- 1Department of Endocrinology and Metabolism, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico
- 2Unidad de Investigación de Enfermedades Metabólicas, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico
- 3Instituto Tecnológico y de Estudios Superiores de Monterrey Tec Salud, Monterrey, Mexico
Interest has been focused on differentiating anatomical, molecular, and physiological characteristics of the types of mammalian adipose tissues. White adipose tissue (WAT) and brown adipose tissue (BAT) are the two main forms of adipose tissue in humans. WAT functions as an endocrine organ and serves as a reservoir of energy in the form of triglycerides. The hormones released by WAT are called adipokines. BAT consists of a group of specialized cells with abundant uncoupling protein 1 (UCP1) in the inner mitochondrial membrane and also fulfills endocrine functions. Following the identification of functional (BAT) in human adults, there has been a great deal of interest in finding out how it is induced, its localization, and the mechanisms by which it regulates thermogenesis. Fibroblast growth factor 21 (FGF21) is a key regulator of the differentiation to brown adipocytes. The main mechanisms occur through enhancing UCP1 expression. In addition, following exposure to cold or exercise, FGF21 induces upregulation of local peroxisome proliferator-activated receptor gamma co-activator (PGC)-1-alfa and thus promotes thermogenesis in adipose tissue and skeletal muscle. FGF21 integrates several pathways allowing the regulation of human energy balance, glucose levels, and lipid metabolism. Such mechanisms and their clinical relevance are summarized in this review.
Introduction
Fibroblast growth factor 21 (FGF21) has important effects on energy balance, glucose metabolism, and lipid metabolism (Kharitonenkov et al., 2005). Initial reports identified the liver as its main source (Nishimura et al., 2000). On secretion, its most important target is white adipose tissue (WAT), where FGF21 increases expression of GLUT1 and consequently glucose uptake (Kharitonenkov et al., 2005). During fasting or starvation, lipolysis is triggered, with a subsequent increment in circulating free fatty acids (FFAs). FFAs induce the activation of the peroxisome proliferator-activated receptor (PPAR)-alfa in the liver, resulting in the synthesis and release of FGF21. Since carbohydrate ingestion is absent during starvation, FGF21 induces ketone body formation in the liver as an additional energy source (Badman et al., 2007; Inagaki et al., 2008). FGF21 may also be considered an adipokine, since it is also synthesized and released from WAT. It shows complementary actions to adiponectin (Lin et al., 2013), increasing insulin sensitivity, improving the lipid profile, reducing glucose levels without causing hypoglycemia, and ensuring energy availability during starvation (Badman et al., 2007; Inagaki et al., 2008). In human insulin resistance states, FGF21 levels have a positive correlation with the number of metabolic syndrome traits, the severity of oxidative stress, and the presence of type 2 diabetes (Zhang et al., 2008; Cuevas-Ramos et al., 2010; Gómez-Sámano et al., 2017). Other human stress-related conditions that increase circulating FGF21 levels are lactation (Schoenberg et al., 2011), exercise (Cuevas-Ramos et al., 2012b), growth hormone treatment (Chen et al., 2011), and anorexia nervosa (Dostálová, 2008). Interestingly, FGF21 is now considered an important mediator for decreasing oxidative stress, and possibly, preventing microvascular diseases such as diabetic nephropathy (Jian et al., 2012).
The role of FGF21 as a therapeutic option in human metabolic diseases is of increasing importance. Currently, there are multiple recombinant FGF21 analogs in phase 2 and phase 3 clinical trials (Mu et al., 2012; Gaich et al., 2013; Talukdar et al., 2016). Interest has increased even more so after the discovery of the crucial role of FGF21 in inducing the proliferation of brown adipose tissue (BAT) (Fisher et al., 2012). This review describes the mechanisms by which FGF21 induces “browning” of adipose tissue and how it may have a role in the treatment of human metabolic diseases, including obesity and type 2 diabetes.
White, Beige, and Brown Subtypes of Adipose Tissues in Humans
WAT and BAT are the two main subtypes of adipose tissue in humans. WAT has important endocrine functions in addition to its role as a reservoir of energy in the form of triglycerides. The hormones released from WAT, namely adipokines, are a well-recognized group of bioactive factors with endocrine actions that act through specific cell-membrane receptors. Adipokines trigger certain intracellular signaling pathways, which modulate human metabolism (Piya et al., 2013). The most important are leptin and adiponectin, but visfatin, chemerin, omentin, hepcidin, apelin, and vaspin have also been described (Piya et al., 2013).
BAT is also an important endocrine organ. It consists of a group of specialized cells with abundant expression of uncoupling protein 1 (UCP1) in the inner mitochondrial membrane (Aherne and Hull, 1966; Cannon and Nedergaard, 2004). The BAT hormones are named “batokines” (Baboota et al., 2015; Booth et al., 2016). The principle function of BAT is to dissipate stored energy in the form of heat by uncoupling energy oxidation from ATP synthesis (Fedorenko et al., 2012). Initially, BAT was only considered as an energy-producing organ in rodents and human infants (Bartness et al., 2010). However, after the development of 18F-fluorodeoxyglucose (FDG) positron emission tomography-computed tomography (PET-CT), BAT has also been identified in human adults (Nedergaard et al., 2007; Cypess et al., 2009). Nevertheless, the origin of BAT is still under debate, although originally it was thought to be derived from skeletal muscle-like lineage (Myf5+) (Seale et al., 2007). In human adults, a type of adipose tissue showing characteristics between that of white and brown adipocytes has been identified; this kind of adipose tissue is known as beige adipose tissue (brite, brown-in-white) (Jespersen et al., 2013). It appears that beige and white adipocytes arise from both Myf5+ and Myf5- progenitor cells. These findings confirm that the skeletal muscle-like lineage is not the only source of BAT (Wu et al., 2012).
The location of the different types of adipose tissue varies. Beige and WAT have mainly visceral (mesenteric, perigonadal or omental adipose tissue surrounding organs) and subcutaneous (under skin) locations. BAT is located only in axillary, subscapular, interscapular, and periaortic regions in rodents; in cervical, supraclavicular, paravertebral, mediastinal regions in humans; and in perirenal regions in both (Park et al., 2014; Sanchez-Gurmaches and Guertin, 2014). Brite or beige adipocytes have basal metabolic actions similar to those seen in white adipocytes, and with the enough stimulus, they are able to transform into thermogenic adipocytes with higher UCP1 expression similar to BAT (Wu et al., 2012). This process is referred as “browning” and it describes the capacity of white adipocytes to acquire a phenotype similar to that of BAT, leading to increased thermogenesis. It is achieved when white adipocytes are exposed to cold or to beta 3-adrenoreceptor agonists (Young et al., 1984, Harms and Seale, 2013). Browning occurs mainly in subcutaneous white adipose fat depots. The underlying molecular mechanisms for this trans-differentiation are currently under intensive research (Luo and Liu, 2016). In addition, there are important structural differences among WAT, brite, and BAT. WAT is a large lipid droplet, with a peripheral nucleus and a small amount of cytoplasm, whereas BAT has a central nucleus with more cytoplasm but smaller lipid droplets. In between these is the brite or beige tissue; this has the mixed structural characteristics of both. Sometimes the different structures are found together; for example, the ectopic expression of UCP1 and the presence of the PR domain containing 16 (PRDM16) suggests that brite adipocytes are mixed with white adipocyte depots (Wu et al., 2013). The balance between WAT and BAT, and their endocrine regulation, are key elements to better understand the development of weight gain and human metabolic diseases.
Molecular Pathways and Clinical Relevance of Browning Induced by FGF21
Since the discovery of FGF21, it has been appreciated that its synthesis is strongly related to cold exposure (Badman et al., 2007; Inagaki et al., 2008). In mice, during hypothermia, FGF21 induces torpor, a short-term hibernation state in which animals can save energy by reducing body temperature and physical activity (Badman et al., 2007). More recently, studies have shown a higher expression of FGF21 in inguinal WAT after cold exposure. The role of FGF21 produced in WAT includes both paracrine and autocrine actions; this results in the local upregulation of peroxisome proliferator-activated receptor gamma co-activator (PGC)-1-alfa and thus an increase in thermogenesis (Hondares et al., 2010; Fisher et al., 2012; Adams et al., 2013; Emanuelli et al., 2014). PGC1-alfa is a protein involved in modulating several effects in post-exercise skeletal muscle, including the improvement of energy and glucose metabolism (Summermatter et al., 2013). Interestingly, PGC1-alfa is also induced after irisin or insulin exposure, both hormones showing a clear interaction with FGF21 post-exercise (Cuevas-Ramos et al., 2010, 2012b; Bostrom et al., 2012; Fisher et al., 2012; Hu and Christian, 2017). Irisin-induced phosphorylation of p38 mitogen-activated protein kinase (p38 MAPK) and extracellular signal-related kinase (ERK) show a positive correlation with shivering intensity (Bostrom et al., 2012; Zhang et al., 2014). FGF21 also shows a direct relationship with exercise intensity (Cuevas-Ramos et al., 2010, 2012b). The consequence of these PGC1-alfa inducers is to promote adaptive thermogenesis with “browning” of WAT (Fisher et al., 2012). The main mechanism following FGF21 action is PPAR-gamma activation in WAT, together with the irisin effect inducing MAPK and ERK pathways. This results in differentiation of pre-adipocytes to mature white adipocytes, which are then available for “browning” (Hondares et al., 2011; Zhang Y. et al., 2016).
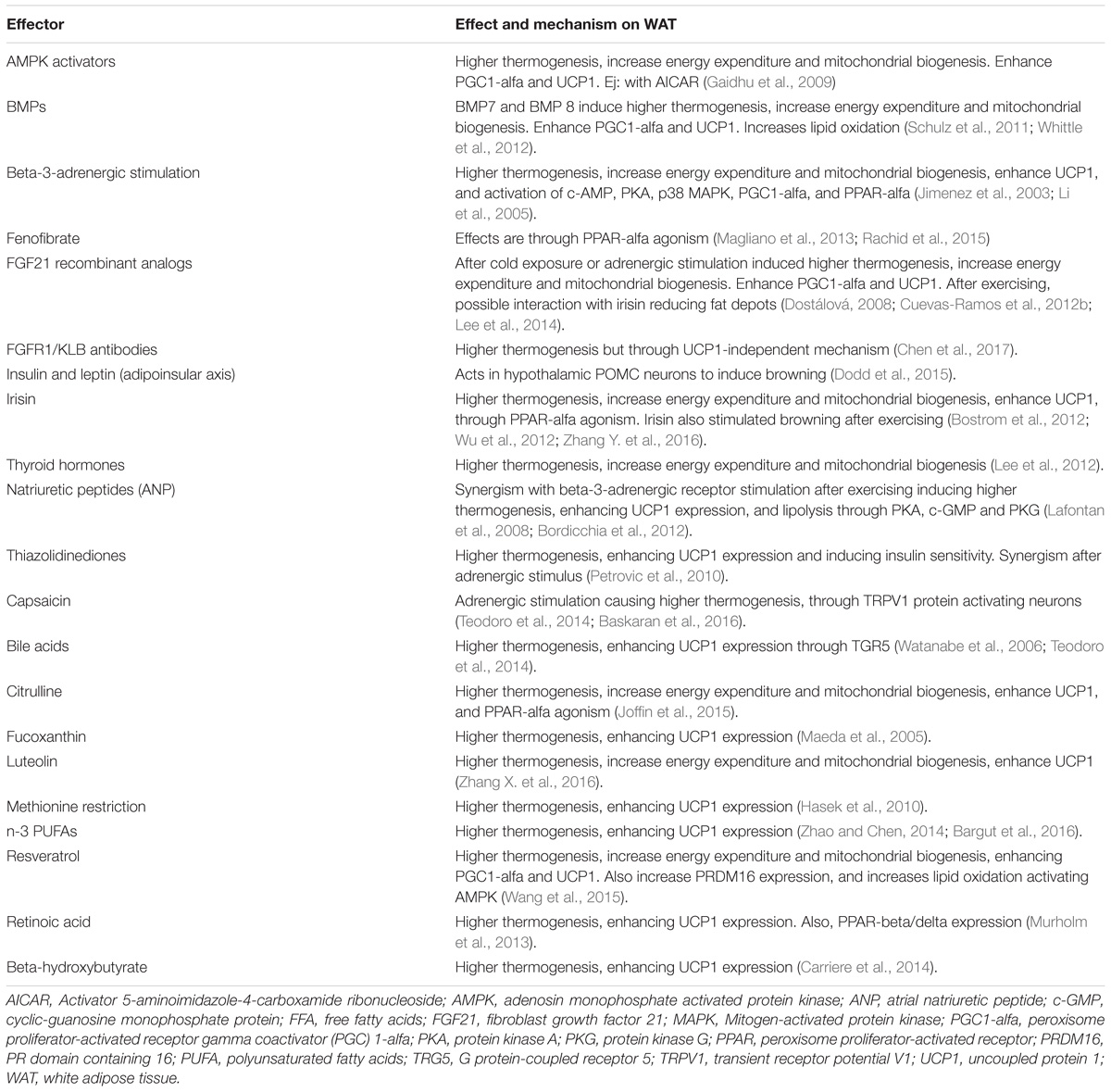
Some animal models have reported findings consistent with these actions. For example, FGF21 deficiency in mice results in increased body weight with excessive adiposity, higher serum cholesterol, insulin resistance, and hyperglycemia (Kharitonenkov et al., 2005). The finding of a 30–40% lower nuclear content of PGC1-alfa at the hepatic mitochondrial level in Fgf21 KO mice compared with WT mice, is a potential explanation for these results (Fletcher et al., 2016). FGF21 induces palmitate oxidation and β-hydroxyacyl-CoA dehydrogenase (β-HAD) activity. In the Fgf21 KO model, these enzymatic activities are decreased, indicating lower lipid oxidation, a reduction in glucose metabolism, and a lower degree of energy waste (Fletcher et al., 2016). In contrast, overexpression of FGF21 effectively decreases weight, adiposity, levels of FFAs, triglycerides, glucose, and insulin, all due to the normalization of mitochondrial oxidation (Fletcher et al., 2016) and the improvement in insulin sensitivity (Kharitonenkov et al., 2005). Interestingly, exercise, irisin, and noradrenaline were necessary to restore PGC1-alfa content in the liver despite overexpression of FGF21, emphasizing the key interaction of such inducers with FGF21 (Fletcher et al., 2016). Insulin, the most important regulator of energy and glucose metabolism, enhanced differentiation of WAT to brite and brown adipocytes through pro-opiomelanocortin (POMC) neurons (Table 1 and Figure 1); (Dodd et al., 2015). Therefore, multiple mechanisms may be inter-connected to improve metabolism in humans, with FGF21 functioning as an important link between them.
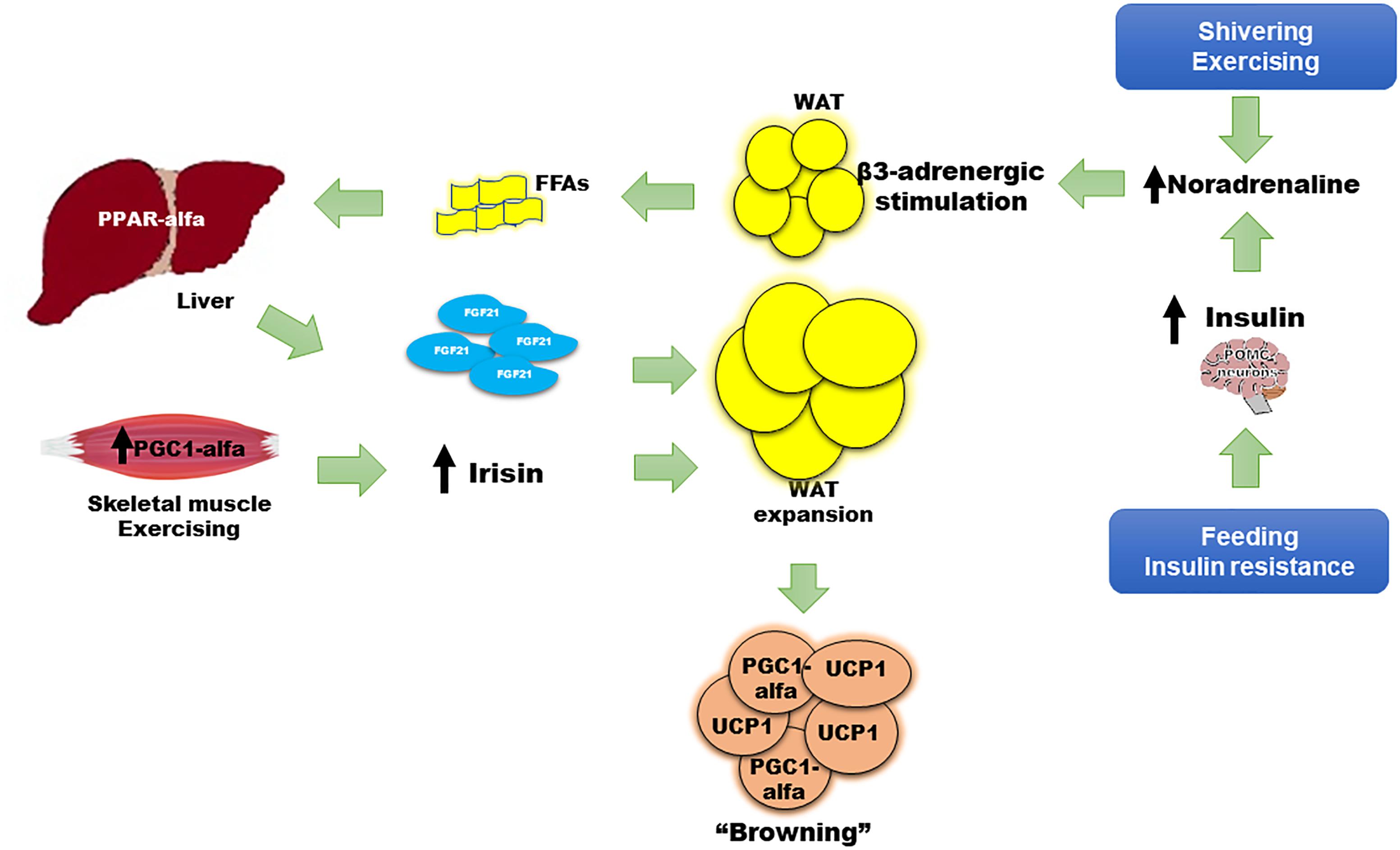
Figure 1. Role of FGF21 in the “browning” of adipose tissue. Adaptive thermogenesis following cold exposure, shivering, or exercise, and physiologic (i.e., feeding) or pathologic (i.e., insulin resistance) states, begins a compensatory process to induce “browning” of WAT, thus enhancing thermogenesis, energy waste, and improving cell metabolism. The principle mechanisms to induce “browning” involve insulin, irisin, and FGF21. Insulin increases adrenergic stimulation and noradrenaline secretion after acting on POMC neurons in the central nervous system. FGF21 also has a WAT-independent mechanism acting directly to the CNS, increasing noradrenaline release. Noradrenaline induces beta 3-adrenergic receptor stimulation and greater lipolysis that produces FFAs as main substrate for PPAR-alfa agonism and FGF21 synthesis and release from liver. Irisin is mainly released from skeletal muscle after shivering or exercise. Noradrenaline, irisin, and FGF21 promote uncoupling protein 1 (UCP1) expression, a protein that increases thermogenesis at the mitochondrial inner membrane, and upregulation of local peroxisome proliferator-activated receptor gamma co-activator (PGC)-1-alfa on white adipocytes, turning them to brite or brown adipose tissue. WAT expansion is also induced by FGF21, increasing insulin sensitivity.
The exogenous administration of an FGF21 analog in animal models has been associated with a thermogenic effect, an improvement in glucose homeostasis, lipid profile, and a reduction in body weight (Wu et al., 2011; Veniant et al., 2012). However, in humans, recombinant FGF21 analogs have not shown these effects. FGF21 is paradoxically increased in insulin-resistant states such as obesity or type 2 diabetes; this suggests either a resistance to FGF21 effects or a compensatory response to these metabolic disarrangements (Zhang et al., 2008; Cuevas-Ramos et al., 2010). Nevertheless, the key metabolic role of FGF21 is clear. Firstly, WAT increases FGF21 expression in response to feeding, which has an autocrine action on PPAR-gamma activity; PPAR-gamma is an important inducer of insulin sensitivity pathways, adipocyte maturation, and function (Dutchak et al., 2012). Secondly, FGF21 is significantly induced after moderate to intensive physical activity (Cuevas-Ramos et al., 2010, 2012b), and interestingly, its release has been correlated with sympathetic nervous system activation and lipolysis markers, such as noradrenaline and serum free fatty acids, respectively (Cuevas-Ramos et al., 2012b). Thirdly, FGF21 regulates metabolism through increasing insulin sensitivity at WAT and BAT, but this may happen in an acute and a chronic manner. Acute and chronic exogenous administration of FGF21 induce insulin sensitivity independently of adiponectin action (BonDurant et al., 2017). However, the chronic effect of FGF21 is not necessarily via a direct effect on WAT since it also induces signaling in the central nervous system via POMC neurons, and on brown adipocytes, enhancing thermogenesis and insulin-sensitivity in mice (Figure 1). These effects require functional insulin signaling in adipose tissue, otherwise the FGF21-benefit is lost. In addition, the action of FGF21 on the central nervous system is important to induce energy expenditure, mainly by increasing noradrenaline release (Figure 1) (Owen et al., 2014). Noradrenaline plays an important role in the regulation of “browning.” It is the most studied activator of thermogenesis, increasing UCP1 transcription, enhancing lipolysis and mitochondrial oxidation (Figure 1; (Puigserver et al., 1996). After cold-induced shivering or physical activity stress, noradrenergic pathways increase thermogenic gene expression through c-AMP-mediated mechanisms (Hondares et al., 2011). Lipolysis and FFAs increase following noradrenaline release. With acute intensive exercise, the increase in beta 3-receptor activity also causes an increment in circulating FGF21 (Cuevas-Ramos et al., 2012b). Increased circulating FFAs induce PPAR-alfa expression in the liver, increasing FGF21 synthesis and release into circulation (Badman et al., 2007; Inagaki et al., 2008). FGF21 can also increase insulin sensitivity by promoting the expansion of subcutaneous WAT. Fgf21 knockout mice show less subcutaneous WAT and a greater degree of insulin resistance. After treatment with recombinant FGF21, subcutaneous adipose tissue was restored with a subsequent improvement in insulin sensitivity (Li et al., 2018). The expression of co-factor beta klotho is necessary to accomplish the FGF21-related expansion of subcutaneous fat (Li et al., 2018). Finally, chronic pharmacologic administration of FGF21 in obese mice has been shown to suppress growth hormone (GH) and the insulin growth factor-1 (IGF1) signaling axis in the liver, increasing lifespan through an improvement in insulin sensitization, normalization of glycemia, and a reduction in body weight (Zhang Y. et al., 2012).
Under normal conditions, FGF21 is synthetized and released from the liver. However, in certain circumstances, such as adaptive thermogenesis induced by cold exposure or exercise, BAT expresses and releasees FGF21 (Chartoumpekis et al., 2011; Hondares et al., 2011; Giralt et al., 2015; Lee et al., 2015). The production of FGF21 by BAT is not negligible; it significantly contributes to systemic FGF21 levels (Hondares et al., 2011). Following exposure to cold, the production of FGF21 by BAT is greater than that of the liver, enhancing thermogenesis, confirming the key roles of BAT in regulating FGF21 levels (Hondares et al., 2011). Moreover, a dramatic rise in Fgf21 expression in BAT has also been reported in Ucp1-null mice or after genetic inactivation of UCP1 protein. In these situations, there is an increase in serum FGF21 levels without changes in FGF21 gene expression in the liver (Keipert et al., 2015; Samms et al., 2015). This suggests thermogenic regulation of FGF21 through both UCP1-dependent as well as UCP1-independent mechanisms (Keipert et al., 2015; Samms et al., 2015).
Taken together, adipose tissue, liver, and skeletal muscle respond to multiple stimuli in order to increase adaptive thermogenesis and induce the browning of WAT (Figure 1). Expression and release of FGF21 by the liver, BAT, and skeletal muscle is induced by shivering (Badman et al., 2007; Inagaki et al., 2008; Hondares et al., 2011), physical activity (Cuevas-Ramos et al., 2010, 2012b; Kim et al., 2013), protein synthesis after growth hormone treatment (Chen et al., 2011), and as a consequence of experimental or clinical mitochondrial dysfunction following DNA mutations (Suomalainen et al., 2011; Keipert et al., 2014). FGF21, together with irisin, insulin, and noradrenaline, provokes metabolically healthy effects that are concomitantly associated with the browning of WAT (Mossenbock et al., 2014; Lee et al., 2015; Villarroya et al., 2017). The higher BAT activity and increased heat production may benefit human health, reducing weight, preventing hyperglycemia, and hyperlipidemia, and protecting against obesity through enhancement of energy waste (Cuevas-Ramos et al., 2012a). These effects explain the association of FGF21 and explain its key role in the “browning” of WAT. This was probably aimed to allow the adaptation of human metabolism to obesity, diabetes, dyslipidemia, metabolic syndrome, and other insulin resistance states (Figure 1).
FGF21 as Potential Medical Treatment to Induce Browning of WAT
The beneficial metabolic consequences of “browning” may be useful to treat metabolic diseases in humans (Barquissau et al., 2016). There are multiple medical drugs or nutritional inducers that may help to stimulate browning (Table 1). Recently, the role of FGF21 in the browning of WAT has been evaluated with the development of recombinant FGF21-analogs. Initially, clinical trials with analogs LY2405319 and PF05231023 were focused on treating human metabolic diseases including obesity, metabolic syndrome and type 2 diabetes (Gaich et al., 2013; Talukdar et al., 2016). However, resistance to their actions, resulting in an inadequate clinical effect, has been a problem; in humans, only a slight glucose, weight or triglyceride reduction was achieved. The subcutaneous depots of WAT are small cells with greater potential to differentiate (Gustafson and Smith, 2015). Therefore, it is feasible to hypothesize a positive effect if “browning” can be induced, following a sufficient stimulus using FGF21-analogs. In addition, the FGF receptor type 1 together with beta klotho cofactor, called the FGFR1/KLB complex, is the functional target for FGF21 (Kolumam et al., 2015). The use of FGF21 agonist antibodies that specifically activate this complex largely mimic the action of recombinant FGF21 in mice (Kolumam et al., 2015). These include the agonist antibodies BFKB8488 and NGM313, which are currently under clinical research (ClinicalTrials.gov, NCT02593331, NCT02708576, and NCT03060538). Both FGF21 recombinant analogs (Gaich et al., 2013; Talukdar et al., 2016) and the FGFR1/KLB complex agonist antibodies induce higher thermogenesis and browning of BAT through UCP1-dependent pathways. Although UCP1 has traditionally been thought of as indispensable for browning and thermogenesis (Golozoubova et al., 2001; Feldmann et al., 2009), recent research suggests a role of a UCP1-independent pathway (Chen et al., 2017). In Ucp1 KO mice, higher thermogenesis with weight loss and beneficial changes in cardiometabolic markers have been reported (Veniant et al., 2015; Chen et al., 2017). The origin of this UCP1-independent thermogenesis is still controversial, with different reports suggesting the opposite (Keipert et al., 2015). Further investigation is warranted to clarify if higher thermogenesis can be obtained without overexpression of UCP1.
In addition to FGF21-recombinant analogs or the FGFR1-KLB complex agonist antibodies, other drugs have been used with similar aims; however, most have shown little clinical utility. For example, PPAR-alfa agonists (fibrates), adrenergic beta-3-receptor stimulators, thyroid hormones, and more recently irisin have been tested (Table 1). There are also certain nutritional inducers of “browning” of WAT that may be considered as therapeutic options. The most important hormones, drugs and nutritional inducers of browning are summarized on Table 1. It is important to mention that although nicotine has been associated with body weight reduction, mainly due to the associated decreased appetite, greater lipolysis, and increased energy waste (Zoli and Picciotto, 2012), it has never been confirmed that smoking cigarettes can induce browning (Chen et al., 2005).
Conclusion
FGF21 is a key regulator of the differentiation of WAT to brown adipocytes, resulting in enhanced thermogenesis and energy waste. The main action seems to be through UCP1-dependent and -independent mechanisms. In addition, after cold exposure or exercising, FGF21-induced upregulation of local peroxisome proliferator-activated receptor gamma co-activator (PGC)-1-alfa increases thermogenesis in adipose tissue and skeletal muscle. Potential mechanisms involve higher noradrenaline levels that act on the WAT-beta-3 adrenergic receptor, inducing lipolysis, and higher serum free fatty acids, which in turn increase PPAR-alfa agonism at liver, and higher FGF21 synthesis and then release into circulation. This effect contributes to other FGF21-related mechanisms that integrate metabolic pathways to regulate human energy balance, glucose and lipid levels. Therefore, the development of better recombinant FGF21 analogs as a potential treatment for metabolic diseases in humans is necessary.
Author Contributions
All authors made substantial contributions to the conception and/or design of the work; acquisition, analysis, and interpretation of data for the work; drafting the work or revising it critically for important intellectual content; and approved the final version of the article to be published.
Funding
This review was supported by Department of Endocrinology and Metabolism at the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Adams, A. C., Coskun, T., Cheng, C. C., Ls, O. F., Dubois, S. L., and Kharitonenkov, A. (2013). Fibroblast growth factor 21 is not required for the antidiabetic actions of the thiazoladinediones. Mol. Metab. 2, 205–214. doi: 10.1016/j.molmet.2013.05.005
Aherne, W., and Hull, D. (1966). Brown adipose tissue and heat production in the newborn infant. J. Pathol. Bacteriol. 91, 223–234. doi: 10.1002/path.1700910126
Baboota, R. K., Sarma, S. M., Boparai, R. K., Kondepudi, K. K., Mantri, S., and Bishnoi, M. (2015). Microarray based gene expression analysis of murine brown and subcutaneous adipose tissue: significance with human. PLoS One 10:e0127701. doi: 10.1371/journal.pone.0127701
Badman, M. K., Pissios, P., Kennedy, A. R., Koukos, G., Flier, J. S., and Maratos-Flier, E. (2007). Hepatic fibroblast growth factor 21 is regulated by PPAR-alfa and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 5, 426–437. doi: 10.1016/j.cmet.2007.05.002
Bargut, T. C., Souza-Mello, V., Mandarim-de-Lacerda, C. A., and Aguila, M. B. (2016). Fish oil diet modulates epididymal and inguinal adipocyte metabolism in mice. Food Funct. 7, 1468–1476. doi: 10.1039/c5fo00909j
Barquissau, V., Beuzelin, D., Pisani, D. F., Beranger, G. E., Mairal, A., Montagner, A., et al. (2016). White-to-brite conversion in human adipocytes promotes metabolic reprogramming towards fatty acid anabolic and catabolic pathways. Mol. Metab. 5, 352–365. doi: 10.1016/j.molmet.2016.03.002
Bartness, T. J., Vaughan, C. H., and Song, C. K. (2010). Sympathetic and sensory innervation of brown adipose tissue. Int. J. Obes. 34(Suppl. 1), S36–S42. doi: 10.1038/ijo.2010.182
Baskaran, P., Krishnan, V., Ren, J., and Thyagarajan, B. (2016). Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms. Br. J. Pharmacol. 173, 2369–2389. doi: 10.1111/bph.13514
BonDurant, L. D., Ameka, M., Naber, M. C., Markan, K. R., Idiga, S. O., Acevedo, M. R., et al. (2017). FGF21 regulates metabolism through adipose-dependent and independent-mechanisms. Cell Metab. 25, 935–944. doi: 10.1016/j.cmet.2017.03.005
Booth, A., Magnuson, A., Fouts, J., and Foster, M. T. (2016). Adipose tissue: an endocrine organ playing a role in metabolic regulation. Horm. Mol. Biol. Clin. Investig. 26, 25–42. doi: 10.1515/hmbci-2015-0073
Bordicchia, M., Liu, D., Amri, E. Z., Ailhaud, G., Dessi-Fulgheri, P., Zhang, C., et al. (2012). Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J. Clin. Invest. 122, 1022–1036. doi: 10.1172/JCI59701
Bostrom, P., Wu, J., Jedrychowski, M. P., Korde, A., Ye, L., Lo, J. C., et al. (2012). A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481, 463–468. doi: 10.1038/nature10777
Cannon, B., and Nedergaard, J. (2004). Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359. doi: 10.1152/physrev.00015.2003
Carriere, A., Jeanson, Y., Berger-Muller, S., Andre, M., Chenouard, V., Arnaud, E., et al. (2014). Browning of white adipose cells by intermediate metabolites: an adaptive mechanism to alleviate redox pressure. Diabetes 63, 3253–3265. doi: 10.2337/db13-1885
Chartoumpekis, D. V., Habeos, I. G., Ziros, P. G., Psyrogiannis, A. I., Kyriazopoulou, V. E., Papavassiliou, A. G., et al. (2011). Brown adipose tissue responds to cold and adrenergic stimulation by induction of FGF21. Mol. Med. 17, 736–740. doi: 10.2119/molmed.2011.00075
Chen, H., Vlahos, R., Bozinovski, S., Jones, J., Anderson, G. P., and Morris, M. J. (2005). Effect of short-term cigarette smoke exposure on body weight, appetite and brain neuropeptide Y in mice. Neuropsychopharmacology 30, 713–719. doi: 10.1038/sj.npp.1300597
Chen, M. Z., Chang, J. C., Zavala-Solorio, J., Kates, L., Thai, M., Ogasawara, A., et al. (2017). FGF21 mimetic antibody stimulates UCP1-independent brown fat thermogenesis via FGFR1/Beta Klotho complex in non-adipocytes. Mol. Metab. 6, 1454–1467. doi: 10.1016/j.molmet.2017.09.003
Chen, W., Hoo, R. L., Konishi, M., Itoh, N., Lee, P. C., Ye, H. Y., et al. (2011). Growth hormone induces hepatic production of fibroblast growth factor 21 through a mechanism dependent on lipolysis in adipocytes. J. Biol. Chem. 286, 34559–34566. doi: 10.1074/jbc.M111.285965
Cuevas-Ramos, D., Aguilar-Salinas, C. A., and Gomez-Perez, F. J. (2012a). Metabolic actions of fibroblast growth factor 21. Curr. Opin. Pediatr. 24, 523–529. doi: 10.1097/MOP.0b013e3283557d22
Cuevas-Ramos, D., Almeda-Valdés, P., Meza-Arana, C. E., Brito-Córdova, G., Gómez-Pérez, F. J., Mehta, R., et al. (2012b). Exercise increases serum fibroblast growth factor 21 (FGF21) levels. PLoS One 7:e38022. doi: 10.1371/journal.pone.0038022
Cuevas-Ramos, D., Almeda-Valdes, P., Gomez-Perez, F. J., Meza-Arana, C. E., Cruz-Bautista, I., Arellano-Campos, O., et al. (2010). Daily physical activity, fasting glucose, uric acid, and body mass index are independent factors associated with serum fibroblast growth factor 21 levels. Eur. J. Endocrinol. 163, 469–477. doi: 10.1530/EJE-10-0454
Cypess, A. M., Lehman, S., Williams, G., Tal, I., Rodman, D., Goldfine, A. B., et al. (2009). Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360, 1509–1517. doi: 10.1056/NEJMoa0810780
Dodd, G. T., Decherf, S., Loh, K., Simonds, S. E., Wiede, F., Balland, E., et al. (2015). Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell 160, 88–104. doi: 10.1016/j.cell.2014.12.022
Dostálová, I., Kaválková, P., Haluzíková, D., Lacinová, Z., Mráz, M., Papezová, H., et al. (2008). Plasma concentrations of fibroblast growth factors 19 and 21 in patients with anorexia nervosa. J. Clin. Endocrinol. Metab. 93, 3627–3632. doi: 10.1210/jc.2008-0746
Dutchak, P. A., Katafuchi, T., Bookout, A. L., Choi, J. H., Yu, R. T., Mangelsdorf, D. J., et al. (2012). Fibroblast growth factor-21 regulates PPARg activity and the antidiabetic actions of thiazolidinediones. Cell 148, 556–567. doi: 10.1016/j.cell.2011.11.062
Emanuelli, B., Vienberg, S. G., Smyth, G., Cheng, C., Stanford, K. I., Arumugam, M., et al. (2014). Interplay between FGF21 and insulin action in the liver regulates metabolism. J. Clin. Investig. 124, 515–527. doi: 10.1172/JCI67353
Fedorenko, A., Lishko, P. V., and Kirichok, Y. (2012). Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 151, 400–413. doi: 10.1016/j.cell.2012.09.010
Feldmann, H. M., Golozoubova, V., Cannon, B., and Nedergaard, J. (2009). UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 9, 203–209. doi: 10.1016/j.cmet.2008.12.014
Fisher, F. M., Kleiner, S., Douris, N., Fox, E. C., Mepani, R. J., Verdeguer, F., et al. (2012). FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 26, 271–281. doi: 10.1101/gad.177857.111
Fletcher, J. A., Linden, M. A., Sheldon, R. D., Meers, G. M., Morris, E. M., Butterfield, A., et al. (2016). Fibroblast growth factor 21 and exercise-induced hepatic mitochondrial adaptations. Am. J. Physiol. Gastrointest. Liver Physiol. 310, G832–G843. doi: 10.1152/ajpgi.00355.2015
Gaich, G., Chien, J. Y., Fu, H., Glass, L. C., Deeg, M. A., Holland, W. L., et al. (2013). The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 18, 333–340. doi: 10.1016/j.cmet.2013.08.005
Gaidhu, M. P., Fediuc, S., Anthony, N. M., So, M., Mirpourian, M., Perry, R. L., et al. (2009). Prolonged AICAR-induced AMP-kinase activation promotes energy dissipation in white adipocytes: novel mechanisms integrating HSL and ATGL. J. Lipid Res. 50, 704–715. doi: 10.1194/jlr.M800480-JLR200
Giralt, M., Gavalda-Navarro, A., and Villarroya, F. (2015). Fibroblast growth factor-21, energy balance and obesity. Mol. Cell. Endocrinol. 418, 66–73. doi: 10.1016/j.mce.2015.09.018
Golozoubova, V., Hohtola, E., Matthias, A., Jacobsson, A., Cannon, B., and Nedergaard, J. (2001). Only UCP1 can mediate adaptive nonshivering thermogenesis in the cold. FASEB J. 15, 2048–2050. doi: 10.1096/fj.00-0536fje
Gómez-Sámano, M. Á., Grajales-Gómez, M., Zuarth-Vázquez, J. M., Navarro-Flores, M. F., Martínez-Saavedra, M., Juárez-León, Ó. A., et al. (2017). Fibroblast growth factor 21 and its novel association with oxidative stress. Redox Biol. 11, 335–341. doi: 10.1016/j.redox.2016.12.024
Gustafson, B., and Smith, U. (2015). Regulation of white adipogenesis and its relation to ectopic fat accumulation and cardiovascular risk. Atherosclerosis 241, 27–35. doi: 10.1016/j.atherosclerosis.2015.04.812
Harms, M., and Seale, P. (2013). Brown and beige fat: development, function and therapeutic potential. Nat. Med. 19, 1252–1263. doi: 10.1038/nm.3361
Hasek, B. E., Stewart, L. K., Henagan, T. M., Boudreau, A., Lenard, N. R., Black, C., et al. (2010). Dietary methionine restriction enhances metabolic flexibility and increases uncoupled respiration in both fed and fasted states. Am. J. Physiol. Regul. Integr. Comp. Physiol. 299, R728–R739. doi: 10.1152/ajpregu.00837.2009
Hondares, E., Iglesias, R., Giralt, A., Gonzalez, F. J., Giralt, M., Mampel, T., et al. (2011). Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J. Biol. Chem. 286, 12983–12990. doi: 10.1074/jbc.M110.215889
Hondares, E., Rosell, M., Gonzalez, F. J., Giralt, M., Iglesias, R., and Villarroya, F. (2010). Hepatic FGF21 expression is induced at birth via PPAR alpha in response to milk intake and contributes to thermogenic activation of neonatal brown fat. Cell Metab. 11, 206–212. doi: 10.1016/j.cmet.2010.02.001
Hu, J., and Christian, M. (2017). Hormonal factors in the control of the browning of white adipose tissue. Horm. Mol. Biol. Clin. Invest. 31, 1868–1891. doi: 10.1515/hmbci-2017-0017
Inagaki, T., Lin, V. Y., Goetz, R., Mohammadi, M., Mangelsdorf, D. J., and Kliewer, S. A. (2008). Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab. 8, 77–83. doi: 10.1016/j.cmet.2008.05.006
Jespersen, N. Z., Larsen, T. J., Peijs, L., Daugaard, S., Homoe, P., Loft, A., et al. (2013). A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab. 17, 798–805. doi: 10.1016/j.cmet.2013.04.011
Jian, W.-X., Peng, W.-H., Jin, J., Chen, X.-R., Fang, W.-J., Wang, W.-X., et al. (2012). Association between serum fibroblast growth factor 21 and diabetic nephropathy. Metabolism 61, 853–859. doi: 10.1016/j.metabol.2011.10.012
Jimenez, M., Barbatelli, G., Allevi, R., Cinti, S., Seydoux, J., Giacobino, J. P., et al. (2003). Beta 3-adrenoceptor knockout in C57BL/6J mice depresses the occurrence of brown adipocytes in white fat. Eur. J. Biochem. 270, 699–705. doi: 10.1046/j.1432-1033.2003.03422.x
Joffin, N., Jaubert, A. M., Bamba, J., Barouki, R., Noirez, P., and Forest, C. (2015). Acute induction of uncoupling protein 1 by citrulline in cultured explants of white adipose tissue from lean and high-fat-diet-fed rats. Adipocyte 4, 129–134. doi: 10.4161/21623945.2014.989748
Keipert, S., Kutschke, M., Lamp, D., Brachthäuser, L., Neff, F., Meyer, C. W., et al. (2015). Genetic disruption of uncoupling protein 1 in mice renders brown adipose tissue a significant source of FGF21 secretion. Mol. Metab. 4, 537–542. doi: 10.1016/j.molmet.2015.04.006
Keipert, S., Ost, M., Johann, K., Imber, F., Jastroch, M., van Schothorst, E. M., et al. (2014). Skeletal muscle mitochondrial uncoupling drives endocrine cross-talk through the induction of FGF21 as a myokine. Am. J. Physiol. Endocrinol. Metab. 306, E469–E482. doi: 10.1152/ajpendo.00330.2013
Kharitonenkov, A., Shiyanova, T. L., Koester, A., Ford, A. M., Micanovic, R., Galbreath, E. J., et al. (2005). FGF-21 as a novel metabolic regulator. J. Clin. Invest. 115, 1627–1635. doi: 10.1172/JCI23606
Kim, K. H., Kim, S. H., Yang, H. M., Lee, J. B., and Lee, M. S. (2013). Acute exercise induces FGF21 expression in mice and healthy humans. PLoS One 8:e63517. doi: 10.1371/journal.pone.0063517
Kolumam, G., Chen, M. Z., Tong, R., Zavala-Solorio, J., Kates, L., van Bruggen, N., et al. (2015). Sustained brown fat stimulation and insulin sensitization by a humanized bispecific antibody agonist for fibroblast growth factor receptor 1/betaKlotho complex. EBioMedicine 2, 730–743. doi: 10.1016/j.ebiom.2015.05.028
Lafontan, M., Moro, C., Berlan, M., Crampes, F., Sengenes, C., and Galitzky, J. (2008). Control of lipolysis by natriuretic peptides and cyclic GMP. Trends Endocrinol. Metab. 19, 130–137. doi: 10.1016/j.tem.2007.11.006
Lee, H. J., Lee, J. O., Kim, N., Kim, J. K., Kim, H. I., Lee, Y. W., et al. (2015). Irisin, a novel myokine, regulates glucose uptake in skeletal muscle cells via AMPK. Mol. Endocrinol. 29, 873–881. doi: 10.1210/me.2014-1353
Lee, J. Y., Takahashi, N., Yasubuchi, M., Kim, Y. I., Hashizaki, H., Kim, M. J., et al. (2012). Triiodothyronine induces UCP-1 expression and mitochondrial biogenesis in human adipocytes. Am. J. Physiol. Cell Physiol. 302, C463–C472. doi: 10.1152/ajpcell.00010.2011
Lee, P., Linderman, J. D., Smith, S., Brychta, R. J., Wang, J., Idelson, C., et al. (2014). Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 19, 302–309. doi: 10.1016/j.cmet.2013.12.017
Li, H., Wu, G., Fang, Q., Zhang, M., Hui, X., Sheng, B., et al. (2018). Fibroblast growth factor 21 increases insulin sensitivity through specific expansion of subcutaneous fat. Nat. Commun. 2:272. doi: 10.1038/s41467-017-02677-9
Li, P., Zhu, Z., Lu, Y., and Granneman, J. G. (2005). Metabolic and cellular plasticity in white adipose tissue II: role of peroxisome proliferator-activated receptor-alpha. Am. J. Physiol. Endocrinol. Metab. 289, E617–E626. doi: 10.1152/ajpendo.00010.2005
Lin, Z., Tian, H., Lam, K. S., Lin, S., Hoo, R. C., Konishi, M., et al. (2013). Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 17, 779–789. doi: 10.1016/j.cmet.2013.04.005
Luo, L., and Liu, M. (2016). Adipose tissue in control of metabolism. J. Endocrinol. 231, R77–R99. doi: 10.1530/JOE-16-0211
Maeda, H., Hosokawa, M., Sashima, T., Funayama, K., and Miyashita, K. (2005). Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys. Res. Commun. 332, 392–397. doi: 10.1016/j.bbrc.2005.05.002
Magliano, D. C., Bargut, T. C., de Carvalho, S. N., Aguila, M. B., Mandarim-de-Lacerda, C. A., and Souza-Mello, V. (2013). Peroxisome proliferator-activated receptors-alpha and gamma are targets to treat offspring from maternal diet-induced obesity in mice. PLoS One 8:e64258. doi: 10.1371/journal.pone.0064258
Mossenbock, K., Vegiopoulos, A., Rose, A. J., Sijmonsma, T. P., Herzig, S., and Schafmeier, T. (2014). Browning of white adipose tissue uncouples glucose uptake from insulin signaling. PLoS One 9:e110428. doi: 10.1371/journal.pone.0110428
Mu, J., Pinkstaff, J., Li, Z., Skidmore, L., Li, N., Myler, H., et al. (2012). FGF21 analogs of sustained action enabled by orthogonal biosynthesis demonstrate enhanced antidiabetic pharmacology in rodents. Diabetes 61, 505–512. doi: 10.2337/db11-0838
Murholm, M., Isidor, M. S., Basse, A. L., Winther, S., Sorensen, C., Skovgaard-Petersen, J., et al. (2013). Retinoic acid has different effects on UCP1 expression in mouse and human adipocytes. BMC Cell Biol. 14:41. doi: 10.1186/1471-2121-14-41
Nedergaard, J., Bengtsson, T., and Cannon, B. (2007). Unexpected evidence for active brown adipose tissue in adult humans. Ame. J. Physiol. Endocrinol. Metabol. 293, E444–E452. doi: 10.1152/ajpendo.00691.2006
Nishimura, T., Nakatake, Y., Konishi, M., and Itoh, N. (2000). Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochem. Biophys. Acta 1492, 203–206. doi: 10.1016/S0167-4781(00)00067-1
Owen, B. M., Ding, X., Morgan, D. A., Coate, K. C., Bookout, A. L., Rahmouni, K., et al. (2014). FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab. 20, 670–677. doi: 10.1016/j.cmet.2014.07.012
Park, A., Kim, W. K., and Bae, K. H. (2014). Distinction of white, beige and brown adipocytes derived from mesenchymal stem cells. World J. Stem Cells 6, 33–42. doi: 10.4252/wjsc.v6.i1.33
Petrovic, N., Walden, T. B., Shabalina, I. G., Timmons, J. A., Cannon, B., and Nedergaard, J. (2010). Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J. Biol. Chem. 285, 7153–7164. doi: 10.1074/jbc.M109.053942
Piya, M. K., McTernan, P. G., and Kumar, S. (2013). Adipokine inflammation and insulin resistance: the role of glucose, lipids, and endotoxin. J. Endocrinol. 216, T1–T15. doi: 10.1530/JOE-12-0498
Puigserver, P., Pico, C., Stock, M. J., and Palou, A. (1996). Effect of selective beta-adrenoceptor stimulation on UCP synthesis in primary cultures of brown adipocytes. Mol. Cell. Endocrinol. 117, 7–16. doi: 10.1016/0303-7207(95)03727-6
Rachid, T. L., Penna-de-Carvalho, A., Bringhenti, I., Aguila, M. B., Mandarim-de-Lacerda, C. A., and Souza-Mello, V. (2015). Fenofibrate (PPARalpha agonist) induces beige cell formation in subcutaneous white adipose tissue from diet-induced male obese mice. Mol. Cell. Endocrinol. 402, 86–94. doi: 10.1016/j.mce.2014.12.027
Samms, R. J., Smith, D. P., Cheng, C. C., Antonellis, P. P., Perfield, J. W. II, Kharitonenkov, A., et al. (2015). Discrete aspects of FGF21 in vivo pharmacology do not require UCP1. Cell Rep. 11, 991–999. doi: 10.1016/j.celrep.2015.04.046
Sanchez-Gurmaches, J., and Guertin, D. A. (2014). Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat. Commun. 5:4099. doi: 10.1038/ncomms5099
Schoenberg, K. M., Giesy, S. L., Harvatine, K. J., Waldron, M. R., Cheng, C., Kharitonenkov, A., et al. (2011). Plasma FGF21 is elevated by the intense lipid mobilization of lactation. Endocrinology 152, 4652–4661. doi: 10.1210/en.2011-1425
Schulz, T. J., Huang, T. L., Tran, T. T., Zhang, H., Townsend, K. L., Shadrach, J. L., et al. (2011). Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc. Natl. Acad. Sci. U.S.A. 108, 143–148. doi: 10.1073/pnas.1010929108
Seale, P., Kajimura, S., Yang, W., Chin, S., Rohas, L. M., Uldry, M., et al. (2007). Transcriptional control of brown fat determination by PRDM16. Cell Metab. 6, 38–54. doi: 10.1016/j.cmet.2007.06.001
Summermatter, S., Shui, G., Maag, D., Santos, G., Wenk, M. R., and Handschin, C. H. (2013). PGC-1 alfa improves glucose homeostasis in skeletal muscle in an activity-dependent manner. Diabetes 62, 85–95. doi: 10.2337/db12-0291
Suomalainen, A., Elo, J. M., Pietiläinen, K. H., Hakonen, A. H., Sevastianova, K., Korpela, M., et al. (2011). FGF-21 as a biomarker for muscle-manifesting mitochondrial respiratory chain deficiencies: a diagnostic study. Lancet Neurol. 10, 806–818. doi: 10.1016/S1474-4422(11)70155-7
Talukdar, S., Zhou, Y., Li, D., Rossulek, M., Dong, J., Somayaji, V., et al. (2016). A long-acting FGF21 molecule, PF-05231023, decreases body weight and improves lipid profile in non-human primates and type 2 diabetic subjects. Cell Metab. 23, 427–440. doi: 10.1016/j.cmet.2016.02.001
Teodoro, J. S., Zouhar, P., Flachs, P., Bardova, K., Janovska, P., Gomes, A. P., et al. (2014). Enhancement of brown fat thermogenesis using chenodeoxycholic acid in mice. Int. J. Obes. 38, 1027–1034. doi: 10.1038/ijo.2013.230
Veniant, M. M., Hale, C., Helmering, J., Chen, M. M., Stanislaus, S., Busby, J., et al. (2012). FGF21 promotes metabolic homeostasis via white adipose and leptin in mice. PLoS One 7:e40164. doi: 10.1371/journal.pone.0040164
Veniant, M. M., Sivits, G., Helmering, J., Komorowski, R., Lee, J., Fan, W., et al. (2015). Pharmacologic effects of FGF21 are independent of the “browning” of white adipose tissue. Cell Metab. 21, 731–738. doi: 10.1016/j.cmet.2015.04.019
Villarroya, F., Cereijo, R., Villarroya, J., and Giralt, M. (2017). Brown adipose tissue as a secretory organ. Nat. Rev. Endocrinol. 13, 26–35. doi: 10.1038/nrendo.2016.136
Wang, S., Liang, X., Yang, Q., Fu, X., Rogers, C. J., Zhu, M., et al. (2015). Resveratrol induces brown-like adipocyte formation in white fat through activation of AMP activated protein kinase (AMPK) alpha1. Int. J. Obes. 39, 967–976. doi: 10.1038/ijo.2015.23
Watanabe, M., Houten, S. M., Mataki, C., Christoffolete, M. A., Kim, B. W., Sato, H., et al. (2006). Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439, 484–489. doi: 10.1038/nature04330
Whittle, A. J., Carobbio, S., Martins, L., Slawik, M., Hondares, E., Vazquez, M. J., et al. (2012). BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell 149, 871–885. doi: 10.1016/j.cell.2012.02.066
Wu, A. L., Kolumam, G., Stawicki, S., Chen, Y., Li, J., Zavala-Solorio, J., et al. (2011). Amelioration of type 2 diabetes by antibody-mediated activation of fibroblast growth factor receptor 1. Sci. Transl. Med. 3:113ra126. doi: 10.1126/scitranslmed.3002669
Wu, J., Bostrom, P., Sparks, L. M., Ye, L., Choi, J. H., Giang, A. H., et al. (2012). Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376. doi: 10.1016/j.cell.2012.05.016
Wu, J., Cohen, P., and Spiegelman, B. M. (2013). Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev. 27, 234–250. doi: 10.1101/gad.211649.112
Young, P., Arch, J. R., and Ashwell, M. (1984). Brown adipose tissue in the parametrial fat pad of the mouse. FEBS Lett. 167, 10–14. doi: 10.1016/0014-5793(84)80822-4
Zhang, X., Yeung, D. C., Karpisek, M., Stejskal, D., Zhou, Z. G., Liu, F., et al. (2008). Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 57, 1246–1253. doi: 10.2337/db07-1476
Zhang, X., Zhang, Q. X., Wang, X., Zhang, L., Qu, W., Bao, B., et al. (2016). Dietary luteolin activates browning and thermogenesis in mice through an AMPK/PGC1alpha pathway-mediated mechanism. Int. J. Obes. 40, 1841–1849. doi: 10.1038/ijo.2016.108
Zhang, Y., Li, R., Meng, Y., Li, S., Donelan, W., Zhao, Y., et al. (2014). Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes 63, 514–525. doi: 10.2337/db13-1106
Zhang, Y., Xie, C., Wang, H., Foss, R. M., Clare, M., George, E. V., et al. (2016). Irisin exerts dual effects on browning and adipogenesis of human white adipocytes. Am. J. Physiol. Endocrinol. Metab. 311, E530–E541. doi: 10.1152/ajpendo.00094.2016
Zhang, Y., Xie, Y., Berglund, E. D., et al. (2012). The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. eLife 1:e00065. doi: 10.7554/eLife.00065
Zhao, M., and Chen, X. (2014). Eicosapentaenoic acid promotes thermogenic and fatty acid storage capacity in mouse subcutaneous adipocytes. Biochem. Biophys. Res. Commun. 450, 1446–1451. doi: 10.1016/j.bbrc.2014.07.010
Keywords: fibroblast growth factor 21, glucose, energy balance, insulin resistance, irisin, exercise, noradrenaline, free fatty acids
Citation: Cuevas-Ramos D, Mehta R and Aguilar-Salinas CA (2019) Fibroblast Growth Factor 21 and Browning of White Adipose Tissue. Front. Physiol. 10:37. doi: 10.3389/fphys.2019.00037
Received: 10 October 2018; Accepted: 14 January 2019;
Published: 05 February 2019.
Edited by:
Rita De Matteis, University of Urbino Carlo Bo, ItalyReviewed by:
Neal Lee Weintraub, Augusta University, United StatesGautham Yepuri, New York University, United States
Patricia Vázquez, Institute of Biomedical Research Alberto Sols (IIBM), Spain
Copyright © 2019 Cuevas-Ramos, Mehta and Aguilar-Salinas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carlos A. Aguilar-Salinas, caguilarsalinas@yahoo.com; ceptamim@gmail.com
 Daniel Cuevas-Ramos
Daniel Cuevas-Ramos R. Mehta1,2
R. Mehta1,2 Carlos A. Aguilar-Salinas
Carlos A. Aguilar-Salinas