- 1Department of Psychiatry, National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan
- 2Department of Behavioral Medicine, National Institute of Mental Health, National Center of Neurology and Psychiatry, Tokyo, Japan
- 3Department of Educational Promotion, Clinical Research and Education Promotion Division, National Center of Neurology and Psychiatry, Tokyo, Japan
- 4Department of Preventive Intervention for Psychiatric Disorders, National Institute of Mental Health, National Center of Neurology and Psychiatry, Tokyo, Japan
Backgrounds: Patients with schizophrenia suffer from cognitive impairment that worsens real-world functional outcomes. We previously reported that multi-session transcranial direct current stimulation (tDCS) delivered to the left dorsolateral prefrontal cortex (DLPFC) improved daily living skills, while stimulation on the left superior temporal sulcus (STS) enhanced performance on a test of social cognition in these patients. To examine the region-dependent influence of tDCS on daily-living skills, neurocognition, and psychotic symptoms, this study compared effects of anodal stimulation targeting either of these two brain areas in patients with schizophrenia.
Methods: Data were collected from open-label, single-arm trials with anodal electrodes placed over the left DLPFC (N = 28) or STS (N = 15). Daily-living skills, neurocognition, and psychotic symptoms were measured with the UCSD performance-based skills assessment-brief (UPSA-B), Brief Assessment of Cognition in Schizophrenia (BACS), and Positive and Negative Syndrome Scale (PANSS), respectively. After baseline evaluation, tDCS (2 mA × 20 min) were delivered two times per day for 5 consecutive days. One month after the final stimulation, clinical assessments were repeated.
Results: Performance on the UPSA-B was significantly improved in patients who received anodal tDCS at the left DLPFC (d = 0.70, p < 0.001), while this effect was absent in patients with anodal electrodes placed on the left STS (d = 0.02, p = 0.939). Significant improvement was also observed for scores on the BACS with anodal tDCS delivered to the DLPFC (d = 0.49, p < 0.001); however, such neurocognitive enhancement was absent when the STS was stimulated (d = 0.05, p = 0.646). Both methods of anodal stimulation showed a significant improvement of General Psychopathology scores on the PANSS (DLPFC, d = 0.50, p = 0.027; STS, d = 0.44, p = 0.001).
Conclusion: These results indicate the importance of selecting brain regions as a target for tDCS according to clinical features of individual patients. Anodal stimulation of the left DLPFC may be advantageous in improving higher level functional outcomes in patients with schizophrenia.
Trial registration: These studies were registered within the University hospital Medical Information Network Clinical Trials Registry [(24), UMIN000015953], and the Japan Registry of Clinical Trials [(28), jRCTs032180026].
1. Introduction
Schizophrenia is one of the most prominent causes of disease burdens worldwide (1), with a prevalence of about 0.75% (2). The main symptoms of the disease include positive symptoms (e.g., hallucinations, delusions), negative symptoms (e.g., apathy, anhedonia, and social withdrawal), and disturbances of several types of cognitive function (e.g., neurocognition and social cognition). Positive symptoms are well treated with antipsychotic drugs, whereas negative symptoms and cognitive dysfunctions are not adequately managed by pharmacotherapy (3). In particular, cognitive impairment leads to a decline in real-world functional outcome, and more than 70% of chronic patients are not employed (4, 5).
Impairments of neurocognition and social cognition have been implicated in social functioning in patients with schizophrenia (6, 7). Neurocognitive domains, such as attention/processing speed, working memory, learning memory, and reasoning and problem solving, are most severely affected (7). On the other hand, social cognition domains affected in schizophrenia includes theory of mind (ToM), emotion recognition, social perception, and attributional bias (8, 9). Several interventional methods, e.g., psychosocial and pharmacological approaches, have been tested to enhance neurocognition and social cognition in patients with schizophrenia (10–16).
Transcranial direct current stimulation (tDCS) is a non-invasive brain stimulation method that modulates neural activity by applying electric currents, usually less than 2 mA, between an anode and cathode electrode for a short period of time (usually less than 30 min per session) (17). Previous meta-analyses reported that tDCS delivered to the dorsolateral prefrontal cortex (DLPFC) alleviates hallucinations (positive symptoms; Hedges’ g = 0.86) and negative symptoms (0.41), and improves neurocognitive function, particularly working memory (0.41), in patients with schizophrenia (18–23). Recently, tDCS targeting the DLPFC has also been reported to improve daily-living skills (functional capacity) (24), insight into the illness (25), and metacognition (26). Regarding social cognition, data from our systematic review indicate tDCS on the prefrontal cortex enhances emotion recognition (27), while stimulation on the left superior temporal sulcus (STS) improved scores on the ToM in these patients (28–30). Therefore, the electrode montage of tDCS, especially the anodal stimulation site, may determine its effect on symptoms and functionality in patients with psychotic conditions, although controversy exists (31–33). Taken together, further considerations are needed to understand which brain regions should be stimulated to improve specific symptoms of schizophrenia (34).
In the present study, we compared effects of anodal tDCS targeting the left DLPFC or left STS on symptoms and functional outcomes in patients with schizophrenia. For this purpose, data from our previous studies targeting either of the two brain regions (24, 28), with the same placement of cathodal electrodes and stimulus frequency and intensity, were analyzed. The previous study targeting the left DLPFC (24) measured three indicators, i.e., psychotic symptoms, neurocognition, and daily-living skills (functional capacity), except social cognition, whereas another previous study targeting the left STS, independently conducted (28), measured all four indicators including social cognition. The latter study did not report the results of daily-living skills (28). Therefore, the present study provides new information, i.e., the effect of anodal tDCS targeting the left STS on daily-living skills, as accumulated evidence suggests the performance on the functional capacity provides important indicator of social functioning (35).
2. Materials and methods
2.1. Participants and procedure
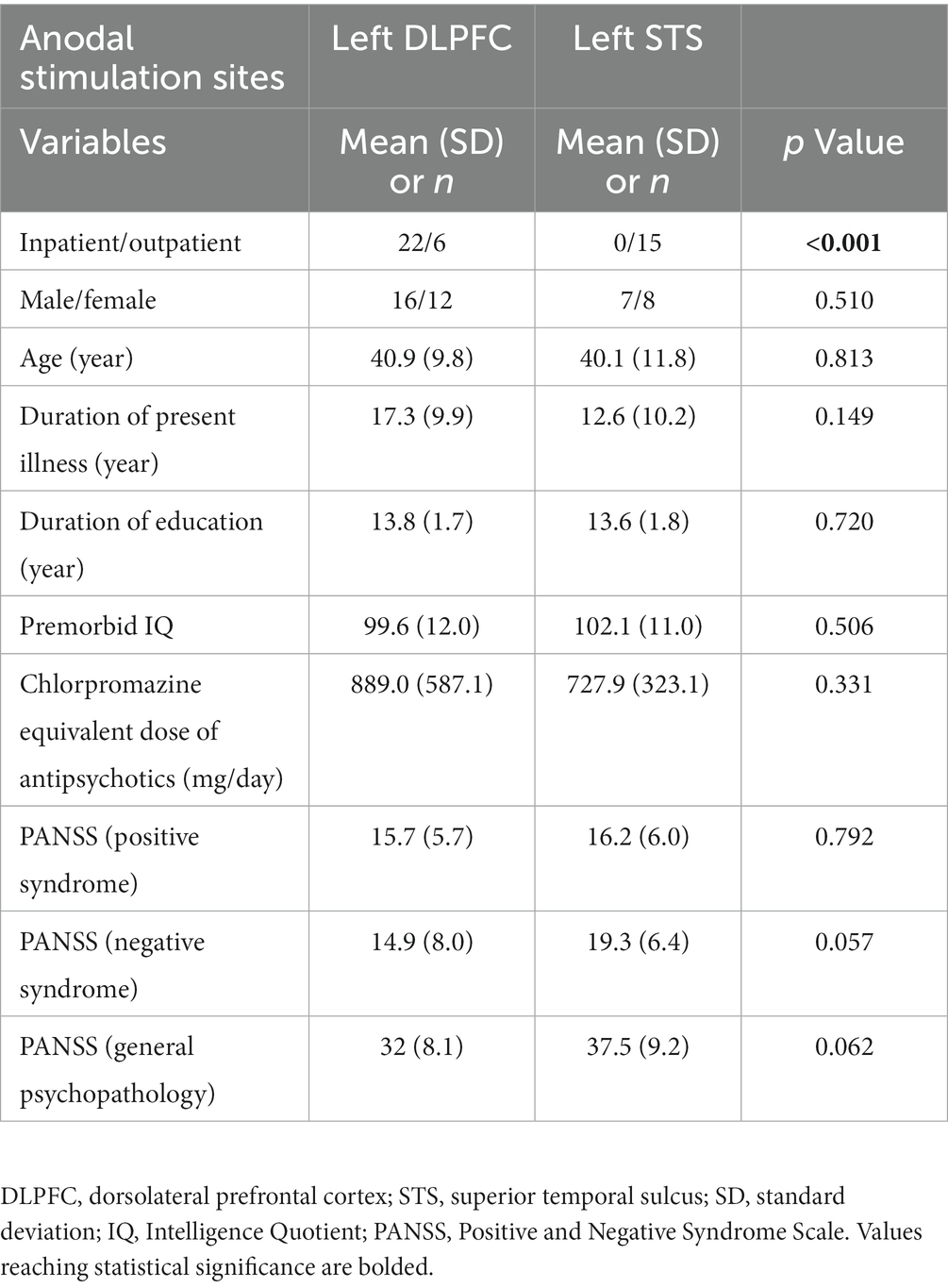
Data were collected from previous studies (Narita et al., UMIN000015953; Yamada et al., jRCTs032180026) (24, 28). The protocols of these studies and demographic data of participants have been reported (24, 28, 29). Both studies were open-label, single-arm trials, conducted in a single-center at the National Center of Neurology and Psychiatry, Japan. Twenty-eight patients with schizophrenia (22 inpatients and 6 outpatients) received anodal stimulation delivered to the left DLPFC (24), while 15 outpatients received the stimulation to the left STS (28). The former study about the anodal stimulation of the DLPFC demonstrated improvements in daily-living skills. Therefore, the latter study targeting the STS implemented a sample size calculation which was based on the results of daily-living skills. Specifically, the minimum number of samples to achieve a value of p of 0.05 and 80% power was found 13, assuming a standard deviation of 15 and a mean difference of 11. Taking drop-outs into account, the sample size was set at 15 (28). Both studies were independent and data were derived from non-controlled trials. Inclusion and exclusion criteria are provided in Supplementary Tables 1, 2. The details of data collection have been described (29).
2.2. Intervention
Direct current was transmitted through 35 cm2 saline-soaked sponge electrodes, and the intervention was performed by a 1 × 1 transcranial direct current low-intensity stimulator (Model 1,300 A; Soterix Medical Inc., New York, NY, United States). For tDCS montage, the anode was placed on F3 (the international 10–20 electroencephalography system) to deliver currents to the left DLPFC or T3 to the left STS, while the cathode was placed on FP2 regions in both cases. We applied 10 sessions direct current of 2 mA for 20 min on 5 consecutive days (twice per day, with an interval of 30 min; Supplementary Table 3).
2.3. Outcomes
After being briefed on the purpose of the study and agreeing to participate, patients received psychological and clinical assessments, including the screening evaluation. Data were collected at baseline and 1 month after the final stimulus (Supplementary Table 3).
2.3.1. Daily-living skills (functional capacity)
Daily-living skills were assessed by the UCSD Performance-Based Skills Assessment-Brief (UPSA-B), which consists of financial and communication skills (36). Subscale scores of the two domains of the UPSA-B (i.e., finances and communication) were converted into the standard score ranging from 0 to 50 to make the maximum of the total score of 100. Higher scores indicate greater functional capacity.
2.3.2. Neurocognition
The Brief Assessment of Cognition in Schizophrenia (BACS) Japanese version was used to evaluate verbal memory (verbal memory list learning), working memory (digit sequencing task), speed of information processing (symbol coding), motor speed (token motor task), executive functions (tower of London) and verbal fluency (37). To provide a standard metric for combining test scores into domains and comparing performance over time, BACS scores were converted to z-scores to represent performance relative to that of healthy people (38).
2.3.3. Psychotic symptoms
The Positive and Negative Syndrome Scale (PANSS) was used to assess psychotic symptoms (39). The PANSS was a structured interview, consisting of positive, negative, and general psychopathology subscales (with scores ranging from 7 to 49, from 7 to 49, and from 16 to 112, respectively). The higher scores represent more severe psychotic symptoms.
2.4. Statistical analysis
Student’s t-test was used for the clinical outcomes to evaluate the efficacy, while effect sizes were calculated as standardized mean difference (Cohen’s d). Categorical variables were compared by the Chi-Squared test. Statistical analysis was conducted using IBM SPSS Statistics version 26.0.
3. Results
3.1. Participants
The demographic variables are shown in Table 1. There were no differences between participants in the two studies in potential parameters affecting cognitive/functional outcomes, i.e., age, duration of present illness, duration of education, estimated premorbid Intelligence Quotient (IQ), dose of antipsychotics, and severity of psychotic symptoms. No medication was changed, nor was cognitive rehabilitation performed during the study period.
3.2. Daily-living skills (functional capacity)
Performance on the UPSA-B was significantly improved in patients who received anodal tDCS at the left DLPFC (d = 0.70, p < 0.001), while this advantage was absent with anodal electrodes placed on the STS (d = 0.02, p = 0.939; Table 2). Similarly, anodal stimulation on the DLPFC significantly improved scores on the tests of financial skills (d = 0.61, p = 0.002) or communication skills (d = 0.59, p = 0.001) from the UPSA-B, while such changes were absent in patients with anodal electrodes for the STS (financial skills, d = 0.14, p = 0.635; communication skills, d = 0.07, p = 0.760; Table 2).
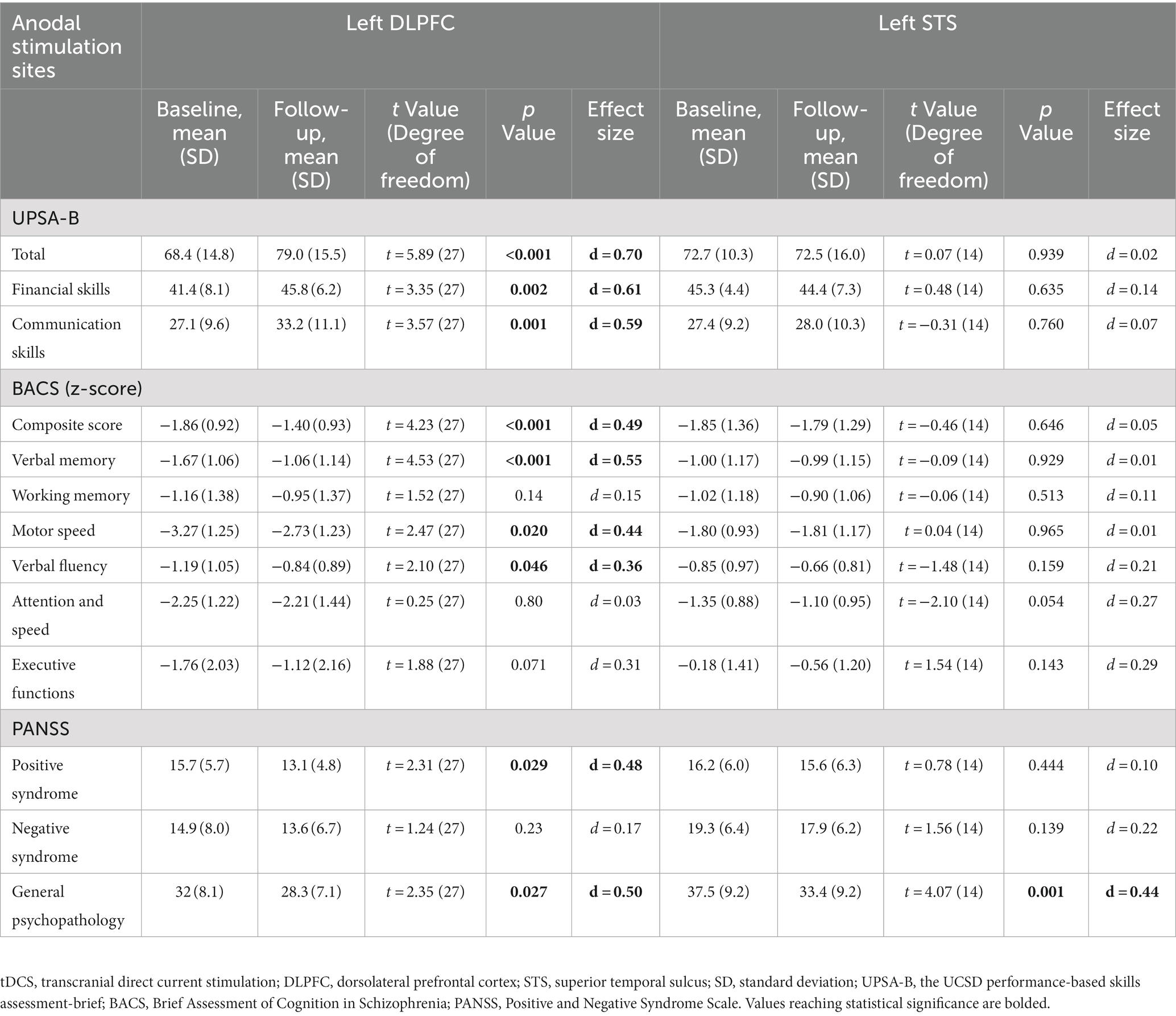
Table 2. Outcome measures at baseline and 1 month after the transcranial direct current stimulation (tDCS).
3.3. Neurocognition
Significant improvement was observed on the composite score of the BACS in patients who received anodal tDCS delivered to the left DLPFC (d = 0.49, p < 0.001), while such neurocognitive enhancement was absent with anodal stimulation targeting the left STS (d = 0.05, p = 0.646; Table 2). Similarly, anodal stimulation delivered to the DLPFC was associated with improvements in verbal memory (d = 0.55, p < 0.001), motor speed (d = 0.44, p = 0.020), and verbal fluency (d = 0.36, p = 0.046), while these benefits were absent when anodal stimulation was targeted to the STS (verbal memory, d = 0.01, p = 0.929; motor speed, d = 0.01, p = 0.965; verbal fluency, d = 0.21, p = 0.159; Table 2).
3.4. Psychotic symptoms
Anodal tDCS to either the left DLPFC (d = 0.50, p = 0.027) or STS (d = 0.44, p = 0.001) showed a significant improvement of general psychopathology scores on the PANSS. On the other hand, anodal stimulation delivered to the DLPFC (d = 0.48, p = 0.029), but not the STS (d = 0.10, p = 0.444) was associated with amelioration of positive symptoms. For negative symptoms, neither stimulation method was effective (DLPFC, d = 0.17, p = 0.230; STS, d = 0.22, p = 0.139).
4. Discussion
The results of the present study indicate that the placement of electrodes influences the ability of tDCS to ameliorate symptoms and improve functional outcome in patients with schizophrenia. This concept is supported by observations that anodal stimulation delivered to the left DLPFC, but not left STS was efficacious in enhancing daily-living skills and alleviating positive symptoms, while stimulation of the latter brain region was associated with improvement of social cognition.
Several neural substrates may explain the ability of anodal tDCS to enhance cognitive function and related functional capacity (daily-living skills). Altered structures in the brain of patients with schizophrenia has been reported, e.g., reduced grey matter in the frontotemporal lobe, thalamus, hippocampus, amygdala, insular cortex, and anterior cingulate cortex (40). Specifically, neurocognitive dysfunction is assumed to result from atrophy of the hippocampus and the DLPFC (40). The degree of change in brain morphology varies among individuals with schizophrenia, yielding variances of electric fields in brain cortical areas produced by tDCS with the same electrode montage (31–33).
Data from the present study indicate differential clinical benefits which depends on the placement of the anodal electrode. Traditionally, social cognitive function has been associated with the neural circuitry involving the STS, medial prefrontal cortex, and middle temporal gyrus (29). Despite previous observations (7, 9, 41, 42), the results of the present study suggest that social cognition does not appear to be directly linked to improvements in functional capacity, as indicated by the lack of efficacy anodal tDCS on the STS (28). In sum, the ability of anodal stimulation on the DLPFC, but not STS to enhance functional capacity (daily-living skills) provides insight into neural mechanisms for therapeutics to improve higher functional outcomes in patients with schizophrenia.
The amelioration of positive symptoms by anodal tDCS delivered to the left DLPFC indicates the role for this brain region in the treatment of psychotic conditions. Specifically, the neural circuit with the hippocampus as a hub may play a role. Thus, anodal stimulation on the DLPFC is assumed to modulate the activities of hippocampus through this neural circuit, possibly via alterations of monoaminergic neurotransmissions (17). This concept is consistent with observations that increased activities of the hippocampus are related to positive symptoms (40). Therefore, the ability of anodal stimulation on the DLPFC to alleviate positive symptoms may be related to modulation of hyperactivity of the hippocampus.
Data from the current study suggest the importance of selecting brain areas to be targeted by tDCS according to clinical features of individual patients. The frontal brain regions, e.g., the left DLPFC, has been used in most studies showing improvement of psychotic symptoms and functionality in patients with schizophrenia (18, 19, 24), although some argued against this concept (31–33, 43, 44). However, these brain areas may not be ideal as a target for alleviating social cognitive impairment (27, 29, 30). Further study is warranted to confirm the present results with randomized controlled trial with target brain regions of the DLPFC or STS.
The limitations of this study should be mentioned. First, the small sample size may raise caution in generalizing the present results. Second, as we compared results from previous studies with different populations, e.g., the ratio of inpatient/outpatient number across the two studies, it cannot be ruled out that the characteristics of participants may have influenced the results. The modest internal validity and low external validity indicate the need for further validation before generalizing the present findings to clinical practice.
5. Conclusion
The results of the present study suggest that anodal stimulation site of tDCS may govern its effect on symptoms and functionality in patients with schizophrenia. For improving daily-living skills associated with neurocognition, the left DLPFC may provide an optimal brain region to be targeted.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the National Center of Neurology and Psychiatry Clinical Research Review Board (CRB3180006). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YYa and TS developed the original concept for the study and designed it. ZN advised on the statistical analysis, while KS advised on the outcome measures. AS, YYa, and ZN administered tDCS. TI and TS recruited participants. TS organized the team for this study. YYa wrote the original draft. All other authors reviewed and commented on the subsequent drafts, and approved the submitted version.
Funding
This work was partially supported by the Japan Society for the Promotion of Science KAKENHI grant no. 20 K16635 to YYa, as well as JSPS KAKENHI grant no. 20H03610, Intramural Research Grants for Neurological and Psychiatric Disorders of NCNP (3-1, 5-3), and Japan Health Research Promotion Bureau Grants (2021-B-01) to TS.
Acknowledgments
We would like to thank Kazuyuki Nakagome and Shinsuke Kito at the National Center of Neurology and Psychiatry for their discussions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1243859/full#supplementary-material
References
1. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/S0140-6736(20)30925-9
2. Moreno-Küstner, B, Martín, C, and Pastor, L. Prevalence of psychotic disorders and its association with methodological issues. A systematic review and meta-analyses. PloS One. (2018) 13:e0195687. doi: 10.1371/journal.pone.0195687
3. Haddad, PM, and Correll, CU. The acute efficacy of antipsychotics in schizophrenia: a review of recent meta-analyses. Ther Adv Psychopharmacol. (2018) 8:303–18. doi: 10.1177/2045125318781475
4. McGurk, SR, Mueser, KT, Harvey, PD, LaPuglia, R, and Marder, J. Cognitive and symptom predictors of work outcomes for clients with schizophrenia in supported employment. Psychiatr Serv. (2003) 54:1129–35. doi: 10.1176/appi.ps.54.8.1129
5. Marwaha, S, and Johnson, S. Schizophrenia and employment - a review. Soc Psychiatry Psychiatr Epidemiol. (2004) 39:337–49. doi: 10.1007/s00127-004-0762-4
6. Yamada, Y, Inagawa, T, Sueyoshi, K, Sugawara, N, Ueda, N, Omachi, Y, et al. Social cognition deficits as a target of early intervention for psychoses: a systematic review. Front Psych. (2019) 10:333. doi: 10.3389/fpsyt.2019.00333
7. Fett, AK, Viechtbauer, W, Dominguez, MD, Penn, DL, van Os, J, and Krabbendam, L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev. (2011) 35:573–88. doi: 10.1016/j.neubiorev.2010.07.001
8. Penn, DL, Sanna, LJ, and Roberts, DL. Social cognition in schizophrenia: an overview. Schizophr Bull. (2008) 34:408–11. doi: 10.1093/schbul/sbn014
9. Brekke, J, Kay, DD, Lee, KS, and Green, MF. Biosocial pathways to functional outcome in schizophrenia. Schizophr Res. (2005) 80:213–25. doi: 10.1016/j.schres.2005.07.008
10. Nijman, SA, Veling, W, van der Stouwe, ECD, and Pijnenborg, GHM. Social cognition training for people with a psychotic disorder: a network Meta-analysis. Schizophr Bull. (2020) 46:1086–103. doi: 10.1093/schbul/sbaa023
11. Yamada, Y, Okubo, R, Tachimori, H, Uchino, T, Kubota, R, Okano, H, et al. Pharmacological interventions for social cognitive impairments in schizophrenia: a protocol for a systematic review and network meta-analysis. Front Psychol. (2022) 13:878829. doi: 10.3389/fpsyg.2022.878829
12. Bürkner, PC, Williams, DR, Simmons, TC, and Woolley, JD. Intranasal oxytocin may improve high-level social cognition in schizophrenia, but not social cognition or Neurocognition in general: a multilevel Bayesian Meta-analysis. Schizophr Bull. (2017) 43:1291–303. doi: 10.1093/schbul/sbx053
13. Gabay, AS, Kempton, MJ, and Mehta, MA. Facial affect processing deficits in schizophrenia: a meta-analysis of antipsychotic treatment effects. J Psychopharmacol. (2015) 29:224–9. doi: 10.1177/0269881114560184
14. Twamley, EW, Jeste, DV, and Bellack, AS. A review of cognitive training in schizophrenia. Schizophr Bull. (2003) 29:359–82. doi: 10.1093/oxfordjournals.schbul.a007011
15. Harvey, PD, Bosia, M, Cavallaro, R, Howes, OD, Kahn, RS, Leucht, S, et al. Cognitive dysfunction in schizophrenia: an expert group paper on the current state of the art. Schizophr Res Cogn. (2022) 29:100249. doi: 10.1016/j.scog.2022.100249
16. Lopez-Morinigo, JD, Leucht, S, and Arango, C. Pharmacological treatment of early-onset schizophrenia: a critical review, evidence-based clinical guidance and unmet needs. Pharmacopsychiatry. (2022) 55:233–45. doi: 10.1055/a-1854-0185
17. Yamada, Y, and Sumiyoshi, T. Neurobiological mechanisms of transcranial direct current stimulation for psychiatric disorders; neurophysiological, chemical, and anatomical considerations. Front Hum Neurosci. (2021) 15:631838. doi: 10.3389/fnhum.2021.631838
18. Narita, Z, Stickley, A, DeVylder, J, Yokoi, Y, Inagawa, T, Yamada, Y, et al. Effect of multi-session prefrontal transcranial direct current stimulation on cognition in schizophrenia: a systematic review and meta-analysis. Schizophr Res. (2020) 216:367–73. doi: 10.1016/j.schres.2019.11.011
19. Kim, J, Iwata, Y, Plitman, E, Caravaggio, F, Chung, JK, Shah, P, et al. A meta-analysis of transcranial direct current stimulation for schizophrenia: "is more better?". J Psychiatr Res. (2019) 110:117–26. doi: 10.1016/j.jpsychires.2018.12.009
20. Palm, U, Keeser, D, Hasan, A, Kupka, MJ, Blautzik, J, Sarubin, N, et al. Prefrontal transcranial direct current stimulation for treatment of schizophrenia with predominant negative symptoms: a double-blind, Sham-controlled proof-of-concept study. Schizophr Bull. (2016) 42:1253–61. doi: 10.1093/schbul/sbw041
21. Lisoni, J, Baldacci, G, Nibbio, G, Zucchetti, A, Butti Lemmi Gigli, E, Savorelli, A, et al. Effects of bilateral, bipolar-nonbalanced, frontal transcranial direct current stimulation (tDCS) on negative symptoms and neurocognition in a sample of patients living with schizophrenia: results of a randomized double-blind sham-controlled trial. J Psychiatr Res. (2022) 155:430–42. doi: 10.1016/j.jpsychires.2022.09.011
22. Smith, RC, Boules, S, Mattiuz, S, Youssef, M, Tobe, RH, Sershen, H, et al. Effects of transcranial direct current stimulation (tDCS) on cognition, symptoms, and smoking in schizophrenia: a randomized controlled study. Schizophr Res. (2015) 168:260–6. doi: 10.1016/j.schres.2015.06.011
23. Jeon, DW, Jung, DU, Kim, SJ, Shim, JC, Moon, JJ, Seo, YS, et al. Adjunct transcranial direct current stimulation improves cognitive function in patients with schizophrenia: a double-blind 12-week study. Schizophr Res. (2018) 197:378–85. doi: 10.1016/j.schres.2017.12.009
24. Narita, Z, Inagawa, T, Sueyoshi, K, Lin, C, and Sumiyoshi, T. Possible facilitative effects of repeated anodal transcranial direct current stimulation on functional outcome 1 month later in schizophrenia: an open trial. Front Psych. (2017) 8:184. doi: 10.3389/fpsyt.2017.00184
25. Adam, O, Blay, M, Brunoni, AR, Chang, HA, Gomes, JS, Javitt, DC, et al. Efficacy of transcranial direct current stimulation to improve insight in patients with schizophrenia: a systematic review and Meta-analysis of randomized controlled trials. Schizophr Bull. (2022) 48:1284–94. doi: 10.1093/schbul/sbac078
26. Chang, CC, Kao, YC, Chao, CY, and Chang, HA. Enhancement of cognitive insight and higher-order neurocognitive function by fronto-temporal transcranial direct current stimulation (tDCS) in patients with schizophrenia. Schizophr Res. (2019) 208:430–8. doi: 10.1016/j.schres.2018.12.052
27. Yamada, Y, Inagawa, T, Hirabayashi, N, and Sumiyoshi, T. Emotion recognition deficits in psychiatric disorders as a target of non-invasive neuromodulation: a systematic review. Clin EEG Neurosci. (2022) 53:506–12. doi: 10.1177/1550059421991688
28. Yamada, Y, Sueyoshi, K, Yokoi, Y, Inagawa, T, Hirabayashi, N, Oi, H, et al. Transcranial direct current stimulation on the left superior temporal sulcus improves social cognition in schizophrenia: an open-label study. Front Psych. (2022) 13:862814. doi: 10.3389/fpsyt.2022.862814
29. Yamada, Y, Inagawa, T, Yokoi, Y, Shirama, A, Sueyoshi, K, Wada, A, et al. Efficacy and safety of multi-session transcranial direct current stimulation on social cognition in schizophrenia: a study protocol for an open-label, single-arm trial. J Pers Med. (2021) 11:317. doi: 10.3390/jpm11040317
30. Yamada, Y, and Sumiyoshi, T. “Transcranial direct current stimulation and social cognition impairments of schizophrenia, current knowledge and future perspectives,” in Horizons in neuroscience research. eds. S. Costa and E. Villalba. vol. 46. New York: Nova Science Publishers (2022). 143–70.
31. Laakso, I, Tanaka, S, Mikkonen, M, Koyama, S, Sadato, N, and Hirata, A. Electric fields of motor and frontal tDCS in a standard brain space: a computer simulation study. Neuroimage. (2016) 137:140–51. doi: 10.1016/j.neuroimage.2016.05.032
32. López-Alonso, V, Cheeran, B, Río-Rodríguez, D, and Fernández-Del-Olmo, M. Inter-individual variability in response to non-invasive brain stimulation paradigms. Brain Stimul. (2014) 7:372–80. doi: 10.1016/j.brs.2014.02.004
33. Chew, T, Ho, KA, and Loo, CK. Inter- and intra-individual variability in response to transcranial direct current stimulation (tDCS) at varying current intensities. Brain Stimul. (2015) 8:1130–7. doi: 10.1016/j.brs.2015.07.031
34. Kostova, R, Cecere, R, Thut, G, and Uhlhaas, PJ. Targeting cognition in schizophrenia through transcranial direct current stimulation: a systematic review and perspective. Schizophr Res. (2020) 220:300–10. doi: 10.1016/j.schres.2020.03.002
35. Harvey, PD, and Isner, EC. Cognition, social cognition, and functional capacity in early-onset schizophrenia. Child Adolesc Psychiatr Clin N Am. (2020) 29:171–82. doi: 10.1016/j.chc.2019.08.008
36. Sumiyoshi, C, Takaki, M, Okahisa, Y, Patterson, TL, Harvey, PD, and Sumiyoshi, T. Utility of the UCSD performance-based skills assessment-brief Japanese version: discriminative ability and relation to neurocognition. Schizophr Res Cogn. (2014) 1:137–43. doi: 10.1016/j.scog.2014.08.002
37. Kaneda, Y, Sumiyoshi, T, Keefe, R, Ishimoto, Y, Numata, S, and Ohmori, T. Brief assessment of cognition in schizophrenia: validation of the Japanese version. Psychiatry Clin Neurosci. (2007) 61:602–9. doi: 10.1111/j.1440-1819.2007.01725.x
38. Kaneda, Y, Ohmori, T, Okahisa, Y, Sumiyoshi, T, Pu, S, Ueoka, Y, et al. Measurement and treatment research toImprove cognition in schizophrenia consensus cognitive battery: validation of the Japanese version. Psychiatry Clin Neurosci. (2013) 67:182–8. doi: 10.1111/pcn.12029
39. Kay, SR, Fiszbein, A, and Opler, LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. (1987) 13:261–76. doi: 10.1093/schbul/13.2.261
40. Yamada, Y, Matsumoto, M, Iijima, K, and Sumiyoshi, T. Specificity and continuity of schizophrenia and bipolar disorder: relation to biomarkers. Curr Pharm Des. (2020) 26:191–200. doi: 10.2174/1381612825666191216153508
41. Halverson, TF, Orleans-Pobee, M, Merritt, C, Sheeran, P, Fett, AK, and Penn, DL. Pathways to functional outcomes in schizophrenia spectrum disorders: Meta-analysis of social cognitive and neurocognitive predictors. Neurosci Biobehav Rev. (2019) 105:212–9. doi: 10.1016/j.neubiorev.2019.07.020
42. Hasson-Ohayon, I, Goldzweig, G, Lavi-Rotenberg, A, Luther, L, and Lysaker, PH. The centrality of cognitive symptoms and metacognition within the interacting network of symptoms, neurocognition, social cognition and metacognition in schizophrenia. Schizophr Res. (2018) 202:260–6. doi: 10.1016/j.schres.2018.07.007
43. Mehta, AR, Pogosyan, A, Brown, P, and Brittain, JS. Montage matters: the influence of transcranial alternating current stimulation on human physiological tremor. Brain Stimul. (2015) 8:260–8. doi: 10.1016/j.brs.2014.11.003
44. Ho, KA, Taylor, JL, Chew, T, Gálvez, V, Alonzo, A, Bai, S, et al. The effect of transcranial direct current stimulation (tDCS) electrode size and current intensity on motor cortical excitability: evidence from single and repeated sessions. Brain Stimul. (2016) 9:1–7. doi: 10.1016/j.brs.2015.08.003
Keywords: neuromodulation, transcranial direct current stimulation (tDCS), schizophrenia, functional capacity, cognition
Citation: Yamada Y, Narita Z, Inagawa T, Yokoi Y, Hirabayashi N, Shirama A, Sueyoshi K and Sumiyoshi T (2023) Electrode montage for transcranial direct current stimulation governs its effect on symptoms and functionality in schizophrenia. Front. Psychiatry. 14:1243859. doi: 10.3389/fpsyt.2023.1243859
Edited by:
Kang Sim, Institute of Mental Health, SingaporeCopyright © 2023 Yamada, Narita, Inagawa, Yokoi, Hirabayashi, Shirama, Sueyoshi and Sumiyoshi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tomiki Sumiyoshi, sumiyot@ncnp.go.jp
 Yuji Yamada
Yuji Yamada Zui Narita2
Zui Narita2 Yuma Yokoi
Yuma Yokoi Naotsugu Hirabayashi
Naotsugu Hirabayashi Aya Shirama
Aya Shirama Kazuki Sueyoshi
Kazuki Sueyoshi Tomiki Sumiyoshi
Tomiki Sumiyoshi