- 1Department of Environmental Science, Zhejiang University, Hangzhou, China
- 2National-Local Joint Engineering Laboratory of Aquatic Animal Genetic Breeding and Nutrition, Zhejiang Provincial Key Laboratory of Aquatic Resources Conservation and Development, College of Life Science, Huzhou University, Huzhou, China
- 3Joint Laboratory of Guangdong Province and Hong Kong Region on Marine Bioresource Conservation and Exploitation, College of Marine Sciences, South China Agricultural University, Guangzhou, China
Microplastics contamination have been extensively reported in aquatic ecosystem and organisms. It is wildly acknowledged that the ingestion, accumulation and elimination of microplastics in fishes are species-specific, which mainly depending on the feeding behavior. This study aimed to investigate the effects of microplastics on the morphology and inflammatory response in intestines of fishes with different feeding types. Largemouth bass (carnivorous fish), grass carp (herbivorous fish) and Jian carp (omnivorous fish) were used as organism model. The contributing concentration and size of microplastics were explored as well as the response time and legacy effect in fishes. Two different sizes of polystyrene microplastics (80 nm and 8 μm) were set at three concentrations. And samples were analyzed at different exposure times and depuration times. Histological analysis indicated that multiple abnormalities in intestines were presented in three species fishes after acute exposure microplastics. The mRNA abundance of immune-related genes in the intestine tissues of fishes were significantly fluctuant. There were differential expressions of genes coping with differential sizes and concentrations of microplastics exposure in different fishes. The reason for the difference effects of microplastics on fishes was still unclear but could be due to the difference in the structure and function of the digestive system. These results provided a theoretical basis to further analysis of the mechanism of fish intestinal pathology caused by microplastics.
1 Introduction
Plastics have been remarkable materials in peoples’ daily life due to its versatile, durable, and incredibly adaptable. Plastics production reached 390 million tonnes in 2021 worldwide with approximately 9% increasing rate every year and China contributed to 32% of world’s plastics production (Plastics Europe, 2022). In the meanwhile, the global total of plastic waste reached 380 Tg in 2018 with an exponential growth every year (Rai et al., 2021). Once entering the environment, plastic would degrade or fragment into microplastics through UV radiation, mechanical transformation or biological degradation by microorganisms (Cole et al., 2011; Alimi et al., 2018). Microplastics are defined as small plastic pieces or fibers smaller than 5 mm (NOAA, 2015). They come in many forms, not only secondary sources, but also primary sources, such as microbeads in personal care products (McDevitt et al., 2017). Microplastics contamination have been extensively reported in marine, freshwater and terrestrial ecosystems (Wang et al., 2020a; Peng et al., 2020; Xu et al., 2020), thus identified as one of the top 10 emerging global environmental problems by the United Nations Environment Program.
Adverse effects of microplastics on fishes have been found in many literatures (Jacob et al., 2020; Anna et al., 2021; Mallik et al., 2021). Due to the attractive color, buoyancy, and food-like properties, fish are particularly prone to ingesting microplastics (Garrido Gamarro et al., 2020). The ingestion of microplastics by fish can cause a variety of consequences: 1) microplastics can lead to physical damage and histopathological alterations (Peda et al., 2016; Jabeen et al., 2018; Ahrendt et al., 2020); 2) microplastics can cause impairments in oxidative, and disorders of inflammatory balance and intestinal microflora (Gu et al., 2020; Huang et al., 2020; Iheanacho and Odo, 2020); 3) microplastics can also lead to fish behavior changes (Brun et al., 2019; Guimarães et al., 2021; Rios-Fuster et al., 2021; Shi et al., 2021); 4) microplastics can act as carriers to intensify further adverse effects of other pollutants on fish (Banaee et al., 2019; Zhang et al., 2019; Li et al., 2023a).
It is wildly acknowledged that the ingestion, accumulation and elimination of microplastics in fishes are species-specific (Mizraji et al., 2017; Xu and Li, 2021). The field investigation found microplastic amounts in filter-feeding and omnivorous fish were higher than that of carnivorous species (Wang et al., 2020b). The laboratory experiment proved that microplastics ingestion in fish larvae was influenced by feeding type of fish, and omnivores fish were less able to eliminate microplastics than filter-feeding fish (Zhang et al., 2021). However, the physiological effects of micro-nano plastics on juvenile fish with different feeding habits have not been reported.
In this study, species-specific effects of microplastics on three commercial fish species with different feeding types were investigated. Largemouth bass, Micropterus salmoides is a typical freshwater carnivorous fish species and widely farmed in China due to its strong adaptability, fast growth, delicious taste, and high economic value (Wang et al., 2020c). Grass carp (Ctenopharyngodon idella), a herbivorous fish species, is one of the most important freshwater cultivars in China, which annual production exceeded 5.53 million tons in 2019 (China Fishery Statistical Yearbook, 2020). Jian carp (Cyprinus carpio var. Jian) is an omnivorous freshwater fish species with an annual production of 24.2 million tons worldwide (Lin et al., 2019; Li et al., 2023b). This study aimed to reveal the effects of microplastics on the morphology and inflammatory response in intestines of fishes with different feeding types. To achieve this goal, histopathological sections were examined, and immune-related genes profiles were used to study the changes in the intestinal tissue of three fishes after microplastics exposure. These results would provide a theoretical basis to further analysis of the mechanism of fish intestinal pathology caused by microplastics.
2 Material and method
2.1 Materials
Polystyrene microplastics with diameters of 80 nm and 8 μm were purchased from Dae Technology Company (Tianjin, China). Largemouth bass, grass carp and Jian carp were bought from a livestock farm in Shunde City (Guangdong, China). Largemouth bass was (5.23 ± 0.62) cm in length and (2.97 ± 0.64) g in weight. Grass carp was (5.81 ± 0.50) cm in length and (3.82 ± 0.91) g in weight. Jian carp was (3.46 ± 0.16) cm in length and (0.93 ± 0.19) g in weight. Fish were acclimatized at 25.2 ± 1.5 °C in culture water (pH 7.1 ± 0.4; dissolved oxygen 6.4 ± 0.5 mg/L) with a 12 h light/dark cycle. Before the experiment, fish were acclimated in 100 L glass tanks for 5 d and were fed with 5.0% body weight fodder (Haid Group, Guangdong, China) twice daily.
2.2 Experimental design
Two different sizes of fluorescent microplastics (80 nm and 8 μm) were set at four concentrations for grass carp and Jian carp: 0, 0.02 mg/L, 0.2 mg/L and 2 mg/L. Based on the previous findings (Zhang et al., 2021), carnivorous fish seemed to be more tolerant to microplastics than other fishes. So, the higher microplastics exposure concentrations (0.05 mg/L, 0.5 mg/L and 5 mg/L) for largemouth bass were set. The concentrations of exposure for MPs were selected based on the other studies (Ding et al., 2018; Li et al., 2020; Zhang et al., 2021). The microplastics with nanometer particle size (80 nm) and micron particle size (8 μm) were compared.
In the exposure experiment, tanks (20 cm × 15 cm × 15 cm) were filled with 2 L of culture water and eight fish. A total of twenty-four tanks were set for each fish species, including control group and replicate group. Each species of fish was tested separately. Three replicate tanks were used for 24 h and 48 h sampling times. After 48 h exposure, the surviving fish were moved to an aquarium with clean water containing no microplastics for 48 h. No feeding was done during exposure and depuration. At 24 and 48 h after exposure and clearance, two fish were dissected from each tank and the intestines were removed for subsequent analysis. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All surgery was performed under anesthesia, and all efforts were made to minimize suffering.
2.3 Histopathological analysis
A total of 24 fish from the control and experimental groups were anesthetized on ice and intestines were dissected. Intestinal tissue fixed in general-purpose tissue fixator (Servicebio, Wuhan, China), embedded in paraffin wax, sectioned at 4 μm thickness, and stained with hematoxylin-eosin (H&E). Tissue slices were examined and photographed by a microscopy (Nikon, Tokyo, Japan) with the Mshot Image Analysis System.
2.4 RNA extraction and cDNA synthesis
The experimental methods of RNA extraction and cDNA synthesis are presented in Supplementary Text S1. The cDNA was stored at −80°C until further analysis.
2.5 Immune and enzyme-related gene expression
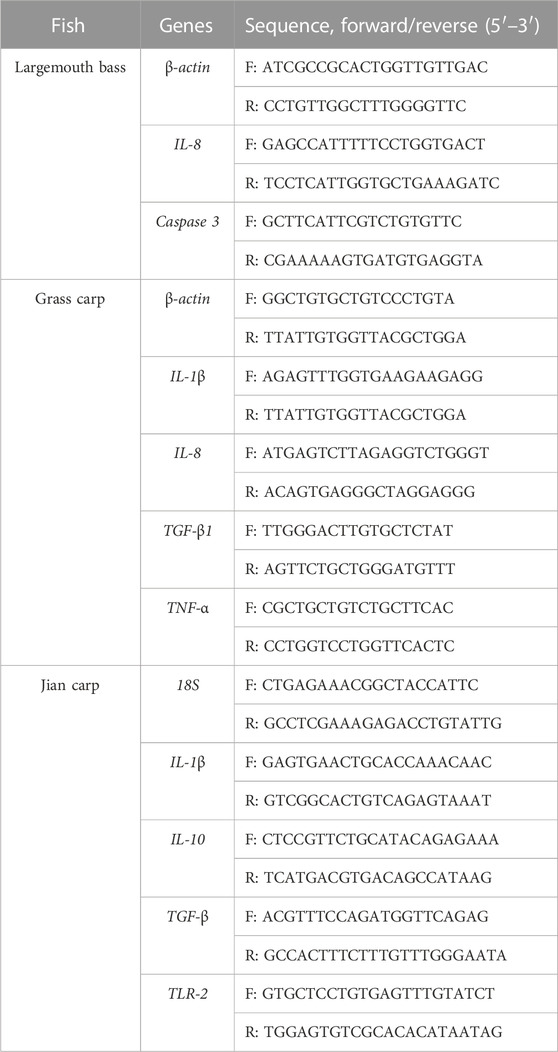
The SYBR green real-time PCR assay was performed on the CFX Connect TM Real-Time System (BIO-RAD, Hercules, CA, USA) using the SYBR® Green Premix Pro Taq HS qPCR kit (Accurate Biotechnology Co., Ltd., Hunan, China) following the manufacturer’s approach. Specific primer sequences are listed in Table 1. Details of the PCR program are presented in Supplementary Text S2. Expression levels of target genes were normalized to the internal reference, and the data were calculated as the fold change in comparison to the control group.
2.6 Statistical analysis
All data were quantified as the mean ± standard error (S.E) and performed by one-way ANOVA using SPSS 17.0 and Excel 2016. Statistical significance between the control and the experimental groups was conducted by the Duncan’s multiple range test. A value of p < 0.05 was set with statistical significance.
3 Results
3.1 Intestinal morphology
After HE staining, intestinal histomorphology of three fishes were examined using a light microscope. Histopathological sections showed that the intestinal folds of largemouth bass juvenile were in disorder and shortened, infiltrated cells, especially when fish were exposure in higher concentration microplastics of micron scale (Figure 1). In the intestine of grass carp juvenile, there was no difference between the control and the treatments for vacuolization, goblet cell hyperplasia or villus shortening (Supplementary Figure S1). After combing all the scored histopathology features together, there was no significant difference in the intestinal muscular thickness and intestinal villi length between the groups (p > 0.05) (Supplementary Figure S2). Juvenile Jian carp showed multiple abnormal intestines after microplastics exposure (Supplementary Figure S3). The intestinal folds in the experimental group were not full or regular. However, no significant difference was found in the muscle thickness or villi length in Jian carp either (p > 0.05). Histopathological data of Jian carp are listed in Supplementary Table S1.
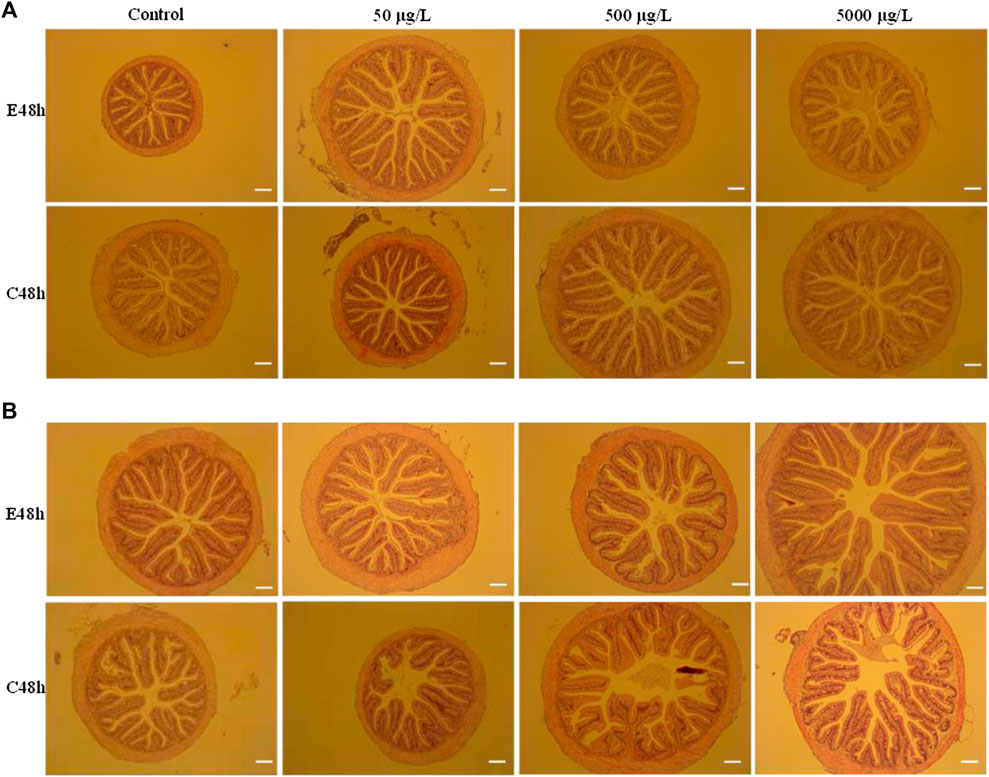
FIGURE 1. Histopathological analysis of intestines of largemouth bass juvenile exposed to polystyrene microspheres of 80 nm (A) and 8 μm (B) after exposure 48 h and clean 48 h. Exposure concentration and time were shown in the picture. Scale bar = 20 μm.
3.2 Transcriptional responses of target genes
After 8 μm microplastics exposure 48 h, the expression levels of the immune-related gene (IL-8) were significantly upregulated in the intestines of largemouth bass juvenile (p < 0.05) (Figure 2C). 80 nm microplastics caused upregulation of IL-8 in 48 h depuration after exposure 48 h (Figure 2A). Whereas the situation of high concentration exposure was different with mid and low concentration exposure (Figures 2A, C). Expression of Caspase 3 gene in the intestines of fish exposed 80 nm microplastics 48 h and cleaned 8 μm microplastics 48 h were significantly lower than that in the intestines of fish in the control group (p < 0.01) (Figures 2B, D).
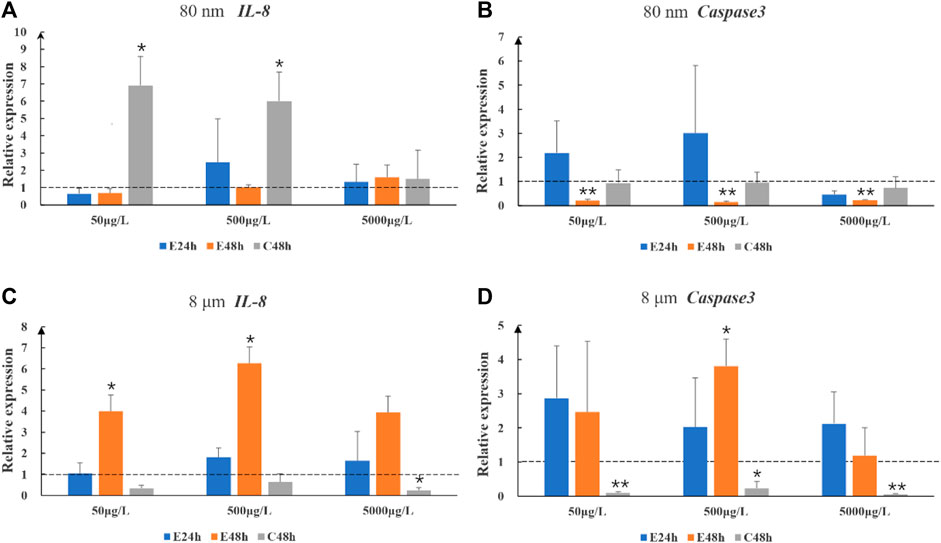
FIGURE 2. The relative gene expression levels (fold change) of IL-8 (A,C) and Caspase 3 (B,D) in intestines of largemouth bass juvenile exposed to microplastics. Data are expressed as mean ± standard deviation. Significant differences from control are shown (*p < 0.05; **p < 0.01).
The effects of microplastics on the expression of levels of immune-related genes in intestine tissues of grass carp are shown in Figure 3. The relative expression levels of IL-1β, IL-8, TGF-β1 and TNF-α were all observably upregulated (p < 0.01) when exposure 80 nm microplastics at low concentration (20 μg/L) in the start of 24 h. TGF-β1 and TNF-α expression level when exposure 80 nm microplastics 24 h at middle and high concentration (200 μg/L and 2000 μg/L) were significantly upregulated, rather than IL-1β and IL-8 expression level. However, there was different gene expression pattern when exposure 8 μm microplastics.
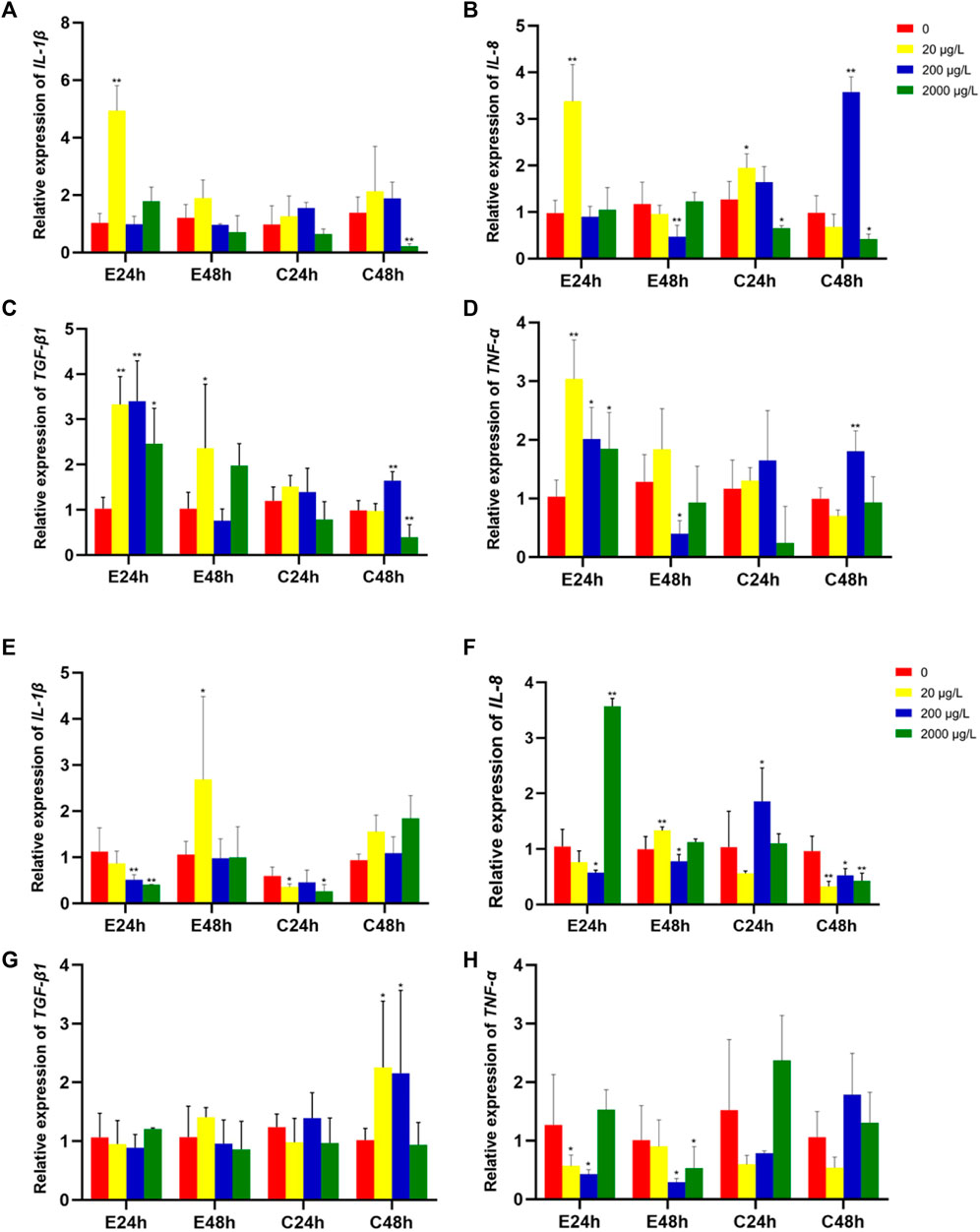
FIGURE 3. The relative gene expression levels (fold change) in intestines of grass carp juvenile exposed to microplastics of 80 nm (A–D) and 8 μm (E–H). Data are expressed as mean ± standard deviation. Significant differences from control are shown (*p < 0.05; **p < 0.01).
The mRNA expression levels of IL-1β, IL-10, TGF-β and TLR-2 in intestines of Jian carp juvenile exposed to microplastics of 80 nm and 8 μm are shown in Figure 4. The upregulation of pro-inflammatory cytokines, such as IL-1β and TLR-2, or/and downregulation of anti-inflammatory cytokines including TGF-β1 and IL-10 could cause inflammation in fish. Noteworthily, Jian carp cured better in 8 μm microplastics treatment than in 80 nm microplastics treatment.
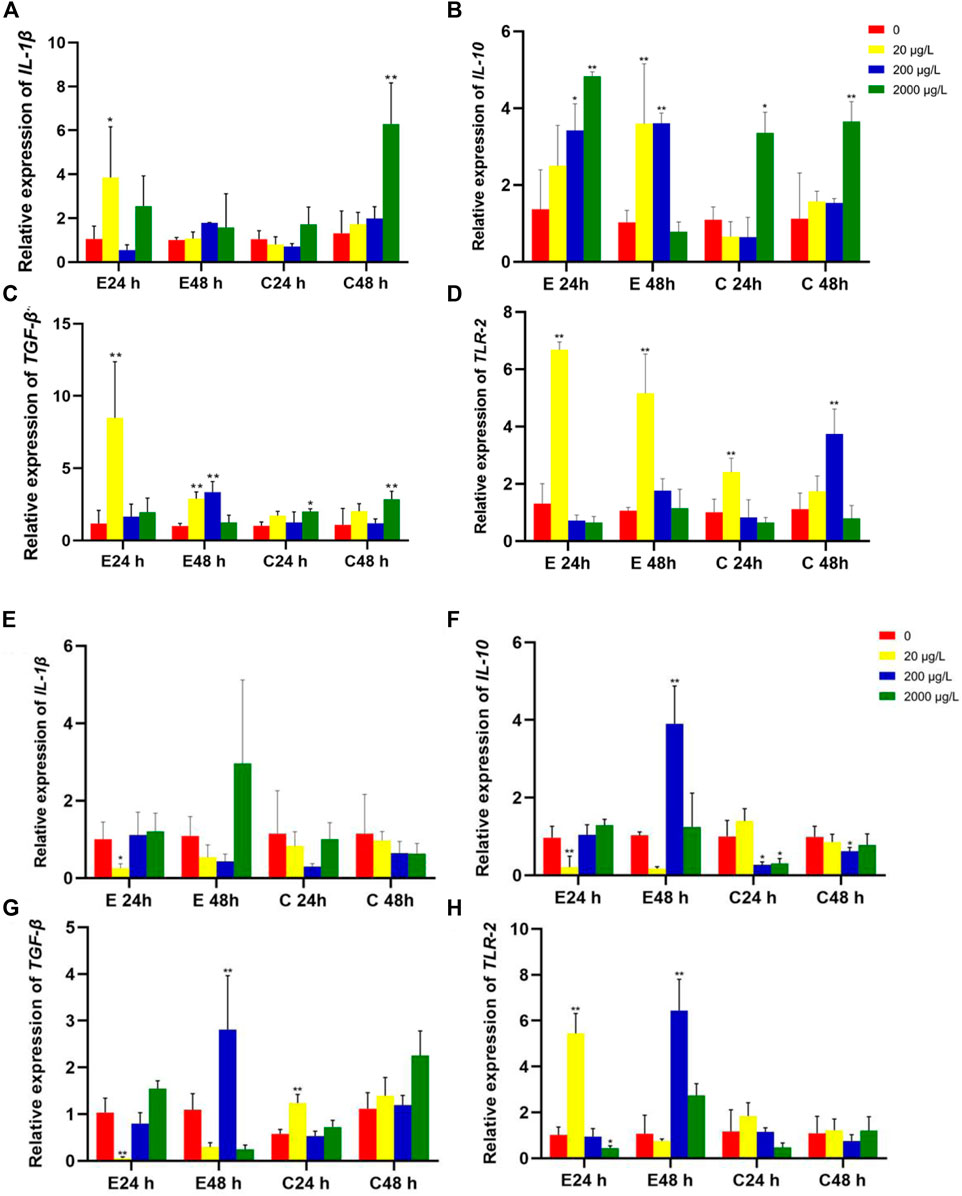
FIGURE 4. The relative gene expression levels (fold change) in intestines of Jian carp juvenile exposed to microplastics of 80 nm (A–D) and 8 μm (E–H). Data are expressed as mean ± standard deviation. Significant differences from control are shown (*p < 0.05; **p < 0.01).
4 Discussion
4.1 Effects of microplastics on intestinal morphology of fish
The intestinal morphological effects of microplastics with a dose-dependent way have been explored in various fishes. Over secretion of goblet cells was found in juvenile guppy (Poecilia reticulata) after exposing microplastics with 32–40 μm diameter, and the higher concentration of microplastics, the more goblet cells secreted (Huang et al., 2020). However, the loss of villus and crypt cells was significantly increased due to microplastic physical abrasion in the intestine of juvenile intertidal fish (Girella laevifrons), and leukocyte infiltration and hyperemia exposure in the high concentration group were more serious than those in the low concentration group (Ahrendt et al., 2020). In the European sea bass (Dicentrarchus labrax L.), intestinal tissues were altered after fish were fed with polyvinyl chloride (PVC) pellets for 90 days (Peda et al., 2016). Another morphometric analyses of sea bass fed polyethylene (PE) microplastics in the diets for 21 days showed a significant reduction in the amounts of goblet cells as well as a decrease in villus height (Espinosa et al., 2019). Histological analysis indicated that multiple abnormalities in intestines are presented in three species fishes after acute exposure microplastics in this study.
As we all known, intestine is vital for the digestion and absorption of nutrients, and intestinal morphology characters, such as muscular layer thickness, villi length, and the number of goblet cells indicate intestine health in fish. To some extent, abnormal in the intestinal sections is an immune response to external stimulus. On one hand, pathological changes of intestinal tract might be the result of microplastics intrusion. On the other hand, it is crucial to determine whether this intrusion outpaces the organism’s ability to repair itself. From histopathological analysis of intestines of largemouth bass juvenile exposed to 8 nm and 8 μm microspheres after exposure 48 h and clean 48 h (Figure 1), we found microplastics of lager size and higher concentration cause more serious damage, and the damage seems to be irreversible. Obviously, this change makes fish more sensitive to infection by pathogens. Compared with the intestinal slices of grass carp and Jian carp, Jian carp with smaller intestinal diameter and less perfect villus structure was more seriously damaged by microplastic invasion.
4.2 Effects of microplastics on immune-related genes expression of fish
Many animal studies have indicated that exposure to microplastics impairs oxidative and inflammatory bowel balance (Choi et al., 2018; Ding et al., 2018). Especially, microplastics cause intestinal inflammation, manifested by a significant increase in IL-1α levels in the intestine (Hirt and Body-Malapel, 2020). The immune function of organs is highly correlated with the inflammatory response, which is generally considered to be a typical defense response that protects the host from pathogens (Zhong et al., 2020). Cytokines mediate the inflammatory response in fish, which are mainly divided into pro-inflammatory factors (e.g., TNF-α, IL-1β and IL-10) and anti-inflammatory factors (e.g., IL-10 and TGF-β). For example, interleukin is a typical class of cytokines which is mainly involved in regulating all kinds of lymphocytes in the immune system. Tumor necrosis factor α (TNF-α), as pleiotropic proinflammatory and potent regulatory cytokines, can regulate cell proliferation, apoptosis or differentiation in the immune system (Cao et al., 2020). Toll-like receptors (TLRs), as a crucial innate receptor, can identify pathogen-associated molecular patterns (PAMPs) of invading microorganisms and induce downstream NF-κB activation and the production of TNF-α, IL-10 and other cytokines (Meng et al., 2021).
Previous research in adult male zebrafish (Danio rerio) showed that exposure to 1,000 μg/L of 0.5 μm microplastics for 14 days significantly upregulated the transcription levels of IL-1α, IL-1β, and Ifn in the intestine (Jin et al., 2018). In the present study, microplastics exposure significantly induced or restrained the mRNA expression of immune-related genes in the intestine tissues of fishes. There were differential expressions of genes coping with differential sizes and concentrations of microplastics in different fishes. Similarly, in other species, such as rats (Wei et al., 2021) and prawn (Li et al., 2023a/b), the mRNA abundance of immune-related genes was increased with microplastics exposure.
4.3 Response time and legacy effect of microplastics with different concentration and size
In terms of damage to intestinal morphology, acute exposure did not cause significant damage at the size and concentration of microplastics exposed in this paper. From the perspective of gene expression level, when exposed to nanoscale microplastics at low concentration, fish can promote self-repair through the upregulation of some inflammatory factors. For micron-scale microplastics, we hypothesized that part of microplastics could be removed by fish excretion after ingestion. Therefore, there was no significant difference in gene expression between the experimental fish and the control group during the recovery period. The effects of microplastics on juvenile fishes are species-specific, the specific mechanism needs to be further studied.
Although time had no significant effect on intestinal morphology, we hypothesized that it was related to exposure conditions. Thankfully, even when exposed to extremely high concentration (mg/L) of microplastics, there is no immediate visible damage to the intestinal morphology of fish. Response time and recovery time of gene expression was species-specific. Grass carp has the longest intestinal tract, followed by Jian carp, and largemouth bass has the shortest intestinal tract, which is related to their feeding habits. We hypothesize that the lag time of microplastics in fish intestine is related to the length of the intestine. A methodology to assess how effective Mediterranean fish species, that are known to have ingested marine plastic, were considered gut length as well, which showed fish with smaller gut length is more representative (Bray et al., 2019).
5 Conclusion
In this study, species-specific effects of microplastics on three fishes with different feeding types were investigated. The contributing concentration and size of microplastics, as well as the response time and legacy effect in fishes were also explored. Two different sizes of fluorescent microplastics (80 nm and 8 μm) were set at four concentrations. Multiple abnormalities in intestines were presented in three species fishes, and there were differential expressions of genes coping with differential sizes and concentrations of microplastics exposure in different fishes. The results of this study would be beneficial for extrapolating microplastics contamination risks to commercial fishes. The reason for the difference effects of microplastics on fishes was still unclear but could be due to the difference in the structure and function of the digestive system. This study will provide a valuable steppingstone for future research, where we hope to address the microplastics research gap between various fish species.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by Animal Ethics and Welfare Committee, South China Agricultural University.
Author contributions
CZ: Data curation, Investigation, Methodology, Project administration, Validation, Writing–original draft, Writing–review and editing. FW: Conceptualization, Supervision, Validation, Visualization, Writing–original draft. QW: Conceptualization, Data curation, Formal Analysis, Software, Writing–original draft. JiZ: Methodology, Project administration, Resources, Validation, Writing–review and editing. JuZ: Funding acquisition, Project administration, Validation, Visualization, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by Key Research and Development Program of Zhejiang Province (2019C02082) and Science and Technology Project of Zhejiang Province (2021YSZX007 and 2021C02069-2-02).
Acknowledgments
We thank the editor and reviewers for their constructive and insightful feedback, which improved the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2023.1256005/full#supplementary-material
References
Ahrendt, C., Perez-Venegas, D. J., Urbina, M., Gonzalez, C., Echeveste, P., Aldana, M., et al. (2020). Microplastic ingestion cause intestinal lesions in the intertidal fish Girella laevifrons. Mar. Pollut. Bull. 151, 110795. doi:10.1016/j.marpolbul.2019.110795
Alimi, O. S., Farner Budarz, J., Hernandez, L. M., and Tufenkji, N. (2018). Microplastics and nanoplastics in aquatic environments: Aggregation, deposition, and enhanced contaminant transport. Environ. Sci. Technol. 52, 1704–1724. doi:10.1021/acs.est.7b05559
Anna, K., Krause, S., Lynch, I., GregorySmith, H. S., and Nel, H. (2021). Nano and microplastic interactions with freshwater biota – current knowledge, challenges and future solutions. Environ. Int. 152, 106504. doi:10.1016/j.envint.2021.106504
Banaee, M., Soltanian, S., Sureda, A., Gholamhosseini, A., Haghi, B. N., Akhlaghi, M., et al. (2019). Evaluation of single and combined effects of cadmium and micro-plastic particles on biochemical and immunological parameters of common carp (Cyprinus carpio). Chemosphere 236, 124335. doi:10.1016/j.chemosphere.2019.07.066
Bray, L., Digka, N., Tsangaris, C., Camedda, A., Gambaiani, D., de Lucia, G. A., et al. (2019). Determining suitable fish to monitor plastic ingestion trends in the Mediterranean Sea. Environ. Pollut. 247, 1071–1077. doi:10.1016/j.envpol.2019.01.100
Brun, N. R., van Hage, P., Hunting, E. R., Haramis, A. G., Vink, S. C., Vijver, M. G., et al. (2019). Polystyrene nanoplastics disrupt glucose metabolism and cortisol levels with a possible link to behavioural changes in larval zebrafish. Commun. Biol. 2, 382. doi:10.1038/s42003-019-0629-6
Cao, S., Xiong, D., Luo, W., Tang, J., Qu, F., Zhou, Y., et al. (2020). Effects of dietary soy isoflavones on growth, antioxidant status, immune response and resistance of juvenile grass carp (Ctenopharyngodon idella) to Aeromonas hydrophila challenge. Aquac. Res. 51, 2472–2482. doi:10.1111/are.14590
Choi, J. S., Jung, Y., Hong, N., Hong, S. H., and Park, J. (2018). Toxicological effects of irregularly shaped and spherical microplastics in a marine teleost, the sheepshead minnow (Cyprinodon variegatus). Mar. Pollut. Bull. 129, 231–234. doi:10.7181/acfs.2018.02033
Cole, M., Lindeque, P., Halsband, C., and Galloway, T. S. (2011). Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 62, 2588–2597. doi:10.1016/j.marpolbul.2011.09.025
Ding, J., Zhang, S., Razanajatovo, R. M., Zou, H., and Zhu, W. (2018). Accumulation, tissue distribution, and biochemical effects of polystyrene microplastics in the freshwater fish red tilapia (Oreochromis niloticus). Environ. Pollut. 238, 1–9. doi:10.1016/j.envpol.2018.03.001
Espinosa, C., Esteban, M. Á., and Cuesta, A. (2019). Dietary administration of PVC and PE microplastics produces histological damage, oxidative stress and immunoregulation in European sea bass (Dicentrarchus labrax L). Fish. Shellfish Immun. 95, 574–583. doi:10.1016/j.fsi.2019.10.072
Garrido Gamarro, E., Ryder, J., Elvevoll, E. O., and Olsen, R. L. (2020). Microplastics in fish and shellfish - a threat to seafood safety? J. Aquat. Food Prod. Trans. 29, 417–425. doi:10.1080/10498850.2020.1739793
Gu, H., Wang, S., Wang, X., Yu, X., Hu, M., Huang, W., et al. (2020). Nanoplastics impair the intestinal health of the juvenile large yellow croaker Larimichthys crocea. J. Hazard. Mat. 397, 122773. doi:10.1016/j.jhazmat.2020.122773
Guimarães, A. T. B., Estrela, F. N., Rodrigues, A. S. D. L., Chagas, T. Q., Pereira, P. S., Silva, F. G., et al. (2021). Nanopolystyrene particles at environmentally relevant concentrations causes behavioral and biochemical changes in juvenile grass carp (Ctenopharyngodon idella). J. Hazard. Mat. 403, 123864. doi:10.1016/j.jhazmat.2020.123864
Hirt, N., and Body-Malapel, M. (2020). Immunotoxicity and intestinal effects of nano- and microplastics: A review of the literature. Part. Fibre Toxicol. 17, 57. doi:10.1186/s12989-020-00387-7
Huang, J. N., Wen, B., Zhu, J. G., Zhang, Y. S., Gao, J. Z., and Chen, Z. Z. (2020). Exposure to microplastics impairs digestive performance, stimulates immune response and induces microbiota dysbiosis in the gut of juvenile guppy (Poecilia reticulata). Sci. Total Environ. 733, 138929. doi:10.1016/j.scitotenv.2020.138929
Iheanacho, S. C., and Odo, G. E. (2020). Dietary exposure to polyvinyl chloride microparticles induced oxidative stress and hepatic damage in Clarias gariepinus (Burchell, 1822). Environ. Sci. Pollut. Res. Int. 27, 21159–21173. doi:10.1007/s11356-020-08611-9
Jabeen, K., Li, B., Chen, Q., Su, L., Wu, C., Hollert, H., et al. (2018). Effects of virgin microplastics on goldfish (Carassius auratus). Chemosphere 213, 323–332. doi:10.1016/j.chemosphere.2018.09.031
Jacob, H., Besson, M., Swarzenski, P. W., Lecchini, D., and Metian, M. (2020). Effects of virgin micro- and nanoplastics on fish: Trends, meta-analysis, and perspectives. Environ. Sci. Technol. 54, 4733–4745. doi:10.1021/acs.est.9b05995
Jin, Y., Xia, J., Pan, Z., Yang, J., Wang, W., and Fu, Z. (2018). Polystyrene microplastics induce microbiota dysbiosis and inflammation in the gut of adult zebrafish. Environ. Pollut. 235, 322–329. doi:10.1016/j.envpol.2017.12.088
Li, Y., Du, X., Li, W., Jiang, Q., Ye, Y., Yang, Y., et al. (2023a). Two genes related to apoptosis in the hepatopancreas of juvenile prawn, Macrobrachium nipponense: Molecular characterization and transcriptional response to nanoplastic exposure. Sci. Total Environ. 877, 162863. doi:10.1016/j.scitotenv.2023.162863
Li, Y., Liu, Z., Li, M., Jiang, Q., Wu, D., Huang, Y., et al. (2020). Effects of nanoplastics on antioxidant and immune enzyme activities and related gene expression in juvenile Macrobrachium nipponense. J. Hazard. Mat. 398, 122990. doi:10.1016/j.jhazmat.2020.122990
Li, Y., Ye, Y., Na, R., Jiang, Q., Liu, X., Zhao, Y., et al. (2023b). Polystyrene nanoplastics decrease nutrient accumulation, disturb sex hormones, and inhibit reproductive development in juvenile Macrobrachium nipponense. Sci. Total Environ. 891, 164481. doi:10.1016/j.scitotenv.2023.164481
Lin, Y., Zeng, D., He, P., Wei, P., Hui, W., Wu, T., et al. (2019). mRNA and microRNA transcriptomics analyses in intermuscular bones of two carp species, rice flower carp (Cyprinus carpio var. Quanzhounensis) and Jian carp (Cyprinus carpio var. Jian). Comp. Biochem. Physiol. Part D. Genomics Proteomics 30, 71–80. doi:10.1016/j.cbd.2019.01.013
Mallik, A., Martin Xavier, K. A., Naidu, B. C., and Nayak, B. B. (2021). Ecotoxicological and physiological risks of microplastics on fish and their possible mitigation measures. Sci. Total Environ. 779, 146433. doi:10.1016/j.scitotenv.2021.146433
McDevitt, J. P., Criddle, C. S., Morse, M., Hale, R. C., Bott, C. B., and Rochman, C. M. (2017). Addressing the issue of microplastics in the wake of the microbead-free waters act—a new standard can facilitate improved policy. Environ. Sci. Technol. 51, 6611–6617. doi:10.1021/acs.est.6b05812
Meng, X., Wu, S., Hu, W., Zhu, Z., Yang, G., Zhang, Y., et al. (2021). Clostridium butyricum improves immune responses and remodels the intestinal microbiota of common carp (Cyprinus carpio L). Aquaculture 530, 735753. doi:10.1016/j.aquaculture.2020.735753
Mizraji, R., Ahrendt, C., Perez-Venegas, D., Vargas, J., Pulgar, J., Aldana, M., et al. (2017). Is the feeding type related with the content of microplastics in intertidal fish gut? Mar. Pollut. Bull. 116, 498–500. doi:10.1016/j.marpolbul.2017.01.008
NOAA (2015). National oceanic and atmospheric administration. Microplastics Available at: https://marinedebris.noaa.gov/sites/default/files/MicroplasticsOnePager_0.pdf.
Peda, C., Caccamo, L., Fossi, M. C., Gai, F., Andaloro, F., Genovese, L., et al. (2016). Intestinal alterations in European sea bass Dicentrarchus labrax (Linnaeus, 1758) exposed to microplastics: Preliminary results. Environ. Pollut. 212, 251–256. doi:10.1016/j.envpol.2016.01.083
Peng, L., Fu, D., Qi, H., Lan, C. Q., Yu, H., and Ge, C. (2020). Micro- and nano-plastics in marine environment: Source, distribution and threats — a review. Sci. Total Environ. 698, 134254. doi:10.1016/j.scitotenv.2019.134254
Plastics Europe (2022). Plastics - the facts 2021: An analysis of European plastics production, demand and waste data. Available at: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/.
Rai, P. K., Lee, J., Brown, R. J. C., and Kim, K. (2021). Environmental fate, ecotoxicity biomarkers, and potential health effects of micro- and nano-scale plastic contamination. J. Hazard. Mat. 403, 123910. doi:10.1016/j.jhazmat.2020.123910
Rios-Fuster, B., Arechavala-Lopez, P., García-Marcos, K., Alomar, C., Compa, M., Álvarez, E., et al. (2021). Experimental evidence of physiological and behavioral effects of microplastic ingestion in Sparus aurata. Aquat. Toxicol. 231, 105737. doi:10.1016/j.aquatox.2020.105737
Shi, W., Sun, S., Han, Y., Tang, Y., Zhou, W., Du, X., et al. (2021). Microplastics impair olfactory-mediated behaviors of goldfish Carassius auratus. J. Hazard. Mat. 409, 125016. doi:10.1016/j.jhazmat.2020.125016
Wang, S., Ge, J., Yu, X., and Li, H. (2020a). Environmental fate and impacts of microplastics in soil ecosystems: Progress and perspective. Sci. Total Environ. 708, 134841. doi:10.1016/j.scitotenv.2019.134841
Wang, S., Ni, J., Nie, Z., Gao, J., Sun, Y., Shao, N., et al. (2020c). Effects of stocking density on growth, serum parameters, antioxidant status, liver and intestine histology and gene expression of largemouth bass (Micropterus salmoides) farmed in the in-pond raceway system. Aquac. Res. 51, 5228–5240. doi:10.1111/are.14862
Wang, S., Zhang, C., Pan, Z., Sun, D., Zhou, A., Xie, S., et al. (2020b). Microplastics in wild freshwater fish of different feeding habits from Beijiang and Pearl River Delta regions, south China. Chemosphere 258, 127345. doi:10.1016/j.chemosphere.2020.127345
Wei, J., Wang, X., Liu, Q., Zhou, N., Zhu, S., Li, Z., et al. (2021). The impact of polystyrene microplastics on cardiomyocytes pyroptosis through NLRP3/Caspase-1 signaling pathway and oxidative stress in Wistar rats. Environ. Toxicol. 36, 935–944. doi:10.1002/tox.23095
Xu, J., and Li, D. (2021). Feeding behavior responses of a juvenile hybrid grouper, Epinephelus fuscoguttatus♀ × E. lanceolatus♂, to microplastics. Environ. Pollut. 268, 115648. doi:10.1016/j.envpol.2020.115648
Xu, S., Ma, J., Ji, R., Pan, K., and Miao, A. (2020). Microplastics in aquatic environments: Occurrence, accumulation, and biological effects. Sci. Total Environ. 703, 134699. doi:10.1016/j.scitotenv.2019.134699
Zhang, C., Wang, J., Zhou, A., Ye, Q., Feng, Y., Wang, Z., et al. (2021). Species-specific effect of microplastics on fish embryos and observation of toxicity kinetics in larvae. J. Hazard. Mat. 403, 123948. doi:10.1016/j.jhazmat.2020.123948
Zhang, S., Ding, J., Razanajatovo, R. M., Jiang, H., Zou, H., and Zhu, W. (2019). Interactive effects of polystyrene microplastics and roxithromycin on bioaccumulation and biochemical status in the freshwater fish red tilapia (Oreochromis niloticus). Sci. Total Environ. 648, 1431–1439. doi:10.1016/j.scitotenv.2018.08.266
Zhong, J., Wu, P., Feng, L., Jiang, W., Liu, Y., Kuang, S., et al. (2020). Dietary phytic acid weakened the antimicrobial activity and aggravated the inflammatory status of head kidney, spleen and skin in on-growing grass carp (Ctenopharyngodon idella). Fish. Shellfish Immun. 103, 256–265. doi:10.1016/j.fsi.2020.05.037
Keywords: microplastics, species-specific, juvenile fish, gene expression, intestinal morphology
Citation: Zhang C, Wang F, Wang Q, Zou J and Zhu J (2023) Species-specific effects of microplastics on juvenile fishes. Front. Physiol. 14:1256005. doi: 10.3389/fphys.2023.1256005
Received: 10 July 2023; Accepted: 20 July 2023;
Published: 04 August 2023.
Edited by:
Yiming Li, Fishery Machinery and Instrument Research Institute, ChinaReviewed by:
Jinran Wu, Australian Catholic University, AustraliaZhenlu Wang, Guizhou University, China
Copyright © 2023 Zhang, Wang, Wang, Zou and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junjie Zhu, Zhjj@zjhu.edu.cn
 Chaonan Zhang1,2,3
Chaonan Zhang1,2,3 Qiujie Wang
Qiujie Wang Jixing Zou
Jixing Zou Junjie Zhu
Junjie Zhu