- 1Guang'anmen Hospital, Chinese Academy of Chinese Medical Sciences, Beijing, China
- 2Key Laboratory of Chinese Internal Medicine of Ministry of Education and Beijing, Dongzhimen Hospital Affiliated to Beijing University of Chinese Medicine, Beijing, China
- 3Department of Cardiology, Beijing An Zhen Hospital of the Capital University of Medical Sciences, Beijing, China
- 4Division of Arrhythmia and Electrophysiology, Department of Cardiovascular Medicine, National Cerebral and Cardiovascular Center, Suita, Osaka, Japan
- 5Masonic Medical Research Laboratory, Utica, NY, United States
- 6Hubei Key Laboratory of Cardiology, Department of Cardiology and Cardiovascular Research Institute, Renmin Hospital of Wuhan University, Wuhan, China
Background: Male gender has been consistently shown to be a risk factor for a greater number of arrhythmic events in patients with Brugada Syndrome (BrS). However, there have been no large-scale comprehensive pooled analyses to statistically and systematically verify this association. Therefore, we conducted a pooled analysis on gender differences in prognosis and risk stratification of BrS with a largest sample capacity at present.
Methods: We searched PubMed, Embase, Medline, Cochrane Library databases, Chinese National Knowledge Infrastructure, and Wanfang Data for relevant studies published from 2002 to 2017. The prognosis and risk stratification of BrS and risk factors were then investigated and evaluated according to gender.
Results: Twenty-four eligible studies involving 4,140 patients were included in the analysis. Male patients (78.1%) had a higher risk of arrhythmic events than female patients (95% confidence interval: 1.46–2.91, P < 0.0001). Among the male population, there were statistical differences between symptomatic patients and asymptomatic patients (95% CI: 2.63–7.86, P < 0.00001), but in the female population, no statistical differences were found. In the female subgroup, electrophysiological study (EPS) positive patients had a tendency toward a higher risk of arrhythmic events than EPS-negative patients (95% CI: 0.93–29.77, P = 0.06).
Conclusions: Male patients are at a higher risk of arrhythmic events than female patients. Within the male population, symptomatic patients have a significantly higher risk profile compared to asymptomatic patients, but no such differences are evident within the female population. Consequently, in the female population, the risk of asymptomatic patterns cannot be underestimated.
Introduction
Brugada syndrome (BrS) is an inherited arrhythmic disorder generally characterized by a distinct electrocardiogram (ECG) pattern: the presence of ST-segment elevation in the right precordial leads (V1–V3), which may carry an increased risk of sudden cardiac death (SCD) due to malignant ventricular arrhythmias (Bayés et al., 2012). That typical “syndrome” was firstly presented by Nava et al. in 1988 at the National Congress of Italian Cardiologists, which subsequently named by Brugada brothers in 1992 (Martini et al., 1988; Nava et al., 1988; Brugada and Brugada, 1992). In current common consensus, BrS was described as a functional abnormality of repolarization, but theory proposed by Nava et al. believed that the true syndrome is not only a primary electrical disease performed particular ECG but a conduction disturbance at the right ventricular outflow tract (RVOT) related to clinical events (Martini and Nava, 2004; Marras et al., 2009). Recent focal therapeutic radiofrequency ablation (RFA) strategy indirectly proved the theory (Brugada et al., 2015). According to the expert consensus in 2013, patients with Brugada type 1 ECG induced by Class I antiarrhythmic drugs are included (Priori et al., 2013). Type 1 ECG induced by drug may occur false positive Brugada (Konigstein et al., 2016; Mizusawa et al., 2016).
Male sex has consistently been shown to be associated with a higher risk of arrhythmic events (Benito et al., 2008; Priori et al., 2013). However, the lack of large-scale samples and systematic comprehensive analysis have contributed to weak conformance and statistical power. In addition, there have been no comprehensive pooled analyses examining prognosis and risk stratification for BrS. Several clinical variables are considered to be potentially associated with worse outcome in patients with BrS. Electrophysiological study (EPS) might be the most controversial factor, and there remains no consensus on whether its inducibility is valuable in predicting outcome (Priori et al., 2002, 2012; Eckardt et al., 2005). Large registries have consistently shown that patients with spontaneous type 1 ECG have a high risk of cardiac arrhythmic events at follow-up (Brugada et al., 2002, 2004, 2005). The presence of symptoms is a significant predictor of arrhythmias (Priori et al., 2002). SCN5A mutation and recent positive family history of SCD have debatable feasibility as risk markers (Kanda et al., 2002). Lack of examination for documented auricular fibrillation (AF) status might lead to new agitation. However, the gender differences between these variables and whether men and women experience disparate outcomes remain indeterminate. Variables differing between the sexes, and how these manifests in certain sex groups, remain to be elucidated.
Therefore, based on a largest sample capacity of 4,140 patients from 24 clinical trials at present, we conducted a comprehensive pooled analysis of gender differences, including the following aspects: risk of arrhythmic events, EPS status, family history of SCD, spontaneous type 1 ECG pattern, SCN5A mutation, diagnosis status, and documented AF status.
Methods
Search Strategy
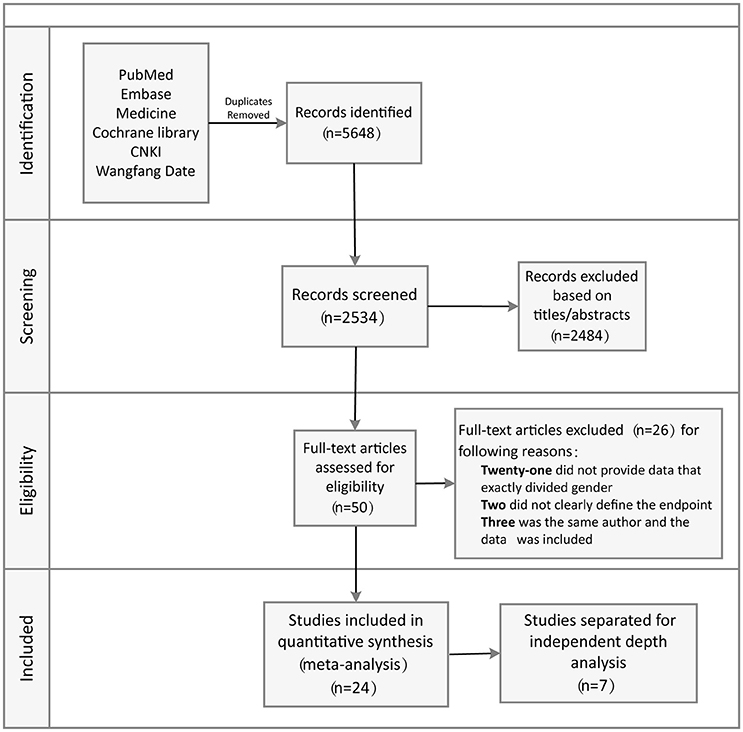
A comprehensive literature search of relevant studies published in PubMed, Embase, Medline, Cochrane Library databases, Chinese National Knowledge Infrastructure, and Wanfang Data was performed by two reviewers independently and systematically. We searched relevant published studies from 2002 to 2017 using the keywords: “Brugada” and “syndrome” or “Brugada syndrome” and “risk stratification.” The titles, abstracts, and reference lists of all articles were carefully reviewed for potential and additional publications regarding this topic. Full text assessment of potential relevant studies was conducted for compliance with the inclusion criteria and to prevent duplication of data by the same group of authors (Figure 1).
Inclusion Criteria
All studies had to meet following criteria for inclusion: (a) full-text English language studies published in peer-reviewed journals; (b) prospective or retrospective observational study design; (c) follow-up duration sufficiently long to detect arrhythmic events; (d) information included regarding clearly defined endpoint events (appropriate shocks, ventricular fibrillation/ventricular tachycardia, and SCD); (e) risk ratio (RR), hazard ratio (HR), odds ratio (OR), corresponding 95% confidence intervals (CIs), or necessary raw data were reported.
Data Collection
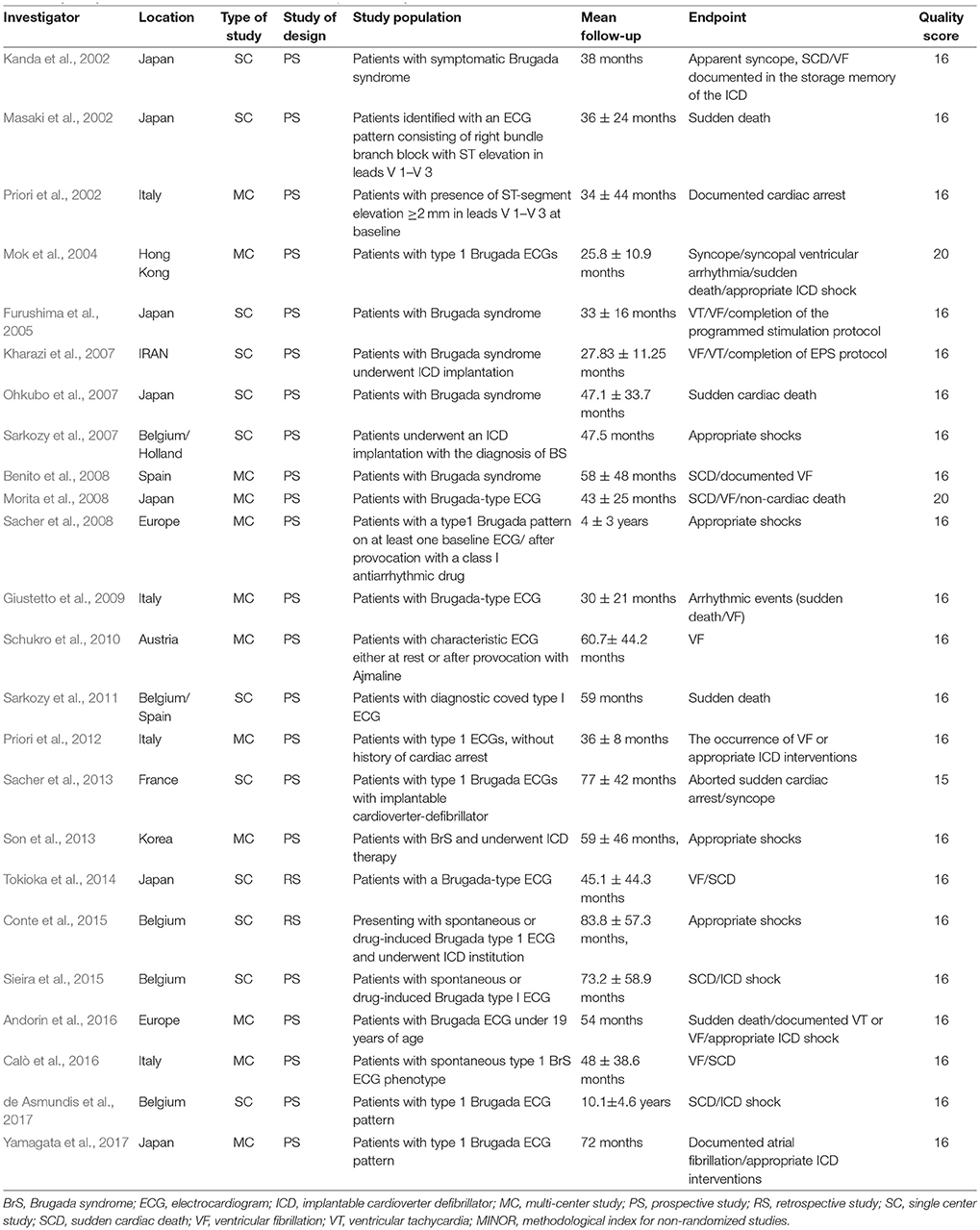
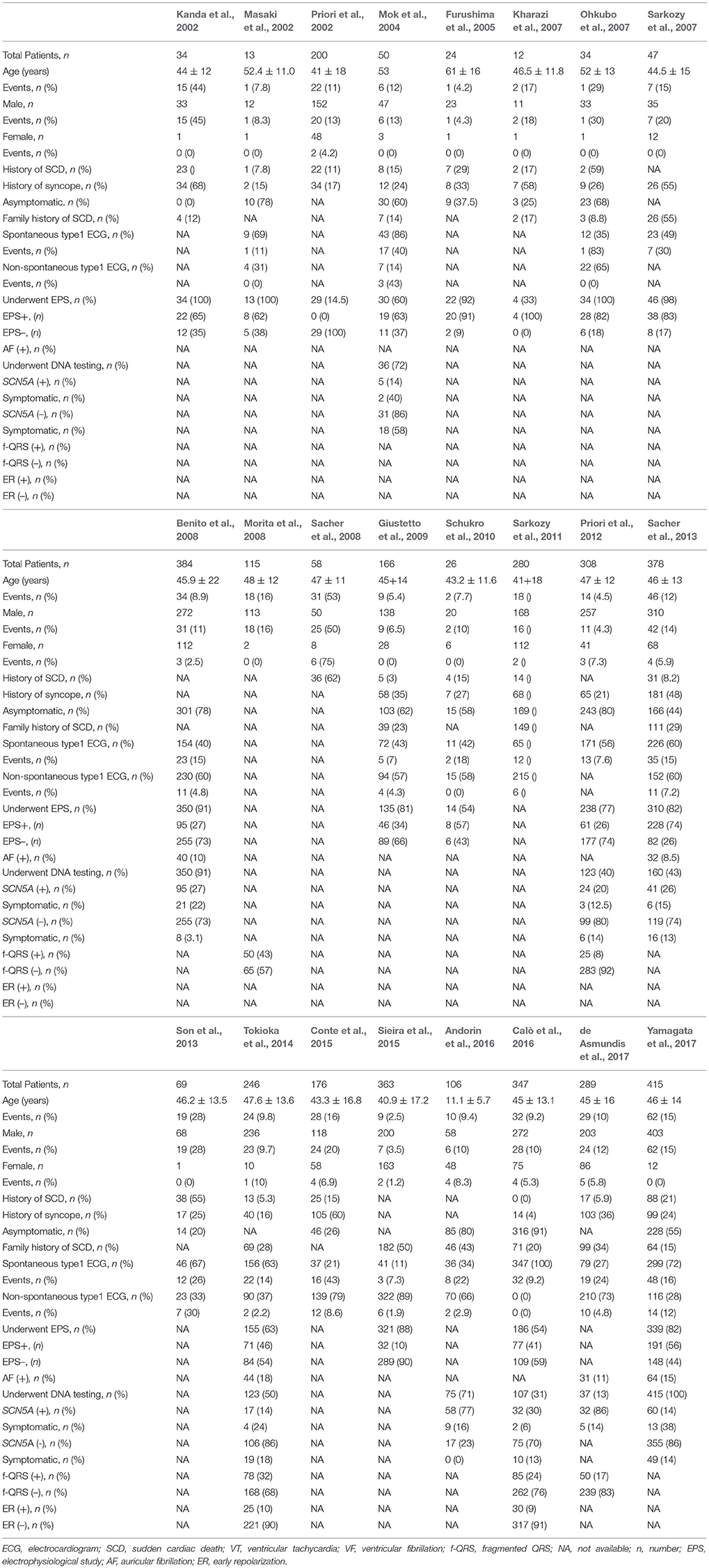
Twenty-four studies (Kanda et al., 2002; Masaki et al., 2002; Priori et al., 2002, 2012; Slim et al., 2003; Mok et al., 2004; Furushima et al., 2005; Kharazi et al., 2007; Ohkubo et al., 2007; Sarkozy et al., 2007, 2011; Benito et al., 2008; Morita et al., 2008; Sacher et al., 2008, 2013; Giustetto et al., 2009; Kamakura et al., 2009; Schukro et al., 2010; Son et al., 2013; Tokioka et al., 2014; Conte et al., 2015; Sieira et al., 2015; Andorin et al., 2016; Calò et al., 2016; de Asmundis et al., 2017; Yamagata et al., 2017) consisting of 4,140 BrS patients were ultimately included in the study analysis. The extracted data elements for the analysis included: surname of first author, publication year, origin of the studied population, type of study, study design, study population, mean duration follow-up, endpoint events, quality score (Table 1); sample size, participants' age and sex, number of subjects with history of SCD or syncope, family history of SCD, spontaneous type 1 ECG pattern, detailed information regarding EPS, positive/negative SCN5A gene mutation, presence of AF, fragmented QRS (f-QRS), and early repolarization (ER) (Table 2).
Upon sending e-mails to the principal authors of identified studies to request data sharing with a standardized form and definitions, we received original data for two of the studies (Sacher et al., 2013; Tokioka et al., 2014). Some of the data could not be found in the articles because the original data might be different from that published, owing to additional patients and longer follow-up times.
Quality Assessment
The Methodological Index for Non-Randomized Studies (MINORS) (Slim et al., 2003) was used to assess the quality of all included studies. The maximum value with this index is 24 points, with each item scored from 0 to 2 on the following aspects: (a) a clearly stated aim; (b) inclusion of consecutive patients; (c) prospective collection of data; (d) endpoints appropriate to the aim of the study; (e) unbiased assessment of the study endpoint; (f) follow-up period appropriate to the aim of the study; (g) loss to follow up < 5%; and (h) prospective calculation of the study size. Both reviewers independently scored the included publications, then used the average MINORS score for final assessment. Based on MINORS scores of <16 and ≥16 points, studies were defined to be low-quality and high-quality studies, respectively.
Statistical Analysis
We estimated heterogeneity between studies using I2, which is derived from the standard chi-square test to represent the variability in effect produced by heterogeneity. An I2 >50% was indicative of significant statistical heterogeneity. We extracted and analyzed all the multivariate adjusted OR with 95% CI for each study. Pooled OR were calculated using the M-H random-effects model and fixed-effects model to take into account within-study and between-study variance. Sensitivity analyses were conducted to evaluate the significance of the final results. We also performed subgroup analysis based on gender (positive vs. negative), EPS status (male vs. female), family history of SCD (male vs. female), spontaneous type 1 ECG (male vs. female), SCN5A (male vs. female), status at diagnosis (male vs. female), and documented AF status (male vs. female), and the OR was also calculated. Publication bias was assessed by means of the funnel plot. Statistical significance was defined as a P-value ≤ 0.05. All analyses were performed using Review Manager, version 5.0.12 (Revman; The Cochrane Collaboration, Oxford, U.K.).
Results
Study Selection
The systematic review of the literature yielded a total of 5,648 potentially relevant studies with our search criteria. After screening of the titles and abstracts, 2,534 studies were excluded, leaving 50 for full-text assessment. Twenty-six duplicate studies were excluded, while 21 did not provide clear data pertaining to sex-related differences. Two studies did not clearly define the endpoint, while three had the same author with data included. Eventually, 24 of the original qualifying studies from the databases were included. Seven of the 24 studies were separated for independent depth analysis (Figure 1).
Male and Female
Overall, among 4,140 patients with BrS, 3,222 male patients (event rate 12.4%) and 918 female patients (event rate 4.4%) were included, because BrS is a male predominance syndrome (Priori et al., 2013). All 24 studies were included in this pooled gender analysis. An increased risk of arrhythmic events was observed in the male population compared to the female population (OR 2.06, 95% CI: 1.46–2.91, P < 0.0001; heterogeneity: P = 0.70, I2 = 0%, Figure 2). The calculations showed a statistically significant difference between the two groups. Males had a higher risk of arrhythmia compared to females. At the same time, we conducted sensitivity analysis, excluding any set of data that would have no effect on the results.
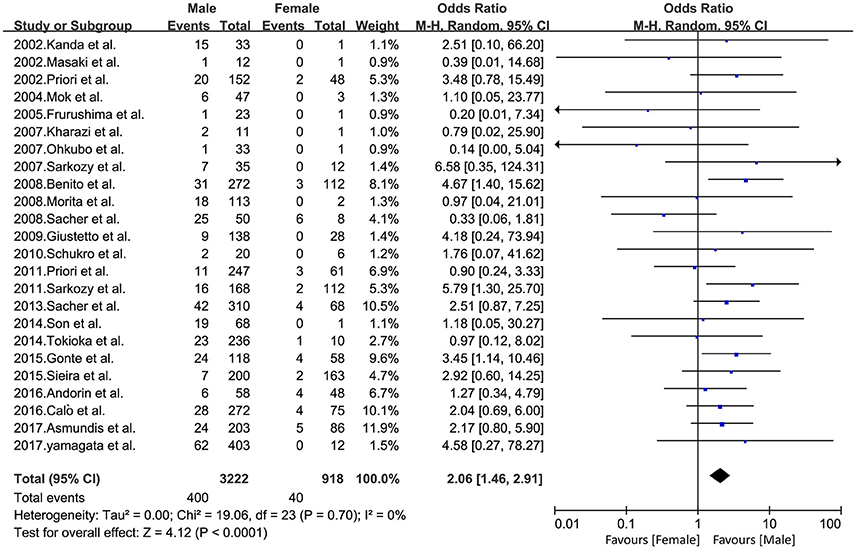
Figure 2. Odds radio for the occurrence of arrhythmic events during follow-up depending on the presence of gender.
EPS Group
A total of 810 patients (men = 704) from eight studies (Kanda et al., 2002; Masaki et al., 2002; Furushima et al., 2005; Ohkubo et al., 2007; Priori et al., 2012; Sacher et al., 2013; Tokioka et al., 2014; Sieira et al., 2015) were included in this group. In the EPS-positive subgroup, no significant gender differences related to cardiac events were found between males and females(OR 0.81, 95% CI: 0.32–2.06, P = 0.65; heterogeneity: P = 0.48, I2 = 0 %, Figure 3A). The result was the same in the EPS-negative subgroup (OR 0.02, 95% CI: −0.02–0.06, P = 0.23; heterogeneity: P = 0.69, I2 = 0%, Figure 3B). In the male subgroup, there was also no statistical difference between EPS-positive patients and EPS-negative patients (OR 1.64, 95% CI: 0.68–3.96, P = 0.28; heterogeneity: P = 0.07, I2 = 49%, Figure 3C). However, in the female subgroup, EPS-positive patients had a tendency toward a higher risk of arrhythmic events (OR 5.26, 95% CI: 0.93–29.77, P = 0.06; heterogeneity: P = 0.48, I2 = 0%, Figure 3D).
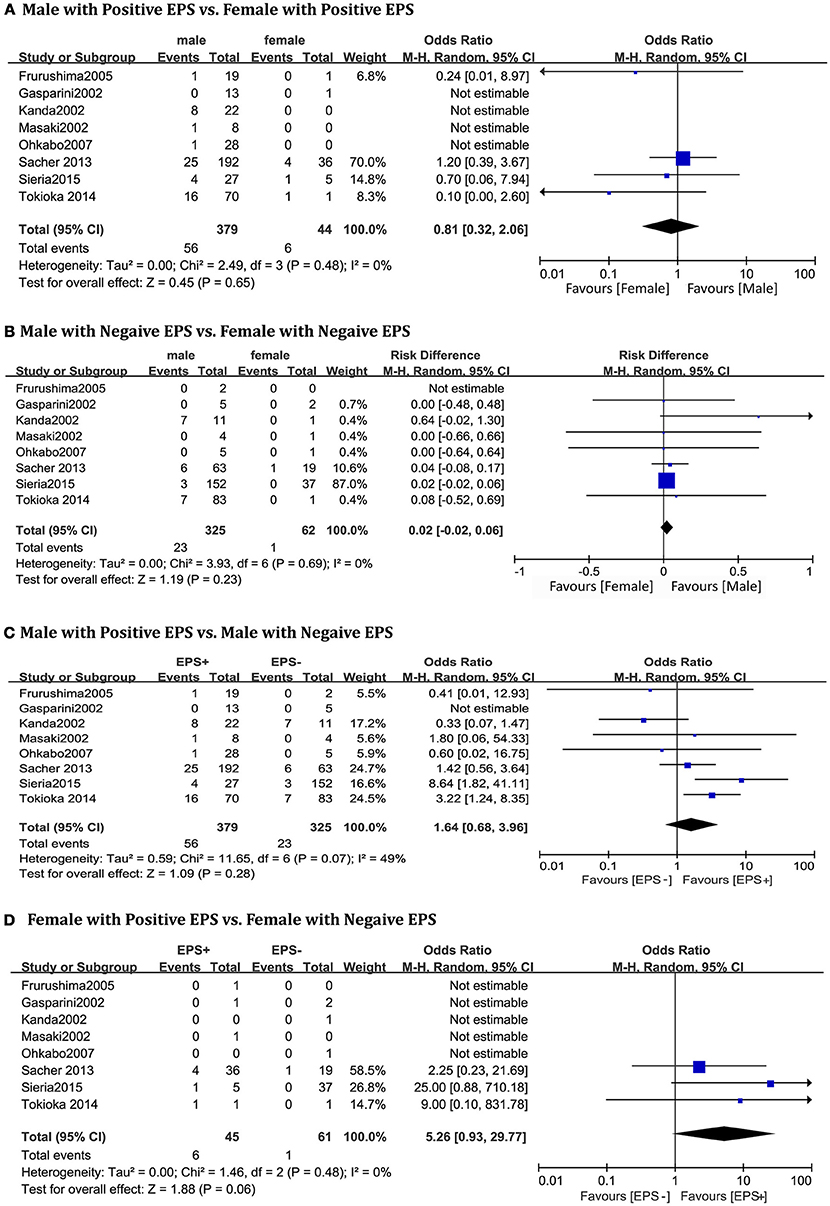
Figure 3. Odds radio for the occurrence of arrhythmic events during follow-up depending on EPS pattern subgroups. (A) Prognosis of male and female in positive EPS subgroup, (B) Prognosis of male and female in negative EPS subgroup, (C) Prognosis of positive EPS and negative EPS in male subgroup, (D) Prognosis of positive EPS and negative EPS in female subgroup.
Family History of SCD
Four studies (Shaowen Liu and Ole Kongstad, 2001; Ohkubo et al., 2007; Bayés et al., 2012; Tokioka et al., 2014), consisting of 634 patients (men = 565) were eligible for this pooled analysis. We did not find significant gender differences in relation to family history of SCD in patients with a positive history (OR 1.92, 95% CI: 0.43–8.56, P = 0.39; heterogeneity: P = 0.13, I2 = 57%, Figure 4A) or in those with a negative history (OR 1.23, 95% CI: 0.44–3.41, P = 0.70; heterogeneity: P = 0.43, I2 = 0%, Figure 4B). There were also no significance differences within the male subgroup (OR 1.23, 95% CI: 0.72–2.09, P = 0.45; heterogeneity: P = 0.02, I2 = 73%, Figure 4C), or within the female subgroup (OR 0.85, 95% CI: 0.16–4.51, P = 0.85; heterogeneity: P = 0.19, I2 = 42%, Figure 4D).
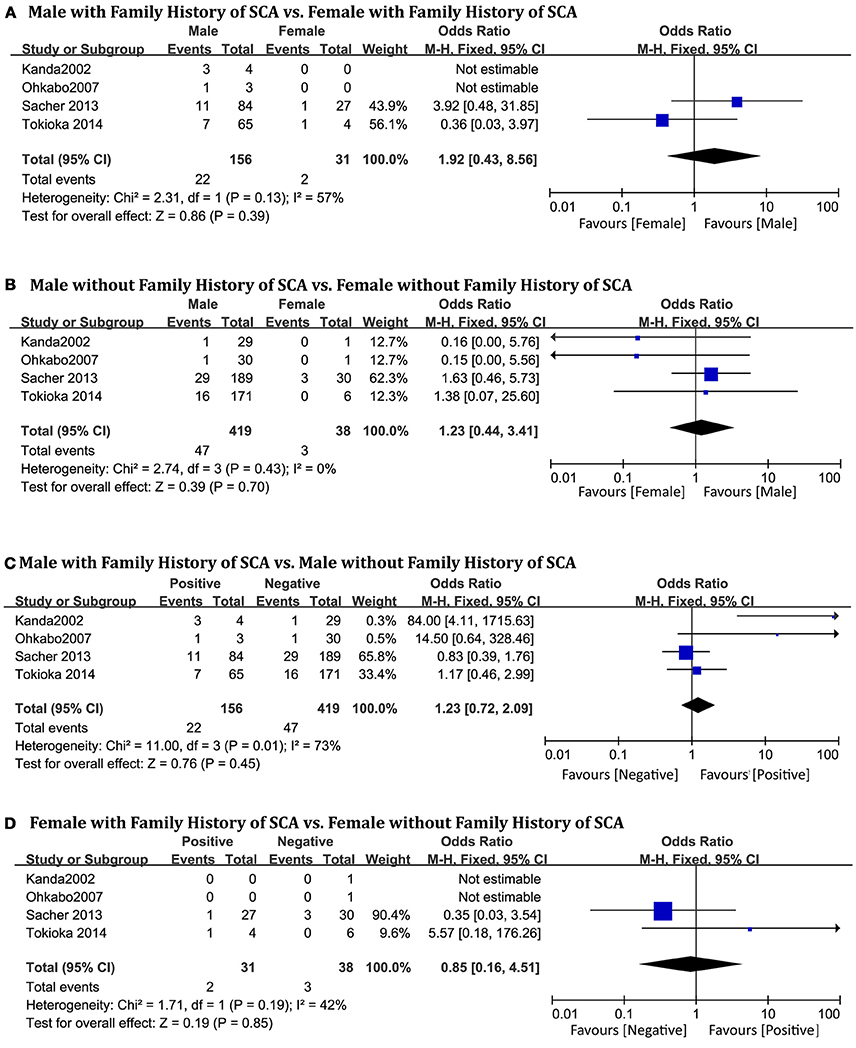
Figure 4. Odds radio for the occurrence of arrhythmic events during follow-up depending on family history of SCA subgroups. (A) Prognosis of male and female in positive family history of SCA subgroup, (B) Prognosis of male and female in negative family history of SCA subgroup, (C) Prognosis of positive family history of SCA and negative family history of SCA in male subgroup, (D) Prognosis of positive family history of SCA and negative family history of SCA in female subgroup.
Spontaneous Type 1 ECG Pattern
A total of 694 patients (men = 420) from five studies (Masaki et al., 2002; Furushima et al., 2005; Ohkubo et al., 2007; Sacher et al., 2013; Tokioka et al., 2014) were included. No statistically significant sex-related differences were observed in the spontaneous type 1 BrS subgroup (OR 1.90, 95% CI: 0.61–5.91, P = 0.27; heterogeneity: P = 0.29, I2 = 18%, Figure 5A). In the non-spontaneous type 1 ECG subgroup, there was also no statistical difference between men and women (OR 0.82, 95% CI: 0.25–2.64, P = 0.74; heterogeneity: P = 0.75, I2 = 0%, Figure 5B).
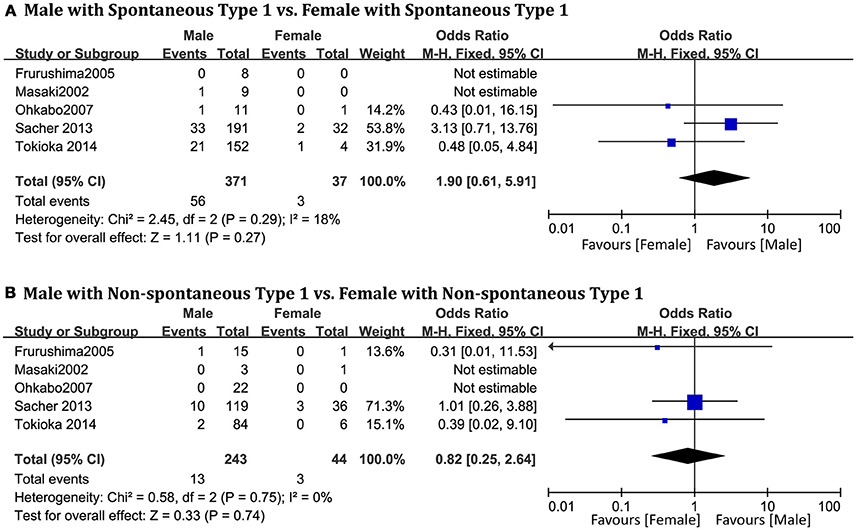
Figure 5. Odds radio for the occurrence of arrhythmic events during follow-up depending on spontaneous type 1 pattern subgroups. (A) Prognosis of male and female in spontaneous type 1 subgroup, (B) Prognosis of male and female in non-spontaneous type 1 subgroup.
SCN5A
Only two original studies (Sacher et al., 2013; Tokioka et al., 2014) including 283 patients (men = 257) were included in this group. In the subgroup positive for SCN5A mutations, we found no significant differences related to SCN5A between men and women (OR 2.53, 95% CI: 0.29–22.18, P = 0.40; heterogeneity: P = 0.56, I2 = 0%, Figure 6A). In the negative subgroup, the outcome was the same (OR 0.43, 95% CI: 0.13–1.41, P = 0.17; heterogeneity: P = 0.22, I2 = 32%, Figure 6B). No significant differences were found within the male subgroup (OR 1.57, 95% CI: 0.70–3.51, P = 0.27; heterogeneity: P = 0.96, I2 = 0%, Figure 6C), nor within the female subgroup (OR 0.19, 95% CI: 0.01–2.53, P = 0.21; heterogeneity: P = 0.80, I2 = 0%, Figure 6D).
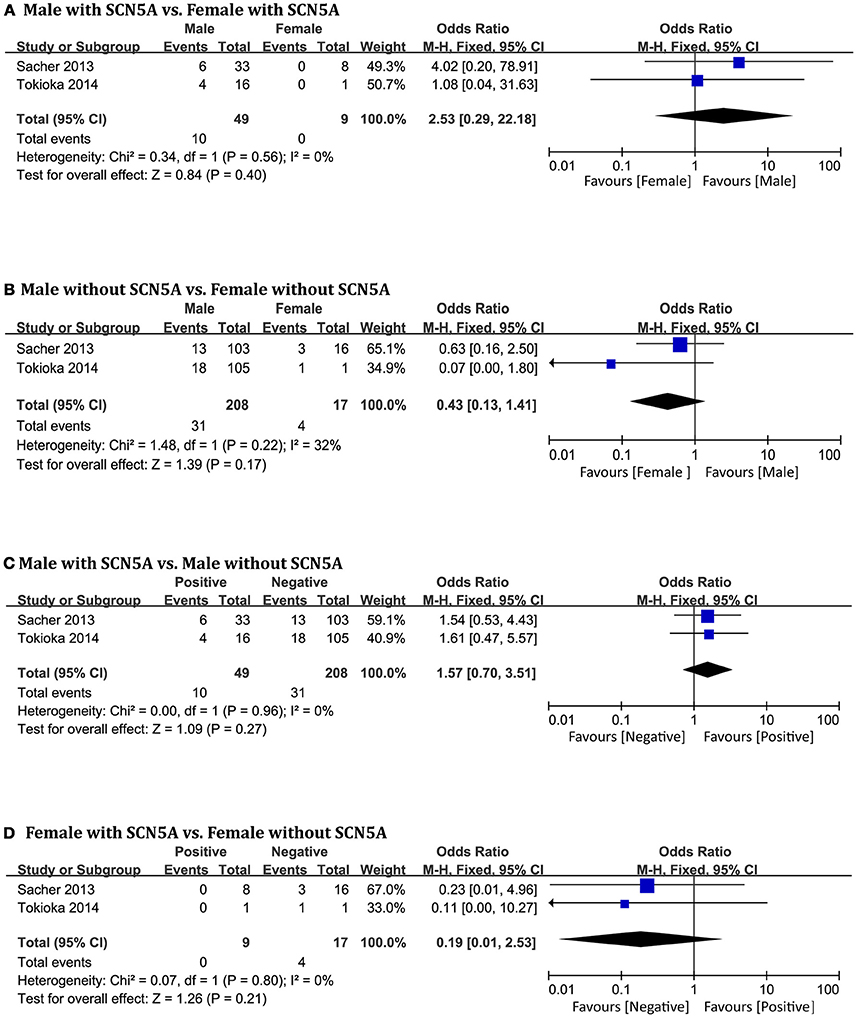
Figure 6. Odds radio for the occurrence of arrhythmic events during follow-up depending on SCN5A pattern subgroups. (A) Prognosis of male and female in positive SCN5A subgroup, (B) Prognosis of male and female in negative SCN5A subgroup, (C) Prognosis of positive SCN5A and negative SCN5A in male subgroup, (D) Prognosis of positive SCN5A and negative SCN5A in female subgroup.
Symptomatic and Asymptomatic
A total of 729 patients (men = 647) in six studies (Kanda et al., 2002; Masaki et al., 2002; Furushima et al., 2005; Ohkubo et al., 2007; Sacher et al., 2013; Tokioka et al., 2014) were eligible for this group. We found that in the male population, symptomatic patients displayed a higher risk of arrhythmic events than asymptomatic patients (OR 4.54, 95% CI: 2.63–7.86, P < 0.00001; heterogeneity: P = 0.002, I2 = 77%, Figure 7C). However, no statistical differences were found within the female population (OR 9.52, 95% CI: 0.85–106.67, P = 0.07; heterogeneity: P = 0.73, I2 = 0%, Figure 7D) (Figure 8). Moreover, there were no significant sex-related differences in the symptomatic subgroup pattern (OR 1.56, 95% CI: 0.62–3.89, P = 0.34; heterogeneity: P = 0.82, I2 = 0%, Figure 7A) or in the asymptomatic subgroup (OR 0.72, 95% CI: 0.06–7.95, P = 0.79; heterogeneity: P = 0.18, I2 = 42%, Figure 7B).
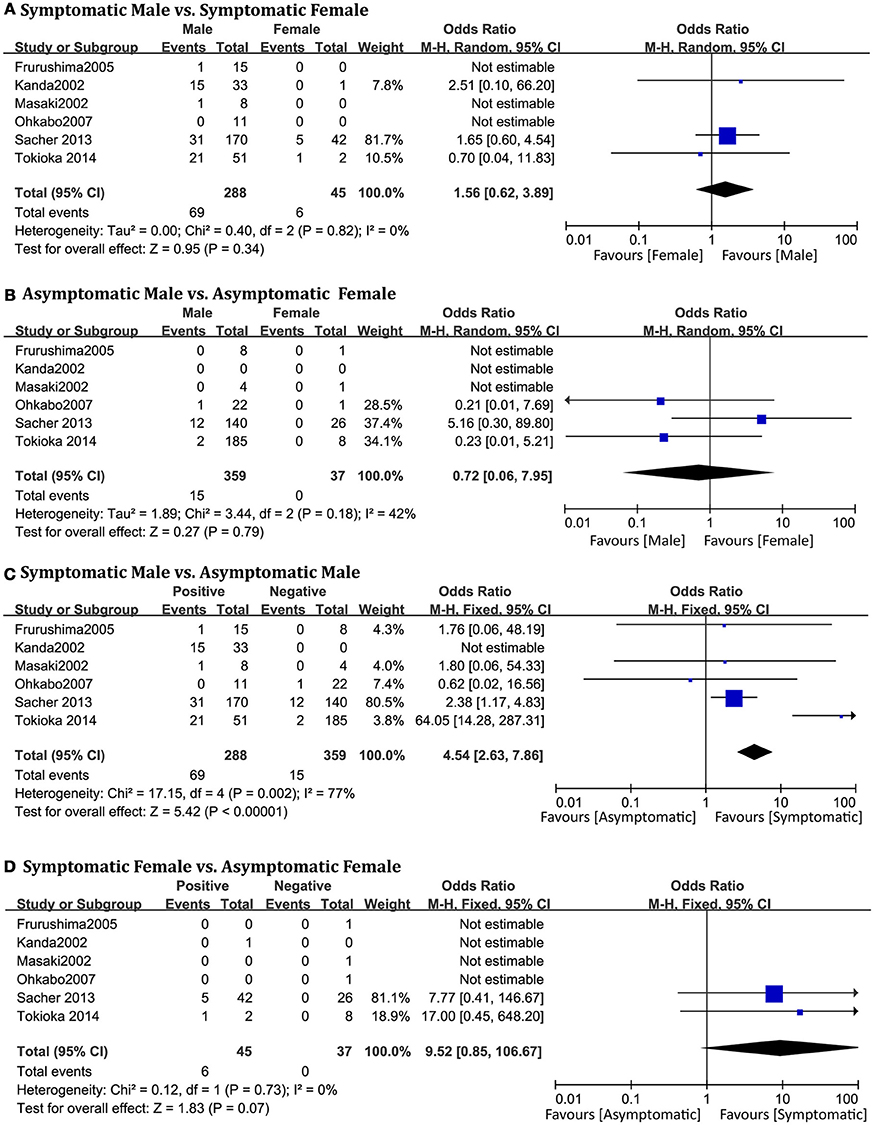
Figure 7. Odds radio for the occurrence of arrhythmic events during follow-up depending on symptomatic pattern subgroups. (A) Prognosis of male and female in symptomatic subgroup, (B) Prognosis of male and female in asymptomatic subgroup, (C) Prognosis of symptomatic and asymptomatic in male subgroup, (D) Prognosis of symptomatic and asymptomatic in female subgroup.
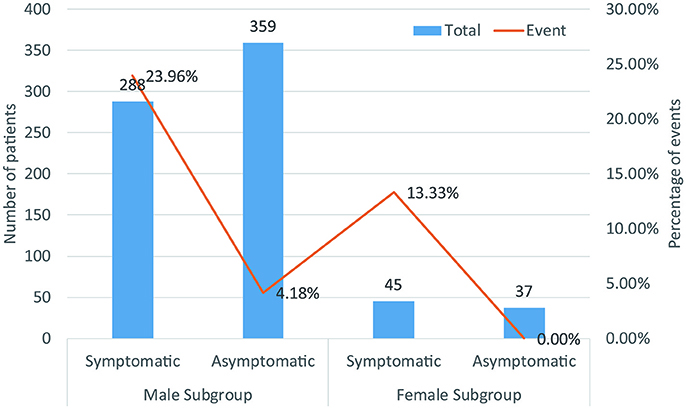
Figure 8. Biaxial diagram depending on number of patients and percentage of events in symptomatic pattern group.
Documented AF Status
The three studies in this analysis (Kanda et al., 2002; Sacher et al., 2013; Tokioka et al., 2014) consisted of 658 patients (men = 579). Sex-related difference was not significantly related to cardiac events in the AF-positive subgroup (OR 2.00, 95% CI: 0.21–18.93, P = 0.55, Figure 9A). In the negative group, male and female patients showed no statistical differences (OR 1.85, 95% CI: 0.73–4.65, P = 0.19; heterogeneity: P = 0.62, I2 = 0%, Figure 9B). In the male subgroup, also, there were no significant differences based on documented AF status (OR 1.67, 95% CI: 0.92–3.04, P = 0.09; heterogeneity: P = 0.12, I2 = 53%, Figure 9C). In the female subgroup, the result was the same (OR 1.50, 95% CI: 0.15–14.99, P = 0.37, Figure 9D). Heterogeneity was not applicable for some outcomes because only one study provided suitable data for documented AF status.
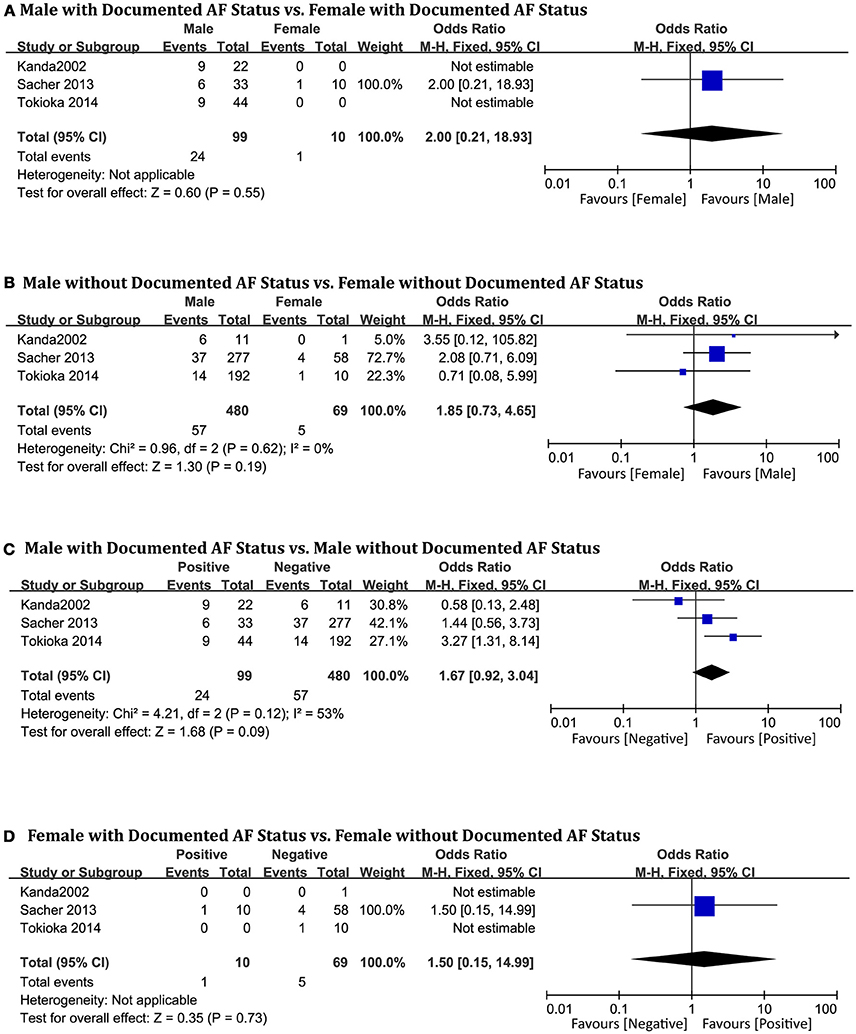
Figure 9. Odds radio for the occurrence of arrhythmic events during follow-up depending on documented AF status subgroups. (A) Prognosis of male and female in positive documented AF subgroup, (B) Prognosis of male and female in negative documented AF subgroup, (C) Prognosis of positive documented AF and negative documented AF in male subgroup, (D) Prognosis of positive documented AF and negative documented AF in female subgroup.
Discussion
We drew the following conclusions from the pooled analysis: (i) male patients display a higher risk of arrhythmic events than female patients; (ii) in the male population, symptomatic patients display a higher risk profile of arrhythmic events compared to asymptomatic patients, but there are no significant differences within the female population. Consequently, in the female population, the risk of asymptomatic patterns cannot be underestimated.
According to our systematically comprehensive analysis of 24 trials, male patients display a higher risk profile compared with female patients. Although this conclusion has been consistently recognized in the HRS/EHRA/APHRS expert consensus statement (Priori et al., 2013), our study is the largest at present, including 4,140 patients, to analyze gender differences in prognosis and risk stratification for BrS. Similar outcomes were found in other studies (Gehi et al., 2006; Benito et al., 2008). New studies have confirmed those acknowledged results, and outlined a complex relationship between sex distribution and patient ethnicity and age (Milman et al., 2018).
Many studies have shown that syncope was an independent predictor of risk, and provided sufficient evidence (Brugada et al., 2004; Priori et al., 2012; Calvo et al., 2016). The presence of symptoms in patients was significantly associated with arrhythmic events (23 vs. 3.8%, P < 0.00001) in our analysis. These results might explain the conclusion that in the male subgroup, symptomatic patients displayed a higher risk of arrhythmic events than asymptomatic patients. Surprisingly, in the female population, there were no significant differences between symptomatic patients and asymptomatic patients. We can infer that symptomatic status might only be a risk factor for men, and that asymptomatic women may be in a potentially dangerous situation. The risk of asymptomatic patterns cannot be underestimated. Although these results may be due to the lower incidence (11%) of women with BrS, the findings offer new insights for further research to combine with the new syncope episodes (Olde Nordkamp et al., 2015).
In our results, EPS-positive patients had a tendency toward a higher risk of arrhythmic events than EPS-negative patients only in the female subgroup(p = 0.06), which presented a potential risk factor to women. We can infer that the result may turn positive when the sample size is enlarged. Whether EPS inducibility is a predictor of arrhythmic events in BrS patients with previous syncope/sudden death or an independent character remains in dispute (Brugada et al., 2002, 2004; Priori et al., 2002; Giustetto et al., 2009). In the 2017 AHA/ACC/HRS guideline for ventricular arrhythmias and SCD, an EPS with programmed ventricular stimulation using single or double extrastimuli may be considered for further risk stratification in asymptomatic and spontaneous type 1patients (Kusumoto et al., 2017). Newly studies suggested that extent of substrate is the only independent predictor of inducibility of VT or VF and may contribute to a new marker for risk stratification and therapy (Pappone et al., 2018). The differences of sex-related cardiac electrophysiological characteristics may be the main reason contributing to the result, that women have lower expression of KChIP2 which is the main accessory subunit of transient outward current in right ventricular epicardium (Tadros et al., 2014). Besides women have greater sinoatrial node automaticity and enhanced atrioventricular node function than men (Burke et al., 1996; Shaowen Liu and Ole Kongstad, 2001).
Spontaneous type 1 ECG was regarded as a risk factor for arrhythmic events in most studies (Brugada and Brugada, 1992; Brugada et al., 1998, 2002, 2004, 2005; Priori et al., 2002, 2012; Benito et al., 2008). Many reporters overserved that men with cardiac events had greater rates of spontaneous type 1 ECG, and among male patients with spontaneous type 1 ECG, cardiac events were more frequent (Benito et al., 2008; Sacher et al., 2013; Shi et al., 2018). Recent studies have indicated that females have less type 1 BrS ECG and lower inducibility rates than males (Milman et al., 2018). However, interestingly no statistically significant sex-related differences were found in our result. In a report by the European Society of Cardiology, family history of SCD is regarded as one of three factors for the events (Priori et al., 2001), but in the family history of the SCD group, we obtained absolutely negative results in all four subgroups. We also observed negative results in the SCN5A group, which was consistent with the HRS/EHRA/APHRS expert consensus statement (Priori et al., 2013).
The limitations of the study should be acknowledged. Although we included 4,140 patients from 24 studies incorporating the original data from two articles, there remain limitations in subgroup analysis to a certain extent. The number of women with BrS is relatively small, especially in some small samples. This situation limits the statistical power.
Author Contributions
YG and YX defined the research theme. Dr. Frédéric Sacher and KK contributed the original data. MY and XL wrote the manuscript. XY, YY, and NL designed the methods. CT and XW analyzed the data. HB-M, DH, and HS interpreted the results. All authors discussed the results and commented on the manuscript.
Funding
The work was supported by the National Natural Science Foundation of China (Grant Nos. 81725024 and 81430098), National high-level talent special support plan (No. W02020052), Clinical base project of State Administration of Traditional Chinese medicine of China (JDZX2015007) and State Key Development Program of China (2017YFC1700400).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors wish to express gratitude to Dr. Frédéric Sacher and KK, for their contributing the original data.
Abbreviations
AF, auricular fibrillation; BrS, Brugada Syndrome; ECG, electrocardiogram; EPS, electrophysiological study; ER, early repolarization; f-QRS, fragmented QRS; ICD, implantable cardioverter-defibrillator; RFA, radiofrequency ablation; RVOT, right ventricular outflow tract; SCD, sudden cardiac death; VF, ventricular fibrillation; VT, ventricular tachycardia.
References
Andorin, A., Behr, E. R., Denjoy, I., Crotti, L., Dagradi, F., Jesel, L., et al. (2016). Impact of clinical and genetic findings on the management of young patients with Brugada syndrome. Heart Rhythm 13, 1274–1282. doi: 10.1016/j.hrthm.2016.02.013
Bayés, d. L. A., Brugada, J., Baranchuk, A., Borggrefe, M., Breithardt, G., Goldwasser, D., et al. (2012). Current electrocardiographic criteria for diagnosis of Brugada pattern: a consensus report. J. Electrocardiol. 45, 433–442. doi: 10.1016/j.jelectrocard.2012.06.004
Benito, B., Sarkozy, A., Mont, L., Henkens, S., Berruezo, A., Tamborero, D., et al. (2008). Gender differences in clinical manifestations of Brugada syndrome. J. Am. Coll. Cardiol. 52, 1567–1573. doi: 10.1016/j.jacc.2008.07.052
Brugada, J., Brugada, R., Antzelevitch, C., Towbin, J., Nademanee, K., and Brugada, P. (2002). Long-term follow-up of individuals with the electrocardiographic pattern of right bundle-branch block and ST-segment elevation in precordial leads V1 to V3. Acc. Curr. J. Rev. 11, 73–78. doi: 10.1016/S1062-1458(02)00629-3
Brugada, J., Brugada, R., and Brugada, P. (1998). Right bundle-branch block and ST-segment elevation in leads V1 through V3: a marker for sudden death in patients without demonstrable structural heart disease. Circulation 97, 457–460. doi: 10.1161/01.CIR.97.5.457
Brugada, J., Brugada, R., and Brugada, P. (2004). Determinants of sudden cardiac death in individuals with the electrocardiographic pattern of Brugada Syndrome and no previous cardiac arrest. Acc. Curr. J. Rev. 13, 3092–3096. doi: 10.1016/j.accreview.2004.03.024
Brugada, J., Pappone, C., Berruezo, A., Vicedomini, G., Manguso, F., Ciconte, G., et al. (2015). Brugada Syndrome Phenotype Elimination by Epicardial Substrate Ablation. Circul. Arrhythmia Electrophysiol. 8:1373. doi: 10.1161/CIRCEP.115.003220
Brugada, P., and Brugada, J. (1992). Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome: a multicenter report. J. Am. Coll. Cardiol. 20, 1391–1396. doi: 10.1016/0735-1097(92)90253-J
Brugada, P., Brugada, R., and Brugada, J. (2005). Should patients with an asymptomatic Brugada electrocardiogram undergo pharmacological and electrophysiological testing? Circulation 112, 279–292. doi: 10.1161/CIRCULATIONAHA.104.485326
Burke, J. H., Goldberger, J. J., Ehlert, F. A., Kruse, J. T., Parker, M. A., and Kadish, A. H. (1996). Gender differences in heart rate before and after autonomic blockade: evidence against an intrinsic gender effect. Am. J. Med. 100:537. doi: 10.1016/S0002-9343(96)00018-6
Calò, L., Giustetto, C., Martino, A., Sciarra, L., Cerrato, N., Marziali, M., et al. (2016). A New Electrocardiographic Marker of Sudden Death in Brugada Syndrome: The S-Wave in Lead, I. J. Am. Coll. Cardiol. 67, 1427–1440. doi: 10.1016/j.jacc.2016.01.024
Calvo, D., Flórez, J. P., Valverde, I., Rubín, J., Pérez, D., Vasserot, M. G., et al. (2016). Surveillance after cardiac arrest in patients with Brugada syndrome without an implantable defibrillator: An alarm effect of the previous syncope. Int. J. Cardiol. 218, 69-74. doi: 10.1016/j.ijcard.2016.05.018
Conte, G., Sieira, J., Ciconte, G., de Asmundis, C., Chierchia, G. B., Baltogiannis, G., et al. (2015). Implantable cardioverter-defibrillator therapy in Brugada syndrome: a 20-year single-center experience. J. Am. Coll. Cardiol. 65, 879–888. doi: 10.1016/j.jacc.2014.12.031
de Asmundis, C., Mugnai, G., Chierchia, G. B., Sieira, J., Conte, G., Rodriguez-Manero, M., et al. (2017). Long-term follow-up of probands with Brugada syndrome. Am. J. Cardiol. 119, 1392–1400. doi: 10.1016/j.amjcard.2017.01.039
Eckardt, L., Probst, V., Smits, J. P., Bahr, E. S., Wolpert, C., Schimpf, R., et al. (2005). Long-term prognosis of individuals with right precordial ST-segment-elevation Brugada syndrome. Circulation 111, 257–263. doi: 10.1161/01.CIR.0000153267.21278.8D
Furushima, H., Chinushi, M., Hirono, T., Sugiura, H., Watanabe, H., Komura, S., et al. (2005). Relationship between dominant prolongation of the filtered QRS duration in the right precordial leads and clinical characteristics in Brugada syndrome. J. Cardiovasc. Electrophysiol. 16, 1311–1317. doi: 10.1111/j.1540-8167.2005.00262.x
Gehi, A. K., Duong, T. D., Metz, L. D., Gomes, J. A., and Mehta, D. (2006). Risk stratification of individuals with the Brugada electrocardiogram: a meta-analysis. J. Cardiovasc. Electrophysiol. 17, 577–583. doi: 10.1111/j.1540-8167.2006.00455.x
Giustetto, C., Drago, S., Demarchi, P. G., Dalmasso, P., Bianchi, F., Masi, A. S., et al. (2009). Risk stratification of the patients with Brugada type electrocardiogram: a community-based prospective study. Europace 11, 507–513. doi: 10.1093/europace/eup006
Kamakura, S., Ohe, T., Nakazawa, K., Aizawa, Y., Shimizu, A., Horie, M., et al. (2009). Brugada Syndrome Investigators in J Long-term prognosis of probands with Brugada-pattern ST-elevation in leads V1-V3. Circ. Arrhythm. Electrophysiol. 2, 495–503. doi: 10.1161/CIRCEP.108.816892
Kanda, M., Shimizu, W., Matsuo, K., Nagaya, N., Taguchi, A., Suyama, K., et al. (2002). Electrophysiologic characteristics and implications of induced ventricular fibrillation in symptomatic patients with Brugada syndrome. J. Am. Coll. Cardiol. 39:1799. doi: 10.1016/S0735-1097(02)01867-3
Kharazi, A., Emkanjoo, Z., Alizadeh, A., Nikoo, M. H., Jorat, M. V., and Sadr-Ameli, M. A. (2007). Mid-term follow-up of patients with Brugada syndrome following a cardioverter defibrillator implantation: a single center experience. Indian. Pac. Electrophysiol. J. 7, 33–39.
Konigstein, M., Rosso, R., Topaz, G., Postema, P. G., Friedensohn, L., Heller, K., et al. (2016). Drug-induced Brugada syndrome: clinical characteristics and risk factors. Heart Rhythm 13, 1083–1087. doi: 10.1016/j.hrthm.2016.03.016
Kusumoto, F. M., Bailey, K. R., Chaouki, A. S., Deshmukh, A. J., Gautam, S., Kim, R. J., et al. (2017). Systematic review for the 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the heart rhythm society. J. Am. Coll. Cardiol. doi: 10.1016/j.jacc.2017.10.052. [Epub ahead of print].
Marras, E., Basso, C., Sciarra, L., and Delise, P. (2009). Unexplained syncope, Brugada-like ECG and minimal structural right ventricular abnormalities: which is the right diagnosis? J. Cardiovasc. Med. 10, 273–275. doi: 10.2459/JCM.0b013e328322fc09
Martini, B., and Nava, A. (2004). 1988-2003. Fifteen years after the first Italian description by Nava-Martini-Thiene and colleagues of a new syndrome (different from the Brugada syndrome?) in the Giornale Italiano di Cardiologia: do we really know everything on this entity? Ital. Heart J. 5:53.
Martini, B., Nava, A., Buja, G. F., and Canciani, B. T. G. (1988). Fibrillazione ventricolare in apparente assenza di cardiopatia. G. Ital. Cardiol. 18 (Suppl 1):136.
Masaki, R., Watanabe, I., Nakai, T., Kondo, K., Oshikawa, N., Sugimura, H., et al. (2002). Role of signal-averaged electrocardiograms for predicting the inducibility of ventricular fibrillation in the syndrome consisting of right bundle branch block and ST segment elevation in leads V1-V3. Jpn. Heart J. 43, 367–378. doi: 10.1536/jhj.43.367
Milman, A., Gourraud, J. B., Andorin, A., Postema, P. G., Sacher, F., Mabo, P., et al. (2018). Gender differences in patients with Brugada syndrome and arrhythmic events: data from a survey on arrhythmic events in 678 patients. Heart Rhythm 97:e10655. doi: 10.1016/j.hrthm.2018.06.019
Mizusawa, Y., Morita, H., Adler, A., Havakuk, O., Thollet, A., Maury, P., et al. (2016). Prognostic significance of fever-induced Brugada syndrome. Heart Rhythm 13, 1515–1520. doi: 10.1016/j.hrthm.2016.03.044
Mok, N. S., Priori, S. G., Napolitano, C., Chan, K. K., Bloise, R., Chan, H. W., et al. (2004). Clinical profile and genetic basis of Brugada syndrome in the Chinese population. Hong Kong Med. J. 10:32.
Morita, H., Kusano, K. F., Miura, D., Nagase, S., Nakamura, K., Morita, S. T., et al. (2008). Fragmented QRS as a marker of conduction abnormality and a predictor of prognosis of Brugada syndrome. Circulation 118, 1697–1704. doi: 10.1161/CIRCULATIONAHA.108.770917
Nava, A., Canciani, B., Martini, B., and Buja, G. F. (1988). La ripolarizzazione precoce nelle precordiali destre. G. Ital. Cardiol. 18:118.
Ohkubo, K., Watanabe, I., Takagi, Y., Okumura, Y., Ashino, S., Kofune, M., et al. (2007). Electrocardiographic and electrophysiologic characteristics in patients with Brugada type electrocardiogram and inducible ventricular fibrillation: single center experience. Circul. J. 71, 1437–1441. doi: 10.1253/circj.71.1437
Olde Nordkamp, L. R., Vink, A. S., Wilde, A. A., de Lange, F. J., de Jong, J. S., Wieling, W., et al. (2015). Syncope in Brugada syndrome: prevalence, clinical significance, and clues from history taking to distinguish arrhythmic from nonarrhythmic causes. Heart Rhythm 12, 367–375. doi: 10.1016/j.hrthm.2014.10.014
Pappone, C., Ciconte, G., Manguso, F., Vicedomini, G., Mecarocci, V., Conti, M., et al. (2018). Assessing the malignant ventricular arrhythmic substrate in patients with Brugada syndrome. J. Am. Coll. Cardiol. 71, 1631–1646. doi: 10.1016/j.jacc.2018.02.022
Priori, S., Napolitano, C., Gasparini, M., Pappone, C., Della Bella, P., Giordano, U., et al. (2002). Natural history of Brugada syndrome: insights for risk stratification and management. Circulation 105, 1342–1347. doi: 10.1161/hc1102.105288
Priori, S. G., Aliot, E., Blomstromlundqvist, C., Bossaert, L., Breithardt, G., Brugada, P., et al. (2001). Task force on sudden cardiac death of the European Society of cardiology. Eur. Heart J. 22, 1374–1450. doi: 10.1053/euhj.2001.2824
Priori, S. G., Gasparini, M., Napolitano, C., Della Bella, P., Ottonelli, A. G., Sassone, B., et al. (2012). Risk stratification in Brugada syndrome: results of the PRELUDE (PRogrammed ELectrical stimUlation preDictive valuE) registry. J. Am. Coll. Cardiol. 59, 37–45. doi: 10.1016/j.jacc.2011.08.064
Priori, S. G., Wilde, A. A., Horie, M., Cho, Y., Behr, E. R., Berul, C., et al. (2013). HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm 10, 1932–1963. doi: 10.1016/j.hrthm.2013.05.014
Sacher, F., Meregalli, P., Veltmann, C., Field, M. E., Solnon, A., Bru, P., et al. (2008). Are women with severely symptomatic brugada syndrome different from men? J. Cardiovasc. Electrophysiol. 19, 1181–1185. doi: 10.1111/j.1540-8167.2008.01223.x
Sacher, F., Probst, V., Maury, P., Babuty, D., Mansourati, J., Komatsu, Y., et al. (2013). Outcome after implantation of a cardioverter-defibrillator in patients with Brugada syndrome: a multicenter study-part 2. Circulation 128, 1739–1747. doi: 10.1161/CIRCULATIONAHA.113.001941
Sarkozy, A., Boussy, T., Kourgiannides, G., Chierchia, G. B., Richter, S., De Potter, T., et al. (2007). Long-term follow-up of primary prophylactic implantable cardioverter-defibrillator therapy in Brugada syndrome. Eur. Heart J. 28, 334–344. doi: 10.1093/eurheartj/ehl450
Sarkozy, A., Sorgente, A., Boussy, T., Casado, R., Paparella, G., Capulzini, L., et al. (2011). The value of a family history of sudden death in patients with diagnostic type I Brugada ECG pattern. Eur. Heart J. 32, 2153–2160. doi: 10.1093/eurheartj/ehr129
Schukro, C., Berger, T., Stix, G., Pezawas, T., Kastner, J., Hintringer, F., et al. (2010). Regional prevalence and clinical benefit of implantable cardioverter defibrillators in Brugada syndrome. Int. J. Cardiol. 144, 191–194. doi: 10.1016/j.ijcard.2009.03.136
Shaowen Liu, S. Y., and Ole Kongstad, S. (2001). Bertil Olsson. Gender differences in the electrophysiological characteristics of atrioventricular conduction system and their clinical implications. Scand J. Thoracic Cardiovasc. Surg. 35, 313–317. doi: 10.1080/140174301317116280
Shi, S., Barajas-Martinez, H., Liu, T., Sun, Y., Yang, B., Huang, C., et al. (2018). Prevalence of spontaneous Brugada ECG pattern recorded at standard intercostal leads: a meta-analysis. Int. J. Cardiol. 254, 151–156. doi: 10.1016/j.ijcard.2017.11.113
Sieira, J., Ciconte, G., Conte, G., Chierchia, G. B., de Asmundis, C., Baltogiannis, G., et al. (2015). Asymptomatic Brugada syndrome: clinical characterization and long-term prognosis. Circ. Arrhythm. Electrophysiol. 8, 1144–1150. doi: 10.1161/CIRCEP.114.003044
Slim, K., Nini, E., Forestier, D., Kwiatkowski, F., Panis, Y., and Chipponi, J. (2003). Methodological index for non-randomized studies (minors): development and validation of a new instrument. Anz. J. Surg. 73, 712–716. doi: 10.1046/j.1445-2197.2003.02748.x
Son, M. K., Byeon, K., Park, S. J., Kim, J. S., Nam, G. B., Choi, K. J., et al. (2013). Prognosis after implantation of cardioverter-defibrillators in Korean patients with Brugada syndrome. Yonsei Med. J. 55, 37–45. doi: 10.3349/ymj.2014.55.1.37
Tadros, R., Ton, A. T., Fiset, C., and Nattel, S. (2014). Sex differences in cardiac electrophysiology and clinical arrhythmias: epidemiology, therapeutics, and mechanisms. Canad J. Cardiol. 30, 783–792. doi: 10.1016/j.cjca.2014.03.032
Tokioka, K., Kusano, K. F., Morita, H., Miura, D., Nishii, N., Nagase, S., et al. (2014). Electrocardiographic parameters and fatal arrhythmic events in patients with Brugada syndrome: combination of depolarization and repolarization abnormalities. J. Am. Coll. Cardiol. 63, 2131–2138. doi: 10.1016/j.jacc.2014.01.072
Yamagata, K., Horie, M., Aiba, T., Ogawa, S., Aizawa, Y., Ohe, T., et al. (2017). Genotype-phenotype correlation of SCN5A mutation for the clinical and electrocardiographic characteristics of probands with Brugada Syndrome: a japanese multicenter registry. Circulation 135:2255. doi: 10.1161/CIRCULATIONAHA.117.027983
Keywords: Brugada syndrome, gender difference, electrophysiological study, prognosis, risk stratification
Citation: Yuan M, Tian C, Li X, Yang X, Wang X, Yang Y, Liu N, Kusano KF, Barajas-Martinez H, Hu D, Shang H, Gao Y and Xing Y (2018) Gender Differences in Prognosis and Risk Stratification of Brugada Syndrome: A Pooled Analysis of 4,140 Patients From 24 Clinical Trials. Front. Physiol. 9:1127. doi: 10.3389/fphys.2018.01127
Received: 18 May 2018; Accepted: 27 July 2018;
Published: 22 August 2018.
Edited by:
Jiashin Wu, University of South Florida, United StatesReviewed by:
Bortolo Martini, Ospedale Alto Vicentino, ItalyKonstantinos Letsas, Evaggelismos General Hospital, Greece
Copyright © 2018 Yuan, Tian, Li, Yang, Wang, Yang, Liu, Kusano, Barajas-Martinez, Hu, Shang, Gao and Xing. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yonghong Gao, gaoyh7088@163.com
Yanwei Xing, xingyanwei12345@163.com
 Mengchen Yuan1,2
Mengchen Yuan1,2 Xinye Li
Xinye Li Nian Liu
Nian Liu Hector Barajas-Martinez
Hector Barajas-Martinez Dan Hu
Dan Hu Yonghong Gao
Yonghong Gao Yanwei Xing
Yanwei Xing