- 1Academy of Scientific and Innovative Research, CSIR-Central Salt and Marine Chemicals Research Institute, Council of Scientific and Industrial Research, Bhavnagar, India
- 2Division of Biotechnology and Phycology, CSIR-Central Salt and Marine Chemicals Research Institute, Council of Scientific and Industrial Research, Bhavnagar, India
NAC proteins are a large family of plant-specific transcription factors which regulate both ABA-dependent and -independent gene expression. These transcription factors participate in biotic and abiotic stress-response through intricate regulation at transcriptional, post-transcriptional and post-translational levels. In the present study, AlNAC4 transcription factor was isolated from a salt excreting halophyte Aeluropus lagopoides. The AlNAC4 has an open reading frame of 936 bp, encoding a protein of 312 amino acid, with an estimated molecular mass of 34.9 kDa. The AlNAC4 showed close homology to monocot NACs in the phylogenetic tree. In silico analysis revealed that AlNAC4 possess the characteristic A-E subdomains within the NAC domain. The AlNAC4 showed sixteen post-translational phosphorylation sites. The AlNAC4 transcript was significantly upregulated with dehydration and H2O2 treatments, showing its role in osmotic and oxidative stress, respectively. The recombinant protein showed binding to mono as well as tandem repeats of NAC recognition sequence (NACRS) of the erd1 promoter. This is the first report mentioning that overexpression of AlNAC4 improved oxidative stress tolerance in tobacco transgenics. The transgenics maintained ROS homeostasis during H2O2 treatment. The transgenics showed regulation of stress-responsive genes including CAT, SOD, LEA5, PLC3, ERD10B, THT1 and transcription factors like AP2, ZFP during oxidative stress.
Key Message: The AlNAC4 transcription factor from recretohalophyte Aeluropus showed regulation with abiotic stresses and binding to NACRS elements of erd1 promoter. The AlNAC4 tobacco transgenics showed improved growth with oxidative stress.
Introduction
The growth and development of sessile autotrophic plants is negatively affected by adverse environmental conditions. Plants manage stress-response via morphological, physiological and biochemical changes, involving expression of functional and regulatory genes for sustainable biological function (Hirayama and Shinozaki, 2010). The transcription factors (TFs) play a pivotal role in complex signaling network by regulating a large number of downstream genes (Zhang et al., 2011). Approximately 5–7% of the total genes comprise TFs in plants, which have expanded largely in plant kingdom due to the complexity of plant metabolism (Shiu et al., 2005).
The NACs are plant-specific TFs, involved in various biological processes including abiotic and biotic stress responses via both ABA-dependent and -independent stress signal transduction pathways (Nuruzzaman et al., 2013). The NAC TF coined by the initials of NAM (Petunia no apical meristem; Souer et al., 1996), ATAF1/2 (Arabidopsis thaliana transcription activation factor) and CUC2 (Arabidopsis cup-shaped cotyledon) proteins (Aida et al., 1997). The NAC TF was originally identified from petunia (Petunia hybrida) as NAM (Souer et al., 1996), which plays a critical role in determining meristem and primordia positions. The N-terminal region, called NAC domain, is divided into five sub-domains A-E and is associated with various functions like nuclear localization, DNA binding and formation of homodimer or heterodimer with other NAC domain-containing proteins (Ooka et al., 2003; Olsen et al., 2005). The C-terminal region is highly variable, acting as transcription regulatory region, having either transmembrane motifs or protein binding activity (Seo et al., 2008). The potential of NAC TFs to perform wide range of functions is tightly regulated at transcriptional, post-transcriptional and post-translational levels. The NAC proteins show binding to NACRS (CATGTG) as multimers, for regulating the transcriptional activity of the NAC proteins (Tran et al., 2004). The NAC TFs are transcriptionally and post-transcriptionally regulated by ABREs (ABA-responsive elements) and DREBs (Dehydration-responsive element binding) and micro-RNAs or alternative splicing, respectively. The post-translational modifications like ubiquitination, dimerization, phosphorylation or proteolysis also regulate NAC TFs activity (Kaneda et al., 2009; Miao et al., 2016).
The Poaceae family includes cultivated staple food crops viz. rice, wheat, maize and a lot of efforts have been made toward understanding physiology, genetics and genomics of these crops for improving yield under varied climatic conditions, nutritional quality, disease manifestations/management etc. Aeluropus lagopoides (L.) trin. Ex Thwis, a C4 salt secreting perennial halophytic grass, belongs to family Poaceae and is a close wild relative of bread wheat. A. lagopoides grows commonly in salt marshes and survives at even 1 M NaCl (Gulzar et al., 2003). It is important to isolate and characterize stress-responsive TFs from halophytes; as halophytes with their distinct genetic constitution have developed potential to survive and complete their life cycle in high salt habitats. These genes can be genetically engineered in crops to upregulate a cascade of stress-responsive genes to enhance stress tolerance. In the present study, we have isolated an abiotic stress-responsive NAC TF and studied its role during multiple stress conditions.
Materials and Methods
Plant Material and Stress Treatments
Aeluropus lagopoides plants were collected from the [CSIR-CSMCRI salt farm, Bhavnagar, Gujarat (N21847′13.5″; E72807′25.7″), India] and the nodal cuttings of 2–3 leaf stage were grown in half-strength Hoagland hydroponic medium (Hoagland and Arnon, 1950) in plastic pots under 300–350 μmol m-2 s-1 of photosynthetically active light with 16/8 h light/dark cycle at 25°C in a growth chamber. For transcript analysis, plantlets (10–12 cm) were acclimatized for 7 days and following treatments were given: (i) 100 μM abscisic acid (ABA), (ii) dehydration by wrapping plants in dry tissue paper at room temperature, (iii) 250 mM NaCl and (iv) 20 mM hydrogen peroxide (H2O2). Another set of seedlings was maintained under control conditions in half-strength Hoagland medium. For all treatments, leaf tissue was collected from three biological replicates after 1, 3, 6, 12, 24, and 48 h of treatments and kept at -80°C until used for RNA isolation.
For stress tolerance study the seeds of tobacco WT and T0 tobacco transgenic lines transformed with AlNAC4 gene (L33, L50 and L64) were germinated on Murashige and Skoog (1962) medium supplemented with NaCl (100, 200, and 300 mM) and mannitol (50, 100, 150, and 200 mM). The seeds of WT and T0 transgenics were also germinated on Whatman filter paper soaked in sterilized Milli-Q water containing 0, 10, and 20 mM H2O2. Different parameters like hypocotyl length, root length, relative water content were studied and, histochemical (DAB and NBT) and biochemical analysis (H2O2 content, CAT and SOD activity) was carried out after 11 days of treatment.
The WT and hygromycin positive T1 transgenic seedlings were also transferred to Hoagland medium for 4 weeks. The uniform plants were treated with 0, 10, and 20 mM H2O2 for 3, 12, and 24 h to analyze the biochemical parameters (H2O2 content, CAT and SOD activity). For all the treatments tissues from three biological replicates were collected for further analyses.
Isolation and Cloning of AlNAC4 cDNA From A. lagopoides
The NAC sequences from Triticum aestivum (AY625683.1), Hordeum vulgare (JX855805.1), Oryza sativa (DQ394702.1), Sorghum bicolor (KC253232.1) and Zea mays (JQ217429.1) were retrieved from NCBI and aligned by DNAMAN for designing degenerate primers (NACFA1, NAC R1, NACFA2 and NAC R2). The total RNA was isolated using Trizol-like reagent (Pyvovarenko and Lopato, 2011), treated with DNaseI (MBI Fermentas) followed by first-strand cDNA synthesis using RevertAid cDNA synthesis kit (Thermo Scientific). The PCR reaction was carried out using cDNA as template, 150 ng degenerate primers, 200 μM dNTPs and 2.5 U Taq DNA polymerase in 50 μl reaction at 94°C for 2 min, 1 cycle; 94°C for 1 min; 55°C for 1 min and 72°C for 2 min, 35 cycles and last 72°C for 7 min, 1 cycle. The amplicon was cloned in pJET 1.2/blunt plasmid vector (Thermo Scientific) and sequenced. The partial length gene sequence was confirmed by NCBI BLAST and 5′ and 3′ RACE (rapid amplification of cDNA ends) was carried out to get full-length sequence of AlNAC4. The NACJ5 F1 : PAoligo-dT, NACJ5 F2: PAR1, NACJ5 F3: PAR2 and NACJ5 R1, NACJ5 R2, NACJ5 R3 primers were used for 3′ RACE and 5′ RACE, respectively. The forward 5′ RACE primers were used as per Invitrogen (United States). The NAC amino acid sequences of different plants were used to construct a phylogenetic tree by DNASTAR Navigator software (version 11.0.0.64). The presence of conserved motifs was analyzed using MEME (Bailey et al., 2006). The sequences of the primers used in the present study are mentioned in Supplementary Table S1.
Isolation of AlNAC4 Genomic Clone
The AlNAC4 genomic fragment was PCR amplified using gene-specific primers (AlNAC4EcoRI F and AlNAC4XhoI R primers) with A. lagopoides genomic DNA as template and 150 ng of each primer, 200 μM dNTPs, 2.5 U Taq DNA polymerase in a 50 μl reaction using following PCR conditions; 94°C, 5 min for 1 cycle; 94°C, 1 min; 55°C, 1 min and 72°C, 2 min for 35 cycles and last 72°C, 7 min for 1 cycle. The PCR product was gel eluted followed by ligation in the pJET1.2 vector and sequenced.
Expression Analysis of AlNAC4 Using Real-Time PCR
For expression analysis of AlNAC4 and downstream genes of the tobacco transgenics, the RNA was isolated (as mentioned above). Five micrograms of RNA was treated with DNaseI and used for cDNA synthesis (Thermo Scientific). The cDNA (1:10 dilution) was used as a template for RT-PCR analysis. The Aeluropus and tobacco actin genes were used as internal control genes for AlNAC4 expression and downstream gene expression, respectively (Supplementary Tables S1, S2). The RT-PCR of AlNAC4 was performed using gene-specific primers (AlNAC4RT F and AlNAC4RT R, Supplementary Table S1), using the first strand cDNA from stress-treated leaf tissue and its corresponding control. To study the regulation of the downstream genes in transgenics, 15-days-old WT and hygromycin positive T1 transgenic seedlings were transferred to Hoagland medium for 4 weeks. The RNA was isolated from leaf tissue of WT and T1 transgenic plants treated with different H2O2 concentrations (0, 10, and 20 mM) for 24 h. List of the primers used for downstream genes study is given in Supplementary Table S2.
The PCR reaction was carried out using SYBR green jumpstart Taq ready mix (Sigma–Aldrich) and 75 ng of each gene-specific primers in CFX96 PCR system (BioRad, United States) at following conditions: 94°C, 2 min for 1 cycle; 94°C, 30 s, 55°C, 30 s and 72°C, 30 s for 45 cycles; 72°C, 7 min for 1 cycle. The specificity of PCR amplification was checked at the end of the PCR cycles, by melt curve analysis. Each reaction was replicated three times and relative-fold expression was calculated by the comparative Ct (2-ΔΔCt) method using actin as an internal reference gene (Livak and Schmittgen, 2001). The expression values are mentioned as the mean ± standard deviation.
Transactivation Assay of AlNAC4
A yeast one-hybrid assay was performed to study the transcriptional activation of the AlNAC4 protein (Shukla et al., 2015). AlNAC4 cDNA was amplified using primers (AlNAC4EcoRI F and AlNAC4SalI R, Supplementary Table S1) with the EcoRI and SalI flanking restriction sites, respectively, and cloned in a pGBKT7 vector (Clontech). AlNAC4 and vector alone plasmids were transformed separately into yeast strain AH109 (Clontech) and grown on synthetic dropout medium lacking tryptophan (SD/-Trp). HIS3 activity was assessed by a viability test on a histidine lacking medium (SD/-Trp/-His/-Ura). The LacZ activity was analyzed by the galactosidase filter lift assay (Ma et al., 2009).
Cloning of AlNAC4 in E. coli Expression Vector and Purification of the Recombinant Protein
The AlNAC4 ORF was PCR amplified using AlNAC4EcoRI F and AlNAC4XhoI R primers flanked with EcoRI and XhoI restriction sites, respectively, and cloned in the pET-28a expression vector (Novagen). The recombinant (pET28a-AlNAC4) plasmid and vector alone were transformed in the BL21 (DE3) star Escherichia coli strain. The recombinant plasmid transformed in BL21 (DE3) star E. coli strain was induced with 1 mM IPTG and cells were harvested after 2, 4, and 6 h of induction. Finally, the recombinant protein was purified to homogeneity using the 2 h harvested cells under the native condition using the Ni-NTA Fast Start Kit (Qiagen) following the manufacturer’s protocol. The AlNAC4 protein was detected by anti-6x-His antibody (Qiagen), followed by alkaline phosphatase conjugated secondary antibody; the signals were developed AP conjugate substrate kit (Bio-Rad).
DNA Probes and Gel Mobility Shift Assay
For electrophoretic mobility shift assay (EMSA), two sets of complementary oligonucleotides for NACRS in tandem (51 bp, NACBST) and NACRS having flanking sequence (69 bp, NACBS) were designed from the Arabidopsis thaliana EARLY RESPONSIVE TO DEHYDRATION STRESS 1 (erd1) promoter (Tran et al., 2004). Two micrograms of complementary oligonucleotides were annealed using the annealing buffer (100 mM Tris-HCl, 5 mM NaCl and 10 mM EDTA) by incubating at 60°C for 5 min and 37°C for 15 min. The binding reaction was carried out in 25 μl reaction volume using 80 ng of probe, 300–2400 ng of purified protein and 4 μg glycogen in binding buffer (15 mM HEPES, 35 mM KCl, 1 mM EDTA pH 8.0, 1 mM DTT, 1 mM MgCl2 and 6% glycerol) at room temperature for 20 min (Gupta et al., 2010). The reaction was fractionated on non-denaturing acrylamide gel (10% acrylamide, 0.5x TBE, 5% glycerol) and the gel was stained with ethidium bromide. The MCS of pBSK+ vector was used as negative control (pBSK+ MCS F and pBSK+ MCS R; primers Supplementary Table S1). The NACBS was also mutated by replacing CATGTG nucleotide with AAAAAA and similarly AACA nucleotide with TTAA in NACBS F, respectively.
Plant Transformation Vector Construction and Tobacco Transformation
The AlNAC4 cDNA was PCR amplified by AlNAC4 F EcoRI and AlNAC4 R KpnI primers and cloned in EcoRI/KpnI sites of pRT101 vector. The pCAMBIA1301 binary vector HindIII site was used for cloning expression cassette containing 35S:AlNAC4:PolyA and mobilized into Agrobacterium tumefaciens (LBA4404). The Agrobacterium cells comprising the 35S:AlNAC4:PolyA was used to transform Nicotiana tabacum L. cv. Petit Havana leaf disks according to Horsch et al. (1985).
Confirmation of Transgenic Plants
The β-glucuronidase reporter gene staining kit (Sigma, United States) was used to visualize glucuronidase gene (GUS) activity in leaf tissue. Genomic DNA was isolated from different T0 lines using CTAB buffer (Doyle and Doyle, 1987) and PCR was carried out to confirm the integration of transgene using the glucuronidase (gus A F and gus A R), hygromycin (hptII F and hptII R) and gene-specific (AlNAC4RT F and AlNAC4RT R) primers (Supplementary Table S1).
Semi-quantitative RT-PCR was used for the determination of the transcript level of AlNAC4 gene in the transgenic plant. The total RNA was isolated from WT and transgenic lines. The cDNA template (1:10 dilution) with gene-specific (AlNAC4RT F and AlNAC4RT R, Supplementary Table S1) and tobacco actin primers (internal control, NtActin F and NtActin R, Supplementary Table S2) were used for semi-quantitative RT-PCR analysis. An agarose gel electrophoresis analysis of the PCR products showed the AlNAC4 mRNA expression in transgenic lines.
The copy number of the transgene was determined by Real-time PCR. The Real-time PCR was optimized for GUS and NRA genes (Nitrate reductase, NCBI accession no. X06134) primers. The PCR reaction was carried out using 7.5 ng primers in 20 μl reactions using SYBR green jumpstart Taq ready mix (Sigma–Aldrich) on 1, 10, and 100 ng of genomic DNA from T1 transgenic lines and the standard curve was plotted using threshold cycle (Ct) value to determine reaction efficiencies for calculating copy number ratio of GUS to NRA (Shepherd et al., 2009) using following formula:
Analysis of Physiological and Biochemical Parameters of Transgenic in Response to Hydrogen Peroxide Treatment
In vivo Localization of O2•- and H2O2
The in vivo localization and quantification of O2•- and H2O2, were performed according to Shukla et al. (2012).
Quantification of H2O2 Content
The H2O2 content in leaf samples was measured as described by Sanadhya et al. (2015a). Leaf tissue extract was prepared with cold acetone to determine the H2O2 levels. 1 ml of the extract was mixed with 0.5 ml of 0.1% titanium dioxide in 20% (v:v) H2SO4 and the mixture was centrifuged at 6,000 g for 15 min. The intensity of the yellow color of the supernatant was measured at 415 nm and the concentration of H2O2 was calculated against the standard curve.
Determination of Enzyme Activities
Leaf tissue (1–2 g) from WT and transgenic plants were homogenized in 50 mM phosphate buffer, pH 7.0, containing 1% polyvinylpyrrolidone. The homogenate was centrifuged at 10,000 g for 10 min and the supernatant obtained was used as enzyme extract.
Superoxide dismutase (EC1.15.1.1)
Superoxide dismutase activity was measured as reported in Dhindsa et al. (1981) with minor modification, based on its potential to inhibit the photoreduction of NBT. The 3 ml reaction mixture contained 100 mM phosphate buffer, pH 7.5, 200 mM methionine, 2.25 mM NBT, 60 μM riboflavin, 3.0 mM EDTA, and 50 μl enzyme extract. The reaction was carried out at room temperature for 15 min under bright light (2 × 15 W fluorescent lamps), and absorbance was recorded at 560 nm. The log A560 was plotted as a function of the volume of enzyme extract (Giannopolitis and Ries, 1977), and the volume of enzyme extract corresponding to 50% inhibition of the reaction was considered as one enzyme unit (Beauchamp and Fridovich, 1971).
Catalase (EC1.11.1.6)
Catalase assay was carried out by measuring the initial rate of disappearance of H2O2 as reported by Dhindsa et al. (1981). The 3 ml reaction mixture contained 100 mM phosphate buffer, pH 7.5, 75 mM H2O2, and 50 μl enzyme extract. The decrease in H2O2 concentration was observed as a decline in O.D. at 240 nm using Epoch spectrophotometer (Biotek, India). The activity was expressed in units (1 unit defines the conversion of one mole of H2O2/minute).
Statistical Analysis
Each experiment was repeated thrice, the mean values and standard deviations were calculated using Microsoft Excel. Analysis of variance was calculated using Fishers Least Significant Difference (LSD) by Infostat software at P ≤ 0.05 to determine the significance of difference between the means of control and different stress treatments. Mean values of treatments that were significantly different from each other were indicated by different alphabets.
Results
Identification and Sequence Analysis of AlNAC4 Gene
An amplicon of 300 bp, having a conserved region of NAC TF, was amplified using degenerate primers. Later, the gene was made full-length by RACE. The 5′ and 3′ RACE, using gene-specific primers, resulted in the amplicons of 352 and 779 bp, respectively. The AlNAC4 cDNA (NCBI accession number KY569078) had an ORF of 936 bp and 191 bp and 202 bp 5′ and 3′ untranslated region, respectively. The AlNAC4 encodes a protein of 312 amino acid (aa), with the calculated molecular weight of 34.9 kDa and isoelectric point (pI) of 6.11.
The AlNAC4 protein shows the presence of highly conserved 153 aa long NAC domain (21–174 aa) comprising of A (20 aa), B (17 aa), C (35 aa), D (28 aa), E (15 aa) sub-domains at N-terminal and a highly variable C-terminal region (Figure 1A and Supplementary Figure S1). The MEME predicted conserved motifs are presented in Table 1. The PROSITE analysis revealed seven protein kinase C phoshphorylation sites (10–12, 81–83, 116–118, 130–132, 160–162, 223–225, 245–247 aa), seven casein kinase II phosphorylation sites (30–33, 81–84, 105–108, 207–210, 217–220, 228–231, 245–248 aa), two amidation sites (10–13 and 155–158 aa), two N-glycosylation motifs (89–92 and 221–224 aa), four myristoylation sites (90–95, 115–120, 121–126, 287–292 aa) and single cAMP- and cGMP-dependent protein kinase phosphorylation sites (157–160 aa). The AlNAC4 amino acid sequence showed 81, 78, and 75% identity to MlNAC4, OsNAC4 and TaNAC4, respectively, indicating relatively high homology with only monocot NAC members, whereas no close homology was found with the dicot NAC members. Phylogenetic relationships among some NAC proteins show that AlNAC4 gets clustered with stress-responsive MlNAC4, OsNAC4 and TaNAC4 proteins of the stress-related NAC (SNAC) group III (Figure 1B).
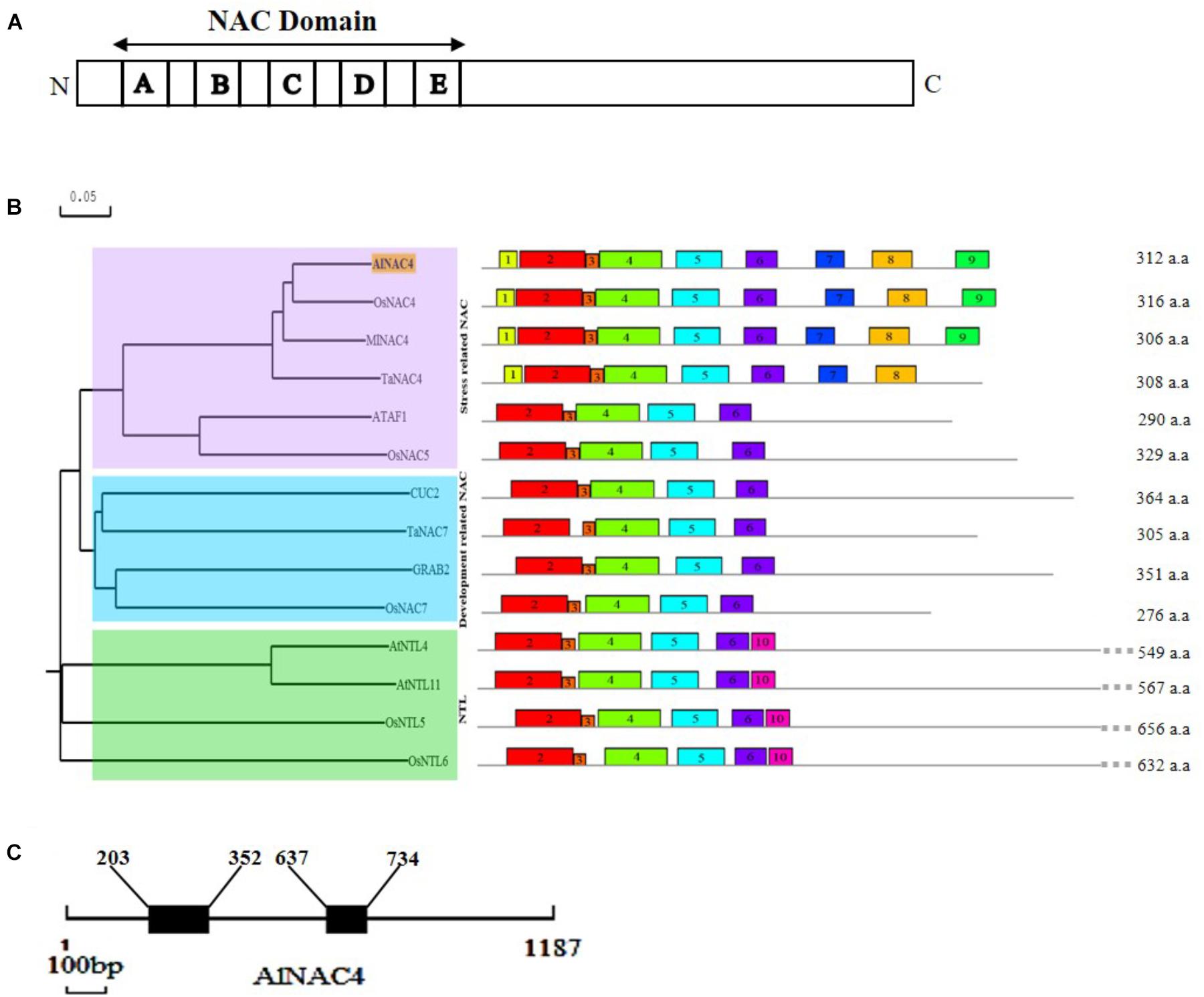
FIGURE 1. In silico analysis of AlNAC4 sequence. (A) Schematic representation of AlNAC4 protein with A-E sub-domains, (B) the relationship among NAC proteins is represented using N-J phylogenetic tree constructed by DNAMAN software. Scale represents the branch length. The sequences used from OsNAC4 (AB028183.1), MlNAC4 (KM017003.1), TaNAC4 (GQ985329), ATAF1 (CAA52771.1), OsNAC5 (AB028184.1), CUC2 (BK007973.1), TaNAC7 (HM027572), GRAB2 (AJ010830), OsNAC7 (BAA89801.1), NTL4 (NM_111885), NTL11 (NM_120523), OsNTL5 (NM_001069053), and OsNTL6 (NM_001055094). Conserved motifs were identified by MEME software (multilevel consensus sequences for the MEME defined motifs are listed in Table 1), (C) diagrammatic representation of the position of introns (203–352; 637–734bp) and exons of AlNAC4. The scale underneath the diagram represents 100 bp.
For the analysis of AlNAC4 genomic clone, 1,187 bp fragment (NCBI accession number KY569079) was PCR amplified using gene-specific primers from A. lagopoides genomic DNA. The genomic DNA sequence was compared with the cDNA sequence to identify the exons and introns positions. The AlNAC4 genomic clone consists of three exons (1–202, 353–636, and 735–1,187 bp) and two introns (203–352 and 637–734 bp, Figure 1C).
The Expression of AlNAC4 Gene at the Transcript Level
The transcript expression of AlNAC4 was studied in the presence of different stresses and stress-related phytohormones (Figures 2A–D). The results showed that AlNAC4 expression was regulated by salinity, dehydration, H2O2 and ABA treatments. The salinity resulted in 1.75-fold transcript accumulation at 12 h duration only. The transcript showed strong expression with dehydration treatment reaching 2.6-fold expression as early as 1 h and showing a maximum transcript expression of 14.1 and 14.6 at 12 and 24 h duration. However, the transcript expression decreased at 48 h (2.7-fold). The H2O2 treatment also showed higher transcript expression, wherein the maximum expression was observed (7-fold) at 6 h and further reduced to 1.5-fold at 48 h. The ABA (100 μM) showed a maximum induction of only 1.74-fold at an early time point of 3 h.
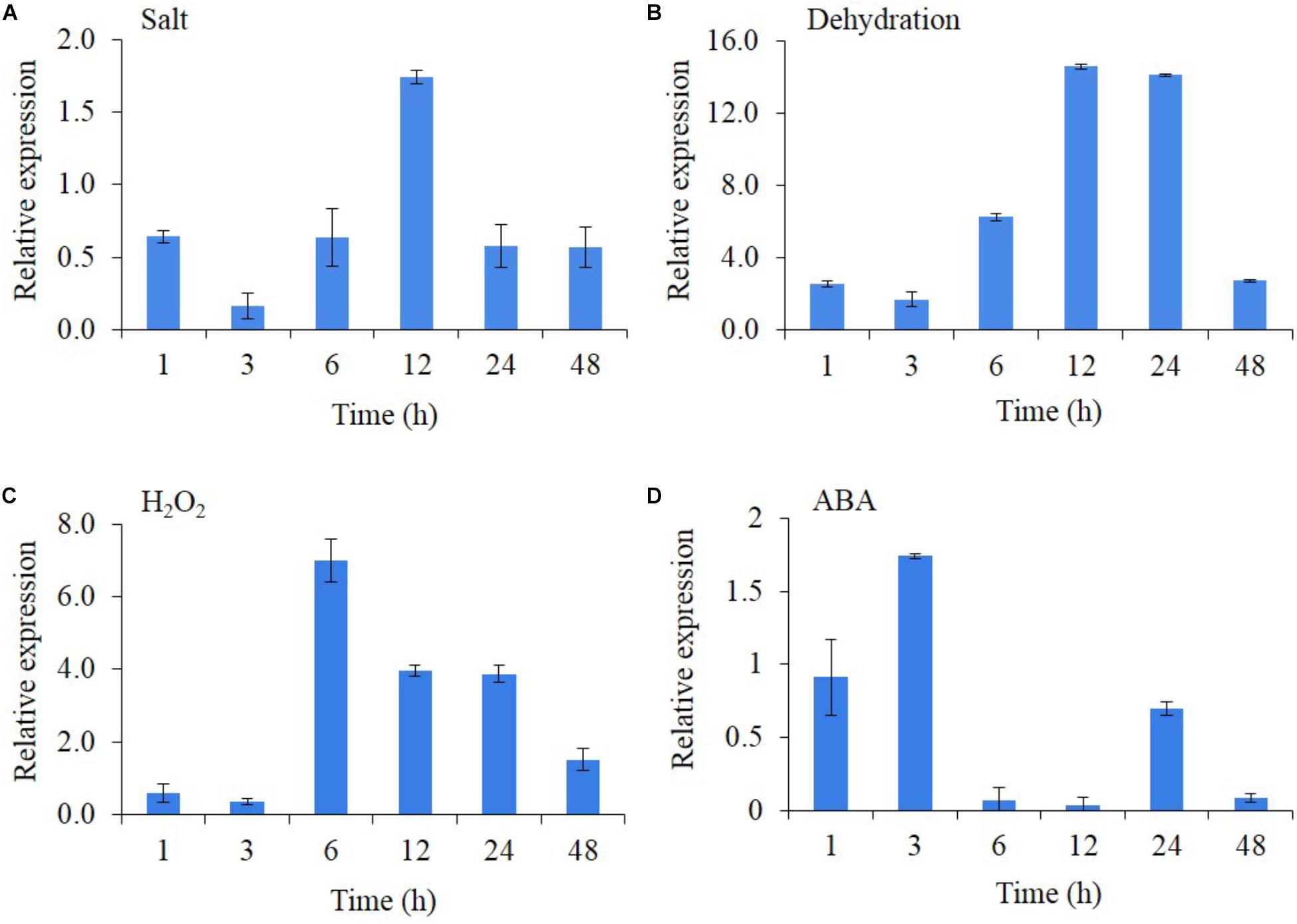
FIGURE 2. Transcript analysis of AlNAC4 gene in response to 250 mM NaCl (A), dehydration (B), 20 mM H2O2 (C) and 100 μM ABA (D). The experiments were replicated three times and the mean values and standard deviations are represented in bars.
Transcriptional Activity and DNA-Binding Assay of AlNAC4 Protein
The transcriptional activity of the AlNAC4 protein was studied using a yeast GAL4 system. The GAL4 DNA-binding domain-AlNAC4 recombinant plasmid was transformed into AH109 yeast strain. The yeast cells activated the transcription of the reporter gene His3 and LacZ, as was evident by the growth of yeast cells on SD/-His medium, and further, development of blue color in X-gal solution (Figures 3A,B), confirmed its activity as a TF.
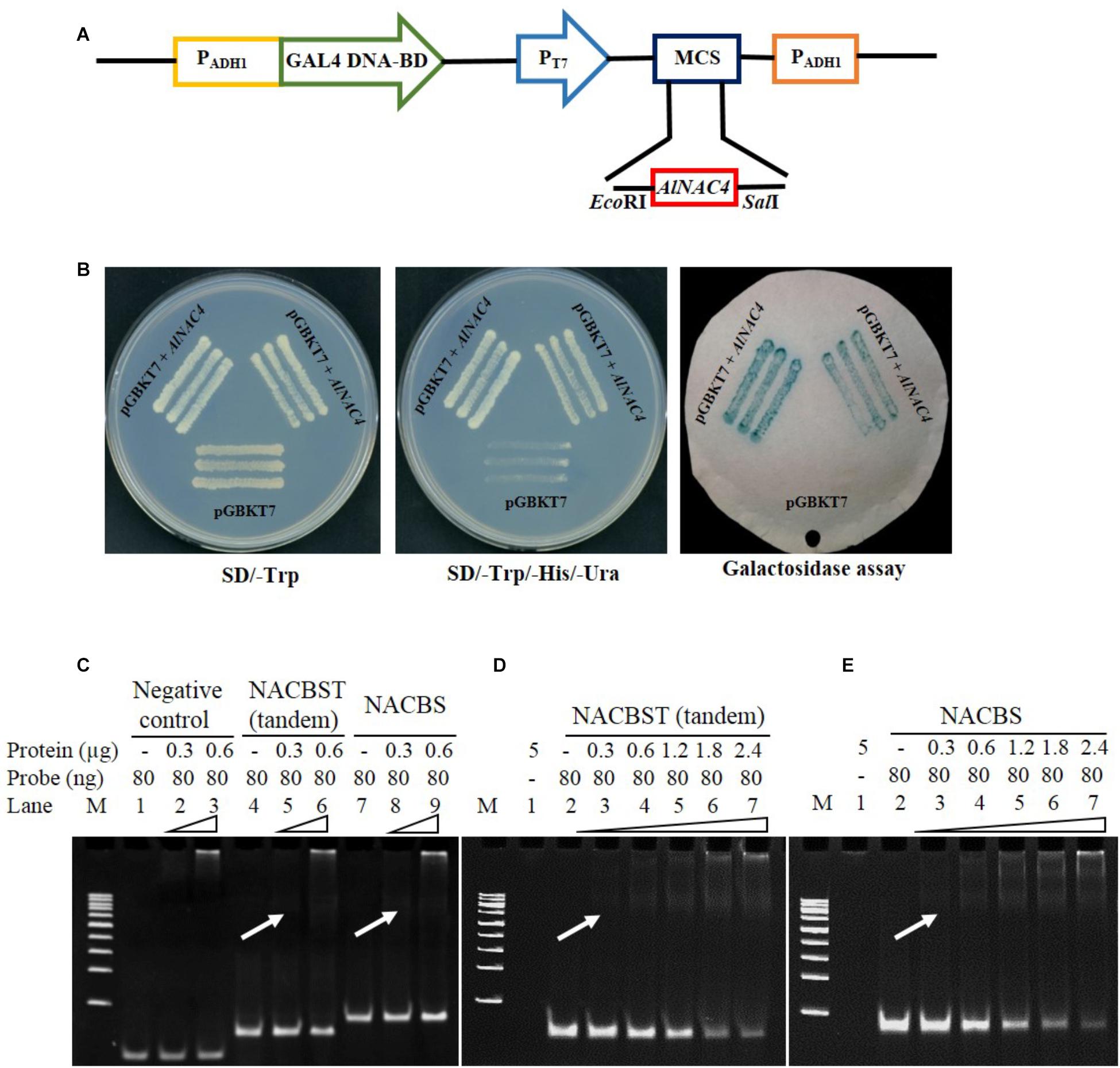
FIGURE 3. (A) Schematic representation of the AlNAC4 ORF cloned in pGBKT7 vector, (B) transactivation assay for AlNAC4 gene, (i) Transformed yeast cell (AH109) containing pGBKT7 + AlNAC4 and pGBKT7 alone grown on SD/-Trp medium, (ii) Transformed yeast cell (AH109) containing pGBKT7 + AlNAC4 showing growth on SD/-Trp/-His/-Ura medium, while only pGBKT7 did not grow, (iii) Yeast cells transferred on filter paper showed β-galactosidase activity using X-gal staining. Electro Mobility Shift Assay of AlNAC4 protein with heterologous, NACBST (tandem) and NACBS probes showing specific binding with NACBST and NACBS (C). The detailed kinetics of AlNAC4 protein with NACBST (tandem, D) and NACBS (E) probes.
The AlNAC4 recombinant protein of 34.9 kDa was induced with 1 mM IPTG for different time periods. The protein showed maximum induction at 2 h; therefore, the protein was induced for 2 h and purified to near homogeneity (Supplementary Figures S3A,B). The purified recombinant protein (without thrombin cleavage) was confirmed by Western analysis using 6x-His antibody (Supplementary Figure S3C). The purified protein was used to study the binding of the AlNAC4 protein with NACBST having three tandem repeats and NACBS having single repeat by EMSA. The heterologous probe (MCS of pBSK+) did not bind to the AlNAC4 recombinant protein (Figure 3C). The recombinant protein showed binding to both the oligos of the erd1 promoter. The strength of binding increased with increasing amount of recombinant protein from 300 to 2400 ng and 80 ng probe (Figures 3D,E). The mutated sequence did not show binding with AlNAC4 protein (data not shown).
Analysis of Transgenic Lines
Confirmation and Transgene Integration in T0 Tobacco Transgenics
To validate the function of AlNAC4 gene in stress tolerance, the cDNA was cloned under the CaMV 35S promoter in pCAMBIA1301 (Figure 4A) and transformed into tobacco via Agrobacterium tumefaciens-mediated transformation. Sixty seven putative transgenic lines of N. tabacum were selected on hygromycin-containing medium and subsequently 20 of them showed positive GUS assay (Figure 4B). Some plants showed proper blue color in leaves while in others the scattered blue spots were seen, whereas no visual staining was observed in WT leaves. The GUS positive transgenic lines were confirmed by PCR using the presence of GUS, hptII and AlNAC4 genes. Ten transgenic lines showed the single product of the expected size of all the genes (Figure 4C).
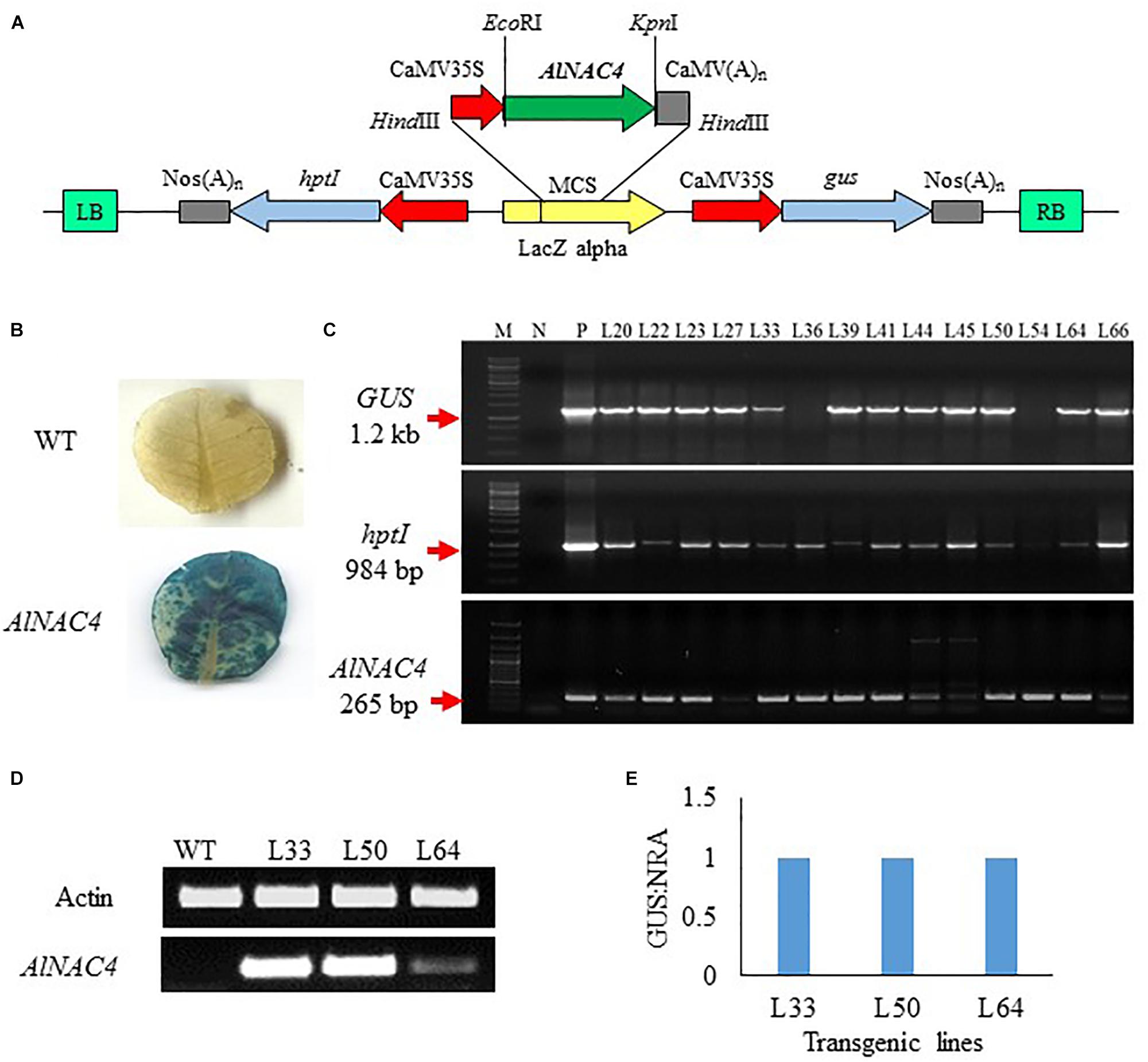
FIGURE 4. (A) Schematic representation of the pCAMBIA1301-35S: AlNAC4 construct used for Nicotiana tabacum transformation, (B) GUS histochemical assay of wild type (WT) and AlNAC4 transgenic leaves, (C) PCR confirmation of transgenic lines by gus (Glucuronidase, 1200 bp), hptI (hygromycin resistant, 984 bp) and AlNAC4 (265 bp) gene specific primers, M denotes 1 kb ladder for upper two panel and 100 bp plus ladder for lower panel, N denotes wild type (WT) tobacco, (D) transcript level of AlNAC4 gene in transgenic lines (L33, L50 and L64) and wild type (WT) plants via semi quantitative RT-PCR, (E) AlNAC4 gene copy number analysis by Real-Time PCR.
Three transgenic lines (L33, L50, and L64) and WT plants were checked by reverse transcriptase PCR to confirm the expression of transgene mRNA. Transgenic lines showed expression of the AlNAC4 gene, but in the WT plants the corresponding band was not observed (Figure 4D). The presence of transcripts indicated that transcription initiation and termination of AlNAC4 mRNA occurred as expected.
Transgene integration analysis was carried out using RT-PCR. The quantitative analysis revealed that L33, L50, and L64 had a GUS: NRA ratio 1.0, thus confirming the single-copy insertion (Figure 4E).
The T0 transgenic plants showed no morphological or growth differences at vegetative and floral stages and was similar to WT plants. Seed set in both transgenics and WT plants was also similar (Supplementary Figures S4A,B).
Analysis of T1 Transgenics for Stress Treatments
Salinity and dehydration stress
The T1 transgenic progeny was studied to establish the stress tolerance potential of tobacco transgenics overexpressing AlNAC4 gene. The WT and transgenics seeds (T0) showed similar germination rate and seedling growth in control conditions as well as in salinity and dehydration conditions (Figures 5A–C).
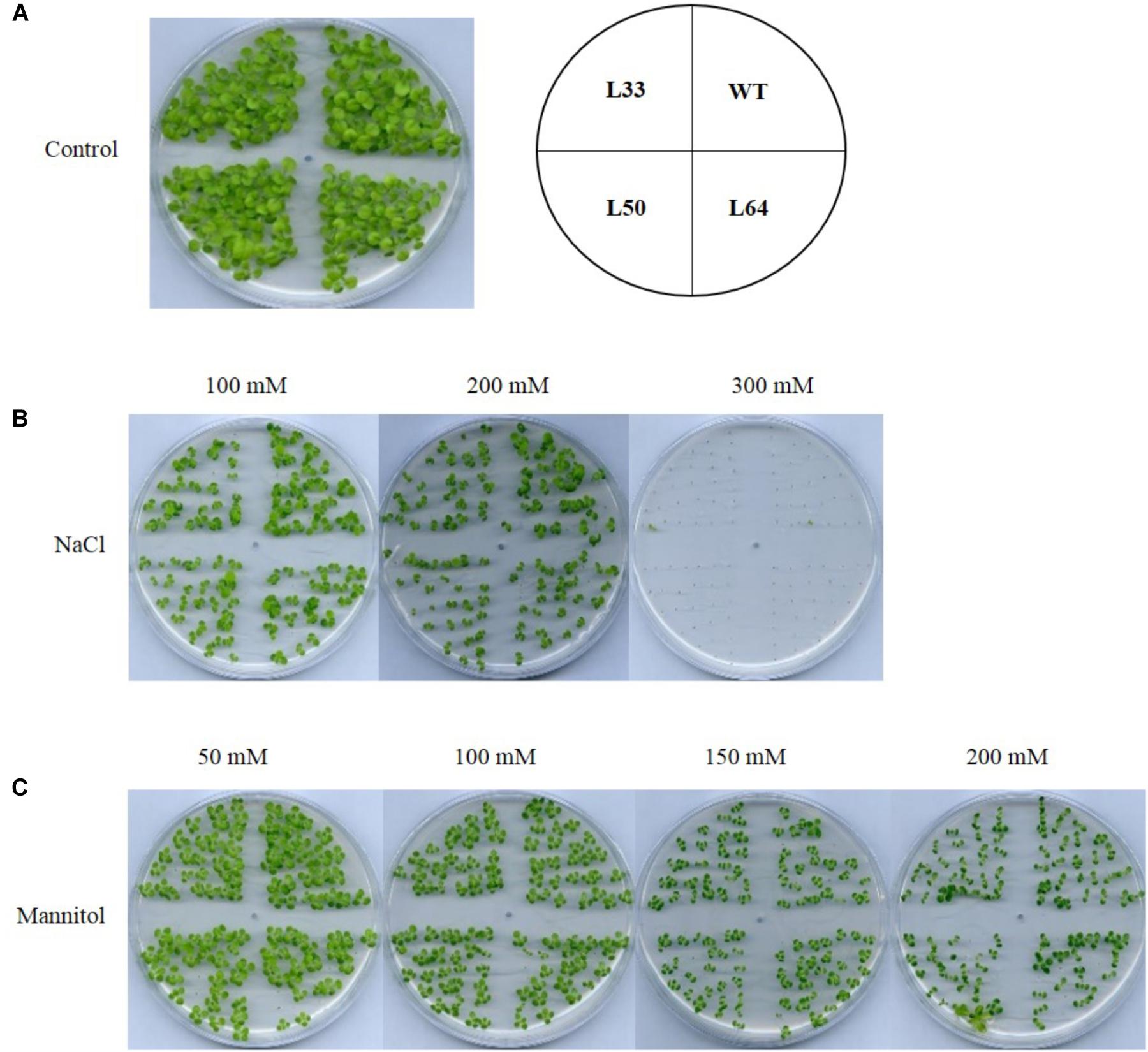
FIGURE 5. Seed germination analysis of wild type (WT) and 35S: AlNAC4 transgenic (L33, L50, and L64) tobacco at 30 days at various stress supplemented medium, (A) control, (B) NaCl, and (C) mannitol.
Oxidative stress
The WT and transgenic lines showed similar germination rate and growth at 0 and 10 mM H2O2 after 6 days. While WT and T1 transgenic lines showed differential germination rate and growth after 6 days at 20 mM H2O2 (Figure 6).
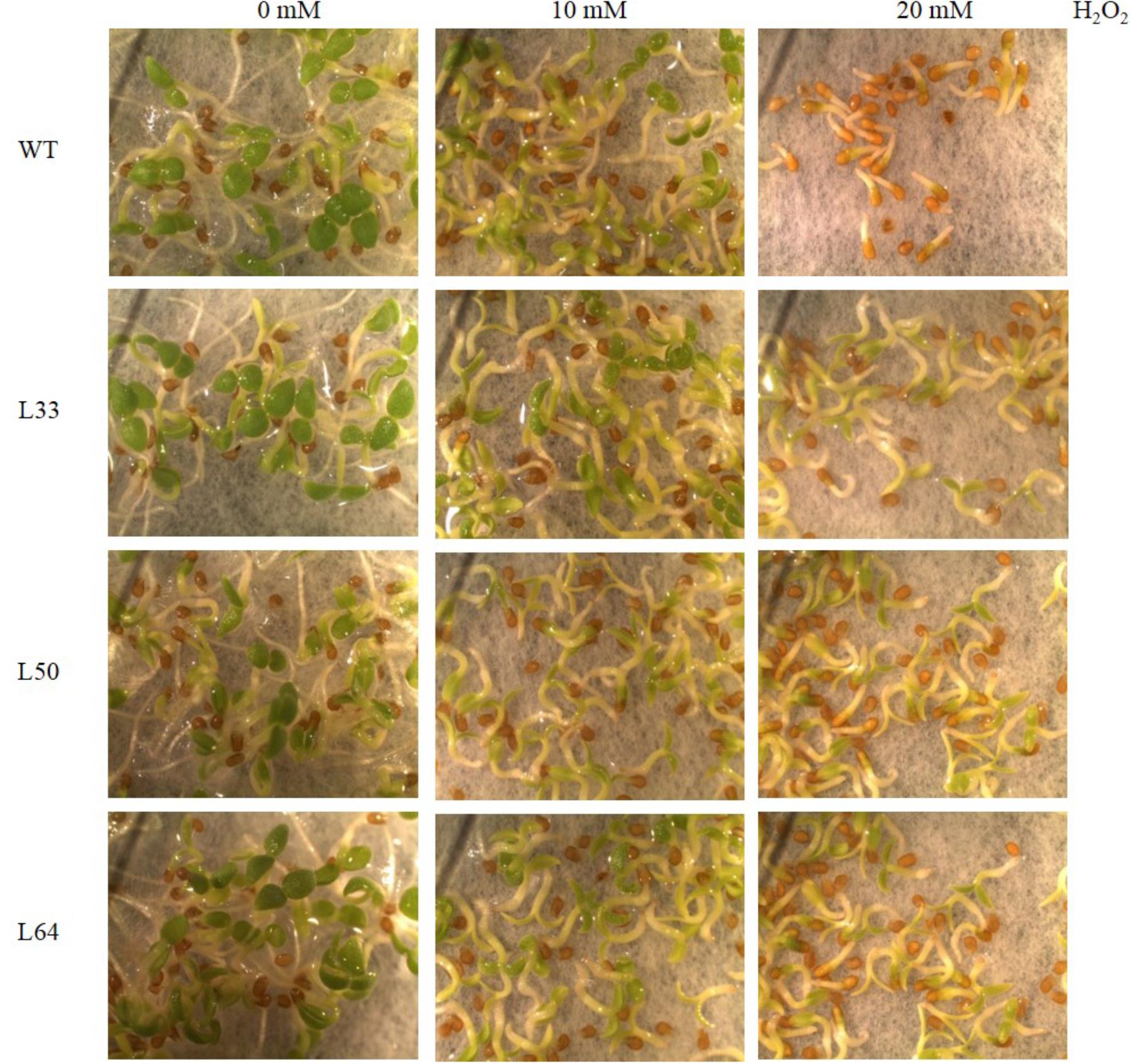
FIGURE 6. Stereomicroscopic study of seed germination of wild type (WT) and 35S: AlNAC4 transgenic (L33, L50, and L64) tobacco on 0, 10, and 20 mM H2O2 at 6th day. The transgenic seeds showed significantly better growth at 20 mM H2O2.
The transgenic lines (L33, L50, and L64) showed enhanced growth (Figure 7A), with longer hypocotyl and roots as compared to WT seedlings with 20 mM H2O2 stress (Figures 7B,C). There was a slight difference in hypocotyl length in WT and transgenics at 10 mM H2O2 compared to the control condition, whereas no difference was observed in root length. An enhanced RWC percentage was exhibited in all transgenic seedlings at 20 mM H2O2 as compared to WT seedlings (Figure 7D).
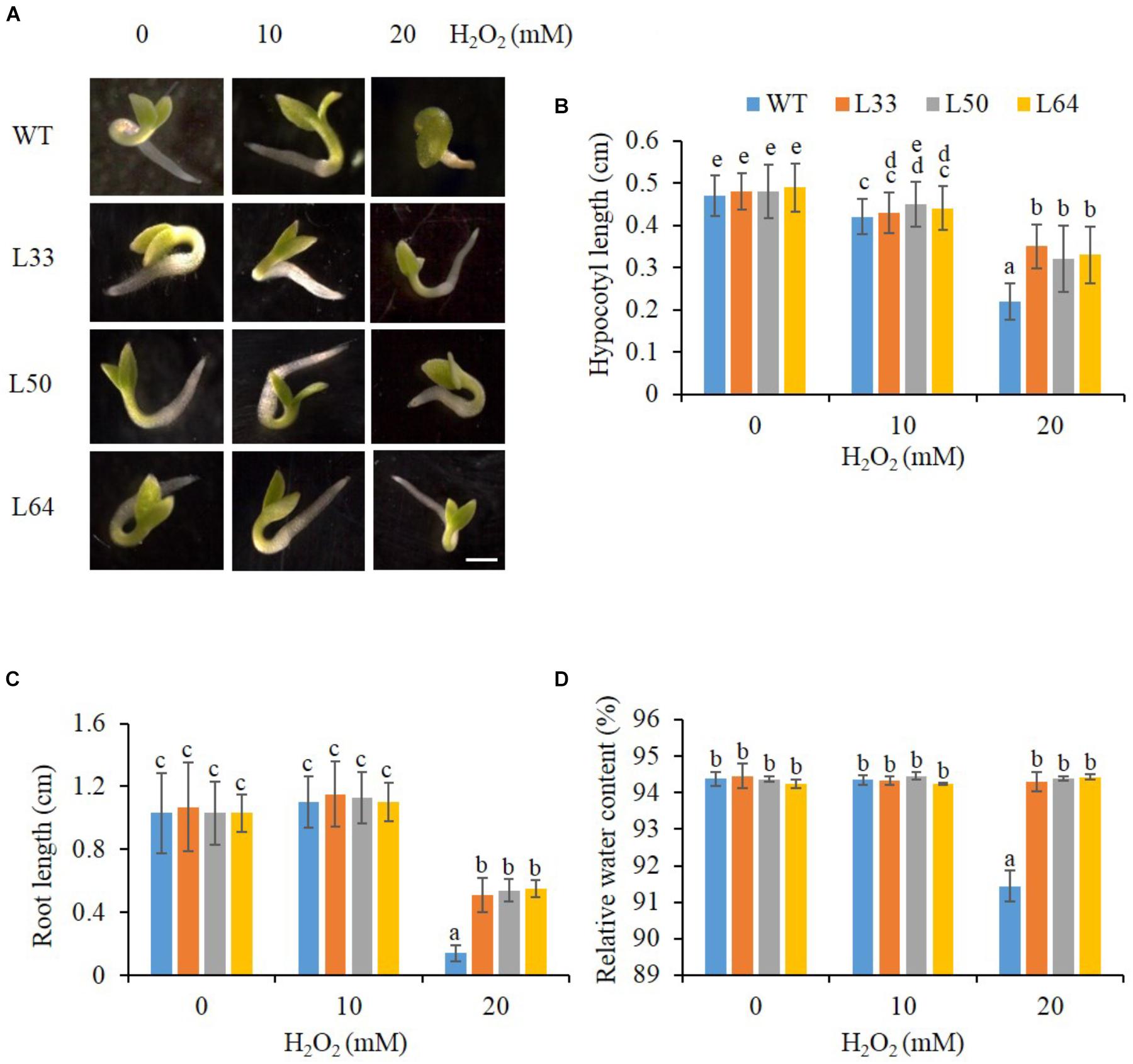
FIGURE 7. (A) Stereomicroscopic study of seed germination of wild type (WT) and 35S: AlNAC4 transgenic (L33, L50 and L64) tobacco plants on 0, 10 and 20 mM H2O2 at 11th day. Scale bar 0.5 cm. Analysis of growth parameters (B) hypocotyl length, (C) root length and (D) relative water content (RWC) of T1 transgenic seedlings germinated in H2O2 stress (0, 10, and 20 mM). The experiment was repeated three times, and the mean values significantly different at P ≤ 0.05 within treatment are indicated by different alphabets.
Physio-Biochemical Analysis of Transgenics on H2O2 Treatment
Seedling stage
The WT and transgenic seedlings (11-day-old) showed similar staining at 0 and 10 mM H2O2 in the presence of DAB and NBT solutions. However, at 20 mM H2O2, WT seedlings accumulated more brown (indicator of H2O2) and blue-colored spots (indicator of O2-) in comparison to transgenics (Figures 8A,B). The WT seedling showed significantly higher accumulation of H2O2 (0.39 μmol/gm FW) at 20 mM H2O2 treatment (Figure 8C). The WT seedlings also showed higher anti-oxidative enzyme (CAT and SOD) activity (114.78 U/gm FW and 0.43 U/mg FW, respectively) at higher concentration of H2O2. While in transgenic seedlings (L33, L50, and L64), significantly lower H2O2 (0.21, 0.18, and 0.24 μmol/gm FW, respectively) as well as CAT (45.69, 47.35, and 49.29 U/gm FW, respectively) and SOD (0.18, 0.22, and 0.23 U/mg FW, respectively) activity was observed at 20 mM H2O2 stress (Figures 8C–E). The transgenic seedlings showed no significant difference in anti-oxidative levels at various H2O2 concentrations (0, 10, and 20 mM).
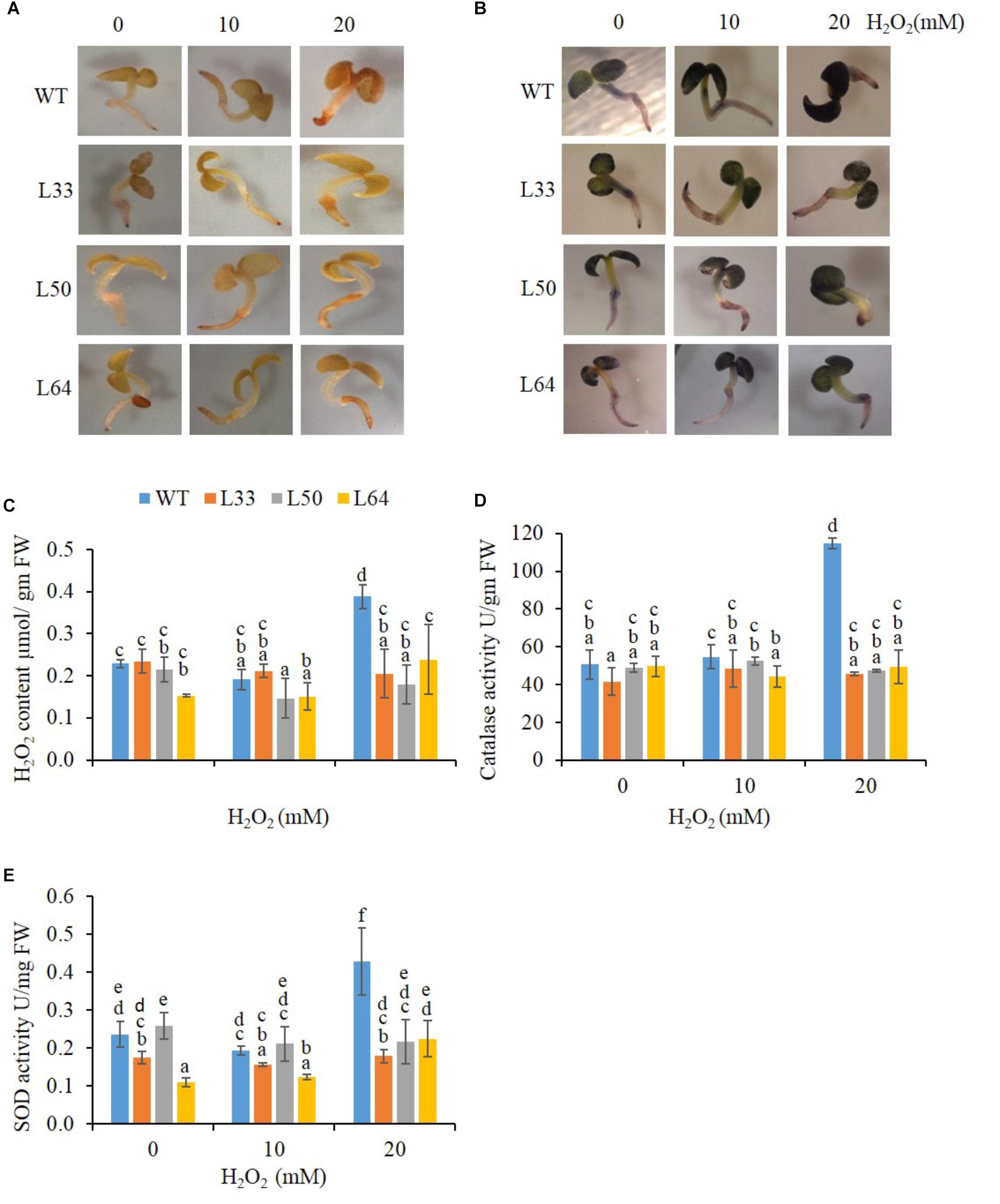
FIGURE 8. Biochemical study of wild type (WT) and transgenic (L33, L50, and L64) seedlings of tobacco at 11th day. In vivo localization of H2O2 (A) and O2- (B), quantification of H2O2 (C) and activity of CAT (D) and SOD (E) in the presence of 0, 10, and 20 mM H2O2 treatments. Values are represented as mean ± SD (n = 3) and marked with different alphabets to indicate significant difference at P ≤ 0.05 probability.
1-month-old plant stage
In plant tissues, exogenously applied H2O2 trigger ROS accumulation leading to oxidative damage. The WT showed significantly higher accumulation of H2O2 content, on exposure to different H2O2 concentrations as compared to the transgenics. In WT, 10 mM H2O2 treatment at 3, 12, and 24 h resulted in higher accumulation of H2O2 e.g., 197.13, 381.9, and 230.6 mmol/gm FW, respectively. Similarly, at 20 mM H2O2, WT showed higher accumulation of H2O2 content at all the duration (182.7, 213.8, and 395.4 mmol/gm FW) compared to transgenics (Figure 9A). The CAT enzyme activity enhanced at the early time point (3 h) in both WT and transgenics at 10 mM and 20 mM H2O2 treatments. However, CAT activity in transgenics was significant low at 12 and 24 h as compared to WT plants. Interestingly, it was observed that transgenic lines (L33, L50, and L64) had significantly higher CAT activity (1127.09, 1286.23 and 1073.01 U/gm FW, respectively) at 3 h during 20 mM H2O2 treatment as compared to WT (548.3 U/gm FW) (Figure 9B).
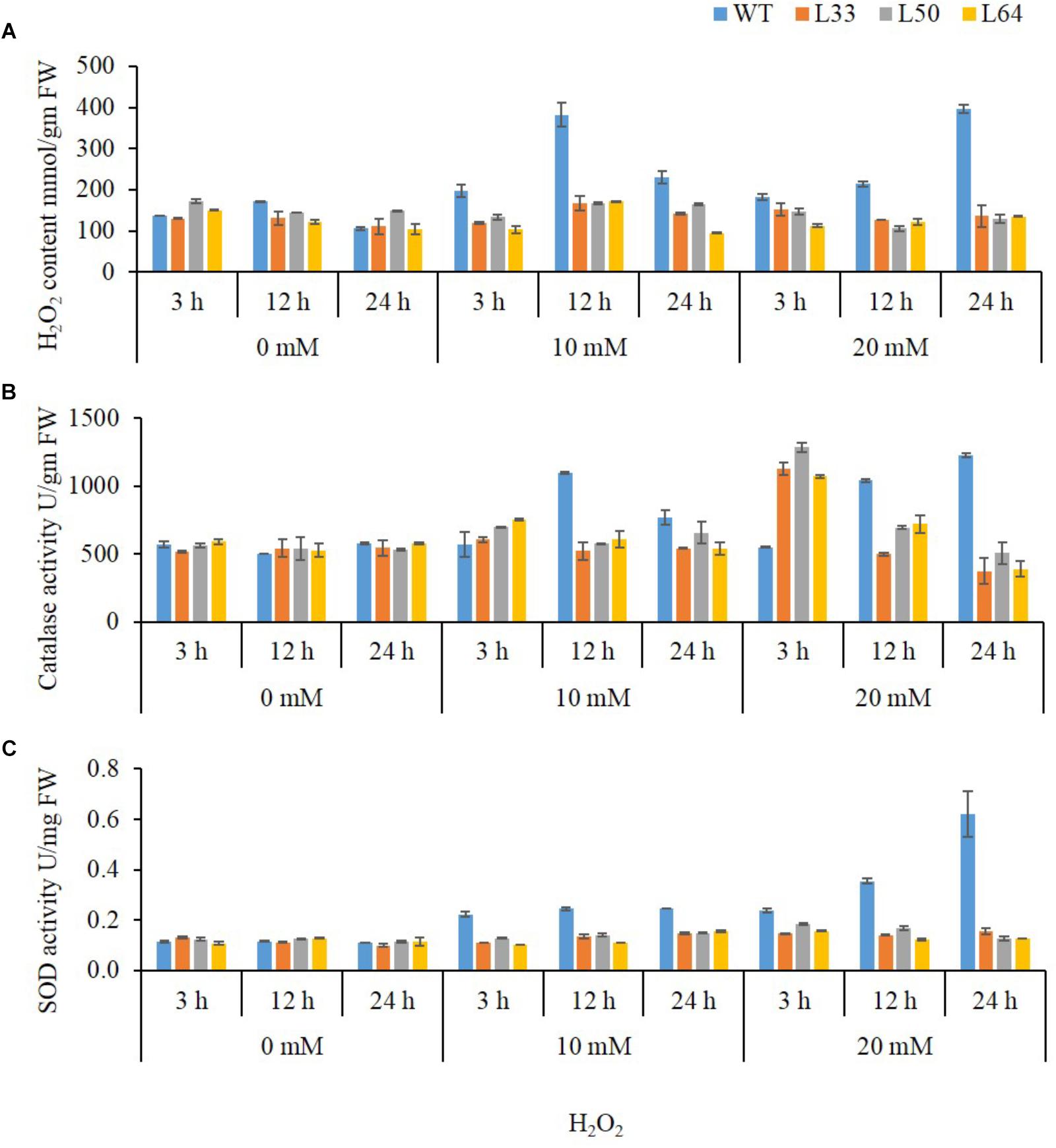
FIGURE 9. Biochemical study of 4-week-old wild type (WT) and transgenic tobacco (L33, L50, and L64) plants at different concentrations of H2O2 for 3, 12, 24 h. Quantification of H2O2 (A) content and activity of CAT (B), and SOD (C) in the presence of 0, 10, and 20 mM H2O2 treatments. Values are represented as mean ± SD (n = 3).
Another important ROS-scavenging enzyme, SOD, activity of WT with various concentrations of H2O2 was considerably high as compared to the transgenics. At 10 mM H2O2 treatment, SOD activity was observed maximum at 12 h (0.41 U/mg FW) and declined at 24 h (0.26 U/mg FW), whereas, with 20 mM H2O2 treatment gradual increase in activity was observed at 3, 12, and 24 h (0.40, 0.59, and 1.03 U/mg FW, respectively) in WT. There was no significant change in SOD activity in transgenics at both 10 and 20 mM H2O2 treatments (Figure 9C).
Regulation of Stress-Responsive Downstream Genes in Transgenics
To get an insight into the gene regulation of AlNAC4 transgenics with enhanced H2O2 tolerance, expression of CAT and SOD antioxidant genes, stress-related genes like LEA5, PLC3, ERD10B, THT1 and TFs like AP2, ZFP were studied. After 24 h, significant accumulation was observed of CAT (2.4-fold), SOD (2.3-fold), LEA5 (3.5-fold), PLC3 (4.1-fold), AP2 (2.4-fold) and ERD10B (4.1-fold) genes at 20 mM H2O2 in WT plants. All the transgenic lines (L33, L50, and L64) showed no enhanced transcript expression of CAT, SOD, LEA5, PLC3, AP2 and ERD10B genes during the same treatments. The higher upregulation of ZFP transcript was observed in transgenics (L33, L50, and L64) during both 10 and 20 mM H2O2 treatments compared to WT. Whereas, significant upregulation of THT1 gene (43.4, 53.6, and 60.7-fold) was observed in transgenics (L33, L50, and L64, respectively) at 20 mM H2O2 treatment compared to WT(14.3-fold) (Figures 10A–H).
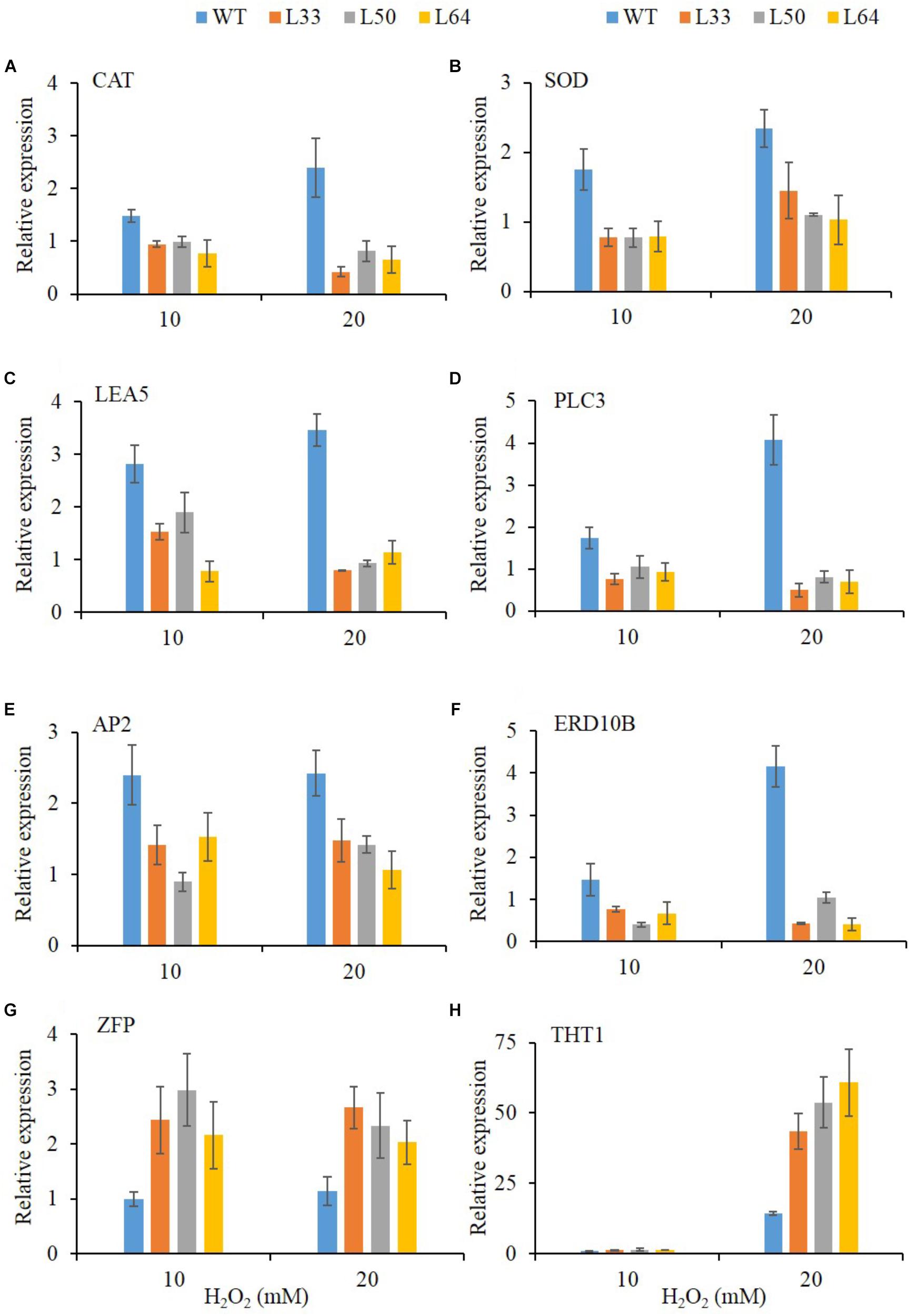
FIGURE 10. Relative-fold expression of downstream genes in AlNAC4 transgenics under 0, 10, and 20 mM H2O2 treatments by Real-Time PCR; (A) CAT, (B) SOD, (C) LEA5, (D) PLC3, (E) AP2, (F) ERD10B, (G) ZFP, and (H) THT1. Values are represented as mean ± SD (n = 3).
Discussion
The NAC superfamily is a largest TF family having multifunctional proteins and play important role in various biological processes and transcriptional regulatory networks. The functions of NACs have been extensively studied in glycophytes but limited studies exist in halophytes. The physiological, biochemical and proteomics studies of Aeluropus lagopoides have been carried out to understand its salinity tolerance mechanism (Gulzar and Khan, 2001; Mohsenzadeh et al., 2006; Sanadhya et al., 2015a,b; Sobhanian et al., 2010), however, limited attempts have been made toward isolation and characterization of its stress-responsive genes/TFs. The occurrence of NAC proteins remains restricted to land plants, largely to angiosperms but also includes moss (Physcomitrella patens), pteridophytes (Selaginella moellendorffii) and conifers (Shen et al., 2009). Here, we identified a NAC gene, AlNAC4, from a recretohalophyte Aeluropus lagopoides. The AlNAC4 contains a highly conserved NAC domain, which participates in DNA binding, while the C-terminal transcription activation region is highly variable and is considered to be involved in conferring specific response to different environmental stimuli. The NAC domain of AlNAC4 comprises of five sub-domains, [A–E; Figure 1A] similar to NAM (petunia no apical meristem), ATAF1/2 (Arabidopsis thaliana transcription activation factor) and CUC2 (Arabidopsis cup-shaped cotyledon) proteins (Souer et al., 1996; Aida et al., 1997). The subdomain A leads to formation of a functional dimer, divergent subdomains B and E provide functional diversity of NAC genes, whereas, the subdomains C and D with a highly conserved positively charged region are responsible for DNA binding (Ernst et al., 2004). The AlNAC4 showed the presence of phosphorylation, glycosylation and other post-translational modification sites. The presence of phosphorylation sites in AlNAC4 suggests the role of phosphorylation in regulating its activity. Phosphorylation is essential for nuclear localization of OsNAC4 (Kaneda et al., 2009), similarly, ATAF1 requires phosphorylation for its subcellular localization, DNA binding activity and protein interactions. Phosphorylation of ZmNAC84 by protein kinase regulates the antioxidant defense in maize (Zhu et al., 2016). The AlNAC4 protein belongs to SNAC group III of stress-responsive NAC genes, as it gets clustered with SNAC group evident from the phylogenetic tree.
The AlNAC4 showed transcript upregulation by dehydration stress and H2O2 exposure. Twenty SNAC genes are reported in rice, which are regulated by at least one stress (Fang et al., 2008), similarly, the transcript upregulation of ZmSNAC1, TaNAC4, TaNAC69-1 and TtNAMB-2 genes is reported by dehydration and salinity (Baloglu et al., 2012; Lu et al., 2012; Tang et al., 2012).
The AlNAC4 protein show binding to the NACRS of the erd1 promoter. Its promoter contains two discrete cis-acting elements viz. myc-like CATGTG and a 14 bp rps1 site involved in dehydration stress (Simpson et al., 2003). The cDNA of ANAC015, ANAC055, ANAC072 were isolated using bait from the erd1 promoter (Tran et al., 2004). The NACBS is also reported from several PR gene promoters (Seo et al., 2010). The flanking sequence of core binding site in the promoter of the downstream gene also participates in defining the specificity of binding of NAC TF. The ATAF1 protein binds to the promoters of ORE1 and GLK1 genes encoding chloroplast maintenance and senescence-promoting TFs, respectively, and promote activation of ORE1 and repression of GLK1 transcription (Garapati et al., 2015). NAC TF bind to a wide array of downstream genes to regulate varied biological functions.
The overexpression of AlNAC4 showed improved resistance toward oxidative stress in tobacco, however, no resistance was observed with salinity or dehydration stress. The low H2O2, (10 mM), concentration generated ROS, resulting in the participation of ROS as signaling molecules, and thereby, not much difference was observed in WT and transgenics. However, higher H2O2 concentration resulted in oxidative stress, whereby, the WT accumulated higher H2O2 as compared to transgenics during seedling (11-day-old) and 1-month-old stage. Similarly, no difference in germination rate and growth was observed among WT and transgenics at low H2O2, whereas, at higher concentration, the germination and growth of WT was inhibited significantly. The H2O2 treatment during seedling stage (11-day-old) was given in Milli-Q water, although it might be variable to stress imposed during natural environment. It is reported that slight increase of ROS serves as signal molecules to activate reversible signal transduction to allow adaptation, whereas, higher levels of ROS cause oxidative stress (Schieber and Chandel, 2014). The NAC TF ZmNTLs is reported to regulate ROS and provide stress tolerance (Wang et al., 2016). The SNAC3 and OsNAC2 provide abiotic stress tolerance by modulating the ROS levels, the OsNAC6 activates expression of peroxidases, involved in defense response toward oxidative stress (Nakashima et al., 2007; Fang et al., 2015; Shen et al., 2017). The ONAC022 confers tolerance against salinity and drought in rice by ABA signaling (Hong et al., 2016). Similarly, wheat TaNAC29 (Xu et al., 2015) and Thellungiella halophila STRESS RELATED NAC1 (TsNAC1, Liu et al., 2018) imparts tolerance to salinity in Arabidopsis. The LcNAC1 protein upregulates the expression of LcAOX1a gene, associated with ROS regulation and energy metabolism (Jiang et al., 2017). The H2O2-inducible JUNGBRUNNEN1 TF (JUB1, NAC TF), lowers the intracellular H2O2 levels by regulating different ROS responsive genes in Arabidopsis (Wu et al., 2012).
The elimination of excess ROS from plants protect cell and sub cellular systems from cytotoxic effect by involving ROS scavenging antioxidant systems (Mittler et al., 2004). The AlNAC4 transgenics showed low CAT and SOD activity at both low and high concentrations of H2O2, as compared to WT. In transgenics, the CAT activity was maximum at 3 h of treatment and consequently reduced with time, however, the WT plants did not show reduction with time. Hsieh et al. (2002) reported that increased catalase activity was associated with decreased H2O2 content in transgenic tomato overexpressing CBF1 TF. The H2O2 potential toward promoting seed germination of cereal plants such as barley, wheat and rice (Naredo et al., 1998) and Zinnia elegans (Ogawa and Iwabuchi, 2001) by removing the antioxidants inhibitors is reported. Interestingly, the maize ZmNAC84 is phosphorylated by H2O2 responsive CCaMK (calcium/calmodulin-dependent protein kinase) and this interaction promotes H2O2 amplification and further, regulating the antioxidant defense activity (Zhu et al., 2016).
The higher tolerance of transgenics is possible due to the regulation of the expression of the different downstream genes. The AlNAC4 transgenics showed lower expression of anti-oxidative genes (CAT, SOD), signal transduction proteins, dehydrins, and AP2 TF. The lower expression of CAT, SOD, PLC3, LEA5 and ERD10B in transgenic lines could be possible as these plants perceive less oxidative stress compared to WT plants by limiting the ROS generation. However, the AlNAC4 transgenics showed upregulation of ZFP and THT1 genes in presence of H2O2. The ZFP182 transient gene expression analysis in rice protoplast suggested its role in ABA-induced anti-oxidative defense (Zhang et al., 2012), also overexpression of AtRZFP enhanced tolerance to salt and osmotic stress with reduced ROS accumulation (Baek et al., 2015). THT1 (Hydroxycinnamoyl-CoA:tyramine N-hydroxycinnamoyl transferase) is an important enzyme involved in synthesis of HCAA (hydroxycinnamic acid amides). The HCAA show antioxidant property and is involved in plant development and defense response (Campos et al., 2014).
Conclusion
We conclude that AlNAC4 is an important stress-responsive gene, as evident by higher transcript accumulation under dehydration and oxidative stress conditions, and thus fall in the stress-related NAC III (SNACIII) group of largest NAC TF family. The binding of AlNAC4 recombinant protein with the EARLY RESPONSIVE TO DEHYDRATION STRESS 1 (erd1) promoter ascertain its potential in regulating downstream genes for modulating stress tolerance. AlNAC4 imparts enhanced oxidative stress tolerance via triggering the antioxidant pathways. The positive effect of AlNAC4 transgenics in promoting resistance to oxidative stresses implies its application in developing stress-tolerant crop plants.
Author Contributions
JK and PA performed the experiments. JK, PA, and PKA were involved in the analysis of data and manuscript writing. PKA and PA coordinated and designed the experiments. All the authors approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
CSIR-CSMCRI Communication No. 016/2017 (as provided by BDIM). The authors are thankful to Department of Science and Technology (DST) for providing the research grant (GAP 2001) and Council of Scientific and Industrial Research (CSIR), New Delhi, India for financial assistance. JK is thankful to CSIR for Junior and Senior research fellowship and also AcSIR. PA is thankful for DST WOS-A fellowship.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2018.01522/full#supplementary-material
FIGURE S1 | The sequence represents 936 bp cDNA of AlNAC4 and its amino acid sequence. The NAC domain is 21–174 amino acids. The sub-domains A-E are marked by colored arrows [sub-domain A-red, B-blue, C-green, D-pink, E-black]. The residues targeted for PKC and CKII phosphorylation are indicated with solid circled and dashed circled, respectively. The amidation sites (A) are indicated in boxes. The N-myristoylation sites (M) are underlined with solid lines and the N-glycosylation site (G) is underlined with a dotted line. The cAMP/cGMP dependent phosphorylation site (cAMP PS) is underlined by dashed bold line.
FIGURE S2 | Sequence logos of different NAC genes. The overall height of each stack indicates the conservation of the sequence at a particular position. The height of letters within each stack represents the relative frequency of the corresponding amino acid.
FIGURE S3 |(A) SDS-PAGE analysis of induced and uninduced AlNAC4 recombinant protein in E. coli BL21 (DE3) star cells. M marker, Lane 1, 3, 5, 7 induced protein with 2 h of vector alone, 2, 4, and 6 h of AlNAC4 culture. Lane 2, 4, 6, 8 uninduced protein with 2 h of vector alone, 2, 4, and 6 h of AlNAC4 culture. (B) Lanes PP represent purified recombinant protein and (C) Western blot (WB) of purified AlNAC4.
FIGURE S4 | The WT and T0 transgenic lines with (A) vegetative and (B) floral stages.
TABLE S1 | Primers sequence list.
TABLE S2 | Downstream genes and primer sequences used for expression analysis by Real-time PCR in transgenic tobacco plants.
References
Aida, M., Ishida, T., Fukaki, H., Fujisawa, H., and Tasaka, M. (1997). Genes involved in organ separation in Arabidopsis: an analysis of the cup-shaped cotyledon mutant. Plant Cell 9, 841–857. doi: 10.1105/tpc.9.6.841
Baek, D., Cha, J. Y., Kang, S., Park, B., Lee, H. J., Hong, H., et al. (2015). The Arabidopsis a zinc finger domain protein ARS1 is essential for seed germination and ROS homeostasis in response to ABA and oxidative stress. Front. Plant Sci. 6:963. doi: 10.3389/fpls.2015.00963
Bailey, T. L., Williams, N., Misleh, C., and Li, W. W. (2006). MEME: discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 34, 369–373. doi: 10.1093/nar/gkl198
Baloglu, M. C., Oz, M. T., Oktem, H. A., and Yucel, M. (2012). Expression analysis of TaNAC69-1 and TtNAMB-2, wheat NAC family transcription factor genes under abiotic stress conditions in durum wheat (Triticum turgidum). Plant Mol. Biol. Rep. 30, 1246–1252. doi: 10.1007/s11105-012-0445-3
Beauchamp, C., and Fridovich, I. (1971). Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 44, 276–287. doi: 10.1016/0003-2697(71)90370-8
Campos, L., Lisón, P., López-Gresa, M. P., Rodrigo, I., Zacarés, L., Conejero, V., et al. (2014). Transgenic tomato plants overexpressing tyramine N-hydroxycinnamoyltransferase exhibit elevated hydroxycinnamic acid amide levels and enhanced resistance to Pseudomonas syringae. MPMI 27, 1159–1169. doi: 10.1094/MPMI-04-14-0104-R
Dhindsa, R. S., Plumb-Dhindsa, P., and Thorpe, T. A. (1981). Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 32, 93–101. doi: 10.1093/jxb/32.1.93
Doyle, J. J., and Doyle, J. L. (1987). A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 19, 11–15.
Ernst, H. A., Olsen, A. N., Skriver, K., Larsen, S., and Leggio, L. L. (2004). Structure of the conserved domain of ANAC, a member of the NAC family of transcription factors. EMBO Rep. 5, 297–303. doi: 10.1038/sj.embor.7400093
Fang, Y., Liao, K., Du, H., Xu, Y., Song, H., Li, X., et al. (2015). A stress-responsive NAC transcription factor SNAC3 confers heat and drought tolerance through modulation of reactive oxygen species in rice. J. Exp. Bot. 66, 6803–6817. doi: 10.1093/jxb/erv386
Fang, Y., You, J., Xie, K., Xie, W., and Xiong, L. (2008). Systematic sequence analysis and identification of tissue-specific or stress-responsive genes of NAC transcription factor family in rice. Mol. Genet. Genomics 280, 547–563. doi: 10.1007/s00438-008-0386-6
Garapati, P., Xue, G.-P., Munné-Bosch, S., and Balazadeh, S. (2015). Transcription factor ATAF1 in Arabidopsis promotes senescence by direct regulation of key chloroplast maintenance and senescence transcriptional cascades. Plant Physiol. 168, 1122–1139. doi: 10.1104/pp.15.00567
Giannopolitis, C. N., and Ries, S. K. (1977). Superoxide dismutases: I. Occurrence in higher plants. Plant physiol. 59, 309–314. doi: 10.1104/pp.59.2.309
Gulzar, S., and Khan, M. A. (2001). Seed germination of a halophytic grass Aeluropus lagopoides. Ann. Bot. 87, 319–324. doi: 10.1006/anbo.2000.1336
Gulzar, S., Khan, M. A., and Ungar, I. A. (2003). Effects of salinity on growth, ionic content, and plant–water status of Aeluropus lagopoides. Commun. Soil Sci. Plant Anal. 34, 1657–1668. doi: 10.1081/CSS-120021303
Gupta, K., Agarwal, P. K., Reddy, M. K., and Jha, B. (2010). SbDREB2A, an A-2 type DREB transcription factor from extreme halophyte Salicornia brachiata confers abiotic stress tolerance in Escherichia coli. Plant Cell Rep. 29, 1131–1137. doi: 10.1007/s00299-010-0896-7
Hirayama, T., and Shinozaki, K. (2010). Research on plant abiotic stress responses in the post-genome era: past, present and future. Plant J. 61, 1041–1052. doi: 10.1111/j.1365-313X.2010.04124.x
Hoagland, D. R., and Arnon, D. I. (1950). The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 347:32.
Hong, Y., Zhang, H., Huang, L., Li, D., and Song, F. (2016). Overexpression of a stress-responsive NAC transcription factor gene ONAC022 improves drought and salt tolerance in rice. Front. Plant Sci. 7:4. doi: 10.3389/fpls.2016.00004
Horsch, R. B., Fry, J. E., Hoffmann, N. L., Eichholtz, D., Rogers, S. G., and Farley, R. T. (1985). A simple and general method for transferring genes into plants. Science 227, 1229–1231. doi: 10.1126/science.227.4691.1229
Hsieh, T. H., Lee, J. T., Charng, Y. Y., and Chan, M. (2002). Tomato plants ectopically expressing Arabidopsis CBF1 show enhanced resistance to water deficit stress. Plant Physiol. 130, 618–626. doi: 10.1104/pp.006783
Jiang, G., Yan, H., Wu, F., Zhang, D., Zeng, W., Qu, H., et al. (2017). Litchi fruit LcNAC1 is a target of LcMYC2 and regulator of fruit senescence through its interaction with LcWRKY1. Plant Cell Physiol. 58, 1075–1089. doi: 10.1093/pcp/pcx054
Kaneda, T., Taga, Y., Takai, R., Iwano, M., Matsui, H., Takayama, S., et al. (2009). The transcription factor OsNAC4 is a key positive regulator of plant hypersensitive cell death. EMBO J. 4, 740–742. doi: 10.1038/emboj.2009.39
Liu, C., Wang, B., Li, Z., Peng, Z., and Zhang, J. (2018). TsNAC1 is a key transcription factor in abiotic stress resistance and growth. Plant Physiol. 176, 742–756. doi: 10.1104/pp.17.01089
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT Methods. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Lu, M., Ying, S., Zhang, D. F., Shi, Y. S., Song, Y. C., Wang, T. Y., et al. (2012). A maize stress-responsive NAC transcription factor, ZmSNAC1, confers enhanced tolerance to dehydration in transgenic Arabidopsis. Plant Cell Rep. 31, 1701–1711. doi: 10.1007/s00299-012-1284-2
Ma, Q., Dai, X., Xu, Y., Guo, J., Liu, Y., Chen, N., et al. (2009). Enhanced tolerance to chilling stress in osmyb3r-2 transgenic rice is mediated by alteration in cell cycle and ectopic expression of stress genes. Plant Physiol. 150, 244–256. doi: 10.1104/pp.108.133454
Miao, M., Niu, X., Kud, J., Du, X., Avila, J., Devarenne, T. P., et al. (2016). The ubiquitin ligase SEVEN IN ABSENTIA (SINA) ubiquitinates a defense-related NAC transcription factor and is involved in defense signaling. New Phytol. 211, 138–148. doi: 10.1111/nph.13890
Mittler, R., Vanderauwera, S., Gollery, M., and Van Breusegem, F. (2004). Reactive oxygen gene network of plants. Trends Plant Sci. 9, 490–498. doi: 10.1016/j.tplants.2004.08.009
Mohsenzadeh, S., Malboobi, M. A., Razavi, K., and Farrahi-Aschtiani, S. (2006). Physiological and molecular responses of Aeluropus lagopoides (Poaceae) to water deficit. Environ. Exp. Bot. 56, 314–322. doi: 10.1016/j.envexpbot.2005.03.008
Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 15, 473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x
Nakashima, K., Tran, L. S. P., Van Nguyen, D., Fujita, M., Maruyama, K., Todaka, D., et al. (2007). Functional analysis of a NAC-type transcription factor OsNAC6 involved in abiotic and biotic stress-responsive gene expression in rice. Plant J. 51, 617–630. doi: 10.1111/j.1365-313X.2007.03168.x
Naredo, M. E. B., Juliano, A. B., Lu, B. R., De Guzman, F., and Jackson, M. T. (1998). Responses to seed dormancy-breaking treatments in rice species (Oryza L.). Seed Sci. Technol. 26, 675–689.
Nuruzzaman, M., Sharoni, A. M., and Kikuchi, S. (2013). Roles of NAC transcription factors in the regulation of biotic and abiotic stress responses in plants. Front. Microbiol. 4:248. doi: 10.3389/fmicb.2013.00248
Ogawa, K. I., and Iwabuchi, M. (2001). A mechanism for promoting the germination of Zinnia elegans seeds by hydrogen peroxide. Plant Cell Physiol. 42, 286–291. doi: 10.1093/pcp/pce032
Olsen, A. N., Ernst, H. A., Leggio, L. L., and Skriver, K. (2005). DNA-binding specificity and molecular functions of NAC transcription factors. Plant Sci. 169, 785–797. doi: 10.1016/j.plantsci.2005.05.035
Ooka, H., Satoh, K., Doji, K., Nagata, T., Otomo, Y., Murakami, K., et al. (2003). Comprehensive Analysis of NAC family genes in Oryza sativa and Arabidopsis thaliana. DNA Res. 247, 239–247. doi: 10.1093/dnares/10.6.239
Pyvovarenko, T., and Lopato, S. (2011). Isolation of plant transcription factors using a yeast one-hybrid system. Methods Protoc. 754, 45–66.
Sanadhya, P., Agarwal, P., and Agarwal, P. K. (2015a). Ion homeostasis in a salt-secreting halophtic grass. AoB Plants 7:plv055. doi: 10.1093/aobpla/plv055
Sanadhya, P., Agarwal, P., Khedia, J., and Agarwal, P. K. (2015b). A low-affinity K + transporter AlHKT2;1 from recretohalophyte Aeluropus lagopoides confers salt tolerance in yeast. Mol. Biotechnol. 57, 489–498. doi: 10.1007/s12033-015-9842-9
Schieber, M., and Chandel, N. S. (2014). ROS function in redox signaling and oxidative stress. Curr. Biol. 24, R453–R462. doi: 10.1016/j.cub.2014.03.034
Seo, P. J., Kim, M. J., Song, J. S., Kim, Y. S., Kim, H. J., and Park, C. M. (2010). Proteolytic processing of an Arabidopsis membrane-bound NAC transcription factor is triggered by cold-induced changes in membrane fluidity. Biochem. J. 367, 359–367. doi: 10.1042/BJ20091762
Seo, P. J., Kim, S. G., and Park, C. M. (2008). Membrane-bound transcription factors in plants. Trends Plant Sci. 13, 550–556. doi: 10.1016/j.tplants.2008.06.008
Shen, H., Yin, Y., Chen, F., Xu, Y., and Dixon, R. A. (2009). A bioinformatic analysis of NAC genes for plant cell wall development in relation to lignocellulosic bioenergy production. Bioenergy Res. 2, 217–232. doi: 10.1007/s12155-009-9047-9
Shen, J., Lv, B., Luo, L., He, J., Mao, C., Xi, D., et al. (2017). The NAC-type transcription factor OsNAC2 regulates ABA-dependent genes and abiotic stress tolerance in rice. Sci. Rep. 7:40641. doi: 10.1038/srep40641
Shepherd, C. T., Lauter, A. N. M., and Scott, M. P. (2009). Determination of transgene copy number by real-time quantitative PCR. Methods Mol. Biol. 526, 129–134. doi: 10.1007/978-1-59745-494-0_11
Shiu, S. H., Shih, M. C., and Li, W. H. (2005). Transcription factor families have much higher expansion rates in plants than in animals. Plant Physiol. 139, 18–26. doi: 10.1104/pp.105.065110
Shukla, P. S., Agarwal, P., Gupta, K., and Agarwal, P. K. (2015). Molecular characterization of a MYB transcription factor from a succulent halophyte involved in stress tolerance. AoB Plants 7:plv054. doi: 10.1093/aobpla/plv054
Shukla, P. S., Agarwal, P. K., and Jha, B. (2012). Improved salinity tolerance of Arachis hypogaea (L.) by the interaction of halotolerant plant-growth-promoting rhizobacteria. J Plant Growth Regul. 31, 195–206. doi: 10.1007/s00344-011-9231-y
Simpson, S. D., Nakashima, K., Narusaka, Y., Seki, M., Shinozaki, K., and Yamaguchi-Shinozaki, K. (2003). Two different novel cis-acting elements of erd1, a clpA homologous Arabidopsis gene function in induction by dehydration stress and dark-induced senescence. Plant J. 33, 259–270. doi: 10.1046/j.1365-313X.2003.01624.x
Sobhanian, H., Motamed, N., Jazii, F. R., Razavi, K., Niknam, V., and Komatsu, S. (2010). Salt stress responses of a halophytic grass Aeluropus lagopoides and subsequent recovery. Russ. J. Plant Physiol. 57, 784–791. doi: 10.1134/S1021443710060063
Souer, E., Houwelingen, A. V., Kloos, D., Mol, J., and Koes, R. (1996). The no apical meristem gene of petunia is required for pattern formation in embryos and flowers and is expressed at meristem and primordia boundaries. Cell 85, 159–170. doi: 10.1016/S0092-8674(00)81093-4
Tang, Y., Liu, M., Gao, S., Zhang, Z., Zhao, X., Zhao, C., et al. (2012). Molecular characterization of novel TaNAC genes in wheat and overexpression of TaNAC2a confers drought tolerance in tobacco. Physiol. Plant. 144, 210–224. doi: 10.1111/j.1399-3054.2011.01539.x
Tran, L. S. P., Nakashima, K., Sakuma, Y., Simpson, S. D., Fujita, Y., Maruyama, K., et al. (2004). Isolation and functional analysis of Arabidopsis stress-inducible NAC transcription factors that bind to a drought-responsive cis-element in the early responsive to dehydration stress 1 promoter. Plant Cell 16, 2481–2498. doi: 10.1105/tpc.104.022699
Wang, D., Yu, Y., Liu, Z., Li, S., Wang, Z., and Xiang, F. (2016). Membrane-bound NAC transcription factors in maize and their contribution to the oxidative stress response. Plant Sci. 250, 30–39. doi: 10.1016/j.plantsci.2016.05.019
Wu, A., Allu, A. D., Garapati, P., Siddiqui, H., Dortay, H., Zanor, M. I., et al. (2012). JUNGBRUNNEN1, a reactive oxygen species–responsive NAC transcription factor, regulates longevity in Arabidopsis. Plant Cell 24, 482–506. doi: 10.1105/tpc.111.090894
Xu, Z., Wang, C., Xue, F., Zhang, H., and Ji, W. (2015). Wheat NAC transcription factor TaNAC29 is involved in response to salt stress. Plant Physiol. Biochem. 96, 356–363. doi: 10.1016/j.plaphy.2015.08.013
Zhang, H., Jin, J., Tang, L., Zhao, Y., Gu, X., Gao, G., et al. (2011). Plant TFDB 2.0: update and improvement of the comprehensive plant transcription factor database. Nucleic Acids Res. 39, 1114–1117. doi: 10.1093/nar/gkq1141
Zhang, H., Ni, L., Liu, Y., Wang, Y., Zhang, A., Tan, M., et al. (2012). The C2H2-type zinc finger protein ZFP182 is involved in abscisic acid-induced antioxidant defense in rice. J. Integr. Plant Biol. 54, 500–510. doi: 10.1111/j.1744-7909.2012.01135.x
Keywords: abiotic stress tolerance, Aeluropus lagopoides, AlNAC4, oxidative stress, transcription factors
Citation: Khedia J, Agarwal P and Agarwal PK (2018) AlNAC4 Transcription Factor From Halophyte Aeluropus lagopoides Mitigates Oxidative Stress by Maintaining ROS Homeostasis in Transgenic Tobacco. Front. Plant Sci. 9:1522. doi: 10.3389/fpls.2018.01522
Received: 06 February 2018; Accepted: 27 September 2018;
Published: 29 October 2018.
Edited by:
Agnieszka Ludwików, Adam Mickiewicz University in Poznań, PolandReviewed by:
Agnieszka Kiełbowicz-Matuk, Institute of Plant Genetics (PAN), PolandLiang Chen, University of Chinese Academy of Sciences (UCAS), China
Taras P. Pasternak, Albert-Ludwigs-Universität Freiburg, Germany
Copyright © 2018 Khedia, Agarwal and Agarwal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Parinita Agarwal, parinitaa@csmcri.org; parinitaa@csmcri.res.in Pradeep K. Agarwal, pagarwal@csmcri.org; pagarwal@csmcri.res.in
 Jackson Khedia1,2
Jackson Khedia1,2 Parinita Agarwal
Parinita Agarwal Pradeep K. Agarwal
Pradeep K. Agarwal