- 1Department of Physiology, University of Alberta, Edmonton, AB, Canada
- 2Department of Obstetrics and Gynecology, University of Alberta, Edmonton, AB, Canada
- 3Women and Children’s Health Research Institute, University of Alberta, Edmonton, AB, Canada
Advanced maternal age (≥35 years old) increases the risk of pregnancy complications such as preeclampsia and fetal growth restriction. We previously demonstrated vascular dysfunction and abnormal pregnancy outcomes in a rat model of advanced maternal age. However, vascular adaptations to pregnancy in aging were not studied. We hypothesize that advanced maternal age is associated with a more vasoconstrictive phenotype due to reduced nitric oxide (NO) and increased activity of matrix metalloproteinases (MMPs), contributing to impaired vascular adaptations to pregnancy. A rat model of advanced maternal age was used: young (4 months) and aged (9.5 months; ∼35 years in humans) non-pregnant and pregnant rats. On gestational day 20 (term = 22 days; non-pregnant rats were aged-matched), blood pressure and heart rate were measured (tail cuff plethysmography) and vascular function was assessed in mesenteric arteries (wire myography). Endothelium-dependent relaxation to methylcholine (MCh) was assessed in the presence/absence of nitric oxide synthase inhibitor (L-NAME), or inhibitors of endothelium-dependent hyperpolarization (EDH; apamin and TRAM-34). Vasoconstriction responses to big endothelin-1 (bigET-1), in the presence/absence of MMPs-inhibitor (GM6001) or endothelin converting enzyme (ECE-1) inhibitor (CGS35066), in addition, ET-1 responsiveness, were measured. Blood pressure was elevated only in aged non-pregnant rats (p < 0.001) compared to all other groups. MCh responses were not different, however, L-NAME decreased maximum vasodilation in young (p < 0.01) and aged pregnant rats (p < 0.001), and decreased MCh sensitivity in young non-pregnant rats (p < 0.01), without effects in aged non-pregnant rats. EDH contribution to relaxation was similar in young non-pregnant, and aged non-pregnant and pregnant rats, while EDH-mediated relaxation was absent in young pregnant rats (p < 0.001). BigET-1 responses were enhanced in aged non-pregnant (p < 0.01) and pregnant rats (p < 0.05). No significant changes in bigET-1 conversion occurred in the presence of MMP-inhibitor, whereas ECE-1 inhibition reduced bigET-1 constriction in aged rats (p < 0.01). No differences in ET-1 sensitivity were observed. In conclusion, contrary to our hypothesis, reduced blood pressure, and an increased EDH-dependent contribution to vasodilation suggest a compensatory mechanism that may reflect beneficial adaptations in these aged rats that were able to maintain pregnancy. These data increase our understanding of how the vascular adaptive pathways in pregnancy compensate for advanced maternal age.
Introduction
Advanced maternal age is defined as maternal age ≥35 years at the time of delivery. The age at which women deliver their first child has been increasing steadily in North America, accounting for 14–18% of total live births from women aged 35 years (Garner and Bushnik, 2008; Martin et al., 2009; Canada, 2012). Studies have shown advanced maternal age increases the risk of pregnancy-related complications such as fetal growth restriction (FGR), preeclampsia, gestational diabetes, hypertension, small for gestational age infants, preterm birth, and stillbirth (Yoon et al., 1996; Salihu et al., 2008; Lamminpaa et al., 2012; Ludford et al., 2012; Kenny et al., 2013; Khalil et al., 2013). A large contemporary cohort study in the United Kingdom revealed an 18.2% prevalence of advanced maternal age (Kenny et al., 2013). In addition, a population based-cohort study in South Australia showed that women of advanced age were prone to have adverse maternal perinatal outcomes (Ludford et al., 2012). These studies clearly delineate that advanced maternal age is becoming a global health challenge and women of advanced age represent a very important and yet understudied population of pregnant women.
Healthy maternal vascular adaptations to pregnancy play an essential role in normal fetal growth and development. To achieve this, several hemodynamic changes occur, such as increased cardiac output, increased heart rate, and increased blood volume, while there is a decrease in blood pressure in early- to mid-gestation due to a reduced systemic vascular resistance during pregnancy (Wang et al., 2015; Donato et al., 2018; Ma et al., 2020). Though the mechanisms of this reduced peripheral resistance are not completely understood, multiple studies have shown that during pregnancy the bioavailability of nitric oxide (NO), a potent endothelium-derived vasodilator, is increased (Dorup et al., 1999; Valdes et al., 2009; Boeldt and Bird, 2017). In addition, vasodilation of small resistance arteries (mesenteric arteries) during pregnancy is also mediated by other factors, such as endothelium-derived hyperpolarization (EDH) (Gerber et al., 1998; Cooke and Davidge, 2003; Mandala et al., 2012). Though EDH is distinct from NO, it has been shown that if NO-mediated relaxation is decreased, EDH may compensate for endothelium-dependent relaxation (Takaki et al., 2008). Moreover, studies have shown upregulation of EDH in normal pregnancy (in resistance-sized myometrial arteries and small subcutaneous arteries), that may be absent in pregnancy-related complication such as preeclampsia (Kenny et al., 2002; Luksha et al., 2004).
In women of advanced maternal age, normal pregnancy-induced vascular adaptations may be impaired, leading to poor pregnancy outcomes and altered vascular function. We have previously demonstrated abnormal pregnancies in aged rats, with reduced capacity to sustain a pregnancy, adverse pregnancy outcomes, and altered vascular function with increased active myogenic responses in both uterine and mesenteric arteries from aged pregnant rats (Care et al., 2015). The maternal uterine arteries undergo significant vascular remodeling during gestation to support the developing fetus and poor vascular adaptations in the uterine vessels have been associated with common pregnancy related complications, including intrauterine growth restriction and preeclampsia (Magness et al., 1996, 1997; Sladek et al., 1997; Osol and Mandala, 2009; Osol and Moore, 2014). Similarly, an impaired adaptation response to pregnancy (hormonal, immunological, and cardiovascular dysfunction) and postnatal growth restriction was shown using a vervet monkey model of advanced maternal age (Plant et al., 2020). Further, in mice, impaired utero-placental vascular function and placental dysfunction were proposed as potential mechanisms for the increased susceptibility to FGR and stillbirth associated with advanced maternal age (Lean et al., 2017; Napso et al., 2019). Overall, these studies suggest that pregnancy-induced vascular adaptations may be suboptimal in advanced maternal age pregnancies, eventually increasing the risk of pregnancy-related complications. However, despite the fact that pregnancies at advanced maternal age are considered high-risk clinically, very little research has focused on understanding the systemic vascular pathways that may be altered in advanced maternal age, leading to abnormal pregnancy adaptations and poor pregnancy outcomes for these women and their children (Ludford et al., 2012; Kenny et al., 2013; Care et al., 2015; Plant et al., 2020).
Aging itself is an independent risk factor for cardiovascular disease (CVD) (Shirasaki et al., 1986; Brandes et al., 2005; Kruger-Genge et al., 2019). The most noticeable characteristics are altered structural and functional properties of the endothelium and vascular smooth muscle cells leading to vascular remodeling, increased vascular stiffness, and endothelial dysfunction (Harvey et al., 2015; Xu et al., 2017; Dong et al., 2019). To maintain vascular homeostasis, there is a fine balance between vasodilator (NO) and vasoconstrictor agents (e.g., endothelin-1 (ET-1)) (Cardillo et al., 2000; Versari et al., 2009); and although the pathophysiological mechanisms leading to endothelial dysfunction are likely multifactorial, a hallmark of endothelial dysfunction in the aging vasculature was shown to be reduced bioavailability of NO and enhanced reactivity of ET-1 (Boss and Seegmiller, 1981; Toda, 2012; Xu et al., 2017; Dong et al., 2019). ET-1 is a potent vasoconstrictor polypeptide and has the capacity to induce vascular remodeling, thus ET-1 signaling is believed to be one of the important contributors to the progression of vascular dysfunction in aging and cardiovascular disease (Bohm and Pernow, 2007; Ergul, 2011). In addition, studies have also shown a significant increase in circulating levels of ET-1 in women with pregnancy-related complications (e.g., preeclampsia and FGR) compared with normal pregnant women (Bernardi et al., 2008; Dieber-Rotheneder et al., 2012). The vasoactive peptide ET-1 is synthesized from its precursor big endothelin-1 (bigET-1), and subsequently cleaved by several enzymes, such as matrix metalloproteases (MMPs) (Abdalvand et al., 2013), endothelin converting enzymes (ECE-1) (Parnot et al., 1997; Kuruppu and Smith, 2012), chymase (Tirapelli et al., 2006), and neutral endopeptidases (Ferro et al., 1998). Studies have demonstrated that MMPs and ECE-1 play essential roles in the processing of bigET-1 to ET-1 under various pathological conditions such as aging (Lekontseva et al., 2010), preeclampsia (Ajne et al., 2003; Abdalvand et al., 2013), and hypoxia (He et al., 2007). In addition, MMPs also maintain the stability of the extracellular matrix by degrading collagen, elastin, and other extracellular proteins as part of the normal physiological and pathological processes (Chen et al., 2013). Although MMPs play a significant role during healthy pregnancies (e.g., in trophoblast invasion of the uterus) (Cohen et al., 2012), several studies showed that with advancing age, dysregulation of MMPs activity may contribute to endothelial dysfunction (Jackson and McArdle, 2011; Donato et al., 2018; Liguori et al., 2018). However, the role of MMPs in vascular adaptations to pregnancy with advanced maternal age is not known.
Both human and animal studies have shown the importance of systemic vascular adaptations to pregnancy (Veerareddy et al., 2002; Cooke and Davidge, 2003; Barry and Anthony, 2008; Amburgey et al., 2010; Kahveci et al., 2018; Albrecht et al., 2021). However, the impact of age-related vascular impairments during pregnancy, such as altered endothelium-dependent vasodilation (reduced NO bioavailability) and increased endothelium-derived contracting factors (e.g., ET-1) due to their processing enzymes (such as MMPs) remains elusive. Thus, the focus of the current study is to increase our understanding of how these vascular pathways impact the systemic vascular adaptations to pregnancy in an established rat model of advanced maternal age. We used mesenteric arteries as they are a systemic, resistance-sized artery that plays an important role in the control of blood pressure (Christensen and Mulvany, 1993). We hypothesize that maternal aging impairs systemic vascular adaptations to pregnancy, due to a more vasoconstrictive phenotype associated with reduced NO contribution and increased activity of MMPs in the aging vasculature.
Materials and Methods
Ethical Approval
All experimental procedures received prior approval by the University of Alberta Health Sciences Animal Policy and Welfare Committee, in accordance with the guidelines of the Canadian Council on Animal Care and the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (AUP #3693).
Animal Model and Experimental Design
Female and male (for breeding) Sprague Dawley (SD) rats were purchased at 3 months of age from Charles River Canada (Saint-Constant, QC, Canada). The rats were housed at an ambient temperature of 22 ± 1°C and a 10:14 h light: dark cycle. All rats were allowed at least 1 week of acclimatization after arrival. We used a rat model of advanced maternal age previously established and characterized in our laboratory (Care et al., 2015; Cooke et al., 2018; Shah et al., 2018). Briefly, aged non-pregnant and pregnant rats were 9.5–10 months of age, which corresponds to ≈35 years of age in humans (considering milestones: weaning, skeletal maturity, sexual maturity, and reproductive senescence) (Quinn, 2005); whereas young non-pregnant and pregnant rats were 3–4 months of age (equivalent to early reproductive maturity in humans) (Quinn, 2005; Cooke et al., 2018). Young rats had ad libitum access to standard rat chow, while aging rats were fed a restricted diet of six pellets per day for caloric and nutrient intake based on National Research Council recommendations, to prevent age-related obesity as a confounding factor (National Research Council (US) Subcommittee on Laboratory Animal Nutrition, 1995). Young and aged female rats were mated with young male rats (aged 3–5 months), and once pregnancy was confirmed by the presence of sperm in a vaginal smear (designated as gestational day GD 0), all rats were fed an ad libitum standard chow diet (Care et al., 2015; Cooke et al., 2018). On GD20 (term = 22 days), blood pressure was assessed, rats were anesthetized using isoflurane and euthanized by exsanguination via cardiac puncture. The mesenteric arcade was immediately excised and placed in ice-cold HEPES-buffered physiological saline solution [PSS; composition (in mM): 10 HEPES, 5.5 glucose, 1.56 CaCl2, 4.7 KCl, 142 NaCl, 1.17 MgSO4, 1.18 KH2PO4, and pH 7.4], after which the mesenteric arteries were isolated for ex vivo vascular function and Western blot analysis.
Blood Pressure and Heart Rate Measurements
Blood pressure and heart rate were measured before euthanasia on GD20 by tail-cuff plethysmography (CODA High Throughput System, Kent Scientific, Torrington, CT, United States). All rats were subjected to a 1-week training period (i.e., to get used to the nose cone holders and occlusion tail cuff) for acclimatization to the system before pregnancy. To obtain the cardiovascular parameters, rats were placed in the nose cone holders for 20 min to warm up (tail skin surface temperature ∼30°C) after which at least 10 consecutive blood pressure measurements (systolic, diastolic, and mean arterial pressure (MAP)) and heart rate measurements were recorded and averaged for each rat (Cooke et al., 2018).
Mesenteric Artery Vascular Function by Wire Myography
Vascular function was assessed ex vivo in isolated systemic mesenteric (small resistance) arteries using wire myography. Second-order mesenteric arteries (150–250 μm) were isolated and cleaned from the perivascular fat in ice-cold PSS and cut into 2 mm pieces. The 2 mm segments of mesenteric artery were mounted onto a wire myograph system (620M DMT, Copenhagen, Denmark) using 40 μm tungsten wires. Isomeric tension of the vessels was recorded using LabChart software (AD Instruments; Colorado Springs, United States). All vessels were normalized through a series of stepwise increases in diameter set their diameter to their optimal resting tension: 0.8 × IC100; 13.3 kPa (the internal circumference equivalent to a transmural pressure of 100 mmHg) (Davidge et al., 1993). After normalization the vessels were exposed to the first wake up dose of phenylephrine (10 μM; Sigma-Aldrich, St. Louis, MO, United States) for 5 min. After washing with PSS thrice and 10 min rest, the vessels were exposed to a second wake up dose of phenylephrine followed by a single dose of methylcholine (MCh; 3 μM; Sigma-Aldrich) to confirm endothelial cell function. After 30 min of rest, vascular responses to MCh were assessed using a cumulative concentration response curve (CCRCs; 0.003 to 3 M MCh) after pre-constriction with the EC80 concentration (3 μM; the mean effective concentration that produces 80% of the maximal response) of phenylephrine. To assess relevant pathways involved in endothelium-dependent vasodilation, the CCRCs to MCh were performed in the absence or presence (30 min pre-incubation prior to phenylephrine EC80 dose) of the following specific inhibitors: NO synthase (NOS) was inhibited with the pan NOS inhibitor N(G)-nitro-L-arginine methyl ester hydrochloride (L-NAME; 100 μM; Sigma-Aldrich) and EDH-induced vasodilation was inhibited with a combination of apamin (100 nM) (Sigma-Aldrich) which blocks small-conductance Ca2+-activated potassium channels (SK), and 1-(2-chlorophenyl)diphenylmethyl-1H-pyrazole (TRAM-34; 10 μM; Sigma-Aldrich), an intermediate-conductance Ca2+-activated potassium channel (IK) inhibitor. In addition, contribution of the myoendothelial gap junctions (MEGJs) to EDH responses was assessed with 18 α-glycyrrhetinic acid (3 μM), which inhibits MEGJs. Simultaneously, a CCRC to the NO donor sodium nitroprusside (0.003 to 2 M SNP; Sigma-Aldrich) was conducted in one of the baths to assess endothelium-independent relaxation. Simultaneously, in separate myography baths, vasoconstriction responses to big endothelin-1 (bigET-1) and endothelin-1 (ET-1) were assessed, using CCRCs to ET-1 (1–200 nM; Sigma-Aldrich) or bigET-1 (3–310 nM; AnaSpec, Fremont, United States) to assess the capacity of the vessel to convert bigET-1 to ET-1. Constriction responses to bigET-1 were assessed in the absence or presence of inhibitors of the bigET-1 converting enzymes: GM6001 (30 μM; Calbiochem), a broad spectrum MMPs inhibitor; CGS35066 (25 μM; Tocris Bioscience, Toronto, Canada), a selective endothelin converting enzyme (ECE-1) inhibitor; chymostatin (100 μM; Sigma-Aldrich), a chymase inhibitor; and DL-Thiorphan (25 μM; Calbiochem), a selective inhibitor of neutral endopeptidase. Finally, all vessels were washed 4 times with PSS, allowed to rest for 15 min, and each bath was exposed to a 124 mmol/L potassium chloride solution (high KCl buffer; containing in mM: 10 HEPES, 24 NaCl, 124 KCl, 2.4 MgSO4, 4.9 CaCl2, 1.18 KH2PO4, and 5.5 glucose; pH 7.4) to assess maximum non-receptor-mediated smooth muscle vasoconstriction responses. All data [maximum relaxation responses to MCh (Emax), the negative log of the mean effective concentration that produces 50% of the maximal response (pEC50) as a measure of sensitivity to the vasodilator/vasoconstrictor, and the area under the cure (AUC)], were analyzed using GraphPad Prism 9 (GraphPad Software, San Diego, United States).
Expression of eNOS and ECE-1 Using Western Blot Analysis
Mesenteric arteries were isolated, cleaned from perivascular fat and homogenized using lysis buffer [concentration in mM: 20 Tris (pH 7.4), 10 sodium pyrophosphates tetrabasic, 100 sodium, 5 EDTA, and 9 fluoride with 1% Nonidet P-40] containing protease inhibitor cocktail (Thermo Fisher Scientific), phosphatase inhibitor (2 mM sodium orthovanadate, Sigma) and 1 mM Phenylmethylsulfonyl fluoride (PMSF; Fluka BioChemika). Total protein concentration of the samples was determined using bicinchoninic acid assay (Pierce, Rockford, IL, United States). Mesenteric artery tissue homogenates (50 μg of protein) were loaded and separated on 7% SDS-polyacrylamide gels for assessment of endothelial nitric oxide synthase (eNOS) and ECE-1 expression. Gels were then transferred to a nitrocellulose membrane (100V, 1.5 h; 0.2 μm, Bio-Rad), after which the membrane was stained with LI-COR Revert 700 Total Protein Stain and imaged using the LI-COR Odyssey system. Following reversal of the total protein staining, membranes were incubated with BlockOut®-Universal Blocking Buffer (Rockland, PA, United States) for 1 h at room temperature to prevent non-specific binding of the antibodies. Membranes were then incubated overnight at 4°C with primary antibodies for phospho-eNOS (Ser1177) (1:500 rabbit polyclonal, Cell signaling technology), total eNOS (1:1000 mouse monoclonal, Thermo Fisher Scientific) and ECE-1 (1:500 mouse monoclonal, Santa Cruz Biotechnology) in phosphate buffered saline with Tween [PBST; NaCl: 137 mM, KCl: 2.7 mM, Na2HPO4: 10 mM, KH2PO4: 1.8 mM, and Tween® 20 detergent: 0.1% (w/v); Thermo Fisher Scientific]. The next day, membranes were incubated with the appropriate secondary antibody: IRDye donkey anti-rabbit IgG (for phospho-eNOS) and IRDye donkey anti-mouse IgM (for total eNOS and ECE-1) at 1:10,000 in PBST buffer (LI-COR Biosciences, Lincoln, NE, United States). Finally, blots were visualized with a LI-COR Odyssey Bioimager (LI-COR Biosciences) and quantified using Image Studio Lite software (LI-COR Biosciences). All data are expressed as percent change compared to the corresponding control (young non-pregnant rats) for each of the separate blots.
Statistical Analyses
Data were plotted using GraphPad Prism 9 (GraphPad Software, San Diego, United States) and presented as mean ± SEM, and were analyzed using a two-way ANOVA with Sidak’s post hoc test for multiple comparisons. P-values < 0.05 were considered statistically significant.
Results
Blood Pressure and Heart Rate Assessment in Young Compared to Aged Non-pregnant and Pregnant Rats
MAP was increased in aged non-pregnant rats compared to aged pregnant, young non-pregnant, and pregnant rats (Figure 1). Pregnancy reduced the MAP in aged pregnant rats compared to aged non-pregnant rats to pressures similar to those of the young pregnant rats. However, no changes in MAP were observed between young non-pregnant and pregnant rats (Figure 1). A similar pattern of changes was observed with the systolic and diastolic blood pressures. Moreover, heart rates tended to be higher in young pregnant rats compare to young non-pregnant rats, while this effect was not observed between the aged non-pregnant and pregnant rats (Supplementary Table 1).
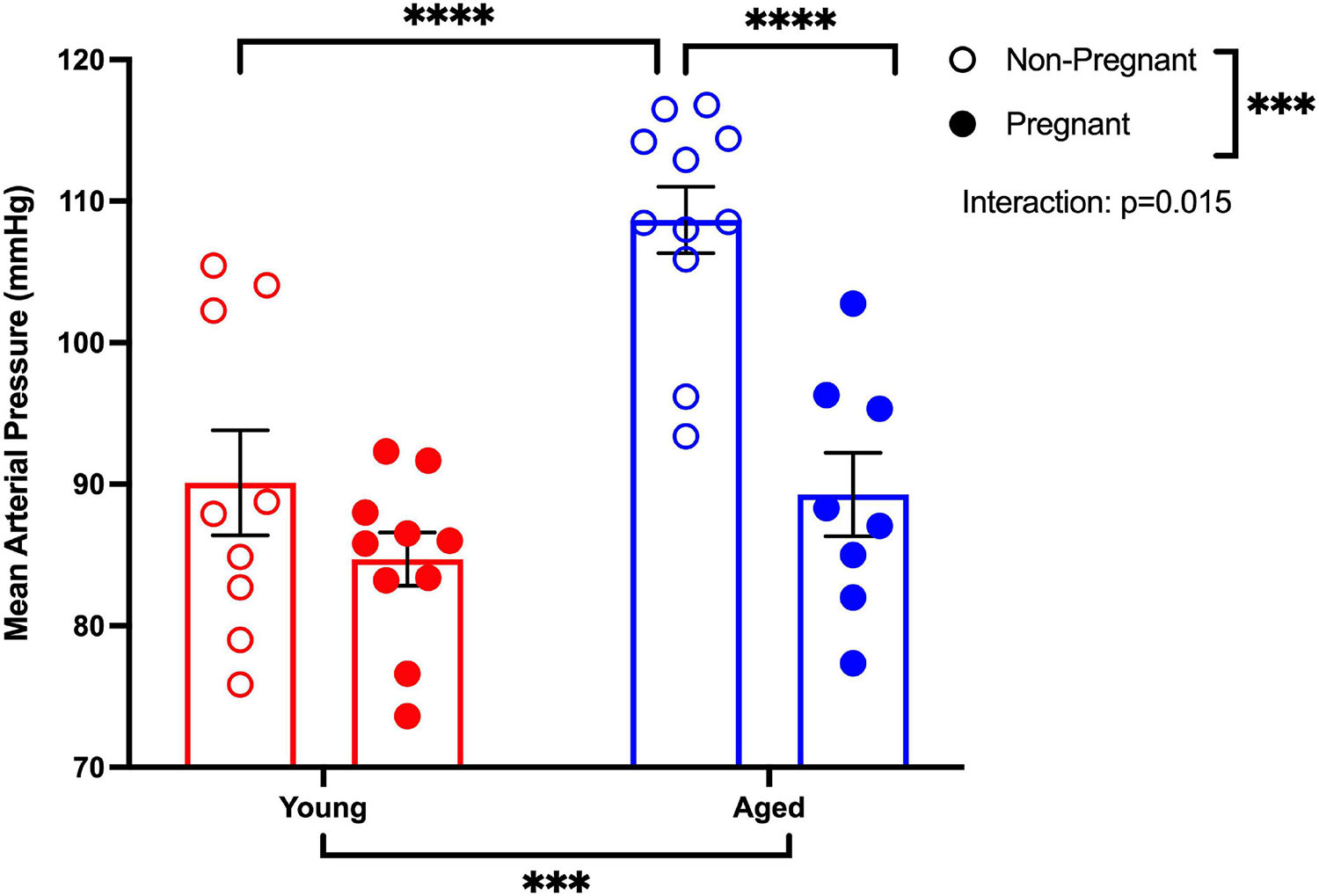
Figure 1. Pregnancy reduced the mean arterial pressure in aged pregnant rats. Mean arterial blood pressure recordings (MAP) of young (3–4 months; in red) and aged (9–9.5 months; in blue) pregnant (on gestational day 20; closed circles) and non-pregnant (age-matched; open circles) rats. Data presented as mean ± SEM; analyzed by two-way ANOVA with Sidak’s multiple comparisons post hoc test; ∗∗∗p < 0.001, ****p < 0.0001; n = 9–10/group.
Vasodilation Pathways
Endothelium-Dependent Vasodilation and NO Contribution to Vasodilation
Methacholine (MCh)-induced vasodilation responses were not different in mesenteric arteries from young or aged, non-pregnant or pregnant rats (Figures 2A,B and Supplementary Table 2). However, pre-incubation with L-NAME (to assess NO contribution) decreased the Emax to MCh in arteries from both young pregnant and aged pregnant rats, while there was no effect of L-NAME on MCh Emax in young and aged non-pregnant rats (Figures 3A,B,D,E). Moreover, L-NAME decreased the sensitivity to MCh (pEC50) only in young non-pregnant (Figure 3C) and aged pregnant rats (Figure 3F). Similarly, a decrease in the AUC was observed after pre-incubation with L-NAME in young non-pregnant, pregnant, and aged pregnant rats, but not in aged non-pregnant rats (Supplementary Table 3). We used Western blot to quantify eNOS protein levels/phosphorylation (at Ser1177) mesenteric arteries. There were no significant differences (p = 0.07) in phosphorylation of eNOS between young non-pregnant and pregnant rats, however, phosphorylation of eNOS was significantly increased in vessels from aged pregnant rats compared to aged non-pregnant rats (Figure 4). Moreover, no changes in total eNOS protein expression were observed between the groups (Supplementary Figure 1).
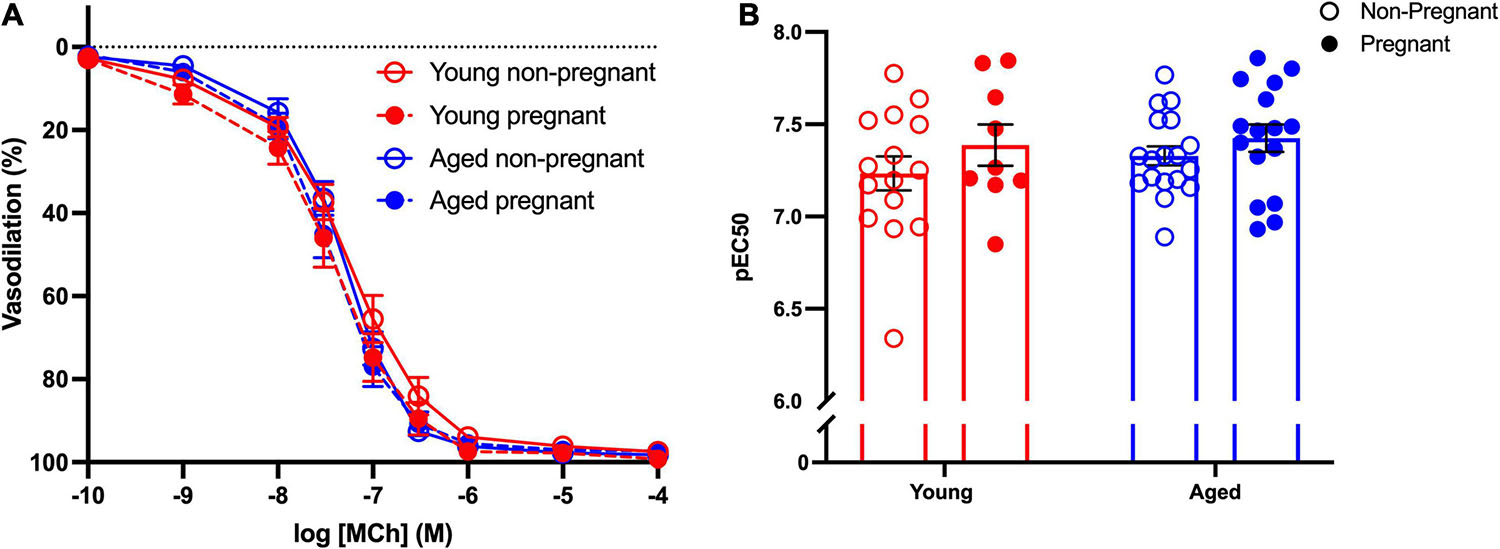
Figure 2. No differences in the endothelium-dependent vasodilation in young and aged non-pregnant and pregnant rats. (A) Endothelium-dependent vasodilation responses to increasing doses of methylcholine (MCh) in mesenteric arteries of young (3–4 months; in red) and aged (9–9.5 months; in blue) pregnant (on gestational day 20; closed circles) and non-pregnant (age-matched; open circles) rats. (B) Data summary of sensitivity to MCh (pEC50). Data are presented as mean ± SEM; analyzed by two-way ANOVA with Sidak’s multiple comparisons post hoc test; n = 9–16/group.
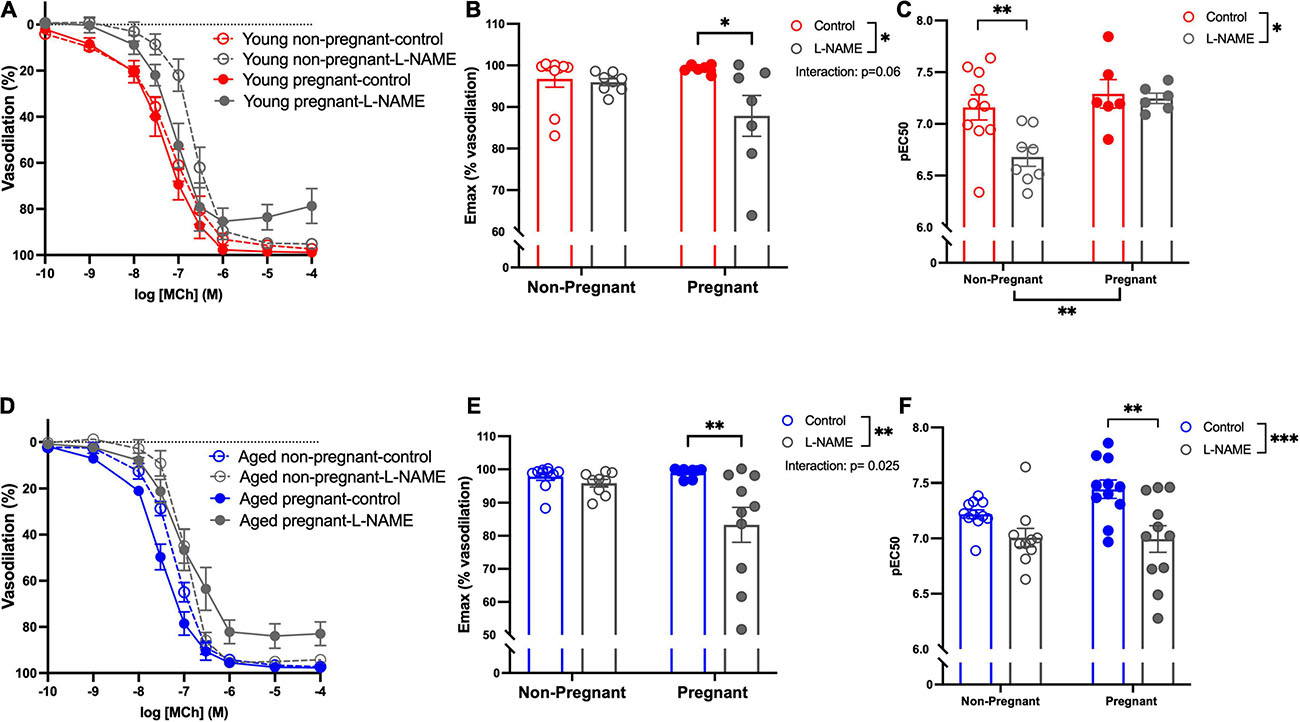
Figure 3. Increased nitric oxide-dependent vasodilation during pregnancy in mesenteric arteries from young and aged pregnant rats. (A,D) Endothelium-dependent vasodilation responses to increasing doses of methylcholine (MCh) in the presence or absence of L-NAME in mesenteric arteries of young [3–4 months; in red; (A–C)] and aged [9–9.5 months; in blue; (D–F)] pregnant (on gestational day 20; closed circles) and non-pregnant (age-matched; open circles) rats. (B,E) Data summaries of the maximal vasodilation responses to MCh (Emax). (C,F) Data summaries of the sensitivity to MCh (pEC50). Data are presented as mean ± SEM; analyzed by two-way ANOVA with Sidak’s multiple comparisons post hoc test; ∗p < 0.05, ∗∗p < 0.01; ∗∗∗p < 0.001; n = 6–11/group.
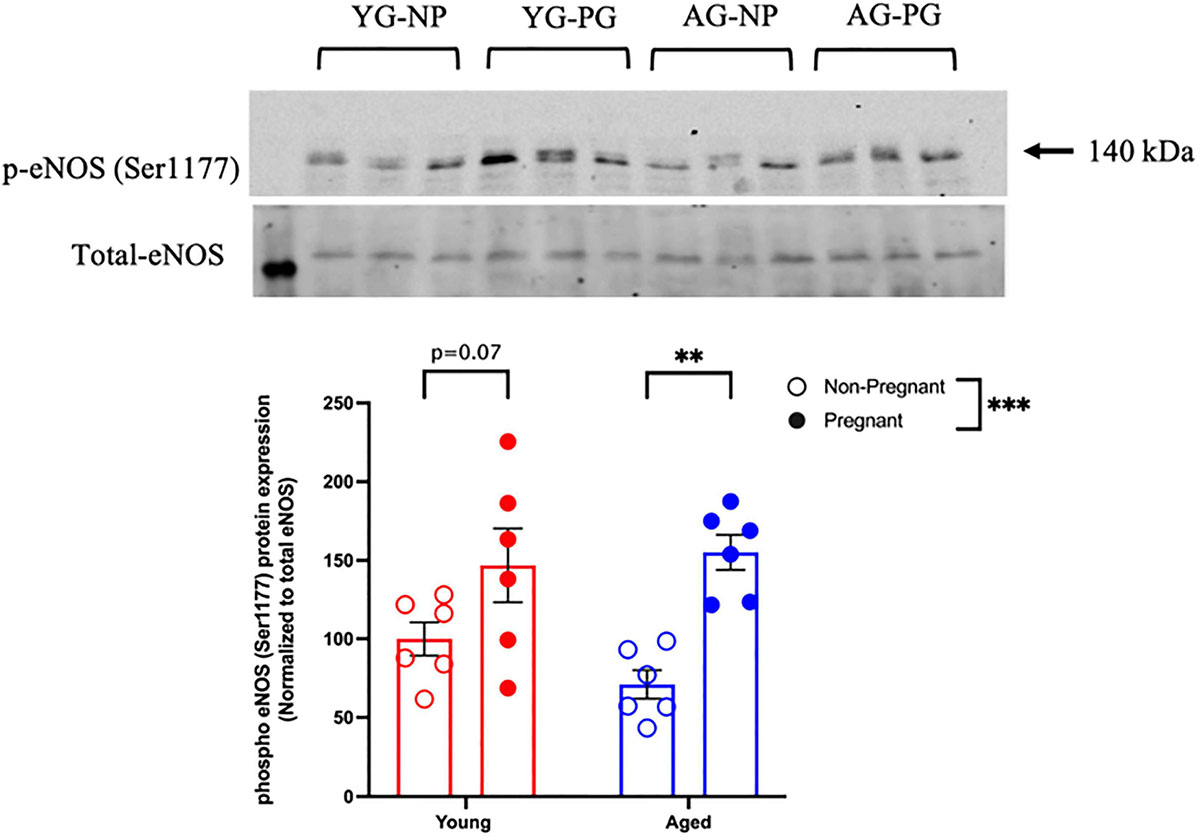
Figure 4. Increased phosphorylation of eNOS protein in young and aged pregnant rats. Western blot analysis of phospho-eNOS (Ser1177) normalized to total eNOS in mesenteric arteries of young (3–4 months; in red) and aged (9–9.5 months; in blue) pregnant (on gestational day 20; closed circles) and non-pregnant (age-matched; open circles) rats. Data are presented as mean ± SEM and expressed as percentage of control (i.e., the mean of the non-pregnant young group); analyzed by two-way ANOVA with Sidak’s multiple comparisons post hoc test; ∗∗p < 0.001; ∗∗∗p < 0.001; n = 6/group. YG-NP, young non-pregnant; YG-PG, young pregnant; AG-NP, aged non-pregnant; AG-PG, aged pregnant.
Endothelium Derived Hyperpolarization (EDH)-Mediated Vasodilation
Assessment of the contribution of EDH (using inhibitors apamin and TRAM-34) showed that the Emax and sensitivity to MCh (pEC50) was decreased after incubation with apamin and TRAM-34 in young non-pregnant, aged non-pregnant and aged pregnant rats while no effect of apamin and TRAM-34 was observed in young pregnant rats (Figures 5A–F). Apamin and TRAM-34 also decreased AUC in aged non-pregnant and pregnant rats (Supplementary Table 4). Inhibition of myoendothelial gap junctions (MEGJs) by 18α-glycyrrhetinic acid had no effect on MCh-induced vasodilation responses in mesenteric arteries from either young or aged, pregnant, or non-pregnant rats (Supplementary Table 5).
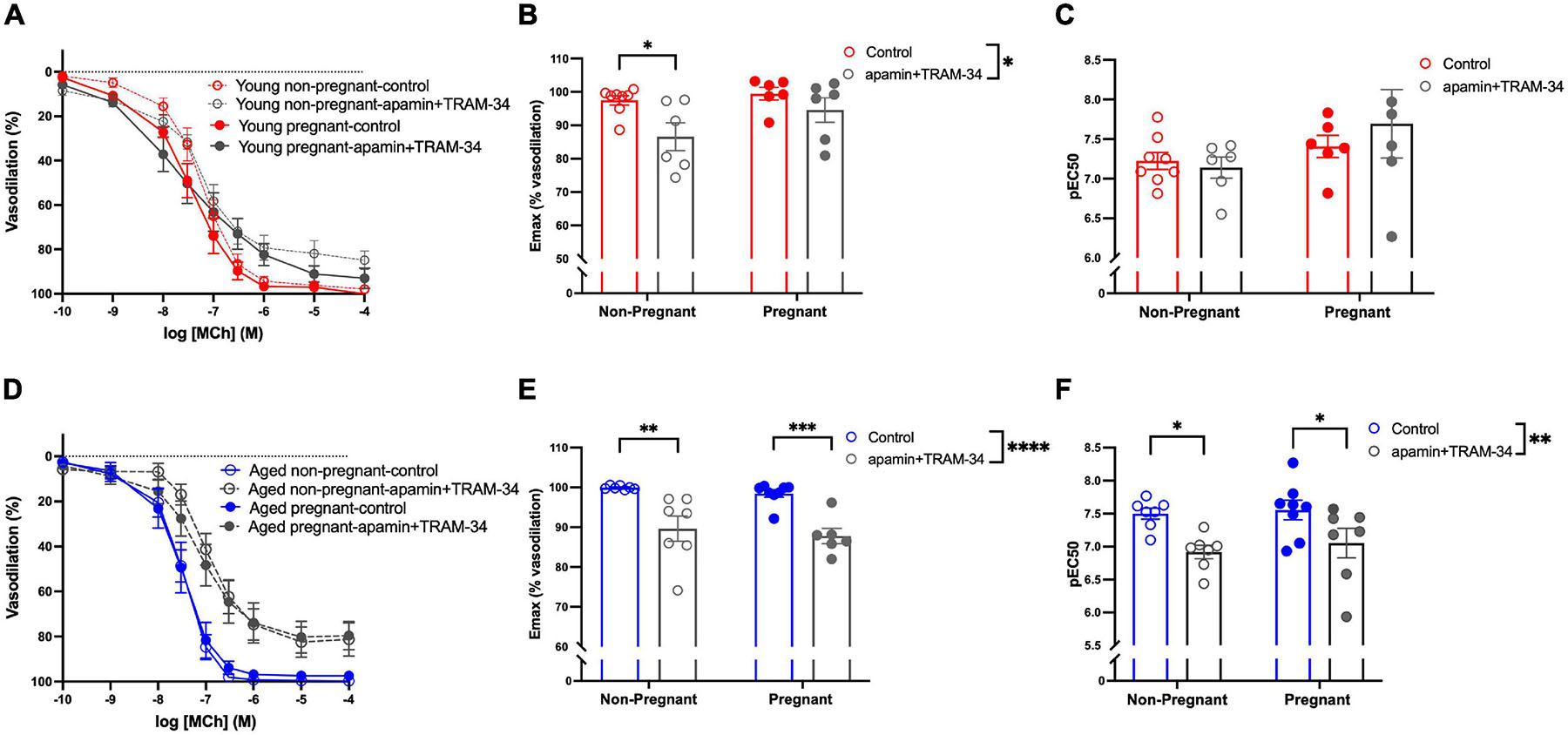
Figure 5. Increased endothelium-derived hyperpolarization-mediated relaxation in mesenteric arteries from aged non-pregnant and pregnant rats. (A,D) Endothelium-dependent vasodilation responses to increasing doses of methylcholine (MCh) in the presence or absence of apamin/TRAM-34 in mesenteric arteries of young [3–4 months; in red; (A–C)] and aged [9–9.5 months; in blue; (D–F)] pregnant (on gestational day 20; closed circles) and non-pregnant (age-matched; open circles) rats. (B,E) Data summaries of the maximal vasodilation responses to MCh (Emax). (C,F) Data summaries of the sensitivity to MCh (pEC50). Data are presented as mean ± SEM; analyzed by two-way ANOVA with Sidak’s multiple comparisons post hoc test; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ****p < 0.0001; n = 6–8/group.
Endothelium-Independent Vasodilation
Sodium nitroprusside (SNP) dose-response curves were conducted to assess potential differences in endothelium-independent relaxation. There were no changes in the vascular responses between the young and aged, non-pregnant, and pregnant rats (Supplementary Table 6).
Vasoconstrictor Pathways
Vascular Responses to BigET-1 in Mesenteric Arteries and Contribution of Converting Enzymes
Vasoconstriction responses to bigET-1 were enhanced in vessels from both aged non-pregnant and pregnant rats compared to the young non-pregnant and pregnant rats, however, there was no significant effect of pregnancy (Figures 6A,B and Supplementary Table 7). The enhanced constriction responses to bigET-1 in aged rats could be a result of increased conversion of bigET-1 to active ET-1, or due to greater vascular smooth muscle sensitivity to ET-1. Since no differences were evident in ET-1 sensitivity/Emax between young and aged non-pregnant and pregnant rats (Figures 6C,D and Supplementary Table 7), it appears that there was a greater capacity for aged vessels to convert bigET-1 to ET-1. To determine which enzymes may be contributing to this enhanced conversion, inhibitors of various bigET-1 conversion enzymes were used. Incubating the vessels with the MMP-inhibitor GM6001 did not alter vasoconstriction responses to bigET-1 in either young or aged non-pregnant and pregnant rats (Figures 7A–D and Supplementary Table 8). We further evaluated the contribution of other enzymes involved in converting bigET-1 into its vasoactive form. Pre-incubation with the ECE-1 inhibitor CGS35066 did not impact bigET-1 responses in young non-pregnant rats but decreased maximum constriction (Emax) was observed in pregnant rats (Figures 8A,B and Supplementary Table 8), while constriction to bigET-1 was significantly reduced in the aged non-pregnant and pregnant rats (Figures 8C,D). However, Western blot analysis showed that there were no changes in ECE-1 protein levels between the groups (Figure 8E). Chymase inhibitor chymostatin and neutral endopeptidase inhibitor thiorphan did not alter bigET-1 vasoconstriction in either young or aged rats (Supplementary Table 9).
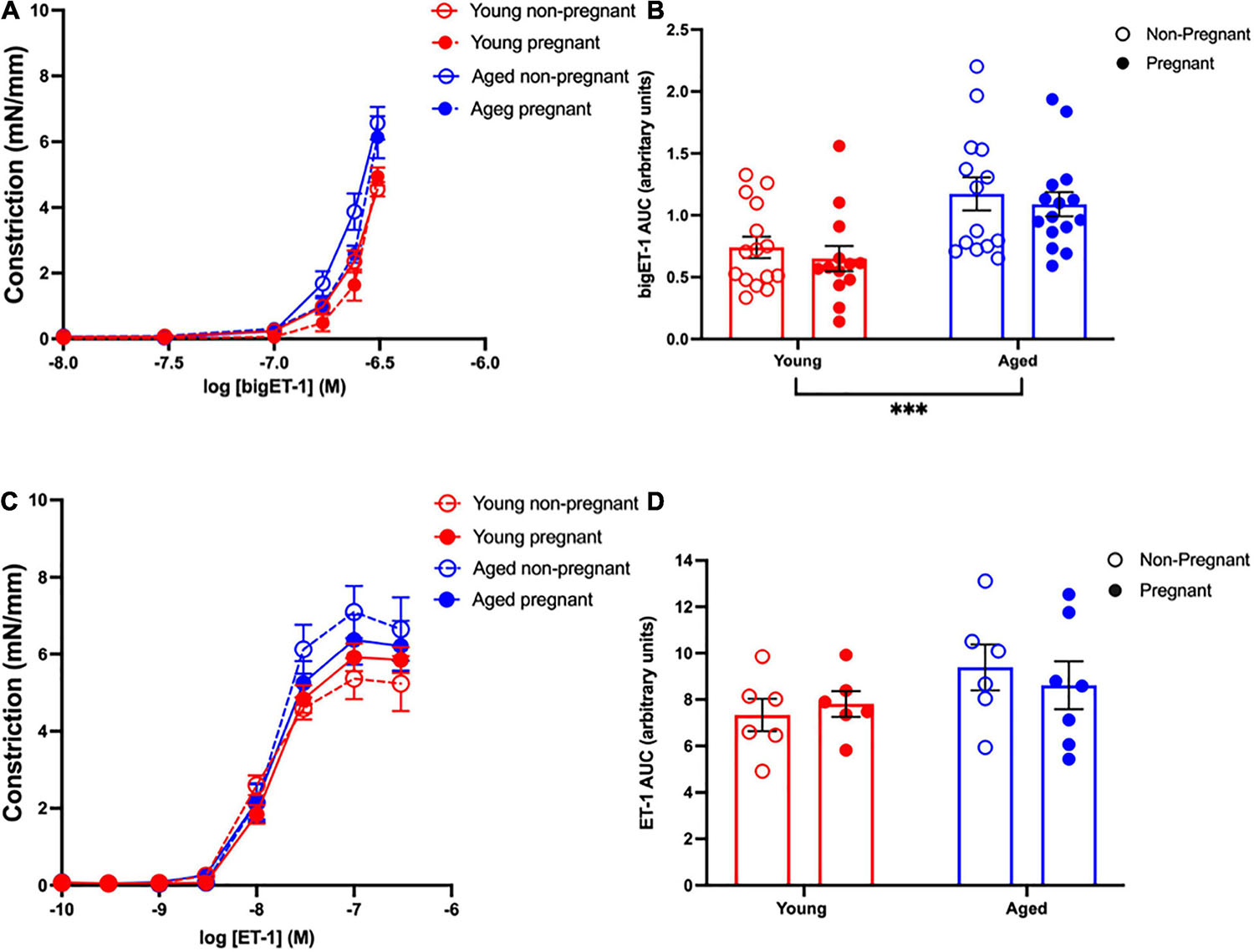
Figure 6. Increased vascular responses to bigET-1 in aged non-pregnant and pregnant rats, and no changes in vascular responses to ET-1. (A,C) Vascular contraction responses to bigET-1 and ET-1 in mesenteric arteries of young (3–4 months; in red) and aged (9–9.5 months; in blue) pregnant (on gestational day 20; closed circles) and non-pregnant (age-matched; open circles) rats. (B,D) Data summaries of area under the curve (AUC) of the bigET-1 (B) and ET-1 (D) responses. Data are presented as mean ± SEM; analyzed by two-way ANOVA with Sidak’s multiple comparisons post hoc test; ∗∗∗p < 0.001; n = 6–16/group.
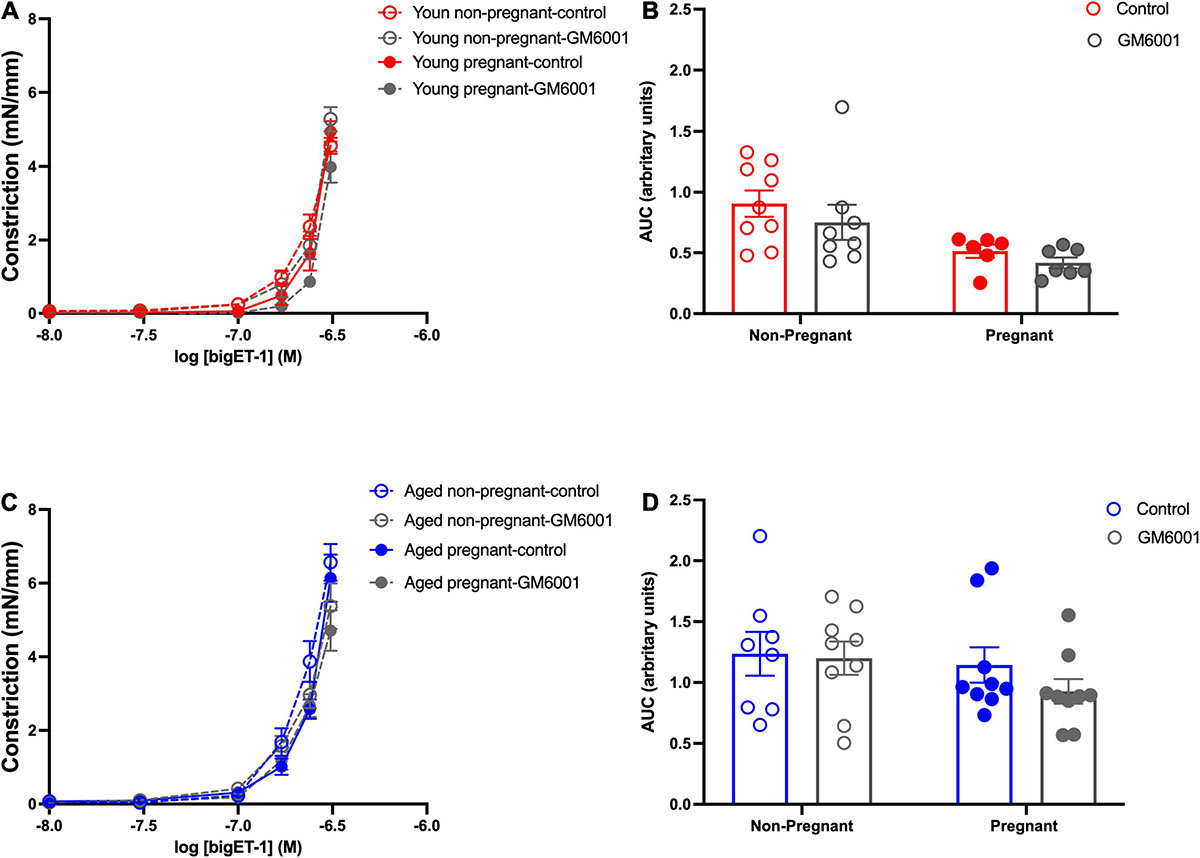
Figure 7. No significant contribution of matrix metalloproteases in converting to bigET-1 in young and aged non-pregnant and pregnant rats. (A,C) Vascular contraction response to bigET-1 in the presence or absence of MMPs inhibitor GM6001 in mesenteric arteries of young [3–4 months; in red; (A,B)] and aged [9–9.5 months; in blue; (C,D)] pregnant (on gestational day 20; closed circles) and non-pregnant (age-matched; open circles) rats. (B,D) Data summaries of area under the curve (AUC) of the bigET-1 responses. Data are presented as mean ± SEM; analyzed by two-way ANOVA with Sidak’s multiple comparisons post hoc test; n = 6-8/group.
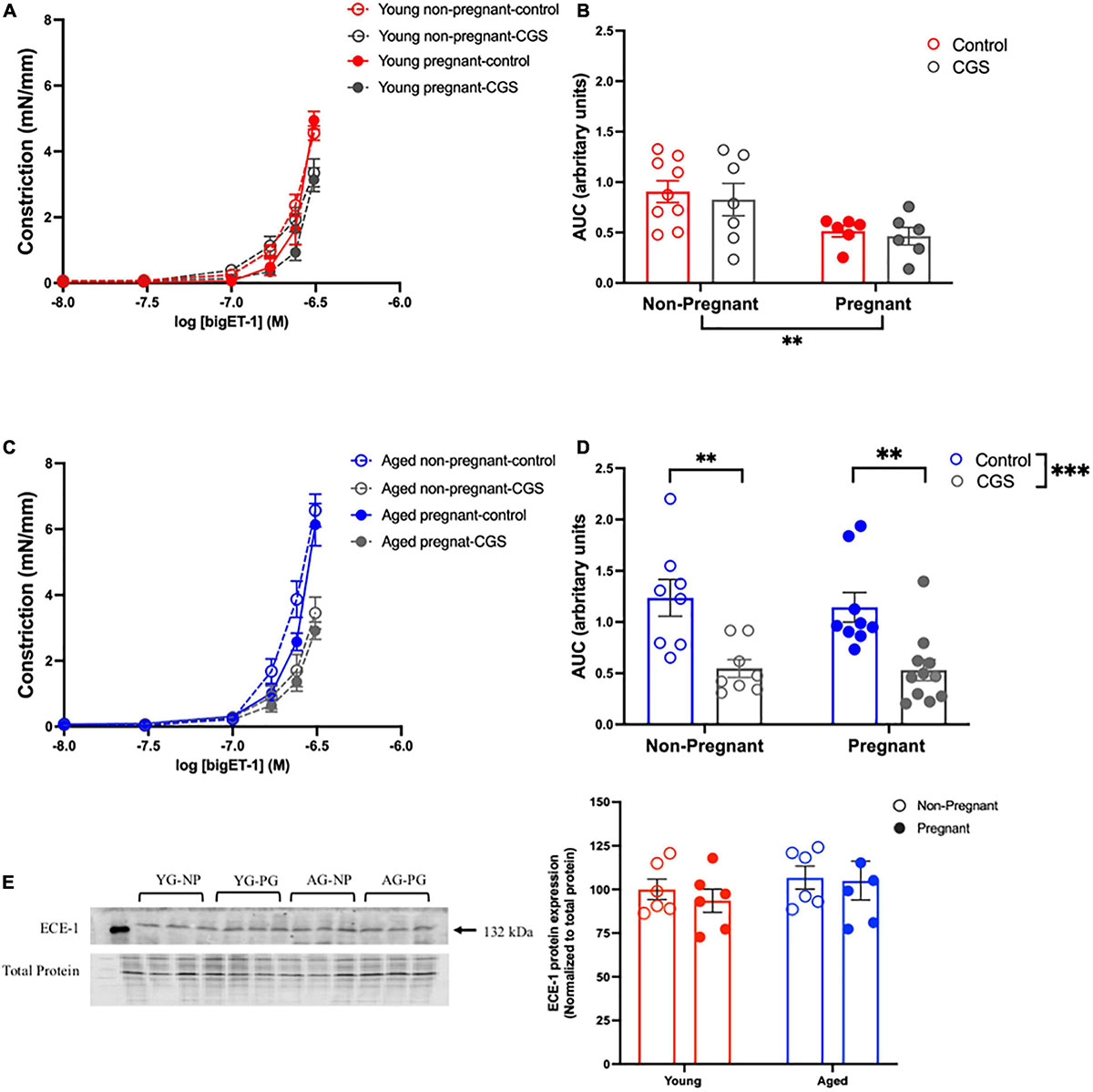
Figure 8. Increased contribution of endothelin converting enzyme (ECE) in converting bigET-1 in aged non-pregnant and pregnant rats. (A,C) Vascular contraction response to bigET-1 in the presence or absence of ECE-1 inhibitor CGS35066 (CGS) in mesenteric arteries of young [3–4 months; in red; (A,B)] and aged [9–9.5 months; in blue; (C,D)] pregnant (on gestational day 20; closed circles) and non-pregnant (age-matched; open circles) rats. (B,D) Data summaries of area under the curve (AUC) of the bigET-1 responses. (E) Western blot analysis of ECE-1 protein normalized to total protein in mesenteric arteries; the ECE-1 data is expressed as percentage of control (the mean of the non-pregnant young group). Data are presented as mean ± SEM; analyzed by two-way ANOVA with Sidak’s multiple comparisons post hoc test; ∗∗p < 0.001; ∗∗∗p < 0.001; n = 6–8/group. YG-NP, young non-pregnant; YG-PG, young pregnant; AG-NP, aged non-pregnant; AG-PG, aged pregnant.
In addition, no changes in the vasoconstriction capacity to KPSS were observed between the young (non-pregnant: 5.17 ± 0.41; n = 16 vs. pregnant: 5.51 ± 0.48 mN/mm; n = 12) and aged rats (non-pregnant; 5.85 ± 0.32; n = 15 vs. pregnant: 5.68 ± 0.38 mN/mm; n = 15), suggesting that overall constrictor capacity of the vascular smooth muscle cells was not different between groups.
Discussion
The goal of the current study was to understand the vascular pathways that may be contributing to impaired vascular adaptations to pregnancy in a rat model of advanced maternal age. Overall, our data demonstrated altered vascular function in the aged non-pregnant rats, while these changes appear to be compensated for during pregnancy with a major reduction in blood pressure accompanied by an increased contribution of NO and EDH-mediated relaxation in aged pregnant rats. Furthermore, we demonstrated that contractile responses to bigET-1 were greater in mesenteric arteries of the aged groups compared with the young groups, independent of pregnancy. In contrast to our hypothesis, no contribution of MMPs in converting bigET-1 to active ET-1 was observed, while an increased contribution with ECE-1 was found in aged rats (both non-pregnant and pregnant). However, a similar contractile response to ET-1 suggests that the vascular smooth muscle sensitivity to ET-1 was not different between the groups. We speculate that, although aging contributes to changes in various pathways of endothelium-dependent vasodilation, pregnancy in aged dams may confer vascular protection.
Advanced Maternal Age and Blood Pressure
It has been well established that aging alters the structural and functional properties of the vascular wall, leading to arterial stiffness with increased blood pressure, resulting in an overall constrictive phenotype (Boss and Seegmiller, 1981; Franklin, 1999; Lee and Oh, 2010; Toda, 2012). In the current study, the significantly higher blood pressure (systolic, diastolic, and mean arterial pressure) in non-pregnant aged rats compared to the other groups was not surprising, considering the prevalence of age-related arterial stiffness and hypertension (Lee and Oh, 2010; Xu et al., 2017). Of note, the systolic blood pressure appears to be a better predictor for cardiovascular disease with advancing age due to an increasing stiffness of large arteries (Franklin, 1999). Moreover, an elevated diastolic blood pressure typically correlates with impaired left ventricular relaxation, while mean arterial pressure in the normal range allows for adequate perfusion of vital organs (Benetos et al., 1997; Domanski et al., 1999; Franklin, 1999). Thus, in our aged non-pregnant rats, elevated blood pressures may be reflective of inherent cardiovascular changes associated with aging. Generally, a mild decrease in blood pressure is observed over the course of a normal pregnancy, being the lowest around mid-gestation, likely reflecting a reduced systemic vascular resistance (Villar and Sibai, 1989). We did not observe a difference in blood pressure between young non-pregnant and pregnant rats, which may, in part, be due to the fact that blood pressure was assessed in late gestation.
Interestingly, a significant reduction in blood pressure was observed in aged pregnant rats compared to the aged non-pregnant rats, such that young and aged pregnant rats had similar blood pressures. In aged pregnancies, this reduction in blood pressure may be due to an increased contribution of endothelium-dependent vasodilator pathways that we observed in aged pregnant rats (i.e., NO and EDH, discussed below). In line with our observations, Gaillard et al. (2011) in a population-based prospective cohort study, showed that blood pressure differences appear to be small and within the normal physiological range between young and older women during pregnancy. In contrast, Plant et al. (2020) showed an increased in diastolic blood pressure, but no changes in the systolic blood pressure in pregnant vervet/African green monkeys of advanced maternal age (3–9 years were considered optimal maternal age, while those 10 years and older were considered to be advanced maternal age). In addition, a trend in increase heart rate was observed in young pregnant rats compare to non-pregnant rats, consistent with the well-characterized cardiovascular adaptation to pregnancy seen in women (May, 2015). Interestingly, this trend was not observed between the aged non-pregnant and pregnant rats. This difference may be due to an impaired cardiac sympathetic activity in aging or may be related to cardiac remodeling that is known to occur in aging (Ferrari et al., 2003; Strait and Lakatta, 2012; Jakovljevic, 2018). While examining the adaptations to cardiac structure and function in our aged rat model was beyond the scope of the current study, future experiments will be designed to further investigate these findings. Overall, our study is unique in that we analyzed blood pressure changes compared to the non-pregnant state thus allowing us to assess the cardiovascular adaptations to pregnancy and with aging. As blood pressure was elevated in the aged non-pregnant rats, these animals may be entering pregnancy with a compromised cardiovascular capacity; and unless significant vascular adaptations to pregnancy occur, the aged cardiovascular system would likely be unable to support normal fetal growth and development.
Advanced Maternal Age and Endothelium-Dependent Vasodilation
Normal pregnancy is associated with an increased endothelium-dependent vascular relaxation, with increased NO contribution during pregnancy (Morris et al., 1995; Williams et al., 1997). In the current study, although we did not observe any overall differences in endothelium-dependent vasodilation to MCh in mesenteric arteries between young and aged non-pregnant and pregnant rats, pre-treatment with the NOS inhibitor L-NAME revealed a significant decrease in Emax in both young and aged pregnant rats. These data suggest that in our model, NO contribution to MCh-induced vasodilation was increased during pregnancy, independent of aging. Interestingly, this greater NO contribution in pregnancy may be due to increased eNOS phosphorylation at Ser1177/activity, which was increased in aged pregnant rats (and tended to be higher in young pregnant rats) compared to both non-pregnant rat groups. Several studies clearly demonstrated increased contribution of NO in young pregnant animals in different vascular beds including mesenteric arteries (Conrad et al., 1993; Pascoal et al., 1995; Ni et al., 1997; Sladek et al., 1997; Choi et al., 2002; Cooke and Davidge, 2003; Hodzic et al., 2017; Owusu Darkwa et al., 2018), but there is paucity of research investigating systemic vascular contribution of NO in advanced maternal age. In non-pregnant animals, the shift in MCh-induced vasodilation by L-NAME in young but not aged rats suggests a reduced NO contribution in aged non-pregnant rats. These data support evidence in the literature that the NO pathway is important in vascular adaptations to pregnancy (Boeldt et al., 2011; Boeldt and Bird, 2017), including in our rat model of advanced maternal age.
In addition to NO, we also observed an EDH contribution to vasodilation (as assessed by inhibiting SK and IK channels with apamin and TRAM-34) in mesenteric arteries of young non-pregnant, aged non-pregnant and aged pregnant rats, however, this contribution of EDH was absent in young pregnant rats. Thus, in young pregnant rats, the EDH contribution to vasodilation (specifically via SK and IK channels) seems minimal, while this pathway may be maintained in aged pregnant rats as an additional endothelium-dependent mechanism that has adapted with aging to compensate for the enhanced constrictor state, thus supporting pregnancy. A variety of other EDH-pathways contribute to vasodilation, such as epoxyeicosatrienoic acids (EETs), hydrogen peroxide, and MEGJs (Chen et al., 1988; Corriu et al., 1998; Vanhoutte, 2004; Ng et al., 2008). We did not observe any contribution of MEGJs to MCh-induced vasodilation. Although assessing all other EDH pathways was beyond the scope of the current study, and it will be interesting to evaluate these specific pathways in future experiments. In contrast to our findings, others have shown a loss of EDH-mediated vasodilation in rat mesenteric arteries with aging and hypertension, in part due to decrease synthesis/release of EDH or a defective electrical coupling between endothelial and smooth muscle cells (Fujii et al., 1993; Goto et al., 2004). Furthermore, in normal pregnancy, EDH-mediated vasodilation was elevated due to increased synthesis/reduced degradation in addition to NO/prostanoid synthesis, contributing to the vascular adaptations (Gerber et al., 1998). Thus, EDH contribution to vasodilation exists based on the species, nature (young vs. aged), vascular bed, and state (pregnant vs. non-pregnant), and the enhanced EDH-dependent relaxation in conditions such as vascular aging may be a compensatory mechanism to maintain a balance of vasoactive factors. Moreover, the overall contribution of NO in young pregnant rats and increased NO and EDH contribution in aged pregnant rats did not account for all of the MCh-induced vasodilation. As such, there might be a significant contribution of other vasodilator pathways, such as prostacyclin, contributing to endothelium-dependent vasodilation, would be interesting to assess in future studies. Overall, our data indicate that NO is involved in endothelium-dependent vasodilation in both young and aged pregnant rats, while enhanced EDH-mediated vasodilation in mesenteric arteries from aged pregnant rats (and a decrease in blood pressure) suggests that beneficial vascular adaptations occur in the aged rats that were able to maintain pregnancy.
Advanced Maternal Age and Vasoconstriction
The ET-1 pathway plays an essential role in the maintenance of vascular tone, however, a greater ET-1 activity is associated with pathological conditions, such as aging, and may impair vascular function (Westby et al., 2011; Wang et al., 2014; Freitas-Rodriguez et al., 2017; Xu et al., 2017). ET-1 production involves enzymatic cleavage of bigET-1. In the current study, constriction responses to bigET-1 were increased in the aged groups, and since the vascular smooth muscle response to ET-1, as well as to KPSS (suggesting no change in non-receptor mediated vasoconstriction responses) was not different among the groups, this enhanced bigET-1 responsiveness appeared to be due to increased bigET-1 processing in aged arteries. We postulated this may be due to upregulation of MMPs in the aged animals. However, we did not observe a significant contribution of MMPs-mediated constriction to bigET-1 in mesenteric arteries from any of our group. It is possible that our aged rat model (9.5 months ∼35 years of human age) is in fact too “young” and do not yet demonstrate an aged-associated increase in MMP expression/activity, which has been described by others at older ages (Wang and Lakatta, 2002; Lindsey et al., 2005; Lekontseva et al., 2010; Wang et al., 2015; Freitas-Rodriguez et al., 2017). Moreover, MMP-contribution may be vascular bed-dependent, as suggested by other studies (Crowther et al., 2000; Wang et al., 2012, 2014). Thus, in the mesenteric arteries of our aged rats, MMPs may not play a significant role, while other enzymes may contribute to bigET-1 conversion, such as ECE-1.
ECE-1 has been shown to play a dominant role in the processing of bigET-1 in aging vasculature (Stauffer et al., 2008; Goel et al., 2010). Indeed, we showed a significant ECE-1 contribution to bigET-1 cleavage in aged mesenteric arteries, independent of pregnancy state, while in young rats there was no role for ECE-1 conversion of bigET-1. Interestingly, we did not observe any changes in the ECE-1 expression between the groups, thus the increased ECE-1 contribution may be due to an enhanced ECE-1 activity rather than expression. Although the cleavage of bigET-1 to active ET-1 is primarily through MMPs and ECE-1, alternative pathways including chymase and neutral endopeptidases are also involved (De Campo et al., 2002; Fecteau et al., 2005; Simard et al., 2009). However, our data did not support a major role for these enzymes. Nevertheless, a constitutive conversion of bigET-1 to the potent vasoconstrictor ET-1 occurs by several enzymes to maintain normal vascular tone, and the to best of our knowledge, we are the first to demonstrate the enhanced contribution of ECE-1 in converting bigET-1 to ET-1 in a rat model of advanced maternal age. Our findings of increased NO and EDH contribution to vasodilation, together with enhanced ECE-1-mediated conversion of bigET-1 to ET-1 in mesenteric arteries, suggests an important link between endothelium-derived NO and EDH signaling and the bigET-1/ET-1 pathways. Indeed, future studies are warranted to determine how these mechanisms are interacting and contributing to systemic vascular adaptations to pregnancy a rat model of advanced maternal age.
Conclusion
The population of women becoming pregnant at an advanced age is increasing globally and poses many health challenges due to their increased risk of pregnancy complications. As such, women of advanced age represent a very important and yet understudied demographic of pregnant women. Our study explores various vascular pathways involved in the adaptations to pregnancy, which were distinct in advanced maternal age compared to young rats. Although NO is involved in the vasodilation in both young and aged pregnant rats, enhanced EDH mediated endothelium-dependent vasodilation in aged mesenteric vasculature and lower MAP suggests a beneficial adaptation in these rats that were able to maintain pregnancy. In addition, increased contribution of ECE-1 may provide a more dominant conversion pathway for bigET-1 in aging vasculature. This study increases our understanding of the vascular pathways involved in the systemic vascular adaptations to pregnancy. However, maternal aging is often associated with co-morbidities, thus future studies including a “second hit” in our animal model may provide more insight. For example, exposing rats to a high fat diet (obesity) or high salt (hypertension) may unmask underlying vascular deficits in the current model of advanced maternal age, and may thus provide additional clinically relevant insights. Given the increasing trend toward delaying pregnancy, understanding the vascular adaptations that may be compromised, thus contributing to an increased risk of adverse pregnancy outcomes in women with advanced maternal age, may help to develop effective treatment and prevention strategies.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by the University of Alberta Health Sciences Animal Policy and Welfare Committee.
Author Contributions
MP, SD, FS, and C-LC: study conception and design, analysis, and interpretation of data. MP, AW, and RK: acquisition of data. MP: drafting of the manuscript. MP, AW, FS, SD, and C-LC: critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by a Canadian Institutes of Health Research (CIHR) Foundation grant (FS154313), the generosity of the Stollery Children’s Hospital Foundation and the Alberta Women’s Health Foundation through the Women and Children’s Health Research Institute (WCHRI), and by the Heart and Stroke Foundation of Canada. SD is a Canada Research Chair in Maternal and Perinatal Cardiovascular Health and a Distinguished University Professor at the University of Alberta. MP was supported by a WCHRI Graduate Studentship award and Faculty of Medicine and Dentistry 75th Anniversary Award from the University of Alberta.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2021.718568/full#supplementary-material
References
Abdalvand, A., Morton, J. S., Bourque, S. L., Quon, A. L., and Davidge, S. T. (2013). Matrix metalloproteinase enhances big-endothelin-1 constriction in mesenteric vessels of pregnant rats with reduced uterine blood flow. Hypertension 61, 488–493. doi: 10.1161/hypertensionaha.111.00055
Ajne, G., Wolff, K., Fyhrquist, F., Carlstrom, K., Hemsen-Mortberg, A., and Nisell, H. (2003). Endothelin converting enzyme (ECE) activity in normal pregnancy and preeclampsia. Hypertens. Pregnancy 22, 215–224. doi: 10.1081/prg-120024025
Albrecht, E. D., Babischkin, J. S., Aberdeen, G. W., Burch, M. G., and Pepe, G. J. (2021). Maternal systemic vascular dysfunction in a primate model of defective uterine spiral artery remodeling. Am. J. Physiol. Heart Circ. Physiol. 320, H1712–H1723.
Amburgey, O. A., Reeves, S. A., Bernstein, I. M., and Cipolla, M. J. (2010). Resistance artery adaptation to pregnancy counteracts the vasoconstricting influence of plasma from normal pregnant women. Reprod. Sci. 17, 29–39. doi: 10.1177/1933719109345288
Barry, J. S., and Anthony, R. V. (2008). The pregnant sheep as a model for human pregnancy. Theriogenology 69, 55–67. doi: 10.1016/j.theriogenology.2007.09.021
Benetos, A., Laurent, S., Asmar, R. G., and Lacolley, P. (1997). Large artery stiffness in hypertension. J. Hypertens. Suppl. 15, S89–S97.
Bernardi, F., Constantino, L., Machado, R., Petronilho, F., and Dal-Pizzol, F. (2008). Plasma nitric oxide, endothelin-1, arginase and superoxide dismutase in pre-eclamptic women. J. Obstet. Gynaecol. Res. 34, 957–963.
Boeldt, D. S., and Bird, I. M. (2017). Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J. Endocrinol. 232, R27–R44.
Boeldt, D. S., Yi, F. X., and Bird, I. M. (2011). eNOS activation and NO function: pregnancy adaptive programming of capacitative entry responses alters nitric oxide (NO) output in vascular endothelium–new insights into eNOS regulation through adaptive cell signaling. J. Endocrinol. 210, 243–258. doi: 10.1530/joe-11-0053
Bohm, F., and Pernow, J. (2007). The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc. Res. 76, 8–18. doi: 10.1016/j.cardiores.2007.06.004
Boss, G. R., and Seegmiller, J. E. (1981). Age-related physiological changes and their clinical significance. West. J. Med. 135, 434–440.
Canada, S. (2012). Births 2009, no. 84F0210X. Available at: http://www.statcan.gc.ca/pub/84f0210x/84f0210x2009000-eng.pdf
Cardillo, C., Kilcoyne, C. M., Cannon, R. O. III, and Panza, J. A. (2000). Interactions between nitric oxide and endothelin in the regulation of vascular tone of human resistance vessels in vivo. Hypertension 35, 1237–1241. doi: 10.1161/01.hyp.35.6.1237
Care, A. S., Bourque, S. L., Morton, J. S., Hjartarson, E. P., and Davidge, S. T. (2015). Effect of advanced maternal age on pregnancy outcomes and vascular function in the rat. Hypertension 65, 1324–1330. doi: 10.1161/hypertensionaha.115.05167
Chen, G., Suzuki, H., and Weston, A. H. (1988). Acetylcholine releases endothelium-derived hyperpolarizing factor and EDRF from rat blood vessels. Br. J. Pharmacol. 95, 1165–1174. doi: 10.1111/j.1476-5381.1988.tb11752.x
Chen, Q., Jin, M., Yang, F., Zhu, J., Xiao, Q., and Zhang, L. (2013). Matrix metalloproteinases: inflammatory regulators of cell behaviors in vascular formation and remodeling. Mediators Inflamm. 2013:928315.
Choi, J. W., Im, M. W., and Pai, S. H. (2002). Nitric oxide production increases during normal pregnancy and decreases in preeclampsia. Ann. Clin. Lab. Sci. 32, 257–263.
Christensen, K. L., and Mulvany, M. J. (1993). Mesenteric arcade arteries contribute substantially to vascular resistance in conscious rats. J. Vasc. Res. 30, 73–79. doi: 10.1159/000158978
Cohen, M., Ribaux, P., Epiney, M., and Irion, O. (2012). Expression of metalloproteinases 1, 2, 7, 9, and 12 in human cytotrophoblastic cells from normal and preeclamptic placentas. Neuro Endocrinol. Lett. 33, 406–411.
Conrad, K. P., Joffe, G. M., Kruszyna, H., Kruszyna, R., Rochelle, L. G., Smith, R. P., et al. (1993). Identification of increased nitric oxide biosynthesis during pregnancy in rats. FASEB J. 7, 566–571. doi: 10.1096/fasebj.7.6.7682524
Cooke, C. L., and Davidge, S. T. (2003). Pregnancy-induced alterations of vascular function in mouse mesenteric and uterine arteries. Biol. Reprod. 68, 1072–1077. doi: 10.1095/biolreprod.102.009886
Cooke, C. M., Shah, A., Kirschenman, R. D., Quon, A. L., Morton, J. S., Care, A. S., et al. (2018). Increased susceptibility to cardiovascular disease in offspring born from dams of advanced maternal age. J. Physiol. 596, 5807–5821. doi: 10.1113/jp275472
Corriu, C., Feletou, M., Puybasset, L., Bea, M. L., Berdeaux, A., and Vanhoutte, P. M. (1998). Endothelium-dependent hyperpolarization in isolated arteries taken from animals treated with NO-synthase inhibitors. J. Cardiovasc. Pharmacol. 32, 944–950. doi: 10.1097/00005344-199812000-00011
Crowther, M., Goodall, S., Jones, J. L., Bell, P. R., and Thompson, M. M. (2000). Increased matrix metalloproteinase 2 expression in vascular smooth muscle cells cultured from abdominal aortic aneurysms. J. Vasc. Surg. 32, 575–583. doi: 10.1067/mva.2000.108010
Davidge, S. T., Hubel, C. A., and McLaughlin, M. K. (1993). Cyclooxygenase-dependent vasoconstrictor alters vascular function in the vitamin E-deprived rat. Circ. Res. 73, 79–88. doi: 10.1161/01.res.73.1.79
De Campo, B. A., Goldie, R. G., Jeng, A. Y., and Henry, P. J. (2002). Role of endothelin-converting enzyme, chymase and neutral endopeptidase in the processing of big ET-1, ET-1(1-21) and ET-1(1-31) in the trachea of allergic mice. Clin. Sci. (Lond.) 103(Suppl. 48), 353S–356S.
Dieber-Rotheneder, M., Beganovic, S., Desoye, G., Lang, U., and Cervar-Zivkovic, M. (2012). Complex expression changes of the placental endothelin system in early and late onset preeclampsia, fetal growth restriction and gestational diabetes. Life Sci. 91, 710–715. doi: 10.1016/j.lfs.2012.04.040
Domanski, M. J., Davis, B. R., Pfeffer, M. A., Kastantin, M., and Mitchell, G. F. (1999). Isolated systolic hypertension : prognostic information provided by pulse pressure. Hypertension 34, 375–380. doi: 10.1161/01.hyp.34.3.375
Donato, A. J., Machin, D. R., and Lesniewski, L. A. (2018). Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circ. Res. 123, 825–848. doi: 10.1161/circresaha.118.312563
Dong, L., Gan, L., Wang, H., and Cai, W. (2019). Age-related impairment of structure and function of iliac artery endothelium in rats is improved by elevated fluid shear stress. Med. Sci. Monit. 25, 5127–5136. doi: 10.12659/msm.916287
Dorup, I., Skajaa, K., and Sorensen, K. E. (1999). Normal pregnancy is associated with enhanced endothelium-dependent flow-mediated vasodilation. Am. J. Physiol. 276, H821–H825.
Ergul, A. (2011). Endothelin-1 and diabetic complications: focus on the vasculature. Pharmacol. Res. 63, 477–482. doi: 10.1016/j.phrs.2011.01.012
Fecteau, M. H., Honore, J. C., Plante, M., Labonte, J., Rae, G. A., and D’Orleans-Juste, P. (2005). Endothelin-1 (1-31) is an intermediate in the production of endothelin-1 after big endothelin-1 administration in vivo. Hypertension 46, 87–92. doi: 10.1161/01.hyp.0000170460.24604.23
Ferrari, A. U., Radaelli, A., and Centola, M. (2003). Invited review: aging and the cardiovascular system. J. Appl. Physiol. (1985) 95, 2591–2597. doi: 10.1152/japplphysiol.00601.2003
Ferro, C. J., Spratt, J. C., Haynes, W. G., and Webb, D. J. (1998). Inhibition of neutral endopeptidase causes vasoconstriction of human resistance vessels in vivo. Circulation 97, 2323–2330. doi: 10.1161/01.cir.97.23.2323
Franklin, S. S. (1999). Ageing and hypertension: the assessment of blood pressure indices in predicting coronary heart disease. J. Hypertens. Suppl. 17, S29–S36.
Freitas-Rodriguez, S., Folgueras, A. R., and Lopez-Otin, C. (2017). The role of matrix metalloproteinases in aging: tissue remodeling and beyond. Biochim. Biophys. Acta Mol. Cell Res. 1864(11 Pt A), 2015–2025. doi: 10.1016/j.bbamcr.2017.05.007
Fujii, K., Ohmori, S., Tominaga, M., Abe, I., Takata, Y., Ohya, Y., et al. (1993). Age-related changes in endothelium-dependent hyperpolarization in the rat mesenteric artery. Am. J. Physiol. 265(2 Pt 2), H509–H516.
Gaillard, R., Bakker, R., Steegers, E. A., Hofman, A., and Jaddoe, V. W. (2011). Maternal age during pregnancy is associated with third trimester blood pressure level: the generation R study. Am. J. Hypertens. 24, 1046–1053. doi: 10.1038/ajh.2011.95
Garner, R. E., and Bushnik, T. (2008). The children of older first-time mothers in Canada: their health and development. Genus 64, 63–81.
Gerber, R. T., Anwar, M. A., and Poston, L. (1998). Enhanced acetylcholine induced relaxation in small mesenteric arteries from pregnant rats: an important role for endothelium-derived hyperpolarizing factor (EDHF). Br. J. Pharmacol. 125, 455–460. doi: 10.1038/sj.bjp.0702099
Goel, A., Su, B., Flavahan, S., Lowenstein, C. J., Berkowitz, D. E., and Flavahan, N. A. (2010). Increased endothelial exocytosis and generation of endothelin-1 contributes to constriction of aged arteries. Circ. Res. 107, 242–251. doi: 10.1161/circresaha.109.210229
Goto, K., Fujii, K., Kansui, Y., and Iida, M. (2004). Changes in endothelium-derived hyperpolarizing factor in hypertension and ageing: response to chronic treatment with renin-angiotensin system inhibitors. Clin. Exp. Pharmacol. Physiol. 31, 650–655. doi: 10.1111/j.1440-1681.2004.04054.x
Harvey, A., Montezano, A. C., and Touyz, R. M. (2015). Vascular biology of ageing-Implications in hypertension. J. Mol. Cell. Cardiol. 83, 112–121. doi: 10.1016/j.yjmcc.2015.04.011
He, J. Z., Quan, A., Xu, Y., Teoh, H., Wang, G., Fish, J. E., et al. (2007). Induction of matrix metalloproteinase-2 enhances systemic arterial contraction after hypoxia. Am. J. Physiol. Heart Circ. Physiol. 292, H684–H693.
Hodzic, J., Izetbegovic, S., Muracevic, B., Iriskic, R., and Stimjanin Jovic, H. (2017). Nitric oxide biosynthesis during normal pregnancy and pregnancy complicated by preeclampsia. Med. Glas. (Zenica) 14, 211–217.
Jackson, M. J., and McArdle, A. (2011). Age-related changes in skeletal muscle reactive oxygen species generation and adaptive responses to reactive oxygen species. J. Physiol. 589(Pt 9), 2139–2145. doi: 10.1113/jphysiol.2011.206623
Jakovljevic, D. G. (2018). Physical activity and cardiovascular aging: physiological and molecular insights. Exp. Gerontol. 109, 67–74. doi: 10.1016/j.exger.2017.05.016
Kahveci, B., Melekoglu, R., Evruke, I. C., and Cetin, C. (2018). The effect of advanced maternal age on perinatal outcomes in nulliparous singleton pregnancies. BMC Pregnancy Childbirth 18:343. doi: 10.1186/s12884-018-1984-x
Kenny, L. C., Baker, P. N., Kendall, D. A., Randall, M. D., and Dunn, W. R. (2002). Differential mechanisms of endothelium-dependent vasodilator responses in human myometrial small arteries in normal pregnancy and pre-eclampsia. Clin. Sci. (Lond.) 103, 67–73. doi: 10.1042/cs1030067
Kenny, L. C., Lavender, T., McNamee, R., O’Neill, S. M., Mills, T., and Khashan, A. S. (2013). Advanced maternal age and adverse pregnancy outcome: evidence from a large contemporary cohort. PLoS One. 8:e56583. doi: 10.1371/journal.pone.0056583
Khalil, A., Syngelaki, A., Maiz, N., Zinevich, Y., and Nicolaides, K. H. (2013). Maternal age and adverse pregnancy outcome: a cohort study. Ultrasound Obstet. Gynecol. 42, 634–643. doi: 10.1002/uog.12494
Kruger-Genge, A., Blocki, A., Franke, R. P., and Jung, F. (2019). Vascular endothelial cell biology: an update. Int. J. Mol. Sci. 20:4411. doi: 10.3390/ijms20184411
Kuruppu, S., and Smith, A. I. (2012). Endothelin converting Enzyme-1 phosphorylation and trafficking. FEBS Lett. 586, 2212–2217. doi: 10.1016/j.febslet.2012.06.020
Lamminpaa, R., Vehvilainen-Julkunen, K., Gissler, M., and Heinonen, S. (2012). Preeclampsia complicated by advanced maternal age: a registry-based study on primiparous women in Finland 1997-2008. BMC Pregnancy Childbirth 12:47. doi: 10.1186/1471-2393-12-47
Lean, S. C., Heazell, A. E. P., Dilworth, M. R., Mills, T. A., and Jones, R. L. (2017). Placental dysfunction underlies increased risk of fetal growth restriction and stillbirth in advanced maternal age women. Sci. Rep. 7:9677.
Lekontseva, O., Chakrabarti, S., and Davidge, S. T. (2010). Endothelin in the female vasculature: a role in aging? Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R509–R516.
Liguori, I., Russo, G., Curcio, F., Bulli, G., Aran, L., Della-Morte, D., et al. (2018). Oxidative stress, aging, and diseases. Clin. Interv. Aging 13, 757–772.
Lindsey, M. L., Goshorn, D. K., Squires, C. E., Escobar, G. P., Hendrick, J. W., Mingoia, J. T., et al. (2005). Age-dependent changes in myocardial matrix metalloproteinase/tissue inhibitor of metalloproteinase profiles and fibroblast function. Cardiovasc. Res. 66, 410–419. doi: 10.1016/j.cardiores.2004.11.029
Ludford, I., Scheil, W., Tucker, G., and Grivell, R. (2012). Pregnancy outcomes for nulliparous women of advanced maternal age in South Australia, 1998-2008. Aust. N. Z. J. Obstet. Gynaecol. 52, 235–241. doi: 10.1111/j.1479-828x.2012.01442.x
Luksha, L., Nisell, H., and Kublickiene, K. (2004). The mechanism of EDHF-mediated responses in subcutaneous small arteries from healthy pregnant women. Am. J. Physiol. Regul. Integr. Comp. Physiol. 286, R1102–R1109.
Ma, Z., Mao, C., Jia, Y., Fu, Y., and Kong, W. (2020). Extracellular matrix dynamics in vascular remodeling. Am. J. Physiol. Cell Physiol. 319, C481–C499.
Magness, R. R., Rosenfeld, C. R., Hassan, A., and Shaul, P. W. (1996). Endothelial vasodilator production by uterine and systemic arteries. I. Effects of ANG II on PGI2 and NO in pregnancy. Am. J. Physiol. 270(6 Pt 2), H1914–H1923.
Magness, R. R., Shaw, C. E., Phernetton, T. M., Zheng, J., and Bird, I. M. (1997). Endothelial vasodilator production by uterine and systemic arteries. II. Pregnancy effects on NO synthase expression. Am. J. Physiol. 272(4 Pt 2), H1730–H1740.
Mandala, M., Gokina, N., Barron, C., and Osol, G. (2012). Endothelial-derived hyperpolarization factor (EDHF) contributes to PlGF-induced dilation of mesenteric resistance arteries from pregnant rats. J. Vasc. Res. 49, 43–49. doi: 10.1159/000329821
Martin, J. A., Hamilton, B. E., Sutton, P. D., Ventura, S. J., Menacker, F., Kirmeyer, S., et al. (2009). Births: final data for 2006. Natl. Vital Stat. Rep. 57, 1–102.
May, L. (2015). Cardiac physiology of pregnancy. Compr Physiol. 5, 1325–1344. doi: 10.1002/cphy.c140043
Morris, N. H., Sooranna, S. R., Learmont, J. G., Poston, L., Ramsey, B., Pearson, J. D., et al. (1995). Nitric oxide synthase activities in placental tissue from normotensive, pre-eclamptic and growth retarded pregnancies. Br. J. Obstet. Gynaecol. 102, 711–714. doi: 10.1111/j.1471-0528.1995.tb11428.x
Napso, T., Hung, Y. P., Davidge, S. T., Care, A. S., and Sferruzzi-Perri, A. N. (2019). Advanced maternal age compromises fetal growth and induces sex-specific changes in placental phenotype in rats. Sci. Rep. 9:16916.
National Research Council (US) Subcommittee on Laboratory Animal Nutrition (1995). Nutrient Requirements of Laboratory Animals: Fourth Revised Edition, 1995. Washington, DC: National Academies Press.
Ng, K. F., Leung, S. W., Man, R. Y., and Vanhoutte, P. M. (2008). Endothelium-derived hyperpolarizing factor mediated relaxations in pig coronary arteries do not involve Gi/o proteins. Acta Pharmacol. Sin. 29, 1419–1424. doi: 10.1111/j.1745-7254.2008.00905.x
Ni, Y., Meyer, M., and Osol, G. (1997). Gestation increases nitric oxide-mediated vasodilation in rat uterine arteries. Am. J. Obstet. Gynecol. 176, 856–864. doi: 10.1016/s0002-9378(97)70611-2
Osol, G., and Mandala, M. (2009). Maternal uterine vascular remodeling during pregnancy. Physiology (Bethesda) 24, 58–71. doi: 10.1152/physiol.00033.2008
Osol, G., and Moore, L. G. (2014). Maternal uterine vascular remodeling during pregnancy. Microcirculation 21, 38–47. doi: 10.1111/micc.12080
Owusu Darkwa, E., Djagbletey, R., Sottie, D., Owoo, C., Vanderpuye, N. M., Essuman, R., et al. (2018). Serum nitric oxide levels in healthy pregnant women: a case- control study in a tertiary facility in Ghana. Matern Health Neonatol. Perinatol. 4:3.
Parnot, C., Le Moullec, J. M., Cousin, M. A., Guedin, D., Corvol, P., and Pinet, F. (1997). A live-cell assay for studying extracellular and intracellular endothelin-converting enzyme activity. Hypertension 30, 837–844. doi: 10.1161/01.hyp.30.4.837
Pascoal, I. F., Lindheimer, M. D., Nalbantian-Brandt, C., and Umans, J. G. (1995). Contraction and endothelium-dependent relaxation in mesenteric microvessels from pregnant rats. Am. J. Physiol. 269(6 Pt 2), H1899–H1904.
Plant, M., Armstrong, C., Ruggiero, A., Sherrill, C., Uberseder, B., Jeffries, R., et al. (2020). Advanced maternal age impacts physiologic adaptations to pregnancy in vervet monkeys. Geroscience 42, 1649–1661. doi: 10.1007/s11357-020-00219-8
Quinn, R. (2005). Comparing rat’s to human’s age: how old is my rat in people years? Nutrition 21, 775–777. doi: 10.1016/j.nut.2005.04.002
Salihu, H. M., Wilson, R. E., Alio, A. P., and Kirby, R. S. (2008). Advanced maternal age and risk of antepartum and intrapartum stillbirth. J. Obstet. Gynaecol. Res. 34, 843–850. doi: 10.1111/j.1447-0756.2008.00855.x
Shah, A., Cooke, C. M., Kirschenman, R. D., Quon, A. L., Morton, J. S., Care, A. S., et al. (2018). Sex-specific effects of advanced maternal age on cardiovascular function in aged adult rat offspring. Am. J. Physiol. Heart Circ. Physiol. 315, H1724–H1734.
Shirasaki, Y., Su, C., Lee, T. J., Kolm, P., Cline, W. H. Jr., and Nickols, G. A. (1986). Endothelial modulation of vascular relaxation to nitrovasodilators in aging and hypertension. J Pharmacol. Exp. Ther. 239, 861–866.
Simard, E., Jin, D., Takai, S., Miyazaki, M., Brochu, I., and D’Orleans-Juste, P. (2009). Chymase-dependent conversion of big endothelin-1 in the mouse in vivo. J. Pharmacol. Exp. Ther. 328, 540–548. doi: 10.1124/jpet.108.142992
Sladek, S. M., Magness, R. R., and Conrad, K. P. (1997). Nitric oxide and pregnancy. Am. J. Physiol. 272(2 Pt 2), R441–R463.
Stauffer, B. L., Westby, C. M., and DeSouza, C. A. (2008). Endothelin-1, aging and hypertension. Curr. Opin. Cardiol. 23, 350–355. doi: 10.1097/hco.0b013e328302f3c6
Strait, J. B., and Lakatta, E. G. (2012). Aging-associated cardiovascular changes and their relationship to heart failure. Heart Fail. Clin. 8, 143–164. doi: 10.1016/j.hfc.2011.08.011
Takaki, A., Morikawa, K., Tsutsui, M., Murayama, Y., Tekes, E., Yamagishi, H., et al. (2008). Crucial role of nitric oxide synthases system in endothelium-dependent hyperpolarization in mice. J. Exp. Med. 205, 2053–2063. doi: 10.1084/jem.20080106
Tirapelli, C. R., Fecteau, M. H., Honore, J. C., Legros, E., Gobeil, F., and D’Orleans-Juste, P. (2006). Enzymatic pathways involved in the generation of endothelin-1(1-31) from exogenous big endothelin-1 in the rabbit aorta. Br. J. Pharmacol. 148, 527–535. doi: 10.1038/sj.bjp.0706735
Toda, N. (2012). Age-related changes in endothelial function and blood flow regulation. Pharmacol. Ther. 133, 159–176.
Valdes, G., Kaufmann, P., Corthorn, J., Erices, R., Brosnihan, K. B., and Joyner-Grantham, J. (2009). Vasodilator factors in the systemic and local adaptations to pregnancy. Reprod. Biol. Endocrinol. 7:79.
Vanhoutte, P. M. (2004). Endothelium-dependent hyperpolarizations: the history. Pharmacol. Res. 49, 503–508.
Veerareddy, S., Cooke, C. L., Baker, P. N., and Davidge, S. T. (2002). Vascular adaptations to pregnancy in mice: effects on myogenic tone. Am. J. Physiol. Heart Circ. Physiol. 283, H2226–H2233.
Versari, D., Daghini, E., Virdis, A., Ghiadoni, L., and Taddei, S. (2009). Endothelium-dependent contractions and endothelial dysfunction in human hypertension. Br. J. Pharmacol. 157, 527–536.
Villar, M. A., and Sibai, B. M. (1989). Clinical significance of elevated mean arterial blood pressure in second trimester and threshold increase in systolic or diastolic blood pressure during third trimester. Am. J. Obstet. Gynecol. 160, 419–423.
Wang, B., Li, B. W., Li, H. W., Li, A. L., Yuan, X. C., Wang, Q., et al. (2014). Enhanced matrix metalloproteinases-2 activates aortic endothelial hypermeability, apoptosis and vascular rarefaction in spontaneously hypertensive rat. Clin. Hemorheol. Microcirc. 57, 325–338.
Wang, M., Kim, S. H., Monticone, R. E., and Lakatta, E. G. (2015). Matrix metalloproteinases promote arterial remodeling in aging, hypertension, and atherosclerosis. Hypertension 65, 698–703.
Wang, M., and Lakatta, E. G. (2002). Altered regulation of matrix metalloproteinase-2 in aortic remodeling during aging. Hypertension 39, 865–873.
Wang, M., Zhang, J., Telljohann, R., Jiang, L., Wu, J., Monticone, R. E., et al. (2012). Chronic matrix metalloproteinase inhibition retards age-associated arterial proinflammation and increase in blood pressure. Hypertension 60, 459–466.
Westby, C. M., Weil, B. R., Greiner, J. J., Stauffer, B. L., and DeSouza, C. A. (2011). Endothelin-1 vasoconstriction and the age-related decline in endothelium-dependent vasodilatation in men. Clin. Sci. (Lond.) 120, 485–491.
Williams, D. J., Vallance, P. J., Neild, G. H., Spencer, J. A., and Imms, F. J. (1997). Nitric oxide-mediated vasodilation in human pregnancy. Am. J. Physiol. 272(2 Pt 2), H748–H752.
Xu, X., Wang, B., Ren, C., Hu, J., Greenberg, D. A., Chen, T., et al. (2017). Age-related impairment of vascular structure and functions. Aging Dis. 8, 590–610.
Keywords: advanced maternal age, mesenteric artery, wire myography, endothelium-dependent relaxation, pregnancy adaptations
Citation: Pasha M, Wooldridge AL, Kirschenman R, Spaans F, Davidge ST and Cooke C-LM (2021) Altered Vascular Adaptations to Pregnancy in a Rat Model of Advanced Maternal Age. Front. Physiol. 12:718568. doi: 10.3389/fphys.2021.718568
Received: 01 June 2021; Accepted: 07 July 2021;
Published: 28 July 2021.
Edited by:
Babbette LaMarca, University of Mississippi Medical Center School of Dentistry, United StatesReviewed by:
Owen Woodman, Monash University, AustraliaDina Gaynullina, Lomonosov Moscow State University, Russia
Sarah H. Lindsey, Tulane University, United States
Copyright © 2021 Pasha, Wooldridge, Kirschenman, Spaans, Davidge and Cooke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christy-Lynn M. Cooke, christyl@ualberta.ca
 Mazhar Pasha
Mazhar Pasha Amy L. Wooldridge
Amy L. Wooldridge Raven Kirschenman
Raven Kirschenman Floor Spaans
Floor Spaans Sandra T. Davidge
Sandra T. Davidge Christy-Lynn M. Cooke
Christy-Lynn M. Cooke