- 1Department of Epidemiology and Biostatistics, School of Public Health, Jilin University, Changchun, China
- 2FAW General Hospital of Jilin Province, Changchun, China
- 3Department of Epidemiology and Biostatistics, School of Public Health, University of Nevada, Las Vegas, NV, United States
Introduction: With the population aging, osteoporosis has become a major public health concern. Elevated oxidative stress is a vital detrimental factor for bone health. Compared to common oxidative stress-related biomarkers, Fluorescent Oxidation Products (FlOPs) reflect the global levels of oxidation from proteins, lipids, and DNA. Nevertheless, whether plasma FlOP levels are related to bone health measured by Quantitative ultrasound (QUS) is unclear. Thus, the present study examined the association between FlOPs and QUS parameters in middle-aged and elderly adults.
Methods: This community-based cross-sectional study was conducted in Changchun, northeast China. Plasma FlOPs were determined by a fluorescent microplate reader at a wavelength of 320/420 nm (excitation/emission). QUS parameters [speed of sound (SOS) and broadband ultrasound attenuation (BUA)] of the calcaneus were assessed by an ultrasound bone densitometer. We used multivariable linear regression to examine the association between FlOPs and QUS parameters.
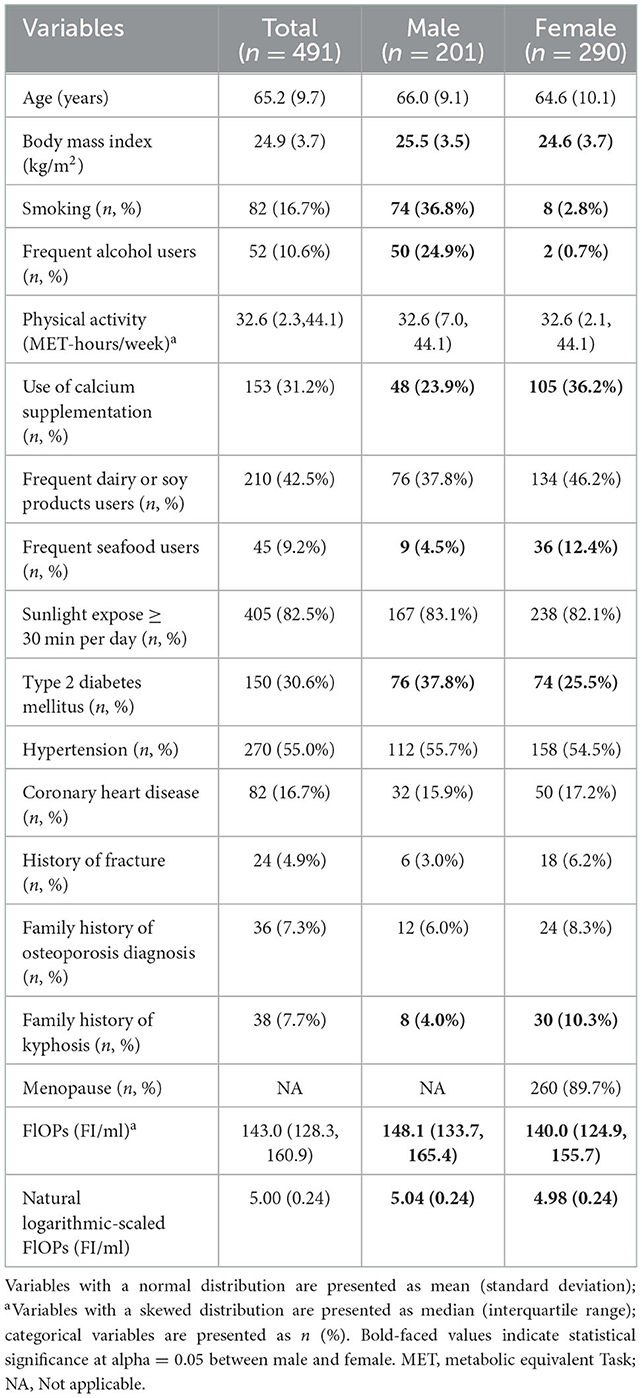
Results: A total of 491 subjects were included in this study. Their average age was 65.2 years (standard deviation [SD]: 9.7 years). FlOPs were inversely associated with SOS (β for an increase of logarithmic interquartile range = −10.64; P = 0.018). Higher FlOP levels were marginally associated with lower SOS in females (β for an increase of logarithmic interquartile range = −9.68, P = 0.066), but not in males (β for an increase of logarithmic interquartile range = −11.84, P = 0.131). No significant relationship between FlOPs and BUA was observed.
Conclusions: Plasma FlOP levels were inversely associated with SOS, but not with BUA in middle-aged and elderly adults.
Introduction
With the aging of the population, osteoporosis has become a major public health concern, leading to lower quality of life and imposing a huge disease burden on patients, their families, and society (1, 2). In China, the prevalence of osteoporosis was 19.2% for people aged 50 years or older, and 32.0% for those over 65 years of age (3). By 2050, it is estimated that the annual number of fragility fractures in China will increase to 5.99 million and the related medical cost will reach $25.43 billion (4). In communities, usage of the quantitative ultrasound device has become a relatively convenient tool for osteoporosis screening (5).
Oxidative stress occurs from the imbalance between the production of free radicals and the antioxidant defense system. The accumulation of excessive reactive oxygen species (ROS) induces oxidative damage to tissue and cellular macromolecules (e.g., protein, lipids, and DNA), leading to increased risk of many diseases such as cardiovascular disease, chronic kidney disease, and osteoporosis (6, 7).
In vivo and in vitro evidence suggests that ROS are involved in the pathogenesis of bone loss, increasing the level of bone resorption by stimulating the differentiation of osteoclasts and reducing bone formation via inhibiting the activity of osteoblasts (8–10). Previous studies on the relationship between oxidative stress and bone health are primarily based on the bone mineral density (BMD) determined by dual-energy X-ray absorptiometry (DXA) (11–13), only a few surveys are based on quantitative ultrasound (QUS) parameters (14, 15). Compared to DXA, QUS is less expensive, portable, and free from ionizing radiation. Several human studies have examined the relationship between traditional oxidative stress-related biomarkers [i.e., malondialdehyde (MDA)] and bone health assessed by BMD or QUS parameters, however, their results were inconsistent (11, 12, 14, 15). A recent study conducted in Iraq suggested that MDA is negatively associated with BMD in postmenopausal women (13). In contrast, Wu et al. observed an insignificant relationship between MDA and BMD in Chinese postmenopausal women (12). This inconsistency is likely due to the fact that traditional oxidative stress biomarkers only capture one specific aspect of oxidative damage. For example, MDA and 8-hydroxyguanosine (8-OHdG) were commonly used to assess the level of lipid peroxidation and DNA oxidation, respectively (16).
Fluorescent Oxidation Products (FlOPs) reflect oxidative damage in terms of protein, lipids, DNA, and carbohydrate, and have been used as a global biomarker of oxidative stress in epidemiologic studies (17, 18). Compared to specific oxidation measurements such as MDA assay via colorimetric thiobarbituric acid, the fluorescence method is 10–100 times more sensitive (19). In our previous study, higher FlOP levels were found to be associated with lower hip BMD in male veterans (20) and an increased risk of hip fracture in postmenopausal women (21). However, whether FlOPs are associated with bone health determined by QUS parameters is unclear. Given the above evidence, we hypothesized that FlOPs are negatively associated with QUS parameters. QUS measurements at the calcaneal could provide information on the structural and mechanical properties of the bone (22, 23). Knowing this relationship would expand our understanding of the impact of oxidative stress on the structural and mechanical properties of bone assessed by QUS.
Materials and methods
Study design and participants
This study is part of the national project entitled “The Comprehensive Demonstration Research Project of Major Chronic Disease Prevention and Control Technology in Northeast China (Health Northeast)” initiated by China Medical University. As one of the important participating units, Jilin University undertook the main work of the project in Jilin. The design and implementation of the study have been previously described (24). In brief, a community-based cross-sectional study was performed between January and December 2019 in Changchun, Jilin, China. We cooperated with more than 20 community health service centers distributed in 10 districts in Changchun. All participants were interviewed face-to-face at the community health service centers using a structured questionnaire. We collected data such as socio-demographic information, medical history, and lifestyle factors. We additionally measured data such as height, weight, fasting blood glucose, and blood pressure. Because we were limited to only one QUS device, we randomly selected two community health service centers from two urban districts in Changchun for the present study. All subjects provided written informed consent. This project was approved by the institutional review board (IRB) of China Medical University.
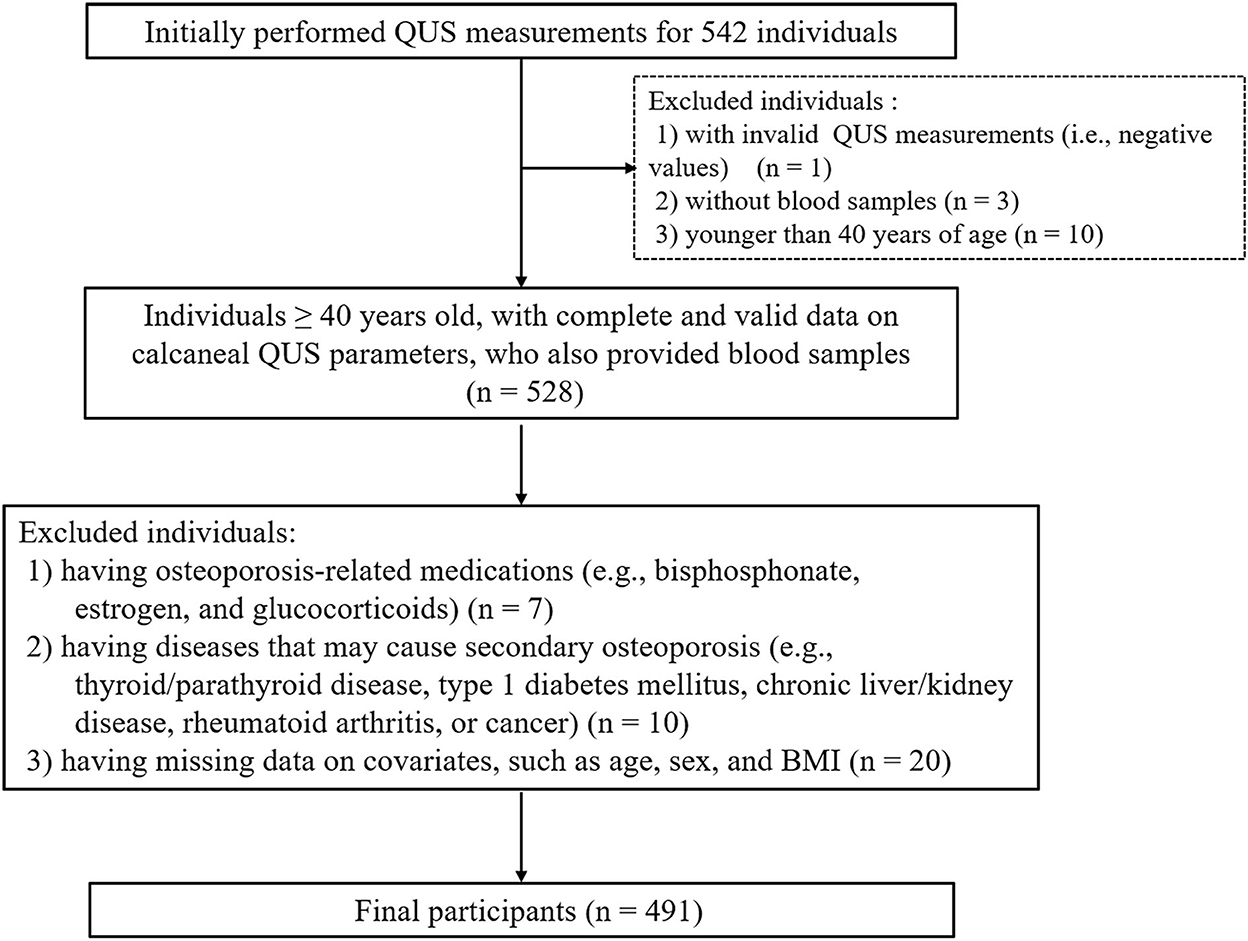
We included all individuals aged 40 years or older, with complete and valid information on QUS parameters, who also provided blood samples. Individuals who met any of the following criteria were excluded: (1) having osteoporosis-related medications, such as bisphosphonates, estrogen, and glucocorticoids; (2) having diseases that may contribute to secondary osteoporosis (e.g., thyroid/parathyroid disorders, type 1 diabetes mellitus, chronic liver/kidney disease, rheumatoid arthritis, or cancer); and (3) having missing data on covariates, such as age, sex, and body mass index (BMI).
Quantitative ultrasound measurement
QUS measurements of the calcaneus were performed using an ultrasound bone densitometer (Osteo KJ3000, Kejin Inc., Nanjing, Jiangsu, China). This device produces two key parameters: speed of sound (SOS, expressed as m/s) and broadband ultrasound attenuation (BUA, expressed as dB/MHz). SOS is the transmission time of sound waves divided by the length of the body part studied; BUA refers to the slope between the attenuation of sound signals and their frequency (23). The daily performed control spine phantom had a coefficient of variation (CV) of <5%. Participants with invalid QUS measurements (i.e., negative values) were excluded.
Blood collection
Fasting blood samples (≥8 h) were collected with anticoagulant tubes (BD, Becton, Dickinson and Company, Franklin Lakes, New Jersey, USA) from all subjects. Within 4 h, these samples were transported to the laboratory of Jilin University using ice boxes for processing and stored at −80°C until assay.
FlOP measurement
We measured FlOPs for all individuals with collected blood samples. Measurement of FlOPs was performed based on the method modified by Shimasaki (25) and Wu (26), and has been previously described (20). In brief, plasma was extracted with ethanol/ether (3:1, v/v) and centrifuged at 3,000 rpm for 10 min at 4°C. Then the supernatant was added to a black 96-well Microplate (Black Fat Bottom Polystyrene High Bind Microplate 3925, Corning) and measured by a fluorescent microplate reader (Cytation 3 Cell Imaging Multi-Mode Reader, BioTek, Vermont, USA) at a wavelength of 320/420 nm (excitation and emission wavelength). The fluorescence of FlOPs was presented as relative fluorescent intensity units per milliliter (FI/ml). The inter-assay and intra-assay CVs for FlOP measurement were 3.3 and 1.7%, respectively. We additionally tested the long-term stability of FlOPs among 16 participants in a pilot study. We found that FlOPs were stable at −80°C for at least 90 days. In the present study, all blood samples were collected between July and September 2019. Plasma FlOP levels were measured in October 2019. The storage duration of the blood samples and subsequent measurement of FlOP levels within 90 days in our study ensured the stability of FlOP levels.
Anthropometric assessment
Body weight and height, without shoes and heavy clothes, were measured using a full automatic ultrasonic height and weight measuring instrument (SK-CK90, SONKA, Shenzhen Shuangjia Electronic Technology Co., Ltd., Guangdong, China). Body weight and height were recorded to the nearest 0.1 kg and 0.1 cm, respectively. BMI was calculated as weight (kg) divided by height (meters) squared. Participants were categorized as non-obese (BMI < 28 kg/m2) or obese (BMI ≥ 28 kg/m2) according to the cut-points recommended by the Working Group on Obesity in China (27).
Ascertainment of other covariates
The covariates included in this study were socio-demographic data (e.g., age and sex), anthropometric variables (e.g., BMI), medical history (e.g., hypertension, type 2 diabetes mellitus status, and family history of kyphosis,), and lifestyle data (e.g., smoking and physical activity). Smoking was defined as the current or past use of tobacco. Frequent alcohol users were defined as a person who consumed an average of 3 or more units of alcohol per day. One unit is equivalent to half a pint (285 ml) of beer, one glass (125 ml) of wine, or a pub measure of spirits (8–10 g pure alcohol). Physical activity was computed using the frequency, duration, and intensity (light, moderate and heavy) of physical activity that was reported by participants and was subsequently expressed as metabolic equivalent hours per week (Met-hours/week) (28). Frequent users of calcium supplementation, dairy or soy products, and seafood were defined as an individual who ate these food items at least three times a week. Fasting blood glucose and blood pressure were obtained by physical examination. Diabetes mellitus was determined by either a fasting blood glucose ≥ 7.0 mmol/L, a self-reported diagnosis by a physician, or taking hypoglycaemic drugs (29). Hypertension was defined as a systolic blood pressure ≥ 140 mmHg or a diastolic blood pressure ≥ 90 mmHg, a self-reported diagnosis of hypertension by a physician, or the use of antihypertensive medication (30). Coronary heart disease, history of fracture, family history of osteoporosis diagnosis, and family history of kyphosis were self-reported.
Statistical analysis
Descriptive data for the total population as well as by sex are provided. Continuous variables with a normal distribution are reported as means and standard deviations (SDs). Categorical variables are reported as frequencies and percentages. Data with a skewed distribution are presented as medians and interquartile ranges. The characteristics of participants by sex were compared using Student's t-test or Wilcoxon non-parametric test for continuous variables or Chi-square test for categorical variables. To test whether there was potential selection bias, we compared the baseline characteristics (i.e., age, sex, and BMI) between the included individuals for this study with the overall population from the 10 districts.
As the effect of oxidative stress on bone homeostasis is regulated by sex hormones and QUS measures were different between sexes (31, 32), all the regression analyses were performed stratified by sex. We used multivariable linear regression models to evaluate the associations of FlOPs with SOS and BUA. To better compare a person with a typical “high” value of FlOPs to a person with a typical “low” value, we rescaled the values of FlOP levels using the interquartile range, defined by the distance between the 25th and the 75th percentiles (33). FlOPs were further transformed into the natural logarithmic scale due to its skewed distribution. The following covariates were considered in all the adjustment models: age, BMI, smoking, frequent alcohol users, physical activity, frequent dairy or soy products, and history of fracture; sex and menopausal status were additionally adjusted for in the analysis of all the participants and in the analysis of only female participants, respectively. We included the covariates for adjustment if they were associated with either SOS or BUA at alpha = 0.1 in the bivariate analysis or were well-known risk factors for bone health. We also examined the relationships between covariates and FlOPs using bivariate analysis. Subgroup linear regression analyses by age (<60 years and ≥60 years), sex (male/female), BMI (<28 kg/m2 and ≥28 kg/m2), smoking (yes/no), frequent dairy or soy products use (yes/no), frequent alcohol use (yes/no), type 2 diabetes mellitus (yes/no), and hypertension (yes/no) were also performed. We conducted these subgroup analyses mainly because these stratified factors were related to FlOPs and/or QUS parameters (17, 32). To test whether there is interaction by these potential factors on FlOPs in relation to QUS parameters, we built the interaction terms (e.g., FlOPs* age, FlOPs*sex, FlOPs*BMI, FlOPs*smoking, FlOPs*frequent dairy or soy products use, FlOPs*frequent alcohol use, FlOPs*type 2 diabetes mellitus, and FlOPs*hypertension) in the linear regression models. All analyses were conducted with SPSS (version 24.0, IBM SPSS Inc., Chicago, IL) or R (version: 4.0.0; R Foundation for Statistical Computing, Vienna, Austria) statistical software.
Results
We measured QUS for all 542 participants. However, we excluded one participant who had a negative BUA measurement value and further excluded ineligible individuals, resulting in a final study sample of 491 eligible subjects for analysis (Figure 1). Our participants were younger than the overall population from the 10 districts (65.2 vs.70.0 years; P < 0.05). However, there was no significant difference in the proportion of females (59.6 vs. 59.1%; P > 0.05) nor BMI (24.9 vs. 24.9 kg/m2) between the two populations. The characteristics of the included participants are shown in Table 1. The participants' median value of FlOPs was 143.0 FI/ml (IQR: 128.3–160.9 FI/ml; range: 97.6–478.8 FI/ml). Compared to males, females had a lower BMI and were less likely to be smokers and frequent alcohol users, and were more likely to consume seafood on a frequent basis (all P < 0.05). Females were also less likely to have type 2 diabetes mellitus, more likely to use calcium supplementation, and have a family history of kyphosis (all P < 0.05). Females also had a significantly lower median level of FlOPs compared to males (P < 0.05).
In the bivariate analyses, we observed significant positive associations between FlOPs with age, BMI, smoking, frequent alcohol use, type 2 diabetes mellitus, hypertension, and coronary heart disease; there was also a negative relationship between FlOPs and family history of kyphosis (all P < 0.05; Supplementary Table 1). As compared to males, females were associated with higher FlOPs. Hypertension and coronary heart disease were positively associated with FlOPs in males, whereas type 2 diabetes mellitus was positively associated with FlOPs in females (all P < 0.05).
In the bivariate analyses (Supplementary Tables 2, 3), SOS and BUA were inversely associated with age, while positively associated with frequent alcohol users. BMI had a positive relationship with SOS, but not with BUA. Frequent dairy or soy product users were only inversely associated with SOS. We only observed a positive association between smoking and BUA. Compared with males, females were associated with lower levels of SOS and BUA. In males, we only observed a statistically significant positive relationship between BMI and SOS. In females, age, coronary heart disease, and menopause were negatively associated with SOS, whereas age, hypertension, and menopause were negatively associated with BUA (all P < 0.05).
In the adjusted multivariable linear regression models (Table 2), we observed an inverse association between FlOPs and SOS (β for an increase of logarithmic interquartile range = −10.64; P = 0.018). Higher FlOP levels were marginally associated with lower SOS in females (β for an increase of logarithmic interquartile range = −9.68, P = 0.066), but not in males (β for an increase of logarithmic interquartile range = −11.84, P = 0.131). No significant association between FlOPs and BUA was noted (all P > 0.05; Table 2).
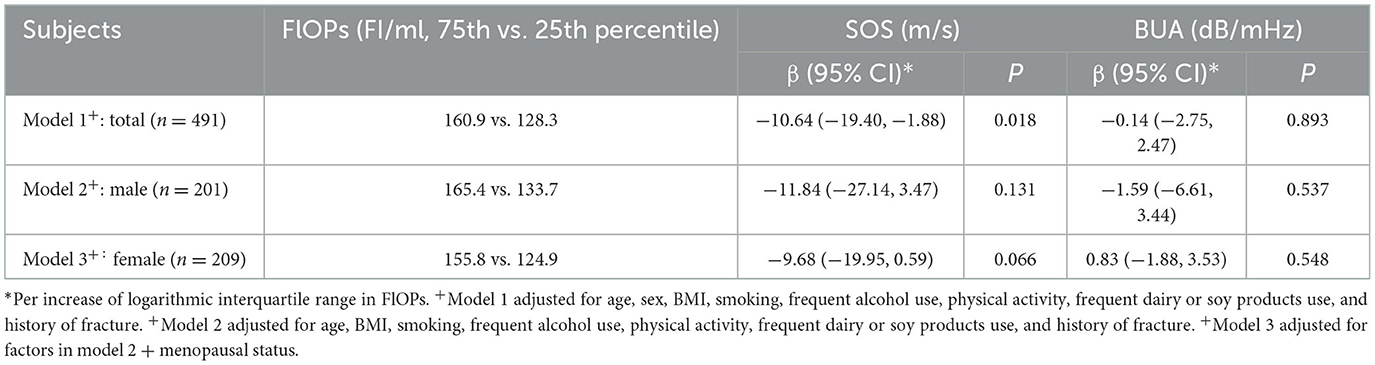
Table 2. Fluorescent oxidation products (FlOPs) and quantitative ultrasound (QUS) parameters: multivariable linear regression analysis.
Subgroup analyses showed that the relationship between FlOPs and SOS was stronger in participants with type 2 diabetes mellitus (β = −24.700, P = 0.003) than in individuals without diabetes (β = −4.389, P = 0.405; P for interaction = 0.028; Table 3). The FlOP-SOS relationship was not modified by age, sex, BMI, smoking, frequent dairy or soy products, frequent alcohol users, or hypertension (all P for interaction > 0.05).
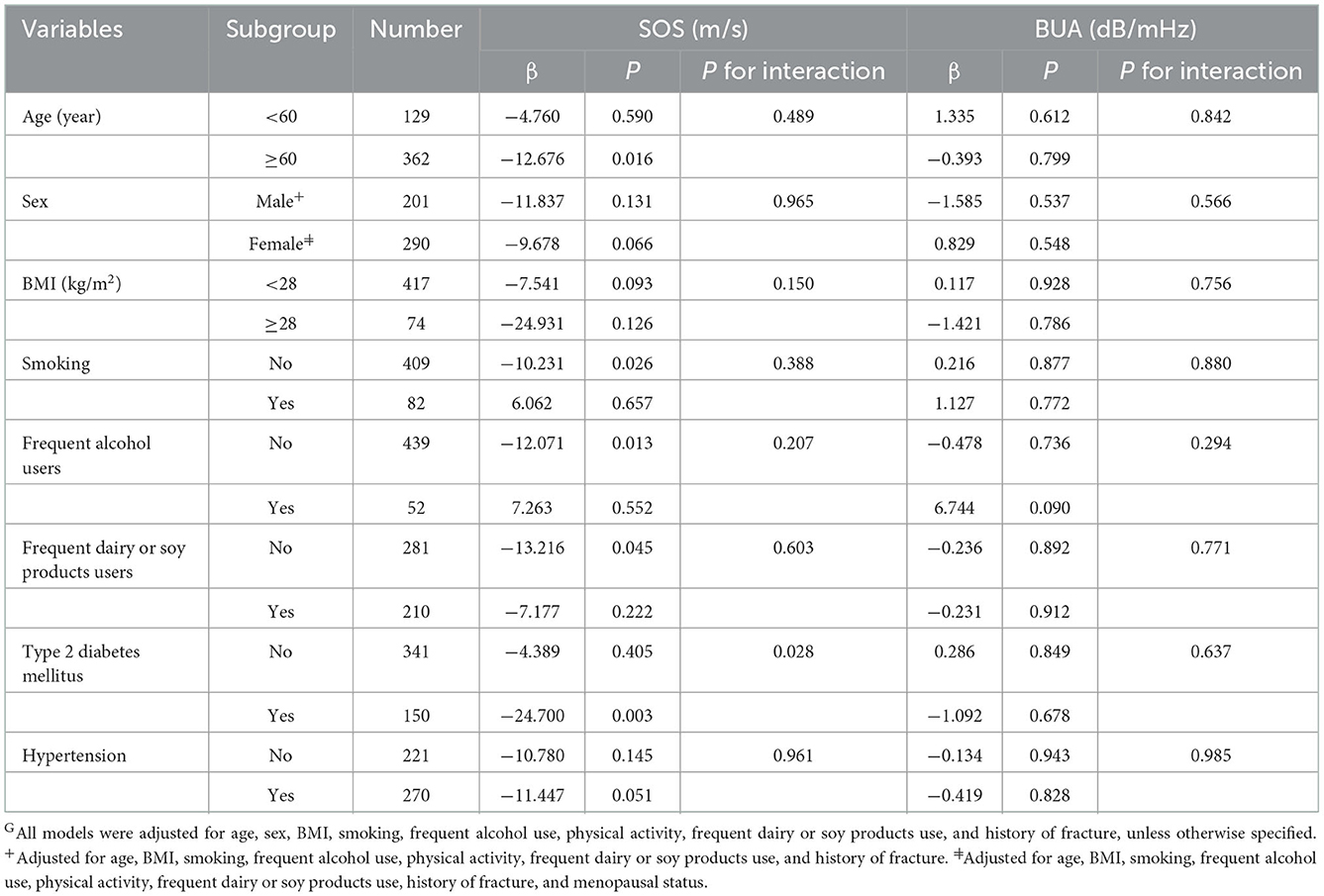
Table 3. Fluorescent oxidation products (FlOPs, Per increase of logarithmic interquartile range) and quantitative ultrasound (QUS) parameters: multivariable linear regression analysis stratified by age, sex, BMI, smoking, frequent alcohol users, frequent dairy or soy products users, type 2 diabetes mellitus, and hypertensionG.
Discussion
In this community-based cross-sectional study, we observed that FlOPs were inversely associated with SOS in middle-aged and elderly adults. Higher FlOP levels were marginally associated with lower SOS in females, but not in males. No significant relationship between FlOPs and BUA was observed in the overall analysis nor by sex. The present findings expand our current knowledge of the relationship between FlOPs and QUS parameters.
As far as we know, this is the first study examining the relationship between FlOPs and QUS parameters. To some extent, our results were in agreement with two previous studies, in which higher FlOP levels are associated with lower BMD in male veterans (20) and an increased risk of hip fracture in postmenopausal women (21). Since QUS reflects the structural and mechanical properties of the bone (22, 23), the present study extended the evidence about the relationship between FlOPs and bone health in males and females. Moreover, among all the interaction terms, we observed that only type 2 diabetes mellitus status significantly modified the relationship between FlOPs and SOS. We did not find evidence to support smoking status as an effect modifier in the association between FlOPs and SOS. This is concordant with a previous study where smoking did not modify the relationship between FlOPs and BMD (20). Nevertheless, the possible explanation for the insignificant interaction terms between some subgroups (i.e., FlOPs*sex, FlOPs*smoking, and FlOPs*BMI) may be due to small sample size.
When compared our results with those of studies using other oxidative stress-related biomarkers, there are both consistencies and inconsistencies. For example, Basu et al. suggested that 8-iso-PGF2α, a lipid peroxidation marker, is negatively associated with SOS and BUA in Swedish adults (34). In the study of 868 Spanish men older than 50 years, Hernández et al. observed that higher levels of serum uric acid, a substance with antioxidant properties, are positively associated with all QUS parameters (14). In the present study, FlOPs were only associated with SOS, but not with BUA. This finding was partially consistent with the pooled results obtained by Enneman et al., which showed a statistically significant inverse association between homocysteine and SOS, but not BUA in older persons (15). Even though SOS and BUA are highly correlated (35), they reflect different aspects of bone properties and are influenced by various factors. SOS reflects the material property of the bone, such as the elastic modulus and compressive strength. BUA reflects bone microarchitecture and bone strength (23, 36). Previous in vivo study suggests that SOS has a stronger association with BMD than BUA as BUA failed to predict the mechanical properties of high-density trabecular bone (37). Further studies are warranted to elucidate this discrepancy.
Several potential mechanisms may explain the association between FlOPs and QUS parameters. Numerous lines of evidence suggest that oxidative stress is involved in the process of bone remodeling, inducing an imbalance between osteoclastic bone resorption and osteoblastic bone formation (7, 38, 39). Excessive ROS affects the differentiation and activity of osteoclasts by regulating mitogen-activated protein kinases (MAPKs), nuclear factor-kappa (NF-κB), and Ca2+-mediated signaling cascades (40). Baek et al. found that the number and activity of osteoclasts, as well as the receptor activator of nuclear factor-kappa B ligand (RANKL)/osteoprotegerin (OPG) ratio, were increased when hydrogen peroxide (H2O2) was added to human marrow mononuclear cells (41). Higher levels of oxidative stress decrease osteoprogenitor differentiation to the osteoblast cell lineage and promote the apoptosis of osteoblasts (7, 42). Bai et al. reported that H2O2-induced oxidative stress suppresses the osteoblastic differentiation process, manifested by a reduction of bone formation markers including alkaline phosphatase (ALP) (43).
Most of the existing studies focused on the relationship between oxidative stress and BMD (11, 12, 20). Bone strength is not only captured by BMD, but also by bone microarchitecture, bone mechanical properties, mineralization degree, and quality of collagens (44–46). In some situations, measurement of DXA is not available due to its high cost, ionizing radiation, and non-portability. QUS measurement of the calcaneus is a suitable method for screening osteoporosis. Moreover, compared to other oxidative stress-related biomarkers (i.e., MDA) that only reflect one specific aspect of oxidative damage (i.e., lipid peroxidation), FlOPs reflect the global level of oxidative damage in vivo. Overall, the present study provided supporting evidence for the association between FlOPs and bone mechanical and structural properties determined by QUS parameters. If our findings are confirmed in further studies, FlOPs may be a better biomarker for assessing the impact of global oxidative damage on BMD at the calcaneus and evaluating the effects of antioxidant use on bone health.
Several limitations of the present study need to be mentioned. First, due to the nature of the cross-sectional design, the temporal association between FlOPs and SOS cannot be determined. Second, our study had a small sample size; this may negatively impact the reliability of our results. Third, the possibility of residual confounding cannot be completely excluded, because some risk factors (i.e., vitamin D intake) were not included in the analysis. However, this limitation is likely to be minor as vitamin D intake is highly correlated with calcium intake (47, 48). Lastly, due to the difference in age between the included individuals selected from two urban districts in Changchun and the overall participants from the 10 districts, the present study may suffer from potential selection bias.
Conclusions
In summary, plasma FlOP levels were inversely associated with SOS, but not with BUA in middle-aged and elderly adults. The present findings support the possibility of using FlOPs as a global biomarker to assess the impact of oxidative stress on the structural and mechanical properties of the bone. It would be worthwhile to conduct further studies to elucidate the roles of FlOPs in QUS parameters with a longitudinal study design.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board (IRB) of China Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
SY and YL designed the study. XS prepared the first draft of the paper. XS, QZ, BL, and YF contributed to the investigation and methodology of the present study. XS, MZ, and SY were responsible for the statistical analysis of the data. SY, MZ, AV, and HC reviewed and edited the manuscript. All authors read and approved the final manuscript.
Funding
The present study was supported by the National Key R&D Program of China (Grant #2018YFC1311600). This work was also partly supported by research grants from the Jilin Scientific and Technological Development Program (Grant Number: 20210101431JC), and the Changchun Science and Technology Planning Project (Grant Numbers: 21ZGM28 and 21ZGM27).
Acknowledgments
We gratefully acknowledge all the medical staff of the community health service centers for their assistance during this survey. We greatly appreciate the cooperation from all participants in the present study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.1032550/full#supplementary-material
References
1. Hopman WM, Berger C, Joseph L, Morin SN, Towheed T, Anastassiades T, et al. Longitudinal assessment of health-related quality of life in osteoporosis: data from the population-based canadian multicentre osteoporosis study. Osteoporosis Int. (2019) 30:1635–44. doi: 10.1007/s00198-019-05000-y
2. Harvey N, Dennison E, Cooper C. Osteoporosis: impact on health and economics. Nat Rev Rheumatol. (2010) 6:99–105. doi: 10.1038/nrrheum.2009.260
3. Chinese Society of Osteoporosis and Bone Mineral Research. The epidemiological survey of osteoporosis in China and release of the results of the “Healthy Bones” special Action Project. Chin J Osteoporos Bone Miner Res. (2019) 12:317–8. doi: 10.3969/j.issn.1674-2591.2019.04.001
4. Si L, Winzenberg TM, Jiang Q, Chen M, Palmer AJ. Projection of osteoporosis-related fractures and costs in China: 2010-2050. Osteoporos Int. (2015) 26:1929–37. doi: 10.1007/s00198-015-3093-2
5. Hans D, Baim S. Quantitative Ultrasound (QUS) in the management of osteoporosis and assessment of fracture risk. J Clin Densitom. (2017) 20:322–33. doi: 10.1016/j.jocd.2017.06.018
6. Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, et al. Oxidative stress, aging, and diseases. Clin Intervent Aging. (2018) 13:757–72. doi: 10.2147/CIA.S158513
7. Kimball JS, Johnson JP, Carlson DA. Oxidative stress and osteoporosis. J Bone Joint Surg Am. (2021) 103:1451–61. doi: 10.2106/JBJS.20.00989
8. Tao H, Ge G, Liang X, Zhang W, Sun H, Li M, et al. Ros signaling cascades: dual regulations for osteoclast and osteoblast. Acta Biochim Biophys Sin. (2020) 52:1055–62. doi: 10.1093/abbs/gmaa098
9. Yao H, Yao Z, Zhang S, Zhang W, Zhou W. Upregulation of SIRT1 inhibits H2O2-induced osteoblast apoptosis via FoxO1/B-Catenin pathway. Mol Med Rep. (2018) 17:6681–90. doi: 10.3892/mmr.2018.8657
10. Xu W, Liu X, He X, Jiang Y, Zhang J, Zhang Q, et al. Bajitianwan attenuates D-galactose-induced memory impairment and bone loss through suppression of oxidative stress in aging rat model. J Ethnopharmacol. (2020) 261:112992. doi: 10.1016/j.jep.2020.112992
11. Zhao F, Guo L, Wang X, Zhang Y. Correlation of oxidative stress-related biomarkers with postmenopausal osteoporosis: a systematic review and meta-analysis. Arch Osteoporos. (2021) 16:4. doi: 10.1007/s11657-020-00854-w
12. Wu Q, Zhong ZM, Pan Y, Zeng JH, Zheng S, Zhu SY, et al. Advanced oxidation protein products as a novel marker of oxidative stress in postmenopausal osteoporosis. Med Sci Monit. (2015) 21:2428–32. doi: 10.12659/MSM.894347
13. Badr Roomi A, Nori W, Mokram Hamed R. Lower serum irisin levels are associated with increased osteoporosis and oxidative stress in postmenopausal. Rep Biochem Mol Biol. (2021) 10:13–9. doi: 10.52547/rbmb.10.1.13
14. Hernández JL, Nan D, Martínez J, Pariente E, Sierra I, González-Macías J, et al. Serum uric acid is associated with quantitative ultrasound parameters in men: data from the camargo cohort. Osteoporos Int. (2015) 26:1989–95. doi: 10.1007/s00198-015-3083-4
15. Enneman AW, Swart KM, Zillikens MC, van Dijk SC, van Wijngaarden JP, Brouwer-Brolsma EM, et al. The association between plasma homocysteine levels and bone quality and bone mineral density parameters in older persons. Bone. (2014) 63:141–6. doi: 10.1016/j.bone.2014.03.002
16. Marrocco I, Altieri F, Peluso I. Measurement and clinical significance of biomarkers of oxidative stress in humans. Oxid Med Cell Longev. (2017) 2017:6501046. doi: 10.1155/2017/6501046
17. Wu T, Willett WC, Rifai N, Rimm EB. Plasma fluorescent oxidation products as potential markers of oxidative stress for epidemiologic studies. Am J Epidemiol. (2007) 166:552–60. doi: 10.1093/aje/kwm119
18. Wu T, Kasper S, Wong RM, Bracken B. Identification of differential patterns of oxidative biomarkers in prostate cancer progression. Clin Genitourinary Cancer. (2020) 18:e174–e9. doi: 10.1016/j.clgc.2019.09.014
19. Frankel EN. Lipid Oxidation. 2nd ed. Dundee: The Oily Press Ltd (2005). doi: 10.1533/9780857097927
20. Shen X, Peng C, Zhao Y, Zhong L, Cai H, Kan B, et al. Plasma fluorescent oxidation products and bone mineral density among male veterans: a cross-sectional study. J Clin Densitom. (2022) 25:141–9. doi: 10.1016/j.jocd.2021.09.003
21. Yang SM, Feskanich D, Willett WC, Eliassen AH, Wu TY. Association between global biomarkers of oxidative stress and hip fracture in postmenopausal women: a prospective study. J Bone Miner Res. (2014) 29:2577–83. doi: 10.1002/jbmr.2302
22. Link TM, Heilmeier U. Bone quality-beyond bone mineral density. Semin Musculoskeletal Radiol. (2016) 20:269–78. doi: 10.1055/s-0036-1592365
23. Chin KY, Ima-Nirwana S. Calcaneal quantitative ultrasound as a determinant of bone health status: what properties of bone does it reflect? Int J Med Sci. (2013) 10:1778–83. doi: 10.7150/ijms.6765
24. Shi J, Guo Y, Li Z, Liang Z, Pan L, Yu Y, et al. Sociodemographic and behavioral influences on multimorbidity among adult residents of northeastern China. BMC Public Health. (2022) 22:342. doi: 10.1186/s12889-022-12722-y
25. Shimasaki H. Assay of fluorescent lipid peroxidation products. Methods Enzymol. (1994) 233:338–46. doi: 10.1016/S0076-6879(94)33039-5
26. Wu T, Rifai N, Roberts LJ II, Willett WC, Rimm EB. Stability of measurements of biomarkers of oxidative stress in blood over 36 hours. Cancer Epidemiol Biomark Prev. (2004) 13:1399–402. doi: 10.1158/1055-9965.1399.13.8
27. Zhou BF. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. (2002) 15:83–96. doi: 10.1046/j.1440-6047.11.s8.9.x
28. Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and met intensities. Med Sci Sports Exerc. (2000) 32:S498–516. doi: 10.1097/00005768-200009001-00009
29. American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. (2021) 44(Suppl 1):S15–33. doi: 10.2337/dc21-S002
30. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. (2003) 289:2560–72. doi: 10.1001/jama.289.19.2560
31. Sibilia V, Bottai D, Maggi R, Pagani F, Chiaramonte R, Giannandrea D, et al. Sex steroid regulation of oxidative stress in bone cells: an in vitro study. Int J Environ Res Public Health. (2021) 18:12168. doi: 10.3390/ijerph182212168
32. Ding Z, Chen Y, Xu Y, Zhou X, Xu Y, Ma Z, et al. Impact of age, gender, and body composition on bone quality in an adult population from the middle areas of China. J Clin Densitom. (2018) 21:83–90. doi: 10.1016/j.jocd.2016.11.001
33. Babyak MA. Rescaling continuous predictors in regression models. Statistical Tips from the Editors of Psychosomatic Medicine (2009). Available online at: http://stattips.blogspot.com/2009/08/rescaling-continuous-predictors-in.html
34. Basu S, Michaelsson K, Olofsson H, Johansson S, Melhus H. Association between oxidative stress and bone mineral density. Biochem Biophys Res Commun. (2001) 288:275–9. doi: 10.1006/bbrc.2001.5747
35. Lee KI. Correlations of linear and nonlinear ultrasound parameters with density and microarchitectural parameters in trabecular bone. J Acoust Soc Am. (2013) 134:El381–6. doi: 10.1121/1.4822420
36. Guglielmi G, Adams J, Link TM. Quantitative ultrasound in the assessment of skeletal status. Eur Radiol. (2009) 19:1837–48. doi: 10.1007/s00330-009-1354-1
37. Töyräs J, Nieminen MT, Kröger H, Jurvelin JS. Bone mineral density, ultrasound velocity, and broadband attenuation predict mechanical properties of trabecular bone differently. Bone. (2002) 31:503–7. doi: 10.1016/S8756-3282(02)00843-8
38. Domazetovic V, Marcucci G, Iantomasi T, Brandi ML, Vincenzini MT. Oxidative stress in bone remodeling: role of antioxidants. Clin Cases Miner Bone Metab. (2017) 14:209–16. doi: 10.11138/ccmbm/2017.14.1.209
39. Agidigbi TS, Kim C. Reactive oxygen species in osteoclast differentiation and possible pharmaceutical targets of ROS-mediated osteoclast diseases. Int J Mol Sci. (2019) 20:3576. doi: 10.3390/ijms20143576
40. Callaway DA, Jiang JX. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J Bone Miner Metab. (2015) 33:359–70. doi: 10.1007/s00774-015-0656-4
41. Baek KH, Oh KW, Lee WY, Lee SS, Kim MK, Kwon HS, et al. Association of oxidative stress with postmenopausal osteoporosis and the effects of hydrogen peroxide on osteoclast formation in human bone marrow cell cultures. Calcif Tissue Int. (2010) 87:226–35. doi: 10.1007/s00223-010-9393-9
42. Huang CX, Lv B, Wang Y. Protein phosphatase 2a mediates oxidative stress induced apoptosis in osteoblasts. Mediat Inflamm. (2015) 2015:804260. doi: 10.1155/2015/804260
43. Bai XC, Lu D, Bai J, Zheng H, Ke ZY, Li XM, et al. oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-kappaB. Biochem Biophys Res Commun. (2004) 314:197–207. doi: 10.1016/j.bbrc.2003.12.073
44. NIH NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. (2001) 285:785–95. doi: 10.1001/jama.285.6.785
45. Garnero P. The role of collagen organization on the properties of bone. Calcif Tissue Int. (2015) 97:229–40. doi: 10.1007/s00223-015-9996-2
46. Fonseca H, Moreira-Gonçalves D, Coriolano HJ, Duarte JA. Bone quality: the determinants of bone strength and fragility. Sports Med. (2014) 44:37–53. doi: 10.1007/s40279-013-0100-7
47. Mazahery H, von Hurst PR. Factors affecting 25-hydroxyvitamin D concentration in response to vitamin D supplementation. Nutrients. (2015) 7:5111–42. doi: 10.3390/nu7075111
Keywords: oxidative stress, fluorescent oxidation products, quantitative ultrasound parameters, speed of sound, broadband ultrasound attenuation, middle-aged and elderly adults
Citation: Shen X, Liu Y, Zhao Q, Cheng H, Li B, Vuong AM, Fan Y, Zhang M and Yang S (2023) Association between global biomarker of oxidative stress and quantitative ultrasound parameters in middle-aged and elderly adults: A cross-sectional study. Front. Public Health 10:1032550. doi: 10.3389/fpubh.2022.1032550
Received: 31 August 2022; Accepted: 15 December 2022;
Published: 06 January 2023.
Edited by:
Wenzhen Li, Huazhong University of Science and Technology, ChinaReviewed by:
Rachel Nadif, INSERM U1018 Centre de Recherche en Épidémiologie et Santé des Populations (CESP), FranceShazia Rehman, Pak-Austria Fachhochschule Institute of Applied Sciences and Technology, Pakistan
Copyright © 2023 Shen, Liu, Zhao, Cheng, Li, Vuong, Fan, Zhang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mengmeng Zhang,  zhmm5866@163.com; Shuman Yang,
zhmm5866@163.com; Shuman Yang,  shumanyang@jlu.edu.cn
shumanyang@jlu.edu.cn
 Xue Shen
Xue Shen Yawen Liu
Yawen Liu Qianqian Zhao1
Qianqian Zhao1 Shuman Yang
Shuman Yang