- 1Department of Neurology, Ahmanson-Lovelace Brain Mapping Center, University of California, Los Angeles, Los Angeles, CA, United States
- 2Department of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, Los Angeles, CA, United States
Background: Total sleep deprivation (TSD) transiently reverses depressive symptoms in a majority of patients with depression. How TSD modulates diffusion tensor imaging (DTI) measures of white matter (WM) microstructure, which may be linked with TSD’s rapid antidepressant effects, remains uncharacterized.
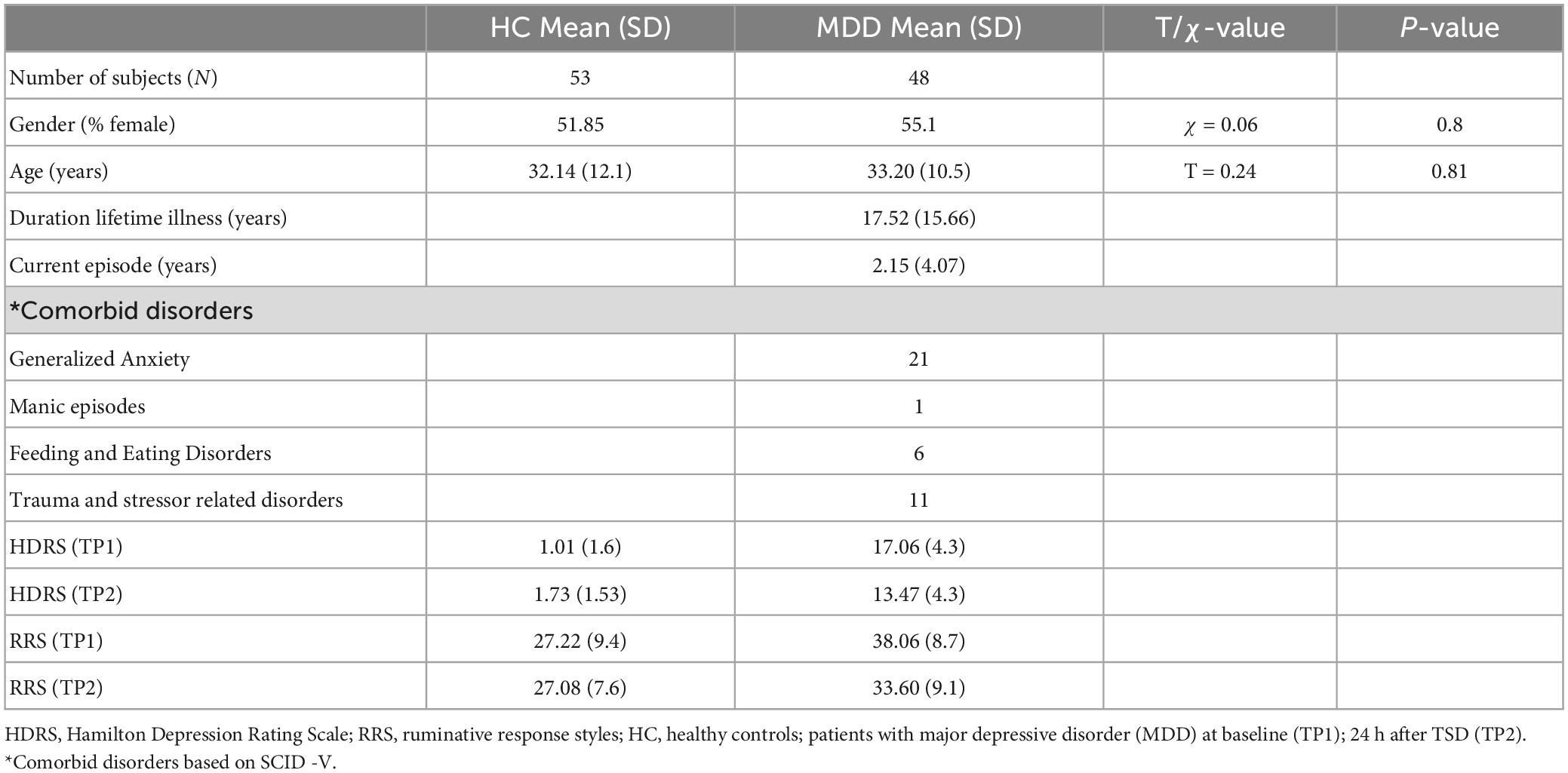
Methods: Patients with depression (N = 48, mean age = 33, 26 women) completed diffusion-weighted imaging and Hamilton Depression Rating (HDRS) and rumination scales before and after >24 h of TSD. Healthy controls (HC) (N = 53, 23 women) completed the same assessments at baseline, and after receiving TSD in a subset of HCs (N = 15). Tract based spatial statistics (TBSS) investigated voxelwise changes in fractional anisotropy (FA) across major WM pathways pre-to-post TSD in patients and HCs and between patients and HCs at baseline. Post hoc analyses tested for TSD effects for other diffusion metrics, and the relationships between change in diffusion measures with change in mood and rumination symptoms.
Results: Significant improvements in mood and rumination occurred in patients with depression (both p < 0.001), but not in HCs following TSD. Patients showed significant (p < 0.05, corrected) decreases in FA values in multiple WM tracts, including the body of the corpus callosum and anterior corona radiata post-TSD. Significant voxel-level changes in FA were not observed in HCs who received TSD (p > 0.05). However, differential effects of TSD between HCs and patients were found in the superior corona radiata, frontal WM and the posterior thalamic radiation (p < 0.05, corrected). A significant (p < 0.05) association between change in FA and axial diffusivity within the right superior corona radiata and improvement in rumination was found post-TSD in patients.
Conclusion: Total sleep deprivation leads to rapid microstructural changes in WM pathways in patients with depression that are distinct from WM changes associated with TSD observed in HCs. WM tracts including the superior corona radiata and posterior thalamic radiation could be potential biomarkers of the rapid therapeutic effects of TSD. Changes in superior corona radiata FA, in particular, may relate to improvements in maladaptive rumination.
1. Introduction
Major depression is a common and debilitating disorder that affects 2–6% of people across the globe each year (1). Though mostly treatable, standard antidepressant medications are slow to act, typically taking weeks to months to yield clinical benefits (2). Identifying brain changes that play a role in depression recovery over these protracted time frames can thus be challenging. Lack of sleep can cause cognitive difficulties, perceptual abnormalities and negatively impact mood and anxiety in healthy individuals (3, 4). Paradoxically, one night of total sleep deprivation (TSD) is known to produce antidepressant response within 24 h in ∼60% of subjects with depression (5, 6). Although the therapeutic effects of TSD are only brief and symptoms return after a full night of recovery sleep (7), its rapid effect on mood serves as a model to understand the biological mechanisms associated with fast-acting antidepressant response. Early neuroimaging studies have linked the clinical response of TSD to metabolic changes in the anterior cingulate (ACC) (8, 9), which is a brain region frequently implicated in other depression treatment studies (10). However, there are limited neuroimaging studies (11, 12) that have attempted to identify the neural correlates of TSD-related therapeutic effects in patients with depression.
Diffusion-weighted imaging (DWI) is a neuroimaging technique that can be used to evaluate the diffusion properties of water within brain tissue. Diffusion tensor imaging (DTI), which relies on the tensor model to estimate the direction and magnitude of diffusion, is typically used to study the microstructural integrity of white matter (WM) tracts in vivo (13, 14). Measures of fractional anisotropy (FA), and axial (AD), and radial diffusivity (RD), which estimate the directionality of diffusion anisotropy, and diffusion along the principle or perpendicular axes of diffusion, respectively, are frequently evaluated with DTI. Mean diffusivity (MD), which represents the magnitude of diffusion independent of direction (15, 16) is also commonly measured. Many previous DTI studies have found abnormalities in WM microstructure in patients with depression, typically reporting decreased FA in tracts within frontal, occipital and parietal WM, and the corpus callosum (17–21). Reduced WM FA is shown to associate with the recurrence or the severity of depression (19, 22). Further, antidepressant response is shown to associate with changes in FA or other diffusion metrics (23–26), including for rapidly acting treatments such as electroconvulsive (ECT) and ketamine therapy (26, 27). Understanding how TSD affects the functional and structural integrity of neural pathways associated with depressive symptoms could provide valuable insights with regard to its rapid antidepressant action and potentially inform the development of other effective fast-acting antidepressant interventions. However, to the best of our knowledge the effect of TSD on WM microstructure in patients with depression is yet uncharacterized.
To better understand the neural processes contributing to the rapid-acting antidepressant action of sleep deprivation, the current study sought to investigate whether TSD elicits changes in cerebral WM microstructure in patients with depression. Since the lack of prior data warrants investigation of all major WM pathways, we used tract-based spatial statistics (TBSS) analysis to examine changes diffusion properties at the voxel-level. However, based on previous findings of TSD in controls (28, 29), we hypothesized TSD would result in change in WM microstructure and this change would be significantly different between controls and patients with depression who received TSD. Furthermore, as rumination is known to have a mediatory effect between mood and sleep deprivation (30), we hypothesized that WM regions associated with TSD response would also relate to changes in rumination.
2. Materials and methods
2.1. Participants
Participants included 48 individuals with depression evaluated using the Structured Clinical Interview for DSM-5-Research Version [SCID-5-RV, (31)] and 53 healthy controls (HC). Subjects were recruited from the Los Angeles area through advertisements and clinician referral. All patients, and a subset of HCs (N = 15) participated in a sleep deprivation session of at least 24 h under controlled conditions at the UCLA Clinical and Translational Research Center. Clinical assessments and MRI brain imaging data was acquired the day before TSD (TP1) and immediately after TSD (TP2). At each time point, depression severity was assessed using the Hamilton Depression Rating Scale (HDRS), 17–item (HDRS sleep scores were adjusted to include the scores of sleep 1-week prior to the study) (32).
Exclusion criteria for all participants included any unstable medical or neurological condition, current substance abuse or dependence (ascertained by laboratory testing) or substance abuse history within the preceding 3-months, current or past history of psychosis, schizophrenia, intellectual disability or other developmental disorder, diagnosis of dementia and any contraindication to scanning (e.g., metal implants or claustrophobia). Prior to treatment, all patients had mild to severe depressive symptoms (HDRS score > = 14) (32). All subjects provided written informed consent following procedures approved by the University of California, Los Angeles (UCLA) Institutional Review Board (IRB).
2.2. TSD procedure
Total sleep deprivation occurred in a private room at the UCLA Clinical and Translational Research Center. During the overnight TSD session, participants were continuously monitored to ensure wakefulness. Subjects were provided regulated meals and snacks, allowed to read, watch movies, use the internet, talk to staff, and walk around the clinic. Subjects were not allowed to lay down or turn off the lights and were restricted from consuming caffeine. Nursing staff frequently checked to ensure that subjects did not fall asleep or appear to be drowsy. MRI scans and clinical/behavioral assessments took place before and directly after the 24 h overnight sleep deprivation session. Pre and post TSD scans and assessments were collected in the late afternoon for all participants. Since subjects were not permitted to sleep on the day of baseline scans, all had experienced at least 36 h of continuous wakefulness by the time of the post-TSD assessments.
2.3. MRI acquisition
Imaging data was acquired using a Siemens 3T Prisma MRI system at UCLA’s Brain Mapping Center and a 32-channel phased array head coil. Image acquisition sequences were identical to those used by the Human Connectome Project (HCP) Lifespan studies for Aging and Development (33). Scans consisted of a T1-weighed (T1w) multi-echo MPRAGE with voxel size (VS) = 0.8 mm isotropic; repetition time (TR) = 2,500 ms; echo time (TE) = 1.81:1.79:7.18 ms; inversion time (TI) = 1,000 ms; flip angle (34) = 8.0o; and acquisition time (TA) = 8:22 min, and a T2-weighted (T2w) acquisition with VS = 0.8 mm isotropic; TR = 3,200 ms; TE = 564 ms; and TA = 6:35 min, both with real-time motion correction (34). Diffusion data were collected using a multiband (MB), echo-planar imaging sequence with 1.5 mm isotropic spatial resolution. Four consecutive diffusion MRI runs were collected with anterior-posterior (AP) (2 runs) and posterior-anterior (PA) (2 runs) phase encoding polarities. Each run contained interleaved shells with two diffusion weightings (b = 1,500 and 3,000 s/mm2), comprising 185 diffusion directions in total across both scans within each phase encoding direction (33).
2.4. DTI preprocessing
Anatomical and diffusion data were visually inspected and minimally preprocessed using the HCP minimal preprocessing pipeline (35–37) implemented using the BIDS-App (38). The HCP Diffusion pipeline included intensity normalization across runs, the “TOPUP” algorithm for EPI distortion correction, the “EDDY” algorithm for eddy current and motion correction, gradient non-linearity correction, registration of mean b0 images to native T1w space with FLIRT BBR and transformation of diffusion data, gradient deviation, and gradient directions to 1.5 mm structural space. Following eddy correction (39), the diffusion scans were again examined visually to screen for potential artifacts. Automated eddy QC tools (40) were subsequently applied to quantify the extent of head motion within and across study participants (average motion: 1.14 mm, 0.056 SD: average relative motion: 0.46 mm, 0.11 SD). No subjects were excluded for motion based on eddy QC outputs. Diffusion tensor models were then fitted independently for each voxel within the brain mask (based on FreeSurfer segmentation) and images of FA, first (λ1), second (λ2), and third (λ3) eigenvalues were generated for each participant using the FSL-FDT toolkit (41).
Using the TBSS tool (42), FA images from each participant were then aligned to the FMRIB58_FA template and transformed into Montreal Neurological Institute 152 1 mm3 standard space. Next, an average FA image was generated and thinned to create a WM skeleton representing the centers of all WM tracts common to all participants. This FA skeleton was then thresholded to FA ≥0.2 to include all major WM pathways. Each participant’s aligned FA image was projected onto the mean FA skeleton. Voxel-wise statistics were subsequently performed on this skeletonized participant data using the FSL randomize tool (43).
2.5. Statistical analysis
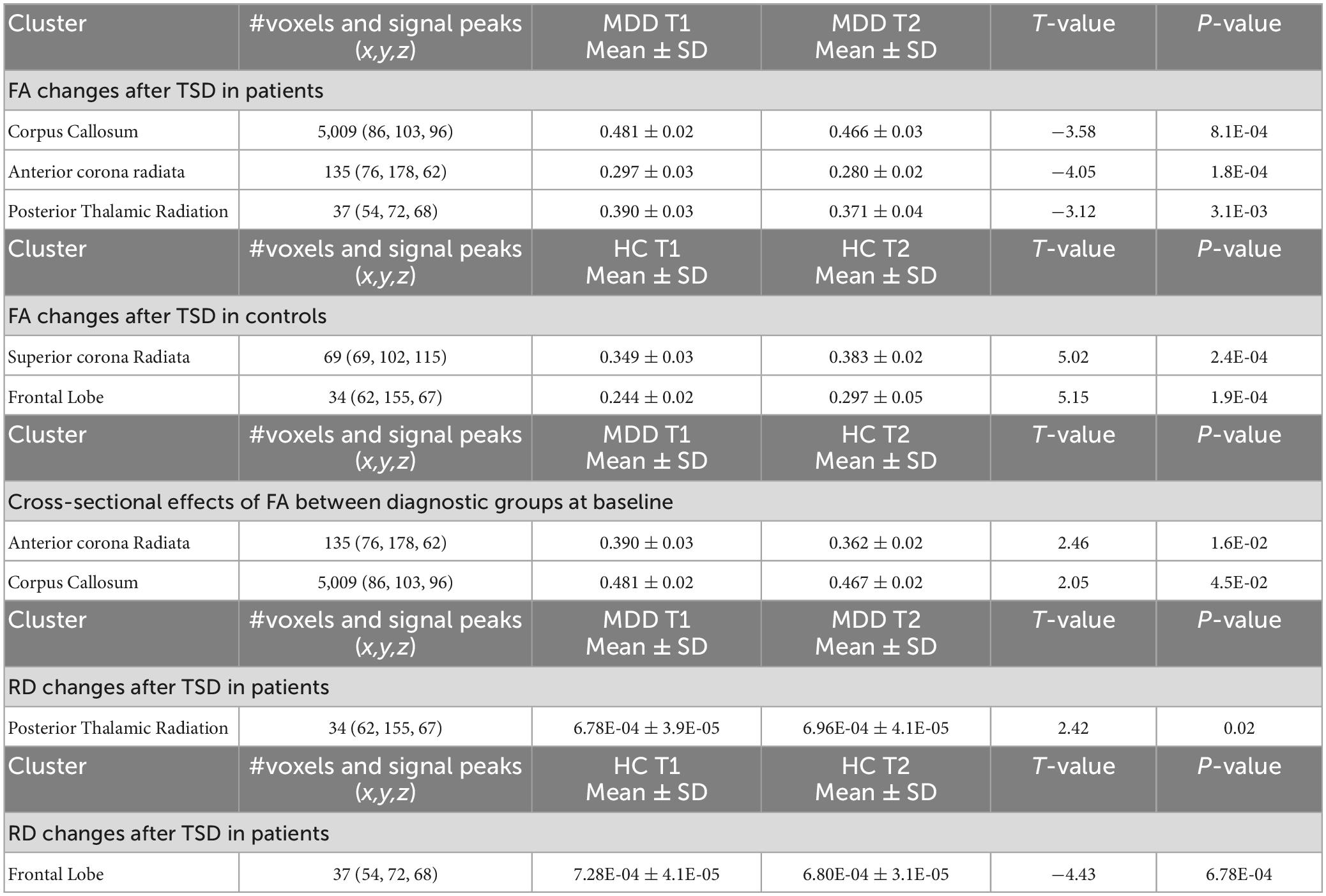
Voxel-level statistical analysis focused on addressing the following comparisons: (1) Effects of time for patients with depression pre to post TSD using a paired t-test, (2) Effects of time for HCs pre to post TSD using a paired t-test, and (3) Differential effects of time (or the interaction) by comparing change in FA (ΔFA) following TSD between patients with depression and HCs, including age and sex as regressors of no interest. For all comparisons, permutation testing included 5,000 permutations with the threshold-free cluster enhancement (TFCE) option (44). Statistical maps were corrected for multiple comparisons (family wise error, FWE P < 0.05). The Johns Hopkins University International Consortium for Brain Mapping (JHU ICBM)-DTI-81 WM labels atlas (45) determined the WM pathways showing significant FA differences. For clusters not labeled using this atlas, assignments were made using the MNI structural template in FSL.
Several post hoc tract-wise regions of interest (ROI) analyses were subsequently performed. These included establishing whether (1) changes in FA in regions showing significant effects of TSD in patients, differed between patients and all controls at baseline, (2) effects of TSD were present for other diffusion metrics (AD, RD, and MD) in regions showing FA changes, and finally if (3) changes in extracted FA, AD, RD, and MD values associated with % change [(TP1–TP2)/TP1] in mood or rumination scores. For post hoc analyses, a binary mask of each significant cluster identified in the whole brain voxel-wise tract analysis was generated to extract the mean FA within the cluster for each participant. The cluster binary mask was similarly used to calculate AD (λ1 values), RD (average of the λ2 and λ3 values), and MD (average diffusion) after the same non-linear warping parameters were used to project the non-FA images onto the TBSS WM skeleton. These tests were conducted using IBM Statistical Packages for the Social Sciences (SPSS v26). A p-value of < 0.05 was used to establish statistical significance for post hoc analyses.
3. Results
3.1. Demographic and clinical results
Age and sex did not significantly differ between HC and patients with depression (Table 1). There was a significant diagnostic group by time interaction for HDRS [F(1,58) = 23.13, p < 0.001] and a trend of an interaction for rumination scores [F(1,58) = 3.47, p = 0.06]. In patients, HDRS [t(47), p < 0.0001] and rumination [t(1, 47), p < 0.0009] scores showed significant improvement after TSD. Rumination scores did not change significantly for HC following TSD, though HDRS scores significantly increased (worsened) [t(14) = 3.02, p = 0.01] (Figure 1).
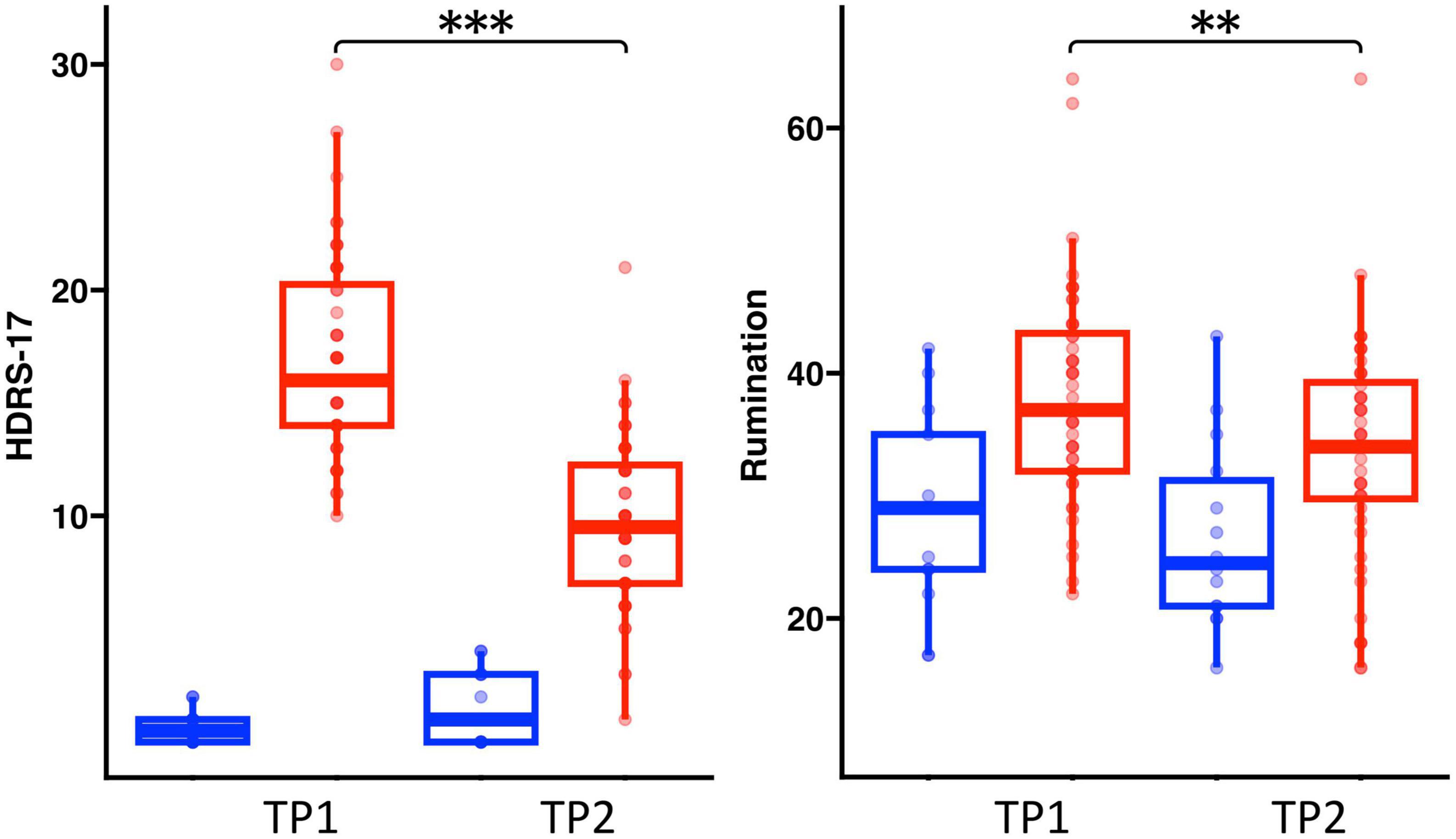
Figure 1. Box plots showing the distribution of HDRS and rumination scores in healthy controls (Blue) and patients (Red) pre-to-post TSD (**p < 0.01 and ***p < 0.001).
3.2. FA changes after TSD
Patients with depression showed significant decreases in FA following TSD in multiple WM tracts (p < 0.05 FWE correction), including in the body of corpus callosum and the anterior corona radiata (Figure 2A). The paired t-test comparing baseline (TP1) and post TSD (TP2) for HCs did not reveal significant FA changes at the voxel level (p > 0.05 FEW) (null results not shown).
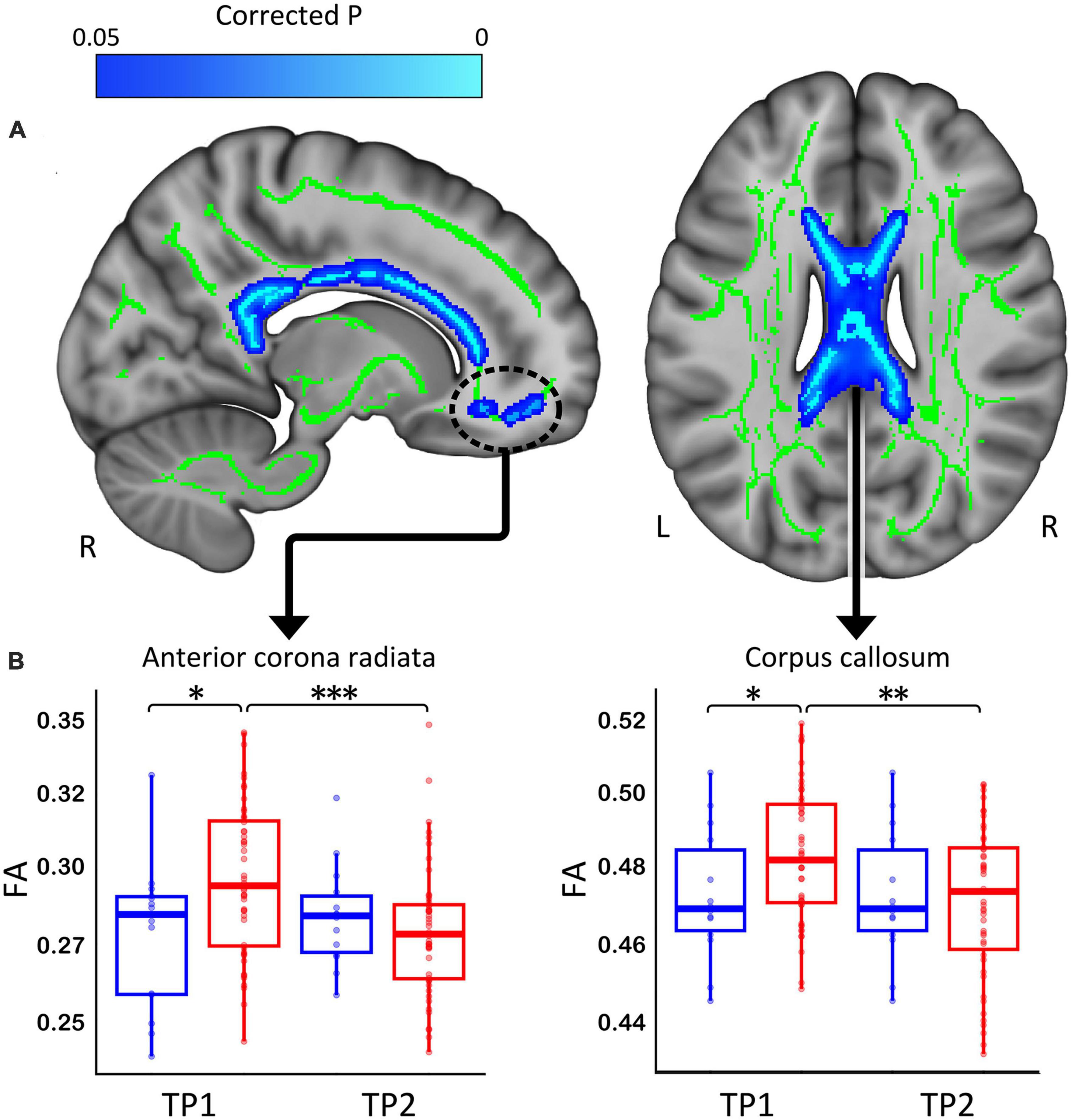
Figure 2. (A) White matter clusters showing significant decrease (in blue, p < 0.05 FWE corrected) in fractional anisotropy (FA) in the corpus callosum and the anterior corona radiata. (B) Box plots showing the distribution of FA values for healthy controls (Blue) and patients (Red) for the corpus callosum and anterior corona radiata (*p < 0.05, **p < 0.01, and ***p < 0.001). R, right, L, left.
3.3. Cross-sectional effects of FA between diagnostic groups at baseline
Post hoc analysis revealed that WM regions showing a significant decrease in FA following TSD in patients differed between patients and HCs at baseline (p < 0.05) (Figure 2B shows these effects graphically and Table 2 provides statistical details for significant follow-up comparisons).
3.4. Differential TSD effects of FA between patients and HCs
At p < 0.05 FWE correction and including age and sex as covariates of no interest, the two-sample t-test comparing ΔFA (TP1–TP2) between HCs and patients with depression at the voxel level revealed multiple significant WM clusters, including the superior corona radiata, a cluster in frontal lobe WM and the posterior thalamic radiation (Figure 3A).
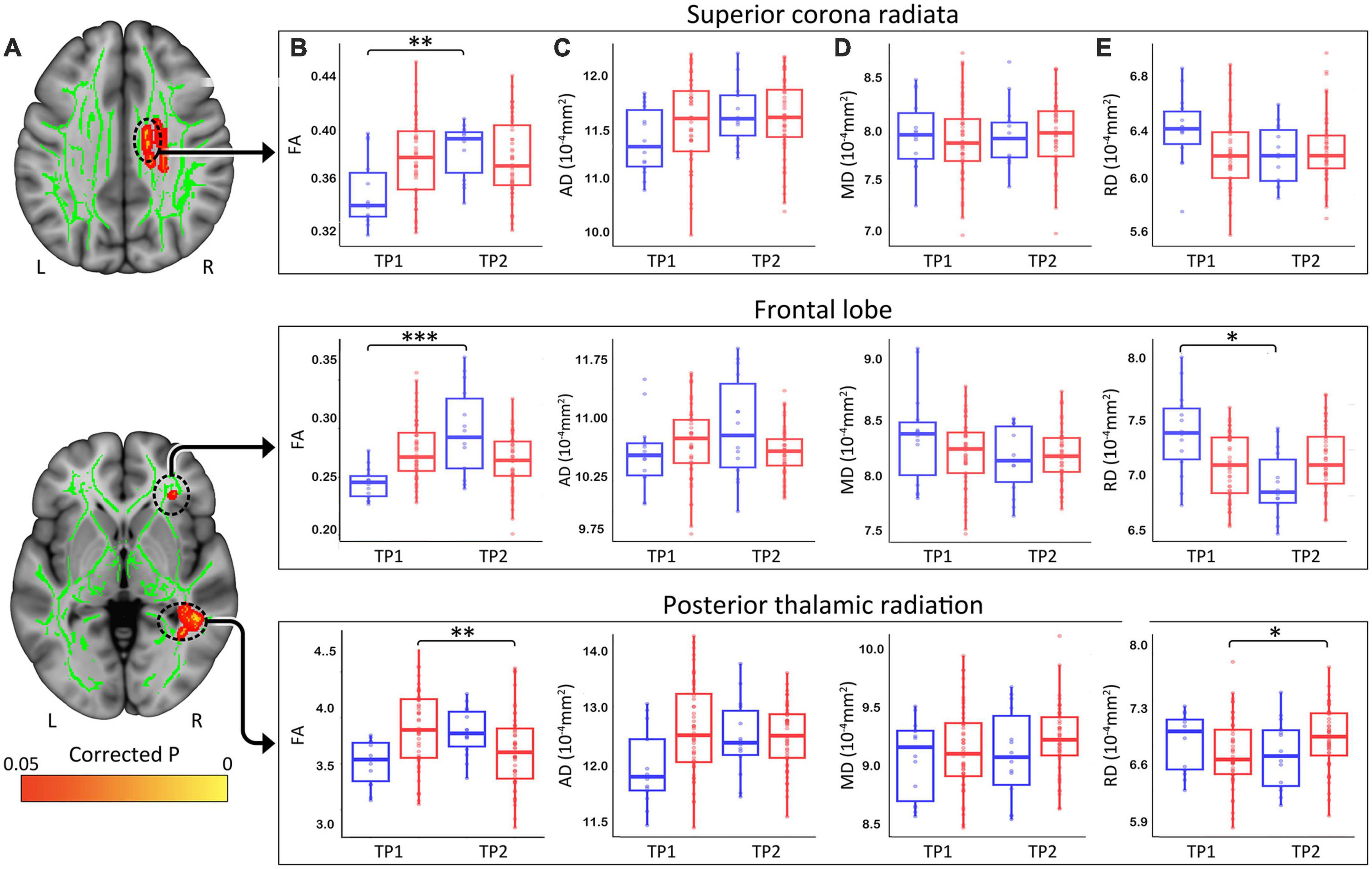
Figure 3. (A) White matter clusters showing significant difference (orange, p < 0.05 FWE corrected) for change in fractional anisotropy (FA) pre-to-post TSD between controls and patients. (B) Box plots showing distribution of FA, axial (C), radial (D), and mean (E) diffusivity values for healthy controls (Blue) and patients (Red) in the superior corona radiata, frontal lobe and the posterior thalamic radiation pre and post TSD. (*p < 0.05, **p < 0.01, and ***p < 0.001). R, right, L, left.
These clusters were used to create ROIs to extract regional FA (Figure 3B), AD (Figure 3C), MD (Figure 3D), and RD (Figure 3E) values for HC and patients. Post TSD, HCs showed a significant increase of mean FA values in the superior corona radiata and a WM cluster in the frontal lobe, while patients showed a significant decrease of mean FA value in the posterior thalamic radiation (Figure 3B). Among the other DTI measures, only RD showed a significant decrease in frontal lobe WM for HCs post TSD, while patients showed a significant increase in RD values in the posterior thalamic radiation (Figure 3E). Table 2 provides statistical details for regional effects observed as significant.
3.5. Associations with mood and rumination in patients
Clusters that survived statistical significance in the whole brain voxel-level tract analysis (ΔFA, p < 0.05 FWE TFCE) were used to create ROIs of average FA, AD, MD, and RD values. None of the DTI measures showed any significant relationship with change in HDRS scores post TSD in patients. However, the change in the superior corona radiata (Figure 4A) for FA (Figure 4B, r = 0.364, p = 0.011) and AD (Figure 4C, r = 0.301, p = 0.038) showed a significant correlation with % change in rumination scores.
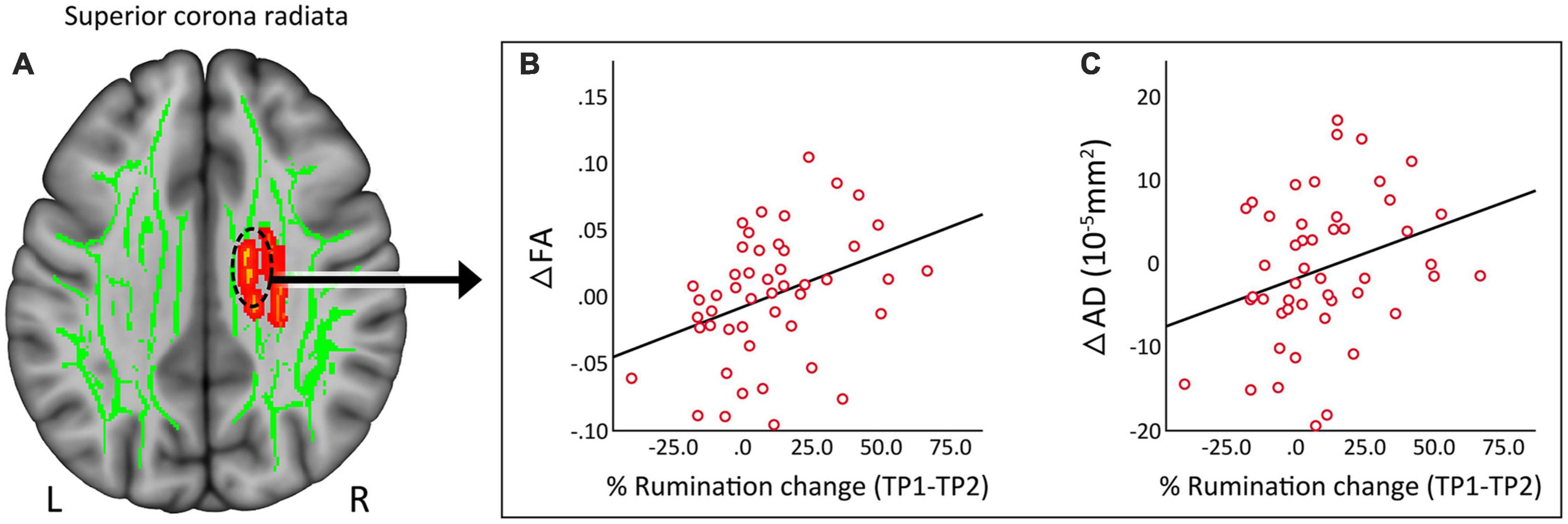
Figure 4. (A) Regional correlates of DTI measures from the superior corona radiata. (B) Change in fractional anisotropy (FA) showed a significant (p = 0.01) positive correlation with change in rumination. (C) Change in axial diffusivity (AD) showed a significant (p = 0.038) positive correlation with change in rumination. R, right, L, left.
4. Discussion
Total sleep deprivation is widely reported to produce rapid onset antidepressant effects within 24–48 h in at least half of depressed patients who receive it (12, 46). Although symptoms return immediately after recovery sleep (6), TSD presents an important opportunity to understand the mechanisms contributing to rapid antidepressant response. How TSD modulates structural networks to influence antidepressant response remains unstudied. By leveraging in vivo diffusion imaging methods, the current study thus sought to investigate whether TSD affects WM microstructure in patients with depression and in controls, and to establish whether observed changes in WM associate with improvements in mood and rumination. Primary findings from this investigation showed that TSD promotes significant reductions of FA within major WM pathways, including the body of corpus callosum and anterior corona radiata in patients with depression. Further, significant interactions of TSD-related change between patients and controls were present for FA (ΔFA) in numerous WM tracts, including the superior corona radiata, frontal WM and the posterior thalamic radiation. Here, patients and HCs mostly exhibited opposite directions of mean change in particular WM pathways. Finally, changes in FA and AD within the superior corona radiata showed a significant positive relationship between improvement in rumination pre-to-post TSD. These findings suggest that while some of observed regional reductions in FA may relate to the vulnerability of insufficient sleep (29), TSD-related changes in WM microstructure positively impact ruminative thought focused on depressive symptoms (47). To the best of our knowledge, this is the first report addressing the immediate effects of TSD on WM microstructure and its behavioral correlates in patients with depression.
4.1. WM microstructural changes after TSD
The current study chose to focus on FA for primary analyses since it reflects the directional selectivity of diffusion and is sensitive to both direction and amount of hindrance/restriction and direction of water molecules (48, 49). We observed significant widespread reduction of FA values in multiple WM tracts in patients with depression post TSD. Regions of FA decreases mainly included the corpus callosum and tracts traversing the frontal WM. Tract-based estimates of FA are shown to be highly reproducible across time when the DWI sequence, scanner software and system remain stable as was the case for DWI acquisition in this study (39, 50, 51). Significant changes in FA observed following TSD in patients (∼4% mean decrease across significant clusters) were of slightly larger magnitude to those reported previously in healthy subjects following 24 h of sleep deprivation (2–3% mean decrease across significant clusters) (28). Decreases in FA observed in a prior study comparing normal sleep-wake cycles to 24–32 h of sleep deprivation in healthy subjects were also similar, though only extra-neurite mean diffusivity (exMD), which is highly correlated with FA, was significant (52).
Though not fully understood at the molecular and cellular level, the antidepressant mechanisms of TSD are suggested to involve the modulation neurotrophic and inflammatory processes and changes in endocrine function (4, 53). TSD’s antidepressant effects are suggested as attributable to some of these same factors, including modulation of brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF), serotonin, cortisol, and tumor necrosis factor-alpha (TNF-α) (54). Some prior evidence suggests that sleep deprivation in healthy populations increases reactivity in mesolimbic reward brain networks in response to positive as well as negative stimuli (55) as well as decreases connectivity between the ACC and reward networks (56), which may contribute to its effects on depression. As potentially relevant to the current findings, previous studies have reported reduced metabolism in frontal regions after sleep deprivation (57, 58) suggesting that frontal executive centers are particularly susceptible to sleep deprivation. Moreover, functional magnetic resonance imaging (fMRI) research has found an overall decrease in regional and global neural activation following sleep deprivation (55, 59). In accordance, sleep deprivation is reported to negatively affect cognitive function, attention, working memory, and psychomotor vigilance (60–62). Though a prior investigation in controls has reported significant decreases in FA following sleep deprivation (28), in the current study reduced FA at the voxel-level was only observed as significant following TSD in patients. Still, these results in the context of limited prior reports may suggest that the decrease in FA observed across commissural and frontal association pathways are an indication of the sleep deprived brain. However, results also suggest that these changes associated with behavioral state after loss of sleep simultaneously act to relieve depressive symptoms. Since FA represents only the direction and magnitude of diffusion in the microenvironment in which water molecules in the WM exist, the exact mechanisms responsible for changes in diffusion signal can only be inferred (48, 49). However, these changes could point to alterations in myelin and axonal microstructure as well as neural swelling associated with brain activity or edema (49, 63), or other biological processes such as glymphatic activity occurring during sleep (64).
4.2. Differential effects of TSD between HC and patients
In line with prior results (7), total sleep deprivation was shown to provide immediate relief of mood symptoms in >44% of patients with depression whereas in HCs mood symptoms tended to increase (see Figure 1). Using independent sample t-tests, we sought to directly compare differences in FA pre-to-post TSD between patients and controls in whole brain voxel-level tract analysis. On comparing the ΔFA maps between HC and patients post TSD, we identified several WM tracts including the superior corona radiata, frontal WM and the posterior thalamic radiation where FA values were modulated in opposite directions in patients relative to HCs. In post hoc analyses, we also evaluated change in AD, MD, and RD, which may reveal further information regarding the influence of TSD on WM microstructural properties. Despite limitations in extrapolating the biological cause of differences in DTI metrics (48, 49), AD has been considered a marker of axonal microstructure, though both increases and decreases may indicate pathology (65–67). In contrast, RD is often considered a marker of myelin though can also signify changes in axonal diameter or density (65–67). Although only RD showed a significant change post TSD for the selected ROIs, as for FA, the diffusivity measures of AD, mean MD and RD were modulated in opposite directions in patients and HCs post TSD. Differences in the modulation of WM microstructure observed between patients with depression and HCs following TSD may suggest that TSDs underlying therapeutic mechanisms are selectively targeted toward differential WM microstructure in patients. Notably, the WM tracts showing changes in FA with TSD in patients with depression have also been implicated in prior cross-sectional studies comparing patients with depression and controls using whole-brain DTI analysis (18–20, 68–70). Some prior evidence further suggests that medication treatments for depression alter or reverse abnormalities in FA. For example, Bracht et al. (71) showed that FA increased in younger patients, but decreased in older patients in the cingulum following successful recovery from depression after standard antidepressant therapy. Ketamine therapy in major depression is also shown to reduce neurite density (correlated with FA) in several WM tracts, including the posterior thalamic radiation, forceps minor (frontal callosal projections) and occipitotemporal pathways (27).
4.3. DTI changes associated with symptoms after TSD
Significant improvements in HDRS and rumination scores were observed post TSD in depressed patients. In post hoc analysis, we also observed a significant positive correlation between change in FA and AD in the superior corona radiata/body of corpus callosum with change in rumination scores. At least one prior study has shown associations between altered FA in WM tracts connecting frontal, parietal and limbic regions and rumination (72). Further, since the superior corona radiata plays an important role in integrating higher-level cognitive, and perceptual processing (18), WM alterations might contribute to the impairments in memory, executive functioning, and emotional regulation reported in patients with depression (73). The significant associations observed between improvement in ruminative state and FA and AD measures suggest that temporary symptom recovery due to TSD is at least partly attributable to the modulation of WM microstructure.
4.4. Limitations
Several limitations need to be acknowledged with regard to the current study. First, only a subset of the HC sample (N = 15) underwent TSD, which led to reduced statistical power for identifying WM microstructural changes perturbed by TSD in the HC group specifically. However, the focus of the current study was to investigate the WM perturbations in patients with depression following TSD and associations with change in mood and rumination. Further, as more impervious to unequal samples we compared the magnitude of change in FA across diagnostic groups and observed changes in diffusion metrics tended to change in opposite directions in patients and HCs. In the current study, patient participants were allowed to continue their current antidepressant medications, which though remaining stable at least 6-weeks prior to TSD invention, may have impacted results.
5. Conclusion
The present study demonstrates that 24 h of sleep deprivation leads to significant decreases in FA in the corpus callosum and the anterior corona radiata in patients with depression. In line with previous findings, these alterations in WM microstructure may be primarily due to lack of sleep. However, changes in FA post TSD observed in certain tracts such as the superior corona radiata, frontal WM and the posterior thalamic radiation differed in patients and controls. WM alterations in these tracts thus appear specific to depression and may be linked with cognitive and emotional deficits as previously suggested. The association between improvement in rumination and change in FA and AD post TSD further indicate that temporary symptom recovery following TSD relates to changes in WM structure. Future studies may address whether changes in diffusion properties within these fiber pathways relate to improvements in mood and rumination following other fast-acting antidepressant therapies.
Data availability statement
Unprocessed and minimally preprocessed imaging data and behavioral data used in this publication are available in the PDC 1.0. Release accessible through the NIMH Data Archive (NDA, https://nda.nih.gov/study.html?id=2010).
Ethics statement
The studies involving human participants were reviewed and approved by The Office of the Human Research Protection Program (OHRPP) at the University of California, Los Angeles. The patients/participants provided their written informed consent to participate in this study.
Author contributions
AK, KN, JL, AS, SJ, RW, and RE contributed to the conception and design of the study. AK, BT, and AZ-P performed the statistical analysis. AK wrote the first draft of the manuscript. BT wrote the sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This work was supported by the National Institute of Mental Health, Grant/Award Number: U01MH110008-01 to KN and RE and the National Institute of Neurological Disorders and Stroke, Grant/Award Number: T32NS048004 to AZ-P. This research was also supported by the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health under the UCLA Clinical and Translational Science Institute (grant number UL1TR001881).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ferrari A, Somerville A, Baxter A, Norman R, Patten S, Vos T, et al. Global variation in the prevalence and incidence of major depressive disorder: a systematic review of the epidemiological literature. Psychol Med. (2013) 43:471–81. doi: 10.1017/S0033291712001511
2. Stassen H, Angst J, Hell D, Scharfetter C, Szegedi A. Is there a common resilience mechanism underlying antidepressant drug response? Evidence from 2848 patients. J Clin Psychiatry. (2007) 68:1195–205. doi: 10.4088/JCP.v68n0805
3. Waters F, Chiu V, Atkinson A, Blom J. Severe sleep deprivation causes hallucinations and a gradual progression toward psychosis with increasing time awake. Front Psychiatry. (2018) 9:303. doi: 10.3389/fpsyt.2018.00303
4. Thompson K, Chau M, Lorenzetti M, Hill L, Fins A, Tartar J. Acute sleep deprivation disrupts emotion, cognition, inflammation, and cortisol in young healthy adults. Front Behav Neurosci. (2022) 16:945661. doi: 10.3389/fnbeh.2022.945661
5. Gillin J. The sleep therapies of depression. Prog Neuropsychopharmacol Biol Psychiatry. (1983) 7:351–64. doi: 10.1016/0278-5846(83)90123-9
6. Wu J, Bunney W. The biological basis of an antidepressant response to sleep deprivation and relapse: review and hypothesis. Am J Psychiatry. (1990) 147:14–21. doi: 10.1176/ajp.147.1.14
7. Boland E, Rao H, Dinges D, Smith R, Goel N, Detre J, et al. Meta-analysis of the antidepressant effects of acute sleep deprivation. J Clin Psychiatry. (2017) 78:e1020–34. doi: 10.4088/JCP.16r11332
8. Wu J, Gillin J, Buchsbaum M, Hershey T, Johnson J, Bunney W Jr. Effect of sleep deprivation on brain metabolism of depressed patients. Am J Psychiatry. (1992) 149:538–43. doi: 10.1176/ajp.149.4.538
9. Ebert D, Feistel H, Barocka A. Effects of sleep deprivation on the limbic system and the frontal lobes in affective disorders: a study with Tc-99m-HMPAO SPECT. Psychiatry Res. (1991) 40:247–51. doi: 10.1016/0925-4927(91)90016-J
10. Mayberg H, Brannan S, Mahurin R, Jerabek P, Brickman J, Tekell J, et al. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. (1997) 8:1057–61. doi: 10.1097/00001756-199703030-00048
11. Clark C, Frank L, Brown G. Sleep deprivation, EEG, and functional MRI in depression: preliminary results. Neuropsychopharmacology. (2001) 25(5 Suppl):S79–84. doi: 10.1016/S0893-133X(01)00324-4
12. Wu J, Gillin J, Buchsbaum M, Schachat C, Darnall L, Keator D, et al. Sleep deprivation PET correlations of Hamilton symptom improvement ratings with changes in relative glucose metabolism in patients with depression. J Affect Disord. (2008) 107:181–6. doi: 10.1016/j.jad.2007.07.030
13. Basser P, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. (1994) 103:247–54. doi: 10.1006/jmrb.1994.1037
14. Sexton C, Mackay C, Ebmeier KP. A systematic review of diffusion tensor imaging studies in affective disorders. Biol Psychiatry. (2009) 66:814–23. doi: 10.1016/j.biopsych.2009.05.024
15. Bennett I, Madden D, Vaidya C, Howard D, Howard J Jr. Age-related differences in multiple measures of white matter integrity: a diffusion tensor imaging study of healthy aging. Hum Brain Mapp. (2010) 31:378–90. doi: 10.1002/hbm.20872
16. Yuan W, Mangano F, Air E, Holland S, Jones B, Altaye M, et al. Anisotropic diffusion properties in infants with hydrocephalus: a diffusion tensor imaging study. AJNR Am J Neuroradiol. (2009) 30:1792–8. doi: 10.3174/ajnr.A1663
17. Ma N, Li L, Shu N, Liu J, Gong G, He Z, et al. White matter abnormalities in first-episode, treatment-naive young adults with major depressive disorder. Am J Psychiatry. (2007) 164:823–6. doi: 10.1176/ajp.2007.164.5.823
18. Kieseppa T, Eerola M, Mantyla R, Neuvonen T, Poutanen V, Luoma K, et al. Major depressive disorder and white matter abnormalities: a diffusion tensor imaging study with tract-based spatial statistics. J Affect Disord. (2010) 120:240–4. doi: 10.1016/j.jad.2009.04.023
19. van Velzen L, Kelly S, Isaev D, Aleman A, Aftanas L, Bauer J, et al. White matter disturbances in major depressive disorder: a coordinated analysis across 20 international cohorts in the ENIGMA MDD working group. Mol Psychiatry. (2020) 25:1511–25.
20. Coloigner J, Batail J, Commowick O, Corouge I, Robert G, Barillot C, et al. White matter abnormalities in depression: a categorical and phenotypic diffusion MRI study. Neuroimage Clin. (2019) 22:101710. doi: 10.1016/j.nicl.2019.101710
21. Liao Y, Huang X, Wu Q, Yang C, Kuang W, Du M, et al. Is depression a disconnection syndrome? Meta-analysis of diffusion tensor imaging studies in patients with MDD. J Psychiatry Neurosci. (2013) 38:49–56. doi: 10.1503/jpn.110180
22. Nobuhara K, Okugawa G, Sugimoto T, Minami T, Tamagaki C, Takase K, et al. Frontal white matter anisotropy and symptom severity of late-life depression: a magnetic resonance diffusion tensor imaging study. J Neurol Neurosurg Psychiatry. (2006) 77:120–2. doi: 10.1136/jnnp.2004.055129
23. Vasavada M, Leaver A, Espinoza R, Joshi S, Njau S, Woods R, et al. Structural connectivity and response to ketamine therapy in major depression: a preliminary study. J Affect Disord. (2016) 190:836–41. doi: 10.1016/j.jad.2015.11.018
24. Alexopoulos G, Murphy C, Gunning-Dixon F, Latoussakis V, Kanellopoulos D, Klimstra S, et al. Microstructural white matter abnormalities and remission of geriatric depression. Am J Psychiatry. (2008) 165:238–44. doi: 10.1176/appi.ajp.2007.07050744
25. Davis A, Hassel S, Arnott S, Harris J, Lam R, Milev R, et al. White matter indices of medication response in major depression: a diffusion tensor imaging study. Biol Psychiatry Cogn Neurosci Neuroimaging. (2019) 4:913–24. doi: 10.1016/j.bpsc.2019.05.016
26. Lyden H, Espinoza R, Pirnia T, Clark K, Joshi S, Leaver A, et al. Electroconvulsive therapy mediates neuroplasticity of white matter microstructure in major depression. Transl Psychiatry. (2014) 4:e380. doi: 10.1038/tp.2014.21
27. Taraku B, Woods R, Boucher M, Espinoza R, Jog M, Al-Sharif N, et al. Changes in white matter microstructure following serial ketamine infusions in treatment resistant depression. Hum Brain Mapp. (2023) 44:2395–406. doi: 10.1002/hbm.26217
28. Elvsashagen T, Norbom L, Pedersen P, Quraishi S, Bjornerud A, Malt U, et al. Widespread changes in white matter microstructure after a day of waking and sleep deprivation. PLoS One. (2015) 10:e0127351. doi: 10.1371/journal.pone.0127351
29. Rocklage M, Williams V, Pacheco J, Schnyer D. White matter differences predict cognitive vulnerability to sleep deprivation. Sleep. (2009) 32:1100–3. doi: 10.1093/sleep/32.8.1100
30. Slavish D, Graham-Engeland J. Rumination mediates the relationships between depressed mood and both sleep quality and self-reported health in young adults. J Behav Med. (2015) 38:204–13. doi: 10.1007/s10865-014-9595-0
31. First MW, Spitzer R. Structured Clinical Interview for DSM-5—Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV). (2015).
32. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. (1960) 23:56–62. doi: 10.1136/jnnp.23.1.56
33. Harms M, Somerville L, Ances B, Andersson J, Barch D, Bastiani M, et al. Extending the human connectome project across ages: imaging protocols for the lifespan development and aging projects. Neuroimage. (2018) 183:972–84. doi: 10.1016/j.neuroimage.2018.09.060
34. Tisdall M, Hess A, Reuter M, Meintjes E, Fischl B, van der Kouwe A. Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. Magn Reson Med. (2012) 68:389–99. doi: 10.1002/mrm.23228
35. Glasser M, Sotiropoulos S, Wilson J, Coalson T, Fischl B, Andersson J, et al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. (2013) 80:105–24. doi: 10.1016/j.neuroimage.2013.04.127
36. Smith S, Beckmann C, Andersson J, Auerbach E, Bijsterbosch J, Douaud G, et al. Resting-state fMRI in the Human Connectome Project. Neuroimage. (2013) 80:144–68. doi: 10.1016/j.neuroimage.2013.05.039
37. Sotiropoulos S, Jbabdi S, Xu J, Andersson J, Moeller S, Auerbach E, et al. Advances in diffusion MRI acquisition and processing in the Human Connectome Project. Neuroimage. (2013) 80:125–43. doi: 10.1016/j.neuroimage.2013.05.057
38. Gorgolewski K, Alfaro-Almagro F, Auer T, Bellec P, Capota M, Chakravarty M, et al. BIDS apps: improving ease of use, accessibility, and reproducibility of neuroimaging data analysis methods. PLoS Comput Biol. (2017) 13:e1005209. doi: 10.1371/journal.pcbi.1005209
39. Pfefferbaum A, Adalsteinsson E, Sullivan E. Replicability of diffusion tensor imaging measurements of fractional anisotropy and trace in brain. J Magn Reson Imaging. (2003) 18:427–33. doi: 10.1002/jmri.10377
40. Bastiani M, Cottaar M, Fitzgibbon S, Suri S, Alfaro-Almagro F, Sotiropoulos S, et al. Automated quality control for within and between studies diffusion MRI data using a non-parametric framework for movement and distortion correction. Neuroimage. (2019) 184:801–12. doi: 10.1016/j.neuroimage.2018.09.073
41. Behrens T, Woolrich M, Jenkinson M, Johansen-Berg H, Nunes R, Clare S, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. (2003) 50:1077–88. doi: 10.1002/mrm.10609
42. Smith S, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols T, Mackay C, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. (2006) 31:1487–505. doi: 10.1016/j.neuroimage.2006.02.024
43. Winkler A, Ridgway G, Webster M, Smith S, Nichols T. Permutation inference for the general linear model. Neuroimage. (2014) 92:381–97. doi: 10.1016/j.neuroimage.2014.01.060
44. Smith S, Nichols T. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. (2009) 44:83–98. doi: 10.1016/j.neuroimage.2008.03.061
45. Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. (2008) 40:570–82. doi: 10.1016/j.neuroimage.2007.12.035
46. Wu J, Kelsoe J, Schachat C, Bunney B, DeModena A, Golshan S, et al. Rapid and sustained antidepressant response with sleep deprivation and chronotherapy in bipolar disorder. Biol Psychiatry. (2009) 66:298–301. doi: 10.1016/j.biopsych.2009.02.018
47. Kovács L, Takacs Z, Tóth Z, Simon E., Schmelowszky Á, Kökönyei G, et al. Rumination in major depressive and bipolar disorder - a meta-analysis. J Affect Disord. (2020) 276:1131–41. doi: 10.1016/j.jad.2020.07.131
48. Jones D, Knösche T, Turner R. White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. Neuroimage. (2013) 73:239–54. doi: 10.1016/j.neuroimage.2012.06.081
49. Alexander A, Lee J, Lazar M, Field A. Diffusion tensor imaging of the brain. Neurotherapeutics. (2007) 4:316–29. doi: 10.1016/j.nurt.2007.05.011
50. Grech-Sollars M, Hales P, Miyazaki K, Raschke F, Rodriguez D, Wilson M, et al. Multi-centre reproducibility of diffusion MRI parameters for clinical sequences in the brain. NMR Biomed. (2015) 28:468–85. doi: 10.1002/nbm.3269
51. Thieleking R, Zhang R, Paerisch M, Wirkner K, Anwander A, Beyer F, et al. Same brain, different look?-The impact of scanner, sequence and preprocessing on diffusion imaging outcome parameters. J Clin Med. (2021) 10:4987. doi: 10.3390/jcm10214987
52. Voldsbekk I, Groote I, Zak N, Roelfs D, Geier O, Due-Tønnessen P, et al. Sleep and sleep deprivation differentially alter white matter microstructure: a mixed model design utilising advanced diffusion modelling. Neuroimage. (2021) 226:117540. doi: 10.1016/j.neuroimage.2020.117540
53. Gorgulu Y, Caliyurt O. Rapid antidepressant effects of sleep deprivation therapy correlates with serum BDNF changes in major depression. Brain Res Bull. (2009) 80:158–62. doi: 10.1016/j.brainresbull.2009.06.016
54. Marek S, Tervo-Clemmens B, Calabro F, Montez D, Kay B, Hatoum A, et al. Towards reproducible brain-wide association studies. bioRxiv [Preprint] (2020). bioRxiv 2020.08.21.257758. doi: 10.1101/2020.08.21.257758
55. Gujar N, Yoo S, Hu P, Walker M. The unrested resting brain: sleep deprivation alters activity within the default-mode network. J Cogn Neurosci. (2010) 22:1637–48. doi: 10.1162/jocn.2009.21331
56. Zhang Y, Dai C, Shao Y, Peng J, Yang Y, Hou Y. Decreased functional connectivity in the reward network and its relationship with negative emotional experience after total sleep deprivation. Front Neurol. (2021) 12:641810. doi: 10.3389/fneur.2021.641810
57. Thomas M, Sing H, Belenky G, Holcomb H, Mayberg H, Dannals R, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. (2000) 9:335–52. doi: 10.1046/j.1365-2869.2000.00225.x
58. Wu J, Gillin J, Buchsbaum M, Chen P, Keator D, Khosla Wu N, et al. Frontal lobe metabolic decreases with sleep deprivation not totally reversed by recovery sleep. Neuropsychopharmacology. (2006) 31:2783–92. doi: 10.1038/sj.npp.1301166
59. Mu Q, Nahas Z, Johnson K, Yamanaka K, Mishory A, Koola J, et al. Decreased cortical response to verbal working memory following sleep deprivation. Sleep. (2005) 28:55–67. doi: 10.1093/sleep/28.1.55
60. Nakashima A, Bouak F, Lam Q, Smith I, Vartanian O. Task switching following 24 h of total sleep deprivation: a functional MRI study. Neuroreport. (2018) 29:123–7. doi: 10.1097/WNR.0000000000000934
61. Zhou X, Wu T, Yu J, Lei X. Sleep deprivation makes the young brain resemble the elderly brain: a large-scale brain networks study. Brain Connect. (2017) 7:58–68. doi: 10.1089/brain.2016.0452
62. Lim J, Dinges D. Sleep deprivation and vigilant attention. Ann N Y Acad Sci. (2008) 1129:305–22. doi: 10.1196/annals.1417.002
63. Kwon D. Brain imaging: fMRI advances make scans sharper and faster. Nature. (2023) 617:640–2. doi: 10.1038/d41586-023-01616-7
64. Reddy O, van der Werf Y. The sleeping brain: harnessing the power of the glymphatic system through lifestyle choices. Brain Sci. (2020) 10:868. doi: 10.3390/brainsci10110868
65. Feldman H, Yeatman J, Lee E, Barde L, Gaman-Bean S. Diffusion tensor imaging: a review for pediatric researchers and clinicians. J Dev Behav Pediatr. (2010) 31:346–56. doi: 10.1097/DBP.0b013e3181dcaa8b
66. Chanraud S, Zahr N, Sullivan E, Pfefferbaum A. MR diffusion tensor imaging: a window into white matter integrity of the working brain. Neuropsychol Rev. (2010) 20:209–25. doi: 10.1007/s11065-010-9129-7
67. Alexander A, Hurley S, Samsonov A, Adluru N, Hosseinbor A, Mossahebi P, et al. Characterization of cerebral white matter properties using quantitative magnetic resonance imaging stains. Brain Connect. (2011) 1:423–46. doi: 10.1089/brain.2011.0071
68. Korgaonkar M, Grieve S, Koslow S, Gabrieli J, Gordon E, Williams L. Loss of white matter integrity in major depressive disorder: evidence using tract-based spatial statistical analysis of diffusion tensor imaging. Hum Brain Mapp. (2011) 32:2161–71. doi: 10.1002/hbm.21178
69. Li L, Ma N, Li Z, Tan L, Liu J, Gong G, et al. Prefrontal white matter abnormalities in young adult with major depressive disorder: a diffusion tensor imaging study. Brain Res. (2007) 1168:124–8. doi: 10.1016/j.brainres.2007.06.094
70. Dinga R, Schmaal L, Penninx B, van Tol M, Veltman D, van Velzen L, et al. Evaluating the evidence for biotypes of depression: methodological replication and extension of. Neuroimage Clin. (2019) 22:101796. doi: 10.1016/j.nicl.2019.101796
71. Bracht T, Jones D, Müller T, Wiest R, Walther S. Limbic white matter microstructure plasticity reflects recovery from depression. J Affect Disord. (2015) 170:143–9. doi: 10.1016/j.jad.2014.08.031
72. Zuo N, Fang J, Lv X, Zhou Y, Hong Y, Li T, et al. White matter abnormalities in major depression: a tract-based spatial statistics and rumination study. PLoS One. (2012) 7:e37561. doi: 10.1371/journal.pone.0037561
Keywords: sleep deprivation, white matter, depression, diffusion imaging, rumination
Citation: Taraku B, Zavaliangos-Petropulu A, Loureiro JR, Al-Sharif NB, Kubicki A, Joshi SH, Woods RP, Espinoza R, Narr KL and Sahib AK (2023) White matter microstructural perturbations after total sleep deprivation in depression. Front. Psychiatry 14:1195763. doi: 10.3389/fpsyt.2023.1195763
Received: 28 March 2023; Accepted: 06 June 2023;
Published: 28 June 2023.
Edited by:
Roberto Esposito, ASUR Marche, ItalyReviewed by:
Justin Minue Kim, Sungkyunkwan University, Republic of KoreaShumei Li, Guangdong Second Provincial General Hospital, China
Copyright © 2023 Taraku, Zavaliangos-Petropulu, Loureiro, Al-Sharif, Kubicki, Joshi, Woods, Espinoza, Narr and Sahib. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katherine L. Narr, narr@ucla.edu
 Brandon Taraku1
Brandon Taraku1 Artemis Zavaliangos-Petropulu
Artemis Zavaliangos-Petropulu Shantanu H. Joshi
Shantanu H. Joshi Randall Espinoza
Randall Espinoza Katherine L. Narr
Katherine L. Narr