- 1National Key Facility for Crop Gene Resources and Genetic Improvement, Institute of Crop Science, Chinese Academy of Agricultural Sciences, Beijing, China
- 2School of Agriculture, Ningxia University, Yinchuan, China
As an important wheat wild relative, the P genome of Agropyron cristatum (L.) Gaertn. (2n = 4x = 28) is very valuable for wheat improvement. A complete set of wheat-A. cristatum disomic addition lines is the basis for studying the genetic behavior of alien homoeologous chromosomes and exploring and utilizing the excellent genes. In this study, a wheat-A. cristatum derivative II-11-1 was proven to contain a pair of 5P chromosomes and a pair of 2P chromosomes with 42 wheat chromosomes by analyzing the fluorescence in situ hybridization (FISH) and expressed sequence tag (EST) markers. Additionally, cytological identification and field investigation showed that the 5P chromosome can weaken the homologous pairing of wheat chromosomes and promote the pairing between homoeologous chromosomes. This provides new materials for studying the mechanism of the alien gene affecting the homologous chromosome pairing and promoting the homoeologous pairing of wheat. In addition, chromosomal structural variants have been identified in the progeny of II-11-1. Therefore, the novel 5P addition line might be used as an important genetic material to widen the genetic resources of wheat.
Introduction
Wheat (Triticum aestivum L., 2n = 42, AABBDD), as one of the most important food crops in the world, plays a significant role in ensuring food production and security. The homogenization of wheat varieties in major regions has narrowed their genetic background, which became the main bottleneck of breeding. Fortunately, there are plenty of beneficial genes in the wheat wild relatives that could be exploited and utilized for wheat improvement (Dong, 2000). The success of distant hybridization made it possible to transfer alien excellent genes to common wheat for creating new germplasms. Wheat disomic addition lines contain a pair of alien chromosomes in the wheat background, which is an important tool and bridge material for transferring alien excellent genes. Furthermore, they are favorable materials to identify the relationship between alien and wheat chromosomes, which could also be used for gene mapping. At present, most of the wheat wild relatives have been successfully hybridized with wheat, such as Aegilops (Gong et al., 2017; Du et al., 2019; Liu et al., 2019; Yi et al., 2019), Secale cereal L. (Li et al., 2016a,2020; Schneider et al., 2016; An et al., 2019), Hordeum vulgare L. (Szakacs and Molnal-Lang, 2010; Fang et al., 2014), Haynaldia villosa (Zhang et al., 2015a, 2018a,b), Leymus racemosus (Yang et al., 2017; Zhang et al., 2017a), and Elytrigia repens (Liu et al., 2017). Meanwhile, a large number of disomic addition lines and substitution lines were identified, which were used to create translocation lines and introgression lines containing desirable genes. Some introgression lines and translocation lines are widely used in wheat production. For instance, the cultivated variety Xiaoyan 6 was bred from the hybridization of wheat and Thinopyrum (Ma et al., 2018), and the crucial translocation lines T1RS⋅1BL and T6VS⋅6AL were from the hybridization of wheat and rye (Su et al., 2006) and H. villosa (Jiang et al., 2014; Gao et al., 2018), respectively.
Agropyron cristatum L. Gaertn. (2n = 28, PPPP) is an important wheat wild relative. It grows in arid grassland, hillside, hill, and desert and contains many desirable traits for wheat improvement, such as resistance to wheat leaf rust, powdery mildew, barley yellow dwarf, and wheat streak mosaic viruses (Dewey, 1984; Sharma et al., 1984; Ochoa et al., 2014) and tolerance to drought and low temperature (Limin and Fowler, 1987; Asay and Johnson, 1990; Dong et al., 1992), as well as with multiple spikelets and florets, small flag leaves, fertile tiller number and strong and tough stem (Dewey, 1984; Wu et al., 2006; Han et al., 2014; Jiang et al., 2018). The acquisition of wheat-A. cristatum disomic addition lines made it possible to utilize these desirable genes to improve wheat variety. This study has been committed to the hybridization of wheat and A. cristatum for a long time and has created a series of wheat-A. cristatum addition, translocation, and deletion lines successfully (Li and Dong, 1991, 1993; Li et al., 1995, 1997, 1998, 2016b; Luan et al., 2010; Song et al., 2013; Ye et al., 2015; Lu et al., 2016; Zhang et al., 2019). So far, wheat-A. cristatum 1P, 2P, 3P, 4P, 6P, and 7P disomic addition lines have been successfully created, and many excellent genes were located in specific chromosomes and transmitted into wheat (Wu et al., 2006; Han et al., 2014; Li et al., 2016a; Lu et al., 2016; Pan et al., 2017; Chen et al., 2018; Zhou et al., 2018). For instance, it has been found that A. cristatum 6P addition line carried gene clusters related to yield, such as multiple florets and grains per spike, and the 2P addition line possessed gene clusters related to disease resistance including powdery mildew, leaf rust, and stripe rust (Wu et al., 2006; Han et al., 2014; Li et al., 2016a). These genes were further mapped by creating translocation lines and introgression lines and developing specific markers for P chromosomes (Liu et al., 2010; Dai et al., 2012; Zhang et al., 2015b,c; Han et al., 2017, 2019; Zhou et al., 2018). However, wheat-A. cristatum 5P disomic addition line has not been obtained.
In this study, a wheat-A. cristatum-derived line II-11-1 with four A. cristatum chromosomes was used as a basic material for backcross and self-cross with recipient parent Fukuhokomugi (Fukuho). The purpose of this study was (1) to analyze the chromosome constitution of wheat-A. cristatum-derived line II-11-1, (2) to obtain wheat-A. cristatum 5P addition line, and (3) to analyze the effects of A. cristatum 5P chromosome on the chromosomes pairing. The obtained wheat-A. cristatum 5P addition line provided basic materials for further systematic study on genetic variation of wheat distant hybrid.
Materials and Methods
Materials
The plant materials included T. aestivum cv. Fukuho (2n = 6x = 42, AABBDD), A. cristatum accession Z559 (2n = 4x = 28, PPPP), wheat-A. cristatum-derived material II-11-1 (2n = 46) and other wheat-A. cristatum homoeologous group addition lines: II-3-1a (1P) (Pan et al., 2017), II-9-3 (2P) (Li et al., 2016a), 7365 (3P) (Zhou et al., 2018), II-21-2 (4P) (Liu et al., 2010), 4844-12 (6P) (Wu et al., 2006), and II-5-1 (7P) (Lu et al., 2016). All the above materials were provided by the Center of Crop Germplasm Resources Research at the Institute of Crop Science, Chinese Academy of Agricultural Sciences, Beijing, China.
Observation on the Mitosis of Root Tip Cells and the Meiosis of Pollen Mother Cells (PMC)
The mitotic metaphase of root tip cells and the meiotic metaphase I of PMC of Fukuho, wheat-A. cristatum-derived material II-11-1, and the newly obtained 5P addition line were observed. Genomic in situ hybridization (GISH) was performed as described by Cuadrado et al. (2000), and the meiotic metaphase I of PMC was observed following the method of Jauhar and Peterson (2006).
Fluorescence in situ Hybridization
The genomic DNA of A. cristatum accession Z559 and common wheat Fukuho were extracted using the cetyl trimethyl ammonium bromide (CTAB) method (Dellaporta et al., 1983). The P chromosome repetitive sequences, pAcTRT1 and pAcpCR2, were used as probes to identify the homologous groups of A. cristatum in II-11-1 and II-11-1b using the method described by Han et al. (2019). The barley clone pHvG38 contains the GAA-satellite sequence (Pedersen and Langridge, 1997), and the clone pAsl contains a 1 kb DNA repetitive sequence from Aegilops tauschii (Rayburn and Gill, 1986). The combination of pAs1 and pHvG38 allowed the discrimination of the three genomes in wheat. The procedure of fluorescence in situ hybridization (FISH) was carried out as described by Han et al. (2004) and Liu et al. (2010). Images were captured using an OLYMPUS AX80 fluorescence microscope (Olympus Corporation, Tokyo, Japan) equipped with a charge-coupled device (CCD) camera (Diagnostic Institute, Inc., Sterling Height, MI, USA) and then were processed with Photoshop CS 3.0.
Molecular Marker Analysis
A total of 236 markers were used to identify the alien P chromatin and to determine its homoeologous group (Supplementary Table 1), of which 160 markers were described by Zhang et al. (2017b) and 76 markers were described by Li et al. (2016b). The PCR amplification procedure was performed as described by Luan et al. (2010). The amplified product was verified using 6% polyacrylamide gel electrophoresis (PAGE).
Evaluation of the Agronomic Traits
All the tested materials were sown in a randomized complete block design with three replicates in the fields at Xinxiang (35°18′13.71″N, 113°55′15.05″E, Henan Province, China) during 2016–2017, 2017–2018, and 2018–2019 growing seasons. A total of 20 grains were evenly planted in 2.0 m rows spaced 0.3 m apart (Zhang et al., 2019). The agronomic traits were measured and quantified including grain number, spikelet number and kernel number per spikelet, thousand-grain weight, and effective tiller number. The Statistical Analysis System (version 9.2, SAS Institute, Cary, NC, USA) software was used for statistical analysis.
Results and Analysis
The Chromosome Composition Analysis of Wheat-A. cristatum II-11-1
In the population composed of 50 individuals of II-11-1, 31 plants containing 42 wheat chromosomes and 4 A. cristatum chromosomes were identified by mitosis observation and GISH detection (Figure 1A). Additionally, pAcTRT1 and pAcpCR2 were used as probes to identify the additional chromosomes of A. cristatum in II-11-1. As shown in Figure 1B, the additional A. cristatum chromosomes were a pair of 2P and a pair of 5P according to the signal characteristics. So, the derivative II-11-1 (2n = 46) was preliminarily determined to be a wheat-A. cristatum 2P and 5P disomic addition line.
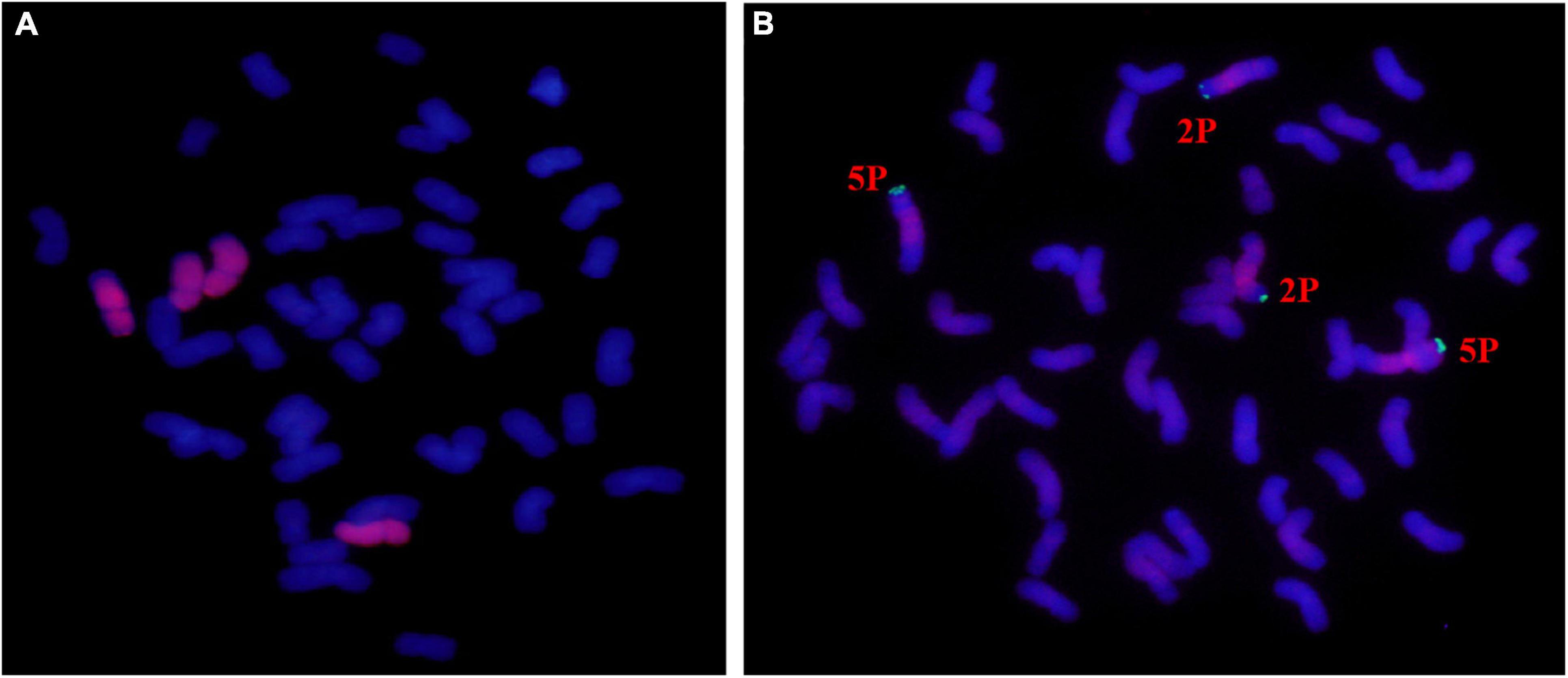
Figure 1. Mitosis genomic in situ hybridization (GISH)/fluorescence in situ hybridization (FISH) identification of II-11-1. (A) The whole-genome DNA probe of Agropyron cristatum was labeled as a red signal, and wheat chromosomes were restained as blue by DAPI. (B) The probes pAcTRT1 and pAcpCR2 were labeled as red and green, respectively, and wheat chromosomes were restained as blue by DAPI.
Identification of II-11-1 With Expressed Sequence Tag (EST)-STS Markers
To further confirm the identity of A. cristatum chromosomes in II-11-1, the EST-STS markers specific to the 2 and 5 homoeologous groups were employed to identify II-11-1 and wheat-A. cristatum disomic addition lines (1P, 2P, 3P 4P, 6P, and 7P). Results showed that 36 pairs of 2P chromosome-specific primers amplified specific bands for the wheat-A. cristatum 2P addition line and II-11-1 (Figure 2A) and 78 pairs of 5P chromosome-specific markers amplified specifically in II-11-1 and II-11-1b (Figure 2B and Supplementary Table 2). Therefore, it was further confirmed that II-11-1 contains 2P and 5P chromosomes, which could be used as a basic material for the separation and identification of wheat-A. cristatum 5P addition line.
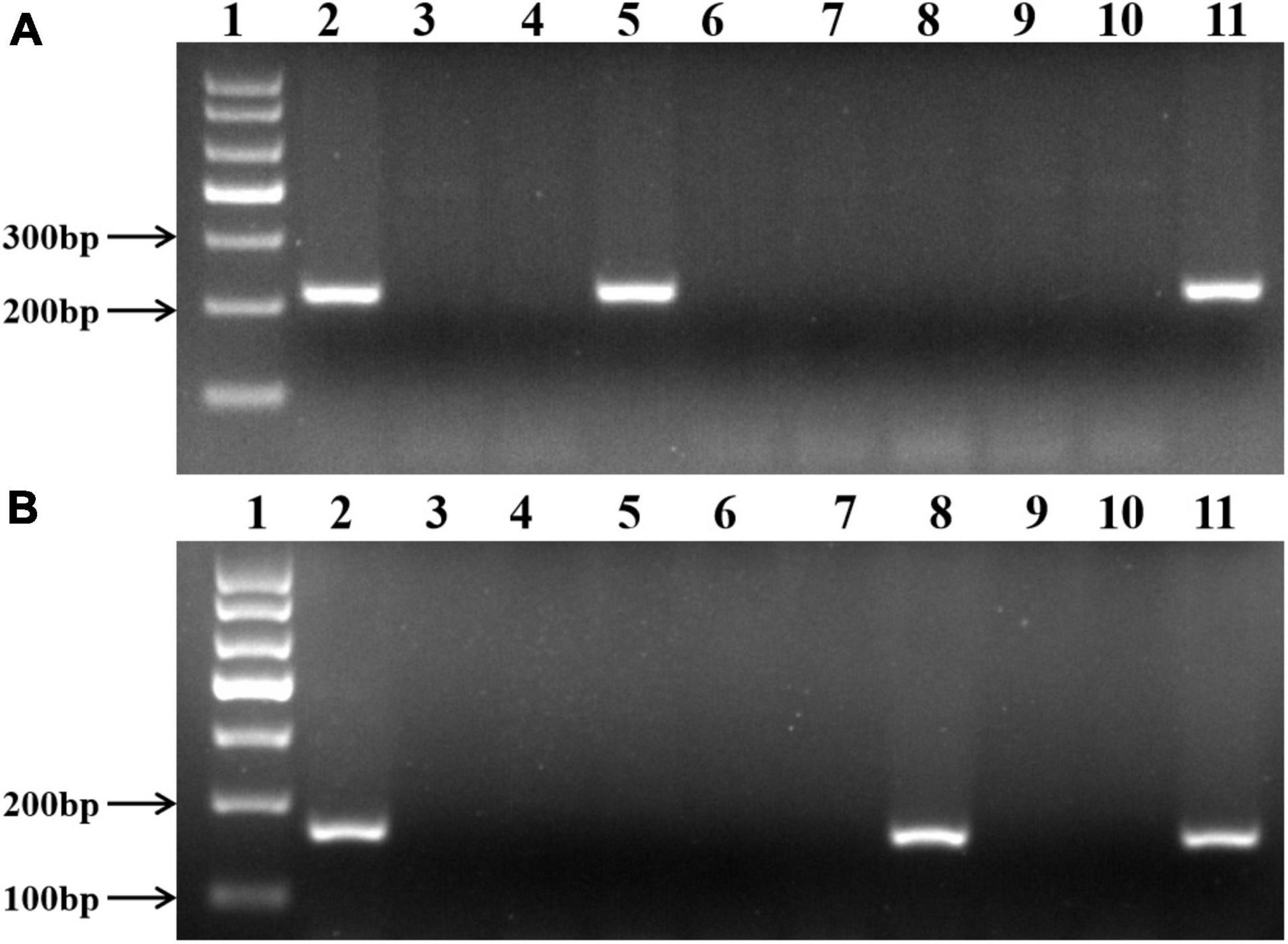
Figure 2. Molecular marker identification of II-11-1. (A,B) 2P chromosome-specific marker, Agc3725, and 5P chromosome-specific marker, Agc737, respectively. 1: Puc19 DNA MSP/I HPA II marker; 2: A. cristatum Z559; 3: common wheat Fukuho; 4: Wheat-A. cristatum 1P addition line II-3-1a; 5: Wheat-A. cristatum 2P addition line II-9-3; 6: Wheat-A. cristatum 3P addition line 7365; 7: Wheat-A. cristatum 4P addition line II-21-2; 8: Wheat-A. cristatum 5P addition line II-11-1b; 9: Wheat-A. cristatum 6P addition line 4844-12; 10: Wheat-A. cristatum 7P addition line II-5-1; 11: Wheat-A. cristatum derivation line II-11-1.
Chromosome Behavior Analysis of II-11-1 During Meiosis
Meiosis pairing was observed in PMCs to analyze the chromosome behavior of II-11-1 during the generation of the gamete. Results indicated that there existed univalent and multivalent chromosomes, chromosome fragments, and ring chromosomes at metaphase (Figures 3A–D). Meanwhile, chromosome lagging and chromosome bridge were found at anaphase (Figure 3E). The chromosome configuration statistics of II-11-1 at metaphase showed that the average univalents, rod bivalents, ring bivalents, trivalents, quadrivalents and chromosome fragments were 3.95, 3.81, 16.85, 0.16, 0.08, and 0.18, respectively (Table 1). The above results indicated that the behaviors of chromosome pairing were abnormal during meiosis in II-11-1.
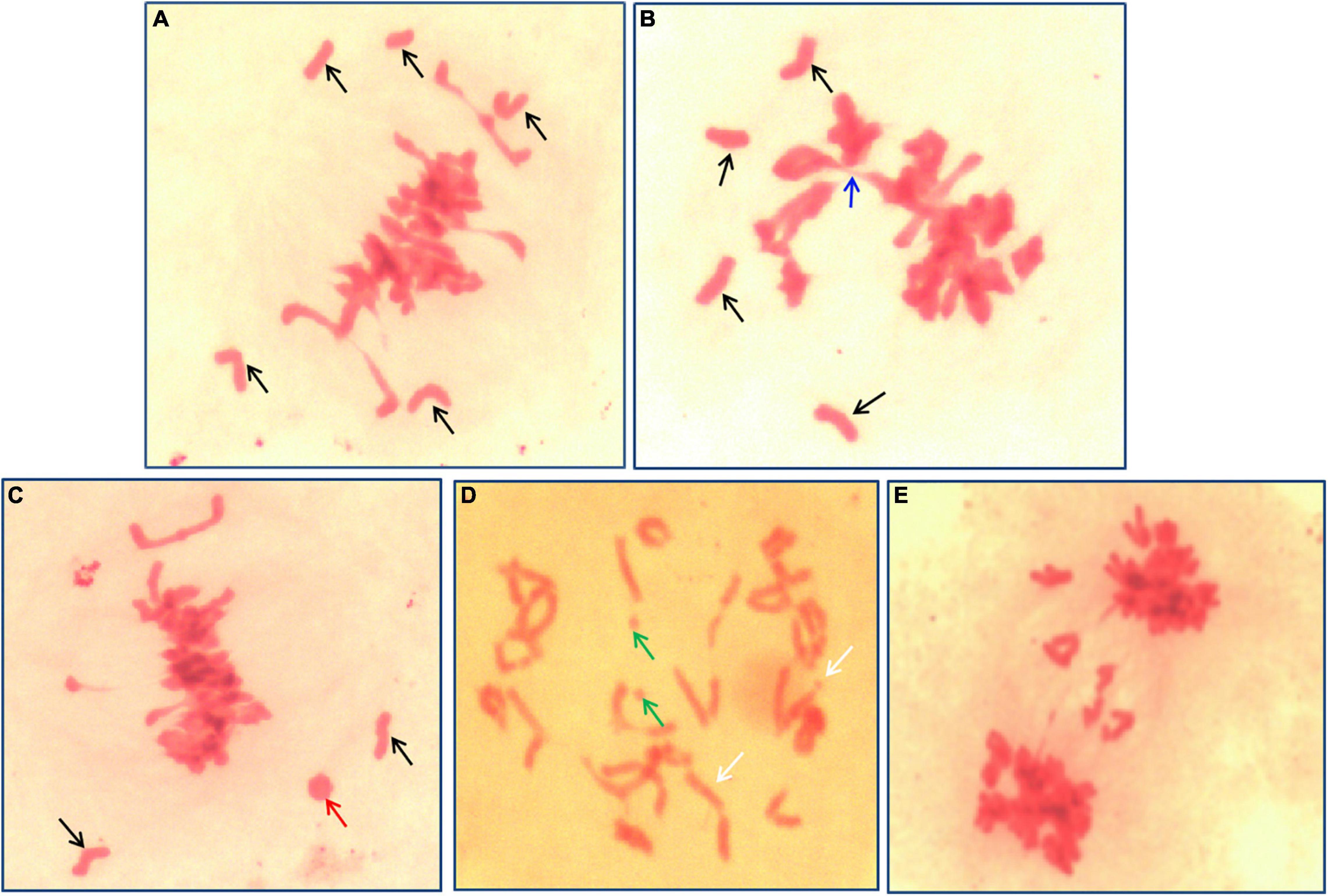
Figure 3. Meiosis identification of II-11-1. (A–D) Meiosis metaphase I; (E) meiosis anaphase I, chromosome bridges, and lagging chromosomes. Black arrows refer to univalents, blue arrows to multivalents, green arrows to chromosome fragments, and red arrows to ring chromosomes.
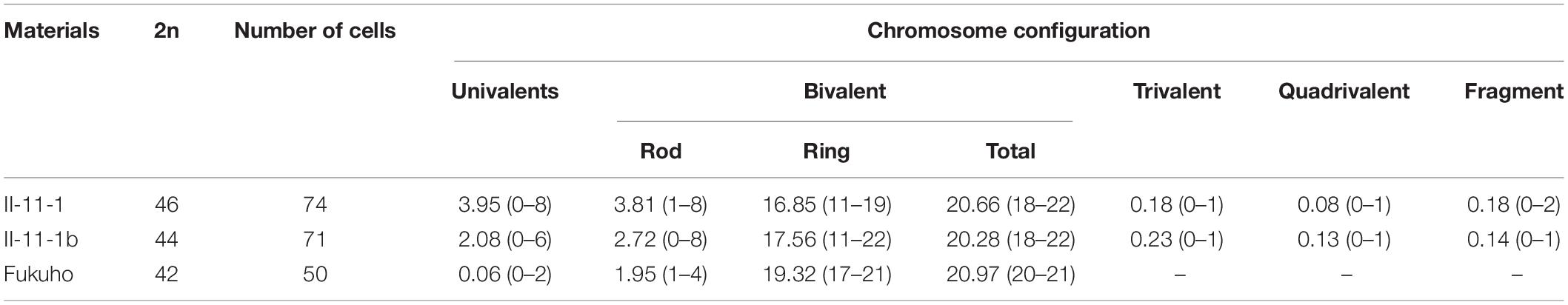
Table 1. Pollen mother cells (PMC) meiosis metaphase I chromosome configuration of wheat-Agropyron cristatum derivatives II-11-1 and 5P addition line II-11-1b.
Identification of the II-11-1 Progenies by Molecular Markers
The markers specific to 2P and 5P chromosomes were used to detect the BC1F2 population of II-11-1 with Fukuho as a recurrent parent. According to the results, 212 BC1F2 individuals were divided into four types, namely, type I, type II, type III, and type IV. There were 47 plants (21.76%) with 5P chromosome only in type I, 54 plants (25.00%) with 2P chromosome only in type II, 66 plants (30.56%) with both 2P and 5P chromosomes in type III, and 45 plants (20.83%) without P chromosomes in type IV. Among them, the type I individuals with the 5P chromosome only provided the candidate materials for further identifying the wheat-A. cristatum 5P addition line.
Identification and Molecular Cytological Detection of Wheat-A. cristatum 5P Addition Line
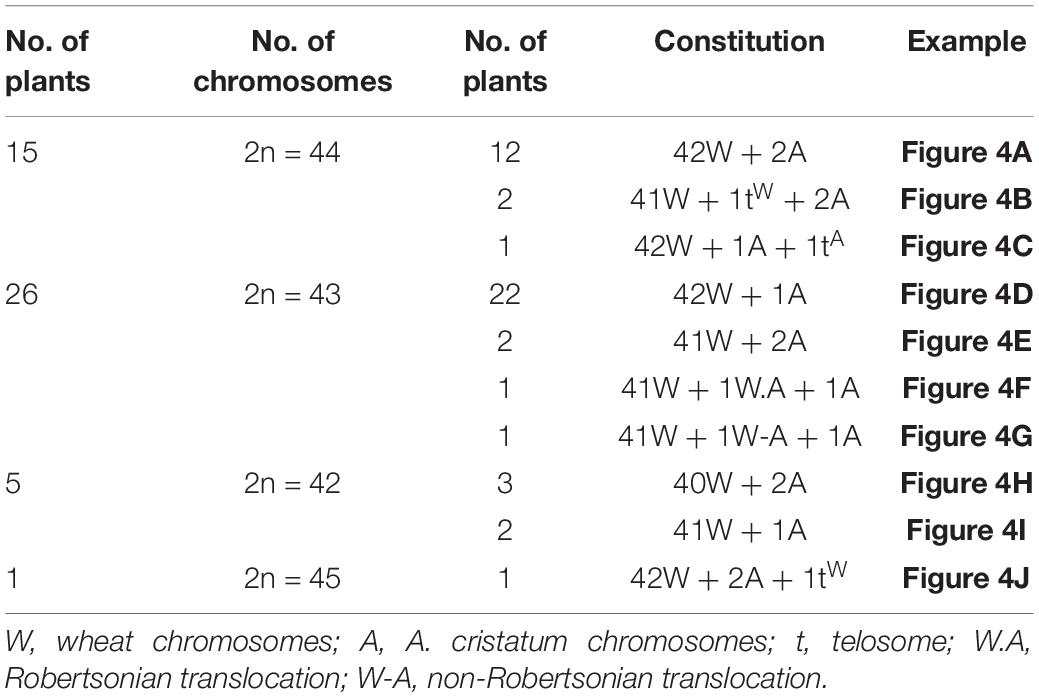
The chromosomal composition of 47 candidate individual plants in type I was further identified using GISH using the root tips (Table 2). Statistical results showed that there were 15 plants with 44 chromosomes, of which 12 plants were composed of 42 wheat chromosomes and 2 A. cristatum chromosomes (Figure 4A); 2 plants composed of 41 wheat chromosomes, 1 wheat telomere, and 2 A. cristatum chromosomes (Figure 4B) and 1 plant consisted of 42 wheat chromosomes, 1 A. cristatum telomere, and 1 A. cristatum chromosome (Figure 4C). There were 26 plants with 43 chromosomes, of which 22 plants were composed of 42 wheat chromosomes and 1 A. cristatum chromosome (Figure 4D); 2 plants contained 41 wheat chromosomes and 2 A. cristatum chromosomes (Figure 4E); 1 plant was composed of 41 wheat chromosomes, 1 whole arm translocation, and 1 A. cristatum chromosome (Figure 4F); 1 plant consisted of 41 wheat chromosomes, 1 small alien segment translocation, and 1 A. cristatum chromosome (Figure 4G). There were 5 plants with 42 chromosomes, of which 3 plants consisted of 40 wheat chromosomes and 2 A. cristatum chromosomes (Figure 4H) and 2 plants consisted of 41 wheat chromosomes and 1 A. cristatum chromosome (Figure 4I). One plant consists of 45 chromosomes with 42 wheat chromosomes, 1 wheat telomere, and 2 A. cristatum chromosomes (Figure 4J). Combined with the results of FISH identification, we found that 6D-7A translocation occurred in wheat chromosomes (Figure 4KSupplementary Figure 1A). Among the 47 individuals of type I, the 12 plants with 42 wheat and 2 A. cristatum chromosomes were further identified using FISH with the pAcTRT1 and pAcpCR2 probes. It was also revealed that the pair of A. cristatum chromosomes was 5P (Figure 4L and Supplementary Figure 1B). Finally, they were identified as a novel wheat-A. cristatum 5P addition line and named as II-11-1b.
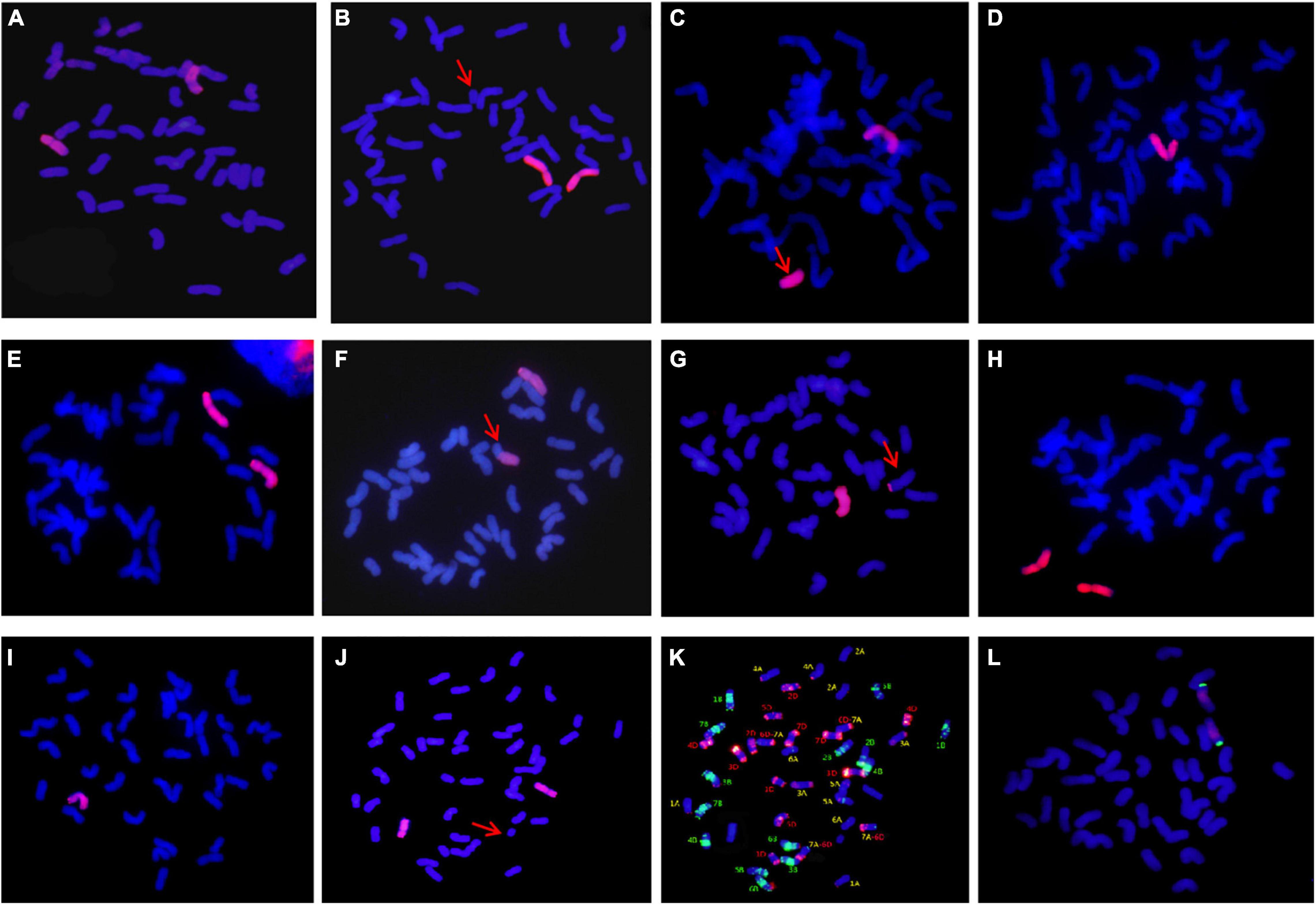
Figure 4. Mitosis GISH/FISH identification of II-11-1b. (A–J) A. cristatum genome DNA probe was labeled as a red signal; (K) pAs1 and pHvG38 were labeled as red and green, respectively; (L) pAcTRT1 and pAcpCR2 were labeled as red and green, respectively, and wheat chromosomes were restained as blue by DAPI.
Meiosis Abnormality and 5P Chromosome Functional Analysis
The meiosis metaphase I chromosome configuration of II-11-1b was observed and counted (Table 1). Except for the normal bivalent formation at metaphase (Figure 5A) and normal separation at anaphase (Figure 5I), chromosome behavioral abnormalities were also identified (Figures 5B–H,J–L). For example, there were chromosome fragments (Figure 5B), univalents of wheat chromosomes (Figure 5C) and A. cristatum 5P chromosomes (Figure 5D), multivalents, including trivalent and tetravalent, formed by wheat chromosomes only or wheat and A. cristatum 5P chromosomes (Figures 5E–G). In addition, the chromosome bridge and the division desynchrony with different numbers of lagging chromosomes were also observed (Figures 5H–L). Statistical analysis of the meiosis metaphase I showed that the 5P addition line II-11-1b had significantly higher frequencies of univalents and multivalents compared with the parent Fukuho (Table 1). The presence of multivalents indicates the existence of some homoeologous chromosome synapses or chromosomal rearrangement. At the anaphase of meiosis, the loss of univalents and the abnormal segregation of multivalents can cause changes in the chromosomal composition of their progenies. Thus, it is predicted that the chromosome 5P of A. cristatum has the function of inducing chromosome breakage, promoting synapses of the homoeologous chromosome.
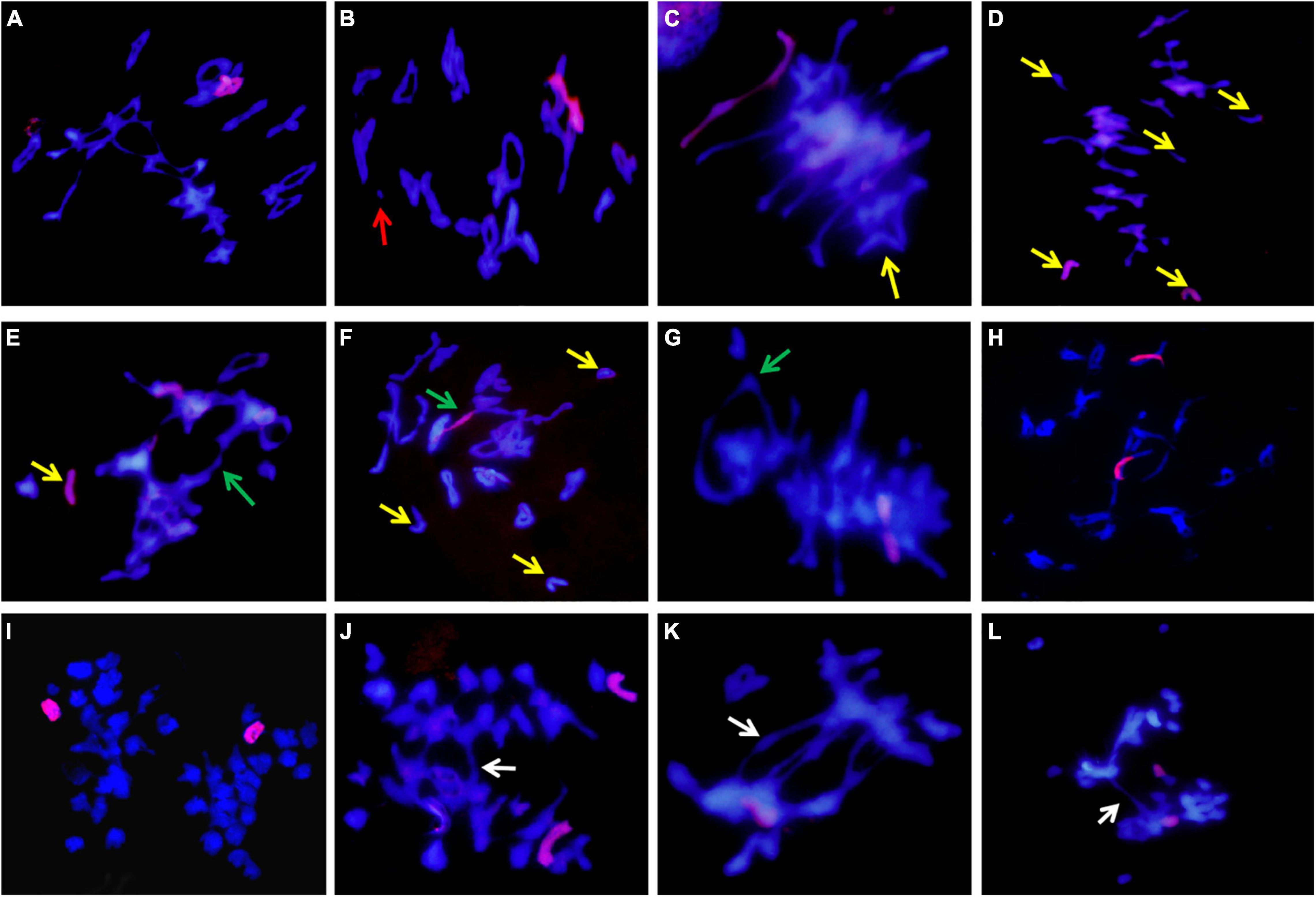
Figure 5. Meiosis GISH identification of II-11-1b. (A–L) A. cristatum genome DNA probe was labeled as a red signal; wheat chromosome restained blue by DAPI. (A–H) Meiotic metaphase I; (I–L) anaphase I. The red arrows refer to chromosome fragments, the yellow arrows refer to univalents, the green arrows refer to polyvalents, and the white arrows refer to chromosome adhesion or chromosome bridge.
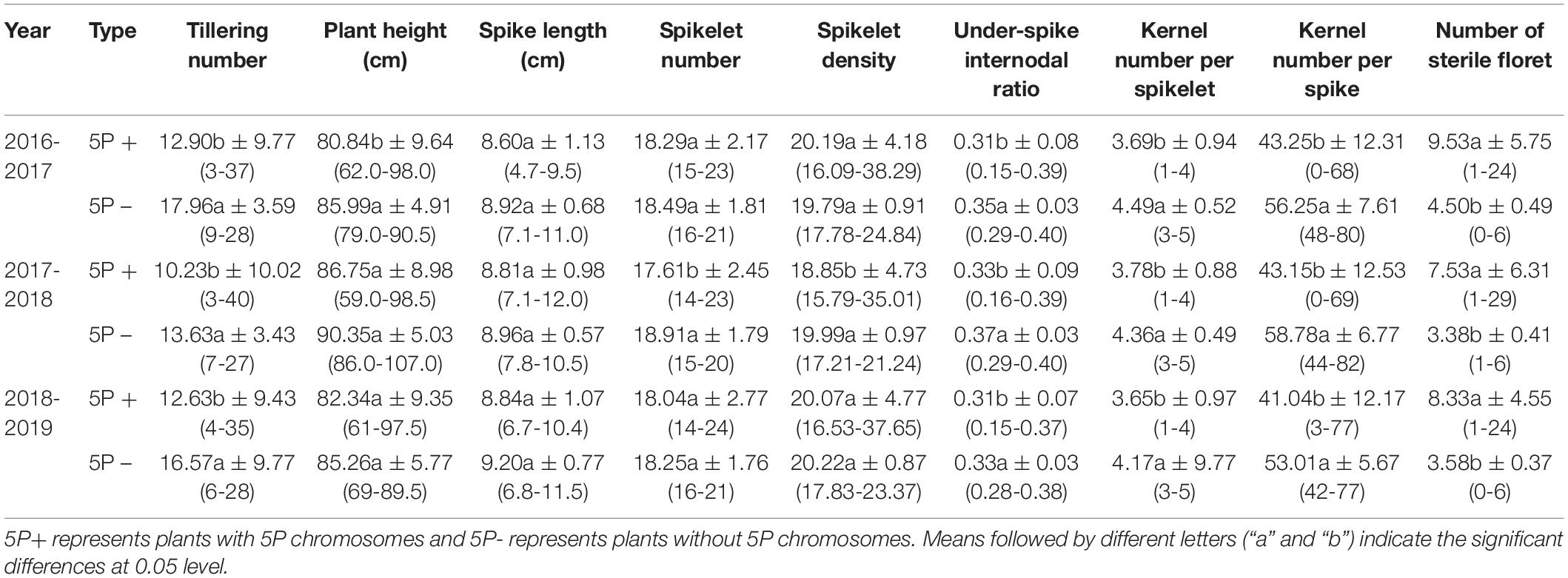
Evaluation of Agronomic Traits
According to the results of observation, statistics, and evaluation in three growing seasons, II-11-1b progenies with 5P chromosomes showed segregation in agronomic traits (Type 1 in Figure 6), and progenies without 5P chromosomes gradually stabilized (Types 2-n in Figure 6). For example, different types of spike traits were detected in II-11-1b progenies with 5P chromosomes (Figure 7). It showed that 5P chromosomes resulted in a decrease in fertility, which was reflected in the significant decrease in the kernel number per spikelet and per spike and the significant increase in the number of sterile florets (p < 0.05) in 5P positive plants compared with negative plants (Table 3). Meanwhile, the variation (statistical standard deviation) in tiller number, plant height, spike length, etc., of 5P positive plants were higher than those of negative plants (Table 3). Combined with the results of traits and molecular cytology, it is speculated that the presence of 5P chromosomes might influence the genetic stability by regulating the homologous and homoeologous chromosome pairing behavior during meiosis, thus leading to fertility decrease and trait separation in the progenies.
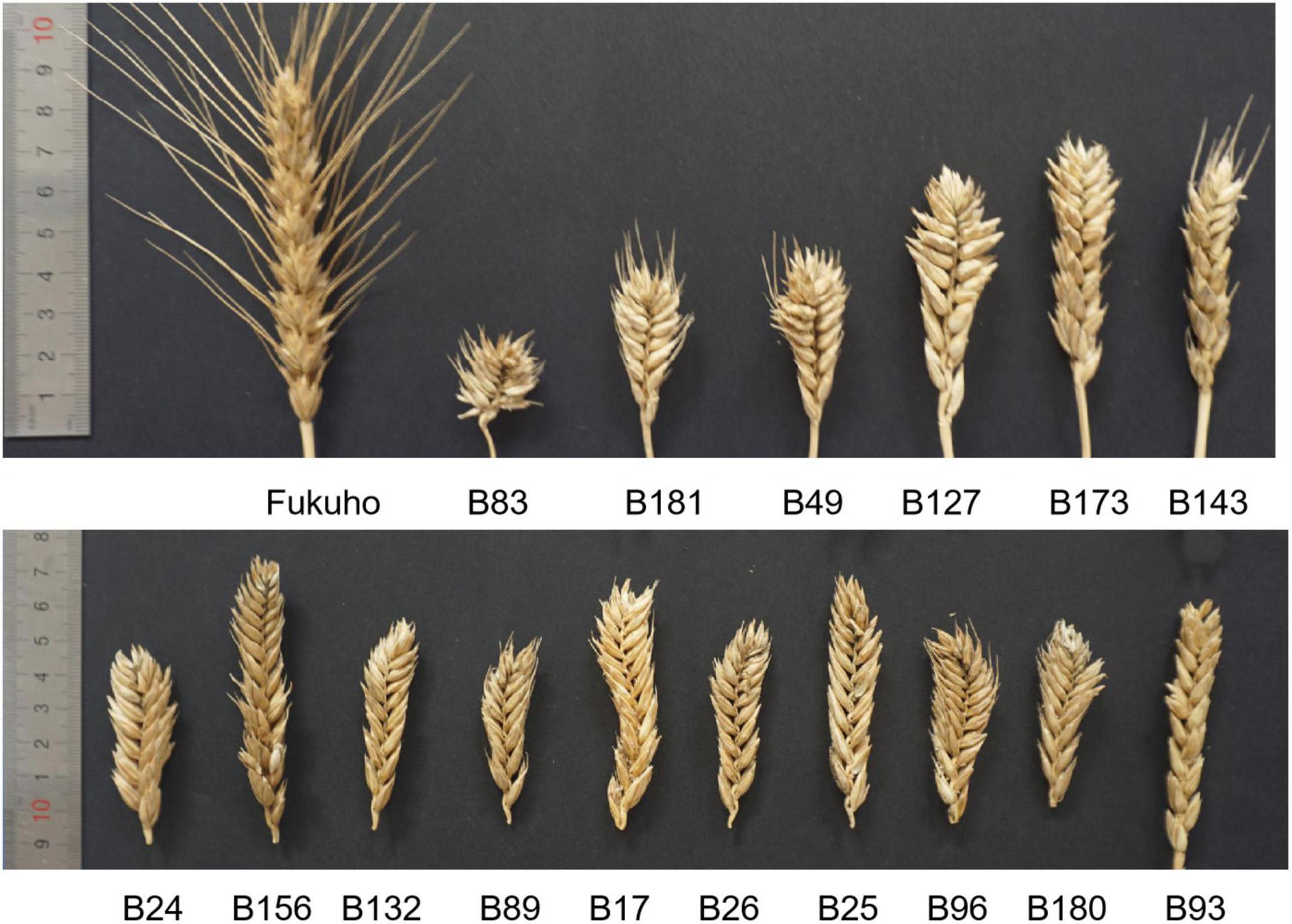
Figure 7. Segregation of spike traits was detected in the F2 population. Fukuho: parent; F2: B83, B181, B49, B127, B173, B143, B24, B156, B132, B89, B17, B26, B25, B96, B180, B93.
Discussion
The Significance of Wheat-Agropyron cristatum 5P Addition Line
Wheat wild relatives as gene resource pools provide abundant genetic resources for wheat improvement. Lots of alien genes of wild relatives have been introgressed into wheat by distant hybridization, which shows great potential in improving yield and quality, disease resistance, and stress resistance. Among the progenies of distant hybridization, wheat-alien disomic addition lines played a very important role in the transfer of alien excellent genes. A complete set of wheat-alien disomic addition lines is an important genetic material for studying the genetic relationship, origin and evolution of species, gene expression, and interaction of chromosomes. To fully explore the desirable genes in A. cristatum, it is necessary to establish a complete wheat-A. cristatum addition line. In the previous studies, all the wheat-A. cristatum addition lines except 5P have been obtained. In this study, we identified the presence of 5P chromosomes in II-11-1. By further backcrossing and selfing, the wheat-A. cristatum 5P addition line was identified. So far, we have obtained a complete set of wheat-A. cristatum addition lines (1P–7P), which provided materials for the systematic study of the excellent exogenous genes from A. cristatum. However, we found that as long as there are 5P chromosomes, there will always be meiosis abnormality and trait separation in the progenies (Table 3). This increases the difficulty in the identification and preservation of the wheat-A. cristatum 5P addition line and also explains its unavailability for a long time. In addition, the 5P addition line is an excellent material for studying the abnormal behavior of chromosomes during meiosis.
The Role and Value of 5P Chromosome: Induced Homoeologous Recombination
To date, some plentiful wild relatives have been successfully hybridized with wheat. However, due to the presence of genes that control homologous chromosome pairing (such as the Ph1 gene on the 5B chromosome), chromosome pairing is difficult to occur between wheat and its wild relatives. This mechanism not only ensures the genetic stability of wheat but also restricts the chromosome recombination and the exogenous gene transfer between wheat and its wild relatives, thus hindering the application of excellent genes in breeding. The discovery of genes that inhibit homologous chromosome pairing has laid a foundation for the transfer of exogenous excellent genes and the creation of new germplasm resources (Ceoloni and Donini, 1993). At present, the Ph suppressor gene has been found in several wild relatives of wheat. For example, the 5Mg chromosome of Aegilops geniculata contained genes that promote synapsis and crossing in prophase I of meiosis in wheat (Tiwari et al., 2015; Koo et al., 2017). Aegilops speltoides 5S chromosome contained a QTL (QPh.ucd-5S) that could increase homeologous chromosome pairing and regulate recombination between homologous chromosomes in T. aestivum × Ae. speltoides hybrids (Dvorak et al., 2006). Genes that affect chromosome pairing during meiosis were also found in the 5U chromosome of Aegilops umbrella (Riley et al., 1973), 4Mg chromosome of A. geniculata (Kynast et al., 2000), and 3S chromosome of A. speltoides Tausch (Li et al., 2019).
The offspring of distant hybridization might exhibit abnormal chromosome pairing behavior during meiosis. Observing the chromosomal configurations during meiosis of the alien addition line is a crucial way to analyze its stability. Based on the previous studies, it is speculated that there may also exist genes affecting homologous pairing in A. cristatum. For instance, by evaluating the Ph-suppressing effect of P chromosomes (1P–6P) and deletion ph1b of the Ph1 gene, it showed that they all displayed a significantly higher level of homoeologous pairing than the control except for 2PL and 2PS, but allosyndetic associations between P and ABD genomes were very rare, which had no prospect in the transfer of alien genes (Jubault et al., 2006). The results of the 5P addition line were inconsistent with this study, which may be due to the different sources of A. cristatum. In this study, the theoretical chromosomal configurations of II-11-1 and II-11-1b should be 21 wheat chromosomes bivalents plus 2 A. cristatum bivalents and 21 wheat chromosomes bivalents plus 1 A. cristatum bivalent, respectively. However, the statistical results showed that the ratio of univalents in II-11-1 and II-11-1b was 9.85 and 2.08, respectively, trivalents was 0.18 and 0.23, respectively, and quadrivalents was 0.08 and 0.13 respectively (Table 1). Meanwhile, the wheat Fukuho had a significantly low univalents ratio of 0.06, and there is no trivalent or quadrivalent. In the anaphase II and telophase II of meiosis, there also existed an abnormal phenomenon including fragments, bridges, lagging chromosomes, and the chromosome adhesions in II-11-1 and II-11-1b. In the progenies of II-11-1b, wheat-A. cristatum 5P translocation lines were identified (Figures 4F–G). Thus, it is supposed that the 5P chromosome might play a comprehensive and complex role in regulating chromosomal behavior during meiosis, including the inhibition of the Ph gene to promote the synapses of the homoeologous chromosome, and the function similar to the gametocidal chromosome, which induces chromosome breakage and recombination and promotes the formation of chromosomal translocation in the progenies.
Meiotic homoeologous recombination could facilitate gene introgression to diversify the wheat genome for germplasm development. Therefore, the wheat-A. cristatum 5P addition line II-11-1b is a potential and valuable material for gene introgression and gene mapping based on recombination between homoeologous chromosomes in wheat. The studies of the 5P chromosome further enhance our understanding of the wheat genome and its homoeologous counterparts A. cristatum and expand the genetic variability of the wheat genome.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
WL and LL conceived the research. CP and QL performed the research and wrote the manuscript. CP, QL, HH, JZ, SZ, XY, and XL participated in the preparation of both the reagents and materials. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the National Key Research and Development Program of China (No. 2016YFD0100102), the National Natural Science Foundation of China (No. 31801349), and the Natural Science Foundation of Ningxia Province (No. 2018AAC03036).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful to Hongjie Li (The Institute of Crop Sciences, Chinese Academy of Agricultural Sciences) and Ainong Gao (The Institute of Crop Sciences, Chinese Academy of Agricultural Sciences) for their useful advice and English language editing of the manuscript. We also thank AJE (https://www.aje.cn/#) for its linguistic assistance and scientific consultation during the preparation of this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.844348/full#supplementary-material
References
An, D. G., Ma, P. T., Zheng, Q., Fu, S. L., Li, L. H., Han, F. P., et al. (2019). Development and molecular cytogenetic identification of a new wheat-rye 4R chromosome disomic addition line with resistances to powdery mildew, stripe rust and sharp eyespot. Theor. Appl. Genet. 132, 257–272. doi: 10.1007/s00122-018-3214-3
Asay, K. H., and Johnson, D. A. (1990). Genetic variances for forage yield in crested wheatgrass at six levels of irrigation. Crop Sci. 30, 79–82. doi: 10.2135/cropsci1990.0011183x003000010018x
Ceoloni, C., and Donini, P. (1993). Combining mutations for the two homoeologous pairing suppressor genes Ph1 and Ph2 in common wheat and in hybrids with alien Triticeae. Genome 36, 377–386. doi: 10.1139/g93-052
Chen, H. X., Han, H. M., Li, Q. F., Zhang, J. P., Lu, Y. Q., Yang, X. M., et al. (2018). Identification and genetic analysis of multiple P chromosomes of Agropyron cristatum in the background of common wheat. J. Integr. Agr. 17, 1697–1705. doi: 10.1016/s2095-3119(17)61861-6
Cuadrado, A., Schwarzacher, T., and Jouve, N. (2000). Identification of different chromatin classes in wheat using in situ hybridization with simple sequence repeat oligonucleotides. Theor. Appl. Genet. 101, 711–717. doi: 10.1007/s001220051535
Dai, C., Zhang, J. P., Wu, X. Y., Yang, X. M., Li, X. Q., Liu, W. H., et al. (2012). Development of EST markers specific to Agropyron cristatum chromosome 6P in common wheat background. Acta Agronomica Sinica 38, 1791–1801.
Dellaporta, S. L., Wood, J., and Hicks, J. B. (1983). A plant DNA minipreparation: version II. Plant Mol. Biol. Rep. 1, 19–21.
Dewey, D. R. (1984). The Genomic System of Classification as a Guide to Intergeneric Hybridization with the Perennial Triticeae//Gene Manipulation in Plant Improvement. Berlin: Springer, 209–279.
Dong, Y. C., Zhou, R. H., Xu, S. J., Li, L. H., Cauderon, Y., and Wang, R. R. C. (1992). Desirable characteristics in perennial Triticeae collected in China for wheat improvement. Hereditas 116, 175–178. doi: 10.1111/j.1601-5223.1992.tb00224.x
Du, X. Y., Jia, Z. Z., Yu, Y., Wang, S., Che, B. J., Ni, F., et al. (2019). A wheat-Aegilops umbellulata addition line improves wheat agronomic traits and processing quality. Breed Sci. 69, 503–507. doi: 10.1270/jsbbs.18200
Dvorak, J., Deal, K. R., and Luo, M. C. (2006). Discovery and mapping of wheat Ph1 suppressors. Genetics 174, 17–27. doi: 10.1534/genetics.106.058115
Fang, Y. H., Yuan, J. Y., Wang, Z. J., Wang, H. Y., Xiao, J., Yang, Z. X., et al. (2014). Development of T. aestivum L.-H. californicum alien chromosome lines and assignment of homoeologous groups of Hordeum californicum chromosomes. J. Genet. Genomics 41, 439–447. doi: 10.1016/j.jgg.2014.06.004
Gao, X. H., Ren, C. C., Song, J., Li, X. J., and Ru, Z. G. (2018). Diversity and detection of 1BL/1RS and Pm21 in wheat cultivars (lines) from regional trial in southern of Yellow and Huai River facultative winter wheat region. Acta Agr. Boreali-occidentalis Sinica 27, 779–785.
Gong, W. P., Han, R. P., Li, H. S., Song, J. M., Yan, H. F., Li, G. Y., et al. (2017). Agronomic traits and molecular marker identification of wheat-Aegilops caudata addition lines. Front. Plant Sci. 8:1743. doi: 10.3389/fpls.2017.01743
Han, F. P., Liu, B., Fedak, G., and Liu, Z. H. (2004). Genomic constitution and variation in five partial amphiploids of wheat-Thinopyrum intermedium as revealed by GISH, multicolor GISH and seed storage protein analysis. Theor. Appl. Genet. 109, 1070–1076. doi: 10.1007/s00122-004-1720-y
Han, H. M., Bai, L., Su, J. J., Zhang, J. P., Song, L. Q., Gao, A. N., et al. (2014). Genetic rearrangements of six wheat-Agropyron cristatum 6P addition lines revealed by molecular markers. PLoS One 9:e9106. doi: 10.1371/journal.pone.0091066
Han, H. M., Liu, W. H., Lu, Y. Q., Zhang, J. P., Yang, X. M., Li, X. Q., et al. (2017). Isolation and application of P genome-specific DNA sequences of Agropyron Gaertn. in Triticeae. Planta 245, 425–437. doi: 10.1007/s00425-016-2616-1
Han, H. M., Liu, W. H., Zhang, J. P., Zhou, S. H., Yang, X. M., Li, X. Q., et al. (2019). Identification of P genome chromosomes in Agropyron cristatum and wheat-A. cristatum derivative lines by FISH. Sci. Rep. 9:9712. doi: 10.1038/s41598-019-46197-6
Jauhar, P. P., and Peterson, T. S. (2006). Cytological analyses of hybrids and derivatives of hybrids between durum wheat and Thinopyrum bessarabicum, using multicolour fluorescent GISH. Plant Breed. 125, 19–26. doi: 10.1111/j.1439-0523.2006.01176.x
Jiang, B., Liu, T. G., Li, H. H., Han, H. M., Li, L. H., Zhang, J. P., et al. (2018). Physical mapping of a novel locus conferring leaf rust resistance on the long arm of Agropyron cristatum chromosome 2P. Front. Plant Sci. 9:817. doi: 10.3389/fpls.2018.00817
Jiang, Z., Wang, Q. L., Wu, J. H., Xue, W. B., Zeng, Q. D., Huang, L. L., et al. (2014). Distribution of powdery mildew resistance gene Pm21 in Chinese winter wheat cultivars and breeding lines based on gene-specific marker. Sci. Agr. Sin. 47, 2078–2087. doi: 10.3864/j.issn.0578-1752.2014.11.002
Jubault, M., Tanguy, A. M., Abélard, P., Coriton, O., Dusautoir, J. C., and Jahier, J. (2006). Attempts to induce homoeologous pairing between wheat and Agropyron cristatum genomes. Genome 49, 190–193. doi: 10.1139/g05-074
Koo, D. H., Liu, W. X., Friebe, B., and Gill, B. S. (2017). Homoeologous recombination in the presence of Ph1 gene in wheat. Chromosoma 126, 531–540. doi: 10.1007/s00412-016-0622-5
Kynast, R. G., Friebe, B., and Gill, B. S. (2000). Fate of multicentric and ring chromosomes induced by a new gametocidal factor located on chromosome 4Mg of Aegilops geniculata. Chromosome Res. 8, 133–139. doi: 10.1023/a:1009294519798
Li, H., Wang, L., Luo, M. C., Nie, F., Zhou, Y., McGuire, P. E., et al. (2019). Recombination between homoeologous chromosomes induced in durum wheat by the Aegilops speltoides Su1-Ph1 suppressor. Theor. Appl. Genet. 132, 3265–3276.
Li, J. B., Dundas, I., Dong, C. M., Li, G. R., Trethowan, R., Yang, Z. J., et al. (2020). Identification and characterization of a new stripe rust resistance gene Yr83 on rye chromosome 6R in wheat. Theor. Appl. Genet. 133, 1095–1107. doi: 10.1007/s00122-020-03534-y
Li, L. H., and Dong, Y. C. (1991). Hybridization between Triticum aestivum L. and Agropyron michnoi Roshev. Theor. Appl. Genet. 81, 312–316.
Li, L. H., and Dong, Y. C. (1993). Progress in studies of Agropyron Gaertner. Acta Genet. Sin. 15, 45–48.
Li, L. H., Dong, Y. C., Zhou, R. H., Li, X. Q., and Li, P. (1995). Cytogenetics and self-fertility of hybrids between Triticum aestivum L. and Agropyron cristatum (L.) Gaertner. Chin. J. Genet. 22, 105–112.
Li, L. H., Li, X., Li, P., Dong, Y. C., and Zhao, G. (1997). Establishment of wheat-Agropyron cristatum alien addition lines. I. Cytology of F3, F2BC1, BC4, and BC3F1 progenies. Acta Genet. Sin. 24, 154–159.
Li, L. H., Yang, X. M., Zhou, R. H., Li, X. Q., and Dong, Y. C. (1998). Establishment of wheat-Agropyron cristatum alien addition lines II. Identification of alien chromosomes and analysis of development approaches. Acta Genet. Sin. 25, 538–544.
Li, Q. F., Lu, Y. Q., Pan, C. L., Zhang, J. P., Liu, W. H., Yang, X. M., et al. (2016a). Characterization of a novel wheat-Agropyron cristatum 2P disomic addition line with powdery mildew resistance. Crop Sci. 56, 2390–2400. doi: 10.2135/cropsci2015.10.0638
Li, H. H., Lv, M. J., Song, L. Q., Zhang, J. P., Gao, A. N., Li, L. H., et al. (2016b). Production and identification of wheat-Agropyron cristatum 2P translocation lines. PLoS One 11:e0145928. doi: 10.1371/journal.pone.0145928
Limin, A. E., and Fowler, D. B. (1987). Cold hardiness of forage grasses grown on the Canadian prairies. Can. J. Plant Sci. 67, 1111–1115. doi: 10.4141/cjps87-150
Liu, C., Gong, W. P., Han, R., Guo, J., Li, G. R., Li, H. S., et al. (2019). Characterization, identification and evaluation of a set of wheat-Aegilops comosa chromosome lines. Sci. Rep. 9:4773. doi: 10.1038/s41598-019-41219-9
Liu, H. P., Dai, Y., Chi, D., Huang, S., Li, H. F., Duan, Y. M., et al. (2017). Production and molecular cytogenetic characterization of a durum wheat-Thinopyrum elongatum 7E disomic addition line with resistance to Fusarium Head Blight. Cytogenet. Genome Res. 153, 165–173. doi: 10.1159/000486382
Liu, W. H., Luan, Y., Wang, J. C., Wang, X. G., Su, J. J., Zhang, J. P., et al. (2010). Production and identification of wheat-Agropyron cristatum (1.4P) alien translocation lines. Genome 53, 472–481. doi: 10.1139/g10-023
Lu, M. J., Lu, Y. Q., Li, H. H., Pan, C. L., Guo, Y., Zhang, J. P., et al. (2016). Transferring desirable genes from Agropyron cristatum 7P chromosome into common wheat. PLoS One 11:e0159577. doi: 10.1371/journal.pone.0159577
Luan, Y., Wang, X. G., Liu, W. H., Li, C. Y., Zhang, J. P., Gao, A. N., et al. (2010). Production and identification of wheat-Agropyron cristatum 6P translocation lines. Planta 232, 501–510. doi: 10.1007/s00425-010-1187-9
Ma, F. F., Xu, Y. F., Ma, Z. Q., Li, L. H., and An, D. G. (2018). Genome-wide association and validation of key loci for yield-related traits in wheat founder parent Xiaoyan 6. Mol. Breed. 38:91.
Ochoa, V., Madrid, E., Said, M., Rubiales, D., and Cabrera, A. (2014). Molecular and cytogenetic characterization of a common wheat Agropyron cristatum chromosome translocation conferring resistance to leaf rust. Euphytica 201, 89–95.
Pan, C. L., Li, Q. F., Lu, Y. Q., Zhang, J. P., Yang, X. M., Li, X. Q., et al. (2017). Chromosomal localization of genes conferring desirable agronomic traits from Agropyron cristatum chromosome 1P. PLoS One 12:e0175265. doi: 10.1371/journal.pone.0175265
Pedersen, C., and Langridge, P. (1997). Identification of the entire chromosome complement of bread wheat by two-colour FISH. Genome 40, 589–593. doi: 10.1139/g97-077
Rayburn, A. L., and Gill, B. S. (1986). Isolation of a D-genome specific repeated DNA sequence from Aegilops squarrosa. Plant Mol. Biol. Rep. 4, 102–109.
Riley, R., Chapman, V., and Miller, T. E. (1973). “The determination of meiotic chromosome pairing,” in Proceeding 4th International Wheat Genetics Symposium, (Columbia, MO: University of Missouri), 713–738.
Schneider, A., Rakszegi, M., Molnár, L. M., and Szakács, É (2016). Production and cytomolecular identification of new wheat-perennial rye (Secale cereanum) disomic addition lines with yellow rust resistance (6R) and increased arabinoxylan and protein content (1R, 4R, 6R). Theor. Appl. Genet. 129, 1045–1059. doi: 10.1007/s00122-016-2682-6
Sharma, H. C., Gill, B. S., and Uyemoto, J. K. (1984). High levels of resistance in Agropyron species to barley yellow dwarf and wheat streak mosaic viruses. J. Phytopathol. 110, 143–147.
Song, L. Q., Jiang, L. L., Han, H. M., Gao, A. N., Yang, X. M., Li, L. H., et al. (2013). Efficient induction of wheat-Agropyron cristatum 6P translocation lines and GISH detection. PLoS One 8:e69501. doi: 10.1371/journal.pone.0069501
Su, Y. R., Wang, Z. C., Zhang, D. L., Gao, H. Y., and Li, S. P. (2006). Genetic diversity of Yellow-Huai River Zone wheat varieties which originated from 1B/1R by SSR. J. Plant Genet. Res. 7, 321–326.
Szakacs, E., and Molnal-Lang, M. (2010). Identification of new winter wheat-winter barley addition lines (6HS and 7H) using fluorescence in situ hybridization and the stability of the whole ‘Martonvasari 9 kr1’- ‘Igri’ addition set. Genome 53, 35–44. doi: 10.1139/g09-085
Tiwari, V. K., Wang, S. C., Danilova, T., Koo, D. H., Vrána, J., Kubaláková, M., et al. (2015). Exploring the tertiary gene pool of bread wheat: sequence assembly and analysis of chromosome 5M(g) of Aegilops geniculata. Plant J. 84, 733–746. doi: 10.1111/tpj.13036
Wu, J., Yang, X. M., Wang, H., Li, H. J., Li, L. H., Li, X. Q., et al. (2006). The introgression of chromosome 6P specifying for increased numbers of florets and kernels from Agropyron cristatum into wheat. Theor. Appl. Genet. 114, 13–20. doi: 10.1007/s00122-006-0405-0
Yang, X. F., Li, X., Wang, C. Y., Chen, C. H., Tian, Z. R., and Ji, W. Q. (2017). Isolation and molecular cytogenetic characterization of a wheat-Leymus mollis double monosomic addition line and its progenies with resistance to stripe rust. Genome 60, 1029–1036. doi: 10.1139/gen-2017-0078
Ye, X. L., Lu, Y. Q., Liu, W. H., Chen, G. Y., Han, H. M., Zhang, J. P., et al. (2015). The effects of chromosome 6P on fertile tiller number of wheat as revealed in wheat-Agropyron cristatum chromosome 5A/6P translocation lines. Theor. Appl. Genet. 128, 797–811. doi: 10.1007/s00122-015-2466-4
Yi, Y. J., Zheng, K., Ning, S. Z., Zhao, L. B., Xu, K., Hao, M., et al. (2019). The karyotype of Aegilops geniculata and its use to identify both addition and substitution lines of wheat. Mol. Cytogenet. 12:15. doi: 10.1186/s13039-019-0428-2
Zhang, A. C., Li, W. Y., Wang, C. Y., Yang, X. F., Chen, C. H., Zhu, C., et al. (2017a). Molecular cytogenetics identification of a wheat -Leymus mollis double disomic addition line with stripe rust resistance. Genome 60, 375–383. doi: 10.1139/gen-2016-0151
Zhang, J. P., Liu, W. H., Lu, Y. Q., Liu, Q. X., Yang, X. M., Li, X. Q., et al. (2017b). A resource of large-scale molecular markers for monitoring Agropyron cristatum chromatin introgression in wheat background based on transcriptome sequences. Sci. Rep. 7:11942. doi: 10.1038/s41598-017-12219-4
Zhang, J., Jiang, Y., Wang, Y., Guo, Y. L., Long, H., Deng, G. B., et al. (2018a). Molecular markers and cytogenetics to characterize a wheat-Dasypyrum villosum 3V (3D) substitution line conferring resistance to stripe rust. PLoS One 13:e0202033. doi: 10.1371/journal.pone.0202033
Zhang, R. Q., Fan, Y. L., Kong, L. N., Wang, Z. J., Wu, J. Z., Xing, L. P., et al. (2018b). Pm62, an adult-plant powdery mildew resistance gene introgressed from Dasypyrum villosum chromosome arm 2VL into wheat. Theor. Appl. Genet. 131, 2613–2620. doi: 10.1007/s00122-018-3176-5
Zhang, R. Q., Hou, F., Feng, Y. G., Zhang, W., Zhang, M. Y., and Chen, P. D. (2015a). Characterization of a Triticum aestivum-Dasypyrum villosum T2VS⋅ 2DL translocation line expressing a longer spike and more kernels traits. Theor. Appl. Genet. 128, 2415–2425.
Zhang, J. P., Liu, W. H., Han, H. M., Song, L. Q., Bai, L. L., Gao, Z. H., et al. (2015b). De novo transcriptome sequencing of Agropyron cristatum to identify available gene resources for the enhancement of wheat. Genomics 106, 129–136. doi: 10.1016/j.ygeno.2015.04.003
Zhang, Y., Zhang, J. P., Huang, L., Gao, A. N., Zhang, J., Yang, X. M., et al. (2015c). A high-density genetic map for P genome of Agropyron Gaertn. based on specific-locus amplified fragment sequencing (SLAF-seq). Planta 242, 1335–1347. doi: 10.1007/s00425-015-2372-7
Zhang, Z., Han, H. M., Liu, W. H., Song, L. Q., Zhang, J. P., Zhou, S. H., et al. (2019). Deletion mapping and verification of an enhanced-grain number per spike locus from the 6PL chromosome arm of Agropyron cristatum in common wheat. Theor. Appl. Genet. 132, 2815–2827. doi: 10.1007/s00122-019-03390-5
Keywords: Agropyron cristatum, 5P chromosomes, molecular cytogenetics, homoeologous pairing, addition line
Citation: Pan C, Li Q, Han H, Zhang J, Zhou S, Yang X, Li X, Li L and Liu W (2022) Identification of 5P Chromosomes in Wheat-Agropyron cristatum Addition Line and Analysis of Its Effect on Homologous Pairing of Wheat Chromosomes. Front. Plant Sci. 13:844348. doi: 10.3389/fpls.2022.844348
Received: 28 December 2021; Accepted: 27 January 2022;
Published: 24 February 2022.
Edited by:
Dayun Tao, Yunnan Academy of Agricultural Sciences, ChinaReviewed by:
Mu Jun Yang, Yunnan Academy of Agricultural Sciences, ChinaLiqiang Song, Agricultural University of Hebei, China
Copyright © 2022 Pan, Li, Han, Zhang, Zhou, Yang, Li, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weihua Liu, liuweihuai@caas.cn; Lihui Li, lilihui@caas.cn
†These authors have contributed equally to this work
 Cuili Pan
Cuili Pan Qingfeng Li
Qingfeng Li Haiming Han
Haiming Han Jinpeng Zhang1
Jinpeng Zhang1 Shenghui Zhou
Shenghui Zhou Lihui Li
Lihui Li Weihua Liu
Weihua Liu