- 1School of Psychology and Public Health, La Trobe University, Melbourne, VIC, Australia
- 2Public Health Genomics, School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia
Public involvement in research occurs when the public, patients, or research participants are actively contributing to the research process. Public involvement has been acknowledged as a key priority for prominent human genomics research initiatives in many different countries. However, to date, there has been no detailed analysis or review of the features, methods, and impacts of public involvement occurring in human genomics research projects worldwide. Here, we review the reported public involvement in 96 human genomics projects (initiatives), based on a database of initiatives hosted by the Global Alliance for Genomics and Health, according to information reported on public domain websites. To conduct the scoping review, we applied a structured categorization of criteria to all information extracted from the search. We found that only a third of all initiatives reported public involvement in any capacity (32/96, 33%). In those reporting public involvement, we found considerable variation in both the methods and tasks of involvement. Some noteworthy initiatives reported diverse and comprehensive ways of involving the public, occurring through different stages of the research project cycle. Three notable initiatives reported a total of eight distinct impacts as a result of involving people. Our findings suggest there would be intrinsic value in having more public involvement occur in human genomics research worldwide. We also suggest that more systematic ways of reporting and evaluating involvement would be highly beneficial, to help develop best practices.
Introduction
In human genomics, there is a growing need to increase involvement of the public in research and policy development. This has been identified as a crucial aspect of responsible research practice (1, 2). The concept of “public involvement” in research is defined as research that is carried out “with” people rather than “on” them (3). Public involvement can also be defined as when the public, patients or research participants actively contribute to the research or policy development process (4).
The number of people involved in genomics research is predicted to grow substantially in coming years (5, 6). By 2025, it is estimated that nearly 2 billion people worldwide will have had their DNA sequenced, creating a global imperative for responsible and effective public involvement in research (7). Many high-profile genomics research initiatives have already made public statements about the importance of involving people, with some governments positioning public involvement as a democratic right (8–10). For example, in the report “Generation Genome,” the UK's Chief Medical Officer suggested that shaping the future of genomic research requires the “active involvement of many stakeholders including patients, health professionals, researchers, policymakers, and wider society,” with a “key role for public engagement and involvement” (10).
The benefits of involving the public in research are wide-ranging. They include improving trust and public influence over research (1, 7, 8); ensuring that research is conducted in an ethical, accessible and transparent manner; and ensuring that research reflects the balance and diversity of priorities within populations (11, 12). However, with the growing interest and importance of large-scale human genomics initiatives worldwide, there has been limited research into how the public are currently being involved. There has also been no structured assessment of the resulting impacts and benefits, including genomics initiatives that have involved the public.
While involving the public in other types of health and medical research has been the subject of previous systematic reviews (13–15), comparable reviews have not been published in human genomics. Many of the existing reviews on other areas of medical research conclude that reports of involvement activities are inconsistent or under-reported (15–19) and that the precise ways in which people are involved in medical research are not well-reported, including any impacts from involving people (7, 14, 16).
Our review provides a summary of reported public involvement in 96 global human genomics projects, listed on a database managed by the Global Alliance for Genomics and Health (GA4GH), a recently formed international organization seeking to enable responsible genomics data sharing within a human rights framework (20). The list provides a representation of the current landscape of human genomics research worldwide, and a snapshot of contemporary practice with regards to public involvement in human genomics research.
This scoping review provides a new perspective by exploring how these genomics initiatives have conducted and reported public involvement to date, including any impacts, facilitators and barriers of involvement. The intention is that resulting data will help inform future directions for integrating public involvement into genomics research and policy development, and inform the development of ways of routinely reporting and evaluating any involvement.
Methods
Source
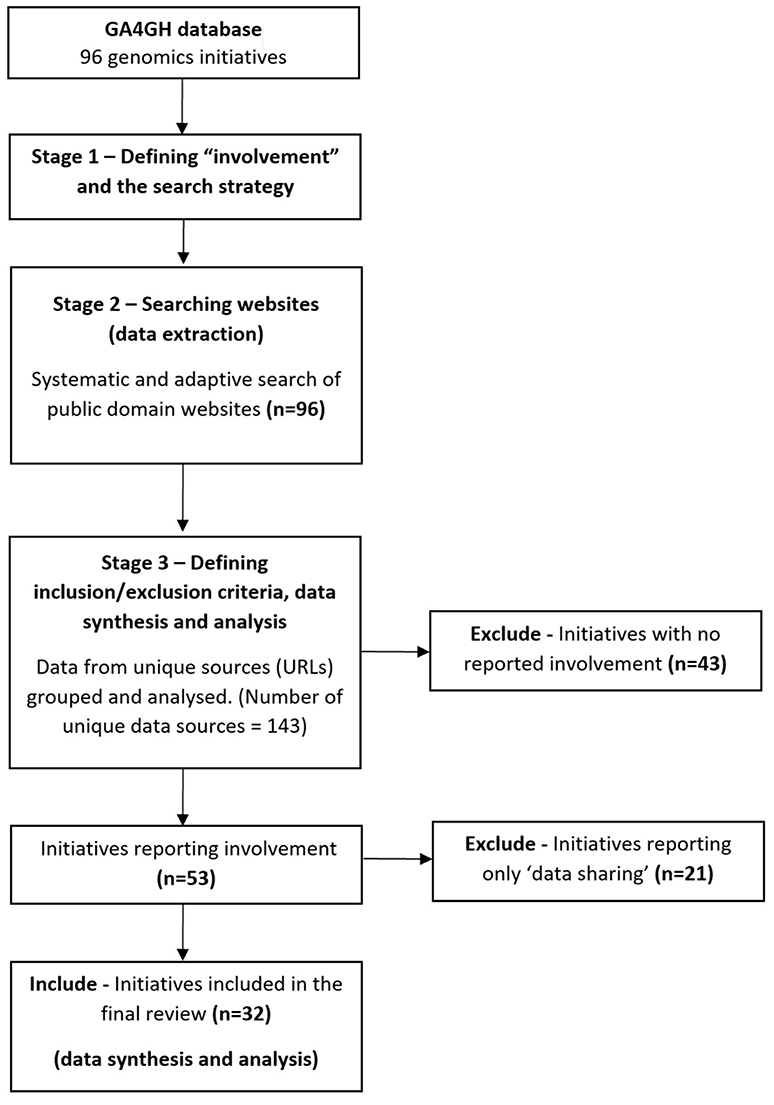
Using a list of human genomics research projects (referred to as “initiatives”) from a database hosted by the GA4GH (see Table S1), we systematically searched public domain websites for information reported on involving the public in research. The database was curated by GA4GH staff, last verified August 2016, and contains information about the type of the genomics research initiative (i.e., consortium, data-sharing initiative, organization(s), repository or research project), the type of data gathered (i.e., whole-genome or whole-exome sequencing), the geographical scope of the initiative, number of participants (cohort size), relevant disease areas, and the public domain URL of the website for the organization or initiative (as some “initiatives” involve a number of organizations). The scoping review methodology can be summarized in three stages (see Figure 1).
Stage 1—Defining “Involvement” and the Search Strategy
We developed a criteria to define “involvement” and refine our search terms, informed by the International Association for Public Participation's participation spectrum and other studies (4, 8, 21, 22). This included reports of “consultation,” “involvement,” “collaboration,” and “empowerment” (23). Involving people in genomics research was defined as the “active involvement” in shaping and guiding research, rather than only providing data (17, 23, 24). We defined specific tasks related to involvement at different stages of the research cycle (3), such as the sharing of views to influence research, or co-creating the research (19, 25, 26). “Consequential” involvement is when involvement contributes to the research process, as distinct from involvement which is ignored or not incorporated (27–29). We could not always determine whether involvement was consequential based on the available information, so an assumption was made that all methods reported were “consequential.”
Stage 2—Searching Websites (Data Extraction)
Public domain websites of all the initiatives in the GA4GH database were searched for reports of involvement and associated impacts. The date range for website searching and data extraction was 16th August to 28th November 2017. The exact text from the URLs where data was extracted from was collected to allow reanalysis, with all relevant URLs archived using an online archive service to preserve the content and the date of extraction (30).
After a manual search of each domain, search engine operators were entered into the Google “site search” function in order to systematically scan the text of each public domain website for relevant phrases, including all grammatical variations of the words used (for example, deriving “involvement,” “involves,” “involved,” and “involving” from the root word “involve”). Grammatical variations of specific phrases (denoted by inverted commas) were generated using tables to systematically create a series of search strings for each domain. For example, this search string returned 4 results:
site:www.ukbiobank.ac.uk/ “public involvement” OR “involves public” OR “public involved” OR “involving the public” OR “involve public”
Reports of involvement were assessed by a member of the research team (JN), then independently assessed by an additional member of the research team, with a random sample assessed by a third investigator (PL). Any disagreements between the team on the data included was discussed until a consensus was reached. Informed by previous reviews, the search terms for the concept of involvement were; “engagement,” “involvement,” and “partnership” (21, 31–34). The search terms to describe the people involved were; “citizen(s),” “community,” “consumer(s),” “lay,” “patient(s),” “public,” “stakeholder(s),” and “user(s).”
In addition to using a standard list of terms, adaptive (context dependent) search terms were sometimes required when searching domains where terms were specific to the region or initiative. Adaptive search terms were; “advocate(s),” “carer(s),” “civil society,” “client(s) (35),” “customer(s) (35),” “group(s),” “participant(s),” “payer(s),” “population(s) (29),” “PPI” (an acronym commonly used in the UK which stands for “patient and public involvement”), “residents” (geographical grouping) (36), “representative(s),” “taxpayer(s),” and “volunteer(s).” For more details on search method, see Systematic search method.
Stage 3—Defining Inclusion/Exclusion Criteria, Data Synthesis and Analysis
Defining the inclusion and exclusion criteria was an iterative process informed by published scoping review methodologies (37, 38). Initiatives reporting no involvement were excluded from further analysis. Initiatives were categorized as “no involvement” if the context of words such as “participation” were used to describe “research participants” (research subjects) only, rather than aligning with the concept of involvement already articulated (4). Reported impacts were excluded if they were phrased as anticipated future impacts (using terms such as “we expect”), rather than reporting real results. Initiatives reporting “data sharing” as the only type of involvement were also excluded. Initiatives reporting any other type of involvement, according to our definition, were included and proceeded to data extraction (structured categorization of extracted search data).
Extracted data was categorized (data synthesis) based on the following types of information; (a) the method of involvement (how people were involved) (24); (b) the tasks they were involved in (what people did) (24); (c) the stage of the research (using an expanded version of an existing framework (15), informed by INVOLVE) (39); (d) who was involved, for example “research participants,” “patients,” and “public” (informed by the Concannon “7Ps Framework” taxonomy) (16); (e) reported facilitators or barriers of involvement; and (f) publicly-reported impacts (informed by section 7 and 8 of the GRIPP2 framework) (16, 24).
As there is currently no standardized way to report and group methods of involving people or descriptions of people involved (24, 29), grouping was informed by methods of previous reviews [for example, grouping similar methods of involving people (24)] and by using previously established nomenclature (26, 33). The initial grouping (JN) was reviewed by other authors (PL). While previous reviews have used frameworks to label the “roles,” “degrees,” or “levels” of involvement or “control” (19, 24), we did not use these frameworks as they require subjective judgements to be made, often with insufficient data (26, 40–42).
Results
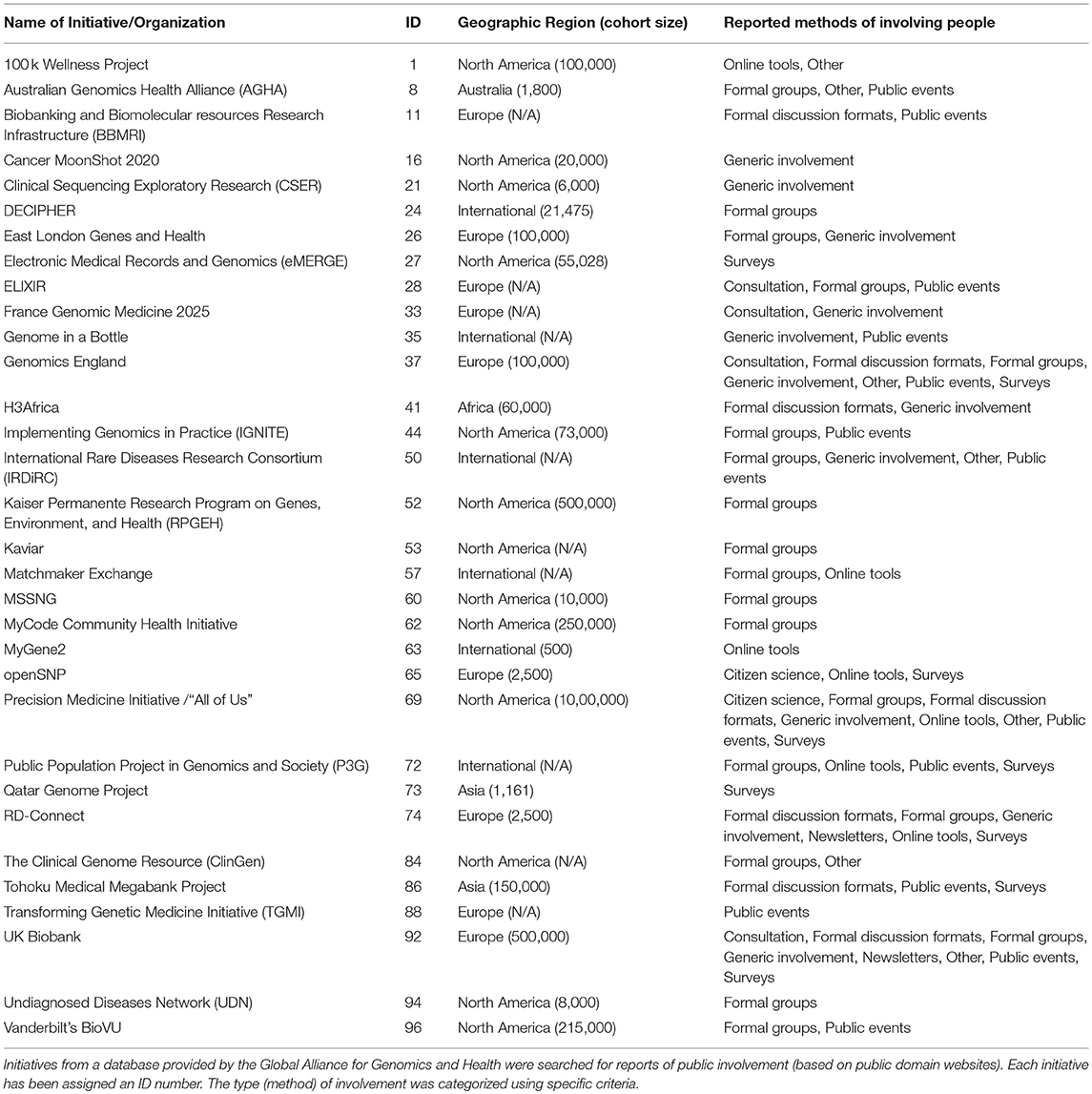
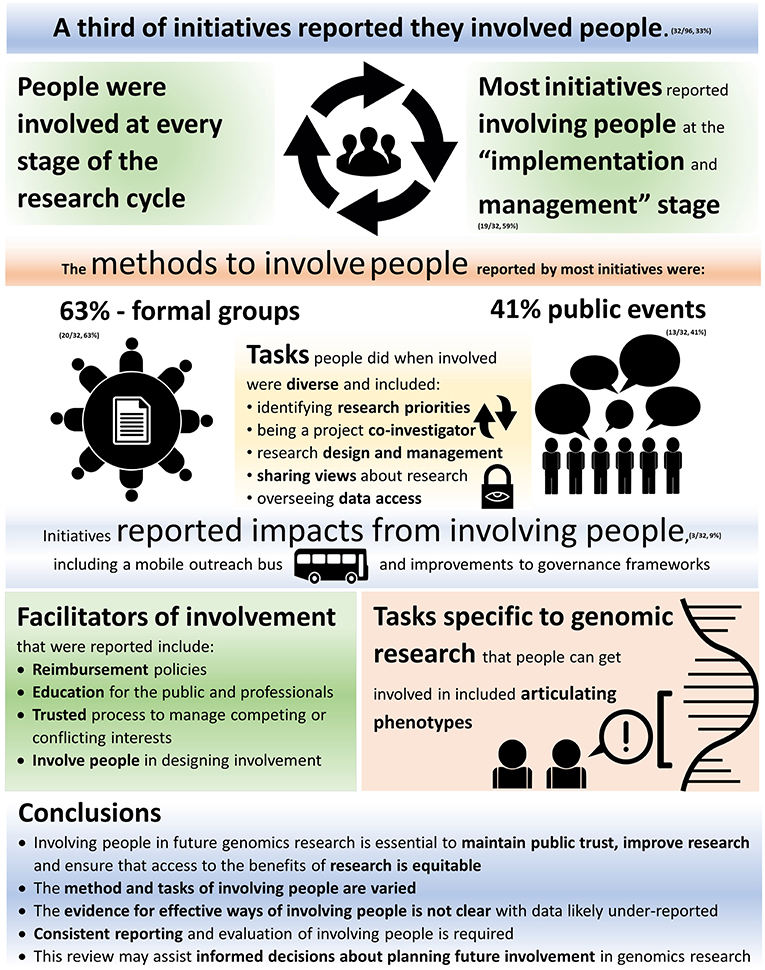
Of the 96 initiatives searched, based on our criteria, only a third reported involving people in some capacity (32/96, 33%) (Table 1). These 32 initiatives were included in the final analysis (data synthesis).
Reported Methods of Involvement
The reported methods of involving people were organized into categories, shown below in bold, with the number of total initiatives reporting each method shown in brackets:
• Citizen science (n = 2)—people involved beyond data collection, research design or data analysis, toward co-creation across all aspects of the scientific process (43);
• Consultation (n = 4)—an organized consultation or dialogue process;
• Formal discussion (n = 8)—formalized “focus groups,” forums or interview structures;
• Formal groups (n = 20)—a working group or committee (including ethics and data access committees, “scientific advisory groups” and “steering groups”);
• Generic involvement (n = 11)—informal, such as meetings, “partnership,” or an unspecified method;
• Newsletters (n = 2)—or mailing lists;
• Online tools (n = 7)—websites, social media, or online community hosting;
• Public events (n = 13)—with discussion—including initiatives hosting public debates, workshops, discussion spaces, or conferences;
• Surveys (n = 10)—including questionnaires; and
• Other (n = 7)—methods not described by other categories.
Some initiatives reported using multiple methods to involve people. Reports of involving people also showed that some methods, for example “formal discussion,” can use different modes of communication, including face to face, online (for example, “massive open online courses”), or a combination of the two.
Figure 2 summarizes overall findings from data synthesis. There was variability in the methods and tasks of involvement reported. This supports previous findings that involvement in biomedical research is diverse, varied, and described using different language (44).
Reported Tasks of Involvement
The tasks people were involved in (what people did when they were involved) were diverse. Tasks included identifying research priorities related to people with specific diseases; communicating priorities to scientists, clinicians and health policy makers [IDs 11, 37, 50, 74]; designing or improving how people will be involved in the research [IDs 41, 50]; educating professionals involved in the research [ID 8]; developing workshops and conferences [IDs 44, 94]; offering culturally appropriate information about research to people in community groups [ID 37]; providing feedback on the cultural and linguistic appropriateness of public domain research documents [ID 96]; and translating information into “lay” language [ID 92]. Tasks also involved sharing views and perspectives about multiple aspects of research projects [ID 37, 92, 96]; articulating phenotypes [ID 65]; and being a project co-investigator [ID 21].
Some initiatives reported involving people in the task of giving feedback and sharing views and perspectives about the “acceptability” of specific aspects of the research design. For example, research management, governance [IDs 27, 41, 92, 72], accountability, planning, policy, protocols, data access, and data use [IDs 37, 74, 84, 92], consent, re-contact, withdrawal, confidentiality, benefit sharing, project closure, and recruitment [IDs 37, 62, 74, 92]. A number of initiatives also involved people in the task of sharing views and perspectives on issues of perceived social and ethical importance (including being told about potentially serious incidental findings) [IDs 37, 74, 96], or to scrutinize a project to ensure it aligned with public interest [ID 11].
While there are commonalities with the principles of involvement in other kinds of biomedical research, the review identified three novel tasks in relation to human genomics research not found in other reviews. These included involving people in phenotype articulation [ID 65], where people can describe the lived-experience of having specific genomic variations; articulating the variation in perspectives of people affected by different rare diseases [ID 74]; and collective governance, problem solving and improving code [ID 53; 65]. For example, Open SNP shared the code for the entire initiative using Github (a platform for sharing open-source code), inviting participants and other members of the public to scrutinize, contribute, and improve the code.
Reported Stages of Involvement
Most reports of involvement were at the “implementation and management” stage of research (19/32, 59%), followed by “dissemination” (12/32, 38%), “evaluation,” and “study design” (both 9/32, 28%) and “data analysis” (8/32, 25%). The stage with the lowest number of initiatives reporting involvement was “funding” (1/32, 3%) with the next lowest being “identifying topics” and “prioritization” (both 4/32, 13%). Four initiatives reported involving people at every stage of research [IDs 21, 50, 69, 74].
Reported Impacts of Involvement
Nearly 10% of the initiatives reporting involvement also reported impacts of the involvement (3/32, 9.4%). Three initiatives reported a total of eight distinct impacts as a direct result of involving people [IDs 37, 73, 92]. The method with the most reported impacts was “public events” (4/8, 50%), followed jointly by “formal discussion formats” and “surveys” (2/8, 25%). Actions taken as a result of involving people (impacts) included the creation of a mobile outreach bus [ID 37]; improvements to ethical and governance frameworks [ID 92] (45); and improved participant information and consent documents [ID 37] (46). Some impacts were reported as being a result of using a combination of methods.
Reported Facilitators and Barriers to Involvement
A number of specific facilitators of involvement were reported, including: reimbursement policies [ID 21], with people involved paid for their time [IDs 92, 94], travel [IDs 74, 94], accommodation [ID 74] and expenses [IDs 74, 92]; education and learning opportunities for the general public [IDs 1, 11, 41]; ensuring people involved are informed and can make informed decisions [ID 11]; education for health professionals [IDs 41, 50]; providing opportunities to learn about how to involve people [IDs 41, 50]; and governance which is trusted by all stakeholders to be able to manage real or perceived competing or conflicting interests [ID 50]. The only barrier reported was limited venue size, which restricted the number of people who could be involved [ID 92]. This also implies a limited budget, which is an important but likely under-reported implicit limitation on all involvement methods.
Discussion
This review provides an overview of reported public involvement occurring in prominent human genomics projects worldwide, during a period of rapid growth for genomics research. We identified significant variability in the way in which involvement occurs and is reported. The variation in reported involvement suggests diversity in both the ways people are being involved in genomics, and in the varied and emergent language used to report and describe involvement, consistent with other areas of biomedical research (8, 21). While there are similarities with the principles of involvement in other kinds of research, this review has identified three different tasks specific to genomics, not found in other reviews (44).
Because the results from this review suggest there is currently no standardized way of reporting involvement in human genomics, and therefore evaluating how people are involved, there is a risk that best-practice will be hard to define or even absent in future evidence reviews. This has implications, as the number of people involved in human genomic research is predicted to grow exponentially. Without a standardized framework to report and transparently evaluate ways people are involved, it will be difficult to create an evidence base to inform best-practice.
While a third of initiatives reported involvement, a majority of projects did not (64/96, 66%). Some prominent initiatives involving the genetic analysis of thousands of people did not refer to public involvement in any way. This is somewhat concerning given that involving the public has been identified as a crucial aspect of responsible research practice in genomics (1). Whilst we acknowledge the probable under-reporting of involvement activities on public-domain websites, we argue public involvement in human genomics research needs to increase.
Findings from this review also suggest it is best-practice to involve multiple stakeholders (including the public) in designing how people will be involved in research (co-design of the involvement plan), and to involve the public throughout the lifetime of a project in certain tasks (such as overseeing data access) and to evaluate the involvement with both qualitative and quantitative data.
Co-design of involvement strategies may improve how appropriate, effective, efficient and equitable they are. Seeking input from people into the design of planned methods of involvement by identifying what is considered “good practice” was reported by H3Africa [ID 41] and the International Rare Diseases Research Consortium (IRDiRC), and reported as a facilitator of involvement by the IRDiRC [ID 50]. The IRDiRC [ID 50] also reported both qualitative and quantitative data should be used to evaluate involvement, although there is currently no way to systematically collect and analyze such activity (47).
If involvement is more effective when the public are invited to help plan it, standardized reporting and evaluation will help make informed decisions at every stage of involvement from co-design through to evaluation.
Implications for Policy and Practice
With the impact of some genomics research data likely to be measured in decades, some of the initiatives offer a useful insight into planning and funding of sustainable (long-term) involvement for the entirety of an initiative's lifetime (9). For example, Genomics England [ID 37] and the UK Biobank [ID92], as exemplars, both reported multiple ways of involving people, at different stages of the research cycle, conducted over a number of years. Other initiatives, such as the International Rare Diseases Research Consortium (IRDiRC) [ID 50] and the Public Population Project in Genomics and Society (P3G) [ID 72], also publicly stated the importance of planning sustainable involvement over the duration of a project. These initiatives demonstrate a standard of involving people which could eventually be used to inform international best practice.
The IRDiRC also reported that involving people throughout an entire project helped maintain trust by scrutinizing and managing competing or conflicting interests [ID 50]. Similarly, the UK Biobank reported that involving people in ethics and governance should not be one-off and must be ongoing [ID 92]. The method of using “formal groups” was more common for more complex or ongoing tasks such as overseeing data access, policy development, research management and improving research protocols.
Some initiatives, such as openSNP, reported tasks that were specific to genomics research, such as articulating phenotypes [ID 65]. Involvement in this kind of task might have important implications when working to usefully describe people's subjective lived-experiences across multiple languages, for example, rare diseases and mental health (48).
Public involvement in articulating phenotypes also suggests that the traditional boundaries between terms such as “research,” “healthcare,” “patients,” “research participants,” and “the public” may be increasingly challenged by the methodology of future genomic research (49). Findings from this review show that both “the public” and “patients” are already involved in every stage of research, including collecting and analyzing data (49). Any future standardized reporting of involvement will need to keep pace with the continually evolving language to describe not only what research is, but who is involved and how.
Limitations
While the database hosted by GA4GH includes many of the most prominent human genomics research initiatives worldwide, the database is not exhaustive. There are several known genomics initiatives which involved people that were not part of the database. Therefore, the GA4GH selection cannot be considered systematic or representative. However, it does provide a reasonable indication and snapshot of the current global landscape in human genomics research up to November 2017. After the review was completed, GA4GH shared a new a database with 220 initiatives (50), presenting an opportunity for future reviews. The addition of so many new projects to the database reflects the rapid pace of growth in human genomics research.
Our data collection was limited to self-reported information reported on English language websites only. This likely under-reports the total amount of public involvement occurring. For example, some initiatives may have conducted involvement, and not reported it publicly. This indicates a current lack of standardization or best-practice in reporting involvement activities in human genomics research, which we feel could be improved.
Of the public involvement activities reported, we did not systematically follow up reports to confirm they had taken place, or if involvement was “consequential” (27–29). While this is a limitation of the review, it also reflects the inconsistent and often incomplete ways genomics research initiatives report impacts of involving people. For example, the impact of how involvement influenced research was only reported by three projects—Genomics England [ID 37], the Qatar Genome Project [ID 73] and the UK Biobank [ID 92].
A number of reported methods did not provide sufficient information to make a clear decision about how to group a method. For example, many reports of involvement simply referred to a “workshop,” “meeting,” or other “public events,” where people were able to get involved by sharing views and perspectives. As a result there is potentially significant overlap between some methods, which could have been articulated more clearly if more data were available. Similarly, while detailed data was extracted about “who” was involved, ways of grouping terms such as “research participants,” “patients,” and “public” requires further development to co-create standardized definitions.
The systematic searching of domains with the Google site search function relies on Google servers having carried out a “website crawl,” where data from the website is indexed (51). As the search and indexing process is partially opaque (not open-source), this method cannot be considered “exhaustive.” However, it is an appropriate supplementary search strategy for this scoping review.
Reports of “data sharing” were excluded, as they were not considered as public involvement. While sharing data may enable people to be involved in some tasks (for example, in analyzing data), data sharing is not necessarily an indicator that people were involved in the analysis of data. The complexity within the term “data sharing” in genomics, and how people can be involved in the analysis and interpretation of data, also requires further consideration (52–54).
Conclusion
Involving people in the future of genomics research is an essential aspect in maintaining public trust, improving research outcomes, and ensuring that access to the benefits of genomics research is equitable (1, 14, 49). The limited number of initiatives reporting public involvement and its impact in this study suggests there would be significant value in developing a more systematic method of both reporting and evaluating how people are involved in human genomics research. Data from such reporting could provide the evidence required to inform future policy around involvement of the public, as human genomics research continues to grow.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2019.00079/full#supplementary-material
Table S1. GA4GH database and results table. This table combines a database of human genomics research projects hosted by the Global Alliance for Genomics and Health with a summary of results from a scoping review of currently reported public involvement reported on public domain websites. The data is organized alphabetically, with organizations reporting involvement listed first.
Systematic search method. This document describes the search method, including how standard and adaptive TERMS were used to search the public domain websites of all the included initiatives in the GA4GH database for reports of involvement and any impacts.
References
1. Burton H, Adams M, Bunton R, Schröder-Bäck P. Developing stakeholder involvement for introducing public health genomics into public policy. Public Health Genomics. (2009) 12:11–19. doi: 10.1159/000153426
2. Lemke AA, Harris-Wai JN. Stakeholder engagement in policy development: challenges and opportunities for human genomics. Genet Med. (2015) 17:949–57. doi: 10.1038/gim.2015.8
3. National Institute for Health Research. Briefing Note Eight: Ways That People Can Be Involved in The Research Cycle. National Institute for Health Research (2017). Available online at: http://web.archive.org/web/20170605035051/http://www.invo.org.uk/posttyperesource/where-and-how-to-involve-in-the-research-cycle/ (accessed June 5, 2017).
4. National Institute for Health Research. Patient and Public Involvement in Health And Social Care Research. (2014). Available online at: https://www.nihr.ac.uk/about-us/CCF/funding/how-we-can-help-you/RDS-PPI-Handbook-2014-v8-FINAL.pdf (accessed February 2, 2018).
5. Sandhu C, Qureshi A, Emili A. Panomics for precision medicine. Trends Mol Med. (2018) 24:85–101. doi: 10.1016/j.molmed.2017.11.001
6. Green ED, Rubin EM, Olson M V. The future of DNA sequencing. Nature. (2017) 550:179–81. doi: 10.1038/550179a
7. Brett J, Staniszewska S, Mockford C, Herron-Marx S, Hughes J, Tysall C, et al. Mapping the impact of patient and public involvement on health and social care research: a systematic review. Health Expect. (2014) 17:637–50. doi: 10.1111/j.1369-7625.2012.00795.x
8. Kelty C, Panofsky A. Disentangling public participation in science and biomedicine. Genome Med. (2014) 6:8. doi: 10.1186/gm525
9. Wagner JK, Peltz-Rauchman C, Rahm AK, Johnson CC. Precision engagement: the PMI/'s success will depend on more than genomes and big data. Genet Med. (2016) 19:620–4. doi: 10.1038/gim.2016.165
10. Sally Davies, et al. Annual Report of the Chief Medical Officer 2016 - “Generation Genome”. (2017). Available online at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/624628/CMO_annual_report_generation_genome.pdf (accessed on February 5, 2019).
11. Crowe S, Fenton M, Hall M, Cowan K, Chalmers I. Patients', clinicians' and the research communities' priorities for treatment research: there is an important mismatch. Res Involve Engage. (2015) 1:2. doi: 10.1186/s40900-015-0003-x
12. World Health Organisation. Declaration of Alma-Ata. (1978). Available online at: http://www.who.int/publications/almaata_declaration_en.pdf?ua=1 (accessed June 25, 2018).
13. Domecq JP, Prutsky G, Elraiyah T, Wang Z, Nabhan M, Shippee N, et al. Patient engagement in research: a systematic review. BMC Health Serv Res. (2014) 14:89. doi: 10.1186/1472-6963-14-89
14. Brett J, Staniszewska S, Mockford C, Herron-Marx S, Hughes J, Tysall C, et al. A systematic review of the impact of patient and public involvement on service users, researchers and communities. Patient. (2014) 7:387–95. doi: 10.1007/s40271-014-0065-0
15. Shippee ND, Domecq Garces JP, Prutsky Lopez GJ, Wang Z, Elraiyah TA, Nabhan M., et al. Patient and service user engagement in research: a systematic review and synthesized framework. Heal Expect. (2015) 18:1151–66. doi: 10.1111/hex.12090
16. Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ. (2017) 358:j3453. doi: 10.1136/bmj.j3453
17. Staniszewska S, Adebajo A, Barber R, Beresford P, Brady LM, Brett J, et al. Developing the evidence base of patient and public involvement in health and social care research: the case for measuring impact. Int J Consum Stud. (2011) 35:628–32. doi: 10.1111/j.1470-6431.2011.01020.x
18. Staley K, Buckland SA, Hayes H, Tarpey M. “The missing links”: understanding how context and mechanism influence the impact of public involvement in research. Heal Expect. (2014) 17:755–64. doi: 10.1111/hex.12017
19. Oliver SR, Rees RW, Clarke-Jones L, Milne R, Oakley AR, Gabbay J., et al. A multidimensional conceptual framework for analysing public involvement in health services research. Heal Expect. (2008) 11:72–84. doi: 10.1111/j.1369-7625.2007.00476.x
20. Terry SF. The global alliance for genomics & health. Genet Test Mol Biomark. (2014) 18:375–6. doi: 10.1089/gtmb.2014.1555
21. Rogers M, Bethel A, Boddy K. Development and testing of a medline search filter for identifying patient and public involvement in health research. Health Info Libr J. (2017) 34:125–33. doi: 10.1111/hir.12157
22. Concannon TW, Meissner P, Grunbaum JA, McElwee N, Guise JM, Santa J, et al. A new taxonomy for stakeholder engagement in patient-centered outcomes research. J Gen Intern Med. (2012) 27:985–91. doi: 10.1007/s11606-012-2037-1
23. International Association for Public Participation. Participation Spectrum. (2014). Available online at: https://www.iap2.org.au/Tenant/C0000004/00000001/files/IAP2_Public_Participation_Spectrum.pdf (accessed July 12, 2018).
24. Oliver S, Clarke-Jones L, Rees R, Milne R, Buchanan P, Gabbay J, et al. Involving consumers in research and development agenda setting for the NHS: developing an evidence-based approach. Health Technol Assess. (2004) 8:1–148. doi: 10.3310/hta8150
25. Woolley JP, McGowan ML, Teare HJ, Coathup V, Fishman JR, Settersten RA Jr, et al. Citizen science or scientific citizenship? Disentangling the uses of public engagement rhetoric in national research initiatives. BMC Med Ethics. (2016) 17:33. doi: 10.1186/s12910-016-0117-1
26. Oliver S, Liabo K, Stewart R, Rees R. Public involvement in research: making sense of the diversity. J Health Serv Res Policy. (2014) 20:45–51. doi: 10.1177/1355819614551848
27. Van Mil A, Hopkins H, Kinsella S. Potential Uses for Genetic Technologies: Dialogue and Engagement Research Conducted on Behalf of the Royal Society Findings Report. (2017). Available online at: https://royalsociety.org/~/media/policy/projects/gene-tech/genetic-technologies-public-dialogue-hvm-full-report.pdf (accessed March 19, 2018).
28. International Finance Corporation. Stakeholder Engagement : A Good Practice Handbook for Companies Doing Business in Emerging Markets. International Finance Corporation (2007). p. 201.
29. Nilsen ES, Myrhaug HT, Johansen M, Oliver S, Oxman AD. Methods of consumer involvement in developing healthcare policy and research, clinical practice guidelines and patient information material. Cochrane Database Syst Rev. (2006) 3:1–25. doi: 10.1002/14651858.CD004563.pub2
30. Dzingle D, May GA, Garland HT. Internet Archive: About IA. (2018). Available online at: https://archive.org/about/ (accessed February 2, 2018).
31. Degeling C, Carter SM, Rychetnik L. Which public and why deliberate? – A scoping review of public deliberation in public health and health policy research. Soc Sci Med. (2015) 131:114–21. doi: 10.1016/j.socscimed.2015.03.009
32. Morley RF, Norman G, Golder S, Griffith P. A systematic scoping review of the evidence for consumer involvement in organisations undertaking systematic reviews: focus on Cochrane. Res Involve Engage. (2016) 2:36. doi: 10.1186/s40900-016-0049-4
33. Pollock A, Campbell P, Struthers C, Synnot A, Nunn J, Hill S, et al. Stakeholder involvement in systematic reviews: a protocol for a systematic review of methods, outcomes and effects. Res Involve Engage. (2017) 3:9. doi: 10.1186/s40900-017-0060-4
34. Lander J, Hainz T, Hirschberg I, Bossert S, Strech D. Do public involvement activities in biomedical research and innovation recruit representatively? A systematic qualitative review. Public Health Genomics. (2016) 19:193–202. doi: 10.1159/000444478
35. Mitton C, Smith N, Peacock S, Evoy B, Abelson J. Public participation in health care priority setting: a scoping review. Health Policy. (2009) 91:219–28. doi: 10.1016/j.healthpol.2009.01.005
36. Collins M. PiiAF The Public Involvement Impact Assessment Framework Guidance. (2014). Available online at: http://piiaf.org.uk/documents/piiaf-guidance-jan14.pdf (accessed October 4, 2017).
37. Levac D, Colquhoun H, O'Brien KK. Scoping studies: advancing the methodology. Implement Sci. (2010) 5:69. doi: 10.1186/1748-5908-5-69
38. Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. (2005) 8:19–32. doi: 10.1080/1364557032000119616
39. National Institute for Health Research. The Research Cycle. (2018). Available online at: https://www.nihr.ac.uk/patients-and-public/how-to-join-in/the-research-cycle/ (accessed June 21, 2018).
40. Ocloo J, Matthews R. From tokenism to empowerment: progressing patient and public involvement in healthcare improvement. BMJ Qual Saf. (2016) 25:1–7. doi: 10.1136/bmjqs-2015-004839
41. Ennis L, Wykes T. Impact of patient involvement in mental health research: longitudinal study. Br J Psychiatry. (2013) 203:381–6. doi: 10.1192/bjp.bp.112.119818
42. PatientPartner. Patient Involvement in Clinical Research: A Guide for Patient Organisations and Patient Representatives. (2011). Available online at: https://www.geneticalliance.org.uk/media/1602/patientspartnerforpatientorgs.pdf (accessed February 20, 2018).
43. Pecl G, Gillies C, Sbrocchi C, Roetman P. Building Australia Through Citizen Science. (2015) Available online at: http://www.chiefscientist.gov.au/wp-content/uploads/Citizen-science-OP_web.pdf (accessed August 24, 2017).
44. Lander J, Hainz T, Hirschberg I, Strech D. Current practice of public involvement activities in biomedical research and innovation: a systematic qualitative review. PLoS ONE. (2014) 9:e113274. doi: 10.1371/journal.pone.0113274
45. UK Biobank. Summary of the UK Biobank Consultation on the Ethics and Governance Framework. (2003). Available online at: http://web.archive.org/web/20170915061800/http://www.ukbiobank.ac.uk/wp-content/uploads/2011/07/Summary-EGF-consultation.pdf?phpMyAdmin=trmKQlYdjjnQIgJ%2CfAzikMhEnx6 (accessed March 3, 2019).
46. Genomics England. Genomics England: 100000 Genomes Project Potential Participant Literature: Research Report. (2014). Available online at: http://web.archive.org/web/20170711065551/https://www.genomicsengland.co.uk/?wpdmdl=5251 (accessed July 11, 2017).
47. International Collaboration for Participatory Health Research (ICPHR). Position Paper 1: What Is Participatory Health Research? Version: May 2013. (2013). Available online at: http://www.icphr.org/uploads/2/0/3/9/20399575/ichpr_position_paper_1_defintion_-_version_may_2013.pdf (accessed June 13, 2017).
48. Uher R. Genomics and the classification of mental illness: focus on broader categories. Genome Med. (2013) 5:97. doi: 10.1186/gm501
49. Nunn JS, Scott CL, Stubbs JW, Cherry SF, Bismark MM. Involving the Public in Rare Cancer Care and Research. Chichester: John Wiley & Sons Ltd (2017). p. 12–18.
50. Global Alliance for Genomics and Health. Catalogue of Genomic Data Initiatives. (2019). Available online at: https://www.ga4gh.org/community/catalogue (accessed March 3, 2019).
51. Google. How Google's Site Crawlers Index Your Site. Available online at: https://www.google.com/search/howsearchworks/crawling-indexing/ (accessed February 26, 2019).
52. Haeusermann T, Greshake B, Blasimme A, Irdam D, Richards M, Vayena E. Open sharing of genomic data: who does it and why? PLoS ONE. (2017) 12:e0177158. doi: 10.1371/journal.pone.0177158
53. Messner DA, Koay P, Al Naber J, Cook-Deegan R, Majumder M, Javitt G, et al. Barriers to clinical adoption of nextgeneration sequencing: a policy Delphi panel's solutions. Per Med. (2017) 14:339–54. doi: 10.2217/pme-2016-0104
Keywords: genomics research, public health, public involvement, scoping review, co-design and co-production, patient and public involvement and engagement, patient participation [MeSH term], public health genomics
Citation: Nunn JS, Tiller J, Fransquet P and Lacaze P (2019) Public Involvement in Global Genomics Research: A Scoping Review. Front. Public Health 7:79. doi: 10.3389/fpubh.2019.00079
Received: 16 January 2019; Accepted: 19 March 2019;
Published: 09 April 2019.
Edited by:
ClarLynda Williams-DeVane, North Carolina Central University, United StatesReviewed by:
Weijian Liu, EMD Serono, United StatesWalter Mazzucco, University of Palermo, Italy
Stefano Ceri, Politecnico di Milano, Italy
Copyright © 2019 Nunn, Tiller, Fransquet and Lacaze. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jack S. Nunn, Jack.Nunn@latrobe.edu.au
 Jack S. Nunn
Jack S. Nunn Jane Tiller
Jane Tiller Peter Fransquet
Peter Fransquet Paul Lacaze
Paul Lacaze