- 1Department of Clinical and Experimental Medicine, Section of Forensic Pathology, University of Foggia, Ospedale Colonnello D'Avanzo, Foggia, Italy
- 2Department of Anatomical, Histological, Forensic Medicine and Orthopedic Sciences, Sapienza University of Rome, Rome, Italy
- 3Polo Sanitario San Feliciano, Rome, Italy
Haemodialysis (HD) is one of the methods for renal replacement therapy in the management of advanced chronic kidney disease through an osmosis process that allows purification of blood in the dialysis machine. The complexity of the dialytic procedure often requires the presence of a multi-specialist, multi-disciplinary team. The dialysis process is an important target for clinical risk management. Failure Mode and Effect Analysis (FMEA) is a proactive technique, considered a purposeful and dynamic tool for clinical risk management. FMEA is noted in five phases that allow a preliminary assessment of a definite process through identification and classification of risk priorities. This study represents the first of a two-phase project where FMEA is applied to HD in the setting of San Feliciano Hospital. The dialysis center performs ~12,000 dialysis sessions per year. The dialysis process is divided into different stages. A total of 31 failure modes were identified in the whole dialysis stages; more than 2/3 of the failure modes were related to the only connecting of the patient to the dialysis machine. The first phase of the study clearly remarked that the most critical step of the dialytic process is represented by the connection between the patient and the machine, as expected. Indeed, in order to have the dialysis set up, an arteriovenous fistula must be surgically created prior to the procedure and it is one of the most important issues in the HD process because of the necessity of a constant revision of it. FMEA application to HD is a useful tool, easy to be implemented and it is likely to nimbly reveal the practical and potential solutions to the critical steps of the procedure.
Introduction
Haemodialysis
Haemodialysis (HD) is one of the methods for renal replacement therapy in the management of advanced chronic kidney disease (1).
To purify the blood, HD uses a dialysis machine and a special filter, called a dialyzer. The operating mechanism of the dialysis machine involves the entry of the patient's blood and its purification through an osmosis process. The filter consists of two compartments separated by a membrane, one in which the blood flows and the other one in which the dialysis solution (i.e., dialysate) flows. The dialysate is a special dialysis fluid similar to plasma which flows counter-currently to the blood, so to maximize the concentration gradient of solutes, thus removing urea, creatinine, and other waste products unusually high in the blood; the dialysate, moreover, being constantly replaced, ensures the correct concentration of several solutes in the blood. The membrane, instead, is semipermeable and its very small pores allow the passage of water and solutes, but not that of proteins and blood cells. Moreover, some solutes like Bicarbonate and Calcium, whose concentration in dialysis solution is high, enter the blood section (2).
The main reason why the purifying efficiency of the dialysis machine does not reach that of the healthy kidney is that—apart from the continuous, organic working of healthy kidneys in human bodies—the hemodialytic procedure can take up to 3–6 h and is usually performed three times a week, hence the social, healthcare-associated and economic costs of the procedure itself (3).
The dialytic session involves several stages:
(i) setting up, which consists of controlling the sanitary conditions of the interested hospital wards, testing and dressing the necessary materials, such as the monitor and the equipment of the dialysis machine;
(ii) actual dialysis procedure, formed by patient's evaluation, connection to the extracorporeal circuit, blood circulation circuit activation and its maintenance;
(iii) disconnecting the patient from the extracorporeal circuit.
It clearly appears that dialysis is a highly complex procedure both from a technological and a clinical point of view. Moreover, dialysis patients are frail because of their healthcare condition and because of the not uncommon, several comorbidities that affect them. The complexity of the dialytic procedure often requires the presence of a multi-specialist, multi-disciplinary team (4–6). Therefore, the interaction of these factors makes the dialysis process an important target in clinical risk management (7, 8).
FMEA and Haemodialysis
Failure Mode and Effect Analysis (FMEA) is a proactive technique, widely used in Human Reliability Analysis (HRA) studies (9–13). HRA's purpose is to examine the activity, process or organizational structure to identify weaknesses and vulnerabilities so that they can be defined and solved. Since its practical use and effective implications, the technique is well-applicable to healthcare practices (14). For instance (15), FMEA has been applied to practical issues such as dosing the right amount of exposition in total body irradiation; it has been used (16) in the diagnostic process, particularly in oncology, where it resulted optimal in the therapeutic decision-making process (17) and, more recently, it showed its potential advantages in surgery (18). In sum, FMEA can be considered a purposeful and dynamic tool for clinical risk management: it is defined as a predictive technique for the identification and classification of risk priorities, which allows a preliminary risks assessment of a process through a qualitative and quantitative analysis aimed at outlining the intervention priorities. The methodological phases of the FMEA are the following:
(i) identification of the target of the analysis;
(ii) identification and description of the activities correlated to the target;
(iii) identification of failure mode(s);
(iv) determination of the risk priority number (RPN) and its analysis;
(v) identification of measures and actions to be implemented as preventive, improvement, and/or corrective acts in order to solve the issue represented by the identified target.
The RPN is the result of the combination of three different assessments as evaluated by a multidisciplinary group; it gathers and accounts for any failure mode or pattern for its severity (S), occurrence (O) and detection (D) ratings. The RPN must be calculated for each recognized cause of failure (19).
In this way, FMEA does not neglect any potential mistake in the execution of the targeted procedure, thus allowing insertion tests and controls, to develop protocols, to prepare countermeasures (20).
Consequently, this study represents the first of a two-phase project where FMEA is applied to HD in a hospital setting. This pilot project is preliminary to the second actualization since in this stage HD was subjected to a descriptive decomposition in order to isolate the several steps which form the dialysis procedure itself with the aim of secluding the most critical stages and approaching them by resolute and/or prophylactic measures. The second phase of the study will evaluate whether the executed implementations have an impact onto the various steps formerly isolated, and the potential magnitude of them.
Materials and Methods
As far as the FMEA is concerned, a multidisciplinary working group has been set up consisting of eight professionals: nephrologists; nurses; the hospital's clinical risk manager; and experts in risk management from the Sapienza University of Rome.
The working group implemented the FMEA and identified five main stages of the dialysis procedure:
(i) dialysis machine preparation
(ii) connecting the patient to the monitor
(iii) dialysis surveillance
(iv) disconnecting the patient from the monitor
(v) central venous catheter management.
Each of the aforementioned stages was further subdivided into single, practical activities; failure modes and consequent, potential repercussions were isolated as well. The fifth stage was inserted as supernumerary since the relevance of it only when present.
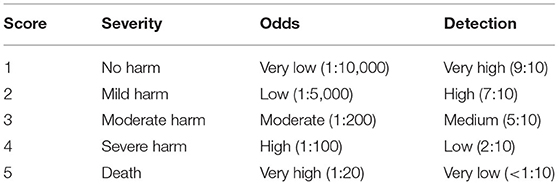
RPN was determined for each failure mode based on a predetermined score, one for each of the three variables included in the calculation (S, O, and D). The categories, the frequencies set up as probability cut-offs, and their related scores are shown in Table 1.
The contingent presence of any control and/or barrier measure was studied where applicable. According to the results of the RPN score, five categories were set up for the classification of the intervention priorities, and labeled as follows: RPN≥40: “very high”;40 < RPN ≤ 30: “high”;30 < RPN ≤ 20: “medium”; 20 < RPN ≤ 11: “low”; and finally, RPN ≤ 10: “monitoring.” For each of these categories, a color code was assigned, as follows: Red (RPN≥40), Orange (40 < RPN ≤ 30), Yellow (30 < RPN ≤ 20), Green (20 < RPN ≤ 11) and White (RPN ≤ 10).
Thus, a specific master list of priorities was built up summarizing the features taken into consideration in the FMEA.
To include in the FMEA the human variables that may represent unavoidable failure mode(s), clinical features of a sample of the patients requiring dialysis at the San Feliciano Hospital were collected from their medical records after they signed informed consent (21).
The San Feliciano Hospital is an Italian contract clinic in Rome. Among its services, it offers a dialysis center, with two dialysis units for a total of 39 beds, divided into two wards (23 beds in ward A and 16 in ward B). Supplementary beds can be added when needed in particular situations like overcrowding.
In the dialysis center, ~12,000 dialysis sessions are carried out per year, hence the relevance of the chosen hospital for the FMEA application.
The only inclusion criterion, signing informed consent and being ≥18 years old apart, was that to have been subjected to dialysis for more than 6 months. Beyond their anamnestic personal data and clinical records, their potential comorbidities were investigated as well. The Charlson Comorbidity Index (CCI) (22), predicting 10-year survival rate in patients affected by multiple comorbidities, was chosen to gather the pathological conditions of the patients and was used as a proxy to reveal other possible failure modes.
The distribution of the main characteristics of the patients was calculated considering it a normally-distributed sample, thus calculating mean and standard deviation, or its proportion, as appropriate. The statistical software chosen was R, version 4.1.2 (23).
Results
A total of 79 patients were included in the pilot project as set up by the multidisciplinary group. Their main characteristics are shown in Table 2.

Table 2. Main characteristics of the patients under dialysis procedure included in the pilot project at san feliciano hospital.
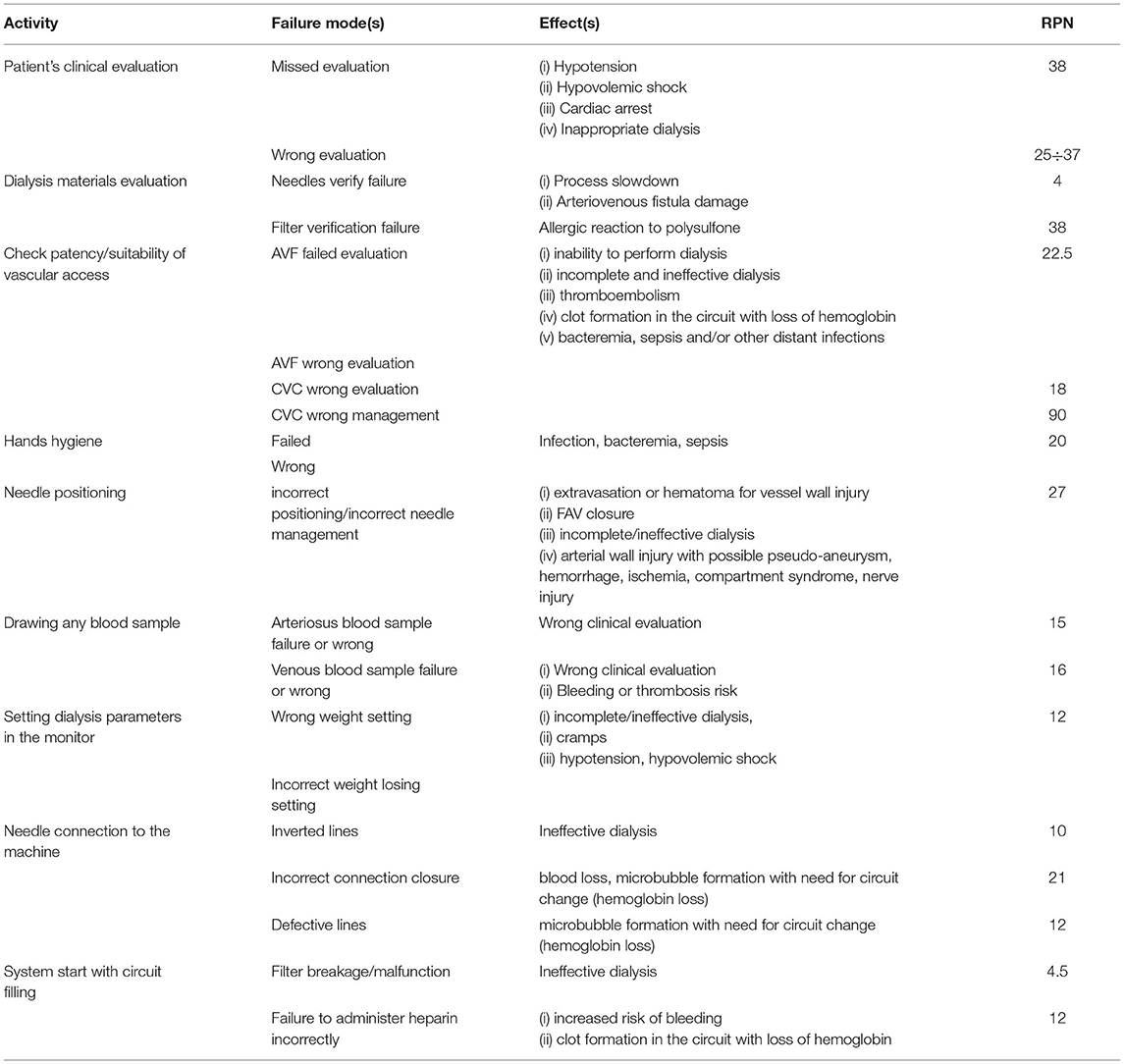
A total of 31 failure modes were identified in the whole dialysis stages; since more than 2/3 of the failure modes were related to the only connecting of the patient to the dialysis machine (stage 2), it was regarded as the most exemplary phase for quantity and variety, and its descriptive results are summarized in Table 3. In this table, the identified Failures Mode(s) are considered as the causes of the potential effects as described in the appropriate column.
The master list directly derived from FMEA, as far as the only second phase is concerned, is shown in Table 4.
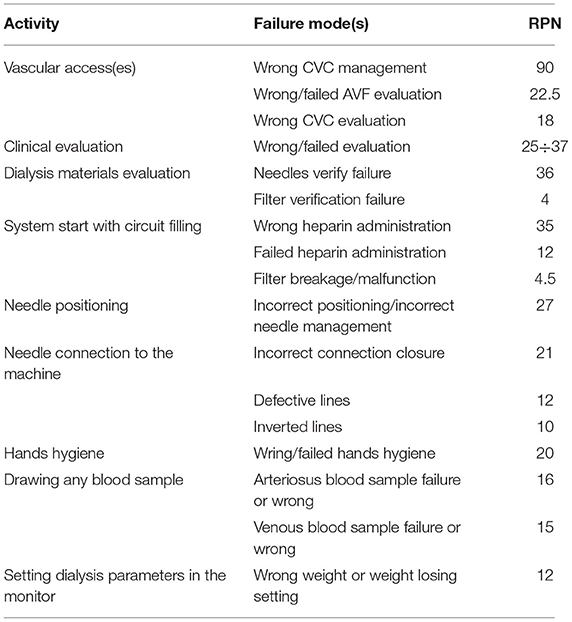
Table 4. Proposed master list as resulted from the failure modes of the FMEA application to the HD process.
Discussion
The first phase of the study clearly remarked that the most critical step of the dialytic process is represented by the connection between the patient and the machine, as expected (24, 25). Contrary to what other Authors found, miscommunication difficulties did not represent significant issues in our case (24, 26). Indeed, in order to have the dialysis set up, an arteriovenous fistula must be surgically created prior to the procedure and it is one of the many issues in the HD process because of the necessity of a constant revision of it (4).
The presence of a CVC is the only red-flagged element in the whole considered stage of HD; in particular, its wrong management has the highest RPN value, while a wrong evaluation of it seemed not to affect as much the procedure, as remarked by others (24, 25). Despite the difference between evaluation and management could actually be idle, what the results of the FMEA highlighted is that when patients carry a CVC and they have to undergo an HD session, they should be regarded as high-risk patients when compared to the other dialyzed. CVCs in fact represent the second discontinuation of the barrier given by skin—being the AVF the first one. This medical device, although useful for liquids and drugs administration, enhances the risks of suffering from infections of any kind, especially those known as healthcare-acquired, thus boosting the risk of incurring in sepsis and death (26, 27). When the variable represented by CVCs is erased from the model, HD's more critical stage is the clinical evaluation. This implicates that an adequate and up-to-date education of the staff, both nurses and physicians, should be enough to guarantee a successful and eventless HD session. In this sense, the correct method of hand sanitization should be one of the first healthcare procedures to be learned by the staff, without considering it irrelevant since the diffusion of its implementation due to the COVID pandemic (20, 28).
Needles management and their correct use is another relevant action liable for what concerns failure modes. Needles prominence is best explained by the high RPN related to the administration of heparin, although it is uncommon: since patients requiring HD are necessarily submitted to anticoagulation drugs (28), if important arteries are damaged during needle insertion, emergency protocols, and blood transfusions must be carried out. Mainly and more frequently, poor needle management makes dialysis inefficient and/or ineffective. Moreover, needles represent another risky device for what concerns healthcare-acquired infections as well as work-related accidents, meaning that potential harm to the staff must be considered, too (29, 30).
What aforementioned openly shows that, although HD is a well-known and established procedure, it can be extremely risky for patients and for hospital staff as well; however, since scarce the critical points appear to be, the prophylactic interventions should be easily carried out in hospital settings.
As far as the patient's sample is considered, several are the implication of it for the second phase of our project. First, we should include patients with some kind of prosthesis, thus adding the presence of it to the FMEA process, to highlight any other critical step. Secondly, female patients should be more thoroughly evaluated, so that women's peculiarities—such as hormonal diversity, compliances differences and any other issue belonging to the so-called gender medicine—are not forgotten and/or neglected (31).
The length of the dialysis treatment for the analyzed sample resulted in being sufficient to consider patients as clinically stable so that their comorbidities could not represent a bias in the evaluation of the FMEA. On the other hand, the not insignificant value of the CCI will undergo a further and deeper analysis in the second phase of the study. Indeed, the 10-year survival rate associated with a CCI value of almost 6 points is equal to 2%. General Italian population coeval to the analyzed sample has a probability of surviving (32) for almost 20 years equal to 90%: this easy comparison emphasizes how the dialytic population is intrinsically prone to get serious complications even from the medical procedures considered as the safest and the most efficient ones.
Nonetheless, at the same time, age could be the more treacherous variable in this sense, because of the comorbidities related to the aging process, along with the condition of frailty in the elderly incur as years go by.
The application of the FMEA process showed that specific strategies for each failure mode, as far as improvement activities from a clinical, organizational, and training point of view, must be listed as well, so that a Risk Management Plan can be built up for HD. In fact, FMEA guarantees an adequate analysis of risk priorities as aforementioned, other than providing a series of relevant information in the selected care setting. Moreover, FMEA results clearly point out where to best allocate the financial resources, in order to prevent risks and improve organizational conditions more effectively.
In this way, the multidisciplinary working group shall enact all the possible directions in order to have the staff and patients informed about the risks and, especially, how to avoid them, with a prophylactic aim. It should be underlined that, recently, legislation changes in Italy have made medical malpractice suits easier to be submitted, hence the huge amount of litigations and costs related to the so-called defensive medicine (33, 34). Furthermore, any tool aimed at anticipating potential claims should be thoroughly considered and implemented, when adequate (35, 36).
Limitations
FMEA application to HD is a useful tool, easy to be implemented and it is likely to nimbly reveal the practical and potential solutions to the critical steps of the procedure. The evaluation of the solutions as identified by the current analysis will be tested, as already mentioned above, in the second phase of the study. However, being dialysis a procedure known and used since the second half of the XX centuries, it is very likely that FMEA results will not be dissimilar if other similar techniques should be implemented; at least, the debate could be about the proposed resolutions and operative arrangements (37, 38).
Another issue is that of applicability: FMEA is a method well-known in other specialties; however, its actual efficacy concerning dialysis has not been recognized yet. For this main reason, further studies investigating the application of this pilot study to broader research will be enlightening.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
RLR, VF, and MF: conceptualization. NDF: methodology. RV: validation. GP: formal analysis. GC: data curation. FM and PF: writing—original draft preparation. VF and MF: writing—review and editing. PF and RLR: supervision. All authors have read and agreed to the to the published version of the manuscript and contributed to the drafting and critical revision of the work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Elliott DA. Hemodialysis. Clin Tech Small Anim Pract. (2000) 15:136–48. doi: 10.1053/svms.2000.18297
2. Eloot S, Van Biesen W, Vanholder R. A sad but forgotten truth: the story of slow-moving solutes in fast hemodialysis. Semin Dial. (2012) 25:505–9. doi: 10.1111/j.1525-139X.2012.01107.x
3. Basile C, Davenport A, Mitra S, Pal A, Stamatialis D, Chrysochou C, et al. Frontiers in hemodialysis: innovations and technological advances. Artificial Organs. (2021) 45:175–82. doi: 10.1111/aor.13798
4. Murea M, Geary RL, Davis RP, Moossavi S. Vascular access for hemodialysis: a perpetual challenge. Semin Dial. (2019) 32:527–34. doi: 10.1111/sdi.12828
5. Ahmadmehrabi S, Wilson Tang WH. Hemodialysis-induced Cardiovascular Disease. Semin Dial. (2018) 31:258–67. doi: 10.1111/sdi.12694
6. See EJ, Polkinghorne KR. Volume management in haemodialysis patients. Curr Opin Nephrol Hypertens. (2020) 29:663–70. doi: 10.1097/MNH.0000000000000642
7. La Russa R, Viola RV, D'Errico S, Aromatario M, Maiese A, Anibaldi P, et al. Analysis of inadequacies in hospital care through medical liability litigation. Int J Environ Res Public Health. (2021) 18:3425. doi: 10.3390/ijerph18073425
8. Treglia M, Pallocci M, Passalacqua P, Giammatteo J, De Luca L, Mauriello S, et al. Medical liability: review of a whole year of judgments of the Civil Court of Rome. Int J Environ Res Public Health. (2021) 18:6019. doi: 10.3390/ijerph18116019
9. Guiñón L, Soler A, Gisell Díaz M, Fernández RM, Rico N, Bedini JL, et al. Analytical performance assessment and improvement by means of the Failure mode and effect analysis (FMEA). Biochem Med. (2020) 30:020703. doi: 10.11613/BM.2020.020703
10. Wetterneck TB, Hundt AS, Carayon P. FMEA team performance in health care: a qualitative analysis of team member perceptions. J Patient Saf . (2009) 5:102–8. doi: 10.1097/PTS.0b013e3181a852be
11. Subriadi AP, Najwa NF. The consistency analysis of failure mode and effect analysis (FMEA) in information technology risk assessment. Heliyon. (2020) 6:e03161. doi: 10.1016/j.heliyon.2020.e03161
12. La Russa R, Ferracuti S. Clinical risk management: As modern tool for prevention and management of care and prevention occupational risk. Int J Environ Res Public Health. (2022) 19:831. doi: 10.3390/ijerph19020831
13. Gatto V, Scopetti M, La Russa R, Santurro A, Cipolloni L, Viola RV, et al. Advanced loss eventuality assessment and technical estimates: An integrated approach for management of healthcare-associated infections. Curr Pharm Biotechnol. (2019) 20:625–34. doi: 10.2174/1389201020666190408095050
14. Najafpour Z, Hasoumi M, Behzadi F, Mohamadi E, Jafary M, Saeedi M. Preventing blood transfusion failures: FMEA, an effective assessment method. BMC Health Serv Res. (2017) 17:453. doi: 10.1186/s12913-017-2380-3
15. Nelson G, Paxton A, Kunz J, Huang J, Szegedi M, Sarkar V, et al. FMEA occurrence values for four failure modes occurring using look-up tables for dose calculations. J Appl Clin Med Phys. (2020) 22:9–12. doi: 10.1002/acm2.13091
16. Kim J, Miller B, Siddiqui MS, Movsas B, Glide-Hurst C. FMEA of MR-only treatment planning in the Pelvis. Adv Radiat Oncol. (2018) 4:168–76. doi: 10.1016/j.adro.2018.08.024
17. Su FF, Huang YJ, Rassiah P, Salter BJ. FMEA-guided transition from microSelectron to Flexitron for HDR brachytherapy. Brachytherapy. (2020) 19:241–8. doi: 10.1016/j.brachy.2020.01.004
18. Xu Y, Wang W, Li Z, Wang Y, Cai Y, Chen Y. Effects of healthcare failure mode and effect analysis on the prevention of multi-drug resistant organisms infections in oral and maxillofacial surgery. Am J Transl Res. (2021) 13:3674–81.
19. Rezaei F, Yarmohammadian MH, Haghshenas A, Fallah A, Ferdosi M. Revised risk priority number in failure mode and effects analysis model from the perspective of healthcare system. Int J Prev Med. (2018) 9:7. doi: 10.4103/2008-7802.224046
20. Pagano AM, Maiese A, Izzo C, Maiese A, Ametrano M, De Matteis A, et al. COVID-19 risk management and screening in the penitentiary facilities of the salerno province in Southern Italy. Int J Environ Res Public Health. (2020) 17:8033. doi: 10.3390/ijerph17218033
21. Viola RV, Di Fazio N, Del Fante Z, Fazio V, Volonnino G, Romano S, et al. Rules on informed consent and advance directives at the end-of-life: the new Italian law. Clin Ter. (2020) 171:e94–e96. doi: 10.7417/CT.2020.2195
22. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. (1987) 40:373–83. doi: 10.1016/0021-9681(87)90171-8
23. R: a language and environment for statistical computing. Available online at: https://www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing (accessed: Oct 11, 2018).
24. Arenas Jiménez MD, Ferre G, Álvarez-Ude F. Strategies to increase patient safety in Hemodialysis: application of the modal analysis system of errors and effects (FEMA system). Nefrologia. (2017) 37:608–21. doi: 10.1016/j.nefroe.2017.11.011
25. Bonfant G, Belfanti P, Paternoster G, Gabrielli D, Gaiter AM, Manes M, et al. Clinical risk analysis with failure mode and effect analysis (FMEA) model in a dialysis unit. J Nephrol. (2010) 23:111–8.
26. Fadili W, Adnouni A, Laouad I. Hemodialysis safety: evaluation of clinical practice. Saudi J Kidney Dis Transpl. (2016) 27:553–6. doi: 10.4103/1319-2442.182398
27. Sahli F, Feidjel R, Laalaoui R. Hemodialysis catheter-related infection: rates, risk factors and pathogens. J Infect Public Health. (2017) 10:403–8. doi: 10.1016/j.jiph.2016.06.008
28. Araghi F, Tabary M, Gheisari M, Abdollahimajd F, Dadkhahfar S. Hand hygiene among health care workers during COVID-19 pandemic: challenges and recommendations. Dermatitis. (2020) 31:233–7. doi: 10.1097/DER.0000000000000639
29. Hutin Y, Hauri A, Chiarello L, Catlin M, Stilwell B, Ghebrehiwet T, et al. Best infection control practices for intradermal, subcutaneous, intramuscular needle injections. Bull World Health Organ. (2003) 81:491–500.
30. Claudel SE, Miles LA, Murea M. Anticoagulation in hemodialysis: a narrative review. Semin Dial. (2021) 34:103–15. doi: 10.1111/sdi.12932
31. Regitz-Zagrosek V. Sex and gender differences in health. EMBO Rep. (2012) 13:596–603. doi: 10.1038/embor.2012.87
32. James SL, Castle CD, Dingels ZV, Fox JT, Hamilton EB, Liu Z, et al. Global injury morbidity and mortality from 1990 to 2017: results from the Global Burden of Disease Study 2017. Inj Prev. (2020) 26:i96–i114. doi: 10.1136/injuryprev-2019-043494
33. Fineschi V, Arcangeli M, Di Fazio N, Fante ZD, Fineschi B, Santoro P, et al. Defensive medicine in the management of cesarean delivery: a survey among Italian Physicians. Healthcare. (2021) 9:1097. doi: 10.3390/healthcare9091097
34. Albolino S, Bellandi T, Cappelletti S, Di Paolo M, Fineschi V, Frati P, et al. New rules on patient's safety and professional liability for the Italian Health Service. Curr Pharm Biotechnol. (2019) 20:615–24. doi: 10.2174/1389201020666190408094016
35. Zanza C, Racca F, Longhitano Y, Piccioni A, Franceschi F, Artico M, et al. Risk management and treatment of coagulation disorders related to COVID-19 infection. Int J Environ Res Public Health. (2021) 18:1268. doi: 10.3390/ijerph18031268
36. Del Fante Z, Di Fazio N, Papale A, Tomao P, Del Duca F, Frati P, et al. Evaluation of physical risk during Necropsy and Morgue activities as risk management strategy. Int J Environ Res Public Health. (2021) 18:8266. doi: 10.3390/ijerph18168266
37. Piccioni A, Cicchinelli S, Saviano L, Gilardi E, Zanza C, Brigida M, et al. Risk management in first aid for acute drug intoxication. Int J Environ Res Public Health. (2020) 17:8021. doi: 10.3390/ijerph17218021
38. Maiese A, Volonnino G, Viola RV, Nelson Cavallari E, Fazio V, Arcangeli M, et al. A rare case of Spinal Epidural Abscess following mesotherapy: a challenging diagnosis and the importance of clinical risk management. Considerations concerning uncommon risk factor for development of Spinal Epidural Abscess and its prevention. Clin Ter. (2020) 170:e15–e18. doi: 10.7417/CT.2020.2183
Keywords: FMEA, haemodialysis, CVC, risk management, failure mode
Citation: La Russa R, Fazio V, Ferrara M, Di Fazio N, Viola RV, Piras G, Ciano G, Micheletta F and Frati P (2022) Proactive Risk Assessment Through Failure Mode and Effect Analysis (FMEA) for Haemodialysis Facilities: A Pilot Project. Front. Public Health 10:823680. doi: 10.3389/fpubh.2022.823680
Received: 28 November 2021; Accepted: 01 March 2022;
Published: 24 March 2022.
Edited by:
Matteo Bolcato, University of Padua, ItalyReviewed by:
Yasamin Molavi Taleghani, Isfahan University of Medical Sciences, IranPeter Kerr, Monash Health, Australia
Copyright © 2022 La Russa, Fazio, Ferrara, Di Fazio, Viola, Piras, Ciano, Micheletta and Frati. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicola Di Fazio, nicola.difazio@uniroma1.it
 Raffaele La Russa
Raffaele La Russa Valentina Fazio
Valentina Fazio Michela Ferrara1
Michela Ferrara1 Nicola Di Fazio
Nicola Di Fazio Gianluca Piras
Gianluca Piras Paola Frati
Paola Frati