- State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, College of Medicine, The First Affiliated Hospital, Zhejiang University, Hangzhou, China
Background: The viral shedding time (VST) of SARS-CoV-2 mainly determines its transmission and duration of infectiousness. However, it was heterogeneous in the existing studies. Here, we performed a meta-analysis to comprehensively summarize the VST of SARS-CoV-2.
Methods: We searched PubMed, Web of Science, MedRxiv, BioRxiv, CNKI, CSTJ, and Wanfang up to October 25, 2020, for studies that reported VSTs of SARS-CoV-2. Pooled estimates and 95% CIs for the VSTs were calculated using log-transformed data. The VSTs in SARS-CoV-2 infections based on different demographic and clinical characteristics, treatments and specimens were stratified by subgroup analysis.
Results: A total of 35 studies involving 3,385 participants met the inclusion criteria. The pooled mean VST was 16.8 days (95% CI: 14.8–19.4, I2 = 99.56%) in SARS-CoV-2 infections. The VST was significantly longer in symptomatic infections (19.7 days, 95% CI: 17.2–22.7, I2 = 99.34%) than in asymptomatic infections (10.9 days, 95% CI: 8.3–14.3, I2 = 98.89%) (P < 0.05). The VST was 23.2 days (95% CI: 19.0–28.4, I2 = 99.24%) in adults, which was significantly longer than that in children (9.9 days, 95% CI: 8.1–12.2, I2 = 85.74%) (P < 0.05). The VST was significantly longer in persons with chronic diseases (24.2 days, 95% CI: 19.2–30.2, I2 = 84.07%) than in those without chronic diseases (11.5 days, 95% CI: 5.3–25.0, I2 = 82.11%) (P < 0.05). Persons receiving corticosteroid treatment (28.3 days, 95% CI: 25.6–31.2, I2 = 0.00%) had a longer VST than those without corticosteroid treatment (16.2 days, 95% CI: 11.5–22.5, I2 = 92.27%) (P = 0.06). The VST was significantly longer in stool specimens (30.3 days, 95% CI: 23.1–39.2, I2 = 92.09%) than in respiratory tract specimens (17.5 days, 95% CI: 14.9–20.6, I2 = 99.67%) (P < 0.05).
Conclusions: A longer VST was found in symptomatic infections, infected adults, persons with chronic diseases, and stool specimens.
Introduction
Coronaviruses (CoVs), belonging to Nidovirales order, have caused three global outbreaks in the past 20 years. The first epidemic was Severe Acute Respiratory Syndrome (SARS) caused by SARS-CoV-1 in 2003, the second outbreak was Middle East Respiratory Syndrome (MERS) caused by MERS-CoV in 2012, and the third and most recent pandemic was Coronavirus Disease 2019 (COVID-19) caused by SARS-CoV-2 (1, 2). As of February 4, 2021, more than 104 million cases of COVID-19 have been reported with over 2.2 million deaths globally (3). Pulmonary clinical manifestations are the most common clinical presentations of COVID-19, such as fever, cough, shortness of breath, sputum production, respiratory failure and even acute respiratory distress syndrome (ARDS). Diarrhea, loss of smell or taste, and other extra-pulmonary clinical manifestations can also be found in some patients (4–7).
Persons infected with SARS-CoV-2 with long viral shedding times (VSTs) have drawn considerable concern, which put greater challenges and difficulties on epidemic prevention and control (8–11). The VST is an important parameter for judging hospital discharge, discontinuation of quarantine and the effect of antiviral treatment for infectious diseases, which mainly determines disease transmission and the duration of infectiousness (12). However, the characteristics of the VST in SARS-CoV-2 infections have not been well-clarified. Although there have been many studies on the VSTs of SARS-CoV-2, the results across studies so far have been heterogeneous (13, 14). A meta-analysis performed by Muge Cevik found that the mean VST of SARS-CoV-2 in the upper respiratory tract, lower respiratory tract, stool and serum was 17.0, 14.6, 17.2, and 16.6 days, respectively (15). However, a comprehensive summary of VSTs in SARS-CoV-2 infections with different demographic and clinical features is still lacking. Therefore, we performed a meta-analysis to estimate the mean VST in SARS-CoV-2 infections and explore the characteristics of VSTs in SARS-CoV-2 infections based on different demographic features, clinical characteristics, treatments and specimens.
Materials and Methods
Our meta-analysis was strictly conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P) guidelines (16).
The Definition of the VST
The definition of the VST varied among the studies, so a unified definition was made. We defined the VST as the time from illness onset to viral shedding cessation. Illness onset was defined as the first appearance of the symptoms for symptomatic infections and the first positive RT-PCR results for asymptomatic infections. Viral shedding cessation referred to the occurrence of the last positive RT-PCR results or negative RT-PCR results.
Search Strategy and Selection Criteria
We searched PubMed, Web of Science, MedRxiv, BioRxiv, the China National Knowledge Infrastructure Database (CNKI), the China Science and Technology Journal Database (CSTJ), and the Wanfang Database up to October 25, 2020, for studies that reported VSTs of SARS-CoV-2. The details of the search strategy are shown in Supplementary Table 1.
In this systematic review with no study design limit, studies meeting the following inclusion criteria were eligible: (i) SARS-CoV-2 infections were based on positive RT-PCR results; (ii) the VSTs of SARS-CoV-2 infections including sample size, mean and standard deviation (SD) could be obtained directly from the original studies or by calculation; and (iii) the definition of the VST in the original studies was consistent with our definition.
We excluded (i) duplicated data; (ii) case reports and case series with <5 participants due to reporting bias; and (iii) studies without original data (e.g., modeling studies and reviews). Studies presenting VSTs with medians and interquartile ranges (IQRs) or ranges were excluded to reduce the errors caused by data conversion.
Screening, Data Extraction, and Quality Assessment
After removing duplicates, two reviewers (DY and XZ) independently performed the initial screening of titles and abstracts to exclude studies that clearly contained no data for VST of SARS-CoV-2. All retained full-text articles were scrutinized against the eligibility criteria by two independent reviewers (DY and XZ). Nine investigators (DY, XZ, CC, DJ, XL, YZ, CH, YZ, and ZG) participated in the data extraction. And data extraction from each study was performed by three independent investigators. Disagreements and uncertainties were consulted by SY to reach a consensus. The following data were extracted: basic information of the studies (first author, publication time, journal name, sample size), VSTs in SARS-CoV-2 infections based on sex, age (adult and child), infection status (symptomatic infection and asymptomatic infection), disease severity (severe infection and non-severe infection), treatments (corticosteroid treatment and antiviral therapy) and specimens (respiratory tract specimens (RTS), upper respiratory tract specimens (URTS), lower respiratory tract specimens (LRTS), stool and serum). The cutoff point for classifying adults and children was 18 years old. Asymptomatic infections referred to the absence of any clinical symptoms throughout the disease course. Non-severe infections included mild and moderate infections, and severe infections included severe and critical infections. Antiviral drugs included interferon, lopinavir/ritonavir, abidor, ribavirin, chloroquine and hydroxychloroquine. URTS included nasopharyngeal, oropharyngeal and oronasopharyngeal swabs, and LRTS included sputum and bronchoalveolar lavage fluid.
The overall VST of the total participants was extracted to estimate the overall pooled VST. If studies did not report the overall VST of the total participants, the stratified VSTs were extracted to estimate the overall pooled VST. If a study included more than one independent study population, each population was extracted as a separate dataset in the meta-analysis. When the same study reported the VSTs of multiple specimens, the VSTs of the URTS were extracted to estimate the overall pooled VST, and the VSTs of other specimens were displayed in the subgroup analysis. In subgroup analysis, only studies having clear population characteristics were included in the corresponding subgroup, and studies having no clear information or mixed population group were included in the unclassified group.
The scale recommended by the Agency for Healthcare Research and Quality was used to assess the quality of the included studies (17). The scale consists of 11 items, and 1 point is given to each item when the conditions are met. It mainly focuses on information source, inclusion and exclusion criteria, study period, selection of participants, evaluation of subjective outcomes/components, quality assurance, possible confounding variables, handling of missing data, participants' response rates and completeness of data collection. According to the total score, the studies were divided into low-(0–3), medium-(4–7) and high-quality (8–11) groups. EndNote (version X9) was used to manage the articles and citations.
Statistical Analysis
We first extracted the individual VSTs from the published articles and found that the distribution type of the VST was approximately in accordance with the log-normal distribution by using P-P plots (Supplementary Figure 1). Then, we used the method developed by McAloon C (18) to transform the original VST data to make the data obey a normal distribution. We used random-effects model to perform the meta-analysis due to the high heterogeneity. Finally, we used the method developed by McAloon C (18) to back-transform the point estimates and their 95% confidence intervals (CIs). The I-squared (I2) statistic was used to evaluate the heterogeneity among the studies. Meta-regression was used to quantify the sources of heterogeneity and to explore the level of significance between subgroup comparisons. We did not assess publication bias because usual appraisal methods are uninformative when studies in the meta-analysis do not include a test of significance. The data cleaning and analysis were performed using the Microsoft Excel 2016 and R version 3.2.3.
Results
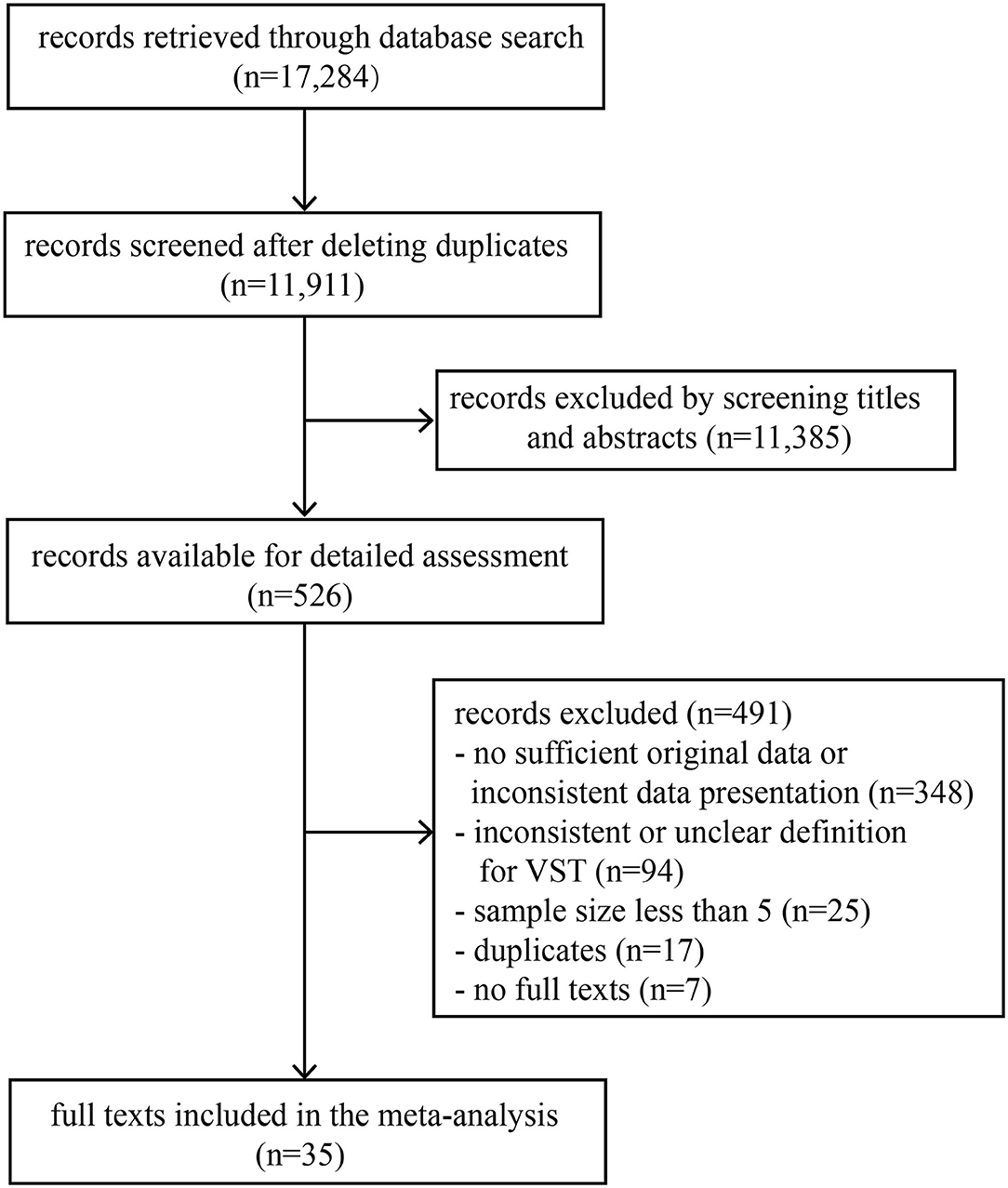
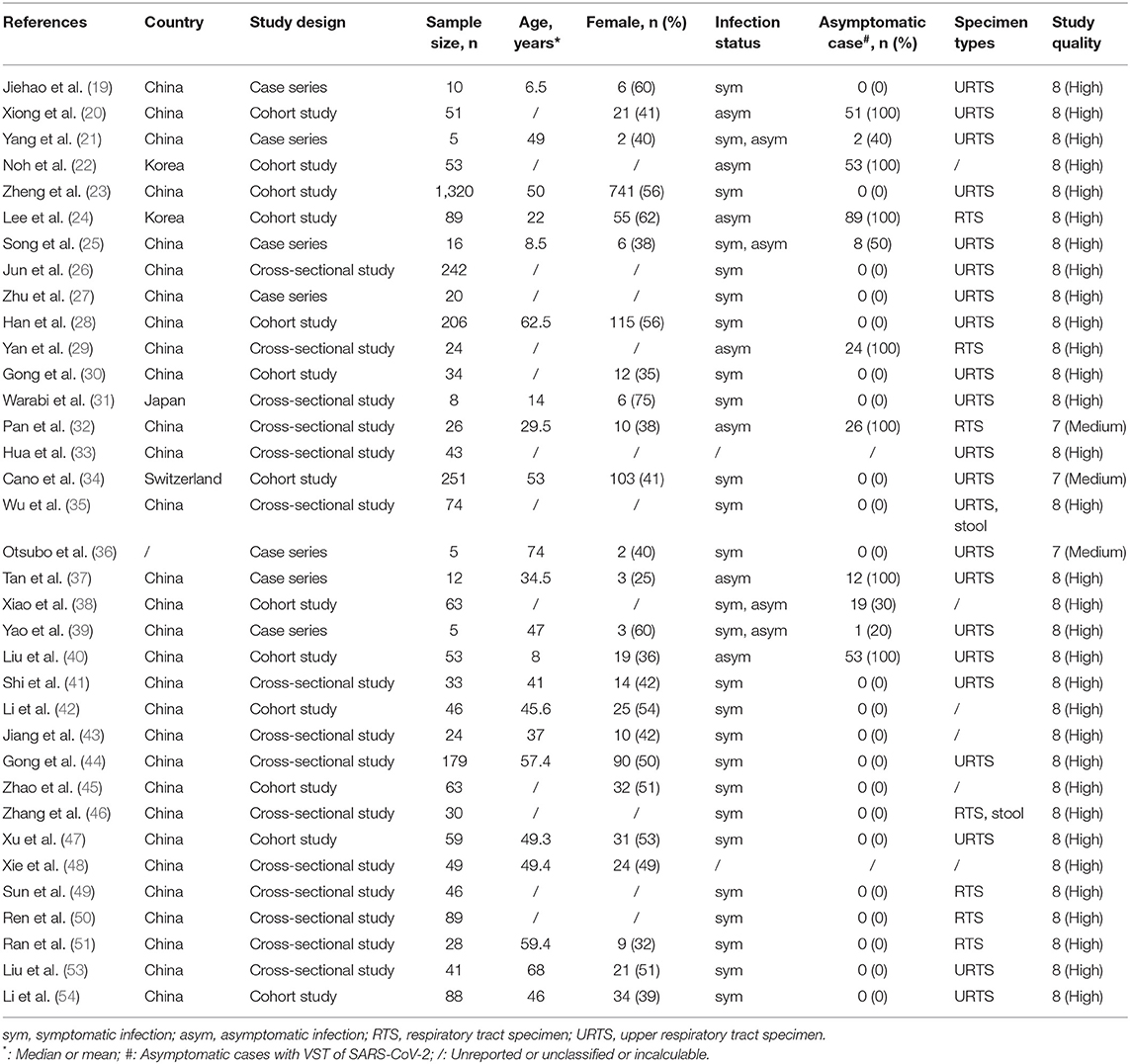
A total of 17,284 records were retrieved through a database search. The titles and abstracts of 11,911 records were screened after deleting duplicates, and then 526 records were selected for full-text review. Finally, 35 full texts met the inclusion criteria (Figure 1). This study included 35 observational studies and involved 3,385 individuals infected with SARS-CoV-2, of which 2,955 were symptomatic infections and 338 were asymptomatic infections (Table 1). According to the scale, 32 studies were of high quality, 3 studies were of medium quality and none were of low quality (Table 1 and Supplementary Table 2).
VSTs in SARS-CoV-2 Infections and Subgroup Results Based on Clinical Characteristics
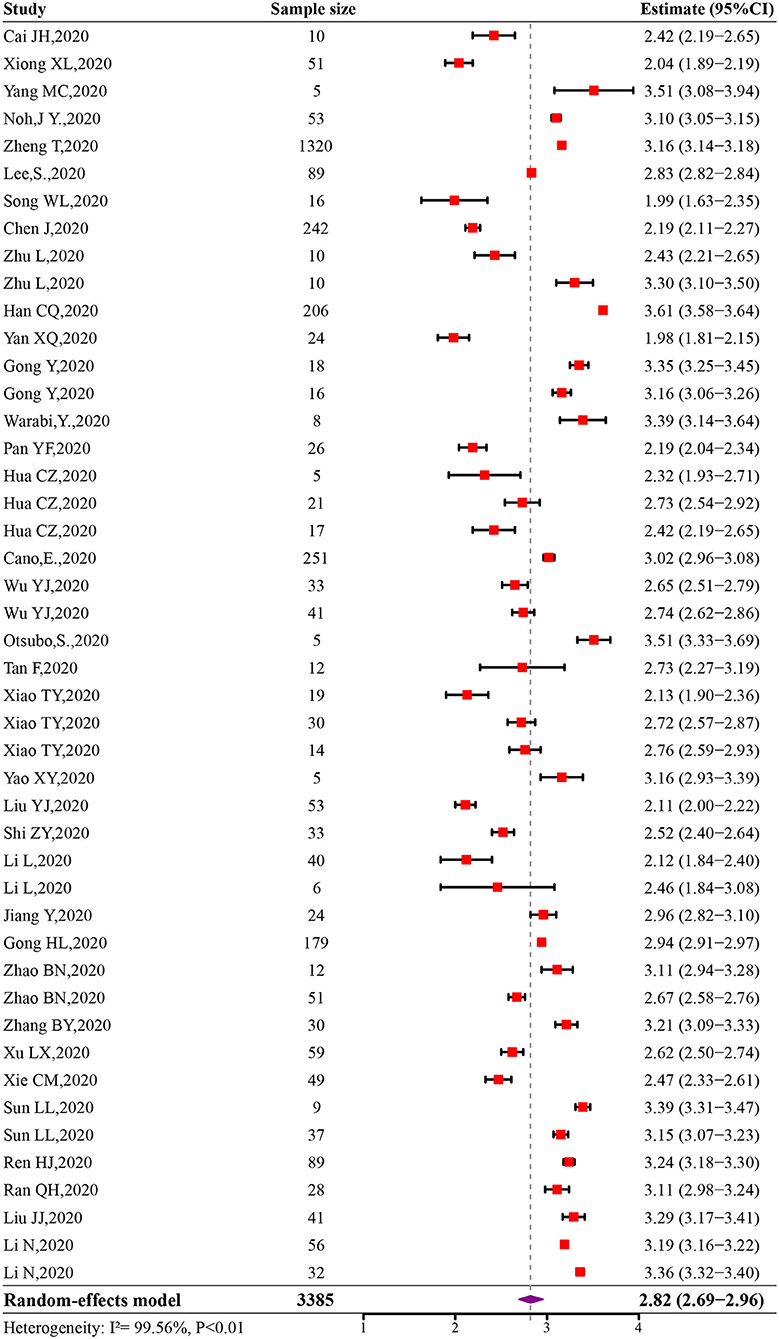
The initial pooled estimate of the log-transformed VST in SARS-CoV-2 infections was 2.82 (95% CI: 2.69–2.96) (Figure 2). The pooled mean VST was 16.8 days (95% CI: 14.8–19.4) in SARS-CoV-2 infections. The mean VST of symptomatic infections was 19.7 days (95% CI: 17.2–22.7), which was significantly longer than that of asymptomatic infections (10.9 days, 95% CI: 8.3–14.3) (P < 0.05). The mean VST was 24.3 days (95% CI: 18.9–31.1) in severe patients and 22.8 days (95% CI: 16.4–32.0) in non-severe patients (Figure 3).
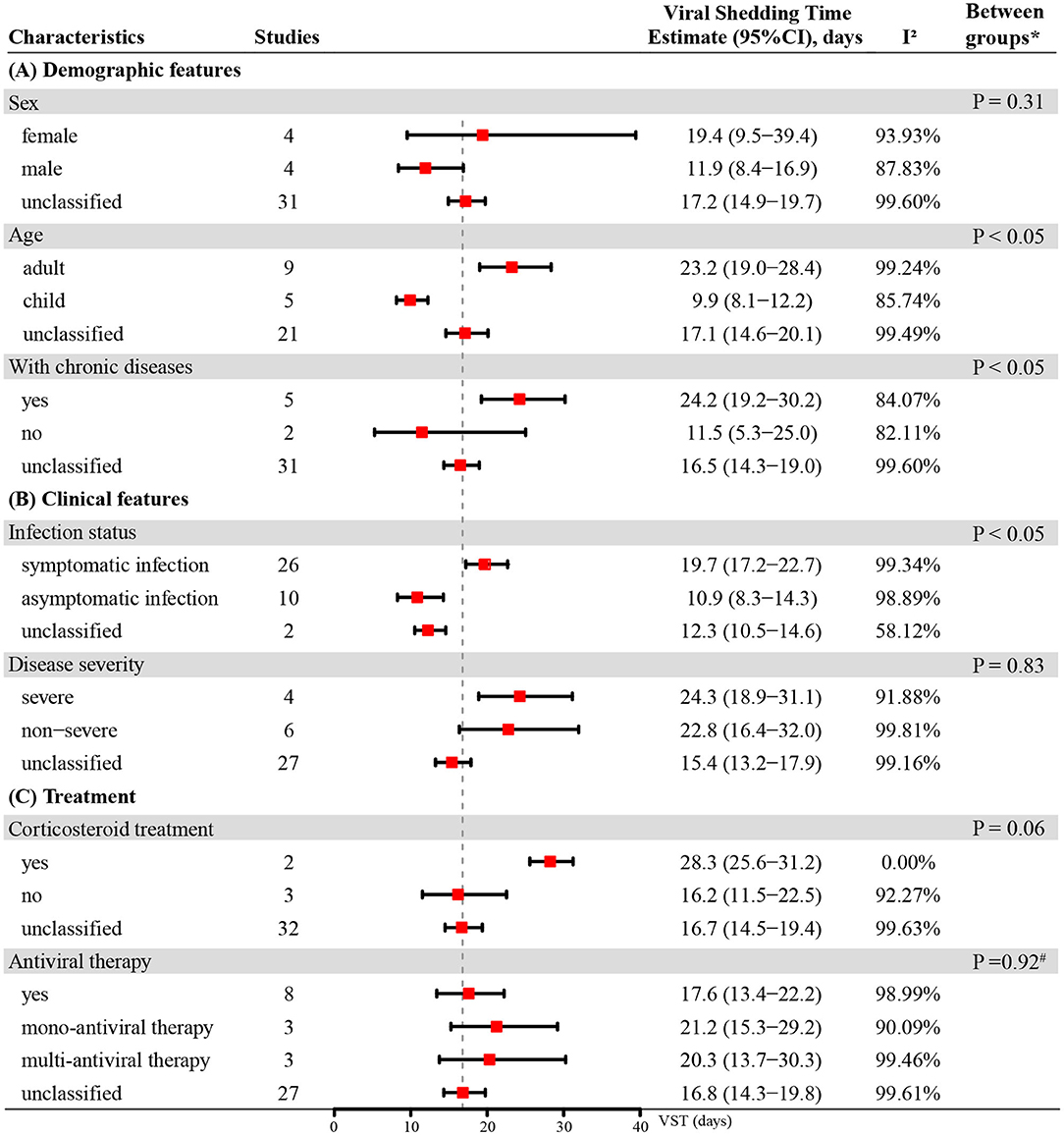
Figure 3. The VST in SARS-CoV-2 infections based on different demographic features, clinical features and treatments. * Unclassified groups were not included in the P-values calculation for the subgroup comparisons. #P-value for comparison between group with antiviral mono-therapy and group with antiviral muti-therapy.
VSTs in SARS-CoV-2 Infections Subgrouped by Demographic Features
The mean VST was 19.4 days (95% CI: 9.5–39.4) in females and 11.9 days (95% CI: 8.4–16.9) in males. The VST was significantly shorter in the infected children (9.9 days, 95% CI: 8.1–12.2) than in the infected adults (23.2 days, 95% CI: 19.0–28.4) (P < 0.05). The VST of persons with chronic diseases was 24.2 days (95% CI: 19.2–30.2), which was significantly longer than that of persons without chronic diseases (11.5 days, 95% CI: 5.3–25.0) (P < 0.05) (Figure 3).
VSTs in SARS-CoV-2 Infections Subgrouped by Treatments
In persons receiving corticosteroid treatment, the VST was 28.3 days (95% CI: 25.6–31.2), which was longer than that in those without corticosteroid treatment (16.2 days, 95% CI: 11.5–22.5). However, there was no statistically significant difference between them (P = 0.06). The VST was 17.6 days (95% CI: 13.4–22.2) in persons receiving antiviral therapy, 21.2 days (95% CI: 15.3–29.2) in persons receiving mono-antiviral therapy and 20.3 days (95% CI: 13.7–30.3) in persons receiving multi-antiviral therapy (Figure 3). Only one study reported the VSTs of 5 patients without antiviral therapy, and the result was 11.2 ± 5.2 days (33).
VSTs in SARS-CoV-2 Infections Subgrouped by Different Specimens
Most studies (63%) reported the VSTs in the URTS. Among the different specimens, the mean VST was 17.5 days (95% CI: 14.9–20.6) in the RTS and 17.5 days (95% CI: 14.6–21.0) in the URTS. Compared with the RTS, a longer VST was found in the stool specimens (30.3 days, 95% CI: 23.1–39.2) (P < 0.05) (Figure 4). No included study reported VSTs in LRTS or serum specimens.
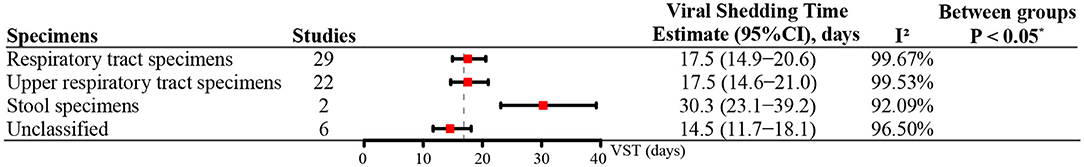
Figure 4. The VST in SARS-CoV-2 infections based on different specimens. *P-value for comparison between respiratory tract specimens and stool specimens.
Meta-Regression for Heterogeneity
The univariate meta-regression model indicated that the mean age (R2 = 35.28%, P < 0.05) and the proportion of the asymptomatic cases (R2 = 22.64%, P < 0.05) could partly explain the overall heterogeneity. By introducing these two variables into the multivariate meta-regression model, nearly half of the heterogeneity could be explained (R2= 44.18%, P < 0.05) (Supplementary Table 3).
Discussion
We performed a meta-analysis to clarify the characteristics of VSTs in SARS-CoV-2 infections, which was important for determining hospital discharge, discontinuation of quarantine and the effect of antiviral treatment for COVID-19. Compared with the meta-analysis conducted by Muge Cevik (15), our study not only estimated the VSTs in different specimens but also summarized the VSTs in SARS-CoV-2 infections based on different demographic features, clinical characteristics and treatments.
Previous studies have shown that the basic reproduction number (R0) of SARS-CoV-2 is between 2 and 6.7, which indicates that SARS-CoV-2 is more infectious than SARS-CoV-1 and MERS-CoV (55–57). In our study, we found that the mean VST of SARS-CoV-2 was 16.8 days (95% CI: 14.8–19.4), which was between that of SARS-CoV-1 (21.0 days) and MERS-CoV (13.2 days) (58, 59). In addition to the VST, the viral load released is also important to evaluate the transmissibility. Some studies have found that the viral load of SARS-CoV-2 is highest during the 1st week after symptom onset and subsequently declines with time (60–62). Based on the above analysis, from the perspective of epidemic prevention and control, strict precautions should be taken throughout the disease course, especially within 1 week after the onset of the disease.
The duration of viral shedding is mainly related to the host immune status (63). Persons with chronic diseases always have relatively low immunity, which might lead to longer viral shedding. In our study, we found that the VST of symptomatic infections was longer than that of asymptomatic infections. One reason is that virus clearance in asymptomatic individuals is indeed faster than that in symptomatic cases (38, 52). Another reason was that the VST for asymptomatic infections was calculated from the first positive PCR results and depended mainly on close contact tracking investigations. These individuals might have begun viral shedding before the first positive PCR results, and were ignored due to the absence of clinical features. A higher proportion of asymptomatic infections and milder clinical symptoms were found in infected children compared with infected adults (64, 65), which might explain the shorter VST of children.
The VST is an important parameter for evaluating the effect of antiviral treatment for infectious diseases. Until now, there have been no specific antiviral drugs for COVID-19, and inhibiting the cytokine storm has been an important treatment for patients with severe COVID-19. Corticosteroids are used because of their rapid, powerful anti-inflammatory effects. In our study, we found that the patients who received corticosteroid treatment had longer VSTs, although no statistically significant difference was found. This phenomenon was also found in severe SARS and MERS, where high-dose corticosteroids could cause prolonged viral clearance, secondary infection and long-term complications (66). Although corticosteroids can inhibit lung inflammation and alleviate possible immune-mediated pulmonary damage, it can also inhibit the systemic immune response dominated by T cell response, resulting in the delayed virus clearance (67). This finding alerted us that high-dose corticosteroids might prolong VSTs in SARS-CoV-2 infections and that appropriate doses of corticosteroids should be used after weighing the advantages and disadvantages according to the patients' condition.
The VST is also an important parameter for determining hospital discharge and discontinuation of quarantine. Two consecutive negative PCR results of RTS are one of the current criteria for hospital discharge or discontinuation of quarantine in China (68). The overexpression of ACE-2 in the gastrointestinal (GI) epithelial cells suggested the replication and shedding of SARS-CoV-2 in GI tract (69). Similar to SARS-CoV-1 (59), the VST of SARS-CoV-2 in stool specimens was longer than that in RTS. One study suggested that the VST in stool specimens could be prolonged by 5 weeks after SARS-CoV-2 had turned negative in RTS (35). Given that, the negative PCR results in RTS might not guarantee that patients no longer shed virus. Recently, several incidents of cold chain food polluted by SARS-CoV-2 have caused widespread concern by indicating that the virus could indeed infect individuals by polluting the environment. Considering the potential risk of oral-fecal transmission (70) and the long VST in stool specimens, more comprehensive protective measures should be taken for high-risk groups of oral-fecal transmission, such as GI endoscopy staff (2, 71), and stool or anal swabs collection and testing staff.
Our results might provide scientific support for the formulation of antiviral treatment and criteria for hospital discharge and discontinuation of quarantine for COVID-19, and help identify which patients need more attention and more effective preventive measures. Based on the mean VST of SARS-CoV-2 infections, hospitals could estimate the number of individuals with COVID-19 who can be admitted in a period of time, and reasonably allocate medical resources, such as the number of beds and medical staff.
This study has several limitations. The mean age, disease severity, treatment regimens, underlying diseases and infection status of individuals infected with SARS-CoV-2 varied in the included studies, which might cause high statistical heterogeneity. In the multivariate meta-regression model, nearly half of the heterogeneity could be explained by mean age and the proportion of the asymptomatic cases (R2= 44.18%, P < 0.05). In some subgroup analyses, the number of included studies was small and most were case series with limited sample sizes, which might make the effect size of some outcomes insufficient. For example, the pooled mean VST in the stool specimens was based on estimates obtained in only two studies. More studies on the VST of SARS-CoV-2 are needed to provide further evidence. It would be better to incorporate as many studies as possible to obtain sufficient subgroup data and to ensure the homogeneity of the studies. Furthermore, the day of symptom onset for symptomatic infections depended on subjective memories and the day of the first positive RT-PCR results for asymptomatic infections relied mainly on close contact tracking investigations. If the individuals' recall was incorrect or close contact tracking investigations were not timely, these would cause the obtained VSTs to deviate from the real values.
Conclusions
This study provided a comprehensive overview of VSTs in SARS-CoV-2 infections, which was important for determining hospital discharge, discontinuation of quarantine and the effect of antiviral treatment for COVID-19. The pooled mean VST was 16.8 days (95% CI: 14.8–19.4) in SARS-CoV-2 infections. Due to the high infectivity of SARS-CoV-2, strict precautions should be taken to reduce the risk of disease transmission, especially for adults, persons with chronic diseases, symptomatic infections and persons with positive RT-PCR results in stool specimens, in whom longer VSTs were found. Given that high-dose corticosteroids could alleviate possible immune-mediated pulmonary damage but might prolong VSTs in SARS-CoV-2 infections, corticosteroids should be used with caution after analyzing the risk of prolonged VST with reducing the disease severity.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
SY, LJL, and JW designed the study and revised the manuscript. DY and XZ independently performed the literature identification and uncertainties were consulted by SY to reach a consensus. DY, XZ, CC, DJ, XL, YZ, CH, YZ, and ZG extracted data. CD, LC, LL, and XF rechecked the data. DY carried out the data analysis. DY and XZ interpreted data and wrote the manuscript. All authors have read and approved the final version of the manuscript for submission.
Funding
This study was supported by grants from the National Natural Science Foundation of China (Grant Nos: 81672005, U1611264, and 81001271), the Mega-Project of National Science and Technology for the 12th and 13th Five-Year Plan of China (Grant Nos: 2018ZX10715-014-002 and 2014ZX10004008).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2021.652842/full#supplementary-material
References
1. Mann R, Perisetti A, Gajendran M, Gandhi Z, Umapathy C, Goyal H. Clinical characteristics, diagnosis, and treatment of major coronavirus outbreaks. Front Med. (2020) 7:766. doi: 10.3389/fmed.2020.581521
2. Perisetti A, Gajendran M, Boregowda U, Bansal P, Goyal H. COVID-19 and gastrointestinal endoscopies: current insights and emergent strategies. Dig Endosc. (2020) 32:715–22. doi: 10.1111/den.13693
3. World Coronavirus Death. Coronavirus Death Toll and Trends –Worldometer. (2020). Available online at: https://www.worldometers.info/coronavirus (accessed February 4, 2021).
4. Perisetti A, Gajendran M, Mann R, Elhanafi S, Goyal H. COVID-19 extrapulmonary illness - special gastrointestinal and hepatic considerations. Dis Mon. (2020) 66:101064. doi: 10.1016/j.disamonth.2020.101064
5. Johnson KD, Harris C, Cain JK, Hummer C, Goyal H, Perisetti A. Pulmonary and extra-pulmonary clinical manifestations of COVID-19. Front Med. (2020) 7:526. doi: 10.3389/fmed.2020.00526
6. Aziz M, Goyal H, Haghbin H, Lee-Smith WM, Gajendran M, Perisetti A. The association of “loss of smell” to COVID-19: a systematic review and meta-analysis. Am J Med Sci. (2020) 361:216–25. doi: 10.1016/j.amjms.2020.09.017
7. Goyal H, Sachdeva S, Perisetti A, Mann R, Inamdar S, Tharian B. Hyperlipasemia and potential pancreatic injury patterns in COVID-19: a marker of severity or innocent bystander? Gastroenterology. (2020) 160:946–8.e2. doi: 10.1053/j.gastro.2020.10.037
8. Tan L, Kang X, Zhang B, Zheng S, Liu B, Yu T, et al. A special case of COVID-19 with long duration of viral shedding for 49 days. medRxiv. (2020). doi: 10.1101/2020.03.22.20040071
9. Yang JR, Deng DT, Wu N, Yang B, Li HJ, Pan XB. Persistent viral RNA positivity during the recovery period of a patient with SARS-CoV-2 infection. J Med Virol. (2020) 92:1681–3. doi: 10.1002/jmv.25940
10. Li Q, Zheng XS, Shen XR, Si HR, Wang X, Wang Q, et al. Prolonged shedding of severe acute respiratory syndrome coronavirus 2 in patients with COVID-19. Emerg Microbes Infect. (2020) 9:2571–7. doi: 10.1080/22221751.2020.1852058
11. Xing YH, Ni W, Wu Q, Li WJ, Li GJ, Wang WD, et al. Prolonged viral shedding in feces of pediatric patients with coronavirus disease 2019. J Microbiol Immunol Infect. (2020) 53:473–80. doi: 10.1016/j.jmii.2020.03.021
12. de Sousa R, Reusken C, Koopmans M. MERS coronavirus: data gaps for laboratory preparedness. J Clin Virol. (2014) 59:4–11. doi: 10.1016/j.jcv.2013.10.030
13. Shi D, Wu W, Wang Q, Xu K, Xie J, Wu J, et al. Clinical characteristics and factors associated with long-term viral excretion in patients with severe acute respiratory syndrome coronavirus 2 infection: a single-center 28-day study. J Infect Dis. (2020) 222:910–8. doi: 10.1093/infdis/jiaa388
14. Shen Y, Zheng F, Sun D, Ling Y, Chen J, Li F, et al. Epidemiology and clinical course of COVID-19 in Shanghai, China. Emerg Microbes Infect. (2020) 9:1537–45. doi: 10.1080/22221751.2020.1787103
15. Cevik M, Tate M, Lloyd O, Maraolo AE, Schafers J, Ho A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe. (2020) 2:e13–22. doi: 10.1016/S2666-5247(20)30172-5
16. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. doi: 10.7326/m14-2385
17. Meyers D. Introduction from the agency for healthcare research and quality. J Am Board Fam Med. (2012) 25(Suppl 1):S1. doi: 10.3122/jabfm.2012.02.120023
18. McAloon C, Collins Á, Hunt K, Barber A, Byrne AW, Butler F, et al. Incubation period of COVID-19: a rapid systematic review and meta-analysis of observational research. BMJ Open. (2020) 10:e039652. doi: 10.1136/bmjopen-2020-039652
19. Jiehao C, Jin X, Daojiong L, Zhi Y, Lei X, Zhenghai Q, et al. A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin infect Dis. (2020) 71:1547–51. doi: 10.1093/cid/ciaa198
20. Xiong X, Chua GT, Chi S, Kwan MYW, Sang Wong WH, Zhou A, et al. A comparison between Chinese children infected with coronavirus disease-2019 and with severe acute respiratory syndrome 2003. J Pediatr. (2020) 224:30–6. doi: 10.1016/j.jpeds.2020.06.041
21. Yang MC, Hung PP, Wu YK, Peng MY, Chao YC, Su WL. A three-generation family cluster with COVID-19 infection: should quarantine be prolonged? Public Health. (2020) 185:31–3. doi: 10.1016/j.puhe.2020.05.043
22. Noh JY, Yoon JG, Seong H, Choi WS, Sohn JW, Cheong HJ, et al. Asymptomatic infection and atypical manifestations of COVID-19: Comparison of viral shedding duration. J Infect. (2020) 81:816–46. doi: 10.1016/j.jinf.2020.05.035
23. Zheng T, Yang C, Wang H-Y, Chen X, Yu L, Wu Z-L, et al. Clinical characteristics and outcomes of COVID-19 patients with gastrointestinal symptoms admitted to Jianghan Fangcang Shelter Hospital in Wuhan, China. J Med Virol. (2020) 92:2735–41. doi: 10.1002/jmv.26146
24. Lee S, Kim T, Lee E, Lee C, Kim H, Rhee H, et al. Clinical course and molecular viral shedding among asymptomatic and symptomatic patients with SARS-CoV-2 infection in a community treatment center in the Republic of Korea. JAMA Inter Med. (2020) 180:1–6. doi: 10.1001/jamainternmed.2020.3862
25. Song W, Li J, Zou N, Guan W, Pan J, Xu W. Clinical features of pediatric patients with coronavirus disease (COVID-19). J Clin Virol. (2020) 127:104377. doi: 10.1016/j.jcv.2020.104377
26. Jun C, Tangkai Q, Li L, Yun L, Zhiping Q, Tao L, et al. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. (2020) 80:e1–6. doi: 10.1016/j.jinf.2020.03.004
27. Zhu L, Gong N, Liu B, Lu X, Chen D, Chen S, et al. Coronavirus disease 2019 pneumonia in immunosuppressed renal transplant recipients: a summary of 10 confirmed cases in Wuhan, China. Euro Urol. (2020) 77:748–54. doi: 10.1016/j.eururo.2020.03.039
28. Han C, Duan C, Zhang S, Spiegel B, Shi H, Wang W, et al. Digestive symptoms in COVID-19 patients with mild disease severity: clinical presentation, stool viral RNA testing, and outcomes. Am J Gastroenterol. (2020) 115:916–23. doi: 10.14309/ajg.0000000000000664
29. Yan X, Han X, Fan Y, Fang Z, Long D, Zhu Y. Duration of SARS-CoV-2 viral RNA in asymptomatic carriers. Crit Care. (2020) 24:245. doi: 10.1186/s13054-020-02952-0
30. Gong Y, Guan L, Jin Z, Chen S, Xiang G, Gao B. Effects of methylprednisolone use on viral genomic nucleic acid negative conversion and CT imaging lesion absorption in COVID-19 patients under 50 years old. J Med Virol. (2020) 92:2551–5. doi: 10.1002/jmv.26052
31. Warabi Y, Tobisawa S, Kawazoe T, Murayama A, Norioka R, Morishima R, et al. Effects of oral care on prolonged viral shedding in coronavirus disease 2019 (COVID-19). Special Care Dentistr. (2020) 40:470–4. doi: 10.1111/scd.12498
32. Pan Y, Yu X, Du X, Li Q, Li X, Qin T, et al. Epidemiological and clinical characteristics of 26 asymptomatic severe acute respiratory syndrome coronavirus 2 carriers. J Infect Dis. (2020) 221:1940–7. doi: 10.1093/infdis/jiaa205
33. Hua CZ, Miao ZP, Zheng JS, Huang Q, Sun QF, Lu HP, et al. Epidemiological features and viral shedding in children with SARS-CoV-2 infection. J Med Virol. (2020) 92:2804–12. doi: 10.1002/jmv.26180
34. Cano E, Corsini Campioli C, O'Horo JC. Nasopharyngeal SARS-CoV-2 viral RNA shedding in patients with diabetes mellitus. Endocrine. (2020) 71:26–7. doi: 10.1007/s12020-020-02516-w
35. Wu Y, Guo C, Tang L, Hong Z, Zhou J, Dong X, et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. (2020) 5:434–5. doi: 10.1016/s2468-1253(20)30083-2
36. Otsubo S, Aoyama Y, Kinoshita K, Goto T, Otsubo Y, Kamano D, et al. Prolonged shedding of SARS-CoV-2 in COVID-19 infected hemodialysis patients. Therap Apher Dial. (2020). doi: 10.1111/1744-9987.13566
37. Tan F, Wang K, Liu J, Liu D, Luo J, Zhou R. Viral transmission and clinical features in asymptomatic carriers of SARS-CoV-2 in Wuhan, China. Front Med. (2020) 7:547. doi: 10.3389/fmed.2020.00547
38. Xiao T, Wang Y, Yuan J, Ye H, Wei L, Liao X, et al. Early viral clearance and antibody kinetics of COVID-19 among asymptomatic carriers. medRxiv. (2020) 2020.04.28.20083139. doi: 10.1101/2020.04.28.20083139
39. Yao X, Zhao X, Wang M, Xu W, Bai H, Ai X. Familial clustering infection caused by asymptomatic COVID-19 (in Chinese). Int J Respi. (2020) 40:982–7. doi: 10.3760/cma.j.cn131368-20200318-00175
40. Liu YJ, Chen P, Liu ZS, Li Y, Du H, Xu JL. Clinical features of asymptomatic or subclinical COVID-19 in children (in Chinese). Zhongguo Dang dai er ke za zhi. (2020) 22:578–82. doi: 10.7499/j.issn.1008-8830.2004088
41. Shi Z, He Y, Yang M, Duan M, Wang Y, Lai M, et al. Influencing factors of negative conversion time of viral nucleic acid of nasopharyngeal swabs in patients with novel coronavirus infected pneumonia (in Chinese). Pract J Clin Med. (2020) 17:45–8. Available online at: http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=syyylczz202002015
42. Li l, Yang H, Gou C, Wang X, Luo X, Zhang J, et al. Traditional Chinese medicine characteristics of 46 patients with COVID-19 (in Chinese). J Capital Med Univ. (2020) 41:155–60. doi: 10.3969/j.issn.1006-7795.2020.02.002
43. Jiang Y, Wang H, Si G, Deng L, Ou C, Rong X. Clinical ananlysis of corona virus disease 2019: a report of 24 cases (in Chinese). J Chongqing Med Univ. (2020) 45:968–70. doi: 10.13406/j.cnki.cyxb.002453
44. Gong H, Huang H, Zhou X, Luo S, Zhang Z, Zhang S, et al. Related factors of the conversion time of virus nucleic acid turning negative in patients with coronavirus disease 2019 and its effect on prognosis (in Chinese). Herald Med. (2020) 39:811–4. doi: 10.3870/j.issn.1004-0781.2020.06.015
45. Zhao B, Liu D, Liu Y, Lan L, Du Q, Chen H, et al. Differences in laboratory indicators and prognosis between COVID-19 patients with or without diabetes mellitus (in Chinese). Sichuan Med J. (2020) 41:913–7. doi: 10.16252/j.cnki.issn1004-0501-2020.09.005
46. Zhang B, Zhang Z, Li Z, Chi Q, Zheng J, Dai X. Retrospective analysis of viral nucleic acid test results of 116 fecal samples from COVID-19 patients (in Chinese). Jinagsu Med J. (2020) 46:571–2. doi: 10.19460/j.cnki.0253-3685.2020.06.009
47. Xu L, Peng P, Li S, Wu P, Han J, Mo X. Clinical characteristics of 59 cases of COVID-19 in Guangzhou (in Chinese). China Trop Med. (2020) 20:871–4. doi: 10.13604/j.cnki.46-1064/r.2020.09.17
48. Xie C, Qin P, Zeng X, Deng H, Cheng S, Xie Y. Dynamic changes of IgM and IgG in confirmed COVID-19 cases: detection results analysis (in Chinese). Chin J Publ Heal. (2020) 36:1396–8. doi: 10.11847/zgggws1130146
49. Sun L, Li X, Li S, Niu C, Pan S, Wang Q, et al. Nucleic acid detection analysis of 71 cases of COVID-19 with biological samples of diferent disease course(in Chinese). China Trop Med. (2020) 20:763–6. doi: 10.13604/j.cnki.46-1064/r.2020.08.16
50. Ren H, Lei H, Sun G, Li H, Bai J, Tang L. Clinical characteristics of 2019 novel coronavirus pneumonia in a mobile cabin hospital in Wuhan: analysis of 94 cases (in Chinese). Chinese J Hygiene Rescue. (2020) 6:209–13. doi: 10.3877/cma.j.issn.2095-9133.2020.04.003
51. Ran Q, Chen H, Zhang L, Zhong Y. Analysis of Factors influencing viral shedding time in severe and critical COVID-19 patients (in Chinese). Adv Clin Med. (2020) 10:1717–24. doi: 10.12677/acm.2020.108258
52. Lu Y, Li Y, Deng W, Liu M, He Y, Huang L, et al. Symptomatic infection is associated with prolonged duration of viral shedding in mild coronavirus disease 2019: a retrospective study of 110 children in Wuhan. Pediatr Infect Dis J. (2020) 39:e95–9. doi: 10.1097/inf.0000000000002729
53. Liu J, Liu X, Yang M, Xiao X, Cen C. Influencing factors for positive duration of viral nucleic acid in patients with severe coronavirus disease 2019 (in Chinese). Acad J Second Military Med Univ. (2020) 41:966–9. doi: 10.16781/j.0258-879x.2020.09.0966
54. Li N, Xie T, Wei X, Yi S, Cao Y, Jiang S, et al. Chloroquine phosphate accelerates the conversion of nucleic acid to negative in 88 common COVID? 19 patients (in Chinese). J Pract Med. (2020) 36:2759–62. doi: 10.3969/j.issn.1006-5725.2020.20.003
55. Tang B, Wang X, Li Q, Bragazzi NL, Tang S, Xiao Y, et al. Estimation of the transmission risk of the 2019-nCoV and its implication for public health interventions. J Clin Med. (2020) 9:462. doi: 10.3390/jcm9020462
56. Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. (2020) 395:689–97. doi: 10.1016/s0140-6736(20)30260-9
57. Zhao S, Lin Q, Ran J, Musa SS, Yang G, Wang W, et al. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: a data-driven analysis in the early phase of the outbreak. Int J Infect Dis. (2020) 92:214–7. doi: 10.1016/j.ijid.2020.01.050
58. Al-Jasser FS, Nouh RM, Youssef RM. Epidemiology and predictors of survival of MERS-CoV infections in Riyadh region, 2014-2015. J Infect Public Health. (2019) 12:171–7. doi: 10.1016/j.jiph.2018.09.008
59. Liu W, Tang F, Fontanet A, Zhan L, Zhao QM, Zhang PH, et al. Long-term SARS coronavirus excretion from patient cohort, China. Emerg Infect Dis. (2004) 10:1841–3. doi: 10.3201/eid1010.040297
60. He X, Lau EHY, Wu P, Deng X, Wang J, Hao X, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. (2020) 26:672–5. doi: 10.1038/s41591-020-0869-5
61. To KK, Tsang OT, Leung WS, Tam AR, Wu TC, Lung DC, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. (2020) 20:565–74. doi: 10.1016/s1473-3099(20)30196-1
62. Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. (2020) 581:465–9. doi: 10.1038/s41586-020-2196-x
63. Hao S, Lian J, Lu Y, Jia H, Hu J, Yu G, et al. Decreased B cells on admission associated with prolonged Viral RNA shedding from the respiratory tract in coronavirus disease 2019: a case-control study. J Infect Dis. (2020) 222:367–71. doi: 10.1093/infdis/jiaa311
64. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the chinese center for disease control and prevention. JAMA. (2020) 323:1239–42. doi: 10.1001/jama.2020.2648
65. Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, et al. SARS-CoV-2 infection in children. N Engl J Med. (2020) 382:1663–5. doi: 10.1056/NEJMc2005073
66. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. (2020) 395:473–5. doi: 10.1016/s0140-6736(20)30317-2
67. Lee N, Allen Chan KC, Hui DS, Ng EK, Wu A, Chiu RW, et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. (2004) 31:304–9. doi: 10.1016/j.jcv.2004.07.006
68. National Health Commission of the People's Republic of China. Diagnosis and Treatment for COVID-19 (Trial Version 8). (2020). Available online at: http://www.nhc.gov.cn (accessed November 16, 2020).
69. Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS Coronavirus. J Virol. (2020) 94:e00127-20. doi: 10.1128/jvi.00127-20
70. Islam MS, Rahman KM, Sun Y, Qureshi MO, Abdi I, Chughtai AA, et al. Current knowledge of COVID-19 and infection prevention and control strategies in healthcare settings: a global analysis. Infect Control Hosp Epidemiol. (2020) 41:1196–206. doi: 10.1017/ice.2020.237
Keywords: viral shedding time, SARS- CoV-2, COVID-19, systematic review, meta-analysis
Citation: Yan D, Zhang X, Chen C, Jiang D, Liu X, Zhou Y, Huang C, Zhou Y, Guan Z, Ding C, Chen L, Lan L, Fu X, Wu J, Li L and Yang S (2021) Characteristics of Viral Shedding Time in SARS-CoV-2 Infections: A Systematic Review and Meta-Analysis. Front. Public Health 9:652842. doi: 10.3389/fpubh.2021.652842
Received: 13 January 2021; Accepted: 22 February 2021;
Published: 19 March 2021.
Edited by:
Walid Alali, Kuwait University, KuwaitReviewed by:
Rami H. Al-Rifai, United Arab Emirates University, United Arab EmiratesAbhilash Perisetti, University of Arkansas for Medical Sciences, United States
Copyright © 2021 Yan, Zhang, Chen, Jiang, Liu, Zhou, Huang, Zhou, Guan, Ding, Chen, Lan, Fu, Wu, Li and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shigui Yang, yangshigui@zju.edu.cn; Lanjuan Li, ljli@zju.edu.cn; Jie Wu, 15955118479@163.com
†These authors have contributed equally to this work and share first authorship
 Danying Yan†
Danying Yan† Cheng Ding
Cheng Ding Jie Wu
Jie Wu Lanjuan Li
Lanjuan Li Shigui Yang
Shigui Yang