- 1College of Bioengineering, Jingchu University of Technology, Jingmen, China
- 2National Key Laboratory of Crop Genetic Improvement, Huazhong Agricultural University, Wuhan, China
- 3Institute of Plant Science of Paris-Saclay (IPS2), CNRS, INRA, University Paris-sud 11, University Paris-Saclay, Orsay, France
Histone lysine acetylation is an essential chromatin modification for epigenetic regulation of gene expression during plant response to stress. On the other hand, enzymes involved in histone acetylation homeostasis require primary metabolites as substrates or cofactors whose levels are greatly influenced by stress and growth conditions in plants. In addition, histone lysine acylation that requires similar enzymes for deposition and removal as histone acetylation has been recently characterized in plant. Results on understanding the intrinsic relationship between histone acetylation/acylation, metabolism and stress response in plants are accumulating. In this review, we summarize recent advance in the field and propose a model of interplay between metabolism and epigenetic regulation of genes expression in plant adaptation to stress.
Introduction
Due to their sessile life style, plants have developed sophisticated signaling pathways by adjusting gene expression, metabolism, cellular activity, and growth to respond to the changing environmental conditions. During the last 2 decades, much progress has been made in deciphering signaling pathways of plant responses to both biotic and abiotic stresses. More recent data indicate that chromatin modification plays an essential role in epigenetic regulation of gene expression and plant adaptation to stress (Kim et al., 2015). Chromatin modifications, including DNA methylation, histone modifications, histone variant deposition, and nucleosome positioning, are the basis of epigenetic regulation. Nucleosome, which is the building block of chromatin, consists of a core histone octamer (H2A, H2B, H3, and H4) and a segment of DNA wrapped around the core. The linker histone H1 binds to the nucleosome at the entry and exit sites of DNA and is involved in the stability of higher-order structure of chromatin. N-terminal tails of core histones are modified with various chemical groups such as methyl, acetyl, acyl, ribosyl, phosphate as well as ubiquitin and SUMO. Histone modification affects nucleosome and chromatin structure, thus plays important roles in fine tuning of gene expression. Histone modification, in particular histone lysine acetylation, has been shown to play an essential role in plant response and tolerance to stress (Servet et al., 2010; Yuan et al., 2013). In this review we focus on summarizing recent progresses in the field and discussing histone acetylation dynamics and its relationship with primary metabolism in plant responses to stress.
Histone Acetylation Dynamics in Plant Responses to Stress
Histone acetylation consists of addition of acetyl group, which is provided by acetyl-CoA, to the ε-amine group of N-terminal lysine residues. Histone lysine acetylation not only neutralizes the positive charge of the amine group, and enhances the hydrophobicity and increases the size of the lysine side chain, but also provides platforms for binding by regulatory proteins of chromatin structure and gene activity to promote genes transcription.
Global change in histone acetylation in response to stress has been reported in various plants. For example, salt, drought, and heat stress increase acetylation of histone H3 lysine 9 (H3K9ac) and histone H4 lysine 5 (H4K5ac) levels in different tissues of maize (Li et al., 2014; Zhao et al., 2014a; Zhao et al., 2014b; Wang et al., 2015). In rice seedlings, drought stress induces H3K9ac, H3K18ac, H3K27ac, H3ac, and H4K5ac (Fang et al., 2014). However, cold stress dramatically reduces global H3K14ac while without altering H3K9ac and H3K27ac in the same plant (Roy et al., 2014). Recent results indicate that in rice plants under submergence or starvation, genome-wide H3K9ac displays dynamic changes which correlates with the stress-induced gene activation (Lu et al., 2018). These data illustrate that histone H3 and H4 lysine acetylation is mostly elevated during plant response to stress. The increase implies that either higher acetylation or lower deacetylation activities are induced during stress responses. The global change of acetylation at specific lysine residues suggests that activity of particular enzymes involved in histone acetylation is induced by the stress. However, despite that acetylation enzymes may have preferential lysine targets in histone H3 or H4 (Henry et al., 2013; Kuo and Andrews, 2013; Zhou et al., 2017), acetylation at a particular site can be spread to neighboring lysine residues (Earley et al., 2007). On the other hand, as primary metabolism is greatly altered by stress or growth conditions, a metabolic control of histone acetylation is not excluded (see below). It is generally thought that the increase of histone acetylation plays an essential role in reprograming gene expression for plant response or tolerance to stress. However, the increase of histone acetylation at some loci may lag behind gene activation, thus appears in this case as the consequence rather than the cause of the stress-induced gene expression.
Function of Histone Acetylation Enzymes in Plant Response to Stress
Histone lysine acetylation is catalyzed by histone acetyltransferases (HATs) and reversed by histone deacetylases (HDACs). In plants, the HATs can be grouped into four classes: General control nondepressible 5 (GCN5)-related Acetyl Transferase (GNAT), MOZ-YBF2/SAS3-SAS2/TIP60 (MYST), cAMP-responsive element Binding Protein (CBP), and TATA-binding protein Associated Factor 1 (TAF1) (Pandey et al., 2002). Histone acetylation at different lysine residues is carried out by specific HATs (Earley et al., 2007). For example, two GNAT class HATs, HAG1 and HAG2, catalyze H3K14 and H4K12 acetylation respectively. Two MYST class HATs, HAM1 and HAM2, redundantly acetylate H4K5. However, multiple HATs are responsible for acetylation of H3K9, H4K8, and H4K16 (Earley et al., 2007). Enzymatic specificities of HATs may reflect their specific roles in the regulation of gene expression, as recognition of different acetylated lysines of histone by distinct reader proteins incur various epigenetic consequences. These proteins include chromatin remodelers, BET proteins, histone lysine methyltransferases and HATs themselves, all of which contain bromodomain that mediates the interaction between the reader protein and acetylated lysine (Marmorstein and Zhou, 2014). Regulatory function of some of these proteins in plant has been characterized. An Arabidopsis ATP-dependent chromatin remodeler, BRAHMA (BRM), is required for the expression of many genes in concert with or counteracting histone variant H2A.Z (Han et al., 2015; Torres and Deal, 2019). The bromodomain of BRM is able to bind histones but its targeting acetylated lysine has not been identified yet (Farrona et al., 2007). The HDACs can be grouped into three families: Reduced Potassium Dependency 3 (RDP3)/Histone DeAcetylase 1 (HDA1), Silent Information Regulator 2 (SIR2), and the plant-specific Histone Deacetylase 2 (HD2) (Pandey et al., 2002). In addition, based on their homology to yeast HDACs the plant RPD3/HDA1 family is divided into three classes (I, II, and IV) and their activity can be suppressed by different HDAC inhibitors respectively (Ueda et al., 2017). Site specificity of HDACs on histones has not been clearly demonstrated. Alteration of rice HDT701 expression level affects global H4K5 and H4K16 acetylation levels, while many HDACs regulates H3 acetylation at specific genes (Ding et al., 2012; Zheng et al., 2016; Liu et al., 2017; Ueda et al., 2017; Wang et al., 2017; Cheng et al., 2018; Park et al., 2018). In addition to histones, non-histone substrates of plant RPD3/HDA1 family HDACs has also been identified (Hartl et al., 2017). These proteins localize either in nucleus or other organelles. The lysine acetylation sites regulated by HDACs account for 10% of total detected lysine acetylation sites (Hartl et al., 2017). These results indicate that HDACs have additional cellular functions aside from regulation of gene expression via histone deacetylation. HAT and HDAC proteins target specific genomic or chromatin regions by interacting with transcription factors and other chromatin proteins for epigenetic regulation of gene expression in response to cellular signals (Servet et al., 2010; Liu et al., 2014). It is also possible that the interactions mediate reversible acetylation of transcription regulators by HATs and HDACs, which is important for the function of the regulators. The mechanism has been testified in mammals but not reported in plant yet (Lee et al., 2012; Sadler et al., 2015). Besides, in rice, moonlight function of Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is modulated by SIR2 family HDAC, SRT1, which will be discussed later. Function of HATs and HDACs in stress-responses was summarized in the previous review (Kim et al., 2015). Here we discuss recent advances in functional analysis of HATs and HDACs in regulating plant response to stress.
GCN5 is a primary histone acetyltransferase in regulating plant gene expression (Servet et al., 2010; Zhou et al., 2017). Recent studies demonstrated a primary role of GCN5 in plant response and tolerance to stress. Mutation or downregulation of GCN5 increases plant sensitivity to varieties of stresses including salt, drought, heat, and disease in different species (Hu et al., 2015; Kong et al., 2017; Zheng et al., 2018; Li et al., 2019). In Arabidopsis, GCN5-mediated salt and heat tolerance is dependent on its regulatory function on stress genes expression (Hu et al., 2015; Zheng et al., 2018). Induction of stress genes is attenuated in gcn5 mutants and overexpression of the stress genes in gcn5 mutant plants can partially restore stress tolerance. In Populus trichocarpa, GCN5 is recruited to promoters of the drought-responsive genes PtrNAC006, PtrNAC007, and PtrNAC120 to activate their expression, thus conferring drought tolerance (Li et al., 2019). In soybean, GCN5-mediated histone acetylation at defense-related genes is important for their activation and plant immunity (Kong et al., 2017). The cytoplasmic effector PsAvh23 produced by the soybean pathogen suppresses histone acetylation by disassembling HAT complex to increase plant susceptibility. The role of the other HATs in plant stress response has not been explored except that HAC1 and HAC5 (HAC1/5) are shown to be essential for salicylic acid (SA) -triggered immunity (Jin et al., 2018). HAC1/5 form a complex with NPR1, which is recruited to PR chromatin by TGACG BINDING FACTORs (TGAs) to induce PR transcription. HAC1/5 mutation diminishes PR induction and plant resistance to pathogen.
Several HDACs have been reported to negatively regulate stress tolerance in Arabidopsis such as HDA9, HDA15, HD2C, AtSRT1, Class I HDAC (HDA19) in Col-0 ecotype, and HDA6 in Ler ecotype (Buszewicz et al., 2016; Mehdi et al., 2016; Zheng et al., 2016; Liu et al., 2017; Ueda et al., 2017; Wang et al., 2017; Park et al., 2018; Ueda et al., 2018; Shen et al., 2019). Consistent with the discovery, treatment with HDACs inhibitor Cyclo(-L-2-amino-8-hydroxamido-suberoyl-aminoisobutylyl-L-phenylalanyl-D-prolyl-), called Ky-2, increases plant tolerance to salt stress, which is dependent on enhanced Na+ efflux by activation of AtSOS1 genes (Sako et al., 2016). Defects in AtSOS1 neutralize salt tolerance caused by ky2 treatment. The phenomenon has also been observed in cassava. Treatment with HDAC inhibitor, suberoylanilide hydroxamic acid (SAHA) induces MeSOS1 expression and reduces Na+ content thus enhancing tolerance to salinity stress in cassava (Patanun et al., 2016). However, other HDACs were found to act as positive regulators of stress tolerance. These include HD2C, HDA19 in Ws ecotype, HDA6 in Ws, and Col-0 ecotype, HD2B and Class II HDACs (HDA5/14/15/18) (Chen et al., 2010; Chen and Wu, 2010; Luo et al., 2012; Latrasse et al., 2017; Ueda et al., 2017). Surprisingly, the same genes in different ecotype appear to function oppositely in stress tolerance. The cause of this functional divergence is unclear. It is suggested that the functional discrepancy between Class I and Class II HDACs may be because they have distinct downstream target genes (Ueda et al., 2017). For instance, there are few common up-regulated genes in hda19 and hda5/14/15/18 mutants before and after salt treatment (Ueda et al., 2017). Moreover, stress tolerance-related and stress sensitivity-related genes are respectively activated in hda19 and hda5/14/15/18 mutants, suggesting mutation of two classes HDACs induces opposite responses to salt stress. There is also possibility that Class II HDACs target non-histone proteins involved in salt tolerance as global histone acetylation remains unchanged in the mutant. Alternatively, HDA19 may act downstream to Class II HDACs because hda5/14/15/18/19 mutants exhibit similar salt tolerance as hda19 (Ueda et al., 2017). To dissect specific function of the different HDACs in stress tolerance, genome-wide identification of their target genes and analysis of changes in histone acetylation at these genes in response to stress are required. In addition, how HDACs respond to stress signaling to control histone acetylation and expression of specific genes remains unclear. The gene or locus specificity of HDACs is at least partly determined by interacting with other partner proteins. It was shown that HDA19 interacts with transcriptional co-repressors such as TOPLESS (TPL), SWI-INDEPENDENT3 (SIN3), and SIN3-LIKE (SNL) proteins and transcription factors such as AP2/EREBP, BES1, SCR-like15, WOX5, etc. to repress genes involved in different developmental and stress-responsive pathways (Song et al., 2005; Long et al., 2006; Krogan et al., 2012; Wang et al., 2013; Ryu et al., 2014; Gao et al., 2015; Pi et al., 2015; Mehdi et al., 2016). HDA9 interacts with POWERDRESS (PWR), a SANT-domain containing protein to regulate heat stress response (Chen et al., 2016; Tasset et al., 2018). HDA15 interacts with the transcription factor HFR1 (long Hypocotyl in Far Red1) to repress warm-temperature response (Shen et al., 2019)
It has been shown that redox modification of HDAC is involved in stress signaling in mammalian cells. In humans, oxidative stress reduces HDAC2 activity by tyrosine nitration, resulting in histone hyperacetylation at the target genes and their activation (Ito et al., 2004). Additionally, in mammals activity of several HDACs is regulated by S-nitrosylation (Nott et al., 2008; Feng et al., 2011; Okuda et al., 2015). In Arabidopsis, treatment with the physiological NO donor S-nitrosoglutathione (GSNO) increases global histone acetylation, which is triggered by inhibition of cellular overall HDAC activity (Mengel et al., 2017). Stress responsive genes are activated owing to increased histone acetylation. Moreover, SA induced abundance of histone acetylation is diminished in the presence of the NO scavenger (Mengel et al., 2017). This indicates that histone hyperacetylation induced by SA is dependent on NO production. However, whether inhibition of HDAC activity by NO production involves direct S-nitrosylation of HDAC proteins has not been demonstrated yet. It is hypothesized that under normal conditions, HDACs maintain repressive chromatin state of stress-responsive genes by histone deacetylation to keep gene transcripts at low levels. SA or stress induces NO accumulation, which subsequently inhibits HDAC activity by redox modification of the proteins. This leads to histone hyperacetylation and activation of stress-responsive genes expression.
Acetyl-CoA Controlled Histone Acetylation in Plant Response to Stress
Histone acetylation consumes acetyl-CoA, which is a precursor for amino acids, lipids, and many secondary metabolites required for plant growth and defense. In addition, acetyl-CoA drives the tricarboxylic acid (TCA) cycle for the production of ATP under aerobic conditions. Acetyl-CoA can be produced by beta-oxidation of fatty acids in peroxisome or by oxidizing pyruvate by the pyruvate dehydrogenase complex (PDC) and can be synthesized by ATP-citrate lyase (ACL) in the cytoplasm and nucleus. Thus, acetyl-CoA level reflects cellular energy status. Recent data firmly demonstrates that histone lysine acetylation is regulated by acetyl-CoA availability. In yeast, unusually high levels of acetyl-CoA have a determinative role in histone acetylation and epigenetic regulation of gene expression (Cai et al., 2011; Shi and Tu, 2013). In yeast or mammals, growth-related genes or genes involved in glucose metabolism are up-regulated by histone hyperacetylation in response to growth factor stimulation or high glucose availability (Etchegaray and Mostoslavsky, 2016). In mammalian cells, up-regulation of these genes relies on ACL that converts glucose-derived citrate into acetyl-CoA in the nucleus (Wellen et al., 2009). Another source of nucleic acetyl-CoA is conversion of acetate by acylCoA synthetase short chain family member 2 (ACSS2). ACSS2-mediated acetyl-CoA production is crucial for histone acetylation as decrease in ACSS2 causes lower level of acetyl-CoA and histone acetylation (Bulusu et al., 2017). Nuclear-localized PDC that generates acetyl-CoA from pyruvate is also required for histone acetylation at specific lysine residues important for expression of cell cycle genes in response to growth factor (Sutendra et al., 2014). Lipid-derived acetyl-CoA promotes histone acetylation at certain lysine residues to activate lipid metabolic genes expression in response to accumulated lipid (Mcdonnell et al., 2016). It is intriguing to disclose how acetyl-CoA originated from distinct precursors promotes acetylation of specific lysine residues of histones and expression of specific genes. Most of enzymes responsible for generation of acetyl-CoA such as ACL, ACSS2 and PDC has been reported to localize in nucleus (Wellen et al., 2009; Sutendra et al., 2014; Li et al., 2017). Additionally, ACSS2 can be recruited to chromatin by interacting with a specific transcription factor (Li et al., 2017). Physical association between ACSS2 and CREB-binding protein (CBP), a histone acetytransferase, has been observed indicating that acetyl-CoA is provided locally to activate histone acetyltransferase that targets specific genes for histone acetylation (Mews et al., 2017). ACL and GCN5 may function in the same pathway to promote histone acetylation despite direct interaction of the two enzymes is not examined (Wellen et al., 2009). These results demonstrate that the acetyl-CoA producing enzymes form complexes with different HATs to enhance acetylation at specific histone lysine residues.
In Arabidopsis, it was shown that increased level of acetyl-CoA, caused by mutation of cytosolic acetyl-CoA carboxylase1 (ACC1), predominantly promotes histone hyperacetylation at H3K27 (Chen et al., 2017). Conversely, loss-of-function of adenosine triphosphate (ATP)-citrate lyase subunit A (ACLA) reduces H3K27ac, supporting a causal relationship between high levels of acetyl-CoA and H3K27ac elevation. It was also observed that increase of H3K27ac at a sub set of genes in acc1 mutant was dependent on GCN5 (Chen et al., 2017). Transcriptome and metabolome analyses indicate that primary metabolism including amino acid biosynthesis is affected in acc1 (Chen et al., 2017), demonstrating that in plants acetyl-CoA also connects metabolic state with epigenetic regulation of gene expression. A recent study showed that the fatty acid β-oxidation pathway enzymes (acyl-CoA oxidase 4, multifunctional protein 2, and 3-ketoacyl-CoA thiolase-2) are required for histone acetylation and anti-silencing at some endogenous transgenic loci in Arabidopsis (Wang et al., 2019). The finding confirms that histone acetylation can be regulated by acetyl-CoA availability in plant cells and indicate that acetyl-CoA produced from β-oxidation in peroxisome participates in histone modification in the nucleus.
Direct evidence supporting how HAT-mediated histone acetylation promoted by acetyl-CoA is involved in stress response has not yet been clearly demonstrated. Plant needs to reduce growth rate to survive from stress conditions, which involves carbon flux from primary metabolism to secondary metabolites (Caretto et al., 2015). The metabolic transition may trigger acetyl-CoA accumulation to enhance global histone acetylation. In addition, glycolysis is generally induced during plant response to stress (Mutuku and Nose, 2012; Lee et al., 2014; Henry et al., 2015), which raises the possibility that pyruvate produced by high glycolytic activity may increase the acetyl-CoA pool. However, it was observed that drought stress induces metabolic flux conversion from glycolysis into acetate synthesis in Arabidopsis (Kim et al., 2017). It was shown that application of exogenous acetic acid enhances stress tolerance by promoting histone H4 acetylation and stimulating jasmonic acid (JA) pathway (Kim et al., 2017). Acetate treatment can also increase stress tolerance in other plants, suggesting a general function of acetate metabolism in stress adaptation (Kim et al., 2017). It can be hypothesized that stress induces accumulation of acetic acid which can be converted to acetyl-CoA to enhance histone acetylation (Figure 1). Nevertheless, more evidence is required to show whether acetate-promoted histone acetylation is mediated by increased levels of acetyl-CoA. Homolog of ACSS2 in Arabidopsis (ACS) catalyzes acetyl-CoA formation in plastid (Lin and Oliver, 2008). Whether this enzyme catalyzes the same reaction in cytosol or nucleus has not yet been demonstrated. Similarly, it remains to show whether ACS loss-of-function lowers acetyl-CoA content and histone acetylation level and affects stress tolerance. The induction of H3K27ac by elevated acetyl-CoA in the acc1 mutant may also play a significant role in stress response, as many genes involved in stress tolerance such as ROS homeostasis, JA response and flavonoid metabolism are up-regulated in the mutant (Chen et al., 2017). However, increased acetyl-CoA from different sources (citrate and acetate) apparently targeted different histone lysine residues (Chen et al., 2017; Kim et al., 2017). One possible explanation may be that specific HATs interact with different enzymatic complexes of acetyl-CoA biosynthesis as described above in animal cells. It is of great interest to uncover how plant histone acetyltransferases discriminate citrate-derived acetyl-CoA from acetate-derived acetyl-CoA to acetylate specific histone lysine residues in response to stress signals. In addition, exploring whether histone acetylation and stress signaling require acetyl-CoA generated from pyruvate and β-oxidation is equally of great importance (Figure 1).
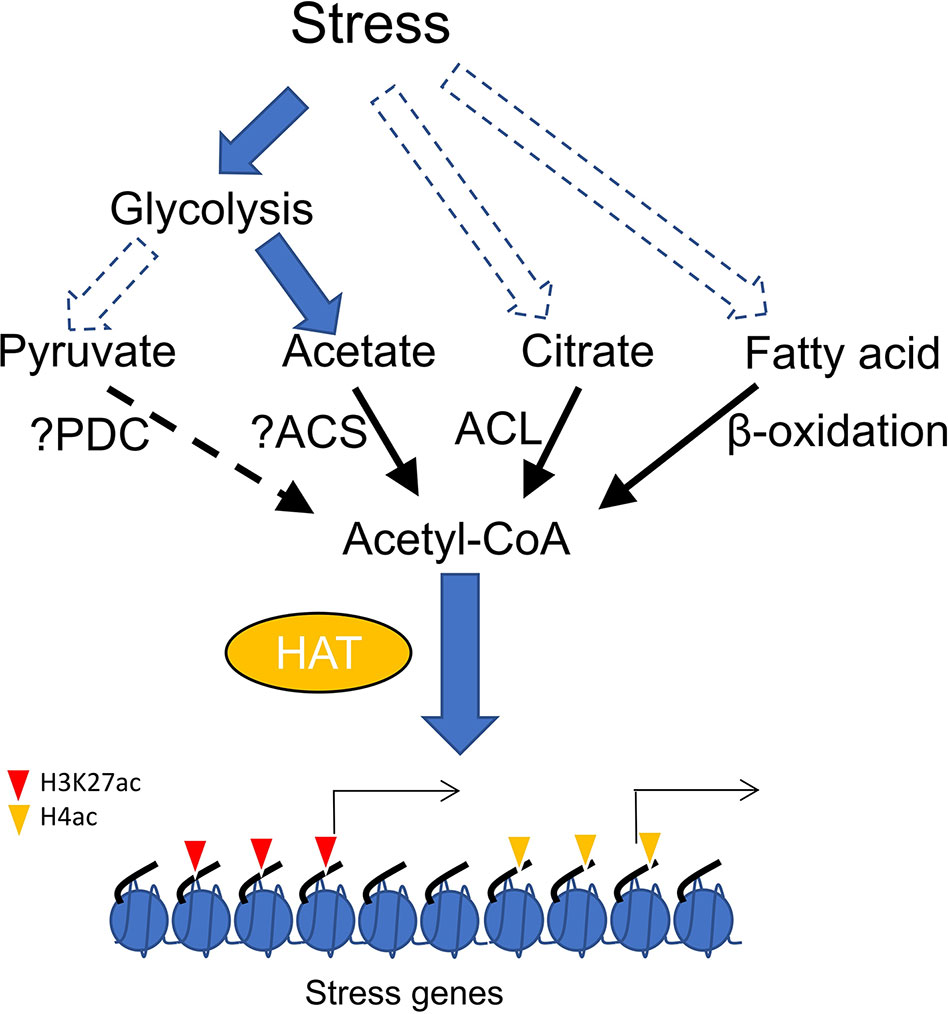
Figure 1 Accumulation of acetyl-CoA stimulates histone acetylation and gene expression in plants, synthesis of acetyl-CoA from different sources including acetate, citrate and fatty acid can be induced by stress. elevation of acetyl-CoA from different sources (citrate and acetate) may stimulate distinct histone acetyl transferases (HAT) to promote histone acetylation at different histone lysine residues (H3K27 and H4) and stress responsive gene expression. PDC, pyruvate dehydrogenase complex; ACS, acetyl-coenzyme A (CoA) synthetase; ACL, ATP-citrate lyase.
NAD+ -Dependent HDAC in Coordinating Metabolism and Stress Response
The sirtuin (SIR2/SIRT)-like proteins require NAD+ to remove acetyl from proteins including histones, suggesting that the activity of sirtuins may be controlled by NAD+ biosynthesis and consumption. NAD+ consumption is likely to be essential in plant response to stress conditions. Downregulation of poly(ADP‐ribose) polymerase (PARP), which encodes enzyme transferring ADP-ribose units from NAD+ to target proteins, enhances stress tolerance (De Block et al., 2005). However, overexpression of NADK encoding enzyme responsible for catalyzing NAD(H) and ATP to produce NADP(H) confers stress tolerance (Li et al., 2018a). Indeed, NADH/NAD+ ratio increases while NAD+ level is not affected in response to stress (Wang et al., 2016). It is postulated that sirtuins may act as a sensor of cellular redox state to modulate histone acetylation and regulate gene expression in plants (Shen et al., 2016). However, there is still no direct evidence showing that NAD+ fluctuation actually regulates SRT1 or SRT2 function in plants.
Glycolysis is enhanced in plants undergoing stress responses (Mutuku and Nose, 2012; Lee et al., 2014; Henry et al., 2015). Interestingly, several glycolytic enzymes also display transcriptional regulatory activities, which are referred to as the moonlighting function of the metabolic enzymes (Boukouris et al., 2016). It was shown that plant SRT1 negatively regulates glycolysis under stress conditions. SRT1 is found to not only deacetylate histones in chromatin of glycolytic genes and reduces the enzymatic activities but to also regulate the moonlighting function of glycolytic enzymes involved in glycolytic gene expression (Liu et al., 2017; Zhang et al., 2017). It was shown that Arabidopsis SRT1 deacetylates and stabilizes Arabidopsis cMyc-Binding Protein 1 (AtMBP-1), a direct transcriptional repressor of both stress master regulatory genes STZ/ZAT10 and LOS2/ENO2. The latter encodes glycolytic enolase and AtMBP-1 itself which is in fact produced from an alternative translational initiation of the LOS2/ENO2 mRNA (Kang et al., 2013; Eremina et al., 2015; Liu et al., 2017). LOS2 is also required for stress tolerance which may be intrinsically related to the alternatively translated AtMBP-1 (Lee et al., 2002; Barkla et al., 2009; Kang et al., 2013). In rice, OsSRT1 represses glycolytic genes expression not only by histone deacetylation of glycolytic genes but also by inhibition of nuclear localization and transcriptional activity of Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) through lysine deacetylation (Zhang et al., 2017). As a rate-limiting enzyme of glycolysis, GAPDH also functions as a transcriptional activator of glycolytic genes under stress, which enhances GAPDH lysine acetylation, nuclear accumulation, and transcriptional activity. These results indicate that SRT1 has a function to coordinate plant sensitivity to abiotic stress and energy metabolism (Figure 2).
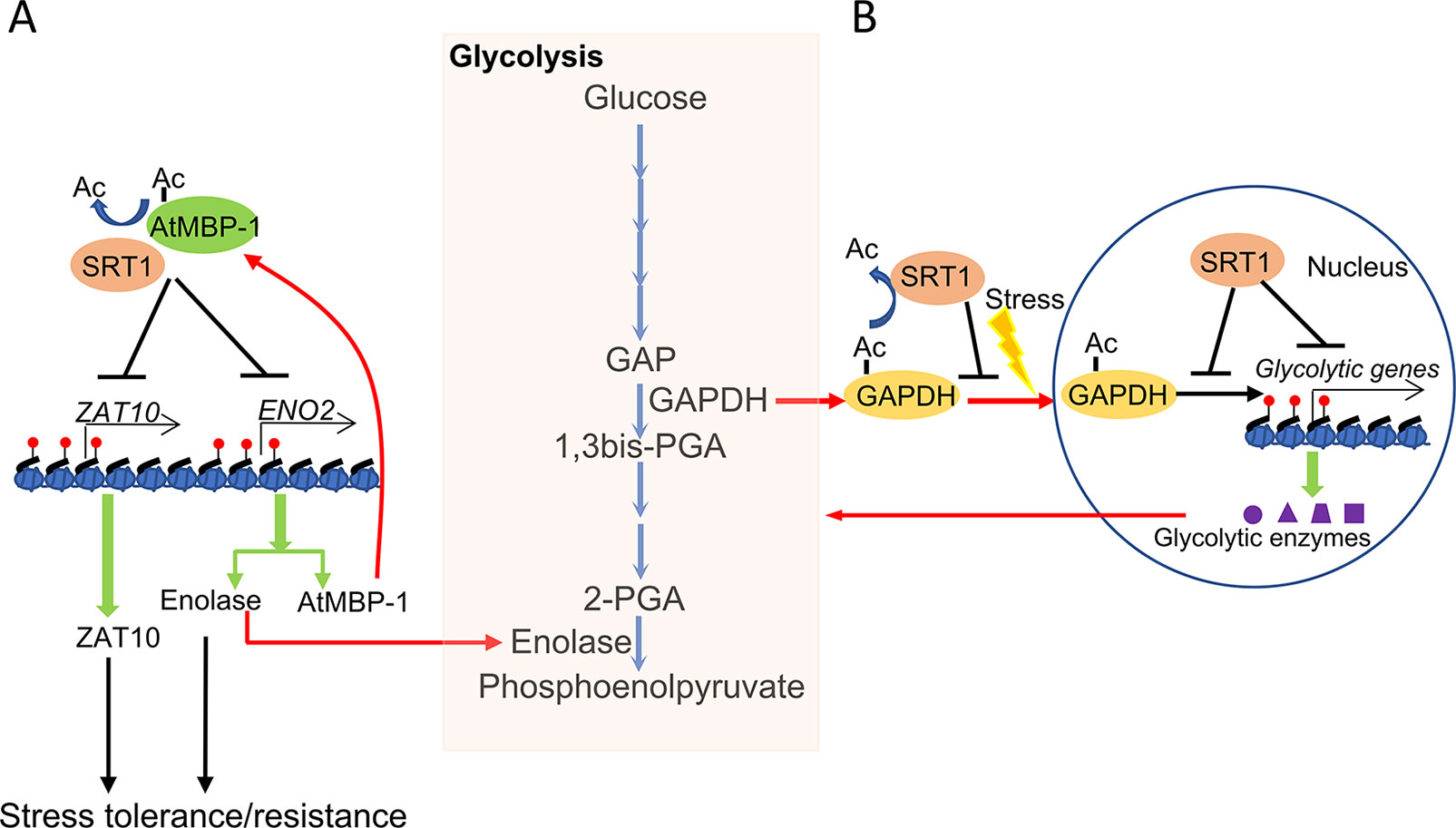
Figure 2 Coordinating metabolism and stress response by plant NAD+-dependent histone deacetylase SRT1. (A) Arabidopsis SRT1 deacetylates and stabilizes AtMBP-1, a direct transcriptional repressor of both stress master regulatory genes ZAT10 and ENO2. ENO2 encodes the glycolytic enolase and AtMBP-1, which are alternative translation products of the ENO2 mRNA and are required for stress tolerance. (B) Rice SRT1 represses glycolytic genes expression not only by histone deacetylation of glycolytic genes but also by inhibition of nuclear localization and transcriptional activity of GAPDH through lysine deacetylation.
As high glycolysis leads to a low NAD+/NADH ratio in the cell, which may eventually inhibit SRT1 activity, it can be suggested that under stress conditions, enhanced glycolysis in turn inhibits SRT1 and consequently reinforces plant stress tolerance or resistance. Thus, SRT1 may play an important role to regulate carbon metabolic flux of the trade-off between growth and stress tolerance, which is essential for plant adaptation to the changing environment (Shen et al., 2016).
Histone Acylation and Stress-Response
Recent results indicate that histones also are acylated by short-chain fatty acids including butyrate, crotonate, 2hydroxyisobutyrate, succinate, malonate, glutarate, etc. (Chen et al., 2007; Tan et al., 2011; Xie et al., 2012). These modifications are similar to lysine acetylation but are distinct in hydrocarbon chain length, hydrophobicity or charge. Studies in animal cells suggest that these histone acylations affect gene expression and are functionally different from histone acetylation (Sabari et al., 2017). In rice plants, most of histone butyrylation (Kbu) and crotonylation (Kcr) sites were shown to overlap with histone acetylation (H3K9ac) sites in highly expressed genes, but subsets of genes show only Kbu and/or Kcr modifications (Lu et al., 2018). The latter genes are most under expressed and stress-inducible. In rice plants under starvation and submergence Kbu and Kcr appear to be less dynamic than H3K9ac and differential changes of the three marks in different sets of genes were observed (Figure 3). Thus, the proportion of histone lysine acetylation (H3K9ac) and acylation (Kbu and Kcr) is regulated by stress, suggesting that environmental and metabolic cues control proportional mixture of histone acetylation and acylations in plants, which has functional consequence in chromatin modification and gene expression. The relative levels between histone acetylation and acylations may be dependent on availability of acetyl-CoA and the short fatty acid pools, as same HATs (i.e. P300 in animal cells) can undergo both acetylation and acylation of histones. The reduction of the cytoplasmic and nuclear pools of acetyl-CoA led to an increase in p300-catalysed Kcr in animal cells and the increase in Kcr could be reduced by replenishing the acetyl-CoA pools (Sabari et al., 2015). Thus, under conditions in which acetyl-CoA is reduced, other acyl-CoA forms will be used more often to modify histones. Additionally, it was showed that histone Kcr could be removed by the NAD+ -dependent histone deacetylase SRT2 to repress gene expression in rice (Lu et al., 2018) (Figure 3). These results indicate that histone acetylation and acylation are at the nexus of the interplay between metabolism, epigenetics, and stress response in plants.
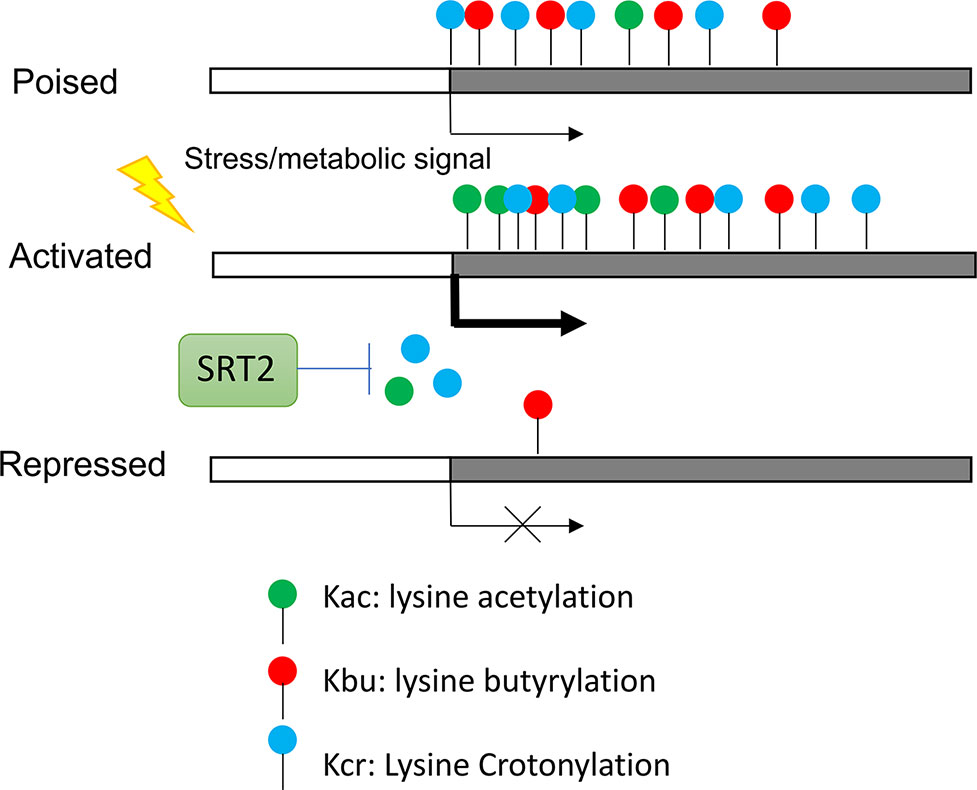
Figure 3 Regulation of stress genes by synergistic effect of histone acetylation and acylation. Genes poised for activation by stress are marked by Kbu and Kcr. Genes activated by stress require histone acetylation. Kcr is removed by NAD+ -dependent histone deacetylase SRT2.
Conclusion
Plant stress-response signaling pathways have been extensively studied. Under stress, plants have to adjust metabolic activities in favor of stress tolerance. Whether and how the altered metabolic activities are linked to gene regulation are still open questions. Recent findings demonstrating that stress induces metabolic conversion to enhance acetyl-CoA supply for histone acetylation or changes of cellular redox states affecting HDAC activities, establish a link between metabolism, epigenetics, and stress response in plants. In addition, the proportion of acetyl-CoA and different forms of acyl-CoA which can be altered by environmental and metabolic changes is also a significant factor in fine-tuning of stress genes expression. Thus, further understanding the precise mechanisms by which HATs and HDACs activities are modulated by metabolic and redox changes will provide new insight into the complex network regulating plant adaptation and tolerance to stress.
Author Contributions
YH and D-XZ wrote the paper. YH and YL prepared the figures. YZ modified the paper.
Funding
This work was supported by grants from the National Key Research and Development Program of China [2016YFD0100802], the National Natural Science Foundation of China [31730049], the Outstanding Young and Middle-aged Science and Technology Innovation Team Project in Higher Education Institutions of Hubei Province [T201621].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Barkla, B. J., Vera-Estrella, R., Hernandez-Coronado, M., Pantoja, O. (2009). Quantitative proteomics of the tonoplast reveals a role for glycolytic enzymes in salt tolerance. Plant Cell 21, 4044–4058. doi: 10.1105/tpc.109.069211
Boukouris, A. E., Zervopoulos, S. D., Michelakis, E. D. (2016). Metabolic enzymes moonlighting in the nucleus: metabolic regulation of gene transcription. Trends Biochem. Sci. 41, 712–730. doi: 10.1016/j.tibs.2016.05.013
Bulusu, V., Tumanov, S., Michalopoulou, E., Van Den Broek, N. J., Mackay, G., Nixon, C., et al. (2017). Acetate recapturing by nuclear acetyl-CoA synthetase 2 prevents loss of histone acetylation during oxygen and serum limitation. Cell Rep. 18, 647–658. doi: 10.1016/j.celrep.2016.12.055
Buszewicz, D., Archacki, R., Palusinski, A., Kotlinski, M., Fogtman, A., Iwanicka-Nowicka, R., et al. (2016). HD2C histone deacetylase and a SWI/SNF chromatin remodelling complex interact and both are involved in mediating the heat stress response in Arabidopsis. Plant Cell Environ. 39, 2108–2122. doi: 10.1111/pce.12756
Cai, L., Sutter, B. M., Li, B., Tu, B. P. (2011). Acetyl-CoA induces cell growth and proliferation by promoting the acetylation of histones at growth genes. Mol. Cell 42, 426–437. doi: 10.1016/j.molcel.2011.05.004
Caretto, S., Linsalata, V., Colella, G., Mita, G., Lattanzio, V. (2015). Carbon fluxes between primary metabolism and phenolic pathway in plant tissues under stress. Int. J. Mol. Sci. 16, 26378–26394. doi: 10.3390/ijms161125967
Chen, C., Li, C., Wang, Y., Renaud, J., Tian, G., Kambhampati, S., et al. (2017). Cytosolic acetyl-CoA promotes histone acetylation predominantly at H3K27 in Arabidopsis. Nat. Plants 3, 814–824. doi: 10.1038/s41477-017-0023-7
Chen, L. T., Luo, M., Wang, Y. Y., Wu, K. (2010). Involvement of Arabidopsis histone deacetylase HDA6 in ABA and salt stress response. J. Exp. Bot. 61, 3345–3353. doi: 10.1093/jxb/erq154
Chen, L. T., Wu, K. (2010). Role of histone deacetylases HDA6 and HDA19 in ABA and abiotic stress response. Plant Signal. Behav. 5, 1318–1320. doi: 10.4161/psb.5.10.13168
Chen, X., Lu, L., Mayer, K. S., Scalf, M., Qian, S., Lomax, A., et al. (2016). POWERDRESS interacts with HISTONE DEACETYLASE 9 to promote aging in Arabidopsis. Elife 5, e17214. doi: 10.7554/eLife.17214
Chen, Y., Sprung, R., Tang, Y., Ball, H., Sangras, B., Kim, S. C., et al. (2007). Lysine propionylation and butyrylation are novel post-translational modifications in histones. Mol. Cell Proteomics 6, 812–819. doi: 10.1074/mcp.M700021-MCP200
Cheng, X., Zhang, S., Tao, W., Zhang, X., Liu, J., Sun, J., et al. (2018). INDETERMINATE SPIKELET1 recruits histone deacetylase and a transcriptional repression complex to regulate rice salt tolerance. Plant Physiol. 178, 824–837. doi: 10.1104/pp.18.00324
De Block, M., Verduyn, C., De Brouwer, D., Cornelissen, M. (2005). Poly(ADP-ribose) polymerase in plants affects energy homeostasis, cell death and stress tolerance. Plant J. 41, 95–106. doi: 10.1111/j.1365-313X.2004.02277.x
Ding, B., Bellizzi Mdel, R., Ning, Y., Meyers, B. C., Wang, G. L. (2012). HDT701, a histone H4 deacetylase, negatively regulates plant innate immunity by modulating histone H4 acetylation of defense-related genes in rice. Plant Cell 24, 3783–3794. doi: 10.1105/tpc.112.101972
Earley, K. W., Shook, M. S., Brower-Toland, B., Hicks, L., Pikaard, C. S. (2007). In vitro specificities of Arabidopsis co-activator histone acetyltransferases: implications for histone hyperacetylation in gene activation. Plant J. 52, 615–626. doi: 10.1111/j.1365-313X.2007.03264.x
Eremina, M., Rozhon, W., Yang, S., Poppenberger, B. (2015). ENO2 activity is required for the development and reproductive success of plants, and is feedback-repressed by AtMBP-1. Plant J. 81, 895–906. doi: 10.1111/tpj.12775
Etchegaray, J. P., Mostoslavsky, R. (2016). Interplay between metabolism and epigenetics: A nuclear adaptation to environmental changes. Mol. Cell 62, 695–711. doi: 10.1016/j.molcel.2016.05.029
Fang, H., Liu, X., Thorn, G., Duan, J., Tian, L. (2014). Expression analysis of histone acetyltransferases in rice under drought stress. Biochem. Biophys. Res. Commun. 443, 400–405. doi: 10.1016/j.bbrc.2013.11.102
Farrona, S., Hurtado, L., Reyes, J. C. (2007). A nucleosome interaction module is required for normal function of Arabidopsis thaliana BRAHMA. J. Mol. Biol. 373, 240–250. doi: 10.1016/j.jmb.2007.07.012
Feng, J. H., Jing, F. B., Fang, H., Gu, L. C., Xu, W. F. (2011). Expression, purification, and S-nitrosylation of recombinant histone deacetylase 8 in Escherichia coli.Biosci. Trends 5, 17–22. doi: 10.5582/bst.2011.v5.1.17
Gao, M. J., Li, X., Huang, J., Gropp, G. M., Gjetvaj, B., Lindsay, D. L., et al. (2015). SCARECROW-LIKE15 interacts with HISTONE DEACETYLASE19 and is essential for repressing the seed maturation programme. Nat. Commun. 6, 7243. doi: 10.1038/ncomms8243
Han, S. K., Wu, M. F., Cui, S., Wagner, D. (2015). Roles and activities of chromatin remodeling ATPases in plants. Plant J. 83, 62–77. doi: 10.1111/tpj.12877
Hartl, M., Fussl, M., Boersema, P. J., Jost, J. O., Kramer, K., Bakirbas, A., et al. (2017). Lysine acetylome profiling uncovers novel histone deacetylase substrate proteins in Arabidopsis. Mol. Syst. Biol. 13, 949. doi: 10.15252/msb.20177819
Henry, E., Fung, N., Liu, J., Drakakaki, G., Coaker, G. (2015). Beyond glycolysis: GAPDHs are multi-functional enzymes involved in regulation of ROS, autophagy, and plant immune responses. PLoS Genet. 11, e1005199. doi: 10.1371/journal.pgen.1005199
Henry, R. A., Kuo, Y. M., Andrews, A. J. (2013). Differences in specificity and selectivity between CBP and p300 acetylation of histone H3 and H3/H4. Biochemistry 52, 5746–5759. doi: 10.1021/bi400684q
Hu, Z., Song, N., Zheng, M., Liu, X., Liu, Z., Xing, J., et al. (2015). Histone acetyltransferase GCN5 is essential for heat stress-responsive gene activation and thermotolerance in Arabidopsis.Plant J. 84, 1178–1191. doi: 10.1111/tpj.13076
Ito, K., Hanazawa, T., Tomita, K., Barnes, P. J., Adcock, I. M. (2004). Oxidative stress reduces histone deacetylase 2 activity and enhances IL-8 gene expression: role of tyrosine nitration. Biochem. Biophys. Res. Commun. 315, 240–245. doi: 10.1016/j.bbrc.2004.01.046
Jin, H., Choi, S. M., Kang, M. J., Yun, S. H., Kwon, D. J., Noh, Y. S., et al. (2018). Salicylic acid-induced transcriptional reprogramming by the HAC-NPR1-TGA histone acetyltransferase complex in Arabidopsis. Nucleic Acids Res. 46, 11712–11725. doi: 10.1093/nar/gky847
Kang, M., Abdelmageed, H., Lee, S., Reichert, A., Mysore, K. S., Allen, R. D. (2013). AtMBP-1, an alternative translation product of LOS2, affects abscisic acid responses and is modulated by the E3 ubiquitin ligase AtSAP5. Plant J. 76, 481–493. doi: 10.1111/tpj.12312
Kim, J. M., Sasaki, T., Ueda, M., Sako, K., Seki, M. (2015). Chromatin changes in response to drought, salinity, heat, and cold stresses in plants. Front. Plant Sci. 6, 114. doi: 10.3389/fpls.2015.00114
Kim, J. M., To, T. K., Matsui, A., Tanoi, K., Kobayashi, N. I., Matsuda, F., et al. (2017). Acetate-mediated novel survival strategy against drought in plants. Nat. Plants 3, 17097. doi: 10.1038/nplants.2017.97
Kong, L., Qiu, X., Kang, J., Wang, Y., Chen, H., Huang, J., et al. (2017). A phytophthora effector manipulates host histone acetylation and reprograms defense gene expression to promote infection. Curr. Biol. 27, 981–991. doi: 10.1016/j.cub.2017.02.044
Krogan, N. T., Hogan, K., Long, J. A. (2012). APETALA2 negatively regulates multiple floral organ identity genes in Arabidopsis by recruiting the co-repressor TOPLESS and the histone deacetylase HDA19. Development 139, 4180–4190. doi: 10.1242/dev.085407
Kuo, Y. M., Andrews, A. J. (2013). Quantitating the specificity and selectivity of Gcn5-mediated acetylation of histone H3. PLoS One 8, e54896. doi: 10.1371/annotation/b2bf9c2e-90a9-4228-9b38-2f1bc977a437
Latrasse, D., Jegu, T., Li, H., De Zelicourt, A., Raynaud, C., Legras, S., et al. (2017). MAPK-triggered chromatin reprogramming by histone deacetylase in plant innate immunity. Genome Biol. 18, 131. doi: 10.1186/s13059-017-1261-8
Lee, D. Y., Lee, C. I., Lin, T. E., Lim, S. H., Zhou, J., Tseng, Y. C., et al. (2012). Role of histone deacetylases in transcription factor regulation and cell cycle modulation in endothelial cells in response to disturbed flow. Proc. Natl. Acad. Sci. U. S. A. 109, 1967–1972. doi: 10.1073/pnas.1121214109
Lee, H., Guo, Y., Ohta, M., Xiong, L., Stevenson, B., Zhu, J. K. (2002). LOS2, a genetic locus required for cold-responsive gene transcription encodes a bi-functional enolase. EMBO J. 21, 2692–2702. doi: 10.1093/emboj/21.11.2692
Lee, K. W., Chen, P. W., Yu, S. M. (2014). Metabolic adaptation to sugar/O2 deficiency for anaerobic germination and seedling growth in rice. Plant Cell Environ. 37, 2234–2244. doi: 10.1111/pce.12311
Li, B. B., Wang, X., Tai, L., Ma, T. T., Shalmani, A., Liu, W. T., et al. (2018a). NAD kinases: Metabolic targets controlling redox co-enzymes and reducing power partitioning in plant stress and development. Front. Plant Sci. 9, 379. doi: 10.3389/fpls.2018.00379
Li, H., Yan, S., Zhao, L., Tan, J., Zhang, Q., Gao, F., et al. (2014). Histone acetylation associated up-regulation of the cell wall related genes is involved in salt stress induced maize root swelling. BMC Plant Biol. 14, 105. doi: 10.1186/1471-2229-14-105
Li, S., Lin, Y.J., Wang, P., Zhang, B., Li, M., Chen, S., et al, (2019). The AREB1 Transcription Factor Influences Histone Acetylation to Regulate Drought Responses and Tolerance in Populus trichocarpa. Plant Cell 31, 663–686. doi: 10.1105/tpc.18.00437
Li, X., Yu, W., Qian, X., Xia, Y., Zheng, Y., Lee, J. H., et al. (2017). Nucleus-translocated ACSS2 promotes gene transcription for lysosomal biogenesis and autophagy. Mol. Cell 66684–697, e689. doi: 10.1016/j.molcel.2017.04.026
Lin, M., Oliver, D. J. (2008). The role of acetyl-coenzyme a synthetase in Arabidopsis. Plant Physiol. 147, 1822–1829. doi: 10.1104/pp.108.121269
Liu, X., Wei, W., Zhu, W., Su, L., Xiong, Z., Zhou, M., et al. (2017). Histone deacetylase AtSRT1 links metabolic flux and stress response in Arabidopsis. Mol. Plant 10, 1510–1522. doi: 10.1016/j.molp.2017.10.010
Liu, X., Yang, S., Zhao, M., Luo, M., Yu, C. W., Chen, C. Y., et al. (2014). Transcriptional repression by histone deacetylases in plants. Mol. Plant 7, 764–772. doi: 10.1093/mp/ssu033
Long, J. A., Ohno, C., Smith, Z. R., Meyerowitz, E. M. (2006). TOPLESS regulates apical embryonic fate in Arabidopsis. Science 312, 1520–1523. doi: 10.1126/science.1123841
Lu, Y., Xu, Q., Liu, Y., Yu, Y., Cheng, Z. Y., Zhao, Y., et al. (2018). Dynamics and functional interplay of histone lysine butyrylation, crotonylation, and acetylation in rice under starvation and submergence. Genome Biol. 19, 144. doi: 10.1186/s13059-018-1533-y
Luo, M., Wang, Y. Y., Liu, X., Yang, S., Lu, Q., Cui, Y., et al. (2012). HD2C interacts with HDA6 and is involved in ABA and salt stress response in Arabidopsis. J. Exp. Bot. 63, 3297–3306. doi: 10.1093/jxb/ers059
Marmorstein, R., Zhou, M. M. (2014). Writers and readers of histone acetylation: structure, mechanism, and inhibition. Cold Spring Harb. Perspect. Biol. 6, a018762. doi: 10.1101/cshperspect.a018762
Mcdonnell, E., Crown, S. B., Fox, D. B., Kitir, B., Ilkayeva, O. R., Olsen, C. A., et al. (2016). Lipids reprogram metabolism to become a major carbon source for histone acetylation. Cell Rep. 17, 1463–1472. doi: 10.1016/j.celrep.2016.10.012
Mehdi, S., Derkacheva, M., Ramstrom, M., Kralemann, L., Bergquist, J., Hennig, L. (2016). The WD40 domain protein MSI1 functions in a histone deacetylase complex to fine-tune abscisic acid signaling. Plant Cell 28, 42–54. doi: 10.1105/tpc.15.00763
Mengel, A., Ageeva, A., Georgii, E., Bernhardt, J., Wu, K., Durner, J., et al. (2017). Nitric oxide modulates histone acetylation at stress genes by inhibition of histone deacetylases. Plant Physiol. 173, 1434–1452. doi: 10.1104/pp.16.01734
Mews, P., Donahue, G., Drake, A. M., Luczak, V., Abel, T., Berger, S. L. (2017). Acetyl-CoA synthetase regulates histone acetylation and hippocampal memory. Nature 546, 381–386. doi: 10.1038/nature22405
Mutuku, J. M., Nose, A. (2012). Changes in the contents of metabolites and enzyme activities in rice plants responding to Rhizoctonia solani Kuhn infection: activation of glycolysis and connection to phenylpropanoid pathway. Plant Cell Physiol. 53, 1017–1032. doi: 10.1093/pcp/pcs047
Nott, A., Watson, P. M., Robinson, J. D., Crepaldi, L., Riccio, A. (2008). S-Nitrosylation of histone deacetylase 2 induces chromatin remodelling in neurons. Nature 455, 411–415. doi: 10.1038/nature07238
Okuda, K., Ito, A., Uehara, T. (2015). Regulation of histone deacetylase 6 activity via S-nitrosylation. Biol. Pharm. Bull. 38, 1434–1437. doi: 10.1248/bpb.b15-00364
Pandey, R., Muller, A., Napoli, C. A., Selinger, D. A., Pikaard, C. S., Richards, E. J., et al. (2002). Analysis of histone acetyltransferase and histone deacetylase families of Arabidopsis thaliana suggests functional diversification of chromatin modification among multicellular eukaryotes. Nucleic Acids Res. 30, 5036–5055. doi: 10.1093/nar/gkf660
Park, J., Lim, C. J., Shen, M., Park, H. J., Cha, J. Y., Iniesto, E., et al. (2018). Epigenetic switch from repressive to permissive chromatin in response to cold stress. Proc. Natl. Acad. Sci. U. S. A. 115, E5400–E5409. doi: 10.1073/pnas.1721241115
Patanun, O., Ueda, M., Itouga, M., Kato, Y., Utsumi, Y., Matsui, A., et al. (2016). The histone deacetylase inhibitor suberoylanilide hydroxamic acid alleviates salinity stress in cassava. Front. Plant Sci. 7, 2039. doi: 10.3389/fpls.2016.02039
Pi, L., Aichinger, E., Van Der Graaff, E., Llavata-Peris, C. I., Weijers, D., Hennig, L., et al. (2015). Organizer-derived WOX5 signal maintains root columella stem cells through chromatin-mediated repression of CDF4 expression. Dev. Cell 33, 576–588. doi: 10.1016/j.devcel.2015.04.024
Roy, D., Paul, A., Roy, A., Ghosh, R., Ganguly, P., Chaudhuri, S. (2014). Differential acetylation of histone H3 at the regulatory region of OsDREB1b promoter facilitates chromatin remodelling and transcription activation during cold stress. PLoS One 9, e100343. doi: 10.1371/journal.pone.0100343
Ryu, H., Cho, H., Bae, W., Hwang, I. (2014). Control of early seedling development by BES1/TPL/HDA19-mediated epigenetic regulation of ABI3. Nat. Commun. 5, 4138. doi: 10.1038/ncomms5138
Sabari, B. R., Tang, Z., Huang, H., Yong-Gonzalez, V., Molina, H., Kong, H. E., et al. (2015). Intracellular crotonyl-CoA stimulates transcription through p300-catalyzed histone crotonylation. Mol. Cell 58, 203–215. doi: 10.1016/j.molcel.2015.02.029
Sabari, B. R., Zhang, D., Allis, C. D., Zhao, Y. (2017). Metabolic regulation of gene expression through histone acylations. Nat. Rev. Mol. Cell Biol. 18, 90–101. doi: 10.1038/nrm.2016.140
Sadler, A. J., Suliman, B. A., Yu, L., Yuan, X., Wang, D., Irving, A. T., et al. (2015). The acetyltransferase HAT1 moderates the NF-kappaB response by regulating the transcription factor PLZF. Nat. Commun. 6, 6795. doi: 10.1038/ncomms7795
Sako, K., Kim, J. M., Matsui, A., Nakamura, K., Tanaka, M., Kobayashi, M., et al. (2016). Ky-2, a histone deacetylase inhibitor, enhances high-salinity stress tolerance in Arabidopsis thaliana. Plant Cell Physiol. 57, 776–783. doi: 10.1093/pcp/pcv199
Servet, C., Conde E Silva, N., Zhou, D. X. (2010). Histone acetyltransferase AtGCN5/HAG1 is a versatile regulator of developmental and inducible gene expression in Arabidopsis. Mol. Plant 3, 670–677. doi: 10.1093/mp/ssq018
Shen, Y., Issakidis-Bourguet, E., Zhou, D. X. (2016). Perspectives on the interactions between metabolism, redox, and epigenetics in plants. J. Exp. Bot. 67, 5291–5300. doi: 10.1093/jxb/erw310
Shen, Y., Lei, T., Cui, X., Liu, X., Zhou, S., Zheng, Y., et al. (2019). Arabidopsis histone deacetylase HDA15 directly represses plant response to elevated ambient temperature. Plant J. Epub ahead of print doi: 10.1111/tpj.14492
Shi, L., Tu, B. P. (2013). Acetyl-CoA induces transcription of the key G1 cyclin CLN3 to promote entry into the cell division cycle in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A. 110, 7318–7323. doi: 10.1073/pnas.1302490110
Song, C. P., Agarwal, M., Ohta, M., Guo, Y., Halfter, U., Wang, P., et al. (2005). Role of an Arabidopsis AP2/EREBP-type transcriptional repressor in abscisic acid and drought stress responses. Plant Cell 17, 2384–2396. doi: 10.1105/tpc.105.033043
Sutendra, G., Kinnaird, A., Dromparis, P., Paulin, R., Stenson, T. H., Haromy, A., et al. (2014). A nuclear pyruvate dehydrogenase complex is important for the generation of acetyl-CoA and histone acetylation. Cell 158, 84–97. doi: 10.1016/j.cell.2014.04.046
Tan, M., Luo, H., Lee, S., Jin, F., Yang, J. S., Montellier, E., et al. (2011). Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell 146, 1016–1028. doi: 10.1016/j.cell.2011.08.008
Tasset, C., Singh Yadav, A., Sureshkumar, S., Singh, R., Van Der Woude, L., Nekrasov, M., et al. (2018). POWERDRESS-mediated histone deacetylation is essential for thermomorphogenesis in Arabidopsis thaliana. PLoS Genet. 14, e1007280. doi: 10.1371/journal.pgen.1007280
Torres, E. S., Deal, R. B. (2019). The histone variant H2A.Z and chromatin remodeler BRAHMA act coordinately and antagonistically to regulate transcription and nucleosome dynamics in Arabidopsis. Plant J. 99, 144–162. doi: 10.1111/tpj.14281
Ueda, M., Matsui, A., Nakamura, T., Abe, T., Sunaoshi, Y., Shimada, H., et al. (2018). Versatility of HDA19-deficiency in increasing the tolerance of Arabidopsis to different environmental stresses. Plant Signal. Behav. 13, e1475808. doi: 10.1080/15592324.2018.1475808
Ueda, M., Matsui, A., Tanaka, M., Nakamura, T., Abe, T., Sako, K., et al. (2017). The distinct roles of Class I and II RPD3-like histone deacetylases in salinity stress response. Plant Physiol. 175, 1760–1773. doi: 10.1104/pp.17.01332
Wang, L., Wang, C., Liu, X., Cheng, J., Li, S., Zhu, J. K., et al. (2019). Peroxisomal beta-oxidation regulates histone acetylation and DNA methylation in Arabidopsis. Proc. Natl. Acad. Sci. U. S. A. 116, 10576–10585. doi: 10.1073/pnas.1904143116
Wang, P., Zhao, L., Hou, H., Zhang, H., Huang, Y., Wang, Y., et al. (2015). Epigenetic changes are associated with programmed cell death induced by heat stress in seedling leaves of Zea mays. Plant Cell Physiol. 56, 965–976. doi: 10.1093/pcp/pcv023
Wang, Q. J., Sun, H., Dong, Q. L., Sun, T. Y., Jin, Z. X., Hao, Y. J., et al. (2016). The enhancement of tolerance to salt and cold stresses by modifying the redox state and salicylic acid content via the cytosolic malate dehydrogenase gene in transgenic apple plants. Plant Biotechnol. J. 14, 1986–1997. doi: 10.1111/pbi.12556
Wang, Y., Hu, Q., Wu, Z., Wang, H., Han, S., Jin, Y., et al. (2017). HISTONE DEACETYLASE 6 represses pathogen defence responses in Arabidopsis thaliana. Plant Cell Environ. 40, 2972–2986. doi: 10.1111/pce.13047
Wang, Z., Cao, H., Sun, Y., Li, X., Chen, F., Carles, A., et al. (2013). Arabidopsis paired amphipathic helix proteins SNL1 and SNL2 redundantly regulate primary seed dormancy via abscisic acid-ethylene antagonism mediated by histone deacetylation. Plant Cell 25, 149–166. doi: 10.1105/tpc.112.108191
Wellen, K. E., Hatzivassiliou, G., Sachdeva, U. M., Bui, T. V., Cross, J. R., Thompson, C. B. (2009). ATP-citrate lyase links cellular metabolism to histone acetylation. Science 324, 1076–1080. doi: 10.1126/science.1164097
Xie, Z., Dai, J., Dai, L., Tan, M., Cheng, Z., Wu, Y., et al. (2012). Lysine succinylation and lysine malonylation in histones. Mol. Cell Proteomics 11, 100–107. doi: 10.1074/mcp.M111.015875
Yuan, L., Liu, X., Luo, M., Yang, S., Wu, K. (2013). Involvement of histone modifications in plant abiotic stress responses. J. Integr. Plant Biol. 55, 892–901. doi: 10.1111/jipb.12060
Zhang, H., Zhao, Y., Zhou, D. X. (2017). Rice NAD+-dependent histone deacetylase OsSRT1 represses glycolysis and regulates the moonlighting function of GAPDH as a transcriptional activator of glycolytic genes. Nucleic Acids Res. 45, 12241–12255. doi: 10.1093/nar/gkx825
Zhao, L., Wang, P., Hou, H., Zhang, H., Wang, Y., Yan, S., et al. (2014a). Transcriptional regulation of cell cycle genes in response to abiotic stresses correlates with dynamic changes in histone modifications in maize. PLoS One 9, e106070. doi: 10.1371/journal.pone.0106070
Zhao, L., Wang, P., Yan, S., Gao, F., Li, H., Hou, H., et al. (2014b). Promoter-associated histone acetylation is involved in the osmotic stress-induced transcriptional regulation of the maize ZmDREB2A gene. Physiol. Plant 151, 459–467. doi: 10.1111/ppl.12136
Zheng, M., Liu, X., Lin, J., Liu, X., Wang, Z., Xin, M., et al. (2018). Histone acetyltransferase GCN5 contributes to cell wall integrity and salt stress tolerance by altering the expression of cellulose synthesis genes. Plant J. 97, 587–602. doi: 10.1111/tpj.14144
Zheng, Y., Ding, Y., Sun, X., Xie, S., Wang, D., Liu, X., et al. (2016). Histone deacetylase HDA9 negatively regulates salt and drought stress responsiveness in Arabidopsis. J. Exp. Bot. 67, 1703–1713. doi: 10.1093/jxb/erv562
Keywords: plant, stress response, histone acetylation, histone acylation, acetyl-CoA, metabolism
Citation: Hu Y, Lu Y, Zhao Y and Zhou D-X (2019) Histone Acetylation Dynamics Integrates Metabolic Activity to Regulate Plant Response to Stress. Front. Plant Sci. 10:1236. doi: 10.3389/fpls.2019.01236
Received: 22 June 2019; Accepted: 05 September 2019;
Published: 04 October 2019.
Edited by:
Paula Casati, CONICET Center for Photosynthetic and Biochemical Studies (CEFOBI), ArgentinaReviewed by:
Yuhai Cui, Agriculture and Agri-Food Canada (AAFC), CanadaToshiro Ito, Nara Institute of Science and Technology (NAIST), Japan
Copyright © 2019 Hu, Lu, Zhao and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dao-Xiu Zhou, dao-xiu.zhou@u-psud.fr
 Yongfeng Hu
Yongfeng Hu Yue Lu
Yue Lu Yu Zhao
Yu Zhao Dao-Xiu Zhou
Dao-Xiu Zhou