for global access to vaccines.
This goes hand in hand with accountability. Those elected by the public to leadership roles should be aware of their responsibilities. They should ensure that everything is done to achieve fair access to vaccines and thus the best public health outcomes. This includes consistently enforcing decisions once they have been made according to ethical standards. Although sanctions are one solution to enforce goals, the example of Orphan Drug Regulation shows that they are not a general panacea for mismanagement. It is not violations by pharmaceutical companies, but unfavorable formulations in the legal text that are responsible for the identified malpractice. As a result, the original intention of the regulation, namely that patients with rare diseases have the same right to good treatment as other patients, is not achieved as well as theoretically possible (11, 33, 35, 44, 72, 84). Such misalignment would have to be avoided in any future worldwide strategy that would include comparable incentives, so that the regulation would not mainly serve economic companies but primarily equitable vaccine allocation.
5. Limitations
It is important to keep in mind that this research is concerned with decision-making processes on global vaccine allocation. How governments can implement these strategies in their countries, include marginalized populations, or address vaccine hesitancy was not the focus of this analysis. Moreover, a limitation of the research is the fact that it was based on the analysis of published literature. Future analysts may have access to documents which are currently not publicly available and therefore could not be assessed at this stage. This may constitute a certain bias of the results; however, the applied search strategy allowed for identification of information pertinent to the research question.
Further research is needed on how comparable legal regulations can be introduced globally. It needs to be investigated how the simultaneous development process of comparable regulations worldwide can be accelerated. Perhaps an initial coordination among the G7 countries would be a start.
6. Conclusions
A legal strategy offering incentives for global vaccine allocation following the Fair Priority Model of Emanuel et al. (14) is an innovative way to achieve global equitable access to vaccines. There is currently no binding, internationally accepted, and successful approach to ensure global, equitable access to healthcare during a pandemic. Current voluntary international initiatives are not sufficiently successful and have weakness concerning ethical decision-making; COVAX, for example, lacks transparency, inclusiveness and accountability.
Instead of relying on a global solution through international organizations (30, 31, 87) and patent issues (39, 51, 53), we argue for a global solution through individual nation states and associations of states that offers incentives for the pharmaceutical industry. The main advantages of the legal approach are that decisions are much more transparent, there are clear responsibilities, representatives of the nation states are legitimized by the population, and regulations can be enforced in court if necessary. Global access to vaccines would no longer depend on the unpredictable goodwill of high-income countries or commercial enterprises and private donors. In particular, we consider the possibility of imposing sanctions in our legal strategy to be advantageous if promises are not kept. This is in significant contrast to strategies currently in use, which do not have an enforcement mechanism. Patent law issues do not arise in our proposal, so that the typical problem of whether interventions in patent law are more likely to harm or benefit pharmaceutical research does not arise. In summary, the research question can be answered as follows: Both strategies need improvement in terms of adherence to ethical values in decision-making. However, COVAX is further away from meeting the central values of ethical decision-making than the examined legal strategy.
Despite the COVID-19 pandemic not being over at the time of writing (June 2022), this proposal is probably too late to implement in the current pandemic. However, our considerations are applicable to vaccine access in other pandemics and can be incorporated into pandemic preparedness guidelines for the future. Our strategy may also be of interest for other allocation issues: Vaccines are not the only medications for which global access is an issue. The same applies to many drugs, such as the new COVID-19 therapeutic Paxlovid (99).
In anticipation of the next pandemic, it is imperative that governments, involving civil society, international organizations and the pharmaceutical industry, develop global solutions that take ethical values into account. Incentives for the pharmaceutical industry to distribute vaccines globally according to the Fair Priority Model (14) could encourage them to act more fairly. However, the strategy proposed here must only be an interim solution on the way to the ideal state envisaged in the Sustainable Development Goals: equitable access to vaccines worldwide through global vaccine research and development (96). Mid-term transitional solutions, such as our legal strategy for equitable access to vaccines, and efforts to find long-term solutions need to be addressed in parallel. This requires all countries to work together in solidarity to find solutions for global vaccine research and development. As a first step, trust would have to be restored. Political will and promises as with COVAX are not sufficient for this; political action is required.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
Conceptualization: KV, CT, and FS. Analysis and discussion of the data: KV, CT, and MO. Writing original draft and visualization: KV. Writing review and editing: KV, CT, MO, and FS. All authors have read and agreed to the published version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bundesministerium für Gesundheit. Fragen und Antworten zur COVID-19-Impfung. (2022). https://www.bundesgesundheitsministerium.de/coronavirus/faq-covid-19-impfung.html (accessed May 31, 2022).
2. Yamey G, Garcia P, Hassan F, Mao W, McDade KK, Pai M, et al. It is not too late to achieve global covid-19 vaccine equity. BMJ. (2022) 376:e070650. doi: 10.1136/bmj-2022-070650
3. Kupferschmidt K. Deaths of health workers in Africa highlight vaccine inequity. Science. (2021) 371:764–5. doi: 10.1126/science.371.6531.764
4. Kennedy RB, Ovsyannikova IG, Palese P, Poland GA. Current challenges in vaccinology. Front Immunol. (2020) 11:1181. doi: 10.3389/fimmu.2020.01181
5. World Trade Organization. TRIPS—Trade-Related Aspects of Intellectual Property Rights. (1994). Available online at: https://www.wto.org/english/docs_e/legal_e/27-trips_02_e.htm (accessed May 31, 2022).
6. Gaviria M, Kilic B. A network analysis of COVID-19 mRNA vaccine patents. Nat Biotechnol. (2021) 39:546–8. doi: 10.1038/s41587-021-00912-9
7. Emanuel EJ, Fabre C, Herzog L, Norheim OF, Persad G, Schaefer GO, et al. Obligations in a global health emergency—Authors' reply. Lancet. (2021) 398:2072. doi: 10.1016/S0140-6736(21)02645-3
8. World Health Organization. Fair Allocation Mechanism for COVID-19 Vaccines Through the COVAX Facility. (2020). Available online at: https://www.who.int/publications/m/item/fair-allocation-mechanism-for-covid-19-vaccines-through-the-covax-facility (accessed May 31, 2022).
9. Sparke M, Levy O. Competing responses to global inequalities in access to COVID vaccines: vaccine diplomacy and vaccine charity vs. vaccine liberty. Clin Infect Dis. (2022) 75:ciac361. doi: 10.1093/cid/ciac361
10. Regulation Regulation (EC) No 1901/2006 of the European Parliament and of the Council of 12 December 2006 on medicinal products for Paediatric use and amending Regulation (EEC) No 1768/92 Directive 2001/20/EC Directive 2001/83/EC and Regulation (EC) No 726/2004. vol. 378. (2006).
11. Regulation (EC) No 141/2000 of the European Parliament and of the Council of 16 December 1999 on orphan medicinal products. vol. 018. (1999).
12. Simoens S, Cassiman D, Dooms M, Picavet E. Orphan drugs for rare diseases. Drugs. (2012) 72:1437–43. doi: 10.2165/11635320-000000000-00000
14. Emanuel EJ, Persad G, Kern A, Buchanan A, Fabre C, Halliday D, et al. An ethical framework for global vaccine allocation. Science. (2020) 369:1309–12. doi: 10.1126/science.abe2803
15. Silverman R, Towse A, Love J, Prashant Y. Would Exempting COVID-19 Vaccines from Intellectual Property Rights Improve Global Access Equity? (2021). Available online at: https://www.cgdev.org/debate/would-exempting-covid-19-vaccines-intellectual-property-rights-improve-global-access (accessed May 31, 2022).
16. European Union. World Trade Organization TRIPS Waiver to Tackle Coronavirus. (2021). Available online at: https://www.europarl.europa.eu/RegData/etudes/ATAG/2021/690649/EPRS_ATA(2021)690649_EN.pdf (accessed May 31, 2022).
17. Daniels N. Accountability for reasonableness: establishing a fair process for priority setting is easier than agreeing on principles. BMJ. (2000) 321:1300–1. doi: 10.1136/bmj.321.7272.1300
18. Joint Centre for Bioethics Pandemic Influenza Working Group. Stand on guard for thee: Ethical considerations in preparedness planning for pandemic influenza. (2005). Available online at: https://jcb.utoronto.ca/wp-content/uploads/2021/03/stand_on_guard.pdf (accessed May 31, 2022).
19. Smith M, Upshur R. “Pandemic Disease, Public Health, and Ethics,” In: Mastroianni AC, Kahn JP, Kass NE, ed. Oxf. Handb. Public Health Ethics. New York: Oxford University Press (2019). p. 797–816. doi: 10.1093/oxfordhb/9780190245191.013.69
20. Thompson AK, Faith K, Gibson JL, Upshur RE. Pandemic influenza preparedness: an ethical framework to guide decision-making. BMC Med Ethics. (2006) 7:12. doi: 10.1186/1472-6939-7-12
21. National Ethics Advisory Committee. Getting Through Together: Ethical values for a pandemic. (2007). Available online at: https://neac.health.govt.nz/publications-and-resources/neac-publications/getting-through-together-ethical-values-for-a-pandemic/ (accessed May 31, 2022).
22. Eli K, Hawkes CA, Fritz Z, Griffin J, Huxley CJ, Perkins GD, et al. Assessing the quality of ReSPECT documentation using an accountability for reasonableness framework. Resusc Plus. (2021) 7:100145. doi: 10.1016/j.resplu.2021.100145
23. Tam V, Allatt RG. Evaluating Toronto hospitals' COVID-19 visitor policy using accountability for reasonableness. Healthc Q. (2022) 25:30–35. doi: 10.12927/hcq.2022.26944
24. Ujewe SJ, van Staden WC. Inequitable access to healthcare in Africa: reconceptualising the “accountability for reasonableness framework” to reflect indigenous principles. Int J Equity Health. (2021) 20:139. doi: 10.1186/s12939-021-01482-7
26. European Parliament. Activity Report: Development Trends of the Ordinary Legislative Procedure (1 July 2014–1 July 2019). (2019). Available online at: http://www.epgencms.europarl.europa.eu/cmsdata/upload/f4c0b9d3-fec4-4d79-815b-6356336be5b9/activity-report-2014-2019_en.pdf (accessed June 2, 2022).
27. Médecins Sans Frontiéres. Open Letter to Gavi Board Members: Inclusion of Civil Society in COVAX Facility COVAX AMC Governance is Essential. Médecins Sans Frontiéres Access Campaign (2020). Available online at: https://msfaccess.org/open-letter-gavi-board-members-inclusion-civil-society-covax-facility-and-covax-amc-governance (accessed June 2, 2022)
28. Storeng KT, de Bengy Puyvallée A, Stein F, COVAX. and the rise of the ‘super public private partnership' for global health. Glob Public Health. (2021) 10:1–17. doi: 10.1080/17441692.2021.1987502
29. Usher AD. Vaccine shortages prompt changes to COVAX strategy. Lancet. (2021) 398:1474. doi: 10.1016/S0140-6736(21)02309-6
30. Li Z, Lu J, Lv J. The inefficient and unjust global distribution of COVID-19 vaccines: from a perspective of critical global justice. Inq J Health Care Organ Provis Financ. (2021) 58:00469580211060992. doi: 10.1177/00469580211060992
31. McAdams D, McDade KK, Ogbuoji O, Johnson M, Dixit S, Yamey G. Incentivising wealthy nations to participate in the COVID-19 vaccine global access facility (COVAX): a game theory perspective. BMJ Glob Health. (2020) 5:e003627. doi: 10.1136/bmjgh-2020-003627
32. Berkley S. COVAX Explained. (2020). Available online at: https://www.gavi.org/vaccineswork/covax-explained (accessed May 31, 2022).
34. Dear JW, Lilitkarntakul P, Webb DJ. Are rare diseases still orphans or happily adopted? The challenges of developing and using orphan medicinal products. Br J Clin Pharmacol. (2006) 62:264–71. doi: 10.1111/j.1365-2125.2006.02654.x
35. Kanavos P, Nicod E. What is wrong with orphan drug policies? Suggestions for ways forward value. Health. (2012) 15:1182–4. doi: 10.1016/j.jval.2012.08.2202
36. Blonda A, Denier Y, Huys I, Simoens S. How to value orphan drugs? A review of European value assessment frameworks. Front Pharmacol. (2021) 12:631527. doi: 10.3389/fphar.2021.631527
37. Westermark K. The Committee for orphan medicinal products and the European medicines agency scientific secretariat. European regulation on orphan medicinal products: 10 years of experience and future perspectives. Nat Rev Drug Discov. (2011) 10:341–9. doi: 10.1038/nrd3445
38. de Bengy Puyvallée A, Storeng KT. COVAX vaccine donations and the politics of global vaccine inequity. Glob Health. (2022) 18:26. doi: 10.1186/s12992-022-00801-z
39. Sung M, Huang Y, Duan Y, Liu F, Jin Y, Zheng Z. Pharmaceutical industry's engagement in the global equitable distribution of COVID-19 vaccines: corporate social responsibility of EUL vaccine developers. Vaccines. (2021) 9:1183. doi: 10.3390/vaccines9101183
40. Wang W, Wu Q, Yang J, Dong K, Chen X, Bai X, et al. Global, regional, and national estimates of target population sizes for covid-19 vaccination: descriptive study. BMJ. (2020) 371:m4704. doi: 10.1136/bmj.m4704
41. Demombynes G. COVID-19 Age-Mortality Curves are Flatter in Developing Countries. Washington, DC: World Bank. (2020). doi: 10.1596/1813-9450-9313
42. Harris J. Combatting Covid-19 Or, “All persons are equal but some persons are more equal than others?” Camb Q Healthc Ethics. (2021) 30:406–14. doi: 10.1017/S096318012000095X
43. Picavet E, Cassiman D, Simoens S. Evaluating and improving orphan drug regulations in Europe: a Delphi policy study. Health Policy. (2012) 108:1–9. doi: 10.1016/j.healthpol.2012.08.023
44. Marselis D, Hordijk L. From blockbuster to “nichebuster”: how a flawed legislation helped create a new profit model for the drug industry. BMJ. (2020) 370:m2983. doi: 10.1136/bmj.m2983
45. World Health Organization. ACT-Accelerator Strategic Plan & Budget: October 2021 to September 2022. (2021). Available online at: https://www.who.int/publications/m/item/act-accelerator-strategic-plan-budget-october-2021-to-september-2022 (accessed June 2, 2022).
46. CEPI. COVAX: CEPI's Response to COVID-19. (2020). Available online at: https://cepi.net/covax/ (accessed June 2, 2022).
47. Gavi. COVAX Facility. (2020). Available online at: https://www.gavi.org/covax-facility (accessed June 2, 2022).
49. European Commission. Communication. (2022). Available online at: https://ec.europa.eu/info/departments/communication_en (accessed June 2, 2022).
50. Gavi. COVAX Pillar: Structure and Principles. (2020). Available online at: https://www.gavi.org/sites/default/files/covid/covax/COVAX_the-Vaccines-Pillar-of-the-Access-to-COVID-19-Tools-ACT-Accelerator.pdf (accessed June 2, 2022).
51. Usher AD. A beautiful idea: how COVAX has fallen short. Lancet. (2021) 397:2322–5. doi: 10.1016/S0140-6736(21)01367-2
52. Bundesregierung. Die Gesetzgebung der Europäischen Union. (2022). Available online at: https://www.bundesregierung.de/breg-de/themen/europa/wie-funktioniert-europa/die-gesetzgebung-der-europaeischen-union (accessed June 3, 2022).
53. Jecker NS. Global sharing of COVID-19 vaccines: a duty of justice, not charity. Dev World Bioeth. (2022). doi: 10.1111/dewb.12342
54. European Commission. Applying EU law. (2022). Available online at: https://ec.europa.eu/info/law/law-making-process/applying-eu-law_en (accessed June 3, 2022).
55. European Commission. Infringement Procedure. (2022). Available online at: https://ec.europa.eu/info/law/law-making-process/applying-eu-law/infringement-procedure_en (accessed June 3, 2022).
56. Editorial. Access to COVID-19 Vaccines: Looking Beyond COVAX. Lancet. (2021) 397:941. doi: 10.1016/S0140-6736(21)00617-6
57. United Nations. Universal Declaration of Human Rights. (1948). Available online at: https://www.un.org/en/about-us/universal-declaration-of-human-rights (accessed May 30, 2022).
58. World Health Organization. WHO SAGE Values Framework for the Allocation and Prioritization of COVID-19 Vaccination, 14 September 2020. (2020). Available online at: https://apps.who.int/iris/handle/10665/334299 (accessed June 3, 2022).
59. Pilkington V, Keestra SM, Hill A. Global COVID-19 Vaccine Inequity: Failures in the First Year of Distribution and Potential Solutions for the Future. Front Public Health. (2022) 10:1–17. doi: 10.3389/fpubh.2022.821117
60. Chinazzi M, Davis JT, Dean NE, Mu K. Estimating the Effect of Cooperative vs. Uncooperative Strategies of COVID-19 Vaccine Allocation: A Modeling Study. (2020). Available online at: www.mobs-lab.org/uploads/6/7/8/7/6787877/global_vax.pdf (accessed May 31, 2022).
61. AstraZeneca. AstraZeneca Takes Next Steps Toward Broad and Equitable Access to Oxford University's Potential COVID-19 Vaccine. (2020). Available online at: https://www.astrazeneca.com/media-centre/articles/2020/astrazeneca-takes-next-steps-towards-broad-and-equitable-access-to-oxford-universitys-potential-covid-19-vaccine.html (accessed June 2, 2022).
62. Shapiro E. Pfizer CEO Albert Bourla raises expectations that the pharmaceutical giant can deliver a COVID-19 vaccine by fall. Time. (2020).
63. Dunn A. The CEO of the buzzy biotech that's working on a potential coronavirus vaccine just pledged he won't set a high price for the shot. Bus Insid. (2020). Available online at: https://www.businessinsider.com/moderna-ceo-stephane-bancel-interview-coronavirus-vaccine-price-2020-3 (accessed June 2, 2022).
64. Rutschman AS. The COVID-19 vaccine race: intellectual property, collaboration(s), nationalism and misinformation. Wash Univ J Law Policy. (2021) 64:167.
65. Usher AD. COVID-19 vaccines for all? Lancet. (2020) 395:1822–3. doi: 10.1016/S0140-6736(20)31354-4
66. Stein F. Risky business: COVAX and the financialization of global vaccine equity. Glob Health. (2021) 17:112. doi: 10.1186/s12992-021-00763-8
67. Storeng KT, Stein F, de Puyvallée AB. COVAX and the many meanings of sharing. BMJ Glob Health. (2021) 6:e007763. doi: 10.1136/bmjgh-2021-007763
68. Kupferschmidt K. Global plan seeks to promote vaccine equity, spread risks. Science. (2020) 369:489–90. doi: 10.1126/science.369.6503.489
69. Manriquez Roa T, Holzer F, Luna F, Biller-Andorno N. Expert views on COVAX and equitable global access to COVID-19 vaccines. Int J Public Health. (2021) 66:114. doi: 10.3389/ijph.2021.1604236
70. Rodwell C, Aymé S. Rare disease policies to improve care for patients in Europe. Biochim Biophys Acta BBA - Mol Basis Dis. (2015) 1852:2329–35. doi: 10.1016/j.bbadis.2015.02.008
71. Bruyaka O, Zeitzmann HK, Chalamon I, Wokutch RE, Thakur P. Strategic corporate social responsibility and orphan drug development: insights from the US and the EU biopharmaceutical industry. J Bus Ethics. (2013) 117:45–65. doi: 10.1007/s10551-012-1496-y
72. Grewal J, Hoppe N. Don't forget the orphans. Eur J Health Law. (2015) 22:107–11. doi: 10.1163/15718093-12341351
73. World Health Organization. Allocation Logic and Algorithm to Support Allocation of Vaccines Secured Through the COVAX Facility. (2021). Available online at: https://www.who.int/publications/m/item/allocation-logic-and-algorithm-to-support-allocation-of-vaccines-secured-through-the-covax-facility (accessed June 2, 2022).
74. World Health Organization. COVAX Working for Global Equitable Access to COVID-19 Vaccines. (2022). Available online at: https://www.who.int/initiatives/act-accelerator/covax (accessed June 2, 2022).
75. Moon S, Armstrong J, Hutler B, Upshur R, Katz R, Atuire C, et al. Governing the access to COVID-19 tools accelerator: toward greater participation, transparency, and accountability. Lancet. (2022) 399:487–94. doi: 10.1016/S0140-6736(21)02344-8
76. Eccleston-Turner M, Upton H. International collaboration to ensure equitable access to vaccines for COVID-19: the ACT-accelerator and the COVAX facility. Milbank Q. (2021) 99:426–49. doi: 10.1111/1468-0009.12503
77. Ramappa S, Reddy A, Dieminger L, Romero Bhathal I, Rhodes N, Nguyen J. Public Medicines for COVID-19. (2021).
78. European Parliament. Members of the European Parliament. (2022). Available online at: https://www.europarl.europa.eu/meps/en/home (accessed June 2, 2022).
79. European Union. Gemeinsamer Leitfaden des Europäischen Parlaments, des Rates und der Kommission für Personen, die an der Abfassung von Rechtstexten der Europäischen Union mitwirken. (2015). Available online at: https://eur-lex.europa.eu/content/techleg/KB0213228DEN.pdf (accessed June 2, 2022).
80. Velásquez G. “COVID-19 vaccines: between ethics, health and economics,” In: Velásquez G, ed. Vaccines Med. COVID-19 Can WHO Be Stronger Voice. Cham: Springer International Publishing (2022). p. 1–6. doi: 10.1007/978-3-030-89125-1_1
81. Bollyky TJ, Gostin LO, Hamburg MA. The equitable distribution of COVID-19 therapeutics and vaccines. JAMA. (2020) 323:2462. doi: 10.1001/jama.2020.6641
82. Vogler S, Haasis MA, van den Ham R, Humbert T, Garner S, Suleman F. European collaborations on medicine and vaccine procurement. Bull World Health Organ. (2021) 99:715–21. doi: 10.2471/BLT.21.285761
83. Geiger S, McMahon A. Analysis of the institutional landscape and proliferation of proposals for global vaccine equity for COVID-19: too many cooks or too many recipes? J Med Ethics. (2021) 107684. doi: 10.1136/medethics-2021-107684
84. Vassal G, Kearns P, Blanc P, Scobie N, Heenen D, Pearson A. Orphan drug regulation: a missed opportunity for children and adolescents with cancer. Eur J Cancer. (2017) 84:149–58. doi: 10.1016/j.ejca.2017.07.021
85. Regulation Regulation (EU) 2020/561 of the European Parliament and of the Council of 23 April 2020 amending Regulation (EU) 2017/745 on medical devices as as regards the dates of application of certain of its provisions (Text with EEA relevance). vol. 130. (2020).
86. European Medicines Agency. EMA Management Board: Highlights of October 2017 Meeting. (2017). Available online at: https://www.ema.europa.eu/en/news/ema-management-board-highlights-october-2017-meeting (accessed June 3, 2022).
87. Luna F, Holzer F. Brief communication International cooperation in a non-ideal world: the example of COVAX. Cad Ibero-Am Direito Sanit Cuad Iberoam Derecho Sanit. (2021) 10:199–210. doi: 10.17566/ciads.v10i3.789
88. Lie RK, Miller FG. Allocating a COVID-19 vaccine: balancing national and international responsibilities. Milbank Q. (2021) 99:450–66. doi: 10.1111/1468-0009.12494
89. Holzer F, Luna F, Roa TM, Biller-Andorno N. A matter of priority: equitable access to COVID-19 vaccines. Swiss Med Wkly. (2021). doi: 10.4414/smw.2021.20488
90. Emanuel EJ, Buchanan A, Chan SY, Fabre C, Halliday D, Heath J, et al. What are the obligations of pharmaceutical companies in a global health emergency? Lancet Lond Engl. (2021) 398:1015–20. doi: 10.1016/S0140-6736(21)01378-7
91. Deters H, Zardo F. The European commission in Covid-19 vaccine cooperation: leadership vs coronationalism? J Eur Public Policy. (2022) 1–21. doi: 10.1080/13501763.2022.2064900
92. Kickbusch I, Liu A. Global health diplomacy—reconstructing power and governance. Lancet. (2022) S0140673622005839:2156–66. doi: 10.1016/S0140-6736(22)00583-9
93. European Commission. State of the Union Address by President von der Leyen. (2020). Available online at: https://ec.europa.eu/commission/presscorner/detail/en/SPEECH_20_1655 (accessed May 30, 2022).
94. European Commission. COVID-19: Next Pandemic Phase. (2022). Available online at: https://ec.europa.eu/commission/presscorner/detail/en/ip_22_2646 (accessed June 2, 2022).
95. Borrell J. Geoeconomics Geopolitics of the COVID-19 Crisis. (2021). Available online at: https://www.eeas.europa.eu/eeas/geoeconomics-and-geopolitics-covid-19-crisis_en (accessed May 30, 2022).
96. United Nations. THE 17 GOALS|Sustainable Development. (2015). Available online at: https://sdgs.un.org/goals (accessed May 30, 2022).
97. Rey-Jurado E, Tapia F, Muñoz-Durango N, Lay MK, Carreño LJ, Riedel CA, et al. Assessing the importance of domestic vaccine manufacturing centers: an overview of immunization programs, vaccine manufacture, and distribution. Front Immunol. (2018) 9:26. doi: 10.3389/fimmu.2018.00026
98. Herzog LM, Norheim OF, Emanuel EJ, McCoy MS. Covax must go beyond proportional allocation of Covid vaccines to ensure fair and equitable access. BMJ. (2021) 372:m4853. doi: 10.1136/bmj.m4853
Keywords: pandemic, COVAX, Fair Priority Model, EU regulation, ethics, equity, accountability for reasonableness, allocation
Citation: Voit K, Timmermann C, Orzechowski M and Steger F (2023) Voluntariness or legal obligation? An ethical analysis of two instruments for fairer global access to COVID-19 vaccines. Front. Public Health 11:995683. doi: 10.3389/fpubh.2023.995683
Received: 05 August 2022; Accepted: 09 January 2023;
Published: 26 January 2023.
Edited by:
Zisis Kozlakidis, International Agency for Research on Cancer (IARC), FranceReviewed by:
Ramesh Mark Nataraja, Monash Children's Hospital, AustraliaSi Man Lei, University of Macau, China
Copyright © 2023 Voit, Timmermann, Orzechowski and Steger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katja Voit,  katja.voit@uni-ulm.de
katja.voit@uni-ulm.de
†ORCID: Katja Voit orcid.org/0000-0001-5091-428X
Cristian Timmermann orcid.org/0000-0001-7935-2823
Marcin Orzechowski orcid.org/0000-0003-4244-7989
Florian Steger orcid.org/0000-0001-8108-1591
 Katja Voit
Katja Voit Cristian Timmermann
Cristian Timmermann Marcin Orzechowski
Marcin Orzechowski Florian Steger
Florian Steger