- Department of Life Sciences and Chemistry, Jacobs University Bremen, Bremen, Germany
Eukaryotic porin, also known as Voltage-Dependent Anion Channel (VDAC), is the most frequent protein in the outer membrane of mitochondria that are responsible for cellular respiration. Mitochondria are most likely descendants of strictly aerobic Gram-negative bacteria from the α-proteobacterial lineage. In accordance with the presumed ancestor, mitochondria are surrounded by two membranes. The mitochondrial outer membrane contains besides the eukaryotic porins responsible for its major permeability properties a variety of other not fully identified channels. It encloses also the TOM apparatus together with the sorting mechanism SAM, responsible for the uptake and assembly of many mitochondrial proteins that are encoded in the nucleus and synthesized in the cytoplasm at free ribosomes. The recognition and the study of electrophysiological properties of eukaryotic porin or VDAC started in the late seventies of the last century by a study of Schein et al., who reconstituted the pore from crude extracts of Paramecium mitochondria into planar lipid bilayer membranes. Whereas the literature about structure and function of eukaryotic porins was comparatively rare during the first 10years after the first study, the number of publications started to explode with the first sequencing of human Porin 31HL and the recognition of the important function of eukaryotic porins in mitochondrial metabolism. Many genomes contain more than one gene coding for homologs of eukaryotic porins. More than 100 sequences of eukaryotic porins are known to date. Although the sequence identity between them is relatively low, the polypeptide length and in particular, the electrophysiological characteristics are highly preserved. This means that all eukaryotic porins studied to date are anion selective in the open state. They are voltage-dependent and switch into cation-selective substates at voltages in the physiological relevant range. A major breakthrough was also the elucidation of the 3D structure of the eukaryotic pore, which is formed by 19 β-strands similar to those of bacterial porin channels. The function of the presumed gate an α-helical stretch of 20 amino acids allowed further studies with respect to voltage dependence and function, but its exact role in channel gating is still not fully understood.
Origin of Bacteria and Cell Organelles
The age of earth is around 4.5 billion years. Stromatolites represent presumably the oldest fossils that date back to something like 3.7 billion years (Garwood, 2012; Nutman et al., 2016). How life on earth started is still a matter of debate. One hypothesis suggests that molecules serving as the basis of life formed from atoms under the input of energy from different sources as proposed by the Miller-Urey experiment (Miller, 1953; Osinski et al., 2020; Takeuchi et al., 2020). Cell-like particles may have formed in the Hadean eon within such a primordial broth from a variety of small molecules. They represent the origin of life, which began about 4 billion years ago (Garwood, 2012; Takeuchi et al., 2020). The first organisms on earth are not known and they did not leave any evidence of their live. What we know are the oldest fossils in the form of sedimentary rocks, known as stromatolites (Margulis et al., 1986; Nutman et al., 2016). They were formed by microbial communities containing photosynthetic bacteria and cyanobacteria and represent the oldest sign for the existence of photosynthesis (Awramik, 1992; Gérard et al., 2009). It is possible that these bacteria were Gram-negative, which means that the cytoplasm was surrounded by two membranes. Their outer membranes may be considered as permeability barriers similar to those of modern Gram-negative bacteria and the mitochondrial outer membrane.
The Last Universal Common Ancestor (LUCA) is a bacteria-like organism, which existed before the bacterial cell lineage divided into the different kingdoms (Theobald, 2010; McInerney, 2016; Weiss et al., 2016). The LUCA is joint ancestor of bacteria and archaea and existed presumably within the time of begin of earth and the oldest fossils, which means that the division of the cell lineage occurred about 4 billion of years ago (Di Giulio, 2011). No fossils of this organism are known but the comparison of the genomes of all modern organisms allowed the identification of a set of about 355 genes, which could have been present in the LUCA (Weiss et al., 2016). The genes code for proteins involved in energy metabolism, synthesis of amino acids, and the equipment for transcription and translation of protein synthesis (Chioccioli et al., 2020; Koonin et al., 2020). It seems to be possible that the life of LUCA depended on hydrogen and metals favoring a deep sea vent environment for its existence (Weiss et al., 2016). Nevertheless, also other environments for the existence of LUCA are possible, for example, in ponds of different salinity and pH and in an N2-CO2 atmosphere together with dry-wet cycles and UV light (Sasselov et al., 2020). The LUCA divided subsequently into the different kingdoms of life: bacteria, eukaryote, and archaea.
The Endosymbiotic Theory and the Development of Mitochondria From Strictly Aerobic Bacteria
A similar concept as described for the LUCA exists also for the LECA, the Last Eukaryotic Common Ancestor (Hedges, 2002; Koonin, 2015; O’Malley et al., 2019). The first eukaryotic cell represents presumably a genomic hybrid of bacteria and archaea (Katz, 2012; Grau-Bové et al., 2015). Special for the LECA is its endosymbiotic capacity because between one and 2 billion years ago specialized Gram-negative bacteria were taken up. This provided a considerable advantage for the eukaryotic host because its energy metabolism was characterized before endosymbiosis by anaerobic fermentation and is now complemented with cellular respiration (Margulis, 1981). The endosymbionts were presumably α-proteobacteria and cyanobacteria (Gray, 2012; Degli, 2014; Nowack and Weber, 2018). This can be concluded from the homology of aerobic respiration between mitochondria and α-proteobacteria and of photosynthesis between chloroplasts and cyanobacteria (Martijn et al., 2018; Nowack and Weber, 2018).
Besides the genes coding for the respiration chain, there exist also other indications for the close relation of mitochondria with Gram-negative bacteria. All proteins residing in the outer mitochondrial membrane and in the intermembrane space are encoded in the nucleus and synthesized on cytoplasmic ribosomes of the host cell and are imported post-translationally into mitochondria with modifications of amino acids in particular of cysteines (Saletti et al., 1859; Grevel et al., 2019). This suggests that since the event of endosymbiosis about 1–2 billion years ago many genes of the protomitochondrion came under control of the eukaryotic host. The mitochondrial proteins produced in the cytoplasm of the host cells are imported via the mitochondrial outer membrane import TOM complex into the intermembrane space using a not yet fully identified import signal, which is presumably related to the β-strands of the imported proteins (Bausewein et al., 2020; Drwesh and Rapaport, 2020; Moitra and Rapaport, 2021). This is based on the observation that the β-hairpin element of eukaryotic porins interacts with the mitochondrial import receptor Tom20 (Jores et al., 2016). From there, the mitochondrial outer membrane proteins are assembled by the multi-subunit TOB/SAM system (sorting and assembly machinery) in the mitochondrial outer membrane with the help of the hexamer of the small TIM chaperones (Moitra and Rapaport, 2021). The SAM system has a high homology to the barrel-assembly machinery BAM of Gram-negative bacteria, which provides also evidence that Gram-negative bacteria were ancestors of mitochondria (Pereira and Lupas, 2018; Diederichs et al., 2020, 2021; Takeda et al., 2021). Accordingly, the bacterial outer membrane represents the ancestor of the mitochondrial outer membrane. However, whereas the porins of the bacterial outer membranes have only passive sieving properties in bacterial metabolism, it seems that the mitochondrial outer membrane including the eukaryotic porin or Voltage-Dependent Anion Channel (VDAC) plays also an active and important role in mitochondrial and cellular metabolism (Benz, 1994a,b; Shoshan-Barmatz et al., 2006, 2010; Gatliff and Campanella, 2012; Grevel and Becker, 2020). It binds different kinases (Fiek et al., 1982; Brdiczka, 1990; Adams et al., 1991; De Pinto et al., 2003) and evidence has been provided that eukaryotic porins play also an important role in mitochondria-mediated apoptosis, protein translocation, and are also involved in response to drugs (Shoshan-Barmatz et al., 2010; Grevel et al., 2019; Grevel and Becker, 2020). This applies also to its interaction with the 18kDa translocator protein, also known as peripheral benzodiazepine receptor, which mediates cholesterol transport between mitochondrial membranes, cytochrome C release, and apoptosis (Gatliff and Campanella, 2012; Bader et al., 2019; Betlazar et al., 2020).
A Voltage-Dependent Pore was Discovered in Crude Extracts of Paramecium Mitochondria
The most interesting property of eukaryotic porins or VDACs is their voltage dependence, which is clearly no reconstitution artifact. The voltage dependence of eukaryotic porins was first discovered in extracts of Paramecium aurelia mitochondria by Schein et al. (1976). They dissolved mitochondrial-rich fractions from Paramecium supplemented with asolectin in hexane and used this lipid-protein mixture for membrane formation. This means that they spread the crude protein-lipid mixture on the surface of electrolyte solutions in a Teflon cell. Membranes were then formed from the protein-lipid layers across a small hole in a thin Teflon by the Montal-Mueller method (Montal and Mueller, 1972). Schein et al. (1976) observed frequently high conductance channels/pores in these solvent-depleted membranes. The number of reconstituted channels in the folded membranes was somewhat dependent how much from the mitochondria-rich fractions were present in the lipid-protein mixtures (Schein et al., 1976). Highest pore-forming activity was observed in fractions 50–55 of the sucrose gradient. The open channels had a conductance of 200 pS in an asymmetric solution of 20mM MgCl2 versus 20mM CaCl2, or 750 pS in an asymmetric solution of 1M KCl versus 0.1M KCl (Schein et al., 1976). The current-voltage behavior of the channel was ohmic, resulting in a linear current-voltage curve (Schein et al., 1976).
At voltages smaller than 10mV, the channels were frequently in the “open” configuration. However, they switched a higher voltages in closed configurations, which could be observed in experiments when the voltage was switched to ±20mV. The maximum voltage dependence was reached at about ±40 to ±50mV transmembrane potential and appeared to be symmetric with respect Vm=0mV (Schein et al., 1976). Figure 1 shows an example of the voltage dependence of human eukaryotic porin 1 (hVDAC1) reconstituted into solvent-containing membranes made of diphytanoyl phosphatidylcholine/n-decane membranes. The experiment started by an application of +10mV to about 50 reconstituted hVDAC1 pores, followed by application of −10mV. At ±20mV applied to the membrane, the current through the pores started to decrease, higher positive, and negative voltages resulted in a stronger decrease of the current and a faster exponential decay from the initial current to the final current level at longer times (see Figure 1). The closing of the eukaryotic porin is a relatively slow process as Figure 1 clearly demonstrates. The invers process, i.e., the reopening of the pores, when the voltage is switched off is much faster, which means that it is difficult to measure it precisely (Schein et al., 1976). It is sometimes so fast that it cannot be resolved properly (Colombini, 1979; Benz, 2004).
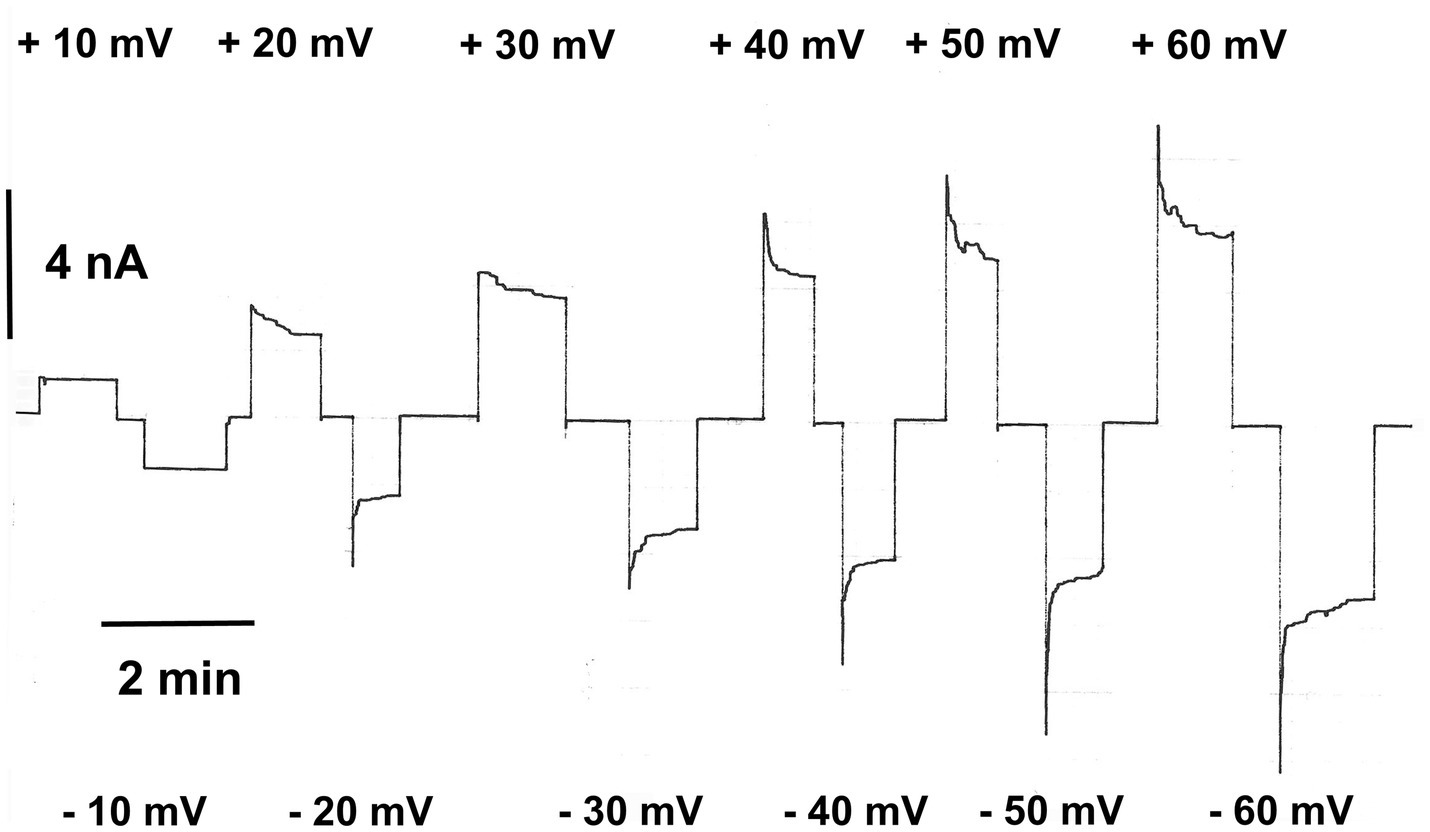
Figure 1. Relaxation of the membrane current in the presence of eukaryotic porin 1 from humans (hVDAC1, Porin 31HL; Benz et al., 1992). The membrane potential was first switched to +10mV and then to −10mV applied to the cis-side of the membrane containing about 50 hVDAC1 pores. Note that the membrane current did not decrease at these voltages. Then, higher positive and negative voltages were applied which resulted in a substantial exponential decrease of the membrane current. The membrane was formed of diphytanoyl phosphatidylcholine/n-decane. The aqueous phase contained 0.5M KCl (pH 7.2); T=20°C.
The decrease of the current at higher positive and negative voltages than ±10mV could be analyzed using a similar approach as proposed by Schein et al. (1976) assuming a Boltzmann distribution of the open and closed states of the pore:
Where F, R, and T are Faraday’s constant, gas constant, and absolute temperature, respectively; n is the number of gating charges moving through the entire membrane potential. V0 is the midpoint potential, where one half of the pores are open and the other half are closed, i.e., = 1. The open to closed ratio of the pores is given by the analysis of the experimental results of experiments similar to Figure 1 according to:
G is the membrane conductance at a given membrane potential Vm. G0 and Gmin are the conductance at zero voltage, when all pores are in the open configuration and when all pores are in the closed one at very high voltage, respectively. The use of the Boltzmann distribution allows also the derivation of the activation energy for the voltage-dependent gating process of eukaryotic porins (Schein et al., 1976). The activation energy is given by W(Vm), which is equivalent to the energy of one mole pores between the open and the closed configuration, i.e., it has the form (Schein et al., 1976):
Comparison of Eqs. (1) and (3) shows that W(Vm) is given by nF(Vm-V0), which means that the activation energy nFV0 is about 5.8kJ/mol (1.38kcal/mol), which is a very low energy that is needed to shift the eukaryotic pores from the open into the closed configuration.
Figure 2 shows the ratio of the conductance, G, at voltages between ±10 and±90mV divided by the initial conductance G0 as a function of the applied voltage for experiments similar to that shown in Figure 1 for 0.5M KCl, 0.5MK-MES, and 0.5M TRIS-Cl (mean values of three experiments taken under the same conditions). The combination of the cations and anions was chosen to show the voltage dependence of human eukaryotic porin 1 (hVDAC1, Porin 31HL) in dependence of cations and anions of different mobility because this provides not only some information on voltage dependence but also on ion selectivity of the open and closed states of the pore. It is obvious that the voltage dependence was in all cases similar. However, G/G0 was found to be dependent on the type of the cation and anion present in the aqueous solution, indicating a selectivity change when the pores switched in the closed configuration at higher voltages. The exact value for the permeability ratio of potassium and chloride was difficult to obtain because the mobility of TRIS and MES inside the pore is not known. However, because of the comparably small mobility of TRIS and MES in the aqueous phase, it is possible that Pcation/Panion of the closed hVDAC1 pore is very high (around 10) in contrast to the selectivity of the open pore where Pcation/Panion is about 0.5 (Benz et al., 1992). The selectivity of the closed state may be even higher if it is impermeable for anions. On the other hand, it is also evident from Figure 2 that potassium is almost equally mobile through the open and the closed state, because of the low mobility of MES in the aqueous phase. This represents another proof that the closed state has completely different properties for the permeation of charged solutes than the open state.
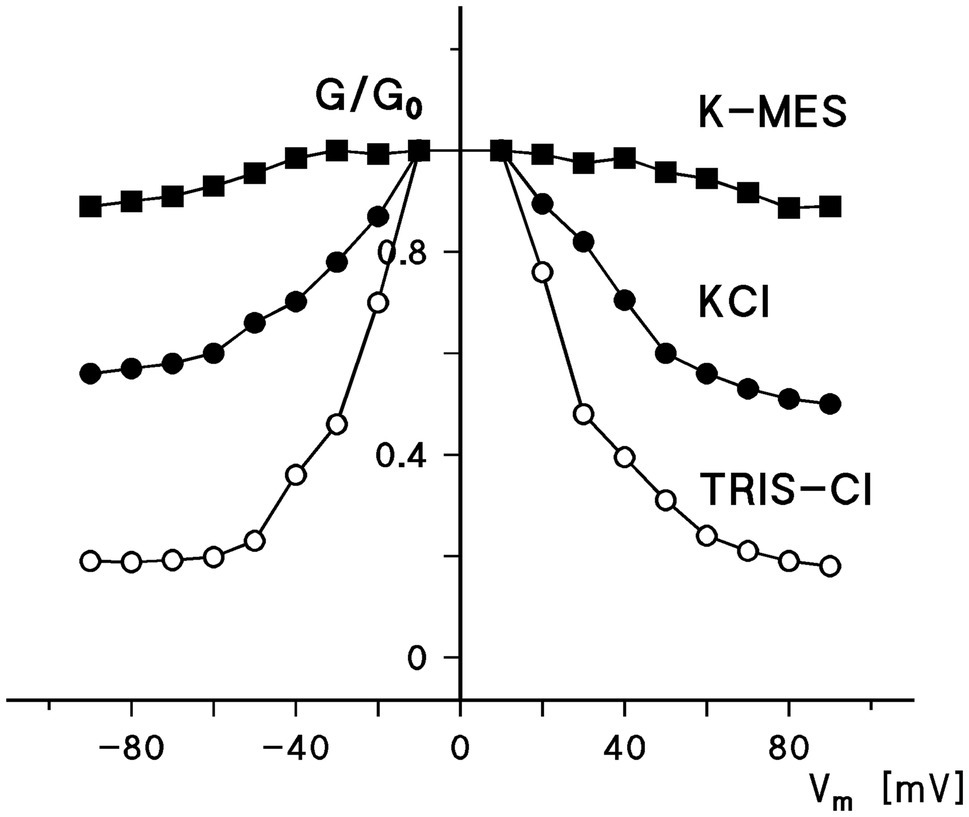
Figure 2. Ratio of the conductance, G, at a given voltage, Vm, divided by the conductance, G0, at 10mV as a function of the voltage. The aqueous phase contained either 0.5M KCI, 0.5MK-MES, or 0.5M TRIS-HCI (pH in all cases 7.2). The cis-side contained about 10ng/ml hVDAC1 [Porin 31 HL (Benz et al., 1992)]. The sign of the voltage is given with respect to the cis-side, the side of the addition of Porin 31HL.
The voltage dependence of the pores formed by Porin 31HL (hVDAC1) can be analyzed using Eq. (1) and semilogarithmic plots of the ratio N0/Nc as a function of the transmembrane potential, Vm, calculated from the experimental results according to Eq. (2) as shown in Figure 3. The slope of the straight line for the application of negative voltages for an e-fold change in N0/Nc was about 12.5mV, which suggested that the number of charges involved in the gating process was approximately 2.0. A similar analysis for positive voltages resulted in a slope for an e-fold change of N0/Nc of 11.9mV, which suggested that the gating charge of the right branch of the voltage dependence of hVDAC1 is about 2.1. The midpoint potentials of the two N0/Nc distributions for negative and positive voltages with respect to the addition of porin 31HL (human eukaryotic porin 1) were−27.4mV and+35mV, respectively. This indicated a slight asymmetry in the midpoint potential, V0, where the number of open and closed channels was balanced, i.e., N0/Nc=1. It is noteworthy that the voltage dependence of eukaryotic porin (VDAC) in the first study of Paramecium mitochondria exhibited a much higher voltage dependence because n was about 4.5 and the midpoint potentials of N0/Nc were around ±20mV (Schein et al., 1976). In a more recent study of eukaryotic porin of Paramecium tetraulia, where the eukaryotic porin was purified to homogeneity, the voltage dependence of the reconstituted pore was lower with about 2 gating charges and a midpoint potential for N0/Nc of 32mV (Ludwig et al., 1989).
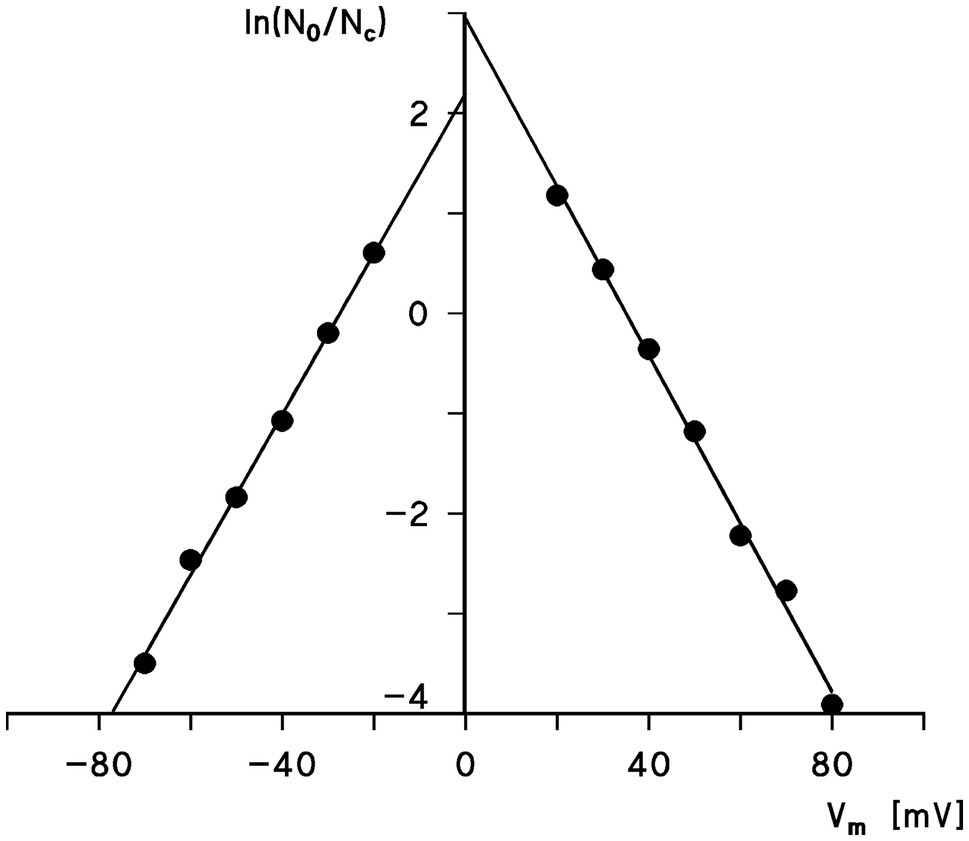
Figure 3. Semilogarithmic plot of the ratio, N0/Nc, as a function of the transmembrane potential Vm. The data were taken from Figure 2. The slope of the straight lines is such that an e-fold change of N0/Nc is produced by a change in Vm of 12.5mV (left side) and 11.9mV (right side), corresponding to gating charges, n=2.0 and 2.1, respectively. The midpoint potential of the N0/Nc distribution (i.e., N0=Nc) was at 27.4mV (left side) and 35mV (right side).
The voltage dependence of eukaryotic porins from a variety of eukaryotic organisms was investigated in detail in many studies: Paramecium (Schein et al., 1976; Doring and Colombini, 1984; Ludwig et al., 1989); Mammals: rat (Roos et al., 1982; Colombini, 1983; Ludwig et al., 1986), rabbit (De Pinto et al., 1987a), bovine (De Pinto et al., 1987a), pig (De Pinto et al., 1987a), and human brain (Bureau et al., 1992); Fish: Anguilla anguilla (De Pinto et al., 1991c); Plants: potato (Heins et al., 1994; Lopes-Rodrigues et al., 2020), pea (Fischer et al., 1994), corn (Smack and Colombini, 1985; Aljamal et al., 1993; Fischer et al., 1994), wheat (Blumenthal et al., 1993), and pea root plastid porin (Fischer et al., 1994; Popp et al., 1997); Other organisms: Neurospora crassa (Freitag et al., 1982c), yeast (Ludwig et al., 1988), and Dictyostelium (Troll et al., 1992); and Flies: Protophormia (Wiesner et al., 1996) and Drosophila (De Pinto et al., 1989a; Aiello et al., 2004; Komarov et al., 2004). Common to all of these studies is that the eukaryotic porins of all these eukaryotes formed high-conducting channels in reconstituted systems. They were all in their open configuration at small transmembrane voltages smaller or equal to 10mV (Benz, 1994b). At higher voltages, they switched into substates. The analysis of the voltage dependence in terms of the above used formalism showed that the number of gating charges for almost all pores formed by these eukaryotic porins was around two, which means that an e-fold change in N0/Nc occurred, when the voltage across the membrane was changed by about 12 mV (De Pinto et al., 1987a; Benz, 1994b, 2004). The midpoint potential for the distribution of the open and closed pores (i.e., N0 =Nc) was in many cases either symmetrical or slightly asymmetrical with values around ±30mV to ±40mV (De Pinto et al., 1987a; Benz, 1994b, 2004).
Isolation and Purification of Eukaryotic Porins
Schein et al. (1976) had already the idea that voltage-dependent pore was present in the mitochondrial outer membrane of the Paramecium mitochondria, because of its high permeability. This was revealed in a study by Colombini (1979), where he could explicitly show that the pore-forming activity came from the outer membrane of rat liver mitochondria, but not from fractions containing inner membranes. The pore had a conductance of 0.45 nS and 4.5 nS in 0.1 and 1M KCl, respectively. The first biochemical evidence for the identity of the pores in the mitochondrial outer membranes of rat liver and mung beans was provided by Hiroshi Nikaido and coworkers (Zalman et al., 1980). In analogy to their work with bacterial porins, they were able to reconstitute fragments of the mitochondrial outer membranes into vesicles from soybean lipids and demonstrated that the vesicles became permeable for low molecular mass carbohydrates but not for high molecular mass dextrans. Following different biochemical procedures, Zalman et al. (1980) were able to identify a protein in the mitochondrial outer membranes of mung bean mitochondria with a molecular mass of about 30kDa, which obviously was responsible for the permeability properties of the reconstituted vesicles. It is quite difficult to establish a potential across vesicles membranes or to study the permeability of charged solutes in the liposome system, which means that the putative voltage dependence of the pore could not be studied. Nevertheless, the diffusion of carbohydrates with a molecular mass up to 8kDa suggested indeed that Zalman et al. (1980) identified the eukaryotic porin of mung beans as a general diffusion pore.
A similar study was performed by Mihara et al. (1982) to identify yeast porin. They isolated yeast porin as a 29kDa polypeptide from the mitochondrial outer membranes of yeast mitochondria. To verify their results, they demonstrated that yeast porin was not accessible for protease treatment as long the protein was localized in the mitochondrial outer membrane (Mihara et al., 1982). When the protease digestion was performed in the presence of detergent yeast porin was no longer protected. They were also the first to notice that in vitro-synthesized yeast porin using yeast total RNA had the same molecular mass as the native protein and did not exhibit any additional leader sequence. It was incorporated directly into intact mitochondria and not into rough endoplasmic reticulum (Mihara et al., 1982). A membrane potential across the inner mitochondrial membrane was not important for this process (Mihara et al., 1982). A similar conclusion was obtained from the import of porin from N. crassa synthesized in homologous or heterologous cell-free systems into mitochondria (Freitag et al., 1982b).
The identification of other eukaryotic porins also proceeded at the same time. Roos et al. (1982) were the first to identify a mammalian eukaryotic porin from rat liver. Rat liver mitochondria were sub fractionated. When the OM fraction obtained by centrifugation steps was treated with detergent it showed pore-forming activity in artificial lipid bilayer membranes (Roos et al., 1982). Rat liver porin was identified as a 32kDa protein using different biochemical methods and the reconstitution of the protein into artificial lipid bilayers. Rat liver porin formed voltage-dependent pores in lipid bilayers with a single-channel conductance of about 4 nS in 1M KCl, which is typical for eukaryotic porins (Colombini, 1979, 1980, 1983). The molecular mass of rat liver porin was confirmed by other groups (Lindén et al., 1982a; Colombini, 1983). However, in contrast to a putative purification of rat liver porin by a Concanavalin A-containing column, eukaryotic porins are pure polypeptides (Benz, 1994b), which means that the 300-fold purification of rat liver porin (rVDAC) by chromatography across this column has presumably nothing to do with eukaryotic porins as glycoproteins Colombini, 1980, 1983).
The purification of eukaryotic porins until 1982 was always dependent on the fractionation of the mitochondrial membranes using different methods, such swelling and shrinking of mitochondria followed by density gradient centrifugation (Roos et al., 1982; Lindén et al., 1982a). This procedure and related methods were always accompanied by a substantial loss of outer membrane material because it is in part tightly associated with the mitochondrial inner membrane (van der Laan et al., 2016). This means that it was a considerable breakthrough when eukaryotic porins could be isolated from detergent-solubilized whole mitochondrial membranes of N. crassa by using the method of Freitag et al. (1982a). In a first step, mitochondria were lysed by an osmotic shock and the total mitochondrial membranes were obtained by centrifugation. Next, the detergent-solubilized mitochondrial membrane proteins were applied to a dry hydroxyapatite (HTP) column and the eluate was passed in a second step through a dry HTP/celite column in a ratio of 1:1 (w/w). Using this method, N. crassa porin was almost pure (Freitag et al., 1982a). Following the isolation and purification of different eukaryotic porins, the method was refined (De Pinto et al., 1987b). Finally, the mitochondrial membrane proteins were dissolved in 3% Triton X-100 using a low protein/detergent ratio and then passed only once through a dry HTP/celite column in a ratio of 2:1 (w/w) (De Pinto et al., 1987b). This procedure resulted in eukaryotic porins in particular from mammals of high purity and was successfully applied many times to the purification of eukaryotic porins by the Bari/Catania group of research into mitochondria (Ludwig et al., 1986, 1988; De Pinto et al., 1987a,b; Carbonara et al., 1996). This easy purification method allowed also further investigations of structure and function of eukaryotic porins and their interaction with different detergents (De Pinto et al., 1989b, 1991a,b; De Pinto and Palmieri, 1992).
Primary Sequences of Eukaryotic Porins
The first two primary sequences of eukaryotic porins that became known were those of yeast and N. crassa (Mihara and Sato, 1985; Kleene et al., 1987). Mammalian porins could not be sequenced from their cDNA at that time because their sequence is only distantly related to the porins of the microorganisms despite a similar length and a relative large fraction of hydrophilic amino acids. That was possible, when human porin (hVDAC1, porin 31HL) was sequenced by direct amino acid sequencing (Kayser et al., 1989). Shortly after, eukaryotic porins from higher eukaryotic cells could be cloned in different organisms, such as mouse and humans (Blachly-Dyson et al., 1993, 1994; Ha et al., 1993; Sampson et al., 1996). Mammalian genomes contain the genes coding for three VDAC species. The genes have the same exon-intron structure (Young et al., 2007). The proteins have the same length but they exhibit some differences, in particular in the number of cysteines. The differences in the primary sequence of the three VDAC isoforms in mammals did not alter the structure of the splicing sequences and the organization of the three genes (Pinto and Messina, 2004; Young et al., 2007; De Pinto, 2021). The most abandoned version of the VDAC isoforms is VDAC1, which was extensively studied in vivo and in vitro (Benz, 1994b, 2004; Báthori et al., 2006; Shoshan-Barmatz et al., 2020). However, also the other two human isoforms were studied in recent years (Checchetto et al., 2014; Gattin et al., 2015; Queralt-Martín et al., 2020). The results of these studies were to some extent controversial, because hVDAC3 was a small channel-forming component in one of the studies (Checchetto et al., 2014), whereas it has in a more recent study quite normal electrophysiological properties (Queralt-Martín et al., 2020). This means that the three human eukaryotic porins have a similar single-channel conductance (see Table 1) and the pores formed by the three human isoforms are all voltage-dependent with some modifications (Benz, 2004; Gattin et al., 2015; Queralt-Martín et al., 2020). The expression of the isoforms may be tissue-specific but their function in mitochondrial outer membrane permeability and in other cellular important functions, such as apoptosis, is still a matter of debate (De Pinto et al., 2016; Shoshan-Barmatz et al., 2020; Shimizu et al., 2021). Three genes coding for analogs of VDAC1 have not only be found in mammals but also in the genome of the fruit fly Drosophila melanogaster (Aiello et al., 2004; Komarov et al., 2004). All of them with one exception code for pore-forming proteins with properties similar to most eukaryotic porins with some modification of the voltage dependence (Aiello et al., 2004; Komarov et al., 2004). Careful analysis of the genes and their comparison with those of other eukaryotic porins from insects suggested that the genes evolved by duplication from an ancestral gene (Komarov et al., 2004).
The genetic organization of eukaryotic porins in plants appears to be even more complicated (Kusano et al., 2009; Homblé et al., 2012). The genome of the popular model organism in plant biology and genetics, Arabidopsis thaliana, contains at least four or five genes coding for eukaryotic porin-like proteins (Lee et al., 2009; Tateda et al., 2011; Berrier et al., 2015). A similar number of eukaryotic porin genes has been found in Lotus japonicus and soybean, where also up to five genes were found (Wandrey et al., 2004). Localization analysis of the different isoforms of eukaryotic porins in Arabidopsis indicated specific functions of the porins including DNA and RNA transport (Tarasenko et al., 2021). Some of the plant porins have similar subcellular localizations (Tateda et al., 2011, 2012). Knockout mutants of AtVDAC2 and AtVDAC4 show despite similar subcellular localizations severe defects in growth indicating that these eukaryotic porins have an important function in Arabidopsis (Tateda et al., 2012). Many eukaryotic porins from plants have been studied in lipid bilayer membranes (see Table 1). Their single-channel conductance seems to be a little smaller than those of porins from other organisms, but plant porins show similar voltage dependences as most eukaryotic porins with some modifications. From the many porins of Arabidopsis, only AtVDAC3 has been studied in lipid bilayers in some detail (Berrier et al., 2015). Again, its properties were quite similar as found for most eukaryotic porins,
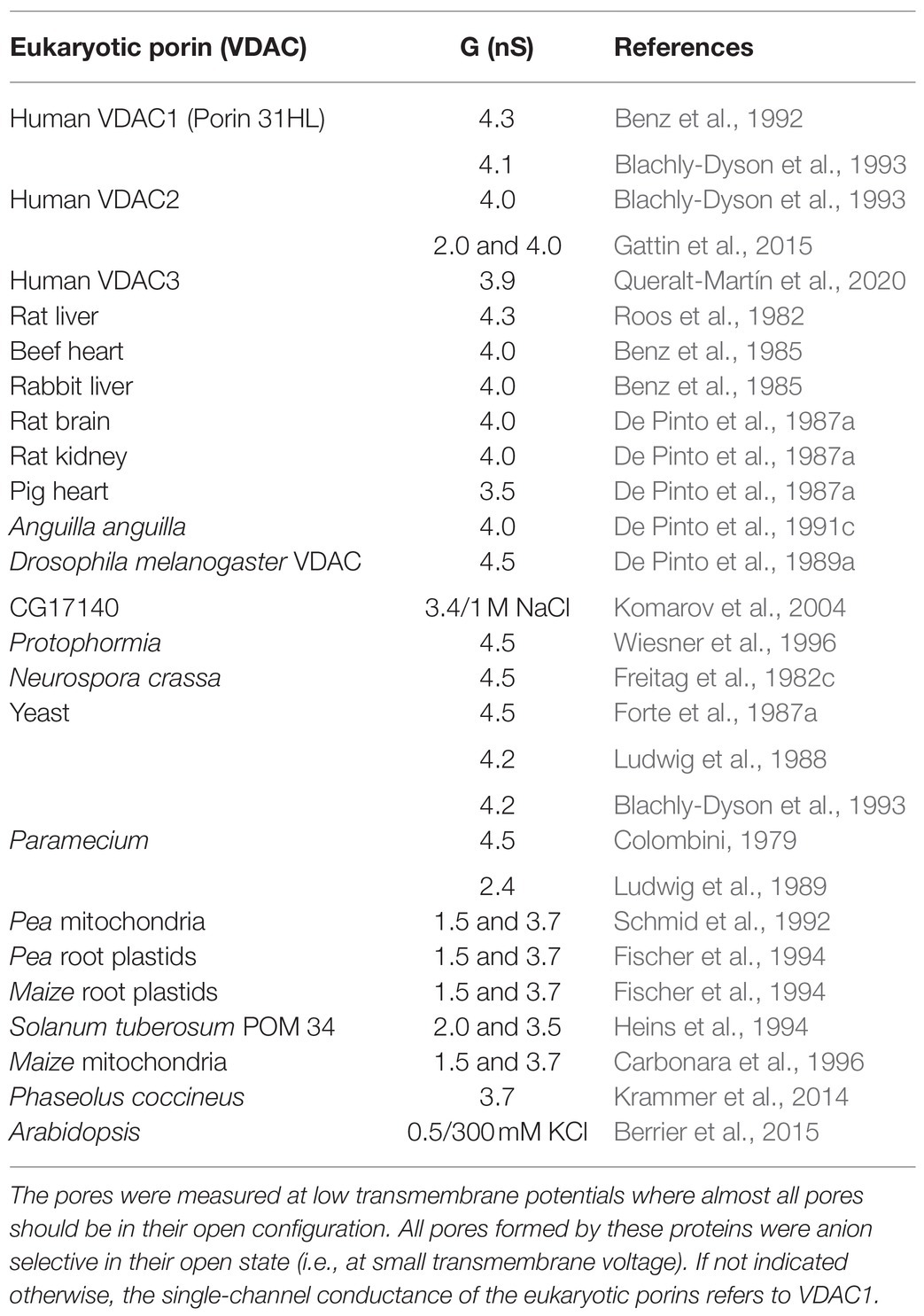
Table 1. Single-channel conductance of eukaryotic (mitochondrial) porins (VDACs) from different eukaryotic organisms in 1M KCI, pH 6, if not indicated otherwise.
Identification of Eukaryotic Porin as Dicyclohexylcarbodiimide (Dccd)-Binding Protein in the Mitochondrial Outer Membrane
When pig heart mitochondria are treated with low doses (1.5nmol/mg of mitochondrial protein) of C14-labeled dicyclohexylcarbodiimide (DCCD), three mitochondrial polypeptides of approximately 9, 16, and 33kDa bound DCCD (Houstĕk et al., 1981; De Pinto et al., 1985). The two smaller DCCD-binding proteins are parts of the F0F1 ATPase localized in the mitochondrial inner membrane (Houstĕk et al., 1981). The 33kDa DCCD-binding protein present in the mitochondrial outer membrane of pig heart was identified as the eukaryotic porin based on biochemical evidence and electrophysiological experiments although DCCD-binding did not change the characteristics of the pore (De Pinto et al., 1985). However, labeling of porin with DCCD resulted in the loss of hexokinase binding to porin (Nakashima et al., 1986; Nakashima, 1989), because porin is the hexokinase-binding protein (Fiek et al., 1982; Lindén et al., 1982b). Fifty percent inhibition of hexokinase binding occurred at very low levels of DCCD by less than 2nmol of DCCD/mg of mitochondrial protein (Nakashima, 1989). Water-soluble carbodiimides had no effect on hexokinase binding on porin, indicating that the binding place was in a hydrophobic environment. DCCD-binding to proteins suggested that a negatively charged amino acids exist in a hydrophobic environment (De Pinto et al., 1985; Nakashima, 1989). This amino acid was identified as glutamate 72 in the sequence of bovine heart eukaryotic porin (De Pinto et al., 1993). The role of this negative charge in mitochondrial metabolism of the three VDAC isoforms in Zebrafish was studied in detail recently because the homologous glutamate 73 is present in VDAC1 and VDAC2 but not in VDAC3 and plays an important role in regulation of Ca2+ uptake (Shimizu et al., 2021). Diafenthiuron is an acaricide and insecticide developed by Ciba-Geigy that cannot longer be used as a pesticide because of its toxicity (Kayser and Ellinger, 2001). Its active form is the carbodiimide CGA 140'408, which labels also components of the mitochondrial ATPase together with the eukaryotic porin of the fly Protophormia (Wiesner et al., 1996). Reconstitution experiments with the CGA 140'408-modified porin of Protophormia showed also no significant effects on the characteristics of channel formation by Protophormia porin similar as described above for the binding of DCCD to pig heart porin (De Pinto et al., 1985; Wiesner et al., 1996).
Reconstitution of Eukaryotic Porins in Lipid Bilayer Membranes
The first reconstitution of eukaryotic porin (VDAC) occurred via the enrichment of mitochondrial particles from Paramecium mitochondria with endogenous asolectin followed by the formation of solvent-depleted membranes according to the Montal-Mueller method (Montal and Mueller, 1972). For this, the protein-lipid mixtures were spread with hexane on the aqueous surface of the membrane cell for membrane formation (Schein et al., 1976). The pores were present in the membranes immediately after its formation. The number of pores incorporated into the membranes by this method depended on the concentration of porin in the protein-lipid mixtures (Schein et al., 1976). The reconstitution of the eukaryotic porins occurred late on similarly as the incorporation of bacterial porins into lipid bilayers (Benz et al., 1978). After isolation and purification of eukaryotic porins, they were added to the aqueous electrolyte solution bathing preexisting membranes (Colombini, 1979; Roos et al., 1982; Freitag et al., 1982c) or to preexisting lipid vesicles (Zalman et al., 1980; Mihara et al., 1982). The characteristics of the pores formed by eukaryotic porins were not dependent on the method of bilayer formation, either painted of folded, although this was occasionally claimed (Colombini, 1983). It seems that these pores, similar as bacterial porins form their own sphere and their properties, are only little dependent on the lipid environment in the membranes.
Figure 4 shows the stepwise increase of membrane current when a eukaryotic porin (Porin 31HL) was added to the aqueous 1M KCl solution bathing a painted black lipid membrane from diphytanoyl phosphatidylcholine/n-decane. The steps were mostly directed upward at low transmembrane potential, as it was already outlined above, when the voltage dependence of the same eukaryotic pore was discussed. The single-channel conductance of Porin 31HL was under these conditions about 4 nS (see the histogram of the current fluctuations obtained with Porin 31HL).
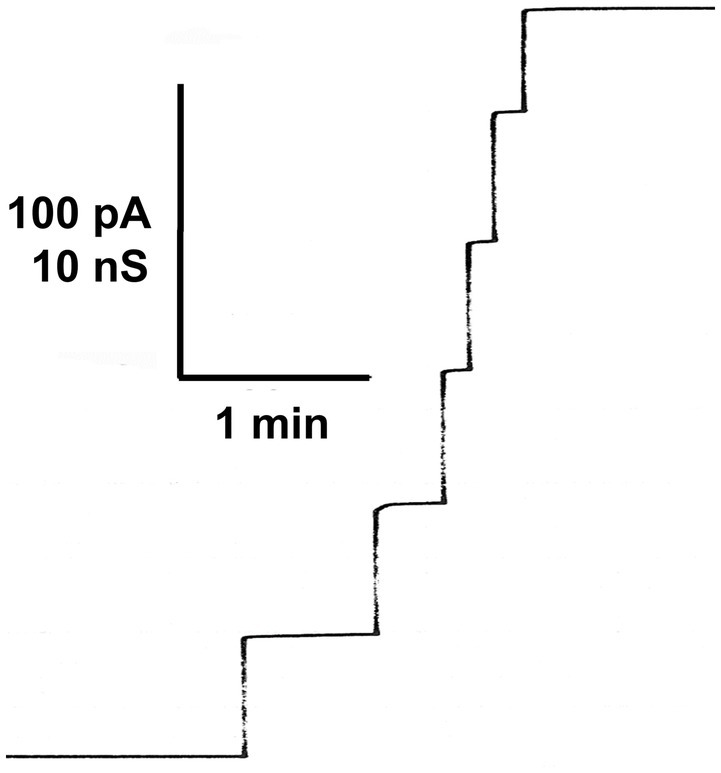
Figure 4. Stepwise increase of the membrane current (given in pA) after the addition of porin 31HL (hVDAC1) to a black lipid bilayer membrane given as a function of time. The aqueous phase contained 5ng/ml Porin 31HL and 1M KCl (Benz et al., 1992). The membrane was formed from diphytanoyl phosphatidylcholine/n-decane. The voltage applied was 10mV; T=20°C.
The histogram of all current fluctuations observed with Porin 31HL showed two maxima for pore distribution (Benz et al., 1992). One is centered on 4 nS and the other one around 2 nS (Figure 5). The higher single-channel conductance refers presumably to the first opening of a Porin 31HL pore by reconstitution of one pore protein into the membrane. The lower conductance represents pores that adopted a sublevel of conductance and reopened again, i.e., these pores reflect the closed state of the Porin 31HL pore, which seems to be around 2 nS.
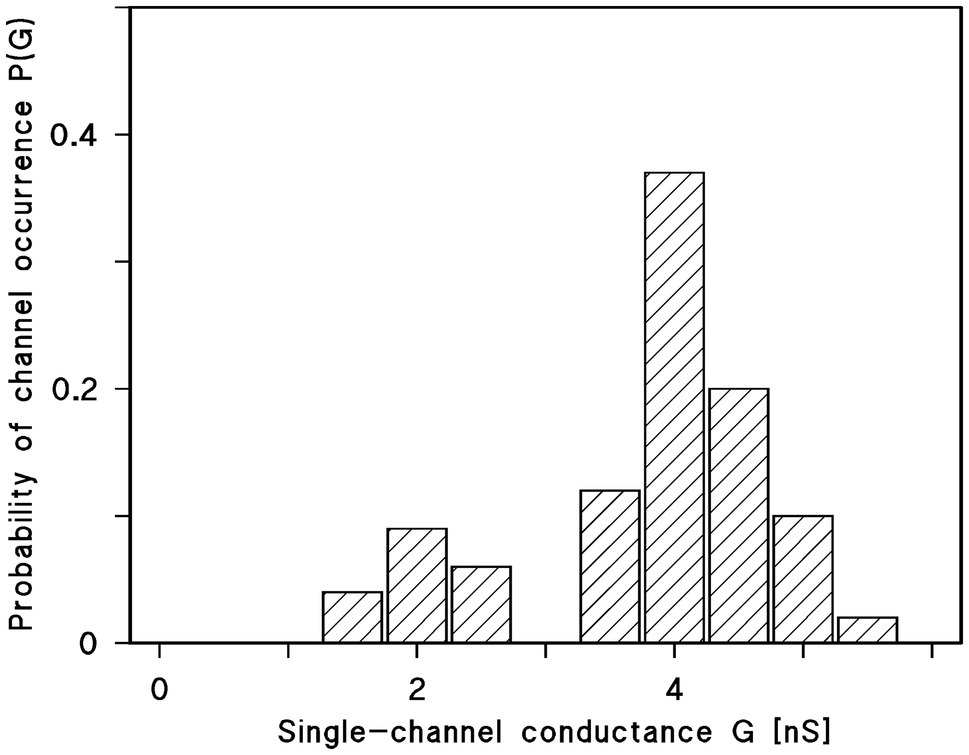
Figure 5. Histogram of conductance fluctuations observed with membranes of diphytanoyl phosphatidylcholine/n-decane in the presence of Porin 31HL (Benz et al., 1992). P(G) is the probability for the occurrence of a conductance step with a certain single-channel conductance (given in nS). The aqueous phase contained 1M KCl. The voltage applied was 10mV. The mean value of all upward directed steps was 4.3 nS for the right-side maximum and 2.4 nS for the left-side maximum (in total 288 single events); T=20°C.
Similar experiments were performed with many eukaryotic porins in different research groups. Typical for the pores formed by the eukaryotic porins is an open state between 4 and 4.5 nS at small voltages. However, histograms of all current fluctuation show also pores around 2 nS, which could refer to substates of the open pore. Plant porins have a somewhat smaller conductance just a little below 4 nS at low voltage. In the case of plant porins, also a second maximum was observed at 1.5 nS. The hypothesis is that this maximum also referred to the closed state or voltage-gated substates of the mitochondrial pore.
Ionic Selectivity of the Open State of Pores Formed by Eukaryotic Porins
The single-channel conductance of pores formed by eukaryotic porins followed in salts composed of different cations and anions approximately the mobility of the different ions in the aqueous phase (Colombini, 1979; Roos et al., 1982). This result suggested together with the high single-channel conductance (see Table 1) that eukaryotic porin pores are wide and water filled. Nevertheless, the pores showed some preference for anions. The ionic selectivity of channels/pores reconstituted into artificial lipid bilayers can be measured by the zero-current membrane potential as the result of a salt gradient applied across the membranes. This could be performed by using a high impedance electrometer connected to electrodes with salt bridges when the gradient is established across the membranes (Benz et al., 1979). It is also possible to measure current-voltage curves and extrapolating the current to zero. The corresponding voltage provides the same information (Schein et al., 1976). Table 2 shows examples of zero-current membrane potentials obtained for several eukaryotic porins using salts composed of anions and cations of different mobility in the aqueous phase. It is evident from the data in Table 2 that the mobility of the ions in the aqueous phase has a substantial influence on the zero-current membrane potential of the pores and the permeability ratio Panion/Pcation. For KCl that is composed of equally mobile potassium ions and chloride, the pores are slightly anion selective. This changes remarkably, when potassium ions or chloride are replaced by lithium ions and acetate, respectively. For LiCl, the potential is more negative, whereas it gets positive for K-acetate. This result is typical for general diffusion pores similar to general diffusion pores of the bacterial outer membranes (Benz, 1994a). The voltage dependence of the eukaryotic porins changes this picture, because all eukaryotic porins are cation-selective in the closed state (Benz, 1994b, 2004). The switch from open-anion selectivity to closed-cation selectivity of eukaryotic porins adopts presumably an important role in regulation of mitochondrial energy metabolism.
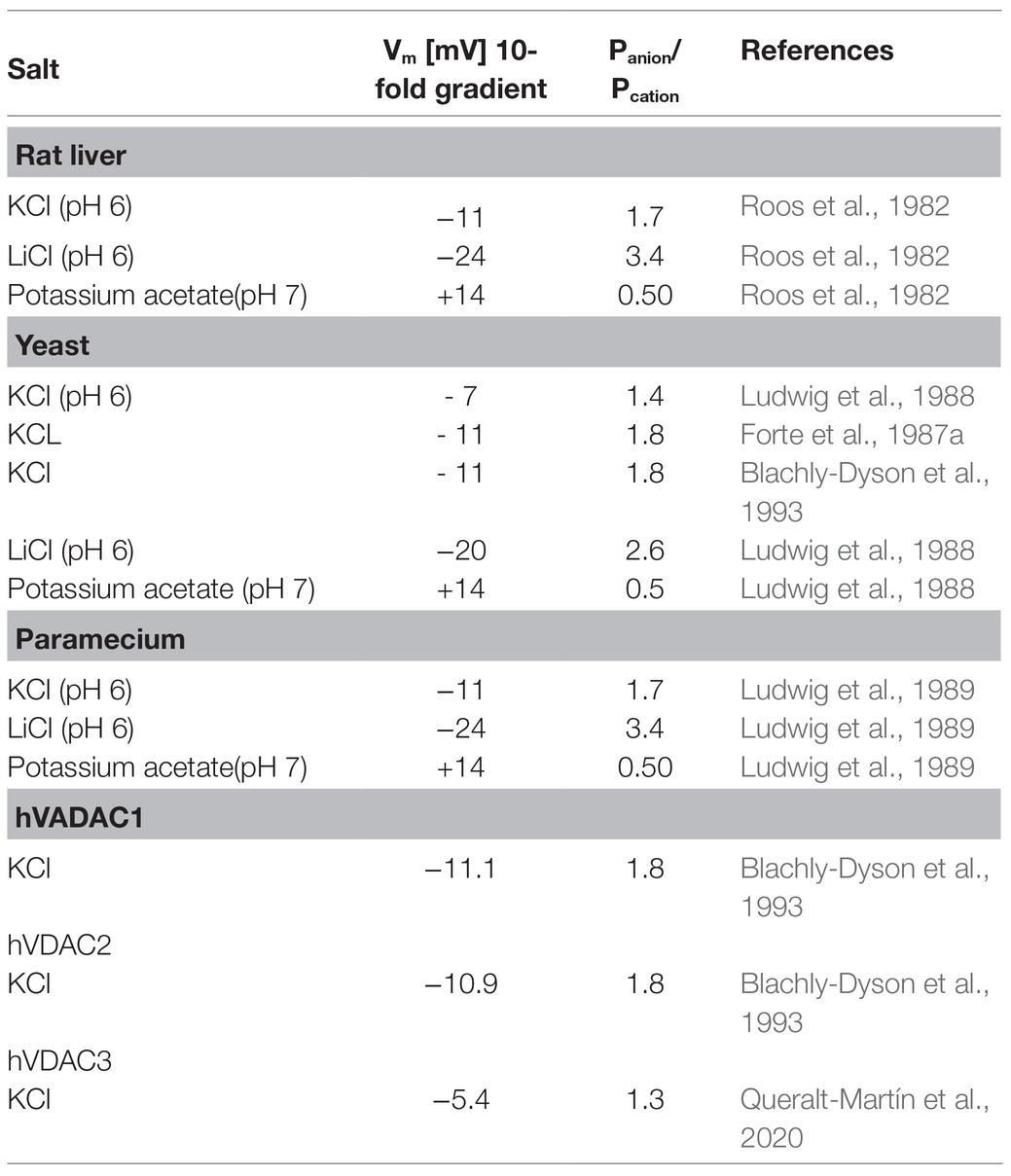
Table 2. Zero-current membrane potentials, Vm, of lipid bilayer membranes the presence of rat liver, yeast, human eukaryotic porin1, human eukaryotic porin2, and Paramecium porins measured for 10-fold gradients of different salts. Vm is defined as the potential of the dilute side (10mM/100mM) relative to that of the concentrated side (100mM/1M); Panion/Pcation was calculated from the Goldman-Hodgkin-Katz equation (Benz et al., 1979).
Conductance of the Closed States of Eukaryotic Porins
The conductance of the closed state of eukaryotic porins could be evaluated from single-channel recordings extended over longer times. At voltages between 20mV and 30mV, the pores close more frequently but not too often, which means that the residual conductance associated with the closing pores could be estimated from the current recordings. This procedure allows a meaningful analysis of the conductance of the closed eukaryotic pores as it is shown in Table 3 for KCl. Included into Table 3 are also the results of this type of experiment when salts composed of different anions and cations were used. The results indicate again (similar to Figure 2) that open and closed states of the eukaryotic pores have different selectivity. This could be concluded from the observation that the single-channel conductance of the closed state of the pore was considerably smaller for Tris-HCl than for K-MES, despite a similar aqueous mobility of K+ and Cl−. This result suggested again that the closed state(s) of mitochondrial porins is cation-selective, otherwise, the relatively small conductance difference for K-MES between open and closed state and the big difference for Tris-HCl cannot be understood.
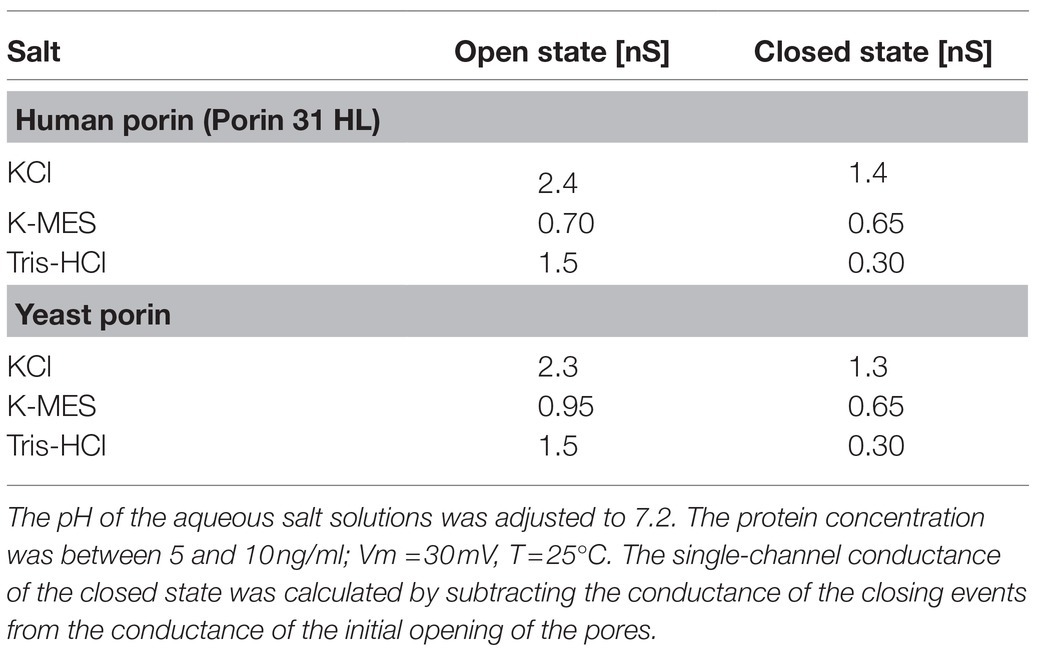
Table 3. Average single-channel conductance of the open and closed states of human (Porin 31HL; Benz et al., 1992) and yeast (Ludwig et al., 1988) porins in different 0.5M salt solutions.
Eukaryotic Pores are Closed In Vitro and in Vivo by a Synthetic Polyanion
König et al. (1977, 1982) described effects of a 10kDa synthetic polyanion (a copolymer of methacrylate, maleate, and styrene in a 1:2:3 proportion) on mitochondrial metabolism. Dependent on its concentration, the polyanion was able to inhibit anion transport, respiration, ATPase activity, and ADP/ATP exchange activity of rat liver mitochondria (König et al., 1977, 1982). The polyanion is by far too big to enter the intermembrane space of mitochondria through the outer membrane pore and to act with inner membrane components, which means that its action on mitochondrial metabolism was something like a mystery. However, reconstitution experiments with eukaryotic porin demonstrated that the polyanion bound to porin and changed its voltage dependence (Colombini et al., 1987; Benz et al., 1988). Application of small voltages of −5mV or less negative to the cis-side of the membranes, where porin and polyanion were added, resulted already in pore closure (Benz et al., 1988; De Pinto et al., 1989a). The mitochondrial pore was always in the open configuration when positive potentials were applied to the cis-side (Benz et al., 1988).
Careful analysis of the polyanion-induced closed state of rat liver porin demonstrated that it showed an interesting analogy to the voltage-mediated closed state (see Table 4). This means that the polyanion, although it is not able to enter the pore, interacts with the gate. It pulls the gate (presumably the α-helical N-terminus) to the side of the polyanion and changes thus the voltage dependence of the gate (Benz et al., 1988). The effect of the polyanion on mitochondrial metabolism was also studied in intact mitochondria, because it allowed the evaluation of the role of the outer membrane pore on different features of mitochondrial metabolism (Benz et al., 1990; Benz and Brdiczka, 1992; Brdiczka, 1993). The addition of 30μg polyanion per mg mitochondria completely blocked adenylate and creatine kinases. Similarly, peripheral kinases, such as hexokinase and glycerolkinase, were also completely inhibited, when mitochondrial, but not external ATP, was used (Benz and Brdiczka, 1992). Disruption of the mitochondrial outer membrane by detergent completely restored the activity of all peripheral kinases, which clearly indicated that compartment formation exists in the intermembrane space of intact mitochondria (Benz and Brdiczka, 1992; Brdiczka, 1993; Gellerich et al., 1993; Ahmadzadeh et al., 1996; Brdiczka et al., 1998). These results suggest that the mitochondrial outer membrane pore could be involved in the control of mitochondrial metabolism via its voltage dependence (Benz and Brdiczka, 1992; Liu and Colombini, 1992). Important for this could be the close apposition of mitochondrial inner and outer membrane that a voltage across the outer membrane is induced via capacitive coupling of inner and outer membranes, in which also the folding of the inner membrane may be involved (Benz, 1985; Mannella et al., 2013).

Table 4. Average single-channel conductance of the open and the polyanion-induced closed state of rat liver porin in different 0.5M salt solutions (Benz et al., 1990).
Renaturation and Reconstitution of Eukaryotic Porins Obtained by Heterologous Expression in Bacteria
During the first time of research into the characteristics of eukaryotic porins, these proteins were always isolated from mitochondria. However, modern research into the properties of channel-forming proteins needs very often mass production and site-directed mutagenesis of the proteins. This is possible in the case of eukaryotic porins but it is very complicated and time consuming to bring the mutated proteins back into mitochondria. Thus, it was of interest to express eukaryotic porins in bacteria and to renature eukaryotic porins for research purposes. This was possible although translation of eukaryotic porins in vivo and in vitro differs considerably. Nevertheless, two early studies describe the renaturation processes of eukaryotic porins in some detail. Pfaller et al. (1985) described for the first time the possibility to make eukaryotic porin from N. crassa water soluble. In this form, the protein binds to the surface of mitochondria and blocks the import of the porin precursors. The water-soluble porin may also be renatured by treatment with low doses of detergents and needs the presence of sterols in the lipid bilayer membranes (Pfaller et al., 1985). In fact, the presence of cholesterol in a ratio of five cholesterol per one polypeptide has been detected in purified eukaryotic porin using different detergents (De Pinto et al., 1989b). Sterols were also necessary when the properties of mutated N. crassa porin were studied in lipid bilayer membranes. Following the renaturation process of different eukaryotic porins, it seems that sterols seem to be necessary, although they may modulate the properties of the pores, in particular of plant porins (Popp et al., 1995; Carbonara et al., 1996; Mlayeh et al., 2010; Lopes-Rodrigues et al., 2020; Saidani et al., 2021). However, in mass production and functional renaturation of two human isoforms of human porin (hVDAC1 and hVDAC2) and of potato VDAC36, no indication for the need of cholesterol/sterol for porin structure and function was observed (Engelhardt et al., 2007; Lopes-Rodrigues et al., 2020; Najbauer et al., 2021). On the other hand, ergosterol interacts with eukaryotic porin of N. crassa (Bay and Court, 2009) and stigmasterol seems to be important for proper function of bean seed VDAC (Saidani et al., 2021) and sterols were found to be important for renaturation of VDAC from pea root plastids (Popp et al., 1995). This means presumably that it is an open question whether sterols are important for porin function and/or could only accelerate the renaturation process but are essentially not needed for the formation of some functional pores.
Structure of the Mitochondrial Outer Membrane Pore
The presence of a voltage-dependent outer membrane pore in mitochondria was an interesting feature in research into mitochondria and attempts were made to visualize the pores. X-ray diffraction of oriented outer mitochondrial membranes from plants suggested a special location of the proteins in the membrane plane (Mannella, 1981). Further electron microscopic analysis of Neurospora mitochondria showed crystalline arrays of putative pores in the outer membranes if the membranes were dialyzed against low salt buffers (Mannella and Frank, 1984; Mannella, 1987). Single repeating units contained three pores, which was revealed by lipid bilayer experiments (Mannella et al., 1983). Analysis of the pores using uranyl acetate suggested that the outer membrane pore formed cylinders with an outer diameter of 5nm and an inner core of about 1.8 to 2nm (Mannella and Frank, 1984). Many attempts were made besides the electron microscopic analyses to resolve the 3D structure of the mitochondrial pore. However, all these attempts were unsuccessful for a very long time presumably because the pore was deeply buried in the outer membrane and did not show intermolecular interactions (De Pinto et al., 1987b).
Starting with eukaryotic porins from yeast (Mihara and Sato, 1985) and N. crassa (Kleene et al., 1987), the primary structure of many eukaryotic porins became known during the last 25years as it is discussed in part 4 of this review. Common to all of them is the length of about 280 amino acids without an obvious leader sequence and the balanced distribution of hydrophobic and hydrophilic amino acids. The genomes of many eukaryotic organisms, in particular of mammals and plants, code very often for several porins with yet not fully understood functions (Anflous and Craigen, 2004; Pinto and Messina, 2004; Graham and Craigen, 2005; Tateda et al., 2011). Secondary structure predictions suggested the existence of many β-strands within the primary sequence of eukaryotic porins similar to the situation in bacterial ones (Benz, 1994a,b). However, all mitochondrial porins known to date contain at the N-terminal end a stretch of about 20 to 25 amino acids that forms an α-helical structure, probably involved in voltage-dependent gating of the pore because its deletion leads to the loss of voltage dependence (Popp et al., 1996; Runke et al., 2006; De Pinto et al., 2007; Zachariae et al., 2012). A comparison of the many primary sequences of eukaryotic porins shows that the sequences have all a similar length, but otherwise, the homology is not very obvious because only a few amino acids are conserved (Benz, 2004). Only near amino acid 90 of most porins a triplet of the form GLK can be found, which is highly conserved but its function is unknown (Runke et al., 2000). The phylogenetic relationship of the β-barrel mitochondrial outer membrane proteins TOM40 (involved in protein transport) and eukaryotic porin was studied in detail by Young et al. (2007) and Bay et al. (2012). The authors suggested from their analysis that these proteins share a common evolutionary origin, meaning that the lineage of both protein families was co-evolutionary and formed later paralogues.
The 3D structure of the mitochondrial pore was for a longer time a matter of debate. Based on secondary structure predictions, electrophysiology and mutagenesis several models were developed (Forte et al., 1987b; Blachly-Dyson et al., 1990, 1993; Peng et al., 1992; Benz, 1994b; Casadio et al., 2002; Anflous and Craigen, 2004). One model assumed that eukaryotic porin is exclusively formed by 16 or 18 β-strands. The other model suggested also that the pore contains β-strands, but only 12–13 strands in combination with the α-helix as part of the channel wall. At the end, three groups were successful to derive simultaneously the 3D structure of eukaryotic porins using different techniques (Bayrhuber et al., 2008; Hiller et al., 2008; Ujwal et al., 2008). Hiller et al. (2008) used the technique of solution NMR to study recombinant hVDAC1 reconstituted in detergent micelles. In this case, the location of the N-terminus was not resolved. Bayrhuber et al. (2008) derived the 3D structure of hVDAC1 from a combination of NMR-spectroscopy and X-ray crystallography. Ujwal et al. (2008) succeeded to crystallize murine VDAC1 (mVDAC1). The three studies agreed in the basic structure of the mitochondrial pore that forms a β-barrel with 19 β-strands. Two structures show the location of the N-terminal α-helix horizontally midway in the pore, restricting its size (Bayrhuber et al., 2008; Ujwal et al., 2008). This means that the α-helix has a strategic position to control the passage of metabolites and ions through the mitochondrial pore. This structure was criticized and Colombini (2012) insisted in the structure of VDAC with one α-helix and 13 β-strands tilted at a 46° angle toward the surface of the mitochondrial outer membrane. However, the structure of VDAC shown in Figure 6 has many times been realized that we can consider it as the real structure of the eukaryotic pore.

Figure 6. Structure of the mitochondrial outer membrane pore (mVDAC1) and an OmpF monomer of E. coli. β-strands within the protein structures are shown in yellow and α-helical stretches in red. The 3D structures of the proteins are shown from the side in direction to the surface of the mitochondrion and the cell (upper structures) and from the surface of the mitochondrion and the bacterial cell (structures down). mVDAC1 (PDB code: 2JK4) is the 3D structure of mouse mitochondrial porin (Ujwal et al., 2008). OmpF (PDB code: 2OMF) represents the structure of the major outer membrane protein of E. coli (Cowan et al., 1992).
Figure 6 shows the schematic structure of mouse VDAC as it was obtained by X-ray crystallography (Ujwal et al., 2008) in comparison with the 3D structure of the most abandoned bacterial porin OmpF of E. coli (Cowan et al., 1992). The mitochondrial pore is formed by 19 β-strands (18 are antiparallel and β-strands one and 19 are parallel) in contrast to 16 antiparallel β-strands of OmpF. It is clear from a comparison of the two 3D structures that the architecture of the two outer membrane pores is quite similar. This has presumably to do with the history of bacterial and mitochondrial outer membrane pores. It has presumably also to do with translation and assembly of both pores. The β-strands of both β-barrel cylinders are tilted by 30–40° toward the surface of the membranes. The dimensions of the eukaryotic porin are 35 Ă for the height and 40 Ă for the width. The N-terminal α-helix (amino acids 1–21) is located inside the β-barrel cylinder and acts there as a gate, but also as a stabilizing element for the mitochondrial pore similar to the external loop 3 of OmpF that is folded inside the bacterial pore (Cowan et al., 1992; Ujwal et al., 2008). Despite the location of the N-terminus inside the eukaryotic pore, it has a high ion permeability. It is approximately the same as OmpF trimers (Benz, 1994a,b). It is noteworthy, that Tom40, the major component of the mitochondrial outer membrane import machinery is also a member of the VDAC-family and shows the same structure of 18 antiparallel β-strands and one pair of parallel β-strands (Zeth and Zachariae, 2018). The most interesting point of the comparison of bacterial and mitochondrial porins is the fact that bacterial outer membrane pores have only passive properties, whereas mitochondrial porins adopted during evolution an active role in mitochondrial metabolism.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
I would like to thank Dieter Brdiczka, Vito De Pinto, Walter Neupert, Ferdinando Palmieri, and Friedrich P. Thinnes for the fruitful and excellent collaboration during many years and my collaborators Otto Ludwig, Elke Maier, Birgit Popp, and Angela Schmid for their excellent work with porins from mitochondria on which this review is largely based. My own research was supported by the Deutsche Forschungsgemeinschaft and by the Fonds der Chemischen Industrie.
References
Adams, V., Griffin, L., Towbin, J., Gelb, B., Worley, K., and McCabe, E. R. (1991). Porin interaction with hexokinase and glycerol kinase: metabolic microcompartmentation at the outer mitochondrial membrane. Biochem. Med. Metab. Biol. 45, 271–291. doi: 10.1016/0885-4505(91)90032-G
Ahmadzadeh, M., Horng, A., and Colombini, M. (1996). The control of mitochondrial respiration in yeast: a possible role of the outer mitochondrial membrane. Cell Biochem. Funct. 14, 201–208. doi: 10.1002/cbf.673
Aiello, R., Messina, A., Schiffler, B., Benz, R., Tasco, G., Casadio, R., et al. (2004). Functional characterization of a second porin isoform in Drosophila melanogaster. DmPorin2 forms voltage-independent cation-selective pores. J. Biol. Chem. 279, 25364–25373. doi: 10.1074/jbc.M310572200
Aljamal, J. A., Genchi, G., De Pinto, V., Stefanizzi, L., De Santis, A., Benz, R., et al. (1993). Purification and characterization of Porin from corn (Zea mays L.) mitochondria. Plant Physiol. 102, 615–621. doi: 10.1104/pp.102.2.615
Anflous, K., and Craigen, W. J. (2004). “Mitochondrial porins in mammals: insight into functional roles from mutant mice and cells,” in Structure and Function of Prokaryotic and Eukaryotic Porins. ed. R. Benz (Wiley-VCh: Weinheim), 285–307.
Awramik, S. M. (1992). The oldest records of photosynthesis. Photosynth. Res. 33, 75–89. doi: 10.1007/BF00039172
Bader, S., Wolf, L., Milenkovic, V. M., Gruber, M., Nothdurfter, C., Rupprecht, R., et al. (2019). Differential effects of TSPO ligands on mitochondrial function in mouse microglia cells. Psychoneuroendocrinology 106, 65–76. doi: 10.1016/j.psyneuen.2019.03.029
Báthori, G., Csordás, G., Garcia-Perez, C., Davies, E., and Hajnóczky, G. (2006). Ca2+−dependent control of the permeability properties of the mitochondrial outer membrane and voltage-dependent anion-selective channel (VDAC). J. Biol. Chem. 281, 17347–17358. doi: 10.1074/jbc.M600906200
Bausewein, T., Naveed, H., Liang, J., and Nussberger, S. (2020). The structure of the TOM core complex in the mitochondrial outer membrane. Biol. Chem. 401, 687–697. doi: 10.1515/hsz-2020-0104
Bay, D. C., and Court, D. A. (2009). Effects of ergosterol on the structure and activity of Neurospora mitochondrial porin in liposomes. Can. J. Microbiol. 55, 1275–1283. doi: 10.1139/W09-088
Bay, D. C., Hafez, M., Young, M. J., and Court, D. A. (2012). Phylogenetic and coevolutionary analysis of the β-barrel protein family comprised of mitochondrial porin (VDAC) and Tom40. Biochim. Biophys. Acta 1818, 1502–1519. doi: 10.1016/j.bbamem.2011.11.027
Bayrhuber, M., Meins, T., Habeck, M., Becker, S., Giller, K., Villinger, S., et al. (2008). Structure of the human voltage-dependent anion channel. Proc. Natl. Acad. Sci. U. S. A. 105, 15370–15375. doi: 10.1073/pnas.0808115105
Benz, R. (1985). Porins from bacterial and mitochondrial outer membranes. CRC Crit. Rev. Biochem. 19, 145–190. doi: 10.3109/10409238509082542
Benz, R. (1994a). “Solute uptake through the bacterial outer membrane,” in Bacterial Cell Wall. eds. J. M. Ghuysen and R. Hakenbeck (Amsterdam: Elsevier Science B.V), 397–423.
Benz, R. (1994b). Permeation of hydrophilic solutes through mitochondrial outer membranes: review on mitochondrial porins. Biochim. Biophys. Acta 1197, 167–196. doi: 10.1016/0304-4157(94)90004-3
Benz, R. (2004). “Structure and function of mitochondrial (eukaryotic) Porins,” in Structure and Function of Prokaryotic and Eukaryotic Porins. ed. R. Benz (Wiley-VCh: Weinheim), 259–284.
Benz, R., and Brdiczka, D. (1992). The cation-selective substate of the mitochondrial outer membrane pore: single-channel conductance and influence on intermembrane and peripheral kinases. J. Bioenerg. Biomembr. 24, 33–39. doi: 10.1007/BF00769528
Benz, R., Janko, K., Boos, W., and Läuger, P. (1978). Formation of large, ion-permeable membrane channels by the matrix protein (porin) of Escherichia coli. Biochim. Biophys. Acta 511, 305–319. doi: 10.1016/0005-2736(78)90269-9
Benz, R., Janko, K., and Läuger, P. (1979). Ionic selectivity of pores formed by the matrix protein (porin) of Escherichia coli. Biochim. Biophys. Acta 551, 238–247. doi: 10.1016/0005-2736(89)90002-3
Benz, R., Kottke, M., and Brdiczka, D. (1990). The cationically selective state of the mitochondrial outer membrane pore: a study with intact mitochondria and reconstituted mitochondrial porin. Biochim. Biophys. Acta 1022, 311–318. doi: 10.1016/0005-2736(90)90279-W
Benz, R., Ludwig, O., De Pinto, V., and Palmieri, F. (1985). “Permeability properties of mitochondrial porins of different eukaryotic cells,” in Achievements and Perspectives of Mitochondrial Research. Vol. 1. ed. E. Quagliariello (Amsterdam: Elsevier), 317–327.
Benz, R., Maier, E., Thinnes, F. P., Götz, H., and Hilschmann, N. (1992). Studies on human porin. VII. The channel properties of the human B-lymphocyte membrane-derived “Porin 31HL” are similar to those of mitochondrial porins. Biol. Chem. Hoppe Seyler 373, 295–303. doi: 10.1515/bchm3.1992.373.1.295
Benz, R., Wojtczak, L., Bosch, W., and Brdiczka, D. (1988). Inhibition of adenine nucleotide transport through the mitochondrial porin by a synthetic polyanion. FEBS Lett. 231, 75–80. doi: 10.1016/0014-5793(88)80706-3
Berrier, C., Peyronnet, R., Betton, J. M., Ephritikhine, G., Barbier-Brygoo, H., Frachisse, J. M., et al. (2015). Channel characteristics of VDAC-3 from Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 459, 24–28. doi: 10.1016/j.bbrc.2015.02.034
Betlazar, C., Middleton, R. J., Banati, R., and Liu, G. J. (2020). The translocator protein (TSPO) in mitochondrial bioenergetics and immune processes. Cell 9:512. doi: 10.3390/cells9020512
Blachly-Dyson, E., Baldini, A., Litt, M., McCabe, E. R., and Forte, M. (1994). Human genes encoding the voltage-dependent anion channel (VDAC) of the outer mitochondrial membrane: mapping and identification of two new isoforms. Genomics 20, 62–67. doi: 10.1006/geno.1994.1127
Blachly-Dyson, E., Peng, S., Colombini, M., and Forte, M. (1990). Selectivity changes in site-directed mutants of the VDAC ion channel: structural implications. Science 247, 1233–1236. doi: 10.1126/science.1690454
Blachly-Dyson, E., Zambronicz, E. B., Yu, W. H., Adams, V., McCabe, E. R., Adelman, J., et al. (1993). Cloning and functional expression in yeast of two human isoforms of the outer mitochondrial membrane channel, the voltage-dependent anion channel. J. Biol. Chem. 268, 1835–1841. doi: 10.1016/S0021-9258(18)53930-2
Blumenthal, A., Kahn, K., Beja, O., Galun, E., Colombini, M., and Breiman, A. (1993). Purification and characterization of the voltage-dependent anion-selective channel protein from wheat mitochondrial membranes. Plant Physiol. 101, 579–587. doi: 10.1104/pp.101.2.579
Brdiczka, D. (1990). Interaction of mitochondrial porin with cytosolic proteins. Experientia 46, 161–167. doi: 10.1007/BF02027312
Brdiczka, D. (1993). Effect of macromolecules on the regulation of the mitochondrial outer membrane pore and the activity of adenylate kinase in the inter-membrane space. Biochim. Biophys. Acta 1142, 217–227. doi: 10.1016/0005-2728(93)90150-E
Brdiczka, D., Beutner, G., Rück, A., Dolder, M., and Wallimann, T. (1998). The molecular structure of mitochondrial contact sites. Their role in regulation of energy metabolism and permeability transition. Biofactors 8, 235–242. doi: 10.1002/biof.5520080311
Bureau, M. H., Khrestchatisky, M., Heeren, M. A., Zambrowicz, E. B., Kim, H., Grisar, T. M., et al. (1992). Isolation and cloning of a voltage-dependent anion channel-like Mr 36,000 polypeptide from mammalian brain. J. Biol. Chem. 267, 8679–8684. doi: 10.1016/S0021-9258(18)42496-9
Carbonara, F., Popp, B., Schmid, A., Iacobazzi, V., Genchi, G., Palmieri, F., et al. (1996). The role of sterols in the functional reconstitution of water-soluble mitochondrial porins from plants. J. Bioenerg. Biomembr. 28, 181–189. doi: 10.1007/BF02110649
Casadio, R., Jacoboni, I., Messina, A., and De Pinto, V. (2002). A 3D model of the voltage-dependent anion channel (VDAC). FEBS Lett. 520, 1–7. doi: 10.1016/S0014-5793(02)02758-8
Checchetto, V., Reina, S., Magrì, A., Szabo, I., and De Pinto, V. (2014). Recombinant human voltage dependent anion selective channel isoform 3 (hVDAC3) forms pores with a very small conductance. Cell. Physiol. Biochem. 34, 842–853. doi: 10.1159/000363047
Chioccioli, S., Del Duca, S., Vassallo, A., Castronovo, L. M., and Fani, R. (2020). Exploring the role of the histidine biosynthetic hisF gene in cellular metabolism and in the evolution of (ancestral) genes: from LUCA to the extant (micro)organisms. Microbiol. Res. 240:126555. doi: 10.1016/j.micres.2020.126555
Colombini, M. (1979). A candidate for the permeability pathway of the outer mitochondrial membrane. Nature 279, 643–645. doi: 10.1038/279643a0
Colombini, M. (1980). Structure and mode of action of a voltage dependent anion-selective channel (VDAC) located in the outer mitochondrial membrane. Ann. N. Y. Acad. Sci. 341, 552–563. doi: 10.1111/j.1749-6632.1980.tb47198.x
Colombini, M. (1980). Pore size and properties of channels from mitochondria isolated from Neurospora crassa. J. Membr. Biol. 53, 79–84. doi: 10.1007/BF01870576
Colombini, M. (1983). Purification of VDAC (voltage-dependent anion-selective channel) from rat liver mitochondria. J. Membr. Biol. 74, 115–121. doi: 10.1007/BF01870500
Colombini, M. (2012). VDAC structure, selectivity, and dynamics. Biochim. Biophys. Acta 1818, 1457–1465. doi: 10.1016/j.bbamem.2011.12.026
Colombini, M., Yeung, C. L., Tung, J., and König, T. (1987). The mitochondrial outer membrane channel, VDAC, is regulated by a synthetic polyanion. Biochim. Biophys. Acta 905, 279–286. doi: 10.1016/0005-2736(87)90456-1
Cowan, S. W., Schirmer, T., Rummel, G., Steiert, M., Ghosh, R., Pauptit, R. A., et al. (1992). Crystal structures explain functional properties of two E. coli porins. Nature 358, 727–733. doi: 10.1038/358727a0
De Pinto, V. (2021). Renaissance of VDAC: new insights on a protein family at the interface between mitochondria and cytosol. Biomol. Ther. 11, 107. doi: 10.3390/biom11010107
De Pinto, V., Al Jamal, J. A., Benz, R., Genchi, G., and Palmieri, F. (1991a). Characterization of SH groups in porin of bovine heart mitochondria. Porin cysteines are localized in the channel walls. Eur. J. Biochem. 202, 903–911. doi: 10.1111/j.1432-1033.1991.tb16450.x
De Pinto, V., Al Jamal, J. A., and Palmieri, F. (1993). Location of the dicyclohexylcarbodiimide-reactive glutamate residue in the bovine heart mitochondrial porin. J. Biol. Chem. 268, 12977–12982. doi: 10.1016/S0021-9258(18)31482-0
De Pinto, V., Benz, R., Caggese, C., and Palmieri, F. (1989a). Characterization of the mitochondrial porin from Drosophila melanogaster. Biochim. Biophys. Acta 987, 1–7. doi: 10.1016/0005-2736(89)90447-1
De Pinto, V., Benz, R., and Palmieri, F. (1989b). Interaction of non-classical detergents with the mitochondrial porin. A new purification procedure and characterization of the pore-forming unit. Eur. J. Biochem. 183, 179–187. doi: 10.1111/j.1432-1033.1989.tb14911.x
De Pinto, V., Ludwig, O., Krause, J., Benz, R., and Palmieri, F. (1987a). Porin pores of mitochondrial outer membranes from high and low eukaryotic cells: biochemical and biophysical characterization. Biochim. Biophys. Acta 894, 109–119.
De Pinto, V., Messina, A., Accardi, R., Aiello, R., Guarino, F., Tomasello, M. F., et al. (2003). New functions of an old protein: the eukaryotic porin or voltage dependent anion selective channel (VDAC). Ital. J. Biochem. 52, 17–24.
De Pinto, V. D., and Palmieri, F. (1992). Transmembrane arrangement of mitochondrial porin or voltage-dependent anion channel (VDAC). J. Bioenerg. Biomembr. 24, 21–26. doi: 10.1007/BF00769526
De Pinto, V., Prezioso, G., and Palmieri, F. (1987b). A simple and rapid method for the purification of the mitochondrial porin from mammalian tissues. Biochim. Biophys. Acta 905, 499–502. doi: 10.1016/0005-2736(87)90480-9
De Pinto, V., Prezioso, G., Thinnes, F., Link, T. A., and Palmieri, F. (1991b). Peptide-specific antibodies and proteases as probes of the transmembrane topology of the bovine heart mitochondrial porin. Biochemistry 30, 10191–10200. doi: 10.1021/bi00106a017
De Pinto, V., Reina, S., Gupta, A., Messina, A., and Mahalakshmi, R. (2016). Role of cysteines in mammalian VDAC isoforms’ function. Biochim. Biophys. Acta Bioenerg. 1857, 1219–1227. doi: 10.1016/j.bbabio.2016.02.020
De Pinto, V., Tomasello, F., Messina, A., Guarino, F., Benz, R., La Mendola, D., et al. (2007). Determination of the conformation of the human VDAC1 N-terminal peptide, a protein moiety essential for the functional properties of the pore. Chembiochem 8, 744–756. doi: 10.1002/cbic.200700009
De Pinto, V., Tommasino, M., Benz, R., and Palmieri, F. (1985). The 35 kDa DCCD-binding protein from pig heart mitochondria is the mitochondrial porin. Biochim. Biophys. Acta 813, 230–242. doi: 10.1016/0005-2736(85)90238-X
De Pinto, V., Zara, V., Benz, R., Gnoni, G. V., and Palmieri, F. (1991c). Characterization of pore-forming activity in liver mitochondria from Anguilla anguilla. Two porins in mitochondria? Biochim. Biophys. Acta 1061, 279–286. doi: 10.1016/0005-2736(91)90293-H
Degli, E. M. (2014). Bioenergetic evolution in proteobacteria and mitochondria. Genome Biol. Evol. 6, 3238–3251. doi: 10.1093/gbe/evu257
Di Giulio, M. (2011). The last universal common ancestor (LUCA) and the ancestors of archaea and bacteria were progenotes. J. Mol. Evol. 72, 119–126. doi: 10.1007/s00239-010-9407-2
Diederichs, K. A., Buchanan, S. K., and Botos, I. (2021). Building better barrels – β-barrel biogenesis and insertion in bacteria and mitochondria. J. Mol. Biol. 433:166894. doi: 10.1016/j.jmb.2021.166894
Diederichs, K. A., Ni, X., Rollauer, S. E., Botos, I., Tan, X., King, M. S., et al. (2020). Structural insight into mitochondrial β-barrel outer membrane protein biogenesis. Nat. Commun. 11:3290. doi: 10.1038/s41467-020-17144-1
Doring, C., and Colombini, M. (1984). On the nature of the molecular mechanism underlying the voltage dependence of the channel-forming protein, voltage-dependent anion-Selective Channel (VDAC). Biophys. J. 45, 44–46. doi: 10.1016/S0006-3495(84)84101-6
Drwesh, L., and Rapaport, D. (2020). Biogenesis pathways of α-helical mitochondrial outer membrane proteins. Biol. Chem. 401, 677–686. doi: 10.1515/hsz-2019-0440
Engelhardt, H., Meins, T., Poynor, M., Adams, V., Nussberger, S., Welte, W., et al. (2007). High-level expression, refolding and probing the natural fold of the human voltage-dependent anion channel isoforms I and II. J. Membr. Biol. 216, 93–105. doi: 10.1007/s00232-007-9038-8
Fiek, C., Benz, R., Roos, N., and Brdiczka, D. (1982). Evidence for identity between the hexokinase-binding protein and the mitochondrial porin in the outer membrane of rat liver mitochondria. Biochim. Biophys. Acta 688, 429–440. doi: 10.1016/0005-2736(82)90354-6
Fischer, K., Weber, A., Brink, S., Arbinger, B., Schünemann, D., Borchert, S., et al. (1994). Porins from plants. Molecular cloning and functional characterization of two new members of the porin family. J. Biol. Chem. 269, 25754–25760. doi: 10.1016/S0021-9258(18)47312-7
Forte, M., Adelsberger-Mangan, D., and Colombini, M. (1987a). Purification and characterization of the voltage-dependent anion channel from the outer mitochondrial membrane of yeast. J. Membr. Biol. 99, 65–72. doi: 10.1007/BF01870622
Forte, M., Guy, H. R., and Mannella, C. A. (1987b). Molecular genetics of the VDAC ion channel: structural model and sequence analysis. J. Bioenerg. Biomembr. 19, 341–350. doi: 10.1007/BF00768537
Freitag, H., Genchi, G., Benz, R., Palmieri, F., and Neupert, W. (1982a). Isolation of mitochondrial porin from Neurospora crassa. FEBS Lett. 145, 72–76. doi: 10.1016/0014-5793(82)81209-X
Freitag, H., Janes, M., and Neupert, W. (1982b). Biosynthesis of mitochondrial porin and insertion into the outer mitochondrial membrane of Neurospora crassa. Eur. J. Biochem. 126, 197–202. doi: 10.1111/j.1432-1033.1982.tb06766.x
Freitag, H., Neupert, W., and Benz, R. (1982c). Purification and characterisation of a pore protein of the outer mitochondrial membrane from Neurospora crassa. Eur. J. Biochem. 123, 629–636. doi: 10.1111/j.1432-1033.1982.tb06578.x
Garwood, R. J. (2012). Patterns in palaeontology: The first 3 billion years of evolution. Palaeontol. Online. 2, 1–14.
Gatliff, J., and Campanella, M. (2012). The 18 kDa translocator protein (TSPO): a new perspective in mitochondrial biology. Curr Mol Med. 12, 356–368. doi: 10.2174/1566524011207040356
Gattin, Z., Schneider, R., Laukat, Y., Giller, K., Maier, E., and Zweckstetter, M. (2015). Solid‐state NMR, electrophysiology and molecular dynamics characterization of human VDAC2. J. Biomol. NMR. 61, 311–320. doi: 10.1007/s10858‐014‐9876‐5
Gérard, E., Moreira, D., Philippot, P., Van Kranendonk, M. J., and López-García, P. (2009). Modern subsurface bacteria in pristine 2.7 Ga-old fossil stromatolite drillcore samples from the Fortescue Group, Western Australia. PLoS One 4:e5298. doi: 10.1371/journal.pone.0005298
Gellerich, F. N., Wagner, M., Kapischke, M., Wicker, U., and Brdiczka, D. (1993). Effect of macromolecules on the regulation of the mitochondrial outer membrane pore and the activity of adenylate kinase in the inter‐membrane space. Biochim Biophys Acta. 6, 217–227. doi: 10.1016/0005‐2728(93)90150‐e
Graham, B. H., and Craigen, W. J. (2005). Mitochondrial voltage-dependent anion channel gene family in Drosophila melanogaster: complex patterns of evolution, genomic organization, and developmental expression. Mol. Genet. Metab. 85, 308–317. doi: 10.1016/j.ymgme.2005.03.009
Grau-Bové, X., Sebé-Pedrós, A., and Ruiz-Trillo, I. (2015). The eukaryotic ancestor had a complex ubiquitin signaling system of archaeal origin. Mol. Biol. Evol. 32, 726–739. doi: 10.1093/molbev/msu334
Gray, M. W. (2012). Mitochondrial evolution. Cold Spring Harb. Perspect. Biol. 4:a011403. doi: 10.1101/cshperspect.a011403
Grevel, A., and Becker, T. (2020). Porins as helpers in mitochondrial protein translocation. Biol. Chem. 401, 699–708. doi: 10.1515/hsz-2019-0438
Grevel, A., Pfanner, N., and Becker, T. (2019). Coupling of import and assembly pathways in mitochondrial protein biogenesis. Biol. Chem. 401, 117–129. doi: 10.1515/hsz-2019-0310
Ha, H., Hajek, P., Bedwell, D. M., and Burrows, P. D. (1993). A mitochondrial porin cDNA predicts the existence of multiple human porins. J. Biol. Chem. 268, 12143–12149. doi: 10.1016/S0021-9258(19)50319-2
Hedges, S. B. (2002). The origin and evolution of model organisms. Nat. Rev. Genet. 3, 838–849. doi: 10.1038/nrg929
Heins, L., Mentzel, H., Schmid, A., Benz, R., and Schmitz, U. K. (1994). Biochemical, molecular, and functional characterization of porin isoforms from potato mitochondria. J. Biol. Chem. 269, 26402–26410. doi: 10.1016/S0021-9258(18)47208-0
Hiller, S., Garces, R. G., Malia, T. J., Orekhov, V. Y., Colombini, M., and Wagner, G. (2008). Solution structure of the integral human membrane protein VDAC-1 in detergent micelles. Science 321, 1206–1210. doi: 10.1126/science.1161302
Homblé, F., Krammer, E. M., and Prévost, M. (2012). Plant VDAC: facts and speculations. Biochim. Biophys. Acta 1818, 1486–1501. doi: 10.1016/j.bbamem.2011.11.028
Houstĕk, J., Svoboda, P., Kopecký, J., Kuzela, S., and Drahota, Z. (1981). Differentiation of dicyclohexylcarbodiimide reactive sites of the ATPase complex bovine heart mitochondria. Biochim. Biophys. Acta 634, 331–339. doi: 10.1016/0005-2728(81)90151-1
Jores, T., Klinger, A., Groß, L. E., Kawano, S., Flinner, N., Duchardt-Ferner, E., et al. (2016). Characterization of the targeting signal in mitochondrial β-barrel proteins. Nat. Commun. 7:12036. doi: 10.1038/ncomms12036
Katz, L. A. (2012). Origin and diversification of eukaryotes. Annu. Rev. Microbiol. 66, 411–427. doi: 10.1146/annurev-micro-090110-102808
Kayser, H., and Ellinger, P. (2001). Metabolism of diafenthiuron by microsomal oxidation: procide activation and inactivation as mechanisms contributing to selectivity. Pest Manage. Sci. 57, 975–980. doi: 10.1002/ps.360
Kayser, H., Kratzin, H. D., Thinnes, F. P., Götz, H., Schmidt, W. E., Eckart, K., et al. (1989). Zur Kenntnis der Porine des menschen. II. Charakterisierung und Primärstruktur eines 31-kDA-Porins aus menschlichen B-Lymphozyten (Porin 31HL) [identification of human porins. II. Characterization and primary structure of a 31-lDa porin from human B lymphocytes (Porin 31HL)]. Biol. Chem. Hoppe Seyler 370, 1265–1278.
Kleene, R., Pfanner, N., Pfaller, R., Link, T. A., Sebald, W., Neupert, W., et al. (1987). Mitochondrial porin of Neurospora crassa: cDNA cloning, in vitro expression and import into mitochondria. EMBO J. 6, 2627–2633. doi: 10.1002/j.1460-2075.1987.tb02553.x
Komarov, A. G., Graham, B. H., Craigen, W. J., and Colombini, M. (2004). The physiological properties of a novel family of VDAC-like proteins from Drosophila melanogaster. Biophys. J. 86, 152–162. doi: 10.1016/S0006-3495(04)74093-X
König, T., Kocsis, B., Mészáros, L., Nahm, K., Zoltán, S., and Horváth, I. (1977). Interaction of a synthetic polyanion with rat liver mitochondria. Biochim. Biophys. Acta 462, 380–389. doi: 10.1016/0005-2728(77)90136-0
König, T., Stipani, I., Horvàth, I., and Palmieri, F. (1982). Inhibition of mitochondrial substrate anion translocators by a synthetic amphipathic polyanion. J. Bioenerg. Biomembr. 14, 297–305. doi: 10.1007/BF00743059
Koonin, E. V. (2015). Origin of eukaryotes from within archaea, archaeal eukaryome and bursts of gene gain: eukaryogenesis just made easier? Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 370:20140333. doi: 10.1098/rstb.2014.0333
Koonin, E. V., Krupovic, M., Ishino, S., and Ishino, Y. (2020). The replication machinery of LUCA: common origin of DNA replication and transcription. BMC Biol. 18:61. doi: 10.1186/s12915-020-00800-9
Krammer, E. M., Saidani, H., Prévost, M., and Homblé, F. (2014). Origin of ion selectivity in Phaseolus coccineus mitochondrial VDAC. Mitochondrion 19, 206–213. doi: 10.1016/j.mito.2014.04.003
Kusano, T., Tateda, C., Berberich, T., and Takahashi, Y. (2009). Voltage-dependent anion channels: their roles in plant defense and cell death. Plant Cell Rep. 28, 1301–1308. doi: 10.1007/s00299-009-0741-z
Lee, S. M., Hoang, M. H., Han, H. J., Kim, H. S., Lee, K., Kim, K. E., et al. (2009). Pathogen inducible voltage-dependent anion channel (AtVDAC) isoforms are localized to mitochondria membrane in Arabidopsis. Mol. Cells 27, 321–327. doi: 10.1007/s10059-009-0041-z
Lindén, M., Gellerfors, P., and Nelson, B. D. (1982a). Purification of a protein having pore forming activity from the rat liver mitochondrial outer membrane. Biochem. J. 208, 77–82. doi: 10.1042/bj2080077
Lindén, M., Gellerfors, P., and Nelson, B. D. (1982b). Pore protein and the hexokinase-binding protein from the outer membrane of rat liver mitochondria are identical. FEBS Lett. 141, 189–192. doi: 10.1016/0014-5793(82)80044-6
Liu, M. Y., and Colombini, M. (1992). Regulation of mitochondrial respiration by controlling the permeability of the outer membrane through the mitochondrial channel, VDAC. Biochim. Biophys. Acta 1098, 255–260. doi: 10.1016/S0005-2728(05)80344-5
Lopes-Rodrigues, M., Matagne, A., Zanuy, D., Alemán, C., Perpète, E. A., and Michaux, C. (2020). Structural and functional characterization of Solanum tuberosum VDAC36. Proteins 88, 729–739. doi: 10.1002/prot.25861
Ludwig, O., Benz, R., and Schultz, J. E. (1989). Porin of paramecium mitochondria isolation, characterization and ion selectivity of the closed state. Biochim. Biophys. Acta 978, 319–327. doi: 10.1016/0005-2736(89)90131-4
Ludwig, O., De Pinto, V., Palmieri, F., and Benz, R. (1986). Pore formation by the mitochondrial porin of rat brain in lipid bilayer membranes. Biochim. Biophys. Acta 860, 268–276. doi: 10.1016/0005-2736(86)90523-7
Ludwig, O., Krause, J., Hay, R., and Benz, R. (1988). Purification and characterization of the pore forming protein of yeast mitochondrial outer membrane. Eur. Biophys. J. 15, 269–276. doi: 10.1007/BF00256477
Mannella, C. A. (1981). Structure of the outer mitochondrial membrane: analysis of X-ray diffraction from the plant membrane. Biochim. Biophys. Acta 645, 33–40. doi: 10.1016/0005-2736(81)90508-3
Mannella, C. A. (1987). Electron microscopy and image analysis of the mitochondrial outer membrane channel, VDAC. J. Bioenerg. Biomembr. 19, 329–340. doi: 10.1007/BF00768536
Mannella, C. A., Colombini, M., and Frank, J. (1983). Structural and functional evidence for multiple channel complexes in the outer membrane of Neurospora crassa mitochondria. Proc. Natl. Acad. Sci. U. S. A. 80, 2243–2247. doi: 10.1073/pnas.80.8.2243
Mannella, C. A., and Frank, J. (1984). Negative staining characteristics of arrays of mitochondrial pore protein: use of correspondence analysis to classify different staining patterns. Ultramicroscopy 13, 93–102. doi: 10.1016/0304-3991(84)90060-3
Mannella, C. A., Lederer, W. J., and Jafri, M. S. (2013). The connection between inner membrane topology and mitochondrial function. J. Mol. Cell. Cardiol. 62, 51–57. doi: 10.1016/j.yjmcc.2013.05.001
Margulis, L., Lopez Baluja, L., Awramik, S. M., and Sagan, D. (1986). Community living long before man: fossil and living microbial mats and early life. Sci. Total Environ. 56, 379–397.
Martijn, J., Vosseberg, J., Guy, L., Offre, P., and Ettema, T. J. G. (2018). Deep mitochondrial origin outside the sampled alphaproteobacteria. Nature 557, 101–105. doi: 10.1038/s41586-018-0059-5
McInerney, J. O. (2016). Evolution: A four billion year old metabolism. Nat. Microbiol. 1:16139. doi: 10.1038/nmicrobiol.2016.139
Mihara, K., Blobel, G., and Sato, R. (1982). In vitro synthesis and integration into mitochondria of porin, a major protein of the outer mitochondrial membrane of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A. 79, 7102–7106. doi: 10.1073/pnas.79.23.7102
Mihara, K., and Sato, R. (1985). Molecular cloning and sequencing of cDNA for yeast porin, an outer mitochondrial membrane protein: a search for targeting signal in the primary structure. EMBO J. 4, 769–774. doi: 10.1002/j.1460-2075.1985.tb03695.x
Miller, S. L. (1953). A production of amino acids Under possible primitive earth conditions. Science 117, 528–529. doi: 10.1126/science.117.3046.528
Mlayeh, L., Chatkaew, S., Léonetti, M., and Homblé, F. (2010). Modulation of plant mitochondrial VDAC by phytosterols. Biophys. J. 99, 2097–2106. doi: 10.1016/j.bpj.2010.07.067
Moitra, A., and Rapaport, D. (2021). The biogenesis process of VDAC – From early cytosolic events to its final membrane integration. Front. Physiol. 12:732742. doi: 10.3389/fphys.2021.732742
Montal, M., and Mueller, P. (1972). Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc. Natl. Acad. Sci. U. S. A. 69, 3561–3566. doi: 10.1073/pnas.69.12.3561
Najbauer, E. E., Becker, S., Giller, K., Zweckstetter, M., Lange, A., Steinem, C., et al. (2021). Structure, gating and interactions of the voltage-dependent anion channel. Eur. Biophys. J. 50, 159–172. doi: 10.1007/s00249-021-01515-7
Nakashima, R. A. (1989). Hexokinase-binding properties of the mitochondrial VDAC protein: inhibition by DCCD and location of putative DCCD-binding sites. J. Bioenerg. Biomembr. 21, 461–470. doi: 10.1007/BF00762518
Nakashima, R. A., Mangan, P. S., Colombini, M., and Pedersen, P. L. (1986). Hexokinase receptor complex in hepatoma mitochondria: evidence from N,N'-dicyclohexylcarbodiimide-labeling studies for the involvement of the pore-forming protein VDAC. Biochemistry 25, 1015–1021. doi: 10.1021/bi00353a010
Nowack, E. C. M., and Weber, A. P. M. (2018). Genomics-informed insights into endosymbiotic organelle evolution in photosynthetic eukaryotes. Annu. Rev. Plant Biol. 69, 51–84. doi: 10.1146/annurev-arplant-042817-040209
Nutman, A. P., Bennett, V. C., Friend, C. R., Van Kranendonk, M. J., and Chivas, A. R. (2016). Rapid emergence of life shown by discovery of 3,700-million-year-old microbial structures. Nature 537, 535–538. doi: 10.1038/nature19355
O’Malley, M. A., Leger, M. M., Wideman, J. G., and Ruiz-Trillo, I. (2019). Concepts of the last eukaryotic common ancestor. Nat. Ecol. Evol. 3, 338–344. doi: 10.1038/s41559-019-0796-3
Osinski, G. R., Cockell, C. S., Pontefract, A., and Sapers, H. M. (2020). The role of meteorite impacts in the origin of life. Astrobiology 20, 1121–1149. doi: 10.1089/ast.2019.2203
Peng, S., Blachly-Dyson, E., Forte, M., and Colombini, M. (1992). Large scale rearrangement of protein domains is associated with voltage gating of the VDAC channel. Biophys. J. 62, 123–131; discussion 131-5. doi: 10.1016/S0006-3495(92)81799-X
Pereira, J., and Lupas, A. N. (2018). The origin of mitochondria-specific outer membrane β-barrels from an ancestral bacterial fragment. Genome Biol. Evol. 10, 2759–2765. doi: 10.1093/gbe/evy216
Pfaller, R., Freitag, H., Harmey, M. A., Benz, R., and Neupert, W. (1985). A water-soluble form of porin from the mitochondrial outer membrane of Neurospora crassa. Properties and relationship to the biosynthetic precursor form. J. Biol. Chem. 260, 8188–8193. doi: 10.1016/S0021-9258(17)39580-7
Pinto, D., and Messina, A. (2004). “Gene family expression and multitopological localization of eukaryotic Porin/voltage dependent Anion-Selective Channel (VDAC): intracellular trafficking and alternative splicing,” in Structure and Function of Prokaryotic and Eukaryotic Porins. ed. R. Benz (Wiley-VCh: Weinheim), 309–337.
Popp, B., Court, D. A., Benz, R., Neupert, W., and Lill, R. (1996). The role of the N and C termini of recombinant Neurospora mitochondrial porin in channel formation and voltage-dependent gating. J. Biol. Chem. 271, 13593–13599. doi: 10.1074/jbc.271.23.13593
Popp, B., Gebauer, S., Fischer, K., Flügge, U. I., and Benz, R. (1997). Study of structure and function of recombinant pea root plastid porin by biophysical methods. Biochemistry 36, 2844–2852. doi: 10.1021/bi961745d
Popp, B., Schmid, A., and Benz, R. (1995). Role of sterols in the functional reconstitution of water-soluble mitochondrial porins from different organisms. Biochemistry 34, 3352–3361. doi: 10.1021/bi00010a026
Queralt-Martín, M., Bergdoll, L., Teijido, O., Munshi, N., Jacobs, D., Kuszak, A. J., et al. (2020). A lower affinity to cytosolic proteins reveals VDAC3 isoform-specific role in mitochondrial biology. J. Gen. Physiol. 152:e201912501. doi: 10.1085/jgp.201912501
Roos, N., Benz, R., and Brdiczka, D. (1982). Identification and characterization of the pore-forming protein in the outer membrane of rat liver mitochondria. Biochim. Biophys. Acta 686, 204–214. doi: 10.1016/0005-2736(82)90114-6
Runke, G., Maier, E., O'Neil, J. D., Benz, R., and Court, D. A. (2000). Functional characterization of the conserved “GLK” motif in mitochondrial porin from Neurospora crassa. J. Bioenerg. Biomembr. 32, 563–570. doi: 10.1023/A:1005618510502
Runke, G., Maier, E., Summers, W. D., Bay, D. C., Benz, R., and Court, D. A. (2006). Deletion variants of Neurospora mitochondrial porin: electrophysiological and spectroscopic analysis. Biophys. 90, 3155–3164. doi: 10.1529/biophysj.105.072520
Saidani, H., Léonetti, M., Kmita, H., and Homblé, F. (2021). The open state selectivity of the bean seed VDAC depends on Stigmasterol and ion concentration. Int. J. Mol. Sci. 22:3034. doi: 10.3390/ijms22063034
Saletti, R., Reina, S., Pittalà, M. G. G., Magrì, A., Cunsolo, V., Foti, S., et al. (1859). Post-translational modifications of VDAC1 and VDAC2 cysteines from rat liver mitochondria. Biochim. Biophys. Acta Bioenerg. 2018, 806–816.
Sampson, M. J., Lovell, R. S., and Craigen, W. J. (1996). Isolation, characterization, and mapping of two mouse mitochondrial voltage-dependent anion channel isoforms. Genomics 33, 283–288. doi: 10.1006/geno.1996.0193
Sasselov, D. D., Grotzinger, J. P., and Sutherland, J. D. (2020). The origin of life as a planetary phenomenon. Sci. Adv. 6:eaax3419. doi: 10.1126/sciadv.aax3419
Schein, S. J., Colombini, M., and Finkelstein, A. (1976). Reconstitution in planar lipid bilayers of a voltage-dependent anion-selective channel obtained from paramecium mitochondria. J. Membr. Biol. 30, 99–120. doi: 10.1007/BF01869662
Schmid, A., Krömer, S., Heldt, H. W., and Benz, R. (1992). Identification of two general diffusion channels in the outer membrane of pea mitochondria. Biochim. Biophys. Acta 1112, 174–180. doi: 10.1016/0005-2736(92)90389-4
Shimizu, H., Huber, S., Langenbacher, A. D., Crisman, L., Huang, J., Wang, K., et al. (2021). Glutamate 73 promotes anti-arrhythmic effects of voltage-dependent anion channel through regulation of mitochondrial Ca2+ uptake. Front. Physiol. 12:724828. doi: 10.3389/fphys.2021.724828
Shoshan-Barmatz, V., De Pinto, V., Zweckstetter, M., Raviv, Z., Keinan, N., and Arbel, N. (2010). VDAC, a multi-functional mitochondrial protein regulating cell life and death. Mol. Asp. Med. 31, 227–285. doi: 10.1016/j.mam.2010.03.002
Shoshan-Barmatz, V., Israelson, A., Brdiczka, D., and Sheu, S. S. (2006). The voltage-dependent anion channel (VDAC): function in intracellular signalling, cell life and cell death. Curr. Pharmacol. Des. 12, 2249–2270. doi: 10.2174/138161206777585111
Shoshan-Barmatz, V., Shteinfer-Kuzmine, A., and Verma, A. (2020). VDAC1 at the intersection of cell metabolism, apoptosis, and diseases. Biomol. Ther. 10:1485. doi: 10.3390/biom10111485
Smack, D. P., and Colombini, M. (1985). Voltage-dependent channels found in the membrane fraction of corn mitochondria. Plant Physiol. 79, 1094–1097. doi: 10.1104/pp.79.4.1094
Takeda, H., Tsutsumi, A., Nishizawa, T., Lindau, C., Busto, J. V., Wenz, L. S., et al. (2021). Mitochondrial sorting and assembly machinery operates by β-barrel switching. Nature 590, 163–169. doi: 10.1038/s41586-020-03113-7
Takeuchi, Y., Furukawa, Y., Kobayashi, T., Sekine, T., Terada, N., and Kakegawa, T. (2020). Impact-induced amino acid formation on Hadean earth and Noachian Mars. Sci. Rep. 10:9220. doi: 10.1038/s41598-020-66112-8
Tarasenko, T. A., Klimenko, E. S., Tarasenko, V. I., Koulintchenko, M. V., Dietrich, A., Weber-Lotfi, F., et al. (2021). Plant mitochondria import DNA via alternative membrane complexes involving various VDAC isoforms. Mitochondrion 60, 43–58. doi: 10.1016/j.mito.2021.07.006
Tateda, C., Kusano, T., and Takahashi, Y. (2012). The Arabidopsis voltage-dependent anion channel 2 is required for plant growth. Plant Signal. Behav. 7, 31–33. doi: 10.4161/psb.7.1.18394
Tateda, C., Watanabe, K., Kusano, T., and Takahashi, Y. (2011). Molecular and genetic characterization of the gene family encoding the voltage-dependent anion channel in Arabidopsis. J. Exp. Bot. 62, 4773–4785. doi: 10.1093/jxb/err113
Theobald, D. L. (2010). A formal test of the theory of universal common ancestry. Nature 465, 219–222. doi: 10.1038/nature09014
Troll, H., Malchow, D., Müller-Taubenberger, A., Humbel, B., Lottspeich, F., Ecke, M., et al. (1992). Purification, functional characterization, and cDNA sequencing of mitochondrial porin from Dictyostelium discoideum. J. Biol. Chem. 267, 21072–21079. doi: 10.1016/S0021-9258(19)36799-7
Ujwal, R., Cascio, D., Colletier, J. P., Faham, S., Zhang, J., Toro, L., et al. (2008). The crystal structure of mouse VDAC1 at 2.3 A resolution reveals mechanistic insights into metabolite gating. Proc. Natl. Acad. Sci. U. S. A. 105, 17742–17747. doi: 10.1073/pnas.0809634105
van der Laan, M., Horvath, S. E., and Pfanner, N. (2016). Mitochondrial contact site and cristae organizing system. Curr. Opin. Cell Biol. 41, 33–42. doi: 10.1016/j.ceb.2016.03.013
Wandrey, M., Trevaskis, B., Brewin, N., and Udvardi, M. K. (2004). Molecular and cell biology of a family of voltage-dependent anion channel porins in Lotus japonicus. Plant Physiol. 134, 182–193. doi: 10.1104/pp.103.031484
Weiss, M. C., Sousa, F. L., Mrnjavac, N., Neukirchen, S., Roettger, M., Nelson-Sathi, S., et al. (2016). The physiology and habitat of the last universal common ancestor. Nat. Microbiol. 1:16116. doi: 10.1038/nmicrobiol.2016.116
Wiesner, P., Popp, B., Schmid, A., Benz, R., and Kayser, H. (1996). Isolation of mitochondrial porin of the fly Protophormia: porin modification by the pesticide CGA 140'408 studied in lipid bilayer membranes. Biochim. Biophys. Acta 1282, 216–224. doi: 10.1016/0005-2736(96)00059-4
Young, M. J., Bay, D. C., Hausner, G., and Court, D. A. (2007). The evolutionary history of mitochondrial porins. BMC Evol. Biol. 7:31. doi: 10.1186/1471-2148-7-31
Zachariae, U., Schneider, R., Briones, R., Gattin, Z., Demers, J. P., Giller, K., et al. (2012). β-Barrel mobility underlies closure of the voltage-dependent anion channel. Structure 20, 1540–1549. doi: 10.1016/j.str.2012.06.015
Zalman, L. S., Nikaido, H., and Kagawa, Y. (1980). Mitochondrial outer membrane contains a protein producing nonspecific diffusion channels. J. Biol. Chem. 255, 1771–1774. doi: 10.1016/S0021-9258(19)85942-2
Keywords: mitochondria, eukaryotic pore, VDAC, single channel, porin, electrophysiology, evolution
Citation: Benz R (2021) Historical Perspective of Pore-Forming Activity Studies of Voltage-Dependent Anion Channel (Eukaryotic or Mitochondrial Porin) Since Its Discovery in the 70th of the Last Century. Front. Physiol. 12:734226. doi: 10.3389/fphys.2021.734226
Edited by:
Radhakrishnan Mahalakshmi, Indian Institute of Science Education and Research, IndiaReviewed by:
Ildikò Szabò, University of Padua, ItalyHarpreet Singh, The Ohio State University, United States
Copyright © 2021 Benz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roland Benz, r.benz@jacobs-university.de
 Roland Benz
Roland Benz