- 1Shandong Institute of Pomology, Shandong Key Laboratory of Fruit Biotechnology Breeding, Taian, China
- 2College of Horticulture, Shenyang Agricultural University, Shenyang, China
Introduction: Substantial previous studies have reported that fulvic acid (FA) application plays an important role in Chinese agricultural production. However, little is known about the mechanisms for using FA to increase apple trees resistance to Cd toxicity. In order to clarify the mechanism underlying FA alleviation in Cd-induced growth inhibition in apple seedlings.
Methods: Herein, we treated M9T337 seedlings to either 0 or 30 µM/L Cd together with 0 or 0.2 g/L FA and analyzed the root growth, antioxidant enzyme activities, carbon (C) assimilation, nitrogen (N) metabolism, and C and N transport.
Results: The results presented that, compared with CK (without Cd addition or FA spraying application), Cd poisoning significantly inhibited the root growth of apple seedlings. However, this Cd-induced root growth inhibition was significantly alleviated by FA spraying relative to the Cd treatment (Cd addition alone). On the one hand, the mitigation of inhibition effects was due to the reduced oxidative damage by enhancing antioxdiant enzyme (SOD, POD, and CAT) activities in leaves and roots. On the other hand, this growth advantage demonstrated compared to the Cd treatment was found to be associated with the strengthen of photosynthetic performance and the elevation of C and N metabolism enzymes activities. Meanwhile, we also found that under Cd stress condition, the distribution of C and N nutrients in apple seedlings was optimised by FA spraying application relative to the Cd treatment, according to the results of 13C and 15N tracing.
Conclusion: Conclusively, our results suggested that the inhibitory effect of Cd on apple seedlings root growth was alleviated by FA through regulating antioxdiant capacities and C and N metabolism.
Introduction
Cadmium (Cd) has become a major concern worldwide, and pollution due to Cd is becoming increasingly severe due to the advances in anthropogenic activities (Duan et al., 2016; Argüello et al., 2019; Kaya et al., 2019);. Numerous studies have shown that excess Cd in plant environments reduces leaf chlorophyll biosynthesis, induces oxidative stress, and inhibits plant growth (Hasan et al., 2015; Luo et al., 2016; Podazza et al., 2016; Zhou et al., 2017). In addition, the Cd accumulated in agricultural products enters the food chain and poses a huge risk to human health (Zhuang et al., 2023). Apple, one of the most widely cultivated fruit in China, plays an important role in regional economic development and rural revitalization. Currently, due to sewage irrigation and chemical fertilizer and pesticide application, the problem of Cd pollution in orchards is becoming more and more prominent, seriously restricting apple plant growth, reducing fruit yields, and causing huge potential health threats to humans (He et al., 2020; Zhuang et al., 2023). Therefore, how to minimize the negative effects induced by Cd poisoning on the growth and development of apple plants through establishing reliable methods to enhance the Cd tolerance of apple plants is currently an urgent problem for the sustainable development of China’s apple industry.
In recent years, considerable research progress has been carried out to explore reliable methods to mitigate Cd-induced negative influence in plants including the exogenous application of materials such as plant growth regulators (Parvaiz et al., 2016; Wang et al., 2016; He et al., 2020). As a reactive, relatively low molecular weight humic acid substance, fulvic acid (FA) has been successful in alleviating the adverse effects induced by external abiotic stresses (Suh et al., 2014; Canellas et al., 2015; Wang et al., 2019; Chen et al., 2022)—for instance, the treatment of FA protected the soybean from heat and salt stresses (Dinler et al., 2016). By increasing the proline levels, FA application in maize reduced the negative effects of drought stress (Anjum et al., 2011). The application of FA also protected Brassica napus from water stress by modulating the antioxidant enzyme activities (Lotfi et al., 2015). In wheat, FA application reduced chromium (Cr) toxicity by regulating the photosynthetic pigments (Ali et al., 2015). However, little is known about the mechanisms for using FA to increase apple trees’ resistance to Cd toxicity and to mitigate the inhibitory effects of Cd stress on apple plants’ growth.
The process of plant growth is the accumulation of plant biomass. As the two basic and metabolic processes in higher plants, how plants grow and develop as well as plant stress tolerance promotion are highly dependent on the close relationship between C and N metabolism (Han et al., 2016; Ren et al., 2020; Liu et al., 2022). Therefore, it is very important to maintain C and N assimilation homeostasis to mitigate the inhibitory effects of external stress conditions on plant growth (Ren et al., 2021). Although numerous studies have shown that FA application could promote plant growth through the regulation of C and N metabolism (Gao et al., 2022; Yu et al., 2023a), details regarding the effects of Cd stress on C and N metabolism in apple plants and whether FA is sufficient to mitigate the inhibitory effects of Cd stress on apple plants growth, from the perspective of the regulation of C and N metabolism, both remain unclear.
Here we used M9T337 (an apple rootstock) seedlings as experiment materials; the effects of FA addition or not on plant growth, photosynthetic indexes, antioxidant enzyme activities, and C and N metabolism-related enzyme activities of M9T337 under Cd stress were investigated. Simultaneously, we used isotopic (13C and 15N) labeling to study the effects of Cd or FA addition on C and N partitioning in apple seedlings. This study’s findings provides a theoretical reference regarding FA alleviation in Cd-induced plant growth inhibition in apple seedlings and promotes the application of FA in apple orchards with excessive soil Cd levels.
Materials and methods
Plant material and growth conditions
The M9T337 seedlings were incubated in a greenhouse at Shandong Institute of Pomology under natural light at 22–26°C and 7–10°C day and night temperatures and 60%–65% relative humidity. The seedlings with uniform height were selected and transplanted into 40 cm × 30 cm × 15 cm plastic basins containing 6 L of half-strength Hoagland’s nutrient solution (Hoagland and Arnon, 1950). Moreover, eight seedlings were maintained per basin in this experiment.
Experimental design and sampling
A hydroponic experiment was carried out by growing the seedlings under four treatments as follows: nutrient solution alone (CK), nutrient solution containing Cd (Cd), nutrient solution along with FA sprayed on the leaves (CK+FA), and nutrient solution containing Cd and FA was sprayed on the leaves (Cd+FA). Here CdCl2·2.5 H2O was used as the only Cd source, and the concentration of the Cd treatment was 30 μM/L. The nutrient solution was changed every 3 days. FA purchased from Bio Aladdin (Shanghai, China) was sprayed on the leaves at every change of nutrient solution. The concentration of FA was 0.2 g/L FA. Plants (seedlings) were harvested after treatment for 15 days (the nutrient solution was changed five times in total). Each treatment contained 36 plastic basins, with eight seedlings in each basin and 12 basins pooled as a repeat, and each treatment was replicated three times. For the purpose of isotope labeling as well as excluding the influence of different treatments on the 15N and 13C natural abundances in seedling organs, each repeat was divided into two groups: one group was used for 15N and 13C labeling as well as the measurement of 15N and 13C abundances (marker group), and the other group (unlabeled group) was used for measuring other indices.
Growth performance indexes
In this study, for each of the normal (unlabeled) groups of each treatment, three seedlings were selected at random for the root morphology measurements, and root total length (RL) and root surface area (RSA) were selected to evaluate the root morphology. After completing the root sample preparations as described by Xing et al. (2021), the values of RL and RSA were measured using the WinRHIZO software (Regent Instruments Canada, Inc.). The samples were prepared according to Xu et al. (2020). Subsequently, a 1/1,000 electronic balance was used to measure the dry weight. Meanwhile, the determination of root activity was also measured using the triphenyltetrazolium chloride (TTC) method of Chen et al. (2018).
Photosynthetic parameter
Following the details presented by Xu et al. (2020), a LI-6400XT portable photosynthesis system (LI-COR, Lincoln, NE, USA) was employed to determine the Pn (net photosynthetic rate) and Gs (stomatal conductance). Moreover, a pulse-modulated chlorophyll fluorescence meter (PAM 2500, Walz, Germany) was used to analyze the chlorophyll fluorescence parameters.
H2O2 and MDA levels
The hydrogen peroxide (H2O2) content was measured following the method of He et al. (2011). The malondialdehyde (MDA) content was measured as described by Lei et al. (2007).
Enzyme activities
In addition to leaf Rubisco (ribulose-1,5-biphosphate carboxylase-oxygenase) measurement based on the method of Hu et al. (2016a), the activities of nitrate reductase (NR), glutamine synthetase (GS), and glutamate synthase (GOGAT) both in roots and leaves were measured using the method presented by Hu et al. (2016b). The method reported by Rufly and Huber (1983) was adopted in this experiment to analyze the activity of sorbitol dehydrogenase (SDH). The activities of fructokinase (FRK) and hexokinase (HK) were measured according to the method of Li et al. (2012). Moreover, the activity levels of SOD, CAT, and POD were analyzed according to He et al. (2011).
13C and 15N isotope labeling
The basins of the marker group in each treatment were used for 15N and 13C labeling. For 15N isotope tracing, 0.5 g Ca(15NO3)2 from Shanghai Chemical Research Institute, China, was added to the nutrient solution at each change of the solution. The total amount of Ca(15NO3)2 used in each basin was 2.5 g. After 12 days of treatment, the 13C isotope tracing was initiated, following the method of Xu et al. (2020). In brief, the basins of the marker group in each treatment, markers (Ba13CO3, 98% independence), fans, and reduced iron powder were placed into a sealed marking room. Each basin corresponded to one marking room, and the dosage of each basin was 2 g. The labeling work lasted for 4 h. Hydrochloric acid was injected into the beaker with a syringe every 0.5 h in order to maintain the concentration of 13CO2. At the same time, three other plants (seedlings) from the unlabeled group were selected to measure the 13C natural abundance. Similar with the unlabeled group, samples of the marker group in each treatment were harvested at the end of the experiment (after 15 days of treatment).
Each plant sample was divided into roots, stems, and leaves and used to measure the 15N and 13C abundance, following the method of Xu et al. (2020). A MAT-251-Stable isotope ratio mass spectrometer was used to measure 15N abundance, while a DELTAVplusXP advantage isotope ratio mass spectrometer was selected to determine 13C abundance. Finally, the 15N- and 13C-related indexes were calculated using the following equations:
Calculation of 15N
The Ndff (%) in Equation (1) Ndff refers to the contribution rate of 15N absorbed from fertilizer and distributed by plant organs relative to the total nitrogen of plant organs and reflects the ability of plant organs to absorb and regulate 15N fertilizer.
The total N content (mg) in Equation (2) refers to organ total N content (mg).
In Equation (3), the total 15N absorption refers to the total amount obtained by adding the 15N of each organ.
Calculation of 13C
In Equation (4), the value of RPBD (standard ratio of carbon isotope) is set to 0.0112372.
In Equation (5), the value of Ci (C content of each organ) is the product of the organ’s dry matter (g) and the organ’s total carbon content (%); Fnl represents the 13C natural abundance of each organ.
In Equation (6), 13Cnet absorption refers to the total amount obtained by adding the 13C of each organ.
RNA isolation and qRT-PCR analysis
Total RNA was extracted from 0.1 g of the roots using the RNAiso Plus extraction kit (Takara, Otsu, Shiga, Japan). Subsequently, RNA extraction, reverse transcription, and qRT-PCR procedures followed a previously reported procedure (Yu et al., 2023a). The relative gene expression levels were normalized according to the 2–ΔΔCT method, and the MdActin gene was used as the internal reference gene. Three biological replicates per treatment and three technical replicates per sample were used in the assay. The primers are listed in Supplementary Table S1.
Statistical analysis
This experiment was conducted using Microsoft Excel for data collection and SPSS 21.0 (SPSS, Inc., Chicago, IL, USA) for data analysis using one-way analysis of variance (ANOVA) and a post hoc test (Duncan’s). The differences were considered statistically significant at a probability level of P< 0.05.
Results
Plant growth
Compared with CK, Cd addition alone (Cd treatment) resulted in a significant decrease in the dry weight of the seedlings’ organ (Figure 1A). In contrast, the Cd-induced decrease was significantly decreased by FA spraying application under Cd exposure condition relative to the Cd treatment. Consistent with Cd-induced root dry weight inhibition, the seedlings’ RL (root total length) and RSA (root surface area) were both significantly decreased under Cd addition (Cd and Cd+FA) conditions relative to CK. In contrast, the RL and RSA were both optimized by FA spraying application under Cd stress, which were 35.55% (RL) and 46.78% (RSA), respectively, higher than that treated by the Cd treatment. Moreover, the Cd treatment significantly lowered the seedling root activities, and FA spraying application could alleviate the Cd-induced adverse effect on root activity, which was 53.52% higher than that of the Cd treatment (Figure 1B).
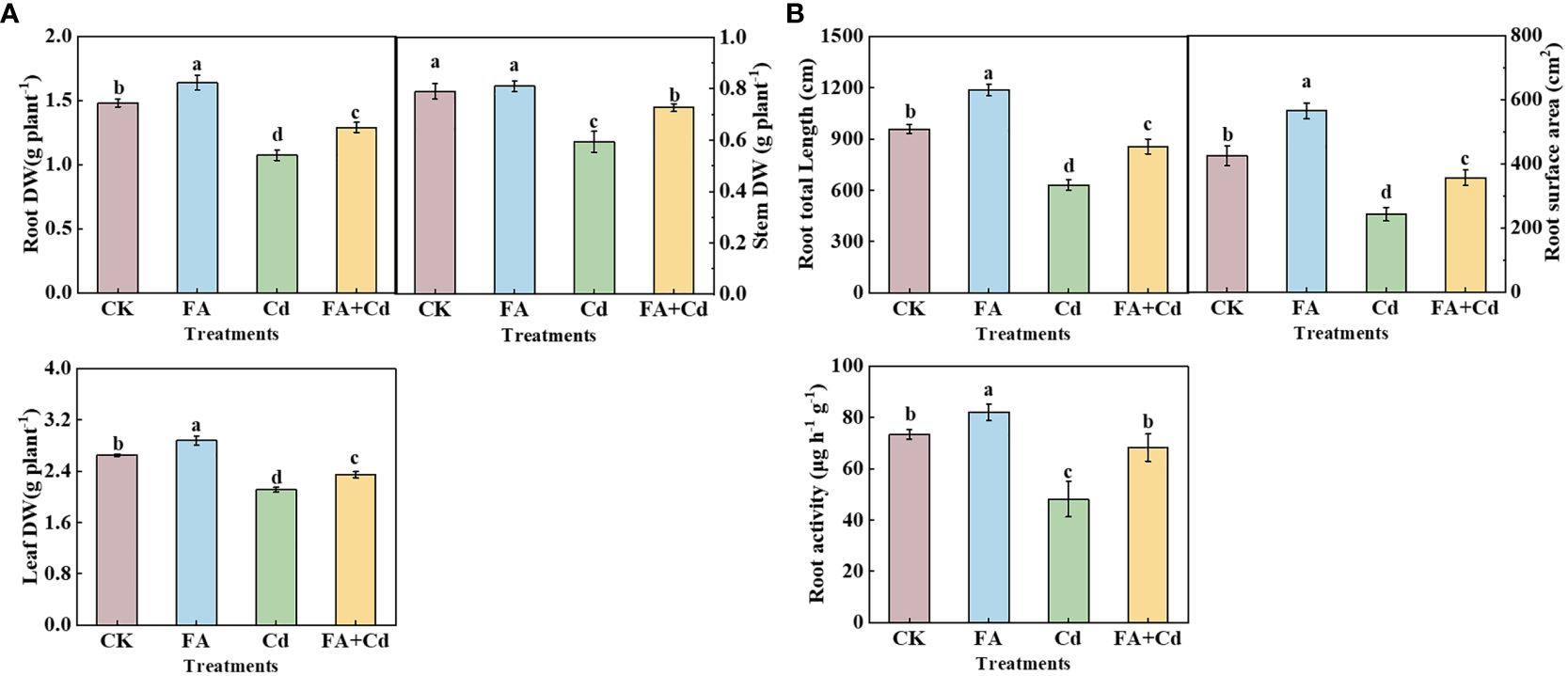
Figure 1 Organs’ dry matter weight (A), root total length, surface area of seedlings as well as root activities (B) under different treatments. CK, control—nutrient solution alone; FA, nutrient solution with FA; Cd, nutrient solution containing Cd; Cd+FA, nutrient solution containing Cd and the spraying application of FA. The vertical bars on the histograms indicate ± SD. The different letters indicate statistically significant differences (P< 0.05).
H2O2, and MDA levels and antioxidant enzyme activity
Irrespective of FA spraying, the addition of Cd (Cd and Cd+FA) treatments both resulted in higher H2O2 and MDA levels in roots than that of CK (Figures 2G-J). The highest H2O2 and MDA levels were observed under the Cd treatment, while the lowest were under CK. However, no significant decrease was observed in root H2O2 and MDA levels between the FA treatment and CK. Relative to CK, the Cd treatment largely elevated the contents of H2O2 and MDA in leaves, which were 2.46 (H2O2) and 1.68 (MDA) times that of CK, respectively. However, under Cd stress condition, the increases of the leaves’ H2O2 and MDA levels induced by Cd stress were weakened by FA spraying application, which were decreased by 35.83% and 23.33%, respectively, compared to that in the Cd treatment.
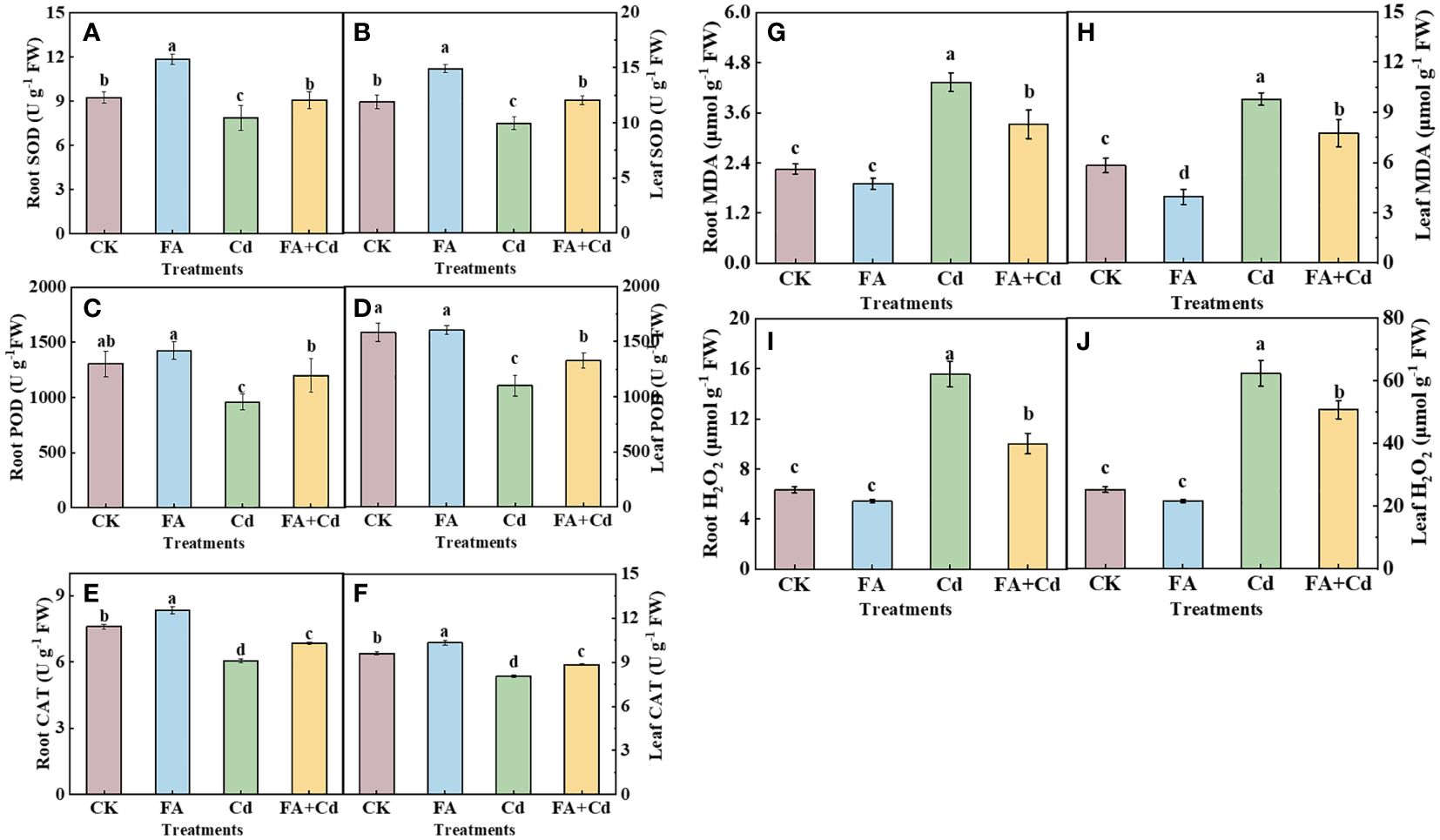
Figure 2 SOD, POD, and CAT activities in roots (A, C, E) and leaves (B, D, F) as well as MDA and H2O2 contents in roots (G, H) and leaves (I, J). The different letters indicate statistically significant differences (P< 0.05).
Subsequently, we determined the SOD, POD, and CAT activities in leaves and roots, respectively, and observed that the activities of enzymes mentioned above were both decreased under Cd exposure condition relative to CK (Figures 2A-F). In contrast, a promoting effect was found under the FA treatment compared to that of CK, showing higher SOD and CAT activities in roots and SOD activity in leaves than that of CK. Meanwhile, the Cd-induced decreases of enzyme activities were both significantly weakened when FA was sprayed on the Cd-treated seedlings’ leaves.
Photosynthetic characteristics
Under conditions without Cd, FA spraying application increased the leaf Pn and Gs by 9.63% (Pn) and 8.10% (Gs) compared to CK (Figures 3A, B). However, 15 days of exposure to Cd stress resulted in an apparent decrease in Pn and Gs compared to CK. This Cd-induced reduction in Pn and Gs was alleviated by FA treatment. The Pn and Gs values under Cd+FA were 25.29% and 17.03% higher than that under Cd alone. Furthermore, Cd significantly decreased the Fv/Fm value compared to CK (0.92 times that of CK), while FA spraying application mitigated the Cd-induced inhibition of Fv/Fm (Figure 3C). Different from the changes in the Fv/Fm among the CK, Cd, and Cd+FA, no significant difference was observed between the CK and the FA treatments. The Cd+FA treatment also elevated the ETR in seedlings compared with the Cd treatment (Cd addition alone); the ETR under the Cd+FA treatment was 31.74% higher than that under the Cd treatment. However, NPQ showed an opposite trend in the apple seedlings. The highest NPQ was detected in the seedlings under Cd treatment (Cd), while the application of FA spraying under Cd stress decreased NPQ compared to Cd alone (Figure 3E).
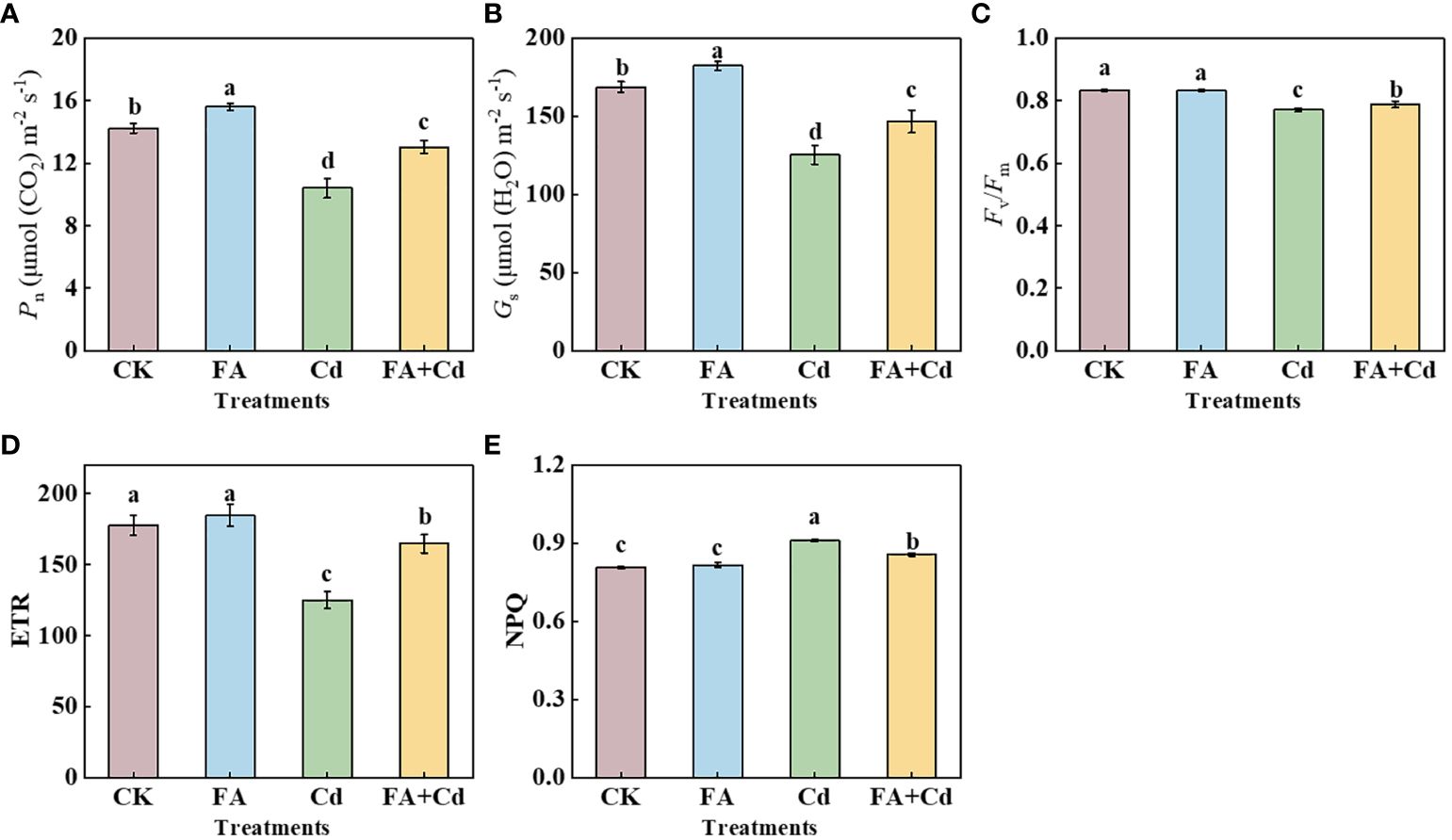
Figure 3 Pn (A), Gs (B), Fv/Fm (C), ETR (D), and NPQ (E) in leaves under different treatments. CK, control—nutrient solution alone; FA, nutrient solution with FA; Cd, nutrient solution containing Cd; Cd+FA, nutrient solution containing Cd and the spraying application of FA. The vertical bars on the histograms indicate ± SD. The different letters indicate statistically significant differences (P< 0.05).
C metabolism-related enzymes
Among all the treatments, the leaves’ Rubisco activity was highest in the FA treatment, and the lowest was obtained under the Cd treatment. Compared with the Cd treatment, the Rubisco activity of leaves was elevated by 20.50% in the Cd+FA treatment (Figure 4A). Moreover, under Cd stress condition, FA spraying application weakened the Cd-induced inhibition in the activity of these enzymes in roots (Figures 4B-D), which were elevated by 22.00% (SDH), 26.53% (HK), and 16.25% (FRK), respectively, compared to the Cd treatment.

Figure 4 Rubisco activity in leaves (A) and SDH, FRK, and HK activities in roots (B–D) under different treatments. CK, control—nutrient solution alone; FA, nutrient solution with FA; Cd, nutrient solution containing Cd; Cd+FA, nutrient solution containing Cd and the spraying application of FA. The vertical bars on the histograms indicate ± SD. The different letters indicate statistically significant differences (P< 0.05).
13C accumulation, distribution, and transportation
The results of 13C labeling indicated that Cd addition and FA spraying applications significantly influenced the 13C accumulation and 13C distribution rate in each organ. As shown in Figures 5A–C, compared with CK, the application of FA spraying significantly elevated the 13C accumulation in seedlings. In contrast, under stress conditions, compared with FA absence treatment (Cd addition alone), the 13C accumulation in seedlings was significantly elevated when FA was sprayed, which was increased by 40.91% (root), 25.58% (stem), and 9.20% (leaf), respectively, compared to that in the Cd treatment. Subsequently, we further observed that, regardless of the treatment of the study, the highest 13C distribution rate was detected in the leaves, followed by the stem, and the lowest in the roots. The Cd treatment resulted in the lowest 13C distribution rate in roots and the highest in leaves, while an opposite trend was observed under FA treatment. Besides this, treating apple seedlings under Cd stress with FA significantly increased the 13C distribution rate in roots and decreased that in leaves compared with the Cd treatment, which was 22.22% (13C distribution rate in roots) higher and 5.30% (13C distribution rate in leaves) lower, respectively, than that of the Cd treatment (Figures 5D, E).
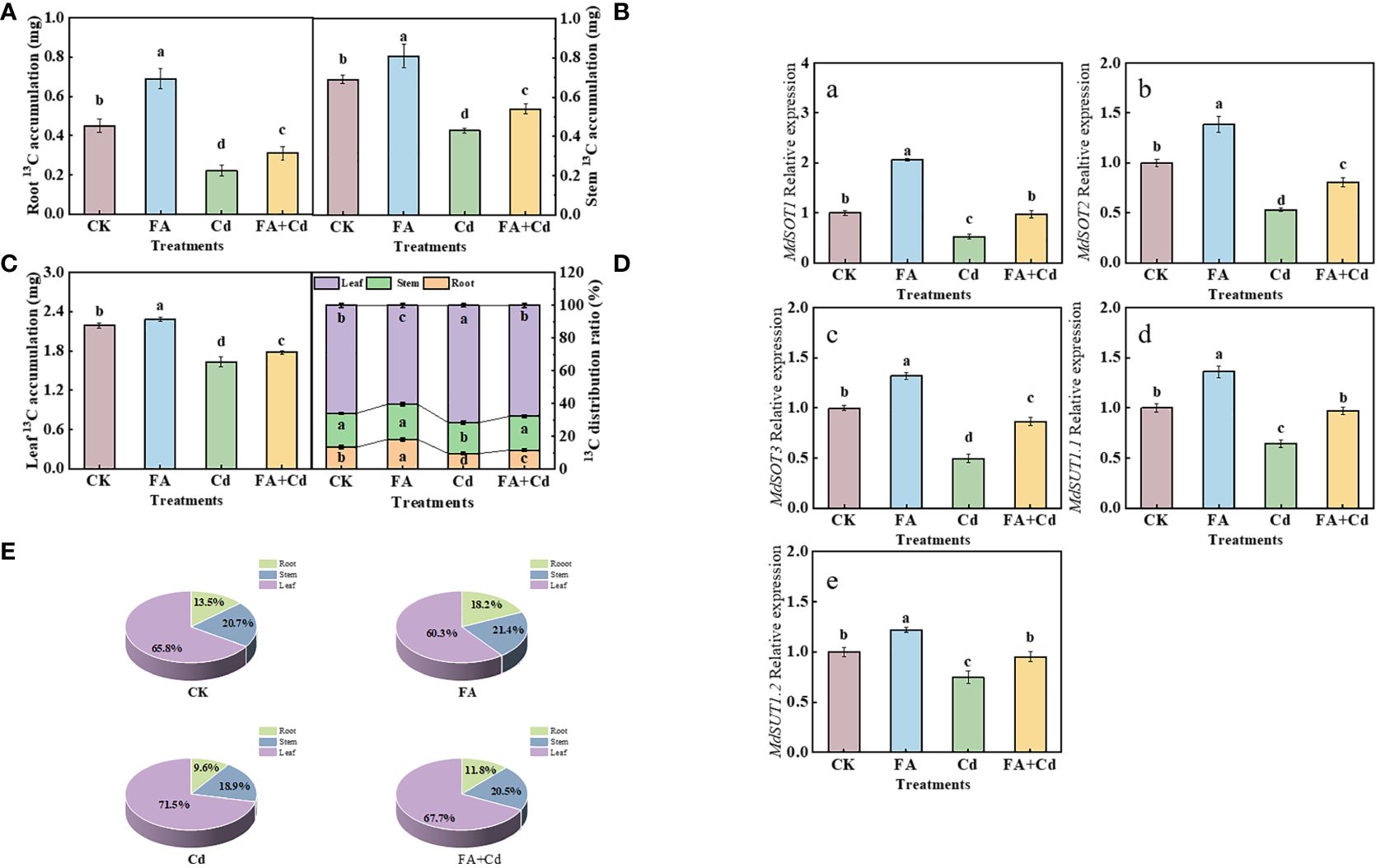
Figure 5 13C accumulation of seedlings (A–C), organs’ 13C distribution rate (D, E) as well as roots’ MdSOTs and MdSUTs gene expression (A–E) under different treatments. CK, control—nutrient solution alone; FA, nutrient solution with FA; Cd, nutrient solution containing Cd; Cd+FA, nutrient solution containing Cd and the spraying application of FA. The vertical bars on the histograms indicate ± SD. The different letters indicate statistically significant differences (P< 0.05).
We measured three MdSOTs (MdSOT1, MdSOT2, and MdSOT3) and two MdSUTs (MdSUT1.1 and MdSUT1.2) expression levels in this study and observed that these five genes’ expression levels were all upregulated by FA spraying application under non-Cd addition condition, relative to CK (Figure 5A-E). Meanwhile, Cd stress downregulated the expression of these genes. However, FA spraying application alleviated this reduction in gene expression under Cd exposure.
N metabolism-related enzymes
Compared with CK, the NR activities in leaves and roots, the GS activity in roots, and the GOGAT activities in the leaves and roots of FA-treated-apple seedlings were 32.00%, 25.24%, 17.65%, 28.03%, and 24.36% higher than those under CK. The Cd treatment also significantly influenced the activity of these enzymes. Compared with CK, Cd stress reduced the activities of NR, GS, and GOGAT in leaves and roots. However, FA spraying application alleviated the negative effect of Cd on the enzymes (Figures 6A-F).
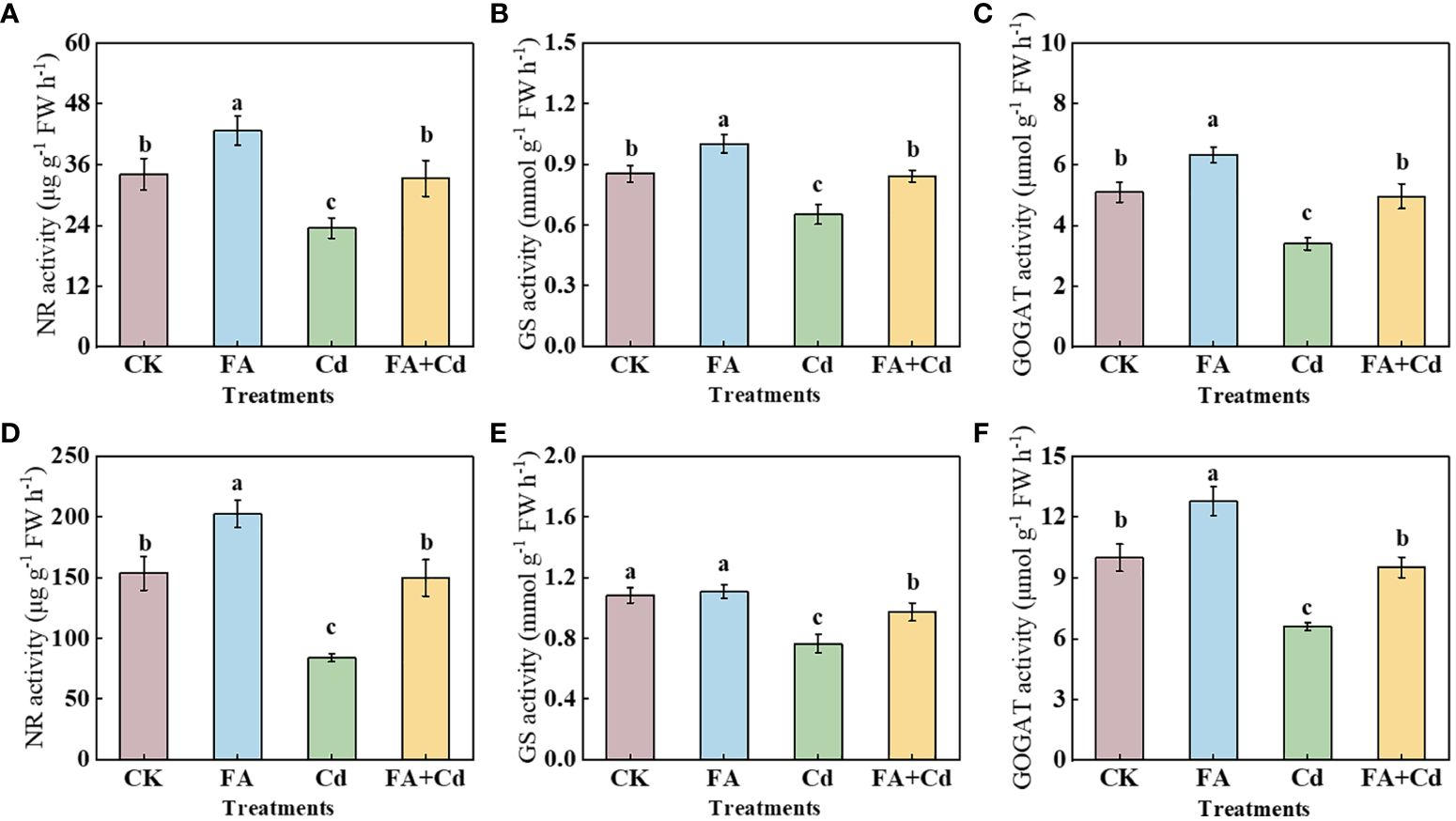
Figure 6 Activities of NR, GS, and GOGAT in leaves (D–F) and roots (A–C) under different treatments. CK, control—nutrient solution alone; FA, nutrient solution with FA; Cd, nutrient solution containing Cd; Cd+FA, nutrient solution containing Cd and the spraying application of FA. The vertical bars on the histograms indicate ± SD. The different letters indicate statistically significant differences (P< 0.05).
NRT gene expressions in roots
Four MdNRTs (MdNRT1.1, MdNRT1.2, MdNRT1.5, and MdNRT2.1) gene expression levels were analyzed in this study. Compared with CK, the FA treatment upregulated the expression of all these genes, in the apple seedlings, while the Cd treatment downregulated their expression (Figures 7A-D). Moreover, although the expression levels of these genes under the Cd+FA treatment were higher than those under the Cd treatment, their expression levels were still lower than that of CK.
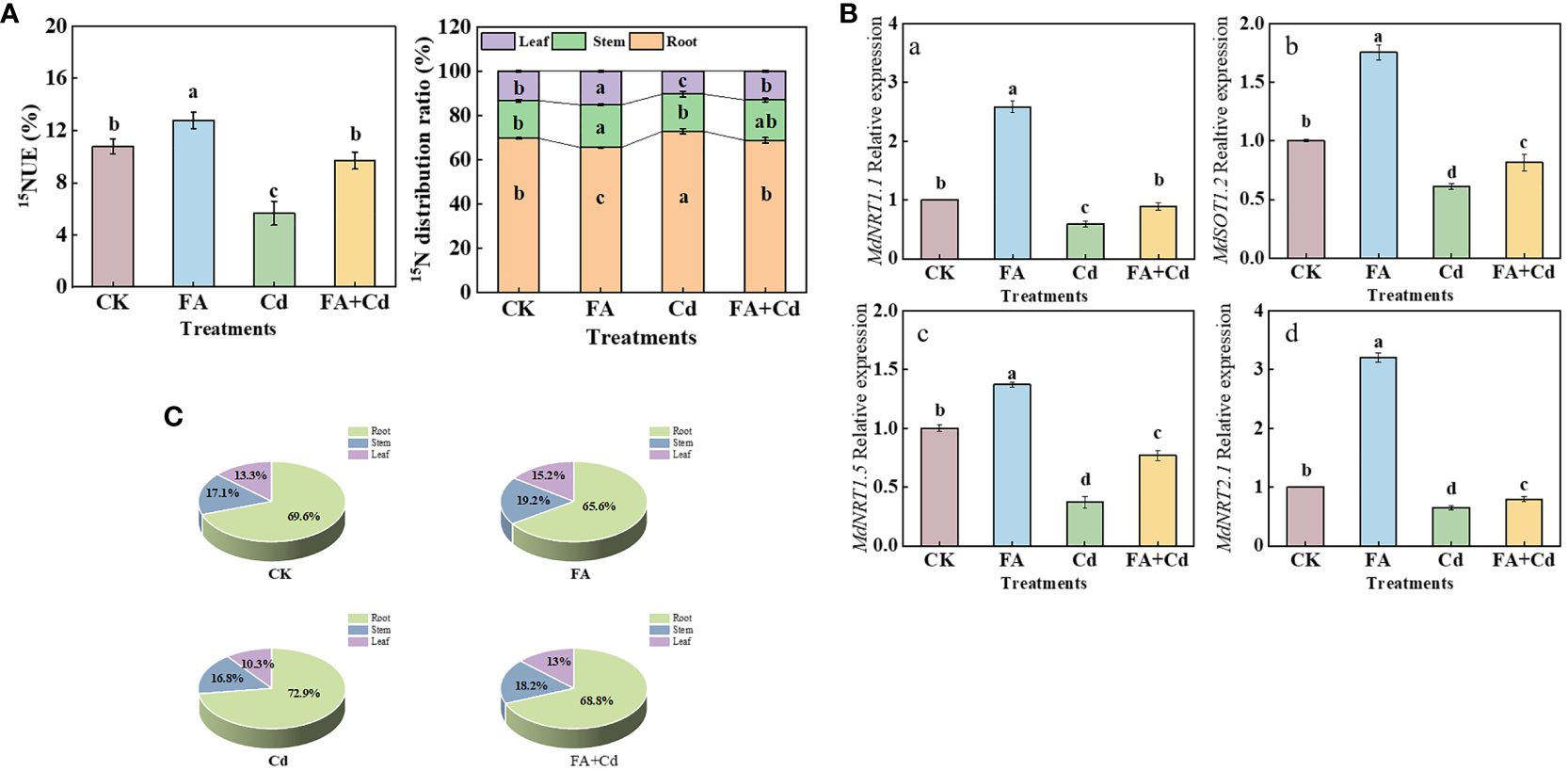
Figure 7 15NUE of seedlings (A), organs’ 15N distribution rate (B, C), and the MdNRTs gene expression (a–e) in roots under different treatments. CK, control—nutrient solution alone; FA, nutrient solution with FA; Cd, nutrient solution containing Cd; Cd+FA, nutrient solution containing Cd and the spraying application of FA. The vertical bars on the histograms indicate ± SD. The different letters indicate statistically significant differences (P< 0.05).
15N accumulation and 15N distribution ratio
As shown in Figure 7A, the FA treatment resulted in the highest 15NUE value (1.18 times that of CK), while the Cd treatment resulted in the lowest (0.52 times that of CK). Compared with the Cd treatment, 15NUE value was increased by the Cd+FA treatment, which was 71.38% higher than that of Cd treatment. Subsequently, we measured the organs’ 15N distribution ratio and observed that, under Cd treatment, the 15N distribution ratio in leaves was significantly lower than that of CK, which was 0.78 times that of CK. However, FA spraying application under Cd exposure condition significantly optimized the 15N distribution ratio in leaves and roots. Compared with the Cd treatment, the Cd+FA treatment significantly increased the 15N distribution ratio in leaves (26.09% higher than that of Cd treatment) and decreased that in roots (Figures 7B, C).
Discussion
FA spraying application improves the antioxidant system in apple plants under Cd stress
Dry weight is an important feature that reflects the growth characteristics of plants. Researchers analyze this crucial feature to assess the influence of different treatments on the growth of seedlings. The present study found that Cd stress decreased the dry weight of roots and all aboveground parts of the apple seedlings, indicating suppressed seedling growth under Cd stress, which is consistent with the findings of Zhou et al. (2016, 2017). In contrast, the negative effect caused by Cd stress was significantly reduced by FA spraying application. Seedlings treated with the Cd+FA treatment presented a higher dry weight than that of the Cd treatment (Cd addition without FA application). These results were similar with those of Wang et al. (2019) in lettuce. The root system provides support for the body of the plant, and the water and nutrients required for the process of plant growth are taken up from the external environment by the root system. Moreover, roots are, in most cases, the first to be exposed to abiotic stressors in the external environment, and the response of roots (the key organ system absorbing nutrients) to changes in the external environment could be the initial driving force for influencing plant growth (Vadez, 2014; Shi et al., 2023). Therefore, improving the growth and development of root under conditions of stress may effectively enhance the tolerance of apple plants. We further analyzed the effect of Cd stress and FA application on the root morphology parameters of apple seedlings. Similar to the changes in root dry weight, the RL and RSA of apple seedlings decreased after exposure to Cd stress (Figure 1B), indicating that root growth was inhibited by Cd stress. Previous studies have pointed out that the production and scavenging of reactive oxygen species (ROS) occur in a relatively stable state (El-Shabrawi et al., 2010). The present study also found that the levels of MDA and H2O2 in roots under Cd stress were obviously higher than that of CK, indicating that Cd stress (the abiotic stressor in this study) increased the ROS production. Besides this, the Cd treatment also decreased the activities of SOD, POD, and CAT in roots, indicating a decrease in ROS scavenging ability and an imbalance in ROS in the roots of the seedlings under Cd stress. However, FA spraying application decreased the levels of MDA and H2O2 but increased the activities of SOD, POD, and CAT in apple seedlings under Cd stress (Figure 2), indicating that FA weakened the Cd-induced root growth inhibition by enhancing the scavenging of ROS and maintaining a balance in ROS production and scavenging. Moreover, FA downregulates the gene expression of transporter proteins and reduces Cd uptake under Cd stress, which could be another important reason to explain the response of root growth and development to Cd stress and FA application (Chen et al., 2022).
FA spraying application enhances C metabolism in apple seedlings under Cd stress condition
As the basic life activity of plants, previous studies had already pointed out that Cd stress could inhibit leaf photosynthesis (He et al., 2020). In this study, the Pn and Gs values of leaves decreased under Cd stress (Figures 3A, B). In contrast, these inhibitions were both reduced when FA was applied in a Cd stress environment, which was consistent with the study of Chen et al. (2022). The Gs under the Cd+FA treatment was higher than that under the Cd treatment, indicating that the leaf CO2 absorption ability could be elevated by FA application in the Cd stress condition to a certain degree. These results may be beneficial to explain the difference in seedling total 13C accumulation treated with Cd or FA spraying (Figure 5A). In the present study, we observed that Cd stress significantly decreased the value of ETR, which was consistent with the findings obtained by Gong et al. (2017), who reported that one of the sites for Cd destruction was the electron transport chain. In addition, the study detected that the ETR was elevated under the Cd+FA treatment compared to the Cd treatment (Figures 3C-E), indicating that the damage Cd stress caused to the electron transfer chain was mitigated by FA spraying application. These benefits (the stability of the photosynthetic apparatus and the efficient transport of electron transfer) caused by FA application under Cd stress condition could provide a basis (a stable reducing force) for the enhancement of photosynthesis and photosynthesis media C (carbon) assimilation.
The CO2 (C) assimilation and the transport of photoassimilates are the basis of plant organ growth (Merlo and Passera, 1991; Lambers et al., 2002; Sha et al., 2020; Xu et al., 2023). Detailed analysis revealed that Cd treatment reduced the activity of Rubisco, indicating that the C assimilation could be somewhat inhibited under Cd stress condition. These results might be favorable to analyze why seedlings treated with the Cd treatment had a lower 13C accumulation than that of CK. According to the results presented by 13C labeling, we found that 13C accumulation of seedlings under the Cd treatment was obviously lower than that of CK. In contrast, this reduction was significantly decreased by FA spraying application under Cd stress condition (Figures 5A-C). The reason might be related with the elevation of Rubisco activity and light energy absorption and harvesting under the Cd+FA treatment (Figure 4A). The transport of photoassimilates has a vital role on root system construction and the ability of nutrient absorption by the root system (Xu et al., 2020; Yu et al., 2023b). Lyu et al. (2023) observed that the inhibition of the transport of photoassimilates and the weakness of leaf C assimilation caused by stress conditions could negatively influence the photosynthetic electron transport chain and result in an elevation of ROS levels. In this study, we found that the Cd treatment resulted in the highest leaf 13C distribution rate, the lowest root 13C distribution rate as well as the lowest seedling 13C accumulation (Figures 5D, E). These results could somewhat help to explain why seedlings treated with the Cd treatment had a higher H2O2 level in leaves and poor photosynthetic performance than other treatments. However, compared with the Cd treatment, the Cd+FA treatment significantly increased the seedlings’ root 13C accumulation. These observations collectively suggest that Cd stress inhibited the leaf-to-root translocation of photoassimilates, while FA spraying application alleviated this inhibition, which could be conducive to explain why seedlings treated with the Cd+FA treatment had a higher root dry weight than those of the Cd treatment to a certain degree (Figure 2B). MdSOTs and MdSUTs are extensively involved in the translocation of photoassimilates (Zhao et al., 2020). We observed that the root MdSOT1, MdSOT2, MdSOT3, MdSUT1.1 and MdSUT1.2 gene expressions were downregulated by Cd addition relative to the CK (Figures 5A-E). Moreover, we noticed that the roots’ SDH, FRK, and HK activities were also decreased by the Cd treatment. In contrast, these reductions were weakened by FA spraying application under Cd stress condition (Figures 4B-D). Combined with relevant research progress obtained in this study, we summarized as follows: Firstly, FA spraying application under Cd stress obviously optimized the leaf photosynthetic performance and reduced the negative influence on leaf C assimilation caused by Cd stress and enhanced the leaf photosynthetic product synthesis capacity, thus elevating source strength. Secondly, root sugar metabolism enzyme activities were elevated by the Cd+FA treatment relative to the Cd treatment, indicating that the process of root sugar metabolism was enhanced, which not only provides energy for root growth but also enhances the competitiveness of the root for photosynthetic products and promoting the translocation of photoassimilates to a certain extent. Finally, the upregulation of root sugar transport-related gene expression under the Cd+FA treatment could also be conducive to explain the enhancement of the leaf to root photoassimilate transport. In conclusion, the role of FA in promoting seedling root growth under Cd stress condition can be expressed through the optimization of leaf photosynthesis, root C metabolism process as well as the distribution of photoassimilates.
FA spraying application enhances N metabolism in apple seedling under Cd stress condition
N, an essential macro-nutrient, is closely related to various physiological and metabolic activities of plants, such as organ construction and leaf photosynthesis (Titus and Kang, 1982; Warner et al., 2004; Tavares et al., 2019; Xu et al., 2020). Improving the absorption and utilization of N by plants promotes growth and enhances stress tolerance under unfavorable conditions (Yuan et al., 2007). In this study, the results of 15N labeling showed that, compared with CK, the Cd treatment obviously decreased the seedling 15NUE value (Figure 7A), indicating that the seedlings’ N absorption and utilization were obviously inhibited under Cd stress condition. However, this inhibition induced by Cd stress was somewhat weakened by FA spraying application. One of the reasons might be due to the optimization of root morphology (RL and RSA) and the elevation of root activity by FA addition under Cd exposure condition (Figure 1B), which improved the absorption of N in nutrient solution by roots. The uptake of N by plants is an energy-consuming process (Bloom, 2015). In this study, we observed that the accumulation of 13C-photoassimilates in roots and sugar metabolism-related enzyme activities was enhanced by FA spraying application under Cd stress condition (Figures 4B–D, 5A), suggesting that the spraying of FA under Cd exposure condition can provide sufficient energy to support N uptake in the root system of apple seedlings by promoting the transport of photosynthetic products to the root system as well as increasing the intensity of sugar metabolism in the root system. Previous studies have reported that external environmental conditions could obviously influence the root NRT gene expression (Xing et al., 2021, 2022). Moreover, Tian et al. (2023) pointed out that the NRT gene expression level in root could determine the strength of nitrate uptake to a certain degree. Thus, changes in root NRT gene expression level in response to Cd exposure or FA spraying application may somewhat explain why FA-treated seedlings have higher 15NUE values than non FA-treated seedlings under Cd stress condition. In this study, under normal condition, compared with CK, FA spraying application promoted the expression of MdNRT1.1, MdNRT1.2, MdNRT1.5, and MdNRT2.1. Moreover, we observed that these genes’ expressions in roots were obviously downregulated under the Cd treatment relative to CK. In contrast, compared with the Cd treatment, the Cd+FA treatment significantly promoted the expression of MdNRT1.1, MdNRT1.2, MdNRT1.5, and MdNRT2.1 (Figures 7A-D), indicating that the strength of nitrate (the only N source in this experiment) absorption was promoted. Earlier studies performed by Xu et al. (2020, 2022) both observed that the accumulation of potassium (K) could be favorable to promote the upregulation of NRT gene expression in roots. Moreover, Bayat et al. (2021) observed that FA application could promote the absorption of K in yarrow (Achillea millefolium L). Therefore, the change of root NRT gene expression level between the Cd and the Cd+FA treatments might be related with the improvement of K absorption by plants under FA spraying application.
After being absorbed by roots, nitrate (NO3−) is assimilated through a range of N metabolism enzymes (Xu et al., 2012). As the key enzymes widely participate in the plants’ N metabolism process, the activities of NR, GS, and GOGAT could somewhat reflect the efficiency of plant N metabolism (Armengaud et al., 2009; Wen et al., 2019; Gao et al., 2022). We observed that, under stress condition, compared with CK, Cd stress treated-seedlings had lower NR, GS, and GOGAT activities in roots and leaves (Figure 6). These results indicated that Cd poisoning could reduce the plant N assimilation process. A further analysis showed that, compared with the Cd treatment, the Cd+FA treatment significantly minimized the negative influence on NR, GS, and GOGAT activities (Figure 6). The elevation of the leaves’ GS and GOGAT activities under the Cd+FA treatment might dissipate excess energy in leaves and reduce photodamage in chloroplasts, which might be favorable to explain why FA-treated seedlings under Cd stress condition had a better photosynthetic performance than that of Cd treatment. The root system is a typical non-photosynthetic tissue and the dominant site for taking up N from the plant. Therefore, the above-mentioned changes in the accumulation of 13C-photosynthetic products and the activities of enzymes involved in root sugar metabolism could, to some extent, explain the differences in the activities of root N metabolism enzymes among various treatments.
N is a massive mineral element that is closely linked to the photosynthesis of the apple plant (Xu et al., 2020). Leaves are the main site of photosynthesis in plants. The enzymes and photosynthetic pigments required for photosynthesis are provided by N metabolism (Reguera et al., 2013). Scholars such as Xu et al. (2022) and Yu et al. (2023b) both pointed out that the alleviation of stress-induced root growth inhibition was closely related to the strength of the root-to-leaf transport of N in plant. The 15N labeling results showed that the lowest leaf 15N distribution ratio was observed under the Cd treatment (Figures 7B, C), indicating that the bottom (root) to top (leaf) transport of N was significantly inhibited. These results could also somewhat explain why Cd stress-treated seedlings had a poor photosynthetic performance. In contrast, the inhibition caused by Cd poisoning was reduced by FA spraying application. Xu et al. (2022) observed a positive correlation between NRT1.5 gene expression level and root-to-leaf translocation of N. Therefore, a higher NRT1.5 gene expression level under the Cd+FA treatment could be conducive to explain why seedlings treated with Cd+FA had a higher leaf N distribution rate than that of the Cd-treated ones (Figure 7D).
Conclusion
In conclusion, the FA alleviation in Cd-induced apple seedlings’ root growth inhibition was associated with the following characteristics (Figure 8): (i) the enhanced antioxidant enzyme activities in roots and leaves, (ii) the enhanced leaf photosynthesis as well as the elevated C and N metabolism-related enzyme activity, and (iii) the more rational distribution of C and N in seedlings. Overall, this study offers a fresh train of thought into the promotion of Cd stress-treated apple plants’ root growth caused by FA spraying application.
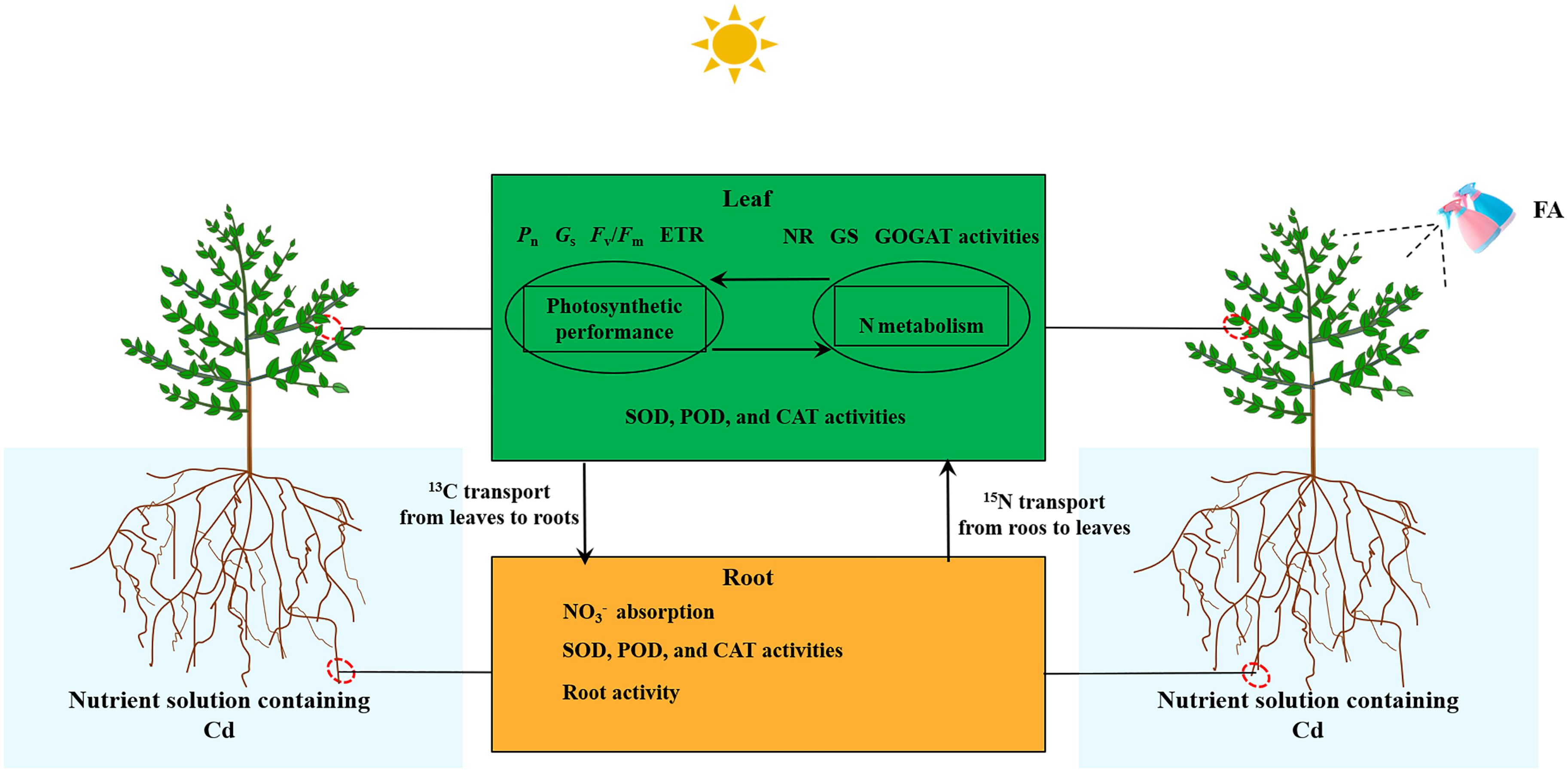
Figure 8 Schematic model displaying the role of FA-mediated alleviation in Cd-induced root growth inhibition in apple seedlings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Files, further inquiries can be directed to the corresponding author.
Author contributions
BY: Methodology, Writing – original draft, Writing – review & editing, Data curation, Formal Analysis. XX: Methodology, Writing – review & editing. PN: Conceptualization, Writing – review & editing. NL: Writing – review & editing. LW: Funding acquisition, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the China Agriculture Research System of MOF and MARA (CARS-27).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2024.1370637/full#supplementary-material
References
Ali, S., Bharwana, S. A., Rizwan, M., Farid, M., Khan, M. D. (2015). Fulvic acid mediates chromium (Cr) tolerance in wheat (Triticum aestivum L.) through lowering of Cr uptake and improved antioxidant defense system. Environ. Sci. pollut. Res. 22, 10601–10609. doi: 10.1007/s11356-015-4271-7
Anjum, S., Wang, L., Farooq, M., Xue, L., Ali, S. (2011). Fulvic acid application improves the maize performance under well-watered and drought conditions. J. Agron. Crop Sci. 197, 409–417. doi: 10.1111/j.1439-037X.2011.00483.x
Argüello, D., Chavez, E., Lauryssen, F., Vanderschueren, R., Smolders, E., Montalvo, D. (2019). Soil properties and agronomic factors affecting cadmium concentrations in cacao beans: a nationwide survey in Ecuador. Sci. Total Environ. 649, 120–127. doi: 10.1016/j.scitotenv.2018.08.292
Armengaud, P., Sulpice, R., Miller, A. J., Stitt, M., Amtmann, A., Gibon, Y. (2009). Multilevel analysis of primary metabolism provides new insights into the role ofpotassium nutrition for glycolysis and nitrogen assimilation in Arabidopsis roots. Plant Physiol. 150, 772–785. doi: 10.1104/pp.108.133629
Bayat, H., Shafie, F., Aminifard, M. H., Daghighi, S. (2021). Comparative effects of humic and fulvic acids on growth, antioxidant activity and nutrient content of yarrow (Achillea millefolium L). Sci. Hortic. 279, 109912. doi: 10.1016/j.scienta.2021.109912
Bloom, A. J. (2015). Photorespiration and nitrate assimilation: a major intersection between plant carbon and nitrogen. Photosynthesis Res. 123, 117–128. doi: 10.1007/s11120-014-0056-y
Canellas, L. P., Olivares, F. L., Aguiar, N. O., Jones, D. L., Nebbioso, A., Mazzei, P., et al. (2015). Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 196, 15–27. doi: 10.1016/j.scienta.2015.09.013
Chen, G. D., Wang, L., Fabrice, M. R., Tian, Y. N., Qi, K. J., Chen, Q., et al. (2018). Physiological and nutritional responses of pear seedlings to nitrate concentrations. Front. Plant Sci. 9, 1679. doi: 10.3389/fpls.2018.01679
Chen, X. J., Zhang, X., Chen, H., Xu, X. M. (2022). Physiology and proteomics reveal Fulvic acid mitigates Cadmium adverse effects on growth and photosynthetic properties of lettuce. Plant Sci. 323, 111418. doi: 10.1016/j.plantsci.2022.111418
Dinler, B. S., Gunduzer, E., Tekinay, T. (2016). Pre-treatment of fulvic acid plays a stimulant role in protection of soybean (Glycine max L.) leaves against heat and salt stress. Acta Biol. Crac. Ser. Bot. 58. doi: 10.1515/abcsb-2016-0002
Duan, Q., Lee, J., Liu, Y., Chen, H., Hu, H. (2016). Distribution of heavy metal pollution in surface soil samples in China: a graphical review. B Environ. Contam Tox 97, 303–309. doi: 10.1007/s00128-016-1857-9
El-Shabrawi, H., Kumar, B., Kaul, T., Reddy, M. K., Singla-Pareek, S. L., Sopory, S. K. (2010). Redox homeostasis, antioxidant defense, and methylglyoxal detoxification asmarkers for salt tolerance in Pokkali rice. Protoplasma 245, 85–96. doi: 10.1007/s00709-010-0144-6
Gao, F., Li, Z., Du, Y., Duan, J., Zhang, T., Wei, Z., et al. (2022). The combined application of urea and fulvic acid solution improved maize carbon and nitrogen metabolism. Agronomy 12, 1400. doi: 10.3390/agronomy12061400
Gong, B. A., Nie, W. J., Yan, Y. Y., Gao, Z. X., Shi, Q. H. (2017). Unravelling cadmium toxicity and nitric oxide induced tolerance in Cucumis sativus: Insight into regulatory mechanisms using proteomics. J. Hazard. Mater 336, 202–213. doi: 10.1016/j.jhazmat.2017.04.058
Han, Y. L., Song, H. X., Liao, Q., Yu, Y., Jian, S. F., Lepo, J. E., et al. (2016). Nitrogen use efficiency is mediated by vacuolar nitrate sequestration capacity in roots of Brassica napus. Plant Physiol. 170, 1684–1698. doi: 10.1104/pp.15.01377
Hasan, M. K., Ahammed, G. J., Yin, L., Shi, K., Xia, X., Zhou, Y., et al. (2015). Melatonin mitigates cadmium phytotoxicity through modulation of phytochelatins biosynthesis, vacuolar sequestration, and antioxidant potential in Solanum lycopersicum L. Front. Plant Sci. 6, 601. doi: 10.3389/fpls.2015.00601
He, J., Qin, J., Long, L., Ma, Y., Li, H., Li, K., et al. (2011). Net cadmium flux and accumulation reveal tissue-specific oxidative stress and detoxification in Populus×canescens. Physiol. Plant 143, 50–63. doi: 10.1111/j.1399-3054.2011.01487.x
He, J., Zhuang, X., Zhou, J., Sun, L., Wan, H., Li, H., et al. (2020). Exogenous melatonin alleviates cadmium uptake and toxicity in apple rootstocks. Tree Physiol. 40, 746–761. doi: 10.1093/treephys/tpaa024
Hoagland, D. R., Arnon, D. I. (1950). The water-culture method for growing plants without soil. Calif. Agric. Exp. Station Circ. 347, 1–32. doi: 10.1016/S0140-6736(00)73482-9
Hu, W., Jiang, N., Yang, J., Meng, Y., Wang, Y., Chen, B., et al. (2016a). Potassium (K) supply affects K accumulation and photosynthetic physiology in two cotton (Gossypium hirsutum L.) cultivars with different K sensitivities. Field Crop Res. 196, 51–63. doi: 10.1016/j.fcr.2016.06.005
Hu, W., Zhao, W., Yang, J., Oosterhuis, D. M., Loka, D. A., Zhou, Z. (2016b). Relationship between potassium fertilization and nitrogen metabolism in the leaf subtending the cotton (Gossypium hirsutum l.) boll during the boll development stage. Plant Physiol. Biochem. 101, 113–123. doi: 10.1016/j.plaphy.2016.01.019
Kaya, C., Okant, M., Ugurlar, F., AlYemeni, M. N., Ashraf, M., Ahmad, P. (2019). Melatonin-mediated nitric oxide improves tolerance to cadmium toxicity by reducing oxidative stress in wheat plants. Chemosphere 225, 627–638. doi: 10.1016/j.chemosphere.2019.03.026
Lambers, H., Atkin, O., Millenaar, F. (2002). “Respiratory patterns in roots in relation totheir functioning,” in Plant Roots, Hidden Half. Eds. Waisel, Y., Eshel, A., Kafkaki, K. (MarcelDekker Inc, NewYork, NY, USA), 521–552.
Lei, Y., Korpelainen, H., Li, C. (2007). Physiological and biochemical responses to high Mn concentrations in two contrasting populus cathayana populations. Chemosphere 68, 686–694. doi: 10.1016/j.chemosphere.2007.01.066
Li, M. J., Feng, P. M., Cheng, L. L. (2012). Expression patterns of genes involved in sugar metabolism and accumulation during apple fruit development. PloS One 7, e33055. doi: 10.1371/journal.pone.0033055
Liu, J., Lyu, M., Xu, X., Liu, C., Qin, H., Tian, G. (2022). Exogenous sucrose promotes the growth of apple rootstocks under high nitrate supply by modulating carbon and nitrogen metabolism. Plant Physiol. Bioch. 192, 196–206. doi: 10.1016/j.plaphy.2022.10.005
Lotfi, R., Pessarakli, M., Gharavi-Kouchebagh, P., Khoshvaghti, H. (2015). Physiological responses of Brassica napus to fulvic acid under water stress: Chlorophyll a fluorescence and antioxidant enzyme activity. Crop J. 3, 434–439. doi: 10.1016/j.cj.2015.05.006
Luo, Z. B., He, J., Polle, A., Rennenberg, H. (2016). Heavy metal accumulation and signal transduction in herbaceous and woody plants: paving the way for enhancing phytoremediation efficiency. Biotechnol. Adv. 34, 1131–1148. doi: 10.1016/j.biotechadv.2016.07.003
Lyu, M. X., Liu, J. Q., Xu, X. X., Liu, C. L., Qin, H. H., Zhang, X. L., et al. (2023). Magnesium alleviates aluminum-induced growth inhibition by enhancing antioxidant enzyme activity and carbon-nitrogen metabolism in apple seedlings. Ecotoxicol. Saf. 249, 114421.
Merlo, L., Passera, C. (1991). Changes in carbohydrate and enzyme levels during development of leaves of prunus persica, a sorbitol synthesizing species. Plant Physiol. 83, 621–626. doi: 10.1111/j.1399-3054.1991.tb02478.x
Parvaiz, A., Abdel, L. A. A., Abd_Allah, E. F., Abeer, H., Maryam, S., Anjum, N. A., et al. (2016). Calcium and potassium supple mentation enhanced growth, osmolyte secondary metabolite production, and enzymatic antioxidant machinery in cadmium-exposed chickpea (Cicer arietinum L.). Front. Plant Sci. 7, 1–12. doi: 10.3389/fpls.2016.00513
Podazza, G., Arias, M., Prado, F. E. (2016). Early interconnectivity between metabolic and defense events against oxidative stress induced by cadmium in roots of four citrus rootstocks. Funct. Plant Biol. 43, 973–985. doi: 10.1071/FP16153
Reguera, M., Peleg, Z., Abdel-Tawab, Y. M., Tumimbang, E. B., Delatorre, C. A., Blumwald, E. (2013). Stress-induced cytokinin synthesis increases drought tolerance through the coordinated regulation of carbon and nitrogen assimilation in Rice. Plant Physiol. 163, 1609–1622. doi: 10.1104/pp.113.227702
Ren, J., Xie, T., Wang, Y., Li, H., Liu, T., Zhang, S., et al. (2020). Coordinated regulation of carbon and nitrogen assimilation confers drought tolerance in maize (Zea mays L.). Environ. Exp. Bot. 176, 104086. doi: 10.1016/j.envexpbot.2020.104086
Ren, J., Yang, X., Ma, C., Wang, Y., Zhao, J. (2021). Melatonin enhances drought stress tolerance in maize through coordinated regulation of carbon and nitrogen assimilation. Plant Physiol. Biochem. 167, 958–969. doi: 10.1016/j.plaphy.2021.09.007
Rufly, T. W., Huber, S. C. (1983). Changes in starch formation and activities of sucrose phosphate synthase and cytoplasmic fructose-1,6-biosphatase in response to source-sink alteration. Plant Physiol. 72, 474–478. doi: 10.1104/pp.72.2.474
Sha, J., Wang, F., Xu, X., Chen, Q., Zhu, Z., Jiang, Y., et al. (2020). Studies on the translocation characteristics of 13C-photoassimilates to fruit during the fruit development stage in ‘fuji’ apple-sciencedirect. Plant Physiol. Biochem. 154, 636–645. doi: 10.1016/j.plaphy.2020.06.044
Shi, J., Xun, M., Song, J., Li, J., Zhang, W., Yang, H. (2023). Multi-walled carbon nanotubes promote the accumulation, distribution, and assimilation of 15N-KNO3 in Malus hupehensis by entering the roots. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1131978
Suh, H. Y., Yoo, K. S., Sang, G. S. (2014). Effect of foliar application of fulvic acid on plant growth and fruit quality of tomato (Lycopersicon esculentum L.). Hortic. Environ. Biotechnol. 55, 455–461. doi: 10.1007/s13580-014-0004-y
Tavares, O. C. H., Santos, L. A., Araújo, O. J. L., Bucher, C. P. C., García, A. C., Arruda, L. N., et al. (2019). Humic acid as a biotechnological alternative to increase N-NO3- or N-NH4+ uptake in rice plants. Biocatalysis Agric. Biotechnol. 20, 101226. doi: 10.1016/j.bcab.2019.101226
Tian, G., Liu, C., Xu, X., Xing, Y., Liu, J., Lyu, M., et al. (2023). Effects of magnesium on nitrate uptake and sorbitol synthesis and translocation in apple seedlings. Plant Physiol. Bioch. 196, 139–151. doi: 10.1016/j.plaphy.2023.01.033
Titus, J. S., Kang, S. M. (1982). Nitrogen metabolism, translocation, and recycling in apple trees. Hortic. Rev. 4, 204–246. doi: 10.1002/9781118060773.ch7
Vadez, V. (2014). Root hydraulics: the forgotten side of roots in drought adaptation. Field Crops Res. 165, 15–24. doi: 10.1016/j.fcr.2014.03.017
Wang, W., Cang, L., Zhou, D. M., Yu, Y. C., et al. (2016). Exogenous amino acids increase antioxidant enzyme activities and tolerance of rice seedlings to cadmium stress. Environ. Prog. Sustain. Energy 36, 155–161. doi: 10.1002/ep.12474
Wang, Y. M., Yang, R. X., Zheng, J. Y., Shen, Z. G., Xu, X. M. (2019). Exogenous foliar application of fulvic acid alleviate cadmium toxicity in lettuce (Lactuca sativa L.). Ecotoxicol. Environ. Saf. 167, 10–19. doi: 10.1016/j.ecoenv.2018.08.064
Warner, J., Zhang, T. Q., Hao, X. (2004). Effects of nitrogen fertilization on fruit yield and quality of processing tomatoes. Can. J. Plant Sci. 84, 865–871. doi: 10.4141/P03-099
Wen, B. B., Li, C., Fu, X. L., Li, D. M., Gao, D. S. (2019). Effects of nitrate deficiency on nitrate assimilation and chlorophyll synthesis of detached apple leaves. Plant Physiol. Biochem. 142, 363–371. doi: 10.1016/j.plaphy.2019.07.007
Xing, J., Wang, Y., Yao, Q., Zhang, Y., Zhang, M., Li, Z. (2022). Brassinosteroids modulate nitrogen physiological response and promote nitrogen uptake in maize (Zea mays L.). Crop J. 10, 166–176. doi: 10.1016/j.cj.2021.04.004
Xing, Y., Zhu, Z., Wang, F., Zhang, X., Li, B., Liu, Z., et al. (2021). Role of calcium as a possible regulator of growth and nitrate nitrogen metabolism in apple dwarf rootstock seedlings. Sci. Hortic. 276, 109740. doi: 10.1016/j.scienta.2020.109740
Xu, G. H., Fan, X. R., Miller, A. J. (2012). Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 63, 153–182. doi: 10.1146/annurev-arplant-042811-105532
Xu, X. X., Du, X., Wang, F., Sha, J. C., Chen, Q., Tian, G., et al. (2020). Effects of potassium levels on plant growth, accumulation and distribution of carbon, and nitrate metabolism in apple dwarf rootstock seedlings. Front. Plant Sci. 11, 904. doi: 10.3389/fpls.2020.00904
Xu, X., Wang, F., Xing, Y., Liu, J., Lv, M., Meng, H., et al. (2022). Appropriate and constant potassium supply promotes the growth of M9T337 apple rootstocks by regulating endogenous hormones and carbon and nitrogen metabolism. Front. Plant Sci. 13, 827478. doi: 10.3389/fpls.2022.827478
Xu, X., Zhang, X., Liu, C., Qin, H., Sun, F., Liu, J., et al. (2023). Appropriate increasing potassium supply alleviates the inhibition of high nitrogen on root growth by regulating antioxidant system, hormone balance, carbon assimilation and transportation in apple. Sci. Hortic. 311, 111828. doi: 10.1016/j.scienta.2023.111828
Yu, B., Wang, L., Cui, D., Gao, W., Xue, X., Nie, P. (2023a). Effects of fulvic acid on growth and nitrogen utilization efficiency in M9T337 seedlings. Plants 12, 3937. doi: 10.3390/plants12233937
Yu, B., Wang, L., Guan, Q., Xue, X., Gao, W., Nie, P. (2023b). Exogenous 24-epibrassinolide promoted growth and nitrogen absorption and assimilation efficiency of apple seedlings under salt stress. Front. Plant Sci. 14, 1178085. doi: 10.3389/fpls.2023.1178085
Yuan, Z. Z., Ou, J. Q., Wang, Z. Q., Zhang, C. F., Zhou, Z. P., Lin, Q. H. (2007). Regulation of carbon and nitrogen metabolisms in rice roots by 2- oxoglutarate at the level of hexokinase. Physiologia plantarum 129, 296–306. doi: 10.1111/j.1399-3054.2006.00806.x
Zhao, H., Sun, S., Zhang, L., Yang, J., Wang, Z., Ma, F., et al. (2020). Carbohydrate metabolism and transport in apple roots under nitrogen deficiency. Plant Physiol. Biochem. 155, 455–463. doi: 10.1016/j.plaphy.2020.07.037
Zhou, J., Wan, H., He, J., Lyu, D., Li, H. (2017). Integration of cadmium accumulation, subcellular distribution, and physiological responses to understand cadmium tolerance in apple rootstocks. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.00966
Zhou, J., Wan, H., Qin, S., He, J., Lyu, D., Li, H. (2016). Net cadmium flux and gene expression in relation to differences in cadmium accumulation and translocation in four apple rootstocks. Environ. Exp. Bot. 130, 95–105. doi: 10.1016/j.envexpbot.2016.05.012
Keywords: apple, cadmium, fulvic acid, antioxidant capacity, photosynthetic performance, C and N metabolism
Citation: Yu B, Xue X, Nie P, Lu N and Wang L (2024) Fulvic acid alleviates cadmium-induced root growth inhibition by regulating antioxidant enzyme activity and carbon–nitrogen metabolism in apple seedlings. Front. Plant Sci. 15:1370637. doi: 10.3389/fpls.2024.1370637
Received: 15 January 2024; Accepted: 15 March 2024;
Published: 02 April 2024.
Edited by:
Dominik K. Großkinsky, Austrian Institute of Technology (AIT), AustriaReviewed by:
Stefanie Maria Primisser, Laimburg Research Centre, ItalyMuhammad Ahsan Asghar, Aarhus University, Denmark
Copyright © 2024 Yu, Xue, Nie, Lu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laiping Wang, wanglaiping666@163.com
†These authors have contributed equally to this work
 Bo Yu
Bo Yu Xiaomin Xue
Xiaomin Xue Peixian Nie
Peixian Nie Ninglin Lu1
Ninglin Lu1 Laiping Wang
Laiping Wang