- 1Department of Psychiatry and Behavioral Sciences, University of Texas Health Science Center at Houston, Houston, TX, United States
- 2University of Texas Harris County Psychiatric Center, Houston, TX, United States
- 3Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX, United States
Peripheral and CNS-localized inflammatory processes are hypothesized to contribute to the complex pathophysiology of schizophrenia. Elevated levels of the acute phase reactant C-reactive protein (CRP) have been observed in schizophrenia, yet relatively few studies have investigated the association between this inflammatory biomarker and psychotic symptoms in schizophrenia. This study is a pilot cross-sectional analysis investigating the relation of plasma CRP levels and the positive and negative symptoms of schizophrenia (the primary aim), assessed by the Positive and Negative Syndrome Scale (PANSS). A secondary analysis was also performed evaluating the potential association of CRP with cognitive function using the NIH Toolbox Cognitive Test Battery. After adjusting for age, sex, race, and body mass index, a positive correlation was observed between CRP and PANSS negative symptoms (rho = 0.37, p = 0.05). There was no correlation between plasma CRP and any of the NIH Toolbox measures of cognitive function in the unadjusted or adjusted analyses. Though limited by a relatively small sample size and the unavailability of longitudinal data, the correlation between CRP and psychopathology in this sample of patients supports a role for inflammation in the pathophysiology of schizophrenia.
Introduction
Peripheral and CNS-localized markers of immunopathology, including cytokine expression and microglial activation, have been associated with schizophrenia (1–5), a psychotic disorder of complex genetic and environmental pathogenesis. Prior studies have indicated that systemic inflammatory mechanisms can influence neural circuits (6–10), and an evolving genetic-vascular-inflammatory theory of schizophrenia suggests that genetically regulated inflammatory reactions cause progressive microvascular damage to the central nervous system in response to environmental stressors (11). Furthermore, animal models have shown that chronically elevated brain levels of pro-inflammatory cytokines may lead to abnormal neural connectivity of the developing brain which could contribute to the emergence of psychosis. Subsequently, and in an attempt to target the low-grade neuroinflammation hypothesized to be present in the illness, adjunctive treatment of schizophrenia with anti-inflammatory agents has been evaluated in a number of studies albeit with mixed results (12–14). However, the relationship between inflammatory processes and schizophrenia remains incompletely understood.
The acute phase reactant C-reactive protein (CRP) is a non-specific serum marker of inflammation. Elevated blood levels of CRP have been observed in schizophrenia (15–17), and elevated CRP in adolescence has been associated with subsequent development of schizophrenia in adulthood (18). A causal association between chronic systemic inflammation and schizophrenia has been suggested (19) but is yet to be confirmed. Moreover, inflammation during prenatal and perinatal neurodevelopment, as measured by elevated levels of IL-8 and TNF-alpha in maternal plasma, has been implicated as a risk factor for subsequent development of schizophrenia in offspring (1, 20, 21). Furthermore, a negative correlation has been observed between several systemic inflammatory markers, including CRP, and general cognitive functioning (22, 23) as well as with all facets of working memory (22, 24) in patients with schizophrenia. Decreased levels of IL-10, an anti-inflammatory cytokine, have also been associated with cognitive impairment in first-episode medication-naïve patients with schizophrenia (25).
Based on the hypothesized link between inflammation and schizophrenia, a few studies have investigated the association between plasma CRP and psychotic symptoms in schizophrenia. Three studies found that patients with schizophrenia and higher levels of serum CRP scored higher on psychotic symptom severity rating scales in comparison to those patients with lower serum CRP levels (26–28). In a study by Akanji and colleagues, higher serum CRP levels were found in patients with catatonic features but, paradoxically, also in patients whose symptoms were in remission (29). In contrast, three separate studies did not find a significant association between CRP levels and positive or negative symptoms in patients with schizophrenia (24, 30, 31). A possible reason for these equivocal results could be the failure to statistically adjust for potential confounders. For example, age and body mass index (BMI) have been shown to be positively correlated with CRP levels (32–36), and it is therefore recommended that these two variables are adjusted for in any analysis evaluating the relationship between CRP levels and psychotic symptoms.
To add to the relatively sparse literature evaluating the association between CRP and psychotic symptoms, we have carried out a cross-sectional analysis of plasma CRP levels and positive and negative symptoms in a pilot sample of 39 patients with schizophrenia. To overcome the primary potential confounds of prior studies, we statistically adjusted for important confounders including age, sex, race, and BMI. We hypothesized that plasma CRP would positively correlate with the positive, negative, general psychopathology, and total psychotic symptoms of schizophrenia. We also carried out a secondary exploratory analysis evaluating the association of CRP with cognitive function as assessed by the NIH Toolbox Cognitive Test Battery (37).
Materials and Methods
Patient Sample
The institutional review board of The University of Texas Health Science Center, Houston, Texas approved this study. This research was completed in conformity with the latest version of the Declaration of Helsinki. All study participants completed a written informed consent form after the study was described to them.
Inpatients with a diagnosis of schizophrenia were recruited from a hospital associated with a university department of psychiatry. All patients had a prior history of multiple episodes of psychosis, and all were being treated with antipsychotic medications at the time of data collection for this study. The following demographic information was obtained: age, sex, race BMI, and level of education. The following inclusion criteria were employed: age ranging from 18 to 60 years, prior documented diagnosis of schizophrenia by DSM-5 criteria, and a negative urine pregnancy test for female patients. The diagnosis of schizophrenia was subsequently confirmed with the Mini-International Neuropsychiatric interview, version 5 (38). The following criteria were employed for exclusion from this study: current endorsement of suicidal or homicidal ideations, prior diagnosis of other cognitive disorders, any current infectious diagnosis, any diagnosis of a primary inflammatory condition, any current use of corticosteroids or non-steroidal anti-inflammatory medications, any recent or current use of warfarin or anticoagulant medications, and a urine drug screen positive for psychostimulant drugs.
Sample Collection and CRP Measurement
Fasting venous blood was collected from patients. Plasma CRP levels were then measured via enzyme-linked immunosorbent assays (Sigma-Aldrich, MO, USA) in conformance with manufacturer instructions. Samples were diluted 1:20,000. Optical density values for the samples were then compared to standard curves ranging from 0 to 600 pg/ml. The minimum detectable dose of human CRP in this assay is 2 pg/ml.
Psychotic Symptom and Cognitive Assessment in Patients
The Positive and Negative Syndrome Scale (PANSS) (39) was used for the psychopathological assessment of the patients, recording the positive syndrome score, negative syndrome score, general psychopathology score, and total PANSS score. Additionally, the NIH Toolbox Cognitive Test Battery (37) was used to assess the following aspects of cognitive function: working memory (via the List Sorting Working Memory Test), episodic memory (via the Picture Sequence Memory Test), processing speed (via the Pattern Comparison Process Speed Test), executive function (via the Dimensional Change Card Sort Test as well as the Flanker Inhibitory Control and Attention Test), and language ability (via the Oral Reading Recognition Test and Picture Vocabulary Test).
Statistical Analysis
Descriptive statistics are presented as mean (with standard deviation) and median as appropriate. The distribution of CRP was skewed and thus for the primary aim (the association between CRP and psychotic symptom severity), Spearman rank correlations were calculated; similar analyses were carried out for the secondary aim (the association of CRP with cognitive measures). Based on prior studies showing positive correlations between systemic inflammation (and hence plasma CRP) and aging (32, 33) as well as BMI (34–36), we calculated nonparametric partial correlations (40) adjusted for age, sex, race, and BMI. By definition, partial correlations are estimates of the strength and direction of the linear relationship (i.e., correlation) between two variables (usually continuous variables) while making adjustment for the effect of one or more other variables. We calculated the nonparametric correlations adjusted for the aforementioned confounders (i.e., nonparametric partial correlations) by running a script (code) in SPSS syntax. For the primary analysis (i.e., primary aim), significance was set at alpha = 0.05. However, to adjust for multiple comparisons in the secondary analysis (eight different cognitive measures), the significance was set at 0.01. All tests were two-tailed. Data analysis was performed using IBM SPSS version 20 (IBM Corp., Armonk, NY, USA).
Results
Demographic and Clinical Characteristics of the Sample
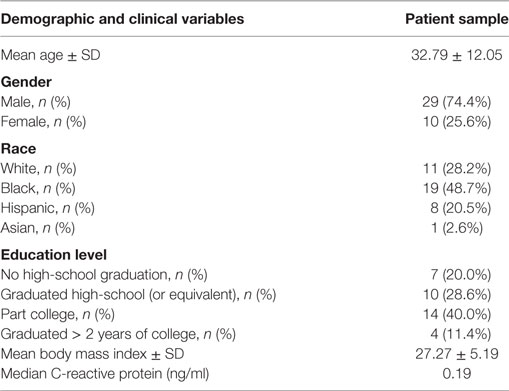
Table 1 presents the demographic and clinical characteristics of the patient sample (n = 39) enrolled in this study. The sample population included more males (74.4%) than females (25.6%). Mean age was 32.7 years old (SD = 12.11), and mean BMI was 27.33 (SD = 5.22). All patients included in this study had a diagnosis of schizophrenia, as well as a history of multiple episodes of psychosis. All were being treated with antipsychotic medications at the time of data collection.
CRP and Psychotic Symptoms
In the unadjusted analyses, a positive correlation was observed between CRP and the PANSS negative symptom subscale (rho = 0.41, p = 0.012) and between CRP and PANSS general psychopathology subscale (Table 2; rho = 0.36, p = 0.029). While a trend toward significance was observed in the correlation between CRP and total PANSS score (rho = 0.32, p = 0.059), there was no correlation between CRP and positive symptoms (rho = −0.21, p = 0.223). Following adjustment for potential confounders (age, sex, race, and BMI), CRP maintained a significant correlation with the PANSS negative symptom subscale (Table 2; rho = 0.37, p = 0.050), but did not correlate with any of the other PANSS subscales or with the total PANSS score (Table 2).
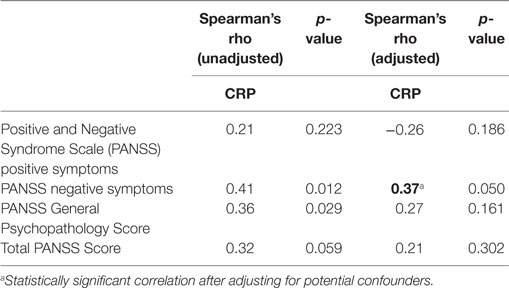
Table 2. Spearman correlation of C-reactive protein (CRP) and psychotic symptom scores, before and after adjustment for age, sex, race, and body mass index.
CRP and Cognitive Function
There was no correlation observed between plasma CRP and any of the NIH Toolbox measures of cognitive function in unadjusted and adjusted analyses (Table 3).

Table 3. Spearman correlation of C-reactive protein (CRP) and scores on the NIH Toolbox Cognitive Test Battery before and after adjustment for age, gender, race, and body mass index.
Discussion
This study found a positive correlation between plasma CRP and the negative symptoms of schizophrenia. We also found a positive correlation between CRP and the PANSS general psychopathology subscale in the unadjusted analysis but this finding was not robust as it did not persist after adjusting for potential confounders.
Based on the research carried out so far on the potential associations between CRP and specific psychotic domains of schizophrenia, no definite conclusions can be drawn and further exploration of this topic is needed. Some studies of CRP and symptom severity in schizophrenia have suggested that CRP levels rise during acute psychotic episodes (16) and that higher CRP levels are associated with heightened severity of psychosis (26–28) and with exacerbation of negative symptoms and general psychopathology (28). In one study, CRP was higher in patients with prominent catatonic features, although the same study found that CRP was highest during remission of symptoms (29). Others have found that CRP was not significantly associated with PANSS scores (24, 30, 31). Of note, prior studies have shown that the use of antipsychotic medications at the time of data collection does not significantly influence the levels of CRP in schizophrenia (17, 29).
Nonetheless, inflammatory processes appear to play a key role in schizophrenia (3, 5, 10, 15, 17), and CRP has been widely regarded as a state marker in schizophrenia alongside other cytokines like tumor necrosis factor-alpha (1). The current genetic-inflammatory-vascular hypothesis of schizophrenia suggests that a chronic systemic inflammatory state causes CNS-localized microvascular damage leading to a dysregulation of CNS blood-flow homeostasis as well as a breach in the blood–brain barrier (11). Findings from animal models also suggest that elevated levels of markers of inflammation including cytokines could alter neural circuits in the prenatal and postnatal developing brain and predispose to the emergence of psychosis in adulthood. Moreover, systemic pro-inflammatory pathways have been observed to directly alter CNS dopaminergic systems and indirectly modify glutamatergic systems via the metabolism of tryptophan and the subsequent increase in CNS-localized kynurenine (secondary to the activity of tryptophan 2,3-dioxygenase and indoleamine 2,3-dioxygenase), which is then converted to kynurenic acid, an NMDA receptor antagonist (1, 5, 41, 42). Importantly, dysregulation of the kynurenine pathway and NMDA dysfunction have been implicated in the pathophysiology of psychosis in schizophrenia and represents an important area of further scientific exploration (5, 41–44). Our findings correlating CRP with psychopathology support and contribute to the hypothesized influence of systemic inflammation upon psychosis in schizophrenia.
Moreover, with regard to prior developmental studies of inflammation and schizophrenia, one would speculate that the current findings are very interesting considering that the negative symptoms often arise early in disease progression, and CRP has been implicated as an early predictor of subsequent development of schizophrenia (18). Some authors have gone so far as to regard CRP as a causative component of schizophrenia (19) alongside other inflammatory molecules such as IL-8 and TNF-alpha, which may alter neurodevelopment (1). Our finding of a correlation between CRP and negative symptoms is also interesting given the overlap of negative symptoms with cognitive symptoms (45), and a relatively consistent finding in several studies is that higher CRP correlates with worse cognitive function in schizophrenia (19, 24, 30, 46). However, our study failed to find an association between CRP and cognition as assessed by the NIH Toolbox Cognitive Test Battery. A possible explanation for the failure to find an association is the relatively small sample size.
The main strength of this study is the adjustment made for potential confounders, an approach lacking in many of the previous studies. The limitations of this study include the cross-sectional design, the relatively small sample size, and the inability to adjust for other potential confounders such as nicotine use or socioeconomic and educational status.
In conclusion, this study investigates a growing area of inquiry within the realm of schizophrenia research, and might be strengthened by increasing sample size, and through the longitudinal study of CRP in patients with schizophrenia over time, ideally from the onset of disease through late progression. If our findings are replicated, and CRP becomes established as a predictor of negative symptom severity, future studies should aim to elucidate the underlying pathophysiological mechanisms of the association, as this could inform the development of novel treatments for negative symptoms of schizophrenia.
Ethics Statement
The institutional review board of The University of Texas Health Science Center, Houston, Texas approved this study. This research was completed in conformity with the latest version of the Declaration of Helsinki. All study participants completed a written informed consent form after the study was described to them.
Author Contributions
All individuals meeting authorship criteria are listed as authors, and all certify that they contributed sufficiently to take responsibility for the content. OO contributed to the conception and design of the work, and with data analysis. OO, TB, AT, and RC contributed to the drafting of the manuscript as well as critical revision and final approval of the version to be published.
Conflict of Interest Statement
This research was conducted in the absence of any commercial or financial relationships that could be regarded as a potential conflict of interest. The authors take complete responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgments
We would like to acknowledge Gabriel Fries, Gabriela Colpo, and Elena Dyukova, who carried out the CRP ELISA assay.
References
1. Kirkpatrick B, Miller BJ. Inflammation and schizophrenia. Schizophr Bull (2013) 39:1174–9. doi:10.1093/schbul/sbt141
2. van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E, et al. Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biol Psychiatry (2008) 64:820–2. doi:10.1016/j.biopsych.2008.04.025
3. Na K-S, Jung H-Y, Kim Y-K. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry (2014) 48:277–86. doi:10.1016/j.pnpbp.2012.10.022
4. Al-Hakeim HK, Al-Rammahi DA, Al-Dujaili AH. IL-6, IL-18, sIL-2R, and TNFα proinflammatory markers in depression and schizophrenia patients who are free of overt inflammation. J Affect Disord (2015) 182:106–14. doi:10.1016/j.jad.2015.04.044
5. Dean B. Understanding the role of inflammatory-related pathways in the pathophysiology and treatment of psychiatric disorders: evidence from human peripheral studies and CNS studies. Int J Neuropsychopharmacol (2011) 14:997–1012. doi:10.1017/S1461145710001410
6. Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur J Neurosci (2004) 20:467–73. doi:10.1111/j.1460-9568.2004.03514.x
7. D’Mello C, Swain MG. Liver-brain interactions in inflammatory liver diseases: implications for fatigue and mood disorders. Brain Behav Immun (2014) 35:9–20. doi:10.1016/j.bbi.2013.10.009
8. Lucas S-M, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol (2009) 147:S232–40. doi:10.1038/sj.bjp.0706400
9. Engelhardt B, Ransohoff RM. Capture, crawl, cross: the T cell code to breach the blood-brain barriers. Trends Immunol (2012) 33:579–89. doi:10.1016/j.it.2012.07.004
10. Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry (2008) 63:801–8. doi:10.1016/j.biopsych.2007.09.024
11. Hanson DR, Gottesman II. Theories of schizophrenia: a genetic-inflammatory-vascular synthesis. BMC Med Genet (2005) 6:7. doi:10.1186/1471-2350-6-7
12. Keller WR, Kum LM, Wehring HJ, Pharm D, Mathew M, Buchanan RW, et al. A review of anti-inflammatory agents for symptoms of schizophrenia. J Psychopharmacol (2013) 27:337–42. doi:10.1177/0269881112467089.A
13. Sommer IE, Van Westrhenen R, Begemann MJH, De Witte LD, Leucht S, Kahn RS. Efficacy of anti-inflammatory agents to improve symptoms in patients with schizophrenia: an update. Schizophr Bull (2014) 40:181–91. doi:10.1093/schbul/sbt139
14. Andrade C. Anti-inflammatory strategies in the treatment of schizophrenia. Expert Rev Clin Pharmacol (2016) 9:161–3. doi:10.1586/17512433.2016.1095086
15. Miller BJ, Culpepper N, Rapaport MH. C-reactive protein levels in schizophrenia: a review and meta-analysis. Clin Schizophr Relat Psychoses (2014) 7:223–30. doi:10.3371/CSRP.MICU.020813
16. Ohaeri JU, Hedo CC, Lagundoye OO. The profile of C-reactive proteins in functional psychotic states in a cohort in Nigeria. Acta Psychiatr Scand (1993) 88:252–5. doi:10.1111/j.1600-0447.1993.tb03452.x
17. Fernandes BS, Steiner J, Bernstein H-G, Dodd S, Pasco JA, Dean OM, et al. C-reactive protein is increased in schizophrenia but is not altered by antipsychotics: meta-analysis and implications. Mol Psychiatry (2016) 21(4):554–64. doi:10.1038/mp.2015.87
18. Metcalf SA, Jones PB, Nordstrom T, Timonen M, Maki P, Miettunen J, et al. Serum C-reactive protein in adolescence and risk of schizophrenia in adulthood: a prospective birth cohort study. Brain Behav Immun (2017) 59:253–9. doi:10.1016/j.bbi.2016.09.008
19. Inoshita M, Numata S, Tajima A, Kinoshita M, Umehara H, Nakataki M, et al. A significant causal association between C-reactive protein levels and schizophrenia. Sci Rep (2016) 6:26105. doi:10.1038/srep26105
20. Miller BJ, Culpepper N, Rapaport MH, Buckley P. Prenatal inflammation and neurodevelopment in schizophrenia: a review of human studies. Prog Neuropsychopharmacol Biol Psychiatry (2013) 42:92–100. doi:10.1016/j.pnpbp.2012.03.010
21. Bilbo SD, Schwarz JM. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav Neurosci (2009) 3:14. doi:10.3389/neuro.08.014.2009
22. Bulzacka E, Boyer L, Schürhoff F, Godin O, Berna F, Brunel L, et al. Chronic peripheral inflammation is associated with cognitive impairment in schizophrenia: results from the multicentric FACE-SZ dataset. Schizophr Bull (2016) 42:1290–302. doi:10.1093/schbul/sbw029
23. Hope S, Hoseth E, Dieset I, Mørch RH, Aas M, Aukrust P, et al. Inflammatory markers are associated with general cognitive abilities in schizophrenia and bipolar disorder patients and healthy controls. Schizophr Res (2015) 165:188–94. doi:10.1016/j.schres.2015.04.004
24. Johnsen E, Fathian F, Kroken RA, Steen VM, Jørgensen HA, Gjestad R, et al. The serum level of C-reactive protein (CRP) is associated with cognitive performance in acute phase psychosis. BMC Psychiatry (2016) 16:60. doi:10.1186/s12888-016-0769-x
25. Xiu MH, Yang GG, Tan YL, Chen DC, Tan SP, Wang ZR, et al. Decreased interleukin-10 serum levels in first-episode drug-naïve schizophrenia: relationship to psychopathology. Schizophr Res (2014) 156:9–14. doi:10.1016/j.schres.2014.03.024
26. Dimitrov DH, Lee S, Yantis J, Honaker C, Braida N, Walss-Bass C. Elevated serum levels of high-sensitivity C-reactive proteins are associated with severe delusional symptoms in a subgroup of patients with schizophrenia. J Clin Psychiatry (2016) 77:131–2. doi:10.4088/JCP.15l09833
27. Fawzi MH, Fawzi MM, Fawzi MM, Said NS. C-reactive protein serum level in drug-free male Egyptian patients with schizophrenia. Psychiatry Res (2011) 190:91–7. doi:10.1016/j.psychres.2011.05.010
28. Fan X, Pristach C, Liu EY, Freudenreich O, Henderson DC, Goff DC. Elevated serum levels of C-reactive protein are associated with more severe psychopathology in a subgroup of patients with schizophrenia. Psychiatry Res (2007) 149:267–71. doi:10.1016/j.psychres.2006.07.011
29. Akanji AO, Ohaeri JU, Al-Shammri S, Fatania HR. Association of blood levels of C-reactive protein with clinical phenotypes in Arab schizophrenic patients. Psychiatry Res (2009) 169:56–61. doi:10.1016/j.psychres.2008.06.010
30. Dickerson F, Stallings C, Origoni A, Boronow J, Yolken R. C-reactive protein is associated with the severity of cognitive impairment but not of psychiatric symptoms in individuals with schizophrenia. Schizophr Res (2007) 93:261–5. doi:10.1016/j.schres.2007.03.022
31. Devanarayanan S, Nandeesha H, Kattimani S, Sarkar S, Jose J. Elevated copper, hs C-reactive protein and dyslipidemia in drug free schizophrenia: relation with psychopathology score. Asian J Psychiatr (2016) 24:99–102. doi:10.1016/j.ajp.2016.08.025
32. Bartlett D, Firth C, Phillips A, Moss P, Baylis D, Syddall H, et al. The age-related increase in low-grade systemic inflammation (inflammaging) is not driven by cytomegalovirus infection. Aging Cell (2012) 11:912–5. doi:10.1111/j.1474-9726.2012.00849.x
33. Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflamm-aging: an evolutionary perspective on immunosenescence. Ann N Y Acad Sci (2000) 908:244–54. doi:10.1111/j.1749-6632.2000.tb06651.x
34. Oliveira A, Oliveira A, Almeida M, Silva A, Adan L, Ladeia A. Alanine aminotransferase and high sensitivity c-reactive protein: correlates of cardiovascular risk factors in youth. J Pediatr (2008) 152:337–42. doi:10.1016/j.jpeds.2007.07.013
35. Bahceci M, Gokalp D, Bahceci S, Tuzcu A, Atmaca S, Arikan S. The correlation between adiposity and adiponectin, tumor necrosis factor alpha, interleukin-6 and high sensitivity C-reactive protein levels. Is adipocyte size associated with inflammation in adults? J Endocrinol Invest (2007) 30:210–4. doi:10.1007/BF03347427
36. Raitakari M, Mansikkaniemi K, Marniemi J, Viikari J, Raitakari O. Distribution and determinants of serum high-sensitive C-reactive protein in a population of young adults: the cardiovascular risk in Young Finns Study. J Intern Med (2005) 258:428–34. doi:10.1111/j.1365-2796.2005.01563.x
37. Casaletto KB, Umlauf A, Beaumont J, Gershon R, Slotkin J, Akshoomoff N, et al. Demographically corrected normative standards for the English version of the NIH toolbox cognition battery. J Int Neuropsychol Soc (2016) 21:378–91. doi:10.1017/S1355617715000351.Demographically
38. Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry (1998) 59:22–33. doi:10.1016/S0924-9338(99)80239-9
39. Kay SR, Fiszbein A, Opier L. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull (1987) 13:261–76. doi:10.1093/schbul/13.2.261
40. Reynolds H. Nonparametric partial correlation and causal analysis. Sociol Methods Res (1974) 2:376–92. doi:10.1177/004912417400200306
41. Miller CL, Llenos IC, Dulay JR, Weis S. Upregulation of the initiating step of the kynurenine pathway in postmortem anterior cingulate cortex from individuals with schizophrenia and bipolar disorder. Brain Res (2006) 1073–1074:25–37. doi:10.1016/j.brainres.2005.12.056
42. Erhardt S, Schwieler L, Imbeault S, Engberg G. The kynurenine pathway in schizophrenia and bipolar disorder. Neuropharmacology (2017) 112:297–306. doi:10.1016/j.neuropharm.2016.05.020
43. Barry S, Clarke G, Scully P, Dinan TG. Kynurenine pathway in psychosis: evidence of increased tryptophan degradation. J Psychopharmacol (2008) 23:287–94. doi:10.1177/0269881108089583
44. Kegel ME, Bhat M, Skogh E, Samuelsson M, Lundberg K, Dahl M-L, et al. Imbalanced kynurenine pathway in schizophrenia. Int J Tryptophan Res (2014) 7:15–22. doi:10.4137/IJTR.S13958.Received
45. Lim J, Lee S, Lam M, Rapisarda A, Kraus M, Keefe R, et al. The relationship between negative symptom subdomains and cognition. Psychol Med (2016) 46:2169–77. doi:10.1017/S0033291716000726
Keywords: schizophrenia, C-reactive protein, inflammation, psychosis, PANSS, cognition, NIH toolbox
Citation: Boozalis T, Teixeira AL, Cho RY-J and Okusaga O (2018) C-Reactive Protein Correlates with Negative Symptoms in Patients with Schizophrenia. Front. Public Health 5:360. doi: 10.3389/fpubh.2017.00360
Received: 02 October 2017; Accepted: 19 December 2017;
Published: 22 January 2018
Edited by:
Richard Eugene Frye, University of Arkansas for Medical Sciences, United StatesReviewed by:
Adonis Sfera, Loma Linda University, United StatesKonstantin Khodosevich, University of Copenhagen, Denmark
Copyright: © 2018 Boozalis, Teixeira, Cho and Okusaga. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Olaoluwa Okusaga, olaokusaga1@yahoo.com
 Ted Boozalis
Ted Boozalis Antonio L. Teixeira1,2
Antonio L. Teixeira1,2 Raymond Young-Jin Cho
Raymond Young-Jin Cho Olaoluwa Okusaga
Olaoluwa Okusaga