- 1Department of Endocrinology and Metabolism, Ningbo First Hospital, Ningbo, China
- 2Department of Engineering Mechanics, Zhejiang University, Hangzhou, China
The viscosity of blood is an indicator in the understanding and treatment of disease. An elevated blood viscosity has been demonstrated in patients with Type 2 Diabetes Mellitus (T2DM), which might represent a risk factor for cardiovascular complications. However, the roles of glycated hemoglobin (HbA1c) and plasma fibrinogen levels on the elevated blood viscosity in subjects with T2DM at different chronic glycemic conditions are still not clear. Here, we evaluate the relationship between the blood viscosity and HbA1c as well as plasma fibrinogen levels in patients with T2DM. The experimental data show that the mean values of the T2DM blood viscosity are higher in groups with higher HbA1c levels, but the correlation between the T2DM blood viscosity and the HbA1c level is not obvious. Instead, when we investigate the influence of plasma fibrinogen level on the blood viscosity in T2DM subjects, we find that the T2DM blood viscosity is significantly and positively correlated with the plasma fibrinogen level. Further, to probe the combined effects of multiple factors (including the HbA1c and plasma fibrinogen levels) on the altered blood viscosity in T2DM, we regroup the experimental data based on the T2DM blood viscosity values at both the low and high shear rates, and our results suggest that the influence of the elevated HbA1c level on blood viscosity is quite limited, although it is an important indicator of glycemic control in T2DM patients. Instead, the elevated blood hematocrit, the enhanced red blood cell (RBC) aggregation induced by the increased plasma fibrinogen level, and the reduced RBC deformation play key roles in the determination of blood viscosity in T2DM. Together, these experimental results are helpful in identifying the key determinants for the altered T2DM blood viscosity, which can be used in future studies of the hemorheological disturbances of T2DM patients.
1. Introduction
Blood is a non-Newtonian fluid that delivers necessary substances—such as nutrients and oxygen—to living cells and removes metabolic waste products (Popel and Johnson, 2005; Secomb, 2017). In vertebrates, it is composed primarily of blood cells suspended in blood plasma. In microcirculation, blood cells are subjected to intense mechanical stimulation from both blood flow and vessel walls; hence, their mechanical and rheological properties are important to their effectiveness in performing their biological functions (Yedgar et al., 2002; Baskurt and Meiselman, 2003; Yao et al., 2003; Chen et al., 2007; Fedosov et al., 2014a; Barshtein et al., 2016, 2018, 2021; Sohrabi et al., 2017; Tan et al., 2018; Xiao et al., 2020; Chien et al., 2021). A change in blood rheological property is usually linked to blood disorders; therefore, understanding the flow dynamics and rheological properties of blood allows us to know how blood viscosity impacts cognitive functions, and provides direction for therapeutic interventions (Chien et al., 1970a; Ballas et al., 1989; Fedosov et al., 2014b; Du et al., 2015; Barshtein et al., 2017; Perakakis et al., 2018). Blood viscosity has been extensively investigated and now it is generally believed that five factors, namely blood hematocrit (Hct), red blood cell (RBC) deformability, RBC aggregation, plasma viscosity, and temperature, primarily determine the rheological behavior of blood (Chien et al., 1966, 1967, 1970b; Berger and King, 1980; Barshtein et al., 2007; Fedosov et al., 2011b; Lei and Karniadakis, 2013). In blood flow, the RBC aggregation is attributed to the macromolecules such as plasma protein fibrinogen and synthetic polymer dextran, which promote the formation of RBC aggregates in the form of rouleaux (Brust et al., 2014; Krüger-Genge et al., 2019) and eventually lead to an increased blood viscosity (Matsuda and Murakami, 1976; Tomaiuolo et al., 2016). The deformability of the RBCs, or the ability of the RBCs to deform their shape under applied stress, plays an important role in the main function of the RBCs. In pathological conditions, these alterations could result in impaired blood flow and other aspects of vascular complications. For example, the malaria-infected RBCs show progressing alteration of their mechanical and adhesive properties as the parasite develops (Zhang et al., 2015; Dearnley et al., 2016; Banas et al., 2021). These changes greatly affect rheological properties of malaria-infected RBCs and lead to obstructions of small capillaries (Shelby et al., 2003; Fedosov et al., 2011a).
Diabetes mellitus (DM), the fastest growing chronic disease worldwide, is a metabolic disease characterized by persistently elevated glucose levels in the blood (Mathers and Loncar, 2006; Deng et al., 2021). Generally, the most widely used indicators of glycemic control for diabetic patients are the blood glucose level and the glycated haemoglobin (HbA1c) level. Compared to the former, the HbA1c level has little biological variability and reflects the average glucose concentration over the preceding 8–12 weeks (Sacks, 2011). In addition, the glycated serum protein (GSP) level, which is the amount of glucose attached to total serum proteins, offers an alternative approach for assessing glycemia in instances where HbA1c may be of limited value such as pregnancy and reduced RBC lifespan. Type 2 diabetes mellitus (T2DM), the most common type of DM, is characterized by relative insulin deficiency caused by pancreatic cell dysfunction and insulin resistance in target organs (Chatterjee et al., 2017). Individuals with T2DM usually suffer from elevated level of HbA1c, which has been identified as an emerging risk factor for developing microvascular and macrovascular complications, (Fowler, 2008) such as diabetic retinopathy, (Brazionis et al., 2008; Cho, 2011; Li et al., 2020) diabetic nephropathy, (Young et al., 1993; Davies et al., 2006; Jeganathan et al., 2008) diabetic peripheral and autonomic neuropathy (Yang et al., 2020).
The viscosity of blood is a direct measure of the resistance of blood to flow through blood vessel. According to Poiseuille's law, the rate of blood flow through a small blood vessel can be calculated from the following algebraic equation: (Phillips et al., 2008).
where r and L are the radius and length of the blood vessel, ΔP is the difference in blood pressure between the ends of the blood vessel, and ηBV is the viscosity of blood. Holding other parameters constant, higher blood viscosity should retard blood flow through blood vessels, which would contribute to insulin resistance and T2DM and eventually lead to diabetic microangiopathy and other circulation problems (Fowler, 2008).
It is generally accepted that the blood viscosity is higher in T2DM patients than in non-diabetic control subjects (Skovborg et al., 1966; Turczynski et al., 2003; Tamariz et al., 2008). Although the reasons for the elevation in blood viscosity are still under investigation, it is believed that the osmotic diuresis, consequence of high HbA1c level, could contribute to increase blood hematocrit and reduce plasma volume (Agrawal et al., 2016). It has also been suggested that the reduced RBC deformability, increased RBC and platelet aggregation, and enhanced platelet adhesion to activated endothelium would contribute to blood hyperviscosity (Schmid-Schönbein and Volger, 1976; McMillan, 1983; Beamer et al., 1997; Cho et al., 2008; Cloutier et al., 2008; Chang et al., 2017, 2018; Li et al., 2018). For example, Turczynski et al. (2003) showed that the T2DM blood viscosity is positively correlated with retinopathy severity, which is attributed to the decreased RBC deformability. Skovborg et al. (1966) revealed that the blood viscosity of diabetic subjects is around 20% higher than that of controls. Ercan et al. (2002) suggested that the elevated plasma cholesterol contributes to increased blood viscosity by an additional effect of hyperglycemia in T2DM patients. Yazdani et al. (2021) integrated blood cell mechanics, platelet adhesive dynamics, and coagulation cascade to model the thrombus formation in diabetic blood. They showed that both the pathological alterations in the biomechanical properties of blood cells and changes in the amount of coagulation factors would contribute to the enhanced platelet adhesion and aggregation in diabetic blood. In addition, it shows that the aggregation of the RBCs is far commoner among the T2DM patients than that among the non-diabetes (MacRury et al., 1993; Babu and Singh, 2004; Deng et al., 2020). It is recognized that the plasma fibrinogen level is one of the dominant factors promoting the formation of RBC aggregates (rouleaux), which could cause an elevated blood viscosity under low shear rate (Krüger-Genge et al., 2019; Deng et al., 2020).
The viscosity of blood has long been used as an indicator in the understanding and treatment of disease (Fedosov et al., 2011a). Previous studies have examined the relationship between blood viscosity, Hct, plasma viscosity, RBC deformability, and platelet adhesion in T2DM patients. However, all studies so far have investigated the effects of just a few of the aforementioned factors in hemorheology and vascular occlusion in certain glucose conditions and for certain time. The roles of hyperglycemia and elevated plasma fibrinogen level on the altered blood viscosity at different chronic glycemic conditions is not completely clear. In this article, we evaluate the influences of both the HbA1c and plasma fibrinogen levels on the T2DM blood viscosity to clarify whether the alterations in blood viscosity are appreciable in these T2DM subjects.
2. Materials and Methods
2.1. Selection of T2DM Blood Samples
In this study, blood samples from 318 patients (199 male and 119 female, mean age 56.80±12.92) with T2DM were collected during fasting glucose tests, following institutional review board (IRB) approvals from the Ningbo City First Hospital. In the fasting glucose tests, the patients were asked to fast for overnight before morning blood collection to avoid the influence of postprandial lipid increase on hemorheological characteristics (Stamos and Rosenson, 1999). To investigate the effect of the HbA1c level on the hemorheological properties of T2DM blood, these blood samples were collected in a wide range of the HbA1c levels. All blood samples were collected into vacuum tubes (5 ml) containing Heparin Lithium salt (75 IU/ml) anticoagulant and stored at 4 °C for in vitro testing within 4 h from blood withdrawal. The levels of HbA1c and GSP, which are the two key parameters for the assessment of long-term glucose control in diabetes, were measured in all 318 subjects. The level of plasma fibrinogen (cFN) and other biochemical and hematologic parameters were also measured using standard methods. The values of Hct were measured as the fraction of the RBCs suspended in plasma following blood centrifugation.
The whole blood viscosity was measured at native Hct using a cone-plate viscometer (SA-6600, Beijing Succeeder Technology Inc, China). The angle between the surface of the cone and the plate was of the order of 1°, where the shear rate was regulated by rotor speed during measurement. Viscosity measurements were completed within 4 h of sample collection by the following procedures: (1) the rheometer was initialized to desired test condition (force and gap) and the turntable and dosing pin were restored to zero; (2) the temperature of the blood rheometer was pre-heated for 30 min to 37 °C; (3) the whole blood was added to the tube with a dosing needle for dilution (100:1) and mixed well; (4) the tube was placed on the pre-heated plate (37 °C); (5) the whole mensuration was then automatically controlled by the computer in a rapid, pointwise, prompt, steady-state method, and the two-dimensional curve for the blood viscosity and shear rate was traced in real-time. The test time for whole blood was within 30 s/sample.
It is known that the blood is a non-Newtonian fluid, which means that its viscosity depends on the shear rate. At low shear rates, the aggregation of RBCs induces a sharp increase in blood viscosity. At high shear rates, the blood becomes less viscous as the RBCs disaggregate, deform and align in the direction of flow. According to previous experimental and computational studies, (Skalak et al., 1981; Fedosov et al., 2011b) the blood viscosity decreased only 10% when the shear rate rises from 200.0 to 1000.0 s-1. In our opinion, the blood viscosity measured over a range of shear rates in 200.0 to 1.0 s-1 can reflect the non-Newtonian behavior of T2DM blood. Hence, in this study, the blood viscosity curves were plotted as a function of shear rate ranging from 200.0 to 1.0 s-1 for whole blood samples. Then, the values of whole blood viscosity (ηBV) were chosen and analyzed at three certain shear rates (i.e., = 1.0 , 50.0 , and 200.0 s-1). The plasma viscosity (ηPV) was measured with a capillary viscometer.
It is generally believed that surface tension plays an important role in the way liquids behave. In general, it is the property of a liquid's surface, which is caused by unbalanced forces on surface molecules that pull toward the main part of the liquid. The blood viscosity is a measure of the resistance of a liquid that is being deformed or moved. As it can be noticed that the origin of the two properties is not directly related to one another. Therefore, there is no conclusive correlation between the surface tension and viscosity (Wesołowski and Młynarczak, 2019). Hence, we did not investigate the effect of blood surface tension in the measurement of blood viscosity at low shear rates.
The ability of the RBCs to deform their shape under applied shear stress are represented by RBC deformability index (Di) or RBC rigidity index (Ri). In past decades, advances in experimental techniques (such as erythrocyte filtration, laser diffraction ellipsometry, micropipette aspiration) have allowed to measure the RBC Di and RBC Ri parameters averaged over a large number of RBCs in blood sample (Dintenfass, 1985; Nash and Meiselman, 1985; Mokken et al., 1992; Kameneva et al., 1999; Spengler et al., 2011). As suggested by previous experimental studies on the measurements of rheological characteristics of the blood, (Spengler et al., 2011) the RBC Ri parameter (i.e., the inverse of RBC deformability) is evaluated according to the following formula,
where ηBV, 200.0 is the whole blood viscosity at shear rate of = 200.0 s-1. Apparently, an increase in the RBC Ri would lead to a reduction in the RBC deformability. Additionally, the RBCs in whole blood aggregate into rouleaux at low shear rates, leading to an obvious increase in blood viscosity, whereas the large RBC aggregates can break down into small structures or isolated RBCs under high shear rates (Fedosov et al., 2011b). Herein, the RBC aggregation index (Ai) is estimated by the ratio of low shear viscosity and high shear viscosity based on the following formula, (Bull et al., 1986).
where ηBV, 1.0 is whole blood viscosity at shear rates of = 1.0 s-1.
2.2. Statistical Analysis
All statistical data analyses were performed using SPSS 25.0 for Windows. One-way Analysis of Variance (ANOVA) and Least Significant Difference (LSD) post-hoc test were used to analyze the difference in studied variables between groups with different HbA1c and plasma fibrinogen levels. Pearson's correlation analysis was used to test the statistical relationship between two continuous variables. A simple correlation analysis was performed to evaluate the independent association of clinical and biochemical variables with the HbA1c and plasma fibrinogen levels. In this study, the significance level was set as 0.05. If the P-value is lower than the significance level, it means that the relationship between two or more variables are statistically significant. Additionally, the R-value and confidence interval (with the confidence level of 95%) were also included in the Pearson's correlation analysis.
3. Results and Discussion
3.1. Effect of Glycated Hemoglobin Level on T2DM Blood Rheology
All T2DM blood subjects were divided into three groups according to their HbA1c levels: Group A (subjects with good glycemic control, HbA1c < 6.5%), Group B (subjects with poor glycemic control, 6.5% ≤ HbA1c <10.0%), and Group C (subjects with the worst glycemic control, HbA1c ≥ 10.0%). Selected biochemical, hematologic and hemorheological parameters in these three different groups are listed in Table 1. It shows that the levels of GSP are significantly higher in the T2DM blood subjects with higher HbA1c levels (Groups B and C) compared to that in the T2DM blood subjects with lower HbA1c level (Group A). The values of Hct are similar in these three different groups but the RBC counts (NRBC) are higher in Groups B and C compared to that in Group A, resulting in lower values of MCV in Groups B and C based on the following equation,
The value of plasma fibrinogen level cFN is slightly higher in Group B compared to those in other two groups; however, there are no significant differences among these three different groups (P = 0.079). Additionally, the values of RBC Ai and Ri are almost the same among the three groups, which indicates that these two factors remain unaffected by the changes in the HbA1c level.
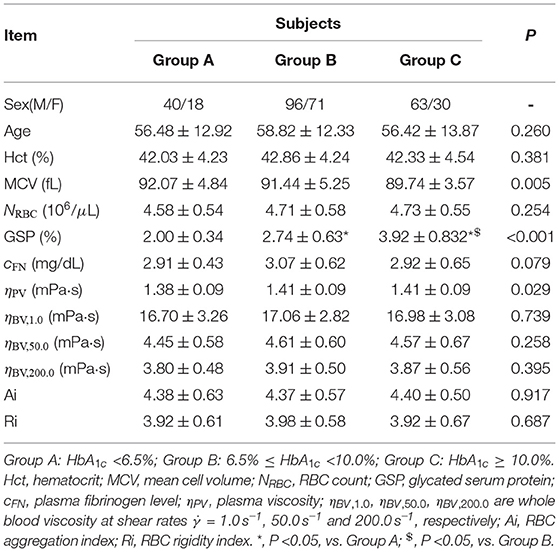
Table 1. Selected biochemical, hematologic and hemorheological characteristics of the T2DM blood subjects by tertile of the HbA1c level.
With regards to the rheological properties of T2DM blood, it shows that the T2DM blood subjects with higher HbA1c levels (Groups B and C) have little bit higher values of plasma viscosity ηPV, compared to that in the T2DM blood subjects with lower HbA1c level (Group A). The values of blood viscosity ηBV at all three selected shear rates ( = 1.0, 50.0, and 200.0 s-1) are also higher in Groups B and C compared to those in Group A; however, there are no statistically significant differences in the mean ηBV values among the three groups (P ≥ 0.258), indicating that the HbA1c level did not seem to have important effect on the blood viscosity.
Next, we investigate the functional dependencies of the GSP level, plasma viscosity ηPV, low-shear-rate blood viscosity ηBV, low ( = 1.0 s-1) and high-shear-rate blood viscosity ηBV, high ( = 200.0 s-1) on the HbA1c level, see Figure 1. It shows that the GSP level is significantly and positively correlated with the HbA1c level (Figure 1A). This agrees with several reported studies (Lapolla et al., 1988; Bahceci et al., 2005). With regards to the rheological properties of T2DM blood, the measurements of plasma viscosity (Figure 1B), low-shear-rate blood viscosity (Figure 1C), and high-shear-rate blood viscosity (Figure 1D) show large fluctuations with the HbA1c level. However, neither the plasma viscosity (Figure 1B) nor the blood viscosity (Figures 1C,D) shows obvious correlation with the HbA1c level. These results further demonstrate that the contribution of the HbA1c level to the blood viscosity seems quite limited, at least in apparently T2DM blood subjects and at the shear rates used in the present study.
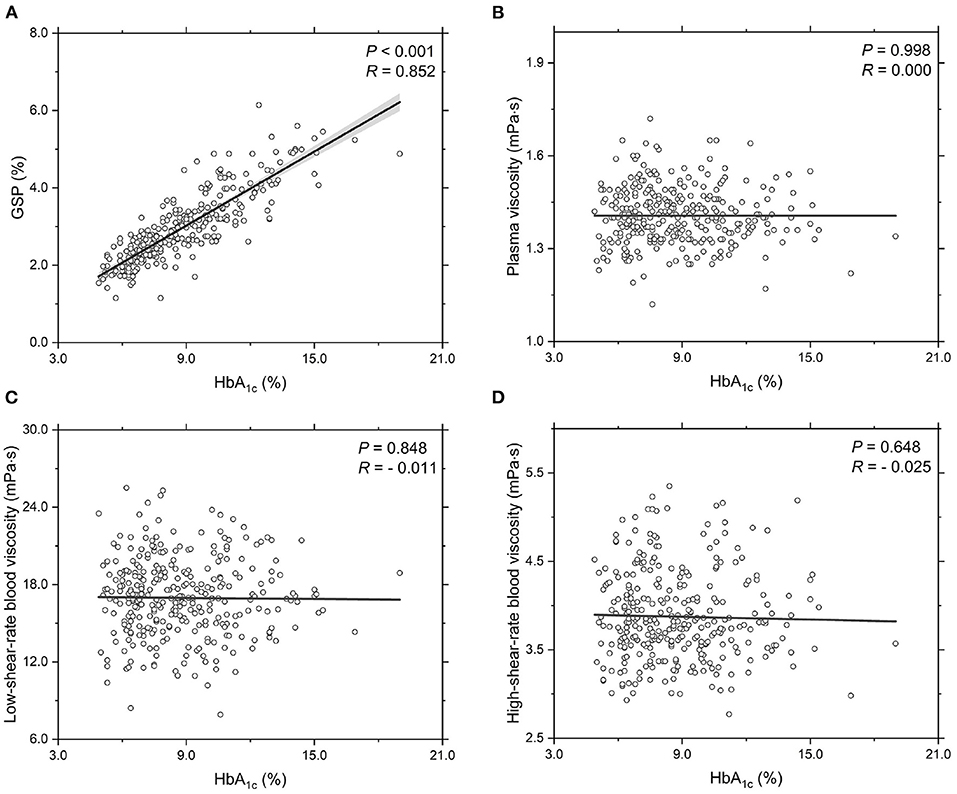
Figure 1. Functional dependencies of (A) GSP, (B) plasma viscosity ηPV, (C) low-shear-rate blood viscosity ηBV, low at = 1.0 s-1, and (D) high-shear-rate blood viscosity ηBV, high at = 200.0 s-1 with respect to HbA1c level. Shadow region represents 95% confidence interval.
3.2. Effect of Plasma Fibrinogen Level on T2DM Blood Rheology
In T2DM patients, the RBC aggregation induced by the plasma fibrinogen level is a key determinant of the non-Newtonian flow behavior of human blood, especially at low shear rates, which has been suggested as a possible contributing factor for the occurrence and progression of diabetic microangiopathy (Krüger-Genge et al., 2019; Deng et al., 2020; Li et al., 2020). Next, we investigate the influence of plasma fibrinogen level on the blood viscosity in T2DM.
All T2DM blood subjects are divided into three groups according to their plasma fibrinogen levels cFN: Group A (cFN <2.5 mg/dL), Group B (2.5 mg/dL ≤ cFN <3.5 mg/dL), and Group C (cFN ≥ 3.5 mg/dL). Selected biochemical, hematologic and hemorheological parameters in these blood subjects are listed in Table 2. It shows that there are no statistically significant differences in the mean values of Hct, MCV, RBC count, and HbA1c level among the three groups (P ≥ 0.168). Additionally, it shows that the RBC Ri remains unaffected by the changes in plasma fibrinogen level cFN. However, our results indeed show a closer correlation between the RBC Ai and the plasma fibrinogen level cFN (P = 0.035), namely, the values of RBC Ai are higher in Groups B and C compared to that in Group A. These results confirm that the plasma fibrinogen mainly affects the RBC aggregation characteristics. With regards to the rheological properties of T2DM blood, it shows that the T2DM blood subjects with higher cFN (Groups B and C) have higher values of plasma viscosity ηPV and blood viscosity ηBV at all three shear rates ( = 1.0, 50.0, and 200.0 s-1), compared to those blood subjects with lower cFN (Group A). For example, the values of low-shear-rate blood viscosity ηBV, low at = 1.0 s-1 in Groups B and C were around 7.6 and 8.7% higher than that in Group A.
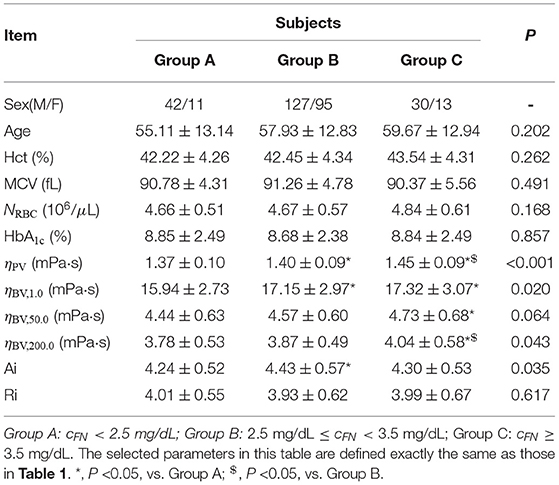
Table 2. Selected biochemical, hematologic and hemorheological characteristics of the T2DM blood subjects by tertile of the plasma fibrinogen level cFN.
To further probe the rheological behavior of T2DM blood, we investigate the functional dependencies of plasma viscosity ηPV and low-shear-rate blood viscosity ηBV, low on the plasma fibrinogen level cFN, see Figure 2. It shows that the plasma viscosity ηPV positively correlated with the plasma fibrinogen level cFN (Figure 2A), which agrees with previous experimental studies that the plasma viscosity increases with plasma fibrinogen level (Brunner, 2007). Regarding the dependence of low-shear-rate blood viscosity on plasma fibrinogen level, the values of ηBV, low show a positive correlation with plasma fibrinogen level (Figure 2B), which is mainly attributed to the enhanced fibrinogen-induced RBC aggregation and the increased plasma viscosity. This result is also consistent with previous experimental study that the blood viscosity elevation is associated with the increased plasma fibrinogen level (Matsuda and Murakami, 1976). Compared to the experimental results presented in Subsection 3.1, we conclude that an increased plasma fibrinogen level is more important to the elevated blood viscosity than the increase in HbA1c level.
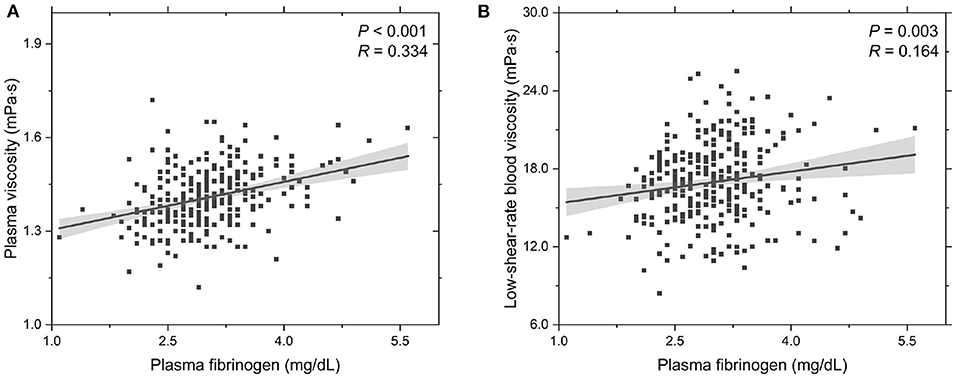
Figure 2. Functional dependencies of (A) plasma viscosity ηPV and (B) whole blood viscosity ηBV, low at = 1.0 s-1 with respect to plasma fibrinogen level cFN. Shadow region represents 95% confidence interval.
3.3. Multi-Factor Analysis in Altered T2DM Blood Rheology
There are several different factors affecting blood viscosity, especially under pathological conditions. Hence, an increased blood viscosity may be the reason why the other biochemical, hematologic and metabolic factors are important, and provides the underlying mechanism through which these other factors convey the pre-inflammatory insult to blood vessel walls (Sloop, 1996). An alternative method of analysis is to group patients into different categories by their blood viscosity levels (Ciuffetti et al., 2005). To probe the combined effects of multiple factors in altered blood rheology in T2DM, we consider to regroup the experimental data based on the values of blood viscosity at both the low and high shear rates. At low shear rate ( = 1.0 s-1), all T2DM blood subjects are divided into three groups according to their blood viscosity values: Group A (ηBV, 1.0 <15.0 mPa·s), Group B (15.0 mPa·s ≤ ηBV, 1.0 <19.0 mPa·s), and Group C (ηBV, 1.0 ≥ 19.0 mPa·s). Selected biochemical, hematologic and hemorheological parameters in these three blood groups are listed in Table 3. It shows that the Hct levels and RBC counts (NRBC) appear opposite trends with the age from Group A to Group C, which is in agreement with previous studies that these two variables decreased in the elderly subjects as they grow older (De Meyer et al., 2008; Zierk et al., 2020). It also shows that there are no statistically significant differences in the mean values of HbA1c, GSP, and RBC Ri. However, different from the results presented above, our results show that the values of Hct, plasma fibrinogen level cFN, and RBC Ai gradually increase from Group A to Group C. In a previous study by Irace et al. (2014), it has been shown that the plasma viscosity is directly associated with the low-density lipoprotein (LDL) cholesterol. Herein, we also consider the mean values of different cholesterol levels. Our results show that the values of LDL cholesterol were increased from Group A to Group C, resulting in a gradual increase in the plasma viscosity from Group A to Group C. To better understand the alteration of the T2DM blood viscosity in response to the changes in the RBC aggregation induced by the plasma fibrinogen level, we evaluate the reduced blood viscosity at low shear rate ( = 1.0 s-1), ηrBV, low, defined by,
From Table 3, we find that the values of ηrBV, low have a remarkable increase from Group A to Group C, which is mainly due to the enhanced RBC aggregation (RBC Ai) induced by the increased level of plasma fibrinogen.
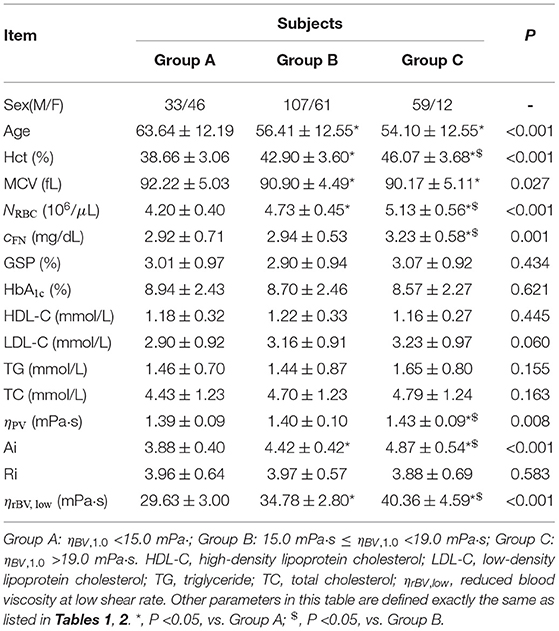
Table 3. Selected biochemical, hematologic and hemorheological characteristics of the T2DM blood subjects by tertile of the whole blood viscosity at low shear rate of = 1.0 s-1.
In addition, we investigate the functional dependencies of Hct, MCV, RBC count and RBC Ai on the low-shear-rate blood viscosity, see Figure 3. It shows that the Hct level increases with increasing low-shear-rate blood viscosity (Figure 3A), indicating that the T2DM patients with an abnormal elevation in low-shear-rate blood viscosity have high Hct levels. From the other point of view, it confirms that the Hct level is one of the major determinants of blood viscosity. As we know, the blood hematocrit reflects the amount of space in the blood that is occupied by the RBCs, which are affected by the size of the RBCs (MCV) and by the number of RBCs (RBC counts). The results on the functional dependence of the MCV and RBC count on the low-shear-rate blood viscosity are shown in (Figures 3B,C). In general, MCV decreases (Figure 3B) while RBC counts grows (Figure 3C) with increasing low-shear-rate blood viscosity, indicating that the increased number of RBCs in the T2BM blood plays a key role in the increased Hct level, causing an elevated low-shear-rate blood viscosity. Additionally, we also probe the functional dependence of RBC Ai on the low-shear-rate blood viscosity (Figure 3D). We find that the RBC Ai is positively associated with the low-shear-rate blood viscosity, indicating that the T2DM patients with higher value of low-shear-rate blood viscosity have enhanced RBC aggregation. Take a look at it another way, we confirm that the RBC aggregation influences the low-shear-rate blood viscosity. In summary, as the values of low-shear-rate blood viscosity are gradually increased from Group A to Group C, we conclude that the T2DM blood viscosity at low shear rate is significantly associated with the increased Hct, plasma viscosity, and RBC aggregation.
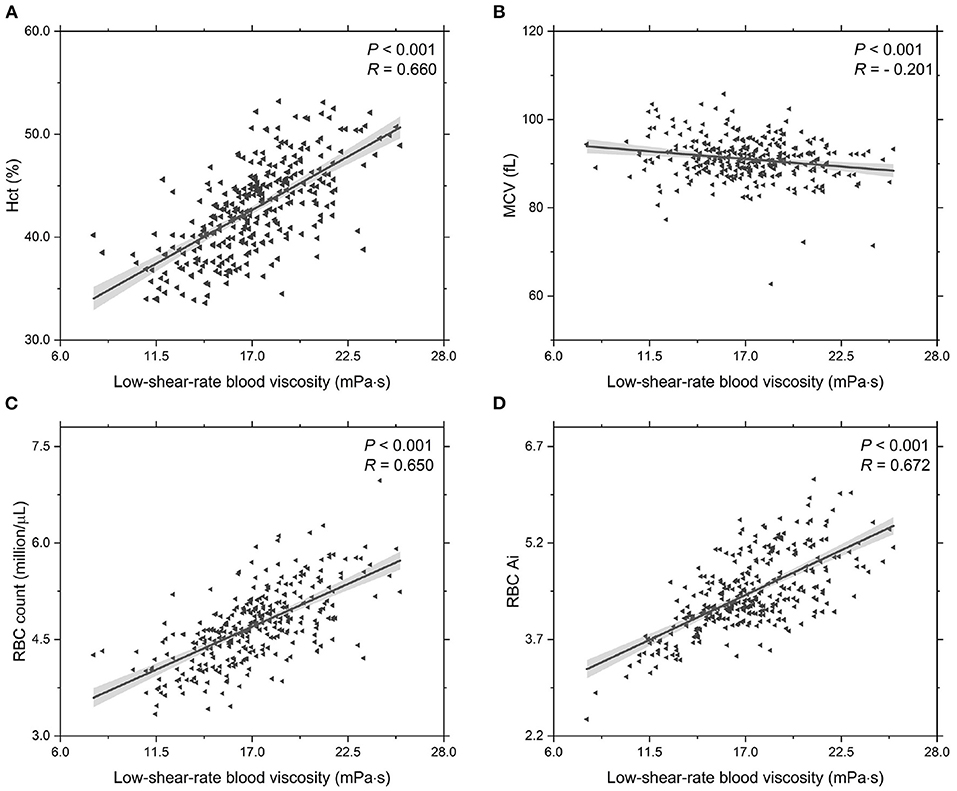
Figure 3. Functional dependencies of (A) Hct, (B) MCV, (C) RBC count, and (D) RBC Ai with respect to low-shear-rate T2DM blood viscosity at = 1.0 s-1. Shadow region represents 95% confidence interval.
At high shear rate ( = 200.0 s-1), all T2DM blood subjects are also divided into three groups according to their blood viscosity values: Group A (ηBV, 200.0 <3.5 mPa·s), Group B (3.5 mPa·s ≤ ηBV, 200.0 <4.5 mPa·s), and Group C (ηBV, 200.0 > 4.5 mPa·s). Selected biochemical, hematologic and hemorheological parameters in these three different groups are listed in Table 4. Similar to the trends obtained at low shear rate, it shows that the values of Hct, RBC count, and plasma fibrinogen level cFN gradually increase from Group A to Group C, and the values of MCV gradually decrease from Group A to Group C. Additionally, our results show that there are no statistically significant differences in the mean values of HbA1c and GSP. In contrast, we find that the values of the RBC Ri gradually increase from Group A to Group C, but the values of the RBC Ai have little changes among the three groups. Next, we evaluate the reduced blood viscosity at high shear rate, ηrBV, high, defined by,
From Table 4, we find that the values of ηrBV, high gradual increase from Group A to Group C, which could attribute to the increased RBC Ri, namely the reduced RBC deformability.
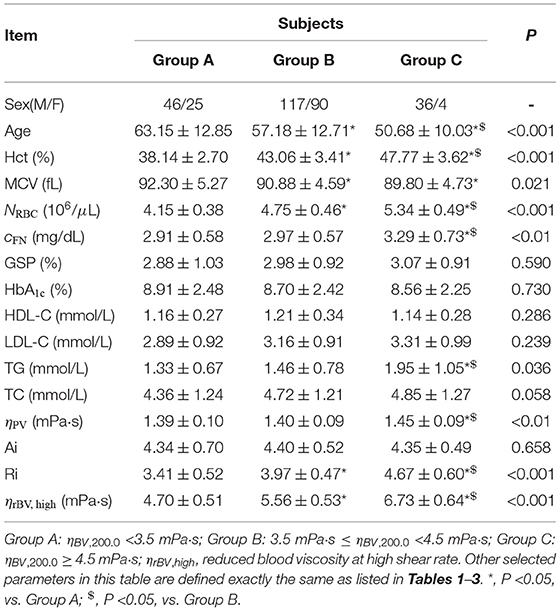
Table 4. Selected biochemical, hematologic and hemorheological characteristics of the T2DM blood subjects by tertile of the whole blood viscosity at high shear rate of = 200.0 s-1.
In addition, we investigate the functional dependencies of both the RBC Ri and RBC Ai on blood viscosity at high shear rate ( = 200.0 s-1), see Figure 4. It shows that the values of the RBC Ri are positively correlated with the blood viscosity (Figure 4A); however, there is no correlation between the RBC Ai and the blood viscosity (Figure 4B). In fact, it is easy to understand because the formation of RBC rouleaux in blood occurs at sufficiently low shear rates. Under high shear stress conditions, the large rouleaux can break down into smaller structures or individual RBCs. Overall, these results confirm that the elevated Hct level and reduced RBC deformability are the two of the most important parameters to the elevated blood viscosity at high shear rate.
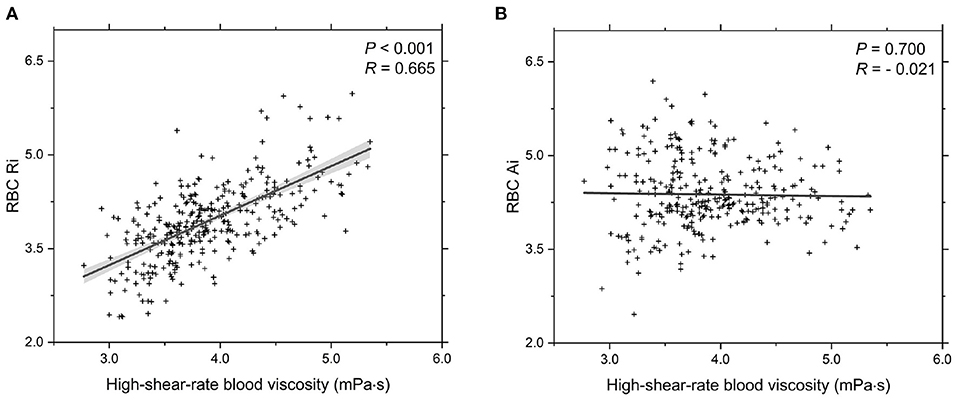
Figure 4. Functional dependencies of (A) RBC Ri and (B) RBC Ai with respect to high-shear-rate T2DM blood viscosity at = 200.0 s-1. Shadow region represents 95% confidence interval.
4. Summary
The viscosity of blood is a direct measure of the resistance of blood to flow, and an increase in blood viscosity would result in retarded blood flow thereby causing reduced delivery of substrates such as oxygen, insulin, and glucose to metabolically active tissues. In this study, we investigate the effects of glycated hemoglobin (HbA1c) and plasma fibrinogen levels on the rheological properties of blood in subjects with type 2 diabetes mellitus (T2DM). Our data suggest that the mean values of blood viscosity are higher in groups with higher HbA1c levels; however, the correction between the blood viscosity and HbA1c level is not obvious. Instead, we find that the T2DM blood viscosity is significantly and positively correlated with the plasma fibrinogen level.
Additionally, to probe the combined effects of multiple factors (including the HbA1c and plasma fibrinogen levels) on the altered blood viscosity in T2DM subjects, we regroup the experimental data based on the blood viscosity values at both low and high shear rates. Our experimental results suggest that the influence of the elevated HbA1c level on blood viscosity is limited, although it is an important indicator of risk for complications in T2DM patients. Instead, the increased blood hematocrit and enhanced RBC aggregation induced by the elevated plasma fibrinogen level are two of the most important parameters that determine the T2DM blood viscosity at low shear rate, and the increased blood hematocrit and reduced RBC deformation mainly contribute to the elevated T2DM blood viscosity at high shear rate.
Overall, in this study, we show that the RBC aggregation is pronounced while the RBC deformability is decreased in T2DM patients, which may cause blood flow abnormality and eventually lead to the development of vascular complications. On the one hand, the RBC hyperaggregability leads to enhanced rouleau formation at low shear rate, causing blood hyperviscosity in capillaries, reducing the delivery of substrates such as oxygen, insulin and glucose to metobolically active tissues, and eventually leading to hemodynamic impairment and vascular occlusion. On the other hand, the RBCs in patients with T2DM are associated with reduced cell deformation, which can also cause blood viscosity elevation contributing to blood flow impairment and other pathophysiological aspects of diabetes-related vascular complications such as the formation of blood clots.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board (IRB) approvals from the Ningbo First Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
JS, KH, MX, JQ, LiL, and XL designed the research. JS and KH performed the experimental measurements and analyzed the data. All authors discussed the results and wrote this article.
Funding
This work was supported by the Major Program of Social Development of Ningbo Science and Technology Bureau (Grant No. 2019C50094) and the Zhejiang Provincial Natural Science Foundation (Grant No. LY22A020004).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agrawal R., Smart T., Nobre-Cardoso J., Richards C., Bhatnagar R., and Tufail A. (2016). Assessment of red blood cell deformability in type 2 diabetes mellitus and diabetic retinopathy by dual optical tweezers stretching technique. Sci Rep. 6:15873. doi: 10.1038/srep15873
Babu N., and Singh M. (2004). Influence of hyperglycemia on aggregation, deformability and shape parameters of erythrocytes. Clin. Hemorheol. Micro. 31, 273–280. doi: 10.3233/CH-2009-1165
Bahceci M, Tuzcu A, Ogun C, Canoruc N, Iltimur K, and Aslan C. (2005). Is serum C-reactive protein concentration correlated with HbA1c and insulin resistance in Type 2 diabetic men with or without coronary heart disease? J. Endocrinol. Invest. 28, 145–150. doi: 10.1007/BF03345357
Ballas S. K., Dover G. J., and Charache S. (1989). Effect of hydroxyurea on the rheological properties of sickle erythrocytes in vivo. Am. J. Hematol. 32, 104–111. doi: 10.1002/ajh.2830320206
Banas A. M., Banas K., Chu T. T. T., Naidu R, Hutchinson P. E., and Agrawal R. (2021). Comparing infrared spectroscopic methods for the characterization of Plasmodium falciparum-infected human erythrocytes. Commun. Chem. 4, 129. doi: 10.1038/s42004-021-00567-2
Barshtein G, Ben-Ami R, and Yedgar, S. (2007). Role of red blood cell flow behavior in hemodynamics and hemostasis. Exp. Rev. Cardiovasc. Ther. 5, 743–752. doi: 10.1586/14779072.5.4.743
Barshtein G, Arbell D, and Yedgar S. (2018). Hemodynamic functionality of transfused red blood cells in the microcirculation of blood recipients. Front Physiol. 9, 41. doi: 10.3389/fphys.2018.00041
Barshtein G, Goldschmidt N, Pries A. R., Zelig O, Arbell D, and Yedgar S. (2017). Deformability of transfused red blood cells is a potent effector of transfusion-induced hemoglobin increment: a study with beta-thalassemia major patients. Am. J. Hematol. 92, E559–E560. doi: 10.1002/ajh.24821
Barshtein G, Pajic-Lijakovi I, and Gural A. (2021). Deformability of stored red blood cells. Front. Physiol. 12:1497. doi: 10.3389/fphys.2021.722896
Barshtei G, Pries A. R., Goldschmidt N., Zukerman A., Orbach A, and Zelig O. (2016). Deformability of transfused red blood cells is a potent determinant of transfusion-induced change in recipient's blood flow. Microcirculation. 23, 479–486. doi: 10.1111/micc.12296
Baskurt O. K., Meiselman H. J. (2003). Blood rheology and hemodynamics. Semin. Thromb. Hemost. 29, 435–450. doi: 10.1055/s-2003-44551
Beamer N., Giraud G., Clark W., Wynn M., Coull B., (1997). Diabetes, hypertension and erythrocyte aggregation in acute stroke. Cerebrovasc Dis. 7, 144–149.
Berger S. A., King W., (1980). The flow of sickle-cell blood in the capillaries. Biophys J. 29, 119–148. doi: 10.1016/S0006-3495(80)85121-6
Brazionis L., Rowley K,. Itsiopoulos C., Harper C. A., O'Dea K. (2008). Homocysteine and diabetic retinopathy. Diabetes Care. 31, 50–56. doi: 10.2337/dc07-0632
Brunner E., (2007). “Fibrinogen and clotting factors,” in Encyclopedia of Stress (2nd Edition). ed G. Fink (New York, NY: Academic Press), 51–55.
Brust M., Aouane O., Thiébaud M.,Flormann D., Verdier C., Kaestner L., (2014). The plasma protein fibrinogen stabilizes clusters of red blood cells in microcapillary flows. Sci. Rep. 4:4348. doi: 10.1038/srep04348
Bull B., Chien S., Dormandy J., Kiesewetter H., Lewis S., Lowe G., (1986). Guidelines for measurement of blood viscosity and erythrocyte deformability. Clin. Hemorheol. Microcirc. 6, 439–453. doi: 10.3233/CH-1986-6510
Chang H. Y., Li X., Karniadakis G. E., (2017). Modeling of biomechanics and biorheology of red blood cells in type 2 diabetes mellitus. Biophys J. 113, 481–490. doi: 10.1016/j.bpj.2017.06.015
Chang H. Y., Yazdani A., Li X., Douglas K. A. Mantzoros C. S. Karniadakis G. E. (2018). Quantifying platelet margination in diabetic blood flow. Biophys J. 115, 1371–1382. doi: 10.1016/j.bpj.2018.08.031
Chatterjee S. Khunti K. Davies M. J. (2017). Type 2 diabetes. Lancet. 389, 2239–2251. doi: 10.1016/S0140-6736(17)30058-2
Chen X. Y. Huang Y. X. Jing L. W. Jian Y. Z. (2007). Membrane surface charge and morphological and mechanical properties of young and old erythrocytes. Curr. Appl. Phys. 7:e94–e96. doi: 10.1016/j.cap.2006.11.024
Chien S. Usami S. Bertles J. F (1970a). Abnormal rheology of oxygenated blood in sickle cell anemia. J. Clin. Invest. 49, 623–634. doi: 10.1172/JCI106273
Chien S. Usami S. Dellenback R. Gregersen M. (1970b). Shear-dependent interaction of plasma proteins with erythrocytes in blood rheology. Am. Physiol.-Legacy Content. 219, 143–153. doi: 10.1152/ajplegacy.1970.219.1.143
Chien S. Usami S. Dellenback R. J. Gregersen M. I. Nanninga L. B. Guest M. M. (1967). Blood viscosity: influence of erythrocyte aggregation. Science. 157, 829–831. doi: 10.1126/science.157.3790.829
Chien S. Usami S. Taylor H. M. Lundberg J. L. Gregersen M. I. (1966). Effects of hematocrit and plasma proteins on human blood rheology at low shear rates. J. Appl. Physiol. 21, 81–87. doi: 10.1152/jappl.1966.21.1.81
Chien W. Gompper G. Fedosov D. A. (2021). Effect of cytosol viscosity on the flow behavior of red blood cell suspensions in microvessels. Microcirculation. 28:e12668. doi: 10.1111/micc.12668
Cho H. C. (2011). The relationship among homocysteine, bilirubin, and diabetic retinopathy. Diabetes Metab. J. 35, 595–601. doi: 10.4093/dmj.2011.35.6.595
Cho Y. I. Mooney M. P. Cho D. J. (2008). Hemorheological disorders in diabetes mellitus. J. Diabetes Sci. Technol. 2, 1130–1138. doi: 10.1177/193229680800200622
Ciuffetti G. Schillaci G. Lombardini R. Pirro M. Vaudo G. Mannarino E. (2005). Prognostic impact of low-shear whole blood viscosity in hypertensive men. Eur. J. Clin. Invest. 35, 93–98. doi: 10.1111/j.1365-2362.2005.01437.x
Cloutier G. Zimmer A. François T. Chiasson J. L. (2008). Increased shear rate resistance and fastest kinetics of erythrocyte aggregation in diabetes measured with ultrasound. Diabetes Care. 31, 1400–1402.
Davies M. Brophy S. Williams R. Taylor A. (2006). The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care. 29, 1518–1522. doi: 10.2337/dc05-2228
De Meyer T. De Buyzere M. L. Langlois M. Rietzschel E. R. Cassiman P. De Bacquer D. (2008). Lower red blood cell counts in middle-aged subjects with shorter peripheral blood leukocyte telomere length. Aging Cell. 7, 700–705. doi: 10.1111/j.1474-9726.2008.00419.x
Dearnley M. Chu T. Zhang Y. Looker O. Huang C. Klonis N. (2016). Reversible host cell remodeling underpins deformability changes in malaria parasite sexual blood stages. Proc. Natl. Acad. Sci. U.S.A. 113, 4800–4805. doi: 10.1073/pnas.1520194113
Deng Y. Lu L. Aponte L. Angelidi A. M. Novak V. Karniadakis G. E. (2021). Deep transfer learning and data augmentation improve glucose levels prediction in type 2 diabetes patients. NPJ Digit. Med. 4:109. doi: 10.1038/s41746-021-00480-x
Deng Y Papageorgiou D. P. Li X. Perakakis N. Mantzoros C. S. Dao M. (2020). Quantifying fibrinogen-dependent aggregation of red blood cells in Type 2 Diabetes Mellitus. Biophys. J. 119, 900–912. doi: 10.1016/j.bpj.2020.07.026
Dintenfass L. (1985). Red cell rigidity, Tk, and filtration. Clin. Hemorheol. Microcirc. 5, 241–244. doi: 10.3233/CH-1985-5308
Du E Diez-Silva M. Kato G. J. Dao M. Suresh S. (2015). Kinetics of sickle cell biorheology and implications for painful vasoocclusive crisis. Proc. Natl. Acad. Sci. U.S.A. 112, 1422–1427. doi: 10.1073/pnas.1424111112
Ercan M. Konukoğlu D. Erdem T. Önen S. (2002). The effects of cholesterol levels on hemorheological parameters in diabetic patients. Clin. Hemorheol. Microcirc. 26, 257–263.
Fedosov D. A. Caswell B. Suresh S. Karniadakis G. E. (2011a). Quantifying the biophysical characteristics of plasmodium-falciparum-parasitized red blood cells in microcirculation. Proc. Natl. Acad. Sci. U.S.A. 108, 35–39. doi: 10.1073/pnas.1009492108
Fedosov D. A. Dao M. Karniadakis G. E. Suresh S. (2014a). Computational biorheology of human blood flow in health and disease. Ann. Biomed. Eng. 42, 368–387. doi: 10.1007/s10439-013-0922-3
Fedosov D. A. Noguchi H. Gompper G.. (2014b). Multiscale modeling of blood flow: from single cells to blood rheology. Biomech. Model Mechanobiol. 13, 239–258. doi: 10.1007/s10237-013-0497-9
Fedosov D. A. Pan W Caswell B. Gompper G. Karniadakis G. E. (2011b). Predicting human blood viscosity in silico. Proc. Natl. Acad. Sci. U.S.A. 108, 11772–11777. doi: 10.1073/pnas.1101210108
Fowler M. J. (2008). Microvascular and macrovascular complications of diabetes. Clin. Diabetes. 26, 77–82. doi: 10.2337/diaclin.26.2.77
Irace C. Carallo C. Scavelli F. Esposito T. (2014). Influence of blood lipids on plasma and blood viscosity. Clin. Hemorheol. Microcirc. 57, 83–290. doi: 10.3233/CH-131705
Jeganathan V. S. E. Wang J. J Wong. T. Y. (2008). Ocular associations of diabetes other than diabetic retinopathy. Diabetes Care. 31, 1905–1912. doi: 10.2337/dc08-0342
Kameneva M. Watach M. Borovetz H. (1999). Gender difference in rheologic properties of blood and risk of cardiovascular diseases. Clin. Hemorheol. Microcirc. 21, 357–363.
Krüger-Genge A. Sternitzky R. Pindur G. Rampling M. Franke R. Jung F. (2019). Erythrocyte aggregation in relation to plasma proteins and lipids. J. Cell. Biotechnol. 5, 65–70. doi: 10.3233/JCB-189014
Lapolla A. Poli T. Meneghini F. Zucchetto M. Franchin A. Barison A. (1988). Glycated serum proteins and glucose tolerance. Acta Diabetol Lat. 25, 325–332.
Lei H Karniadakis G. E. (2013). Probing vasoocclusion phenomena in sickle cell anemia via mesoscopic simulations. Proc. Natl. Acad. Sci. U.S.A. 110, 11326–11330. doi: 10.1073/pnas.1221297110
Li H Papageorgiou D. P. Chang H. Y. Lu L. Yang J. Deng Y. (2018). Synergistic integration of laboratory and numerical approaches in studies of the biomechanics of diseased red blood cells. Biosensors. 8, 76. doi: 10.3390/bios8030076
Li H. Sampani K. Zheng X. Papageorgiou D. P. Yazdani A. Bernabeu M. O. (2020). Predictive modelling of thrombus formation in diabetic retinal microaneurysms. R. Soc. Open Sci. 7:201102. doi: 10.1098/rsos.201102
MacRury S. Lennie S. McColl P. Balendra R. MacCuish A. Lowe G. (1993). Increased red cell aggregation in diabetes mellitus: association with cardiovascular risk factors. Diabetic Med. 10, 21–26.
Mathers C. D. Loncar D. (2006). Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 3:e442. doi: 10.1371/journal.pmed.0030442
Matsuda T. Murakami M. (1976). Relationship between fibrinogen and blood viscosity. Thromb Res. 8, 25–33. doi: 10.1016/0049-3848(76)90044-X
Mokken F. C. Kedaria M. Henny C. P. Hardeman M. Gelb A. (1992). The clinical importance of erythrocyte deformability, a hemorrheological parameter. Ann. Hematol. 64, 113–122.
Nash G. B. Meiselman H. J. (1985). Alteration of red cell membrane viscoelasticity by heat treatment: effect on cell deformability and suspension viscosity. Biorheology. 22, 73–84.
Perakakis N. Yazdani A. Karniadakis G. E. Mantzoros C. (2018). Omics, big data and machine learning as tools to propel understanding of biological mechanisms and to discover novel diagnostics and therapeutics. Metabolism. 87, A1–A9. doi: 10.1016/j.metabol.2018.08.002
Phillips R. Kondev J. Theriot J. (2008). Physical Biology of the Cell. New York, NY: Garland Science.
Popel A. S. Johnson P. C. (2005). Microcirculation and hemorheology. Annu. Rev. Fluid Mech. 37, 43–69. doi: 10.1146/annurev.fluid.37.042604.133933
Sacks D. B. (2011). A1C versus glucose testing: a comparison. Diabetes Care. 34, 518–523. doi: 10.2337/dc10-1546
Schmid-Schönbein H. Volger E. (1976). Red-cell aggregation and red-cell deformability in diabetes. Diabetes. 25, 897–902. doi: 10.2337/dc07-1802
Secomb T. W. (2017). Blood flow in the microcirculation. Annu. Rev. Fluid Mech. 49, 443–461. doi: 10.1146/annurev-fluid-010816-060302
Shelby J. P. White J. Ganesan K. Rathod P. K. Chiu D. T. (2003). A microfluidic model for single-cell capillary obstruction by Plasmodium falciparum-infected erythrocytes. Proc. Natl. Acad. Sci. U.S.A. 100, 14618–14622. doi: 10.1073/pnas.2433968100
Skovborg F. Nielsen A. Schlichtkrull J. Ditzel J. (1966). Blood-viscosity in diabetic patients. Lancet. 287, 129–131.
Sloop G. D. (1996). A unifying theory of atherogenesis. Med. Hypotheses. 47, 321–325. doi: 10.1016/s0306-9877(96)90073-0
Sohrabi S. Wang S. Tan J. Xu J. Yang J. Liu Y. (2017). Nanoparticle transport and delivery in a heterogeneous pulmonary vasculature. J Biomech. 50, 240–247. doi: 10.1016/j.jbiomech.2016.11.023
Spengler M. Svetaz M. Leroux M. Bertoluzzo S. Carrara P. Van Isseldyk F. (2011). Erythrocyte aggregation in patients with systemic lupus erythematosus. Clin. Hemorheol. Microcirc. 47, 279–285. doi: 10.20944/preprints201907.0090.v1
Stamos T. D. Rosenson R. S. (1999). Low high density lipoprotein levels are associated with an elevated blood viscosity. Atherosclerosis. 146, 161–165.
Tamariz L. J. Young J. H. Pankow J. S. Yeh H. C. Schmidt M. I. Astor B. (2008). Blood viscosity and hematocrit as risk factors for type 2 diabetes mellitus: the atherosclerosis risk in communities (ARIC) study. Am. J. Epidemiol. 168, 1153–1160.
Tan J Sohrabi S. He R. Liu Y. (2018). Numerical simulation of cell squeezing through a micropore by the immersed boundary method. Proc. Inst. Mech. Eng. C J. Mec Eng. Sci. 232, 502–514. doi: 10.1177/0954411918764508
Tomaiuolo G. Carciati A. Caserta S. Guido S. (2016). Blood linear viscoelasticity by small amplitude oscillatory flow. Rheol Acta. 55, 485–495. doi: 10.1007/s00397-015-0894-3
Turczynski B. Michalska-Malecka K. Slowinska L. Szczesny S. Romaniuk W. (2003). Correlations between the severity of retinopathy in diabetic patients and whole blood and plasma viscosity. Clin. Hemorheol. Microcirc. 29, 129–137.
Wesołowski A. Młynarczak A. (2019). Surface tension and viscosity of blood. Preprint. doi: 10.20944/preprints2019070090v1
Xiao L. L. Lin C. S. Chen S. Liu Y. Fu B. M. Yan W. W. (2020). Effects of red blood cell aggregation on the blood flow in a symmetrical stenosed microvessel. Biomech. Model Mechanobiol. 19, 159–171. doi: 10.1007/s10237-019-01202-9
Yang H. Sloan G. Ye Y. Wang S. Duan B. Tesfaye S. (2020). New perspective in diabetic neuropathy: from the periphery to the brain, a call for early detection, and precision medicine. Front Endocrinol. 10, 929. doi: 10.3389/fendo.2019.00929
Yao C Huang Y. Li X. Ruan P. (2003). Effects of pH on structure and function of single living erythrocyte. Chin. Sci. Bull. 48, 1342–1346. doi: 10.1007/BF03184176
Yazdani A. Deng Y. Li H. Javadi E. Li Z. Jamali S. (2021). Integrating blood cell mechanics, platelet adhesive dynamics and coagulation cascade for modeling thrombus formation in normal and diabetic blood. J. R. Soc. Interface. 18:20200834. doi: 10.1098/rsif.2020.0834
Yedgar S. Koshkaryev A, and Barshtein G. (2002). The red blood cell in vascular occlusion. Pathophysiol. Haemostasis. Thromb. 32, 263–268. doi: 10.1159/000073578
Young M. Boulton A .MacLeod A. Williams D. Sonksen P. (1993). A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia. 36, 150–154.
Zhang Y. Huang C. Kim S. Golkaram M. Dixon M. W. A. Tilley L. (2015). Multiple stiffening effects of nanoscale knobs on human red blood cells infected with Plasmodium falciparum malaria parasite. Proc. Natl. Acad. Sci. U.S.A. 112, 6068–6073. doi: 10.1073/pnas.1505584112
Keywords: red blood cell, blood flow, blood viscosity, diabetes mellilus, RBC aggregation, RBC deformation
Citation: Sun J, Han K, Xu M, Li L, Qian J, Li L and Li X (2022) Blood Viscosity in Subjects With Type 2 Diabetes Mellitus: Roles of Hyperglycemia and Elevated Plasma Fibrinogen. Front. Physiol. 13:827428. doi: 10.3389/fphys.2022.827428
Received: 02 December 2021; Accepted: 20 January 2022;
Published: 25 February 2022.
Edited by:
Gregory Barshtein, Hebrew University of Jerusalem, IsraelReviewed by:
Herbert J. Meiselman, Independent Researcher, Los Angeles, United StatesGiovanna Tomaiuolo, University of Naples Federico II, Italy
Pedro Cabrales, University of California, San Diego, United States
Copyright © 2022 Sun, Han, Xu, Li, Qian, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin Qian, jqian@zju.edu.cn; Li Li, lilyningbo@163.com; Xuejin Li, xuejin_li@zju.edu.cn
†These authors have contributed equally to this work
 Jiehui Sun
Jiehui Sun Keqin Han
Keqin Han Miao Xu
Miao Xu Lujuan Li
Lujuan Li Jin Qian
Jin Qian Li Li
Li Li Xuejin Li
Xuejin Li