- 1Institute of Geriatrics, Jiangxi Provincial People’s Hospital, The First Affiliated Hospital of Nanchang Medical College, Nanchang, China
- 2Department of Pharmacology, School of Pharmaceutical Science, Nanchang University, Nanchang, China
- 3The Second Department of Neurology, Jiangxi Provincial People’s Hospital, The First Affiliated Hospital of Nanchang Medical College, Nanchang, China
- 4Department of Critical Care Medicine, Jiangxi Provincial People’s Hospital, The First Affiliated Hospital of Nanchang Medical College, Nanchang, China
The human eye is susceptible to various disorders that affect its structure or function, including glaucoma, age-related macular degeneration (AMD) and diabetic retinopathy (DR). Mitochondrial dysfunction has been identified as a critical factor in the pathogenesis and progression of eye disorders, making it a potential therapeutic target in the clinic. Natural products have been used in traditional medicine for centuries and continue to play a significant role in modern drug development and clinical therapeutics. Recently, there has been a surge in research exploring the efficacy of natural products in treating eye disorders and their underlying physiological mechanisms. This review aims to discuss the involvement of mitochondrial dysfunction in eye disorders and summarize the recent advances in the application of natural products targeting mitochondria. In addition, we describe the future perspective and challenges in the development of mitochondria-targeting natural products.
1 Introduction
The human eye is responsible for detecting light and transmitting visual signals to the brain. Eye disorders encompass a range of pathological conditions that affect the function or structure of the eye. Common examples include AMD, glaucoma and DR. These disorders may stem from various factors, such as aging, genetics, inflammation and physical trauma (Miyamoto et al., 1999; Weinreb et al., 2014; Dieguez et al., 2019). It is worth noting that certain eye disorders can have multiple etiologies, and the precise cause of a specific condition may remain elusive. Nonetheless, mitochondrial dysfunction is believed to be critical in the pathogenesis and progression of most eye disorders.
Mitochondria are essential cellular organelles involved in multiple metabolic processes and are often referred as cell’s powerhouse due to their role in energy production (Han et al., 2011). Key mitochondrial functions include ATP synthesis via oxidative phosphorylation, redox regulation, calcium buffering and apoptosis control in response to extracellular and intracellular stimuli or stress. Mitochondrial quality is stringently regulated by interconnected mechanisms, such as biogenesis, dynamics and mitophagy. Given their significance in cellular homeostasis, mitochondrial dysfunctions can lead to a wide array of diseases (Lin and Beal, 2006). Numerous studies have established strong links between mitochondrial dysfunction and eye disorders, including AMD, blue light-induced damage and corneal chemical injuries (Li et al., 2018; Zhang et al., 2021; Zou et al., 2023). Insight into mitochondrial dysfunction may provide new therapeutical targets for understanding the pathophysiology of eye disorders and facilitate the development of innovative treatments.
Natural products, defined as substances or metabolites produced by living organisms, have long been used in traditional medicine and have also played pivotal role in modern drug development and clinical therapeutics. Generally considered safer than synthetic metabolites due to their reduced side effects, not all natural products have been extensively researched (Ekiert and Szopa, 2020). Recently, a growing number of studies have focused on examining the efficacy of natural products in treating eye disorders. Natural products may serve as potential therapeutic agents by targeting mitochondrial dysfunction through various signaling pathways (Guo et al., 2021; Park et al., 2021; Xu et al., 2021; Yang et al., 2022). This review aims to summarize the involvement and molecular mechanisms of mitochondrial dysfunction in eye disorders. In addition, we discuss the application of natural products in eye disorders by targeting mitochondria as well as its challenges and future perspective.
2 Involvement of mitochondrial dysfunction in eye disorders
2.1 Mitochondrial biogenesis
Mitochondrial biogenesis, the cellular mechanism responsible for augmenting mitochondrial quantity and size, is paramount to maintaining energy homeostasis. Deficiencies in mitochondria biogenesis can contribute to various eye disorders (Figure 1), such as Leber’s hereditary optic neuropathy (LHON), an affliction attributed to mt-DNA mutation (Stenton et al., 2021). Decreased expression of NRF1, TFAMB1, and TFAMA in mitochondrial biogenesis may lead to optic neuropathies (Iyer et al., 2012). In diabetic conditions, while TFAM gene transcription seems to escalate, the converse is true for its mitochondrial protein levels, which diminish, leading to subpar mitochondria copy numbers (Koh et al., 2019). Santos et al. shed light on the regulatory dynamics of TFAM, uncovering that TFAM’s ubiquitination hampers its translocation to mitochondria, thus impinging on mt-DNA transcription and impairing mitochondrial function (Santos et al., 2014). Remarkably, mitigation of TFAM ubiquitination reestablished mitochondrial homeostasis. This suggests that focusing on the post-translational modulation of TFAM may offer a novel approach to safeguard mitochondrial equilibrium and potentially alleviate the burden of DR.
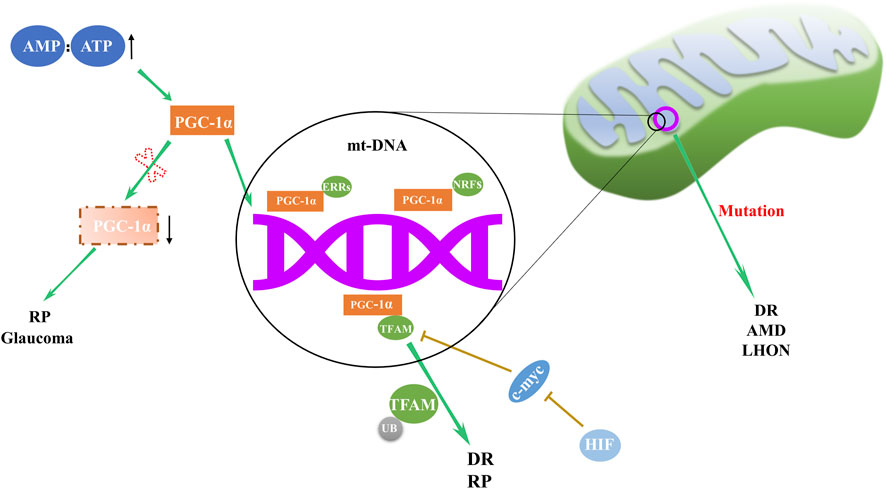
Figure 1. Regulation and abnormalities of mitochondrial biogenesis in ocular disorders. The orchestration of mitochondrial biogenesis involves the cooperative activity of PGC-1α and downstream transcription factors, including NRFs, ERRs, and TFAM. Deviations in this complex network are implicated in a range of eye disorders, with abnormal expression of PGC-1α and TFAM, along with mt-DNA mutations, contributing to the pathogenesis of RP, DR, AMD, LHON and glaucoma.
In DR, retinal mitochondria become dysfunctional, and their mt-DNA is damaged (Kowluru, 2020). Mitochondrial biogenesis in the retina of patients with DR is impaired due to decreased transport of TFAM to the mitochondria (Santos et al., 2011). Modulating biogenesis through pharmaceutical or molecular approaches may offer a potential strategy to delay DR progression.
In AMD patients, retinal pigment epithelial (RPE) cells exhibit structurally and functionally defective mitochondria as well as deficient expression of Dicer enzyme (Kaneko et al., 2011; Lewis Lujan et al., 2022). Alu RNA expression is upregulated by Dicer deficiency, inducing mitochondrial membrane potential loss, reactive oxygen species (ROS) generation and release of mt-DNA into cytoplasm. This cytoplasmic mt-DNA, along with oxidative stress, activates the NLRP3 inflammasome, leading to interleukin-18 production and RPE cell apoptosis (Lewis Lujan et al., 2022).
Retinitis pigmentosa (RP), a retinal disorder rooted in mitochondrial dysfunction, manifests as progressive rod and cone cell degeneration. This process ultimately precipitates the loss of retinal light sensitivity and culminates in blindness (Pagano et al., 2021). The etiology of RP is associated with anomalies in mitochondrial biogenesis, including alterations in the regulatory factors and dynamic proteins such as PGC-1α, TFAM, Fis1, Mfn1, and Mfn2 (Ozawa et al., 2022).
In the context of neurodegenerative diseases, a common thread appears to be fluctuations in the expression of PGC-1α, master regulator of mitochondrial biogenesis (Lin et al., 2004; Leone et al., 2005; Ma et al., 2010). Significant decrease in PGC-1α expression was observed in the ganglion cell layer of inherited glaucoma model DBA/2J mice (Guo et al., 2014). This evidence underscores the pervasive role of mitochondrial dysregulation across a spectrum of retinal disorders.
2.2 Mitochondrial dynamics
Mitochondria continuously undergo fission and fusion, collectively known as mitochondrial dynamics (Figure 2), to acclimate to shifting cellular environments (Suen et al., 2008). Mitochondrial dynamics disturbance has been observed in various eye disorders (Table 1). Light-induced mitochondrial fragmentation in retina has been reported, with blue light exposure increasing Drp1 expression and decreasing Mfn2 expression (Knels et al., 2011; Marie et al., 2018). Agustina et al. reported that blue light decreased expression of OPA1 and increased expression of Drp1 in ARPE-19 cells (Alaimo et al., 2019). Dominant optic atrophy (DOA), a neuro-ophthalmic condition typified by bilateral optic nerve degeneration, is connected to mutations in OPA1 gene (Del Dotto et al., 2018). Intriguingly, heterozygous OPA1 mutations have also been linked to extra-ocular symptoms, including mitochondrial myopathy, sensorineural deafness, axonal sensory-motor polyneuropathy, chronic progressive external ophthalmoplegia, and ataxia (Amati-Bonneau et al., 2003; Li et al., 2005).
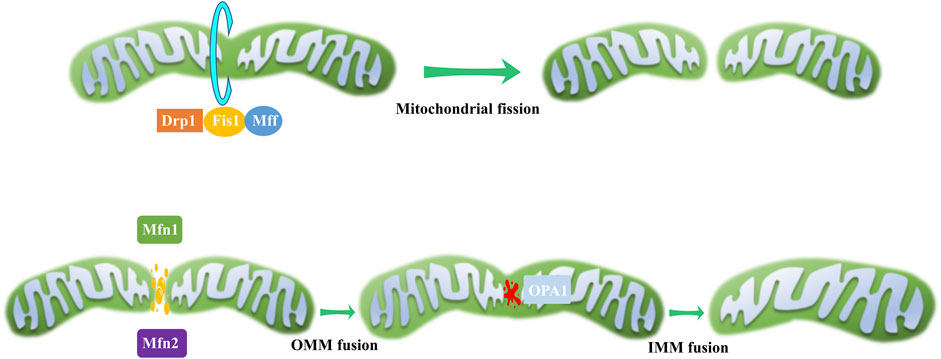
Figure 2. Mitochondrial dynamics. During mitochondrial fission, a scission complex composed of Drp1, Fis1, and Mff facilitates mitochondrial division. In the process of mitochondrial fusion, OMM fusion is mediated by Mfn1 and Mfn2, while IMM fusion is governed by OPA1.
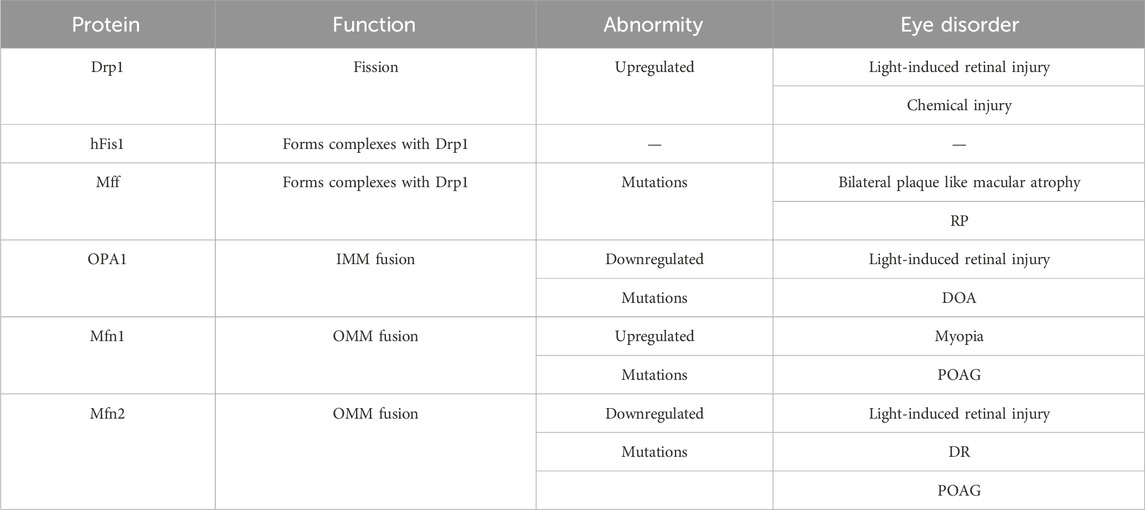
Table 1. The function of mitochondrial dynamic regulatory proteins and their abnormity in eye disorders.
The retina’s active metabolism makes it particularly vulnerable to genetic and environmental alterations causing mitochondrial dysfunction. For example, disturbances in mitochondrial dynamics and quality control system increase susceptibility of photoreceptor and RGC to cell death, contributing to retinitis pigmentosa onset (Narayan et al., 2017; Eells, 2019; Mirra and Marfany, 2019).
Mitochondrial dynamics are also central to DR development and its associated “metabolic memory” phenomenon. Drp1 is central to maintaining mitochondrial homeostasis under these conditions and is associated with the disease’s continued progression (Mohammad and Kowluru, 2022). In diabetic milieu, retinal mitochondria exhibit swolling and damag, and Mfn2 expression decreases. Mfn2 overexpression prevents glucose-induced mitochondrial fragmentation (Duraisamy et al., 2019). Therefore, modulating Mfn2 expression and its epigenetic alterations, through molecular or pharmacological strategies, may offer potential avenues for preserving mitochondrial homeostasis and mitigating the development of DR.
In the case of corneal alkali burns, considered as a severe ophthalmic emergency and difficult to manage conservatively, Drp1-dependent mitochondrial fission has been implicated (Shi et al., 2023). It appears to mediate alkali burn-induced corneal injury by regulating inflammatory responses, oxidative stress, and corneal neovascularization (Zhang et al., 2021). These insights highlight the diverse and critical roles of mitochondrial dynamics in retinal health and various ocular disorders.
2.3 Mitophagy
Mitophagy is a critical cellular process that involves the selective degradation of damaged or excess mitochondria through autophagy (Su et al., 2023). Proper functioning of mitochondria is essential for cellular energy homeostasis, and mitophagy prevents the accumulation of damaged organelles, which can lead to cellular damage and disease. Mitophagy has been implicated in a host of ocular disorders, as well as in neurodegenerative, metabolic, and aging-related diseases (Figure 3). The dysregulation of spliceosome-mediated mitophagy, for instance, contributes to the pathogenesis of RP (Xu et al., 2018). High glucose environments, such as those seen in DR, have been reported to inhibit cell proliferation and mitophagy via the ROS-mediated inactivation of the ROS/PINK1/Parkin signaling pathway (Zhang et al., 2019). Moreover, TXNIP and associated oxidative stress have been proposed as mechanisms for mitophagy in retinal RPE cells under sustained high glucose conditions (Singh et al., 2021). Furthermore, blue light exposure has been observed to stimulate mitophagy, as evidenced by the conversion of autophagy marker LC3B and the overexpression of mitophagy sensor PINK1 (Li et al., 2018). These findings underscore the extensive role of mitophagy in ocular health.
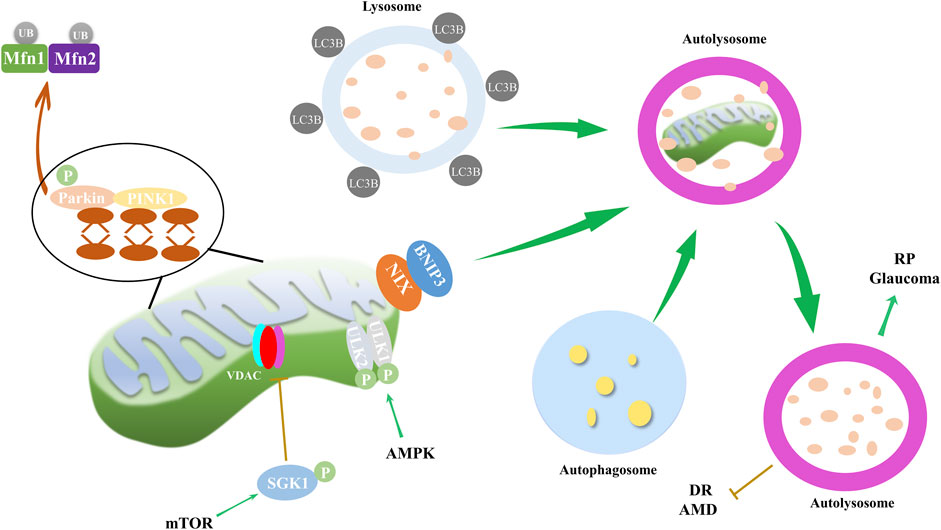
Figure 3. Mitophagy and its impact on ocular disorders. Mitophagy, regulated through mTOR and AMPK pathways, along with PINK1/Parkin and NIX/BNIP3 pathways, is implicated in the progression of eye disorders such as DR, RP, AMD and glaucoma. During mitophagy, PINK1 recruits and activates Parkin, and then mitochondrial Mfn1/2 are ubiquitinated by Parkin. PINK1 also recruits autophagy receptor proteins NIX and BNIP3 to mitochondria, and receptor proteins recruit LC3B. Finally, autophagosome, mitochondria and lysosome form autolysosome to dissolve and recycle damaged mitochondria. AMPK pathway mediates mitophagy through phosphorylation of ULK1/2, while mTOR inhibits VDAC by phosphorylating SGK1, thereby reducing mitophagy.
2.4 Mitochondrial redox homeostasis
Mitochondria, as the primary source of ROS in the cell, can cause damage to cellular components. Mitochondrial redox homeostasis is tightly controlled by antioxidants, including superoxide dismutases, catalases and glutathione (Seminotti et al., 2022). Dysregulation of this homeostasis can lead to oxidative stress and eye disorders (Figure 4). Various studies have shown that visible light exposure in cell cultures can trigger an overproduction of ROS, including peroxynitrite, hydroxyl free radicals, nitric oxide, hydrogen peroxide, and singlet oxygen (Godley et al., 2005; Lascaratos et al., 2007; Wood et al., 2007). This perturbation of mitochondrial redox homeostasis can subsequently lead to cataract formation (Hightower et al., 1999; Bantseev and Youn, 2006).
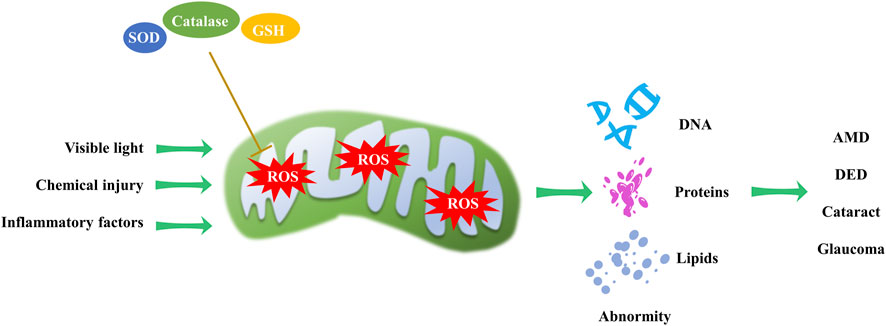
Figure 4. Mitochondrial redox homeostasis and its impact on eye disorders. Visible light, chemical injury and inflammatory factors induce ROS production, while antioxidants can reduce ROS levels. An imbalance in mitochondrial redox homeostasis can result in DNA, protein, and lipid abnormalities, contributing to the progression of AMD, DED, cataract and glaucoma.
Intracellular redox reactions are crucial for maintaining cellular homeostasis. However, the disruption of these reactions has been implicated in the onset and progression of DR (Sharma et al., 2022). In the context of AMD, excessive ROS oxidizes macromolecules such as nucleic acids, lipids, and proteins, potentially causing structural and functional alterations. Excessive ROS and oxidized lipoproteins can trigger protein misfolding, aggregation, and chronic activation of the innate immune response (Ferrington et al., 2016; Jadeja and Martin, 2021; Tang et al., 2021).
The trabecular meshwork, the anterior chamber tissue responsible for aqueous humor drainage, is fortified with antioxidant defenses. Despite this, it remains vulnerable to mitochondrial oxidative damage that can impair its endothelial function, increase intraocular pressure, and initiate glaucoma (Nair et al., 2021). Additionally, in dry eye disease (DED), augmented oxidative stress has been strongly linked to the etiology of corneal epithelial alterations. Chronic oxidative stress exposure activates cell regulatory molecules involved in corneal surface disorders associated with dry eye conditions (Nakamura et al., 2007; Cejkova et al., 2008). These examples highlight the far-reaching implications of mitochondrial redox homeostasis in ocular diseases.
2.5 Apoptosis
Apoptosis, also known as programmed cell death, is an orderly and controlled process that is activated by many cell stresses, including mitochondrial damage, growth factor deprivation, disruption of the cytoskeleton, accumulation of unfolded proteins, and hypoxia. The two major pathways leading to apoptosis are extrinsic pathway and intrinsic pathway. The intrinsic pathway commences with mitochondrial outer membrane permeabilization, releasing cytochrome c into the cytoplasm and activating caspases-3 and caspases-9 (Sparrow and Cai, 2001). B-cell lymphoma 2 (Bcl-2) family proteins critically regulate mitochondrial permeability, with pro-apoptotic members (Bax and Bak) facilitating the release of pro-apoptotic factors and anti-apoptotic members (Bcl-2 and Bcl-xl) inhibiting this process (Ma et al., 2020).
Apoptosis dysregulation contributes to a range of ocular disorders (Figure 5). For instance, blue light exposure was reported to induced apoptosis in RGC cells, with continuous activation of JNK and p38 pathways leading to c-jun and c-fos phosphorylation, which subsequently triggers apoptosis (Huang et al., 2014; Li et al., 2018). In retinal endothelial cells (RECs), several stressors can induce apoptosis, such as high glucose levels and inflammatory factors (Hu et al., 2022; Kong et al., 2022).
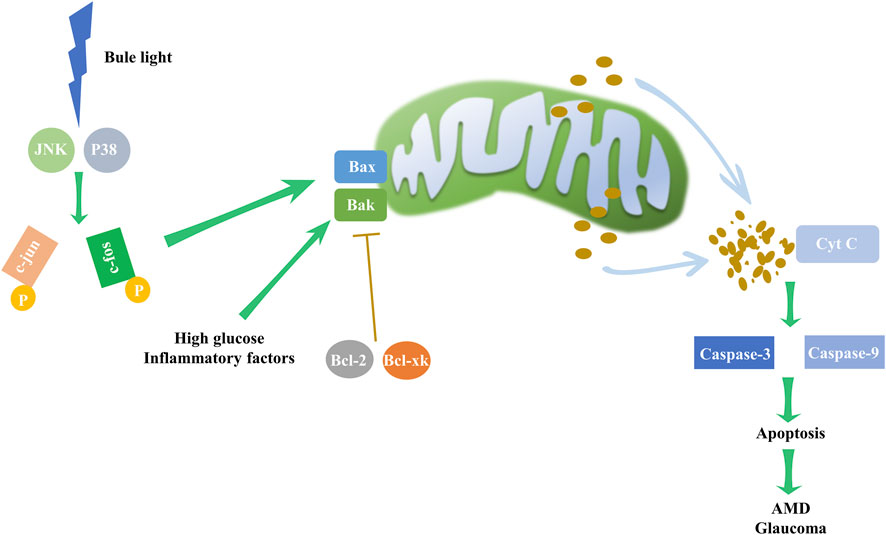
Figure 5. Intrinsic apoptosis pathway and its impact on eye disorders. Environmental factors, such as high glucose levels and inflammation, may upregulate the expression of Bax and Bak. In particular, blue light may elevate the levels of JNK and P38, boosting c-jun and c-fos phosphorylation, subsequently driving the expression of Bax and Bak, which are pivotal to intrinsic apoptosis pathway initiation. The pathway, however, is inhibited by the counteracting activity of Bcl-2 and Bcl-xl. Disruption of this delicate balance activates the mitochondrial apoptosis pathway, triggering cytochrome c release and escalating caspase-3 and caspase-9 activity, resulting in apoptosis. These mechanistic disruptions may underpin the development of AMD and glaucoma.
Glaucoma-related RGC death is primarily caused by apoptosis, which can be initiated through neurotrophin deprivation (Osborne et al., 1999; Weinreb and Khaw, 2004; Erdurmus et al., 2011). In primary open-angle glaucoma (POAG) patients, significantly reduced brain-derived neurotrophic factor (BDNF) levels adversely affect RGC and trabecular meshwork cell survival, correlating with disease severity (Ghaffariyeh et al., 2011).
Mitogen-activated protein kinase (MAPK) signaling intermediates’ alterations in expression or function contribute to AMD pathogenesis (Kyosseva, 2016). For example, increased JNK1 or its activation leads to apoptosis in a mouse model of exudative AMD (Du et al., 2013) and the high level of phosphorylated ERK may cause more Choroidal neovascularization (CNV) in neovascular AMD (Li Y. et al., 2022). These findings further underscore the integral role of apoptosis regulation in ocular diseases.
3 Modulation of mitochondrial function in eye disorders by natural products
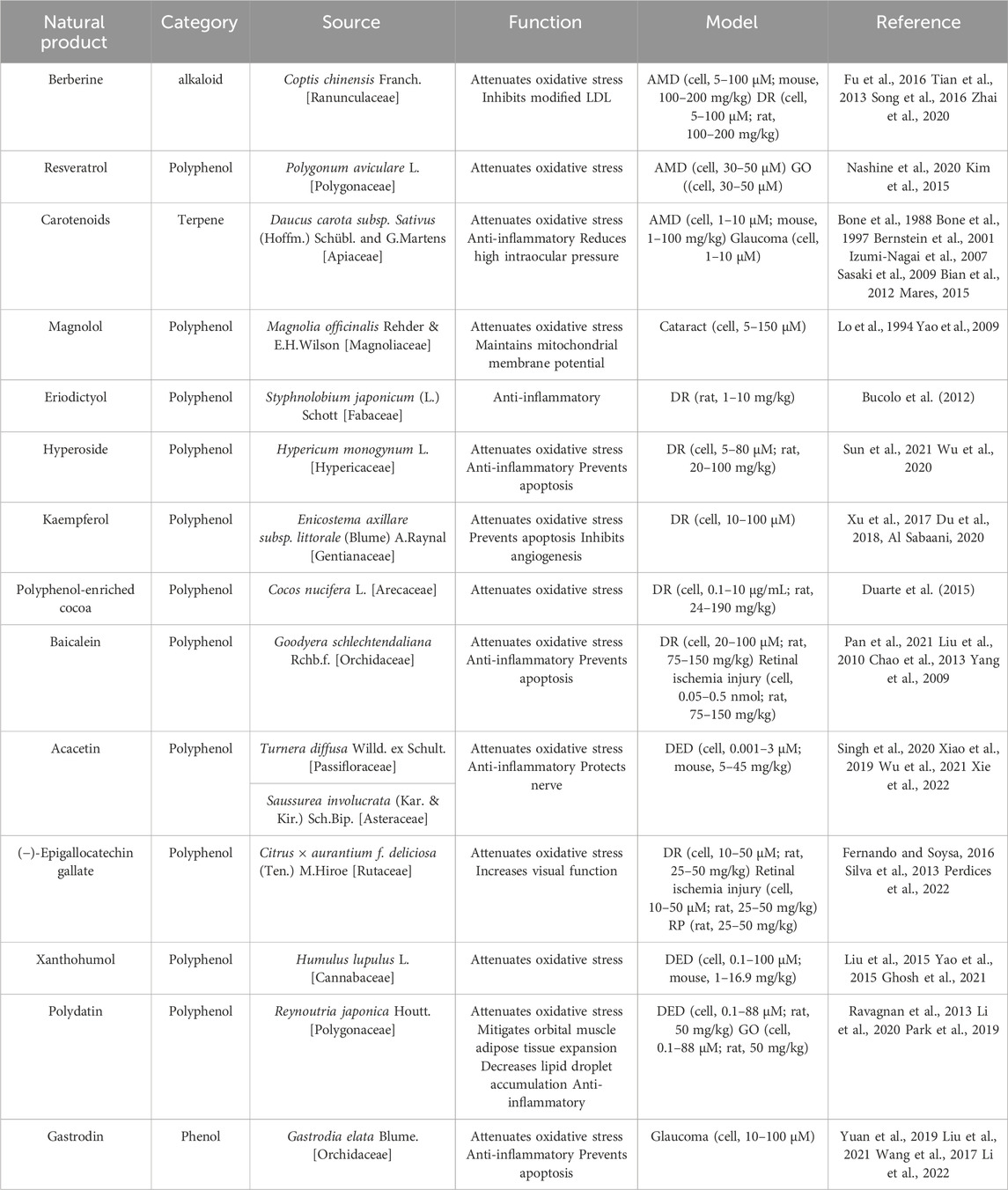
The therapeutic potential of natural metabolites, particularly plant extracts, has garnered significant attention in recent years as alternative solutions for various health and wellness concerns. As shown in Table 2, these metabolites exhibit potent biological effects and have potential applications in the treatment of numerous diseases and conditions.
For AMD and DR, berberine (BBR) derived from Coptis chinensis Franch. [Ranunculaceae] has shown therapeutic promise. It inhibits modified low-density lipoprotein (LDL)-induced Müller cell injury by activating the AMPK signaling pathway (Fu et al., 2016). Moreover, BBR mitigates leukocyte-mediated vascular endothelial damage, decreases antioxidant enzyme activities, and combats DR and AMD involving oxidative stress (Tian et al., 2013; Song et al., 2016). BBR’s capability to inhibit oxidative stress and cell apoptosis via NF-κB signaling pathway deactivation highlights its therapeutic potential for DR (Zhai et al., 2020). Resveratrol, a plant polyphenol, is noted for reducing intracellular ROS levels and enhancing mitochondrial quality in an AMD model (Nashine et al., 2020), and attenuating oxidative stress in Graves’ orbitopathy (GO) (Kim et al., 2015).
3.1 AMD, cataract and glaucoma
Carotenoids, namely, lutein (L), zeaxanthin (Z), and meso-zeaxanthin (meso-Z), are known to confer retinal protection (Bone et al., 1988; Bone et al., 1997; Bernstein et al., 2001). Ubiquitously found in plants, L and Z have been shown to mitigate oxidative stress and inflammation, thus playing a crucial role in preventing AMD (Izumi-Nagai et al., 2007; Sasaki et al., 2009; Bian et al., 2012). Significantly, these are the only two carotenoids documented to offer protection against lens opacities and cataract formation, attributable to their antioxidative properties (Mares, 2015). Additionally, L and Z may also exert protective effects on the trabecular meshwork, thereby decreasing the risk of high intraocular pressure (IOP) and subsequent glaucoma (Bernstein et al., 2001).
Magnolol, a metabolite isolated from the Chinese botanical drug Magnolia officinalis Rehder & E.H.Wilson [Magnoliaceae], has received increasing attention due to its antioxidant activity (Lo et al., 1994). Magnolol has been shown to inhibit ROS generation, prevent loss of mitochondrial membrane potential, and curtail cytochrome c release from mitochondria in H2O2-treated HLE B-3 cells. (Yao et al., 2009). By thwarting oxidative stress, magnolol implies a promising therapeutic strategy for cataract prevention.
Eriodictyol demonstrates potent anti-inflammatory properties, attenuating plasma lipid peroxidation and preserving the integrity of the blood-retinal barrier, thereby safeguarding retinal health (Bucolo et al., 2012). Hyperoside (Hyp), a plant-derived flavonoid, possesses multifaceted properties, including anti-cancer, anti-inflammatory, and anti-oxidative effects (Sun et al., 2021). It exhibits a protective role in diabetes-induced retinopathy, as evidenced in diabetic rat models, through the mitigation of oxidative stress, cell damage inhibition, and apoptosis prevention (Wu et al., 2020). Polyphenol-enriched cocoa offers retinal protection by enhancing the SIRT1 pathway in streptozotocin-induced diabetic rats, safeguarding the retina from oxidative stress damage (Duarte et al., 2015).
Kaempferol, a beneficial flavonoid in retinal protection, impedes angiogenesis in human retinal endothelial cells. This effect is mediated by downregulating the Src-Akt1-Erk1/2 signaling pathway and the placental growth factor, and vascular endothelial growth factor (VEGF) (Xu et al., 2017). Moreover, it bolsters cell survival, shields RPE cells from H2O2-induced oxidative damage and apoptosis by suppressing ROS generation, downregulating VEGF, and upregulating superoxide dismutase (Du et al., 2018). The protective influence of kaempferol against H2O2-induced ARPE-19 damage is attributed to its antioxidant and anti-inflammatory attributes, facilitated partly through the stimulation of nuclear accumulation, activation, and deacetylase ability of SIRT1, while concurrently inhibiting PARP1 (Al Sabaani, 2020).
3.2 DR and retinal ischemia injury
Baicalein, an active metabolite extracted from botanical drugs, has therapeutic potential due to its antioxidative and anti-inflammatory properties (Pan et al., 2021). Liu et al., 2010 highlighted the antioxidative capabilities of baicalein in the context of retinal ischemia. Moreover, the pre-treatment of baicalein has shown efficacy in modulating apoptotic factors, including Bax and Bcl-2, thus attenuating retinal cell apoptosis in a rat retinal ischemia/reperfusion model (Chao et al., 2013). Furthermore, baicalein, when administered orally, safeguards retinal vessels and neurons from DR-induced dysfunction and apoptosis. This protective effect is attributed to its ability to curb retinal inflammatory processes governed by microglia and Müller cells and to attenuate the release of pro-inflammatory cytokines such as IL-18, TNF-α, and IL-1β (Yang et al., 2009).
(−)-Epigallocatechin gallate (EGCG), the most prevalent catechin-based flavonoid in green tea leaves, has demonstrated substantial potential as a retinal protective agent (Fernando and Soysa, 2016). EGCG confers protection against ischemia injury (Peng et al., 2008) and DR (Silva et al., 2013). Its protective scope also extends to RP, where it not only lessens the visual function loss in P23H rats but also elevates the levels of antioxidant enzymes and reduces oxidative damage (Perdices et al., 2022).
Acacetin, a naturally occurring flavone found in various plants, including Turnera diffusa Willd. ex Schult. [Passifloraceae] and Saussurea involucrata (Kar. & Kir.) Sch.Bip. [Asteraceae], shows a gamut of pharmacological and biochemical activities (Singh et al., 2020). These encompass antioxidant, anti-inflammatory, and neuroprotective effects (Xiao et al., 2019; Wu et al., 2021). Importantly, Acacetin has demonstrated the ability to inhibit inflammatory responses by augmenting NLRP3 ubiquitination, thereby suggesting potential therapeutic benefits for depression-associated DED (Xie et al., 2022).
Xanthohumol, a naturally occurring prenylated chalconoid derived from Humulus lupulus L. [Cannabaceae], is a known promoter of the transcription of phase II antioxidant enzymes. It achieves this through the facilitation of the dissociation of Kelch-like ECH-associated protein 1 (Keap1) from nuclear factor erythroid 2-related factor 2 (NRF2) (Liu et al., 2015). Additionally, the chalconoid structure of Xanthohumol confers direct ROS scavenging activity, thereby broadening its therapeutic potential (Yao et al., 2015). As a result, Xanthohumol has exhibited cytoprotective effects in human corneal epithelial cells and a mouse desiccating stress/scopolamine model, further validating its prospective role in ocular therapeutics (Ghosh et al., 2021).
Polydatin (resveratrol-3-O-β-mono-D-glucoside, PD), a resveratrol glycoside found in Reynoutria japonica Houtt. [Polygonaceae], exhibits notable effects on orbital muscle adipose tissue expansion and lipid droplet accumulation. (Ravagnan et al., 2013). It employs a NRF2-mediated oxidative stress response involving the Keap1/NRF2/ARE pathway (Li et al., 2020). Furthermore, PD impedes hyperosmolar stress-induced inflammation by attenuating NF-κB translocation to the nucleus and diminishing the expression of pro-inflammatory markers such as TNF-α, IL-6, IL-1β, and MMP9. Importantly, PD also inhibits the hyperosmolar stress-induced NLRP3 inflammasome pathway and ROS production, suggesting its promising therapeutic potential for DED and GO (Park et al., 2019).
On the other hand, Gastrodin, an active metabolite of the traditional Chinese botanical drug Gastrodia elata Blume. [Orchidaceae], possesses anti-inflammatory, antioxidative, and anti-apoptotic properties (Yuan et al., 2019; Liu et al., 2021). This points to its potential role in treating retinal neurodegenerative diseases marked by retinal ganglion cell death (Wang et al., 2017). Notably, Gastrodin can protect retinal ganglion cells from hypoxia/reoxygenation injury by activating the PI3K/AKT/NRF2 pathway, thus offering potential avenues for glaucoma therapy (Li S. et al., 2022).
The increasing application of natural products in ophthalmology signifies their untapped pharmacological potential. However, hurdles such as under targeting, low bioavailability, subpar pharmacological activity, high metabolic decomposition rates, and uncertain pharmacological mechanisms persist. These challenges impede the translation of plant-derived natural products from basic research to clinical practice (Mahran et al., 2017; Rao et al., 2019; Takke and Shende, 2019). Hence, further research is paramount to enhance structural modifications and develop novel pharmaceutical agents based on natural products (Gunasekaran et al., 2014; Gaston et al., 2020).
4 Conclusion
As cellular powerhouses, mitochondria are vital for an array of cellular activities, producing the energy necessary for these processes. A growing body of research suggests a close association between mitochondrial dysfunction and common ocular disorders, including glaucoma, AMD and DR. Encouragingly, certain treatment modalities are transitioning from experimental stages to clinical applications. Particularly, the utilization of natural metabolites shows considerable promise, as numerous studies have demonstrated their potential efficacy in treating diverse ocular disorders. However, it is important to note that further investigations are necessary to comprehensively elucidate the underlying mechanisms of their biological effects. Additionally, a critical aspect that warrants attention is the safety assessments of these natural metabolites in the context of ocular disorders. Addressing these aspects will not only enhance our understanding of their therapeutic potential but also contribute to their safe and effective translation into medical practices. In light of the accumulating evidence, it is evident that natural metabolites could assume a significant role in the future of medical and personal care domains.
Author contributions
G-FS: Visualization, Writing–original draft. X-HQ: Writing–original draft. L-PJ: Writing–original draft. Z-PC: Writing–original draft, Supervision. TW: Conceptualization, Writing–review and editing. X-JH: Writing–review and editing, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by National Natural Science Foundation of China (82060177), Jiangxi Province Natural Science Foundation (20224ACB206014), the Research Fund for Jiangxi Geriatric Clinical Medical Research Center (2020BCG74003) and The Key Science and Technology Innovation Project of Jiangxi Provincial Health Commission (2023ZD001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alaimo, A., Linares, G. G., Bujjamer, J. M., Gorojod, R. M., Alcon, S. P., Martinez, J. H., et al. (2019). Toxicity of blue led light and A2E is associated to mitochondrial dynamics impairment in ARPE-19 cells: implications for age-related macular degeneration. Arch. Toxicol. 93 (5), 1401–1415. doi:10.1007/s00204-019-02409-6
Al Sabaani, N. (2020). Kaempferol protects against hydrogen peroxide-induced retinal pigment epithelium cell inflammation and apoptosis by activation of SIRT1 and inhibition of PARP1. J. Ocul. Pharmacol. Ther. 36 (7), 563–577. doi:10.1089/jop.2019.0151
Amati-Bonneau, P., Odent, S., Derrien, C., Pasquier, L., Malthiery, Y., Reynier, P., et al. (2003). The association of autosomal dominant optic atrophy and moderate deafness may be due to the R445H mutation in the OPA1 gene. Am. J. Ophthalmol. 136 (6), 1170–1171. doi:10.1016/s0002-9394(03)00665-2
Bantseev, V., and Youn, H. Y. (2006). Mitochondrial "movement" and lens optics following oxidative stress from UV-B irradiation: cultured bovine lenses and human retinal pigment epithelial cells (ARPE-19) as examples. Ann. N. Y. Acad. Sci. 1091, 17–33. doi:10.1196/annals.1378.051
Bernstein, P. S., Khachik, F., Carvalho, L. S., Muir, G. J., Zhao, D. Y., and Katz, N. B. (2001). Identification and quantitation of carotenoids and their metabolites in the tissues of the human eye. Exp. Eye Res. 72 (3), 215–223. doi:10.1006/exer.2000.0954
Bian, Q., Qin, T., Ren, Z., Wu, D., and Shang, F. (2012). Lutein or zeaxanthin supplementation suppresses inflammatory responses in retinal pigment epithelial cells and macrophages. Adv. Exp. Med. Biol. 723, 43–50. doi:10.1007/978-1-4614-0631-0_7
Bone, R. A., Landrum, J. T., Fernandez, L., and Tarsis, S. L. (1988). Analysis of the macular pigment by HPLC: retinal distribution and age study. Investig. Ophthalmol. Vis. Sci. 29 (6), 843–849.
Bone, R. A., Landrum, J. T., Friedes, L. M., Gomez, C. M., Kilburn, M. D., Menendez, E., et al. (1997). Distribution of lutein and zeaxanthin stereoisomers in the human retina. Exp. Eye Res. 64 (2), 211–218. doi:10.1006/exer.1996.0210
Bucolo, C., Leggio, G. M., Drago, F., and Salomone, S. (2012). Eriodictyol prevents early retinal and plasma abnormalities in streptozotocin-induced diabetic rats. Biochem. Pharmacol. 84 (1), 88–92. doi:10.1016/j.bcp.2012.03.019
Cejkova, J., Ardan, T., Simonova, Z., Cejka, C., Malec, J., Dotrelova, D., et al. (2008). Decreased expression of antioxidant enzymes in the conjunctival epithelium of dry eye (Sjogren's syndrome) and its possible contribution to the development of ocular surface oxidative injuries. Histol. Histopathol. 23 (12), 1477–1483. doi:10.14670/HH-23.1477
Chao, H. M., Chuang, M. J., Liu, J. H., Liu, X. Q., Ho, L. K., Pan, W. H., et al. (2013). Baicalein protects against retinal ischemia by antioxidation, antiapoptosis, downregulation of HIF-1α, VEGF, and MMP-9 and upregulation of HO-1. J. Ocul. Pharmacol. Ther. 29 (6), 539–549. doi:10.1089/jop.2012.0179
Del Dotto, V., Fogazza, M., Musiani, F., Maresca, A., Aleo, S. J., Caporali, L., et al. (2018). Deciphering OPA1 mutations pathogenicity by combined analysis of human, mouse and yeast cell models. Biochim. Biophys. Acta Mol. Basis Dis. 1864 (10), 3496–3514. doi:10.1016/j.bbadis.2018.08.004
Dieguez, H. H., Romeo, H. E., Alaimo, A., Gonzalez Fleitas, M. F., Aranda, M. L., Rosenstein, R. E., et al. (2019). Oxidative stress damage circumscribed to the central temporal retinal pigment epithelium in early experimental non-exudative age-related macular degeneration. Free Radic. Biol. Med. 131, 72–80. doi:10.1016/j.freeradbiomed.2018.11.035
Du, H., Sun, X., Guma, M., Luo, J., Ouyang, H., Zhang, X., et al. (2013). JNK inhibition reduces apoptosis and neovascularization in a murine model of age-related macular degeneration. Proc. Natl. Acad. Sci. U. S. A. 110 (6), 2377–2382. doi:10.1073/pnas.1221729110
Du, W., An, Y., He, X., Zhang, D., and He, W. (2018). Protection of kaempferol on oxidative stress-induced retinal pigment epithelial cell damage. Oxid. Med. Cell. Longev. 2018, 1610751. doi:10.1155/2018/1610751
Duarte, D. A., Rosales, M. A., Papadimitriou, A., Silva, K. C., Amancio, V. H., Mendonca, J. N., et al. (2015). Polyphenol-enriched cocoa protects the diabetic retina from glial reaction through the sirtuin pathway. J. Nutr. Biochem. 26 (1), 64–74. doi:10.1016/j.jnutbio.2014.09.003
Duraisamy, A. J., Mohammad, G., and Kowluru, R. A. (2019). Mitochondrial fusion and maintenance of mitochondrial homeostasis in diabetic retinopathy. Biochim. Biophys. Acta Mol. Basis Dis. 1865 (6), 1617–1626. doi:10.1016/j.bbadis.2019.03.013
Eells, J. T. (2019). Mitochondrial dysfunction in the aging retina. Biol. (Basel) 8 (2), 31. doi:10.3390/biology8020031
Ekiert, H. M., and Szopa, A. (2020). Biological activities of natural products. Molecules 25 (23), 5769. doi:10.3390/molecules25235769
Erdurmus, M., Yagci, R., Atis, O., Karadag, R., Akbas, A., and Hepsen, I. F. (2011). Antioxidant status and oxidative stress in primary open angle glaucoma and pseudoexfoliative glaucoma. Curr. Eye Res. 36 (8), 713–718. doi:10.3109/02713683.2011.584370
Fernando, C. D., and Soysa, P. (2016). Simple isocratic method for simultaneous determination of caffeine and catechins in tea products by HPLC. Springerplus 5 (1), 970. doi:10.1186/s40064-016-2672-9
Ferrington, D. A., Sinha, D., and Kaarniranta, K. (2016). Defects in retinal pigment epithelial cell proteolysis and the pathology associated with age-related macular degeneration. Prog. Retin Eye Res. 51, 69–89. doi:10.1016/j.preteyeres.2015.09.002
Fu, D., Yu, J. Y., Connell, A. R., Yang, S., Hookham, M. B., McLeese, R., et al. (2016). Beneficial effects of berberine on oxidized LDL-induced cytotoxicity to human retinal muller cells. Investig. Ophthalmol. Vis. Sci. 57 (7), 3369–3379. doi:10.1167/iovs.16-19291
Gaston, T. E., Mendrick, D. L., Paine, M. F., Roe, A. L., and Yeung, C. K. (2020). Natural" is not synonymous with "Safe": toxicity of natural products alone and in combination with pharmaceutical agents. Regul. Toxicol. Pharmacol. 113, 104642. doi:10.1016/j.yrtph.2020.104642
Ghaffariyeh, A., Honarpisheh, N., Heidari, M. H., Puyan, S., and Abasov, F. (2011). Brain-derived neurotrophic factor as a biomarker in primary open-angle glaucoma. Optom. Vis. Sci. 88 (1), 80–85. doi:10.1097/OPX.0b013e3181fc329f
Ghosh, A. K., Thapa, R., Hariani, H. N., Volyanyuk, M., Ogle, S. D., Orloff, K. A., et al. (2021). Poly(lactic-co-glycolic acid) nanoparticles encapsulating the prenylated flavonoid, xanthohumol, protect corneal epithelial cells from dry eye disease-associated oxidative stress. Pharmaceutics 13 (9), 1362. doi:10.3390/pharmaceutics13091362
Godley, B. F., Shamsi, F. A., Liang, F. Q., Jarrett, S. G., Davies, S., and Boulton, M. (2005). Blue light induces mitochondrial DNA damage and free radical production in epithelial cells. J. Biol. Chem. 280 (22), 21061–21066. doi:10.1074/jbc.M502194200
Gunasekaran, T., Haile, T., Nigusse, T., and Dhanaraju, M. D. (2014). Nanotechnology: an effective tool for enhancing bioavailability and bioactivity of phytomedicine. Asian Pac J. Trop. Biomed. 4 (Suppl. 1), S1–S7. doi:10.12980/APJTB.4.2014C980
Guo, W., Liu, S., Zheng, X., Xiao, Z., Chen, H., Sun, L., et al. (2021). Network pharmacology/metabolomics-based validation of AMPK and PI3K/AKT signaling pathway as a central role of shengqi fuzheng injection regulation of mitochondrial dysfunction in cancer-related fatigue. Oxid. Med. Cell. Longev. 2021, 5556212. doi:10.1155/2021/5556212
Guo, X., Dason, E. S., Zanon-Moreno, V., Jiang, Q., Nahirnyj, A., Chan, D., et al. (2014). PGC-1α signaling coordinates susceptibility to metabolic and oxidative injury in the inner retina. Am. J. Pathol. 184 (4), 1017–1029. doi:10.1016/j.ajpath.2013.12.012
Han, X. J., Tomizawa, K., Fujimura, A., Ohmori, I., Nishiki, T., Matsushita, M., et al. (2011). Regulation of mitochondrial dynamics and neurodegenerative diseases. Acta Med. Okayama 65 (1), 1–10. doi:10.18926/AMO/43824
Hightower, K. R., Duncan, G., Dawson, A., Wormstone, I. M., Reddan, J., and Dziedizc, D. (1999). Ultraviolet irradiation (UVB) interrupts calcium cell signaling in lens epithelial cells. Photochem Photobiol. 69 (5), 595–598. doi:10.1562/0031-8655(1999)069<0595:uiiccs>2.3.co;2
Hu, Y., Xu, Q., Li, H., Meng, Z., Hao, M., Ma, X., et al. (2022). Dapagliflozin reduces apoptosis of diabetic retina and human retinal microvascular endothelial cells through ERK1/2/cPLA2/AA/ROS pathway independent of hypoglycemic. Front. Pharmacol. 13, 827896. doi:10.3389/fphar.2022.827896
Huang, C., Zhang, P., Wang, W., Xu, Y., Wang, M., Chen, X., et al. (2014). Long-term blue light exposure induces RGC-5 cell death in vitro: involvement of mitochondria-dependent apoptosis, oxidative stress, and MAPK signaling pathways. Apoptosis 19 (6), 922–932. doi:10.1007/s10495-014-0983-2
Iyer, S., Bergquist, K., Young, K., Gnaiger, E., Rao, R. R., and Bennett, J. P. (2012). Mitochondrial gene therapy improves respiration, biogenesis, and transcription in G11778A Leber's hereditary optic neuropathy and T8993G Leigh's syndrome cells. Hum. Gene Ther. 23 (6), 647–657. doi:10.1089/hum.2011.177
Izumi-Nagai, K., Nagai, N., Ohgami, K., Satofuka, S., Ozawa, Y., Tsubota, K., et al. (2007). Macular pigment lutein is antiinflammatory in preventing choroidal neovascularization. Arterioscler. Thromb. Vasc. Biol. 27 (12), 2555–2562. doi:10.1161/ATVBAHA.107.151431
Jadeja, R. N., and Martin, P. M. (2021). Oxidative stress and inflammation in retinal degeneration. Antioxidants (Basel) 10 (5), 790. doi:10.3390/antiox10050790
Kaneko, H., Dridi, S., Tarallo, V., Gelfand, B. D., Fowler, B. J., Cho, W. G., et al. (2011). DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration. Nature 471 (7338), 325–330. doi:10.1038/nature09830
Kim, C. Y., Lee, H. J., Chae, M. K., Byun, J. W., Lee, E. J., and Yoon, J. S. (2015). Therapeutic effect of resveratrol on oxidative stress in Graves' orbitopathy orbital fibroblasts. Investig. Ophthalmol. Vis. Sci. 56 (11), 6352–6361. doi:10.1167/iovs.15-16870
Knels, L., Valtink, M., Roehlecke, C., Lupp, A., de la Vega, J., Mehner, M., et al. (2011). Blue light stress in retinal neuronal (R28) cells is dependent on wavelength range and irradiance. Eur. J. Neurosci. 34 (4), 548–558. doi:10.1111/j.1460-9568.2011.07790.x
Koh, J. H., Johnson, M. L., Dasari, S., LeBrasseur, N. K., Vuckovic, I., Henderson, G. C., et al. (2019). TFAM enhances fat oxidation and attenuates high-fat diet-induced insulin resistance in skeletal muscle. Diabetes 68 (8), 1552–1564. doi:10.2337/db19-0088
Kong, H., Zhao, H., Chen, T., Song, Y., and Cui, Y. (2022). Targeted P2X7/NLRP3 signaling pathway against inflammation, apoptosis, and pyroptosis of retinal endothelial cells in diabetic retinopathy. Cell. Death Dis. 13 (4), 336. doi:10.1038/s41419-022-04786-w
Kowluru, R. A. (2020). Retinopathy in a diet-induced type 2 diabetic rat model and role of epigenetic modifications. Diabetes 69 (4), 689–698. doi:10.2337/db19-1009
Kyosseva, S. V. (2016). Targeting MAPK signaling in age-related macular degeneration. Ophthalmol. Eye Dis. 8, 23–30. doi:10.4137/OED.S32200
Lascaratos, G., Ji, D., Wood, J. P., and Osborne, N. N. (2007). Visible light affects mitochondrial function and induces neuronal death in retinal cell cultures. Vis. Res. 47 (9), 1191–1201. doi:10.1016/j.visres.2006.12.014
Leone, T. C., Lehman, J. J., Finck, B. N., Schaeffer, P. J., Wende, A. R., Boudina, S., et al. (2005). PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 3 (4), e101. doi:10.1371/journal.pbio.0030101
Lewis Lujan, L. M., McCarty, M. F., Di Nicolantonio, J. J., Galvez Ruiz, J. C., Rosas-Burgos, E. C., Plascencia-Jatomea, M., et al. (2022). Nutraceuticals/drugs promoting mitophagy and mitochondrial biogenesis may combat the mitochondrial dysfunction driving progression of dry age-related macular degeneration. Nutrients 14 (9), 1985. doi:10.3390/nu14091985
Li, C., Kosmorsky, G., Zhang, K., Katz, B. J., Ge, J., and Traboulsi, E. I. (2005). Optic atrophy and sensorineural hearing loss in a family caused by an R445H OPA1 mutation. Am. J. Med. Genet. A 138A (3), 208–211. doi:10.1002/ajmg.a.30794
Li, H., Min, J., Chen, Y., Li, H., and Zhang, Y. (2020). Polydatin attenuates orbital oxidative stress in Graves' orbitopathy through the NRF2 pathway. Chem. Biol. Interact. 315, 108894. doi:10.1016/j.cbi.2019.108894
Li, J. Y., Zhang, K., Xu, D., Zhou, W. T., Fang, W. Q., Wan, Y. Y., et al. (2018). Mitochondrial fission is required for blue light-induced apoptosis and mitophagy in retinal neuronal R28 cells. Front. Mol. Neurosci. 11, 432. doi:10.3389/fnmol.2018.00432
Li, S., Yang, Q., Zhou, Z., Yang, X., Liu, Y., Hao, K., et al. (2022a). Gastrodin protects retinal ganglion cells from ischemic injury by activating phosphatidylinositol 3-kinase/protein kinase B/nuclear factor erythroid 2-related factor 2 (PI3K/AKT/Nrf2) signaling pathway. Bioengineered 13 (5), 12625–12636. doi:10.1080/21655979.2022.2076499
Li, Y., Ou, K., Wang, Y., Luo, L., Chen, Z., and Wu, J. (2022b). TLR9 agonist suppresses choroidal neovascularization by restricting endothelial cell motility via ERK/c-Jun pathway. Microvasc. Res. 141, 104338. doi:10.1016/j.mvr.2022.104338
Lin, J., Wu, P. H., Tarr, P. T., Lindenberg, K. S., St-Pierre, J., Zhang, C. Y., et al. (2004). Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell. 119 (1), 121–135. doi:10.1016/j.cell.2004.09.013
Lin, M. T., and Beal, M. F. (2006). Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443 (7113), 787–795. doi:10.1038/nature05292
Liu, F. Y., Wen, J., Hou, J., Zhang, S. Q., Sun, C. B., Zhou, L. C., et al. (2021). Gastrodia remodels intestinal microflora to suppress inflammation in mice with early atherosclerosis. Int. Immunopharmacol. 96, 107758. doi:10.1016/j.intimp.2021.107758
Liu, J. H., Wann, H., Chen, M. M., Pan, W. H., Chen, Y. C., Liu, C. M., et al. (2010). Baicalein significantly protects human retinal pigment epithelium cells against H₂O₂-induced oxidative stress by scavenging reactive oxygen species and downregulating the expression of matrix metalloproteinase-9 and vascular endothelial growth factor. J. Ocul. Pharmacol. Ther. 26 (5), 421–429. doi:10.1089/jop.2010.0063
Liu, M., Hansen, P. E., Wang, G., Qiu, L., Dong, J., Yin, H., et al. (2015). Pharmacological profile of xanthohumol, a prenylated flavonoid from hops (Humulus lupulus). Molecules 20 (1), 754–779. doi:10.3390/molecules20010754
Lo, Y. C., Teng, C. M., Chen, C. F., Chen, C. C., and Hong, C. Y. (1994). Magnolol and honokiol isolated from Magnolia officinalis protect rat heart mitochondria against lipid peroxidation. Biochem. Pharmacol. 47 (3), 549–553. doi:10.1016/0006-2952(94)90187-2
Ma, D., Li, S., Lucas, E. K., Cowell, R. M., and Lin, J. D. (2010). Neuronal inactivation of peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) protects mice from diet-induced obesity and leads to degenerative lesions. J. Biol. Chem. 285 (50), 39087–39095. doi:10.1074/jbc.M110.151688
Ma, K., Zhang, Z., Chang, R., Cheng, H., Mu, C., Zhao, T., et al. (2020). Dynamic PGAM5 multimers dephosphorylate BCL-xL or FUNDC1 to regulate mitochondrial and cellular fate. Cell. Death Differ. 27 (3), 1036–1051. doi:10.1038/s41418-019-0396-4
Mahran, R. I., Hagras, M. M., Sun, D., and Brenner, D. E. (2017). Bringing curcumin to the clinic in cancer prevention: a review of strategies to enhance bioavailability and efficacy. AAPS J. 19 (1), 54–81. doi:10.1208/s12248-016-0003-2
Mares, J. (2015). Food antioxidants to prevent cataract. JAMA 313 (10), 1048–1049. doi:10.1001/jama.2014.15301
Marie, M., Bigot, K., Angebault, C., Barrau, C., Gondouin, P., Pagan, D., et al. (2018). Light action spectrum on oxidative stress and mitochondrial damage in A2E-loaded retinal pigment epithelium cells. Cell. Death Dis. 9 (3), 287. doi:10.1038/s41419-018-0331-5
Mirra, S., and Marfany, G. (2019). Mitochondrial gymnastics in retinal cells: a resilience mechanism against oxidative stress and neurodegeneration. Adv. Exp. Med. Biol. 1185, 513–517. doi:10.1007/978-3-030-27378-1_84
Miyamoto, K., Khosrof, S., Bursell, S. E., Rohan, R., Murata, T., Clermont, A. C., et al. (1999). Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc. Natl. Acad. Sci. U. S. A. 96 (19), 10836–10841. doi:10.1073/pnas.96.19.10836
Mohammad, G., and Kowluru, R. A. (2022). Mitochondrial dynamics in the metabolic memory of diabetic retinopathy. J. Diabetes Res. 2022, 3555889. doi:10.1155/2022/3555889
Nair, K. S., Srivastava, C., Brown, R. V., Koli, S., Choquet, H., Kang, H. S., et al. (2021). GLIS1 regulates trabecular meshwork function and intraocular pressure and is associated with glaucoma in humans. Nat. Commun. 12 (1), 4877. doi:10.1038/s41467-021-25181-7
Nakamura, S., Shibuya, M., Nakashima, H., Hisamura, R., Masuda, N., Imagawa, T., et al. (2007). Involvement of oxidative stress on corneal epithelial alterations in a blink-suppressed dry eye. Investig. Ophthalmol. Vis. Sci. 48 (4), 1552–1558. doi:10.1167/iovs.06-1027
Narayan, D. S., Chidlow, G., Wood, J. P., and Casson, R. J. (2017). Glucose metabolism in mammalian photoreceptor inner and outer segments. Clin. Exp. Ophthalmol. 45 (7), 730–741. doi:10.1111/ceo.12952
Nashine, S., Nesburn, A. B., Kuppermann, B. D., and Kenney, M. C. (2020). Role of resveratrol in transmitochondrial AMD RPE cells. Nutrients 12 (1), 159. doi:10.3390/nu12010159
Osborne, N. N., Wood, J. P., Chidlow, G., Bae, J. H., Melena, J., and Nash, M. S. (1999). Ganglion cell death in glaucoma: what do we really know? Br. J. Ophthalmol. 83 (8), 980–986. doi:10.1136/bjo.83.8.980
Ozawa, Y., Toda, E., Homma, K., Osada, H., Nagai, N., Tsubota, K., et al. (2022). Effects of epigenetic modification of PGC-1α by a chemical chaperon on mitochondria biogenesis and visual function in retinitis pigmentosa. Cells 11 (9), 1497. doi:10.3390/cells11091497
Pagano, G., Pallardo, F. V., Lyakhovich, A., Tiano, L., and Trifuoggi, M. (2021). Mitigating the pro-oxidant state and melanogenesis of Retinitis pigmentosa: by counteracting mitochondrial dysfunction. Cell. Mol. Life Sci. 78 (23), 7491–7503. doi:10.1007/s00018-021-04007-1
Pan, L., Cho, K. S., Yi, I., To, C. H., Chen, D. F., and Do, C. W. (2021). Baicalein, baicalin, and wogonin: protective effects against ischemia-induced neurodegeneration in the brain and retina. Oxid. Med. Cell. Longev. 2021, 8377362. doi:10.1155/2021/8377362
Park, B., Jo, K., Lee, T. G., Hyun, S. W., Kim, J. S., and Kim, C. S. (2019). Polydatin inhibits NLRP3 inflammasome in dry eye disease by attenuating oxidative stress and inhibiting the NF-κB pathway. Nutrients 11 (11), 2792. doi:10.3390/nu11112792
Park, S. H., Kim, D. S., Oh, J., Geum, J. H., Kim, J. E., Choi, S. Y., et al. (2021). Matricaria chamomilla (chamomile) ameliorates muscle atrophy in mice by targeting protein catalytic pathways, myogenesis, and mitochondrial dysfunction. Am. J. Chin. Med. 49 (6), 1493–1514. doi:10.1142/S0192415X21500701
Peng, P. H., Ko, M. L., and Chen, C. F. (2008). Epigallocatechin-3-gallate reduces retinal ischemia/reperfusion injury by attenuating neuronal nitric oxide synthase expression and activity. Exp. Eye Res. 86 (4), 637–646. doi:10.1016/j.exer.2008.01.008
Perdices, L., Fuentes-Broto, L., Segura, F., Cavero, A., Orduna-Hospital, E., Insa-Sanchez, G., et al. (2022). Systemic epigallocatechin gallate protects against retinal degeneration and hepatic oxidative stress in the P23H-1 rat. Neural Regen. Res. 17 (3), 625–631. doi:10.4103/1673-5374.320990
Rao, T., Tan, Z., Peng, J., Guo, Y., Chen, Y., Zhou, H., et al. (2019). The pharmacogenetics of natural products: a pharmacokinetic and pharmacodynamic perspective. Pharmacol. Res. 146, 104283. doi:10.1016/j.phrs.2019.104283
Ravagnan, G., De Filippis, A., Carteni, M., De Maria, S., Cozza, V., Petrazzuolo, M., et al. (2013). Polydatin, a natural precursor of resveratrol, induces β-defensin production and reduces inflammatory response. Inflammation 36 (1), 26–34. doi:10.1007/s10753-012-9516-8
Santos, J. M., Mishra, M., and Kowluru, R. A. (2014). Posttranslational modification of mitochondrial transcription factor A in impaired mitochondria biogenesis: implications in diabetic retinopathy and metabolic memory phenomenon. Exp. Eye Res. 121, 168–177. doi:10.1016/j.exer.2014.02.010
Santos, J. M., Tewari, S., Goldberg, A. F., and Kowluru, R. A. (2011). Mitochondrial biogenesis and the development of diabetic retinopathy. Free Radic. Biol. Med. 51 (10), 1849–1860. doi:10.1016/j.freeradbiomed.2011.08.017
Sasaki, M., Ozawa, Y., Kurihara, T., Noda, K., Imamura, Y., Kobayashi, S., et al. (2009). Neuroprotective effect of an antioxidant, lutein, during retinal inflammation. Investig. Ophthalmol. Vis. Sci. 50 (3), 1433–1439. doi:10.1167/iovs.08-2493
Seminotti, B., Brondani, M., Ribeiro, R. T., Leipnitz, G., and Wajner, M. (2022). Disturbance of mitochondrial dynamics, endoplasmic reticulum-mitochondria crosstalk, redox homeostasis, and inflammatory response in the brain of glutaryl-CoA dehydrogenase-deficient mice: neuroprotective effects of bezafibrate. Mol. Neurobiol. 59 (8), 4839–4853. doi:10.1007/s12035-022-02887-3
Sharma, I., Yadav, K. S., and Mugale, M. N. (2022). Redoxisome and diabetic retinopathy: pathophysiology and therapeutic interventions. Pharmacol. Res. 182, 106292. doi:10.1016/j.phrs.2022.106292
Shi, X., Zhou, T., Huang, S., Yao, Y., Xu, P., Hu, S., et al. (2023). An electrospun scaffold functionalized with a ROS-scavenging hydrogel stimulates ocular wound healing. Acta Biomater. 158, 266–280. doi:10.1016/j.actbio.2023.01.016
Silva, K. C., Rosales, M. A., Hamassaki, D. E., Saito, K. C., Faria, A. M., Ribeiro, P. A., et al. (2013). Green tea is neuroprotective in diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 54 (2), 1325–1336. doi:10.1167/iovs.12-10647
Singh, L. P., Yumnamcha, T., and Devi, T. S. (2021). Mitophagy, ferritinophagy and ferroptosis in retinal pigment epithelial cells under high glucose conditions: implications for diabetic retinopathy and age-related retinal diseases. JOJ Ophthalmol. 8 (5), 77–85. doi:10.19080/jojo.2021.08.555748
Singh, S., Gupta, P., Meena, A., and Luqman, S. (2020). Acacetin, a flavone with diverse therapeutic potential in cancer, inflammation, infections and other metabolic disorders. Food Chem. Toxicol. 145, 111708. doi:10.1016/j.fct.2020.111708
Song, D., Song, J., Wang, C., Li, Y., and Dunaief, J. L. (2016). Berberine protects against light-induced photoreceptor degeneration in the mouse retina. Exp. Eye Res. 145, 1–9. doi:10.1016/j.exer.2015.10.005
Sparrow, J. R., and Cai, B. (2001). Blue light-induced apoptosis of A2E-containing RPE: involvement of caspase-3 and protection by Bcl-2. Investig. Ophthalmol. Vis. Sci. 42 (6), 1356–1362.
Stenton, S. L., Sheremet, N. L., Catarino, C. B., Andreeva, N. A., Assouline, Z., Barboni, P., et al. (2021). Impaired complex I repair causes recessive Leber's hereditary optic neuropathy. J. Clin. Investig. 131 (6), e138267. doi:10.1172/JCI138267
Su, L., Zhang, J., Gomez, H., Kellum, J. A., and Peng, Z. (2023). Mitochondria ROS and mitophagy in acute kidney injury. Autophagy 19 (2), 401–414. doi:10.1080/15548627.2022.2084862
Suen, D. F., Norris, K. L., and Youle, R. J. (2008). Mitochondrial dynamics and apoptosis. Genes. Dev. 22 (12), 1577–1590. doi:10.1101/gad.1658508
Sun, K., Luo, J., Jing, X., Xiang, W., Guo, J., Yao, X., et al. (2021). Hyperoside ameliorates the progression of osteoarthritis: an in vitro and in vivo study. Phytomedicine 80, 153387. doi:10.1016/j.phymed.2020.153387
Takke, A., and Shende, P. (2019). Nanotherapeutic silibinin: an insight of phytomedicine in healthcare reformation. Nanomedicine 21, 102057. doi:10.1016/j.nano.2019.102057
Tang, Z., Ju, Y., Dai, X., Ni, N., Liu, Y., Zhang, D., et al. (2021). HO-1-mediated ferroptosis as a target for protection against retinal pigment epithelium degeneration. Redox Biol. 43, 101971. doi:10.1016/j.redox.2021.101971
Tian, P., Ge, H., Liu, H., Kern, T. S., Du, L., Guan, L., et al. (2013). Leukocytes from diabetic patients kill retinal endothelial cells: effects of berberine. Mol. Vis. 19, 2092–2105.
Wang, J. W., Liu, Y. M., Zhao, X. F., and Zhang, H. (2017). Gastrodin protects retinal ganglion cells through inhibiting microglial-mediated neuroinflammation in an acute ocular hypertension model. Int. J. Ophthalmol. 10 (10), 1483–1489. doi:10.18240/ijo.2017.10.01
Weinreb, R. N., Aung, T., and Medeiros, F. A. (2014). The pathophysiology and treatment of glaucoma: a review. JAMA 311 (18), 1901–1911. doi:10.1001/jama.2014.3192
Weinreb, R. N., and Khaw, P. T. (2004). Primary open-angle glaucoma. Lancet 363 (9422), 1711–1720. doi:10.1016/S0140-6736(04)16257-0
Wood, J. P., Lascaratos, G., Bron, A. J., and Osborne, N. N. (2007). The influence of visible light exposure on cultured RGC-5 cells. Mol. Vis. 14, 334–344.
Wu, W., Xie, Z., Zhang, Q., Ma, Y., Bi, X., Yang, X., et al. (2020). Hyperoside ameliorates diabetic retinopathy via anti-oxidation, inhibiting cell damage and apoptosis induced by high glucose. Front. Pharmacol. 11, 797. doi:10.3389/fphar.2020.00797
Wu, Y., Song, F., Li, Y., Li, J., Cui, Y., Hong, Y., et al. (2021). Acacetin exerts antioxidant potential against atherosclerosis through Nrf2 pathway in apoE(-/-) Mice. J. Cell. Mol. Med. 25 (1), 521–534. doi:10.1111/jcmm.16106
Xiao, W. Z., Zhou, W. H., Ma, Q., Cui, W. G., Mei, Q. Y., and Zhao, X. (2019). Serotonergically dependent antidepressant-like activity on behavior and stress axis responsivity of acacetin. Pharmacol. Res. 146, 104310. doi:10.1016/j.phrs.2019.104310
Xie, M., Wang, H., Peng, J., Qing, D., Zhang, X., Guo, D., et al. (2022). Acacetin protects against depression-associated dry eye disease by regulating ubiquitination of NLRP3 through gp78 signal. Front. Pharmacol. 13, 984475. doi:10.3389/fphar.2022.984475
Xu, G., Li, T., Chen, J., Li, C., Zhao, H., Yao, C., et al. (2018). Autosomal dominant retinitis pigmentosa-associated gene PRPF8 is essential for hypoxia-induced mitophagy through regulating ULK1 mRNA splicing. Autophagy 14 (10), 1818–1830. doi:10.1080/15548627.2018.1501251
Xu, Q., Fu, Q., Li, Z., Liu, H., Wang, Y., Lin, X., et al. (2021). The flavonoid procyanidin C1 has senotherapeutic activity and increases lifespan in mice. Nat. Metab. 3 (12), 1706–1726. doi:10.1038/s42255-021-00491-8
Xu, X. H., Zhao, C., Peng, Q., Xie, P., and Liu, Q. H. (2017). Kaempferol inhibited VEGF and PGF expression and in vitro angiogenesis of HRECs under diabetic-like environment. Braz J. Med. Biol. Res. 50 (3), e5396. doi:10.1590/1414-431X20165396
Yang, L. P., Sun, H. L., Wu, L. M., Guo, X. J., Dou, H. L., Tso, M. O., et al. (2009). Baicalein reduces inflammatory process in a rodent model of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 50 (5), 2319–2327. doi:10.1167/iovs.08-2642
Yang, S., Li, F., Lu, S., Ren, L., Bian, S., Liu, M., et al. (2022). Ginseng root extract attenuates inflammation by inhibiting the MAPK/NF-κB signaling pathway and activating autophagy and p62-Nrf2-Keap1 signaling in vitro and in vivo. J. Ethnopharmacol. 283, 114739. doi:10.1016/j.jep.2021.114739
Yao, J., Zhang, B., Ge, C., Peng, S., and Fang, J. (2015). Xanthohumol, a polyphenol chalcone present in hops, activating Nrf2 enzymes to confer protection against oxidative damage in PC12 cells. J. Agric. Food Chem. 63 (5), 1521–1531. doi:10.1021/jf505075n
Yao, K., Zhang, L., Ye, P. P., Tang, X. J., and Zhang, Y. D. (2009). Protective effect of magnolol against hydrogen peroxide-induced oxidative stress in human lens epithelial cells. Am. J. Chin. Med. 37 (4), 785–796. doi:10.1142/S0192415X09007247
Yuan, X., Li, Z., Wang, X. T., Li, X. Y., Hua, H., Li, X. C., et al. (2019). Roles and mechanisms of traditional Chinese medicine and its active ingredients in treating epilepsy. Zhongguo Zhong Yao Za Zhi 44 (1), 9–18. doi:10.19540/j.cnki.cjcmm.20181012.006
Zhai, J., Li, Z., Zhang, H., Ma, L., Ma, Z., Zhang, Y., et al. (2020). Berberine protects against diabetic retinopathy by inhibiting cell apoptosis via deactivation of the NF‑κB signaling pathway. Mol. Med. Rep. 22 (5), 4227–4235. doi:10.3892/mmr.2020.11505
Zhang, K., Guo, M. Y., Li, Q. G., Wang, X. H., Wan, Y. Y., Yang, Z. J., et al. (2021). Drp1-dependent mitochondrial fission mediates corneal injury induced by alkali burn. Free Radic. Biol. Med. 176, 149–161. doi:10.1016/j.freeradbiomed.2021.09.019
Zhang, Y., Xi, X., Mei, Y., Zhao, X., Zhou, L., Ma, M., et al. (2019). High-glucose induces retinal pigment epithelium mitochondrial pathways of apoptosis and inhibits mitophagy by regulating ROS/PINK1/Parkin signal pathway. Biomed. Pharmacother. 111, 1315–1325. doi:10.1016/j.biopha.2019.01.034
Zou, G. P., Wang, T., Xiao, J. X., Wang, X. Y., Jiang, L. P., Tou, F. F., et al. (2023). Lactate protects against oxidative stress-induced retinal degeneration by activating autophagy. Free Radic. Biol. Med. 194, 209–219. doi:10.1016/j.freeradbiomed.2022.12.004
Glossary
Keywords: eye disorder, mitochondria, mitochondrial dysfunction, natural product, oxidative stress
Citation: Sun G-F, Qu X-H, Jiang L-P, Chen Z-P, Wang T and Han X-J (2024) The mechanisms of natural products for eye disorders by targeting mitochondrial dysfunction. Front. Pharmacol. 15:1270073. doi: 10.3389/fphar.2024.1270073
Received: 31 July 2023; Accepted: 10 April 2024;
Published: 25 April 2024.
Edited by:
Patricia Rijo, Lusofona University, PortugalCopyright © 2024 Sun, Qu, Jiang, Chen, Wang and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-Jian Han, hanxiaojian@hotmail.com; Tao Wang, wangtaoalepellis@gmail.com
 Gui-Feng Sun
Gui-Feng Sun Xin-Hui Qu1,3
Xin-Hui Qu1,3 Li-Ping Jiang
Li-Ping Jiang Tao Wang
Tao Wang Xiao-Jian Han
Xiao-Jian Han