- 1Lebanese American University, School of Medicine, Beirut, Lebanon
- 2Department of Internal Medicine, Lebanese American University-Rizk Hospital, Beirut, Lebanon
- 3Department of Internal Medicine, SUNY Upstate Medical University Hospital, Syracuse, NY, United States
- 4Georgetown University School of Medicine, Washington, DC, United States
- 5Faculty of Medicine and Medical Sciences, University of Balamand, Tripoli, Lebanon
- 6Department of Internal Medicine, Saint George Hospital-University Medical Center, Beirut, Lebanon
- 7Department of Laboratory, Makased General Hospital, Beirut, Lebanon
- 8Department of Laboratory, Mount Liban Hospital, Hazmiyeh, Lebanon
- 9Department of Laboratory, Rafic Hariri University Hospital, Beirut, Lebanon
- 10Department of Laboratory, The Middle East Institute of Health University Hospital, Mount Lebanon, Lebanon
- 11Saint Georges Ajaltoun Hospital, Ajaltoun, Lebanon
- 12Haykal Hospital, Tripoli, Lebanon
- 13Department of Internal Medicine, Notre Dame de Secours University Hospital, Byblos, Lebanon
- 14College of Medicine, Central Michigan University, Saginaw, MI, United States
- 15Department of Clinical Microbiology and Infection Prevention, Michigan Health Clinics, Saginaw, MI, United States
Background: Invasive fungal infections have presented a challenge in treatment. In the past, it was known that the frontrunner in such infections is Candida albicans with little emphasis placed on non-albicans Candida species (NAC). Studies worldwide have shown a rise in fungal infections attributed to non-albicans Candida species. The aim of this study is to describe the epidemiology of NAC infections along with an overview of resistance in Lebanese hospitals.
Methods: This is a two-year observational multi-central descriptive study. Between September 2016 and May of 2018, a total of 1000 isolates were collected from 10 different hospitals distributed all over the country. For the culture, Sabouraud Dextrose Agar was used. Antifungal Susceptibility was evaluated by determining the Minimum Inhibitory Concentration (MIC) in broth (microdilution) of the different antifungal treatments.
Results: Out of the 1000 collected isolates, Candida glabrata, being the most isolated species (40.8%), followed by Candida tropicalis: 231(23.1%), Candida parapsilosis: 103(10.3%), and other NAC species at lower percentage. Most of these isolates (88.67%) were susceptible to posaconazole, 98.22% were susceptible to micafungin, and 10% were susceptible to caspofungin.
Conclusion: The change of etiology of fungal infections involving a significant increase in NAC cases is alarming due to the different antifungal susceptibility patterns and the lack of local guidelines to guide the treatment. In this context, proper identification of such organisms is of utmost importance. The data presented here can help in establishing guidelines for the treatment of candida infections to decrease morbidity and mortality. Future surveillance data are needed.
1. Introduction
The incidence and burden of fungal infections is rising globally. Fungal infections are a major concern for clinicians because it is associated with high morbidity and mortality, mainly in critical and immunocompromised patients. Serious and invasive Candida infections are usually hospital acquired. In the hospital setting, Non-albicans Candida species (NAC) are more frequently isolated (1).
Invasive candidiasis includes a variety of infectious conditions caused by Candida species. Invasive candidiasis is a serious infection that causes high mortality and morbidity. In the United States (US), around 25,000 cases of invasive candidiasis are reported annually (2). The most common and studied form of invasive candidiasis is candidemia, especially in intensive care patients (3). It remains a challenge to estimate the global incidence of candidemia and this is due to many factors including diagnostic techniques as well as the lack of surveillance systems for fungal infections (4). New diagnostic techniques are developing including Polymerase chain reaction and specific rapid antigen. Nevertheless, positive predictive values of non-culture techniques remain low while negative predictive values are high. Therefore, clinical suspicion of invasive fungal infections in combination with Candida diagnostics should be used in patients care. However, the reported annual incidence of candidemia in the US is around 9 cases per 100,000 (5). Candida species rank as the fourth most common cause of hospital-acquired bloodstream infections, after coagulase-negative staphylococci (CNS), staphylococcus aureus, and enterococcus spp. (6).
Candida albicans is the predominant isolate from patients with invasive candidiasis worldwide (7). However, a new threat has emerged over the last few decades, as NAC are increasingly recovered from patients. The most reported species of NAC include C. glabrata, C. tropicalis, C. parapsilosis, and C. krusei (8). Collectively along with C. albicans, these species are responsible for over 90% of the cases of invasive candidiasis (9). The frequency of each species varies with geographic differences in different countries (10–14), the local hospital epidemiology within the same country (15–17), the different units within the same hospital, underlying patient characteristics, and the antimicrobial treatment strategies and protocols (18, 19). Nevertheless, the clinical importance of NAC species lies in the potential antifungal resistance which can lead to treatment failure and its consequences.
Several studies (20–25) have estimated the incidence rates of candidemia in the Middle East and North Africa countries. Candidemia incidence rate was estimated to be the highest in Qatar, with a calculated rate of (15.4/100,000) (21) and the lowest in Iran (0.34/100,000) (20). In a study done by Koehler et al., European incidence of candidemia was estimated to be 79 cases per day, of which an estimated 29 patients might have fatal outcome at Day 30 (26). There was a higher proportion of Candida spp. other than C. albicans in the decade from 2010 till 2019 in population-based data (26).
Echinocandin and azole-resistance is increasingly reported in non-albicans Candida from cases of invasive candidiasis (27, 28). Exceptional resistance to antifungals in some Candida species, such as in Candida auris, constitutes a major threat to patients and has a significant impact worldwide. Candida's ability to form biofilm represents a problem in the context of antifungal drug-resistance.
Lebanon is a small country in the Middle East Region where a prominent level of antimicrobial use has been documented (29). The current compiled antimicrobial susceptibility data have shed light on increasing bacterial resistance trends in this country, which were found to be comparable with data from some Eastern and Southern European countries (29). For that reason, it was important to understand the local epidemiology and subsequently to establish guidelines for the appropriate identification and treatment of such infections as well as for their prevention. This multicenter study aimed at describing the epidemiology and distribution of NAC species in the context of the global data, as well as identifying and determining the antifungal susceptibility profiles of 1000 NAC clinical specimens collected from various clinical infections.
2. Methods
2.1. Samples and study population
A total of 1,000 clinical samples including urine, vaginal swabs, sputum, blood, cerebrospinal fluid (CSF) and miscellaneous samples were collected prospectively from all patients having a positive fungal culture and presenting to 10 hospitals located in different geographic areas of the country between September 2016 and May of 2018 according to standard procedures. More than one clinical sample from the same patient with the same identification and same susceptibility profile were considered duplicates, and therefore only the first isolate was included. All clinical samples were inoculated on Sabouraud dextrose agar (Oxoid, Basingstoke, UK) to which 50 μg/ml of Gentamycin was added to suppress the growth of bacterial contaminants. Inoculated plates were incubated at 37° C for 72 hours aerobically, extended incubation was performed when needed. Isolates were identified by conventional methods using microscopic examination using KOH preparation, colonial morphology, and carbohydrate assimilation method using the API 20C Aux system (bioMerieux-Vitek, Hazelwood, Mo.).
2.2. Antifungal susceptibility testing
Antifungal Susceptibility testing was evaluated by determining the Minimum Inhibitory Concentration (MIC) in broth (microdilution method) of 7 different antifungals after 24 and 72 hours of incubation according to the CLSI M27 and M60 documents “Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard—Second Edition- CLSI) (30) and re-analyzed according to CLSI M60 “Performance Standards for Antifungal Susceptibility Testing of Yeasts” after the second version of this document was issued (2020) (31). Each sample (Candida isolate) was run in duplicate to ensure accuracy of the results. The MICs were considered in Essential and Categorical agreement when their values fell within one dilution. When disagreement was observed, the experiment was repeated.
Antifungal standard reference powders were obtained commercially or directly from the drug manufacturer. After preparation, antifungal solutions were stored as recommended. All antifungal agents were assayed for standard units of activity. Antifungal solutions were standardized based on assays of the lots of antifungal powders.
Antifungal stock solutions were prepared at concentrations of at least 1280 μg/mL or ten times the highest concentration to be tested, whichever was greater.
The antifungal agents tested were: Amphotericin B, Micafungin, Caspofungin, Anidulafungin, Voriconazole, Fluconazole, and Posaconazole). Antifungal powders were dissolved depending on the chemical properties of each one. Some were dissolved in DMSO diluted in RPMI (Amphotericin B, Ketoconazole, Itraconazole, Posaconazole, Voriconazole). The concentrations to be tested were based on the breakpoint concentrations and the expected results for the quality control strains. Based on previous studies, the following drug concentration ranges were used: amphotericin B, 0.0313 to 16 μg/mL; flucytosine, 0.125 to 64 μg/mL; ketoconazole, 0.0313 to 16 μg/mL; itraconazole, 0.0313 to 16 μg/mL; fluconazole, 0.125 to 64 μg/mL; and new triazoles, 0.0313 to 16 μg/mL.
Quality control strains included C. parapsilosis ATCC 22019, C. albicans ATCC 90028, and C. krusei ATCC 6258. RPMI 1640 medium was used as a Synthetic Medium for susceptibility testing. Zwitterion buffers were used to buffer the media to a pH of 7.0 ± 0.1 at 25 °C. All organisms were sub-cultured from sterile vials onto Sabouraud Dextrose Agar.
2.3. Data analysis and interpretation
Patients' privacy and Identities were not revealed, all data were coded for that purpose. Statistical analysis was performed using SPSS version 20. Descriptive statistics such as frequency and percentage of Candida species were calculated.
2.4. Ethical clearance
All ethical deliberations and responsibilities were appropriately addressed, and the study was conducted after the approval of the Institutional Review Board (IRB) of the Lebanese American University. (IRB# LAU.SOM.RH1.26/Apr/2016).
3. Results
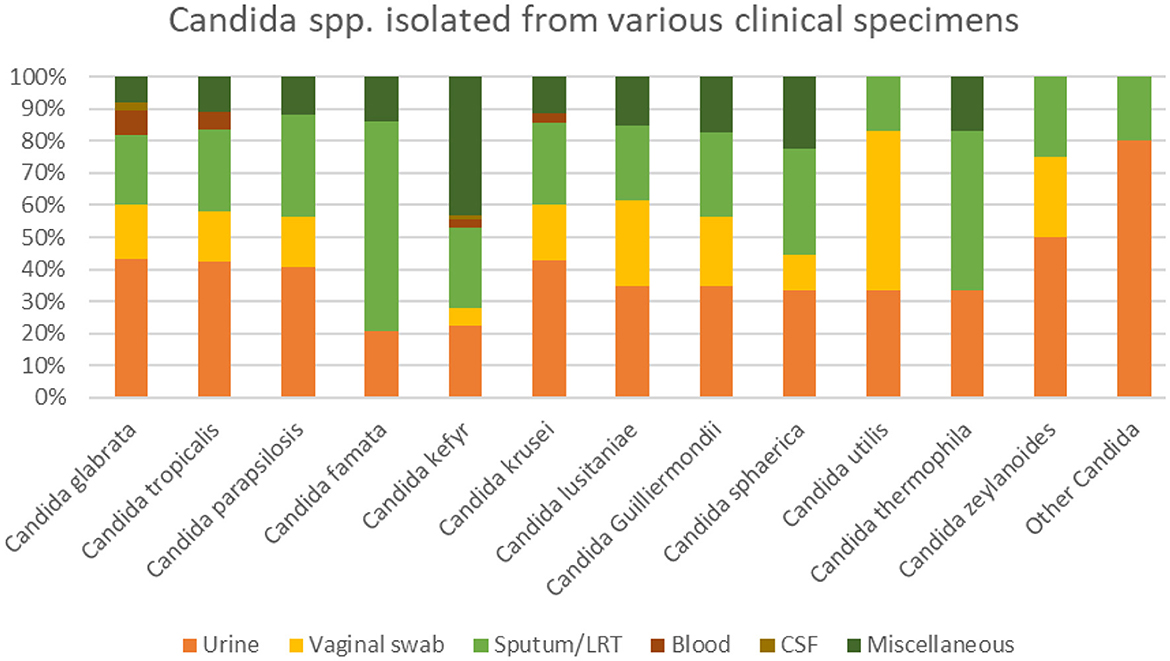
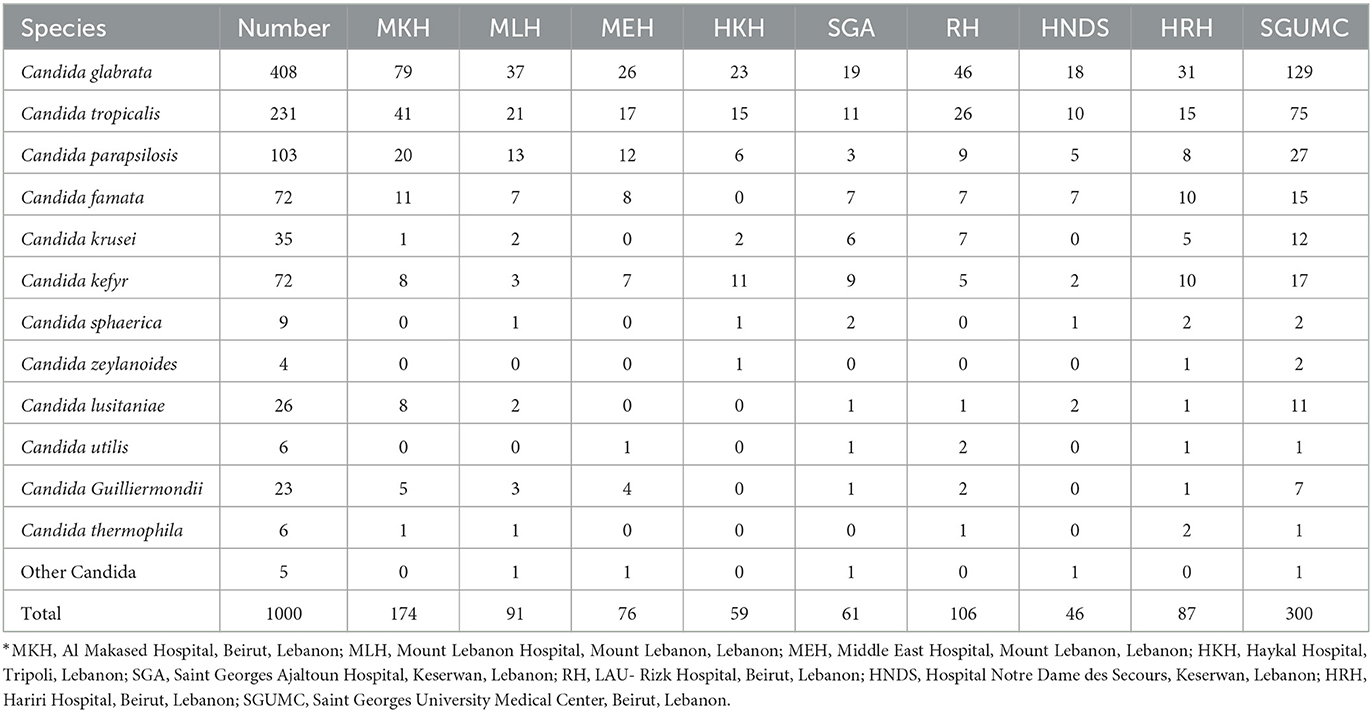
A total of 1,000 yeast non-duplicates isolates were collected from different clinical samples (Figure 1). Among the isolates, 147 (14.7%) were recovered from vaginal swab, and 393 (39.3%) from urinary samples. The remaining 460 (46%) were isolated from sputa, blood, CSF, and miscellaneous sources. The distribution of Candida species was split between Candida glabrata (40.8%/ 408), Candida tropicalis (23.1%/ 231), Candida parapsilosis (10.3 %/ 103), Candida famata (7.2 %/ 72), Candida kefyr (7.2 %/ 72), Candida krusei (3.5%/ 35), Candida lusitaniae (2.6%/ 26), and Candida guilliermondii (2.3%/ 23). The remaining species were found to represent 3% of the total number of isolates found. The distribution of the isolates among the different hospitals are in Table 1.
Among the 48 candidemia cases, 66.7 % had C. glabrata. Similarly, C. glabrata grew in 9 specimens among the 10 CSF specimens. Similarly, in the miscellaneous group (mostly abdominal and skin infections) the most common pathogens were C. kefyr, Candida glabrata, C. tropicalis and C. parapsilosis (Figure 1). Candida auris was not isolated in any of the specimen.
Susceptibility profile:
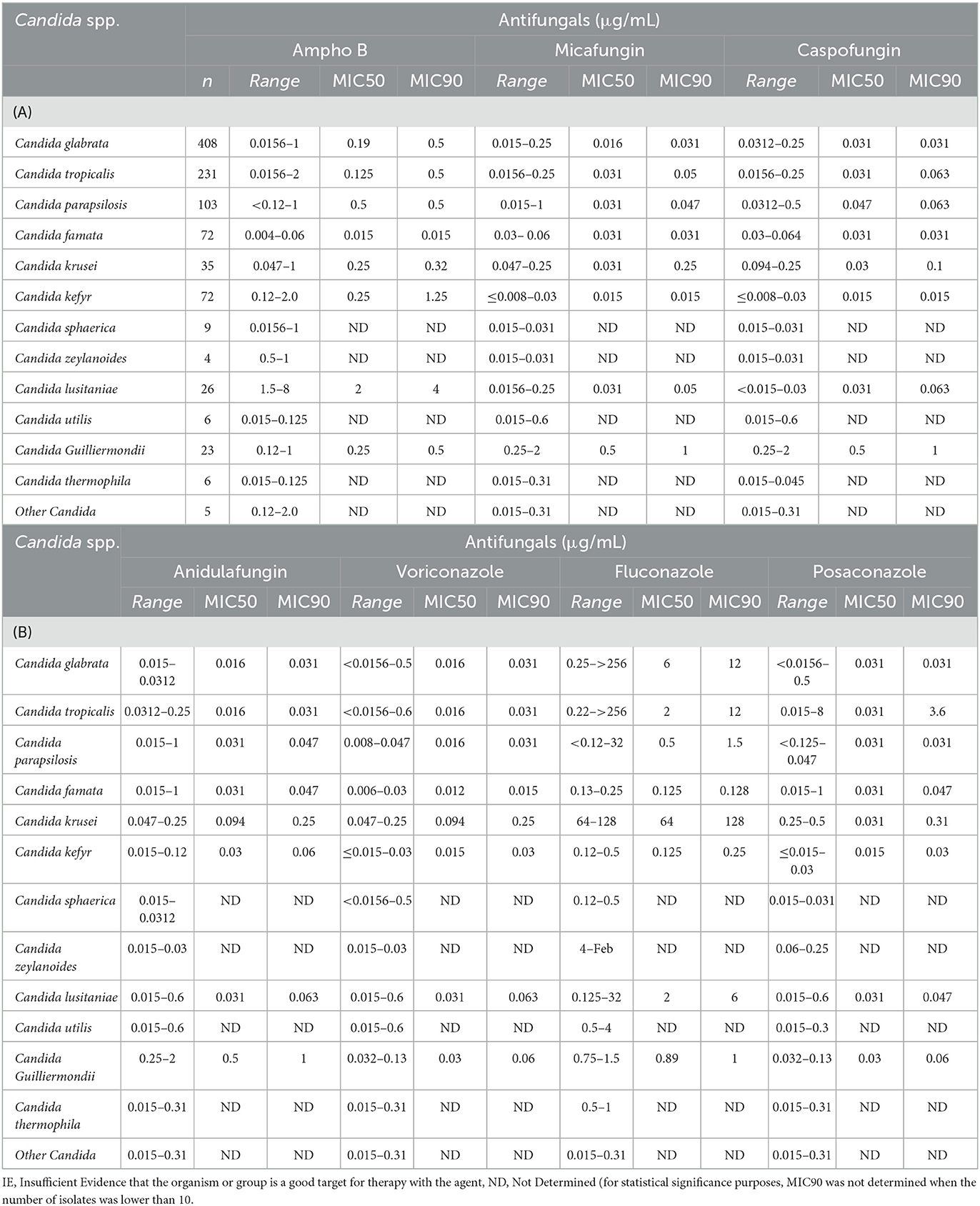
Table 2 shows Candida spp. Isolates susceptibility to various antifungals. C. glabrata isolates were highly 100% susceptible to Anidulafungin, and Amphotericin B, 98.5 % susceptible to micafungin, but none was susceptible to Fluconazole (Table 2). C. tropicalis isolates were 100% susceptible to Anidulafungin and Voriconazole and 99.6% to Amphotericin B. Only 4.3 % of C. tropicalis were susceptible to Fluconazole and 3.9 % to Pozaconazole. C. parapsilosis isolates were 100 % susceptible to Micafungin, Voriconazole, Anidulafungin and Amphotericin B. Only 6.8% were susceptible to Fluconazole and none to Pozaconazole. Multidrug resistance was not seen among any of the pathogens cultured. The data showed that the isolates found in blood and CSF were mostly C. Glabrata and C. tropicalis. These species had the highest pattern of resistance.
4. Discussion
Fungi are increasingly recognized as important pathogens in critically ill and immunocompromised patients (32–36). The incidence of invasive candidiasis has increased over the past decade due to the increasing prevalence of immunosuppressive therapy, invasive surgical procedures, and use of indwelling medical devices (13). In addition, the increased use of broad-spectrum antibiotics leads to changes in the microbiome, shifting the balance toward fungi and more resistant strains of bacteria (37). Antifungal susceptibility is not uniform among different candida species, and some species are innately resistant while others acquire resistance to the first line of antifungals, Fluconazole and Echinocandins (38, 39). Because of this increase in resistance, candida speciation and Surveillance of Candida infection has become a must for every country as well as each hospital. Accordingly, the Clinical and Laboratory Standards Institute (CLSI) has recently adopted species-specific minimum inhibitory concentration (MIC) breakpoints for Candida species and recommends speciation and antifungal susceptibility of candida species isolated from sterile sites and causing invasive fungal infections. High rates of morbidity and mortality are associated with invasive Candida infections. The rate of mortality from candidemia is about 30%, while directly attributable mortality is between 19 and 24% (40, 41). Treating these infections requires antifungals that are expensive, and this is considered a burden in our country.
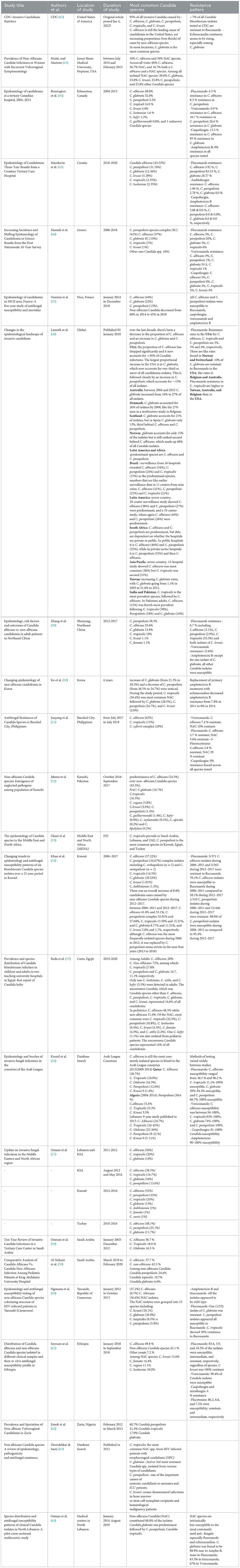
Table 3 summarizes the most common species in different countries around the world. Looking at the most common species in Lebanon, C. tropicalis was dominant in Lebanon with percentage ranging from 20 % to 45 % in some studies (56, 57). However, our study showed that C. Glabrata was the most common pathogen detected in all sites.
In a study done in one region in Lebanon on 93 Candida isolates, C. glabrata was the most common, followed by C. parapsilosis, and C. tropicalis which is similar to our results (64).
While C. tropicalis and C. parapsilosis are the most common species found in many countries with variable percentages in African countries: Nigeria: C. parapsilosis 60.7% and C. tropicalis 21.4 % (62), Algeria: C. parapsilosis 36.6% and C. tropicalis 23.3 % (56), Cairo: C. parapsilosis 16.7% and C. tropicalis 27.8 % (55), South Africa C. parapsilosis 35% (48). Similar percentages are also seen in South America C. parapsilosis 24% and C. tropicalis 15 % (48) and the Middle East and Arab countries; Saudi Arabia: C. parapsilosis 13.6% and C. tropicalis 16.7 % (57), Kuwait: C. parapsilosis 32 to 34 % and C. tropicalis 14.5 to 20% (54, 57), Turkey: C. parapsilosis 25.1% (57) and Qatar: C. parapsilosis 12.6% and C. tropicalis 18.9 % (56). In Europe, some countries have similar percentages with C. parapsilosis like Greece 41 % (46). Thus, understanding the local epidemiology of resistance of NAC and their susceptibility profiles provided by our data has an important role in guiding care of patients with the adequate choice of antifungal.
Invasive Candidiasis is a major healthcare problem associated with high mortality and cost. According to the country's susceptibility pattern described above, non-albicans species are increasing and are associated with reduced antifungal susceptibility. Thus, Echinocandins are the drug of choice in empirical treatment for these patients with risk factors for invasive candida infection. However, according to the literature de-escalation and the use of oral therapy are acceptable strategies to follow in the management of such patients. Voriconazole is also an acceptable alternative if the patient did not receive prior azoles therapy whether prophylaxis or therapeutic. Clearly, this data sheds light on proper management of patients with fungal infections. However, patients with vaginal infection who have C. glabrata need further studies and consideration of treatment since oral medications might not be the best choice as seen in our data. In addition, CNS infections should be treated with amphoteric B not Echinocandins because of lack of concentration in the CNS (65).
Newer technologies such as Maldi-tof-MS and molecular techniques are considered the most reliable for microbial identification. However, sugar fermentation-based techniques are still reliable and commonly used for yeast identification. In a study by Arastehfar (66), API 20C AUX correctly identified 83.7% of yeast isolates. Another study Using sequencing as a standard technique for NAC identification, 78.9% of the isolates were correctly identified by API 20C AUX while the Vitek 2 YST ID Card system yielded 71.8% and Bruker and Vitek proteomic techniques yielded 90.1% and 80.3% of correct identification (67). These studies, in addition to many others, show a high accuracy of yeast identification of sugar fermentation-based methods and support their use for yeast identification.
Invasive Candida infections has high mortality and the yield of culture remains low. Mucocutaneaous Candida infection and colonization have a high positive predictive correlation with invasive infection. Thus, any patient with risk factors of invasive candidiasis should be empirically or preemptively treated before susceptibility pattern in determined. This is why it is important to know the epidemiology and resistance patterns in order to direct our treatment properly especially in the ICU and in immunocompromised patients.
The importance of such studies is obvious. It can help in establishing guidelines of treatment for such infections. However, this should be complemented by continuous proper surveillance system to interpret the dynamic changes of the epidemiology. For example, it is important to note that lately Candida auris was reported in one of the tertiary centers in our country but not in others. Moreover, further studies about the epidemiology from animals and environmental candida species are needed as part of the One Health approach to decrease morbidity and mortality associated with this infection.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board (IRB) of the Lebanese American University (IRB# LAU.SOM.RH1.26/Apr/2016). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work received Lebanese American University Grant.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Taei M, Chadeganipour M, Mohammadi R. An alarming rise of non-albicans Candida species and uncommon yeasts in the clinical samples; a combination of various molecular techniques for identification of etiologic agents. BMC Res Notes. (2019) 12:1–7. doi: 10.1186/s13104-019-4811-1
2. Suleyman G, Alangaden GJ. Nosocomial fungal infections: epidemiology, infection control, and prevention. Infectious Disease Clinics. (2016) 30:1023–52. doi: 10.1016/j.idc.2016.07.008
3. Lim C-Y, Rosli R, Seow H, Chong P. Candida, and invasive candidiasis: back to basics. Eur J Clin Microbiol Infect Dis. (2012) 31:21–31. doi: 10.1007/s10096-011-1273-3
4. Benedict K, Richardson M, Vallabhaneni S, Jackson BR, Chiller T. Emerging issues, challenges, and changing epidemiology of fungal disease outbreaks. Lancet Infect Dis. (2017) 17:e403–e11. doi: 10.1016/S1473-3099(17)30443-7
5. Johnson NB Hayes LD Brown K Hoo EC Ethier KA; Centers for Disease Control and Prevention (CDC). CDC National Health Report: leading causes of morbidity and mortality and associated behavioral risk and protective factors–United States, 2005–2013. MMWR Suppl. (2014) 63:3–27.
6. Kim EJ, Lee E, Kwak YG, Yoo HM, Choi JY, Kim SR, et al. Trends in the epidemiology of candidemia in intensive care units from 2006 to 2017: results from the Korean national healthcare-associated infections surveillance system. Front Med. (2020) 7:974. doi: 10.3389/fmed.2020.606976
7. Papon N, Courdavault V, Clastre M, Bennett RJ. Emerging and emerged pathogenic Candida species: beyond the Candida albicans paradigm. PLoS Pathog. (2013) 9:e1003550. doi: 10.1371/journal.ppat.1003550
8. Kunyeit L, Kurrey NK, Anu-Appaiah K, Rao RP. Probiotic yeasts inhibit virulence of non-albicans Candida species. MBio. (2019) 10: e02307–19. doi: 10.1128/mBio.02307-19
9. Moran G, Coleman D, Sullivan D. An introduction to the medically important Candida species. Candida Candidiasis. (2011) 7:9–25. doi: 10.1128/9781555817176.ch2
10. Turner SA, Butler G. The Candida pathogenic species complex. Cold Spring Harb Perspect Med. (2014) 4:a019778. doi: 10.1101/cshperspect.a019778
11. Quindós G. Epidemiology of candidaemia and invasive candidiasis. Rev Iberoamericana Micol. (2014) 31:42–8. doi: 10.1016/j.riam.2013.10.001
12. Drago M, Scaltrito M, Morace G. In vitro activity of voriconazole and other antifungal agents against clinical isolates of Candida glabrata and Candida krusei. Eur J Clin Microbiol Infect Dis. (2004) 23:619–24. doi: 10.1007/s10096-004-1174-9
13. Silva S, Negri M, Henriques M, Oliveira R, Williams DW, Azeredo J. Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity, and antifungal resistance. FEMS Microbiol Rev. (2012) 36:288–305. doi: 10.1111/j.1574-6976.2011.00278.x
14. Savastano C. Silva EdO, Gonçalves LL, Nery JM, Silva NC, Dias ALT. Candida glabrata among Candida spp from environmental health practitioners of a Brazilian Hospital. Braz J Microbiol. (2016) 47:367–72. doi: 10.1016/j.bjm.2015.05.001
15. Ghaddar N, Anastasiadis E, Halimeh R, Ghaddar A, Dhar R, AlFouzan W, et al. Prevalence and antifungal susceptibility of Candida albicans causing vaginal discharge among pregnant women in Lebanon. BMC Infect Dis. (2020) 20:1–9. doi: 10.1186/s12879-019-4736-2
16. Halawi MH, Nasser R, Yassine W, Yusef H, Borjac J, Zeaiter Z. first case of identification of candida kefyr and pichia kluyveri in lebanese water. Water Air Soil Pollut. (2020) 231:1–11. doi: 10.1007/s11270-020-4460-y
17. Araj GF, Asmar RG, Avedissian AZ. Candida profiles and antifungal resistance evolution over a decade in Lebanon. J Inf Dev Countries. (2015) 9:997–1003. doi: 10.3855/jidc.6550
18. Awad L, Tamim H, Ibrahim A, Abdallah D, Salameh M, Mugharbil A, et al. Correlation between antifungal consumption and distribution of Candida spp. in different departments of a Lebanese hospital. J Inf Dev Countries. (2018) 12:33S–S. doi: 10.3855/jidc.10105
19. Bitar I, Khalaf RA, Harastani H, Tokajian S. Identification, typing, antifungal resistance profile, and biofilm formation of Candida albicans isolates from Lebanese hospital patients. Biomed Res Int. (2014) 1:931372. doi: 10.1155/2014/931372
20. Hedayati M, Tagizadeh M, Charati JY, Denning D. Burden of Serious Fungal Infection in Iran. Hoboken, NJ: Wiley-Blackwell (2013).
21. Taj-Aldeen SJ, Chandra P, Denning DW. Burden of fungal infections in Qatar. Mycoses. (2015) 58:51–7. doi: 10.1111/myc.12386
22. Wadi J, Denning DW. Burden of serious fungal infections in Jordan. J Fungi. (2018) 4:15. doi: 10.3390/jof4010015
23. Zaki SM, Denning DW. Serious fungal infections in Egypt. Eur J Clin Microbiol Infect Dis. (2017) 36:971–4. doi: 10.1007/s10096-017-2929-4
24. Chekiri-Talbi M, Denning D. Burden of fungal infections in Algeria. Eur J Clin Microbiol Infect Dis. (2017) 36:999–1004. doi: 10.1007/s10096-017-2917-8
25. Hilmioglu-Polat S, Seyedmousavi S, Ilkit M, Hedayati MT, Inci R, Tumbay E, et al. Estimated burden of serious human fungal diseases in Turkey. Mycoses. (2019) 62:22–31. doi: 10.1111/myc.12842
26. Koehler P, Stecher M, Cornely OA, Koehler D, Vehreschild MJ, Bohlius J, et al. Morbidity and mortality of candidaemia in Europe: an epidemiologic meta-analysis. Clin Microbiol Infect. (2019) 25:1200–12. doi: 10.1016/j.cmi.2019.04.024
27. Morace G, Perdoni F, Borghi E. Antifungal drug resistance in Candida species. J Global Antimicrob Resistance. (2014) 2:254–9. doi: 10.1016/j.jgar.2014.09.002
28. Pristov KE, Ghannoum MA. Resistance of Candida to azoles and echinocandins worldwide. Clin Microbiol Infect. (2019) 25:792–8. doi: 10.1016/j.cmi.2019.03.028
29. Moghnieh R, Araj GF, Awad L, Daoud Z, Mokhbat JE, Jisr T, et al. A compilation of antimicrobial susceptibility data from a network of 13 Lebanese hospitals reflecting the national situation during 2015–2016. Antimicrob Resist Infect Control. (2019) 8:1–17. doi: 10.1186/s13756-019-0487-5
30. Wayne P. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, Approved Standard-Second Edition. (2002).
31. Knabl L, Lass-Flörl C. Antifungal susceptibility testing in Candida species: current methods and promising new tools for shortening the turnaround time. Expert Rev Anti Infect Ther. (2020) 18:779–87. doi: 10.1080/14787210.2020.1760841
32. Sydnor ER, Perl TM. Hospital epidemiology and infection control in acute-care settings. Clin Microbiol Rev. (2011) 24:141–73. doi: 10.1128/CMR.00027-10
33. Abu-Elteen KH, Hamad MA. Changing epidemiology of classical and emerging human fungal infections: a review. Jordan J Biol Sci. (2012) 5:215–30.
34. Al-Jasser AM, Elkhizzi NA. Distribution of Candida species among bloodstream isolates. Saudi Med J. (2004) 25:566–9.
35. Kullberg BJ, Arendrup MC. Invasive candidiasis. N Engl J Med. (2015) 373:1445–56. doi: 10.1056/NEJMra1315399
36. Kåhrström CT. Resistance is costly for Candida. Nat Rev Microbiol. (2015) 13:189. doi: 10.1038/nrmicro3454
37. Yoon MY, Yoon SS. Disruption of the gut ecosystem by antibiotics. Yonsei Med J. (2018) 59:4–12. doi: 10.3349/ymj.2018.59.1.4
38. Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. (2016) 62: e1–50. doi: 10.1093/cid/civ933
39. C Deorukhkar S, Saini S. Echinocandin susceptibility profile of fluconazole resistant Candida species isolated from blood stream infections. Inf Disorders Drug Targets. (2016) 16:63–8. doi: 10.2174/1871526516666151209155447
40. Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. (2007) 20:133–63. doi: 10.1128/CMR.00029-06
41. Vincent JL, Anaissie E, Bruining H, Demajo W, El-Ebiary M, Haber J, et al. Epidemiology, diagnosis, and treatment of systemic Candida infection in surgical patients under intensive care. Intensive Care Med. (1998) 24:206–16. doi: 10.1007/s001340050552
42. Statistics. Invasive Candidiasis, Candidiasis, Types of Diseases, Fungal Diseases, CDC. (2022). Available online at: https://www.cdc.gov/fungal/diseases/candidiasis/invasive/statistics.html (accessed January 4, 2022)
43. Mintz JD, Martens MG. Prevalence of non-albicans candida infections in women with recurrent vulvovaginal symptomatology. Adv Inf Dis. (2013) 3:238–42. doi: 10.4236/aid.2013.34035
44. Remington TL, Isaac A, Vickers DM, Fuller J, Wrenn Smith S. Epidemiology of candidemia at a tertiary Canadian hospital, 2004–2013. Off J Assoc Med Microbiol Inf Dis. (2018) 3:14–23. doi: 10.3138/jammi.3.1.04
45. Mareković I, Pleško S, Rezo Vranješ V, Herljević Z, Kuliš T, Jandrlić M. Epidemiology of Candidemia: three-year results from a croatian tertiary care hospital. J Fungi. (2021) 7:267. doi: 10.3390/jof7040267
46. Increasing Incidence Shifting Epidemiology of Candidemia in Greece: Results from the First Nationwide 10-Year Survey—PMC. (2022). Available online at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8879520/ (accessed October 1, 2022).
47. Vannini M, Emery S, Lieutier-Colas F, Legueult K, Mondain V, Retur N. Epidemiology of candidemia in NICE area, France: a five-year study of antifungal susceptibility and mortality. J Med Mycol. (2022) 32:101210. doi: 10.1016/j.mycmed.2021.101210
48. Lamoth F, Lockhart SR, Berkow EL, Calandra T. Changes in the epidemiological landscape of invasive candidiasis. J Antimicrob Chemother. (2018) 73:4–13. doi: 10.1093/jac/dkx444
49. Zhang W, Song X, Wu H, Zheng R. Epidemiology, risk factors and outcomes of Candida albicans vs. Non-albicans candidaemia in adult patients in Northeast China. Epidemiol Inf. (2019) 147:e277. doi: 10.1017/S0950268819001638
50. Ko JH, Jung DS, Lee JY, Kim HA, Ryu SY, Jung SI. Changing epidemiology of non-albicans candidemia in Korea. J Inf Chemother. (2019) 25:388–91. doi: 10.1016/j.jiac.2018.09.016
51. Juayang A, Lim J, de los Reyes Z, Tuante M, Batiles Z, Guino-o JF, Villanueva F, de los Reyes G. Antifungal resistance of Candida species in Bacolod City, Philippines. J Infect Dis Epidemiol. (2019) 5:76 doi: 10.23937/2474-3658/1510076
52. Jabeen G, Naz SA, Jabeen N, Shafique M, Sharafat S, Baig S. Non-albicans Candida species: Emergence of neglected pathogens among population of Karachi. Pakistan J Pharm Sci. (2019) 32:1185–1192.
53. Ghazi S, Rafei R, Osman M, El Safadi D, Mallat H, Papon N. The epidemiology of Candida species in the Middle East and North Africa. J Mycol Méd. (2019) 29:245–52. doi: 10.1016/j.mycmed.2019.07.006
54. Khan Z, Ahmad S, Al-Sweih N, Mokaddas E, Al-Banwan K, Alfouzan W. Changing trends in epidemiology and antifungal susceptibility patterns of six bloodstream Candida species isolates over a 12-year period in Kuwait. PLoS ONE. (2019) 14:e0216250. doi: 10.1371/journal.pone.0216250
55. Reda NM, Hassan RM, Salem ST, Yousef RHA. Prevalence and species distribution of Candida bloodstream infection in children and adults in two teaching university hospitals in Egypt: first report of Candida kefyr. Infection. (2022)26:1–7. doi: 10.1007/s15010-022-01888-7
56. Kmeid J, Jabbour JF, Kanj SS. Epidemiology and burden of invasive fungal infections in the countries of the Arab League. J Inf Pub Health. (2020) 13:2080–2086. doi: 10.1016/j.jiph.2019.05.007
57. Osman M, Al Bikai A, Rafei R, Mallat H, Dabboussi F, Hamze M. Update on invasive fungal infections in the Middle Eastern and North African region. Braz J Microbiol. (2020) 51:1771–89. doi: 10.1007/s42770-020-00325-x
58. Omrani AS, Makkawy EA, Baig K, Baredhwan AA, Almuthree SA, Elkhizzi NA. Ten-year review of invasive Candida infections in a tertiary care center in Saudi Arabia. Saudi Med J. (2014) 35:821–6.
59. Al-Sofyani K, Uddin M, Alghamdi H, El-Hossary D. Comparative Analysis of Candida albicans Versus Candida Non-albicans Infection among Pediatric Patients at King Abdulaziz University Hospital. (2022). Available online at: https://www.researchgate.net/publication/346617671_Comparative_Analysis_of_Candida_albicans_Versus_Candida_Non-albicans_Infection_among_Pediatric_Patients_at_King_Abdulaziz_University_Hospital (accessed October 1, 2022).
60. Ngouana TK, Toghueo RM, Kenfack IF, Lachaud L, Nana AK, Tadjou L. Epidemiology and antifungal susceptibility testing of non-albicans Candida species colonizing mucosae of HIV-infected patients in Yaoundé (Cameroon). J Mycol Méd. (2019) 29:233–8. doi: 10.1016/j.mycmed.2019.06.003
61. Seyoum E, Bitew A, Mihret A. Distribution of Candida albicans and non-albicans Candida species isolated in different clinical samples and their in vitro antifungal suscetibity profile in Ethiopia. BMC Inf Dis. (2020) 20:231. doi: 10.1186/s12879-020-4883-5
62. Jimoh O, Inabo HI, Yakubu SE, Ankuma SJ, Olayinka AT. Prevalence and speciation of non-albican vulvovaginal candidiasis in Zaria. J Nat Sci Res. (2016) 6:51–6.
63. Deorukhkar S, Saini S. Non albicans Candida species: A review of epidemiology, pathogenicity and antifungal resistance. Pravara Med Rev. (2015) 7:7–15.
64. Osman M, Al Bikai A, Rafei R, Mallat H, Dabboussi F, Hamze M. Species distribution and antifungal susceptibility patterns of clinical Candida isolates in North Lebanon: a pilot cross-sectional multi centric study. J Mycol Med. (2020) 30:100986. doi: 10.1016/j.mycmed.2020.100986
65. Ashley ED. Antifungal drugs: special problems treating central nervous system infections. J Fungi. (2019) 5:97. doi: 10.3390/jof5040097
66. Arastehfar A, Daneshnia F, Kord M, Roudbary M, Zarrinfar H, Fang W. Comparison of 21-Plex PCR and API 20C AUX, MALDI-TOF MS, and rDNA sequencing for a wide range of clinically isolated yeast species: improved identification by combining 21-Plex PCR and API 20C AUX as an alternative strategy for developing countries. Front Cell Infect Microbiol. (2019) 9:21. doi: 10.3389/fcimb.2019.00021
Keywords: fungal infection, non-albicans Candida, infection, microbiology, pathogens
Citation: Husni R, Bou Zerdan M, Samaha N, Helou M, Mahfouz Y, Saniour R, Hourani S, Kolanjian H, Afif C, Azar E, El Jisr T, Mokhbat J, Abboud E, Feghali R, Abboud E, Matta H, Karayakouboglo G, Matar M, Moghnieh R and Daoud Z (2023) Characterization and susceptibility of non-albicans Candida isolated from various clinical specimens in Lebanese hospitals. Front. Public Health 11:1115055. doi: 10.3389/fpubh.2023.1115055
Received: 03 December 2022; Accepted: 21 February 2023;
Published: 10 March 2023.
Edited by:
Marwan Osman, Cornell University, United StatesReviewed by:
Samar Kabbara, Conservatoire National des Arts et Métiers (CNAM), FranceImad Al Kassaa, Fonterra, New Zealand
Copyright © 2023 Husni, Bou Zerdan, Samaha, Helou, Mahfouz, Saniour, Hourani, Kolanjian, Afif, Azar, El Jisr, Mokhbat, Abboud, Feghali, Abboud, Matta, Karayakouboglo, Matar, Moghnieh and Daoud. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rola Husni, roula.husni@lau.edu.lb
 Rola Husni
Rola Husni Maroun Bou Zerdan
Maroun Bou Zerdan Nadia Samaha4
Nadia Samaha4 Mariana Helou
Mariana Helou Youssef Mahfouz
Youssef Mahfouz Jacques Mokhbat
Jacques Mokhbat Rita Feghali
Rita Feghali Edmond Abboud
Edmond Abboud Gilbert Karayakouboglo
Gilbert Karayakouboglo Madonna Matar
Madonna Matar Rima Moghnieh
Rima Moghnieh Ziad Daoud
Ziad Daoud