- 1Department of Crop and Soil Science, Oregon State University, Corvallis, OR, United States
- 2Natural Products Utilization Research Unit, Agricultural Research Service, United States Department of Agriculture, University, MS, United States
As a staple crop, potatoes (Solanum tuberosum) play an important role in meeting daily caloric needs. To ensure adequate supplies for year-round consumption, potato quality must be maintained throughout lengthy storage periods. Towards this end, potato sprouting during storage must be minimized. Due to changing regulations regarding chemical means of potato sprout suppression, increased focus has turned to alternative products including essential oils (EO) as sprout suppressants in recent years. The complex composition of various EOs promises numerous options for sprout suppression. Furthermore, blends of several EOs may achieve enhanced sprout suppressant properties if synergistic interactions are present. We evaluated Syzygium aromaticum, Artemisia herba-alba, and Laurus nobilis EOs and blends thereof as sprout suppressants in potato cultivar Ranger Russet stored at room temperature and also tested for their antifungal activity against Colletotrichum fragariae, a causal organism of anthracnose disease in strawberries including other vegetables and fruits. A. herba-alba EO was an effective sprout suppressant when used alone and suppressed sprouting over the 90-day storage period. Interactions between A. herba-alba and S. aromaticum affected sprout length whereas interactions between A. herba-alba and L. nobilis EOs affected sprout number. An optimum blend of 50% - 82.31% A. herba-alba, 17.69% - 50% L. nobilis, and 0% - 1.01% S. aromaticum EOs could more effectively minimize tuber sprout length and number than any of the three whole EOs used alone. Among these three EOs, only S. aromaticum EO showed antifungal activity against C. fragariae in bioautography assay. These results exhibit the potential of EOs blends as a novel tactic in potato sprout suppression as well as potential natural product-based fungicides in managing C. fragariae.
1 Introduction
Cultivated in over 100 countries, potato (Solanum tuberosum) is the most important non-cereal crop globally (Boivin et al., 2020). A staple in the diets of many, potatoes can be sold fresh or processed into numerous products (Plant Production and Protection Division, 2009). 2019 saw the production of over 370 million tons of potato tubers, 19 million of which were produced in the United States (FAO, 2021). Sales of U.S. grown potatoes exceeded $3.6 billion in 2020, demonstrating the crop’s national and global significance (USDA NASS, 2021).
It is common for potatoes to be stored for several months before use (Paul et al., 2016a). Immediately after harvest, potatoes are in a natural state of dormancy and will not sprout for several days to weeks, depending on environmental, physiological, and hormonal factors as well as cultivar (Sonnewald & Sonnewald, 2014; Visse-Mansiaux et al., 2022). Unfortunately, the length of this innate dormancy is often unable to meet market needs (Boivin et al., 2020). Extended storage times are associated with increased water loss, disease occurrence, and sprouting in stored tubers (Magdalena & Dariusz, 2018). Moreover, appropriate management of sprouting is necessary as sprouted tubers show undesirable changes in weight, texture, and nutritional value, and sprouting is associated with formation of toxic alkaloids such as solanine (Sorce et al., 2005; Sonnewald & Sonnewald, 2014). Because sprouted potatoes are considered undesirable and inedible, sprouting during storage leads to lost revenue and increased food waste (Plant Production and Protection Division, 2009).
Strategies including storage at low temperatures and the use of chemical sprout suppressants are regularly implemented to limit tuber sprouting. For example, processing potato quality may be preserved for up to 9 months when stored between 8-12°C at 85–90% relative humidity (Paul et al., 2016a). However, these conditions alone are unable to completely suppress sprouting and sprout elongation once the period of innate dormancy has ended (Paul et al., 2016a). Though storage between 0-7°C is possible, these temperatures are known to alter reducing sugar content within the potato flesh. This is associated with darkening of potato products and the formation of acrylamide during frying (Wiltshire & Cobb, 1996). While this darkening is considered undesirable, the potential toxicity of acrylamide to humans makes cold storage of processing potatoes inadvisable (Paul et al., 2016b). Therefore, chemical sprout suppressants are commonly combined with low temperature storage to achieve adequate sprout control.
Isopropyl N-(3-chlorophenyl) carbamate (chlorpropham or CIPC) is the most widely used chemical sprout suppressant since the mid-20th century (Boivin et al., 2020). Its relatively low cost and high efficacy underlies its popularity; a single application can achieve complete sprout suppression for up to 5 months (Vaughn & Lehnen, 1991; Kleinkopf et al., 2003). However, due to environmental and health concerns of CIPC and its metabolites, CIPC was banned in the European Union in 2020 (Authority (EFSA) et al., 2017; Briddon & Stroud, 2019). While CIPC is still a legal sprout suppressant in many countries including the United States, the domestic potato industry could suffer considerable economic losses if American-grown potatoes are unable to be sold to countries with zero-tolerance policies for CIPC residues. Meanwhile, the growing popularity of organically produced foods and products in recent years suggests significant emerging economic opportunities in the potato industry; organic sales totaled $62 billion in the U.S. in 2021 (Organic Trade Association, 2022). Several essential oil (EO) sprout suppressants are commercially available including Biox-M, Biox-C, and Talent®. Biox-M contains 100% spearmint (Mentha spicata L.) EO, whereas Biox-C and Talent® contain 100% clove (Syzygium aromaticum L.) and caraway (Carum carvi L.) EOs, respectively (Boivin et al., 2020). Because EOs can be produced organically, the potential for their expanded use as alternative chemical sprout suppressants in potato storage is high. Greater use of these alternatives could reduce the economic strain that recent regulations may have on the U.S. potato industry while also providing organic potato growers and processors more control over sprouting in their operations.
As plant EOs are often composed of numerous constituents, it is possible that multiple compounds may act synergistically to achieve sprout suppression. It follows that blends of different EOs may achieve better sprout suppression than a single EO used alone. This study was conducted to determine the optimum blend of clove (Syzygium aromaticum), armoise (Artemisia herba-alba), and bay laurel (Laurus nobilis) EOs to maximize sprout suppression in one cultivar, Ranger Russet, stored at room temperature. These EOs were chosen based on preliminary experiments showing sprout suppressant capabilities of A. herba-alba and L. nobilis EOs and the commercial use of S. aromaticum EO in sprout suppressant formulations.
2 Materials and methods
2.1 Plant material
Potato tubers of cultivar Ranger Russet were obtained from Hermiston Agricultural Research and Extension Center in Hermiston, OR, USA. Potatoes were harvested in September 2021 and subsequently stored in 22.5 kg mesh bags at 4°C before use in January 2022. All tubers were left untreated by any chemicals prior to the experiment.
2.2 Whole essential oils and EO blends
Essential oils (EOs) of Syzygium aromaticum, Artemisia herba-alba, and Laurus nobilis were used. A. herba-alba and L. nobilis EOs were purchased from Mountain Rose Herbs, Eugene, Oregon, USA, https://mountainroseherbs.com/. S. aromaticum EO was purchased from The Essential Oil Company, Milwaukie, Oregon, USA, https://www.essentialoil.com/collections/essential-oils/. Essential oil blends were created according to the design in Table 1.
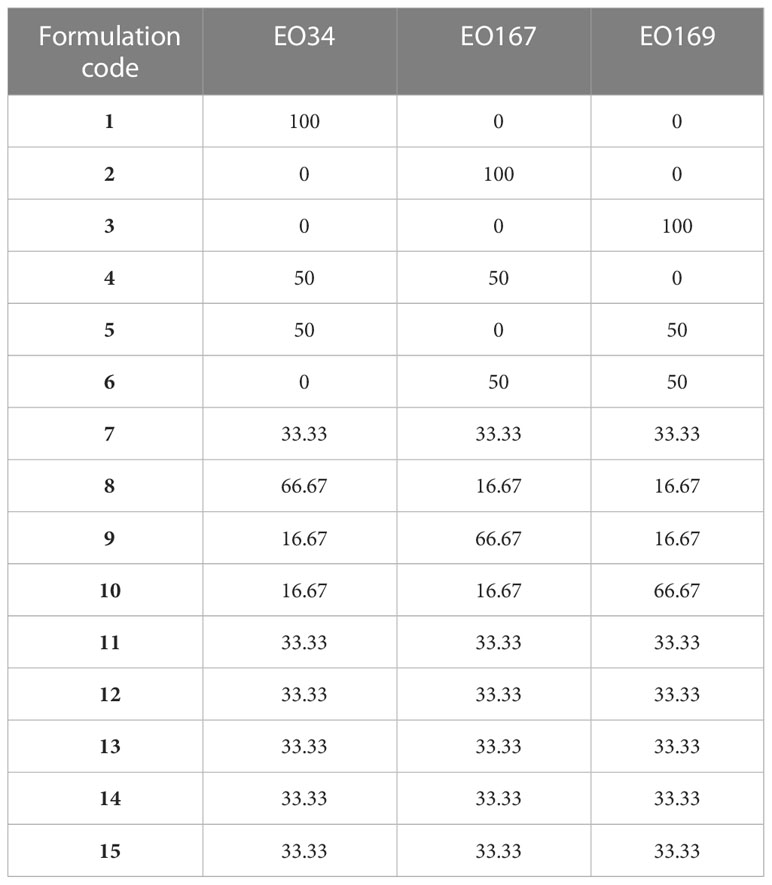
Table 1 Mixture design with 3 components (S. aromaticum EO: EO34, A. herba-alba EO: EO167, L. nobilis EO: EO169).
2.3 Tuber treatment and storage conditions
Tubers were treated with essential oils (EOs) and EO blends and stored in a similar manner as previously described by Thoma et al. (2022). One (1) mL of each whole EO or EO blend was placed on to a cotton ball sitting in a plastic Petri dish lined with filter paper in the center of a new black 20 L plastic container. A control with 1 mL distilled water was included. Four randomly selected tubers were placed in each container after which the containers were sealed with aluminum foil for fumigation with the EO vapor. The Petri dish holding the EO had no direct contact with the tubers. Containers were topped with lids, stacked, and left undisturbed at room temperature. The experiment lasted 90 days.
2.3.1 Observations
Sprout length and number of sprouts were used to evaluate treatment effects. Sprout length was measured by recording the longest sprout on each tuber in millimeters. Sprout number was measured as the total number of germinated (≥ 1 mm) eyes on each tuber. Observations were taken at 30, 45, 60, 75, and 90 days of storage. Averages for both observations were calculated for each replication and used in mixture design analysis.
2.4 Mixture design and statistical analysis
R software, Version 3.6.3, was used for statistical analysis. Linear mixed models were used to analyze both sprout length and number (R Core Team, 2021). To address wide variability in the data and to satisfy the assumptions for ANOVA, a square root transformation was applied to the sprout number data to achieve normality of residuals. Multiple comparisons were made using a post-hoc Tukey’s HSD test to identify differences in sprout length and number by treatment across all time points. Estimated marginal means and confidence intervals for the sprout number data were back-transformed for reporting and graphics. Multiple R-packages (“tidyverse”, “ggpubr”, “rstatix”, “nlme”, “emmeans”, and “ggplot2”) were used in the analysis, data summary, and graphics (Kassambara, 2020, 2020, p. 2, Kassambara, 2021; Wickham and RStudio, 2021; Lenth et al., 2022; Pinheiro et al., 2022).
Analysis of the simplex centroid mixture design was conducted using Minitab statistical software (version 16, Minitab Inc.) The three EOs (S. aromaticum, A. herba-alba, and L. nobilis) were included as the independent variables. A mixture experiment with three components has a simplex structure and is depicted as a triangle. The composition of each mixture can be displayed on a triangular region with its composition indicated by its position. For example, pure, single ingredients are located at the vertices of the triangle (BahramParvar et al., 2015). Component combinations in all mixtures must have the same final weight (EO34+EO167+EO169 = 100). The experimental design is shown in Table 1. The studied responses were sprout length and sprout number.
2.5 Direct bioautography bioassay
The antifungal activity of the three EOs: S. aromaticum, A. herba-alba, and L. nobilis against Colletotrichum fragariae, a causal organisms of anthracnose disease in strawberries was evaluated with the direct bioautography assay. The inoculum was prepared as described previously. Briefly, fresh conidia of C. fragariae was harvested from a 7-10 days old fungal culture grown on a ½ strength potato dextrose agar (PDA) by flooding the plate with 10 mL sterile water. The spore suspension was filtered through a sterile double Mira cloth (Calbiochem-Novabiochem Corp., La Jolla CA) and was centrifuged at 1968 rcf for 10 min. The supernatant was discarded, and the spores were counted in Countess 3 (Invitrogen). The spore concentration was adjusted to 3 x 105 spores mL-1 with the required volume of PDB-TLC media (12.5 g PDB, 0.5 g agar, 0.5 ml tween 80 in 500 ml water) to obtain the inoculum for bioautography assay.
A 10 µL volume of each EOs in ethyl alcohol (10 mg mL-1) was spotted twice onto a silica gel plate (Analtech, Inc. Silica Gel GHLF, 250 micron). The inoculum was then sprayed uniformly onto the spotted silica gel plate with a hand sprayer. The plate was placed in a moisture chamber (99.9% humidity) and incubated at ~26 °C for 4 days. The antifungal activity was evaluated with the presence of clear zones (no fungal growth) on the TLC plate. One µL volume of technical grade fungicides captan (>98%, Chem Service, Inc., West Chester, PA), and fluodioxonil (>99.5%, Chem Service, Inc., West Chester, PA) (stock concentration 2 mg/mL in ethanol) were used as a positive control.
2.6 Gas chromatography mass spectrometry flame ionization detection essential oil analysis
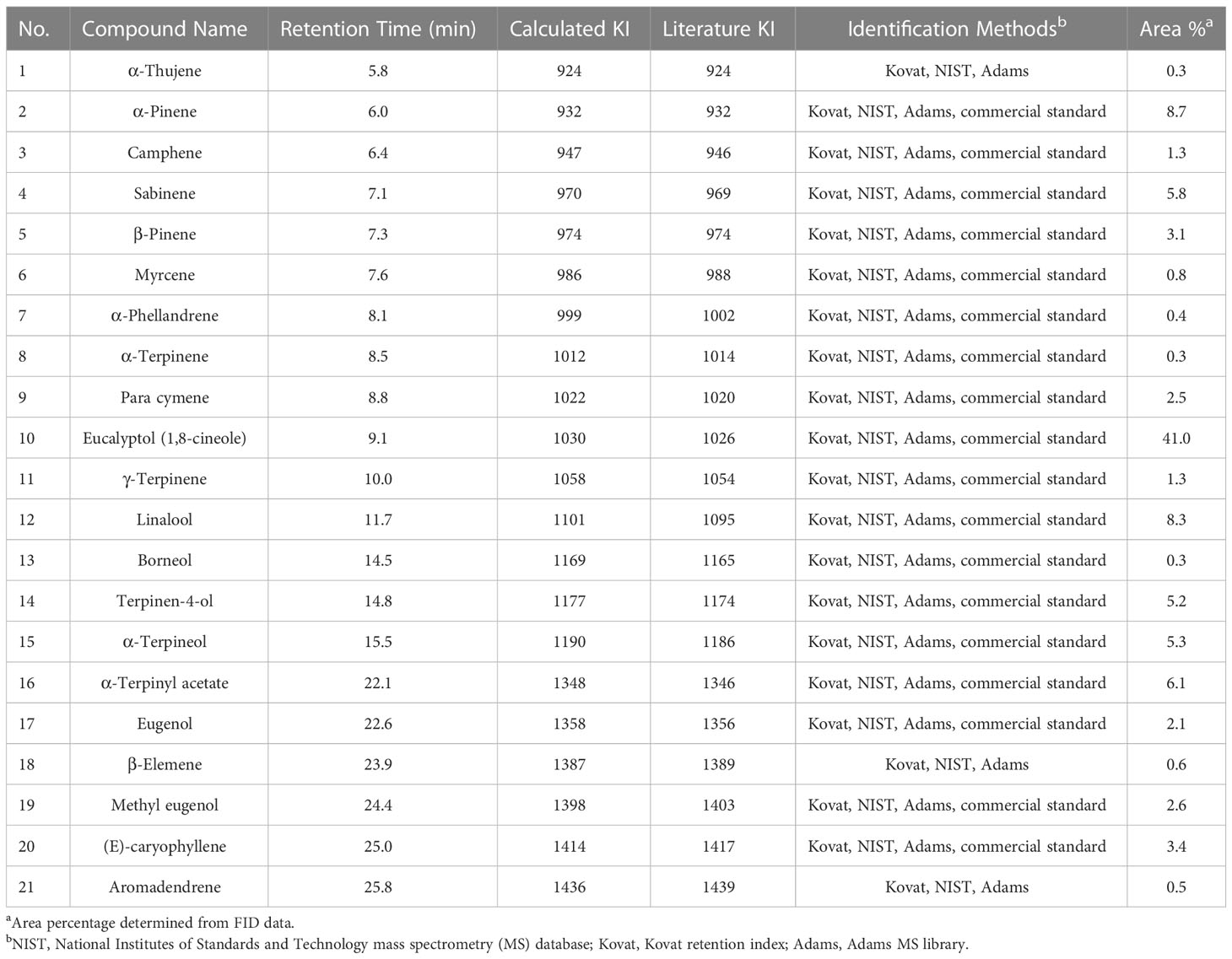
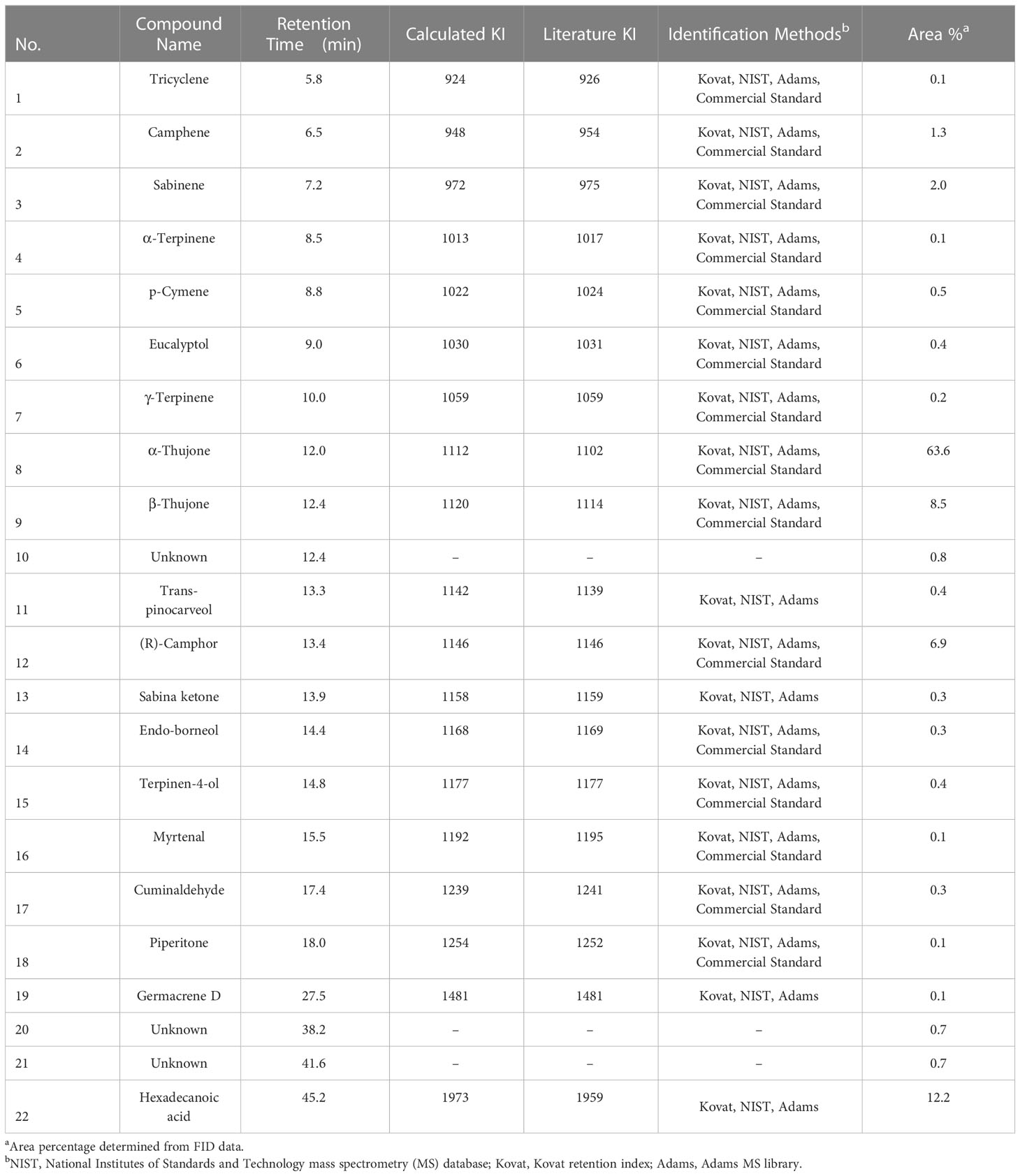
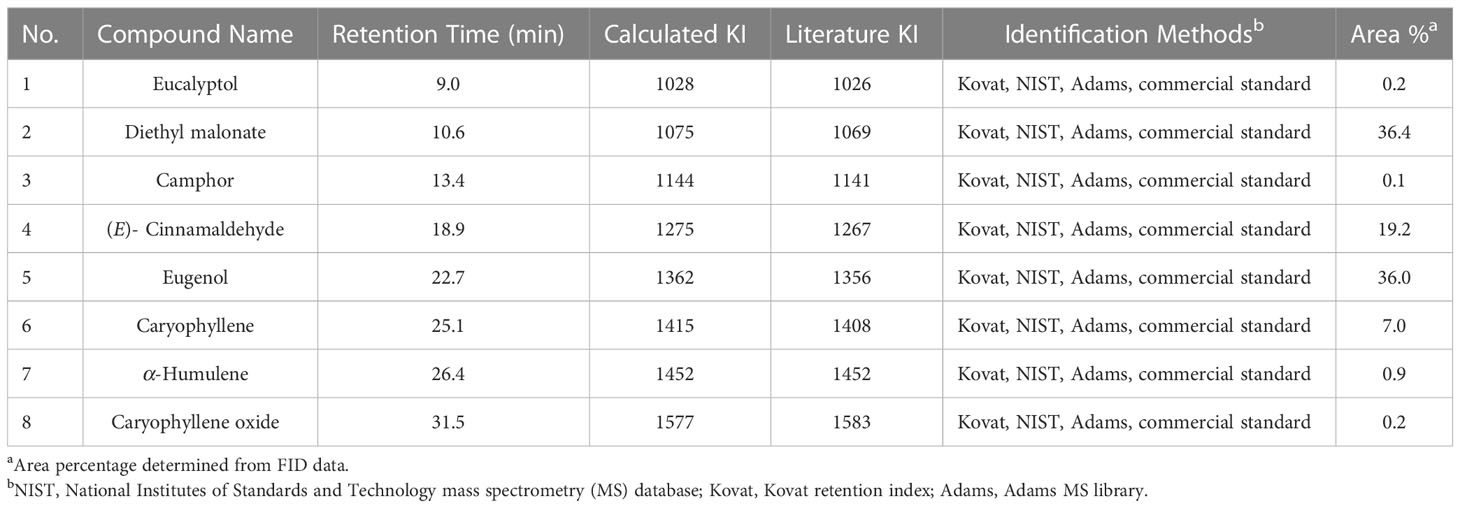
Gas chromatography (GC)—mass spectroscopy (MS)—flame ionization detection (FID) analysis of Syzygium aromaticum, Artemisia herba-alba, and Laurus nobilis EOs was performed by dissolving 50 μL of oil (weight also recorded) from each sample in chloroform in a 10 mL volumetric flask. Oil samples were analyzed by GC–MS–FID on an Agilent (Santa Clara, CA, USA) 7890A GC system coupled to an Agilent 5975C inert XL MSD as previously described by Thoma et al., 2022. Compounds were identified by Kovats Index analyses, direct comparison of MS and retention time to authentic standards, and comparison of mass spectra with those reported in the Adams and NIST mass spectra databases, unless otherwise noted in Tables 2–4. Commercial standards were obtained from Sigma-Aldrich (St. Louis, MO, USA) and used for direct comparison with retention time and MS data providing unequivocal identification where noted in Tables 2–4.
Compounds were quantified by performing area percentage calculations based on the total combined FID area. For example, the area for each reported peak was divided by total integrated area from the FID chromatogram from all reported peaks and multiplied by 100 to arrive at a percentage. The percentage of a peak is a percentage relative to all other constituents integrated in the FID chromatogram.
3 Results and discussion
3.1 Effects of whole essential oils on tuber sprout length and number
The EOs of A. herba-alba and L. nobilis significantly suppressed sprout length relative to the control throughout the 90-day storage period (Figure 1). Artemisia herba-alba EO was the most effective at reducing sprout length, limiting this response to less than 1 mm for up to 90 days (Table 5). Laurus nobilis EO limited sprout length to 3.2 mm for up to 45 days (Table 5). Only A. herba-alba EO significantly reduced sprout number, limiting this response to less than 1 germinated sprout per tuber for up to 90 days (Table 6). Syzygium aromaticum EO treatment did not significantly affect either sprout length or sprout number relative to the control at any time point (Figures 1, 2).
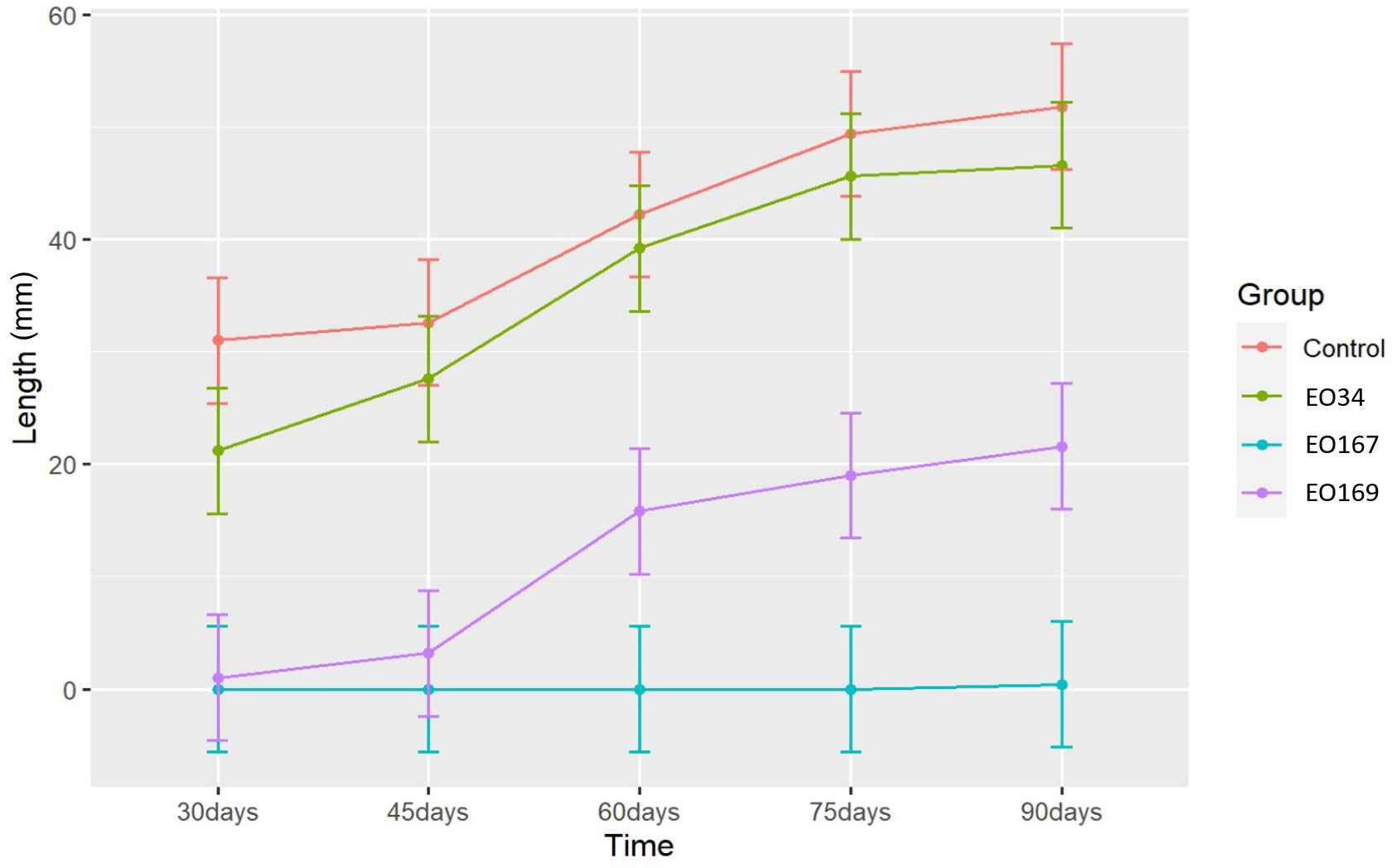
Figure 1 Sprout length (mm) over time of potatoes treated with distilled water (control), S. aromaticum (EO34), A. herba-alba (EO167), and L. nobilis (EO169) EOs. Error bars represent the 95% confidence level of the estimated means (emmeans method).
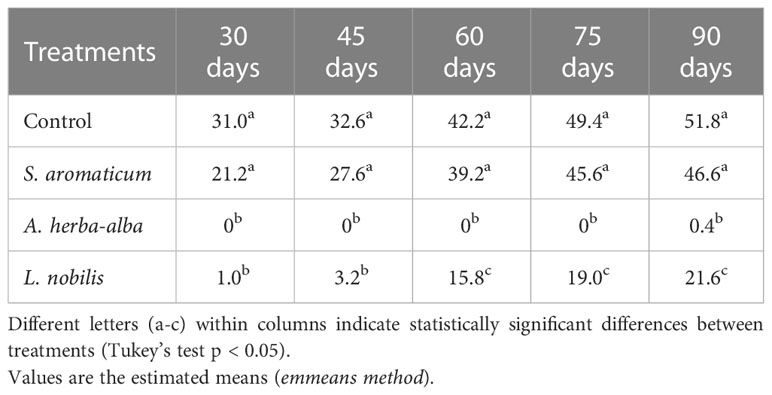
Table 5 Longest sprout length (mm) of potato tubers treated with different essential oils at different time points.
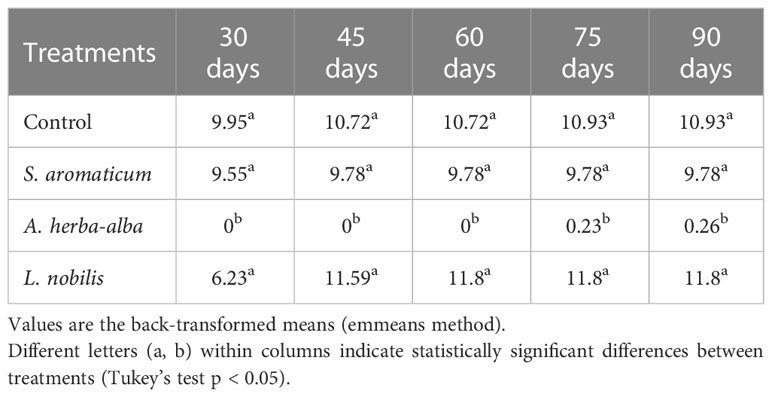
Table 6 Number of germinated eyes of potato tubers treated with different essential oils at different time points.
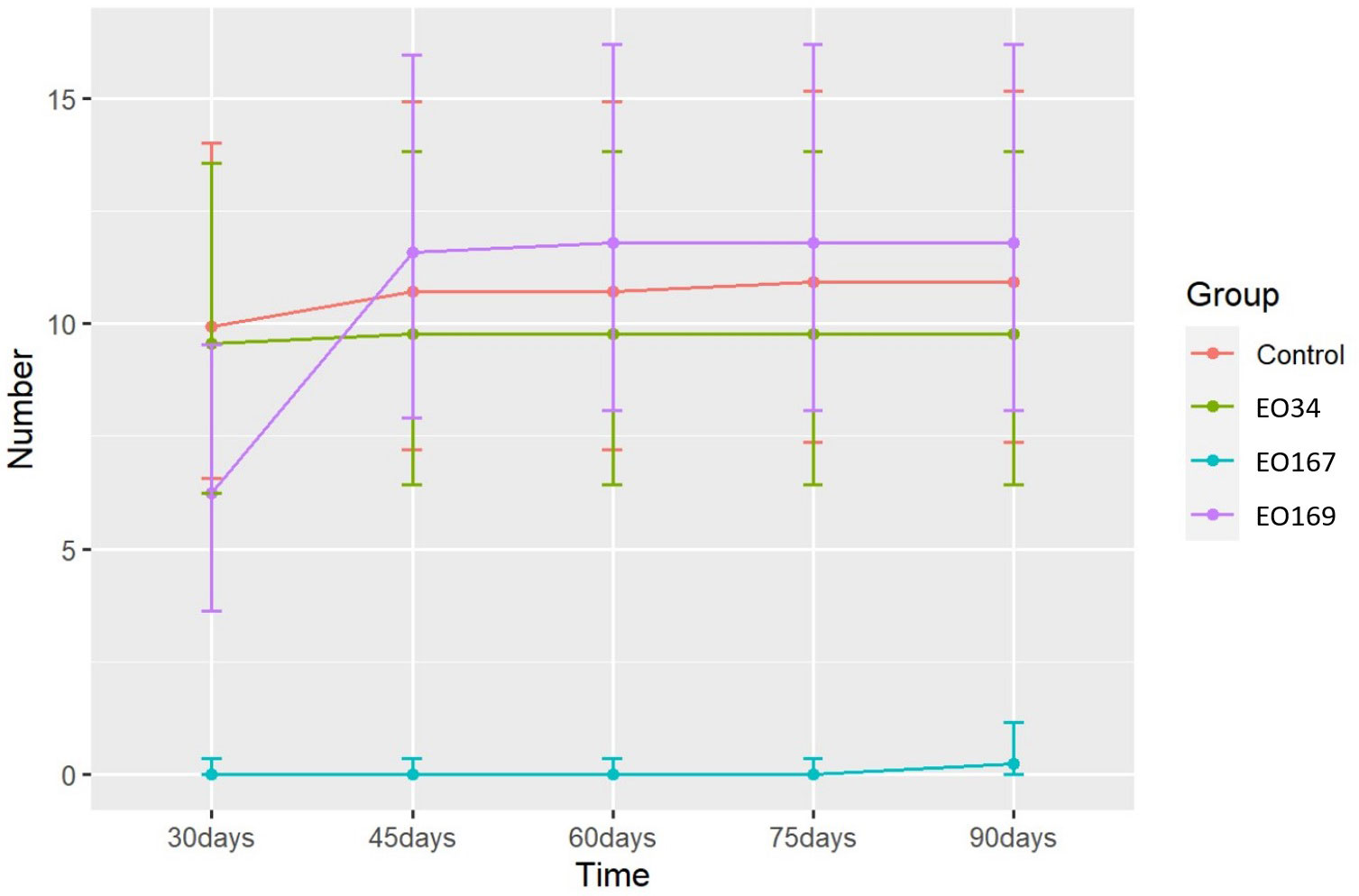
Figure 2 Number of germinated eyes over time of potatoes treated with distilled water (control), S. aromaticum (EO34), A. herba-alba (EO167), and L. nobilis (EO169) EOs. Error bars represent the 95% confidence level of the back-transformed means (emmeans method).
3.2 Effects of essential oil blends on tuber sprout length and number
ANOVA results and relevant p-values for the different models fitted to the responses are summarized in Tables 7, 8. A significant interaction between S. aromaticum and A. herba-alba EOs on sprout length was observed up to 75 days (p ≤ 0.05), whereas a significant interaction between A. herba-alba and L. nobilis EOs on sprout length was observed at 60 days (Table 7). A significant interaction between all three EOs on sprout length was observed at 45 days (p ≤ 0.05) (Table 7). A significant interaction between A. herba-alba and S. aromaticum EOs was observed on sprout number for up to 45 days (p ≤ 0.05), whereas a significant interaction between A. herba-alba and L. nobilis EOs was observed for up to 75 days (Table 8).
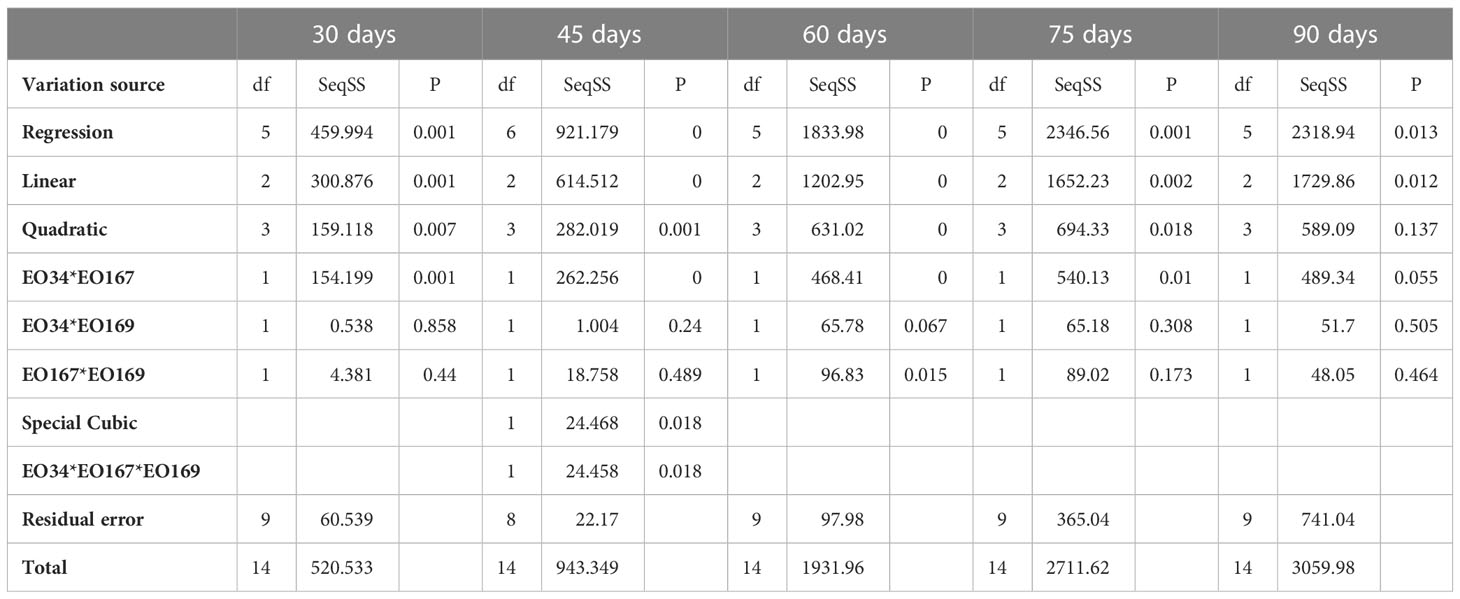
Table 7 Analysis of variance for the different models fitted to the sprout length data. EO34 corresponds to S. aromaticum EO, EO167 corresponds to A. herba-alba EO, and EO169 corresponds to L. nobilis EO.
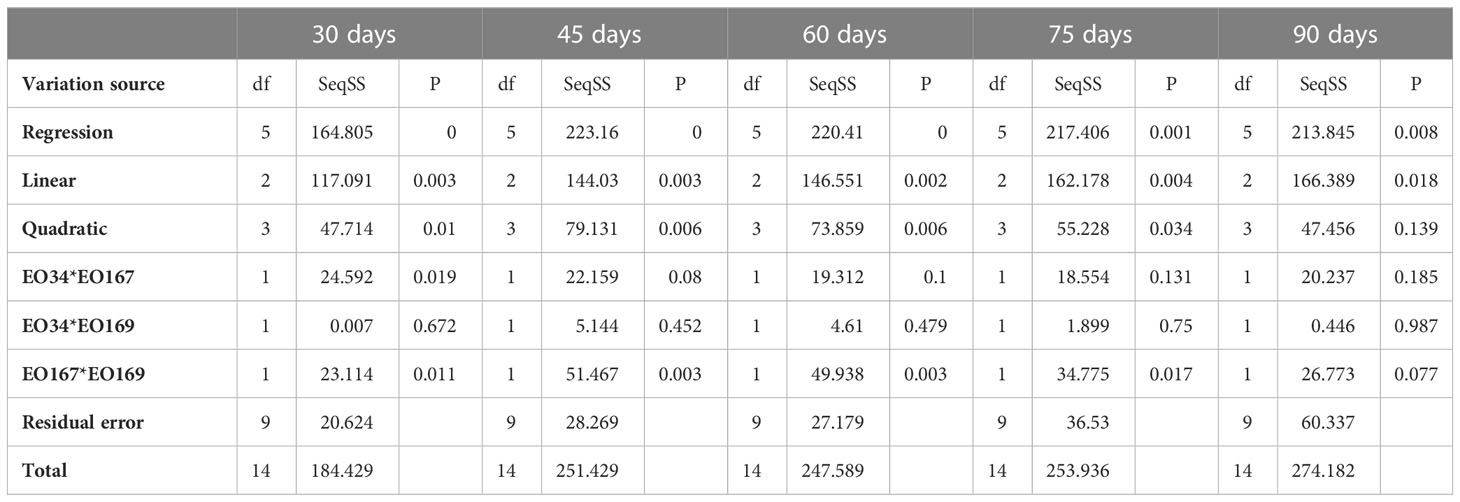
Table 8 Analysis of variance for the different models fitted to the sprout number data. EO34 corresponds to S. aromaticum EO, EO167 corresponds to A. herba-alba EO, and EO169 corresponds to L. nobilis EO.
Quadratic models were chosen for analysis of sprout number at all time points as simple cubic models were not significant (not shown, p > 0.05). Quadratic models were chosen for analysis of sprout length for every time point except 45 days for which a simple cubic model was used. The triangular mixture contour plots of the responses are depicted in Figures 3, 4 to display the interaction between components on sprout length and number, respectively, across time.
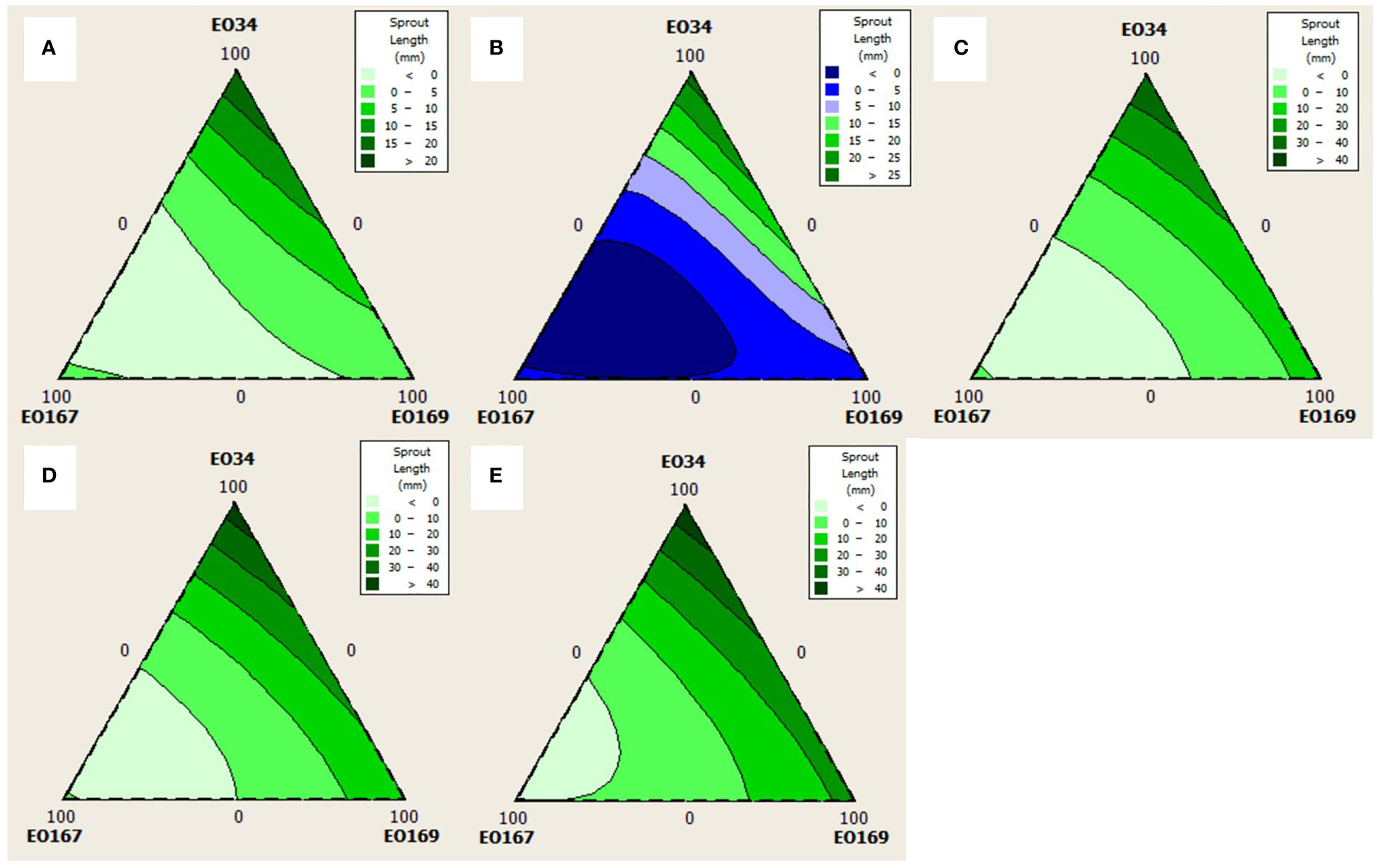
Figure 3 Contour plots for the effect of different combinations of S. aromaticum (EO34), A herba-alba (EO167), and L. nobilis (EO169) EOs on sprout length at (A) 30 days, (B) 45 days, (C) 60 days, (D) 75 days, and (E) 90 days.
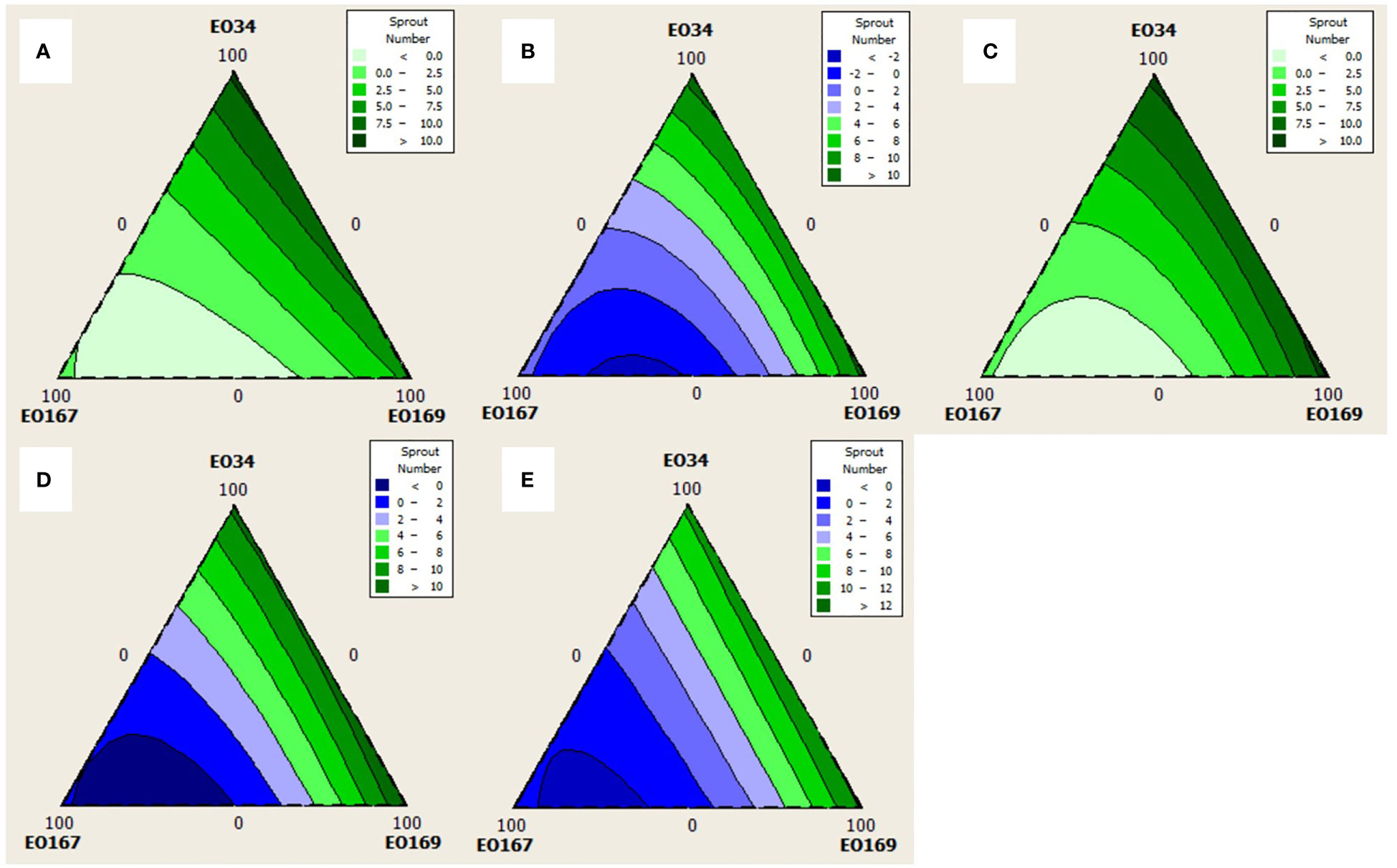
Figure 4 Contour plots for the effect of different combinations of S. aromaticum (EO34), A herba-alba (EO167), and L. nobilis (EO169) EOs on sprout number at (A) 30 days, (B) 45 days, (C) 60 days, (D) 75 days, and (E) 90 days.
3.3 Formulation optimization
A response optimizer was used to identify the optimum blend to minimize both responses at the various time points. The parameters used in the optimization are shown in Table 9. Minimization of both sprout length and number was preferred. The optimum blend consists of 50% - 82.31% A. herba-alba EO, 0% - 1.01% S. aromaticum EO, and 17.69% - 50% L. nobilis EO depending on storage length (Table 10).
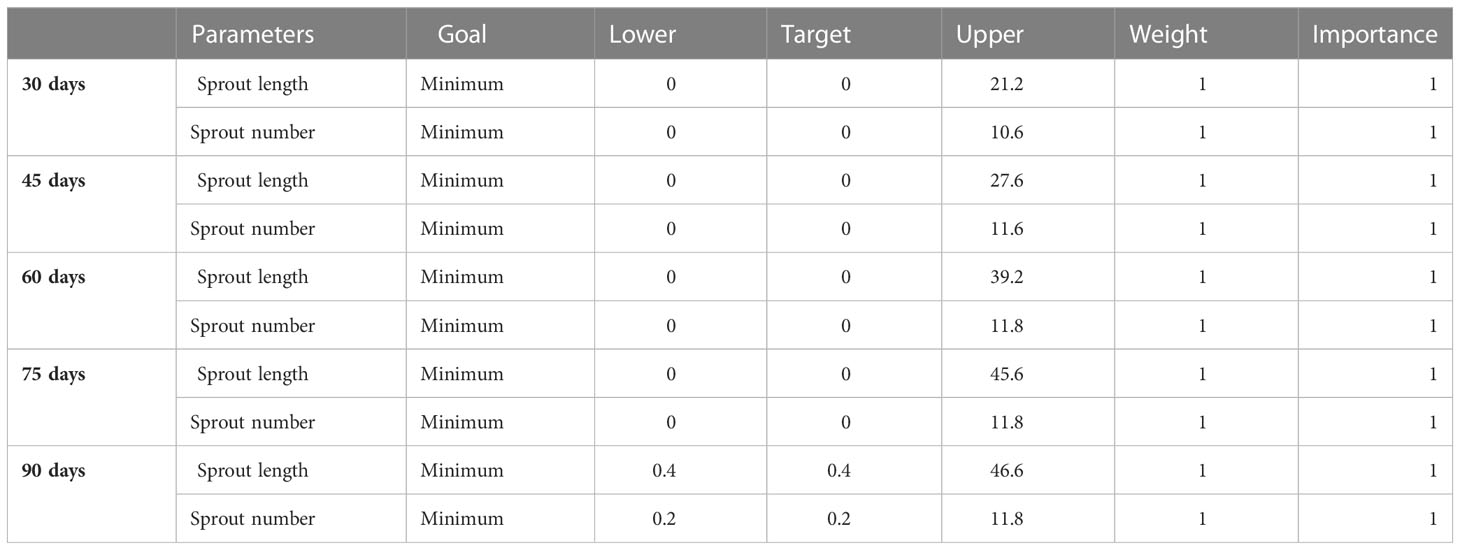
Table 9 The desirable ranges for each response to determine the optimum EO blend at each time point.
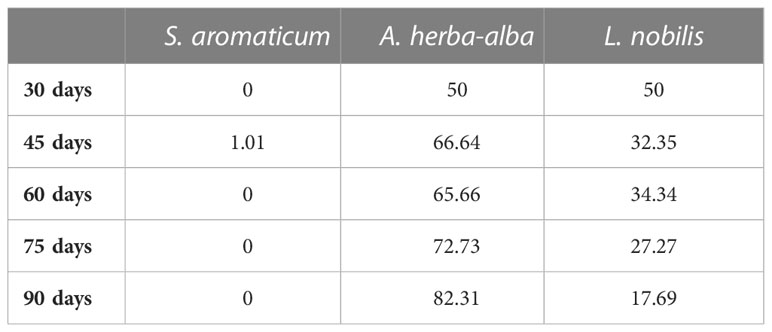
Table 10 Proportions of each EO predicted to achieve optimal suppression of both sprout length and number at each time point.
3.4 Gas chromatography– mass spectroscopy– flame ionization detection
Results of gas chromatography (GC) – mass spectroscopy (MS) – flame ionization detection (FID) analysis of A. herba-alba, S. aromaticum, and L. nobilis EOs are presented in Tables 2–4, respectively. Compounds making up > 1% of A. herba-alba EO included α-thujone (63.6%), β-thujone (8.5%), hexadecenoic acid (12.2%), (R)-camphor (6.9%), sabinene (2.0%), and camphene (1.3%) (Table 3). Compounds making up > 1% of S. aromaticum EO included diethyl malonate (36.4%), eugenol (36%), (E)-cinnamaldehyde (19.2%), and caryophyllene (7.0%) (Table 4). Compounds making up > 1% of L. nobilis EO included eucalyptol (41.0%), α-pinene (8.7%), linalool (8.3%), α-terpinyl acetate (6.1%), sabinene (5.8%), α-terpineol (5.3%), terpinene-4-ol (5.2%), (E)- caryophyllene (3.4%), β-pinene (3.1%), methyl eugenol (2.6%), para cymene (2.5%), eugenol (2.1%), camphene (1.3%), and γ-terpinene (1.3%) (Table 2).
3.5 Bioautography assay
Among the three whole EOs, only S. aromaticum EO showed antifungal activity against C. fragariae in bioautography assay with a clear zone of fungal growth inhibition (Figure 5). The average ± standard deviation from two replications was 8.3 ± 0.35 mm for S. aromaticum EO. The EOs from A. herba-alba and L. nobilis did not show any antifungal activity against C. fragariae in our study.
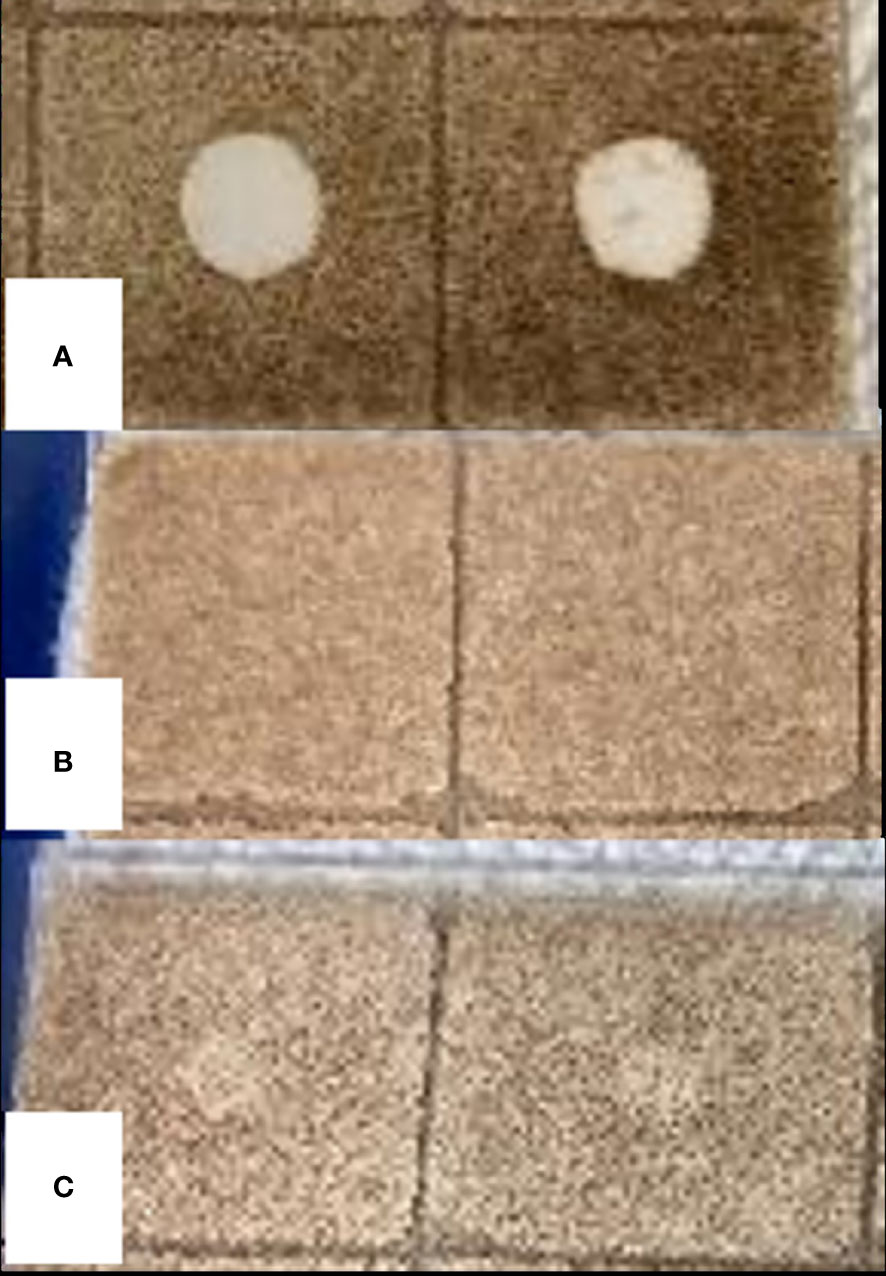
Figure 5 Bioautography assay of EOs from S. aromaticum (A), A herba-alba (B), and L. nobilis (C). The antifungal activity against C fragariae is represented by “zone of fungal growth inhibition”.
4 Discussion
Sprout length is one of many important traits used to grade potatoes in the United States. For example, processing potatoes with sprout lengths longer than half an inch (~13 mm) or one inch (~25 mm) are considered “damaged” and “seriously damaged”, respectively (USDA AMS, 2011). Potatoes with sprouts are considered defects, and greater degrees of damage result in lower grades and increased food waste (Pedreschi et al., 2008).
Sprout number is another important attribute that affects potato grading in the United States. Unlike sprout length, there is no specific limit on the allowable number of sprouts per tuber. However, if numerous individual or clusters of sprouts are present, lower potato grades may result (USDA AMS, 2011). Individual tubers possess numerous eyes from which sprouts can develop. Therefore, limiting sprout number in addition to sprout length is crucial for maintaining tuber quality.
Average longest sprout length varied between 0 and 46.6 mm depending on the EO blend with higher concentrations of A. herba-alba EO showing the strongest negative relationship with sprout length. A significant 3-way interaction on sprout length at 45 days suggests that a blend of the three EOs could achieve better suppression of sprout length than when any of the EOs is used alone. Indeed, response optimization suggested a blend of all three EOs for storage lengths of 45 days, though the efficacy of this blend has yet to be tested.
Average sprout number varied between 0 and 11.8, with higher concentrations of A. herba-alba EO showing the strongest negative relationship with sprout number. The interactions between A. herba-alba and S. aromaticum EOs and A. herba-alba and L. nobilis EOs for up to 45 days and 75 days, respectively, suggests that a combination of these EOs could obtain better control of sprout number than when any of the EOs are used alone. At all timepoints, response optimization suggests that a blend of A. herba-alba and L. nobilis EOs could result in better sprout suppression than A. herba-alba EO alone, although for longer storage periods, greater proportions of A. herba-alba EO are suggested.
Essential oils are known to contain numerous compounds, some of which have demonstrated sprout suppressant activity (Boivin et al., 2020). Of the three EOs, L. nobilis contained the most compounds previously reported to be effective sprout suppressants at amounts >1% including α-pinene (8.7%), eucalyptol (41.0%), and terpinen-4-ol (5.2%) (Boivin et al., 2020). A. herba-alba EO contained camphor (6.9%), previously reported as a somewhat effective sprout suppressant, but its primary constituent α-thujone (63.6%) has been reported as an ineffective sprout suppressant when used alone (Boivin et al., 2020). The higher efficacy of A. herba-alba EO over L. nobilis EO as a sprout suppressant could be due to synergistic interactions between its various constituents. Furthermore, the superior performance of an EO blend over any of the three EOs used individually could be due to compounds within the EOs acting synergistically.
Of the three whole EOs, S. aromaticum EO was the least effective as a sprout suppressant. These findings are corroborated by previous findings demonstrating A. herba-alba as an effective sprout suppressant in Ranger Russet potato storage (Thoma et al., 2022). Though S. aromaticum EO is the main component of Biox-C, a commercial sprout suppressant, treatment with S. aromaticum EO did not result in adequate sprout suppression in the present study. This could be due to only a single application of EO when repeated applications are often necessary, or the amount applied may have been too low.
S. aromaticum EO was reported to have antimicrobial properties against both fungi and bacteria (Burt and Reinders, 2003; Fu et al., 2007; Pinto et al., 2009; Gupta et al., 2014; Aguilar-González et al., 2015; Harčárová et al., 2021). Harčárová et al. (2021) has reported 100% growth inhibition in both in-vivo and in-vitro study of Fusarium graminearum at 500 and 1000 µg/ml. Similarly, S. aromaticum EO demonstrated antifungal activity with a minimum inhibitory concentration (MIC) of 92.56 µL/Lair against gray mold disease of fruits and vegetables caused by Botrytis cinerea (Aguilar-González et al., 2015). The MIC of S. aromaticum EO was even lower with 46.28 µL/Lair in in-vitro and 11.57 µL/Lair in in-vivo when combined with Mustard EO. Neither of these studies have further investigated the EO to determine the major bioactive constituents in S. aromaticum. The antifungal activity was also reported against human pathogens like Candida sp., Aspergillus sp. and dermatophyte species (Pinto et al., 2009) which reported eugenol is the major component of this EO (85.3%).
The EO from A. herba-alba did not show any antifungal activity against C. fragariae in our study. However, A. dracunculus L. var. dracunculus and A. douglasiana has shown antifungal activity against C. acutatum, C. fragariae, C. gloesporioides and Botrytis cinerea (Meepagala et al., 2002 and Meepagala et al., 2003). Vulgarone B was isolated from A. douglasiana as an active compound (Meepagala et al., 2003).
L. nobilis EO has been reported to have antifungal properties against post-harvest pathogens in cherry tomatoes (Xu et al., 2014), seed borne fungi of cucurbits (Moumni et al., 2021) and citrus black rot disease (Soylu and Kose, 2015) which are all caused by Alternaria alternata. However, its antifungal activity against C. fragariae has not been reported.
Additional studies verifying the ability of the optimum blend to suppress sprouting in Ranger Russet and other potato cultivars are warranted. Prior to industry adoption of this novel treatment, the optimum blend’s efficacy should be evaluated at semi-commercial or commercial scale using industrial infrastructure and application methods. In addition, pure compounds from L. nobilis and A. herba-alba EOs may be blended and tested to reveal which components are acting synergistically. Results from the present study suggest that EO blends may provide even greater sprout control than single EOs used alone. Furthermore, EO blends may even outperform commercially available EO sprout suppressant products, requiring lower concentrations or less frequent applications, which could offset the costs of using EOs in this industry. Therefore, experiments comparing this blend to currently available EO sprout suppressants and conventional products are justified.
5 Conclusion
As international restrictions on dominant sprout suppressant chemicals tighten, the need for effective alternatives is only expected to grow. Essential oils (EOs) have a history of use as potato sprout suppressants and may achieve varying rates of suppression depending on potato cultivar, storage temperature, and method and timing of application. Due to variability in EO compositions, it is possible that these constituents may interact to produce enhanced sprout suppression. This study demonstrated that A. herba-alba and L. nobilis EOs can significantly suppress sprouting in Ranger Russet tubers when used alone. A simplex centroid mixture design was used to determine the optimum blend of three EOs (S. aromaticum, A. herba-alba, and L. nobilis) to achieve sprout suppression in Ranger Russet potato tuber storage. The optimum blend to minimize both sprout length and number over a 90-day storage period consists of 50% - 82.31% A. herba-alba EO, 17.69% - 50% L. nobilis EO, and 0% - 1.01% S. aromaticum EO. This suggests that a blend of two or more EOs could achieve better sprout suppression than a single EO alone. Though the efficacy of this blend remains to be verified, the results illustrate the promise of mixture experiments in the development of novel sprout suppressant treatments. EO blends could provide increased control over potato sprouting and may even perform better than currently available EO sprout suppressant products. EOs therefore have a significant role to play in increasing the economic resilience of the potato industry while reducing food waste. Also, the result from bioautography assay of S. aromaticum EO’s exhibited antifungal activity against C. fragariae, and it could serve as a potential natural-product based fungicide as well as a sprout suppressant in potato.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
JT: Formal analysis, Investigation, Writing – original draft, Writing – review and editing, Visualization. CC: Formal analysis, Investigation, Writing – review and editing. PT: Formal analysis, Investigation, Writing – original draft, Writing – review and editing. VZ: Conceptualization, Methodology, Resources, Writing – review and editing, Supervision, Project administration, Funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This work was partially supported by the Oregon Potato Commission, the Oregon Department of Agriculture – Specialty Crops BGP ODA6024GR, and by USDA-NIFA 2021-51106-35584 grants awarded to Valtcho Zheljazkov (Jeliazkov).
Acknowledgments
We acknowledge the help from Sagar Suthuvalli’s potato breeding program and the OSU Hermiston Agricultural Research and Extension Center crew for growing and storing the potato for us. Authors also thank Solomon Yilma from the OSU potato breeding program for his support and advice.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aguilar-González, A. E., Palou, E., López-Malo, A. (2015). Antifungal activity of essential oils of clove (Syzygium aromaticum) and/or mustard (Brassica nigra) in vapor phase against gray mold (Botrytis cinerea) in strawberries. Innovative Food Sci. Emerging Technol. 32, 181–185. doi: 10.1016/j.ifset.2015.09.003
Authority (EFSA), E. F. S, Arena, M., Auteri, D., Barmaz, S., Bellisai, G., et al. (2017). Peer review of the pesticide risk assessment of the active substance chlorpropham. EFSA J. 15 (7), e04903. doi: 10.2903/j.efsa.2017.4903
BahramParvar, M., Tehrani, M. M., Razavi, S. M. A., Koocheki, A. (2015). Application of simplex-centroid mixture design to optimize stabilizer combinations for ice cream manufacture. J. Food Sci. Technol. 52 (3), 1480–1488. doi: 10.1007/s13197-013-1133-5
Boivin, M., Bourdeau, N., Barnabé, S., Desgagné-Penix, I. (2020). Sprout suppressive molecules effective on potato (Solanum tuberosum) tubers during storage: a review. Am. J. Potato Res. 97 (5), 451–463. doi: 10.1007/s12230-020-09794-0
Briddon, A., Stroud, G. P. (2019) Efficacy of sprout suppressants used alone, or in combination, to control sprouting of stored potato. Available at: https://projectblue.blob.core.windows.net/media/Default/Potato%20knowledge%20library/S1043%202018-19%20Yr%202%20Report.pdf.
Burt, S. A., Reinders, R. D. (2003). Antibacterial activity of selected plant essential oils against Escherichia coli O157:H7. Lett. Appl. Microbiol. 36 (3), 162–167. doi: 10.1046/j.1472-765x.2003.01285.x
FAO (2021). World food and agriculture – statistical yearbook 2021 (Rome: FAO). doi: 10.4060/cb4477en
Fu, Y., Zu, Y., Chen, L., Shi, X., Wang, Z., Sun, S., et al. (2007). Antimicrobial activity of clove and rosemary essential oils alone and in combination. Phytother. Research: PTR 21 (10), 989–994. doi: 10.1002/ptr.2179
Gupta, N., Parashar, P., Mittal, M., Mehra, V., Khatri, M., Rajguru, S. (2014). Antibacterial potential of Elletaria cardamomum, Syzygium aromaticum and Piper nigrum, their synergistic effects and phytochemical determination. J. Pharm. Res. 8 (8):1091–1097.
Harčárová, M., Čonková, E., Proškovcová, M., Váczi, P., Marcinčáková, D., Bujňák, L. (2021). Comparison of antifungal activity of selected essential oils against Fusarium graminearum in vitro. Ann. Agric. Environ. Medicine: AAEM 28 (3), 414–418. doi: 10.26444/aaem/137653
Kassambara, A. (2020) Ggpubr: “ggplot2” based publication ready plots (0.4.0). Available at: https://CRAN.R-project.org/package=ggpubr.
Kassambara, A. (2021) Rstatix: pipe-friendly framework for basic statistical tests (0.7.0). Available at: https://CRAN.R-project.org/package=rstatix.
Kleinkopf, G. E., Oberg, N. A., Olsen, N. L. (2003). Sprout inhibition in storage: current status, new chemistries and natural compounds. Am. J. Potato Res. 80 (5), 317. doi: 10.1007/BF02854316
Lenth, R. V., Buerkner, P., Herve, M., Love, J., Miguez, F., Riebl, H., et al. (2022) Emmeans: estimated marginal means, aka least-squares means (R package version 1.7.3). Available at: https://CRAN.R-project.org/package=emmeans.
Magdalena, G., Dariusz, M. (2018). Losses during storage of potato varieties in relation to weather conditions during the vegetation period and temperatures during long-term storage. Am. J. Potato Res. 95 (2), 130–138. doi: 10.1007/s12230-017-9617-x
Meepagala, K., Kuhajek, J., Sturtz, G., Wedge, D. (2003). Vulgarone b, the antifungal constituent in the steam-distilled fraction of artemisia douglasiana. J. Chem. Ecol. 29 (8), 1771–1780. doi: 10.1023/A:1024842009802
Meepagala, K. M., Sturtz, G., Wedge, D. E. (2002). Antifungal constituents of the essential oil fraction of Artemisia dracunculus l. var. dracunculus. J. Agric. Food Chem. 50 (24), 6989–6992. doi: 10.1021/jf020466w
Moumni, M., Romanazzi, G., Najar, B., Pistelli, L., Ben Amara, H., Mezrioui, K., et al. (2021). Antifungal activity and chemical composition of seven essential oils to control the main seedborne fungi of cucurbits. Antibiotics 10 (2), 104. doi: 10.3390/antibiotics10020104
Organic Trade Association (2022) U.S. organic sales soar to new high of nearly $62 billion in 2020 | OTA. Available at: https://ota.com/news/press-releases/21755.
Paul, V., Ezekiel, R., Pandey, R. (2016a). Sprout suppression on potato: need to look beyond CIPC for more effective and safer alternatives. J. Food Sci. Technol. 53 (1), 1–18. doi: 10.1007/s13197-015-1980-3
Paul, V., Ezekiel, R., Pandey, R. (2016b). Acrylamide in processed potato products: progress made and present status. Acta Physiologiae Plantarum 38 (12), 276. doi: 10.1007/s11738-016-2290-8
Pedreschi, F., Mery, D., Marque, T. (2008). “Grading of potatoes,” in Computer vision technology for food quality evaluation, 305–317. doi: 10.1016/B978-012373642-0.50016-8
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D., Heisterkamp, S., Van Willigen, B., et al. (2022) Nlme: linear and nonlinear mixed effects models (3.1-157). Available at: https://CRAN.R-project.org/package=nlme.
Pinto, E., Vale-Silva, L., Cavaleiro, C., Salgueiro, L. (2009). Antifungal activity of the clove essential oil from Syzygium aromaticum on candida, aspergillus and dermatophyte species. J. Med. Microbiol. 58 (Pt 11), 1454–1462. doi: 10.1099/jmm.0.010538-0
Plant Production and Protection Division (2009). International year of the potato 2008 - new light on a hidden treasure: an end-of-year review (Rome: FAO). Available at: https://www.fao.org/publications/card/en/c/c55eefde-ab51-5e8b-8237-7e8ed9796b93/.
R Core Team (2021). R: a language and environment for statistical computing (3.6.3) (R Foundation for Statistical Computing). Available at: https://www.R-project.org/.
Sonnewald, S., Sonnewald, U. (2014). Regulation of potato tuber sprouting. Planta 239 (1), 27–38. doi: 10.1007/s00425-013-1968-z
Sorce, C., Lorenzi, R., Parisi, B., Ranalli, P. (2005). Physiological mechanisms involved in potato (Solanum tuberosum) tuber dormancy and the control of sprouting by chemical suppressants. Acta Hortic. 24 (684), 177–186. doi: 10.17660/ActaHortic.2005.684.24
Soylu, E. M., Kose, F. (2015). Antifungal activities of essential oils against citrus black rot disease agent Alternaria alternata. J. Essential Oil Bearing Plants 18 (4), 894–903. doi: 10.1080/0972060X.2014.895158
Thoma, J. L., Cantrell, C. L., Zheljazkov, V. D. (2022). Effects of essential oil fumigation on potato sprouting at room-temperature storage. Plants 11 (22):3109. doi: 10.3390/plants11223109
USDA AMS (2011) United states standards for grades of potatoes. Available at: https://www.ams.usda.gov/sites/default/files/media/Potato_Standard%5B1%5D.pdf.
USDA NASS (2021) Press release. Available at: https://www.nass.usda.gov/Statistics_by_State/Alaska/Publications/Current_News_Release/PT09_1.pdf.
Vaughn, K. C., Lehnen, L. P. (1991). Mitotic disrupter herbicides. Weed Sci. 39 (3), 450–457. doi: 10.1017/S0043174500073215
Visse-Mansiaux, M., Soyeurt, H., Herrera, J. M., Torche, J.-M., Vanderschuren, H., Dupuis, B. (2022). Prediction of potato sprouting during storage. Field Crops Res. 278, 108396. doi: 10.1016/j.fcr.2021.108396
Wickham, H., RStudio (2021) Tidyverse: easily install and load the “Tidyverse” (1.3.1). Available at: https://CRAN.R-project.org/package=tidyverse.
Wiltshire, J. J. J., Cobb, A. H. (1996). A review of the physiology of potato tuber dormancy. Ann. Appl. Biol. 129 (3), 553–569. doi: 10.1111/j.1744-7348.1996.tb05776.x
Keywords: potato storage, essential oils, sprout suppressant, organic agriculture, room temperature, fungicide
Citation: Thoma JL, Cantrell CL, Tamang P and Zheljazkov VD (2023) Determining the optimum mixture of three essential oils for potato sprout suppression at room temperature storage. Front. Plant Sci. 14:1199117. doi: 10.3389/fpls.2023.1199117
Received: 03 April 2023; Accepted: 23 May 2023;
Published: 14 June 2023.
Edited by:
Agnieszka Synowiec, University of Agriculture in Krakow, PolandReviewed by:
Porfirio Gutierrez-Martinez, Instituto Tecnológico de Tepic, MexicoAditi Kundu, Indian Agricultural Research Institute (ICAR), India
Copyright © 2023 Thoma, Cantrell, Tamang and Zheljazkov. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jena L. Thoma, Jena.Thoma@oregonstate.edu
 Jena L. Thoma
Jena L. Thoma Charles L. Cantrell2
Charles L. Cantrell2 Prabin Tamang
Prabin Tamang Valtcho D. Zheljazkov
Valtcho D. Zheljazkov