- 1Guangdong Provincial Key Laboratory of Coronary Heart Disease Prevention, Department of Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China
- 2The School of Clinical Medicine, Fujian Medical University, Fuzhou, China
- 3The Second School of Clinical Medicine, Southern Medical University, Guangzhou, China
- 4Guangdong Provincial People’s Hospital, School of Medicine, South China University of Technology, Guangzhou, China
- 5Longyan First Affiliated Hospital of Fujian Medical University, Longyan, China
Background: Acute kidney injury (AKI) is a common complication after coronary angiography (CAG) and associated with heart failure (HF). Left ventricular (LV) remodeling is a vital process in the progression of HF. However, few studies investigate the relationship between AKI and LV remodeling.
Methods: We included consecutive patients undergoing CAG from January 2007 to December 2018 at Guangdong Provincial People’s Hospital (NCT04407936). AKI was defined as an absolute increase in serum creatinine (Scr) of ≥ 0.3mg/dl or a ≥ 50% increase in Scr from baseline within the first 48–72 h after the procedure. LV remodeling was defined as: (1) an absolute decrease in left ventricular ejection fraction (LVEF) of ≥ 10% compared to baseline, or (2) a follow-up LVEF < 40%. Univariate and multivariate logistical regressions were used to assess the association between AKI and LV remodeling.
Results: Of the 1,573 patients (62.2 ± 9.7 years, female 36.7%) included in the study, 231 (14.7%) had AKI. The incidence of LV remodeling was higher in patients with AKI than in those without AKI (24.7% vs. 14.5%). After adjusting for confounding, multivariate logistic regression showed that AKI was associated with a significantly higher risk of LV remodeling [adjusted odds ratio (aOR) 1.87; 95% CI, 1.30–2.66; p < 0.001]. In addition, LV remodeling patients had higher all-cause mortality compared to non-LV remodeling patients (9.7% vs. 19.1%).
Conclusion: Our data suggested that AKI is present in up to 15% of patients after CAG and that nearly a quarter of AKI patients suffered LV remodeling and AKI patients have a two-fold risk of developing LV remodeling than non-AKI patients. Our findings suggest that more active measures be taken not only to prevent AKI patient developing into LV remodeling, but to prevent patients undergoing CAG from developing AKI.
Introduction
Acute kidney injury (AKI) is a common complication after coronary angiography (CAG) and interventional procedures, with an estimated incidence of up to 12% (Lun et al., 2021).
Previous studies have shown that AKI is strongly associated with the development of heart failure (HF). Odutayo A’s meta-analysis showed that AKI was associated with a 58% increased risk of HF (Odutayo et al., 2017). Studies also found that the patient with AKI had higher levels of oxidative stress, inflammation, calcium transport abnormalities, and other pathophysiological mechanisms through activation of the renin-angiotensin system (Robinson et al., 1992; Alge et al., 2013a; Ni et al., 2018). In addition, oxidative stress, inflammatory responses, calcium transport abnormalities and especially the renin-angiotensin system also play an essential part in the pathophysiology of left ventricular (LV) remodeling (Azevedo et al., 2016). However, it remains unclear whether AKI is independently associated with LV remodeling. LV remodeling is one of the most important processes in the occurrence and development of HF. Early diagnosis and treatment can further reduce the development to HF.
The study aims to investigate the association between AKI and LV remodeling in a large Chinese population who underwent CAG. On this basis, clinicians can be guided to identify and intervene early in LV remodeling and reduce the incidence of HF after AKI.
Materials and Methods
Study Population
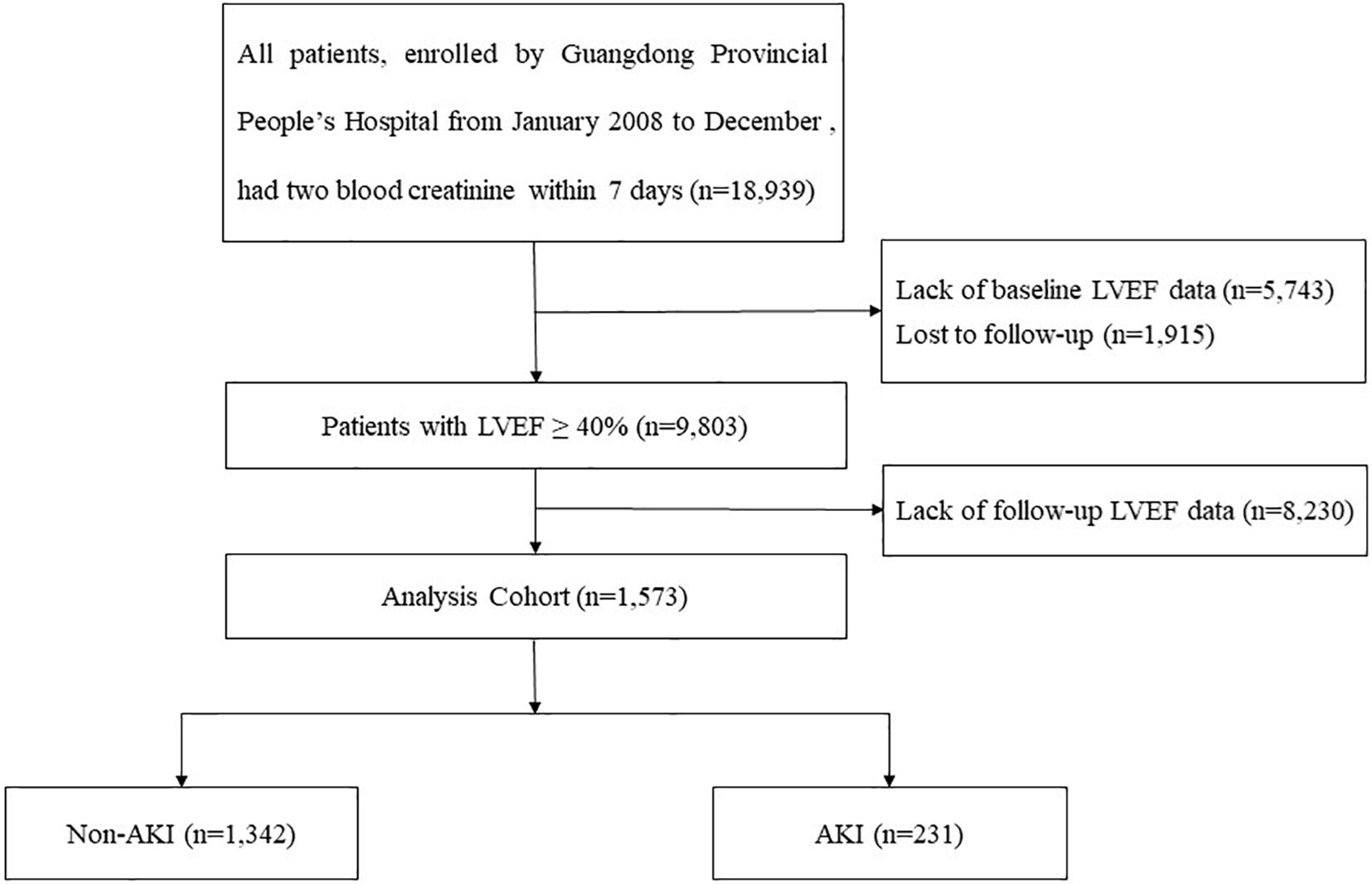
We obtain data from the registry of Cardiorenal Improvement (CIN) study (ClinicalTrials.gov; NCT04407936) from January 2007 to December 2018 at Guangdong Provincial People’s Hospital. A total of 1,573 CAG patients were included in the final analysis after excluding patients combined with conditions as follow: (a) LVEF ≤ 40% at baseline; (b) follow-up echocardiography was not acquired within 3–12 mouths; (c) lost serum creatinine (Scr) before and after surgery (Figure 1). The cohort were divided into AKI and non-AKI groups on the basis of Scr changes. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Research Ethics Committee of Guangdong Provincial People’s Hospital (No. GDREC2019555H). CAG or percutaneous coronary intervention (PCI) was performed in accordance with standard clinical practice guidelines (Kushner et al., 2009; Jneid et al., 2012; Levine et al., 2016).
Clinical Data and Measurement
In this study, we retrieved all of clinical data of 1,573 patients during their first hospitalization from data base of Guangdong Provincial People’s Hospital, including demographic characteristics, medical comorbidities, laboratory examinations, echocardiography and medications at discharge. Echocardiography was performed by a trained cardiac ultrasound doctor for all patients at the time of admission. The calculations for LVEF used the biplane-Simpson method by the end diastolic and end systolic apical 4- and 2- chamber views. Follow-up echocardiography was assessed over 3–12 months after hospitalization by the same method, the median follow-up echocardiography time was 3.5 months.
Definition of Deterioration and Outcomes
The primary endpoint was LV remodeling, defined as: (1) an absolute decrease in LVEF of ≥ 10% compared to baseline, or (2) a follow-up LVEF < 40% (Puls et al., 2020). AKI was defined as an absolute Scr increase ≥ 0.3 mg/dl or a relative increase in Scr ≥ 50% within 48 h after contrast-medium exposure (van der Molen et al., 2018). Estimated glomerular filtration rate (eGFR) was estimated by the Modification of Diet in Renal Disease (MDRD) formula, and chronic kidney disease (CKD) was defined as eGFR < 60 ml/min/1.73 m2 (Levey et al., 1999). Congestive heart failure (CHF) was defined as New York Heart Association (NYHA) functional class > 2, Killip class > 1 or pulmonary edema.
Statistical Analysis
The study population was divided into two groups AKI and non-AKI. Data was presented as the mean with standard deviation (SD) or median with interquartile range (IQR) for continuous variables and quantity and frequency (%) for categorical variables. Categorical variables were compared by Pearson chi-squared test, and continuous variables by t-test. The association between AKI and LV remodeling was tested by univariable and multivariable logistic regression. Model 1 was unadjusted, Model 2 was adjusted with demographic characteristics (age and gender), and Model 3 was adjusted with medical comorbidities and treatment based on Model 2 [acute myocardial infarction (AMI), PCI, diabetes (DM), hypertension (HT), CKD, CHF, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker (ACEI/ARB) and contrast medium volume (CMV)]. All analyses were performed by R software (version 4.0.3; R Foundation for Statistical Computing, Vienna, Austria). A two-sided P-value < 0.05 indicated the significance for all analyses.
Results
Clinical and Procedural Characteristics
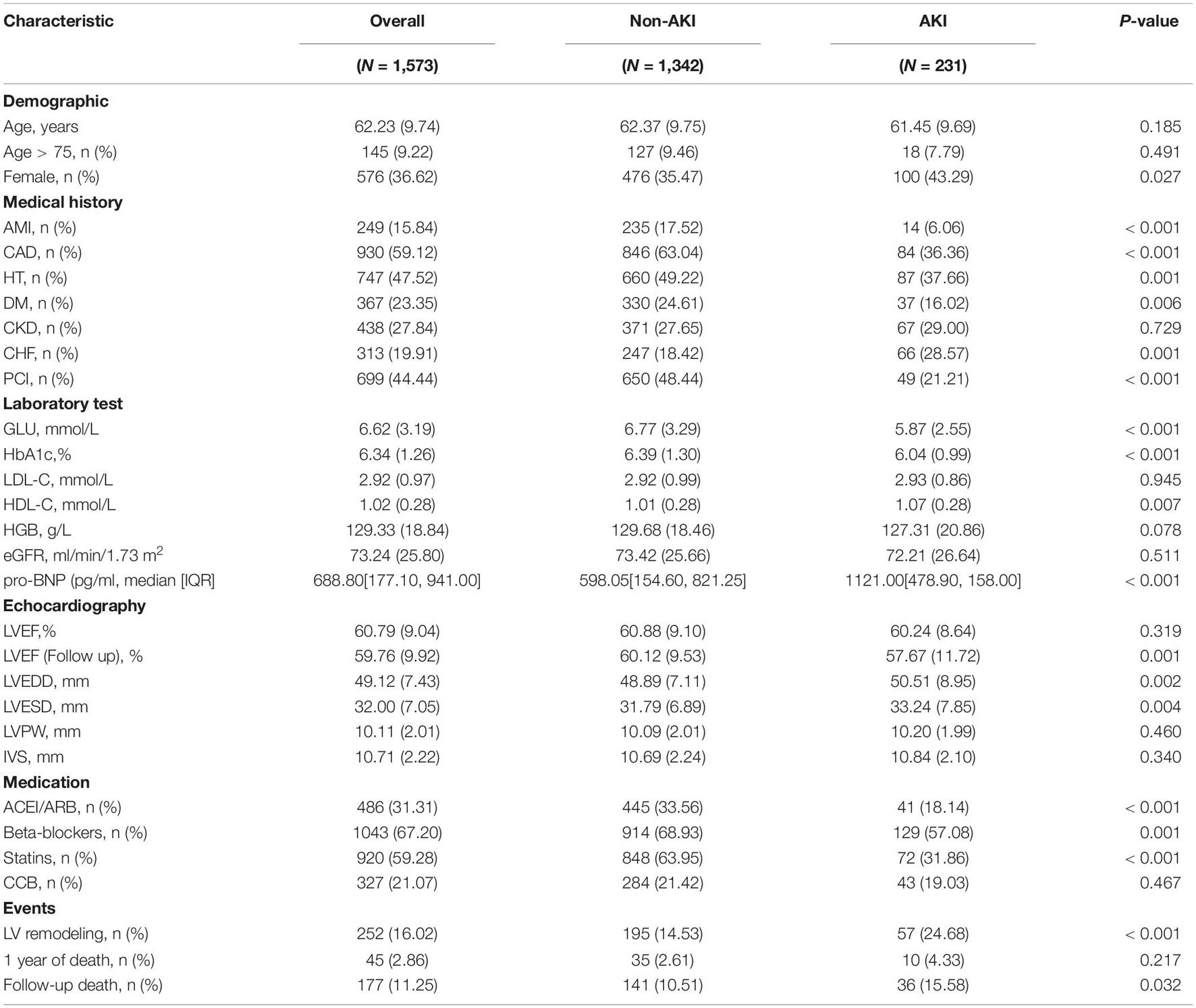
The patient population selected for the study consisted of 1,573 patients, of whom 997 were male and 576 were females (mean age 62.2 ± 9.7 years). In total, 930 (59.1%) had coronary artery disease (CAD), 367 (23.4%) had DM, 313 (19.9%) had CHF and 438 (27.8%) had CKD. The population was divided into 2 groups subsequently: AKI (n = 231) and non-AKI (n = 1,342). Compared to the non-AKI patients, those in the AKI group had higher pro-brain natriuretic peptide (pro-BNP), a larger left ventricular end-diastolic dimension (LVEDD), left ventricular end-systolic dimension (LVESD) and more likely combined with CHF. On the contrast, the AKI group was less likely to be combined with DM, HT, and had fewer uses of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker (ACEI/ARB). The detailed patients’ clinical characteristics are listed in Table 1.
On the basis of the diagnosis of LV remodeling, 252 patients (16.0%) had LV remodeling, the patients developed LV remodeling were elder (62.0 ± 9.8 vs. 63.4 ± 9.4, p = 0.04), more likely to combine CHF (18.9% vs. 25.1%, p = 0.03), and had lower eGFR (73.8 vs. 70.1 ml/min/1.73 m2, p = 0.04). Demographics, risk factors, and clinical and laboratory characteristics of the 1,573 patients according to the presence of LV remodeling are summarized in Supplementary Table 1.
Incidence of Ventricular Remodeling
Follow-up echocardiography data showed that there were 252 cases of LV remodeling (16.0%), 57 cases of AKI (3.6%), and 195 cases of non-AKI (12.4%). Among patients with AKI, the incidence of LV remodeling was 24.7%, accounting for nearly a quarter of AKI patients. In contrast, the incidence of LV remodeling was only 14.5% in non-AKI patients (Figure 2). And the baseline LVEF results showed no significant difference between the patient with AKI and non-AKI (60.24% vs. 60.88%, p > 0.05), while during the 1-year follow-up, the reduction in LVEF was more pronounced in the patient with AKI compared to non-AKI (LVEF: 57.67% in AKI vs. 60.12% in non-AKI, p < 0.05), and the difference was statistically significant (Figure 3).
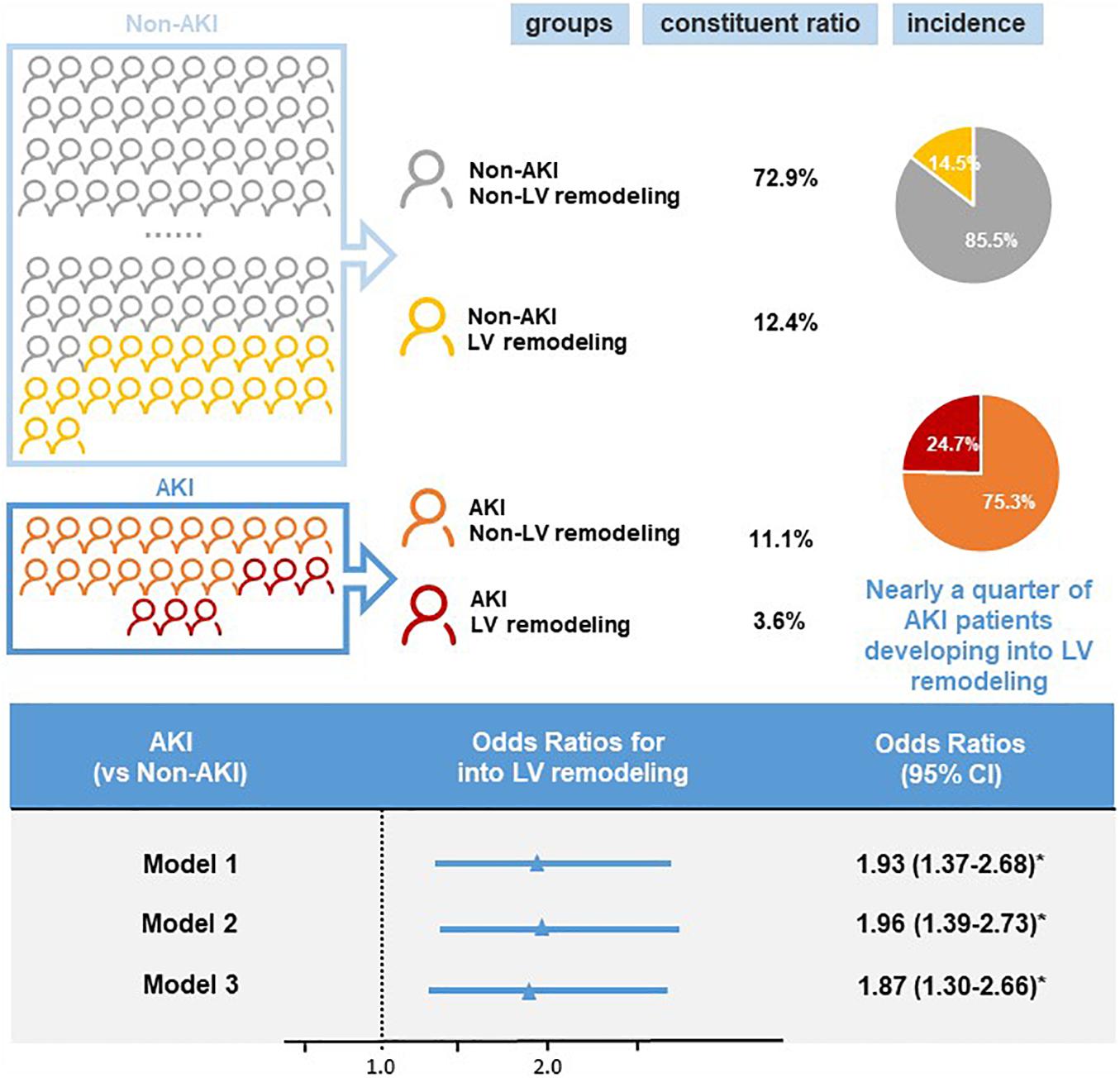
Figure 2. Incidence and risk of left ventricular (LV) remodeling by acute kidney injury (AKI) status. Logistic regression was used to calculate the odds ratios (OR) and 95% confidence intervals (CI) for left ventricular remodeling. Model 1: unadjusted odds ratios for acute kidney injury. Model 2: odds ratios adjusted for age and gender. Model 3: odds ratios adjusted for multiple variables (age, gender, acute myocardial infarction, percutaneous coronary intervention, diabetes, hypertension, chronic kidney disease, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, contrast medium volume). *p < 0.05.
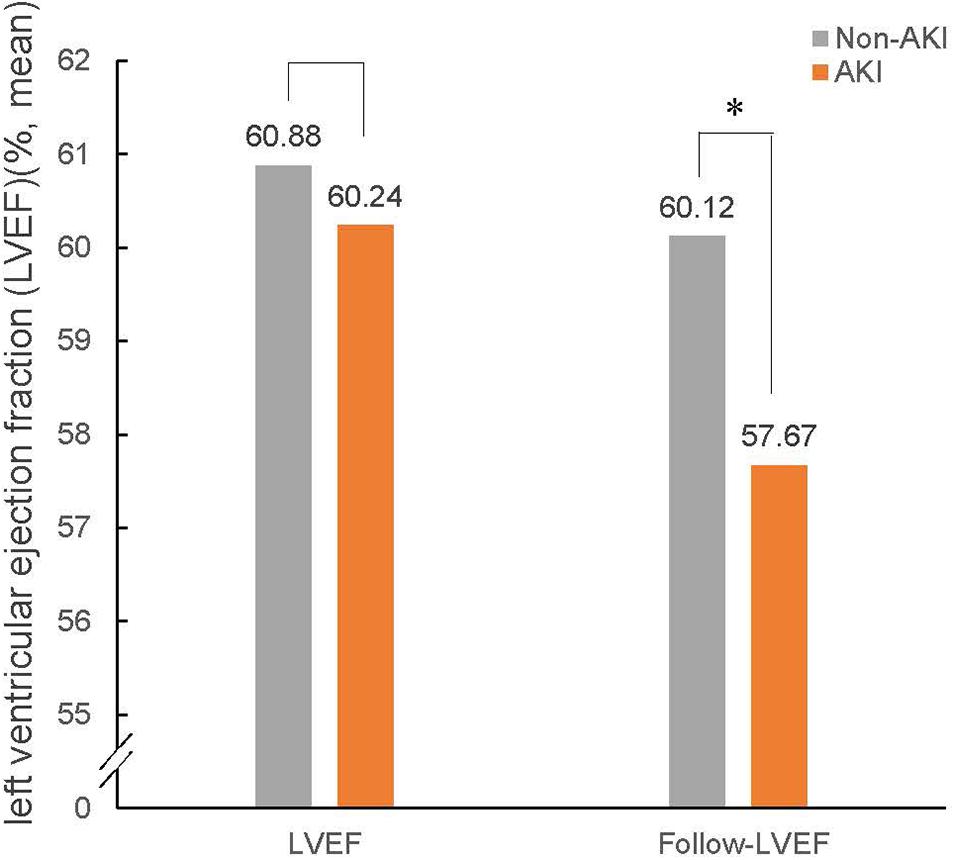
Figure 3. LVEF at baseline and follow-up in patients with non-AKI and AKI. The bar graphs show LVEF at baseline and follow-up in patients with non-AKI (gray) and AKI (orange). AKI, acute kidney injury; LVEF, left ventricular ejection fraction. ∗P < 0.05.
Association Between Acute Kidney Injury and Ventricular Remodeling
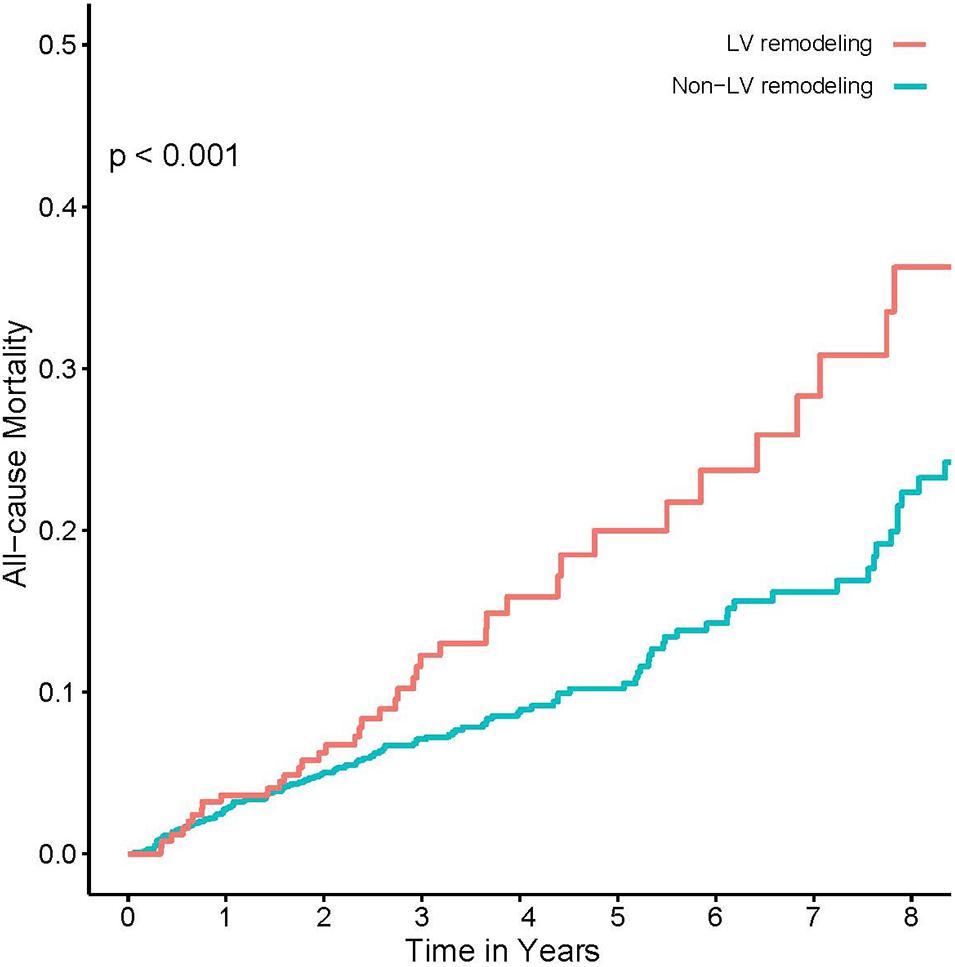
Univariate logistic regression analysis showed a significant correlation between LV remodeling and AKI [odds ratio (OR), 1.93; 95% CI, 1.37–2.68; p < 0.001]. After adjusting age and gender, there was still a significant correlation between LV remodeling and AKI [adjusted odds ratio (a OR) 1.96; 95% CI, 1.39–2.73; p < 0.001]. After adjusting demographic characteristics, medical comorbidities and treatment (age, gender, AMI, PCI, DM, HT, CKD, ACEI/ARB, and CMV) (Figure 2), LV remodeling remained significantly associated with AKI [adjusted odds ratio (a OR) 1.87; 95% CI, 1.30–2.66; p < 0.001]. In addition, Kaplan-Meier curves showed that LV remodeling patients had higher all-cause mortality compared to non-LV remodeling patients (Log-rank test, p < 0.001) (Figure 4).
Discussion
To the best of our knowledge, this is the first study aiming to investigate the association between AKI and LV remodeling as well as to assess the incidence of AKI combined with LV remodeling and changes in related indicators. According to our results, AKI is an independent risk factor for the development of LV remodeling, and patients with LV remodeling after AKI may have a worse clinical outcome. Therefore, it is necessary to pay attention to the occurrence of LV remodeling in AKI patients, early identification and intervention, and prevention of adverse clinical outcomes.
LV remodeling is a vital process in the progression of HF, and it is defined as genomic expression, molecular, cellular and interstitial changes that clinically manifest as changes in heart size, shape and function after cardiac injury (Cohn et al., 2000). Current thinking is that LV remodeling confers short-term benefits. However, neglect can lead to maladaptation of these remodeling events and increase cardiovascular morbidity and mortality. HF has been thought to be causally related to LV remodeling. Recent studies have found that except for HF, a number of other cardiovascular diseases are also associated with LV remodeling, such as AMI, HT, valvular disease, and hypertrophic cardiomyopathy (Pfeffer and Braunwald, 1990; Harris et al., 2006; Berk et al., 2007; Chin et al., 2017). Furthermore, a growing number of studies have shown that LV remodeling is associated not only with cardiovascular disease, but also with other diseases. Alpert et al. (2018) suggest that obesity produces hemodynamic changes can cause LV remodeling. In another article on LV remodeling, Jia et al. (2016) concluded that DM mellitus is also a risk factor for LV remodeling In a clinical trial, Bajaj et al. (2020) demonstrated that CKD is associated with the development of LV remodeling and is mediated by coronary microvascular dysfunction Similarly, our study was the first to connect AKI with LV remodeling and identified AKI as an independent predictor of the occurrence of LV remodeling.
There are several possible mechanisms that could explain the link between AKI and the development of LV remodeling. First, AKI causes sustained oxidative stress and promotes dysregulation of the renin-angiotensin-aldosterone system, which induces macrophage infiltration, cardiac inflammation, and myocardial fibrosis, and leads to endothelial dysfunction and cardiac fibrosis as well as ventricular dysfunction. Induction of this system is a hallmark of the cardiovascular response to AKI (Alge et al., 2013a, b; Crowley and Rudemiller, 2017). Second, oxidative stress appears to play an important pathophysiological role in LV remodeling, and the high oxidative stress state of AKI may contribute to the occurrence of LV remodeling after AKI (Azevedo et al., 2016; Ni et al., 2018). Third, AKI causes changes in the dihydropyridine receptors on L-type calcium channels, leading to distortions in intracellular calcium homeostasis. In turn, alterations in the proteins that transport calcium may lead to cardiac dysfunction in the remodeling heart (Luo and Anderson, 2013; Feridooni et al., 2015; Burtscher et al., 2020). Fourth, deleterious upregulation of the renin-angiotensin-aldosterone system resulting in fluid retention and peripheral arterial vasoconstriction, thereby increasing the preload and afterload of the left ventricle, which is one of the important mechanisms leading to ventricular remodeling (Murphy et al., 2020).
This study had several important clinical significances and research implications. Our results demonstrated that AKI was an independent predictor of LV remodeling among patients undergoing CAG. It is estimated that there are approximately 2 million cases of hospitalized AKI each year (Goldstein et al., 2013). Based on the disparities observed in our study, this translates to approximately 50,000 AKI patients per year with combined LV remodeling, and they have a much higher mortality rate than those who do not. Therefore, this reminds clinicians that ancillary tests related to the assessment of cardiovascular disease, such as cardiac ultrasound, deserve equal attention in the follow-up of patients with AKI. Routine measurements such as LVEF can provide clinicians with useful information to identify patients at high risk for LV remodeling after AKI and thus provide more attention and prevention.
Limitations
Our study has some limitations. First, our study used only an LVEF decrease greater than 10% for the determination of LV remodeling, which has different criteria for determination. However, an LVEF decrease greater than 10% still proved to be a good proxy for LV remodeling. Second, our study did not investigate the effect of recovery after the occurrence of AKI on LV remodeling. However, our study still demonstrates that whenever AKI occurs, there is a significantly higher probability of LV remodeling in patients with a poor prognosis. Third, patients with AKI tend to be hypervolemic, a state that could have a negative impact on the LV. Due to the lack of echocardiography examination during AKI/at discharge, our study could not confirm whether AKI patients were hypervolemic at the time. This will be improved in our future study designs. Finally, despite the use of rigorous statistical methods, there is a possibility of residual confounding, and the observational nature of this study precludes definitive conclusions about causality. It is necessary for clinicians to identify and intervene in LV remodeling early to reduce the incidence of HF after AKI.
Conclusion
Our data suggested that AKI is present in up to 15% of patients after CAG and that nearly a quarter of AKI patients suffered LV remodeling and AKI patients have a twofold risk of LV remodeling than non-AKI patients. Additionally, LV remodeling patients had higher mortality compared to non-LV remodeling patients. It is necessary for clinicians to identify and intervene in LV remodeling early to reduce the occurrence of HF after AKI, and more importantly, to prevent patients undergoing CAG from developing AKI.
Data Availability Statement
The data analyzed in this study is subject to the following licenses/restrictions: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. Requests to access these datasets should be directed to YL, liuyong@gdph.org.cn.
Ethics Statement
The studies involving human participants were reviewed and approved by the Guangdong Provincial People’s Hospital Ethics Committee. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
YL, JL, SC, and JC: research idea and study design. QL, WC, SS, HH, WL, LL, MY, BW, HL, ZH, and LC: data acquisition. JL and YL: data analysis and interpretation. SC and QL: statistical analysis. YL, JL, SC, and JC: supervision and mentorship. All authors contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy and integrity of any portion of the work are appropriately investigated and resolved.
Funding
This research was funded and supported by the National Key Research and Development Program of China, Grant (2016YFC1301202), the Multi-Center Study on Key Techniques for Prevention, Diagnosis and Treatment of High Risk Coronary Artery Disease (DFJH2020026), the Study on the Function and Mechanism of the Potential Target for Early Warning of Cardiorenal Syndrome after Acute Myocardial Infarction based on Transmoomics (DFJH201919), the Natural Science Foundation of Guangdong Province General Project (020A1515010940), the Beijing Lisheng Cardiovascular Health Foundation, Guangdong Provincial People’s Hospital Foundation (LHJJ20141751), and the Guangdong Provincial Key Laboratory of Coronary Heart Disease Prevention (No. 2017B030314041).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This study was approved by Guangdong Provincial People’s Hospital Ethics Committee and the study was performed according to the declaration of Helsinki. Informed consent was not required for this study by the Guangdong Provincial People’s Hospital Ethics Committee.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2021.744735/full#supplementary-material
Abbreviations
AKI, acute kidney injury; AMI, acute myocardial infarction; CAD, coronary artery disease; HT, hypertension; DM, diabetes; CKD, chronic kidney disease; CHF, congestive heart failure; PCI, percutaneous coronary intervention; GLU, glucose; HbA1c, hemoglobin A1c; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; HGB, hemoglobin; eGFR, estimated glomerular filtration rate; pro-BNP, pro-brain natriuretic peptide; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic dimension; LVESD, left ventricular end-systolic dimension; LVPW, left ventricular posterior wall; IVS, interventricular septal thickness; ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; CCB, calcium channel blockers; LV, left ventricular.
References
Alge, J. L., Karakala, N., Neely, B. A., Janech, M. G., Tumlin, J. A., Chawla, L. S., et al. (2013a). Association of elevated urinary concentration of renin-angiotensin system components and severe AKI. Clin. J. Am. Soc. Nephrol. 8, 2043–2052. doi: 10.2215/cjn.03510413
Alge, J. L., Karakala, N., Neely, B. A., Janech, M. G., Velez, J. C., and Arthur, J. M. (2013b). Urinary angiotensinogen predicts adverse outcomes among acute kidney injury patients in the intensive care unit. Crit. Care 17:R69.
Alpert, M. A., Karthikeyan, K., Abdullah, O., and Ghadban, R. (2018). Obesity and cardiac remodeling in adults: mechanisms and clinical implications. Prog. Cardiovasc. Dis. 61, 114–123. doi: 10.1016/j.pcad.2018.07.012
Azevedo, P. S., Polegato, B. F., Minicucci, M. F., Paiva, S. A., and Zornoff, L. A. (2016). Cardiac remodeling: concepts, clinical impact, pathophysiological mechanisms and pharmacologic treatment. Arq. Bras. Cardiol. 106, 62–69.
Bajaj, N. S., Singh, A., Zhou, W., Gupta, A., Fujikura, K., Byrne, C., et al. (2020). Coronary microvascular dysfunction, left ventricular remodeling, and clinical outcomes in patients with chronic kidney impairment. Circulation 141, 21–33. doi: 10.1161/circulationaha.119.043916
Berk, B. C., Fujiwara, K., and Lehoux, S. (2007). ECM remodeling in hypertensive heart disease. J. Clin. Invest. 117, 568–575. doi: 10.1172/jci31044
Burtscher, J., Millet, G. P., and Burtscher, M. (2020). Cardiovascular consequences of acute kidney injury. N. Engl. J. Med. 383:1093. doi: 10.1056/nejmc2023901
Chin, C. W. L., Everett, R. J., Kwiecinski, J., Vesey, A. T., Yeung, E., Esson, G., et al. (2017). Myocardial fibrosis and cardiac decompensation in aortic stenosis. JACC Cardiovasc. Imaging 10, 1320–1333. doi: 10.1016/j.jcmg.2016.10.007
Cohn, J. N., Ferrari, R., and Sharpe, N. (2000). Cardiac remodeling–concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. behalf of an international forum on cardiac remodeling. J. Am. Coll. Cardiol. 35, 569–582. doi: 10.1016/s0735-1097(99)00630-0
Crowley, S. D., and Rudemiller, N. P. (2017). Immunologic effects of the renin-angiotensin system. J. Am. Soc. Nephrol. 28, 1350–1361. doi: 10.1681/asn.2016101066
Feridooni, H. A., Dibb, K. M., and Howlett, S. E. (2015). How cardiomyocyte excitation, calcium release and contraction become altered with age. J. Mol. Cell. Cardiol. 83, 62–72. doi: 10.1016/j.yjmcc.2014.12.004
Goldstein, S. L., Jaber, B. L., Faubel, S., and Chawla, L. S. (2013). AKI transition of care: a potential opportunity to detect and prevent CKD. Clin. J. Am. Soc. Nephrol. 8, 476–483. doi: 10.2215/cjn.12101112
Harris, K. M., Spirito, P., Maron, M. S., Zenovich, A. G., Formisano, F., Lesser, J. R., et al. (2006). Prevalence, clinical profile, and significance of left ventricular remodeling in the end-stage phase of hypertrophic cardiomyopathy. Circulation 114, 216–225. doi: 10.1161/circulationaha.105.583500
Jia, G., DeMarco, V. G., and Sowers, J. R. (2016). Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat. Rev. Endocrinol. 12, 144–153. doi: 10.1038/nrendo.2015.216
Jneid, H., Anderson, J. L., Wright, R. S., Adams, C. D., Bridges, C. R., and Casey, D. E. Jr., et al. (2012). 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the american college of cardiology foundation/american heart association task force on practice guidelines. J. Am. Coll. Cardiol. 60, 645–681. doi: 10.1016/j.jacc.2012.06.004
Kushner, F. G., Hand, M., Smith, S. C. Jr., King, S. B. III, Anderson, J. L., Antman, E. M., et al. (2009). 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the american college of cardiology foundation/american heart association task force on practice guidelines. J. Am. Coll. Cardiol. 54, 2205–2241. doi: 10.1016/j.jacc.2009.10.015
Levey, A. S., Bosch, J. P., Lewis, J. B., Greene, T., Rogers, N., and Roth, D. (1999). A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. modification of diet in renal disease study group. Ann. Intern. Med. 130, 461–470. doi: 10.7326/0003-4819-130-6-199903160-00002
Levine, G. N., Bates, E. R., Blankenship, J. C., Bailey, S. R., Bittl, J. A., Cercek, B., et al. (2016). 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with st-elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction. J. Am. Coll. Cardiol. 67, 1235–1250.
Lun, Z., Liu, L., Chen, G., Ying, M., Liu, J., Wang, B., et al. (2021). Correction to: the global incidence and mortality of contrast-associated acute kidney injury following coronary angiography: a meta-analysis of 1.2 million patients. J. Nephrol. [Epub ahead of print].
Luo, M., and Anderson, M. E. (2013). Mechanisms of altered Ca2+ handling in heart failure. Circ. Res. 113, 690–708.
Murphy, S. P., Ibrahim, N. E., and Januzzi, J. L. Jr. (2020). Heart failure with reduced ejection fraction: a review. JAMA 324, 488–504.
Ni, D., Jiang, D., Kutyreff, C. J., Lai, J., Yan, Y., Barnhart, T. E., et al. (2018). Molybdenum-based nanoclusters act as antioxidants and ameliorate acute kidney injury in mice. Nat. Commun. 9:5421.
Odutayo, A., Wong, C. X., Farkouh, M., Altman, D. G., Hopewell, S., Emdin, C. A., et al. (2017). AKI and long-term risk for cardiovascular events and mortality. J. Am. Soc. Nephrol. 28, 377–387. doi: 10.1681/asn.2016010105
Pfeffer, M. A., and Braunwald, E. (1990). Ventricular remodeling after myocardial infarction. experimental observations and clinical implications. Circulation 81, 1161–1172. doi: 10.1161/01.cir.81.4.1161
Puls, M., Beuthner, B. E., Topci, R., Vogelgesang, A., Bleckmann, A., Sitte, M., et al. (2020). Impact of myocardial fibrosis on left ventricular remodelling, recovery, and outcome after transcatheter aortic valve implantation in different haemodynamic subtypes of severe aortic stenosis. Eur. Heart J. 41, 1903–1914. doi: 10.1093/eurheartj/ehaa033
Robinson, S. C., Bowmer, C. J., and Yates, M. S. (1992). Cardiac function in rats with acute renal failure. J. Pharm. Pharmacol. 44, 1007–1014.
van der Molen, A. J., Reimer, P., Dekkers, I. A., Bongartz, G., Bellin, M. F., Bertolotto, M., et al. (2018). Post-contrast acute kidney injury - part 1: definition, clinical features, incidence, role of contrast medium and risk factors : recommendations for updated ESUR contrast medium safety committee guidelines. Eur. Radiol. 28, 2845–2855. doi: 10.1007/s00330-017-5246-5
Keywords: coronary angiography, incidence, cardiac remodeling, acute kidney injury, left ventricular remodeling
Citation: Li Q, Chen W, Shi S, Huang H, Lai W, Liu L, Ying M, Wang B, Li H, Huang Z, Chen L, Chen J, Chen S, Liu J and Liu Y (2021) Acute Kidney Injury Increase Risk of Left Ventricular Remodeling: A Cohort of 1,573 Patients. Front. Physiol. 12:744735. doi: 10.3389/fphys.2021.744735
Received: 20 July 2021; Accepted: 02 September 2021;
Published: 27 September 2021.
Edited by:
Michael Lichtenauer, Paracelsus Medical University, AustriaReviewed by:
Christoph Edlinger, University Hospital Salzburg, AustriaMartin Busch, University Hospital Jena, Germany
Copyright © 2021 Li, Chen, Shi, Huang, Lai, Liu, Ying, Wang, Li, Huang, Chen, Chen, Chen, Liu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shiqun Chen, shiqunchen@126.com; Jin Liu, ljaw397017568@163.com; Yong Liu, liuyong@gdph.org.cn
†These authors have contributed equally to this work
 Qiang Li
Qiang Li Weihua Chen2†
Weihua Chen2† Haozhang Huang
Haozhang Huang Ming Ying
Ming Ying Bo Wang
Bo Wang Huanqiang Li
Huanqiang Li Jiyan Chen
Jiyan Chen Shiqun Chen
Shiqun Chen Yong Liu
Yong Liu