- 1Department of Biomedical Engineering, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, Netherlands
- 2Department of Physiology, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, Netherlands
- 3Division of Heart and Lungs, Department of Cardiology, University Medical Center Utrecht, Utrecht, Netherlands
- 4Department of Cardiology, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University Medical Center, Maastricht, Netherlands
- 5Department of Cardiology, Radboud University Medical Center, Nijmegen, Netherlands
Introduction: Computational modeling of cardiac mechanics and hemodynamics in ischemic heart disease (IHD) is important for a better understanding of the complex relations between ischemia-induced heterogeneity of myocardial tissue properties, regional tissue mechanics, and hemodynamic pump function. We validated and applied a lumped two-compartment modeling approach for IHD integrated into the CircAdapt model of the human heart and circulation.
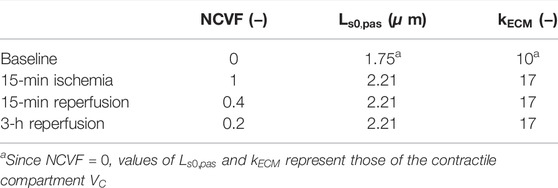
Methods: Ischemic contractile dysfunction was simulated by subdividing a left ventricular (LV) wall segment into a hypothetical contractile and noncontractile compartment, and dysfunction severity was determined by the noncontractile volume fraction (
Results: Simulation of 20% of
Conclusion: We successfully used a two-compartment model for simulation of the ventricular pump and tissue mechanics in IHD. Patient-specific optimizations using regional myocardial deformation estimated the
1 Introduction
Computational modeling of cardiac mechanics and hemodynamics in ischemic heart disease (IHD) is important for better understanding of the complex relations between ischemia-induced heterogeneity of myocardial tissue properties, regional tissue mechanics, and hemodynamic pump function. Spatially detailed three-dimensional models based on the finite element method (FEM) are most frequently used for simulation of myocardial infarction (MI) and its effects on cardiac geometry, tissue mechanics, and electrophysiology. While many studies have successfully used FEM models for investigating the pathophysiology and potential treatment of MI (Fomovsky et al., 2011; Fomovsky et al., 2012; Rouillard and Holmes, 2014; Veress et al., 2015; Leong et al., 2017; Haddad and Samani, 2018; Wang et al., 2018; Chan et al., 2019; Fan et al., 2019; Zhuan et al., 2019; Estrada et al., 2020; Zhang et al., 2020; Zhang et al., 2021), complexity of these models can provide a problem when performing simulations on a patient-specific level (Moulton et al., 2017).
Reduced-order modeling approaches with sufficient spatial and physiological details to accurately simulate global and regional tissue mechanics are important for patient-specific simulation of MI (Moulton et al., 2017; Holmes and Lumens, 2018). The CircAdapt lumped-parameter model of the human heart and circulation is a closed-loop model which simulates real-time, beat-to-beat hemodynamics and mechanics of the heart and blood vessels (Arts et al., 2004; Lumens et al., 2009). Previous studies using CircAdapt have shown that the model realistically simulates global ventricular hemodynamics and regional myocardial mechanics in various pathological conditions (Lumens et al., 2009; Walmsley et al., 2015). However, its ability to simulate the effects of ischemia-induced contractile dysfunction on both global pump and regional tissue mechanics has not been evaluated yet.
In this study, we presented and tested a modeling approach for MI-induced myocardial contractile dysfunction, which is integrated into the CircAdapt modeling framework. Following previous observations by Sunagawa et al. (1982), who showed that global ventricular mechanics during acute regional ischemia were best described using a two-compartment modeling approach, we subdivided an ischemic wall segment into an active and a passive compartment. We validated this two-compartment implementation by comparing its simulated effects of regional ischemia on the global LV pump function against the results of a one-compartment implementation and by tuning parameters of the two-compartment model to mimic existing data on regional myocardial deformation in dogs with coronary artery ligation. We then evaluated whether the model could be used to estimate the severity of regional contractile dysfunction in MI patients by applying an automatic optimization algorithm to measurements of regional myocardial deformation performed within 72 h and after 6 months following MI and by comparing estimated severities to percentage late gadolinium enhancement (LGE) after 6 months.
2 Materials and Methods
A brief description of the CircAdapt model components, which are most relevant for this study, are provided as follows. For further details on the CircAdapt model, we referred to previously published validation studies (Walmsley et al., 2015; Lumens et al., 2009). Thereafter, the one- and two-compartment models for ischemia-induced contractile dysfunction were described, and we explained how simulated global mechanics were compared between both modeling approaches. Then, we described how model simulations using the two-compartment model were tuned using existing experimental measurements. Finally, we introduced the clinical data and computational methods used for imaging-based estimation of regional contractile dysfunction in patient hearts acutely and 6 months after MI.
2.1 CircAdapt Model
The CircAdapt computational model of the human heart and circulation is a closed-loop lumped-parameter model that simulates beat-to-beat hemodynamics and mechanics of the heart and blood vessels (Arts et al., 2004; Lumens et al., 2009). The model uses a simplified ventricular geometry, where cardiac walls are represented by thick-walled spherical shells consisting of myofibers. The TriSeg module allows for interventricular interaction by coupling the left (LV) and right ventricular (RV) walls through the interventricular septum (Lumens et al., 2009). Walls can be subdivided into patches using the MultiPatch module (Walmsley et al., 2015), which enables heterogeneity of myocardial tissue properties within the walls. Myofiber active and passive stress generation is modeled using a three-element Hill model (Arts et al., 2003). In brief, the series contractile element with the time-dependent length
Passive myocardial tissue behavior is described by the parallel elastic element, which captures both extracellular and intracellular structures. Therefore, total passive stress
The extracellular matrix stress
Here,
2.2 Simulating Ischemia-Induced Contractile Dysfunction: One-Compartment Vs. Two-Compartment Model
In a combined experimental and modeling study, Sunagawa et al. (1982) showed that the LV systolic function during regional ischemia was best captured by a two-compartment model including an active and a passive compartment. The rationale behind this two-compartment model is that the active compartment lumps all of the normally functioning tissues within the nonischemic and ischemic region, while the passive compartment lumps all of the nonfunctional parts of the ischemic region. As mentioned before, we hypothesized that we could use a similar modeling approach not only for simulating global LV pump mechanics during regional ischemia but also to simulate average regional LV tissue mechanics (i.e., the myofiber stress and strain) in ischemic myocardial segments, which are defined by deformation imaging techniques, for example, speckle tracking echocardiography (STE). In the CircAdapt model, tissue properties are defined per myocardial patch, and by default, each myocardial wall includes one myocardial patch. The active and passive stress of a patch is calculated using Eqs 1–4. To simulate reduced patch contractility, one approach is to reduce parameter
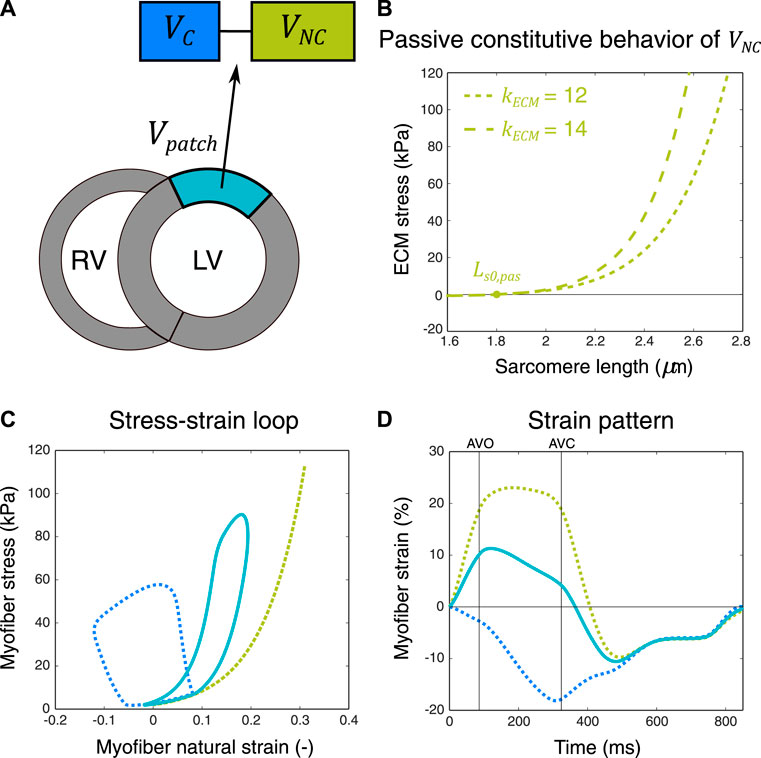
FIGURE 1. Schematic representation of the two-compartment modeling approach used for simulation of ischemic myocardial dysfunction. A myocardial patch with volume
2 3.Myofiber Strain Calculations
The calculated strain
where
For the two-compartment model, the sarcomere length
2.4 Simulation Protocol
2.4.1 Global Myocardial Mechanics: One-Compartment Vs. Two-Compartment Model
In line with the study of Sunagawa et al. (1982), we compared simulated LV pump mechanics during acute regional ischemia when using a two-compartment model against a one-compartment model by constructing end-systolic pressure volume relations (ESPVRs). We started with a baseline reference simulation with a heart rate of 71 bpm, stroke volume (SV) of 72 ml, and a mean arterial pressure (MAP) of 92 mmHg. Homeostatic pressure-flow regulation was enabled, meaning that MAP and SV were kept constant through regulation of the peripheral arterial resistance and circulating blood volume. First, for the two-compartment simulation, the
2.4.2 Regional Myocardial Mechanics: Acute Ischemia and Reperfusion
We hypothesized that acute ischemia and subsequent reperfusion could realistically be simulated by manipulation of the
Parameters
2.4.3 Regional Myocardial Mechanics: Prolonged Ischemia and Reperfusion
To evaluate whether the two-compartment model could also accurately simulate regional mechanics in ischemic dysfunction with increased stiffness, potentially indicating MI, we attempted to calibrate parameters
2.5 Patient-Specific
2.5.1 Patient Cohort
Six patients were selected from the DEFI-MI (DEtection of cardiac FIbrosis by LGE magnetic resonance imaging (MRI) and circulating biomarkers in patients with myocardial infarction) cohort. This was a prospective study on first-time MI patients, approved by the local ethics committee (METC: NL45241.041.13) and in accordance with the Declaration of Helsinki. Patients in this study were included after an acute MI, when urgent revascularization was performed. From the measured cohort, we selected patients with narrow QRS (<120 ms), who had a minimum infarct size of 10% of the LV wall mass and a minimum relative LGE percentage of 25% in at least one myocardial AHA segment.
2.5.2 Echocardiographic Measurements
Echocardiography was performed at the baseline (i.e., within 72 h after admission) and at the 6-month follow-up using a commercially available system (Vivid E9, GE Vingmed Ultrasound AS, Horten, Norway). LV volumes and ejection fraction (EF) were acquired by Simpson’s biplane method. Focused loops of the apical four-chamber view, two-chamber view, and three-chamber view were stored for post-processing (GE EchoPAC version 203). Speckle tracking deformation imaging of the LV was performed in 18 segments according to current clinical standards while blinded to the MRI results (Voigt et al., 2015).
2.5.3 Cardiac Magnetic Resonance
At a median of 6 months after primary MI, all patients underwent contrast-enhanced 1.5 Tesla cardiac magnetic resonance (CMR) imaging (Philips Healthcare, Best, Netherlands). LGE image acquisition was performed 15 min after administration of 0.2 ml/kg gadobutrol (Gadovist, Bayer Vital GmbH, Leverkusen, Germany), using prospective ECG-gated sequences of the short axis views from the base to apex, with 5 mm slice thickness. Images were analyzed off-line using Philips ISP9 software (Philips Healthcare, Best, Netherlands). Using the RV insertion points to the interventricular septum as anatomical landmarks, the heart was subdivided into 16 segments according to the model of the American Heart Association (AHA) (Cerqueira et al., 2002), excluding the apical cap. The LGE was quantitatively assessed using the full width at half maximum (FWHM) method, providing a percentage for each of the analyzed segments and the total infarct (scar) size (global %) of the whole LV.
2.5.4 Optimization Algorithm
To estimate the
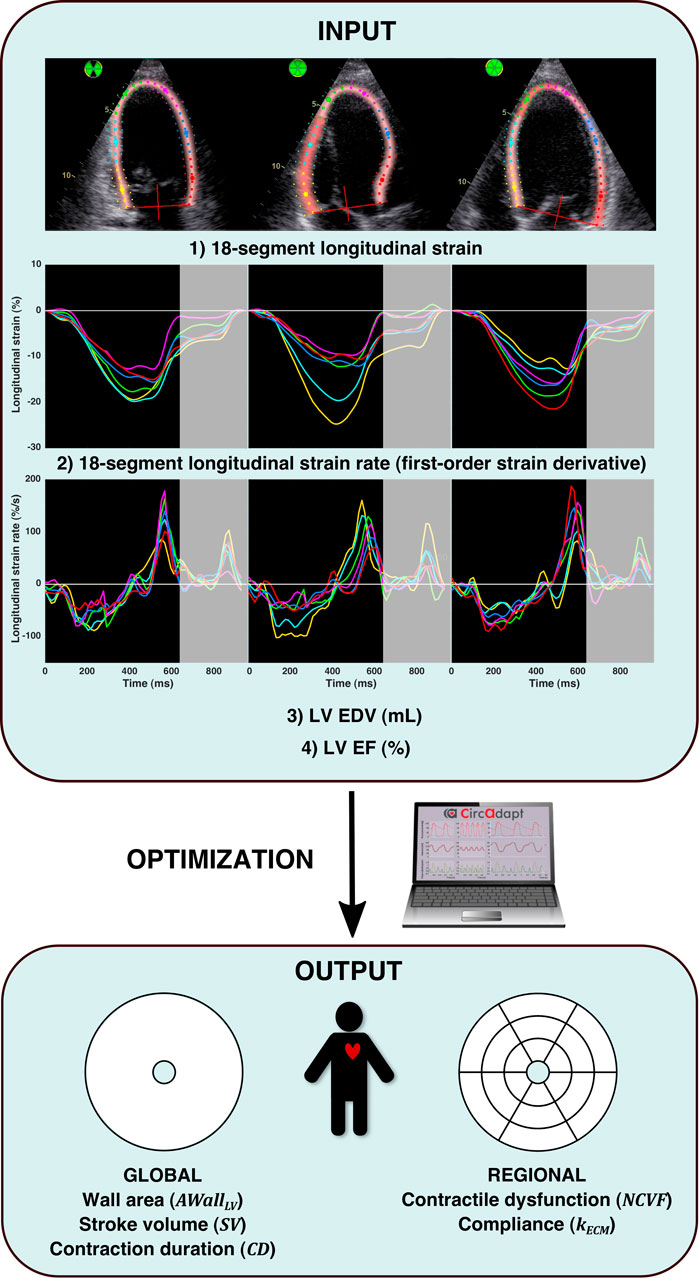
FIGURE 2. Patient-specific parameter estimation protocol. The input of the optimization algorithm (INPUT) consisted of 1) 18-segment longitudinal strain, 2) 18-segment longitudinal strain rate (calculated as the first-order time derivative of strain), 3) LV end-diastolic volume (EDV), and 4) LV ejection fraction (EF). Strain and strain rate signals were analyzed until early diastole, as indicated by the gray-enhanced parts of the strain and strain rate signals. For numerical optimization (OPTIMIZATION), a multi-swarm particle swarm optimization (MSPSO) algorithm similar to the one previously described in the study by Van Osta et al. (2021) was run, which estimated (OUTPUT): global LV wall area, global stroke volume, global ventricular contraction duration, regional contractile dysfunction, and regional compliance.
2.5.5 Model Implementation
The CircAdapt model used in this study has been published before in more detail (Walmsley et al., 2015). To reduce the computational cost, a C++ implementation of this version was used as published before (Van Osta et al., 2020). Equations were linearized using the Newton–Raphson method, and 61 ordinary differential equations were time-integrated using the Adams–Bashford method, with a variable timestep Δt with max (Δt) = 2 ms. MSPSO was performed in MATLAB 2019a (MathWorks, Natick, MA, United States). Simulations ran in parallel on an AMD Ryzen Threadripper 3970X.
3 Results
3.1 Global Myocardial Mechanics: One-Compartment Vs. Two-Compartment Model
Constructed ESPVRs for the one- and two-compartment simulations of LV acute regional ischemia are shown in Figure 3. The associated values of
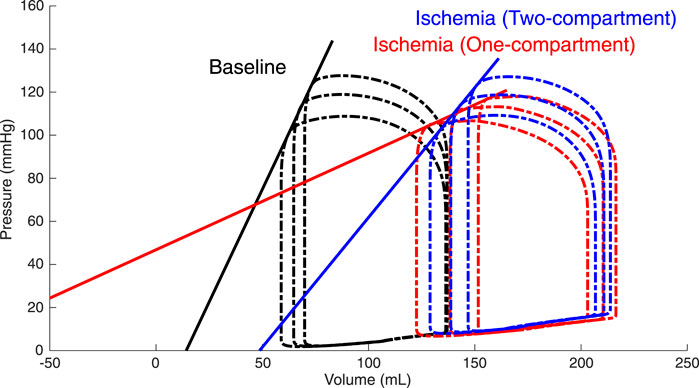
FIGURE 3. Simulated left ventricular (LV) end-systolic pressure-volume relations (ESPVRs) for a baseline healthy LV (black loops and line) and for an ischemic LV affecting 20% of the LV wall mass modeled by a two-compartment approach (blue loops and line) and a one-compartment approach (red loops and line). ESPVRs were constructed by afterload manipulations through reducing and increasing arterial resistance by 20% compared to the reference resistance. The two-compartment simulation of ischemia demonstrated a rightward shift of the ESPVR with a moderate reduction in the slope, while the one-compartment simulation demonstrated a severe reduction in both the slope and zero-pressure volume.
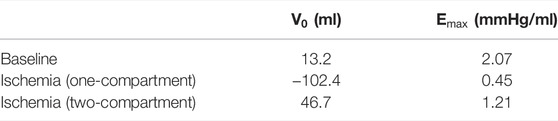
TABLE 1. Characteristics of the left ventricular (LV) end-systolic pressure–volume relation (ESPVR).
3.2 Regional Myocardial Mechanics: Acute Ischemia and Reperfusion
Simulated LV pressure-segment length loops and corresponding strain patterns at the baseline (
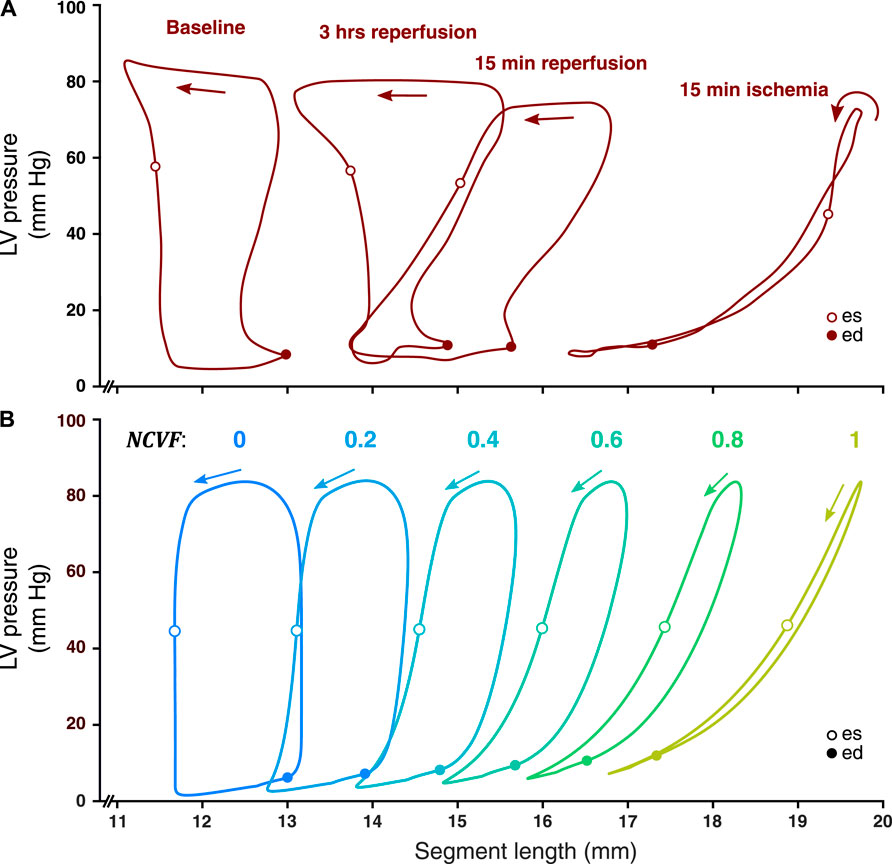
FIGURE 4. Comparison between measured LV pressure-segment length loops in a representative dog after 15 min of left anterior descending (LAD) coronary artery occlusion, followed by 15 min and 3 h of reperfusion [(A), resketched from Lyseggen et al. (2005)] and simulated LV pressure-segment length loops (B) for varying regional contractile dysfunction (
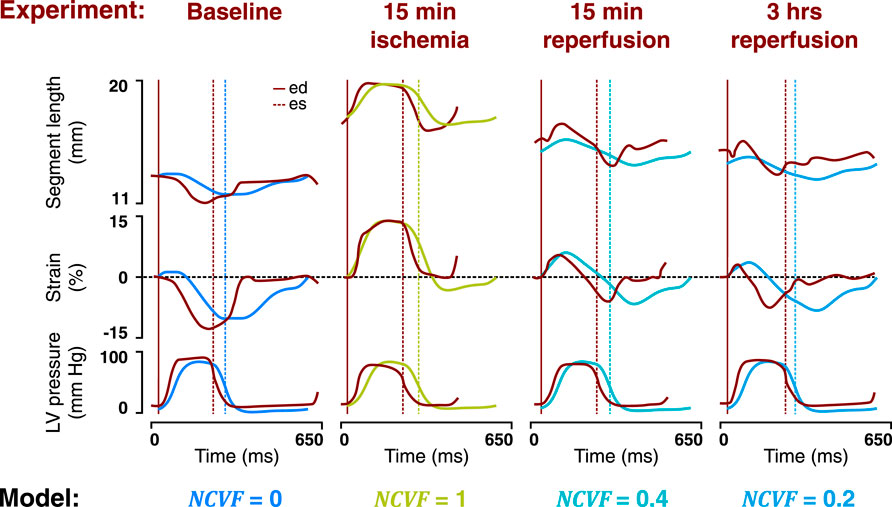
FIGURE 5. Comparison between the temporal behavior of the measured segment length, strain, and LV pressure in the same representative dog as in Figure 4 after 15 min of left anterior descending (LAD) coronary artery occlusion followed by 15 min and 3 h of reperfusion (dark red tracings, resketched from Lyseggen et al. (2005)) and the simulated segment length, strain, and LV pressure (blue and green tracings) for the best matching severities of regional contractile dysfunction (
3.3 Regional Myocardial Mechanics: Prolonged Ischemia and Reperfusion
When starting from the calibrated simulation of acute ischemia, increasing
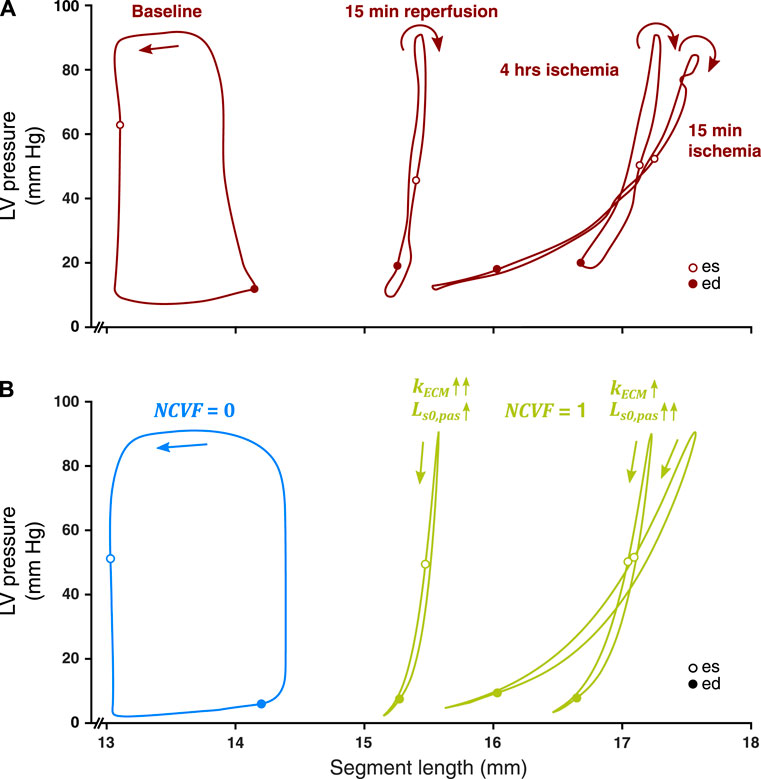
FIGURE 6. Comparison between measured LV pressure-segment length loops in a representative dog after 15 min and 4 h of left anterior descending (LAD) coronary artery occlusion, followed by 15 min of reperfusion [(A), resketched from Lyseggen et al. (2005)] and simulated LV pressure-segment length loops (B) for no contractile dysfunction (
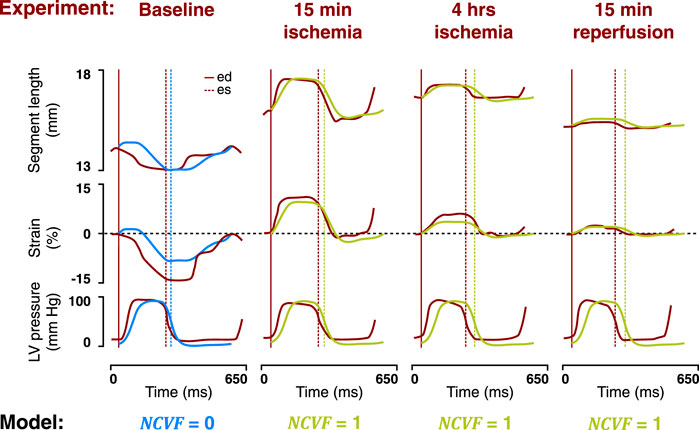
FIGURE 7. Comparison between the temporal behavior of the measured segment length, strain, and LV pressure in the same representative dog as in Figure 6 after 15 min and 4 h of left anterior descending (LAD) coronary artery occlusion, followed by 15 min of reperfusion (dark red tracings, resketched from Lyseggen et al. (2005)) and the simulated segment length, strain, and LV pressure (blue and green tracings) for no contractile dysfunction (
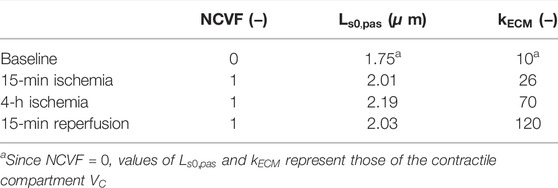
TABLE 3. Calibrated parameter values for the simulations of acute ischemia, prolonged ischemia, and reperfusion.
3.4 Patient-specific
Patient characteristics are summarized in Table 4. Results of the patient-specific estimation of the
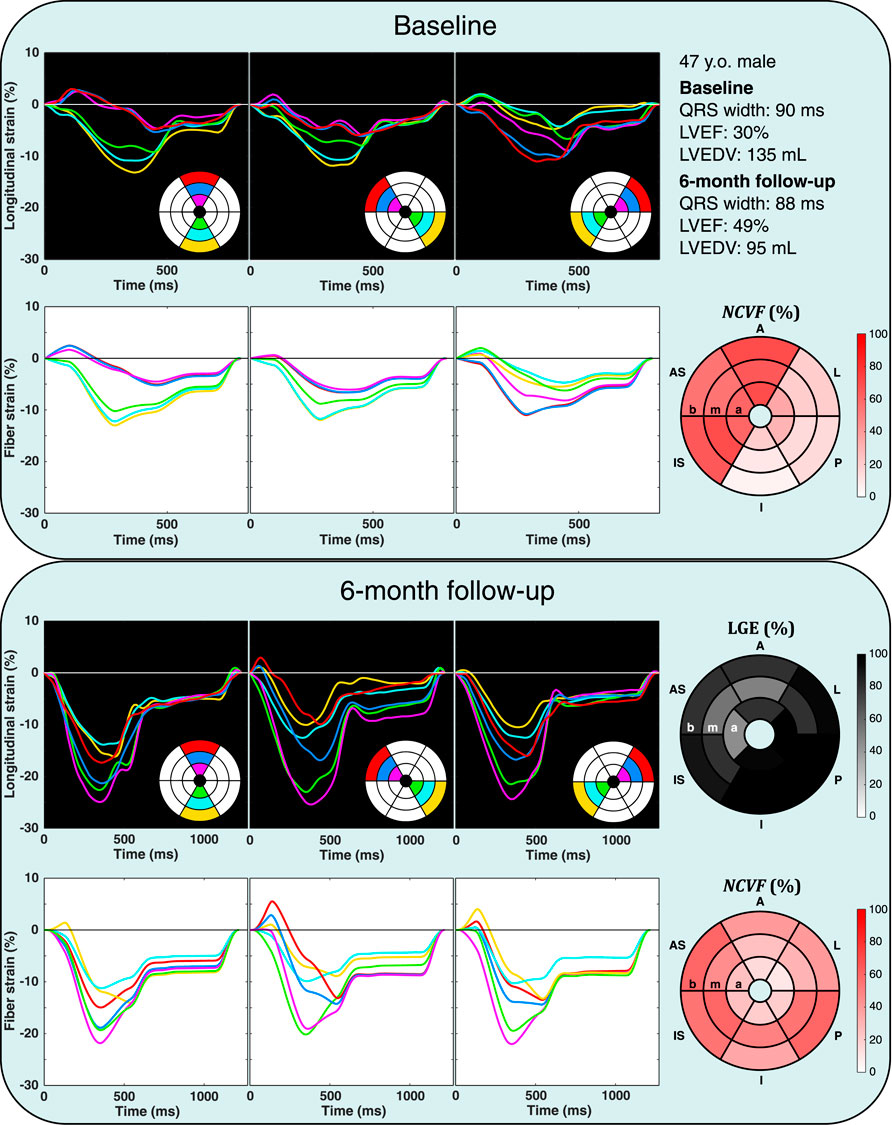
FIGURE 8. Echocardiographic strain measurements (black panels) and simulated strain patterns obtained using an optimization algorithm (white panels) at the baseline and 6-month follow-up in one patient from the DEFI-MI subcohort (patient 1, a 47-year-old male). Estimated
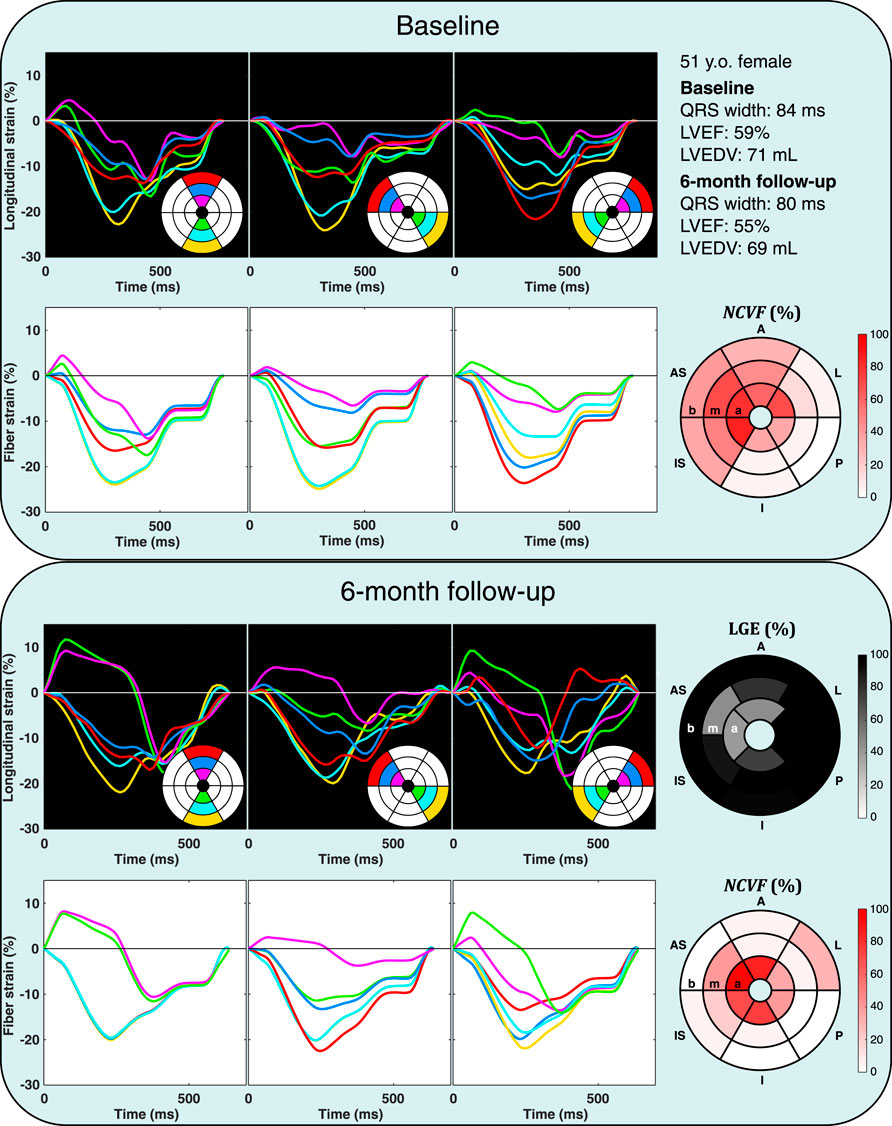
FIGURE 9. Echocardiographic strain measurements (black panels) and simulated strain patterns obtained using an optimization algorithm (white panels) at the baseline and 6-month follow-up in one patient from the DEFI-MI subcohort (patient 2, a 51-year-old female). Estimated
Analysis of all 22 myocardial segments with LGE>25% at 6-month follow-up revealed that in most segments (68%), the
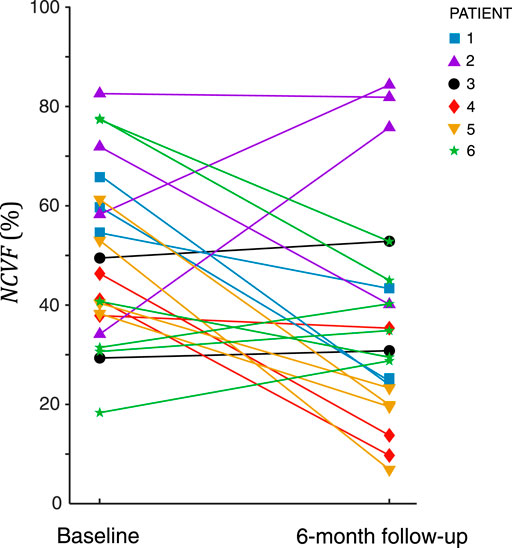
FIGURE 10. Estimated
4 Discussion
In this computational study, we used the CircAdapt model of the human heart and circulation to test the ability of a lumped two-compartment model to realistically simulate the effects of ischemia-induced contractile dysfunction on ventricular pump and myocardial tissue mechanics. The modeling approach was first evaluated on the level of global LV mechanics by constructing ESPVRs at the baseline and during acute regional ischemia using simulations with a two- and one-compartment modeling approach. Then, simulated regional strains and pressure-length loops for increasing degrees of contractile dysfunction were compared with existing gold standard myocardial deformation measurements in dogs with acute and prolonged ischemia followed by reperfusion. Finally, patient-specific optimizations were performed to estimate the noncontractile volume fraction
4.1 Two-Compartment Model Realistically Simulates Global LV Mechanics
Constructed LV ESPVRs at baseline and during acute regional ischemia using both a one- and two-compartment modeling approach demonstrated that
4.2 Two-Compartment Model Reproduces Regional Mechanics During Acute Ischemia and Reperfusion
Simulated strain patterns and LV pressure-segment length loops for
4.3 Two-Compartment Model Reproduces Regional Mechanics During Prolonged Ischemia and Reperfusion
Our simulations of prolonged ischemia and subsequent reperfusion demonstrated that
4.4
The parameter
4.5 Limitations
The experimental measurements of Lyseggen et al. (2005) used in our study were acquired by ultrasonic crystals which were aligned parallel to the LV long axis, thereby closely resembling the LV longitudinal strain. However, in our model, we used the one-fiber model to simulate the myofiber strain within a spherical LV geometry; therefore, there can be a systematic discrepancy between simulated and experimental strain values. In the patient-specific optimizations, we corrected for this model discrepancy by normalization of measured and simulated strains to global strain amplitude, but global strain values were not measured in the experiment; hence, no correction was performed. However, the temporal behavior of the experimental and simulated strains was similar; therefore, we expected that this potential mismatch could have had consequences for the quantitative values of calibrated parameters, but the qualitative results remained unaffected. We also assumed that the measurements were obtained from the anterior portion of the LV free wall, implying that we compared these data to simulated strains in the ischemic region located within the LV free wall, thereby disregarding septal deformation. This assumption, if not fully valid, could additionally have caused a minor mismatch in the strain data between the model and experiment. Furthermore, a sensitivity analysis (data not shown) demonstrated that correlations between the
5 Conclusion
We successfully used a two-compartment model for simulation of ventricular pump and myocardial tissue mechanics in ischemic heart disease. Patient-specific optimizations using regional myocardial deformation successfully estimated
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the METC: NL45241.041.13. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
TK and JL conceived the study. TK performed the simulations and wrote the first version of the manuscript. BK, FK and AT obtained the clinical data. NO, TL, FN, FP, BK, FK,AT, KV and TD helped with analysis and interpretation of the data. All co-authors critically read the manuscript and approved it.
Funding
This study was funded by the Dutch Heart Foundation (grant no. 2015T082 to JL), the Netherlands Organisation for Scientific Research (grant 016.176.340), and the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement no. 860745.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors acknowledge the support from the Dutch Heart Foundation, the Netherlands Organisation for Scientific Research, and the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2022.782592/full#supplementary-material
References
Arts T., Delhaas T., Bovendeerd P., Verbeek X., Prinzen F. W. (2004). Adaptation to Mechanical Load Determines Shape and Properties of Heart and Circulation: the CircAdapt Model. Am. J. Physiol. Heart Circ. Physiol. 288 (4), H1943–H1954. doi:10.1152/ajpheart.00444.2004
Arts T., Bovendeerd P., Delhaas T., Prinzen F. (2003). Modeling the Relation between Cardiac Pump Function and Myofiber Mechanics. J. Biomech. 36 (5), 731–736. doi:10.1016/s0021-9290(02)00451-7
Becker M., Ocklenburg C., Altiok E., Futing A., Balzer J., Krombach G., et al. (2009). Impact of Infarct Transmurality on Layer-specific Impairment of Myocardial Function: A Myocardial Deformation Imaging Study. Eur. Heart J. 30 (12), 1467–1476. doi:10.1093/eurheartj/ehp112
Braunwald E., Kloner R. A. (1982). The Stunned Myocardium: Prolonged, Postischemic Ventricular Dysfunction. Circulation 66 (6 I), 1146–1149. doi:10.1161/01.cir.66.6.1146
Cerqueira M. D., Weissman N. J., Dilsizian V., Jacobs A. K., Kaul S., Laskey W. K., et al. (2002). Standardized Myocardial Segmentation and Nomenclature for Tomographic Imaging of the Heart. J. Cardiovasc. Magn. Reson. 4 (2), 203–210. doi:10.1081/jcmr-120003946
Chan B. T., Ahmad Bakir A., Al Abed A., Dokos S., Leong C. N., Ooi E. H., et al. (2019). Impact of Myocardial Infarction on Intraventricular Vortex and Flow Energetics Assessed Using Computational Simulations. Int. J. Numer. Method. Biomed. Eng. 35 (6), e3204–17. doi:10.1002/cnm.3204
Chan J., Hanekom L., Wong C., Leano R., Cho G.-Y., Marwick T. H. (2006). Differentiation of Subendocardial and Transmural Infarction Using Two-Dimensional Strain Rate Imaging to Assess Short-Axis and Long-Axis Myocardial Function. J. Am. Coll. Cardiol. 48 (10), 2026–2033. doi:10.1016/j.jacc.2006.07.050
Estrada A. C., Yoshida K., Clarke S. A., Holmes J. W. (2020). Longitudinal Reinforcementof Acute Myocardial Infarcts Improves Function by Transmurally Redistributing Stretch and Stress. J. Biomech. Eng. 142 (2), 1–10. doi:10.1115/1.4044030
Fan Y., Ronan W., Teh I., Schneider J. E., Varela C. E., Whyte W., et al. (2019). A Comparison of Two Quasi-Static Computational Models for Assessment of Intra-myocardial Injection as a Therapeutic Strategy for Heart Failure. Int. J. Numer. Method. Biomed. Eng. 35 (9), e3213. doi:10.1002/cnm.3213
Ferferieva V., Van den Bergh A., Claus P., Jasaityte R., Veulemans P., Pellens M., et al. (2012). The Relative Value of Strain and Strain Rate for Defining Intrinsic Myocardial Function. Am. J. Physiol. Heart Circ. Physiol. 302 (1), H188–H195. doi:10.1152/ajpheart.00429.2011
Fomovsky G. M., MacAdangdang J. R., Ailawadi G., Holmes J. W. (2011). Model-based Design of Mechanical Therapies for Myocardial Infarction. J. Cardiovasc. Trans. Res. 4 (1), 82–91. doi:10.1007/s12265-010-9241-3
Fomovsky G. M., Rouillard A. D., Holmes J. W. (2012). Regional Mechanics Determine Collagen Fiber Structure in Healing Myocardial Infarcts. J. Mol. Cell Cardiol. 52 (5), 1083–1090. doi:10.1016/j.yjmcc.2012.02.012
Gao W. D., Atar D., Backx P. H., Marban E. (1995). Relationship between Intracellular Calcium and Contractile Force in Stunned Myocardium. Circ. Res. 76 (6), 1036–1048. doi:10.1161/01.res.76.6.1036
Haddad S. M. H., Samani A. (2018). A Finite Element Model of Myocardial Infarction Using a Composite Material Approach. Comput. Methods Biomech. Biomed. Eng. 21 (1), 33–46. doi:10.1080/10255842.2017.1416355
Holmes J. W., Lumens J. (2018). Clinical Applications of Patient-specific Models: The Case for a Simple Approach. J. Cardiovasc. Transl. Res. 11 (1–9), 71–79. doi:10.1007/s12265-018-9787-z
Holmes J. W., Borg T. K., Covell J. W. (2005). Structure and Mechanics of Healing Myocardial Infarcts. Annu. Rev. Biomed. Eng. 7 (1), 223–253. doi:10.1146/annurev.bioeng.7.060804.100453
Huttin O., Marie P.-Y., Benichou M., Bozec E., Lemoine S., Mandry D., et al. (2016). Temporal Deformation Pattern in Acute and Late Phases of ST-Elevation Myocardial Infarction: Incremental Value of Longitudinal post-systolic Strain to Assess Myocardial Viability. Clin. Res. Cardiol. 105 (10), 815–826. doi:10.1007/s00392-016-0989-6
Kihlberg J., Haraldsson H., Sigfridsson A., Ebbers T., Engvall J. E. (2015). Clinical Experience of Strain Imaging Using DENSE for Detecting Infarcted Cardiac Segments. J. Cardiovasc. Magn. Reson. 17 (1), 50–59. doi:10.1186/s12968-015-0155-8
Leong C. N., Lim E., Andriyana A., Al Abed A., Lovell N. H., Hayward C., et al. (2017). The Role of Infarct Transmural Extent in Infarct Extension: A Computational Study. Int. J. Numer. Method. Biomed. Eng. 33 (2), 1–18. doi:10.1002/cnm.2794
Lumens J., Tayal B., Walmsley J., Delgado-Montero A., Huntjens P. R., Schwartzman D., et al. (2015). Differentiating Electromechanical from Non-electrical Substrates of Mechanical Discoordination to Identify Responders to Cardiac Resynchronization Therapy. Circ. Cardiovasc. Imaging 8 (9), e003744–12. doi:10.1161/CIRCIMAGING.115.003744
Lumens J., Delhaas T., Kirn B., Arts T. (2009). Three-wall Segment (TriSeg) Model Describing Mechanics and Hemodynamics of Ventricular Interaction. Ann. Biomed. Eng. 37 (11), 2234–2255. doi:10.1007/s10439-009-9774-2
Lyseggen E., Skulstad H., Helle-Valle T., Vartdal T., Urheim S., Rabben S. I., et al. (2005). Myocardial Strain Analysis in Acute Coronary Occlusion. Circulation 112 (25), 3901–3910. doi:10.1161/circulationaha.105.533372
McComb C., Carrick D., McClure J. D., Woodward R., Radjenovic A., Foster J. E., et al. (2015). Assessment of the Relationships between Myocardial Contractility and Infarct Tissue Revealed by Serial Magnetic Resonance Imaging in Patients with Acute Myocardial Infarction. Int. J. Cardiovasc. Imaging 31 (6), 1201–1209. doi:10.1007/s10554-015-0678-y
Moulton M. J., Hong B. D., Secomb T. W. (2017). Simulation of Left Ventricular Dynamics Using a Low-Order Mathematical Model. Cardiovasc. Eng. Tech. 8 (4), 480–494. doi:10.1007/s13239-017-0327-9
P. Reant (2016). Impact of Afterload Increase on Left Ventricular Myocardial Deformation Indices. J. Am. Soc. Echocardiog. 29, 1217–1228. doi:10.1016/j.echo.2016.09.006
Richardson W. J., Clarke S. A., Quinn T. A., Holmes J. W. (2015). Physiological Implications of Myocardial Scar Structure. Compr. Physiol. 5 (4), 1877–1909. doi:10.1002/cphy.c140067
Rouillard A. D., Holmes J. W. (2014). Coupled Agent-Based and Finite-Element Models for Predicting Scar Structure Following Myocardial Infarction. Prog. Biophys. Mol. Biol. 115 (2–3), 235–243. doi:10.1016/j.pbiomolbio.2014.06.010
Skulstad H., Edvardsen T., Urheim S., Rabben S. I., Stugaard M., Lyseggen E., et al. (2002). Postsystolic Shortening in Ischemic Myocardium. Circulation 106 (6), 718–724. doi:10.1161/01.cir.0000024102.55150.b6
Sunagawa K., Maughan W. L., Sagawa K. (1982). Effect of Regional Ischemia on the Left Ventricular End- Systolic Pressure-Volume Relationship of Isolated Canine Hearts, 170–179.
Theroux P., Ross J., Franklin D., Covell J. W., Bloor C. M., Sasayama S. (1977). Regional Myocardial Function and Dimensions Early and Late after Myocardial Infarction in the Unanesthetized Dog. Circ. Res. 40 (2), 158–165. doi:10.1161/01.res.40.2.158
Ugander M., Cain P. A., Johnsson P., Palmer J., Arheden H. (2010). Chronic Non-transmural Infarction Has a Delayed Recovery of Function Following Revascularization. BMC Cardiovasc. Disord. 10, 4. doi:10.1186/1471-2261-10-4
Van Osta N., Kirkels F., Lyon A., Koopsen T., van Loon T., Cramer M. J., et al. (2021). Electromechanical Substrate Characterization in Arrhythmogenic Cardiomyopathy Using Imaging-Based Patient-specific Computer Simulations. Europace 23, I153–I160. doi:10.1093/europace/euaa407
Van Osta N., Lyon A., Kirkels F., Koopsen T., van Loon T., Cramer M. J., et al. (2020). Parameter Subset Reduction for Patient-specific Modelling of Arrhythmogenic Cardiomyopathy-Related Mutation Carriers in the CircAdapt Model. Philos. Trans. A. Math. Phys. Eng. Sci. 378, 20190347. doi:10.1098/rsta.2019.0347
Veress A. I., Fung G. S., Lee T. S., Tsui B. M., Kicska G. A., Paul Segars W., et al. (2015). The Direct Incorporation of Perfusion Defect Information to Define Ischemia and Infarction in a Finite Element Model of the Left Ventricle. J. Biomech. Eng. 137 (5), 051004–051010. doi:10.1115/1.4028989
Voigt J.-U., Pedrizzetti G., Lysyansky P., Marwick T. H., Houle H., Baumann R., et al. (2015). Definitions for a Common Standard for 2D Speckle Tracking Echocardiography: Consensus Document of the EACVI/ASE/Industry Task Force to Standardize Deformation Imaging. Eur. Heart J. - Cardiovasc. Imaging 16 (1), 1–11. doi:10.1093/ehjci/jeu184
Walmsley J., Arts T., Derval N., Bordachar P., Cochet H., Ploux S., et al. (2015). Fast Simulation of Mechanical Heterogeneity in the Electrically Asynchronous Heart Using the MultiPatch Module. Plos Comput. Biol. 11 (7), e1004284–23. doi:10.1371/journal.pcbi.1004284
Wang H., Rodell C. B., Zhang X., Dusaj N. N., Gorman J. H., Pilla J. J., et al. (2018). Effects of Hydrogel Injection on Borderzone Contractility post-myocardial Infarction. Biomech. Model. Mechanobiol. 17 (5), 1533–1542. doi:10.1007/s10237-018-1039-2
Zhang Y., Wang V. Y., Morgan A. E., Kim J., Ge L., Guccione J. M., et al. (2020). A Novel MRI-Based Finite Element Modeling Method for Calculation of Myocardial Ischemia Effect in Patients with Functional Mitral Regurgitation. Front. Physiol. 11, 158. doi:10.3389/fphys.2020.00158
Zhang Y., Chan A. K. Y., Yu C.-M., Yip G. W. K., Fung J. W. H., Lam W. W. M., et al. (2005). Strain Rate Imaging Differentiates Transmural from Non-transmural Myocardial Infarction. J. Am. Coll. Cardiol. 46 (5), 864–871. doi:10.1016/j.jacc.2005.05.054
Zhang Y., Wang V. Y., Morgan A. E., Kim J., Tafreshi R., Wallace A. W., et al. (2021). Finite-element Based Optimization of Left Ventricular Passive Stiffness in normal Volunteers and Patients after Myocardial Infarction: Utility of an Inverse Deformation Gradient Calculation of Regional Diastolic Strain. J. Mech. Behav. Biomed. Mater. 119, 104431. doi:10.1016/j.jmbbm.2021.104431
Keywords: myocardial infarction, computational modeling and simulation, deformation imaging, contractile dysfunction, strain
Citation: Koopsen T, Van Osta N, Van Loon T, Van Nieuwenhoven FA, Prinzen FW, Van Klarenbosch BR, Kirkels FP, Teske AJ, Vernooy K, Delhaas T and Lumens J (2022) A Lumped Two-Compartment Model for Simulation of Ventricular Pump and Tissue Mechanics in Ischemic Heart Disease. Front. Physiol. 13:782592. doi: 10.3389/fphys.2022.782592
Received: 24 September 2021; Accepted: 10 March 2022;
Published: 11 May 2022.
Edited by:
Alexander Y. Mitrophanov, National Cancer Institute at Frederick (NIH), United StatesReviewed by:
Vicky Y. Wang, The University of Auckland, New ZealandPer Lav Madsen, University of Copenhagen, Denmark
Copyright © 2022 Koopsen, Van Osta, Van Loon, Van Nieuwenhoven, Prinzen, Van Klarenbosch, Kirkels, Teske, Vernooy, Delhaas and Lumens. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tijmen Koopsen, t.koopsen@maastrichtuniversity.nl
 Tijmen Koopsen
Tijmen Koopsen Nick Van Osta
Nick Van Osta Tim Van Loon
Tim Van Loon Frans A. Van Nieuwenhoven
Frans A. Van Nieuwenhoven Frits W. Prinzen
Frits W. Prinzen Bas R. Van Klarenbosch3
Bas R. Van Klarenbosch3 Arco J. Teske
Arco J. Teske Kevin Vernooy
Kevin Vernooy Tammo Delhaas
Tammo Delhaas Joost Lumens
Joost Lumens