- 1Neuroscience Research Center, Faculty of Medical Sciences, Lebanese University, Hadath, Lebanon
- 2Institut Mondor de Recherche Biomedicale (IMRB)-Inserm U955, Ecole Doctorale Science de la Vie et de la Santé, Université Paris-Est, Creteil, Paris, France
- 3Faculty of Pharmacy, Lebanese University, Hadath, Lebanon
- 4Institut National de Sante Publique, Epidémiologie Clinique et Toxicologie (INSPECT-LB), Beirut, Lebanon
- 5University of Nicosia Medical school, Nicosia, Cyprus
- 6College of Health Sciences, Abu Dhabi University, Abu Dhabi, United Arab Emirates
Introduction: Stroke continues to be a common and debilitating medical condition which has a significant effect on public health as the second primary source of mortality and the third major root of disability worldwide. A wide range of complications affecting the survivor's life and interfering with the recovery process usually follows stroke; anxiety and depression are considered one of the major complications post-stroke. This study sought to investigate the short-term psychological consequences of stroke among Lebanese survivors and to identify their correlates.
Methods: This study is a prospective observational epidemiological study. 143 stroke patients admitted to hospitals in Mount Lebanon and Beirut between February and May 2018.were included in this study. Assessments of complications were carried out at 3 months post-stroke by completing a 30-min face-to-face interview questionnaire. The survey included the socio-demographic -characteristics of the patients, their lifestyle, health indicators, the severity of stroke, and the post-stroke consequences disturbing their quality of life.
Results: Complications were recorded for 117 stroke survivors (mean age, 72.46 years; 60.7% male). The analysis of results 3 months post stroke showed that 29 survivors suffered from neuropathic pain (24.8%), 110 (94%) suffered from fatigue, and 81 (69.2%) from cognitive impairment. High rates of anxiety (51.3%), and depression (76.1%) were recorded as well. Multivariate logistic regression confirmed that there is a significant association between depression and the following variables: anxiety (OR = 4.814, p-value = 0.017), pain (OR = 6.868, p-value = 0.002), and physical activity, which acts as a protective factor against depression (OR = 0.261; p-value = 0.029). However, the results of the multivariate logistic regression analysis for anxiety indicated that immobility-related complications increase the risk of anxiety by 8.457 in sedentary duration longer than 12 h (ORa = 8.457, p-value = 0.01). Furthermore, patients with neuropathic pain (24.8%) are 3.858 times more likely to have anxiety compared to patients without neuropathic pain (ORa = 3.858, p-value = 0.019).
Conclusion: Using a patient-centered structure more interventions should take place to evaluate stroke survivors' outcomes, and organize rehabilitation services that deal with stroke consequences, particularly high anxiety and depression levels, which are prevalent and persistent among the Lebanese stroke survivors.
Introduction
Stroke is described as a neurological impairment due to a non-traumatic brain injury caused by cerebral infarction, or hemorrhage, which is categorized into intracerebral and subarachnoid bleeding (Easton et al., 2009). According to the “Global Burden of Diseases” (GBD), this cerebrovascular accident is rated as the second source of death and as the third most mutual root for disability worldwide (Cheah et al., 2016).
Stroke leads to various complications including musculoskeletal pain, pulmonary embolism, deep vein thrombosis, shoulder subluxation, pressure sores, spasticity, swallowing difficulties, urinary infections, and psychological problems (Kuptniratsaikul et al., 2013). According to the study of Sackley et al. (2008) that was conducted over a year after stroke, the prevalent types of complication experienced were falls (73%), contractures (60%), pain (55%), shoulder pain (52%), emotional distress (50%), and pressure sores (22%) (Sackley et al., 2008). Moreover, hemiparesis and hemiplegia are frequent and widely recognized impairments of stroke where they affect over 65% of stroke survivors (Bindawas et al., 2017). Following this cerebrovascular accident, the ability to perform daily life activities is greatly affected by physical disability, essentially upper extremities involvement, and cognitive impairment fluctuating between minor vascular cognitive impairment (VCI) and vascular dementia (VaD) (Arauz et al., 2014; Kwakkel et al., 2015). Another consequence is participation restrictions represented by loss or decline in social engagement (Scott et al., 2012). The inability to perform daily life activities and social isolation might lead to increased psychiatric complications (Bartoli et al., 2013; Hara, 2015). Diagnostic and Statistical Manual (DSM) IV defined Post-stroke depression (PSD) as a “mood disorder with the specifiers of depressive features, major depressive-like episodes, manic features or mixed features” (American Psychiatric Association, 2000; Shi et al., 2017). PSD is the most common post-stroke neuropsychiatric disorder. Depression in these patients is more prevalent than in the general population (Hackett et al., 2014; Shi et al., 2017). PSD negatively affects functional recovery, survival, cognitive and social functions. It is associated with disorders affecting attention, memory, psychomotor speed, orientation, language, and executive/motor functions (Bolla-Wilson et al., 1989; Kauhanen et al., 1999). It was demonstrated that the reported prevalence of PSD ranges between ~20 and 40% (Barker-Collo, 2007; De Wit et al., 2008; Ayerbe et al., 2011, 2013; Vansimaeys et al., 2017).
Stroke severity, deficiency of communal or household support, anxiety, and genetic factors are predisposing factors for developing depression in stroke survival patients (Ayerbe et al., 2013). Depression represents a significant load on the rehabilitation process efficacy, where it leads to more limitations in daily life activities, higher risk of intellectual deficiency, elevated chance of recurrent stroke, and greater death chance (Gillen et al., 1999; Yuan et al., 2012; Bartoli et al., 2013).
Post-stroke anxiety is another common neuropsychiatric outcome of stroke characterized by excessive anxiousness or worries, and difficulties in controlling worries. According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (Kim, 2016), post-stroke anxiety is associated with the following symptoms: restlessness, insomnia, fatigue, discomfort, low concentration, and nervous tension (Castillo et al., 1995; Schultz et al., 1997; Kim, 2016). Many studies mentioned the persistence and mutuality of post-stroke anxiety (Cumming et al., 2016). Stroke survival patients are at risk of recurrent strokes, disabilities, lack of independence, and mortality, therefore, predisposing patients to develop anxiety (Johnson, 1991). Moreover, anxiety can have a detrimental effect on someone's life and lead to depression (Rafsten et al., 2018). An rate of 20% of stroke patients develop anxiety within the first 30 days following a cerebrovascular event, up to 23% in 5 months, and it reaches a value of 24% within a 6 months' length (Campbell Burton et al., 2013). Therefore, the anxiety level increases in the direction of the enduring phase of stroke (Burton et al., 2013; D'Aniello et al., 2014). Anxiety severity in this population is independent of gender. However, a negatively affected lifestyle following a stroke is linked to more severe anxiety (Rafsten et al., 2018).
Regarding the MENA (Middle East and North Africa) region, 34 studies stated the prevalence of post-stroke depression (Kaadan and Larson, 2017). The lowest rates are reported in Saudi Arabia (17%) (Hamad et al., 2011) and Iran (18% to one-third of stroke patients) (Ahangar and Hosseini, 2009; Iranmanesh, 2010), whereas higher rates are reported in Algeria (56.1%) (Layadi et al., 2013), Israel (63%) (Heruti et al., 2002), Jordan (64%) (Alghwiri, 2016), and Morocco (73.2%) (Riah et al., 2013).
Regarding Lebanon, several published studies shed the light on the prevalence, incidence, symptoms, and the needed therapeutics for stroke (Jurjus et al., 2009; El Sayed et al., 2014; Farah et al., 2015). Smoking, hypertension, diabetes mellitus, and physical inactivity were linked to a higher risk of stroke in published Lebanese studies (Lahoud et al., 2017; El-Hajj et al., 2019). Death is known as one of the major consequences of stroke, a recent study done by Abdo et al. (2019) confirmed that the cumulative mortality rates of Lebanese stroke patients increased from ~1 over 7 at 1 month to 1 over 5 at 1 year post-stroke (Abdo et al., 2019). However, no study has shed the light on the challenges faced by Lebanese surviving stroke patients.
In the light of the aforementioned literature, this study explores post-stroke neuropsychiatric complications in Lebanon and identifies their potential correlations.
Materials and Methods
Study Design, Settings, and Participants
An observational prospective study was performed among stroke patients survivors admitted to eight hospitals from Mount Lebanon and Beirut including Hôtel-Dieu De France, Clinique Du Levant, Mount Lebanon, Middle East Institute of Health, Al Sahel General, Al Zahraa, Rafic Al Hariri University, and Al Hayat.
Starting from February 1st, 2018, and for the subsequent 3 months, all consecutive patients admitted to the participating hospitals with a confirmed diagnosis of ischemic or hemorrhagic stroke were eligible to participate in the study. The inclusion criteria of our study considered Lebanese patients of either sex, aged over 18 years with a clinical diagnosis of first ischemic or hemorrhagic stroke confirmed by a brain CT scan or MRI and that is well-identified by the following codes based on the “International Classification of Diseases” 10 (ICD-10) (I63-I61) (“ICD-10 Version: 2016,” 2016), and classified into cerebrovascular accident, stroke, ischemic stroke, hemorrhagic stroke, intracerebral hemorrhage or embolic/thrombotic stroke. The exclusion criteria were: patient admitted for a recurrent stroke or a transient ischemic attack (TIA), patient with neuropsychiatric and/or cognitive disorders prior to the onset of the stroke such as previously diagnosed depression, anxiety, seizures, dementia, aphasia, memory and attention disorders, patients who refused to participate in the study, and patients who accepted participation at the beginning of the study and decided to stop during the first 3 months of follow-up. This article complies with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [44].
Procedure
All the eligible patients were contacted via phone within the first 3 months post-stroke after collecting the baseline characteristics including socio-demographic characteristics, current health behaviors and clinical data from the medical records in the hospitals. They were informed about the purpose of the study, invited to participate in the study and to sign a consent form. After receiving the signed informed consents, a face to face interview was performed by two trained researchers in the place of residence of the patients at the 3 months after the acute event. Data gathering was performed through structured questionnaires directly from the patients or their family members and from hospital records. The questionnaire used for data collection was composed of several parts:
1. Socio-demographic characteristics: age, gender, marital status, education level, professional status, and Insurance.
2. Current health behaviors: cigarette or waterpipe smoking (yes/no) and physical activity (yes/no)
3. Clinical data: Clinical data were collected from the medical records to detect patients diagnosed with Hypertension (HTN), Atrial Fibrillation (AF), Dyslipidemia prior to stroke onset, and to specify the type and location of the stroke. Hypertension is defined as the elevation of blood pressure revealing a value equals or more than 140 mmHg for systolic blood pressure, or equals or greater than 90 mmHg for diastolic blood pressure (Chobanian et al., 2003). Atrial fibrillation (AF) is) is defined by discharge diagnosis coded from hospitals (ICD-8 codes 4279, 42793, and ICD-10 codes I48) (Gundlund et al., 2016). Dyslipidemia is defined by a history of the total level of cholesterol more than or equals 200 mg/dL or in case of the presence of lipid-lowering therapy (Matz et al., 2016).
4. A battery of tools was also administered to the patients 3 months post-stroke: Mini-Mental State Examination (MMSE) for cognitive function assessment, Hospital Anxiety and Depression Scale (HADS), Fatigue Severity Scale (FSS), “Douleur Neuropathique 4” (DN4) to evaluate neuropathic pain, National Institutes of Health Stroke Scale (NIHSS) to measure the neurological function. The required information of patients with mute; global aphasia; no usable speech or auditory comprehension were provided by their caregivers.
Data Measurements
Mini-Mental State Examination(MMSE)
The MMSE was used to evaluate the cognitive impairment in research (Arevalo-Rodriguez et al., 2015). MMSE is divided into 11 criteria for the assessment of cognitive function that mainly include attention and orientation, memory, registration, recall, calculation, language and ability to draw a complex polygon. Scores fluctuate between 1 and 30 depending on each case. Lower scores designate severe cases with higher impairment. Previous research has validated the use of the Arabic version of MMSE among the Lebanese population and recommended the use of 23 as a cut off to describe “normal” intellectual function (El-Hayeck et al., 2019).
Hospital Anxiety and Depression Scale (HADS)
HADS is designed to measure the severity of depression and anxiety post stroke through a concise self-assessment questionnaire of 14 items. It is divided into two scales of seven components: a scale for depression and a scale for anxiety. Classically, a score of eight or more for each subscale is used as a cut-off score in studies to distinguish between cases of depression and anxiety and the unharmed. This cut-off point is also frequently used in the population of stroke and has adequate specificity and sensitivity in recognition of depression and anxiety disorders after stroke (Vansimaeys et al., 2017). The Arabic validated version of HADS was used in our study (Al Aseri et al., 2015).
Fatigue Severity Scale (FSS)
FSS is a self-assessed scale of nine elements to examine the severity of fatigue among the various circumstances throughout the last week. Items are answerable on a Likert scale from “1” “strong disagreement” to “7” “strong agreement.” The FSS, a measure created by adding all the items of the score, ranged from 1 to 63 with higher scores indicating greater fatigue. A value more than 4 indicates the presence of fatigue (Valko et al., 2008). The Arabic validated version of the FSS was used in our study (Al-Sobayel et al., 2016).
Douleur Neuropathique 4 (DN4)
DN4 is a 4-item questionnaire of Neuropathic pain designed by Bouhassira and colleagues in 2005 to diagnose central pain. It is characterized by sensitivity and specificity in recognizing chronic neuropathic pain of different etiology. DN4 includes ten elements (burning, painful cold, electric shocks, tingling, pins and needles, numbness, itching, touch hypoesthesia with a soft brush, disposable examination pins used for pinprick hypoesthesia, a soft brush used to test tactile dynamic allodynia) answerable by yes (presence) or no (absence). The total score ranges from 0 to 10. Neuropathic pain is diagnosed if the total value is 4; which is the cut-off value (Spallone et al., 2012; Bashir et al., 2017). The Arabic validated version Dn4 was used in our study (Terkawi et al., 2017).
National Institutes of Health Stroke Scale (NIHSS)
The outcome of stroke in the medical practice, especially the measure of the neurological function is widely evaluated through the NIHSS. It involves 11 graded criteria comprising: “level of consciousness, language function, visual fields, eye movements, facial symmetry, motor strength, sensation, coordination and so on, which has undergone extensive validation and reliable assessments” (Cao et al., 2015). This scale is marked from zero (without alteration) to a maximum of 42. Severe cases usually compromise scores of 21 or more (Harrison et al., 2013). It is grouped into four categories concerning variations in severity. “0–5 is mild,” “6–10 is moderate,” “11–15 is moderate to severe,” and “equals or more than 16 is severe impairment” (Roth et al., 2001).
Study Size
The Epi-info 7 program was used to calculate the minimum sample size needed in the study. Considering a prevalence of stroke in Lebanon of 3.9% depending on the study of Jurjus and collaborators (Jurjus et al., 2009), assuming a confidence interval of 95% and a margin of error of 5%, the minimum sample size calculated for our study was estimated at 116 patients. To account for an estimated 10% of lost to follow-up, it is necessary to include 128 patients.
Statistical Methods
The data entry and analyses were performed using the “Statistical Package for Social Sciences (SPSS) software version 22 (SPSSTM Inc., Chicago, IL USA).” Means with their standard deviations and percentages were used to describe continuous and categorical variables, respectively. Statistical bivariate analysis was performed. The Pearson chi-square (χ2) test was used for categorical variables.
Multivariable analyses using logistic regression were carried out to identify the correlates of post-stroke depression and post-stroke anxiety, 3 months after stroke. Dependent variables were anxiety and depression. Independent variables for Anxiety were gender, educational level, physical activity, sedentary duration, hypertension, atrial fibrillation, dyslipidemia, neuropathic pain (Dn4), National Institutes of Health Stroke Scale (NHISS). Independent variables for Depression were gender, physical activity, educational level, employment status, cognitive impairment (MMSE), anxiety (HADS), neuropathic pain (Dn4), Fatigue (FSS), National Institutes of Health Stroke Scale (NHISS). The independent variables introduced in multivariable analyses were those showing a p-value <0.2 in bivariate analyses. The final logistic regression model was reached after ensuring the adequacy of our data using the Hosmer and Lemeshow test. Adjusted odds ratios and their 95% confidence intervals were reported. In all cases, a P-value <0.05 is considered significant.
Results
Participants
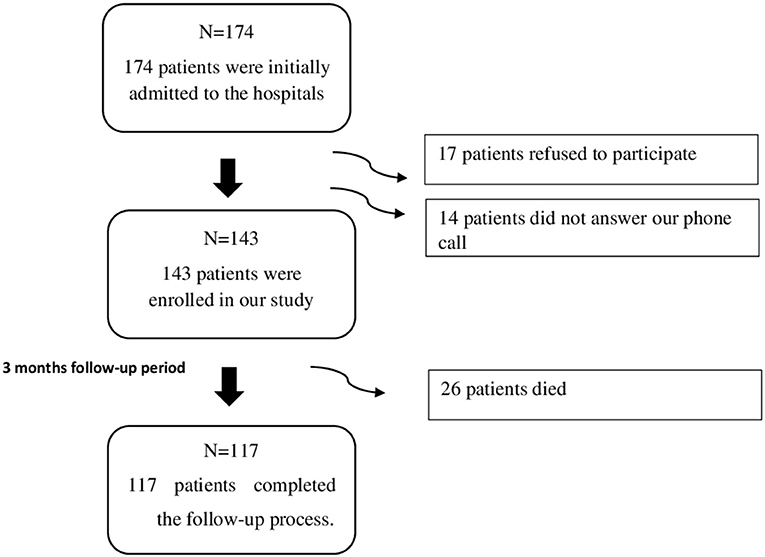
Among the 174 patients admitted to the hospitals between February and June 2018, 143 patients were enrolled in our study, where 17 patients refused to participate, and 14 did not answer our phone call. The mean length of stay in the hospital of the patients was 9.78 (SD = 8.52) days. The patients recruited in our study were followed up for a 3 months' duration, through which 26 patients died, and the remaining 117 patients completed the follow-up process as shown in Figure 1.
Baseline Characteristics of the Study Participants
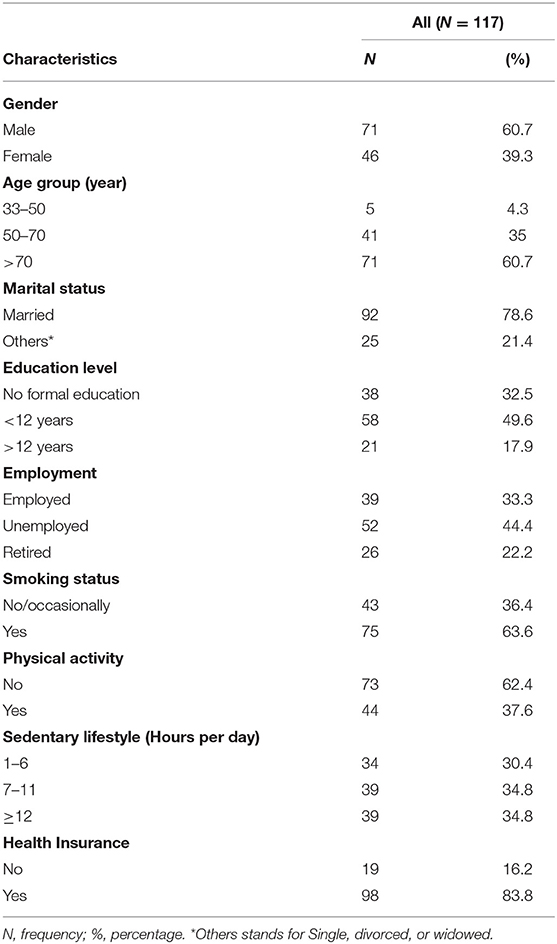
Table 1 depicts the socio-demographic characteristics of the study sample. Of the total, 60.7% were males. The mean age was 72.46 (SD = 12.44) ranging from 33 to 95 and 60.7% of our sample were aged more than 70 years old, which confirm that stroke mainly occur in the elders. The majority (78.6%) were married. Concerning the education level, about one third (32.5%) did not receive any formal education and 49.6% had <12 years of education. Concerning the work status (44.4%) were unemployed and 22.2% were retired. Regarding current lifestyle, 63.6% of participants were smokers and 62.4% did not practice any physical activity. The majority of our sample (83.8%) had social security or insurance.
Clinical Features of the Stroke Among the Patients
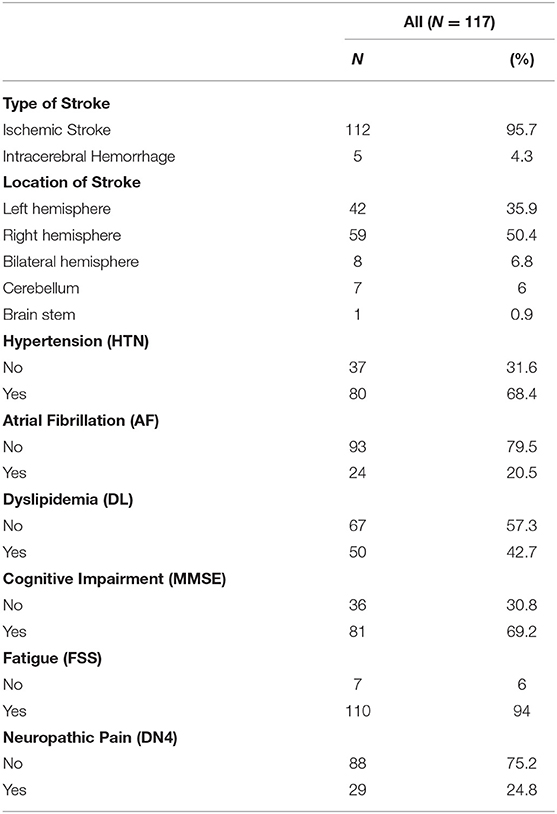
Concerning the type of stroke distributed among the patients, 112 of the 117 patients included (95.7%) were diagnosed with ischemic stroke, and 5 (4.3%) were diagnosed with intracerebral hemorrhage as shown in Table 2. Regarding the different locations of stroke among the patients, 42 patients (35.9%) had their stroke in their left hemisphere, 59 patients (50.4%) in the right hemisphere, 8 patients (6.8%) in their bilateral hemisphere, 7 patients (6%) in their cerebellum, and one patient in his brain stem (0.9%). When checking on their medical conditions, 80 patients (68.4%) have hypertension, 24 patients (20.5%) suffer from atrial fibrillation, and 50 patients (42.7%) suffer from dyslipidemia. Three months following stroke, 81 (69.2%, with a 95% CI of 60–77.4%), suffered from cognitive impairment, 110 (94%, with a 95% CI of 88.1–97.6%) from fatigue, and 29 (24.8%, with a 95% CI of 17.3–33.6%) from neuropathic pain (Table 2).
Neurological Status of the Study Group at 3 Months After Stroke
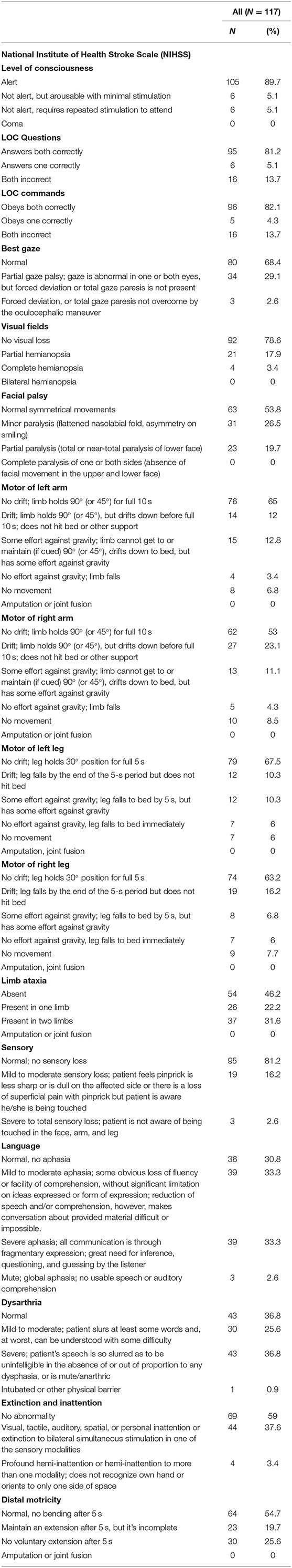
Regarding the Neurological (NHISS) status of our sample size, the results showed that the majority of our patients were alert n = 105(89.7%), answer LOC Questions both correctly n = 95(81.2%), obey LOC commands both correctly n = 96(82.1%), have normal gaze n = 80(68.4%), have no visual loss n = 92(78.6%), have normal symmetrical movements of facial palsy n = 63(53.8%), have no drift; limb holds 90° (or 45°) for full 10 s of motor of left arm n = 76(65%), have no drift; limb holds 90° (or 45°) for full 10 s of motor of right arm n = 62(53%), have no drift; leg holds 30° position for full 5 s of motor of left leg n = 79(67.5%), have no drift; leg holds 30° position for full 5 s of motor of right leg n = 74(63.2%), have absent limb ataxia n = 54(46.2%), have normal; no sensory loss n = 95(81.2%), have mild to moderate aphasia n = 39(33.3%) and severe aphasia n = 39(33.3%), have normal dysarthria n = 43(36.8%), and severe dysarthria n = 43(36.8%), and have normal, no bending after 5 s of distal motricity n = 64(54.7%) (Table 3).
Frequency of Post-Stroke Psychological Complications Among Patients
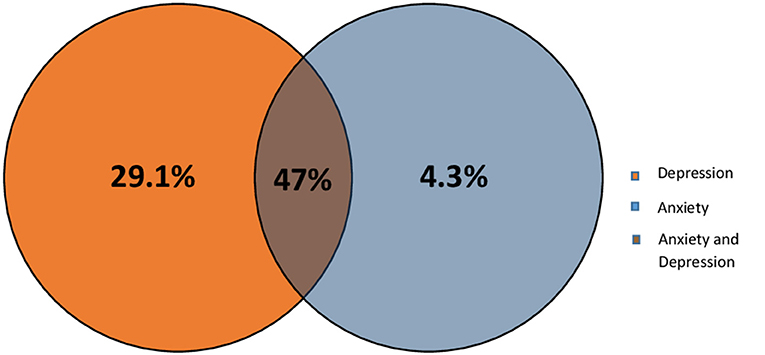
Previous studies have shown that most of the stroke patients suffer from depression and/or anxiety (Barker-Collo, 2007). Therefore, we wanted to check the level of each of these conditions within our Lebanese patients using HADS scale. Results shows that at the end of the first 3 months, 34 (29.1%, 95% CI 21–38.2%) of the patients experienced only depression, and 4.3%, with a 95% CI of 1.4–9.7% experienced only anxiety, however 55 (47%, 95% CI 37.7–56.5%) of the patients experienced both anxiety and depression (Based on HADS scale; score ≥8 for depression and anxiety subscales) as shown in Figure 2.
Correlates of Depression 3 Months After Stroke Among Lebanese Patients
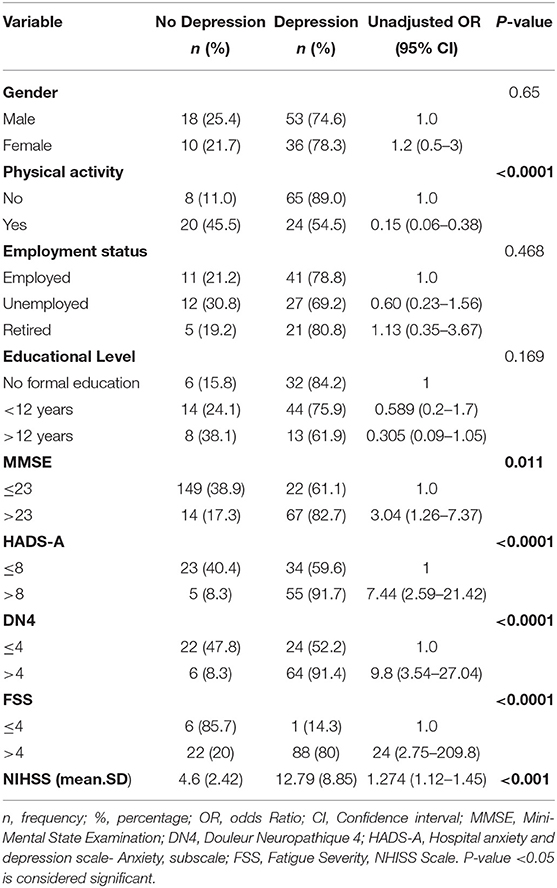
Depression, a frequent neuropsychiatric disorder after stroke, is commonly encountered by stroke survivors. In addition, its prevalence and severity are affected with several factors. The results of the bivariate analysis of the HADS depression scores with variables related to post stroke depression (based on literature) are presented in Table 4. Physical activity, Mini-Mental State Examination (MMSE), Hospital Anxiety and Depression Scale (HADS-A), Douleur Neuropathique 4 (DN4), Fatigue Severity Scale (FSS), and National Institutes of Health Stroke Scale (NIHSS) were associated with depression in bivariate analysis (P-value <0.05). However, logistic regression analysis revealed that HADS-A, DN4, NHISS, and physical activity were significantly associated with depression (Table 5). The odds of depression in patients that experienced post stroke anxiety was 4.814 times the odds of depression in non-anxious patients (OR = 4.814, p-value = 0.017), and the odds of depression in patients that experienced post stroke pain was 6.868 times more than that in patients that didn't experienced pain (OR = 6.868, p-value = 0.002), while physical activity was found to be inversely correlated with depression (OR = 0.261; p-value = 0.029). Moreover, NHISS increases the odds of depression by 1.274 times.
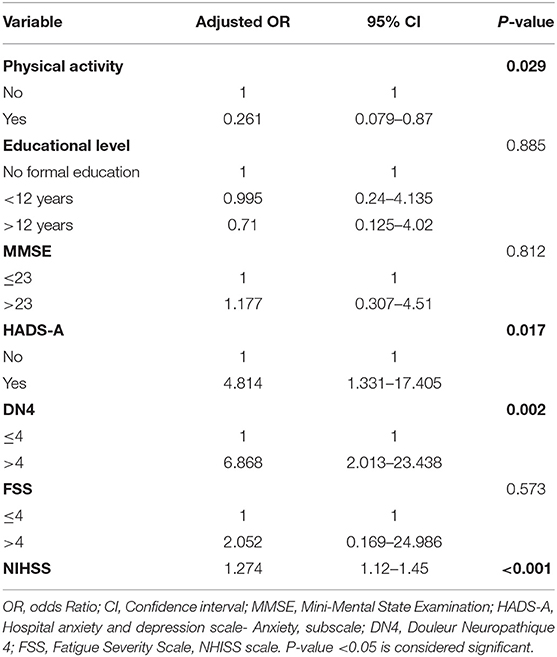
Table 5. Adjusted odds ratios with their 95% confidence intervals from the logistic regression of depression at 3 months after stroke in the study group.
Correlates of Anxiety 3 Months After Stroke Among Lebanese Patients
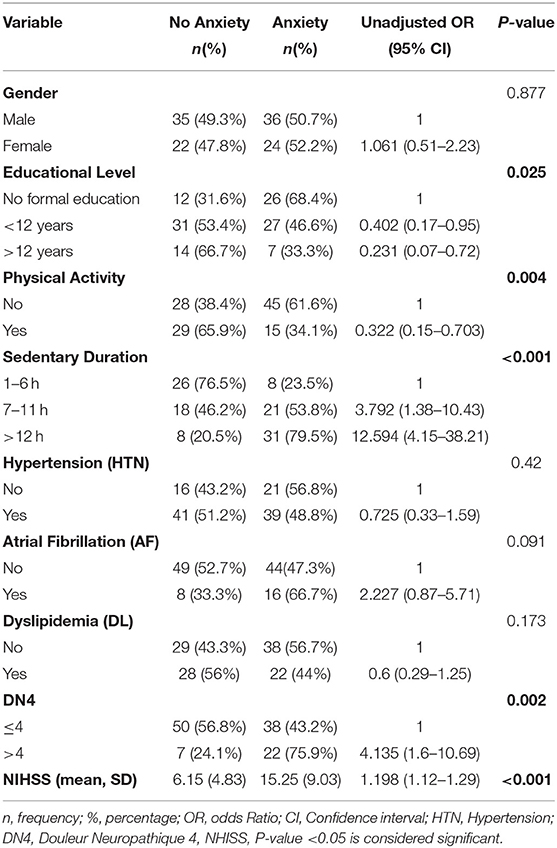
Anxiety is one of the prominent consequences of stroke. Several factors play important roles in affecting its existence and severity post stroke. The results of the bivariate analysis of the HADS anxiety scores with variables related to post stroke depression (based on literature) are presented in Table 6. Educational level, physical activity, sedentary duration, AF, DL, neuropathic pain, and NHISS scale were associated with anxiety in bivariate analysis (P-value <0.05). Additionally, a multivariate logistic regression analysis was performed in order to adjust the independent effects of the variables. Non-significant Hosmer and Lomeshow Test (0.103) were found, and Nagelkerke R square: R2 = 0.391: 39.1% of variance of the dependent variables was explained by the independent variables. The results of the multivariate logistic regression analysis as shown in Table 7 revealed that sedentary life style, neuropathic pain (DN4 ≥ 4), and NHISS scale during the first 3 months post-stroke were significantly associated with anxiety. Anxiety is more likely to occur in case of extended sedentary duration. Patients with longer sedentary duration (between 7 and 11 h per day) were 4.123 more likely to develop post stroke anxiety compared to those with shorter sedentary duration (1–6 h per day). Moreover, patients with sedentary duration longer than 12 h were 8.457 times more likely to develop post stroke anxiety compared to their counterparts with sedentary duration of 1–6 h per day. Furthermore, patients with neuropathic pain are 3.858 times more likely to have anxiety compared to patients without neuropathic pain (ORa = 3.858, p-value = 0.019). Moreover, NHISS increases the odds of anxiety by 1.198 times.
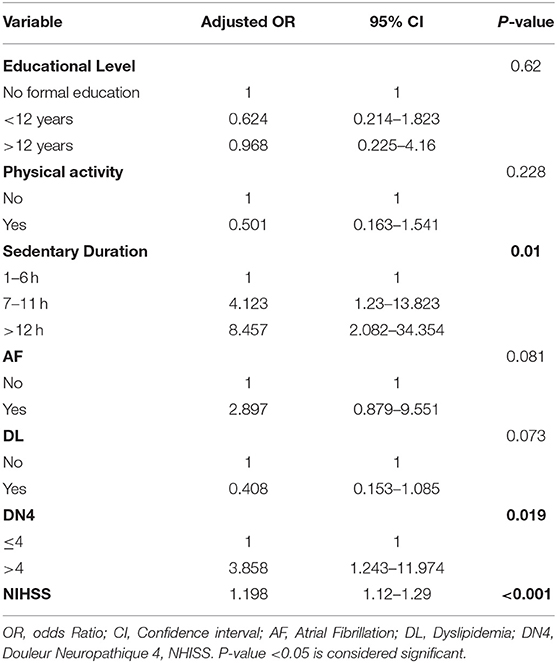
Table 7. Adjusted odds ratios with their 95% confidence intervals from the logistic regression of anxiety at 3 months after stroke in the study group.
Discussion
Anxiety and depression are common and major consequences following stroke. About 30% of stroke survivors experience depression, while anxiety prevalence is around 20–25% (Fang et al., 2017). This study aimed to investigate the psychological consequences of a stroke among Lebanese survivors 3 months following the event and to identify their correlates. At the end of the first 3 months, 89 (76.1%) of the patients experienced depression and 51.3% experienced anxiety. Anxiety, neuropathic pain, physical activity, and NHISS scale were correlated with depression. However, sedentary life style, neuropathic pain, and NHISS scale during the first 3 months post-stroke were significantly associated with anxiety.
Previous studies indicated that post stroke depression is the commonest mental disorder in survivors. Our study indicated a high level of depression (76.1%) recorded 3 months following stroke compared to other studies conducted in western countries which indicated a prevalence of 45.6% (Barker-Collo, 2007), and 39% (Hosking et al., 2000). A possible explanation for this difference could be the general poor feature of life following stroke, especially among the Lebanese population, which are deprived of good care and rehabilitation facilities, physical and social restrictions following stroke, and lack of additional training for healthcare professionals on the symptoms of depression (Kaadan and Larson, 2017). Another possible explanation could be the usage of different methods of assessment (Hackett et al., 2005).
Depression has negative effects on functional recovery, survival, cognitive and social functions, where it is usually associated with several factors (Bolla-Wilson et al., 1989; Kauhanen et al., 1999). We further investigated the elements affecting depression. Our results revealed that physical activity acts as a protective factor against depression 3 months following stroke. It improves the patient's self-esteem, increases their sense of comfort, and happiness due to elevated serotonin and noradrenergic synthesis following exercise, which consequently diminish depression levels (Anderson and Shivakumar, 2013). Similarly, these results have been reported by two recent systematic reviews, thus spotting the light on the importance of intervention to increase physical activity (Pengpid and Peltzer, 2019). Neuropathic pain revealed a significant association with depression as well. These findings are similar to a previous review of 40 studies indicating that pain is a causative factor for depression (Woo, 2010). This could be clarified by the fact that pain could lead to impaired functioning, and thus causes social isolation, which in turn upsurges depression levels (Mushtaq et al., 2014). Furthermore, our study indicated a correlation between anxiety and depression, which was consistent with previous studies (Fang et al., 2017). Researchers have tested hypotheses of shared genetic etiologies as a potential basis of this relationship (Hettema, 2008). Moreover, our results showed that there is a significant association between NHISS scale and depression 3 months after stroke (p-value <0.001), which was consistent with the study of Ilut et al. (2017) that showed that higher NIHSS scores were associated with severe depression (Ilut et al., 2017).
Post-stroke anxiety is another common neuropsychiatric consequence prevalent after stroke. About one-quarter of stroke survivors experience post-stroke anxiety that affects their rehabilitation due to its negative impact on quality of life (QOL) (Li et al., 2019). It is associated with immobility, fatigue and poor self-control (Fang et al., 2017). Many studies indicated the persistence and mutuality of post-stroke anxiety (Cumming et al., 2016). Through our investigation, the frequency of anxiety (51.7%) was not consistent with other studies that demonstrated lower frequency after 3 months of stroke, with an estimated prevalence ranging between 18 and 38% (Barker-Collo, 2007; Ayerbe et al., 2014; Vansimaeys et al., 2017). The high level of anxiety in Lebanon could be related to the lack of rehabilitative services following stroke, the lack of social and psychological support for the survivor, and the high burden of physical disability on them.
The severity of anxiety syndrome after a stroke is usually linked to a general poor feature of life following stroke (Rafsten et al., 2018). Our study indicated a relationship between anxiety and sedentary duration (p-value = 0.01), which was confirmed by a previous systematic review (Teychenne et al., 2015). Long sedentary behavior seems to be related to anxiety at the biological and the psychosocial levels. Biologically, sedentary behavior might lead to central nervous system arousal, sleep disturbances, or poor metabolic health. Alternatively, prolonged sedentary behaviors may be linked to social withdrawal theory. For example, prolonged television viewing, may lead to social solitude and withdrawing from interpersonal relationships, which has been linked to increased feelings of social anxiety (Teychenne et al., 2015). Furthermore, our study confirmed a direct relation between anxiety and pain. This is explained by the fact that stroke survivors who suffer from pain usually pay increased attention toward threat, which increases their anxiety levels toward any physical work (de Heer et al., 2014). Moreover, our results showed that there is a significant association between NHISS scale and anxiety three months after stroke (p-value <0.001), which was consistent with the study of Lee et al. (2019) that revealed that post stroke anxiety was significantly associated with a higher NIHSS score.
To the best of our knowledge, this is the first prospective study examining post-stroke consequences done among first-ever stroke survivors in Lebanon. The data is collected through a face-to-face interview and completed by the investigator himself, and thus, the degree of bias usually resulting from self-completed questionnaires due to a misunderstanding of the questions was declined. However, regarding the limitations, our study had a small sample size, and the risk of selection bias due to the convenience sampling method used to select patients might have restricted the capacity to generalize our findings to all the Lebanese stroke survivors. It should be mentioned we have used the Arabic validated version of the HADS (Al Aseri et al., 2015), Dn4 (Terkawi et al., 2017), FSS (Al-Sobayel et al., 2016), NHISS (Hussein et al., 2015) which is considered a strength with regard to information bias. Nevertheless, additional studies are required to confirm the current study results.
Conclusion
Neuropsychological disorders are generally debilitating for patients and they impose a socioeconomic burden on these patients and their caregivers. The current study provides pioneer information about the consequences of stroke 3 months following the event, mainly neuropsychiatric events affecting functional recovery. Special care should be provided for patients presenting risk factors for anxiety and depression, such as extended sedentary durations, low physical activity, and high pain rates. These psychiatric events represent a major complication in front of the recovery mechanism where they could lead to less compliance to medications, higher fatigue levels, and therefore poorer outcomes. The high frequency of neuropsychiatric complications provided by this study in Lebanon will give much more attention on post-stroke complications and highlight the need for further investigations into the pathogenesis, prevention, and treatment. Thus, necessary protocols should be established for early detection and treatment, and an action plan should be conducted to prevent, manage stroke during the acute phase, evaluate its outcome, and organize the rehabilitation services concerning the consequences following stroke. The rehabilitation process needs to be considered in its broadest context, and include an equal focus on the social, psychological and physical aspects. Additionally, the inclusion of community-based rehabilitation workers is critical to the success of any comprehensive, cost-effective rehabilitation paradigm.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Neuroscience Research Committee, at the NRC, Lebanon. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
WK, MT, and CB undertook the data collection process. WK and MT undertook data management, statistical analysis, and drafted the manuscript. LA-A critically revised the data analysis part. NS, PS, and LA-A contributed to the final draft of the manuscript and gave final approval for submission. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Neuroscience Research Center (NRC) directed by Dr. Yousef Fares, and the contributing authors.
Abbreviations
WHO, World Health Organization; GBD, Global Burden of Diseases; VCI, Vascular Cognitive Impairment; VaD, Vascular Dementia; PSD, Post-stroke depression; DSM, Diagnostic and Statistical Manual; ICD-10, International Classification of Diseases 10; TIA, Transient Ischemic Attack; IRB, Institutional Review Board; HTN, Hypertension; AF, Atrial Fibrillation; DL, Dyslipidemia; MMSE, Mini-Mental State Examination; HADS, Hospital Anxiety and Depression Scale; FSS, Fatigue Severity Scale; DN4, Douleur Neuropathique 4; SPSS, Statistical Package for Social Sciences; QOL, Quality of Life.
References
Abdo, R., Abboud, H., Salameh, P., El Hajj, T., and Hosseini, H. (2019). Mortality and predictors of death poststroke: data from a multicenter prospective cohort of lebanese stroke patients. J. Stroke Cerebrovasc. Dis. 28, 859–868. doi: 10.1016/j.jstrokecerebrovasdis.2018.11.033
Ahangar, A. A., and Hosseini, S. (2009). Epidemiological evaluation of post stroke depression in Babol, Northern Iran. Neurosciences 14, 102–103.
Al Aseri, Z. A., Suriya, M. O., Hassan, H. A., Hasan, M., Sheikh, S. A., Al Tamimi, A., et al. (2015). Reliability and validity of the Hospital Anxiety and Depression Scale in an emergency department in Saudi Arabia: a cross-sectional observational study. BMC Emerg. Med. 15:28. doi: 10.1186/s12873-015-0051-4
Alghwiri, A. A. (2016). The correlation between depression, balance, and physical functioning post stroke. J. Stroke Cerebrovasc. Dis. 25, 475–479. doi: 10.1016/j.jstrokecerebrovasdis.2015.10.022
Al-Sobayel, H. I., Al-Hugail, H. A., AlSaif, R. M., Albawardi, N. M., Alnahdi, A. H., Daif, A. M., et al. (2016). Validation of an Arabic version of fatigue severity scale. Saudi Med. J. 37, 73–78. doi: 10.15537/smj.2016.1.13055
American Psychiatric Association (2000). Diagnostic and Statistical Manual-Text Revision (DSM-IV-TRim, 2000). Washington, DC: American Psychiatric Association.
Anderson, E., and Shivakumar, G. (2013). Effects of exercise and physical activity on anxiety. Front. Psychiatry 4:27. doi: 10.3389/fpsyt.2013.00027
Arauz, A., Rodríguez-Agudelo, Y., Sosa, A. L., Chávez, M., Paz, F., González, M., et al. (2014). Vascular cognitive disorders and depression after first-ever stroke: the Fogarty-Mexico Stroke Cohort. Cerebrovasc. Dis. 38, 284–289. doi: 10.1159/000366471
Arevalo-Rodriguez, I., Smailagic, N., i Figuls, M. R., Ciapponi, A., Sanchez-Perez, E., Giannakou, A., et al. (2015). Mini-Mental State Examination (MMSE) for the detection of Alzheimer's disease and other dementias in people with mild cognitive impairment (MCI). Cochr. Database Syst. Rev. 2015:CD010783. doi: 10.1002/14651858.CD010783.pub2
Ayerbe, L., Ayis, S., Crichton, S., Wolfe, C., and Rudd, A. (2014). The long-term outcomes of depression up to 10 years after stroke; the South London Stroke Register. J. Neurol. Neurosurg. Psychiatry 85, 514–521. doi: 10.1136/jnnp-2013-306448
Ayerbe, L., Ayis, S., Rudd, A. G., Heuschmann, P. U., and Wolfe, C. D. (2011). Natural history, predictors, and associations of depression 5 years after stroke: the South London Stroke Register. Stroke 42, 1907–1911. doi: 10.1161/STROKEAHA.110.605808
Ayerbe, L., Ayis, S., Wolfe, C. D., and Rudd, A. G. (2013). Natural history, predictors and outcomes of depression after stroke: systematic review and meta-analysis. Br. J. Psychiatry 202, 14–21. doi: 10.1192/bjp.bp.111.107664
Barker-Collo, S. L. (2007). Depression and anxiety 3 months post stroke: prevalence and correlates. Arch. Clin. Neuropsychol. 22, 519–531. doi: 10.1016/j.acn.2007.03.002
Bartoli, F., Lillia, N., Lax, A., Crocamo, C., Mantero, V., Carrà, G., et al. (2013). Depression after stroke and risk of mortality: a systematic review and meta-analysis. Stroke Res. Treat. 2013:862978. doi: 10.1155/2013/862978
Bashir, A. H., Abdullahi, A., Abba, M. A., and Mukhtar, N. B. (2017). Central poststroke pain: its profile among stroke survivors in Kano, Nigeria. Behav. Neurol. 2017:9318597. doi: 10.1155/2017/9318597
Bindawas, S. M., Mawajdeh, H. M., Vennu, V. S., and Alhaidary, H. M. (2017). Functional recovery differences after stroke rehabilitation in patients with uni- or bilateral hemiparesis. Neurosciences (Riyadh) 22, 186–191. doi: 10.17712/nsj.2017.3.20170010
Bolla-Wilson, K., Robinson, R. G., Starkstein, S. E., Boston, J., and Price, T. R. (1989). Lateralization of dementia of depression in stroke patients. Am. J. Psychiatry 146, 627–634. doi: 10.1176/ajp.146.5.627
Burton, C. A. C., Murray, J., Holmes, J., Astin, F., Greenwood, D., and Knapp, P. (2013). Frequency of anxiety after stroke: a systematic review and meta-analysis of observational studies. Int. J. Stroke 8, 545–559. doi: 10.1111/j.1747-4949.2012.00906.x
Cao, K. G., Fu, C. H., Li, H. Q., Xin, X. Y., and Gao, Y. (2015). A new prognostic scale for the early prediction of ischemic stroke recovery mainly based on traditional Chinese medicine symptoms and NIHSS score: a retrospective cohort study. BMC Comp. Altern. Med. 15:407. doi: 10.1186/s12906-015-0903-1
Castillo, C. S., Schultz, S. K., and Robinson, R. G. (1995). Clinical correlates of early-onset and late-onset poststroke generalized anxiety. Am. J. Psychiatry 152, 1174–1179. doi: 10.1176/ajp.152.8.1174
Cheah, W. K., Hor, C. P., Zariah, A. A., and Looi, I. (2016). A review of stroke research in Malaysia from 2000 - 2014. Med. J. Malaysia 71, 58–69.
Chobanian, A. V., Bakris, G. L., Black, H. R., Cushman, W. C., Green, L. A., Izzo Jr, J. L., et al. (2003). The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. Jama 289, 2560–2571. doi: 10.1001/jama.289.19.2560
Cumming, T. B., Blomstrand, C., Skoog, I., and Linden, T. (2016). The high prevalence of anxiety disorders after stroke. Am. J. Geriatr. Psychiatry 24, 154–160. doi: 10.1016/j.jagp.2015.06.003
D'Aniello, G. E., Scarpina, F., Mauro, A., Mori, I., Castelnuovo, G., Bigoni, M., et al. (2014). Characteristics of anxiety and psychological well-being in chronic post-stroke patients. J. Neurol. Sci. 338, 191–196. doi: 10.1016/j.jns.2014.01.005
de Heer, E. W., Gerrits, M. M., Beekman, A. T., Dekker, J., van Marwijk, H. W., de Waal, M. W., et al. (2014). The association of depression and anxiety with pain: a study from NESDA. PLoS ONE 9:e0106907. doi: 10.1371/journal.pone.0106907
De Wit, L., Putman, K., Baert, I., Lincoln, N. B., Angst, F., Beyens, H., et al. (2008). Anxiety and depression in the first six months after stroke. A longitudinal multicentre study. Disabil. Rehabil. 30, 1858–1866. doi: 10.1080/09638280701708736
Easton, J. D., Saver, J. L., Albers, G. W., Alberts, M. J., Chaturvedi, S., Feldmann, E., et al. (2009). Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease: the American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke 40, 2276–2293. doi: 10.1161/STROKEAHA.108.192218
El Sayed, M. J., El Zahran, T., and Tamim, H. (2014). Acute stroke care and thrombolytic therapy use in a tertiary care center in Lebanon. Emerg. Med. Int. 2014:438737. doi: 10.1155/2014/438737
El-Hajj, M., Salameh, P., Rachidi, S., Al-Hajje, A., and Hosseini, H. (2019). Cigarette and waterpipe smoking are associated with the risk of stroke in Lebanon. J. Epidemiol. Global Health 9, 62–70. doi: 10.2991/jegh.k.181231.002
El-Hayeck, R., Baddoura, R., Wehbé, A., Bassil, N., Koussa, S., Abou Khaled, K., et al. (2019). An Arabic version of the mini-mental state examination for the Lebanese population: reliability, validity, and normative data. J. Alzheimer Dis. 71, 525–540. doi: 10.3233/JAD-181232
Fang, Y., Mpofu, E., and Athanasou, J. (2017). Reducing depressive or anxiety symptoms in post-stroke patients: pilot trial of a constructive integrative psychosocial intervention. Int. J. Health Sci. 11:53.
Farah, R., Zeidan, R. K., Chahine, M. N., Asmar, R., Chahine, R., Salameh, P., et al. (2015). Prevalence of stroke symptoms among stroke-free residents: first national data from Lebanon. Int. J. Stroke 10, 83–88. doi: 10.1111/ijs.12563
Gillen, R., Eberhardt, T. L., Tennen, H., Affleck, G., and Groszmann, Y. (1999). Screening for depression in stroke: relationship to rehabilitation efficiency. J. Stroke Cerebrovasc. Dis. 8, 300–306. doi: 10.1016/S1052-3057(99)80004-4
Gundlund, A., Christiansen, M. N., Hansen, M. L., Olesen, J. B., Zahir, D., Køber, L., et al. (2016). Familial clustering and subsequent incidence of atrial fibrillation among first-degree relatives in Denmark. Ep Europace 18, 658–664. doi: 10.1093/europace/euv274
Hackett, M. L., Köhler, S. T, O'Brien, J., and Mead, G. E. (2014). Neuropsychiatric outcomes of stroke. Lancet Neurol. 13, 525–534. doi: 10.1016/S1474-4422(14)70016-X
Hackett, M. L., Yapa, C., Parag, V., and Anderson, C. S. (2005). Frequency of depression after stroke: a systematic review of observational studies. Stroke 36, 1330–1340. doi: 10.1161/01.STR.0000165928.19135.35
Hamad, A. M., Siddiqui, K. A., Al-Mansoor, N. M., Al-Senani, F. M., and Sinha, S. (2011). Post stroke depression in acute stroke: correlating with site and stroke severity. Neurosciences 16, 382–383.
Hara, Y. (2015). Brain plasticity and rehabilitation in stroke patients. J. Nippon Med. School 82, 4–13. doi: 10.1272/jnms.82.4
Harrison, J. K., McArthur, K. S., and Quinn, T. J. (2013). Assessment scales in stroke: clinimetric and clinical considerations. Clin. Interv. Aging 8, 201–211. doi: 10.2147/CIA.S32405
Heruti, R. J., Lusky, A., Dankner, R., Ring, H., Dolgopiat, M., Barell, V., et al. (2002). Rehabilitation outcome of elderly patients after a first stroke: effect of cognitive status at admission on the functional outcome. Arch. Phys. Med. Rehabil. 83, 742–749. doi: 10.1053/apmr.2002.32739
Hettema, J. M. (ed.). (2008). What is the genetic relationship between anxiety and depression? Am. J. Med. Genet. 148C, 140–146. doi: 10.1002/ajmg.c.30171
Hosking, S. G., Marsh, N. V., and Friedman, P. J. (2000). Depression at 3 months poststroke in the elderly: predictors and indicators of prevalence. Aging Neuropsychol. Cogn. 7, 205–216. doi: 10.1076/anec.7.4.205.798
Hussein, H. M., Abdel Moneim, A., Emara, T., Abd-elhamid Yousry, A., Salem, H. H., Abd-Allah, F., et al. (2015). Arabic cross cultural adaptation and validation of the National Institutes of Health Stroke Scale. J. Neurol. Sci. 357, 152–156. doi: 10.1016/j.jns.2015.07.022
Ilut, S., Stan, A., Blesneag, A., Vacaras, V., Vesa, S., and Fodoreanu, L. (2017). Factors that influence the severity of post-stroke depression. J. Med. Life 10, 167–171.
Iranmanesh, F. (2010). Post-stroke depression and hospital admission. A need for nursing care partition according to the clinical condition. Neurosciences (Riyadh) 15, 33–36.
Johnson, G. (1991). Research into psychiatric disorder after stroke: the need for further studies. Aust. N. Z. J. Psychiatry 25, 358–370. doi: 10.3109/00048679109062637
Jurjus, A. R., Tohme, R. A., Ephrem, G., Hussein, I. A. H., and Jurjus, R. (2009). Incidence and prevalence of circulatory diseases in Lebanon: a physician's inquiry. Ethn. Dis. 19:1.
Kaadan, M. I., and Larson, M. J. (2017). Management of post-stroke depression in the Middle East and North Africa: too little is known. J. Neurol. Sci. 378, 220–224. doi: 10.1016/j.jns.2017.05.026
Kauhanen, M.-L., Korpelainen, J., Hiltunen, P., Brusin, E., Mononen, H., Maatta, R., et al. (1999). Poststroke depression correlates with cognitive impairment and neurological deficits. Stroke 30, 1875–1880. doi: 10.1161/01.STR.30.9.1875
Kim, J. S. (2016). Post-stroke mood and emotional disturbances: pharmacological therapy based on mechanisms. J. Stroke 18, 244–255. doi: 10.5853/jos.2016.01144
Kuptniratsaikul, V., Kovindha, A., Suethanapornkul, S., Manimmanakorn, N., and Archongka, Y. (2013). Long-term morbidities in stroke survivors: a prospective multicenter study of Thai stroke rehabilitation registry. BMC Geriatr. 13:33. doi: 10.1186/1471-2318-13-33
Kwakkel, G., Veerbeek, J. M., van Wegen, E. E., and Wolf, S. L. (2015). Constraint-induced movement therapy after stroke. Lancet Neurol. 14, 224–234. doi: 10.1016/S1474-4422(14)70160-7
Lahoud, N., Abbas, M.-H., Salameh, P., Saleh, N., Abes, S., Hosseini, H., et al. (2017). A retrospective analysis of 254 acute stroke cases admitted to two University hospitals in Beirut: classification and associated factors. Funct. Neurol. 32:41. doi: 10.11138/FNeur/2017.32.1.041
Layadi, K., Abderrahim, A., Kahli, M., Douba, N., Moulay, M., and Rémaoun, M. (2013). Depression after stroke: what characteristics? Ann. Phys. Rehabil. Med. 2013:e62. doi: 10.1016/j.rehab.2013.07.064
Lee, E. H., Kim, J. W., Kang, H. J., Kim, S. W., Kim, J. T., Park, M. S., et al. (2019). Association between anxiety and functional outcomes in patients with stroke: a 1-year longitudinal study. Psychiatr. Invest. 16, 919–925. doi: 10.30773/pi.2019.0188
Li, W., Xiao, W.-M., Chen, Y.-K., Qu, J.-F., Liu, Y.-L., Fang, X.-W., et al. (2019). Anxiety in patients with acute ischemic stroke: risk factors and effects on functional status. Front. Psychiatry 10:257. doi: 10.3389/fpsyt.2019.00257
Matz, K., Seyfang, L., Dachenhausen, A., Teuschl, Y., Tuomilehto, J., and Brainin, M. (2016). Post-stroke pneumonia at the stroke unit–a registry based analysis of contributing and protective factors. BMC Neurol. 16:107. doi: 10.1186/s12883-016-0627-y
Mushtaq, R., Shoib, S., Shah, T., and Mushtaq, S. (2014). Relationship between loneliness, psychiatric disorders and physical health? A review on the psychological aspects of loneliness. J. Clin. Diagn. Res. 8:WE01. doi: 10.7860/JCDR/2014/10077.4828
Pengpid, S., and Peltzer, K. (2019). High sedentary behaviour and low physical activity are associated with anxiety and depression in Myanmar and Vietnam. Int. J. Environ. Res. Public Health 16:1251. doi: 10.3390/ijerph16071251
Rafsten, L., Danielsson, A., and Sunnerhagen, K. S. (2018). Anxiety after stroke: a systematic review and meta-analysis. J. Rehabil. Med. 50, 769–778. doi: 10.2340/16501977-2384
Riah, L., Elbouchikhi, M., Lmidmani, F., and Elfatimi, A. (2013). Fluoxetine for post stroke depression: experience of rehabilitation unit in Ibn Rochd University hospital of Casablanca. J. Neurol. Sci. 333:e543. doi: 10.1016/j.jns.2013.07.1910
Roth, E. J., Lovell, L., Harvey, R. L., Heinemann, A. W., Semik, P., and Diaz, S. (2001). Incidence of and risk factors for medical complications during stroke rehabilitation. Stroke 32, 523–529. doi: 10.1161/01.STR.32.2.523
Sackley, C., Brittle, N., Patel, S., Ellins, J., Scott, M., Wright, C., et al. (2008). The prevalence of joint contractures, pressure sores, painful shoulder, other pain, falls, and depression in the year after a severely disabling stroke. Stroke 39, 3329–3334. doi: 10.1161/STROKEAHA.108.518563
Schultz, S. K., Castillo, C. S., Rosier, J. T., and Robinson, R. G. (1997). Generalized anxiety and depression: assessment over 2 years after stroke. Am. J. Geriatr. Psychiatry 5, 229–237. doi: 10.1097/00019442-199700530-00007
Scott, C. L., Phillips, L. H., Johnston, M., Whyte, M. M., and MacLeod, M. J. (2012). Emotion processing and social participation following stroke: study protocol. BMC Neurol. 12:56. doi: 10.1186/1471-2377-12-56
Shi, Y., Yang, D., Zeng, Y., and Wu, W. (2017). Risk factors for post-stroke depression: a meta-analysis. Front. Aging Neurosci. 9:218. doi: 10.3389/fnagi.2017.00218
Spallone, V., Morganti, R., D'amato, C., Greco, C., Cacciotti, L., and Marfia, G. (2012). Validation of DN4 as a screening tool for neuropathic pain in painful diabetic polyneuropathy. Diabetic Med. 29, 578–585. doi: 10.1111/j.1464-5491.2011.03500.x
Terkawi, A. S., Abolkhair, A., Didier, B., Alzhahrani, T., Alsohaibani, M., Terkawi, Y. S., et al. (2017). Development and validation of Arabic version of the douleur neuropathique 4 questionnaire. Saudi J. Anaesth. 11:S31. doi: 10.4103/sja.SJA_97_17
Teychenne, M., Costigan, S. A., and Parker, K. (2015). The association between sedentary behaviour and risk of anxiety: a systematic review. BMC Public Health 15:513. doi: 10.1186/s12889-015-1843-x
Valko, P. O., Bassetti, C. L., Bloch, K. E., Held, U., and Baumann, C. R. (2008). Validation of the fatigue severity scale in a Swiss cohort. Sleep 31, 1601–1607. doi: 10.1093/sleep/31.11.1601
Vansimaeys, C., Zuber, M., Pitrat, B., Join-Lambert, C., Tamazyan, R., Farhat, W., et al. (2017). Combining standard conventional measures and ecological momentary assessment of depression, anxiety and coping using smartphone application in minor stroke population: a longitudinal study protocol. Front. Psychol. 8:1172. doi: 10.3389/fpsyg.2017.01172
Woo, A. K. (2010). Depression and anxiety in pain. Rev. Pain 4, 8–12. doi: 10.1177/204946371000400103
Keywords: depression, anxiety, stroke survivors, rehabilitation, stroke
Citation: Khazaal W, Taliani M, Boutros C, Abou-Abbas L, Hosseini H, Salameh P and Sadier NS (2021) Psychological Complications at 3 Months Following Stroke: Prevalence and Correlates Among Stroke Survivors in Lebanon. Front. Psychol. 12:663267. doi: 10.3389/fpsyg.2021.663267
Received: 02 February 2021; Accepted: 13 May 2021;
Published: 10 June 2021.
Edited by:
Nicola Canessa, University Institute of Higher Studies in Pavia, ItalyReviewed by:
Catherine Mary Sackley, King's College London, United KingdomChiara Pavese, University of Pavia, Italy
Copyright © 2021 Khazaal, Taliani, Boutros, Abou-Abbas, Hosseini, Salameh and Sadier. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Najwane Said Sadier, joanne.sadier@adu.ac.ae
†These authors have contributed equally to this work and share first authorship
 Walaa Khazaal
Walaa Khazaal Maram Taliani
Maram Taliani Celina Boutros
Celina Boutros Linda Abou-Abbas
Linda Abou-Abbas Hassan Hosseini2
Hassan Hosseini2 Pascale Salameh
Pascale Salameh Najwane Said Sadier
Najwane Said Sadier