- 1Student Research Committee, Shiraz University of Medical Sciences, Shiraz, Iran
- 2Transplant Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
Stress-induced mental health disorders are affecting many people around the world. However, effective drug therapy for curing psychiatric diseases does not occur sufficiently. Many neurotransmitters, hormones, and mechanisms are essential in regulating the body's stress response. One of the most critical components of the stress response system is the hypothalamus-pituitary-adrenal (HPA) axis. The FKBP prolyl isomerase 51 (FKBP51) protein is one of the main negative regulators of the HPA axis. FKBP51 negatively regulates the cortisol effects (the end product of the HPA axis) by inhibiting the interaction between glucocorticoid receptors (GRs) and cortisol, causing reduced transcription of downstream cortisol molecules. By regulating cortisol effects, the FKBP51 protein can indirectly regulate the sensitivity of the HPA axis to stressors. Previous studies have indicated the influence of FKBP5 gene mutations and epigenetic changes in different psychiatric diseases and drug responses and recommended the FKBP51 protein as a drug target and a biomarker for psychological disorders. In this review, we attempted to discuss the effects of the FKBP5 gene, its mutations on different psychiatric diseases, and drugs affecting the FKBP5 gene.
1. Introduction
A mental health disorder may be defined as a condition in which a person experiences abnormal thoughts, perceptions, feelings, behaviors, and distress (1). An estimated one billion people worldwide suffer from mental illness, and projections indicate that this number will continue to rise (2). Every 40 s, a suicide occurs, and ~160 million individuals require humanitarian assistance due to mental health concerns (3). Individuals suffering from mental health issues often experience a compromised quality of life marked by diminished self-control, impaired judgment, reduced self-assurance, feelings of demoralization, and hopelessness (4).
Despite many people being affected by psychiatric disorders, there is a substantial lack of effective cures for psychiatric diseases (5–8). The etiology of psychological manifestations is often unclear, particularly concerning molecular aspects (5, 6, 9). However, despite the availability of monoaminergic anti-depression drugs, less than half of the patients experience full rehabilitation after taking medications (5). Furthermore, pharmacogenetic studies have highlighted the associations between an individual's genetic variations and treatment outcomes. Therefore, pharmacogenetic findings indicate the potential for more effective molecular interventions (10). These pharmacogenetic studies, alongside twin studies, have highlighted the importance of genetic and molecular factors in psychiatric diseases (10, 11). Moreover, approximately one-third of schizophrenic patients are diagnosed as “treatment resistant” (12). Psychiatric treatment resistance is characterized by a correct diagnosis, sufficient and suitable treatment, and an inadequate symptomatic response. Treatment resistance is currently defined in a variety of psychiatric diseases, including obsessive-compulsive disorder (OCD) (13), bipolar affective disorder (14), schizophrenia (15), and major depressive disorder (MDD) (16, 17). The number of published studies indicates the growing rate of treatment resistance year by year (18). Under some circumstances, a patient's specific condition can create barriers to achieving the full potential of treatments. For instance, in both MDD and schizophrenia, liver drug metabolism (19, 20), tobacco smoking (21, 22), and genetic variations are predisposing factors to inadequate response to treatment (18). Furthermore, pharmacogenetic studies have demonstrated the relationships between an individual's genetic variations and treatment outcomes. Thus, as a consequence, pharmacogenetic findings could be applied to more effective molecular interventions (10). One of the mechanisms that are considered to potentially trigger psychological disorders underlying psychoneuroendocrinology is hormone and transmitter fluctuation in the central nervous system (CNS) (23). Endocrine-produced steroid hormones can passively enter the CNS, acting as a transcriptional factor leading to the upregulation of some genes, neuron excitability modification, and mood and behavior alterations (24). The glucocorticoid receptors (GRs) are omnipresent and expressed in almost all cells, including CNS cells. Previous studies have shown that GR expression in specific CNS parts could regulate mood and behavior (25). Dysregulation of steroid hormone receptors, such as the upregulation of the hypothalamus-pituitary-adrenal (HPA) axis, can lead to mood disorders such as depression (26). In this regard, restoring factors such as FK-506 binding proteins 51 (FKBP51) and 52(FKBP52) can be promising targets (27).
FKBP51 acts as an inhibitor for GRs' translocation to the cellular nucleus and is also associated with the negative feedback mechanism of these receptors. GR activation affects both inhibitory and excitatory synapses in the hippocampus. However, this regulatory function is dependent on the presence of FKBP51, as evidenced by studies indicating that such mediation does not occur in its absence (28). Additionally, Genomic Wide Association Studies (GWAS) have revealed a correlation between certain allelic variants of FKBP5 and various mental health conditions, including but not limited to aggression (29), bipolar disorders (30), suicide (31–34), post-traumatic stress disorder (PTSD) (35), negative personality traits (36), and peritraumatic dissociation (37). An animal study on mice indicated that FKBP5 may have a crucial role in morphine addiction induction (38). FKBP5 also has a significant association with a higher probability of severe depressive disorder in subjects with methamphetamine use disorders (39). Besides some other genes, FKBP5 has an effect on the electroconvulsive therapy effect in resistant major depression cases (40). Recently, an association between mutations of FKBP5 and functional seizures, another stress-induced disease whose biological aspects are not well known, has been found (41, 42). Moreover, in another study, potential drugs for targeting the FKBP51 protein have been investigated and reported (43) because the inhibition of FKBP51 can promote the regulation of the HPA axis in stress-induced disorders (41, 43). In this study, we attempted to explain the role of FKBP5 in the human body, especially in the HPA axis and stress-induced disorders, the effects of the most important genetic mutations of this gene in stress-induced disorders, and the potential therapeutic effects affecting FKBP51.
2. FKBP5's mechanism of action
The FKBP5 gene is located on chromosome 6 (6p21.3) in humans and consists of 13 exons. The FKBP5 gene encodes the FKBP51 protein, a member of the immunophilin protein family (44), which derives its name from its capacity to bind to immunosuppressive drugs (45). The peptidyl-prolyl isomerase (PPIase) domain, present in all members of this family, allows them to bind to different drugs such as rapamycin, cyclosporine A (a subfamily of cyclophilins), and FK506 (an FKBP subfamily protein), based on their ability to recognize them selectively (45, 46).
The FKBP51 and FKBP52 proteins of the immunophilin family are named for their high molecular weights (51 and 52 kDa, respectively) (47). FKBP51 and FKBP52 are homologous and share 75% similarity. The remaining 25% difference between these two proteins potentially affects their conformation and domain orientation and, consequently, their divergent effects on steroid hormone receptor (SHR) activities (48, 49). Further, FKBP51 and FKBP52 contain tetratricopeptide-repeated (TPR) domains, which allow them to bind to heat shock protein 90 (HSP90) dimers (50). The HSP90 protein plays an important role in the maturation and folding of SHRs, allowing them to bind to their ligands (51). Furthermore, the FKBP51 protein functions as a negative regulator of GRs in association with the HPA axis (52). As a substantial regulatory factor of the HPA axis, GR (Nuclear Receptor Subfamily 3 Group C Member 1 (NR3C1) gene) is a ligand-dependent transcription factor that modulates gene transcription (activation or repression) in the nucleus for diverse genes, including the FKBP5 gene (53–55). GCs, by interacting with GRs, can induce stress-related actions such as vasoconstriction, lipolysis, and suppression of reproduction, preparing the human body for “fight or flight” actions. Chronic stress can disturb the resilience of neurons, causing susceptibility to many human psychiatric disorders (56). This is the reason for the importance of the negative regulatory effects of FKBP51 on GRs.
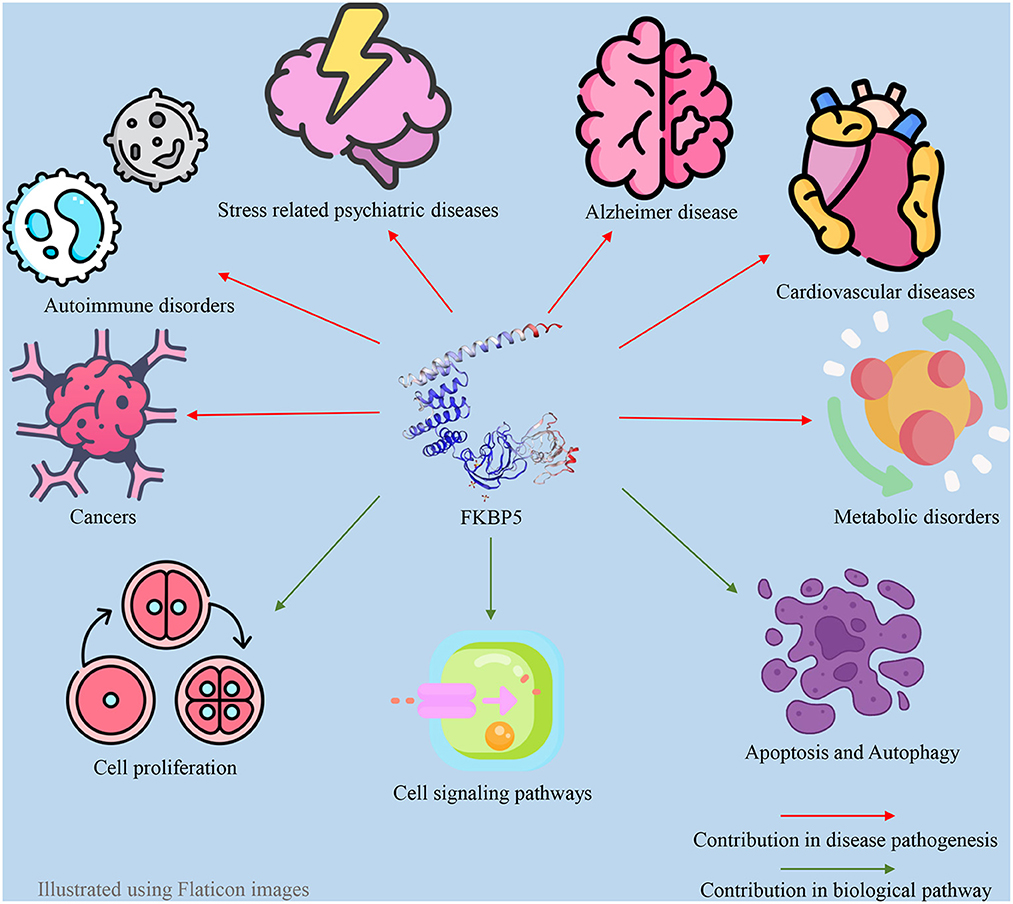
FKBP51 plays a pivotal role as a regulatory factor in numerous biological processes within both the central and peripheral nervous systems. FKBP51 exerts its effect through a variety of direct and indirect mechanisms and Hsp90-dependent or Hsp90-independent pathways (57). The impact of FKBP51 on critical cellular signaling pathways is well established, such as the modulation of Akt and the mammalian target of rapamycin (AKT/mTOR) pathway (58), Tau formation (59), nuclear factor kappa B (NF-κB) pathway (60), microtubule dynamics (61), epigenetic remodeling (62), metabolism (63), apoptosis (64), autophagy (64), and reproduction (27). Thus, changes in FKBP51 expression and function are associated with the occurrence of a wide variety of disorders, including metabolic syndrome (65), Alzheimer's disease (66), the development of different types of cancer, cardiovascular disease, and immune disturbances, in addition to stress-induced psychiatric disorders (67, 68). FKBP5 also has an association with atrial fibrillation. Indeed, the loss of function of the FKBP5 gene has a significant impact on atrial fibrillation development and cardiac function (69). In addition to responding to stress, the HPA axis can regulate sleep mechanisms via the FKBP5 gene. As a result, a disturbed FKBP51 protein level can act as a pathogenic factor for sleep disturbance (70). Additionally, since androgens and androgen receptors (ARs) directly and indirectly regulate the FKBP5 gene, this gene may also be associated with polycystic ovary syndrome (PCOS) and hyperandrogenism (71). These associations between the FKBP5 gene and various diseases make FKBP51 a promising drug target. For example, FKBP5 is a target for anti-depressants and anti-cancer drugs. Furthermore, FKBP51 may serve as a biomarker for cancer diagnosis and chemotherapy response in patients with varying levels of FKBP51 changes (67, 72, 73). Many studies have indicated a significant negative association between FKBP5 and the severity of different types of cancer (73–76). Furthermore, previous studies have indicated that FKBP51 could be a potential diagnostic marker for psychiatric diseases (77, 78). Some of the roles of FKBP51 in different aspects of human physiology can be observed in Figure 1.
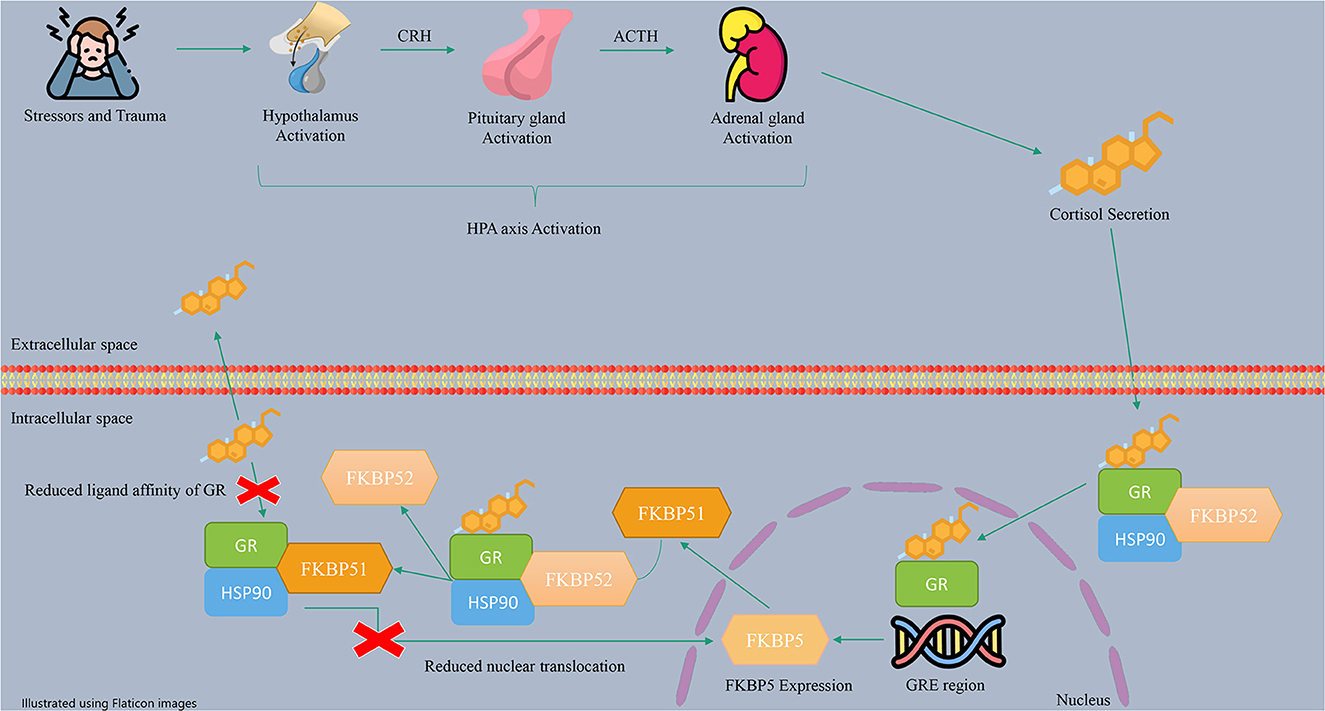
The HPA axis is a neuroendocrine stress response system activated by exposure to physical or psychological stress. The HPA axis regulates many human body systems, including the metabolic, cardiovascular, and immune systems, resulting in adaptation to the human environment (56). In more detail, stress triggers the hypothalamus to release the corticotropin-releasing hormone (CRH), which stimulates the pituitary gland to produce adrenocorticotropic hormone (ACTH). ACTH stimulates the adrenal glands to release glucocorticoid hormones (GCs), such as cortisol, which circulate throughout the body and bind to GRs, leading to negative feedback control (65, 79). Besides, in the cells, GR and other regulatory proteins, including HSP90, make a protein complex which bindes to cortisol. The role of regulatory proteins is to change the formation of GR to a conformation with more affinity to bind hormones (27). The activated GRs with cortisol induce the transcription and translation of the FKBP5 gene (57). FKBP51 expression is regulated by the interaction of GR with glucocorticoid response elements (GREs) (44). These enhancer elements can bind to the FKBP5 gene in the 2, 5, and 7 introns and the promoter, which are regions enriched with cytosine phosphate guanine (CpG) islands (80). The FKBP51 protein can inhibit GR function through a negative feedback loop (44, 81). This inhibition occurs through a complex interplay involving the replacement of FKBP51 with FKBP52 in the receptor heterocomplex and the modification of GR folding and conformation. As a result, the affinity of GR for cortisol decreases, leading to GR resistance and an increase in circulating cortisol levels.
Additionally, by enhancing the FKBP51 level, FKBP51 can inhibit the translocation of GRs to the nucleus, resulting in a reduction in the expression of GR target genes (82, 83). Therefore, FKBP51 helps maintain HPA axis balance during periods of stress. A summary of the described mechanism of action of the HPA axis and FKBP5 is shown in Figure 2.
The HPA axis and its regulators are intricately connected; any imbalance among these components may result in psychiatric disorders (84). Any dysregulation of the HPA axis caused by genetic, epigenetic, or environmental factors may result in the altered expression of the FKBP51 protein (44, 85, 86). However, genetic and epigenetic variations of the GREs and the FKBP5 gene can influence the expression level of the FKBP51 protein (80). Some single nucleotide polymorphisms (SNPs) of GREs and the FKBP5 gene are associated with increased expression of FKBP51, while others are associated with a decreased expression of FKBP51 (87, 88). There is a positive correlation between these changes in FKBP5 gene expression levels and mental disorders (28).
In terms of translation regulation, it has been demonstrated that DNA methylation is the most common epigenetic modification studied for FKBP5 compared with post-translational modifications and non-coding RNAs. In CpGs, the methylation process contributes to chromatin compression and usually reduces gene expression by inhibiting transcription factor binding. It has been demonstrated that long-lasting methylation of genes associated with the glucocorticoid signaling pathway may alter the function of these genes and contribute to a variety of mental health disorders, particularly during early-life prolonged stress conditions and childhood adversity (89, 90). Additionally, methylation of FKBP5 leads to accelerated DNA methylation aging and, consequently, cardiometabolic risk (91). Recent studies have shown that FKBP51 plays a direct role in epigenetic processes by controlling phosphorylation and activating the DNA methyltransferase 1 (DNMT1) enzyme (62). Genetic polymorphisms and DNA methylation of FKBP51 proteins are transcriptional modifications (92, 93). The expression of FKBP51 can also be regulated at the post-transcriptional level through interference with FKBP51 mRNA by micro-RNAs (94) and post-translational modifications such as protein phosphorylation (95). Studies have shown that micro RNAs (miRNAs) and the FKBP5 gene interact at a posttranscriptional level. For example, non-coding RNA molecules such as circular RNAs (circRNAs) and long non-coding RNAs (lncRNAs) can contribute to the progression of autoimmune diseases and cancer through miRNA/FKBP5 axis regulation (96, 97).
3. FKBP5 genetic variations and psychiatric diseases
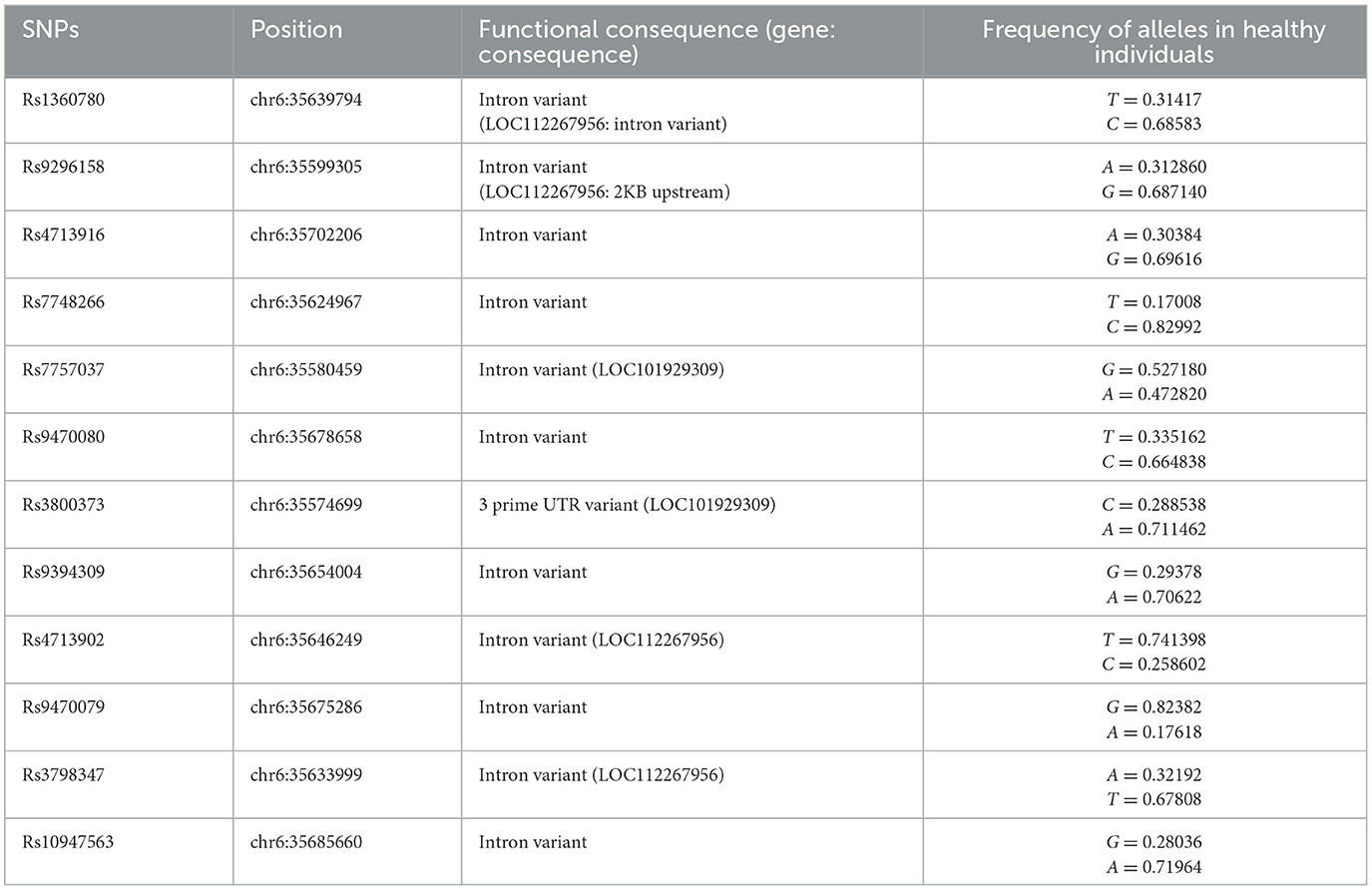
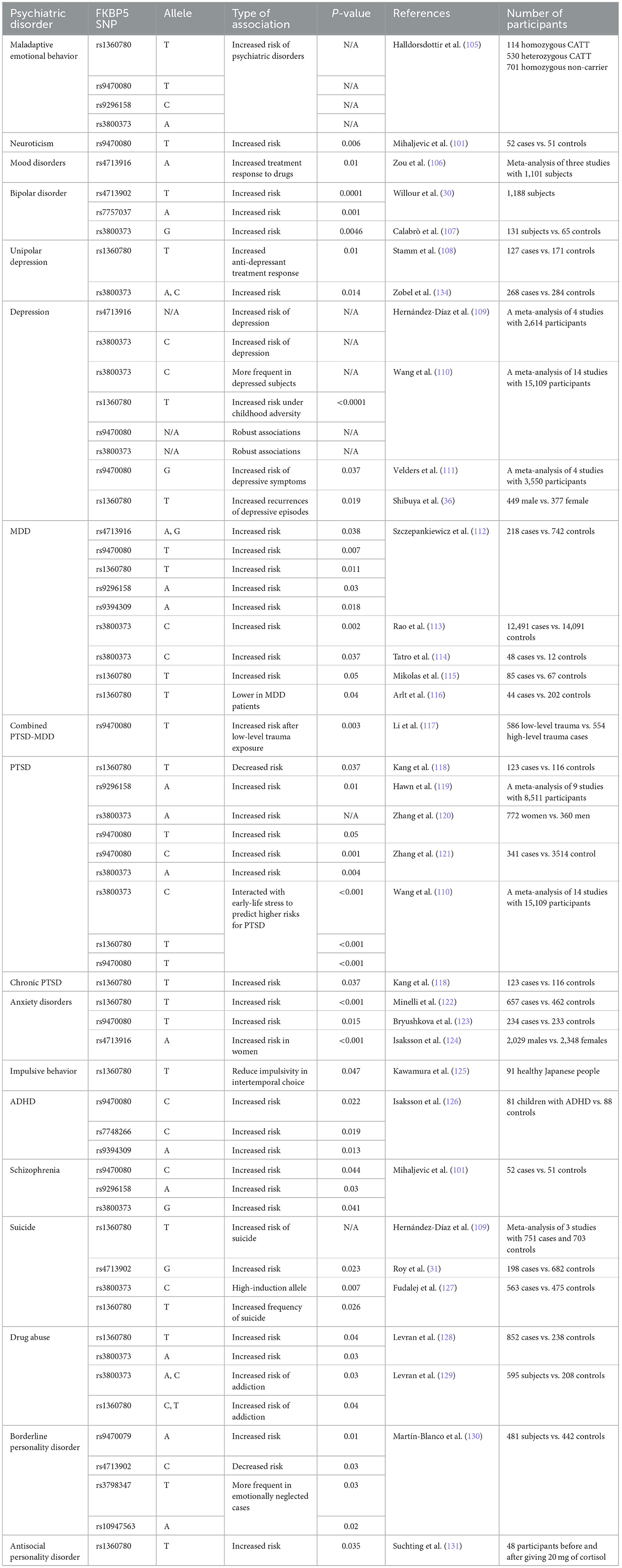
The HPA axis is one of the most important biological components of the stress response (98, 99). Thus, genetic factors regulating the HPA axis may affect individual HPA axis responses and stress-related psychological issues (100, 101). Many studies have examined the effects of genetic polymorphisms and epigenetic modifications on the HPA axis' response to stress (102). In this regard, FKBP5 has attracted much interest due to its regulatory effect on the HPA axis by regulating GC sensitivity (103). Variants of the FKBP5 gene can cause dysregulation of the stress response in healthy individuals through decreased negative feedback efficiency between FKBP5, GRs, and GCs, as well as the dysregulation of GR sensitivity (87, 88). In this section, we have attempted to demonstrate the association between the most studied SNPs of the FKBP5 gene and various mental problems. Information concerning SNP regions in the human genome and the frequency of each allele was obtained from the database of SNPs (dbSNP) and is presented in Table 1 (104). Additionally, a summary of relevant studies investigating the association between FKBP5 SNPs and different mental health conditions has been provided in Table 2.
3.1. Rs1360780
Anxiety can cause the upregulation of FKBP5, resulting in a lowered affinity of GRs to GCs when sustained, leading to clinical symptoms of anxiety and MDD (122). Observations have shown that emotionally neglected or abused children with the T allele of rs136780 have lower DNA methylation in the seventh intron of FKBP5 (88). The presence of the T allele of rs1360780 is associated with a higher risk of developing PTSD after child abuse and medical trauma (132); thus, this particular allele is referred to as the “high induction” allele of FKBP5 since it can cause an increase in FKBP5 mRNA levels (132). Conversely, the C allele of rs136780 can cause lower contact of GREs with FKBP5's transcription initiation site and less expression of this gene (132). Controversial studies have shown an increased rate of the T allele in depressive episodes (133) and the C allele in MDD patients (134). Thus, further studies, specifically on MDD patients, are required to determine the exact role of rs136780 in psychiatric disorders.
Rs136780 can also affect the pharmacogenetic aspects of drugs. In the Munich Anti-depressant Response Signature (MARS) project, patients with psychiatric disorders and depressive symptoms who have rs1360780 have shown better responses to treatment with citalopram (135). However, patients who have schizophrenia have a greater probability of not responding to clozapine when they have TT homozygotes of the rs13860780 than the C allele carries (136). However, in patients with rs1360780, no evidence demonstrated a relationship between paroxetine and mirtazapine with treatment outcomes (137). As a result, rs1360780 is an important SNP, and its features for predicting the treatment outcome can be helpful.
3.2. Rs9296158
The SNP rs9296158, located in the FKBP5 gene, has been identified as a risk factor for suicide after childhood trauma (31) and the severity of PTSD symptoms (132). Rs9296158 has also been identified as a susceptibility factor for anxiety sensitivity (138) and MDD (112). Patients with cancer who possess rs9296158 after prolonged stress exposure may experience heightened levels of anxiety and depression (139). In addition to the effect of rs9296158 on the expression of FKBP5, this SNP can affect the signal transducer and activator of transcription 5B (STAT5B) mRNA levels. STAT5B is a protein involved in the translocation of GRs to the nucleus of cells. The reduction of STAT5B can cause excessive signaling induced by GRs, exacerbating stress-related effects (140). A recent study has demonstrated that traumatized individuals with the rs9296158 G allele exhibit much more severe dissociation symptoms than those with the rs9296158 A allele (136). Further details outlining the association between rs9296158 and various psychological conditions are presented in Table 2.
3.3. Rs4713916
Rs4713916 is associated with MDD (112, 141), psychotic symptoms (142), and higher levels of anxiety after social stressors (143). Rs4713916 can affect MDD patients by decreasing the hippocampal volume and causing a lower response to anti-depressants (112). Moreover, A allele carriers respond better to treatment in patients with mood disorders than those with the G allele (106). In the German population, carriers of the A allele of rs4713916 have a lower incidence of depression (141). Patients could be examined by the Trier Social Stress Test (TSST), measuring the cortisol level after acute stressors to determine the normal and abnormal responses of the HPA axis against acute stress. The result of the TSST can be changed from person to person, because the serum cortisol levels can be genetically influenced by polymorphisms of the FKBP5 gene (144). The result of the TSST in people who have rs4713916, rs380373, and rs1360780 SNPs demonstrated impaired cortisol responses to social stressors. This observation demonstrated that the impaired negative feedback of the HPA axis could result in chronically high levels of GRs and susceptibility to stress-induced psychiatric diseases (143). Finally, more accurate studies are needed to clarify the effect of rs4713916 on the regulation of stress responses.
3.4. Rs7748266
The T allele of rs7748266 is associated with an increased risk of attention deficit hyperactivity disorder (ADHD) in children (145), susceptibility to developing depressive symptoms (146), and decreased cortisol levels (111). Rs7748266 is also associated with an increased amygdala reaction in childhood emotional neglect by decreasing the basal cortisol levels (147). The amygdala is the brain's emotional processing center, and its structure could be altered by early life adversity. The amygdala can affect the HPA axis via neurotransmitters that should be noticed in stress-induced psychiatric disorders (148). In addition, rs7748266 is associated with lithium effectiveness and response to stressful situations in bipolar individuals (149). The C allele of rs7748266, by changing the regulation of GR activity, could influence the efficacy of lithium (149).
3.5. Rs4713902
Rs4713902 is associated with an increased risk of bipolar disorder, borderline personality disorder, and suicidal behaviors (30, 31, 130). There is also evidence of an association between the C allele of rs4713902 and higher baseline cortisol levels in psychological stress than homozygote T carriers (150). A higher cortisol level may lead to neuropsychiatric disorders by altering neural circuitry (150). GC negative feedback can be reduced when FKBP5 expression or function increases (151). Thus, the improvement in the expression or function of FKBP5 could explain the role of the C allele of rs4713902.
3.6. Rs9470079
Martín-Blanco et al. reported that rs9470079 might be involved in the etiology of bipolar disorder despite the polymorphisms associated with bipolar disorder being intronic variants with unknown functions (130). The rs9470079 variant was significantly associated with decreased cortisol suppression, indicating a potential independent relationship between this variant and altered HPA axis negative feedback (152). Previous studies have shown that alleles associated with MDD increase FKBP5 expression, which induces enhanced binding to GR (82, 87, 112), and Ferrer et al. indicated that a similar mechanism might be involved in rs9470079 modifying HPA axis negative feedback as well (152). This decreased cortisol suppression caused by the A allele of rs9470079 can increase the susceptibility of people to stressors (152). Therefore, additional research is needed to identify vulnerable individuals carrying this allele to provide better mental health care.
3.7. Rs9394309
In female subjects with breast cancer, certain genetic variations of FKBP5, such as rs9394309, are associated with increased fatigue, depression, and anxiety (153). Rs9394309 is also linked to MDD (112), higher levels of anxiety in female subjects (124), ADHD, and lower cortisol levels in ADHD patients (126). A significant effect on left dorsal amygdala reactivity can be observed in patients with rs9394309 and childhood emotional neglect. This reactivity bias is one reason why people carrying FKBP5 risk alleles (“i.e., rs1360780 T allele, rs9296158 A allele, rs9470080 T allele, rs3800373 G allele, rs7748266 T allele, rs9394309 G allele”) are more likely to suffer from stress-related psychopathologies. In detail, heightened threat-related amygdala reactivity is due to FKBP5 risk genotypes that cause an elevated and prolonged cortisol response to stress (147). However, extreme emotional neglect in childhood can cause higher levels of HPA hyperresponsiveness (154, 155). In addition, anxiety and negative experiences can induce the overexpression of FKBP5 (82, 103, 132). This overexpression of FKBP5 and HPA hyperresponsiveness with adverse experiences (156, 157) can cause HPA negative feedback impairment (158). As a result, both the FKBP5 risk genotypes and a history of emotional neglect are linked to dysregulation of the HPA axis. The interaction of FKBP5 risk genotypes and a history of emotional neglect can increase the amygdala's reactivity and sensitivity to environmental hazards, which increases the risk of stress-related psychopathology (147).
3.8. Rs3800373
Rs3800373 is located in the 3′-untranslated region (3′-UTR) of the FKBP5 gene, which may affect the mRNA stability and half-life of FKBP5 (114). SNP rs3800373 CC is more common in patients with MDD and MDD/psychosis. FKBP51 protein levels are higher in these patients than in control groups, but transcription levels do not differ significantly (114). There is a possibility that translation efficiency may be affected by this 3′UTR polymorphism. A study on miRNA binding in 3′UTRs indicates that miRNA binding can influence the stability of mRNA, affecting the half-lives of a specific transcript (159). However, the G allele of rs3800373 can induce the overexpression of the FKBP51 protein, resulting in reduced GR availability and sensitivity to GCs and disrupting the negative feedback loop of the HPA axis. This impaired inhibition of negative feedback can cause susceptibility to psychological diseases (107).
Studies have reported conflicting results about the association of rs3800373 with psychopathology. Binder et al. observed an interaction between childhood trauma and rs3800373, linked to an increased risk of PTSD in African-American populations. However, allelic variation in rs3800373 was not associated with PTSD in an analysis involving predominantly European-Caucasian participants. The results suggest that ethnicity might be an essential factor affecting the functions of the FKBP5 SNPs (160).
According to White et al.'s study, FKBP5 genotypes interacted with childhood emotional neglect to predict increased threat-related dorsal amygdala reactivity. As described in the explanation of the role of rs9394309, individuals with these FKBP5 alleles may be more susceptible to stress-related psychopathology through this reactivity bias in the context of emotional neglect (147). In conclusion, rs3800373 is one of the most important SNPs of the FKBP5 gene, and its association with several diseases has been found before. Moreover, some hypotheses regarding the molecular mechanism of the polymorphism were made before, as were signs about the effect of SNPs on the amygdala. Further research and analysis are required to fully understand the comprehensive role of this SNP and its different alleles.
3.9. Rs9470080
The genetic variant rs9470080 has been associated with various mental health conditions such as depressive disorders and symptoms, drug abuse, anxiety disorders, ADHD, rumination disorder, schizophrenia, borderline personality disorder, and PTSD (101, 105, 110, 117, 123, 126, 161). Several studies have indicated that individuals with the rs9470080 TT genotype exhibit reduced FKBP5 mRNA expression, resulting in lower plasma cortisol levels. These patients were more likely to experience depressive and PTSD symptoms after experiencing low-level trauma. Therefore, low cortisol levels may constitute a vulnerability factor that might partially explain the high comorbidity of PTSD and depression. Moreover, the C allele of Rs9470080 appears to play a protective role against developing psychopathologies like PTSD in severe trauma cases. According to Li et al.'s findings, some alleles associated with a certain disorder in one environment may confer protection against that disorder in a different environment and condition (117).
3.10. Rs7757037
Rs7757037 is associated with an increased risk for bipolar disorder. The G allele, with higher frequencies in the population, was linked with the risk of bipolar disorder (30). In lupus patients, the FKBP5 gene variant rs7757037 was linked to depression (162), but no similar finding was achieved in an adolescent Chinese population in which the association between depressive symptoms and parenting styles was measured (163).
3.11. Rs3798347 and rs10947563
Mahon et al. (150) found an association between rs3798347-T and rs10947563-A alleles and a history of physical abuse and emotional neglect in borderline personality disorder subjects in comparison to patients who had never experienced these traumas and the control group. These two variants are intronic SNPs of the FKBP5 gene. To the best of our knowledge, few studies have been conducted on them, and further studies are needed to determine their exact action.
4. Drugs associated with FKBP51
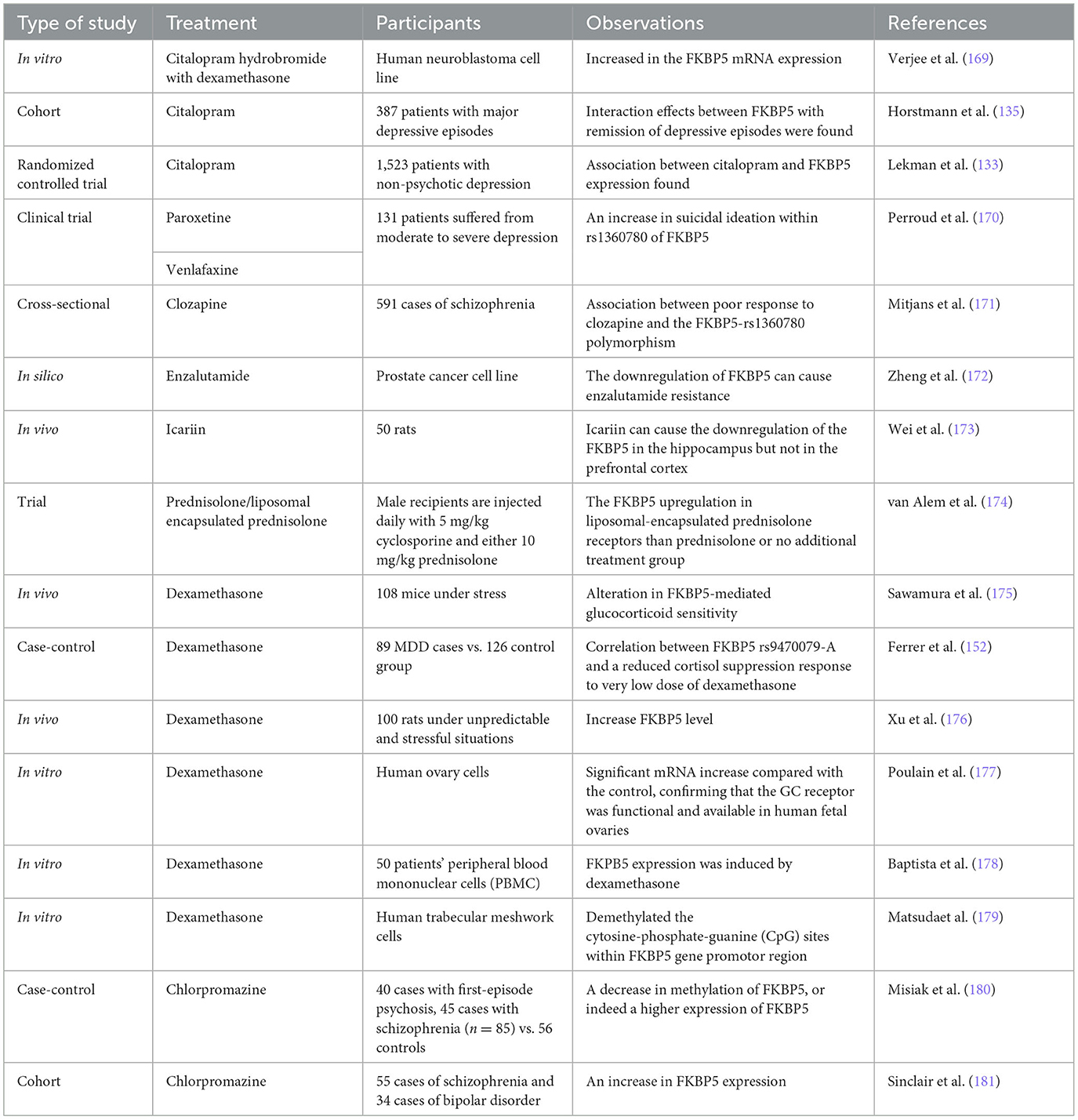
FKBP51 is an essential mediator in the HPA axis of stress responsiveness due to its regulatory effect on GR activity (52, 79). Previous studies have demonstrated how the epigenetic process of DNA methylation is involved in response to psychological therapies, particularly the methylation of FKBP5 at its promoter region, which is associated with PTSD and anti-depressant therapy responses (135, 164). Indeed, the reduction of FKBP5 methylation was strongly correlated with a greater response to therapy (165). Moreover, pharmacogenetic studies on FKBP5 showed promising results in psychopharmacology drug usage (137). FKBP5 can be affected by many substances, and it has been found that many drugs can cross the blood–brain barrier and affect the FKBP51 protein level by methylating the FKBP5 gene (166, 167). The importance of these findings lies in the possible role of FKBP51 as a potential pharmacologic target for stress-induced psychiatric diseases due to its importance in HPA axis regulation (168). In this section, we attempt to describe the relationship between FKBP51 and different drugs and compounds that affect FKBP51 in different aspects. The drugs discussed in this section are the most promising drugs that can inhibit the FKBP51 protein based on our previous in-silico study. In a previous study, multiple databases were screened, and those therapeutics that can cross the blood–brain barrier (BBB) and have capable pharmacologic and bioinformatic features for interacting with and inhibiting the FKBP5 gene were reported (43). In addition, the effects of different drugs on FKBP51 are listed in Table 3.
4.1. FKBP51 ligands
4.1.1. SAFit1 and SAFit2
SAFits are selective inhibitors of the FKBP51 protein, and their anti-depressant and neurogenic effects have been previously demonstrated (182, 183). Previous studies have shown that SAFits can be utilized for immunosuppression in heart transplant donors by reducing programmed death ligand 1 (PD-L1) (184). Balsevich et al. demonstrated that SAFit2 treatment affects glucose tolerance and reduces body weight, which is specific to FKBP51 inhibition (183).
Besides, in various in vivo studies, SAFit2 has also exhibited anti-anxiolytic (185), anti-stress (186), and anti-alcohol consumption (187) effects. Based on a study performed by Pöhlmann et al. (188), it has been revealed that SAFit2 could be a valuable co-medication in the case of depression disorders that could alter the effect of other anti-depressant drugs. In 2014, Gaali et al. (183) used SAFit2 in the combined dexamethasone/corticotropin-releasing factor (Dex/CRF) test to investigate its effect on HPA axis regulation. They reported that SAFit2 improved the elimination of corticosterone levels after applying dexamethasone. Their results are associated with an enhancement of GR sensitivity by FKBP51 inhibition. Researchers demonstrated that FKBP51 inhibitors would improve the regulation of the HPA axis. It has also been noted that the Dex/CRF test could act as a functional biomarker throughout the clinical improvement of selective FKBP51 antagonists (183). However, Connelly et al. proposed that SAFit2 would debilitate stress-induced reinstatement of cocaine seeking in mice through increasing HPA axis negative feedback. Based on this study, SAFit2 would improve not only HPA axis negative feedback managed by GRs but also progesterone receptor sensitivity (189).
While SAFit1 and SAFit2 are two major selective inhibitors of the FKBP51 protein, they are unsuitable therapeutic compounds in clinical and pharmacological aspects. This is because the pharmacological features of these drugs, such as bioavailability and solubility, are far from their pharmacological usage in humans (190). As previously mentioned, finding other drugs for psychological disorder treatment is essential. The promising effects of selective inhibitors of FKBP51 showed the need for developing new pharmacologically suitable drugs for the selective inhibition of FKBP51.
4.1.2. Rapamycin and tacrolimus
Tacrolimus (FK506) and rapamycin (sirolimus) are widely utilized antiproliferative and immunosuppressive drugs that are commonly used to manage cancer and prevent acute graft rejection. They inhibit mTOR activity, which is known for its significant impact on cell growth and proliferation (191, 192). Clinical findings have demonstrated that patients undergoing immunosuppressive drug therapy frequently experience affective disorders such as anxiety or depression. In an investigation by Hadamitzky et al., it was observed that the acute administration of rapamycin upregulated the level of FKBP51 in the brain of mice experiencing psychiatric diseases, while repeated administration did not affect FKBP51 levels (191, 192). Tacrolimus can also bind to immunophilin FK-binding protein 12 (FKBP12) and form FKBP/FK506 complexes that can change the PPIase activity of FKBPs and result in immunosuppressants (193), which indicates the potentiality of tacrolimus to affect FKBP51. To the best of our knowledge, few studies have been conducted on the effect of tacrolimus and sirolimus on psychiatric diseases via FKBP51 modulation. Further research is required to determine the impact of these medications on psychiatric diseases owing to the importance of FKBP51 in stress-induced psychiatric diseases and the binding capacity of these two drugs to FKBP51.
4.2. Anti-depressants
4.2.1. Fluoxetine
It has been identified that treatment with selective serotonin reuptake inhibitors (SSRIs) can decrease the activity of CRH neurons and consequently contribute to SSRI's therapeutic activity. It has been propounded that the downregulation of the activity of CRH is the common step of anti-depressant activity. Many investigations have found that anti-depressants of different classes suppress CRH gene expression and HPA activity in depressed and healthy humans (29). In a study on mice exposed to chronic stress, fluoxetine could downregulate the overexpression of FKBP5 mRNA levels in the hippocampus and the prefrontal cortex (173). Besides, in another study that attempted to find an association between fluoxetine efficacy and patients who were carriers of two SNPs of FKBP5, no association was found. However, Maruf et al. (194) demonstrated pharmacogenetic associations between FKBP5 and fluoxetine. However, further research to confirm the absolute gene-antidepressant relationship is needed.
4.2.2. Citalopram
Citalopram is an SSRI whose association between drug response and different FKBP5 SNPs has been studied before (87, 133). In 2010, Zobel et al. (134) selected 110 German patients with unipolar depression after accounting for dropouts and 284 controls. The anti-depressant used was citalopram with or without lorazepam. The anti-depressant response was assessed using a Dex/CRF test. A previous study demonstrated the associations between rs3800373 and rs4713916 and lower citalopram responses (134). Furthermore, only the relation between FKBP5 and Citalopram response in the STAR*D sample (135) of the MARS project has been tested to date. A better response to Citalopram was found in a combination of protective SNPs of FKBP5, rs1360780, and glutamate receptor inotropic kainite 4 (GRIK4), rs12800734 (195). Therefore, anti-depressant drugs potentially influence the HPA axis, especially the expression level and genetic alteration of FKBP5. As a result, finding the exact mechanism of action of anti-depressants on FKBP5 should be considered in further research.
4.2.3. Icariin
Icariin, a major component of Herba Epimedii, has been indicated to possess potential anti-depressant effects (159). Icariin could reduce inflammatory cytokines and increase the cortisol level in chronically stressed rats (196). Additionally, in socially defeated mice, the administration of Icariin also renormalized the GR binding affinity and expression levels (197). These results indicated the various effects of Icariin on the HPA axis. Icariin administration in depressed individuals downregulates the FKBP5 gene in the prefrontal cortex and the hippocampus (173), which is similar to the action of anti-depressants such as fluoxetine. This downregulation of FKBP51 is involved in normalizing GR distribution in cells, which may ultimately restore HPA-axis negative regulation (173, 198). While the upregulation of FKBP51 in chronic stress is a normal reaction of the body, FKBP51 blockade could worsen the adverse effects of stress (44). However, the mechanism that explained the role of anti-depressants in the HPA axis in chronic depression was the renormalization of the upregulated FKBP51 level (199).
4.2.4. Paroxetine, venlafaxine, and clomipramine
Paroxetine, venlafaxine, and clomipramine are three anti-depressants, and high levels of any one of the three anti-depressants in the blood were associated with greater suicidal ideation. These anti-depressants could influence FKBP5 expression. The T allele of rs1360780 on FKBP5 was higher in these patients, indicating the involvement of the HPA axis in suicidality (170). As described in a previous study, T alleles of rs136780 are considered “high induction” alleles, resulting in higher FKBP5 mRNA levels (132). As a result, FKBP5 genetic variation could predispose individuals taking anti-depressants such as venlafaxine, paroxetine, and clomipramine to suicidal ideation.
4.2.5. Serotonin and noradrenaline reuptake inhibitors and tricyclic anti-depressants
Duloxetine is an SNRI that serves as an anti-depressant (200). A study conducted in 2013 measured the mRNA and protein levels of FKBP5 in chronic mild stress (CMS). The study found a significant increase in FKBP5 mRNA levels in the ventral and dorsal hippocampus of CMS mice. These changes, which come from CMS, reproduce a depressive-like phenotype. Duloxetine led to controversial results; while it increased FKBP5 mRNA levels in control rats, it controversially decreased the levels of FKBP5 mRNA when administered to CMS rats. These findings suggest that CMS-exposed animals alter FKBP5-GR activity, which is a crucial step in the HPA axis and GR function. It has been proposed that duloxetine treatment can normalize some of these changes (19). Another study on the MDD mouse models revealed that treatment with venlafaxine and sertraline had no impact on the expression of the FKBP5 gene (201). Furthermore, it has been shown that treatment with tricyclic anti-depressants (TCAs) would increase GR binding affinity and GR mRNA expression in rat hypothalamic and hippocampal neurons. These results propose that anti-depressants may enhance GC sensitivity and function, particularly in the brain, and revitalize GR-mediated feedback prevention on the HPA axis (202).
4.3. Antipsychotics
4.3.1. Chlorpromazine
Many studies have demonstrated that patients experiencing first-episode psychosis and schizophrenia may exhibit a flattened HPA axis response to stress (203). Although FKBP5 hypermethylation has been detected in patients with schizophrenia (204), Misiak et al. (180) demonstrated that FKBP5 methylation could not be detected after accounting for potential confounding factors. It was suggested that confounders, including medication usage, such as chlorpromazine, could influence FKBP5 methylation and expression in schizophrenia patients. Thus, further investigation of the effects of chlorpromazine on the methylation and expression of FKBP5 should be undertaken in the future.
4.3.2. Clozapine
Clozapine is another atypical antipsychotic drug with a benzodiazepine derivative and is more effective in curing treatment-resistant schizophrenic patients (205). GCs significantly induct the FKBP5-rs1360780 with clozapine consumption in schizophrenia patients. Clozapine is the only FDA-approved drug for treating resistant schizophrenia (TRS), and several variants, such as FKBP5 variants, have been involved in clozapine response. Furthermore, FKBP51 could be a treatment target for clozapine (171).
4.4. Corticosteroids and glucocorticoid ligands
4.4.1. Dexamethasone
As HPA axis hyperactivity is the most consistent abnormality in depressive disorders, and GCs maintain and reduce HPA axis activity, dexamethasone as a glucocorticoid agonist potentially positively affects mood disorders (206). A study conducted by Bekhbat et al. reported that an increased gene expression of FKBP5 was associated with depressive symptoms in dexamethasone-administered cases (207). Dexamethasone also upregulates the expression of the FKBP5 gene in osteogenesis, chondrogenesis (208), and choroidal endothelial cells, modulated by the GR (209). Furthermore, hypermethylation of the FKBP5 gene induced by dexamethasone may involve the HPA axis, which has a substantial role in psychotic disorders (210). Dexamethasone can induce FKBP5 mRNA and protein expression (211). Xu et al. (176) also proved that an increase in FKBP5 expression and a decrease in GR expression in the hippocampus and prefrontal cortex had been detected due to dexamethasone administration in adult rats. The potential effects of dexamethasone on FKBP5 expression impact the HPA axis, and its promising antipsychotic effect should be noticed.
4.4.2. Prednisolone
Previous studies have indicated that FKBP51 can be a glucocorticoid sensitivity biomarker due to being the negative regulator of GRs (212, 213). In this regard, a recent study (174) evaluated prednisolone administration's effect on the FKBP5 level. For 24 h, the overexpression of FKBP5 mRNA was found under the effect of liposomal-encapsulated prednisolone (LP). As a result, similar to dexamethasone, the upregulation of FKBP5 can result in GR downregulation. Therefore, the potential effects of dexamethasone could be considered for prednisolone, and further studies are needed.
4.4.3. BI653048
GCs intervene through the GR, a member of the nuclear receptor family of intracellular receptors that are generally placed in the cytoplasm. Recent data indicate that glucocorticoid receptor ligand (GRL) is functionally selective among transrepression and transactivation. These compounds are known as selective glucocorticoid receptor agonists (SEGRAs). Trifluoromethyl carbinol-derived compounds are an extremely well-profiled nonsteroidal class of GRLs. In the study of Harcken et al. (214), a compound named BI653048 was identified. BI653048 is in the SERGA class, which binds with high affinity to the human GR. They revealed that treatment with 200 mg of BI653048 can be associated with a decreased expression of FKBP5 (214).
5. Discussion and conclusion
The current study sheds light on the vital role of FKBP5 in stress-induced psychiatric disorders, the HPA axis, and the human body. Despite the limited knowledge of their biological dimensions, it is evident that FKBP5 mutations can significantly contribute to the manifestation and progression of personality disorders and functional seizures by influencing the expression of FKBP51 and the amygdala. The contribution of FKBP5 mutations to many stress-induced psychiatric disorders was found. This significant influence of FKBP5 on stress-induced psychiatric disorders highlights the importance of this gene as a potential drug target. Although the included studies have explored the potential of FKBP51 as a drug target, further investigation is essential to clarify the direct inhibitory effects of the drugs on this gene. If confirmed, psychiatric drugs targeting FKBP51 may not only impact neurotransmitters but also directly modulate the HPA axis. Such significant implications call for pharmaceutical companies to formulate optimized drugs capable of efficiently and simultaneously regulating both neurotransmitters and FKBP51. Hence, the findings from this study establish the significance of FKBP51 as a promising therapeutic target for stress-induced psychiatric disorders.
Author contributions
MM contributed to study conceptualization, writing, and reviewing. DS and NE contributed to writing and reviewing. MS, SS, MJ, and SM contributed to data acquisition and writing. NA contributed to the study conceptualization and review. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kesari KK, Agarwal A, Henkel R. Radiations and male fertility. Reprod Biol Endocrinol. (2018) 16:1–16. doi: 10.1186/s12958-018-0431-1
2. Evaluation IoHMa. Global Health Data Exchange (GHDx). Available online at: https://vizhub.healthdata.org/gbd-results/ (accessed March 03, 2023).
3. Kovacevic R. Mental Health: Lessons Learned in 2020 for 2021 and Forward 2021. Available online at: https://blogs.worldbank.org/health/mental-health-lessons-learned-2020-2021-and-forward (accessed March 03, 2023).
4. Wdowiak A, Skrzypek M, Stec M, Panasiuk L. Effect of ionizing radiation on the male reproductive system. Ann Agricul Environ Med. (2019) 26:85. doi: 10.26444/aaem/106085
5. Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am. (1996) 19:179–200. doi: 10.1016/S0193-953X(05)70283-5
6. Ivanov I, Schwartz JM. Why psychotropic drugs don't cure mental illness-but should they? Front Psychiatry. (2021) 12:579566. doi: 10.3389/fpsyt.2021.579566
7. Collaborators GMD. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. (2022) 9:137–50. doi: 10.1016/S2215-0366(21)00395-3
8. Ghaemi SN. Symptomatic versus disease-modifying effects of psychiatric drugs. Acta Psychiatr Scand. (2022) 146:251–7. doi: 10.1111/acps.13459
9. Troisi A, Dieguez G. Etiology of psychiatric disorders: lay beliefs and the role of gender, field of study and personality traits. Clin Neuropsychiatry. (2022) 19:150–5. doi: 10.36131/cnfioritieditore20220303
10. Serretti A, Artioli P, Quartesan R. Pharmacogenetics in the treatment of depression: pharmacodynamic studies. Pharmacogenet Genom. (2005) 15:61–7. doi: 10.1097/01213011-200502000-00001
11. Uher R, Zwicker A. Etiology in psychiatry: embracing the reality of poly-gene-environmental causation of mental illness. World Psychiatry. (2017) 16:121–9. doi: 10.1002/wps.20436
12. McCutcheon R, Beck K, Bloomfield MA, Marques TR, Rogdaki M, Howes OD. Treatment resistant or resistant to treatment? Antipsychotic plasma levels in patients with poorly controlled psychotic symptoms. J Psychopharmacol. (2015) 29:892–7. doi: 10.1177/0269881115576688
13. Bloch MH, Storch EA. Assessment and management of treatment-refractory obsessive-compulsive disorder in children. J Am Acad Child Adolesc Psychiatry. (2015) 54:251–62. doi: 10.1016/j.jaac.2015.01.011
14. Gitlin M. Treatment-resistant bipolar disorder. Mol Psychiatry. (2006) 11:227–40. doi: 10.1038/sj.mp.4001793
15. Howes OD, McCutcheon R, Agid O, de Bartolomeis A, van Beveren NJ, Birnbaum ML, et al. Treatment-resistant schizophrenia: treatment response and resistance in psychosis (TRRIP) working group consensus guidelines on diagnosis and terminology. Am J Psychiatry. (2017) 174:216–29. doi: 10.1176/appi.ajp.2016.16050503
16. Schosser A, Serretti A, Souery D, Mendlewicz J, Zohar J, Montgomery S, et al. European Group for the Study of Resistant Depression (GSRD)–where have we gone so far: review of clinical and genetic findings. Eur Neuropsychopharmacol. (2012) 22:453–68. doi: 10.1016/j.euroneuro.2012.02.006
17. Hidalgo-Mazzei D, Berk M, Cipriani A, Cleare AJ, Florio AD, Dietch D, et al. Treatment-resistant and multi-therapy-resistant criteria for bipolar depression: consensus definition. Br J Psychiatry. (2019) 214:27–35. doi: 10.1192/bjp.2018.257
18. Howes OD, Thase ME, Pillinger T. Treatment resistance in psychiatry: state of the art and new directions. Mol Psychiatry. (2022) 27:58–72. doi: 10.1038/s41380-021-01200-3
19. Skogh E, Reis M, Dahl ML, Lundmark J, Bengtsson F. Therapeutic drug monitoring data on olanzapine and its N-demethyl metabolite in the naturalistic clinical setting. Ther Drug Monit. (2002) 24:518–26. doi: 10.1097/00007691-200208000-00010
20. Leinonen E, Lillsunde P, Laukkanen V, Ylitalo P. Effects of carbamazepine on serum antidepressant concentrations in psychiatric patients. J Clin Psychopharmacol. (1991) 11:313–8. doi: 10.1097/00004714-199110000-00007
21. Wagner E, McMahon L, Falkai P, Hasan A, Siskind D. Impact of smoking behavior on clozapine blood levels - a systematic review and meta-analysis. Acta Psychiatr Scand. (2020) 142:456–66. doi: 10.1111/acps.13228
22. Augustin M, Schoretsanitis G, Hiemke C, Gründer G, Haen E, Paulzen M. Differences in duloxetine dosing strategies in smoking and nonsmoking patients: therapeutic drug monitoring uncovers the impact on drug metabolism. J Clin Psychiatry. (2018) 79:86. doi: 10.4088/JCP.17m12086
23. Fink G. “Neural control of the anterior lobe of the pituitary gland (pars distalis).” In: Handbook of neuroendocrinology. Academic Press, (2012) 97–137.
24. Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci. (2005) 6:565–75. doi: 10.1038/nrn1703
25. de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. (2005) 6:463–75. doi: 10.1038/nrn1683
26. Varghese FP, Brown ES. The hypothalamic-pituitary-adrenal axis in major depressive disorder: a brief primer for primary care physicians. Prim Care Companion J Clin Psychiatry. (2001) 3:151–5. doi: 10.4088/PCC.v03n0401
27. Criado-Marrero M, Rein T, Binder EB, Porter JT, Koren J, Blair LJ. Hsp90 and FKBP51: complex regulators of psychiatric diseases. Philosop Trans R Soc B Biol Sci. (2018) 373:20160532. doi: 10.1098/rstb.2016.0532
28. Zhang S, Cheon M, Park H, Kim T, Chung C. Interaction between glucocorticoid receptors and FKBP5 in regulating neurotransmission of the hippocampus. Neuroscience. (2021) 483:95–103. doi: 10.1016/j.neuroscience.2021.12.020
29. Bevilacqua L, Carli V, Sarchiapone M, George DK, Goldman D, Roy A, et al. Interaction between FKBP5 and childhood trauma and risk of aggressive behavior. Arch Gen Psychiatry. (2012) 69:62–70. doi: 10.1001/archgenpsychiatry.2011.152
30. Willour VL, Chen H, Toolan J, Belmonte P, Cutler DJ, Goes FS, et al. Family-based association of FKBP5 in bipolar disorder. Mol Psychiatry. (2009) 14:261–8. doi: 10.1038/sj.mp.4002141
31. Roy A, Gorodetsky E, Yuan Q, Goldman D, Enoch MA. Interaction of FKBP5, a stress-related gene, with childhood trauma increases the risk for attempting suicide. Neuropsychopharmacology. (2010) 35:1674–83. doi: 10.1038/npp.2009.236
32. Brent D, Melhem N, Ferrell R, Emslie G, Wagner KD, Ryan N, et al. Association of FKBP5 polymorphisms with suicidal events in the treatment of resistant depression in adolescents (TORDIA) study. Am J Psychiatry. (2010) 167:190–7. doi: 10.1176/appi.ajp.2009.09040576
33. Roy A, Hodgkinson CA, Deluca V, Goldman D, Enoch MA. Two HPA axis genes, CRHBP and FKBP5, interact with childhood trauma to increase the risk for suicidal behavior. J Psychiatr Res. (2012) 46:72–9. doi: 10.1016/j.jpsychires.2011.09.009
34. Supriyanto I, Sasada T, Fukutake M, Asano M, Ueno Y, Nagasaki Y, et al. Association of FKBP5 gene haplotypes with completed suicide in the Japanese population. Prog Neuropsychopharmacol Biol Psychiatry. (2011) 35:252–6. doi: 10.1016/j.pnpbp.2010.11.019
35. Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Farrer LA, et al. Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharmacology. (2010) 35:1684–92. doi: 10.1038/npp.2010.37
36. Shibuya N, Suzuki A, Sadahiro R, Kamata M, Matsumoto Y, Goto K, et al. Association study between a functional polymorphism of FK506-binding protein 51 (FKBP5) gene and personality traits in healthy subjects. Neurosci Lett. (2010) 485:194–7. doi: 10.1016/j.neulet.2010.09.010
37. Koenen KC, Saxe G, Purcell S, Smoller JW, Bartholomew D, Miller A, et al. Polymorphisms in FKBP5 are associated with peritraumatic dissociation in medically injured children. Mol Psychiatry. (2005) 10:1058–9. doi: 10.1038/sj.mp.4001727
38. Jiang Y, Wei D, Xie Y. Functional modular networks identify the pivotal genes associated with morphine addiction and potential drug therapies. BMC Anesthesiol. (2023) 23:151. doi: 10.1186/s12871-023-02111-2
39. Fang T, Liu MN, Tian XY, Lu GY, Li F, Zhang X, et al. The association of FKBP5 polymorphisms with the severity of depressive disorder in patients with methamphetamine use disorders. Front Psychiatry. (2023) 14:1147060. doi: 10.3389/fpsyt.2023.1147060
40. Castro SCC, Bicca C, Bicca B, Araujo S, Viola TW, A. systematic mini-review of epigenetic mechanisms associated with electroconvulsive therapy in humans. Front Hum Neurosci. (2023) 17:1143332. doi: 10.3389/fnhum.2023.1143332
41. Asadi-Pooya AA, Simani L, Asadollahi M, Rashidi FS, Ahmadipour E, Alavi A, et al. Potential role of FKBP5 single nucleotide polymorphisms in functional seizures. Epilepsia Open. (2023) 8:479–86. doi: 10.1002/epi4.12716
42. Malekpour M, Jafari A, Kashkooli M, Salarikia SR, Negahdaripour M. A systems biology approach for discovering the cellular and molecular aspects of psychogenic non-epileptic seizure. Front Psychiatry. (2023) 14:1116892. doi: 10.3389/fpsyt.2023.1116892
43. Asadi-Pooya AA, Malekpour M, Zamiri B, Kashkooli M, Firouzabadi N. FKBP5 blockade may provide a new horizon for the treatment of stress-associated disorders: an in-silico study. Epilepsia Open. (2023) 8:633–40. doi: 10.1002/epi4.12749
44. Zannas AS, Wiechmann T, Gassen NC, Binder EB. Gene–stress–epigenetic regulation of FKBP5: clinical and translational implications. Neuropsychopharmacology. (2016) 41:261–74. doi: 10.1038/npp.2015.235
45. Kang CB, Hong Y, Dhe-Paganon S, Yoon HS, FKBP. family proteins: immunophilins with versatile biological functions. Neurosignals. (2008) 16:318–25. doi: 10.1159/000123041
46. Schiene-Fischer C. Multidomain peptidyl prolyl cis/trans isomerases. Biochim Biophys Acta Gen Subj. (2015) 1850:2005–16. doi: 10.1016/j.bbagen.2014.11.012
47. Callebaut I, Renoir J-M, Lebeau M-C, Massol N, Burny A, Baulieu E-E, et al. An immunophilin that binds M (r) 90,000 heat shock protein: main structural features of a mammalian p59 protein. Proc Nat Acad Sci. (1992) 89:6270–4. doi: 10.1073/pnas.89.14.6270
48. Riggs DL, Cox MB, Tardif HL, Hessling M, Buchner J, Smith DF. Noncatalytic role of the FKBP52 peptidyl-prolyl isomerase domain in the regulation of steroid hormone signaling. Mol Cell Biol. (2007) 27:8658–69. doi: 10.1128/MCB.00985-07
49. Wu B, Li P, Liu Y, Lou Z, Ding Y, Shu C, et al. 3D structure of human FK506-binding protein 52: implications for the assembly of the glucocorticoid receptor/Hsp90/immunophilin heterocomplex. Proc Nat Acad Sci. (2004) 101:8348–53. doi: 10.1073/pnas.0305969101
50. Smith DF. Tetratricopeptide repeat cochaperones in steroid receptor complexes. Cell Stress Chaperones. (2004) 9:109. doi: 10.1379/CSC-31.1
51. Echeverria PC, Picard D. Molecular chaperones, essential partners of steroid hormone receptors for activity and mobility. Biochim Biophys Acta Mol Cell Res. (2010) 1803:641–9. doi: 10.1016/j.bbamcr.2009.11.012
52. Pérez-Ortiz JM, García-Gutiérrez MS, Navarrete F, Giner S, Manzanares J. Gene and protein alterations of FKBP5 and glucocorticoid receptor in the amygdala of suicide victims. Psychoneuroendocrinology. (2013) 38:1251–8. doi: 10.1016/j.psyneuen.2012.11.008
53. Kumar R, Thompson EB. Gene regulation by the glucocorticoid receptor: structure: function relationship. J Steroid Biochem Mol Biol. (2005) 94:383–94. doi: 10.1016/j.jsbmb.2004.12.046
54. Merkulov VM, Merkulova TI. Structural variants of glucocorticoid receptor binding sites and different versions of positive glucocorticoid responsive elements: analysis of GR-TRRD database. J Steroid Biochem Mol Biol. (2009) 115:1–8. doi: 10.1016/j.jsbmb.2009.02.003
55. Meijsing SH. Mechanisms of glucocorticoid-regulated gene transcription. Adv Exp Med Biol. (2015) 872, 59–81. doi: 10.1007/978-1-4939-2895-8_3
56. Sheng JA, Bales NJ, Myers SA, Bautista AI, Roueinfar M, Hale TM, et al. The hypothalamic-pituitary-adrenal axis: development, programming actions of hormones, and maternal-fetal interactions. Front Behav Neurosci. (2021) 14:601939. doi: 10.3389/fnbeh.2020.601939
57. Juszczak GR, Stankiewicz AM. Glucocorticoids, genes and brain function. Prog Neuro-Psychopharmacol Biol Psychiatry. (2018) 82:136–68. doi: 10.1016/j.pnpbp.2017.11.020
58. Hausch F, Kozany C, Theodoropoulou M, Fabian A-K. FKBPs and the Akt/mTOR pathway. Cell Cycle. (2013) 12:2366–70. doi: 10.4161/cc.25508
59. Zannas A, Binder E. Gene–environment interactions at the FKBP5 locus: sensitive periods, mechanisms and pleiotropism. Genes Brain Behav. (2014) 13:25–37. doi: 10.1111/gbb.12104
60. Erlejman AG, De Leo SA, Mazaira GI, Molinari AM, Camisay MF, Fontana V, et al. NF-κB transcriptional activity is modulated by FK506-binding proteins FKBP51 and FKBP52: a role for peptidyl-prolyl isomerase activity. J Biol Chem. (2014) 289:26263–76. doi: 10.1074/jbc.M114.582882
61. Jinwal UK, Koren J, Borysov SI, Schmid AB, Abisambra JF, Blair LJ, et al. The Hsp90 cochaperone, FKBP51, increases Tau stability and polymerizes microtubules. J Neurosci. (2010) 30:591–9. doi: 10.1523/JNEUROSCI.4815-09.2010
62. Gassen NC, Fries GR, Zannas AS, Hartmann J, Zschocke J, Hafner K, et al. Chaperoning epigenetics: FKBP51 decreases the activity of DNMT1 and mediates epigenetic effects of the antidepressant paroxetine. Sci Signal. (2015) 8:ra119-ra. doi: 10.1126/scisignal.aac7695
63. Zannas AS, Balsevich G, Gassen NC. The emerging role of FKBP5 in the regulation of metabolism and body weight. Surg Obes Relat Dis. (2016) 12:1560–1. doi: 10.1016/j.soard.2016.05.016
64. Gassen NC, Hartmann J, Zschocke J, Stepan J, Hafner K, Zellner A, et al. Association of FKBP51 with priming of autophagy pathways and mediation of antidepressant treatment response: evidence in cells, mice, and humans. PLoS Med. (2014) 11:e1001755. doi: 10.1371/journal.pmed.1001755
65. Häusl AS, Balsevich G, Gassen NC, Schmidt MV. Focus on FKBP51: a molecular link between stress and metabolic disorders. Mol Metabol. (2019) 29:170–81. doi: 10.1016/j.molmet.2019.09.003
66. Blair LJ, Nordhues BA, Hill SE, Scaglione KM, O'Leary JC, Fontaine SN, et al. Accelerated neurodegeneration through chaperone-mediated oligomerization of tau. J Clin Invest. (2013) 123:4158–69. doi: 10.1172/JCI69003
67. Pirkl F, Buchner J. Functional analysis of the Hsp90-associated human peptidyl prolyl cis/trans isomerases FKBP51, FKBP52 and Cyp40. J Mol Biol. (2001) 308:795–806. doi: 10.1006/jmbi.2001.4595
68. Sanchez ER. Hsp56: a novel heat shock protein associated with untransformed steroid receptor complexes. J Biol Chem. (1990) 265:22067–70. doi: 10.1016/S0021-9258(18)45667-0
69. Wang X, Song J, Yuan Y, Li L, Abu-Taha I, Heijman J, et al. Downregulation of FKBP5 promotes atrial arrhythmogenesis. Circ Res. (2023). doi: 10.1161/CIRCRESAHA.122.322213
70. Li P, Wang Y, Liu B, Wu C, He C, Lv X, et al. Association of job stress, FK506 binding protein 51 (FKBP5) gene polymorphisms and their interaction with sleep disturbance. PeerJ. (2023) 11:e14794. doi: 10.7717/peerj.14794
71. Ma X, Wang Z, Zhang C, Bian Y, Zhang X, Liu X, et al. Association of SNPs in the FK-506 binding protein (FKBP5) gene among Han Chinese women with polycystic ovary syndrome. BMC Med Genomics. (2022) 15:149. doi: 10.1186/s12920-022-01301-0
72. Mazaira GI, Camisay MF, De Leo S, Erlejman AG, Galigniana MD. Biological relevance of Hsp90-binding immunophilins in cancer development and treatment. Int J Cancer. (2016) 138:797–808. doi: 10.1002/ijc.29509
73. Pei H, Li L, Fridley BL, Jenkins GD, Kalari KR, Lingle W, et al. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer Cell. (2009) 16:259–66. doi: 10.1016/j.ccr.2009.07.016
74. Yu J, Qin B, Wu F, Qin S, Nowsheen S, Shan S, et al. Regulation of serine-threonine kinase akt activation by NAD(+)-dependent deacetylase SIRT7. Cell Rep. (2017) 18:1229–40. doi: 10.1016/j.celrep.2017.01.009
75. Daneri-Becerra C, Zgajnar NR, Lotufo CM, Ramos Hryb AB, Piwien-Pilipuk G, Galigniana MD. Regulation of FKBP51 and FKBP52 functions by post-translational modifications. Biochem Soc Trans. (2019) 47:1815–31. doi: 10.1042/BST20190334
76. Zgajnar NR, De Leo SA, Lotufo CM, Erlejman AG, Piwien-Pilipuk G, Galigniana MD. Biological actions of the Hsp90-binding immunophilins FKBP51 and FKBP52. Biomolecules. (2019) 9:52. doi: 10.3390/biom9020052
77. Li H, Su P, Lai TK, Jiang A, Liu J, Zhai D, et al. The glucocorticoid receptor–FKBP51 complex contributes to fear conditioning and posttraumatic stress disorder. J Clin Invest. (2020) 130:877–89. doi: 10.1172/JCI130363
78. Syed SA, Zannas AS. Chapter 29 - Epigenetics in psychotherapy. In:Peedicayil J, Grayson DR, Avramopoulos D, , editors. Epigenetics in Psychiatry, 2nd ed. Academic Press (2021). p. 701–9.
79. Bremner JD, Vythilingam M, Vermetten E, Adil J, Khan S, Nazeer A, et al. Cortisol response to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroendocrinology. (2003) 28:733–50. doi: 10.1016/S0306-4530(02)00067-7
80. Paakinaho V, Makkonen H, Jaaskelainen T, Palvimo JJ. Glucocorticoid receptor activates poised FKBP51 locus through long-distance interactions. Mol Endocrinol. (2010) 24:511–25. doi: 10.1210/me.2009-0443
81. Reynolds P, Ruan Y, Smith D, Scammell J. Glucocorticoid resistance in the squirrel monkey is associated with overexpression of the immunophilin FKBP51. J Clin Endocrinol Metabol. (1999) 84:663–9. doi: 10.1210/jc.84.2.663
82. Wochnik GM, Rüegg Jl, Abel GA, Schmidt U, Holsboer F, Rein T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J Biol Chem. (2005) 280:4609–16. doi: 10.1074/jbc.M407498200
83. R Fries G, C Gassen N, Schmidt U, Rein T. The FKBP51-glucocorticoid receptor balance in stress-related mental disorders. Curr Mol Pharmacol. (2016) 9:126–40. doi: 10.2174/1874467208666150519114435
84. Matosin N, Halldorsdottir T, Binder EB. Understanding the molecular mechanisms underpinning gene by environment interactions in psychiatric disorders: the FKBP5 model. Biol Psychiatry. (2018) 83:821–30. doi: 10.1016/j.biopsych.2018.01.021
85. Naughton M, Dinan TG, Scott LV. Corticotropin-releasing hormone and the hypothalamic–pituitary–adrenal axis in psychiatric disease. Handb Clin Neurol. (2014) 124:69–91. doi: 10.1016/B978-0-444-59602-4.00005-8
86. Jarcho MR, Slavich GM, Tylova-Stein H, Wolkowitz OM, Burke HM. Dysregulated diurnal cortisol pattern is associated with glucocorticoid resistance in women with major depressive disorder. Biol Psychol. (2013) 93:150–8. doi: 10.1016/j.biopsycho.2013.01.018
87. Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Pütz B, et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. (2004) 36:1319–25. doi: 10.1038/ng1479
88. Saito T, Shinozaki G, Koga M, Tanichi M, Takeshita S, Nakagawa R, et al. Effect of interaction between a specific subtype of child abuse and the FKBP5 rs1360780 SNP on DNA methylation among patients with bipolar disorder. J Affect Disord. (2020) 272:417–22. doi: 10.1016/j.jad.2020.03.120
89. Shenker N, Flanagan J. Intragenic DNA methylation: implications of this epigenetic mechanism for cancer research. Br J Cancer. (2012) 106:248–53. doi: 10.1038/bjc.2011.550
90. Castro-Vale I, Carvalho D, editors. The Pathways Between Cortisol-Related Regulation Genes and PTSD Psychotherapy. Healthcare: Multidisciplinary Digital Publishing Institute (Basel) (2020) 8:376. doi: 10.3390/healthcare8040376
91. Beach SR, Ong ML, Lei M-K, Carter SE, Simons RL, Gibbons FX, et al. Methylation of FKBP5 is associated with accelerated DNA methylation ageing and cardiometabolic risk: replication in young-adult and middle-aged Black Americans. Epigenetics. (2022) 17:982–1002. doi: 10.1080/15592294.2021.1980688
92. Pelleymounter LL, Moon I, Johnson JA, Laederach A, Halvorsen M, Eckloff B, et al. A novel application of pattern recognition for accurate SNP and indel discovery from high-throughput data: targeted resequencing of the glucocorticoid receptor co-chaperone FKBP5 in a Caucasian population. Mol Genet Metab. (2011) 104:457–69. doi: 10.1016/j.ymgme.2011.08.019
93. Lee RS, Tamashiro KL, Yang X, Purcell RH, Harvey A, Willour VL, et al. Chronic corticosterone exposure increases expression and decreases deoxyribonucleic acid methylation of Fkbp5 in mice. Endocrinology. (2010) 151:4332–43. doi: 10.1210/en.2010-0225
94. Fries GR, Carvalho AF, Quevedo J. The miRNome of bipolar disorder. J Affect Disord. (2018) 233:110–6. doi: 10.1016/j.jad.2017.09.025
95. Cox MB, Riggs DL, Hessling M, Schumacher F, Buchner J, Smith DF. FK506-binding protein 52 phosphorylation: a potential mechanism for regulating steroid hormone receptor activity. Mol Endocrinol. (2007) 21:2956–67. doi: 10.1210/me.2006-0547
96. Zhao P, Ma G, Ma L. Circ_0000479 promotes proliferation, invasion, migration and inflammation and inhibits apoptosis of rheumatoid arthritis fibroblast-like synoviocytes via miR-766/FKBP5 axis. J Orthop Surg Res. (2023) 18:1–10. doi: 10.1186/s13018-023-03700-0
97. Liu H, Chen Q, Zheng W, Zhou Y, Bai Y, Pan Y, et al. LncRNA CASC19 enhances the radioresistance of nasopharyngeal carcinoma by regulating the miR-340-3p/FKBP5 Axis. Int J Mol Sci. (2023) 24:3047. doi: 10.3390/ijms24033047
98. Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. (1992) 267:1244–52. doi: 10.1001/jama.267.9.1244
99. Johnson EO, Kamilaris TC, Chrousos GP, Gold PW. Mechanisms of stress: a dynamic overview of hormonal and behavioral homeostasis. Neurosci Biobehav Rev. (1992) 16:115–30. doi: 10.1016/S0149-7634(05)80175-7
100. Yehuda R, Cai G, Golier JA, Sarapas C, Galea S, Ising M, et al. Gene expression patterns associated with posttraumatic stress disorder following exposure to the World Trade Center attacks. Biol Psychiatry. (2009) 66:708–11. doi: 10.1016/j.biopsych.2009.02.034
101. Mihaljevic M, Zeljic K, Soldatovic I, Andric S, Mirjanic T, Richards A, et al. The emerging role of the FKBP5 gene polymorphisms in vulnerability-stress model of schizophrenia: further evidence from a Serbian population. Eur Arch Psychiatry Clin Neurosci. (2017) 267:527–39. doi: 10.1007/s00406-016-0720-7
102. Borges S, Gayer-Anderson C, Mondelli V. A systematic review of the activity of the hypothalamic-pituitary-adrenal axis in first episode psychosis. Psychoneuroendocrinology. (2013) 38:603–11. doi: 10.1016/j.psyneuen.2012.12.025
103. Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. (2009) 34:S186–S95. doi: 10.1016/j.psyneuen.2009.05.021
104. Sherry ST, Ward MH, Kholodov M, Baker J, Phan L, Smigielski EM, et al. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res. (2001) 29:308–11. doi: 10.1093/nar/29.1.308
105. Halldorsdottir T, de Matos APS, Awaloff Y, Arnarson E, Craighead WE, Binder EB. FKBP5 moderation of the relationship between childhood trauma and maladaptive emotion regulation strategies in adolescents. Psychoneuroendocrinology. (2017) 84:61–5. doi: 10.1016/j.psyneuen.2017.06.012
106. Zou YF, Wang F, Feng XL, Li WF, Tao JH, Pan FM, et al. Meta-analysis of FKBP5 gene polymorphisms association with treatment response in patients with mood disorders. Neurosci Lett. (2010) 484:56–61. doi: 10.1016/j.neulet.2010.08.019
107. Calabrò M, Crisafulli C, Di Nicola M, Colombo R, Janiri L, Serretti A. FKBP5 gene variants may modulate depressive features in bipolar disorder. Neuropsychobiology. (2019) 78:104–12. doi: 10.1159/000499976
108. Stamm TJ, Rampp C, Wiethoff K, Stingl J, Mössner R, O Malley G, et al. The FKBP5 polymorphism rs1360780 influences the effect of an algorithm-based antidepressant treatment and is associated with remission in patients with major depression. J Psychopharmacol. (2016) 30:40–7. doi: 10.1177/0269881115620459
109. Hernández-Díaz Y, González-Castro TB, Tovilla-Zárate CA, Juárez-Rojop IE, López-Narváez ML, Pérez-Hernández N, et al. Association between FKBP5 polymorphisms and depressive disorders or suicidal behavior: a systematic review and meta-analysis study. Psychiatry Res. (2019) 271:658–68. doi: 10.1016/j.psychres.2018.12.066
110. Wang Q, Shelton RC, Dwivedi Y. Interaction between early-life stress and FKBP5 gene variants in major depressive disorder and post-traumatic stress disorder: a systematic review and meta-analysis. J Affect Disord. (2018) 225:422–8. doi: 10.1016/j.jad.2017.08.066
111. Velders FP, Kuningas M, Kumari M, Dekker MJ, Uitterlinden AG, Kirschbaum C, et al. Genetics of cortisol secretion and depressive symptoms: a candidate gene and genome wide association approach. Psychoneuroendocrinology. (2011) 36:1053–61. doi: 10.1016/j.psyneuen.2011.01.003
112. Szczepankiewicz A, Leszczyńska-Rodziewicz A, Pawlak J, Narozna B, Rajewska-Rager A, Wilkosc M, et al. FKBP5 polymorphism is associated with major depression but not with bipolar disorder. J Affect Disord. (2014) 164:33–7. doi: 10.1016/j.jad.2014.04.002
113. Rao S, Yao Y, Ryan J, Li T, Wang D, Zheng C, et al. Common variants in FKBP5 gene and major depressive disorder (MDD) susceptibility: a comprehensive meta-analysis. Sci Rep. (2016) 6:32687. doi: 10.1038/srep32687
114. Tatro ET, Everall IP, Masliah E, Hult BJ, Lucero G, Chana G, et al. Differential expression of immunophilins FKBP51 and FKBP52 in the frontal cortex of HIV-infected patients with major depressive disorder. J Neuroimmune Pharmacol. (2009) 4:218–26. doi: 10.1007/s11481-009-9146-6
115. Mikolas P, Tozzi L, Doolin K, Farrell C, O'Keane V, Frodl T. Effects of early life adversity and FKBP5 genotype on hippocampal subfields volume in major depression. J Affect Disord. (2019) 252:152–9. doi: 10.1016/j.jad.2019.04.054
116. Arlt S, Demiralay C, Tharun B, Geisel O, Storm N, Eichenlaub M, et al. Genetic risk factors for depression in Alzheimer's disease patients. Curr Alzheimer Res. (2013) 10:72–81. doi: 10.2174/156720513804871435
117. Li G, Wang L, Zhang K, Cao C, Cao X, Fang R, et al. FKBP5 genotype linked to combined PTSD-depression symptom in Chinese earthquake survivors. Can J Psychiatry. (2019) 64:863–71. doi: 10.1177/0706743719870505
118. Kang JI, Kim TY, Choi JH, So HS, Kim SJ. Allele-specific DNA methylation level of FKBP5 is associated with post-traumatic stress disorder. Psychoneuroendocrinology. (2019) 103:1–7. doi: 10.1016/j.psyneuen.2018.12.226
119. Hawn SE, Sheerin CM, Lind MJ, Hicks TA, Marraccini ME, Bountress K, et al. GxE effects of FKBP5 and traumatic life events on PTSD: a meta-analysis. J Affect Disord. (2019) 243:455–62. doi: 10.1016/j.jad.2018.09.058
120. Zhang K, Wang L, Li G, Cao C, Fang R, Liu P, et al. Correlation between hypothalamic-pituitary-adrenal axis gene polymorphisms and posttraumatic stress disorder symptoms. Horm Behav. (2020) 117:104604. doi: 10.1016/j.yhbeh.2019.104604
121. Zhang L, Hu XZ Yu T, Chen Z, Dohl J, Li X, et al. Genetic association of FKBP5 with PTSD in US service members deployed to Iraq and Afghanistan. J Psychiatr Res. (2020) 122:48–53. doi: 10.1016/j.jpsychires.2019.12.014
122. Minelli A, Maffioletti E, Cloninger CR, Magri C, Sartori R, Bortolomasi M, et al. Role of allelic variants of FK506-binding protein 51 (FKBP5) gene in the development of anxiety disorders. Depress Anxiety. (2013) 30:1170–6. doi: 10.1002/da.22158
123. Bryushkova L, Zai C, Chen S, Pappa I, Mileva V, Tiemeier H, et al. FKBP5 interacts with maltreatment in children with extreme, pervasive, and persistent aggression. Psychiatry Res. (2016) 242:277–80. doi: 10.1016/j.psychres.2015.09.052
124. Isaksson J, Comasco E, Åslund C, Rehn M, Tuvblad C, Andershed H, et al. Associations between the FKBP5 haplotype, exposure to violence and anxiety in females. Psychoneuroendocrinology. (2016) 72:196–204. doi: 10.1016/j.psyneuen.2016.07.206
125. Kawamura Y, Takahashi T, Liu X, Nishida N, Tokunaga K, Ukawa K, et al. DNA polymorphism in the FKBP5 gene affects impulsivity in intertemporal choice. Asia Pac Psychiatry. (2013) 5:31–8. doi: 10.1111/appy.12009
126. Isaksson J, Allen M, Nilsson KW, Lindblad F. Polymorphisms in the FK506 binding protein 5 gene are associated with attention deficit hyperactivity disorder and diurnal cortisol levels. Acta Paediatr. (2015) 104:910–5. doi: 10.1111/apa.13056
127. Fudalej S, Kopera M, Wołyńczyk-Gmaj D, Fudalej M, Krajewski P, Wasilewska K, et al. Association between FKBP5 functional polymorphisms and completed suicide. Neuropsychobiology. (2015) 72:126–31. doi: 10.1159/000441659
128. Levran O, Peles E, Randesi M, Li Y, Rotrosen J, Ott J, et al. Stress-related genes and heroin addiction: a role for a functional FKBP5 haplotype. Psychoneuroendocrinology. (2014) 45:67–76. doi: 10.1016/j.psyneuen.2014.03.017
129. Levran O, Randesi M, Li Y, Rotrosen J, Ott J, Adelson M, et al. Drug addiction and stress-response genetic variability: association study in African Americans. Ann Hum Genet. (2014) 78:290–8. doi: 10.1111/ahg.12064
130. Martín-Blanco A, Ferrer M, Soler J, Arranz MJ, Vega D, Calvo N, et al. The role of hypothalamus-pituitary-adrenal genes and childhood trauma in borderline personality disorder. Eur Arch Psychiatry Clin Neurosci. (2016) 266:307–16. doi: 10.1007/s00406-015-0612-2
131. Suchting R, Gowin JL, Green CE, Walss-Bass C, Lane SD. Genetic and psychosocial predictors of aggression: variable selection and model building with component-wise gradient boosting. Front Behav Neurosci. (2018) 12:89. doi: 10.3389/fnbeh.2018.00089
132. Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. (2008) 299:1291–305. doi: 10.1001/jama.299.11.1291
133. Lekman M, Laje G, Charney D, Rush AJ, Wilson AF, Sorant AJM, et al. The FKBP5-gene in depression and treatment response—an association study in the sequenced treatment alternatives to relieve depression (STAR*D) cohort. Biol Psychiatry. (2008) 63:1103–10. doi: 10.1016/j.biopsych.2007.10.026
134. Zobel A, Schuhmacher A, Jessen F, Höfels S, von Widdern O, Metten M, et al. DNA sequence variants of the FKBP5 gene are associated with unipolar depression. Int J Neuropsychopharmacol. (2010) 13:649–60. doi: 10.1017/S1461145709991155
135. Horstmann S, Lucae S, Menke A, Hennings JM, Ising M, Roeske D, et al. Polymorphisms in GRIK4, HTR2A, and FKBP5 show interactive effects in predicting remission to antidepressant treatment. Neuropsychopharmacology. (2010) 35:727–40. doi: 10.1038/npp.2009.180
136. Stramecki F, Frydecka D, Gaweda L, Prochwicz K, Klosowska J, Samochowiec J, et al. The Impact of the FKBP5 gene polymorphisms on the relationship between traumatic life events and psychotic-like experiences in non-clinical adults. Brain Sci. (2021) 11:561. doi: 10.3390/brainsci11050561
137. Sarginson JE, Lazzeroni LC, Ryan HS, Schatzberg AF, Murphy GM, Jr. FKBP5 polymorphisms and antidepressant response in geriatric depression. Am J Med Genet B Neuropsychiatr Genet. (2010) 153b:554–60. doi: 10.1002/ajmg.b.31019
138. Womersley JS, Martin LI, van der Merwe L, Seedat S, Hemmings SMJ. Hypothalamic-pituitary-adrenal axis variants and childhood trauma influence anxiety sensitivity in South African adolescents. Metab Brain Dis. (2018) 33:601–13. doi: 10.1007/s11011-017-0138-6
139. Kang JI, Chung HC, Jeung HC, Kim SJ, An SK, Namkoong K. FKBP5 polymorphisms as vulnerability to anxiety and depression in patients with advanced gastric cancer: a controlled and prospective study. Psychoneuroendocrinology. (2012) 37:1569–76. doi: 10.1016/j.psyneuen.2012.02.017
140. Moraitis AG, Block T, Nguyen D, Belanoff JK. The role of glucocorticoid receptors in metabolic syndrome and psychiatric illness. J Steroid Biochem Mol Biol. (2017) 165:114–20. doi: 10.1016/j.jsbmb.2016.03.023
141. Fan B, Ma J, Zhang H, Liao Y, Wang W, Zhang S, et al. Association of FKBP5 gene variants with depression susceptibility: a comprehensive meta-analysis. Asia Pac Psychiatry. (2021) 13:e12464. doi: 10.1111/appy.12464
142. Collip D, Myin-Germeys I, Wichers M, Jacobs N, Derom C, Thiery E, et al. FKBP5 as a possible moderator of the psychosis-inducing effects of childhood trauma. Br J Psychiatry. (2013) 202:261–8. doi: 10.1192/bjp.bp.112.115972
143. Ising M, Depping AM, Siebertz A, Lucae S, Unschuld PG, Kloiber S, et al. Polymorphisms in the FKBP5 gene region modulate recovery from psychosocial stress in healthy controls. Eur J Neurosci. (2008) 28:389–98. doi: 10.1111/j.1460-9568.2008.06332.x
144. Allen AP, Kennedy PJ, Dockray S, Cryan JF, Dinan TG, Clarke G. The trier social stress test: principles and practice. Neurobiol Stress. (2017) 6:113–26. doi: 10.1016/j.ynstr.2016.11.001
145. Kim HJ, Jin HJ. Polymorphisms in the FKBP5 gene are associated with attention deficit and hyperactivity disorder in Korean children. Behav Brain Res. (2021) 414:113508. doi: 10.1016/j.bbr.2021.113508
146. Keijser R, Olofsdotter S, Nilsson KW, Åslund C. Three-way interaction effects of early life stress, positive parenting and FKBP5 in the development of depressive symptoms in a general population. J Neural Transm. (2021) 128:1409–24. doi: 10.1007/s00702-021-02405-0
147. White MG, Bogdan R, Fisher PM, Munoz KE, Williamson DE, Hariri AR. FKBP5 and emotional neglect interact to predict individual differences in amygdala reactivity. Genes Brain Behav. (2012) 11:869–78. doi: 10.1111/j.1601-183X.2012.00837.x
148. Weidenfeld J, Ovadia H. The role of the amygdala in regulating the hypothalamic-pituitary-adrenal axis. In:Ferry B, , editors. The Amygdala: Where Emotions Shape Perception, Learning and Memories. London: IntechOpen (2017). p. 173–86.
149. Szczepankiewicz A, Narozna B, Rybakowski JK, Kliwicki S, Czerski P, Dmitrzak-Weglarz M, et al. Genes involved in stress response influence lithium efficacy in bipolar patients. Bipolar Disord. (2018) 20:753–60. doi: 10.1111/bdi.12639
150. Mahon PB, Zandi PP, Potash JB, Nestadt G, Wand GS. Genetic association of FKBP5 and CRHR1 with cortisol response to acute psychosocial stress in healthy adults. Psychopharmacology. (2013) 227:231–41. doi: 10.1007/s00213-012-2956-x
151. Hartmann J, Wagner KV, Liebl C, Scharf SH, Wang XD, Wolf M, et al. The involvement of FK506-binding protein 51 (FKBP5) in the behavioral and neuroendocrine effects of chronic social defeat stress. Neuropharmacology. (2012) 62:332–9. doi: 10.1016/j.neuropharm.2011.07.041
152. Ferrer A, Costas J, Labad J, Salvat-Pujol N, Segalàs C, Urretavizcaya M, et al. FKBP5 polymorphisms and hypothalamic-pituitary-adrenal axis negative feedback in major depression and obsessive-compulsive disorder. J Psychiatr Res. (2018) 104:227–34. doi: 10.1016/j.jpsychires.2018.08.003
153. Li H, Marsland AL, Conley YP, Sereika SM, Bender CM. Genes involved in the HPA axis and the symptom cluster of fatigue, depressive symptoms, and anxiety in women with breast cancer during 18 months of adjuvant therapy. Biol Res Nurs. (2020) 22:277–86. doi: 10.1177/1099800419899727
154. McCrory E, De Brito SA, Viding E. Research review: the neurobiology and genetics of maltreatment and adversity. J Child Psychol Psychiatry. (2010) 51:1079–95. doi: 10.1111/j.1469-7610.2010.02271.x
155. Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. (2009) 10:434–45. doi: 10.1038/nrn2639
156. Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. (2001) 24:1161–92. doi: 10.1146/annurev.neuro.24.1.1161
157. Heim C, Mletzko T, Purselle D, Musselman DL, Nemeroff CB. The dexamethasone/corticotropin-releasing factor test in men with major depression: role of childhood trauma. Biol Psychiatry. (2008) 63:398–405. doi: 10.1016/j.biopsych.2007.07.002
158. Scharf SH, Liebl C, Binder EB, Schmidt MV, Müller MB. Expression and regulation of the Fkbp5 gene in the adult mouse brain. PLoS ONE. (2011) 6:e16883. doi: 10.1371/journal.pone.0016883
159. Lai EC. Micro RNAs are complementary to 3′ UTR sequence motifs that mediate negative post-transcriptional regulation. Nat Genet. (2002) 30:363–4. doi: 10.1038/ng865
160. van Zuiden M, Geuze E, Willemen HL, Vermetten E, Maas M, Amarouchi K, et al. Glucocorticoid receptor pathway components predict posttraumatic stress disorder symptom development: a prospective study. Biol Psychiatry. (2012) 71:309–16. doi: 10.1016/j.biopsych.2011.10.026
161. Amad A, Ramoz N, Peyre H, Thomas P, Gorwood P. FKBP5 gene variants and borderline personality disorder. J Affect Disord. (2019) 248:26–8. doi: 10.1016/j.jad.2019.01.025
162. Lou QY Li Z, Teng Y, Xie QM, Zhang M, Huang SW, et al. Associations of FKBP4 and FKBP5 gene polymorphisms with disease susceptibility, glucocorticoid efficacy, anxiety, depression, and health-related quality of life in systemic lupus erythematosus patients. Clin Rheumatol. (2021) 40:167–79. doi: 10.1007/s10067-020-05195-0
163. Guo L, Wang W, Guo Y, Du X, Shi G, Lu C. Associations of FKBP5 polymorphisms and methylation and parenting style with depressive symptoms among Chinese adolescents. BMC Psychiatry. (2021) 21:552. doi: 10.1186/s12888-021-03576-6
164. Yehuda R, Daskalakis NP, Desarnaud F, Makotkine I, Lehrner AL, Koch E, et al. Epigenetic biomarkers as predictors and correlates of symptom improvement following psychotherapy in combat veterans with PTSD. Front Psychiatry. (2013) 4:118. doi: 10.3389/fpsyt.2013.00118
165. Roberts S, Keers R, Breen G, Coleman JRI, Jöhren P, Kepa A, et al. DNA methylation of FKBP5 and response to exposure-based psychological therapy. Am J Med Genet B Neuropsychiatr Genet. (2019) 180:150–8. doi: 10.1002/ajmg.b.32650
166. Bailus BJ, Scheeler SM, Simons J, Sanchez MA, Tshilenge K-T, Creus-Muncunill J, et al. Modulating FKBP5/FKBP51 and autophagy lowers HTT (huntingtin) levels. Autophagy. (2021) 17:4119–40. doi: 10.1080/15548627.2021.1904489
167. Schreiber KH, Ortiz D, Academia EC, Anies AC, Liao CY. Kennedy BKJAc. Rapamycin-mediated mTORC 2 inhibition is determined by the relative expression of FK 506-binding proteins. Aging Cell. (2015) 14:265–73. doi: 10.1111/acel.12313
168. Bauder M, Meyners C, Purder PL, Merz S, Sugiarto WO, Voll AM, et al. Structure-based design of high-affinity macrocyclic FKBP51 inhibitors. J Med Chem. (2021) 64:3320–49. doi: 10.1021/acs.jmedchem.0c02195
169. Verjee S, Weston A, Kolb C, Kalbhenn-Aziz H, Butterweck V. Hyperforin and miquelianin from St. John's wort attenuate gene expression in neuronal cells after dexamethasone-induced stress. Planta Med. (2018) 84:696–703. doi: 10.1055/a-0581-5286
170. Perroud N, Bondolfi G, Uher R, Gex-Fabry M, Aubry JM, Bertschy G, et al. Clinical and genetic correlates of suicidal ideation during antidepressant treatment in a depressed outpatient sample. Pharmacogenomics. (2011) 12:365–77. doi: 10.2217/pgs.10.189
171. Mitjans M, Catalán R, Vázquez M, González-Rodríguez A, Penadés R, Pons A, et al. Hypothalamic-pituitary-adrenal system, neurotrophic factors and clozapine response: association with FKBP5 and NTRK2 genes. Pharmacogenet Genomics. (2015) 25:274–7. doi: 10.1097/FPC.0000000000000132
172. Zheng L, Dou X, Ma X, Qu W, Tang X. Identification of potential key genes and pathways in enzalutamide-resistant prostate cancer cell lines: a bioinformatics analysis with data from the gene expression omnibus (GEO) database. Biomed Res Int. (2020) 2020:8341097. doi: 10.1155/2020/8341097
173. Wei K, Xu Y, Zhao Z, Wu X, Du Y, Sun J, et al. Icariin alters the expression of glucocorticoid receptor, FKBP5 and SGK1 in rat brains following exposure to chronic mild stress. Int J Mol Med. (2016) 38:337–44. doi: 10.3892/ijmm.2016.2591
174. van Alem CMA, Schmidbauer M, Rong S, Derlin K, Schmitz J, Bräsen JH, et al. Liposomal delivery improves the efficacy of prednisolone to attenuate renal inflammation in a mouse model of acute renal allograft rejection. Transplantation. (2020) 104:744–53. doi: 10.1097/TP.0000000000003060
175. Sawamura T, Klengel T, Armario A, Jovanovic T, Norrholm SD, Ressler KJ, et al. Dexamethasone treatment leads to enhanced fear extinction and dynamic Fkbp5 regulation in amygdala. Neuropsychopharmacology. (2016) 41:832–46. doi: 10.1038/npp.2015.210
176. Xu J, Wang R, Liu Y, Wang W, Liu D, Jiang H, et al. Short- and long-term alterations of FKBP5-GR and specific microRNAs in the prefrontal cortex and hippocampus of male rats induced by adolescent stress contribute to depression susceptibility. Psychoneuroendocrinology. (2019) 101:204–15. doi: 10.1016/j.psyneuen.2018.11.008
177. Poulain M, Frydman N, Duquenne C, N'Tumba-Byn T, Benachi A, Habert R, et al. Dexamethasone induces germ cell apoptosis in the human fetal ovary. J Clin Endocrinol Metab. (2012) 97:E1890–7. doi: 10.1210/jc.2012-1681
178. Baptista MJ, Muntañola A, Calpe E, Abrisqueta P, Salamero O, Fernández E, et al. Differential gene expression profile associated to apoptosis induced by dexamethasone in CLL cells according to IGHV/ZAP-70 status. Clin Cancer Res. (2012) 18:5924–33. doi: 10.1158/1078-0432.CCR-11-2771
179. Matsuda A, Asada Y, Takakuwa K, Sugita J, Murakami A, Ebihara N, et al. Methylation analysis of human trabecular meshwork cells during dexamethasone stimulation. Invest Ophthalmol Vis Sci. (2015) 56:3801–9. doi: 10.1167/iovs.14-16008
180. Misiak B, Karpiński P, Szmida E, Grazlewski T, Jabłoński M, Cyranka K, et al. Adverse childhood experiences and methylation of the FKBP5 gene in patients with psychotic disorders. J Clin Med. (2020) 9:792. doi: 10.3390/jcm9123792
181. Sinclair D, Fillman SG, Webster MJ, Weickert CS. Dysregulation of glucocorticoid receptor co-factors FKBP5, BAG1 and PTGES3 in prefrontal cortex in psychotic illness. Sci Rep. (2013) 3:3539. doi: 10.1038/srep03539
182. Jagtap PKA, Asami S, Sippel C, Kaila VRI, Hausch F, Sattler M. Selective inhibitors of FKBP51 employ conformational selection of dynamic invisible states. Angew Chem Int Ed Engl. (2019) 58:9429–33. doi: 10.1002/anie.201902994
183. Gaali S, Kirschner A, Cuboni S, Hartmann J, Kozany C, Balsevich G, et al. Selective inhibitors of the FK506-binding protein 51 by induced fit. Nat Chem Biol. (2015) 11:33–7. doi: 10.1038/nchembio.1699
184. Luo X, Du G, Chen B, Yan G, Zhu L, Cui P, et al. Novel immunosuppressive effect of FK506 by upregulation of PD-L1 via FKBP51 in heart transplantation. Scand J Immunol. (2022) 96:e13203. doi: 10.1111/sji.13203
185. Hartmann J, Wagner KV, Gaali S, Kirschner A, Kozany C, Rühter G, et al. Pharmacological inhibition of the psychiatric risk factor FKBP51 has anxiolytic properties. J Neurosci. (2015) 35:9007–16. doi: 10.1523/JNEUROSCI.4024-14.2015
186. Codagnone MG, Kara N, Ratsika A, Levone BR, van de Wouw M, Tan LA, et al. Inhibition of FKBP51 induces stress resilience and alters hippocampal neurogenesis. Mol Psychiatry. (2022) 27:4928–38. doi: 10.1038/s41380-022-01755-9
187. König L, Kalinichenko LS, Huber SE, Voll AM, Bauder M, Kornhuber J, et al. The selective FKBP51 inhibitor SAFit2 reduces alcohol consumption and reinstatement of conditioned alcohol effects in mice. Addict Biol. (2020) 25:e12758. doi: 10.1111/adb.12758
188. Pöhlmann ML, Häusl AS, Harbich D, Balsevich G, Engelhardt C, Feng X, et al. Pharmacological modulation of the psychiatric risk factor FKBP51 alters efficiency of common antidepressant drugs. Front Behav Neurosci. (2018) 12:262. doi: 10.3389/fnbeh.2018.00262
189. Connelly KL, Wolsh CC, Barr JL, Bauder M, Hausch F. Sex differences in the effect of the FKBP5 inhibitor SAFit2 on anxiety and stress-induced reinstatement following cocaine self-administration. Neurobiol Stress. (2020) 13:100232. doi: 10.1016/j.ynstr.2020.100232
190. Hähle A, Merz S, Meyners C, Hausch F. The many faces of FKBP51. Biomolecules. (2019) 9:35. doi: 10.3390/biom9010035
191. Hadamitzky M, Herring A, Kirchhof J, Bendix I, Haight MJ, Keyvani K, et al. Repeated systemic treatment with rapamycin affects behavior and amygdala protein expression in rats. Int J Neuropsychopharmacol. (2018) 21:592–602. doi: 10.1093/ijnp/pyy017
192. Hadamitzky M, Herring A, Keyvani K, Doenlen R, Krügel U, Bösche K, et al. Acute systemic rapamycin induces neurobehavioral alterations in rats. Behav Brain Res. (2014) 273:16–22. doi: 10.1016/j.bbr.2014.06.056
193. Xu X, Su B, Barndt RJ, Chen H, Xin H, Yan G, et al. FKBP12 is the only FK506 binding protein mediating T-cell inhibition by the immunosuppressant FK506. Transplantation. (2002) 73:1835–8. doi: 10.1097/00007890-200206150-00023
194. Maruf AA, Greenslade A, Arnold PD, Bousman C. Antidepressant pharmacogenetics in children and young adults: a systematic review. J Affect Disord. (2019) 254:98–108. doi: 10.1016/j.jad.2019.05.025
195. Laje G, Perlis RH, Rush AJ, McMahon FJ. Pharmacogenetics studies in STAR*D: strengths, limitations, and results. Psychiatr Serv. (2009) 60:1446–57. doi: 10.1176/ps.2009.60.11.1446
196. Pan Y, Zhang W-Y, Xia X, Kong L-D. Effects of icariin on hypothalamic-pituitary-adrenal axis action and cytokine levels in stressed sprague-dawley rats. Biol Pharmaceut Bull. (2006) 29:2399–403. doi: 10.1248/bpb.29.2399
197. Wu J, Du J, Xu C, Le J, Xu Y, Liu B, et al. Icariin attenuates social defeat-induced down-regulation of glucocorticoid receptor in mice. Pharmacol Biochem Behav. (2011) 98:273–8. doi: 10.1016/j.pbb.2011.01.008
198. Ising M, Maccarrone G, Brückl T, Scheuer S, Hennings J, Holsboer F, et al. FKBP5 gene expression predicts antidepressant treatment outcome in depression. Int J Mol Sci. (2019) 20:485. doi: 10.3390/ijms20030485
199. Guidotti G, Calabrese F, Anacker C, Racagni G, Pariante CM, Riva MA. Glucocorticoid receptor and FKBP5 expression is altered following exposure to chronic stress: modulation by antidepressant treatment. Neuropsychopharmacology. (2013) 38:616–27. doi: 10.1038/npp.2012.225
200. Frampton JE, Plosker GL. Duloxetine: a review of its use in the treatment of major depressive disorder. CNS Drugs. (2007) 21:581–609. doi: 10.2165/00023210-200721070-00004
201. Banach E, Szczepankiewicz A, Leszczyńska-Rodziewicz A, Pawlak J, Dmitrzak-Weglarz M, Zaremba D, et al. Venlafaxine and sertraline does not affect the expression of genes regulating stress response in female MDD patients. Psychiatr Pol. (2017) 51:1029–38. doi: 10.12740/PP/76329
202. Kinoshita T, Inagaki CJ. Long-term treatment with antidepressants increases glucocorticoid receptor binding and gene expression in cultured rat hippocampal neurones. J Neuroendocrinol. (1999) 11:887–95. doi: 10.1046/j.1365-2826.1999.00405.x
203. Berger M, Kraeuter AK, Romanik D, Malouf P, Amminger GP, Sarnyai Z. Cortisol awakening response in patients with psychosis: systematic review and meta-analysis. Neurosci Biobehav Rev. (2016) 68:157–66. doi: 10.1016/j.neubiorev.2016.05.027
204. Lee CH, Sinclair D, O'Donnell M, Galletly C, Liu D, Weickert CS, et al. Transcriptional changes in the stress pathway are related to symptoms in schizophrenia and to mood in schizoaffective disorder. Schizophr Res. (2019) 213:87–95. doi: 10.1016/j.schres.2019.06.026
205. Reynolds GP. The pharmacogenetics of symptom response to antipsychotic drugs. Psychiatry Investig. (2012) 9:1–7. doi: 10.4306/pi.2012.9.1.1
206. Pariante CM. Risk factors for development of depression and psychosis: glucocorticoid receptors and pituitary implications for treatment with antidepressant and glucocorticoids. Ann N Y Acad Sci. (2009) 1179:144. doi: 10.1111/j.1749-6632.2009.04978.x
207. Bekhbat M, Mehta CC, Kelly SD, Vester A, Ofotokun I, Felger J, et al. HIV and symptoms of depression are independently associated with impaired glucocorticoid signaling. Psychoneuroendocrinology. (2018) 96:118–25. doi: 10.1016/j.psyneuen.2018.06.013
208. Della Bella E, Menzel U, Basoli V, Tourbier C, Alini M, Stoddart MJ. Differential regulation of circRNA, miRNA, and piRNA during early osteogenic and chondrogenic differentiation of human mesenchymal stromal cells. Cells. (2020) 9:398. doi: 10.3390/cells9020398
209. Brinks J, van Dijk EHC, Habeeb M, Nikolaou A, Tsonaka R, Peters HAB, et al. The effect of corticosteroids on human choroidal endothelial cells: a model to study central serous chorioretinopathy. Invest Ophthalmol Vis Sci. (2018) 59:5682–92. doi: 10.1167/iovs.18-25054
210. Chatzittofis A, Boström ADE, Ciuculete DM, Öberg KG, Arver S, Schiöth HB, et al. HPA axis dysregulation is associated with differential methylation of CpG-sites in related genes. Sci Rep. (2021) 11:20134. doi: 10.1038/s41598-021-99714-x
211. Kageyama K, Iwasaki Y, Watanuki Y, Niioka K, Daimon M. Differential effects of Fkbp4 and Fkbp5 on regulation of the proopiomelanocortin gene in murine AtT-20 corticotroph cells. Int J Mol Sci. (2021) 22:724. doi: 10.3390/ijms22115724
212. Faiz A, Postma DS, Koppelman GH, Hiemstra PS, Sterk PJ, Timens W, et al. FKBP5 a candidate for corticosteroid insensitivity in COPD. Eur Respir J. (2016) 48:OA1779. doi: 10.1183/13993003.congress-2016.OA1779
213. Starvaggi Cucuzza L, Pregel P, Biolatti B, Cannizzo FT. FKBP5 gene expression in skeletal muscle as a potential biomarker for illegal glucocorticoid treatment in veal calves. Res Vet Sci. (2020) 133:157–62. doi: 10.1016/j.rvsc.2020.09.018
Keywords: FKBP5, psychiatric diseases, stress induced psychiatric disorders, FKBP5 genetic, drug discovery
Citation: Malekpour M, Shekouh D, Safavinia ME, Shiralipour S, Jalouli M, Mortezanejad S, Azarpira N and Ebrahimi ND (2023) Role of FKBP5 and its genetic mutations in stress-induced psychiatric disorders: an opportunity for drug discovery. Front. Psychiatry 14:1182345. doi: 10.3389/fpsyt.2023.1182345
Received: 08 March 2023; Accepted: 24 May 2023;
Published: 16 June 2023.
Edited by:
Long Li, Icahn School of Medicine at Mount Sinai, United StatesReviewed by:
Evgeniia Y. Chibikova, Samara Regional Clinical Psychiatric Hospital, RussiaChong Chen, Yamaguchi University Graduate School of Medicine, Japan
Copyright © 2023 Malekpour, Shekouh, Safavinia, Shiralipour, Jalouli, Mortezanejad, Azarpira and Ebrahimi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Negar Azarpira, negarazarpira@gmail.com
†These authors share first authorship
 Mahdi Malekpour
Mahdi Malekpour Dorsa Shekouh1†
Dorsa Shekouh1† Mohammad Ebrahim Safavinia
Mohammad Ebrahim Safavinia Negar Azarpira
Negar Azarpira