- 1Department of Plant Physiology, Kerala Agricultural University-College of Agriculture, Vellayani, Thiruvananthapuram, Kerala, India
- 2Department of Genetics and Plant Breeding, University of Agricultural Sciences, Bangalore, Karnataka, India
- 3Division of Crop Production, Indian Council of Agricultural Research-National Rice Research Institute, Cuttack, Odisha, India
- 4Department of Plant Physiology, Kerala Agricultural University-College of Agriculture, Thrissur, Kerala, India
- 5Mulberry Breeding & Genetics Section, Central Sericultural Research and Training Institute-Berhampore, Central Silk Board, Murshidabad, West Bengal, India
- 6Department of Plant Physiology, Kerala Agricultural University-Regional Agricultural Research Station, Kumarakom, Kerala, India
- 7Department of Plant Physiology, Indira Gandhi Krishi Vishwavidyalaya, Raipur, India
- 8The University of Western Australia Institute of Agriculture, The University of Western Australia, Perth, WA, Australia
Abiotic stresses adversely affect rice yield and productivity, especially under the changing climatic scenario. Exposure to multiple abiotic stresses acting together aggravates these effects. The projected increase in global temperatures, rainfall variability, and salinity will increase the frequency and intensity of multiple abiotic stresses. These abiotic stresses affect paddy physiology and deteriorate grain quality, especially milling quality and cooking characteristics. Understanding the molecular and physiological mechanisms behind grain quality reduction under multiple abiotic stresses is needed to breed cultivars that can tolerate multiple abiotic stresses. This review summarizes the combined effect of various stresses on rice physiology, focusing on grain quality parameters and yield traits, and discusses strategies for improving grain quality parameters using high-throughput phenotyping with omics approaches.
1. Introduction
Global warming and accompanying climate variabilities adversely impact global agricultural output, dwindling the production of food grains such as rice (Ramegowda and Senthil-Kumar, 2015). Abiotic stresses such as heat or temperature stress, submergence, drought, or nutritional deficiency create suboptimal environments (Jeyasri et al., 2021) that impair germination, seedling establishment, vegetative growth, flower initiation, panicle growth, grain filling, and productivity (Banerjee and Roychoudhury, 2020; Beena et al., 2021a). In rice, these attributes severely compromise crop establishment, growth (Beena et al., 2021b; Anie et al., 2022; Stephen et al., 2022), grain quality, and productivity (Pravallika et al., 2020; Pathak et al., 2021). Some abiotic pressures in rice-growing environments spur the development and infection of biotic causal agents, aggravating the losses in productivity (Narsai and Whelan, 2013) and grain quality.
As the major staple food crop in the world, reductions in rice production due to climate change will have serious socioeconomic impacts. Many paddy growers experience frequent crop failure, resulting in unprecedented hardships such as starvation and financial pressure (Rejeth et al., 2020). Exposure to multiple abiotic stresses leads to physical and biochemical alterations in crop produce (Manikanta et al., 2020; Ali et al., 2022; Manikanta et al., 2022). Concurrent abiotic stresses damage rice crops more than individual stresses (Pandey et al., 2017), posing various physiological effects that trigger cross-talk reactions that affect rice phenology (Ramu et al., 2016; Ali et al., 2022). While not an abiotic stress component, elevated CO2 (eCO2) can alleviate or aggravate the stress effects.
Rice grain quality is measured primarily on the physical appearance of the grain, mineral content, proportion of amylose and amylopectin starch, aroma, and cooking quality (Chakraborti et al., 2021). Abiotic stresses during grain filling affect milling quality, grain chalkiness, starch composition, and cooking quality (Lanning and Siebenmorgen, 2013). According to ; Liu et al. (2021), high-temperature stress has the greatest impact on grain quality attributes, including reducing the sensory qualities of milled rice. Numerous studies have investigated the fundamentals of rice grain biochemistry, but few have examined how multiple abiotic stresses affect grain quality (Liu et al., 2013; Kadam et al., 2014).
Among abiotic stresses, high temperatures are particularly devastating, decreasing productivity and grain biochemical components. High temperatures decrease photosynthesis and photorespiration, decreasing total biomass production (Moore et al., 2021). High temperatures post-anthesis affect grain quality and appearance and decrease grain production (Dong et al., 2014). Similarly, high temperatures reduce pollen viability and increase spikelet sterility, decreasing grain production and quality (Rang et al., 2011). Extreme temperature stress at the maturity stage abates grain chalkiness, physical appearance, and biochemical properties such as amylose content and protein composition (Ahmed et al., 2015).
Excessive water stresses such as waterlogging and submergence adversely affect rice growth and grain yield. While some historical rice cultivars exhibit notable resilience to submergence, their total yield suffers (Singh et al., 2014). In contrast, modern rice cultivars are sensitive to flooding, often resulting in farmers losing their whole crop. Rice plants can perish soon after flooding due to high energy expenditure and protein hydrolysis during submergence. Flooding degrades the quality of endosperm reserves, adversely affecting the nutritional value and milling and cooking properties of rice grain (Zhou et al., 2020). Flooding at harvest-ready stages results in pre-harvest sprouting, compromising the marketable grain quality (Nonogaki et al., 2018) and reducing the grain’s eating and cooking quality (Zhou et al., 2020).
In recent decades, rice researchers have been working to improve crop yield and quality under stressful situations (Patra et al., 2020). Genomic techniques have been used to investigate how abiotic stresses affect grain development (Verma et al., 2021), with several genetic regulators of tolerance identified and successfully used to improve rice cultivars. For example, genetic loci controlling salinity stress have been discovered and pyramided to develop green super rice types (Pang et al., 2017). Using marker-assisted breeding, Kumar et al. (2018) combined quantitative trait loci (QTL) for submergence and drought tolerance to identify varieties with high yield potential, validating their performance by exposing them to various stresses. However, little information is available on combining stress tolerance and grain quality traits to fulfill food security (Ali et al., 2021).
Another major concern affecting plant growth is eCO2, with carbon dioxide levels expected to reach 685 ppm by 2050, raising the global mean temperature by 3–6°C relative to the pre-industrial era (Kilkis and Caglar, 2022). At the global level, crop models suggest that eCO2 levels could increase precipitation, but large spatial and temporal variabilities exist at the regional scale. Rainfall occurrence and intensity can be unpredictable, creating patches of drought and waterlogging (Walter, 2018). Various experiments have indicated that optimum levels of eCO2 can mitigate the effects of drought stress.
Candidate gene markers can be used to identify genes or QTL for grain production (Azharudheen et al., 2022). Anabolic gene expression requires favorable environmental conditions. Increased temperature impairs starch production, slowing sugar and starch metabolism and thus reducing grain filling and the number of filled grains per panicle (Fahad et al., 2019); a similar response occurs under salt stress (Hussain et al., 2017). Furthermore, significant QTL identified for drought tolerance are crucial for normal reproduction in paddy under drought (Catolos et al., 2017; Feng et al., 2018). The effects of combined mild salinity stress (75 mM NaCl) and moderately high temperatures (30/26°C day/night) were not additive when compared to the individual stresses. The combined stress had longer seedling roots and higher relative water content and Chl b than the salinity treatment alone. He et al. (2018) reported that ABA treatment mimicked protein perturbations in rice subjected to combined salinity stress and desiccation. In another study, Wytynck et al., 2021 reported similar ultrastructural changes in young leaf cells of rice seedlings subjected to salinity or high temperature stress, including the enhanced formation of rough endoplasmic reticulum assembly, reduced cristae formation in mitochondria, and disorganized cell wall fibrils.
QTL conferring tolerance to drought (qDTY1.1, qDTY2.1), salinity (Saltol), and submergence (Sub1) were introgressed by marker-assisted breeding, resulting in a climate-ready rice genotype, Improved White Ponni, a classic example of how information from multiple studies can assist in pyramiding traits for crop improvement (Muthu et al., 2020). The basal methylation patterns in the genomes of Pokkali (salinity tolerant), Nagina 22 (drought tolerant), and IR64 (susceptible) revealed that various stress-associated transcription factors (TFs) and signaling intermediates hypermethylated and thus downregulated to impart stress tolerance relative to IR64 (Garg et al., 2015). In addition, submergence-tolerant rice (FR13A) could withstand the compromise in photosynthetic traits despite lacking innate salinity tolerance (Sarkar et al., 2016). Several combined salinity and submergence stress experiments have revealed various physiological responses in rice. In one study, one week of this combined stress had little impact, while two weeks had detrimental effects on paddy rice, decreasing the relative growth rate, increasing the time to flowering, and decreasing yield (Kurniasih et al., 2021).
This review investigates the individual and interactive effects of various abiotic stresses (e.g., drought, salinity, high temperature, eCO2, submergence, nutrient deficiency) on rice growth, agronomy, and physiological traits, including grain quality and production, and the benefits of genomics for improving rice productivity and grain quality.
2. Physiological and molecular implications of individual stresses in rice yield and quality
2.1. Impact of drought stress on paddy
Climate change disrupts the regularity and magnitude of hydrological events, threatening crop production and affecting food security. The major regions affected include South and Southeast Asia, Sub-Saharan Africa, and Latin America, with unbunded and bunded uplands and shallow rainfed lowlands. Globally, drought stress events account for up to 40% of overall crop and livestock output losses, totaling nearly USD 28 billion (FAO, 2017). In Asia, frequent drought stress affects about 34 million ha of rainfed lowland rice and 8 million ha of upland rice (Barik et al., 2019). Drought stress frequently affects an area of 27 million ha of rainfed rice area (Shamsudin et al., 2016). In 2002, severe drought and depleted soil moisture affected over 65% of South Asia, resulting in considerable rice yield losses (~400 kg ha–1).
Water deficit causes numerous unfavorable changes in rice (Nithya et al., 2020). For example, 15 days of drought stress during reproductive stage reduced rice yields by up to 70%, increasing to up to 88% during flowering and 52% during grain filling. Drought stress at the flowering stage resulted in incomplete panicle exertion, 30% spikelet sterility, and a 20–46% reduction in seed set in a set of rice cultivars (Bahuguna et al., 2018). Drought stress during grain filling stage increases the proportion of chalky grains (Yang et al., 2018). The imposition of drought stress at the onset of anthesis for 30 days reduced the grain yield and harvest index of 25 rice genotypes, with reduced pollen fertility and test weight of grains for most genotypes, compared to irrigated conditions (Ahmad et al., 2022). While leaf rolling is considered a defense mechanism against drought stress, its promptness correlated with anatomical traits rather than water deficit (Nithya et al., 2021. While more leaf rolling occurred in genotypes such as Dangar, water deficit did not affect transpiration (Cal et al., 2019). Drought stress also affects the root system, with the ill-effects on root architecture and yield genotype-dependent (Prince et al., 2015; Beena et al., 2017; Beena et al., 2018c).
Plants have developed numerous adaptive responses to drought stress that aid their survival, including deeper roots, reduced water loss from shoots due to thick cuticle deposition, reduced leaf area, and osmotic adjustment, primarily by maintaining a high internal water status (Beena et al., 2018b; Manikanta et al., 2020; Rejeth et al., 2020). Beena et al. (2012) reported that root architecture, water uptake, and osmotic adjustment are important traits for drought tolerance screening. Physiological and biochemical changes in rice under drought are given in Supplementary Table 1. In rice, QTL mapping has revealed regions responsible for physiological traits, yield, and yield components. Table 1 lists QTL/genes introgressed into rice for drought stress tolerance.
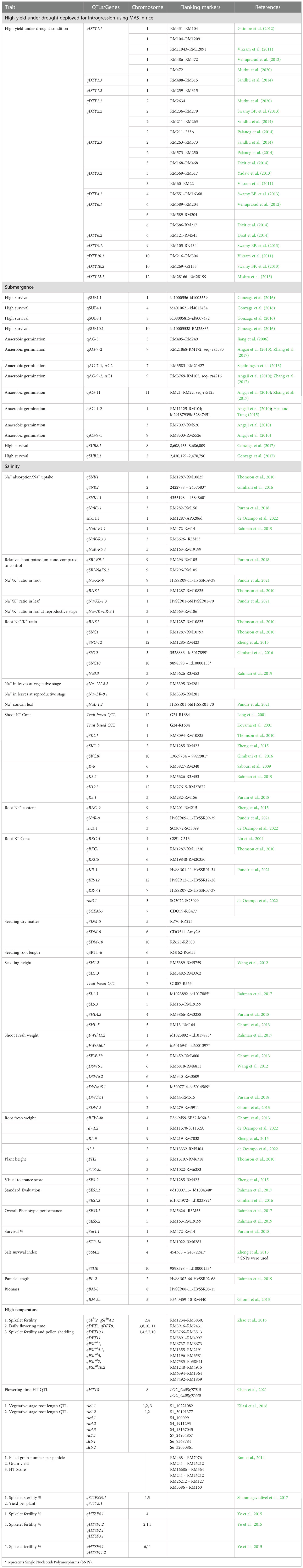
Table 1 Major QTLs reported for physio-morphological traits under various abiotic stress conditions in rice.
2.2. Impacts of submergence on paddy
Rice is adapted to stagnant conditions because its well-developed aerenchyma promotes oxygen transport through roots. However, submergence caused by recurrent flooding can adversely affect plant growth and productivity. In lowland and deep-water rice areas, flooding occurs on more than 16 million ha, with annual economic losses estimated to exceed $600 USD million (www.knowledgebank.irri.org). In addition, unpredictable flash floods can occur at any stage of paddy development.
Submergence reduces the quality and quantity of rice, especially when it occurs during the reproductive and maturity stages. Submergence significantly delays flowering and maturity, reducing grain yield, shoot biomass, harvest index, and yield components (Marndi et al., 2022). Reductions in grain filling, grain number per panicle, and grain weight are primarily responsible for decreased grain production due to submergence (Kato et al., 2014). Submergence during the vegetative stage affects critical grain quality parameters, with a higher proportion of hull, brown rice, and bran in rough rice compared to non-stressed counterparts, as well as chalky grains, breakage during hulling, and reduced proportion of amylose, but increased in crude protein content. Starch accumulation negatively correlated with ADP-glucose pyrophosphorylase activity in submerged rice. ADP-glucose pyrophosphorylase (AGPase) catalyzes the first committed reaction in the pathway of starch synthesis. ADP-glucose pyrophosphorylase is activated by posttranslational redox-modification in response to light and to sugars in leaves of wheat and other plant species (Ferrero et al., 2020).
Yield losses due to submergence are attributable to a smaller sink size/capacity and reduced carbohydrate metabolism and thus reduced partitioning into grain. Djali et al. (2012) reported that submerged rice had higher protein, moisture, and amylase contents than the control plants but lower yield, hardness, stickiness, and brightness. Physiological and biochemical changes in rice under submergence/flash flooding is given in Supplementary Table 1. Further, increased starch and non-structural carbohydrate accumulation positively correlated with survival percentage under submerged conditions (Panda and Sarkar, 2014). Table 1 lists QTL/genes identified in rice for submergence tolerance.
2.3. Impact of salt stress on paddy
Rice is sensitive to soil salinity, which occurs in 25–30% of irrigated regions of rice, equating to more than 1 billion ha of saline or sodic land (Shahid et al., 2018). Rice is more resistant to salt during the germination and vegetative stages than the seedling and reproductive stages. High-yielding rice cultivars at salinity levels >3 dS m–1 suffered yield losses of ~12%, which increased to ~50% at 6 dS m–1 (Kumar and Sharma, 2020). Plants subjected to salt stress have delayed seed germination and seed set, sterile spikelets, and reduced leaf dry matter, leaf area, tiller number, grains per panicle, pollen viability (Reshma et al., 2021).
Salt-stressed rice plants suffer from a reduced water potential, poor nutrient uptake, and increased sodium (Na+) and chlorine (Cl–) uptake. Salinity stress also affects proline and anthocyanin contents, peroxidase activity, and Ca2+, Na+, K+, chlorophyll, and H2O2 concentrations (Negrão et al., 2017). Salt stress significantly reduced amylose concentration in a salt-tolerant rice genotype but not a semi-tolerant genotype, even at low EC (4 mS cm–1) and alkalinity (pH 9.5), while high EC (8 dS m–1) and alkalinity (pH 9.8) significantly reduced starch content in both genotypes, but not the susceptible genotype (Rao et al., 2013). Details of physiological and biochemical changes in rice under salinity is listed in Supplementary Table 1. In addition, salinity (EC 4 and 8 mS/cm) and high alkalinity (pH 9.8) affected gel consistency in the salt-susceptible genotype (Rao et al., 2013). Table 1 lists QTL/genes identified in rice for salinity-related traits.
2.4. Impact of high temperature on paddy
Heat stress in rice is related to specific morphological, physiological, biochemical, and molecular changes. Morphological aspects include genotypes that shield the panicles with their foliage to maintain a lower spikelet temperature for increased spikelet fertility (Beena et al., 2018a). An early morning flowering habit also plays a vital role in plants avoiding high temperatures later in the day (Hirabayashi et al., 2015; Raghunath and Beena, 2021).
Physiological mechanisms that provide heat stress tolerance in rice include an increased membrane stability index, which reduces reactive oxygen species (ROS) damage to biological membranes (Kumar et al., 2016). Increased pollen viability ensures increased fertilization success, maintaining a higher photosynthetic rate to offset yield losses due to excess transpiration rate under heat stress (Sinha et al., 2022). An increased transpiration rate ensures transpirational cooling to prevent ROS increases (Xiong et al., 2014). Physiological adaptations play a critical role in protecting membrane integrity and the biological compounds required to maintain cellular homeostasis. Heat shock proteins (HSPs), which maintain the tertiary structure of proteins, are also critical players in cellular tolerance (Khan and Shahwar, 2020). In addition, enzymatic and non-enzymatic antioxidants such as superoxide dismutase (SOD), peroxidase (POD), glutathione peroxidase (GPX), catalase (CAT), ascorbic acid, phenolic compounds, and carotenoids are crucial for negating the toxic effects of ROS (Irato and Santovito, 2021). Physiological and biochemical changes in rice under high temperature stress is given in Supplementary Table 1.
Marker-assisted introgression of QTL controlling spikelet fertility (Vivitha et al., 2018) and early morning anthesis traits (Ishimaru et al., 2022) under high-temperature conditions have contributed greatly to crop improvement. Table 1 lists QTL/genes identified for physiological and yield traits in rice under high-temperature stress.
2.5. Impact of elevated CO2 on paddy
CO2 levels have risen from 270 ppm during the pre-industrial era (1850s) to 400 ppm. At this rate, atmospheric CO2 (aCO2) will reach eCO2 levels by 2050, estimated at 550 ppm, affecting the morphology, physiology and biochemistry of rice (Abdelhakim et al., 2022). A meta-analysis involving 125 studies on the effect of eCO2 in rice showed that hybrid cultivars respond with higher biomass and yield over popular indica and japonica types, primarily due to increased panicle and spikelet numbers, followed by tiller number. eCO2 levels increase the accumulation of root biomass more than shoot biomass (Wang et al., 2018). A three-year experiment in a free-air CO2 enrichment (FACE) facility revealed a declining proportion of brown, milled, and head rice under eCO2 (200 ppm above ambient) relative to aCO2 (Gao et al., 2021). In addition, the eCO2 increased grain chalkiness, viscosity, and stickiness but, improving palatability; however, the eCO2 compromised the processing quality and nutritional attributes such as protein and mineral contents (Ca, Cu and S; except for K) (Gao et al., 2021). A comparative study at eCO2 (700 ppm) improved seedling emergence, C/N ratio, and biomass in two rice genotypes (IR20 and ADT46). Changes in physiological traits under elevated CO2 is given in Supplementary Table 1. When subjected to brown plant hopper infestation, the eCO2-grown plants had greater insect attack, but insect survival decreased by several days, relative to the control plants (SenthilNathan, 2021). Thus eCO2 poses several ecological effects on rice-based agri-ecosystem.
2.6. Impact of soil nutrient deficit on paddy
Since the green revolution, fertilizer application is essential due to the unintentional emergence of fertilizer-responsive, high-yielding semi-dwarf rice cultivars (Neeraja et al., 2021). Reported poor nutrient use efficiencies in rice, with 30−50% for nitrogen, 30% for phosphorous, and 26% for potassium. In addition to macronutrients, breeders are now paying close attention to micronutrient deficits (‘hidden hunger’) due to human health concerns. The most common micronutrient disorders are Fe insufficiency, Zn deficiency, and B toxicity for wetland rice and Fe and B deficiency and Mn toxicity for upland rice (Shrestha et al., 2020).
Rice is the primary source of nutrition for much of the world’s population. However, rice is deficient in essential fatty acids, vitamins, minerals, phytochemicals, and amino acids (Sultana et al., 2022). Zhou et al. (2018) reported positive effects of nitrogen on the milling and nutritional quality of rice. Increased nitrogen application increased protein content but decreased milling quality, appearance, amylose content, gel consistency, cooking/eating quality, and rice flour viscosity (Zhu et al., 2017). The nitrogen‐efficient line (OsNRT2.3b‐overexpressing (O8) and wild type (WT) were treated with different levels of nitrogen and carbon fertilizers under field conditions to study the effects of different fertilization treatments on rice quality. The results showed that the appearance, nutrition, and taste qualities of O8 were generally high compared with WT under various fertilization treatment conditions (Zhang et al., 2022).
Rice is particularly vulnerable to nutrient deficit stress at the seedling emergence, tillering, panicle initiation, booting, heading, and maturity stages (Shrestha et al., 2020). During the early and mid-phases of grain filling, K and Ca control root exudation, which affects grain quality characteristics such as the proportion of chalky kernels, chalkiness, and amylose content (Lijun et al., 2011). N fertilization can affect micronutrient concentrations.
3. Physiological and molecular implications of combined abiotic stresses on rice yield and quality
3.1. Effect of combined drought and temperature
Drought and high-temperature stress often occur simultaneously in the field, drastically affecting plant growth, development, and yield by inducing physiological, biochemical, and molecular changes and responses that impact various cellular and whole plant functions (Figure 1). Combined effect of drought and high temperature is more severe than individual effects (Dreesen et al., 2012).
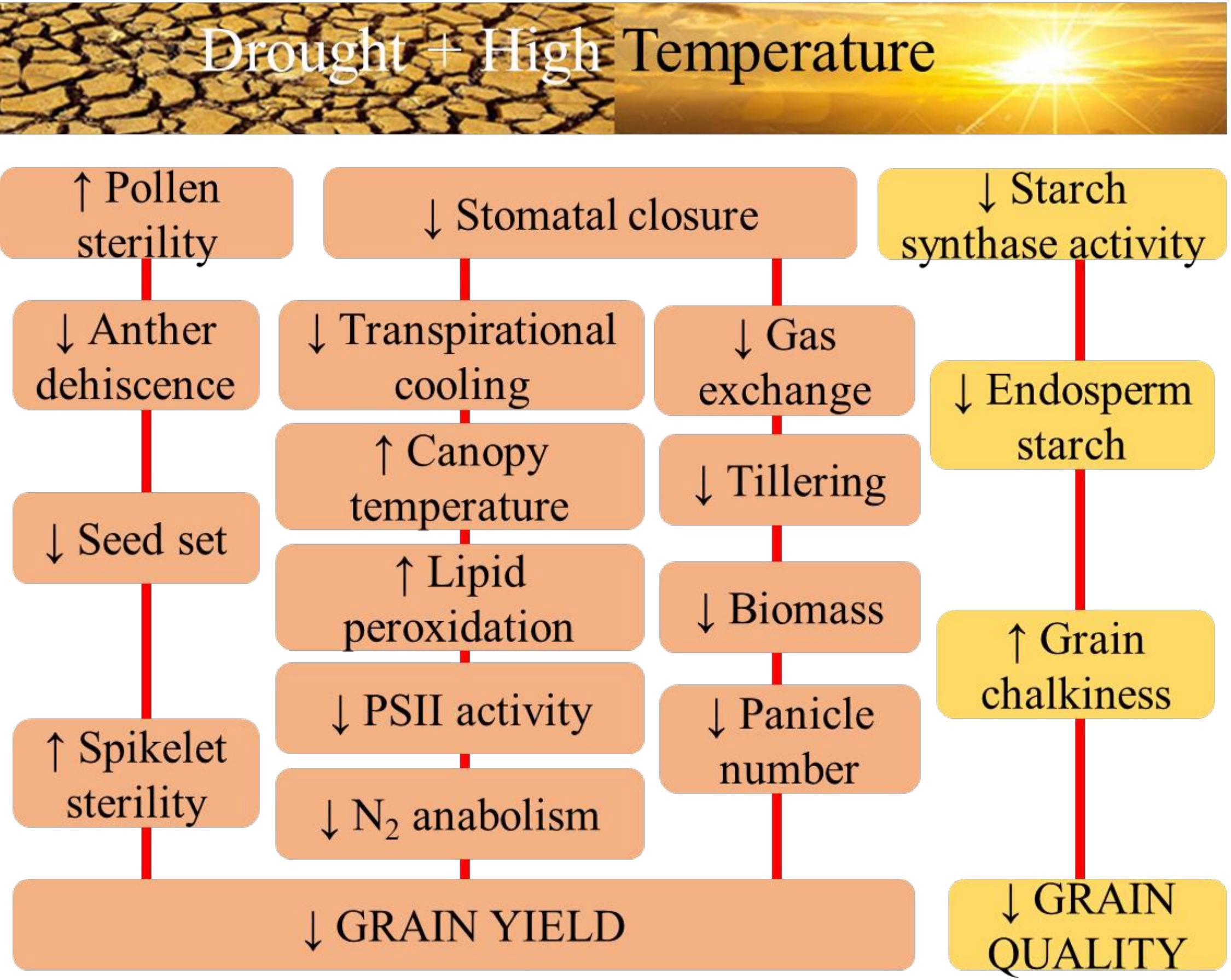
Figure 1 The physiological aspects of sensitivity to combined drought and high temperature stress in rice with respect to grain yield, and quality.
3.1.1. Physiological and genetic components of sensitivity
Drought and heat stress combined affect rice crops at the cell, organ, plant, and canopy level, ultimately reducing growth and yield. The combined stress often has conflicting or antagonistic responses dissimilar to their individual effects. Vapor pressure deficit (VPD) naturally increases during heat waves and droughts, impacting rice physiology (Williams et al., 2014). During heat stress, plants open stomata to cool their leaves by transpiration but cannot open them when faced with combined heat and drought stress (Sinha et al., 2022). In perennial grasses, combined heat and drought stress reduces PSII function, weakens N anabolism, strengthens protein catabolism, and increases lipid peroxidation. Long-term combined heat and drought stress affects growth, leaf gas exchange, and water use efficiency (WUE) in rice, severely reducing total biomass relative to individual stresses (Perdomo et al., 2015; Perdomo et al., 2016).
Rice is more sensitive to drought, heat, and combined stress during the reproductive stage, specifically flowering, than the vegetative stage. Combined heat and drought stress at the seedling and tillering stages resulted in the absence of panicles for seven African rice cultivars (Mukamuhirwa et al., 2019). The number of germinated pollens on the stigma decreased when exposed to heat (81%), drought (59%) and concomitant stress (Rang et al., 2011). Combined heat and drought stress at flowering significantly affected peduncle length, anther dehiscence, pollen number, pollen germination, spikelet fertility, and thus yield in rice (Li et al., 2015; Rang et al., 2010). Heat and drought stress hinder the accumulation of various seed constituents in rice by inhibiting starch processes and protein synthesis. Grain quality is more susceptible to combined stress than yield. High temperature (30°C) inhibited starch metabolism by decreasing starch synthase activity due to thermal denaturation (Pravallika et al., 2020). Reduced grain endosperm starch content is a leading cause of reduced quality and yield in crops subjected to drought and heat (Worch et al., 2011). Similar to drought, heat stress decreases starch content but increases grain protein and mineral concentrations (Mariem et al., 2021). Heat stress reduced amylose content and partially altered the fine structure of amylopectin, indicating that the abnormal expression of the starch synthesizing enzymes is a key factor causing chalkiness (Nakata et al., 2017).
The changing climate is adversely affecting the nutritional quality in terms of mineral content and protein, which will impact human health (Mariem et al., 2021). Higher temperatures also decrease aroma quality in rice. Basmati rice had excellent aroma when grown under relatively cool temperatures in the afternoon (25–32°C) and night (20–25°C) and 70–80% humidity during the primordial and grain-filling stages (Singh et al., 2000). It is important to understand the physiological, biochemical and genetic mechanisms governing the response to combined heat and drought stress to develop strategies to improve stress tolerance.
3.1.2. Physiological and genetic components of tolerance
Plants cope with drought and heat stress through cellular tolerance via metabolic homeostasis, osmotic adjustment, cellular membrane stability, oxidative stress management, production of stress proteins (e.g., late embryogenesis abundant proteins and HSPs) and secondary metabolites, and reducing fatty acid desaturation. Sucrose accumulated in the anthers of rice genotype Nagina 22 under combined drought and high-temperature stress (Li et al., 2015). Heat shock factors (HSFs) and HSPs showed differential upregulation in rice, with HSF7A upregulated under drought stress, HSF2a upregulated under heat stress, and HSP74.8, HSP80.2, and HSP24.1 upregulated under the combined stress (Piveta et al., 2020).
He et al. (2018) noted that a complex regulatory network mobilizes these defenses by involving upstream signaling molecules that transmit the stress signal via hormones, ROS, and nitric oxide (NO). Under drought and heat stress condition, overexpression of the gene Rab7 (OsRab7) improved tolerance in rice by high survival rate, relative water content, chlorophyll content, gas-exchange characteristics, soluble protein content, soluble sugar content, proline content, and activities of antioxidant enzymes (CAT, SOD, APX, POD) than that of the wild-type. In contrast, the levels of hydrogen peroxide, electrolyte leakage, and malondialdehyde of the transgenic lines were significantly reduced when compared to wild-type. Furthermore, the expression of four genes encoding reactive oxygen species (ROS)-scavenging enzymes (OsCATA, OsCATB, OsAPX2, OsSOD-Cu/Zn) and eight genes conferring abiotic stress tolerance (OsLEA3, OsRD29A, OsSNAC1, OsSNAC2, OsDREB2A, OsDREB2B, OsRAB16A, OsRAB16C) was significantly up-regulated in the transformed rice lines as compared to their expression in wild-type (El- Esawi and Alayafi, 2019).
Combined heat and drought stress studies have been undertaken on a few cultivars in rice, with one study identifying Nagina 22 as the only tolerant cultivar (Reshma et al., 2021). Therefore, systematic screening of rice germplasm and mapping populations are needed to identify and introgress QTL into elite cultivars. Genome-wide association studies can identify QTL/genes for dissecting the genetic basis of combined stress tolerance. The grain-filling stage is one of the most important phases that determine yield. Stay green traits can be used as an indicator of sustainable assimilate supply and stem reserve utilization to promote seed filling under stressful conditions (Abdelrahman et al., 2017). There is an immense need to identify plant species and genotypes tolerant to combined stresses (Zandalinas et al., 2018) and tailor genotypes with acceptable performance under combined drought and high-temperature stress for sustainable crop production.
3.2. Effect of combined drought and elevated CO2
3.2.1. Physiological and genetic components of sensitivity
Rice requires 5000 L of water to produce 1 kg biomass and 3,000–5,000 L for 1 kilo grain (Mainuddin et al., 2020). Studies in controlled environment chambers showed that eCO2 reduced evapotranspiration, allowing photosynthesis to continue for 1–2 days longer than aCO2 under drought stress (Supplementary Figure 1). While the saturation point for CO2 is 500 ppm in rice, the down regulation of photosynthesis occurred beyond 900 ppm. In addition, eCO2 attenuated the canopy dark respiration. Dark respiration has physiological relevance, as the energy derived is used for plant growth and metabolism (Zou and Xu, 2021). The reduced stomatal aperture increased the canopy temperature due to the suppression of transpiration. Prolonged exposure to eCO2 also reduced the net photosynthetic rate. The resultant decrease or increase in yield will be location specific, influenced by regional temperatures. Drought stress increases ABA content, which affects CO2 intake. Drought stress also reduces the levels of RuBisCo large and small subunits at the proteomic level. Thus plants cannot harness all of the benefits of CO2 fertilization under drought stress (Perdomo et al., 2017).
Prolonged drought stress significantly decreases some core physiological traits. The eCO2 treatment increased RuBisCo activity by 17.5% compared to the aCO2 treatment. One study showed that eCO2 (700 ppm) treated plants under drought stress had a 40% lower CO2 exchange rate than drought-stressed plants under aCO2 (350 ppm). The Km of RuBisCo also decreased compared to irrigated and drought-stressed plants under aCO2. Plants raised in a CO2-enriched atmosphere had higher RuBisCo content and activity after ~20 days of drought stress, but this comparative advantage did not occur after ~30 days of stress. In this situation, eCO2 plants had inferior physiological traits. Prabnakorn et al. (2018) reported that rice production would suffer more under climate change events, where increases in CO2 cannot mitigate the adverse effects on rice productivity.
3.2.2. Physiological and genetic components of tolerance
Rice grown under eCO2 has more tillers and higher grain yield (Cho and Oki, 2012). eCO2 increased biomass by 5.7% due to an increased leaf area index and leaf water potential in rice (Kumar et al., 2017). Certain simulation models have highlighted the significance of CO2 fertilization in assisting crops to withstand water deficits (Kang et al., 2021). A meta-analysis study on rice, wheat, and maize under increased CO2 levels and drought stress revealed that the CO2 component alone increased grain yield and starch content but decreased protein and mineral contents. The inevitable consequence of stomatal conductance for CO2 leads to loss of water, affecting the proportion of net photosynthesis to transpiration rate (i.e., transpiration efficiency), as a function of leaf anatomical features that determine the utilization of CO2 levels in the atmosphere (Ouyang et al., 2017). Under eCO2 (700 ppm), the imposition of drought stress had less effect on yield attributes than aCO2 and reduced water use by 10% (Shanker et al., 2022) Similarly, combined eCO2 and drought stress maintained canopy net photosynthesis by 6−12%. CO2 supply extended the maintenance of mid-day photosynthesis for a few days, which had an ameliorative effect on rice.
In rice, a soil matric potential of –40 kPa (~43% moisture) or below results in water deficit stress (Kumar et al., 2019). An eCO2 (550 ppm) treatment at a 2°C elevated temperature imparted intrinsic drought (–40 kPa) stress tolerance traits in aerobic rice genotypes (CR-143-2-2, APO, and CR Dhan 201), reducing antioxidant enzyme (SOD, POX, CAT) activities in leaves (Padhy et al., 2018). Drought stress also decreased the aboveground biomass and yield in IR72. However, an eCO2 (700 ppm) treatment maintained higher rice biomass and yield than aCO2 (350 ppm), with both CO2 regimes maintaining a comparable harvest index in corresponding treatments. Both CO2 regimes increased sucrose and reduced starch content in drought-stressed IR72, reducing grain quality. Plants raised under aCO2 conditions exposed to drought stress had more pronounced reductions (45%) in sucrose phosphate synthase activity (sucrose biosynthesis enzyme) than those raised under eCO2 (Wang et al., 2022).
Under drought stress, ABA acts as the primary regulator of stomatal closure, eCO2 delays the sunthesis of ABA. Crosstalk also occurs between these two components at the aquaporin level (Li et al., 2020). A brassinosteroid (BR) treatment ameliorated the ill-effects of drought stress by improving CO2 assimilation (Raghunath et al., 2021; Lakshmi et al., 2022). The induction of endogenous BR under drought stress might help accumulate carbon. A study on d1 mutants for the Gα subunit (of heterotrimeric G protein complex) gene RGA1 (Rice Gα subunit 1) reported that Nipponbare and Taichung 65 had higher mesophyll conductance for CO2 than the wild type and likely had higher WUE and productivity under drought stress (Zait et al., 2021). Overexpression of the OsEPF1 (Epidermal Patterning Factor 1) gene reduced stomatal density in rice, enhancing drought tolerance but compromising yield, which improved with 450–480 ppm CO2 supply. Such a plant type will benefit future climate scenarios with scant rainfall and elevated CO2 (Caine et al., 2019).
3.3. Effect of combined high temperature and eCO2
3.3.1. Physiological and genetic components of sensitivity
Periods of high temperature and eCO2 concentration due to anthropogenic activities threaten rice production (Supplementary Table 2). eCO2 should enhance the photosynthetic rate, increasing total yield and productivity (Kant et al., 2012; Hasegawa et al., 2013) because CO2 is directly involved in major physiological processes such as photosynthesis and stomatal conductance. Rising temperatures reduce rice yield alone or in combination with eCO2 (Wang et al., 2020). A higher respiration rate and declining membrane thermostability reduce rice yield under high night temperature (HNT) conditions (Mohammed and Tarpley, 2010). The decreased membrane stability index in susceptible rice varieties under elevated temperature was related to the extent of lipid peroxidation by ROS (Das et al., 2014; Kumar et al., 2016).
The most sensitive stages to high-temperature stress in rice are booting, anthesis, and fertilization. Several studies have investigated the effect of high temperature and eCO2 concentrations in rice in growth or open-top chambers. The closed chamber experiments revealed that rice is highly susceptible to heat stress and heat-induced spikelet sterility (HISS) at flowering, resulting in yield losses. eCO2 cannot ameliorate yield losses due to the high temperature (Wang et al., 2018). Cai et al. (2016) and Wang et al., (2018, 2020) reported that rising temperatures decreased panicle number per unit area and spikelet number per panicle, decreasing rice yields; these effects escalated under eCO2. eCO2 alone exacerbates HISS as stomatal closure increases the canopy temperature, with a stimulatory effect on biomass production, but an increase in night temperatures counteracts this effect. Significant compromises in yield occur due to the higher respiratory cost of the increased biomass. Night respiration increased by 4–18 mg C hill–1 h–1 in rice genotypes under eCO2 and HNT at various crop stages before heading (Shanker et al., 2022).
The interactive effect of heat stress and eCO2 adversely impacts rice growth, development, and pollen viability (Mittler et al., 2012). Decreased anther dehiscence, poor pollen shedding, poor pollen grain germination on stigmas, and decreased pollen tube elongation led to spikelet sterility under heat stress. Raised night temperatures have more adverse effects than raised day temperatures due to deprived anther dehiscence, impaired pollination, abnormal pollen germination, and floret sterility (Das et al., 2014; Fahad et al., 2018). Floral sterility under high temperatures reduces sink demand due to the reduction in carbohydrate transfer from shoots to grain (Madan et al., 2012). Active selection and breeding for the eCO2 response and HNT-resilient rice are needed to compensate for yield losses.
Heat stress during the reproductive and grain-filling stage reduces rice yield by diminishing the proportion of fertile spikelets (Beena et al., 2018a), shortening the grain-filling period (Ahmed et al., 2015), and reduction in sink activity (Kim et al., 2011). Thus, elevated CO2 and high-temperature stress during flowering and early grain filling significantly reduce rice seed set and thousand-grain weight (Chaturvedi et al., 2017). eCO2 and high temperature also shorten the phenology of rice. Rice grain quality is reflected in parameters such as head and chalky rice rate, amylose and protein contents, and edible quality, as indicated by gel consistency. As CO2 and temperature increased, rice grain appearance initially declined but then improved (Liu et al., 2017). Exposure to high temperature during ripening causes abnormal morphology and grain discoloration in rice, probably due to reduced enzymatic activity related to grain filling, respiratory consumption of assimilation products, and decreased sink activity. Combined eCO2 and high-temperature stress significantly affects amylose content and gel consistency (Supplementary Figure 2). Madan et al. (2012) reported a slight decrease in amylose content and gel consistency in the sensitive genotype IR64, which carries one of two heat-sensitive alleles responsible for amylose accumulation during grain filling.
Soluble protein is the principal holder of plant nitrogen and an important index for measuring leaf aging. Liu et al. (2017) documented that soluble protein content did not vary widely across rice growth stages under eCO2 and high-temperature conditions. In another study, eCO2 stimulated grain production and starch accumulation but negatively affected nutritional traits such as protein and mineral contents (Mariem et al., 2021). The severity of eCO2 and high-temperature stress increases when the stress period coincides with flowering and grain filling and further intensified by high canopy temperatures associated with stomatal opening. Elevated CO2 combined with canopy warming affects plant C, N, and P ratios due to insufficient N uptake and allocation (Wang et al., 2019). The whole plant C/N ratio will remain unaffected if C assimilation and N absorption both increase under eCO2 and HNT conditions (Cheng et al., 2010).
3.3.2. Physiological and genetic components of tolerance
Being a C3 crop, rice theoretically will benefit from the eCO2 fertilization effect, whereas the concomittent increase in temperature will negate the positive benefit of eCO2 (Chaturvedi et al., 2017). At the cellular level, the photosynthetic response to eCO2 will be greater at higher temperatures due to the reduction in RuBisCo activity. In addition, canopy photosynthesis will significantly increase with eCO2, which could negate the adverse effects of high-temperature stress on the C3 pathway (Kadam et al., 2014).
In contrast to high day temperature (HDT) stress, rice lacks an escape or avoidance mechanism under HNT stress (Bahuguna et al., 2014; Bahuguna et al., 2015; Hirabayashi et al., 2015). However, rice may have an enhanced ability to meet the increased carbon demand under increased night respiration, minimizing the negative impact of HNT on grain yield and quality (Impa et al., 2020). The usefulness of increased crop responsiveness to eCO2 under warmer nights has not been investigated. Bahuguna et al. (2022) reported that rice cultivars with significantly higher CO2 responsiveness could fix the additional carbon available under future scenarios.
FACE experiments revealed that eCO2 significantly reduced rice grain quality. However, newly developed heat-tolerant rice cultivars retained high grain quality under eCO2 (Usui et al., 2014), suggesting that current breeding efforts for heat tolerance will be useful for the projected climate change scenarios. Under climate change, the photosynthetic apparatus should be improved and some physiological responses such as stomatal conductance and transpiration rate should be maintained. The sensitivity of rice to HNT could be overcome by surveying germplasm to develop climate-resilient varieties for eCO2 responsiveness through marker development and genomic mapping (Silva et al., 2020; Bahuguna et al., 2022). Supplementary Figure 2 shows the interactive effect of high temperature, and eCO2.
3.4. Effect of combined salinity and drought stress
3.4.1. Physiological and genetic components of sensitivity
Salinity and drought stress disrupt morphological features and physiological and biochemical processes in rice. While these stresses have their respective domains and scopes, drought and salinity stress often co-occur in natural field environments (Fan et al., 2015; Paul et al., 2019; Yadav et al., 2022). The severity and occurrence of combined drought and salinity stress are expected to increase with global environmental changes, which could have profound implications on the food supply. This combined stress is a major limiting factor for rice cultivation and productivity (Landi et al., 2017), triggering oxidative, osmotic, and temperature stresses leading to cellular dehydration and reduced cytosolic and vacuolar volume (Fan et al., 2015). ROS production under combined salinity and drought stress amplifies the damage to proteins, DNA, and membranes (Landi et al., 2017), reducing the photosynthetic rate and efficiency and inducing programmed cell death; thus reducing yields by more than 30% each year (Bhar, 2020).
Several studies have shown that drought and salt stress share similar initial plant responses, resulting in ion toxicity in the long term. Salinity and drought stress both cause physiological water deficits that affect all plant organs to varying degrees. However, plants react to hyper-ionic and hyper-osmotic stress under extended salt stress. Concomitantly large VPD also increases under drought stress. The effect of drought and salinity on photosynthesis ranges from restricted CO2 diffusion into chloroplasts, limited stomatal opening mediated by shoot and root-generated hormones and CO2 transport through the mesophyll, and changes in leaf photochemistry and carbon metabolism (Ma et al., 2020). The combined effect of drought and salinity at early stages (germination, seedling establishment, and tillering) delays transplantation (in rainfed lowlands) or crop establishment (in uplands) and stunts growth, resulting in poor stand establishment and ultimately reducing the number of panicles per unit area and panicle size. The combined stresses at the reproductive stage (panicle initiation, flowering, and grain filling) cause varying degrees of spikelet sterility and poor grain filling, with greater detrimental effects on grain yield (Ali et al., 2022).
3.4.2. Physiological and genetic components of tolerance
Most drought and salt stress studies focus on roots and shoots, with measurements of physiological and genetic parameters (Qin et al., 2020; Hao et al., 2022). Among them, ABA plays an important role in plant responses to abiotic stresses (Zhao et al., 2021). The overexpression of OsPYL5 can improve drought and salt tolerance through ABA-mediated processes (Ruiz et al., 2021). Secondary messengers such as Ca2+ and ROS can alleviate osmotic stress damage and improve drought and salt tolerance through ABA-dependent/independent pathways. In addition, H2O2 plays a vital role in stomatal closure through ABA-dependent and ABA-independent pathways (Chen et al., 2021). Under drought and salt stress, stress-response genes increase plant resistance by activating the associated proteins and accumulating protective metabolites. Downregulating the expression of DST1 (DROUGHT AND SALT TOLERANT 1), ABIL2 (ABL INTERACTOR-LIKE PROTEIN 2), and HDA704 (histone deacetylase) positively regulates drought and stress tolerance in rice. hda704 knockdown mutants exhibited susceptibility to drought and salinity stress. HDA704 imparts drought tolerance by promoting stomatal closure (Zhao et al., 2021). Shikimate pathway is known to be activated under abiotic stress conditions, such as drought and salinity, resulting in the accumulation of high levels of aromatic amino acids and related secondary metabolites (Francini et al., 2019). Overexpression of OsSKL2 in rice increased tolerance to salinity, drought and oxidative stress by increasing antioxidant enzyme activity, and reducing levels of H2O2, malondialdehyde, and relative electrolyte leakage (Jiang et al., 2022).
3.5. Effect of combined salinity and submergence stress
The changing climate and resultant rise in sea water levels lead to unexpected spells of multiple abiotic stresses at different stages of paddy production. In coastal areas, increasing temperatures, erratic rainfall, and inundation of saline water due to sea-level rises can change the micro-environment in fields. Studies are limited in this arena for rice. Tolerant rice genotypes adapt to combined salinity and submergence due to the presence of well-developed constitutive aerenchyma and increased ethylene production and respiratory burst oxidase homolog (RBOH) signaling. RBOH-mediated ROS production resulted in the development of constitutive aerenchyma in a saline and flooding tolerant rice variety, Rashpanjor (Chakraborty et al., 2021). Chlorophyll fluorescence imaging identified tolerant varieties under combined salinity and partial submergence (Pradhan et al., 2018).
3.6. Effect of combined salinity and high temperature
High temperature and salinity in tropical coastal belts derail rice productivity. Exposure to salinity and high temperature, in combination or in tandem, changes rice growth patterns, defense mechanisms, reproduction, and survival functions, reducing shoot fresh weight, relative water content, photosynthetic pigments, and protein content and increasing proline and SOD activities. A saline-tolerant rice variety, YNU31-2-4, under combined high temperature and salinity stress, downregulated K+ transporter OsHKT1;5 and upregulated OsHSP18, OsP5CS, and Na+/H+ antiporter OsNHX (Nahar et al., 2022). However, under combined stress condition Nagina-22 performed well than other genotypes in terms of proline content, cell membrane stability index, SOD activity, pollen viability, spikelet fertility, and yield per plant and lower lipid peroxidation and Na+/K+ ratio than susceptible genotypes (Ali et al., 2021). Combined effects of various abiotic stresses on physio-biochemical traits in rice is given in Supplementary Table 2. Figure 2 shows the interactive effect of high temperature, eCO2. and drought.
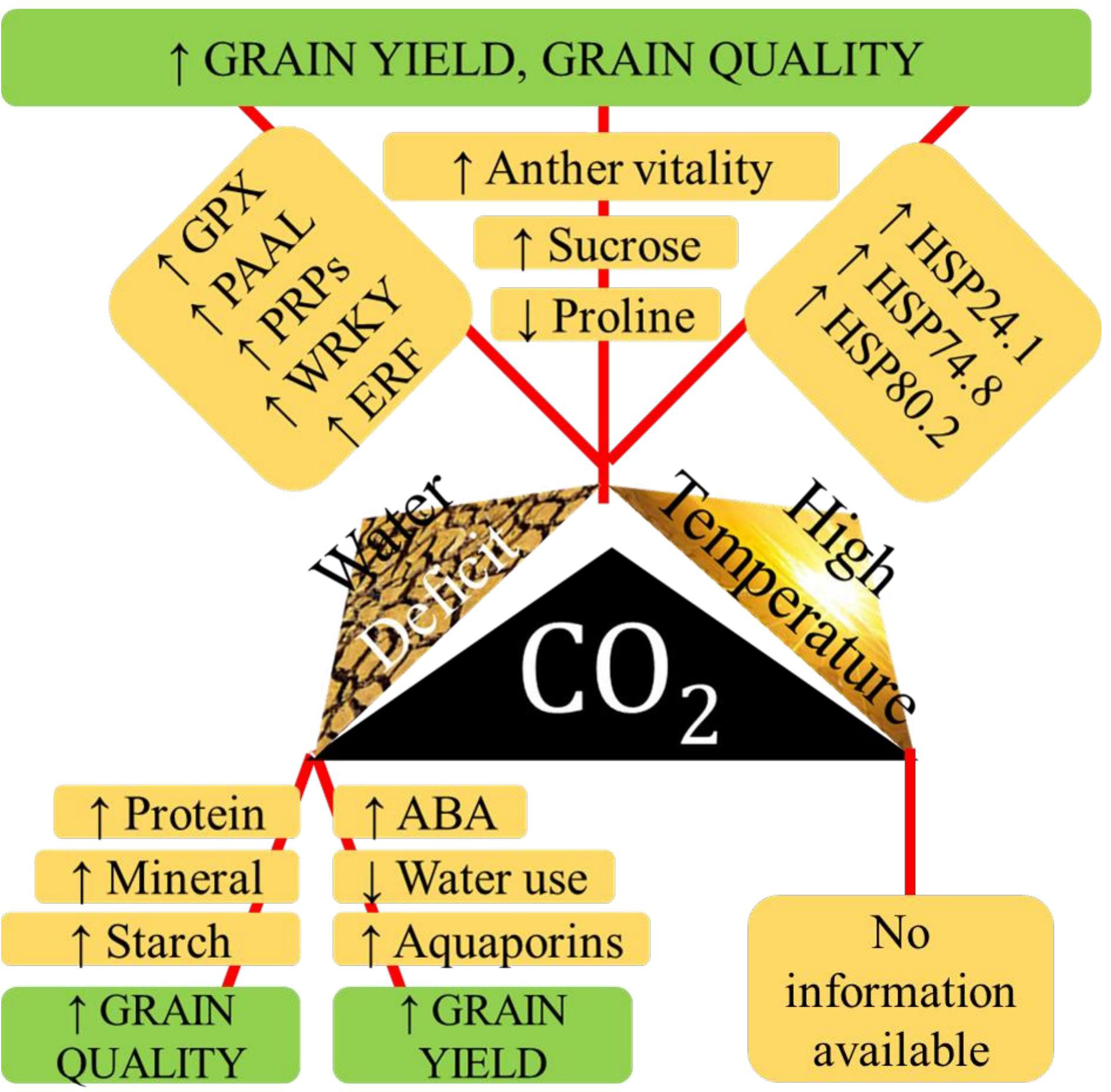
Figure 2 The physiological aspects of tolerance to various paired combinations of high temperature, water deficit stress and elevated CO2 with respect to grain yiled, and quality in rice.
4. Conclusion
Rice (Oryza sativa L.) is the staple food crop consumed by much of the world’s population. Projected rice statistics for 2021–22 estimated global production of 505.4 million tons, an increase of 1.9 million tons than previous year, mainly attributed to China, Bangladesh, South Korea, and Taiwan. Paddy is cultivated primarily in tropical climates, where water scarcity, high temperatures, salinity, and nutrient deficits can significantly reduce yields. Rapid fluctuations in environmental conditions can impact the adaptive ability of rice, further impairing its productivity. Various abiotic stresses affect seed germination, seedling establishment, shoot and root lengths, plant height, days to flowering, grain filling, maturity, and grain quality. Abiotic stresses during both vegetative and reproductive stage compromise panicle development and grain filling, impacting overall grain production and jeopardizing global food security. Genomics and QTL-based approaches have helped identify genes and loci responsible for abiotic stress tolerance in rice. Introgressing these newly identified molecular candidates can improve rice physiological growth under suboptimal conditions and stimulate reproductive development and grain production. However, further studies involving next-generation sequencing platforms and high-throughput phenotyping will help identify novel candidate genes responsible for regulating grain development in combined stress situations and pave the way for developing climate-ready crops.
Author contributions
BR, RS and GK conceived and designed the study; GK prepared the figures; All authors review the literature, synthesize the data/material and draft the review; KS critically edited the manuscript; RS, MT, GK, DU, ST, CA, BS, BM and BR, helped in developing main andsupplementary tables. All authors have read and agreed to the published version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.996514/full#supplementary-material
References
Abdelrahman, M., El-Sayed, M., Jogaiah, S., Burritt, D. J., Tran, L. S. P. (2017). The “Stay-green” trait and phytohormone signaling networks in plants under heat stress. Plant Cell Rep. 36, 1009–1025. doi: 10.1007/s00299-017-2119-y
Abdelrahman, L. O. A., Zhou, R., Ottosen, C.O. (2022). Physiological Responses of Plants to Combined Drought and Heat under Elevated CO2. Agronomy 12, 2526. doi: 10.3390/agronomy12102526
Abdelhakim, L. O. A., Zhou, R., Ottosen, C.-O.. (2022). Physiological Responses of Plants to Combined Drought and Heat under Elevated CO2. Agronomy 12, 2526. doi: 10.3390/agronomy12102526
Ahmad, H., Zafar, S. A., Naeem, M. K., Shokat, S., Inam, S., Rehman, M. A., et al. (2022). Impact of preanthesis drought stress on physiology, yield-related traits, and drought-responsive genes in green super rice. Front. Genet. 13. doi: 10.3389/fgene.2022.832542
Ahmed, N., Tetlow, I. J., Nawaz, S., Iqbal, A., Mubin, M., Nawaz ul Rehman, M. S., et al. (2015). Effect of high temperature on grain filling period, yield, amylose content and activity of starch biosynthesis enzymes in endosperm of basmati rice. J. Sci. Food Agric. 95 (11), 2237–2243. doi: 10.1002/jsfa.6941
Ali, A., Beena, R., Manikanta, Ch L. N., Swapna, A., Soni, K. B., Viji, M. M. (2022). Molecular characterization and varietal identification for multiple abiotic stress tolerance in rice (Oryza sativa l.). Oryza -An Int. J. rice. 59 (1), 140–15). doi: 10.35709/ory.2022.59.1.7
Ali, B. S. A., Beena, R., Stephen, K. (2021). Combined effect of high temperature and salinity on growth and physiology of rice (Oryza sativa l.). Agric. Res. J. 58 (5), 783–788. doi: 10.5958/2395-146X.2021.00111.3
Angaji, S. A., Septiningsih, E. M., Mackill, D. J., Ismail, A. M. (2010). QTLs associated with tolerance of flooding during germination in rice (Oryza sativa l.).". Euphytica 172 (2), 159–168. doi: 10.1007/s10681-009-0014-5
Anie, T., Beena, R., Lakshmi, G., Soni, K. B., Swapna, A., Viji, M. M. (2022). Changes in sucrose metabolic enzymes to water stress in contrasting rice genotypes. Plant Stress 5. doi: 10.1016/j.stress.2022.100088
Azharudheen, M. T. P., Nayak, A. K., Behera, S., Anilkumar, C., Marndi, B. C., Moharana, D., et al. (2022). Genome-wide association analysis for plant type characters and yield using cgSSR markers in rice (Oryza sativa l.). Euphytica 218 (69). doi: 10.1007/s10681-022-03021-z
Bahuguna, R. N., Chaturvedi, A. K., Pal, M., Viswanathan, C., Jagadish, S. K., Pareek, A. (2022). Carbon dioxide responsiveness mitigates rice yield loss under high night temperature. Plant Physiol. 188 (1), 285–300. doi: 10.1093/plphys/kiab470
Bahuguna, R. N., Jagadish, K. S. V., Coast, O., Wassmann, R. (2014). Plant abiotic stress: Temperature extremes. Encyclopedia Agric. Food Syst., 330–334. doi: 10.1016/B978-0-444-52512-3.00172-8
Bahuguna, R. N., Jha, J., Pal, M., Shah, D., Lawas, L. M., Khetarpal, S., et al. (2015). Physiological and biochemical characterization of NERICA-L-44: a novel source of heat tolerance at the vegetative and reproductive stages in rice. Physiologia plantarum. 154 (4), 543–559. doi: 10.1111/ppl.12299
Bahuguna, R. N., Tamilselvan, A., Muthurajan, R., Solis, C. A., Jagadish, S. V. K. (2018). Mild preflowering drought priming improves stress defences, assimilation and sink strength in rice under severe terminal drought. Funct. Plant Biol. 45, 827–839. doi: 10.1071/FP17248
Banerjee, A., Roychoudhury, A. (2020). “Rice grain quality and abiotic stress: Genomics and biotechnological perspectives,” in Rice research for quality improvement: Genomics and genetic engineering: Volume 1: Breeding techniques and abiotic stress tolerance. Ed. Roychoudhury, A. (Singapore: Springer), 747–752. doi: 10.1007/978-https://doi.org/10.1007/978-981-15-4120-9_30
Barik, S. R., Pandit, E., Pradhan, S. K., Mohanty, S. P., Mohapatra, T. (2019). Genetic mapping of morpho-physiological traits involved during reproductive stage drought tolerance in rice. PloS One 14, e0214979. doi: 10.1371/journal.pone.0214979
Beena, R., Praveenkumar, V. P., Vighneswaran, V., Narayankutty, M. C. (2018c). Bulked line analysis: A useful tool to identify microsatellite markers linked to drought tolerance in rice. Indian J. Plant Physiol. 23 (1), 7–15. doi: 10.1007/s40502-017-0321-0
Beena, R., Praveenkumar, V. P., Vighneswaran, V., Sindhumol, P., Narayankutty, M. C. (2017). Phenotyping for root traits and carbon isotope in rice genotypes of kerala. Oryza Int. J. Rice. 54 (3), 282–289. doi: 10.5958/2249-5266.2017.00039.X
Beena, R., Silvas, K., Nithya, N., Manickavelu, A., Sah, R. P., Abida, P. S., et al. (2021a). Association mapping of drought tolerance and agronomic traits in rice (Oryza sativa l.) landraces. BMC Plant Biol. 21 (1), 1–21. doi: 10.1186/s12870-021-03272-3
Beena, R., Thandapani, V., Chandrababu, R. (2012). Physio-morphological and biochemical characterization of selected recombinant inbred lines of rice for drought resistance. Indian J. Plant Physiol. 17 (2), 189–193.
Beena, R., Veena, V., Jaslam, M. P. K., Nithya, N., Adarsh, V. S. (2021b). Germplasm innovation for high temperature tolerance from traditional rice accessions of kerala using genetic variability, genetic advance, path coefficient analysis and principal component analysis. J. Crop Sci. Biotechnol. 24 (5), 555–566. doi: 10.1007/s12892-021-00103-7
Beena, R., Veena, V., Narayankutty, M. C. (2018b). Evaluation of rice genotypes for acquired thermo-tolerance using temperature induction response technique. Oryza-An Int. J. Rice. 55 (2), 285–291. doi: 10.5958/2249-5266.2018.00035.8
Beena, R., Vighneswaran, V., Sindumole, P., Narayankutty, M. C., Voleti, S. R. (2018a). Impact of high temperature stress during reproductive and grain filling stage in rice. Oryza Int. J. Rice. 55 (1), 126–133. doi: 10.5958/2249-5266.2018.00015.2
Bhar, A. (2020). “Rice tolerance to multiple abiotic stress: Genomics and genetic engineering,” in Rice research for quality improvement: Genomics and genetic engineering (Singapore: Springer), 591–615.
Buu, B. C., Ha, P. T. T., Tam, B. P., Nhien, T. T., Hieu, N. V., Phuoc, N. T., et al. (2014). Quantitative trait loci associated with heat tolerance in rice (Oryza sativa l.). Plant Breed. Biotech. 2 (1), 14~24. doi: 10.9787/PBB.2014.2.1.014
Caine, R. S., Yin, X., Sloan, J., Harrison, E. L., Mohammed, U., Fulton, T., et al. (2019). Rice with reduced stomatal density conserves water and has improved drought tolerance under future climate conditions. New Phytol. 221, 371–384. doi: 10.1111/nph.15344
Cai, C., Yin, X., He, S., Jiang, W., Si, C., Struik, P. C., et al. (2016). Responses of wheat and rice to factorial combinations of ambient and elevated CO2 and temperature in FACE experiments. Global Change Biol. 22 (2), 856–874. doi: 10.1111/gcb.13065
Cal, A. J., Sanciangco, M., Rebolledo, M. C., Luquet, D., Torres, R. O., McNally, K. L., et al. (2019). Leaf morphology, rather than plant water status, underlies genetic variation of rice leaf rolling under drought. Plant Cell Environ. 42 (5), 1532–1544. doi: 10.1111/pce.13514
Catolos, M., Sandhu, N., Dixit, S., Shamsudin, N. A. A., Naredo, M. E. B., McNally, K. L., et al. (2017). Genetic loci governing grain yield and root development under variable rice cultivation conditions. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.01763
Chakraborti, M., Anilkumar, C., Verma, R. L., Abdul Fiyaz, R., Raj, K. R., Patra, B. C., et al. (2021). Rice breeding in India: eight decades of journey towards enhancing the genetic gain for yield, nutritional quality, and commodity value. Oryza 58, 69–88. doi: 10.35709/ory.2021.58.spl.2
Chakraborty, K., Ray, S., Vijayan, J., Molla, K., Nagar, R., Jene, P., et al. (2021). Preformed aerenchyma determines the differential tolerance response under partial submergence imposed by fresh and saline water flooding in rice. Physiologia Plantarum 173 (4). doi: 10.1111/ppl.13536
Chaturvedi, A. K., Bahuguna, R. N., Shah, D., Pal, M., Jagadish, S. V. (2017). High temperature stress during flowering and grain filling offsets beneficial impact of elevated CO2 on assimilate partitioning and sink-strength in rice. Sci. Rep. 7 (1), 1–13. doi: 10.1038/s41598-017-07464-6
Cheng, W., Sakai, H., Yagi, K., Hasegawa, T. (2010). Combined effects of elevated CO2 and high night temperature on carbon assimilation, nitrogen absorption and the allocations of c and n by rice (Oryza sativa l.). Agri For. meteorology 150 (9), 1174–1181. doi: 10.1016/j.agrformet.2010.05.001
Chen, L., Wang, Q., Tang, M., Zhang, X., Pan, Y., Yang, X., et al. (2021). QTL mapping and identification of candidate genes for heat tolerance at the flowering stage in rice. Front. Plant Sci. 11:621871. doi: 10.3389/fgene.2020.621871
Cho, J., Oki, T. (2012). Application of temperature, water stress, CO2 in rice growth models. Rice. 5 (1), 10. doi: 10.1186/1939-8433-5-10
Das, S., Krishnan, P., Nayak, M., Ramakrishnan, B. (2014). High temperature stress effects on pollens of rice (Oryza sativa l.) genotypes. Environ. Exp. Bot. 101, 36–46. doi: 10.1016/j.envexpbot.2014.01.004
de Ocampo, M. P., Thomson, M. J., Mitsuya, S., Yamauchi, A., Ismail, A. M. (2022). QTL mapping under salt stress in rice using a kalarata–azucena population. Euphytica 218 (6), 1–15. doi: 10.1007/s10681-022-03026-8
Dixit, S., Singh, A., Sta Cruz, M. T., Maturan, P. T., Amante, M., Kumar, A. (2014). Multiple major QTL lead to stable yield performance of rice cultivars across varying drought intensities. BMC Genet. 15, 1–13. doi: 10.1186/1471-2156-15-16
Djali, M., Nurhasanah, S., Lembong, E., Rahmat, R. (2012). The effect of flooding on rice characteristics of’Cilamaya muncul’on various days after planting during the last reproductive and maturation phase. II Asia Pacific Symposium Postharvest Res. Educ. Extension: APS2012 1011, 285–291. doi: 10.17660/ActaHortic.2013.1011.35
Dong, W., Chen, J., Wang, L., Tian, Y., Zhang, B., Lai, Y., et al. (2014). Impacts of nighttime post-anthesis warming on rice productivity and grain quality in East China. Crop J. 2, 63–69. doi: 10.1016/j.cj.2013.11.002
Dreesen, F. E., De Boeck, H. J., Janssens, I. A., Nijs, I. (2012). Summer heat and drought extremes trigger unexpected changes in productivity of a temperate annual/biannual plant community. Environ. Exp. Bot. 79, 21–30. doi: 10.1016/j.envexpbot.2012.01.005
El- Esawi, M. A., Alayafi, A. A. (2019). Overexpression of rice Rab7 gene improves drought and heat tolerance and increases grain yield in rice (Oryza sativa l.). Genes 10, 56. doi: 10.3390/genes10010056
Fahad, S., Ihsan, M. Z., Khaliq, A., Daur, I., Saud, S., Alzamanan, S., et al. (2018). Consequences of high temperature under changing climate optima for rice pollen characteristics-concepts and perspectives. Arch. Agron. Soil Sci. (Singapore: Springer) 64 (11), 1473–1488. doi: 10.1080/03650340.2018.1443213
Fahad, S., Noor, M., Adnan, M., Khan, M. A., Rahman, I. U., Alam, M., et al. (2019). “Abiotic stress and rice grain quality,” in In advances in rice research for abiotic stress tolerance (Woodhead Publishing), 571–583. doi: 10.1016/B978-0-12-814332-2.00028-9
Fan, Y., Shabala, S., Ma, Y., Xu, R., Zhou, M. (2015). Using QTL mapping to investigate the relationships between abiotic stress tolerance (drought and salinity) and agronomic and physiological traits. BMC Genomics 16 (1), 1–11. doi: 10.1186/s12864-015-1243-8
FAO (2017). The impact of natural hazards and disasters on agriculture and food and nutrition security: A call for action to build resilient livelihoods.
Feng, B., Chen, K., Cui, Y., Wu, Z., Zheng, T., Zhu, Y., et al. (2018). Genetic dissection and simultaneous improvement of drought and low nitrogen tolerances by designed QTL pyramiding in rice. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.00306
Ferrero, D. M. L., Piattoni, C. V., Asencion Diez, M. D., Rojas, B. E., Hartman, M. D., Ballicora, M. A., et al. (2020). Phosphorylation of ADP-glucose pyrophosphorylase during wheat seeds development. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.01058
Francini, A., Giro, A., Ferrante, A. (2019). Biochemical and molecular regulation of phenylpropanoids pathway under abiotic stresses. Plant Sign Mole 2019, 183–192. doi: 10.1016/B978-0-12-816451-8.00011-3
Gao, B., Hu, S., Jing, L., Wang, Y., Zhu, J., Wang, K., et al. (2021). Impact of elevated CO2 and reducing the source- sink ratio by partial defoliation on rice grain quality - a 3-year free-air CO2 enrichment study. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.788104
Garg, R., Chevala, V. V. S. N., Shankar, R., Mukesh Jain, M. (2015). Divergent DNA methylation patterns associated with gene expression in rice cultivars with contrasting drought and salinity stress response. Sci. Rep. 5, 14922. doi: 10.1038/srep14922
Ghimire, K. H., Quiatchon, L. A., Vikram, P., Swamy, B. M., Dixit, S., Ahmed, H., et al. (2012). Identification and mapping of a QTL (qDTY1. 1) with a consistent effect on grain yield under drought. Field Crops Res. 131, 88–96. doi: 10.1016/j.fcr.2012.02.028
Ghomi, K., Rabiei, B., Sabouri, H., Sabouri, A. (2013). Mapping QTLs for traits related to salinity tolerance at seedling stage of rice (Oryza sativa l.): an agrigenomics study of an Iranian rice population. Omics: J. Integr. Biol. 17 (5), 242–251. doi: 10.1089/omi.2012.0097
Gimhani, D. R., Gregorio, G. B., Kottearachchi, N. S., Samarasinghe, W. L. G. (2016). SNP-based discovery of salinity-tolerant QTLs in a bi-parental population of rice (Oryza sativa). Mol. Genet. Genomics 291 (6), 2081–2099. doi: 10.1007/s00438-016-1241-9
Gonzaga, Z. J. C., Carandang, J., Sanchez, D. L., Mackill, D. J., Septiningsih, E. M. (2016). Mapping additional QTLs from FR13A to increase submergence tolerance in rice beyond SUB1. Euphytica 209 (3), 627–636. doi: 10.1007/s10681-016-1636-z
Gonzaga, Z. J. C., Carandang, J., Singh, A., Collard, B. C. Y., Thomson, M. J., Septiningsih, E. M. (2017). Mapping QTLs for submergence tolerance in rice using a population fixed for SUB1A tolerant allele. Mol. breeding. 37 (4), 1–10. doi: 10.1007/s11032-017-0637-5
Hao, Z., Ma, S., Liang, L., Feng, T., Xiong, M., Lian, S., et al. (2022). Candidate genes and pathways in rice co-responding to drought and salt identified by gcHap network. Int. J. Mol. Sci. 23 (7), 4016. doi: 10.3390/ijms23074016
Hasegawa, T., Sakai, H., Tokida, T., Nakamura, H., Zhu, C., Usui, Y., et al. (2013). Rice cultivar responses to elevated CO2 at two free-air CO2 enrichment (FACE). Japan. Funct. Plant Biol. 40, 148–159. doi: 10.1071/FP12357
He, M., He, C. Q., Ding, N. Z. (2018). Abiotic stresses: General defenses of land plants and chances for engineering multistress tolerance. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.01771
Hirabayashi, H., Sasaki, K., Kambe, T., Gannaban, R. B., Miras, M. A., Mendioro, M. S., et al. (2015). qEMF3, a novel QTL for the early-morning flowering trait from wild rice, oryza officinalis, to mitigate heat stress damage at flowering in rice, o. sativa. J. Exp. Bot. 66 (5), 1227–1236. doi: 10.1093/jxb/eru474
Hussain, S., Jun-hua, Z., Chu, Z., Lian-feng, H., Xiao-chuang, C., Sheng-miao, Y., et al. (2017). Effects of salt stress on rice growth, development characteristics, and the regulating ways: A review. J. Integr. Agric. 16 (11), 2357–2374. doi: 10.1016/S2095-3119(16)61608-8
Hsu, S.-K., Chih-Wei, T. (2015). "Genetic mapping of anaerobic germination-associated QTLs controlling coleoptile elongation in rice.". Rice 8 (1), 1–12. doi: 10.1186/s12284-015-0072-3
Impa, S. M., Vennapusa, A. R., Bheemanahalli, R., Sabela, D., Boyle, D., Walia, H., et al. (2020). High night temperature induced changes in grain starch metabolism alters starch, protein, and lipid accumulation in winter wheat. Plant Cell Environ. 43 (2), 431–447. doi: 10.1111/pce.13671
Irato, P., Santovito, G. (2021). Enzymatic and non-enzymatic molecules with antioxidant function. Antioxidants 10, 579. doi: 10.3390/antiox10040579
Ishimaru, T., Hlaing, K. T., Oo, Y. M., Lwin, T. M., Sasaki, K., Lumanglas, P. D., et al. (2022). An early-morning flowering trait in rice can enhance grain yield under heat stress field conditions at flowering stage. Field Crops Res. doi: 10.1016/j.fcr.2021.108400
Jeyasri, R., Muthuramalingam, P., Satish, L., Pandian, S. K., Chen, J.-T., Ahmar, S., et al. (2021). An overview of abiotic stress in cereal crops: Negative impacts, regulation, biotechnology and integrated omics. Plants. 10, 1472. doi: 10.3390/plants10071472.https://doi.org/10.3390/plants10071472
Jiang, L., Shijia, L., Mingyu, H., Jiuyou, T., Liangmin, C., Huqu, Z., et al. (2006). Analysis of QTLs for seed low temperature germinability and anoxia germinability in rice (Oryza sativa l.). Field Crops Res. 98 (1), 68–75. doi: 10.1016/j.fcr.2005.12.015
Jiang, Y., Peng, X., Zhang, Q., Liu, Y., Li, A., Cheng, et al. (2022). Regulation of drought and salt tolerance by OsSKL2 and OsASR1 in rice. l. Rice 15, 46. doi: 10.1186/s12284-022-00592-2
Kadam, N. N., Xiao, G., Melgar, R. J., Bahuguna, R. N., Quinones, C., Tamilselvan, A., et al. (2014). Agronomic and physiological responses to high temperature, drought and elevated CO2 interactions in cereals. Adv. Agron. 127, 111–156. doi: 10.1016/B978-0-12-800131-8.00003-0
Kang, H., Sridhar, V., Mainuddin, M., Trung, L. D. (2021). Future rice farming threatened by drought in the lower Mekong basin. Sci. Rep. 11 (1), 1–15. doi: 10.1038/s41598-021-88405-2
Kant, S., Seneweera, S., Rodin, J., Materne, M., Burch, D., Rothstein, S. J., et al. (2012). Improving yield potential in crops under elevated CO2: integrating the photosynthetic and nitrogen utilization efficiencies. Front. Plant Sci. 3. doi: 10.3389/fpls.2012.00162
Kato, Y., Collard, B. C., Septiningsih, E. M., Ismail, A. M. (2014). Physiological analyses of traits associated with tolerance of long-term partial submergence in rice. AoB Plants 6, plu058. doi: 10.1093/aobpla/plu058
Kilasi, N. L., Singh, J., Vallejos, C. E., Ye, C., Jagadish S.V, K., Paul Kusolwa, P., et al. (2018). Heat stress tolerance in rice (Oryza sativa l.): Identification of quantitative trait loci and candidate genes for seedling growth under heat stress. Front. Plant Sci 9:1578. doi: 10.3389/fpls.2018.01578
Kilkis, B., Caglar, M. (2022). “May. exergy guided optimization model for decarbonized solar districts,” in CLIMA 2022 conference.
Kim, J., Shon, J., Lee, C. K., Yang, W., Yoon, Y., Yang, W. H., et al. (2011). Relationship between grain filling duration and leaf senescence of temperate rice under high temperature. Field Crops Res. 122 (3), 207–213. doi: 10.1016/j.fcr.2011.03.014
Koyama, M. L., Levesley, A., Koebner, R. M., Flowers, T. J., Yeo, A. R. (2001). Quantitative trait loci for component physiological traits determining salt tolerance in rice. Plant Physiol. 125 (1), 406–422. doi: 10.1104/pp.125.1.406
Kumar, A., Nayak, A. K., Das, B. S., Panigrahi, N., Dasgupta, P., Mohanty, S., et al. (2019). Effects of water deficit stress on agronomic and physiological responses of rice and greenhouse gas emission from rice soil under elevated atmospheric CO2. Sci. Total Environ. 650, .2032–.2050. doi: 10.1016/j.scitotenv.2018.09.332
Kumar, A., Sandhu, N., Dixit, S., Yadav, S., Swamy, B. P. M., Shamsudin, N. A. A. (2018). Marker-assisted selection strategy to pyramid two or more QTLs for quantitative trait-grain yield under drought. Rice 11, 35. doi: 10.1186/s12284-018-0227-0
Kumar, N., Shankhdhar, S. C., Shankhdhar, D. (2016). Impact of elevated temperature on antioxidant activity and membrane stability in different genotypes of rice (Oryza sativa l.). Indian J. Plant Physiol. 21 (1), 37–43. doi: 10.1007/s40502-015-0194-z
Kumar, P., Sharma, P. K. (2020). Soil salinity and food security in India. Front. Sustain. Food Syst. 4, 533781. doi: 10.3389/fsufs.2020.533781
Kurniasih, B., Tarigan, I., Firmansyah, E., Indradewa, D. (2021). “Rice growth in a combined submergence and salinity stresses,” in IOP Conference Series: Earth and Environmental Science, Vol. 012012 (Indonesia: IOP Publishing).
Kumar, A., Nayak, A. K., Sah, R. P., Das, B. S. (2017). Effects of elevated CO 2 concentration on water productivity and antioxidant enzyme activities of rice (Oryza sativa l.) under water deficit stress. Field Crops Res. 212 (2), 61–72. doi: 10.1016/j.fcr.2017.06.020
Lang, N. T., Yanagihara, S., Buu, B. C. (2001). A microsatellite marker for a gene conferring salt tolerance on rice at the vegetative and reproductive stages. SABRAO J. Breed. Genet. 33, 1–10.
Lakshmi, G., Beena, R., Soni, K. B., Viji, M. M., Uday, C. J. (2022). Exogenously applied plant growth regulator protects rice from heat induced damage by modulating plant defense mechanism. J. Crop Sci. Biotechnol. doi: 10.1007/s12892-022-00162-4
Landi, S., Hausman, J. F., Guerriero, G., Esposito, S. (2017). Poaceae vs. abiotic stress: focus on drought and salt stress, recent insights and perspectives. Front. Plant Sci. 8, 1–9. doi: 10.3389/fpls.2017.01214
Lanning, S., Siebenmorgen, T. (2013). Effects of preharvest nighttime air temperatures on whiteness of head rice. Cereal Chem. 90, 218–222. doi: 10.1094/cchem-07-12-0082-r
Li, X., Lawas, L. M., Malo, R., Glaubitz, U., Erban, A., Mauleon, R., et al. (2015). Metabolic and transcriptomic signatures of rice floral organs reveal sugar starvation as a factor in reproductive failure under heat and drought stress. Plant Cell Environ. 38, 2171–2192. doi: 10.1111/pce.12545
Li, S., Li, X., Wei, Z., Liu, F. (2020). ABA-mediated modulation of elevated CO2 on stomatal response to drought. Curr. Opin. Plant Biol. 56, 174–180. doi: 10.1016/j.pbi.2019.12.002
Lijun, L., Chang, E. H., Fan, M. M., Zhang, J. (2011). Effects of potassium and calcium on root exudates and grain quality during grain filling. Acta agronomica Sin. 37 (4), 661–669. doi: 10.1016/S1875-2780(11)60018-7
Lin, H. X., Zhu, M. Z., Yano, M., Gao, J. P., Liang, Z. W., Su, W. A., et al. (2004). QTLs for na+ and k+ uptake of the shoots and roots controlling rice salt tolerance. Theor. Appl. Genet. 108 (2), 253–260. doi: 10.1007/s00122-003-1421-y
Liu, S., Waqas, M. A., Wang, S. H., Xiong, X. Y., Wan, Y. F. (2017). Effects increased levels atmospheric CO2 High temperatures Rice Growth quality. PloS One 12 (11), e0187724. doi: 10.1371/journal.pone.0187724
Liu, W., Yin, T., Zhao, Y., Wang, X., Wang, K., Shen, Y., et al. (2021). Effects of high temperature on rice grain development and quality formation based on proteomics comparative analysis under field warming. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.746180
Liu, L., Waters, D. L., Rose, T. J., Bao, J., King, G. J. (2013). Phospholipids in rice: Significance in grain quality and health benefits: A review. Food Chem. 139, 1133–1145. doi: 10.1016/j.foodchem.2012.12.046
Ma, Y., Dias, M. C., Freitas, H. (2020). Drought and salinity stress responses and microbe-induced tolerance in plants. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.591911
Madan, P., Jagadish, S. V. K., Craufurd, P. Q., Fitzgerald, M., Lafarge, T., Wheeler, T. R. (2012). Effect of elevated CO2 and high temperature on seedset and grain quality of rice. J. Exp. Bot. 63 (10), 3843–3852. doi: 10.1093/jxb/ers077
Mainuddin, M., Maniruzzaman, M. D., Alam, M. M., Mojid, M. A., Schmidt, E. J., Islam, M. T., et al. (2020). Water usage and productivity of boro rice at the field level and their impacts on the sustainable groundwater irrigation in the north-West Bangladesh. Agric. Water Manage. 240, 106294. doi: 10.1016/j.agwat.2020.106294
Manikanta, C. L. N., Beena, R., Rejeth, R. (2022). Root anatomical traits influence water stress tolerance in rice (Oryza sativa l.). J. Crop Sci. Biotechnol. doi: 10.1007/s12892-022-00142-8
Manikanta, C., Beena, R., Roy, S., Manju, R. V., Viji, M. M., Swapna, A. (2020). Physio-morphological plasticity of rice (Oryza sativa l.) genotypes exposed to water stress. J. Trop. Agriculture. 58 (1), 139–145.
Mariem, S. B., Soba, D., Zhou, B., Loladze, I., Morales, F., Aranjuelo, I. (2021). Climate change, crop yields, and grain quality of C3 cereals: A meta-analysis of CO2, temperature and drought effects. Plants 10, 6–1052. doi: 10.3390/plants10061052`
Marndi, B. C., Anilkumar, C., Muhammed, Azharudheen, T. P., Sah, R. P., Moharana, D., et al. (2022). “Cataloguing of rice varieties of NRRI suitable for different abiotic stress-prone ecologies. Climate resilient technologies for rice based production systems in Eastern india. ICARNational rice research institute,”. Bhattacharyya, P., Chakraborty, K., Molla, K. A., Poonam, A., Bhaduri, D., Sah, R. P., et al (Eds.) (India: Cuttack, Odisha), 408.
Mishra, K. K., Vikram, P., Yadaw, R. B., Swamy, B. P., Dixit, S., Cruz, M. T. S., et al. (2013). qDTY12. 1: a locus with a consistent effect on grain yield under drought in rice. BMC Genet. 14, 1–10. doi: 10.1186/1471-2156-14-1
Mittler, R., Finka, A., Goloubinoff, P. (2012). How do plants feel the heat? Trends Biochem. Sci. 37 (3), 118–125. doi: 10.1016/j.tibs.2011.11.007
Mohammed, A. R., Tarpley, L. (2010). Effects of high night temperature and spikelet position on yield-related parameters of rice (Oryza sativa l.) plants. Eur. J. Agron. 33, 117–123. doi: 10.1016/j.eja.2009.11.006
Moore, C. E., Hensold, K. M., Lemonnier, P., Slattery, R. A., Benjamin, C., Bernacchi, C. J., et al. (2021). The effect of increasing temperature on crop photosynthesis: From enzymes to ecosystems. J. Exp. Bot. 72 (8), 2822–2844. doi: 10.1093/jxb/erab090
Muthu, V., Abbai, R., Nallathambi, J., Rahman, H., Ramasamy, S., Kambale, R., et al. (2020). Pyramiding QTLs controlling tolerance against drought, salinity, and submergence in rice through marker assisted breeding. PloS One 15, e0227421. doi: 10.1371/journal.pone.0227421
Mukamuhirwa, A., Hovmalm, H. P., Bolinsson, H., Ortiz, R., Nyamangyoku, O., Johansson, E. (2019). Concurrent drought and temperature stress in rice–a possible result of the predicted climate change: Effects on yield attributes, eating characteristics, and health promoting compounds. Int. J. Environ. Res. Public Health 16, 1043. doi: 10.3390/ijerph16061043
Nahar, L., Aycan, M., Hanamata, S., Marouane Baslam, M., Mitsui, T. (2022). Impact of single and combined salinity and high-temperature stresses on agro-physiological, biochemical, and transcriptional responses in rice and stress-release. Plants. 11 (501), 1–23. doi: 10.3390/plants11040501
Nakata, M., Fukamatsu, Y., Miyashita, T., Hakata, M., Kimura, R., Nakata, Y., et al. (2017). High temperature-induced expression of rice α-amylases in developing endosperm produces chalky grains. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.02089
Narsai, R., Whelan, J. (2013). How unique is the low oxygen response? Ananalysis of the an aerobic response during germination and comparison with abiotic stress in rice and Arabidopsis. Front. Plant science. 4 (349). doi: 10.3389/fpls.2013.00349
Neeraja, C. N., Voleti, S. R., Desiraju, S., Kuchi, S., Bej, S., Talapanti, K., et al. (2021). “Molecular breeding for improving nitrogen use efficiency in rice: Progress and perspectives,” in Molecular breeding for rice abiotic stress tolerance and nutritional quality (USA: John Wiley & Sons, Ltd), 234–248. doi: 10.1002/9781119633174.ch12
Negrão, S., Schmöckel, S. M., Tester, M. (2017). Evaluating physiological responses of plants to salinity stress. Ann. Bot. 119, 1–11. doi: 10.1093/aob/mcw191
Nithya, N., Beena, R., Abida, P. S., Sreekumar, J., Roy, S., Jayalekshmi, V. G., et al. (2021). Genetic diversity and population structure analysis of bold type rice collection from southern India. Cereal Res. Commun. 49 (2), 311–328. doi: 10.1007/s42976-020-00099-w
Nithya, N., Beena, R., Stephen, R., Abida, P. S., Jayalekshmi, V. G., Viji, M. M., et al. (2020). Genetic variability, heritability, correlation coefficient and path analysis of morpho-physiological and yield related traits of rice under drought stress. Chem. Sci. Rev. Lett. 9 (33), 48–54. doi: 10.37273/chesci.cs142050122
Nonogaki, H., Barrero, J. M., Li, C. (2018). Editorial: Seed dormancy, germination, and pre-harvest sprouting. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.01783
Ouyang, W., Struik, P. C., Yin, X., Yang, J. (2017). Stomatal conductance, mesophyll conductance, and transpiration efficiency in relation to leaf anatomy in rice and wheat genotypes under drought. J. Exp. Bot. 68 (18). doi: 10.1093/jxb/erx314
Padhy, S. R., Nayak, S., Dash, P. K., Das, M., Roy, K. S., Nayak, A. K., et al. (2018). Elevated carbon dioxide and temperature imparted intrinsic drought tolerance in aerobic rice system through enhanced exopolysaccharide production and rhizospheric activation. Agriculture Ecosyst. Environ. 268, 52–60. doi: 10.1016/j.agee.2018.08.009
Palanog, A. D., Swamy, B. M., Shamsudin, N. A. A., Dixit, S., Hernandez, J. E., Boromeo, T. H., et al. (2014). Grain yield QTLs with consistent-effect under reproductive-stage drought stress in rice. Field Crops Res. 161, 46–54. doi: 10.1016/j.fcr.2014.01.004
Panda, D., Sarkar, R. K. (2014). Mechanism associated with nonstructural carbohydrate accumulation in submergence tolerant rice (Oryza sativa l.) cultivars. J. Plant Interact. 9, 62–68. doi: 10.1080/17429145.2012.763000
Pandey, P., Irulappan, V., Bagavathiannan, M. V., Senthil-Kumar, M. (2017). Impact of combined abiotic and biotic stresses on plant growth and avenues for crop improvement by exploiting physio-morphological traits. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.00537
Pang, Y., Chen, K., Wang, X., Wang, W., Xu, J., Ali, J., et al. (2017). Simultaneous improvement and genetic dissection of salt tolerance of rice (Oryza sativa l.) by designed QTL pyramiding. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.01275
Pathak, H., Kumar, M., Molla, K. A., Chakraborty, K. (2021). Abiotic stresses in rice production: impacts and management. Oryza 58, 103–125. doi: 10.35709/ory.2021.58.spl.4
Patra, B. C., Anilkumar, C., Chakraborti, M. (2020). Rice breeding in India: A journey from phenotype-based pure-line selection to genomics assisted breeding. Agric. Res. J. 57, 816–825. doi: 10.5958/2395-146X.2020.00120.9
Paul, K., Pauk, J., Kondic-Spika, A., Grausgruber, H., Allahverdiyev, T., Sass, L., et al. (2019). Co-Occurrence of mild salinity and drought synergistically enhances biomass and grain retardation in wheat. Front. Plant Sci. 10, 501. doi: 10.3389/fpls.2019.00501
Perdomo, J. A., Capó-Bauçà, S., Carmo-Silva, E., Galmés, J. (2017). Rubisco and rubisco activase play an important role in the biochemical limitations of photosynthesis in rice, wheat, and maize under high temperature and water deficit. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.00490
Perdomo, J. A., Carmo-Silva, E., Hermida-Carrera, C., Flexas, J., Galmés, J. (2016). Acclimation of biochemical and diffusive components of photosynthesis in rice, wheat, and maize to heat and water deficit: Implications for modeling photosynthesis. Front. Plant Sci. 7, 1719. doi: 10.3389/fpls.2016.01719
Perdomo, J. A., Conesa, M.À, Medrano, H., Ribas-Carbó, M., Galmés, J. (2015). Effects of long-term individual and combined water and temperature stress on the growth of rice, wheat and maize: Relationship with morphological and physiological acclimation. Physiol. Plant 155 (2), 149–165. doi: 10.1111/ppl.12303
Piveta, L. B., Roma-Burgos, N., Noldin, J. A., Viana, V. E., Oliveira, C., Lamego, F. P., et al. (2020). Molecular and physiological responses of rice and weedy rice to heat and drought stress. Agriculture 11, 9. doi: 10.3390/agriculture11010009
Prabnakorn, S., Maskey, S., Suryadi, F., de Fraiture, C. (2018). Rice yield in response to climate trends and drought index in the mun river basin, Thailand. Sci. Total Environ. 621, 108–119. doi: 10.1016/j.scitotenv.2017.11.136
Pradhan, B., Chakraborty, K., Prusty, N., Deepa, M. A. K., Chattopadhyay, K., et al. (2019). Distinction and characterisation of rice genotypes tolerant to combined stresses of salinity and partial submergence, proved by a high-resolution chlorophyll fluorescence imaging system. Funct. Plant Biol. 46, 248–261. doi: 10.1071/FP18157
Pravallika, K., Arunkumar, C., Vijayakumar, A., Beena, R., Jayalekshmi, V. G. (2020). Effect of high temperature stress on seed filling and nutritional quality of rice (Oryza sativa l.). J. Crop Weed 16 (2), 18–23. doi: 10.22271/09746315.2020.v16.i2.1310
Prince, S. J., Beena, R., Michael Gomez, S., Senthivel, S., Chandra Babu, R. (2015). Mapping consistent yield QTLs under drought stress in target rainfed environments. Rice. 8 (1), 53. doi: 10.1186/s12284-015-0053-6
Pundir, P., Devi, A., Krishnamurthy, S. L., Sharma, P. C., Vinaykumar, N. M. (2021). QTLs in salt rice variety CSR10 reveals salinity tolerance at reproductive stage. Acta Physiologiae Plantarum 43 (2), 1–15. doi: 10.1007/s11738-020-03183-0
Puram, V. R. R., Ontoy, J., Subudhi, P. K. (2018). Identification of QTLs for salt tolerance traits and prebreeding lines with enhanced salt tolerance in an introgression line population of rice. Plant Mol. Biol. Rep. 36 (5), 695–709. doi: 10.1007/s11105-018-1110-2
Qin, H., Li, Y., Huang, R. (2020). Advances and challenges in the breeding of salt-tolerant rice. Int. J. Mol. Sci. 21 (21), 8385. doi: 10.3390/ijms21218385
Raghunath, M. P., Beena, R. (2021). Manipulation of flowering time to mitigate high temperature stress in rice (Oryza sativa l.). Indian J. Agric. Res. doi: 10.18805/IJARe.A-5707
Raghunath, M. P., Beena, R., Vipin, M., Viji, M. M., Manju, R. V., Roy, S. (2021). High temperature stress mitigation in rice: foliar application of plant growth regulators and nutrients. J. Crop Weed. 17 (1), 34–47. doi: 10.22271/09746315.2021.v17.i1.1404
Rahman, M. A., Bimpong, I. K., Bizimana, J. B., Pascual, E. D., Arceta, M., Swamy, B. P., et al. (2017). Mapping QTLs using a novel source of salinity tolerance from hasawi and their interaction with environments in rice. Rice. 10 (1), 1–17. doi: 10.1186/s12284-017-0186-x
Rahman, M. A., Thomson, M. J., De Ocampo, M., Egdane, J. A., Salam, M. A., Ismail, A. M. (2019). Assessing trait contribution and mapping novel QTL for salinity tolerance using the Bangladeshi rice landrace capsule. Rice. 12 (1), 1–18. doi: 10.1186/s12284-019-0319-5
Ramegowda, V., Senthil-Kumar, M. (2015). The interactive effects of simultaneous biotic and abiotic stresses on plants: Mechanistic understanding from drought and pathogen combination. J. Plant Physiol. 176, 47–54. doi: 10.1016/j.jplph.2014.11.008
Ramu, V. S., Paramanantham, A., Ramegowda, V., Mohan-Raju, B., Udayakumar, M., Senthil-Kumar, M. (2016). Transcriptome analysis of sunflower genotypes with contrasting oxidative stress tolerance reveals individual- and combined- biotic and abiotic stress tolerance mechanisms. PloS One 11, e0157522. doi: 10.1371/journal.pone.0157522
Rang, Z. W., Jagadish, S. V. K., Zhoua, Q. M., Craufurd, P. Q., Heuer, S. (2010). Effect of high temperature and water stress on pollen germination and spikelet fertility in rice. Environ. Exp. Botany. 70 (1), 58–65. doi: 10.1016/j.envexpbot.2010.08.009
Rejeth, R., Manikanta, C., Beena, R., Roy, S., Manju, R. V., Viji, M. M. (2020). Water stress mediated root trait dynamics and identification of microsatellite markers associated with root traits in rice (Oryza sativa l.). Physiol. Mol. Biol. Plants. 26 (6), 1225–1236. doi: 10.1007/s12298-020-00809
Reshma, M., Beena, R., Viji, M. M., Manju, R. V., Roy, S. (2021). Validation of temperature induction response technique on combined effect of drought and heat stress in rice (Oryza sativa l.). J. Crop Weed. 17 (2), 119–128. doi: 10.22271/09746315.2021.v17.i2.1461
Ruiz, P. R., Rosario, S. M., Lozano-Juste, J. (2021). An update on crop ABA receptors. Plants 10 (6), 1087. doi: 10.3390/plants10061087
Sabouri, H., Rezai, A. M., Moumeni, A., Kavousi, A., Katouzi, M., Sabouri, A. (2009). QTLs mapping of physiological traits related to salt tolerance in young rice seedlings. Biol. Plantarum 53 (4), 657–662. doi: 10.1007/s10535-009-0119-7
Sandhu, N., Singh, A., Dixit, S., Sta Cruz, M. T., Maturan, P. C., Jain, R. K., et al. (2014). Identification and mapping of stable QTL with main and epistasis effect on rice grain yield under upland drought stress. BMC Genet. 15, 1–15. doi: 10.1186/1471-2156-15-63
Sarkar, R. K., Ray, A. (2016). Submergence-tolerant rice withstands complete submergence even in saline water: Probing through chlorophyll a fluorescence induction O-J-I-P transients. Photosynthetica 54 (2), 275–287. doi: 10.1007/s11099-016-0082-4
SenthilNathan, S. (2021). Effects of elevated CO2 on resistant and susceptible rice cultivar and its primary host, brown planthopper (BPH), Nilaparvata lugens (Stål). Sci. Rep. 11, 8905. doi: 10.1038/s41598-021-87992-4
Septiningsih, E. M., Ignacio, J. C. I., Sendon, P., Sanchez, D. L., Ismail, A. M., Mackill, D. J. (2013). QTL mapping and confirmation for tolerance of anaerobic conditions during germination derived from the rice landrace ma-zhan red. Theor. Appl. Genet. 12 (6), 1357–1366. doi: 10.1007/s00122-013-2057-1
Shahid, S. A., Zaman, M., Heng, L. (2018). “Soil salinity: Historical perspectives and a world overview of the problem,” in In guideline for salinity assessment, mitigation and adaptation using nuclear and related techniques (Cham, Switzerland: Springer International Publishing), 43–53.
Shamsudin, N. A. A., Swamy, B. P. M., Ratnam, W., Cruz, M. T. S., Sandhu, N., Raman, A. K., et al. (2016). Pyramiding of drought yield QTLs into a high quality Malaysian rice cultivar MRQ74 improves yield under reproductive stage drought. Rice. 9, 21. doi: 10.1186/s12284-016-0093-6
Shanker, A. K., Gunnapaneni, D., Bhanu, D., Vanaja, M., Lakshmi, N. J., Yadav, S. K., et al. (2022). Elevated CO2 and water stress in combination in plants: Brothers in arms or partners in crime? Biology 11, 1330. doi: 10.3390/biology11091330
Shanmugavadivel, P. S., Mithra, S. V. A., Prakash, C., Ramkumar, M. K., Tiwari, R., Mohapatra, T., et al. (2017). High resolution mapping of QTLs for heat tolerance in rice using a 5K SNP array. Rice 10 (28). doi: 10.1186/s12284-017-0167-0
Shrestha, J., Kandel, M., Subedi, S., Shah, K. K. (2020). Role of nutrients in rice (Oryza sativa l.): A review. Agrica 9, 53–62. doi: 10.5958/2394-448X.2020.00008.5
Singh, R. K., Singh, U. S., Khush, G. S., Rohilla, R. (2000). Genetics and biotechnology of quality traits in aromatic rices. Aromatic Rices (Manila, the Philippines), 47–70.
Silva, R. G. D., Alves, R. D. C., Zingaretti, S. M. (2020). Increased [CO2] causes changes in physiological and genetic responses in C4 crops: A brief review. Plants 9 (11), 1567. doi: 10.3390/plants9111567
Singh, S., Mackill, D. J., Ismail, A. M. (2014). Physiological basis of tolerance to complete submergence in rice involves genetic factors in addition to the SUB1 gene. AoB Plants 6. doi: 10.1093/aobpla/plu060
Sinha, R., Zandalinas, S. I., Fichman, Y., Sen, S., Zeng, S., Cadenas, A. G., et al. (2022). Differential regulation of flower transpiration during abiotic stress in annual plants. New Phytologist. 235, 611–629. doi: 10.1111/nph.18162
Stephen, K., Beena, R., Kiran, A. G., Shanija, S., Saravanan, R. (2022). Changes in physiological traits and expression of key genes involved in sugar signaling pathway in rice under high temperature stress. 3 Biotech. 12 (2), 183. doi: 10.1007/s13205-022-03242-y
Sultana, S., Faruque, M., Islam, M. R. (2022). Rice grain quality parameters and determination tools: a review on the current developments and future prospects. Int. J. Food Properties 25, 1063–1078. doi: 10.1080/10942912.2022.2071295
Swamy BP, M., Ahmed, H. U., Henry, A., Mauleon, R., Dixit, S., Vikram, P., et al. (2013). Genetic, physiological, and gene expression analyses reveal that multiple QTL enhance yield of rice mega-variety IR64 under drought. PloS One 8. doi: 10.1371/journal.pone.0062795
Thomson, M. J., de Ocampo, M., Egdane, J., Rahman, M. A., Sajise, A. G., Adorada, D. L., et al. (2010). Characterizing the saltol quantitative trait locus for salinity tolerance in rice. Rice. 3 (2), 148–160. doi: 10.1007/s12284-010-9053-8
Usui, Y., Sakai, H., Tokida, T., Nakamura, H., Nakagawa, H., Hasegawa, T. (2014). Heat-tolerant rice cultivars retain grain appearance quality under free-air CO2 enrichment. Rice 7 (1), 1–9. doi: 10.1186/s12284-014-0006-5
Venuprasad, R., Bool, M. E., Quiatchon, L., Sta Cruz, M. T., Amante, M., Atlin, G. N. (2012). A large-effect QTL for rice grain yield under upland drought stress on chromosome 1. Mol. Breed. 30, 535–547. doi: 10.1007/s11032-011-9642-2
Verma, R., Katara, J., Anilkumar, C., Devanna, B. N., Chidambaranathan, P., Dash, B., et al. (2021). “Advanced breeding strategies for rice improvement,“ 263.
Vikram, P., Swamy, B. P., Dixit, S., Ahmed, H. U., Teresa Sta Cruz, M., Singh, A. K., et al. (2011). qDTY 1.1, a major QTL for rice grain yield under reproductive-stage drought stress with a consistent effect in multiple elite genetic backgrounds. BMC Genet. 12, p. 1–15. doi: 10.1186/1471-2156-12-89
Vivitha, P., Raveendran, M., Vijayalakshmi, C., Vijayalakshmi, D. (2018). Genetic dissection of high temperature stress tolerance using photosynthesis parameters in QTL introgressed lines of rice cv. Improved White Ponni. Indian J. Plant Physiol. 23 (4), 741–747. doi: 10.1007/s40502-018-0408-2
Walter, J. (2018). Effects of changes in soil moisture and precipitation patterns on plant-mediated biotic interactions in terrestrial ecosystems. Plant Ecol. 219, 1449–1462. doi: 10.1007/s11258-018-0893-4
Wang, W., Cai, C., He, J., Gu, J., Zhu, G., Zhang, W., et al. (2020). Yield, dry matter distribution and photosynthetic characteristics of rice under elevated CO2 and increased temperature conditions. Field Crops Res. 248, 107605. doi: 10.1016/j.fcr.2019.107605
Wang, W., Cai, C., Lam, S. K., Liu, G., Zhu, J. (2018). Elevated CO2 cannot compensate for japonica grain yield losses under increasing air temperature because of the decrease in spikelet density. Eur. J. Agron. 99, 21–29. doi: 10.1016/j.eja.2018.06.005
Wang, Z., Cheng, J., Chen, Z., Huang, J., Bao, Y., Wang, J., et al. (2012). Identification of QTLs with main, epistatic and QTL× environment interaction effects for salt tolerance in rice seedlings under different salinity conditions. Theor. Appl. Genet. 125 (4), 807–815. doi: 10.1007/s00122-012-1873-z
Wang, J., Liu, X., Zhang, X., Li, L., Lam, S. K., Pan, G. (2019). Changes in plant c, n and p ratios under elevated (CO2) and canopy warming in a rice-winter wheat rotation system. Sci. Rep. 9 (1), 1–9. doi: 10.1038/s41598-019-41944-1
Wang, X., Liu, H., Zhang, D., Zou, D., Wang, J., Zheng, H., et al. (2022). Photosynthetic carbon fixation and sucrose metabolism supplemented by weighted gene Co-expression network analysis in response to water stress in rice with overlapping growth stages. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.864605
Williams, A. P., Seager, R., Berkelhammer, M., Macalady, A. K., Crimmins, M. A., Swetnam, T. W., et al. (2014). Causes and implications of extreme atmospheric moisture demand during the record-breaking 2011 wildfire season in the southwestern united states. J. Appl. Meteorology Climatology 53, 2671–2684. doi: 10.1175/JAMC-D-14-0053.1
Worch, S., Rajesh, K., Harshavardhan, V. T., Pietsch, C., Korzun, V., Kuntze, L., et al. (2011). Haplotyping, linkage mapping and expression analysis of barley genes regulated by terminal drought stress influencing seed quality. BMC Plant Biol. 11, 1–14.
Wytynck, P., Lambin, J., Chen, S., Asci, S. D., Verbeke, I., De Zaeytijd, J., et al. (2021). Effect of RIP overexpression on abiotic stress tolerance and development of rice. Int. J. Mol. Sci. 22, 1434. doi: 10.3390/ijms22031434
Xiong, D., Yu, T., Ling, X., Fahad, S., Peng, S., Li, Y., et al. (2014). Sufficient leaf transpiration and nonstructural carbohydrates are beneficial for high-temperature tolerance in three rice (Oryza sativa) cultivars and two nitrogen treatments. Funct. Plant Biol. 42, 347–356. doi: 10.1071/FP14166
Yadav, C., Bahuguna, R. N., Dhankher, O. P., Singla-Pareek, S. L., Pareek, A. (2022). Physiological and molecular signatures reveal differential response of rice genotypes to drought and drought combination with heat and salinity stress. Physiol. Mol. Biol. Plants 28 (4), 899–910. doi: 10.1007/s12298-022-01162-y
Yadaw, R. B., Dixit, S., Raman, A., Mishra, K. K., Vikram, P., Swamy, B. M., et al. (2013). A QTL for high grain yield under lowland drought in the background of popular rice variety sabitri from Nepal. Field Crops Res. 144, 281–287. doi: 10.1016/j.fcr.2013.01.019
Yang, X., Wang, B., Chen, L., Li, P., Cao, C. (2018). The different influences of drought stress at the flowering stage on rice physiological traits, grain yield, and quality. Sci. Rep. 9, 3742. doi: 10.1038/s41598-019-40161-0
Ye, C., Tenorio, F. A., Argayoso, M. A., Laza, M. A., Koh, H. J., Redoña, E. D., et al. (2015). Identifying and confirming quantitative trait loci associated with heat tolerance at flowering stage in different rice populations. BMC Genet. 16, 41. doi: 10.1186/s12863-015-0199-7
Zait, Y., Ferrero-Serrano, ,. A., Assmann, S. M. (2021). The α subunit of the heterotrimeric G protein regulates mesophyll CO2 conductance and drought tolerance in rice. New Phytologist. 232, 2324–2338. doi: 10.1111/nph.17730
Zandalinas, S. I., Mittler, R., Balfagon, D., Arbona, V., Gomez-Cadenaz, A. (2018). Plant adaptations to the combination of drought and high temperatures. Physiolgia Plantarum 162, 2–12. doi: 10.1111/ppl.12540
Zhang, M., Lu, Q., Wu, W., Niu, X., Wang, C., Feng, Y., et al. (2017). Association mapping reveals novel genetic loci contributing to flooding tolerance during germination in indica rice. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.00678
Zhang, R., Wang, Y., Hussain, S., Yang, S., Li, R., Liu, S., et al. (2022). Study on the effect of salt stress on yield and grain quality among different rice varieties. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.918460
Zhao, L., Lei, J., Huang, Y., Zhu, S., Chen, H., Renliang Huang, R., et al. (2016). Mapping quantitative trait loci for heat tolerance at anthesis in rice using chromosomal segment substitution lines. Breed. Sci. 66, 358–366. doi: 10.1270/jsbbs.15084.4
Zhao, J., Zhang, W., da Silva, J. A. T., Liu, X., Duan, J. (2021). Rice histone deacetylase HDA704 positively regulates drought and salt tolerance by controlling stomatal aperture and density. Planta 254 (4), 1–15. doi: 10.1007/s00425-021-03729-7
Zheng, H., Zhao, H., Liu, H., Wang, J., Zou, D. (2015). QTL analysis of na+ and k+ concentrations in shoots and roots under NaCl stress based on linkage and association analysis in japonica rice. Euphytica. doi: 10.1007/s10681-014-1192-3
Zhou, C., Huang, Y., Jia, B., Wang, Y., Wang, Y., Xu, Q., et al. (2018). Effects of cultivar, nitrogen rate, and planting density on rice-grain quality. Agronomy 8, 246. doi: 10.3390/agronomy8110246
Zhou, L., Lu, Y., Zhang, Y., Zhang, C., Zhao, L., Yao, S., et al. (2020). Characteristics of grain quality and starch fine structure of japonica rice kernels following preharvest sprouting. J. Cereal Sci. 95, 103023. doi: 10.1016/j.jcs.2020.103023
Zhu, D., Zhang, H., Guo, B., Xu, K., Dai, Q., Wei, H., et al. (2017). Effects of nitrogen level on yield and quality of japonica soft super rice. J. Integr. Agric. 16, 1018–1027. doi: 10.1016/S2095-3119(16)61577-0
Keywords: multiple abiotic stresses, physiology, high temperature, salinity, drought, eCO2, sensitivity, tolerance
Citation: Radha B, Sunitha NC, Sah RP, T. P. MA, Krishna GK, Umesh DK, Thomas S, Anilkumar C, Upadhyay S, Kumar A, Ch L. N. M, S. B, Marndi BC and Siddique KHM (2023) Physiological and molecular implications of multiple abiotic stresses on yield and quality of rice. Front. Plant Sci. 13:996514. doi: 10.3389/fpls.2022.996514
Received: 17 July 2022; Accepted: 05 December 2022;
Published: 11 January 2023.
Edited by:
Pradeep Sharma, Indian Institute of Wheat and Barley Research (ICAR), IndiaReviewed by:
Reetika Mahajan, Sher-e-Kashmir University of Agricultural Sciences and Technology, IndiaRuchi Bansal, National Bureau of Plant Genetic Resources (ICAR), India
Copyright © 2023 Radha, Sunitha, Sah, T. P., Krishna, Umesh, Thomas, Anilkumar, Upadhyay, Kumar, Ch L. N., S., Marndi and Siddique. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Beena Radha, beena.r@kau.in
 Beena Radha
Beena Radha Nagenahalli Chandrappa Sunitha2
Nagenahalli Chandrappa Sunitha2 Rameswar P. Sah
Rameswar P. Sah Md Azharudheen T. P.
Md Azharudheen T. P. G. K. Krishna
G. K. Krishna Sini Thomas
Sini Thomas Chandrappa Anilkumar
Chandrappa Anilkumar Sameer Upadhyay
Sameer Upadhyay Awadhesh Kumar
Awadhesh Kumar Manikanta Ch L. N.
Manikanta Ch L. N. Kadambot H. M. Siddique
Kadambot H. M. Siddique