- Department of Dermatology, Incheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea
The interplay between the microbes and the skin barrier holds pivotal significance in skin health and aging. The skin and gut, both of which are critical immune and neuroendocrine system, harbor microbes that are kept in balance. Microbial shifts are seen with aging and may accelerate age-related skin changes. This comprehensive review investigates the intricate connection between microbe dynamics, skin barrier, and the aging process. The gut microbe plays essential roles in the human body, safeguarding the host, modulating metabolism, and shaping immunity. Aging can perturb the gut microbiome which in turn accentuates inflammaging by further promoting senescent cell accumulation and compromising the host’s immune response. Skin microbiota diligently upholds the epidermal barrier, adeptly fending off pathogens. The aging skin encompasses alterations in the stratum corneum structure and lipid content, which negatively impact the skin’s barrier function with decreased moisture retention and increased vulnerability to infection. Efficacious restoration of the skin barrier and dysbiosis with strategic integration of acidic cleansers, emollients with optimal lipid composition, antioxidants, and judicious photoprotection may be a proactive approach to aging. Furthermore, modulation of the gut-skin axis through probiotics, prebiotics, and postbiotics emerges as a promising avenue to enhance skin health as studies have substantiated their efficacy in enhancing hydration, reducing wrinkles, and fortifying barrier integrity. In summary, the intricate interplay between microbes and skin barrier function is intrinsically woven into the tapestry of aging. Sound understanding of these interactions, coupled with strategic interventions aimed at recalibrating the microbiota and barrier equilibrium, holds the potential to ameliorate skin aging. Further in-depth studies are necessary to better understand skin-aging and develop targeted strategies for successful aging.
1 Introduction
Aging is a gradual deterioration of bodily functions as one grows older (Kirkwood and Austad, 2000). It is an inevitable biological process that affects all living organisms, including humans (Ostojić et al., 2009). While aging is complex and individualized, research suggests that inflammation may be a strong contributor. In fact, older adults tend to develop a pro-inflammatory status which is fittingly called inflammaging (Franceschi et al., 2000). Potential factors contributing to inflammaging encompass genetic preposition, enhanced gut permeability, central obesity, alteration in microbial composition, oxidative stress, and cellular senescence (Ferrucci and Fabbri, 2018).
As one age, the skin undergoes numerous changes. A key feature of the aging skin is decline in its barrier function, with decreased ability to protect the body from external aggressors, maintain hydration, and preserve the overall skin integrity (Forslind et al., 1997). Over the years, extensive research has shed light on the significance of the skin microbiota as an integral part of the skin barrier and its interaction with other components of the skin barrier such as the keratinocytes, immune cells and the nerve (Baldwin et al., 2017; Kim and Yosipovitch, 2020; Harris-Tryon and Grice, 2022; Lee and Kim, 2022).
The skin surface is the habitat of a diverse community of microorganisms, collectively known as the skin microbiota. These microbes establish a complex interaction with the skin cells and contribute to its defense mechanisms (Belkaid and Segre, 2014). Grasping the intricate interplay between the skin microbiota and the skin barrier is of paramount importance in pursuit of effective skincare strategies and interventions addressing the aging skin. The gut microbiome is recognized for its substantial influence on the skin’s overall balance by modulating the skin barrier (Lee and Kim, 2022).
This comprehensive review thoroughly explores the intricate interplay between the human microbiota (i.e., skin, gut) and skin barrier function during the aging process. We will examine the pattern of dysbiosis with advancing age, analyze the mechanisms by which these microbial communities impact the skin’s overall health, and explore potential therapeutic approaches to fortify the microbes and skin barrier function. By synthesizing the latest findings from a multitude of studies, this review seeks to offer valuable insights into the power of the microbes in supporting and maintaining the integrity of the skin barrier as we age.
2 Microbial alteration and skin aging
Both the skin and gut play intricate roles as an immune and neuroendocrine system, regularly interacting with the external environment and harboring diverse microbes (Salem et al., 2018). Their proper functioning is essential for organisms to maintain balance and survive (Salem et al., 2018). Notably, the skin, being the largest organ, acts as a protective barrier against injuries and microbial attacks (Salem et al., 2018). The gut also contains trillions of microbial communities which contribute to host’s health and longevity (Ellis et al., 2019; ONeill et al., 2016; Mohajeri et al., 2018).
2.1 Gut microbes and skin aging
The gut microbes play three crucial roles starting from birth, which are safeguarding the host, carrying out metabolic activities, and contributing to the development and regulation of the immune system (Renz et al., 2012). However, the composition of the gut microbe can be altered by a range of factors, including the individual’s lifestyle, use of antibiotics, and degree of inflammation (Álvarez et al., 2021). Gut dysbiosis is associated with increased intestinal permeability, diminished nutrient absorption, and dysregulated immune response which are detrimental to the host (Myers and Hawrelak, 2004; Tomasello et al., 2016; Takiishi et al., 2017).
The function and composition of the gut microbiota also change with age (Lephart and Naftolin, 2022). In the elderly population, alteration of the gut microbial community is evident, characterized by a decrease in microbial diversity (Sekirov et al., 2010; Kho and Lal, 2018). However, studies have yielded conflicting results in terms of the composition of intestinal microbiota in older adults. Some reported a decline in Bifidobacteria and Lactobacilli, coupled with an increase in facultative anaerobes and Bacteroidetes among older individuals (Hopkins et al., 2001; Claesson et al., 2011), whereas another group indicated higher levels of Ruminococcus, Bifidobacterium, and Eubacterium in the elderly compared to their younger counterparts (He et al., 2003). One study also revealed significant reduction in Firmicutes and an increase in Bacteroidetes across adulthood, indicating a decline in the Firmicutes-to-Bacteroidetes (F/B) ratio in old age (Vaiserman et al., 2020). Notably, the F/B ratio plays a pivotal role in the generation of short-chain fatty acids (SCFAs), which is a crucial component in maintaining health beyond the gut (Vaiserman et al., 2020). Based on the finding, age-related gut dysbiosis is believed to accelerate aging, inflammation, and frailty, thereby compromising the host’s overall health and longevity (Vaiserman et al., 2020).
Many proposed and have identified that the variation of the gut microbiota is not attributed solely to chronological age but is associated with a broader decline in the overall health status (i.e., fragility) (Jackson et al., 2016; Jeffery et al., 2016; Ratanapokasatit et al., 2022). To identify the link between cellular senescence and microbial imbalance, a recent study examined the microbial composition in mice models of cellular senescence. Here, scientists specifically focused on gut microbiome signatures associated with cellular senescence markers and senescence-associated secretory phenotype (SASP), which encompass inflammatory factors (Saccon et al., 2021). While Clostridiales, Staphylococcus, and Lachnospiraceae were found to have positive correlation with cellular senescence and inflammatory markers, Akkermansia and Coriobacteriaceae, showed negative correlation (Saccon et al., 2021).
Gut dysbiosis associated with senescence results in a “leaky gut” condition (where the gut mucosa becomes more permeable due to disruptions in tight junctions), permitting periodic, small-scale movement of bacterial components into the systemic circulation (Ratanapokasatit et al., 2022). Bacterial antigens, particularly lipopolysaccharide, trigger the release of pro-inflammatory cytokines like, IL-1β, TNFα, and IL-6. It is theorized that extended exposure to these bacterial antigens may lead to cell aging and immune system aging, both contributing to a persistent, low-level systemic inflammation (Biragyn and Ferrucci, 2018; Ratanapokasatit et al., 2022). This combination of inflammation and aging (inflammaging) is thought to be the basis of immune dysregulation and dysbiosis of the skin (Ratanapokasatit et al., 2022).
The human intestine harbors a wide array of microbes that have a crucial role in upholding the balance between the gut and skin. As such, disruptions of the gut-skin axis can potentially contribute to the emergence of skin problems. Various skin conditions, including acne, atopic dermatitis, psoriasis, and rosacea, have been linked with gut dysbiosis (Petersen et al., 2019; Sikora et al., 2020; Tutka et al., 2020; Siddiqui et al., 2022). Intestinal microbes are thought to affect the skin barrier directly through their metabolic products and indirectly via their impact on the innate and adaptive immune system (Salem et al., 2018; ONeill et al., 2016; De Pessemier et al., 2021). For instance, the gut microbes are known to provide skin stability by regulating T-cell differentiation (Salem et al., 2018).
2.2 Skin microbes and skin aging
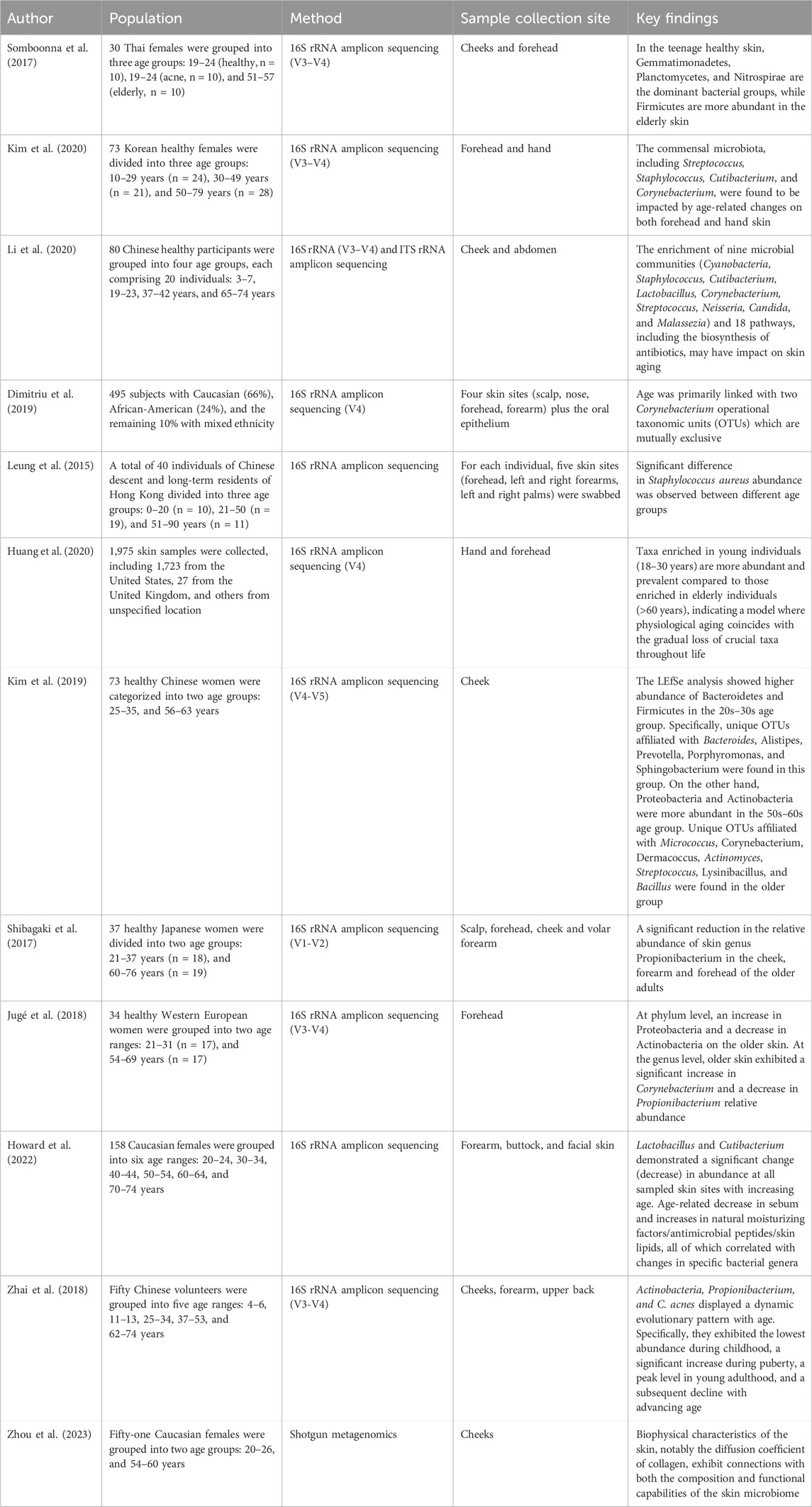
Besides the gut, studies have recognized the involvement of the skin microbiome in various dermatological conditions such as acne, eczema, rosacea, prurigo nodularis, and skin cancer (Dekio et al., 2007; Murillo and Raoult, 2013; Kim and Kim, 2019; Kim, 2020; Tutka et al., 2020; Woo et al., 2022; Kim et al., 2023). Naturally, there is also a growing interest in the association between the skin microbiota and the aging process. Studies have explored how the skin microbes change across age which offer us insight to age-related microbial shift (Table 1). Alpha diversity was increased in older individuals which was consistent over the studies (Shibagaki et al., 2017; Somboonna et al., 2017; Zhai et al., 2018; Kim et al., 2019; Li et al., 2020; Howard et al., 2022). Overall, the four main phyla on the skin were Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria (Leung et al., 2015; Shibagaki et al., 2017; Jugé et al., 2018; Kim et al., 2019).
A Japanese cohort revealed bacterial species difference between younger adults (21–37 years old) and older adults (60–76 years old) at multiple skin sites (Shibagaki et al., 2017). Actinobacteria showed lower abundance in the older age group’s cheek, forearm, and forehead, while the other three phyla increased (Shibagaki et al., 2017). Actinobacteria was most significantly reduced on the forearm (Shibagaki et al., 2017). The elderly had higher levels of Proteobacteria on the forearm and scalp, and an increase in Firmicutes and Bacteroidetes on the forehead and cheek (Shibagaki et al., 2017). At the genus level, the older population showed a significant increase in Acinetobacter on the scalp and Corynebacterium on the cheeks and forehead, while having significant decrease in Cutibacterium on the forehead, cheeks, and forearms (Shibagaki et al., 2017). Furthermore, the presence of Staphylococcus on the forearm was notably lower in the older age group compared to younger adults (Shibagaki et al., 2017).
A study conducted in West European women found that older individuals (54–69 years old) exhibited a significant decrease in the proportion of Actinobacteria compared to younger individuals (21–31 years old) (mean abundance: older 46.7% vs. younger 59.2%) (Jugé et al., 2018). Conversely, the phylum Proteobacteria showed a significantly higher abundance in the older age group compared to the younger age group (mean abundance: older 13.9% vs. younger 3.5%) (Jugé et al., 2018).
A North American study found that both chronological age and skin aging is best predicted by 2 mutually co-excluding Corynebacterial operational taxonomic units (OTUs), Corynebacterium kroppenstedtii and Corynebacterium amycolatum (Dimitriu et al., 2019). West European women exhibited higher alpha diversity in the older age group, with a decrease in Acinetobacter and Cutibacterium and an increase in Proteobacteria and Corynebacterium (Jugé et al., 2018).
Findings from the Chinese cohort study was different from others, where the abundance of Bacteroidetes and Firmicutes was significantly higher in the 20s–30 s age group (Kim et al., 2019). Within the Bacteroidetes phylum, OTUs associated with Bacteroides, Alistipes, Prevotella, Porphyromonas, and Sphingobacterium were exclusively identified in the younger age group (Kim et al., 2019). Similarly, within the Firmicutes phylum, specific OTUs attributed to Aerococcus, Lactobacillus, Oscillospira, and Ruminococcus were observed solely in the 20s–30 s age group (Kim et al., 2019). On the contrary, the Proteobacteria and Actinobacteria were more abundant in the 50s–60 s age group (Kim et al., 2019). Among the Actinobacteria-related OTUs, those specific to Micrococcus, Corynebacterium, Dermacoccus, and Actinomyces were exclusively present in the older group (Kim et al., 2019). Moreover, within the Firmicutes phylum, OTUs primarily belonging to Streptococcus, Lysinibacillus, and Bacillus predominated in the 50s–60s age group (Kim et al., 2019).
Howard et al. (Howard et al., 2022) reported a decrease in Cutibacterium and Lactobacillus across different body sites including the forearm, face, and buttock in Caucasian women (Howard et al., 2022). There was an increase in Corynebacterium and Enhydrobacter on the elderly face (Howard et al., 2022), whereas on the elderly buttocks, Streptococcus and Anaerococcus increased, while Staphylococcus decreased (Howard et al., 2022). The elderly forearm showed an increase in Anaerococcus, Enhydrobacter, and Acinetobacter, and a decrease in Methylobacterium-Methylorubrum (Howard et al., 2022).
The researches have shown noteworthy changes in the skin microbiota composition of the elderly, characterized by a reduction in Cutibacterium, accompanied by an increase in Corynebacterium, and Proteobacteria. This shift raises questions about its potential impact on the prevalence of specific skin disorders in this demographic. Studies have suggested an increased incidence of erythrasma, with increased abundance of Corynebacterium species in the elderly (Zarrin et al., 2010; Polat and Nilhan, 2015; Salemi et al., 2022). These findings suggest a direct connection between age-related shifts in skin microbiota and the increased incidence of specific dermatological conditions, underscoring the importance of microbiota composition in skin health and disease manifestation in the elderly.
With regards to the fungal microbiota of the skin, a study was conducted on 80 Chinese participants across four age groups, (Kirkwood and Austad, 2000), 3–7 years, (Ostojić et al., 2009), 19–23 years, (Franceschi et al., 2000), 37–42 years (middle-aged), and (Ferrucci and Fabbri, 2018) 65–74 years (eldery) (Li et al., 2020). The dominant fungal phyla in all four age groups were Ascomycota and Basidiomycota (Li et al., 2020). When categorized into photoaged (cheek) and intrinsically aged (abdomen) skin sites, the relative abundance of Malassezia on the cheeks of the middle-aged and elder groups was greater in the middle-aged and elderly groups (Li et al., 2020).
Age-related changes in skin microenvironment influence skin microbial composition. In aged skin, there is a size reduction of small blood vessels, sebaceous glands, and sweat glands, as well as a decrease in nutrient supply (Hurlow and Bliss, 2011; Kianmehr et al., 2022). Howard et al. (Howard et al., 2022) found a notable reduction in facial sebaceous glands in the elderly, using histomorphometry analysis. A positive correlation (Cutibacterium) and a negative correlation (Streptococcus, Acinetobacter, Enhydrobacter, Corynebacterium, Methylobacterium‒Methylorubrum) of skin microbiota with sebum were identified through correlation analysis (Howard et al., 2022). During the aging process, the skin pH tends to rise, rendering it to be more alkaline (Zlotogorski, 1987; Thune et al., 1988; Farage et al., 2008). This shift creates a more conducive environment for certain pathogenic microorganisms (Howard et al., 2022), which can be detrimental to skin health. Overall in aged skin, the skin becomes more dry and alkaline, which leads to a decrease in once dominant microorganisms such Cutibacterium and Staphylococcus (Zhai et al., 2018), and a simultaneous increase in the less abundant microbiota (Zhai et al., 2018).
Skin lipids produced by epidermal cells (i.e., ceramides, free fatty acids, cholesterol) have a potential to act as a viable nutrient for bacteria. One particular study found an age-related increase of 8 types of ceramide, which positively correlated with the abundance of Streptococcus (Howard et al., 2022). While this suggests a possible influence of these specific ceramides on Streptococcus abundance, it is also plausible that Streptococcus might impact the production of these skin ceramides, as Streptococcus was shown to elevate ceramide levels in the stratum corneum (SC), particularly in aged individuals (Di Marzio et al., 1999).
As the integrity of the skin barrier falls with aging, its capacity to retain moisture diminish, resulting in compensatory elevation of natural moisturizing factors(NMFs) (Woolery-Lloyd et al., 2023). NMFs play a dual role in absorbing water and facilitating bacterial proliferation and adherence to the skin (Miajlovic et al., 2010; Feuillie et al., 2018), which explains the positive correlation between most bacterial genera and NMFs in advanced age (Howard et al., 2022). Specifically, NMFs have been linked with the heightened presence Corynebacterium, Micrococcus, Streptococcus, and Anaerococcus, and a reduction in Cutibacterium (Kim et al., 2019; Howard et al., 2022), with expansion in overall diversity (Shibagaki et al., 2017; Somboonna et al., 2017; Zhai et al., 2018; Kim et al., 2019; Li et al., 2020; Howard et al., 2022). However, the relationship between individual bacteria and NMFs requires further investigation.
Two antimicrobial peptides (AMPs), lysozyme, and RNAse7 were also shown to increase with age in both combined and individual skin-site data, except for lysozyme at the buttocks (Howard et al., 2022). Correlation analysis revealed that some bacterial genera positively correlate with AMPs, while others exhibit negative correlation. Both the modulation of host AMP by cutaneous microbes and the host’s ability to influence skin microbes through AMP production have been reported in numerous studies (Brogden et al., 2012; Gallo and Hooper, 2012).
The direct damage of cellular DNA, RNA, and proteins by UV radiation (UVR), results in photoaging. UVR also stimulates intracellular reactive oxygen species (ROS) production, which further inflicts damage upon DNA and contributes to the aging process (Teng et al., 2022). Furthermore, UVR triggers pathways associated with mitochondrial dysfunction, photooxidative stress, inflammatory cascades, and immune suppression within the skin, which accelerate skin aging (Teng et al., 2022). Preliminary evidence suggests that aging may induce changes in the composition of the skin microbiota (Li et al., 2020) with a decrease in those (i.e., Cyanobacteria) with a protective mechanism against UVR.
Alteration in skin pH, NMFs, AMPs, and skin lipids across age and their correlation with specific bacterial genera explain age-related changes in the composition of the cutaneous microbiota. However, the field of skin microbial research is relatively nascent, and contribution of this microbial shift to aging remains largely unknown. As microbes show distinct skin-site specificity, further studies are warranted to understand the true role of the skin microbiome in the complex phenomenon of skin aging.
3 Skin barrier alteration and skin aging
Structural alterations in aged skin stem from the cumulative impact of a multitude of factors (Fisher et al., 2002). Skin aging is classified into two types, where intrinsic aging, also known as chronological aging, primarily involves functional changes with lesser degree of morphological alteration (Fisher et al., 2002). On the other hand, extrinsic aging, also referred to as photoaging, results from chronic sun exposure and is characterized by structural and physiological changes (Fisher et al., 2002).
In the elderly, the integrity of the SC is compromised, and its ability to recover after acute disturbance, such as tape stripping, is delayed compared to younger individuals (Ghadially et al., 1995; Choi et al., 2007; Choi, 2019). Preserving ideal hydration of the SC is a key role of the epidermis, relying on multiple elements. This involves the layered arrangement of SC intercellular lipids that retain water in corneocytes, the quantity of NMFs—a diverse blend of low molecular-weight substances formed in corneocytes through filaggrin breakdown, and the glycerol content in the SC. In the aged epidermis, a number of factors are compromised, resulting in reduced SC hydration (i.e., dry skin) which further weakens the epidermal barrier function (Verdier-Sévrain and Bonté, 2007; White-Chu and Reddy, 2011; Choi, 2019).
The epidermal calcium gradient, which plays a crucial role in various epidermal functions, undergoes significant change with aging, leading to an abnormally broad distribution of calcium in the epidermis (Forslind et al., 1999). In the elderly patients, calcium ions were found to be distributed throughout the epidermis, with a shallow epidermal calcium gradient (Denda et al., 2003). The loss of the epidermal calcium gradient in aged skin may be attributed to a reduced number of ion channels, ion pumps, or ionotropic receptors in this population (Denda et al., 2001). Epidermal calcium gradient flattening in aged skin adversely affects the epidermal permeability barrier by hindering the delivery of lamella bodies to the SC, with a decrease in extracellular lipid bilayers (Choi, 2019).
With regards to epidermal lipids, aged skin show significant reduction in all 3 major lipid components: ceramide, cholesterol, and fatty acids (Ghadially et al., 1995). The structural anomalies observed in the SC intercellular lipid membrane in aged skin, is due to a global decline in SC lipids with around one-third less lipid by weight percentage compared to young skin (Choi, 2019). Moreover, specific changes are observed in SC ceramides, such as a decline in ceramide 2 (N-lingocerylsphingosine), and concurrent increase in ceramide 3 (nonhydroxy N-acyl fatty acid and phytosphingosine) within the aged epidermis (Denda et al., 1993). The molecular basis for these age-related findings have been partially explored. Possible mechanisms include aberrations in sterol regulatory element binding proteins, which regulate cholesterol and fatty acid synthesis, and disruption in the signaling pathways that regulate the epidermal lipid metabolic pathway (Gilhar et al., 1992; Harris et al., 1998).
Disruption of the barrier triggers the production of epidermal cytokines as a homeostatic signal response to start the process of barrier restoration (Choi, 2019). Acute treatment with cytokines helps improve epidermal barrier function and accelerate permeability barrier reformation (Choi, 2019). However, in aged skin, the repair mechanisms might be impaired, leading to the prolonged persistence of barrier disruption (Choi, 2019). This in turn may result in persistent upregulation of proinflammatory cytokines and chronic inflammation in the skin. Hu et al. (2017) identified elevated cytokine levels (proinflammatory cytokines TNF-α, IL-1α, IL-1β, and IL-6) in both the serum and skin of aged mice and showed that daily rehydration of the epidermis (i.e., barrier improvement) normalizes age-associated increase in systemic cytokine levels. The findings suggest that epidermal-derived signals play an important role in the rise of circulating inflammatory cytokines in aged mice. Aging in humans is also accompanied by increased inflammation (Franceschi et al., 2000; Brodin and Davis, 2017) and it is likely that improving the epidermal barrier too would reduce inflammaging in humans (Velarde, 2017).
As we age, the functionality of the immune system (i.e., immune barrier) undergoes a gradual decline (Simon et al., 2015; Brodin and Davis, 2017; Feuillie et al., 2018). Within the skin, the presence of Langerhans cells in the epidermis gradually diminishes (Hasegawa et al., 2020). Additionally, cutaneous dendritic cells, along with the remaining Langerhans cells, demonstrate impaired migratory capacity to lymph nodes and compromised antigen presentation to T-cells (Grolleau-Julius et al., 2008; Pilkington et al., 2018). Consequently, this perturbed antigen presentation, combined with systemic and local deficiencies in immune signaling, ultimately lead to continuing inflammation with accelerated aging (Chambers and Vukmanovic-Stejic, 2020).
4 The multifaceted role of microbiota in enhancing the skin barrier & attenuating skin aging
The skin barrier may be conceptualized as 4 intertwining factors consisting of microbial, immune, chemical, and physical barrier which are tightly orchestrated by the commensal microbiota (Eyerich et al., 2018). The fundamental mechanism whereby the microbiota regulate skin barrier formation and repair has far-reaching implication for aging skin characterized by epidermal barrier dysfunction.
Cutaneous microbes primarily act as a protective barrier against foreign and pathogenic microbes (Parlet et al., 2019). Within poly-microbial communities, skin microbes have evolved mechanisms to compete and directly antagonize potential rivals (Harris-Tryon and Grice, 2022). Coagulase negative Staphylococci (CoNS) species, such as Staphylococcus hominis and Staphylococcus capitis, antagonize the major skin pathogen Staphylococcus aureus (Nakatsuji et al., 2017), either through the production of unique antibiotics or interference with specific quorum sensing pathways essential for S. aureus virulence (Paharik et al., 2017; Williams et al., 2019). Additionally, some of these antagonistic mechanisms synergize with the host’s antimicrobial responses, maximizing the defense. Certain strains of Cutibacterium acnes secrete a thiopeptide antibiotic called cutimycin, which effectively restricts the colonization of S. aureus (Claesen et al., 2020). The role of these skin commensals as a microbial barrier is important since S. aureus has powerful resources to damage the skin barrier and contribute to a pro-inflammatory status which can theoretically exacerbate inflammaging/skin aging (Kim and Kim, 2019).
Commensal microbes also interact and modulate other functional levels of the skin barrier (Uberoi et al., 2021). First, skin microbes play a fundamental role in the induction, training, and homeostasis of the skin immune barrier (Belkaid and Segre, 2014). Under optimal conditions, the skin’s immune system seamlessly integrates the innate and adaptive branches of immunity, engaging in a dialogue that guides the selection, fine-tuning, and cessation of responses as needed. A key aspect of this process is tissue repair, wherein acute skin damage triggers the release of ligands that activate keratinocytes and lead to the emission of inflammatory mediators (Lai et al., 2009). In such conditions, a specific component of S. epidermidis, known as lipoteichoic acid, helps to reduce inflammation and aid in wound healing through its interaction with the innate immune receptor, Toll-like receptor 2 (TLR2) (Lai et al., 2009). In the gastrointestinal system, certain microbes and microbial metabolic products contribute to immune balance by influencing the regulatory immune network (Belkaid and Hand, 2014). Similarly, Vitreoscilla filiformis, a Gram-negative bacterium found in spa waters, is known to encourage the development of tissue-resident regulatory T cells and curb T cell proliferation during skin inflammation in mice (Nakatsuji and Gallo, 2014; Volz et al., 2014). Microbes endowed such with regulatory and protective properties would be of great benefit in maintaining the immune barrier homeostasis and controlling skin aging/inflammaging.
Additionally, C. acnes and Corynebacterium spp., produce lipases that break down triglycerides in sebum to release free fatty acids. Free fatty acids maintain the acidic surface pH of the skin, which dictates the chemical barrier (Schmid-Wendtner and Korting, 2006; Elias, 2007).
Lastly, Uberoi et al., have shown that skin commensals (a collection consisting of members of Firmicutes phylum, i.e., Staphylococcus epidermidis, S. warneri, S. hemolyticus, and members of Actinobacteria phylum, i.e., Micrococcus luteus and Corynebacterium aurimucosum) play a pivotal role in restoring the physical barrier by stimulating the aryl hydrocarbon receptor (AHR) in keratinocytes (Uberoi et al., 2021). C. acnes also induces a prominent increase in lipids and barrier protein (i.e., filaggrin and loricrin) in the epidermis, further contributing to improvement of the physical barrier (Almoughrabie et al., 2023). S. epidermidis, another commensal skin bacteria, help maintain the physical barrier by producing sphingomyelinase, which plays a role in the production of ceramide (Zheng et al., 2022).
In the elderly, long-term antibiotic use can also lead to a microbial dysbiosis, reducing microbial diversity (Zapata and Quagliarello, 2015). The mode of antibiotic administration affects the skin microbiota differently (Woo et al., 2020; Weiss et al., 2022). Topical antibiotics cause immediate, localized changes, whereas oral antibiotics impact the entire microbiome, including the gut-skin axis, influencing skin health more broadly (Vaughn et al., 2017; Xu and Li, 2019). In the elderly, antibiotic use is associated with increased dryness (Jiang et al., 2022), which is likely due to microbiome alterations affecting skin homeostasis and immune responses.
UVR is one of the most concerning environmental factors that accelerate skin aging. Interestingly, a study has shown that bacterial molecules can either shield against UVR or counteract its damaging effects (Souak et al., 2021). Mycosporine-like amino acids (MAAs), stable under sunlight and capable of absorbing UV, are secondary metabolites produced by Cyanobacteria in response to solar UVR exposure (Colabella et al., 2014). MAAs can convert UV energy into heat without generating free oxygen radicals and can prevent the UV-induced creation of pyrimidine dimers (Colabella et al., 2014). S. epidermidis generates 6-N hydroxyaminopurine (6-HAP), which inhibits UV-induced new cell growth (Nakatsuji et al., 2018). Additionally, Micrococcus luteus produces an endonuclease that enhances the effectiveness of DNA repair enzymes (Skotarczak et al., 2015). A recent discovery showed that C. acnes secrete an antioxidant enzyme Radical oxygenase of Propionibacterium acnes (RoxP) which reduces oxidative stress linked to UV exposure (Allhorn et al., 2016; Andersson et al., 2019).
As described above, commensal microbes are regarded as a source of compounds that provide indirect photoprotection. Some even have direct UVR blocking or absorbing effects, as well as anti-inflammatory and anti-oxidative activities which can attenuate photoaging.
5 Restoring skin barrier function and dysbiosis in aging skin
The aging process of the skin can be modulated and potentially attenuated by reestablishing the normal functions of its protective barrier (An et al., 2017). The quest for healthy and resilient skin in the face of aging has sparked a surge of interest in novel therapeutic strategies. Among these approaches, the utilization of a proper moisturizer, antioxidants, probiotics, prebiotics, postbiotics, and effective UV protection has emerged as a captivating frontier in bolstering the aging skin barrier and correcting dysbiosis (Figure 1).
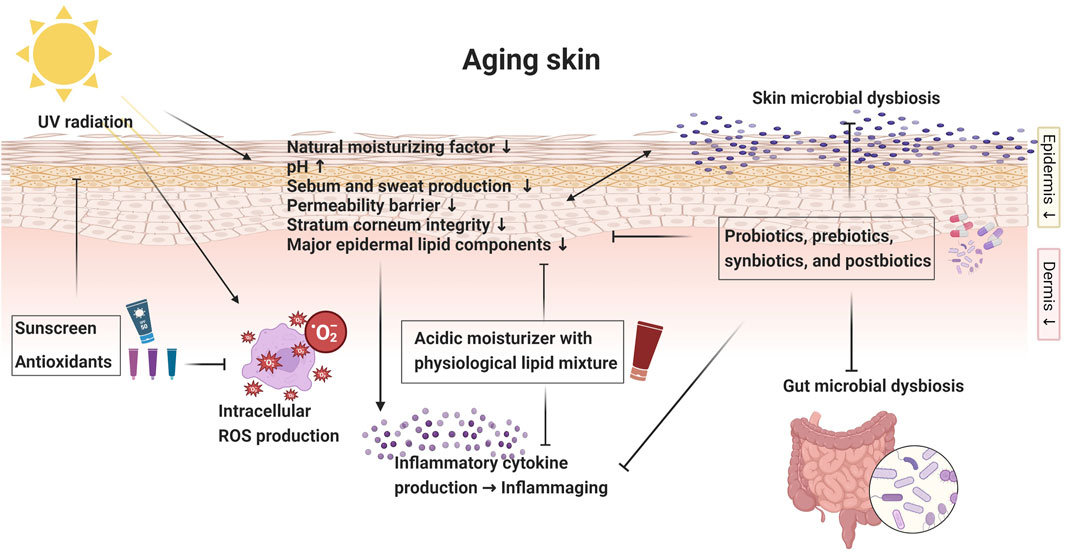
FIGURE 1. Diagrammatic overview of aging skin: skin barrier changes and intervention strategies. Created with Biorender.com.
5.1 Moisturizer
Given that the acidity is diminished in aged skin, one would expect an acidic moisturizer to be beneficial to the aging skin (Choi et al., 2007). Topical acidification of the SC has in fact shown to restore barrier abnormalities where noteworthy enhancement in SC integrity was seen among elderly patients following a 4-week skin care regimen with pH 4.0 products (Jürgen et al., 2011). Acidified skin surface pH correlated with SC integrity, prompting the authors to recommend skincare products with pH within the range of 3.5–4.0 to the elderly (Jürgen et al., 2011).
A 3:1:1:1 mixture of SC lipids (cholesterol: ceramide: essential FFA, nonessential FFA) was shown to accelerate barrier recovery in both chronologically aged murine and human skin, and is considered optimal for aging skin (Zettersten et al., 1997). Topical mevalonic acid also has a potential to be incorporated in the moisturizer for the elderly as it was shown to enhance barrier recovery in aged mice by stimulating de novo cholesterol synthesis (Ikenaga et al., 2000). Interestingly, mevalonic acid and the optimized ratio of physiologic lipids had little impact on barrier recovery in young mice limiting their use in aging skin (Ikenaga et al., 2000).
The emollient plus, which refers to emollients having active and non-medicated ingredients, are recently reported to positively impact the skin barrier and skin microbiota especially in atopic dermatitis (AD) patients (Wollenberg et al., 2018). These emollients feature active ingredients like flavonoids, riboflavins, and bacterial lysates, effective in managing AD by maintaining skin barrier integrity and balancing skin microflora (Wollenberg et al., 2020). Emollients plus have proven effective in restoring barrier function and microbial diversity in mild to moderate AD (Bianchi et al., 2016; Quadri et al., 2021; Zelenkova et al., 2023). Given these benefits, emollients plus could show promis for application in aging skin, potentially addressing barrier disruptions and microbial imbalances typical in older skin.
5.2 Antioxidant and UV protection
The aging process is notably characterized by a weakened endogenous defense mechanism and an increased production of ROS, leading to accelerated skin aging (Masaki, 2010). Oxidative stress induced by ROS, especially those exacerbated by UV radiation (Beak et al., 2004), plays a significant role in skin barrier alteration (Chiba et al., 2003; Fujita et al., 2007; Biniek et al., 2012).
As such, topical antioxidants have emerged as a potent therapeutic strategy to strengthen the skin barrier in aged individuals. While α-tocopherol (free vitamin E) is renowned for stabilizing the lamellar lipid layers in the SC (Thiele, 2001), other antioxidants like vitamin C have also demonstrated clinical improvements in aging skin when applied topically (Thiele and Ekanayake-Mudiyanselage, 2007). Moreover, the advancements in skin care have introduced a variety of other topical antioxidants, such as ferulic acid, resveratrol, and niacinamide (Baxter, 2008; Boo, 2021; Zduńska-Pęciak et al., 2022), which, when used individually or synergistically, offer a robust defense against oxidative stress and enhance the skin’s resilience against environmental aggressors like UV radiation (Baxter, 2008; Farris et al., 2014; Hahn et al., 2016; Rattanawiwatpong et al., 2020; Boo, 2021; Zduńska-Pęciak et al., 2022).
The use of sunscreens would be a direct approach to minimize the harmful effects of UVR to the skin. By employing effective UV protection measures, individuals can shield their skin from UV radiation and help restore the skin barrier’s function (Shanbhag et al., 2019). Sunscreen mixture with barrier-enforcing lipid formulations (i.e., ceramide-containing sunscreens) (Dumbuya et al., 2021; Cao et al., 2023) and antioxidants (i.e., suncreens containing pre-tocopheryl) (Boisnic et al., 2005) were found to better in enhancing the skin barrier compared to sunscreen alone.
5.3 Probiotics, prebiotics, synbiotics, and postbiotics
Probiotics, prebiotics, synbiotics, and postbiotics have demonstrated efficacy in enhancing SC hydration, reducing wrinkle depth, and offering photoprotective properties. Clinical studies confirm these benefits following their oral administration or topical application (Table 2).
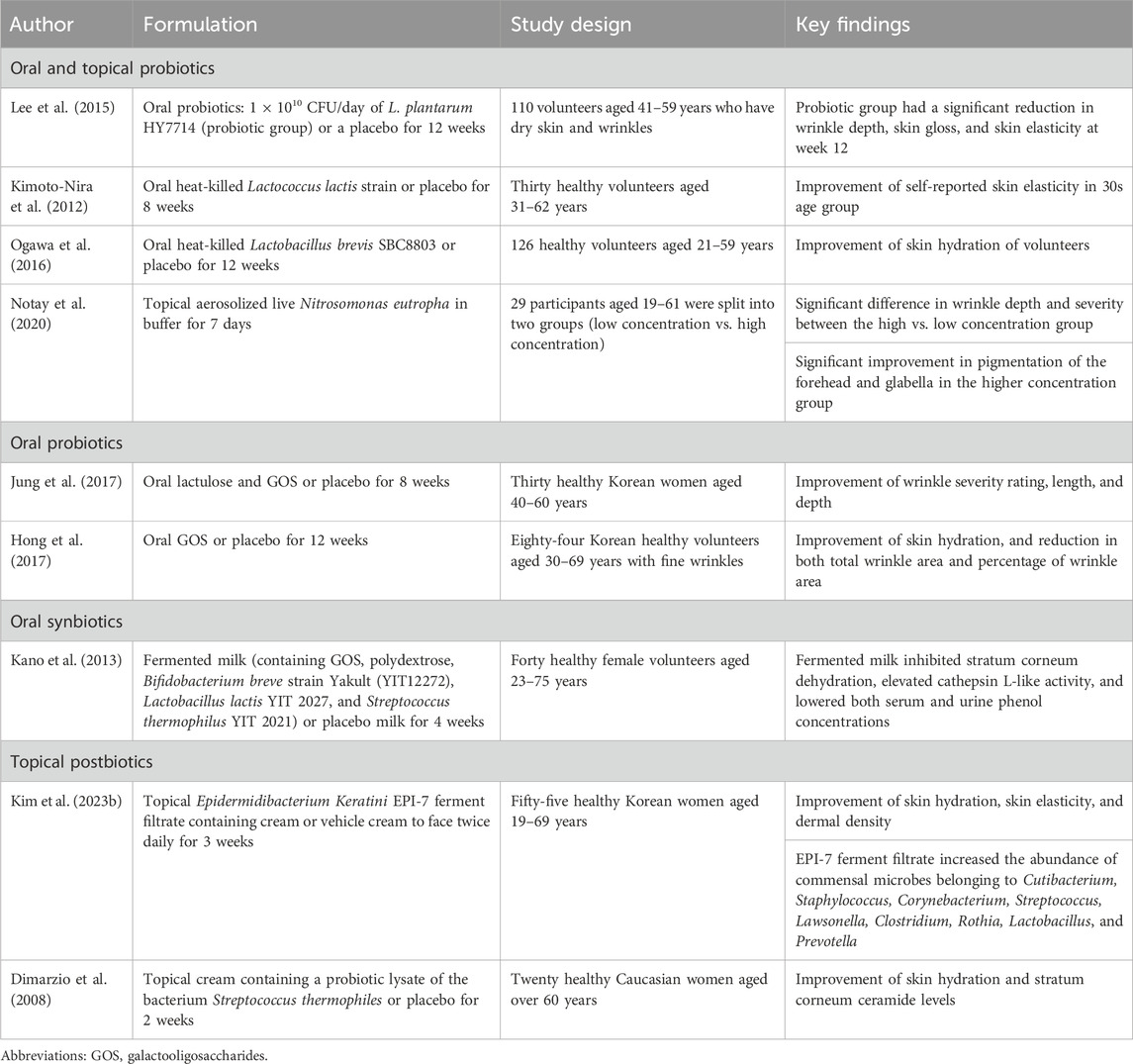
TABLE 2. Summary of clinical studies investigating the impact of prebiotics, probiotics, synbiotics and postbiotics on skin aging.
Probiotics confer health benefits to the host when administered in adequate amounts (Schrezenmeir and de Vrese, 2001). Specific strains enrich the composition of the intestinal microflora, playing a pivotal role in promoting overall health (Fuller, 1989). Both oral and topical probiotics have also been explored as a potential therapeutic strategy for skin aging, showing promising outcomes.
A variety of bacterial strains from the gut (i.e., Lactobacillus and Bifidobacterium), skin (i.e., Staphylococcus and Cutibacterium), and environment (i.e., Cyanobacteria) are recognized as potential senotherapeutic agents, effectively modulating pH imbalance, oxidative stress, photodamage, inflammation and impaired skin barrier function (Ishii et al., 2014; Kober and Bowe, 2015; Sharma et al., 2016; Shin et al., 2018; Im et al., 2019; Keshari et al., 2019; Kang et al., 2020; Khmaladze et al., 2020; Lim et al., 2020; Chen et al., 2022). Im et al. (Im et al., 2019) identified the efficacy of topical Lactobacillus acidophilus IDCC 3302 in increasing skin antioxidant enzymes, promoting hydration, and suppressing MMP. Ishii et al. (Ishii et al., 2014) elucidated the potential of oral Bifidobacterium breve Yakult in preventing UV-induced ROS, consequently reducing oxidative stress and skin barrier damage in animal models. The butyric acid from Staphylococcus epidermidis downregulated the UV-induced pro-inflammatory IL-6 cytokine via short-chain fatty acid receptors (Keshari et al., 2019). Moreover, Lactobacillus paracasei was recognized to play a vital role in maintaining immune equilibrium by regulating T regulatory cells (Tregs) and averting UVR-induced immune disruptions (Kober and Bowe, 2015). C. acnes (Allhorn et al., 2016; Andersson et al., 2019) and Cyanobacteria (Chaubey et al., 2021) also possess antioxidant potential and may replace synthetic ingredients in cosmetic formulations.
Few clinical studies examined the impact of probiotics on aging skin. A randomized, double-blind, placebo-controlled study involving 110 middle-aged participants, showed Lactobacillus plantarum HY7714 ingestion for 12 weeks resulted in significant improvements in skin hydration, gloss, elasticity, and a noticeable reduction in facial wrinkles (Lee et al., 2015). Another study highlighted the effectiveness of a probiotic facial mist containing Nitrosomonas eutropha in reducing wrinkles and improving skin pigmentation (Notay et al., 2020). Here, the active component, N. eutropha, which is a non-pathogenic bacterium, enzymatically converted ammonia in sweat into nitrite and nitric oxide, exhibiting anti-inflammatory properties (Notay et al., 2020).
Prebiotics, when selectively utilized by host microorganisms, can be beneficial to the skin (Gibson and Roberfroid, 1995). A clinical trial showed that an oral prebiotic formulation containing lactulose and galactooligosaccharides (GOS) significantly reduced the depth and length of facial wrinkles (Jung et al., 2017).
Synbiotics, which are combined products of probiotics and prebiotics, are also thought to be beneficial to aging skin. In a randomized, double-blind, placebo-controlled study of 600 healthy Japanese women, a daily regimen of B. breve strain Yakult and GOS for 4 weeks led to significant improvement in skin hydration, heightened cathepsin L-like activity (indicative of keratinocyte differentiation), and a notable reduction in serum and urine phenol levels in the active intervention group (Kano et al., 2013).
Postbiotics, the bioactive compounds produced during the fermentation process, are promising for skin aging (Malagón-Rojas et al., 2020). A topical postbiotic cream containing a lysate sourced from Streptococcus thermophilus was shown to enhance the SC lipid barrier and reduce water loss (Dimarzio et al., 2008). In addition, a 3-week (twice daily) application of a topical cream infused with Epidermidibacterium Keratini EPI-7 ferment filtrate demonstrated notable enhancements in skin hydration, elasticity, and dermal density (Kim J. et al., 2023). The application of EPI-7 ferment filtrate was associated with an increase in beneficial commensal microorganisms such as Cutibacterium, Corynebacterium, Staphylococcus, Streptococcus, Clostridium, Lawsonella, Rothia, Lactobacillus, and Prevotella (Kim J. et al., 2023).
In summary, pre-, pro-, syn-, and postbiotics have potential as an alternative therapeutic option for aging skin by targeting multiple pathways involved in skin aging.
6 Conclusion
An emerging risk factor for aging-related diseases is the microbiome, based on its ubiquity and central role in immunity and metabolism. Since the skin is the beacon for general health, successful management of aging skin carries great importance. The fundamental mechanism whereby the microbes regulate the skin barrier has far-reaching implication for aging skin and offers innovative strategies to bolster skin health. As we continue to learn, we anticipate the emergence of novel senotherapeutic, presenting fresh pathways to nurture vibrant and resilient skin in the aging population.
Author contributions
YW: Writing–original draft. HK: Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Catholic Medical Center Research Foundation made in the program year of 2023, the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (grant number: 2023R1A2C1007759) and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HI23C0860).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Allhorn M., Arve S., Brüggemann H., Lood R. (2016). A novel enzyme with antioxidant capacity produced by the ubiquitous skin colonizer Propionibacterium acnes. Sci. Rep. 6, 36412. doi:10.1038/srep36412
Almoughrabie S., Cau L., Cavagnero K., O’Neill A. M., Li F., Roso-Mares A., et al. (2023). Commensal Cutibacterium acnes induce epidermal lipid synthesis important for skin barrier function. Sci. Adv. 9 (33), eadg6262. doi:10.1126/sciadv.adg6262
Álvarez J., Real J. M. F., Guarner F., Gueimonde M., Rodríguez J. M., de Pipaon M. S., et al. (2021). Gut microbes and health. Gastroenterol. Hepatol. (English Ed. 44 (7), 519–535. doi:10.1016/j.gastrohep.2021.01.009
An S., Cha H. J., Ko J.-M., Han H., Kim S. Y., Kim K.-S., et al. (2017). Kinetin improves barrier function of the skin by modulating keratinocyte differentiation markers. Ann. Dermatology 29 (1), 6–12. doi:10.5021/ad.2017.29.1.6
Andersson T., Ertürk Bergdahl G., Saleh K., Magnúsdóttir H., Stødkilde K., Andersen C. B. F., et al. (2019). Common skin bacteria protect their host from oxidative stress through secreted antioxidant RoxP. Sci. Rep. 9 (1), 3596. doi:10.1038/s41598-019-40471-3
Baldwin H. E., Bhatia N., Friedman A., Prunty T., Martin R., Seite S. (2017). The role of cutaneous microbiota harmony in maintaining a functional skin barrier. SKIN J. Cutan. Med. 1, s139. doi:10.25251/skin.1.supp.138
Baxter R. A. (2008). Anti-aging properties of resveratrol: review and report of a potent new antioxidant skin care formulation. J. Cosmet. dermatology 7 (1), 2–7. doi:10.1111/j.1473-2165.2008.00354.x
Beak S. M., Lee Y. S., Kim J. A. (2004). NADPH oxidase and cyclooxygenase mediate the ultraviolet B-induced generation of reactive oxygen species and activation of nuclear factor-kappaB in HaCaT human keratinocytes. Biochimie 86 (7), 425–429. doi:10.1016/j.biochi.2004.06.010
Belkaid Y., Hand T. W. (2014). Role of the microbiota in immunity and inflammation. Cell 157 (1), 121–141. doi:10.1016/j.cell.2014.03.011
Belkaid Y., Segre J. A. (2014). Dialogue between skin microbiota and immunity. Science 346 (6212), 954–959. doi:10.1126/science.1260144
Bianchi P., Theunis J., Casas C., Villeneuve C., Patrizi A., Phulpin C., et al. (2016). Effects of a new emollient-based treatment on skin microflora balance and barrier function in children with mild atopic dermatitis. Pediatr. Dermatol. 33 (2), 165–171. doi:10.1111/pde.12786
Biniek K., Levi K., Dauskardt R. H. (2012). Solar UV radiation reduces the barrier function of human skin. Proc. Natl. Acad. Sci. 109 (42), 17111–17116. doi:10.1073/pnas.1206851109
Biragyn A., Ferrucci L. (2018). Gut dysbiosis: a potential link between increased cancer risk in ageing and inflammaging. Lancet Oncol. 19 (6), e295–e304. doi:10.1016/S1470-2045(18)30095-0
Boisnic S., Branchet-Gumila M. C., Merial-Kieny C., Nocera T. (2005). Efficacy of sunscreens containing pre-tocopheryl in a surviving human skin model submitted to UVA and B radiation. Skin. Pharmacol. Physiol. 18 (4), 201–208. doi:10.1159/000085866
Boo Y. C. (2021). Mechanistic basis and clinical evidence for the applications of nicotinamide (niacinamide) to control skin aging and pigmentation. Antioxidants 10 (8), 1315. doi:10.3390/antiox10081315
Brodin P., Davis M. M. (2017). Human immune system variation. Nat. Rev. Immunol. 17 (1), 21–29. doi:10.1038/nri.2016.125
Brogden N., Mehalick L., Fischer C., Wertz P., Brogden K. (2012). The emerging role of peptides and lipids as antimicrobial epidermal barriers and modulators of local inflammation. Skin Pharmacol. physiology 25 (4), 167–181. doi:10.1159/000337927
Cao Y., Zhang X., He X., Wang W., Yi Y., Ai Y. (2023). Efficacy of ceramide-containing sunscreen on skin barrier. J. Cosmet. Dermatol. doi:10.1111/jocd.15977
Chambers E. S., Vukmanovic-Stejic M. (2020). Skin barrier immunity and ageing. Immunology 160 (2), 116–125. doi:10.1111/imm.13152
Chaubey M. G., Patel S. N., Sonani R. R., Singh N. K., Rastogi R. P., Madamwar D. (2021). “Antioxidant, anti-aging and anti-neurodegenerative biomolecules from Cyanobacteria,” in Ecophysiology and biochemistry of Cyanobacteria. Editor R. P. Rastogi (Singapore: Springer Nature Singapore), 327–350.
Chen H., Li Y., Xie X., Chen M., Xue L., Wang J., et al. (2022). Exploration of the molecular mechanisms underlying the anti-photoaging effect of limosilactobacillus fermentum XJC60. Front. Cell. Infect. Microbiol. 12, 838060. doi:10.3389/fcimb.2022.838060
Chiba K., Kawakami K., Sone T., Onoue M. (2003). Characteristics of skin wrinkling and dermal changes induced by repeated application of squalene monohydroperoxide to hairless mouse skin. Skin. Pharmacol. Appl. Skin. Physiol. 16 (4), 242–251. doi:10.1159/000070847
Choi E. H. (2019). Aging of the skin barrier. Clin. dermatology 37 (4), 336–345. doi:10.1016/j.clindermatol.2019.04.009
Choi E.-H., Man M.-Q., Xu P., Xin S., Liu Z., Crumrine D. A., et al. (2007). Stratum corneum acidification is impaired in moderately aged human and murine skin. J. Investigative Dermatology 127 (12), 2847–2856. doi:10.1038/sj.jid.5700913
Claesen J., Spagnolo J. B., Ramos S. F., Kurita K. L., Byrd A. L., Aksenov A. A., et al. (2020). A Cutibacterium acnes antibiotic modulates human skin microbiota composition in hair follicles. Sci. Transl. Med. 12 (570), eaay5445. doi:10.1126/scitranslmed.aay5445
Claesson M. J., Cusack S., O’Sullivan O., Greene-Diniz R., de Weerd H., Flannery E., et al. (2011). Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl. Acad. Sci. 108 (1), 4586–4591. doi:10.1073/pnas.1000097107
Colabella F., Moline M., Libkind D. (2014). UV sunscreens of microbial origin: mycosporines and mycosporine-like aminoacids. Recent Pat. Biotechnol. 8 (3), 179–193. doi:10.2174/1872208309666150102104520
Dekio I., Sakamoto M., Hayashi H., Amagai M., Suematsu M., Benno Y. (2007). Characterization of skin microbiota in patients with atopic dermatitis and in normal subjects using 16S rRNA gene-based comprehensive analysis. J. Med. Microbiol. 56 (12), 1675–1683. doi:10.1099/jmm.0.47268-0
Denda M., Ashida Y., Inoue K., Kumazawa N. (2001). Skin surface electric potential induced by ion-flux through epidermal cell layers. Biochem. Biophysical Res. Commun. 284 (1), 112–117. doi:10.1006/bbrc.2001.4925
Denda M., Koyama J., Hori J., Horii I., Takahashi M., Hara M., et al. (1993). Age-and sex-dependent change in stratum corneum sphingolipids. Archives dermatological Res. 285, 415–417. doi:10.1007/BF00372135
Denda M., Tomitaka A., Akamatsu H., Matsunaga K. (2003). Altered distribution of calcium in facial epidermis of aged adults. J. investigative dermatology 121 (6), 1557–1558. doi:10.1111/j.1523-1747.2003.12619.x
De Pessemier B., Grine L., Debaere M., Maes A., Paetzold B., Callewaert C. (2021). Gut–skin axis: current knowledge of the interrelationship between microbial dysbiosis and skin conditions. Microorganisms 9 (2), 353. doi:10.3390/microorganisms9020353
Dimarzio L., Cinque B., Cupelli F., De Simone C., Cifone M., Giuliani M. (2008). Increase of skin-ceramide levels in aged subjects following a short-term topical application of bacterial sphingomyelinase from Streptococcus thermophilus. Int. J. Immunopathol. Pharmacol. 21 (1), 137–143. doi:10.1177/039463200802100115
Di Marzio L., Cinque B., De Simone C., Cifone M. G. (1999). Effect of the lactic acid BacteriumStreptococcus thermophilus on ceramide levels in human Keratinocytesin vitro and stratum corneum in vivo. J. investigative dermatology 113 (1), 98–106. doi:10.1046/j.1523-1747.1999.00633.x
Dimitriu P. A., Iker B., Malik K., Leung H., Mohn W., Hillebrand G. G. (2019). New insights into the intrinsic and extrinsic factors that shape the human skin microbiome. MBio 10 (4), e00839-19. doi:10.1128/mbio.00839-19
Dumbuya H., Yan X., Chen Y., Wangari-Olivero J., Lynch S., Brieva P., et al. (2021). ARTICLE: efficacy of ceramide-containing formulations on UV-induced skin surface barrier alterations. J. Drugs Dermatol 20 (4), s29–s35. doi:10.36849/JDD.2021.589E
Elias P. M. (2007). The skin barrier as an innate immune element. Semin. Immunopathol. 29 (1), 3–14. doi:10.1007/s00281-007-0060-9
Ellis S. R., Nguyen M., Vaughn A. R., Notay M., Burney W. A., Sandhu S., et al. (2019). The skin and gut microbiome and its role in common dermatologic conditions. Microorganisms 7 (11), 550. doi:10.3390/microorganisms7110550
Eyerich S., Eyerich K., Traidl-Hoffmann C., Biedermann T. (2018). Cutaneous barriers and skin immunity: differentiating A connected network. Trends Immunol. 39 (4), 315–327. doi:10.1016/j.it.2018.02.004
Farage M. A., Miller K. W., Elsner P., Maibach H. I. (2008). Functional and physiological characteristics of the aging skin. Aging Clin. Exp. Res. 20, 195–200. doi:10.1007/BF03324769
Farris P., Yatskayer M., Chen N., Krol Y., Oresajo C. (2014). Evaluation of efficacy and tolerance of a nighttime topical antioxidant containing resveratrol, baicalin, and vitamin e for treatment of mild to moderately photodamaged skin. J. drugs dermatology JDD 13 (12), 1467–1472.
Ferrucci L., Fabbri E. (2018). Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 15 (9), 505–522. doi:10.1038/s41569-018-0064-2
Feuillie C., Vitry P., McAleer M. A., Kezic S., Irvine A. D., Geoghegan J. A., et al. (2018). Adhesion of Staphylococcus aureus to corneocytes from atopic dermatitis patients is controlled by natural moisturizing factor levels. MBio 9 (4), e01184. doi:10.1128/mbio.01184-18
Fisher G. J., Kang S., Varani J., Bata-Csorgo Z., Wan Y., Datta S., et al. (2002). Mechanisms of photoaging and chronological skin aging. Archives dermatology 138 (11), 1462–1470. doi:10.1001/archderm.138.11.1462
Forslind B., Engström S., Engblom J., Norlén L. (1997). A novel approach to the understanding of human skin barrier function. J. dermatological Sci. 14 (2), 115–125. doi:10.1016/s0923-1811(96)00559-2
Forslind B., Werner-Linde Y., Lindberg M., Pallon J. (1999). Elemental analysis mirrors epidermal differentiation. Acta dermato-venereologica. 79 (1), 12–17. doi:10.1080/000155599750011624
Franceschi C., Bonafè M., Valensin S., Olivieri F., De Luca M., Ottaviani E., et al. (2000). Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 908, 244–254. doi:10.1111/j.1749-6632.2000.tb06651.x
Fujita H., Hirao T., Takahashi M. (2007). A simple and non-invasive visualization for assessment of carbonylated protein in the stratum corneum. Skin. Res. Technol. 13 (1), 84–90. doi:10.1111/j.1600-0846.2007.00195.x
Fuller R. (1989). Probiotics in man and animals. J. Appl. Bacteriol. 66 (5), 365–378. doi:10.1111/j.1365-2672.1989.tb05105.x
Gallo R. L., Hooper L. V. (2012). Epithelial antimicrobial defence of the skin and intestine. Nat. Rev. Immunol. 12 (7), 503–516. doi:10.1038/nri3228
Ghadially R., Brown B. E., Sequeira-Martin S. M., Feingold K. R., Elias P. M. (1995). The aged epidermal permeability barrier. Structural, functional, and lipid biochemical abnormalities in humans and a senescent murine model. J. Clin. investigation 95 (5), 2281–2290. doi:10.1172/JCI117919
Gibson G. R., Roberfroid M. B. (1995). Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 125 (6), 1401–1412. doi:10.1093/jn/125.6.1401
Gilhar A., Aizen E., Pillar T., Eidelman S. (1992). Response of aged versus young skin to intradermal administration of interferon gamma. J. Am. Acad. Dermatology 27 (5), 710–716. doi:10.1016/0190-9622(92)70243-9
Grolleau-Julius A., Harning E. K., Abernathy L. M., Yung R. L. (2008). Impaired dendritic cell function in aging leads to defective antitumor immunity. Cancer Res. 68 (15), 6341–6349. doi:10.1158/0008-5472.CAN-07-5769
Hahn H. J., Jung H. J., Schrammek-Drusios M. C., Lee S. N., Kim J. H., Kwon S. B., et al. (2016). Instrumental evaluation of anti-aging effects of cosmetic formulations containing palmitoyl peptides, Silybum marianum seed oil, vitamin E and other functional ingredients on aged human skin. Exp. Ther. Med. 12 (2), 1171–1176. doi:10.3892/etm.2016.3447
Harris I. R., Farrell A. M., Holleran W. M., Jackson S., Grunfeld C., Elias P. M., et al. (1998). Parallel regulation of sterol regulatory element binding protein-2 and the enzymes of cholesterol and fatty acid synthesis but not ceramide synthesis in cultured human keratinocytes and murine epidermis. J. lipid Res. 39 (2), 412–422. doi:10.1016/s0022-2275(20)33902-x
Harris-Tryon T. A., Grice E. A. (2022). Microbiota and maintenance of skin barrier function. Science 376 (6596), 940–945. doi:10.1126/science.abo0693
Hasegawa T., Feng Z., Yan Z., Ngo K. H., Hosoi J., Demehri S. (2020). Reduction in human epidermal Langerhans cells with age is associated with decline in CXCL14-mediated recruitment of CD14+ monocytes. J. Investigative Dermatology 140 (7), 1327–1334. doi:10.1016/j.jid.2019.11.017
He T., Harmsen H. J., Raangs G. C., Welling G. W. (2003). Composition of faecal microbiota of elderly people. Microb. Ecol. Health & Dis. 15 (4), 153–159. doi:10.1080/08910600310020505
Hong Y. H., Chang U. J., Kim Y. S., Jung E. Y., Suh H. J. (2017). Dietary galacto-oligosaccharides improve skin health: a randomized double blind clinical trial. Asia Pac. J. Clin. Nutr. 26 (4), 613–618. doi:10.6133/apjcn.052016.05
Hopkins M., Sharp R., Macfarlane G. (2001). Age and disease related changes in intestinal bacterial populations assessed by cell culture, 16S rRNA abundance, and community cellular fatty acid profiles. Gut 48 (2), 198–205. doi:10.1136/gut.48.2.198
Howard B., Bascom C. C., Hu P., Binder R. L., Fadayel G., Huggins T. G., et al. (2022). Aging-associated changes in the adult human skin microbiome and the host factors that affect skin microbiome composition. J. Investigative Dermatology 142 (7), 1934–1946. doi:10.1016/j.jid.2021.11.029
Hu L., Mauro T. M., Dang E., Man G., Zhang J., Lee D., et al. (2017). Epidermal dysfunction leads to an age-associated increase in levels of serum inflammatory cytokines. J. Investigative Dermatology 137 (6), 1277–1285. doi:10.1016/j.jid.2017.01.007
Huang S., Haiminen N., Carrieri A.-P., Hu R., Jiang L., Parida L., et al. (2020). Human skin, oral, and gut microbiomes predict chronological age. Msystems 5 (1), 006300–e719. doi:10.1128/mSystems.00630-19
Hurlow J., Bliss D. Z. (2011). Dry skin in older adults. Geriatr. Nurs. 32 (4), 257–262. doi:10.1016/j.gerinurse.2011.03.003
Ikenaga K., Katoh N., Hirano S., Yasuno H., Haratake A., Uchiwa H. (2000). Topical mevalonic acid stimulates de novo cholesterol synthesis and epidermal permeability barrier homeostasis in aged mice. J. investigative dermatology 114 (2), 247–252. doi:10.1046/j.1523-1747.2000.00875.x
Im A., Lee B., Kang D. J., Chae S. (2019). Protective effects of tyndallized Lactobacillus acidophilus IDCC 3302 against UVB-induced photodamage to epidermal keratinocytes cells. Int. J. Mol. Med. 43 (6), 2499–2506. doi:10.3892/ijmm.2019.4161
Ishii Y., Sugimoto S., Izawa N., Sone T., Chiba K., Miyazaki K. (2014). Oral administration of Bifidobacterium breve attenuates UV-induced barrier perturbation and oxidative stress in hairless mice skin. Archives dermatological Res. 306, 467–473. doi:10.1007/s00403-014-1441-2
Jackson M. A., Jeffery I. B., Beaumont M., Bell J. T., Clark A. G., Ley R. E., et al. (2016). Signatures of early frailty in the gut microbiota. Genome Med. 8, 8–11. doi:10.1186/s13073-016-0262-7
Jeffery I. B., Lynch D. B., O’toole P. W. (2016). Composition and temporal stability of the gut microbiota in older persons. ISME J. 10 (1), 170–182. doi:10.1038/ismej.2015.88
Jiang Q., Wang Y., Liu Y., Zhu D., Xie Y., Zhao J., et al. (2022). Prevalence and associated factors of dry skin among older inpatients in hospitals and nursing homes: a multicenter cross-sectional study. Int. J. Nurs. Stud. 135, 104358. doi:10.1016/j.ijnurstu.2022.104358
Jugé R., Rouaud-Tinguely P., Breugnot J., Servaes K., Grimaldi C., Roth M. P., et al. (2018). Shift in skin microbiota of Western European women across aging. J. Appl. Microbiol. 125 (3), 907–916. doi:10.1111/jam.13929
Jung E. Y., Kwon J. I., Hong Y. H., Suh H. J. (2017). Evaluation of anti-wrinkle effects of duoligo, composed of lactulose and galactooligosaccharides. Prev. Nutr. food Sci. 22 (4), 381–384. doi:10.3746/pnf.2017.22.4.381
Jürgen B., Rainer W., Nanna Y. S. (2011). Treatment of aged skin with a pH 4 skin care product normalizes increased skin surface pH and improves barrier function: results of a pilot study. J. Cosmet. Dermatological Sci. Appl. 2011.
Kang Y.-M., Hong C.-H., Kang S.-H., Seo D.-S., Kim S.-O., Lee H.-Y., et al. (2020). Anti-photoaging effect of plant extract fermented with Lactobacillus buchneri on CCD-986sk fibroblasts and HaCaT keratinocytes. J. Funct. Biomaterials 11 (1), 3. doi:10.3390/jfb11010003
Kano M., Masuoka N., Kaga C., Sugimoto S., Iizuka R., Manabe K., et al. (2013). Consecutive intake of fermented milk containing Bifidobacterium breve strain Yakult and galacto-oligosaccharides benefits skin condition in healthy adult women. Biosci. microbiota, food health 32 (1), 33–39. doi:10.12938/bmfh.32.33
Keshari S., Balasubramaniam A., Myagmardoloonjin B., Herr D. R., Negari I. P., Huang C.-M. (2019). Butyric acid from probiotic staphylococcus epidermidis in the skin microbiome down-regulates the ultraviolet-induced pro-inflammatory IL-6 cytokine via short-chain fatty acid receptor. Int. J. Mol. Sci. 20 (18), 4477. doi:10.3390/ijms20184477
Khmaladze I., Leonardi M., Fabre S., Messaraa C., Mavon A. (2020). The skin interactome: a holistic “genome-microbiome-exposome” approach to understand and modulate skin health and aging. Clin. Cosmet. Investigational Dermatology 13, 1021–1040. doi:10.2147/CCID.S239367
Kho Z. Y., Lal S. K. (2018). The human gut microbiome–a potential controller of wellness and disease. Front. Microbiol. 9, 1835. doi:10.3389/fmicb.2018.01835
Kianmehr S., Jahani M., Moazzen N., Ahanchian H., Khameneh B. (2022). The potential of Probiotics for treating skin Disorders: a concise review. Curr. Pharm. Biotechnol. 23 (15), 1851–1863. doi:10.2174/1389201023666220411090301
Kim H.-J., Kim J. J., Myeong N. R., Kim T., Kim D., An S., et al. (2019). Segregation of age-related skin microbiome characteristics by functionality. Sci. Rep. 9 (1), 16748. doi:10.1038/s41598-019-53266-3
Kim H. S. (2020). Microbiota in rosacea. Am. J. Clin. Dermatol 21 (1), 25–35. doi:10.1007/s40257-020-00546-8
Kim H. S., Keum H. L., Chung I. Y., Nattkemper L., Head C. R., Koh A., et al. (2023a). Characterization of a perturbed skin microbiome in prurigo nodularis and lichen simplex chronicus. J. Invest. Dermatol 143 (10), 2082–2085.e5. doi:10.1016/j.jid.2023.03.1669
Kim H. S., Yosipovitch G. (2020). The skin microbiota and itch: is there a link? J. Clin. Med. 9 (4), 1190. doi:10.3390/jcm9041190
Kim J., Lee Y. I., Mun S., Jeong J., Lee D.-G., Kim M., et al. (2023b). Efficacy and safety of Epidermidibacterium Keratini EPI-7 derived postbiotics in skin aging: a prospective clinical study. Int. J. Mol. Sci. 24 (5), 4634. doi:10.3390/ijms24054634
Kim J. E., Kim H. S. (2019). Microbiome of the skin and gut in atopic dermatitis (AD): understanding the pathophysiology and finding novel management strategies. J. Clin. Med. 8 (4), 444. doi:10.3390/jcm8040444
Kim M., Park T., Yun J. I., Lim H. W., Han N. R., Lee S. T. (2020). Investigation of age-related changes in the skin microbiota of Korean women. Microorganisms 8 (10), 1581. doi:10.3390/microorganisms8101581
Kimoto-Nira H., Aoki R., Sasaki K., Suzuki C., Mizumachi K. (2012). Oral intake of heat-killed cells of Lactococcus lactis strain H61 promotes skin health in women. J. Nutr. Sci. 1, e18. doi:10.1017/jns.2012.22
Kirkwood T. B., Austad S. N. (2000). Why do we age? Nature 408 (6809), 233–238. doi:10.1038/35041682
Kober M.-M., Bowe W. P. (2015). The effect of probiotics on immune regulation, acne, and photoaging. Int. J. women’s dermatology 1 (2), 85–89. doi:10.1016/j.ijwd.2015.02.001
Lai Y., Di Nardo A., Nakatsuji T., Leichtle A., Yang Y., Cogen A. L., et al. (2009). Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat. Med. 15 (12), 1377–1382. doi:10.1038/nm.2062
Lee D. E., Huh C.-S., Ra J., Choi I.-D., Jeong J.-W., Kim S.-H., et al. (2015). Clinical evidence of effects of Lactobacillus plantarum HY7714 on skin aging: a randomized, double blind, placebo-controlled study. J. Microbiol. Biotechnol. 25 (12), 2160–2168. doi:10.4014/jmb.1509.09021
Lee H.-J., Kim M. (2022). Skin barrier function and the microbiome. Int. J. Mol. Sci. 23 (21), 13071. doi:10.3390/ijms232113071
Lephart E. D., Naftolin F. (2022). Menopause and the skin: old favorites and new innovations in cosmeceuticals for estrogen-deficient skin. Dermatology Ther. 12 (7), 53–69. doi:10.1007/s13555-020-00468-7
Leung M. H., Wilkins D., Lee P. K. (2015). Insights into the pan-microbiome: skin microbial communities of Chinese individuals differ from other racial groups. Sci. Rep. 5 (1), 11845. doi:10.1038/srep11845
Li Z., Bai X., Peng T., Yi X., Luo L., Yang J., et al. (2020). New insights into the skin microbial communities and skin aging. Front. Microbiol. 11, 565549. doi:10.3389/fmicb.2020.565549
Lim H. Y., Jeong D., Park S. H., Shin K. K., Hong Y. H., Kim E., et al. (2020). Antiwrinkle and antimelanogenesis effects of tyndallized Lactobacillus acidophilus KCCM12625P. Int. J. Mol. Sci. 21 (5), 1620. doi:10.3390/ijms21051620
Malagón-Rojas J. N., Mantziari A., Salminen S., Szajewska H. (2020). Postbiotics for preventing and treating common infectious diseases in children: a systematic review. Nutrients 12 (2), 389. doi:10.3390/nu12020389
Masaki H. (2010). Role of antioxidants in the skin: anti-aging effects. J. Dermatol Sci. 58 (2), 85–90. doi:10.1016/j.jdermsci.2010.03.003
Miajlovic H., Fallon P. G., Irvine A. D., Foster T. J. (2010). Effect of filaggrin breakdown products on growth of and protein expression by Staphylococcus aureus. J. Allergy Clin. Immunol. 126 (6), 1184–1190. doi:10.1016/j.jaci.2010.09.015
Mohajeri M. H., Brummer R. J., Rastall R. A., Weersma R. K., Harmsen H. J., Faas M., et al. (2018). The role of the microbiome for human health: from basic science to clinical applications. Eur. J. Nutr. 57, 1–14. doi:10.1007/s00394-018-1703-4
Murillo N., Raoult D. (2013). Skin microbiota: overview and role in the skin diseases acne vulgaris and rosacea. Future Microbiol. 8 (2), 209–222. doi:10.2217/fmb.12.141
Myers S. P., Hawrelak J. (2004). The causes of intestinal dysbiosis: a review. Altern. Med. Rev. 9 (2), 180–197.
Nakatsuji T., Chen T. H., Butcher A. M., Trzoss L. L., Nam S. J., Shirakawa K. T., et al. (2018). A commensal strain of Staphylococcus epidermidis protects against skin neoplasia. Sci. Adv. 4 (2), eaao4502. doi:10.1126/sciadv.aao4502
Nakatsuji T., Chen T. H., Narala S., Chun K. A., Two A. M., Yun T., et al. (2017). Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci. Transl. Med. 9 (378), eaah4680. doi:10.1126/scitranslmed.aah4680
Nakatsuji T., Gallo R. L. (2014). Dermatological therapy by topical application of non-pathogenic bacteria. J. Invest. Dermatol 134 (1), 11–14. doi:10.1038/jid.2013.379
Notay M., Saric-Bosanac S., Vaughn A. R., Dhaliwal S., Trivedi M., Reiter P. N., et al. (2020). The use of topical Nitrosomonas eutropha for cosmetic improvement of facial wrinkles. J. Cosmet. dermatology 19 (3), 689–693. doi:10.1111/jocd.13060
Ogawa M., Saiki A., Matsui Y., Tsuchimoto N., Nakakita Y., Takata Y., et al. (2016). Effects of oral intake of heat-killed Lactobacillus brevis SBC8803 (SBL88™) on dry skin conditions: a randomized, double-blind, placebo-controlled study. Exp. Ther. Med. 12 (6), 3863–3872. doi:10.3892/etm.2016.3862
ONeill C. A., Monteleone G., McLaughlin J. T., Paus R. (2016). The gut-skin axis in health and disease: a paradigm with therapeutic implications. Bioessays 38 (11), 1167–1176. doi:10.1002/bies.201600008
Ostojić S., Pereza N., Kapović M. (2009). A current genetic and epigenetic view on human aging mechanisms. Coll. Antropol. 33 (2), 687–699.
Paharik A. E., Parlet C. P., Chung N., Todd D. A., Rodriguez E. I., Van Dyke M. J., et al. (2017). Coagulase-negative staphylococcal strain prevents Staphylococcus aureus colonization and skin infection by blocking quorum sensing. Cell host microbe 22 (6), 746–756. doi:10.1016/j.chom.2017.11.001
Parlet C. P., Brown M. M., Horswill A. R. (2019). Commensal Staphylococci influence Staphylococcus aureus skin colonization and disease. Trends Microbiol. 27 (6), 497–507. doi:10.1016/j.tim.2019.01.008
Petersen E. B., Skov L., Thyssen J. P., Jensen P. (2019). Role of the gut microbiota in atopic dermatitis: a systematic review. Acta dermato-venereologica. 99 (1), 5–11. doi:10.2340/00015555-3008
Pilkington S. M., Ogden S., Eaton L. H., Dearman R. J., Kimber I., Griffiths C. E. (2018). Lower levels of interleukin-1β gene expression are associated with impaired Langerhans’ cell migration in aged human skin. Immunology 153 (1), 60–70. doi:10.1111/imm.12810
Polat M., Nilhan M. (2015). Dermatological complaints of the elderly attending a dermatology outpatient clinic in Turkey: a prospective study over a one-year period. Acta Dermatovenerol. Croat. 23 (4), 277–281.
Quadri M., Lotti R., Bonzano L., Ciardo S., Guanti M. B., Pellacani G., et al. (2021). A novel multi-action emollient plus cream improves skin barrier function in patients with atopic dermatitis: in vitro and clinical evidence. Skin Pharmacol. Physiology 34 (1), 8–18. doi:10.1159/000513055
Ratanapokasatit Y., Laisuan W., Rattananukrom T., Petchlorlian A., Thaipisuttikul I., Sompornrattanaphan M. (2022). How microbiomes affect skin aging: the updated evidence and current perspectives. Life 12 (7), 936. doi:10.3390/life12070936
Rattanawiwatpong P., Wanitphakdeedecha R., Bumrungpert A., Maiprasert M. (2020). Anti-aging and brightening effects of a topical treatment containing vitamin C, vitamin E, and raspberry leaf cell culture extract: a split-face, randomized controlled trial. J. Cosmet. dermatology 19 (3), 671–676. doi:10.1111/jocd.13305
Renz H., Brandtzaeg P., Hornef M. (2012). The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nat. Rev. Immunol. 12 (1), 9–23. doi:10.1038/nri3112
Saccon T. D., Nagpal R., Yadav H., Cavalcante M. B., Nunes A. D. C., Schneider A., et al. (2021). Senolytic combination of dasatinib and quercetin alleviates intestinal senescence and inflammation and modulates the gut microbiome in aged mice. Journals Gerontology Ser. A 76 (11), 1895–1905. doi:10.1093/gerona/glab002
Salem I., Ramser A., Isham N., Ghannoum M. A. (2018). The gut microbiome as a major regulator of the gut-skin axis. Front. Microbiol. 9, 1459. doi:10.3389/fmicb.2018.01459
Salemi S. Z., Memar M. Y., Kafil H. S., Sadeghi J., Ghadim H. H., Alamdari H. A., et al. (2022). The prevalence and antibiotics susceptibility patterns of Corynebacterium minutissimum isolates from skin lesions of patients with suspected erythrasma from tabriz, Iran. Can. J. Infect. Dis. Med. Microbiol. 2022, 4016173. doi:10.1155/2022/4016173
Schmid-Wendtner M. H., Korting H. C. (2006). The pH of the skin surface and its impact on the barrier function. Skin. Pharmacol. Physiol. 19 (6), 296–302. doi:10.1159/000094670
Schrezenmeir J., de Vrese M. (2001). Probiotics, prebiotics, and synbiotics—approaching a definition. Am. J. Clin. Nutr. 73 (2), 361S–4s. doi:10.1093/ajcn/73.2.361s
Sekirov I., Russell S. L., Antunes L. C. M., Finlay B. B. (2010). Gut microbiota in health and disease. Physiol. Rev. 90, 859–904. doi:10.1152/physrev.00045.2009
Shanbhag S., Nayak A., Narayan R., Nayak U. Y. (2019). Anti-aging and sunscreens: paradigm shift in cosmetics. Adv. Pharm. Bull. 9 (3), 348–359. doi:10.15171/apb.2019.042
Sharma D., Kober M.-M., Bowe W. P. (2016). Anti-aging effects of probiotics. J. drugs dermatology JDD 15 (1), 9–12.
Shibagaki N., Suda W., Clavaud C., Bastien P., Takayasu L., Iioka E., et al. (2017). Aging-related changes in the diversity of women’s skin microbiomes associated with oral bacteria. Sci. Rep. 7 (1), 10567. doi:10.1038/s41598-017-10834-9
Shin D., Lee Y., Huang Y.-H., Lim H.-W., Jang K., Kim D.-D., et al. (2018). Probiotic fermentation augments the skin anti-photoaging properties of Agastache rugosa through up-regulating antioxidant components in UV-B-irradiated HaCaT keratinocytes. BMC Complementary Altern. Med. 18 (1), 196–210. doi:10.1186/s12906-018-2194-9
Siddiqui R., Makhlouf Z., Khan N. A. (2022). The increasing importance of the gut microbiome in acne vulgaris. Folia Microbiol. 67 (6), 825–835. doi:10.1007/s12223-022-00982-5
Sikora M., Stec A., Chrabaszcz M., Knot A., Waskiel-Burnat A., Rakowska A., et al. (2020). Gut microbiome in psoriasis: an updated review. Pathogens 9 (6), 463. doi:10.3390/pathogens9060463
Simon A. K., Hollander G. A., McMichael A. (2015). Evolution of the immune system in humans from infancy to old age. Proc. R. Soc. B Biol. Sci. 282 (1821), 20143085. doi:10.1098/rspb.2014.3085
Skotarczak K., Osmola-Mańkowska A., Lodyga M., Polańska A., Mazur M., Adamski Z. (2015). Photoprotection: facts and controversies. Eur. Rev. Med. Pharmacol. Sci. 19 (1), 98–112.
Somboonna N., Wilantho A., Srisuttiyakorn C., Assawamakin A., Tongsima S. (2017). Bacterial communities on facial skin of teenage and elderly Thai females. Archives Microbiol. 199, 1035–1042. doi:10.1007/s00203-017-1375-0
Souak D., Barreau M., Courtois A., André V., Duclairoir Poc C., Feuilloley M. G. J., et al. (2021). Challenging cosmetic innovation: the skin microbiota and probiotics protect the skin from UV-induced damage. Microorganisms 9 (5), 936. doi:10.3390/microorganisms9050936
Takiishi T., Fenero C. I. M., Câmara N. O. S. (2017). Intestinal barrier and gut microbiota: shaping our immune responses throughout life. Tissue barriers 5 (4), e1373208. doi:10.1080/21688370.2017.1373208
Teng Y., Huang Y., Danfeng X., Tao X., Fan Y. (2022). The role of probiotics in skin photoaging and related mechanisms: a review. Clin. Cosmet. Investigational Dermatology 15, 2455–2464. doi:10.2147/CCID.S388954
Thiele J. (2001). Oxidative targets in the stratum corneum. A new basis for antioxidative strategies. Skin Pharmacol. physiology 14 (1), 87–91. doi:10.1159/000056395
Thiele J. J., Ekanayake-Mudiyanselage S. (2007). Vitamin E in human skin: organ-specific physiology and considerations for its use in dermatology. Mol. aspects Med. 28 (5-6), 646–667. doi:10.1016/j.mam.2007.06.001
Thune P., Nilsen T., Hanstad I., Gustavsen T., Lövig Dahl H. (1988). The water barrier function of the skin in relation to the water content of stratum corneum, pH and skin lipids. The effect of alkaline soap and syndet on dry skin in elderly, non-atopic patients. Acta dermato-venereologica. 68 (4), 277–283.
Tomasello G., Mazzola M., Leone A., Sinagra E., Zummo G., Farina F., et al. (2016). Nutrition, oxidative stress and intestinal dysbiosis: influence of diet on gut microbiota in inflammatory bowel diseases. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 160 (4), 461–466. doi:10.5507/bp.2016.052
Tutka K., Żychowska M., Reich A. (2020). Diversity and composition of the skin, blood and gut microbiome in rosacea—a systematic review of the literature. Microorganisms 8 (11), 1756. doi:10.3390/microorganisms8111756
Uberoi A., Bartow-McKenney C., Zheng Q., Flowers L., Campbell A., Knight S. A., et al. (2021). Commensal microbiota regulates skin barrier function and repair via signaling through the aryl hydrocarbon receptor. Cell host microbe 29 (8), 1235–1248. doi:10.1016/j.chom.2021.05.011
Vaiserman A., Romanenko M., Piven L., Moseiko V., Lushchak O., Kryzhanovska N., et al. (2020). Differences in the gut Firmicutes to Bacteroidetes ratio across age groups in healthy Ukrainian population. BMC Microbiol. 20 (1), 221–228. doi:10.1186/s12866-020-01903-7
Vaughn A. R., Notay M., Clark A. K., Sivamani R. K. (2017). Skin-gut axis: the relationship between intestinal bacteria and skin health. World J. Dermatology 6 (4), 52–58. doi:10.5314/wjd.v6.i4.52
Velarde M. C. (2017). Epidermal barrier protects against age-associated systemic inflammation. J. Invest. Dermatol 137 (6), 1206–1208. doi:10.1016/j.jid.2017.02.964
Verdier-Sevrain S., Bonte F. (2007). Skin hydration: a review on its molecular mechanisms. J. Cosmet. dermatology 6 (2), 75–82. doi:10.1111/j.1473-2165.2007.00300.x
Volz T., Skabytska Y., Guenova E., Chen K. M., Frick J. S., Kirschning C. J., et al. (2014). Nonpathogenic bacteria alleviating atopic dermatitis inflammation induce IL-10-producing dendritic cells and regulatory Tr1 cells. J. Invest. Dermatol 134 (1), 96–104. doi:10.1038/jid.2013.291
Weiss A., Delavenne E., Matias C., Lagler H., Simon D., Li P., et al. (2022). Topical niclosamide (ATx201) reduces Staphylococcus aureus colonization and increases shannon diversity of the skin microbiome in atopic dermatitis patients in a randomized, double-blind, placebo-controlled phase 2 trial. Clin. Transl. Med. 12 (5), e790. doi:10.1002/ctm2.790
White-Chu E. F., Reddy M. (2011). Dry skin in the elderly: complexities of a common problem. Clin. dermatology 29 (1), 37–42. doi:10.1016/j.clindermatol.2010.07.005
Williams M. R., Costa S. K., Zaramela L. S., Khalil S., Todd D. A., Winter H. L., et al. (2019). Quorum sensing between bacterial species on the skin protects against epidermal injury in atopic dermatitis. Sci. Transl. Med. 11 (490), eaat8329. doi:10.1126/scitranslmed.aat8329
Wollenberg A., Barbarot S., Bieber T., Christen-Zaech S., Deleuran M., Fink-Wagner A., et al. (2018). Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J. Eur. Acad. Dermatology Venereol. 32 (5), 657–682. doi:10.1111/jdv.14891
Wollenberg A., Christen-Zäch S., Taieb A., Paul C., Thyssen J., de Bruin-Weller M., et al. (2020). ETFAD/EADV Eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J. Eur. Acad. Dermatology Venereol. 34 (12), 2717–2744. doi:10.1111/jdv.16892
Woo Y. R., Cho S. H., Lee J. D., Kim H. S. (2022). The human microbiota and skin cancer. Int. J. Mol. Sci. 23 (3), 1813. doi:10.3390/ijms23031813
Woo Y. R., Lee S. H., Cho S. H., Lee J. D., Kim H. S. (2020). Characterization and analysis of the skin microbiota in rosacea: impact of systemic antibiotics. J. Clin. Med. 9 (1), 185. doi:10.3390/jcm9010185
Woolery-Lloyd H., Andriessen A., Day D., Gonzalez N., Green L., Grice E., et al. (2023). Review of the microbiome in skin aging and the effect of a topical prebiotic containing thermal spring water. J. Cosmet. dermatology 22 (1), 96–102. doi:10.1111/jocd.15464
Xu H., Li H. (2019). Acne, the skin microbiome, and antibiotic treatment. Am. J. Clin. dermatology 20 (3), 335–344. doi:10.1007/s40257-018-00417-3
Zapata H. J., Quagliarello V. J. (2015). The microbiota and microbiome in aging: potential implications in health and age-related diseases. J. Am. Geriatrics Soc. 63 (4), 776–781. doi:10.1111/jgs.13310
Zarrin M., Arab S. E., Yaghoobi R. (2010). The prevalence of superficial fungal infections in the elderly. Pak. J. Med. Sci. 26 (2), 364–367.
Zduńska-Pęciak K., Dębowska R., Kołodziejczak A., Rotsztejn H. (2022). Ferulic acid–A novel topical agent in reducing signs of photoaging. Dermatol. Ther. 35 (7), e15543. doi:10.1111/dth.15543
Zelenkova H., Kerob D., Salah S., Demessant-Flavigny A. L. (2023). Impact of daily use of emollient ‘plus’ on corticosteroid consumption in patients with atopic dermatitis: an open, randomized controlled study. J. Eur. Acad. Dermatology Venereol. 37, 27–34. doi:10.1111/jdv.18947
Zettersten E. M., Ghadially R., Feingold K. R., Crumrine D., Elias P. M. (1997). Optimal ratios of topical stratum corneum lipids improve barrier recovery in chronologically aged skin. J. Am. Acad. Dermatology 37 (3), 403–408. doi:10.1016/s0190-9622(97)70140-3
Zhai W., Huang Y., Zhang X., Fei W., Chang Y., Cheng S., et al. (2018). Profile of the skin microbiota in a healthy Chinese population. J. Dermatology 45 (11), 1289–1300. doi:10.1111/1346-8138.14594
Zheng Y., Hunt R. L., Villaruz A. E., Fisher E. L., Liu R., Liu Q., et al. (2022). Commensal Staphylococcus epidermidis contributes to skin barrier homeostasis by generating protective ceramides. Cell Host Microbe 30 (3), 301–313.e9. doi:10.1016/j.chom.2022.01.004
Zhou W., Fleming E., Legendre G., Roux L., Latreille J., Gendronneau G., et al. (2023). Skin microbiome attributes associate with biophysical skin ageing. Exp. Dermatol. 32, 1546–1556. doi:10.1111/exd.14863
Keywords: aging, skin, microbe, skin barrier, gut
Citation: Woo YR and Kim HS (2024) Interaction between the microbiota and the skin barrier in aging skin: a comprehensive review. Front. Physiol. 15:1322205. doi: 10.3389/fphys.2024.1322205
Received: 16 October 2023; Accepted: 03 January 2024;
Published: 19 January 2024.
Edited by:
Alexandra P. Marques, University of Minho, PortugalReviewed by:
Piotr Konopelski, Medical University of Warsaw, PolandKarolina Chilicka-Hebel, Opole University, Poland
Copyright © 2024 Woo and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hei Sung Kim, hazelkimhoho@gmail.com
 Yu Ri Woo
Yu Ri Woo Hei Sung Kim
Hei Sung Kim