- Environmental and Life Sciences, Faculty of Science, Universiti Brunei Darussalam, Bandar Seri Begawan, Brunei
The theory for thermal plasticity of tropical ectotherms has centered on terrestrial and open-water marine animals which experience reduced variation in diurnal and seasonal temperatures, conditions constraining plasticity selection. Tropical marine intertidal animals, however, experience complex habitat thermal heterogeneity, circumstances encouraging thermal plasticity selection. Using the tropical rocky-intertidal gastropod, Echinolittorina malaccana, we investigated heat tolerance plasticity in terms of laboratory acclimation and natural acclimatization of populations from thermally-dissimilar nearby shorelines. Laboratory treatments yielded similar capacities of snails from either population to acclimate their lethal thermal limit (LT50 variation was ∼2°C). However, the populations differed in the temperature range over which acclimatory adjustments could be made; LT50 plasticity occurred over a higher temperature range in the warm-shore snails compared to the cool-shore snails, giving an overall acclimation capacity for the populations combined of 2.9°C. In addition to confirming significant heat tolerance plasticity in tropical intertidal animals, these findings reveal two plasticity forms, reversible (laboratory acclimation) and non-reversible (population or shoreline specific) plasticity. The plasticity forms should account for different spatiotemporal scales of the environmental temperature variation; reversible plasticity for daily and tidal variations in microhabitat temperature and non-reversible plasticity for lifelong, shoreline temperature conditions. Non-reversible heat tolerance plasticity, likely established after larvae settle on the shore, should be energetically beneficial in preventing heat shock protein overexpression, but also should facilitate widespread colonization of coasts that support thermally-diverse shorelines. This first demonstration of different plasticity forms in benthic intertidal animals supports the hypothesis that habitat heterogeneity (irrespective of latitude) drives thermal plasticity selection. It further suggests that studies not making reference to different spatial scales of thermal heterogeneity, nor seeking how these may drive different thermal plasticity forms, risk misinterpreting ectothermic responses to environmental warming.
Introduction
Thermal plasticity enables ectothermic animals to modify their lifetime responses to environmental temperature. In the context of climate warming, a complex theory for this plasticity has emerged, which considers its energetic benefits, environmental drivers and evolutionary constraints (see the beneficial acclimation hypothesis, and hypotheses for thermal variability, predictability and latitudinal effects; Leroi et al., 1994; Kingsolver and Huey, 1998; Wilson and Franklin, 2002; Angilletta et al., 2006; Deere and Chown, 2006; Gunderson and Stillman, 2015). This theory has, however, been developed with taxonomic and ecological biases toward terrestrial insects, lizards, amphibians, and marine fishes (Huey et al., 1999; Mitchell et al., 2011; Overgaard et al., 2011; Kingsolver et al., 2013; Phillips et al., 2015), to the exclusion largely of animals inhabiting marine intertidal zones (but see Stillman, 2003; Somero, 2010). Marine intertidal circumstances are important as the theory may not always apply to them, despite its assumed generality. For example, studies investigating environmental temperature variation as the primary driver of thermal plasticity selection, commonly consider latitudinal and seasonal effects (Angilletta, 2009). These studies frequently conclude that temperate species, which typically experience greater thermal variation possess a greater capacity for thermal acclimation than tropically-distributed species, which experience relatively limited thermal variation (Gunderson and Stillman, 2015; Rohr et al., 2018). Although this may be true for most tropical animals and habitats, tropical marine intertidal habitats often promote extreme and variable temperature conditions, likely to drive thermal plasticity selection (Helmuth et al., 2006a,b; Marshall et al., 2010, 2018; Denny et al., 2011; Gedan et al., 2011).
Thermal heterogeneity in benthic tropical intertidal ecosystems derives from multiple within- and between-shore effects (Figure 1). Within-shore temperature regimes depend primarily on the vertical position on the shore (low- to high-shore), which determines the degree of tidal inundation by relatively cool, thermally-stable seawater and concomitantly the period of exposure to warm, thermally-variable air. At any shore height, the microclimate at scales of below 1 m varies in relation to topography, slope, texture and color of the rocky substratum (Helmuth and Hofmann, 2001; Marshall et al., 2010; Denny et al., 2011; Gedan et al., 2011; Dong et al., 2017). Nearby shorelines which experience similar ambient air temperatures can, however, differ greatly in heat-loading in relation to aspect (north, south, east or west facing), slope, and level of protection from the prevailing winds and swells (Figure 1; Helmuth et al., 2006a,b).
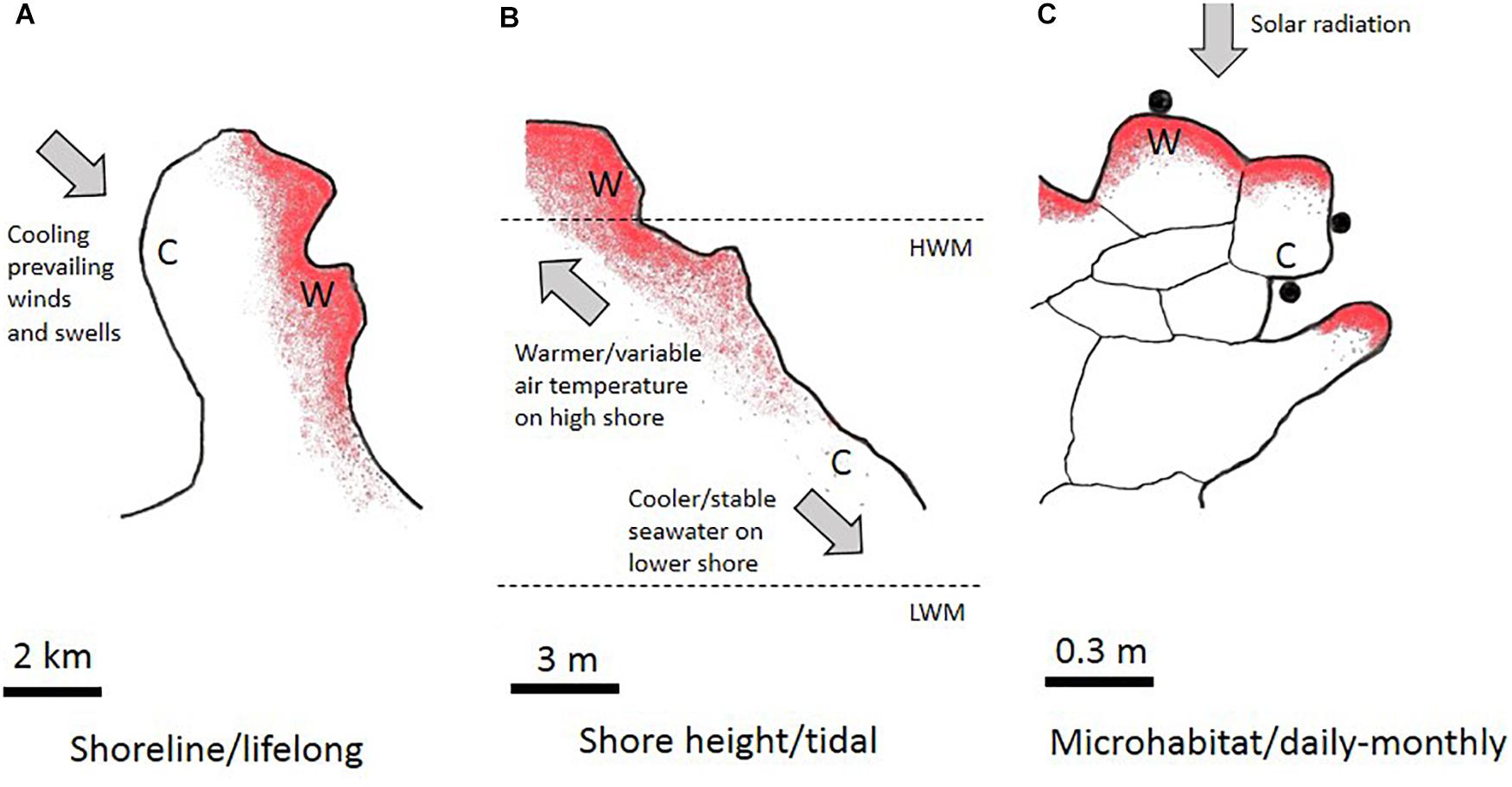
Figure 1. Concept for spatiotemporal scaling of rocky-shore thermal heterogeneity. (A) Aerial view of a coastal headland (2 km scale) showing the heat-load distribution (more intense red shading represents greater heat-load). One side of the headland (C) is exposed to prevailing winds and swells whereas the other side (W) is sheltered. Organisms experience either cooler or warmer shoreline conditions throughout their lifetimes. (B) Vertical profile of a rocky-shore (3 m scale), showing greater heat-loading in the higher shore (W) due to the variable air temperature. Relatively stable, cooler conditions (C) are experienced in the lower-shore. HWM, high water mark; LWM, low water mark. (C) Microhabitats of resting snails (0.3 m scale) showing the effect of solar heating on heat loads. Snails (solid circles) may retain these positions for months in the supratidal or may be redistributed after splashing during spring high-water or high monsoon swell conditions. Snails are primarily heated from the rock surface (see Marshall and Chua, 2012).
From a temporal perspective, tropical rocky-shore thermal heterogeneity is encompassed within daily and tidal timeframes (Figure 1), rather than a seasonal timeframe as in temperate regions (Helmuth and Hofmann, 2001; Marshall et al., 2010; Denny et al., 2011; Gedan et al., 2011). Whereas conspecific individuals on the same shoreline often experience different thermal regimes in relation to different vertical or microhabitat distributions (see above), behavioral idiosyncrasies of some species result in the same individual undergoing rapid regime change within the narrow timeframe of a tidal cycle. This is exemplified by high-shore snails whose settling positions when the tide recedes are preferentially determined by desiccation risk avoidance rather than by thermal cues causing snails to rapidly stop crawling when rock surfaces become hot and dry (Monaco et al., 2017). New resting sites can be retained for weeks and can be much hotter (25 to >45°C in full sun-exposed individuals) or much cooler (25–35°C in individuals settling the shade) than the original sites occupied before tidal wetting and activity (Marshall et al., 2013). The exceptional habitat heterogeneity and dynamic variability of the thermal regimes experienced by many tropical intertidal animals should drive plasticity selection.
Because high-shore animals are particularly threatened by acute overheating (Williams and Morritt, 1995; Harley, 2008; Garrabou et al., 2009), heat tolerance plasticity should be under significant selection pressure, especially where behavioral thermoregulatory capabilities are limited. Although reversible plasticity is better known with respect to seasonality, this plasticity should also benefit intertidal animals facing variations in daily maximum temperatures. Superimposed on microhabitat thermal regimes are shoreline-specific thermal conditions, resulting in hotter microhabitats on hotter shores and vice versa, throughout an individual’s lifetime. Between-shore temperature differences present circumstances likely to drive non-reversible (or developmental) plasticity selection (Hoffmann et al., 2003; Angilletta, 2009; Seebacher et al., 2012, 2014; Beaman et al., 2016). Despite the important contributions of Somero and Stillman to ecological and evolutionary perspectives for intertidal thermal plasticity (Stillman, 2003; Somero, 2010), no previous studies have investigated different plasticity forms in benthic intertidal animals. Most of the research considering non-reversible thermal plasticity (developmental and transgenerational) concerns insects and fishes, and refers more commonly to performance than tolerance traits (Angilletta et al., 2006; Angilletta, 2009; Donelson et al., 2011, 2012; Beaman et al., 2016; Sørensen et al., 2016).
Gastropods represent a dominant ecological component of rocky intertidal zones, and nearly exclusively inhabit the uppermost shore level. Consequently, they have evolved complex behavioral and physiological mechanisms to endure energy gain constraints and resist extreme heat and desiccation exposures (Marshall and McQuaid, 2011; Marshall et al., 2011, 2013, 2015; Marshall and Chua, 2012; Verberk et al., 2016; Ng et al., 2017). The ubiquitous tropical high-shore gastropod, Echinolittorina malaccana, is emerging as a model species for exploring molecular heat stress innovations (Dong et al., 2011, 2017; Liao et al., 2017; Somero et al., 2017; Han et al., 2019). Contrary to the general theory predicting a trade-off between thermal acclimation capacity and basal heat tolerance, this thermophilic snail has been shown to exhibit substantial heat tolerance plasticity (Gunderson and Stillman, 2015; Marshall et al., 2018).
The present study aimed to determine whether the heat tolerance plasticity of E. malaccana snails could be described in terms of reversibility and non-reversibility. Reversible plasticity of the lethal temperature (LT50) was investigated from laboratory acclimation experiments. Non-reversible plasticity was assessed by comparing the thermal bands for lethal temperature acclimation of snail populations from warmer or cooler shorelines. Because reversible laboratory acclimation is expected to eliminate the effects of recent field temperature exposures, non-reversible acclimatization was assumed in cases where the thermal acclimation bands varied between the populations.
Materials and Methods
Snail Habitats and Thermal Regimes
Echinolittorina malaccana (Philippi 1847) occurs abundantly on rocky-shores throughout the Indo-Pacific (Reid, 2007). The local shores of Brunei Darussalam sustain two morphologically-distinct ecotypes, occupying different vertical zones. A brown ecotype inhabits the upper intertidal zone (roughly 1–2.5 m Chart Datum) and experiences tidal wetting, whereas a pale blue ecotype inhabits the supratidal zone (2.5- above 5 m Chart Datum) and is only wetted during high seas and monsoon swells. After periods of wetting and feeding, snails stop moving as the tide recedes, glue their shells to the rock surface, and withdraw into the shell (Marshall et al., 2011; Monaco et al., 2017). Because avoidance of desiccation while moving over hot dry rocks supersedes behavioral selection of thermally suitable resting (aestivating) sites, individuals often settle under direct sunlight (Marshall and Chua, 2012; Marshall et al., 2013; Monaco et al., 2017). Isolated resting snails undergo temperature-insensitive metabolic rate depression to overcome the energetic problem of high temperature exposure (Marshall and McQuaid, 2011; Marshall et al., 2011).
This study considered the intertidal brown ecotype. We determined the thermal regimes experienced at their upper distribution on a cool and a warm shoreline at Pantai Tungku, Brunei Darussalam (4.974°N, 114.867°E), between 21 June and 22 July 2018 (30 days during the warmest time of the year; Supplementary Figure S1). Pantai Tungku comprises a man-built promontory with artificial seawalls having diametrically-opposed orientations and carrying very different heat-loads. Study sites were established on the seawalls around 2 km apart, along the contour of the coast; the cool shoreline (CS) comprised a north-west-facing seawall exposed to prevailing monsoon winds and swells (#1, Supplementary Figure S1), whereas the warm shoreline comprised a north-east-facing sheltered seawall (#2, Supplementary Figure S1). To assess the potential range of temperature conditions experienced in snail microhabitats on either shoreline, temperature-loggers (see details in Monaco et al., 2017) were deployed on rocks under direct solar exposure (a 45° angled surface), or in total shade under the rocks. Loggers were set to record temperatures every 30 min.
Snail Collection and Laboratory Treatments
Snails (7–9 mm) were collected from both shorelines within the proximity of the temperature loggers, while awash and feeding. In the laboratory they were rinsed in freshly-collected seawater to rehydrate the snails before starting the acclimation treatments. Prior to field-fresh thermal tolerance determinations, snails were exposed to blown air (30°C for 20 min; Memmert UFE 500, Schwabach, Germany) to inactivate them, dry shells and induce withdrawal into the shell (Marshall et al., 2018). Field-fresh tolerance experiments were carried out within 12 h of collection of the snails.
Four (4) primary laboratory temperature acclimation treatments were conducted on both warm-shore (WS, warm-acclimatized) and cool-shore (CS, cool-acclimatized) snails. The first set of experiments involved using a programmable Memmert Peltier-cooled (IPP400) incubator to cool-acclimate (CA) one group of randomly selected snails in air at 22–23°C, which approximates the coolest thermal regimes naturally experienced by tropical populations of E. malacccana (Marshall et al., 2010; Monaco et al., 2017; Figure 2 and Supplementary Figure S2). A second group was warm-acclimated (WA) to a daily 25–45°C thermal cycle, holding the temperature at 45°C for 4 h (midday) and at 25°C for 12 h (night-time), to mimic hot daily field conditions (Figure 2 and Supplementary Figure S2). Notably, whereas sun-exposure associates with high daily temperature fluctuations, cool-shaded conditions are relatively stable. These acclimations proceeded for 10 d, with snails, unfed and resting, immersed each day in flowing seawater for 5 min, to simulate tidal wetting, maintain full hydration and prevent deep aestivation (Marshall and McQuaid, 2011). Another set of experiments, which tested whether complete acclimation occurred during the above thermal treatments, involved 10 d more severe cooling [near-constant 20°C; extra-cool acclimation (ECA)] and warming (25–50°C cycle, 2 h at 50°C; extra-warm acclimation, EWA; Supplementary Figure S2). We further tested whether full acclimation occurred within a 10 d period, by assessing the effect of WA for 20 d.
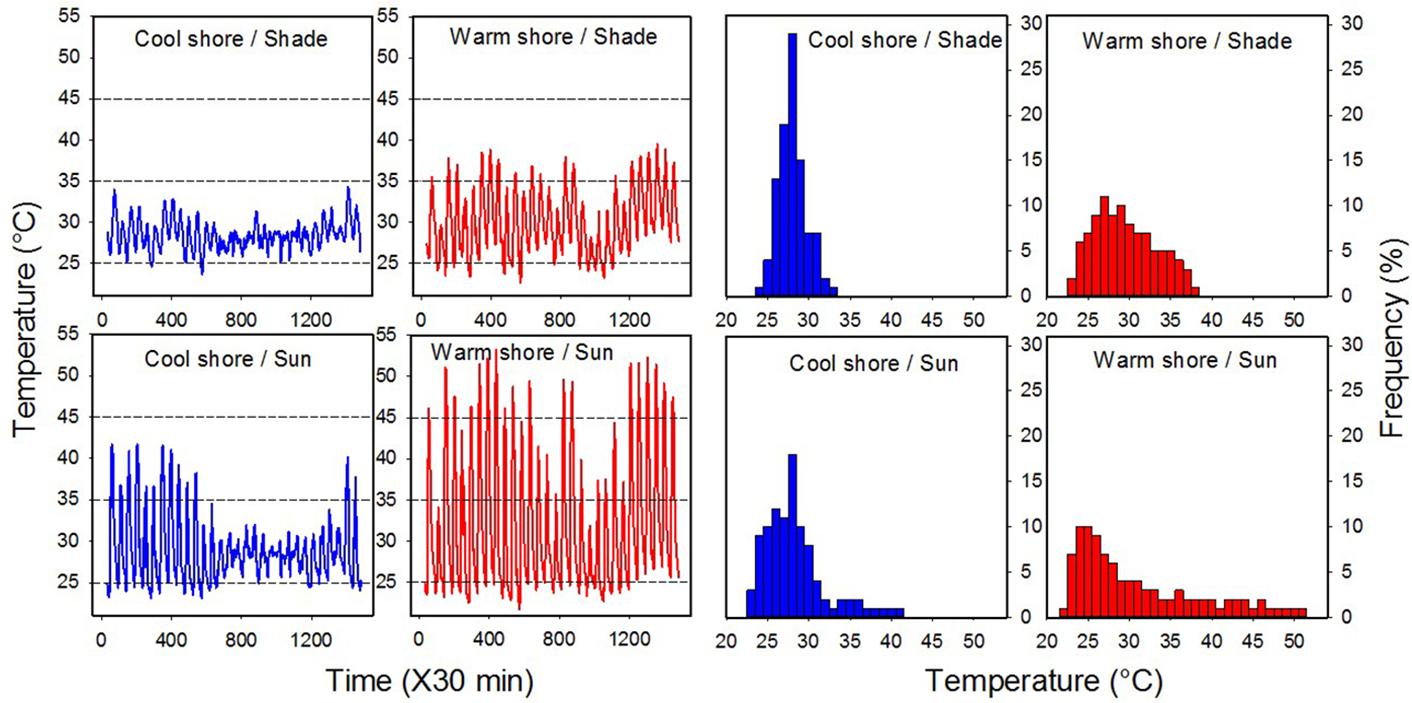
Figure 2. Daily field temperature regimes and thermal frequencies for the four habitats (cool shore, CS, sun and shaded, and warm shore, WS, sun and shaded). All data were logged using DS1923-F5# Hygrochron I-buttons over 30 days. Temperatures were recorded between 21 June and 22 July 2018 at the sites where snails were collected (see Supplementary Figure S1).
Heat Ramping and LT50 Determination
We assayed the effect of acute heating on mortality using the median lethal temperature (LT50; Marshall and McQuaid, 2011; Marshall et al., 2011, 2015, 2018). Acute overheating, rather than chronic temperature conditions that involve energetics, is the most likely cause of thermal stress related mortality in high-shore animals (Williams and Morritt, 1995; Marshall and McQuaid, 2011; Marshall et al., 2011). The critical thermal maximum (CTmax), the temperature at which neuromuscular co-ordination fails, which is commonly used in ectotherm experiments, is unsuitable for determining gastropod lethality. This is because inactivity in high-shore littorinid snails relates to desiccation-risk-avoidance, and snails with shells glued to rock surfaces withstand temperatures well above those limiting foot physiological performance (Marshall and McQuaid, 2011; Marshall et al., 2013, 2015; Monaco et al., 2017). Much controversy surrounds the effects of ramping on acute heat tolerance determination (Terblanche et al., 2011). We selected a ramp rate of 0.25°C.min-1 or slightly slower, appropriate to the heating experienced in the field (Figure 2; Marshall et al., 2011). Prior to determining heat tolerance of acclimated snails, individuals were rehydrated and their shells dried as in the case of the field-fresh snails.
To determine heat tolerance, acclimated snails were placed in dry 50 ml glass tubes in a programmable bath (Grant TXF200, Cambridge, United Kingdom) and equilibrated at 30°C for 10 min, before being heated at 0.25°C.min-1. To maintain water bath temperature stability during the LT50 experiment, the heating rate was slowed to 0.12°C.min-1 between 50 and 60°C. Naturally, the apparent lethal temperature will be affected by time at different temperatures. Temperatures inside the test tubes were recorded every minute using calibrated K-type thermocouples connected to a TC-08 interface and Picolog software (Pico Technology, Cambridge, United Kingdom). Lethality (LT50) was determined for groups of 10 snails that were randomly removed from the water bath at 1°C intervals between 55 and 60°C, and allowed to recover at 28°C in wetted Petri dishes. Snails that emerged from their shells, extended their foot, and remained attached to the surface after 12 h were scored as alive. Alive but unattached snails, which are ecologically non-functional and vulnerable, were scored dead. All experiments were repeated either three or six times based on logistic constraints, with numbers of individuals limited for conservation purposes. One thousand nine hundred individual snails were used in the experiments. The study was approved by the Faculty of Science Ethics Committee, Universiti Brunei Darussalam. The effect of acclimation on LT50 was statistically compared between paired treatments using Generalized Linear Models (GLZM) for a binomial distribution, with a logit-link function (Statistica v12, StatSoft, New York, United States). Actual values of LT50 were computed from three parameter logistic regressions, which were plotted using Sigmaplot v14 (Systat Software, Inc., New York, United States).
Results
Field Temperature Conditions
The daily temperature conditions and thermal frequencies for four habitats, the coolest in the shade and the hottest in the sun, are shown for each shoreline in Figure 2. There was little difference between the habitats in average temperature for this period (28.5–30.1°C; Table 1). Likewise, absolute and mean daily minimum temperatures were largely invariable among the habitats (21.7–23.7 and 24.2–26.0°C, respectively). However, the maxima differed greatly (34.3–53.3 and 30.6–45.8°C, respectively), being markedly elevated in the sun-exposed habitats. Notably, in the three cooler habitats, daily temperatures did not rise above 45°C, the temperature around which a heat shock response (HSR) is induced (Marshall et al., 2011; Han et al., 2019), whereas peak temperatures in the warmest habitat surpassed 45°C on 18 (of the 30) days (Figure 2). Daily temperature variation (ΔT) was greatest in the warmest habitat (sun-exposed, WS; 21.6°C) and lowest in the coolest habitat (shade, CS; 4.6°C; Table 1); the warmest habitat exhibited the highest maximum and the lowest minimum temperatures (Figure 2 and Table 1). The stark difference in temperatures between the shores is highlighted by similar measures (means, maxs, mins, and ΔT) for the sun-exposed, CS and the shaded, WS habitats (Figure 2 and Table 1). Although several factors influence long-term temperature variations in these tropical habitats, including seasonal and El Nino effects, our recordings for a narrow timeframe are representative of relative daily thermal regime differences between the habitats and shores.
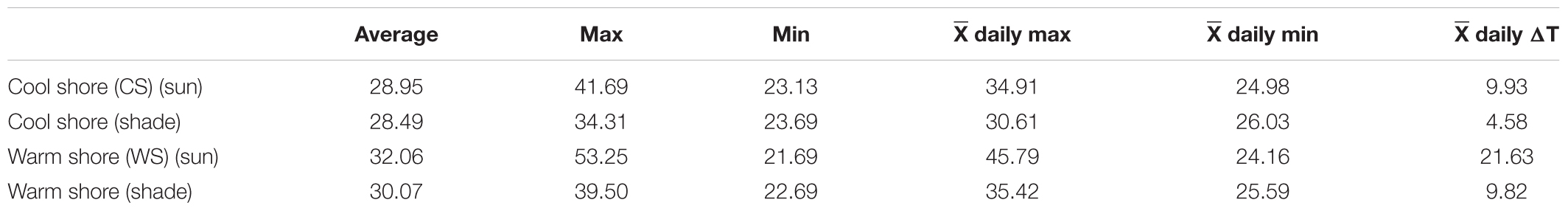
Table 1. Field temperatures in the four habitats, logged every 30 min for 30 days using DS1923-F Hygrochron I-buttons (see Figure 2).
Heat Tolerance Plasticity
Substantial heat tolerance plasticity was observed in E. malaccana snails. The overall magnitude of their LT50 adjustment was 2.9°C, when accounting for all laboratory-acclimated and field-acclimatized conditions (mean LT50 ranged from 56.1 to 59.0; Figures 3, 4 and Table 2). Whereas snails from either shore exhibited similar magnitudes in reversible acclimation (∼2°C), the thermal bands over which acclimatory adjustments were made differed between the shores. The acclimation band for WS snails was shifted to a hotter temperature range compared to that for CS snails (Figure 4).
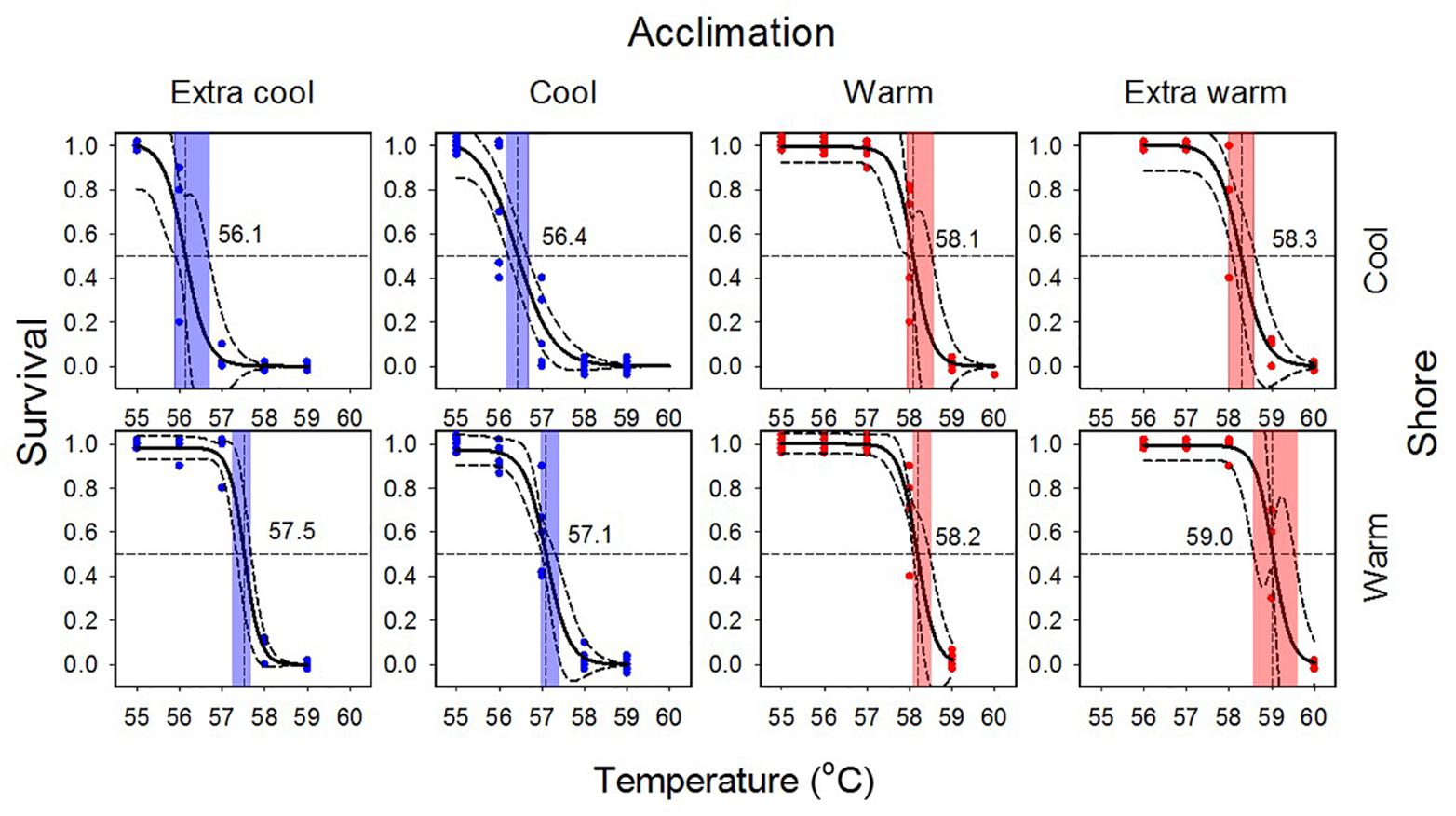
Figure 3. Survival curves for laboratory-acclimated Echinolittorina malaccana snails from the cool (CS) and the warm shore (WS). Solid black logistic regressions are based on the combined data for each trial. Dashed lines associated with regressions represent 95% CIs. Colored symbols refer to individual trials for cool and warm acclimation (CA and WA, 5 trials) and extra cool and extra warm acclimation (ECA and EWA, 3 trials) using 50 snails per trial. Vertical lines indicate LT50 values and their associated colored bands, their 95% CIs.
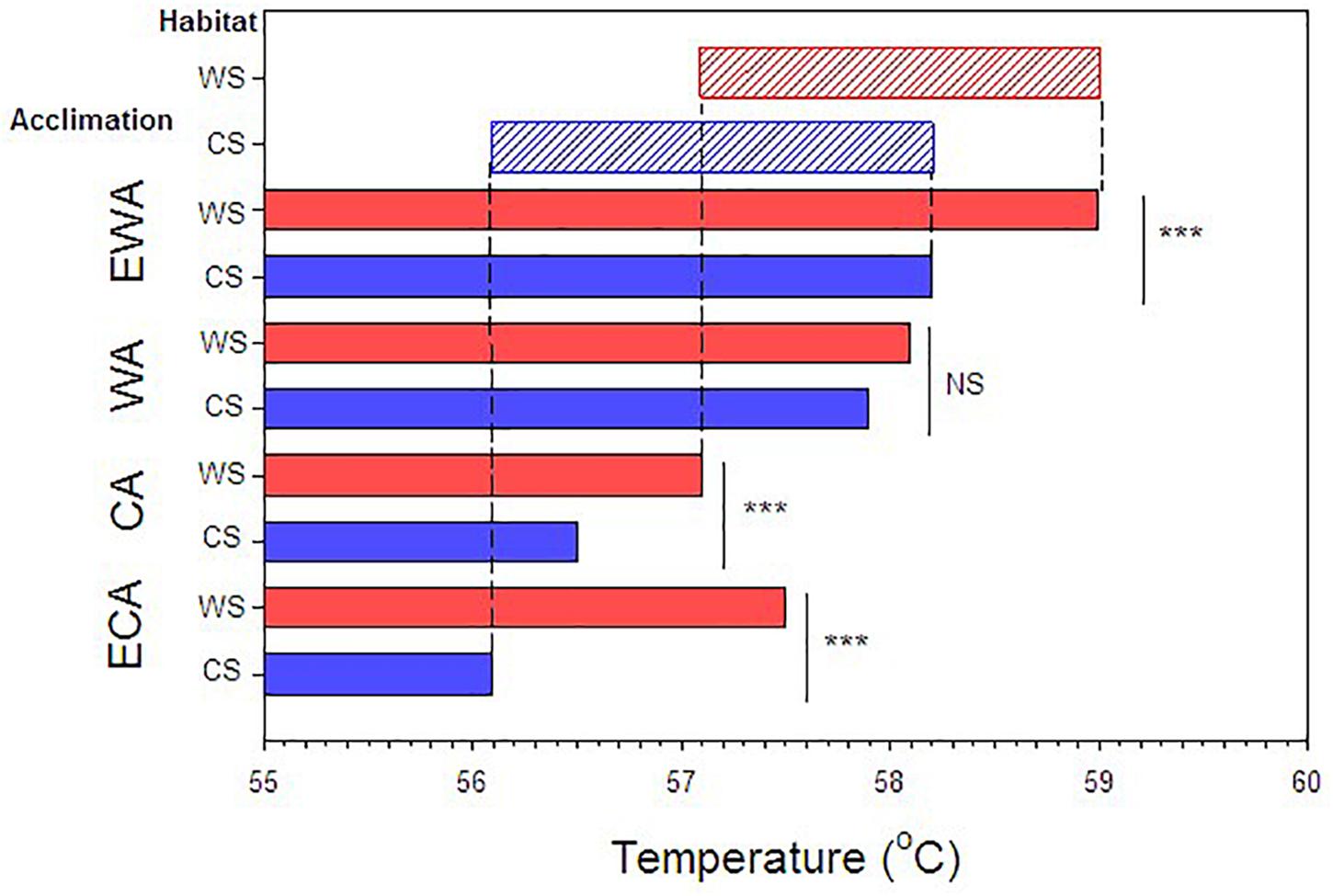
Figure 4. Compilation of results showing the LT50 values for the various acclimation and acclimatization (habitat) conditions. Red bars indicate warm shore acclimatization (WS) and blue bars, cool shore acclimatization (CS). ECA, CA, WA, EWA refer to extra-cool, cool, warm and extra-warm acclimation, respectively. Asterisk indicates P < 0.01 and NS, non-significant difference. Lightly shaded upper bars indicate acclimation capacities and ranges for warm shore (red) and cool shore (blue) acclimatized snails.
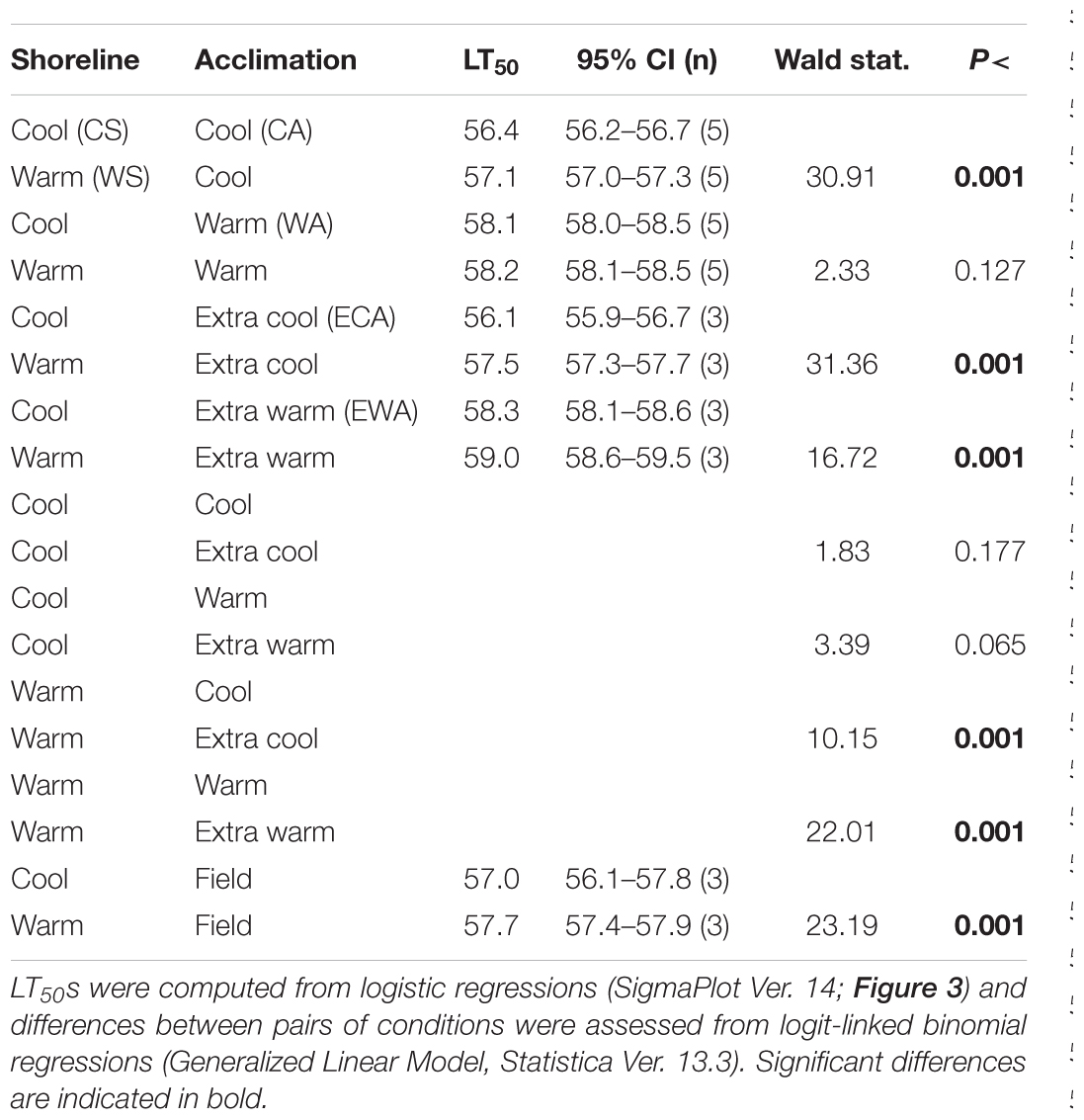
Table 2. Statistical information comparing effects of field acclimatization (shoreline) and laboratory acclimation temperature conditions on the LT50 of Echinolittorina malaccana.
Cool acclimation (CA) significantly lowered the mean LT50 of CS snails below that of WS snails (p < 0.001; Table 2). Because further cooling (ECA) of CS snails did not reduce the LT50 further, we consider the mean hard lower limit to heat tolerance of this snail population to be 56.1°C (p = 0.177; Table 2). Similarly, the hard lower limit for WS snails is apparently 57.1°C (Figure 4 and Table 2). Warm acclimation (WA) markedly raised the mean LT50 of CS snails (58.1°C), but no further increase in heat tolerance was observed for this population with further warming (EWA, extra-warm acclimation; p = 0.065; Table 2), suggesting that their hard upper heat tolerance boundary is reached under WA (Figure 4). There was also no significant difference between the two shoreline populations under WA (p = 0.127; Table 2). However, WA apparently did not result in complete thermal acclimation of the WS snails, as their heat tolerance rose following extra-warm temperature acclimation (EWA, LT50 = 59.0°C; p < 0.001, Table 2).
Mean field-fresh LT50 was greater in WS snails (57.7°C) compared to CS snails (57.0°C; p < 0.001; Table 2), reflecting the different recent thermal histories experienced on the different shorelines. There was no difference in LT50 between the 10 d and 20 d WA treatment for warm shore snails, indicating that 10 d is sufficiently long to produce complete compensation (p = 0.295). When the cool and warm habitat data were combined (CS and WS), and compared with the data for WS snails, the combined data set showed significantly lower LT50 values for ECA, CA, and EWA (p < 0.007; Table 3), but not for the WA laboratory treatments (p = 0.369; Table 3). While it was not investigated in the present study, individual variation can be as important an endpoint as the mean value in any study investigating temperature responses.
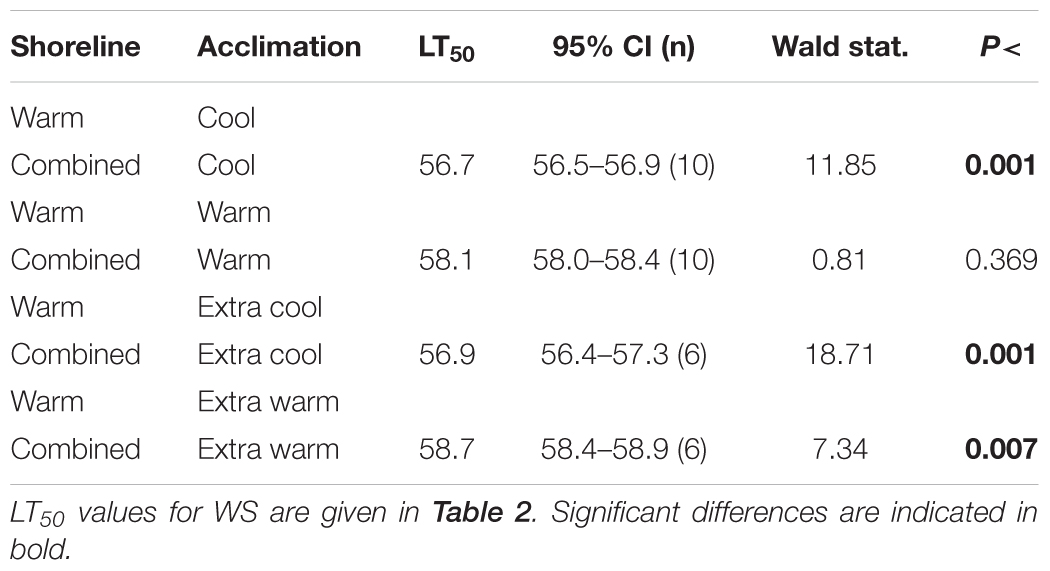
Table 3. Statistical information comparing effects of warm-shore (WS) and combined-shore (warm and cool) on the LT50 of Echinolittorina malaccana for the various acclimation treatments.
Discussion
Ectotherms vary widely in ability to adjust physiological performances and tolerances in response to lifetime changes in environmental temperature (Angilletta, 2009). Contrary to the prediction of mainstream theory that thermal acclimation is relatively constrained in tropical and high-intertidal animals (Stillman and Somero, 2000; Stillman, 2002; Somero, 2005, 2010; Gunderson and Stillman, 2015; Rohr et al., 2018), we found substantial plasticity in the lethal thermal limit (LT50) of Echinolittorina snails (see also, Marshall et al., 2018). However, we additionally, show that heat tolerance plasticity of these snails comprises both a reversible and a non-reversible component. Reversible plasticity was induced by laboratory acclimation and non-reversible plasticity was shown by differences in the thermal bands for lethal temperature (LT50) acclimation between populations from thermally-different shorelines (see Figure 4). Snails from the warmer shore were found to shift the band for thermal acclimation to a higher range of temperatures compared to the CS snails. These different forms of plasticity align with different spatiotemporal scales of the environmental temperature variation. Reversible plasticity facilitates thermal tolerance adjustments in response to daily or tidal habitat temperature variation, whereas non-reversibility canalizes or fixes the thermal tolerance to shoreline-specific temperature conditions throughout the individual’s lifetime.
Reversible and Non-reversible Plasticity
Reversible plasticity enables individual organisms to adjust performances and tolerances in response to cycling temperatures for timeframes from seasons to days. In comparative studies, laboratory acclimation is typically performed to eliminate the effect of recent field temperatures on the physiological performance of individuals. Our observed persistence of phenotypic differences after laboratory acclimation in populations from thermally-different shorelines (Figure 3 and Table 2) suggests an effect of heritable differences between the populations or non-reversible plasticity. Because the snail larvae settling on either shoreline were randomly drawn from the same planktotrophic pool, comprising individuals derived from multiple different parents, we disregard local adaptation or other inherited effects as the cause of the observed population difference (Schmidt and Rand, 1999; Williams and Reid, 2004; Kelly et al., 2011; Foo and Byrne, 2016). Non-reversible plasticity has been described as transgenerational, such as non-genetic heat-hardening transferred to the embryo from the parent, or as developmental, such as post-embryonic heat-hardening (Angilletta, 2009; Donelson et al., 2011, 2012; Reusch, 2014). Using the same argument as above for random shoreline recruitment of larvae, the shoreline (population) differences in thermal plasticity can also not be explained by a transgenerational response to heat exposure (Williams and Reid, 2004). Our findings therefore suggest that non-reversible heat tolerance is most likely founded after larval snails have settled on the shore; thus, shoreline differences are best described as developmental plasticity (Angilletta, 2009). Because the crawling snails occupy meter-size habitats, cross-shore migrations can be discounted. Notably, the apparent developmental plasticity should be reinforced by the shoreline-specific temperature conditions during the lifetime of each snail, from the crawling juvenile to the adult (Angilletta, 2009; see Slotsbo et al., 2016 on reversibility of developmental plasticity).
Environmental Temperature and Molecular Underpinnings of Plasticity
Molecular processes underlying thermal plasticity are cued by different components of the thermal regime (mean, maxima, and minima) for different durations of thermal cycling (daily or seasonal). Whereas seasonal acclimatization, regulated by isozyme expression (Hochachka and Somero, 2002), is typically cued by mean temperature change (Angilletta, 2009), acute daily heating of rocky-shores elicits thermal plasticity through a HSR, triggered by peak (maximum) temperatures (Hofmann and Somero, 1996; Feder and Hofmann, 1999; Tomanek and Somero, 1999; Somero et al., 2017). Although HSRs are complex, involving upregulation of multiple heat shock proteins (Hsps), much information can be gleaned from individual gene thermal expression profiles (relative levels and thermal ranges of expression) and thermal thresholds (Tomanek and Somero, 1999; Wang et al., 2017; Han et al., 2019). Previous studies on E. malaccana showed that hsp70 expression profiles differ among geographically-separated populations (Wang et al., 2017; Han et al., 2019), such that reduced expression correlates with populations from cooler locations and vice versa (Wang et al., 2017; Han et al., 2019). The same mechanism in a spatially scaled-down form could underlie the heat tolerance differences observed between our cool and warm shore populations. Whereas these findings imply plasticity of hsp expression profiles, studies for diverse populations of E. malacanna suggest that the HSR thermal threshold for this species may otherwise be fixed at around 45°C (Marshall et al., 2011; Han et al., 2019; see also Hoffmann et al., 2003). Interestingly, whereas our cool and warm shore snails adjusted heat tolerance to the same level when acclimated to a daily maximum of 45°C, only the WS snails that commonly experience temperatures above 45°C responded positively to the extra-warm acclimation treatment (EWA; for which the daily thermal peak was 50°C; Figure 3 and Table 2).
Whereas daily maximum temperatures varied greatly across the snail habitats, mean field temperatures (which typically drive seasonal acclimatization) were largely similar across habitats (sunned and shaded) and shorelines (cooler and warmer; Table 1). Clearly, mean temperatures contribute insignificantly to heat tolerance plasticity selection in tropical Echinolittorina snails. Likewise, minimum field temperatures were largely invariable across the habitats and shores, which excludes these temperatures as potential determinants of the lower boundary for heat tolerance plasticity. The limit to this boundary, assessed during CA for both shores, possibly relates to the loss of heat-hardening, a mechanism which should be beneficial by eliminating costs associated with the upregulating and functioning of Hsps (Angilletta, 2009).
Benefits of Dual Heat Tolerance Plasticity
Plastic responses involving Hsps incur significant energetic and fitness costs, a topic extensively critiqued in the framework of the beneficial acclimation hypothesis (Leroi et al., 1994; Huey et al., 1999; Wilson and Franklin, 2002; Woods and Harrison, 2002; Deere and Chown, 2006). In their high-shore habitat, Echinolittorina snails face severe energy intake restrictions due to limited food availability and limited time in which to feed. Consequently, they have evolved multifaceted behavioral and physiological mechanisms to conserve energy resources (Marshall et al., 2011, 2013; Marshall and Chua, 2012). Among the most impressive energy-conserving mechanism is deep temperature-independent metabolic rate depression (10% of resting metabolism; Marshall et al., 2011; Verberk et al., 2016). In view of their energetic constraints, advantage should be gained by minimizing the use of costly physiological processes, including HSRs.
Non-reversible plasticity should be energetically beneficial by ensuring that the range for reversible heat tolerance plasticity matches the habitat temperatures of a particular shore. Such matching should prevent snails on warmer shores from experiencing excessive HSR induction and hsp overexpression (Feder and Hofmann, 1999; Sørensen et al., 2003). Non-reversible heat tolerance plasticity should enable reversible acclimatization to occur in similar ways and at similar costs on thermally-different shores under the same regional change in ambient air temperature. Furthermore, this plasticity should yield species-level benefits by enabling colonization of a broader range of shores (warmer and cooler shores) along a coastline. Importantly, these findings accounting for non-reversibility supersede an earlier suggestion that E. malaccana is unlikely to benefit from reversible plasticity (Marshall et al., 2018).
Taxonomic Generalization and Habitat Heterogeneity
Our understanding of thermal acclimation of rocky-intertidal animals in the context of contemporary theory (Angilletta, 2009) is encompassed by the influential work of Somero and Stillman (see Stillman, 2002; Somero, 2005, 2010). This work suggesting a reduced acclimation capacity in higher-shore species compared to their lower-shore congeners, arises from generalization of data for porcelain crabs (Stillman, 2002; Somero, 2005, 2010). These crabs, however, behaviourally thermoregulate by sheltering in the shade under rocks during air emersion, limiting their exposure to the full spectrum of the shoreline’s thermal heterogeneity. The substantial heat tolerance plasticity observed in high-shore Echinolittorina snails contradicts this theory, and we suggest that the difference between the crab and snail responses relates to the snails using a broader spectrum of the shoreline thermal heterogeneity. They experience relatively great habitat temperature variation through the behavior of settling when air-exposed in thermally-divergent microhabitats, including those under direct solar heating (Marshall et al., 2010, 2013). This habitat temperature variation persists despite the phenomenal variety of morphological and behavioral thermoregulatory attributes of littorinid snails (Miller and Denny, 2011; Marshall and Chua, 2012; Marshall et al., 2013; Ng et al., 2017), primarily because the most effective thermoregulatory behavior, shade-seeking, can be undermined by desiccation risk avoidance behavior (Monaco et al., 2017).
An arising question is whether capacity for non-reversible plasticity (the shoreline effect) is restricted to high-shore species or whether it is independent of vertical distribution on the shore. Because lower-shore habitats are strongly stabilized by the seawater temperature, and because the seawater temperature is largely invariable across nearby shorelines, the shoreline effect should be lessened in the lower-shore. In addition to suggesting that seawater temperature stability is likely to restrict thermal acclimation selection in lower-shore tropical species, we propose as a testable hypothesis that non-reversible plasticity should also be more constrained in lower-shore compared to higher-shore species.
Methodological Implications
Acclimation capacity (the degree of change in a trait following cool or warm laboratory acclimation) is becoming a key measure of the vulnerability of ectothermic animals to future warming (Gunderson and Stillman, 2015; Rohr et al., 2018). An earlier study revealed that laboratory experiments alone (without field-referencing) may underestimate this vulnerability in animals living in near-completely acclimatized states, which are unable to improve heat hardening with further warming (Marshall et al., 2018). The present study adds to this caveat by showing that inaccuracies in determining heat-tolerance acclimation capacity can potentially arise from not accounting for shoreline-specific temperature differences. Whereas the capacity for reversible acclimation was similar for each shoreline (∼2°C), this became greater when the data for the shores were combined (∼2.9°C; Table 3 and Figure 4). This highlights the importance, when assessing acclimation capacities, of prior knowledge of the habitat thermal heterogeneity of experimental animals.
Emerging from this study is a second methodological issue relating to diel cycling of the laboratory acclimation temperature. This is not only important considering that such cycling occurs naturally, but also in terms of initiating an acclimatory HSR. If the primary heat-hardening response requires that exposure temperature surpasses an HSR induction threshold, then acclimation temperatures that are stable or fluctuate below this threshold will conceivably not yield an acclimatory response, leading to erroneous conclusions. This further highlights the need to determine the HSR threshold temperature prior to developing acclimation protocols in marine intertidal animal studies.
Conclusion
Our study adds an important dimension to the existing theory proposing that thermal tolerance plasticity is relatively constrained in tropical ectotherms. In particular, it reveals that this plasticity can be complex in thermally-heterogeneous tropical marine intertidal ecosystems. Whereas the contribution of marine intertidal circumstances to a body of theory for thermal plasticity developed largely from terrestrial and subtidal animals might be questionable, our findings nonetheless caution against the indiscriminate use of this theory when interpreting intertidal data. We further show that without critical consideration of the thermal heterogeneity at the scale of the organism and how this heterogeneity may drive different forms of thermal tolerance plasticity, investigations risk generating misleading conclusions.
Author Contributions
AB and DM developed the hypothesis and prepared the manuscript. AB and NM carried out the experimental laboratory work. DM undertook the field recordings. NM reviewed the manuscript.
Funding
DM was supported by a Universiti Brunei Darussalam grant (UBD/RSCH/1.4/FICBF(b)/2018/016).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2018.01909/full#supplementary-material
References
Angilletta, M. J. (2009). Thermal Adaptation: a Theoretical and Empirical Synthesis. Oxford: Oxford University Press. doi: 10.1093/acprof:oso/9780198570875.001.1
Angilletta, M. J., Bennett, A. F., Guderley, H., Navas, C. A., Seebacher, F., and Wilson, R. S. (2006). Coadaptation: a unifying principle in evolutionary thermal biology. Physiol. Biochem. Zool. 79, 282–294. doi: 10.1086/499990
Beaman, J. E., White, C. R., and Seebacher, F. (2016). Evolution of plasticity: mechanistic link between development and reversible acclimation. Trends. Ecol. Evol. 31, 237–249. doi: 10.1016/j.tree.2016.01.004
Deere, J. A., and Chown, S. L. (2006). Testing the beneficial acclimation hypothesis and its alternatives for locomotor performance. Am. Nat. 168, 630–644. doi: 10.1086/508026
Denny, M. W., Dowd, W. W., Bilir, L., and Mach, K. J. (2011). Spreading the risk: small-scale body temperature variation among intertidal organisms and its implications for species persistence. J. Exp. Mar. Biol. Ecol. 400, 175–190. doi: 10.1016/j.jembe.2011.02.006
Donelson, J. M., Munday, P. L., McCormick, M. I., and Nilsson, G. E. (2011). Acclimation to predicted ocean warming through developmental plasticity in a tropical reef fish. Glob. Change Biol. 17, 1712–1719. doi: 10.1371/journal.pone.0097223
Donelson, J. M., Munday, P. L., McCormick, M. I., and Pitcher, C. R. (2012). Rapid transgenerational acclimation of a tropical reef fish to climate change. Nat. Clim. Change 2, 30. doi: 10.1038/nclimate1323
Dong, Y. W., Li, X., Choi, F. M. P., Williams, G. A., Somero, G. N., and Helmuth, B. (2017). Untangling the roles of microclimate, behaviour and physiological polymorphism in governing vulnerability of intertidal snails to heat stress. Proc. R. Soc. Lond. B. Biol. Sci. 284:20162367. doi: 10.1098/rspb.2016.2367
Dong, Y. W., Yu, S. S., Wang, Q. L., and Dong, S. L. (2011). Physiological responses in a variable environment: relationships between metabolism, HSP and thermotolerance in an intertidal-subtidal species. PLoS One 6:e26446. doi: 10.1371/journal.pone.0026446
Feder, M. E., and Hofmann, G. E. (1999). Heat-shock proteins, molecular chaperones, and the stress response. Annu. Rev. Physiol. 61, 243–282. doi: 10.1146/annurev.physiol.61.1.243
Foo, S. A., and Byrne, M. (2016). “Acclimatization and adaptive capacity of marine species in a changing ocean,” in Advances in Marine Biology, Vol. 74, ed. E. C. Barbara (Cambridge, MA: Academic Press), 69–116. doi: 10.1016/bs.amb.2016.06.001
Garrabou, J., Coma, R., Bensoussan, N., Bally, M., Chevaldonné, P., Cigliano, M., et al. (2009). Mass mortality in Northwestern Mediterranean rocky benthic communities: effects of the 2003 heat wave. Glob. Change Biol. 15, 1090–1103. doi: 10.1111/j.1365-2486.2008.01823.x
Gedan, K. B., Bernhardt, J., Bertness, M. D., and Leslie, H. M. (2011). Substrate size mediates thermal stress in the rocky intertidal. Ecology 92, 576–582. doi: 10.1890/10-0717.1
Gunderson, A. R., and Stillman, J. H. (2015). Plasticity in thermal tolerance has limited potential to buffer ectotherms from global warming. Proc. R. Soc. Lond. B. Biol. Sci. 282:20150401. doi: 10.1098/rspb.2015.0401
Han, G. D., Cartwright, S. R., Ganmanee, M., Chan, B. K., Adzis, K. A., Hutchinson, N., et al. (2019). High thermal stress responses of Echinolittorina snails at their range edge predict population vulnerability to future warming. Sci. Total. Environ. 647, 763–771. doi: 10.1016/j.scitotenv.2018.08.005
Harley, C. D. (2008). Tidal dynamics, topographic orientation, and temperature-mediated mass mortalities on rocky shores. Mar. Ecol. Prog. Ser. 371, 37–46. doi: 10.3354/meps07711
Helmuth, B., Broitman, B. R., Blanchette, C. A., Gilman, S., Halpin, P., Harley, C. D., et al. (2006a). Mosaic patterns of thermal stress in the rocky intertidal zone: implications for climate change. Ecol. Monogr. 76, 461–479. doi: 10.1890/0012-9615 (2006)076[0461:MPOTSI]2.0.CO;2
Helmuth, B., and Hofmann, G. E. (2001). Microhabitats, thermal heterogeneity, and patterns of physiological stress in the rocky intertidal zone. Biol. Bull. 201, 374–384. doi: 10.2307/1543615
Helmuth, B., Mieszkowska, N., Moore, P., and Hawkins, S. J. (2006b). Living in the edge of two changing worlds: forecasting the responses of rocky intertidal ecosystems to climate change. Annu. Rev. Ecol. Evol. Syst. 37, 373–404. doi: 10.1146/annurev.ecolsys.37.091305.110149
Hochachka, P. W., and Somero, G. N. (2002). Biochemical Adaptation: Mechanism and Process in Physiological Evolution. New York, NY: Oxford University Press.
Hoffmann, A. A., Sørensen, J. G., and Loeschcke, V. (2003). Adaptation of Drosophila to temperature extremes: bringing together quantitative and molecular approaches. J. Therm. Biol. 28, 175–216. doi: 10.1016/S0306-4565(02)00057-8
Hofmann, G. E., and Somero, G. N. (1996). Interspecific variation in thermal denaturation of proteins in the congeneric mussels Mytilus trossulus and M. galloprovincialis: evidence from the heat shock response and protein ubiquitination. Mar. Biol. 126, 65–75. doi: 10.1007/BF00571378
Huey, R. B., Berrigan, D., Gilchrist, G. W., and Herron, J. C. (1999). Testing the adaptive significance of acclimation: a strong inference approach. Am. Zool. 39, 323–336. doi: 10.1093/icb/39.2.323
Kelly, M. W., Sanford, E., and Grosberg, R. K. (2011). Limited potential for adaptation to climate change in a broadly distributed marine crustacean. Proc. Biol. Sci. 279, 349–356. doi: 10.1098/rspb.2011.0542
Kingsolver, J. G., Diamond, S. E., and Buckley, L. B. (2013). Heat stress and the fitness consequences of climate change for terrestrial ectotherms. Funct. Ecol. 27, 1415–1423. doi: 10.1098/rspb.2013.1149
Kingsolver, J. G., and Huey, R. B. (1998). Evolutionary analyses of morphological and physiological plasticity in thermally variable environments. Am. Zool. 38, 545–560. doi: 10.1093/icb/38.3.545
Leroi, A. M., Bennett, A. F., and Lenski, R. E. (1994). Temperature acclimation and competitive fitness: an experimental test of the beneficial acclimation assumption. Proc. Natl. Acad. Sci. U.S.A. 91, 1917–1921. doi: 10.1073/pnas.91.5.1917
Liao, M. L., Zhang, S., Zhang, G. Y., Chu, Y. M., Somero, G. N., and Dong, Y. W. (2017). Heat-resistant cytosolic malate dehydrogenases (cMDHs) of thermophilic intertidal snails (genus Echinolittorina): protein underpinnings of tolerance to body temperatures reaching 55°C. J. Exp. Biol. 220, 2066–2075. doi: 10.1242/jeb.156935
Marshall, D. J., Baharuddin, N., and McQuaid, C. D. (2013). Behaviour moderates climate warming vulnerability in high-rocky-shore snails: interactions of habitat use, energy consumption and environmental temperature. Mar. Biol. 160, 2525–2530. doi: 10.1007/s00227-013-2245-1
Marshall, D. J., Baharuddin, N., Rezende, E., and Helmuth, B. (2015). Thermal tolerance and climate warming sensitivity in tropical snails. Ecol. Evol. 5, 5905–5919. doi: 10.1002/ece3.1785
Marshall, D. J., Brahim, A., Mustapha, N., Dong, Y. W., and Sinclair, B. J. (2018). Substantial heat-tolerance acclimatory capacity in tropical thermophilic snails, but to what benefit? J. Exp. Biol 221:jeb187476. doi: 10.1242/jeb.187476
Marshall, D. J., and Chua, T. (2012). Boundary layer convective heating and thermoregulatory behaviour during aerial exposure in the rocky eulittoral fringe snail Echinolittorina malaccana. J. Exp. Mar. Biol. Ecol. 430, 25–31. doi: 10.1016/j.jembe.2012.06.011
Marshall, D. J., Dong, Y. W., Williams, G. A., and McQuaid, C. D. (2011). Thermal adaptation in the intertidal snail Echinolittorina malaccana contradicts current theory by revealing the crucial roles of resting metabolism. J. Exp. Biol. 214, 3649–3657. doi: 10.1242/jeb.059899
Marshall, D. J., and McQuaid, C. D. (2011). Warming reduces metabolic rate in marine snails: adaptation to fluctuating high temperatures challenges the metabolic theory of ecology. Proc. R. Soc. Lond. B Biol. Sci. 278, 281–288. doi: 10.1098/rspb.2010.1414
Marshall, D. J., McQuaid, C. D., and Williams, G. A. (2010). Non-climatic thermal adaptation: implications for species’ responses to climate warming. Biol. Lett. 6, 669–673. doi: 10.1098/rsbl.2010.0233
Miller, L. P., and Denny, M. W. (2011). Importance of behavior and morphological traits for controlling body temperature in littorinid snails. Biol. Bull. 220, 209–223. doi: 10.1086/BBLv220n3p209
Mitchell, K. A., Sgrò, C. M., and Hoffmann, A. A. (2011). Phenotypic plasticity in upper thermal limits is weakly related to Drosophila species distributions. Funct. Ecol. 25, 661–670. doi: 10.1111/j.1365-2435.2010.01821.x
Monaco, C. J., McQuaid, C. D., and Marshall, D. J. (2017). Decoupling of behavioural and physiological thermal performance curves in ectothermic animals: a critical adaptive trait. Oecologia 185, 583–593. doi: 10.1007/s00442-017-3974-5
Ng, T. P. T., Lau, S. L. Y., Seuront, L., Davies, M. S., Stafford, R., Marshall, D. J., et al. (2017). Linking behaviour and climate change in intertidal ectotherms: insights from littorinid snails. J. Exp. Mar. Biol. Ecol. 492, 121–131. doi: 10.1016/j.jembe.2017.01.023
Overgaard, J., Kristenses, T. N., Mitchell, K. A., and Hoffmann, A. A. (2011). Thermal tolerance in widespread and tropical Drosophila species: Does phenotypic plasticity increase with latitude? Am. Nat. 178, S80–S96. doi: 10.1086/661780
Phillips, B. L., Munoz, M. M., Hatcher, A., Macdonald, S. L., Llewelyn, J., Lucy, V., et al. (2015). Heat hardening in a tropical lizard: geographic variation explained by the predictability and variance in environmental temperatures. Funct. Ecol. 30, 1161–1168. doi: 10.1111/1365-2435.12609
Reid, D. (2007). The genus Echinolittorina (Habe, 1956) (Gastropoda: Littorinidae) in the Indo-West Pacific Ocean. Zootaxa 1420, 1–161. doi: 10.11646/zootaxa.1420.1.1
Reusch, T. B. H. (2014). Climate change in the oceans: evolutionary versus phenotypically plastic responses of marine animals and plants. Evol. Appl. 7, 104–122. doi: 10.1111/eva.12109
Rohr, J. R., Civitello, D. J., Cohen, J. M., Roznik, E. A., Sinervo, B., and Dell, A. I. (2018). The complex drivers of thermal acclimation and breadth in ectotherms. Ecol. Lett. 21, 1425–1439. doi: 10.1111/ele.13107
Schmidt, P. S., and Rand, D. M. (1999). Intertidal microhabitat and selection at Mpi: interlocus contrasts in the northern acorn barnacle, Semibalanus balanoides. Evolution 53, 135–146. doi: 10.1111/j.1558-5646.1999.tb05339.x
Seebacher, F., Beaman, J., and Little, A. G. (2014). Regulation of thermal acclimation varies between generations of the short-lived mosquitofish that developed in different environmental conditions. Funct. Ecol. 28, 137–148. doi: 10.1111/1365-2435.12156
Seebacher, F., Holmes, S., Roosen, N. J., Nouvian, M., Wilson, R. S., and Ward, A. J. (2012). Capacity for thermal acclimation differs between populations and phylogenetic lineages within a species. Funct. Ecol. 26, 1418–1428. doi: 10.1111/j.1365-2435.2012.02052.x
Slotsbo, S., Schou, M. F., Kristensen, T. N., Loeschcke, V., and Sørensen, J. G. (2016). Reversibility of developmental heat and cold plasticity is asymmetric and has long-lasting consequences for adult thermal tolerance. J. Exp. Biol. 219, 2726–2732. doi: 10.1242/jeb.143750
Somero, G. N. (2005). Linking biogeography to physiology: evolutionary and acclimatory adjustments of thermal limits. Front. Zool. 2:1. doi: 10.1186/1742-9994-2-1
Somero, G. N. (2010). The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine “winners” and “losers”. J. Exp. Biol. 213, 912–920. doi: 10.1242/jeb.037473
Somero, G. N., Lockwood, B. L., and Tomanek, L. (2017). Biochemical Adaptation: Response to Environmental Challenges from Life’s Origins to the Anthropocene. Sunderland, MA: Sinauer Associates, Inc.
Sørensen, J. G., Kristensen, T. N., and Loeschcke, V. (2003). The evolutionary and ecological role of heat shock proteins. Ecol. Lett. 6, 1025–1037. doi: 10.1046/j.1461-0248.2003.00528.x
Sørensen, J. G., Kristensen, T. N., and Overgaard, J. (2016). Evolutionary and ecological patterns of thermal acclimation capacity in Drosophila: is it important for keeping up with climate change? Curr. Opin. Insect. Sci. 17, 98–104. doi: 10.1016/j.cois.2016.08.003
Stillman, J. H. (2002). Causes and consequences of thermal tolerance limits in rocky intertidal porcelain crabs, genus Petrolisthes. Integr. Comp. Biol. 42, 790–796. doi: 10.1093/icb/42.4.790
Stillman, J. H. (2003). Acclimation capacity underlies susceptibility to climate change. Science 301:65. doi: 10.1126/science.1083073
Stillman, J. H., and Somero, G. N. (2000). A comparative analysis of the upper thermal tolerance limits of eastern Pacific porcelain crabs, genus Petrolisthes: influences of latitude, vertical zonation, acclimation, and phylogeny. Physiol. Biochem. Zool. 73, 200–208. doi: 10.1086/316738
Terblanche, J. S., Hoffmann, A. A., Mitchell, K. A., Rako, L., le Roux, P. C., and Chown, S. L. (2011). Ecologically relevant measures of tolerance to potentially lethal temperatures. J. Exp. Biol. 214, 3713–3725. doi: 10.1242/jeb.061283
Tomanek, L., and Somero, G. N. (1999). Evolutionary and acclimation-induced variation in the heat-shock responses of congeneric marine snails (genus Tegula) from different thermal habitats: implications for limits of thermotolerance and biogeography. J. Exp. Biol. 202, 2925–2936.
Verberk, W. C., Bartolini, F., Marshall, D. J., Pörtner, H. O., Terblanche, J. S., White, C. R., et al. (2016). Can respiratory physiology predict thermal niches? Ann. N. Y. Acad. Sci. 1365, 73–88. doi: 10.1111/nyas.12876
Wang, W., Ding, M. W., Li, X. X., Wang, J., and Dong, Y. W. (2017). Divergent thermal sensitivities among different life stages of the pulmonate limpet Siphonaria japonica. Mar. Biol. 164:125. doi: 10.1007/s00227-017-3157-2
Williams, G. A., and Morritt, D. (1995). Habitat partitioning and thermal tolerance in a tropical limpet, Cellana grata. Mar. Ecol. Prog. Ser. 124, 89–103. doi: 10.3354/meps124089
Williams, S. T., and Reid, D. G. (2004). Speciation and diversity on tropical rocky shores: a global phylogeny of snails of the genus Echinolittorina. Evolution 58, 2227–2251. doi: 10.1111/j.0014-3820.2004.tb01600.x
Wilson, R. S., and Franklin, C. E. (2002). Testing the beneficial acclimation hypothesis. Trends Ecol. Evol. 17, 66–70. doi: 10.1016/S0169-5347(01)02384-9
Keywords: developmental plasticity, Echinolittorina malaccana, habitat heterogeneity, heat resistance, thermal acclimation
Citation: Brahim A, Mustapha N and Marshall DJ (2019) Non-reversible and Reversible Heat Tolerance Plasticity in Tropical Intertidal Animals: Responding to Habitat Temperature Heterogeneity. Front. Physiol. 9:1909. doi: 10.3389/fphys.2018.01909
Received: 06 September 2018; Accepted: 18 December 2018;
Published: 14 January 2019.
Edited by:
Carlos Rosas, National Autonomous University of Mexico, MexicoReviewed by:
Mikko Juhani Nikinmaa, University of Turku, FinlandFernando Diaz, Ensenada Center for Scientific Research and Higher Education (CICESE), Mexico
Copyright © 2019 Brahim, Mustapha and Marshall. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David J. Marshall, davidmarshall11@gmail.com
 Amalina Brahim
Amalina Brahim Nurshahida Mustapha
Nurshahida Mustapha David J. Marshall
David J. Marshall