- 1Guangdong Engineering Research Center for Marine Algal Biotechnology, Guangdong Provincial Key Laboratory for Plant Epigenetics, Shenzhen Engineering Laboratory for Marine Algal Biotechnology, Longhua Innovation Institute for Biotechnology, College of Life Sciences and Oceanography, Shenzhen University, Shenzhen, China
- 2Key Laboratory of Optoelectronic Devices and Systems of Ministry of Education and Guangdong Province, College of Physics and Optoelectronic Engineering, Shenzhen University, Shenzhen, China
- 3Key Laboratory of Precision Nutrition and Food Quality, Department of Nutrition and Health, China Agricultural University, Beijing, China
Introduction
Human mitochondrial diseases are commonly caused by mutations in mitochondrial DNA (mtDNA). The severity of mitochondrial disease is associated with heteroplasmy, which is defined as the coexistence of two or more different mtDNA variants within one cell (Taylor and Turnbull, 2005). Although mitochondria-targeted zinc-finger nucleases (mitoZFNs) or mitochondria-targeted transcription activator-like effector nucleases (mitoTALENs) can be used for mitochondrial genome editing, they have limitations that include the laborious design and assembly of the monomers, their limited sequence specificity and large size. The CRISPR/Cas genome editing system is a powerful tool to precisely edit the genomes of a wide range of mammals and plants. However, the biggest challenge in utilizing this system in mitochondria is the delivery of exogenous guide RNA (gRNA) into mitochondria. Previous attempts at delivering gRNA via stem-loop motifs have been reported but there is no robust evidence to show the success of this approach. In the future, the efficient delivery of gRNA, with a mitochondrial localization signal (MLS), and the effective cleavage activity of the modified gRNA/Cas complex will be necessary for mitochondrial genome editing by CRISPR/Cas system.
Current Methods for Mitochondrial Genome Editing
Mitochondrial Genome and mtDNA Disorder
Mitochondria are double membrane-bounded organelles that are known as the “powerhouse of the cell.” They contain maternally inherited, double-stranded, multi-copy DNA of 16.5 kb. The genome is circular and encodes 13 protein subunits involved in the respiratory chain, 22 transfer RNAs (tRNAs) and two ribosomal RNAs (rRNAs) (Falkenberg et al., 2007).
Several mtDNA mutations have been associated with mitochondrial dysfunction (Wallace and Chalkia, 2013). As there are multiple copies of mtDNA within a cell, pathogenic mutations in human mtDNA are commonly heteroplasmic. When the percentage of mutated mtDNA molecules exceeds the threshold that compromises the mitochondrial function, thereby disrupting the overall cellular function, mtDNA disorder will occur (Vafai and Mootha, 2012). It has been reported that the A3243G mutation, at 50%–90% mtDNA heteroplasmy, may cause the severe mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes (MELAS) syndrome (Goto et al., 1990; Picard et al., 2014).
Mitochondria-Targeted DNA Nucleases
For clinical applications that target mtDNA disorder, it is essential to achieve homoplasmic status in a cell by eliminating the mutated mtDNA. In contrast to nuclear DNA (nDNA) repair pathways, the efficient DNA double-strand break (DSB) repair and homologous recombination (HR) mechanisms are lacking in mammalian mitochondria (Nissanka et al., 2019; Carvalho et al., 2021). Once cut on both strands, mtDNA molecules are not repaired, which results in rapid degradation of mtDNA in mammalian cells (Peeva et al., 2018). Hence, several approaches have been developed to use mitochondria-targeted and site-specific DNA nucleases that will quickly degrade the mutant mtDNA in heteroplasmic cells, to adjust the heteroplasmy ratio (Patananan et al., 2016; Silva-Pinheiro and Minczuk, 2022). The expression of mitochondria-targeted restriction endonucleases (mtREs) has been used to shift the mtDNA heteroplasmy ratio from mutant to wild-type in the mouse model and patient somatic cells. Results indicated that mtREs specifically reduce the amount of mutated mtDNA molecules (Srivastava and Moraes, 2001; Tanaka et al., 2002). However, this mtRE approach has obvious limitations. For example, only one unique restriction site (XmaI) has been found to arise in approximately 200 different mtDNA mutations (Reddy et al., 2015). To overcome the limitation of mtREs, other alternative approaches have been developed. The use of mitoZFNs or mitoTALENs (Figure 1A), which are composed of a mitochondrial targeting sequence (MTS), the specific DNA recognition modules and a non-specific FokI nuclease, has successfully altered the heteroplasmy ratio in previous studies (Minczuk et al., 2008; Bacman et al., 2013; Gammage et al., 2014; Reddy et al., 2015; Gammage et al., 2016; Bacman et al., 2018; Gammage et al., 2018b). Zinc-finger proteins, as the recognition modules of the mitoZFN monomer, can recognize a 12 bp sequence. Unlike mitoZFN, the mitoTALEN monomer utilizes TAL effector proteins as the recognition modules that can recognize approximately 17 nucleotides. In addition to the conventional mitoTALEN monomer, a new monomer, mitoTev-TALE, has been successfully used to manipulate mutant mtDNA in cybrid cells (Pereira et al., 2018). The TAL effector proteins are linked to the I-TevI nuclease in mitoTev-TALE, rather than the FokI nuclease, as in mitoTALEN. Recently, a radically different approach for mtDNA editing has been reported, namely double-stranded DNA deaminase (DddA)-derived cytosine base editors (DdCBEs) (Mok et al., 2020; Lee et al., 2021; Silva-Pinheiro et al., 2022). The DdCBE is composed of mitoTALE proteins, the interbacterial toxin DddA and an uracil glycosylase inhibitor. It is able to precisely catalyze C•G-to-T•A conversions in human mtDNA with high target specificity.
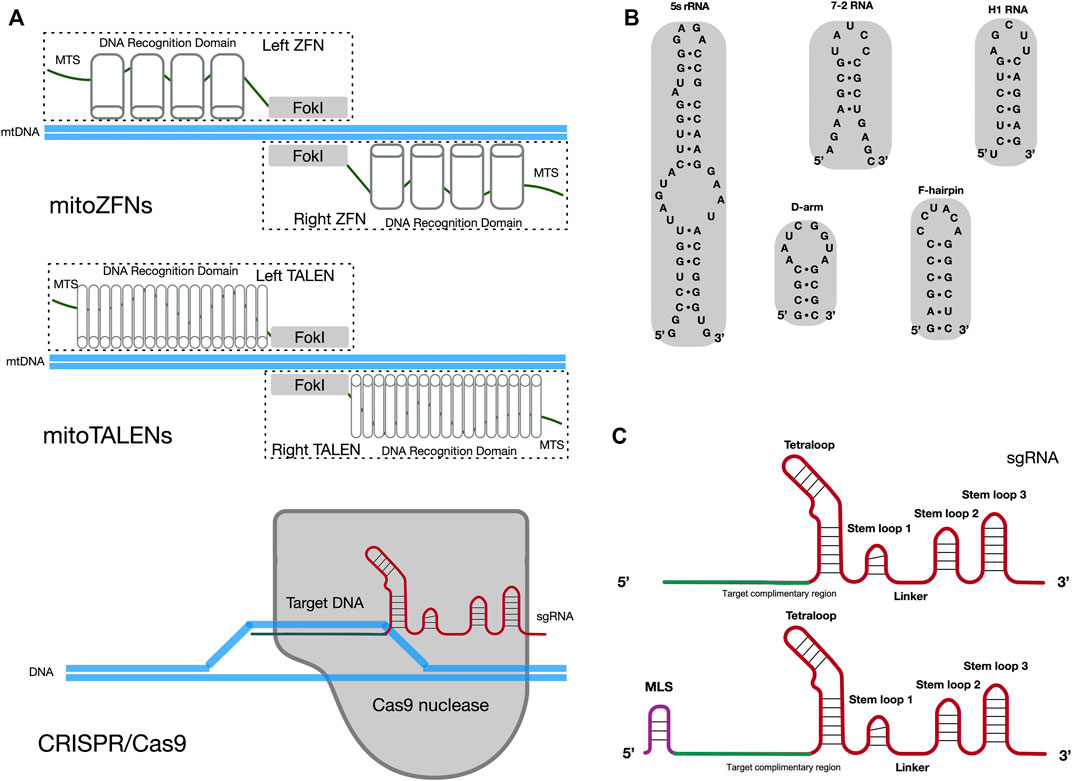
FIGURE 1. Potential methods for sgRNA importation into mitochondria. (A) Diagram illustrating the cleavage mechanism of mitoZFNs, mitoTALENs and the CRISPR/Cas9 system. MTS, mitochondrial targeting sequence; FokI, non-specific FokI nuclease; sgRNA, single guide RNA. (B) Stem-loop motifs identified in human 5s rRNA, 7-2 RNA, H1 RNA,D-arm and F-hairpin motifs identified in yeast tRNALys (CUU). (C) The secondary structures of the original sgRNA and sgRNA with MLS. The original sgRNA is comprised of three stem loops and one tetraloop. When compared with the original sgRNA, the potential mitochondria-targeted sgRNA may be have an additional MLS at the 5′-end. The target complimentary region, which the RNA sequence for recognized target DNA, is shown in green. The backbone of the sgRNA is shown in red. MLS is the mitochondrial localization signal and is shown in purple.
Despite the promising results of mitoZFNs and mitoTALENs in mtDNA editing, these methods have limitations. Since each mitoZFN or mitoTALEN monomer needs to be designed and engineered to recognize a specific range of mtDNA sequences, they require enormous labor and cost-intensive protein production. In addition, the size of the coding nuclease sequences exceeds the capacity of current virus-based (adeno-associated virus, AAV) delivery systems (Moraes, 2014). The CRISPR/Cas9 genome editing system may solve this issue as it relies on the single guide RNA (sgRNA) to recognize a specific 20 bp DNA sequence and only Cas9 nuclease can cleave this specific DNA sequence (Figure 1A) (Hsu et al., 2013; Ran et al., 2013; Doudna and Charpentier, 2014). Currently, reliable chloroplast and mitochondrial genome editing methods by CRISPR/Cas9 system exist only in yeast and green algae (Yoo et al., 2020). Yoo et al. introduced plasmids, which contained a cassette for the organelle-specific expression of Cas9 nuclease and a cassette for the expression of sgRNA, into yeast mitochondria and Chlamydomonas chloroplasts through microprojectile transformation. The authors confirmed the insertion of donor DNA at the target sites of organelle DNA, facilitated by HR, only in the presence of Cas9/sgRNA activity. In mammals, two independent studies have attempted to edit mtDNA through the utilization of a CRISPR/Cas9 genome editing system (Jo et al., 2015; Bian et al., 2019). In these studies, mitochondria-targeted Cas9 nucleases (mitoCas9) and unmodified sgRNAs in cells resulted in mtDNA cleavage. These data implied that the original sgRNA, without any modifications, was spontaneously delivered into mitochondria from the cytosol. This occurred despite the double-membrane structure of mitochondria making it difficult for exogenous RNA and proteins to spontaneously enter mammalian mitochondria. However, attempts to repeat this work, by other research groups, have not been successful (Pereira and Moraes, 2017). Therefore, it is not clear whether the CRISPR/Cas9 genome editing system is able to cleave or edit mammalian mtDNA, due to the challenge of importing the exogenous sgRNA into the mitochondria.
In contrast to mitochondrial protein, the mechanism of importing nucleus-encoded RNA into mitochondria is still not fully understood (Gammage et al., 2018a). A controversial mechanism of importing RNA into mammalian mitochondria, mediated by polynucleotide phosphorylase (PNPase), is not widely accepted (Wang et al., 2010; Wang et al., 2012a). If the delivery of exogenous sgRNA into mitochondria can be achieved, the CRISPR/Cas9 genome editing system could be widely used to edit the mitochondrial genome.
Delivery of Exogenous sgRNA Into Mitochondria
There are numerous endogenous RNAs reported to have functional roles in mammalian mitochondria, such as H1 RNA (RNase P), 7-2 RNA (RNase MRP) and 5S rRNA (Puranam and Attardi, 2001; Mercer et al., 2011; Smirnov et al., 2011). Notably, some distinct stem-loop motifs have been found at the 5′ or 3′ -end of these RNA molecules and could mediate the import of nucleus-encoded RNA molecules into mitochondria (Figure 1B). For example, it has been suggested that stem-loop motifs identified in H1 RNA and 5S rRNA are MLS for mitochondrial import of RNA molecules (Wang et al., 2012b; Towheed et al., 2014; Zelenka and Ježek, 2016). Meanwhile, other studies have claimed that the delivery of synthetic RNA into mammalian mitochondria can be achieved through the utilization of F-arm or D-hairpin motifs from yeast cytosolic tRNALys (CUU) (Gowher et al., 2013; Tonin et al., 2014). In the CRISPR/Cas9 system, the sgRNA is created by fusing the CRISPR RNA (crRNA) and trans-activating crRNA (tracrRNA) sequences together into a single RNA chimera. It forms a T-shaped architecture, with one tetraloop and three stem-loops. Stem-loop 1 is essential for the formation of a functional Cas9/sgRNA complex. Stem-loop 2 and stem-loop 3 support the stable complex formation and enhance the stability of the sgRNA (Jinek et al., 2012; Anders et al., 2014; Nishimasu et al., 2014). Another study has suggested that sgRNA, with an elongated 5’ end or insertions in the tetraloop, should not significantly disrupt the CRISPR/Cas9 system (Tang et al., 2017).
The above studies provide some inspiration for methods of delivery of exogenous sgRNA into mitochondria. The potential mitochondria-targeted sgRNA architecture may be comprised of additional MLS at the 5′-end of the sgRNA and conserved structures in the sgRNA (three stem-loops and one tetraloop), to ensure that it is imported into mitochondria and to meet the requirement for Cas9/sgRNA complex formation, respectively (Figure 1C). The varied stem-loop motifs (20–40 nt RNA sequences) identified in mitochondria-targeted RNAs act as the MLS for sgRNA and may provide a potential pathway for importing sgRNA into mammalian mitochondria.
Mitochondrial Genome Editing by CRISPR/Cas9 System
Attempts to develop a mitochondria-adapted CRISPR/Cas9 system, using mitoCas9 and sgRNA that contains the stem-loop motif, have been reported. One such study declared that the stem-loop motif from H1 RNA can mediate delivery of sgRNA into mitochondria. In mouse cells, modified sgRNA/mitoCas9 complexes were able to reduce the amount of mtDNA that carried the 11205G mutation in the mitochondrial ND4 gene (mtND4). Both modified sgRNAs, with the stem-loop motif from H1 RNA, and non-modified sgRNAs were present in the mitochondrial fraction. Modified sgRNAs were highly enriched in the mitochondria of transfected cells versus non-modified controls (Hussain et al., 2021). However, the process used in this study for mitochondria isolation did not remove the outer mitochondrial membrane to eliminate outer-membrane contaminants. Consequently, the levels of sgRNAs detected in the mitochondrial fraction may be over-estimated, due to contamination by cytosolic RNAs and RNAs associated with the outer membrane. In addition, analysis and quantification of mtDNA content, 48 and 72 h after cells were transfected, in the control and experimental groups, have not been performed in this study. The additional long-term analysis of mtDNA content may strengthen the conclusion that the mitochondria-adapted CRISPR/Cas9 system reduces the mitochondrial genomes.
Another study claimed that using a pair of modified sgRNAs, with an F-arm motif from yeast cytosolic tRNALys (CUU), reduced the mtDNA content in Kearns Sayre Syndrome cybrids, through incorporation of mitoCas9. No changes in mtDNA content were observed when using a single modified sgRNA. Moreover, any significant shift of mtDNA heteroplasmy was not observed (Loutre et al., 2018). Loutre et al. detected either the modified sgRNAs, with an F-arm/D-hairpin, or original sgRNAs that lacked the stem-loop motif in the enriched mitochondrial RNA samples. The sgRNAs bearing the F-arm motif and the original sgRNAs had a similar ability (approximately 35%) to enter mitochondria. These data implied that mitochondria-adapted CRISPR/Cas9 system was unbale to specifically reduce mutant mtDNA content, and the F-arm motif was unable to improve the mitochondrial import of sgRNA. In addition, the cleavage activity of the sgRNA (with an F-arm or D-hairpin motif)/Cas9 complex had been partially or completely blocked, which meant that the stem-loop motif may have influenced the binding of sgRNA and Cas9 nuclease or the recognition process of the sgRNA and targeted DNA.
A different study found that numerous insertion/deletion (InDel) events in mtDNA were generated using the unmodified sgRNA/mitoCas9 complex (Wang et al., 2021). Interestingly, in this study, the InDel frequencies generated by the mitochondria-adapted CRISPR/Cas9 system were low, at <0.05%, which was much lower than the elimination efficacies of mutated mtDNA by mitoTALENs/ZFNs. Moreover, modified sgRNAs, with the stem-loop motif from 7-2 RNA or H1 RNA, were unable to increase the frequencies of InDel, when compared with the original sgRNA. These data implied that stem-loop motifs from 7-2 RNA and H1 RNA were unable to improve the import of sgRNA into mitochondria. Generally, homology between nuclear mitochondrial DNA sequences (NUMTs) and mtDNA causes problems for the detection of mtDNA variants from Next Generation Sequencing (NGS) data because the origin of sequences cannot be determined (Maude et al., 2019). Hence, the InDel events determined by NGS may be over-estimated in this study. Wang et al. need to prove that the observed InDel events happened in mtDNA and not in NUMTs. If this is not properly addressed, it will undermine the credibility of reports of CRISPR-mediated mtDNA manipulation.
Mitochondrial Genome Editing by CRISPR/Cas12a System
In contrast to the CRISPR/Cas9 system, the CRISPR/Cas12a system only requires a single crRNA (42–44 nt) as gRNA (Zetsche et al., 2015; Fonfara et al., 2016). Recently, the use of a mitochondria-adapted CRISPR/Cas12a (mitoCRISPR/Cas12a) system for mtDNA manipulation has been reported (Antón et al., 2020). Antón et al. demonstrated that LbCas12a nucleases were localized to mitochondria with high efficiency and caused less mitochondrial damage than the other Cas nucleases, such as SpCas9 and SaCas9. These data suggested that LbCas12a had a much higher predisposition to be imported into mitochondria. In this study, unmodified sgRNA, sgRNA with the stem-loop motif from H1 RNA and sgRNA with the stem-loop motif from yeast tRNALys (CUU) were detected in the nuclear fraction and, to a lesser extent, the mitochondrial fraction. These data showed that sgRNA can be imported into mitochondria regardless of whether an MLS was present. As with the Hussain et al. work, the levels of sgRNAs observed in mitochondria may be over-estimated, because the outer-membrane contaminants had not yet been fully removed from the mitochondrial fraction. Although the authors had not performed any assessment of the delivery efficiency of crRNAs (with or without an MLS) into mitochondria, they still tested the activity of mitoLbCas12a/crRNA (with or without the stem-loop motif from H1 RNA) complexes in the MELAS cybrids. In theory, mitoLbCas12a/crRNA complexes would reduce the mtDNA content. However, instead both modified and unmodified crRNAs seem to have increased the amount of mtDNA in a mitoLbCas12a-dependent manner. The authors did not explain the reasons for the mitoCRISPR/Cas12a system effects. These confusing results mean that it remains unclear whether the CRISPR/Cas12a genome editing system is able to edit mammalian mtDNA.
Discussion
Although these reported data are not perfect and are unable to provide reasonable evidence to show that stem-loop motifs (D-hairpin, F-arm, 7-2 RNA and H1 RNA) enhance the delivery efficiency of gRNA (sgRNA and crRNA) into mammalian mitochondria, these attempts can provide many new perspectives on the import of gRNA. Meanwhile, several research reports have shown that nuclear non-coding RNAs (ncRNAs) act as important mediators of the crosstalk between the nucleus and the mitochondria. These ncRNAs could directly impact mitochondria by affecting transcripts of the mitochondrial genome (Kim et al., 2017; Vendramin et al., 2017). Further modification of the gRNA scaffold, by integrating the recent knowledge of mitochondrial ncRNA transport, may show promise for increasing the delivery efficiency of gRNA into mitochondria. The CRISPR screening strategy may efficiently identify appropriate architectures of gRNA with an MLS, for mitochondria-adapted CRISPR/Cas genome editing. For specific mutations associated with mtDNA disorder, the cleavage activity of the modified gRNA/Cas complex may require validation by exploration and confirmation in vivo and in vitro, as MLS may affect the binding of gRNA and Cas nucleases or the recognition of gRNA and targeted DNA sequences. Therefore, the utilization of potential MLS from mitochondria-targeted RNAs, for the import of gRNAs into mitochondria, can provide a potential future for mitochondrial genome editing by CRISPR/Cas system, which remains a relatively optimum strategy.
Human mtDNA mutations often affect mitochondrial function and cause mitochondrial diseases. Manipulation of the mutated mitochondrial genome is the most direct and thorough approach to resolve mitochondrial dysfunction. Currently, owing to the lack of an efficient delivery system to import gRNA into the mitochondria, the popular CRISPR/Cas genome editing method is still restricted to nuclear genomes and cannot efficiently be used to edit mitochondrial genomes. Early attempts to deliver gRNA into mitochondria were unsuccessful but may bring inspiration to follow-up researchers. Success at delivering exogenous gRNA into mammalian mitochondria may create new possibilities for mtDNA editing and will also help to prevent mitochondria disorder.
Author Contributions
TY prepared the image and wrote the manuscript. TY, JJ-L, and DQ-H joined the discussion for the revision. DQ-H and JJ-L revised the manuscript. TY and HL designed the review article. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the National Natural Science Foundation of China (No. 31970366) and China National Key Research and Development Project (No.2018YFA0902500).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Anders C., Niewoehner O., Duerst A., Jinek M. (2014). Structural Basis of PAM-dependent Target DNA Recognition by the Cas9 Endonuclease. Nature 513 (7519), 569–573. doi:10.1038/nature13579
Antón Z., Mullally G., Ford H., van der Kamp M. W., Szczelkun M. D., Lane J. D. (2020). Mitochondrial Import, Health and mtDNA Copy Number Variability Using Type II and Type V CRISPR Effectors. J. Cel Sci. 133 (18), 248468. doi:10.1242/jcs.248468
Bacman S. R., Kauppila J. H. K., Pereira C. V., Nissanka N., Miranda M., Pinto M., et al. (2018). MitoTALEN Reduces Mutant mtDNA Load and Restores tRNAAla Levels in a Mouse Model of Heteroplasmic mtDNA Mutation. Nat. Med. 24 (11), 1696–1700. doi:10.1038/s41591-018-0166-8
Bacman S. R., Williams S. L., Pinto M., Peralta S., Moraes C. T. (2013). Specific Elimination of Mutant Mitochondrial Genomes in Patient-Derived Cells by mitoTALENs. Nat. Med. 19 (9), 1111–1113. doi:10.1038/nm.3261
Bian W.-P., Chen Y.-L., Luo J.-J., Wang C., Xie S.-L., Pei D.-S. (2019). Knock-in Strategy for Editing Human and Zebrafish Mitochondrial DNA Using Mito-CRISPR/Cas9 System. ACS Synth. Biol. 8 (4), 621–632. doi:10.1021/acssynbio.8b00411
Carvalho G., Repolês B. M., Mendes I., Wanrooij P. H. (2021). Mitochondrial DNA Instability in Mammalian Cells. Antioxid. Redox Signaling. Advance online publication. doi:10.1089/ars.2021.0091
Doudna J. A., Charpentier E. (2014). The New Frontier of Genome Engineering with CRISPR-Cas9. Science 346 (6213), 1258096. doi:10.1126/science.1258096
Falkenberg M., Larsson N.-G., Gustafsson C. M. (2007). DNA Replication and Transcription in Mammalian Mitochondria. Annu. Rev. Biochem. 76, 679–699. doi:10.1146/annurev.biochem.76.060305.152028
Fonfara I., Richter H., Bratovič M., Le Rhun A., Charpentier E. (2016). The CRISPR-Associated DNA-Cleaving Enzyme Cpf1 Also Processes Precursor CRISPR RNA. Nature 532 (7600), 517–521. doi:10.1038/nature17945
Gammage P. A., Gaude E., Van Haute L., Rebelo-Guiomar P., Jackson C. B., Rorbach J., et al. (2016). Near-complete Elimination of Mutant mtDNA by Iterative or Dynamic Dose-Controlled Treatment with mtZFNs. Nucleic Acids Res. 44 (16), 7804–7816. doi:10.1093/nar/gkw676
Gammage P. A., Moraes C. T., Minczuk M. (2018a). Mitochondrial Genome Engineering: The Revolution May Not Be CRISPR-Ized. Trends Genet. 34 (2), 101–110. doi:10.1016/j.tig.2017.11.001
Gammage P. A., Rorbach J., Vincent A. I., Rebar E. J., Minczuk M. (2014). Mitochondrially Targeted ZFN S for Selective Degradation of Pathogenic Mitochondrial Genomes Bearing Large‐scale Deletions or point Mutations. EMBO Mol. Med. 6 (4), 458–466. doi:10.1002/emmm.201303672
Gammage P. A., Viscomi C., Simard M.-L., Costa A. S. H., Gaude E., Powell C. A., et al. (2018b). Genome Editing in Mitochondria Corrects a Pathogenic mtDNA Mutation In Vivo. Nat. Med. 24 (11), 1691–1695. doi:10.1038/s41591-018-0165-9
Goto Y.-i., Nonaka I., Horai S. (1990). A Mutation in the tRNALeu(UUR) Gene Associated with the MELAS Subgroup of Mitochondrial Encephalomyopathies. Nature 348 (6302), 651–653. doi:10.1038/348651a0
Gowher A., Smirnov A., Tarassov I., Entelis N. (2013). Induced tRNA Import into Human Mitochondria: Implication of a Host aminoacyl-tRNA-synthetase. PLoS One 8 (6), e66228. doi:10.1371/journal.pone.0066228
Hsu P. D., Scott D. A., Weinstein J. A., Ran F. A., Konermann S., Agarwala V., et al. (2013). DNA Targeting Specificity of RNA-Guided Cas9 Nucleases. Nat. Biotechnol. 31 (9), 827–832. doi:10.1038/nbt.2647
Hussain S.-R. A., Yalvac M. E., Khoo B., Eckardt S., McLaughlin K. J. (2021). Adapting CRISPR/Cas9 System for Targeting Mitochondrial Genome. Front. Genet. 12, 627050. doi:10.3389/fgene.2021.627050
Jinek M., Chylinski K., Fonfara I., Hauer M., Doudna J. A., Charpentier E. (2012). A Programmable Dual-RNA-Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 337 (6096), 816–821. doi:10.1126/science.1225829
Jo A., Ham S., Lee G. H., Lee Y.-I., Kim S., Lee Y.-S., et al. (2015). Efficient Mitochondrial Genome Editing by CRISPR/Cas9. Biomed. Res. Int. 2015, 1–10. doi:10.1155/2015/305716
Kim K. M., Noh J. H., Abdelmohsen K., Gorospe M. (2017). Mitochondrial Noncoding RNA Transport. BMB Rep. 50 (4), 164–174. doi:10.5483/bmbrep.2017.50.4.013
Lee H., Lee S., Baek G., Kim A., Kang B.-C., Seo H., et al. (2021). Mitochondrial DNA Editing in Mice with DddA-TALE Fusion Deaminases. Nat. Commun. 12 (1), 1190. doi:10.1038/s41467-021-21464-1
Loutre R., Heckel A.-M., Smirnova A., Entelis N., Tarassov I. (2018). Can Mitochondrial DNA Be CRISPRized:ProandContra. IUBMB Life 70 (12), 1233–1239. doi:10.1002/iub.1919
Maude H., Davidson M., Charitakis N., Diaz L., Bowers W. H. T., Gradovich E., et al. (2019). NUMT Confounding Biases Mitochondrial Heteroplasmy Calls in Favor of the Reference Allele. Front. Cel Dev. Biol. 7, 201. doi:10.3389/fcell.2019.00201
Mercer T. R., Neph S., Dinger M. E., Crawford J., Smith M. A., Shearwood A.-M. J., et al. (2011). The Human Mitochondrial Transcriptome. Cell 146 (4), 645–658. doi:10.1016/j.cell.2011.06.051
Minczuk M., Papworth M. A., Miller J. C., Murphy M. P., Klug A. (2008). Development of a Single-Chain, Quasi-Dimeric Zinc-finger Nuclease for the Selective Degradation of Mutated Human Mitochondrial DNA. Nucleic Acids Res. 36 (12), 3926–3938. doi:10.1093/nar/gkn313
Mok B. Y., de Moraes M. H., Zeng J., Bosch D. E., Kotrys A. V., Raguram A., et al. (2020). A Bacterial Cytidine Deaminase Toxin Enables CRISPR-free Mitochondrial Base Editing. Nature 583 (7817), 631–637. doi:10.1038/s41586-020-2477-4
Moraes C. T. (2014). A Magic Bullet to Specifically Eliminate Mutated Mitochondrial Genomes from Patients' Cells. EMBO Mol. Med. 6 (4), 434–435. doi:10.1002/emmm.201303769
Moraes C. T., Moraes C. T. (2017). Current Strategies towards Therapeutic Manipulation of mtDNA Heteroplasmy. Front. Biosci. 22 (6), 991–1010. doi:10.2741/4529
Nishimasu H., Ran F. A., Hsu P. D., Konermann S., Shehata S. I., Dohmae N., et al. (2014). Crystal Structure of Cas9 in Complex with Guide RNA and Target DNA. Cell 156 (5), 935–949. doi:10.1016/j.cell.2014.02.001
Nissanka N., Minczuk M., Moraes C. T. (2019). Mechanisms of Mitochondrial DNA Deletion Formation. Trends Genet. 35 (3), 235–244. doi:10.1016/j.tig.2019.01.001
Patananan A. N., Wu T.-H., Chiou P.-Y., Teitell M. A. (2016). Modifying the Mitochondrial Genome. Cel Metab. 23 (5), 785–796. doi:10.1016/j.cmet.2016.04.004
Peeva V., Blei D., Trombly G., Corsi S., Szukszto M. J., Rebelo-Guiomar P., et al. (2018). Linear Mitochondrial DNA Is Rapidly Degraded by Components of the Replication Machinery. Nat. Commun. 9 (1), 1727. doi:10.1038/s41467-018-04131-w
Pereira C. V., Bacman S. R., Arguello T., Zekonyte U., Williams S. L., Edgell D. R., et al. (2018). mitoTev‐TALE: a Monomeric DNA Editing Enzyme to Reduce Mutant Mitochondrial DNA Levels. EMBO Mol. Med. 10 (9), e8084. doi:10.15252/emmm.201708084
Picard M., Zhang J., Hancock S., Derbeneva O., Golhar R., Golik P., et al. (2014). Progressive Increase in mtDNA 3243A>G Heteroplasmy Causes Abrupt Transcriptional Reprogramming. Proc. Natl. Acad. Sci. U.S.A. 111 (38), E4033–E4042. doi:10.1073/pnas.1414028111
Puranam R. S., Attardi G. (2001). The RNase P Associated with HeLa Cell Mitochondria Contains an Essential RNA Component Identical in Sequence to that of the Nuclear RNase P. Mol. Cel Biol 21 (2), 548–561. doi:10.1128/MCB.21.2.548-561.2001
Ran F. A., Hsu P. D., Wright J., Agarwala V., Scott D. A., Zhang F. (2013). Genome Engineering Using the CRISPR-Cas9 System. Nat. Protoc. 8 (11), 2281–2308. doi:10.1038/nprot.2013.143
Reddy P., Ocampo A., Suzuki K., Luo J., Bacman S. R., Williams S. L., et al. (2015). Selective Elimination of Mitochondrial Mutations in the Germline by Genome Editing. Cell 161 (3), 459–469. doi:10.1016/j.cell.2015.03.051
Silva-Pinheiro P., Minczuk M. (2022). The Potential of Mitochondrial Genome Engineering. Nat. Rev. Genet. 23 (4), 199–214. doi:10.1038/s41576-021-00432-x
Silva-Pinheiro P., Nash P. A., Van Haute L., Mutti C. D., Turner K., Minczuk M. (2022). In Vivo mitochondrial Base Editing via Adeno-Associated Viral Delivery to Mouse post-mitotic Tissue. Nat. Commun. 13 (1), 750. doi:10.1038/s41467-022-28358-w
Smirnov A., Entelis N., Martin R. P., Tarassov I. (2011). Biological Significance of 5S rRNA Import into Human Mitochondria: Role of Ribosomal Protein MRP-L18. Genes Dev. 25 (12), 1289–1305. doi:10.1101/gad.624711
Srivastava S., Moraes C. T. (2001). Manipulating Mitochondrial DNA Heteroplasmy by a Mitochondrially Targeted Restriction Endonuclease. Hum. Mol. Genet. 10 (26), 3093–3099. doi:10.1093/hmg/10.26.3093
Tanaka M., Borgeld H.-J., Zhang J., Muramatsu S.-i., Gong J.-S., Yoneda M., et al. (2002). Gene Therapy for Mitochondrial Disease by Delivering Restriction endonucleaseSmaI into Mitochondria. J. Biomed. Sci. 9 (6), 534–541. doi:10.1007/bf02254980
Tang W., Hu J. H., Liu D. R. (2017). Aptazyme-embedded Guide RNAs Enable Ligand-Responsive Genome Editing and Transcriptional Activation. Nat. Commun. 8, 15939. doi:10.1038/ncomms15939
Taylor R. W., Turnbull D. M. (2005). Mitochondrial DNA Mutations in Human Disease. Nat. Rev. Genet. 6 (5), 389–402. doi:10.1038/nrg1606
Tonin Y., Heckel A.-M., Dovydenko I., Meschaninova M., Comte C., Venyaminova A., et al. (2014). Characterization of Chemically Modified Oligonucleotides Targeting a Pathogenic Mutation in Human Mitochondrial DNA. Biochimie 100, 192–199. doi:10.1016/j.biochi.2013.08.020
Towheed A., Markantone D. M., Crain A. T., Celotto A. M., Palladino M. J. (2014). Small Mitochondrial-Targeted RNAs Modulate Endogenous Mitochondrial Protein Expression In Vivo. Neurobiol. Dis. 69, 15–22. doi:10.1016/j.nbd.2014.04.017
Vafai S. B., Mootha V. K. (2012). Mitochondrial Disorders as Windows into an Ancient Organelle. Nature 491 (7424), 374–383. doi:10.1038/nature11707
Vendramin R., Marine J. C., Leucci E. (2017). Non‐coding RNA S: the Dark Side of Nuclear-Mitochondrial Communication. EMBO J. 36 (9), 1123–1133. doi:10.15252/embj.201695546
Wallace D. C., Chalkia D. (2013). Mitochondrial DNA Genetics and the Heteroplasmy Conundrum in Evolution and Disease. Cold Spring Harbor Perspect. Biol. 5 (11), a021220. doi:10.1101/cshperspect.a021220
Wang B., Lv X., Wang Y., Wang Z., Liu Q., Lu B., et al. (2021). CRISPR/Cas9-mediated Mutagenesis at Microhomologous Regions of Human Mitochondrial Genome. Sci. China Life Sci. 64 (9), 1463–1472. doi:10.1007/s11427-020-1819-8
Wang G., Chen H.-W., Oktay Y., Zhang J., Allen E. L., Smith G. M., et al. (2010). PNPASE Regulates RNA Import into Mitochondria. Cell 142 (3), 456–467. doi:10.1016/j.cell.2010.06.035
Wang G., Shimada E., Koehler C. M., Teitell M. A. (2012a). PNPASE and RNA Trafficking into Mitochondria. Biochim. Biophys. Acta (Bba) - Gene Regul. Mech. 1819 (9-10), 998–1007. doi:10.1016/j.bbagrm.2011.10.001
Wang G., Shimada E., Zhang J., Hong J. S., Smith G. M., Teitell M. A., et al. (2012b). Correcting Human Mitochondrial Mutations with Targeted RNA Import. Proc. Natl. Acad. Sci. U.S.A. 109 (13), 4840–4845. doi:10.1073/pnas.1116792109
Yoo B.-C., Yadav N. S., Orozco E. M., Sakai H. (2020). Cas9/gRNA-mediated Genome Editing of Yeast Mitochondria and Chlamydomonas Chloroplasts. PeerJ 8, e8362. doi:10.7717/peerj.8362
Zelenka J., Ježek P. (2016). “Import of Fluorescent RNA into Mitochondria of Living Cells,” in Mitochondrial DNA: Methods and Protocols. Editor M. McKenzie (New York, NYNew York: Springer), 175–181. doi:10.1007/978-1-4939-3040-1_13
Keywords: mitochondria, mtDNA, RNA import, CRISPR/Cas, gRNA
Citation: Yin T, Luo J, Huang D and Li H (2022) Current Progress of Mitochondrial Genome Editing by CRISPR. Front. Physiol. 13:883459. doi: 10.3389/fphys.2022.883459
Received: 25 February 2022; Accepted: 18 April 2022;
Published: 02 May 2022.
Edited by:
Zhiying Zhang, Northwest A&F University, ChinaReviewed by:
Sathees C. Raghavan, Indian Institute of Science (IISc), IndiaAlexandre Smirnov, UMR7156 Génétique moléculaire, génomique et microbiologie (GMGM), France
Copyright © 2022 Yin, Luo, Huang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Li, lihui80@szu.edu.cn
 Tao Yin
Tao Yin Junjie Luo
Junjie Luo Danqiong Huang
Danqiong Huang Hui Li
Hui Li