- 1Department of Botany, Aligarh Muslim University, Aligarh, India
- 2Department of Agricultural and Environmental Sciences, Università degli Studi di Milano, Milan, Italy
- 3Department of Botany, Jamia Hamdard, New Delhi, India
- 4Department of Plant Biology, University of Szeged, Szeged, Hungary
Editorial on the Research Topic
Ethylene: a key regulatory molecule in plants, Volume II
Ethylene (ET) is a gaseous hormone that regulates plant developmental processes and tolerance to biotic and abiotic stresses (Abeles et al., 1992; Chen et al., 2022; Fatma et al., 2022). The use of ET for crop management and genetic enhancement has substantially expanded with advancements in our understanding of functional genomics. This article collection consists of two reviews and five original research articles that provide scientific references, valuable clues, and molecular and transcriptomic strategies for coping with a range of crop disasters such as metal toxicity, nutrient deficiency, and oxidative imbalance not only in angiosperms but also in liverworts.
The biosynthesis of this plant hormone starts with methionine that through the activities of specific enzymes leads to ET production. The two key enzymes are the 1-aminocyclopropane-1-carboxylate synthase (ACS), which is encoded by ACS genes, and 1-aminocyclopropane-1-carboxylate oxidase (ACO), encoded by ACO genes. The 1-aminocyclopropane-1-carboxylate (ACC) is considered the direct precursor of ET (Sauter et al., 2013). The linear signaling pathway is as follows:
ET—||ET receptors → CTR1—||EIN2 → EIN3/EILs → ERFs → ET responses
The biological function of ET depends on the CONSTITUTIVE TRIPLE RESPONSE1 (CTR1), which is a kinase, the ETHYLENE INSENSITIVE2 (EIN2) that is a protein located in the endoplasmic reticulum (ER) membrane, EIN3, ETHYLENE INSENSITIVE LIKEs (EILs), and ETHYLENE RESPONSE FACTORs (ERFs) that are transcription factors (Binder, 2020). In the absence of ET, CTR1 phosphorylates EIN2, preventing the cleavage and translocation of the C-terminal end (CEND) of EIN2 into the nucleus. In the presence of ET, CTR1 is inactivated, resulting in the dephosphorylation of EIN2 and its cleavage. CEND is then translocated into the nucleus, enhancing EIN3/EIL1 binding to target genes and promoting transcriptional changes (Binder, 2020; Angulo et al., 2021).
ET-mediated abiotic stress regulation in angiosperms and liverworts
Industrial runoff contains heavy metals, contaminating soil and poisoning crops. Sehar et al. investigated the role of ET in overcoming arsenic (As) phytotoxicity in the presence of selenium in mustard. The higher accumulation of As in leaves than in roots was the primary cause of reduced photosynthetic performance and reduced growth in mustard. ET application alone and in the presence of Se resulted in adaptive responses to As toxicity through increased expression of ASCORBATE PEROXIDASE (APX) and GLUTATHIONE REDUCTASE (GR), accumulation of reduced glutathione (GSH) and the suppression of abscisic acid (ABA)-mediated stomatal closure. Evolutionary insights into ET action were presented by Bharadwaj et al. The mechanism of ET action in liverwort (M. polymorpha) in response to a range of abiotic stresses was found to be markedly similar to that of angiosperms against a range of abiotic stresses. Mutant lines Mpein3 and Mpctr1 defective in positive and negative regulators of ET were compared with respective wild types against a range of abiotic stresses such as heat, salinity, nutrient deficiency, and far-red light. According to a previous study (Li et al., 2020), lines defective in the EIN3 transcription factor would be insensitive to ET response, indicating that EIN3 is a positive regulator of ET signaling, whereas the CTR1 mutants would display constitutive ET responses, suggesting that it is a negative regulator of ET perception. As hypothesized, Mpctr1 mutant lines showed prominent ET response and more resilience under sublethal conditions such as 29°C, 10 mM NaCl, 1/20 Gamborg’s dilution, or 50 days of exposure to far-red light, whereas Mpein3 mutant showed lower resilience than wild type.
Strategic control over nutrient deficiency and post-harvest ripening
Two comprehensive reviews dealing with TARGET OF RAPAMYCIN (TOR) and ET interconnection under nutrient deficiency and strategic post-harvest processing for increasing the shelf life of fruits and vegetables showcased detailed reports on the ET action and control mechanisms. Garcia et al. set ground on the so-called “stop growing” and “searching for nutrient” schemes to deal with nutrient deficit through TOR and ET interaction. Authors described reports where TOR and ET share antagonistic relationships: TOR blocks ET signaling alongside CTR1 by phosphorylating EIN2 and EIN3. However, nutrient deprivation inactivates TOR reactivating ET signaling.
In their review of post-harvest practices, Cocetta and Natalini describe how apart from conventional means such as chilling treatments and antisense approaches for ACS and POLYGALACTURONASE (PG) genes, transgenic approaches have been applied to elucidate ET biochemical and metabolic pathways. Ripening-related transcription factors of the APETALA2/ETHYLENE-RESPONSE FACTOR (AP2/ERF) family have been studied in tomatoes for their role in regulating ripening-responsive genes (Fenn and Giovannoni, 2021). Genetic analysis has led to the identification of ripening mutants like ripening inhibitor (rin), non-ripening (nor), colorless non-ripening (Cnr), green-ripe (Gr), green flesh (gf), high pigmnet1 (hp1), high pigment2 (hp2), and never ripe (Nr) in tomato (Osorio et al., 2020). These mutants are used commercially to develop new hybrids with longer shelf life. The CRISPR/Cas9 approach is promising, and it has been applied to regulatory protein genes CNR and NOR and to transcription factors AP2a, FUL1, and FUL2 to achieve targeted deletion or substitution (Wang et al., 2019).
ACC-DI peptide (di-ACC) and AP2/ERFs regulating ET responses
Vaughan-Hirsch et al. investigated the biological function of custom-synthesized 1- aminocyclopropane-1-carboxylic-acid dipeptide (di-ACC) molecule taken up by Arabidopsis just as ACC, in part via LYSINE HISTIDINE TRANSPORTERS (e.g., LHT1). Once taken up, the ACC dimer can evoke a triple response phenotype in dark-grown seedlings, reminiscent of ET responses induced by ACC itself, albeit less efficiently than ACC. Moreover, di-ACC operates via the known ET signaling pathway and not through ACC. Altogether, the study supported that di-ACC appears to be transported and processed into ET like ACC, and it can increase ET production levels and trigger further ET reactions in Arabidopsis.
Considering the importance of AP2/ERF in resilience responses, Ding et al. conducted a genome-based study in barley to explore the role of AP2/ERFs in starch synthesis. After re-examining the available genome database (Morex), these researchers identified 64 new genes in the HvAP2/ERF gene family and corrected some previously misannotated and duplicated genes. HvAP2-18 was identified as a promising candidate gene to shed light on the mechanism governing the production of starch in barley.
The role of the AP2/ERF gene family in resistance of N. benthamiana against P. infestans infection after INF1 (elicitin from P. infestans) treatment has been confirmed by Imano et al. The authors successfully demonstrated that NbERF-IX-33 transcription factors are responsible for the accumulation of the sesquiterpenoid phytoalexin capsidiol that serves as an important protective compound against P. infestans infection.
The articles in this collection contribute to our understanding of ET as a regulatory signal in plants. ET is again demonstrated to serve as an important mediator of stress in liverworts and angiosperms. Future studies in this exciting area will help to unravel stress response networks and how they interact with ET to determine which pathways may have existed in the ancestor of early land plants and to create strategies for reducing the adverse effects of abiotic stresses, which are becoming more common as a result of climate change. A graphical summary of the articles in the volume is presented in Figure 1.
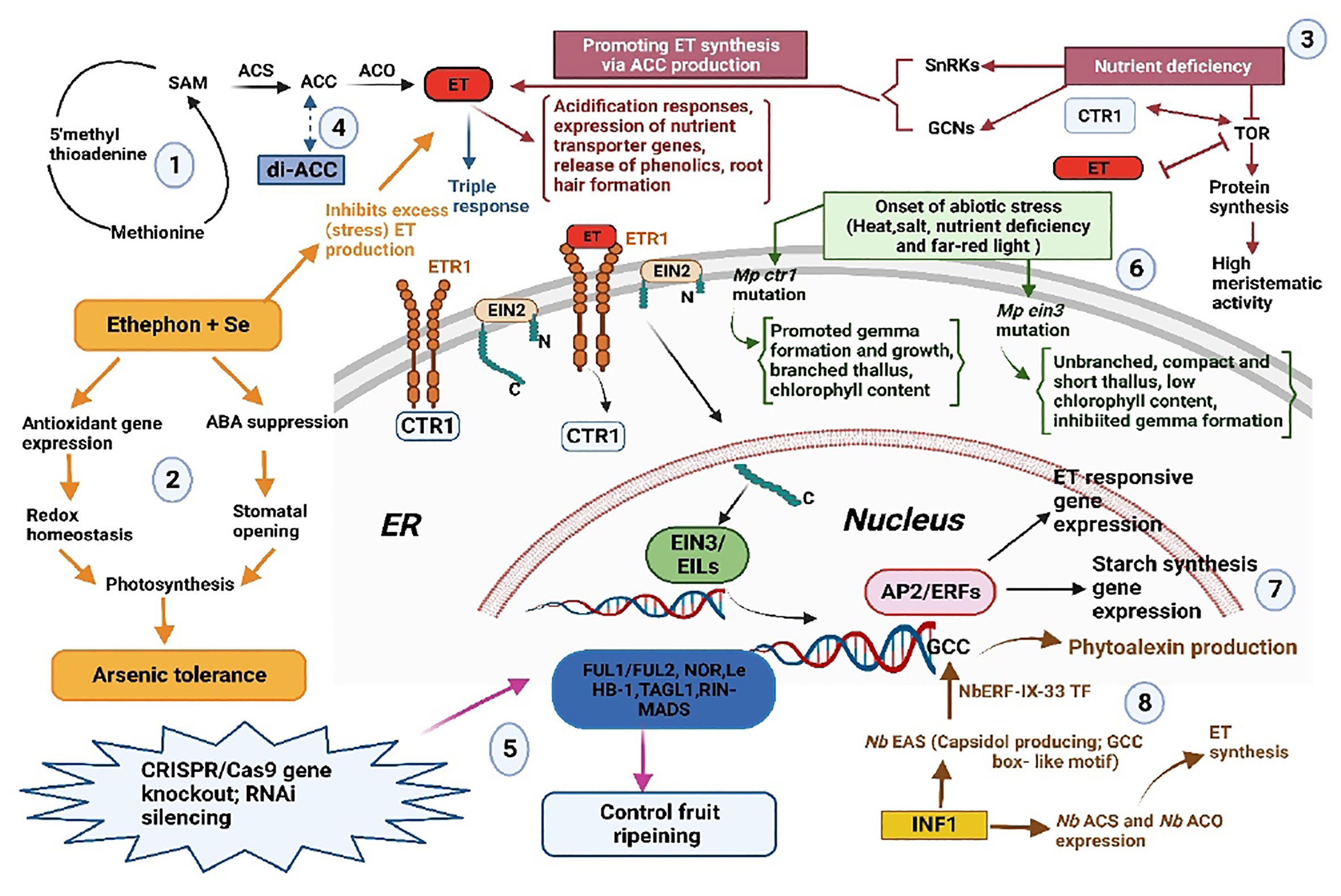
Figure 1 Summary of the articles published in the volume. (1) ET biosynthesis and perception leading to various ET-related responses; (2) Findings of Zebus et al. on Sc, ET and ABA interaction for As tolerance; (3) Garcia et al. report on inter-relationship between ET and TOR system; (4) Vanughan-Hirsch et al. report on di-ACC action; (5) Cocetta & Natalini review on fruit ripening; (6) Bharadawaj et al. report on Marchantia polymorpha mutants Mp ein3 and Mp ctrl; (7) Ding et al. finding on the role of AP2/ ERFS in starch synthesis; and (8) Finding of Imano et al. on phytoalexin production against Phytophthora infestans through NbERF-IX-33 in Nicotiana benthamiana. Arrows indicate  promotion;
promotion;  Similarity in function;
Similarity in function;  similarity but less substrate affinity than ACC;
similarity but less substrate affinity than ACC;  inhibition;
inhibition;  antagonistic action; ABA, Abscisic acid; ACO, ACC oxidase; ACC, 1-aminocyclopropane-1-carboxylic acid, ACS, ACC synthase; AP2/ERFs, APETALA2/ETHYLENE RESPONSE FACTOR, As, arsenic, CTR1, CONSTITUTIVE TRIPLE RESPONSE; di-ACC, 1- aminocyclopropane-1-carboxylic acid-dipeptide; EIN, ETHYLENE INSENSITIVE; EIL, EIN3-LIKE, ET, ethylene, ETR, ETHYLENE RESPONSE, ER, Endoplasmic reticulum; FUL1/FUL2; NOR; Le HB-1,TAGL.RIN-MADS, Genes for fruit ripening; GCN, GENERAL CONTROL NON-DEREPRESSIBLE; SAM, S- adenosyl-l-methionine, Se, Selenium; SnRKS, SUCROSE NON-FERMENTING1- RELATED PROTEIN KINASEA; INF, Elicitin from Phytophthora infestans.
antagonistic action; ABA, Abscisic acid; ACO, ACC oxidase; ACC, 1-aminocyclopropane-1-carboxylic acid, ACS, ACC synthase; AP2/ERFs, APETALA2/ETHYLENE RESPONSE FACTOR, As, arsenic, CTR1, CONSTITUTIVE TRIPLE RESPONSE; di-ACC, 1- aminocyclopropane-1-carboxylic acid-dipeptide; EIN, ETHYLENE INSENSITIVE; EIL, EIN3-LIKE, ET, ethylene, ETR, ETHYLENE RESPONSE, ER, Endoplasmic reticulum; FUL1/FUL2; NOR; Le HB-1,TAGL.RIN-MADS, Genes for fruit ripening; GCN, GENERAL CONTROL NON-DEREPRESSIBLE; SAM, S- adenosyl-l-methionine, Se, Selenium; SnRKS, SUCROSE NON-FERMENTING1- RELATED PROTEIN KINASEA; INF, Elicitin from Phytophthora infestans.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abeles, F. B., Morgan, P. W., Saltveit, M. E., Jr. (1992). Ethylene in plant biology (Amsterdam: Elsevier).
Angulo, M., García, M. J., Alcántara, E., Pérez-Vicente, R., Romera, F. J. (2021). Comparative study of several fe deficiency responses in the arabidopsis thaliana ethylene insensitive mutants ein2-1 and ein2-5. Plants 10, 262. doi: 10.3390/plants10020262
Binder, B. M. (2020). Ethylene signaling in plants. J. Biol. Chem. 295, 7710–7725. doi: 10.1074/jbc.REV120.010854
Chen, H., Bullock, D. A., Alonso, J. M., Stepanova, A. N. (2022). To fight or to grow: the balancing role of ethylene in plant abiotic stress responses. Plants 11, 33. doi: 10.3390/plants11010033
Fatma, M., Asgher, M., Iqbal, N., Rasheed, F., Sehar, Z., Sofo, A., et al. (2022). Ethylene signaling under stressful environments: analyzing collaborative knowledge. Plants 11, 2211. doi: 10.3390/plants11172211
Fenn, M.A., Giovannoni, J.J. (2021). Phytohormones in fruit development and maturation. Plant J. 105, 446–458. doi: 10.111/tpj.15112
Li, D., Flores-Sandoval, E., Ahtesham, U., Coleman, A., Clay, J. M., Bowman, J. L., et al. (2020). Ethylene-independent functions of the ethylene precursor ACC in marchantia polymorpha. Nat. Plants 6, 1335–1344. doi: 10.1038/s41477-020-00784-y
Osorio, S., Carneiro, R. T., Lytovchenko, A., Mcquinn, R., Sørensen, I. (2020). Genetic and metabolic effects of ripening mutations and vine detachment on tomato fruit quality. Plant Biotechnol. J. 18, 106–118. doi: 10.1111/pbi.13176
Sauter, M., Moffatt, B., Saechao, M. C., Hell, R., Wirtz, M. (2013). Methionine salvage and s-adenosylmethionine: essential links between sulfur, ethylene and polyamine biosynthesis. Biochem. J. 451, 145–154. doi: 10.1042/BJ20121744
Keywords: ethylene, physiology, metabolism, phytohormones, signaling molecules
Citation: Khan NA, Ferrante A, Khan MIR and Poor P (2023) Editorial: Ethylene: a key regulatory molecule in plants, Volume II. Front. Plant Sci. 14:1222462. doi: 10.3389/fpls.2023.1222462
Received: 14 May 2023; Accepted: 05 June 2023;
Published: 16 June 2023.
Edited and Reviewed by:
Anna N Stepanova, North Carolina State University, United StatesCopyright © 2023 Khan, Ferrante, Khan and Poor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nafees A. Khan, naf9.amu@gmail.com
 Nafees A. Khan
Nafees A. Khan Antonio Ferrante
Antonio Ferrante M. Iqbal R. Khan
M. Iqbal R. Khan Peter Poor
Peter Poor