- 1Department of Pharmacy, Aerospace Center Hospital, Beijing, China
- 2College of Pharmacy, Hebei Medical University, Shijiazhuang, China
- 3Aerospace School of Clinical Medicine, Peking University, Beijing, China
Aims: This study aimed to investigate the association between the use of sodium-glucose transporter 2 inhibitors (SGLT-2i) and the risk of diabetic ketoacidosis (DKA), lower limb amputation (LLA), urinary tract infections (UTI), genital tract infections (GTI), bone fracture, and hypoglycemia in cohort studies.
Methods: A systematic search was conducted in the PubMed and Embase databases to identify cohort studies comparing the safety of SGLT-2i versus other glucose-lowering drugs (oGLD) in patients with type 2 diabetes mellitus (T2DM). The quality of the studies was assessed using the Newcastle-Ottawa Scale. Primary endpoints were DKA and LLA, while secondary endpoints included UTI, GTI, bone fracture, and hypoglycemia. Hazard ratios (HR) with 95% confidence intervals (CI) were calculated.
Results: A total of 9,911,454 patients from 40 cohort studies were included in the analysis. SGLT-2i use was associated with a higher risk of DKA (HR: 1.21, 95% CI: 1.07–1.38, p = 0.003) and GTI (HR: 2.72, 95% CI: 2.48–2.98, p < 0.01). However, it was not associated with an increased risk of LLA (HR: 1.06, 95% CI: 0.92–1.23, p = 0.42), UTI (HR: 0.99, 95% CI: 0.89–1.10, p = 0.83), or bone fracture (HR: 0.99, 95% CI: 0.94–1.04, p = 0.66). Furthermore, SGLT-2i was associated with a reduced risk of hypoglycemia. Furthermore, compared to dipeptidyl peptidase 4 inhibitors, SGLT-2i as a class and individually was associated with an increased risk of DKA. Canagliflozin specifically increased the risk of LLA (HR: 1.19, 95% CI: 1.04–1.36, p = 0.01). The subgroup analysis suggested that SGLT-2i increased the risk of LLA among patients with a history of cardiovascular disease.
Conclusion: SGLT-2i versus oGLD was associated with a similar occurrence of LLA, UTI, and bone fracture. However, SGLT-2i was associated with a higher risk of DKA and GTI than oGLD. These findings provide valuable information on the safety profile of SGLT-2i in patients with T2DM and can help inform clinical decision-making.
1 Introduction
Individuals diagnosed with type 2 diabetes mellitus (T2DM) face an increased risk of mortality and cardiovascular problems. SGLT2 inhibitors (SGLT-2i) have received approval to reduce blood glucose levels in adults with T2DM when used in conjunction with diet and exercise. In addition to lowering glucose, SGLT-2i offers additional benefits in addressing various aspects of metabolic syndrome, such as improving blood pressure, weight management, and lipid profiles. Furthermore, they have shown promising outcomes in reducing cardiovascular events and overall mortality. SGLT-2i is effective in lowering cardiovascular risk in individuals with T2DM by interrupting or mitigating several processes involved in atherosclerosis development, resulting in fewer cardiovascular complications. Additionally, promising outcomes in reducing cardiovascular events and overall mortality have been reported in studies conducted by (Hu et al., 2020; Li et al., 2021; Andreadi et al., 2023). Despite their apparent advantages, a degree of controversy has arisen regarding the potential link between SGLT-2i and an elevated risk of adverse events, including diabetic ketoacidosis (DKA), lower limb amputations (LLA), genital tract infections (GTI), and others.
Several meta-analyses and cohort studies have investigated the risk of DKA, urinary tract infections (UTI), GTI, and hypoglycemia in individuals with T2DM who received SGLT-2i treatment. Multiple meta-analyses and cohort studies have been performed to evaluate the risk of DKA, UTI, or GTI in T2DM patients receiving SGLT-2i treatment. The findings have exhibited some degree of inconsistency. Concerning the risk of DKA, three meta-analyses that synthesized data from randomized controlled trials (RCTs) (Tang et al., 2016; Monami et al., 2017; Donnan et al., 2019) indicated that SGLT-2i did not increase the risk of DKA in T2DM patients. In contrast, two additional meta-analyses (Liu et al., 2020; Salah et al., 2021) reported an elevated risk of DKA in these individuals due to SGLT-2i use. Likewise, three cohort studies (Wang et al., 2017; Kim et al., 2018; Han et al., 2021) found no increased risk of DKA associated with SGLT-2i use, while three different cohort studies (Douros et al., 2020; Fralick et al., 2021a; Patorno et al., 2021) identified an increased risk of DKA in T2DM patients treated with SGLT-2i. A study by Mantovani suggested that newer hypoglycemic medications with reduced potential for drug-induced hypoglycemia can mitigate the occurrence of severe hypoglycemia and related adverse effects, particularly among more susceptible patients (Mantovani et al., 2016).
Concerning UTI and GTI, several meta-analysis of RCTs (Storgaard et al., 2016; Wu et al., 2016; Tang et al., 2017; Zhang et al., 2018) revealed that SGLT-2i increased the risk of these infections when compared to the placebo group. In contrast, two additional meta-analyses (Zhang et al., 2018; Donnan et al., 2019) reported that the incidence of UTI and GTI in the SGLT-2i group closely resembled that in the placebo group.
Multiple meta-analyses of RCTs have explored the relationship between SGLT-2i and the risk of LLA, demonstrating that SGLT-2i use elevated the LLA risk compared to the placebo group (Zaccardi et al., 2016; Rådholm et al., 2018). In contrast, additional meta-analyses (Dorsey-Treviño et al., 2020; Kumar et al., 2020) did not identify a statistically significant increase in LLA risk associated with SGLT-2i use compared to control groups. The evidence of observational studies that examine the relationship between SGLT-2i and the risk of LLA presents a broader range of findings. Seven observational studies (Chang et al., 2018; Ryan et al., 2018; Udell et al., 2018; Ueda et al., 2018; Yuan et al., 2018; Dawwas et al., 2019; Yang et al., 2019) have generated varied outcomes concerning the link between SGLT-2i use and the risk of LLA.
Observational studies using real-world data offer valuable insight into the safety and efficacy of medications in clinical practice. However, when evaluating the risk of adverse events linked to SGLT-2i, the results of these studies have displayed inconsistency. While one study (Caparrotta et al., 2021) systematically assessed the safety of SGLT-2i based on observational studies, it is important to note that the studies included were published before 2020. Because medical practices and patient demographics can evolve, the applicability of older studies in capturing current safety patterns may be constrained. Another concern is that the baseline characteristics of participants in the included studies were not balanced through propensity score matching (PSM), a statistical technique used to mitigate confounding in observational research. In the absence of PSM, there is the possibility of imbalances in the baseline characteristics between the SGLT-2i and control groups, which introduces confounding variables that might impact the study’s outcomes. Moreover, in some cases, participants in the included studies may not have been accurately classified as individuals with T2DM. Such misclassification could introduce additional confounding variables, potentially compromising the accuracy and reliability of the study’s findings. Given these constraints, conducting updated meta-analyses of real-world data and employing more robust methodologies is imperative to gain a deeper understanding of the safety profile of SGLT-2i in individuals with T2DM.
2 Methods
This meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Kumpf et al., 2023). The protocol was registered in the International Prospective Register of Systematic Review (PROSPERO, registration number CRD42021235831).
2.1 Data sources
Cohort studies investigating the safety or adverse events of SGLT-2i in patients with T2DM were identified by searching the PubMed and Embase databases. All eligible studies in English published up to 11 July 2023, were considered. Literature searches used specific keywords related to SGLT-2i, such as DKA, LLA, UTI, GTI, bone fracture, hypoglycemia, safety, and adverse effects. RCTs were excluded from the search strategy. The customized search strategy for each database is shown in Supplementary Appendix S2.
2.2 Study selection
2.2.1 Criteria for inclusion
Studies that met the following criteria were included in this meta-analysis:
1. Types of study: Prospective or retrospective cohort studies.
2. Study populations: Patients with T2DM without restrictions on age, sex, or ethnicity.
3. Study design: For retrospective cohort studies, baseline information from the observation and control groups had to be essentially the same, achieved through PSM. For prospective cohort studies, the comparability between baseline information from the observation and control groups was necessary.
4. Interventions: The observation group received treatment with SGLT-2i as a class or as individual agents, while the control group received other glucose-lowering drugs (oGLD).
5. Sample size: Studies with a sample size of 1,000 or more were included to minimize the heterogeneity of the pooled study arising from small sample size studies.
The primary outcomes were the occurrences of DKA and LLA. Secondary outcomes were the occurrences of GTI, UTI, hypoglycemia, and bone fractures. Studies were included if they reported at least one of these outcome measures.
2.2.2 Criteria for exclusion
The following were the exclusion criteria for this meta-analysis:
1. RCTs, reviews, systematic reviews, meta-analyses, case reports, case studies, case series, letters, opinions, audits, protocols, and methodologies.
2. Studies in which the intervention did not meet the specified outcome measures.
Two investigators (T.T.L. and Q.Z.) independently screened the literature. In cases of discrepancies or uncertainties, consensus was reached through discussion with the other author (C.X.L.).
2.3 Data extraction
A standardized extraction form was used to collect the study data. The following data were independently extracted by three authors (T.T.L., Q. Z, and Q.X.): first author, year of publication, country, study population, age, sex, number of patients, intervention measure, follow-up time, and outcome measures. The data extraction forms were cross-checked to verify the accuracy and consistency of the extracted data. The third author (C.X.L.) checked all data and disagreements were resolved by discussion.
2.4 Quality assessment of the study
The quality assessment of the studies was conducted independently by three authors (T.T.L., Q.Z., and Q.X.) using the Newcastle-Ottawa Scale (Yu et al., 2018). This scale evaluates studies based on three main domains: selection, comparability, and exposure. Within the selection and exposure categories, each numbered item can be awarded a maximum of 1 point, while comparability can receive a maximum of 2 points. The total score ranges from 0 to 9, with higher scores indicating higher study quality. Studies were classified into low quality (scores 0–3), moderate quality (scores 4–6), and high quality (scores 7–9).
2.5 Statistical analysis
Meta-analysis was performed using Stata 16.0 software (StataCorp, College Station, TX, United States). Statistical heterogeneity between studies was assessed using the Cochran chi-square test complemented with the I2 statistic. I2 values of 25%, 50%, and 75% indicate low, moderate, and high heterogeneity, respectively (Higgins et al., 2003). The random-effects model was used for the analysis. The hazard ratio (HR) and the 95% confidence interval (95% CI) were used to describe categorical variables, where the p-value < 0.05 is considered significantly different.
To examine the sources of heterogeneity, we performed a meta-regression analysis with intervention, study region, study year, sex proportion, follow-up time, and sample size as independent variables, and DKA, LLA, UTI, GTI, bone fracture, and hypoglycemia as dependent variables, respectively. To assess the stability of the results, a sensitivity analysis was systematically performed, excluding one study at a time to examine its impact on overall findings.
We utilized Confunnel plots for qualitative evaluation and Egger’s test for quantitative analysis to assess publication bias. Confunnel plots display areas of statistical significance on a funnel plot, and it help distinguish publication bias from other causes of asymmetry. If the missing studies are in areas of non-significant, the asymmetry observed in the confunnel plot is caused by publication bias. In contrast, if the absent studies are statistically significant, the observed asymmetry is more likely due to factors other than publication bias based on statistical significance (e.g., variable study quality or non-statistical significance-based publication bias mechanisms) (Peters et al., 2008). P < 0.05 indicates a possible publication bias.
3 Results
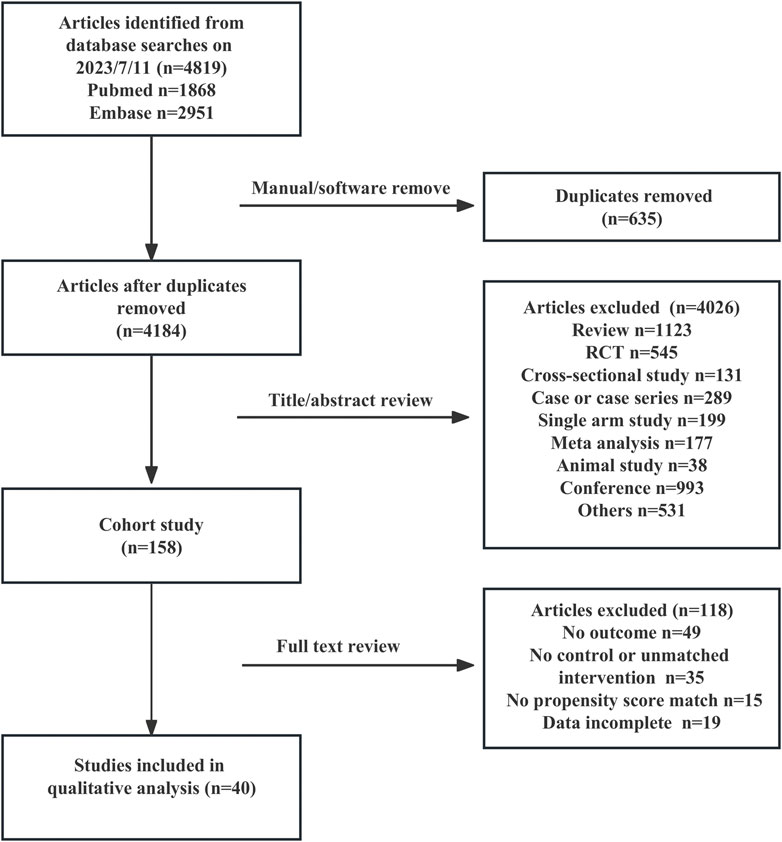
3.1 Literature retrieval and selection
A total of 4,819 studies were retrieved, and 40 (n = 9,911,454) were included in the analysis (Birkeland et al., 2017; Nyström et al., 2017; Tang et al., 2017; Wang et al., 2017; Persson et al., 2018; Ryan et al., 2018; Toulis et al., 2018; Udell et al., 2018; Yuan et al., 2018; Kim et al., 2018; Dave et al., 2019a; Dawwas et al., 2019; Wang et al., 2019; Yang et al., 2019; Dave et al., 2019b; Norhammar et al., 2019; Douros et al., 2020; Fralick et al., 2020; Lee et al., 2020; Udell et al., 2020; Laursen et al., 2021; Yu et al., 2020; Han et al., 2021; Patorno et al., 2021; Han et al., 2021; Fralick et al., 2021b; Fralick et al., 2021b; Laursen et al., 2021; Ryan et al., 2018; Caparrotta et al., 2021; Bhosle et al., 2022; Laursen et al., 2021; Fralick et al., 2020; Schneeweiss et al., 2021, Al-et al. 2022, Ha et al., 2022; van Dalem et al., 2022; D'Andrea et al., 2023; Fu et al., 2023; Htoo et al., 2023). Figure 1 shows the literature selection flowchart.
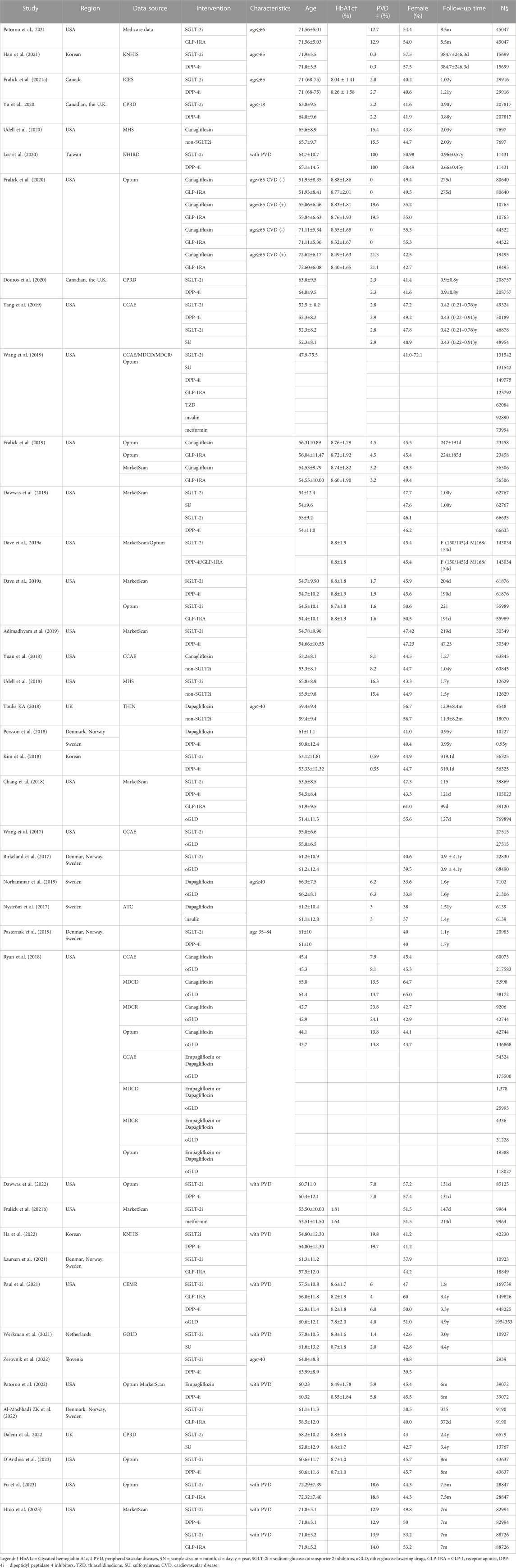
3.2 Study characteristics and quality assessment
The included studies spanned from 2017 to 2023, 22 published in 2020 or later. Among these studies, 23 were conducted in the US, 7 in northern Europe, three in Canada, and 3 in Asia. The proportion of women in the included studies ranged from 33.6% to 64.7%. The median duration of follow-up was 10.8 months (3.3–58.8). Baseline glycosylated hemoglobin (HbA1c) was reported in 13 studies, while the baseline prevalence of peripheral vascular diseases (PVD) was reported in 26 studies. A comprehensive summary of the characteristics of the included studies is presented in Table 1.
3.3 Primary outcomes
3.3.1 DKA
Fourteen studies compared the safety of the SGLT-2i class and oGLD in terms of DKA among the 40 trials that met the inclusion criteria (Kim et al., 2018; Han et al., 2021; Fralick et al., 2021a; Douros et al., 2020; Patorno et al., 2021; Tang et al., 2017; Laursen et al., 2021; Fralick et al., 2021b; Toulis et al., 2018; Patorno et al., 2021; Laursen et al., 2021; Bhosle et al., 2022; D'Andrea et al., 2023; Fu et al., 2023). A total of 2,665 DKA cases were reported among 1,233,569 patients treated with SGLT-2i (mean incidence rate 2.86 per 1,000 person-years). In contrast, 2,063 DKA cases were reported among 1,306,053 patients treated with oGLD (mean incidence rate DKA 1.87 per 1,000 person-years) (Supplementary Appendix S4.1).
The SGLT-2i class was associated with an increased risk of DKA compared to oGLD (HR: 1.21, 95% CI: 1.07–1.38, p = 0.003, I2 = 61.0%; Figure 2A). Subgroup analysis revealed that the SGLT-2i class did not significantly increase the risk of DKA compared to glucagon-like peptide-1 receptor agonists (GLP-1RA). Among 208,609 patients, there were 393 DKA events in the SGLT-2i group and 348 in the GLP-1RA group [mean incidence rates 2.20 vs. 1.76 per 1,000 person-years (Supplementary Appendix S4.1); HR: 1.15; 95% CI: 0.96–1.37; p = 0.125; I2 = 3.4% (Supplementary Appendix S5.1). However, the analysis found an increased risk of DKA in the SGLT-2i group compared to DPP-4i. Among 627,019 patients, there were 1,224 DKA events in the SGLT-2i group and 799 events in the DPP-4i group [mean incidence rates 2.46 vs. 1.48 per 1,000 person-years (Supplementary Appendix S4.1); HR: 1.38; 95% CI: 1.10–1.74; p = 0.006; I2 = 72.8% (Supplementary Appendix S5.1).
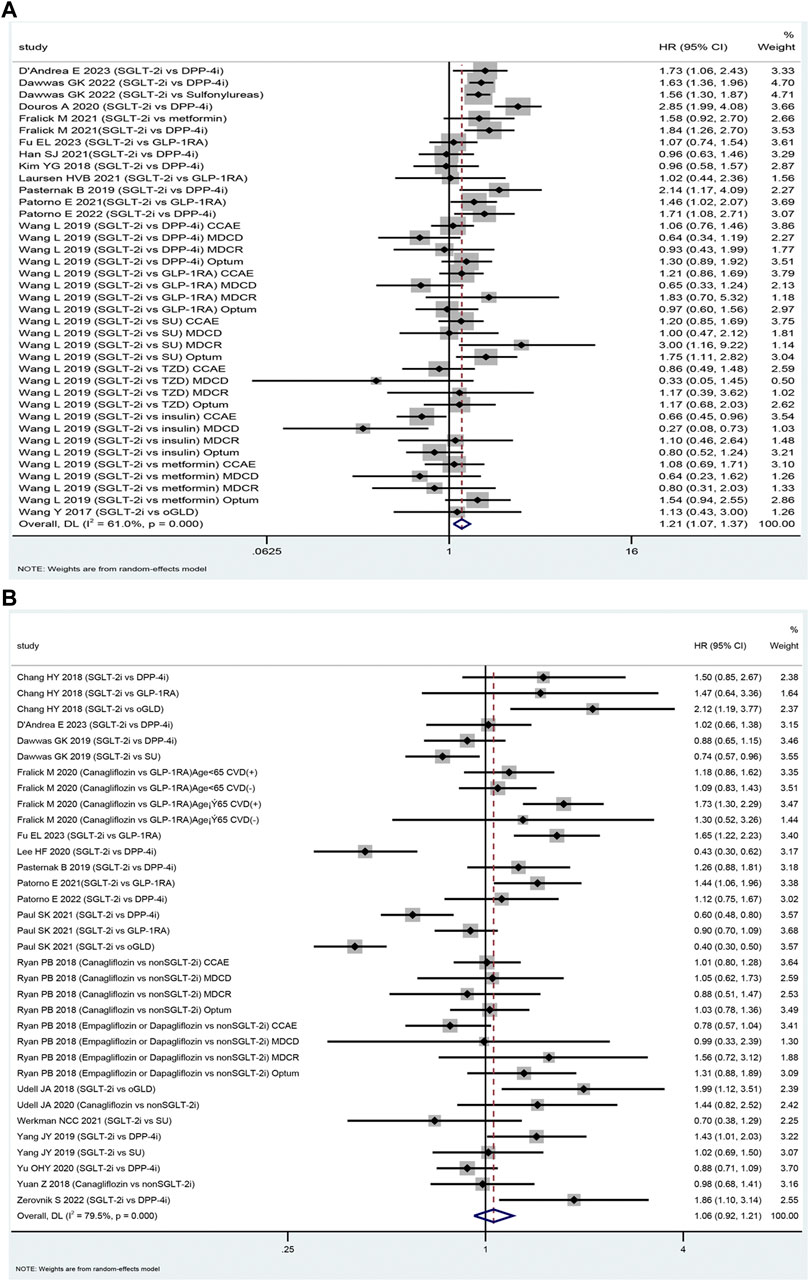
FIGURE 2. The forest plot of diabetic ketoacidosis (A) and lower limb amputation (B) Legend: SGLT-2i = sodium-glucose cotransporter 2 inhibitors, GLP-1RA = GLP-1 receptor agonist, DPP-4i = dipeptidyl peptidase 4 inhibitors, SU = sulfonylureas, TZD = thiazolidinedione, oGLD = other glucose-lowering drugs, CVD = cardiovascular disease.
Among 224,186 patients, canagliflozin had 653 DKA events compared to 376 events with DPP-4i [mean incidence rate 4.93 vs. 2.54 per 1,000 person-years; HR: 1.57; 95% CI: 1.12–2.19; p = 0.008; I2 = 67.4%]. Among 96, 077 patients, dapagliflozin had 171 DKA events compared to 117 events with DPP-4i [mean incidence rate 5.17 vs. 1.94 per 1,000 person-years; HR: 1.54; 95% CI: 1.14–2.087; p = 0.004; I2 = 0.0%]. Among 151,625 patients, empagliflozin had 241 DKA events compared to 192 events with DPP-4i [mean incidence rate 3.16 vs. 2.03 per 1,000 person-years; HR: 1.50; 95% CI: 1.14–1.97; p = 0.004; I2 = 23.7%]. Details are shown in Supplementary Appendix S5.2.
3.3.2 LLA
Eighteen studies were included in the assessment of the safety of SGLT-2i versus oGLD in terms of LLA (Fralick et al., 2021b; Fralick et al., 2019; Yang et al., 2019;Ueda et al., 2018; Udell et al., 2018; Ryan et al., 2018; Dawwas et al., 2019; Chang et al., 2018; Caparrotta et al., 2021; Wang et al., 2019; Persson et al., 2018; Udell et al., 2020; Wang et al., 2019; Dawwas et al., 2019; Dave et al., 2019a; Dave et al., 2019b; D'Andrea et al., 2023; Fu et al., 2023). Among these studies, 2,025 LLA cases were reported in the SGLT-2i group (mean incidence rate 2.30 per 1,000 person-years). In comparison, the oGLD group had 4,142 LLA cases (mean incidence rate of 1.98 per 1,000 person-years) (Supplementary Appendix S4.2). The pooled analysis indicated that the use of SGLT-2i was not associated with a significantly higher risk of LLA compared to oGLD (HR: 1.06, 95% CI: 0.92–1.21, p = 0.42, I2 = 79.5%; Figure 2B).
The meta-analysis showed that the SGLT-2i class increased the risk of LLA compared to GLP-1RA (629 vs. 442 events among 269,183 patients; mean incidence rate 5.28 vs. 3.56 per 1,000 person-years; HR: 1.22; 95% CI: 1.00–1.45; p = 0.048; I2 = 72.5%; Supplementary Appendix S5.3). However, there was no significant difference in LLA risk between the SGLT-2i class and DPP-4i (670 vs. 830 events among 471,139 patients; mean incidence rate 3.33 vs. 2.75 per 1,000 person-years; HR: 0.98, 95% CI: 0.77–1.26; p = 0.89; I2 = 81.2%; Supplementary Appendix S5.3). In contrast, the SGLT-2i class showed a lower risk of LLA compared to sulfonylurea (164 events among 122,648 patients vs. 415 events among 131,372 patients; mean incidence rate 1.46 vs. 1.70 per 1,000 person-years; HR: 0.80, 95% CI: 0.65–0.99; p = 0.04; I2 = 1.40%; Supplementary Appendix S5.3).
Canagliflozin increased the risk of LLA compared to oGLD (HR: 1.19; 95% CI: 1.04–1.36, p = 0.01, I2 = 36.8%; Supplementary Appendix S5.4). Regarding the prevalence of PVD in the included studies, the analysis revealed that SGLT-2i did not increase the risk of LLA when the baseline PVD prevalence was less than 10% (1,190 events among 735,546 patients vs. 3,093 events among 1,324,812 patients; mean incidence rate 1.66 vs. 1.61 per 1,000 person-years; HR: 0.87; 95% CI: 0.72–1.06; p = 0.18; I2 = 79.8%; Supplementary Appendix S5.5). The prevalence of PVD in these studies ranged from 0% to 8.1% (Ryan et al., 2018; Yang et al., 2019; Yuan et al., 2018; Fralick et al., 2020; Yu et al., 2020; Paul et al., 2021; Werkman et al., 2021; Patorno et al., 2022). Similarly, when the baseline PVD prevalence was greater than 10% (768 events among 264,885 patients vs. 2,347 events among 845,427 patients; mean incidence rate 7.39 vs. 5.42 per 1,000 person-years), SGLT-2i was not associated with an increased risk of LLA (HR: 1.21, 95% CI: 0.96–1.52, p = 0.22, I2 = 75.8%; Supplementary Appendix S5.5). The prevalence of PVD in these six studies ranged from 12.7% to 23.8% (Ryan et al., 2018; Udell et al., 2018; Fralick et al., 2020; Lee et al., 2020; Udell et al., 2020; Patorno et al., 2021; Fu et al., 2023).
In patients with previous cardiovascular disease, SGLT-2i increased the risk of LLA compared to oGLD (HR: 1.24, 95% CI: 1.05–1.46, p = 0.046, I2 = 37.6%; Supplementary Appendix S5.6). Specifically, there were 772 LLA events among 248,685 patients in the SGLT-2i group (mean incidence rate 8.42 per 1,000 person-years). In comparison, the oGLD group had 2,350 LLA events among 829,227 patients (mean incidence rate 5.91 per 1,000 person-years). In contrast, SGLT-2i did not increase the risk of LLA in patients without cardiovascular disease at baseline compared to oGLD (HR: 0.90, 95% CI: 0.48–1.68, p = 0.74, I2 = 83.2%, Supplementary Appendix S5.6). There were 198 LLA events among 125,162 patients in the SGLT-2i group (mean incidence rate 2.01 per 1,000 person-years). In comparison, the oGLD group had 151 LLA events among 125,162 patients (mean incidence rate 1.58 per 1,000 person-years).
3.4 Secondary outcomes
In the SGLT-2i group, 615 cases of UTI (mean incidence rate 9.58 per 1,000 person-years), 10,178 GTI cases (median incidence rate 58.67 per 1,000 person-years), 3,751 cases of bone fractures (mean incidence rate 5.18 per 1,000 person-years), and 302 cases of hypoglycemia (mean incidence rate 6.58 per 1,000 person-years) were identified (Supplementary Appendix S4.3-4.5). In comparison, the oGLD group had 305 cases of UTI (mean incidence rate 7.31 per 1,000 person-years), 2,390 cases of GTI (median incidence rate 15.64 per 1,000 person-years), 1,299 cases of bone fractures (mean incidence rate 4.34 per 1,000 person-years), and 2,167 cases of hypoglycemia (mean incidence rate 9.42 per 1,000 person-years) (Supplementary Appendix S4.3-4.5).
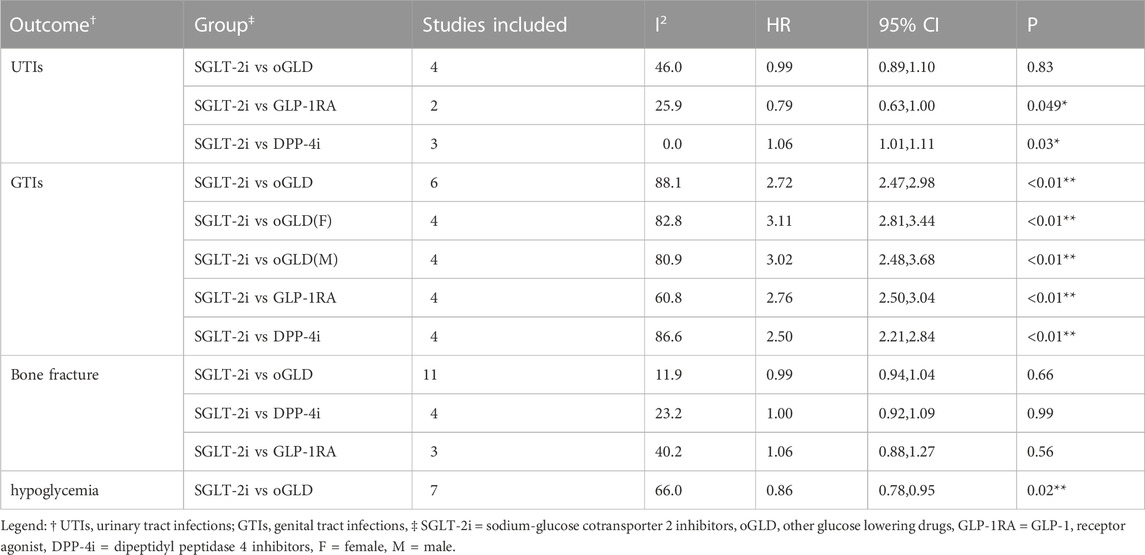
Table 2 presents the pooled results of various outcomes from multiple studies. The use of SGLT-2i was not associated with an increased risk of UTI (HR: 0.99, 95% CI: 0.89–1.10, p = 0.83; Figure 3B) based on these studies (Dave et al., 2019b; Lee et al., 2020; Han et al., 2021; Patorno et al., 2021; D'Andrea et al., 2023; Fu et al., 2023) or bone fractures (HR: 0.99, 95% CI: 0.94–1.04, p = 0.66; Figure 3C) compared to oGLD based on these studies (Toulis et al., 2018; Adimadhyam et al., 2019; Fralick et al., 2019; Lee et al., 2020; Han et al., 2021; Patorno et al., 2021; Al-Mashhadi et al., 2022; Ha et al., 2022; Patorno et al., 2022; van Dalem et al., 2022; D'Andrea et al., 2023; Fu et al., 2023). However, SGLT-2i showed a 2.72-fold increase in GTI risk (HR: 2.72, 95% CI: 2.47–2.98, p < 0.01; Figure 3A) based on these studies (Dave et al., 2019b; Fralick et al., 2021a; Han et al., 2021; Patorno et al., 2021; D'Andrea et al., 2023; Fu et al., 2023) and a reduced risk of hypoglycemia (HR: 0.86, 95% CI: 0.78–0.95, p = 0.002; Figure 3D) compared to oGLD based on these studies (Birkeland et al., 2017; Nyström et al., 2017; Persson et al., 2018; Norhammar et al., 2019; Fralick et al., 2021b; Han et al., 2021; Htoo et al., 2023).
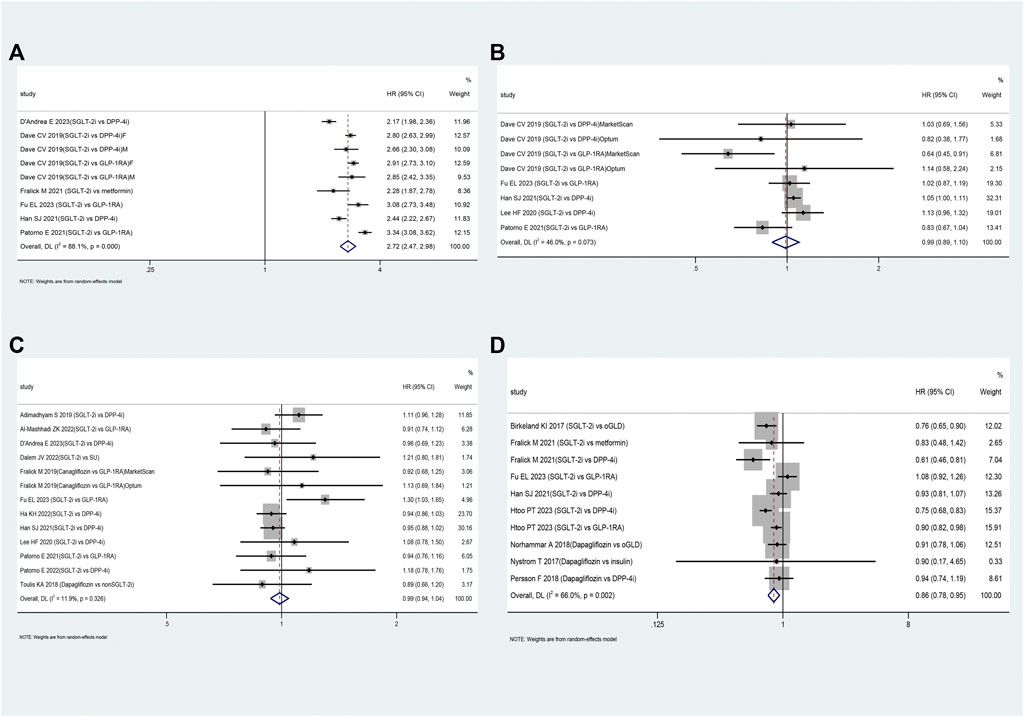
FIGURE 3. The forest plot of secondary outcomes. Legend: (A): genital tract infections; (B): urinary tract infections; (C): bone fractures; (D): hypoglycemia. SGLT-2i = sodium-glucose cotransporter 2 inhibitors, GLP-1RA = GLP-1 receptor agonist, DPP-4i = dipeptidyl peptidase 4 inhibitors, SU = sulfonylureas, oGLD = other glucose-lowering drugs, F=female, M=male.
Subgroup analysis revealed that SGLT-2i increased the risk of UTI by approximately 6% compared to DPP-4i (HR: 1.06, 95% CI: 1.01–1.11, p < 0.01; Supplementary Appendix S5.7). Compared to DPP-4i and GLP-1RA, SGLT-2i showed a 2.50-fold and 3.06-fold increased risk of GTI, respectively (Supplementary Appendix S5.8). Furthermore, the risk of GTI associated with SGLT-2i was consistent between patients of different sex (Supplementary Appendix S5.9). However, SGLT-2i was not associated with an increased risk of bone fractures compared to DPP-4i or GLP-1RA (Supplementary Appendix S5.10).
3.5 Meta-regression
Meta-regression indicated that none of the above independent variables was identified as the source of heterogeneity (Supplementary Appendix S6). For the subgroup analysis of DKA and LLA, we conducted separate evaluations based on different study regions (Supplementary Appendix S9). In studies in northern Europe, SGLT-2i did not show a significant increase in the risk of DKA (HR: 1.56, 95% CI: 0.76–3.20, p = 0.22). However, studies in the US showed a slightly elevated risk of DKA associated with SGLT-2i use (HR: 1.16, 95% CI: 1.02–1.31, p = 0.022). Similarly, studies conducted in Canada revealed a significantly higher risk of DKA with SGLT-2i use (HR: 2.30, 95% CI: 1.50–3.53, p < 0.01). Regarding LLA risk, SGLT-2i did not demonstrate a significant increase in the US (HR: 1.12, 95% CI: 0.92–1.35, p = 0.26) or in northern Europe (HR: 1.03, 95% CI: 0.91–1.16, p = 0.63).
3.6 Sensitivity analysis
Sensitivity analysis demonstrated that the combined effect values remained consistent before and after excluding any study for the above outcomes (Supplementary Appendix S7). This consistency suggests that the study results were stable.
3.7 Publication bias analysis
Confunnel plots for DKA, genital infections, and hypoglycemia showed that missing studies were in areas of statistical significance, which suggests that asymmetry is more likely to be due to factors other than publication bias. Confunnel plots for LLA revealed that absent studies were distributed across regions of statistical significance, as well as those yielding inconclusive results, suggesting that the detected asymmetry may be attributable to publication bias and other confounding factors. (Supplementary Appendix S8). Regarding Egger’s test, the p values for UTI, bone fracture, GTI, and hypoglycemia were 0.27, 0.14, 0.39, and 0.93, respectively, suggesting no significant publication bias, except for LLA (p = 0.045) and DKA (p = 0.01).
4 Discussion
A total of 9,911,454 patients with T2DM were included in this real-world data study. When assessing SGLT-2i as a class and as individual agents, DPP-4i and GLP-1RA were the two most used active comparators.
The mean or median incidence rates per 1,000 person-years for various adverse events in the SGLT-2i group were the following: 2.86 for DKA, 2.30 for LLA, 9.58 for UTI, 15.64 for GTI, 5.18 for bone fractures, and 9.42 for hypoglycemia. Compared to the control group, the mean incidence rates of DKA and GTI were significantly higher in the SGLT-2i group. In contrast, the rates of LLA, UTI, bone fractures, and hypoglycemia were similar to those of the control group. In a meta-analysis of five RCTs, the mean spontaneous rates of DKA, GTI, and amputations were 0.3, 4, and 4 per 1,000 person-years, respectively (Marilly et al., 2022). Furthermore, a matched incidence rate ranging from 33.58 to 35.66 per 1,000 person-years for GTI was reported in five comparative cohorts (Alkabbani et al., 2022). The absolute rate of DKA ranged from 0.6 to 2.2 per 1,000 person-years in RCTs and ranged from 0.6 to 4.9 per 1,000 person-years in observational studies (Colacci et al., 2022). Interestingly, our real-world study indicated that adverse events such as DKA and GTI were slightly higher than observed in RCTs.
The SGLT-2i class was associated with an increased risk of DKA by approximately 21% compared to oGLD (Colacci et al., 2022). However, compared to GLP-1RA, the SGLT-2i class did not show an increased risk of DKA. On the other hand, compared to DPP-4i, the SGLT-2i class increased the risk of DKA. The subgroup analysis also revealed that specific SGLT-2 inhibitors, namely canagliflozin, empagliflozin, and dapagliflozin, could increase the risk of DKA compared to DPP-4i. Interestingly, SGLT-2i increased the risk of DKA 2.3 times in Canadians but not in patients from Nordic countries. The reason for this disparity can be attributed to the fact that the two studies conducted in Canada (Douros et al., 2020; Fralick et al., 2021b) compared SGLT-2i with DPP-4i, while the two studies in Nordic countries compared SGLT-2i with both DPP-4i and GLP-1RA. Our findings are consistent with previous studies (Colacci et al., 2022; Marilly et al., 2022).
Studies have indicated a higher risk of DKA in older patients and those with a history of insulin use or poor glycemic control (Fralick et al., 2021a). However, a subgroup analysis of these factors was not feasible due to the limited data provided by the included studies on baseline insulin use, fasting glucose levels, postprandial glucose levels, and HbA1c values.
The CANVAS trial reported that the use of canagliflozin increased the risk of LLA nearly twice (HR 1.97; 95% CI 1.41–2.75) compared to placebo, raising concerns about the possible risk of LLA with SGLT-2i (Neal et al., 2017). We did not find an increased risk of LLA with the SGLT-2i class compared to oGLD, DPP-4i, or sulfonylureas. However, we observed that patients taking canagliflozin had a 19% higher LLA risk than those taking oGLD. Unfortunately, due to limited data, we could not analyze the association between dapagliflozin or empagliflozin and the risk of LLA.
A previous study revealed that preexisting PVD was the main factor influencing the risk of amputation (Paul et al., 2021). Although the studies included in our analysis did not support grouping based on the presence or absence of previous PVD, we performed a subgroup analysis categorizing baseline PVD prevalence into two groups: PVD incidence <10% and PVD incidence >10%. The results of the subgroup analysis indicated that SGLT-2i decreased the risk of LLA by 13% among patients with a preexisting PVD incidence <10%, while it increased the risk of LLA by 21% among patients with a PVD incidence >10%. However, although these differences were not statistically significant, the findings were consistent with the analysis results conducted on the entire study group. Furthermore, we also examined patients with and without cardiovascular disease at baseline as subgroups. The analysis suggested that SGLT-2i increased the risk of LLA in patients with cardiovascular disease at baseline. However, these subgroup analysis results should be interpreted cautiously as they were based on fewer studies (3–4) included. More research and verification are required to confirm these findings.
Studies have indicated that preexisting LLA can increase the risk of future LLA (Arnott et al., 2020). In the studies we included, researchers matched the baseline incidence of LLA between the groups using PSM or directly excluded patients with preexisting LLA. This approach helps minimize the influence of this confounding factor on our results. However, it is crucial to acknowledge that publication bias could potentially affect estimates of the association between the use of SGLT2i and the risk of LLA.
This study showed that SGLT-2i did not increase the risk of UTI or bone fractures. Several meta-analyses of RCTs have consistently shown that the risk of UTI did not increase with SGLT-2i compared to the placebo or active comparator groups (Storgaard et al., 2016; Wu et al., 2016; Puckrin et al., 2018). However, a subgroup analysis revealed a 6% increased risk of UTI when SGLT-2i was compared to DPP-4i. It is essential to interpret this result with caution, as it was mainly influenced by a study of a Korean study conducted in 2021 (Han et al., 2021). This study included older patients over 65 years of age, with a significant proportion of women (57.5%). T2DM itself is a known risk factor for UTI, regardless of the treatment regimen (Nitzan et al., 2015), and older women are particularly susceptible to UTI (Nicolle et al., 2014; Fioretto et al., 2016). Additionally, the study did not match the baseline incidence of UTI. Therefore, we must consider these specific factors and potential confounders when interpreting the association between SGLT-2i use and the risk of UTI in our study.
Our study revealed that SGLT-2i did not increase the risk of bone fractures, even in patients with T2DM over 65 years of age. These findings are consistent with the results of a previous study (Wu et al., 2016). However, concerning GTI, SGLT-2i use was associated with a three-fold increased risk, regardless of sex. This result remained consistent across different subgroup analyses based on various active comparators. Several meta-analyses have also reported an increased risk of GTI with SGLT-2i (Jabbour et al., 2018; Zhang et al., 2018). Fortunately, GTI is considered an adverse event of lesser severity than other safety outcomes and can be mitigated by educating patients to increase their water intake during medication. Furthermore, the hypoglycemic effect of SGLT-2i is independent of insulin, resulting in a 14% lower risk of hypoglycemia than other medications.
5 Strength and limitation of the study
Our investigation has a large cohort of 9,911,454 patients with T2DM and included data from 40 cohort studies conducted in various countries. Rigorous inclusion and exclusion criteria were used to ensure the robustness of the study. The PSM matched the baseline information of included studies and some excluded patients with preexisting LLA, further reducing potential confounding variables and increasing the reliability of our results. To address the study heterogeneity, we used meta-regression and subgroup analysis. Fortunately, our sensitivity analyses did not show significant differences compared to our primary analyses, reinforcing the reliability of our findings. We also assessed potential publication bias using Egger’s test, which indicated no evidence of bias except for LLA and DKA.
However, our study has the following limitations. First, although all included studies had comparable demographic characteristics between treatment groups through PSM, there may still be residual confounding from some unmeasured or not fully measured factors (e.g., HbA1c level, diabetes duration, prior insulin use) that cannot be completely ruled out. Second, certain pooled studies showed high heterogeneity. Third, the discussion of the safety of SGLT-2i as an individual agent was limited due to the availability of limited data. Furthermore, the subgroup analysis included only a few studies included, and inconsistencies in the results of some subgroup analyses must be verified by more high-quality studies with larger sample sizes.
6 Conclusion
In a comprehensive population-based cohort study comprising 9,911,454 patients with T2DM, the use of SGLT-2i compared to oGLD showed a similar incidence of LLA, UTI, and bone fractures. However, SGLT-2i was associated with a higher risk of DKA and GTI than oGLD. The subgroup analysis indicated that the use of SGLT-2i was associated with an increased risk of LLA among patients with a history of CVD. Specifically, canagliflozin, empagliflozin, and dapagliflozin increased the risk of DKA compared to DPP-4i. Canagliflozin was associated with an elevated risk of LLA.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author’s contributions
CL: Supervision, Writing–review and editing, Data curation, Formal Analysis, Investigation, Methodology, Software, Writing–original draft. TL: Data curation, Investigation, Writing–original draft, Writing–review and editing. QZ: Data curation, Investigation, Writing–review and editing. QX: Data curation, Investigation, Writing–review and editing. XG: Investigation, Writing–review and editing. CM: Investigation, Writing–review and editing. JL: Investigation, Writing–review and editing. XM: Investigation, Writing–review and editing. YQ: Writing–review and editing. HL: Writing–review and editing, Conceptualization, Supervision, Validation.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1275060/full#supplementary-material
References
Adimadhyam, S., Lee, T. A., Calip, G. S., Smith Marsh, D. E., Layden, B. T., and Schumock, G. T. (2019). Sodium-glucose co-transporter 2 inhibitors and the risk of fractures: A propensity score-matched cohort study. Pharmacoepidemiol Drug Saf. 28 (12), 1629–1639. doi:10.1002/pds.4900
Al-Mashhadi, Z. K., Viggers, R., Starup-Linde, J., Vestergaard, P., and Gregersen, S. (2022). SGLT2 inhibitor treatment is not associated with an increased risk of osteoporotic fractures when compared to GLP-1 receptor agonists: A nationwide cohort study. Front. Endocrinol. (Lausanne) 13, 861422. doi:10.3389/fendo.2022.861422
Alkabbani, W., Zongo, A., Minhas-Sandhu, J. K., Eurich, D. T., Shah, B. R., Alsabbagh, M. W., et al. (2022). Five comparative cohorts to assess the risk of genital tract infections associated with sodium-glucose cotransporter-2 inhibitors initiation in type 2 diabetes mellitus. Diabet. Med. 39 (8), e14858. doi:10.1111/dme.14858
Andreadi, A., Muscoli, S., Tajmir, R., Meloni, M., Muscoli, C., Ilari, S., et al. (2023). Recent Pharmacological Options in Type 2 Diabetes and Synergic Mechanism in Cardiovascular Disease. Int. J. Mol. Sci. 24 (2), 1646. doi:10.3390/ijms24021646
Arnott, C., Huang, Y., Neuen, B. L., Di Tanna, G. L., Cannon, C. P., Oh, R., et al. (2020). The effect of canagliflozin on amputation risk in the CANVAS program and the CREDENCE trial. Diabetes Obes. Metab. 22 (10), 1753–1766. doi:10.1111/dom.14091
Bhosle, D., Indurkar, S., Quadri, U., and Chandekar, B. (2022). A Comparative Study of efficacy and safety of different Sodium Glucose Co-transporter 2 (SGLT-2) Inhibitors in the Management of Patients with Type II Diabetes Mellitus. J. Assoc. Physicians India 70 (6), 11–12. doi:10.5005/japi-11001-0001
Birkeland, K. I., Jørgensen, M. E., Carstensen, B., Persson, F., Gulseth, H. L., Thuresson, M., et al. (2017). Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium-glucose co-transporter-2 inhibitors versus other glucose-lowering drugs (CVD-REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol. 5 (9), 709–717. doi:10.1016/s2213-8587(17)30258-9
Caparrotta, T. M., Greenhalgh, A. M., Osinski, K., Gifford, R. M., Moser, S., Wild, S. H., et al. (2021). Sodium-Glucose Co-Transporter 2 Inhibitors (SGLT2i) Exposure and Outcomes in Type 2 Diabetes: A Systematic Review of Population-Based Observational Studies. Diabetes Ther. Res. Treat. Educ. diabetes Relat. Disord. 12 (4), 991–1028. doi:10.1007/s13300-021-01004-2
Chang, H. Y., Singh, S., Mansour, O., Baksh, S., and Alexander, G. C. (2018). Association Between Sodium-Glucose Cotransporter 2 Inhibitors and Lower Extremity Amputation Among Patients With Type 2 Diabetes. JAMA Intern Med. 178 (9), 1190–1198. doi:10.1001/jamainternmed.2018.3034
Colacci, M., Fralick, J., Odutayo, A., and Fralick, M. (2022). Sodium-Glucose Cotransporter-2 Inhibitors and Risk of Diabetic Ketoacidosis Among Adults With Type 2 Diabetes: A Systematic Review and Meta-Analysis. Can. J. Diabetes 46 (1), 10–15.e2. e2. Epub 2021/06/13. doi:10.1016/j.jcjd.2021.04.006
D'Andrea, E., Wexler, D. J., Kim, S. C., Paik, J. M., Alt, E., and Patorno, E. (2023). Comparing Effectiveness and Safety of SGLT2 Inhibitors vs DPP-4 Inhibitors in Patients With Type 2 Diabetes and Varying Baseline HbA1c Levels. JAMA Intern Med. 183 (3), 242–254. doi:10.1001/jamainternmed.2022.6664
Dave, C. V., Schneeweiss, S., Kim, D., Fralick, M., Tong, A., and Patorno, E. (2019b). Sodium-Glucose Cotransporter-2 Inhibitors and the Risk for Severe Urinary Tract Infections: A Population-Based Cohort Study. Ann. Intern Med. 171 (4), 248–256. doi:10.7326/m18-3136
Dave, C. V., Schneeweiss, S., and Patorno, E. (2019a). Comparative risk of genital infections associated with sodium-glucose co-transporter-2 inhibitors. Diabetes, Obes. Metabolism 21 (2), 434–438. doi:10.1111/dom.13531
Dawwas, G. K., Flory, J. H., Hennessy, S., Leonard, C. E., and Lewis, J. D. (2022). Comparative Safety of Sodium-Glucose Cotransporter 2 Inhibitors Versus Dipeptidyl Peptidase 4 Inhibitors and Sulfonylureas on the Risk of Diabetic Ketoacidosis. Diabetes Care 45 (4), 919–927. doi:10.2337/dc21-2177
Dawwas, G. K., Smith, S. M., and Park, H. (2019). Cardiovascular outcomes of sodium glucose cotransporter-2 inhibitors in patients with type 2 diabetes. Diabetes, Obes. Metabolism 21 (1), 28–36. doi:10.1111/dom.13477
Donnan, J. R., Grandy, C. A., Chibrikov, E., Marra, C. A., Aubrey-Bassler, K., Johnston, K., et al. (2019). Comparative safety of the sodium glucose co-transporter 2 (SGLT2) inhibitors: a systematic review and meta-analysis. BMJ Open 9 (1), e022577. doi:10.1136/bmjopen-2018-022577
Dorsey-Treviño, E. G., González-González, J. G., Alvarez-Villalobos, N., González-Nava, V., Contreras-Garza, B. M., Díaz González-Colmenero, A., et al. (2020). Sodium-glucose cotransporter 2 (SGLT-2) inhibitors and microvascular outcomes in patients with type 2 diabetes: systematic review and meta-analysis. J. Endocrinol. investigation 43 (3), 289–304. doi:10.1007/s40618-019-01103-9
Douros, A., Lix, L. M., Fralick, M., Dell'Aniello, S., Shah, B. R., Ronksley, P. E., et al. (2020). Sodium-Glucose Cotransporter-2 Inhibitors and the Risk for Diabetic Ketoacidosis: A Multicenter Cohort Study. Ann. Intern Med. 173 (6), 417–425. doi:10.7326/m20-0289
Fioretto, P., Mansfield, T. A., Ptaszynska, A., Yavin, Y., Johnsson, E., and Parikh, S. (2016). Long-Term Safety of Dapagliflozin in Older Patients with Type 2 Diabetes Mellitus: A Pooled Analysis of Phase IIb/III Studies. Drugs Aging 33 (7), 511–522. doi:10.1007/s40266-016-0382-1
Fralick, M., Colacci, M., Thiruchelvam, D., Gomes, T., and Redelmeier, D. A. (2021a). Sodium-glucose co-transporter-2 inhibitors versus dipeptidyl peptidase-4 inhibitors and the risk of heart failure: A nationwide cohort study of older adults with diabetes mellitus. Diabetes, Obes. Metabolism 23 (4), 950–960. doi:10.1111/dom.14300
Fralick, M., Kim, S. C., Schneeweiss, S., Everett, B. M., Glynn, R. J., and Patorno, E. (2020). Risk of amputation with canagliflozin across categories of age and cardiovascular risk in three US nationwide databases: cohort study. Bmj 370, m2812. doi:10.1136/bmj.m2812
Fralick, M., Kim, S. C., Schneeweiss, S., Kim, D., Redelmeier, D. A., and Patorno, E. (2019). Fracture Risk After Initiation of Use of Canagliflozin: A Cohort Study. Ann. Intern Med. 170 (3), 155–163. doi:10.7326/m18-0567
Fralick, M., Schneeweiss, S., Redelmeier, D. A., Razak, F., Gomes, T., and Patorno, E. (2021b). Comparative effectiveness and safety of sodium-glucose cotransporter-2 inhibitors versus metformin in patients with type 2 diabetes: An observational study using data from routine care. Diabetes Obes. Metab. 23 (10), 2320–2328. doi:10.1111/dom.14474
Fu, E. L., D'Andrea, E., Wexler, D. J., Patorno, E., and Paik, J. M. (2023). Safety of Sodium-Glucose Cotransporter-2 Inhibitors in Patients with CKD and Type 2 Diabetes: Population-Based US Cohort Study. Clin. J. Am. Soc. Nephrol. 18, 592–601. doi:10.2215/CJN.0000000000000115
Ha, K. H., Kim, D. J., and Choi, Y. J. (2022). Sodium-glucose cotransporter 2 inhibitors do not increase the risk of fractures in real-world clinical practice in Korea: A national observational cohort study. J. Diabetes Investig. 13 (6), 986–996. doi:10.1111/jdi.13768
Han, S. J., Ha, K. H., Lee, N., and Kim, D. J. (2021). Effectiveness and safety of sodium-glucose co-transporter-2 inhibitors compared with dipeptidyl peptidase-4 inhibitors in older adults with type 2 diabetes: A nationwide population-based study. Diabetes, Obes. Metabolism 23 (3), 682–691. doi:10.1111/dom.14261
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. Bmj 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Htoo, P. T., Paik, J. M., Alt, E., Kim, D. H., Wexler, D. J., Kim, S. C., et al. (2023). Risk of severe hypoglycemia with newer second-line glucose-lowering medications in older adults with type 2 diabetes stratified by known indicators of hypoglycemia risk. J. Gerontol. A Biol. Sci. Med. Sci., glad075. doi:10.1093/gerona/glad075
Hu, M., Cai, X., Yang, W., Zhang, S., Nie, L., and Ji, L. (2020). Effect of Hemoglobin A1c Reduction or Weight Reduction on Blood Pressure in Glucagon-Like Peptide-1 Receptor Agonist and Sodium-Glucose Cotransporter-2 Inhibitor Treatment in Type 2 Diabetes Mellitus: A Meta-Analysis. J. Am. Heart Assoc. 9 (7), e015323. doi:10.1161/jaha.119.015323
Jabbour, S., Seufert, J., Scheen, A., Bailey, C. J., Karup, C., and Langkilde, A. M. (2018). Dapagliflozin in patients with type 2 diabetes mellitus: A pooled analysis of safety data from phase IIb/III clinical trials. Diabetes Obes. Metab. 20 (3), 620–628. doi:10.1111/dom.13124
Kim, Y. G., Jeon, J. Y., Han, S. J., Kim, D. J., Lee, K. W., and Kim, H. J. (2018). Sodium-glucose co-transporter-2 inhibitors and the risk of ketoacidosis in patients with type 2 diabetes mellitus: A nationwide population-based cohort study. Diabetes, Obes. Metabolism 20 (8), 1852–1858. doi:10.1111/dom.13297
Kumar, K., Kheiri, B., Simpson, T. F., Osman, M., and Rahmouni, H. (2020). Sodium-Glucose Cotransporter-2 Inhibitors in Heart Failure: A Meta-Analysis of Randomized Clinical Trials. Am. J. Med. 133 (11), e625–e630. doi:10.1016/j.amjmed.2020.04.006
Kumpf, U., Palm, U., Eder, J., Ezim, H., Stadler, M., Burkhardt, G., et al. (2023). TDCS at home for depressive disorders: an updated systematic review and lessons learned from a prematurely terminated randomized controlled pilot study. Eur. Arch. Psychiatry Clin. Neurosci. 273, 1403–1420. doi:10.1007/s00406-023-01620-y
Laursen, H. V. B., Røikjer, J. B., Dal, J., and Jensen, M. H. (2021). Sodium Glucose Cotransporter-2 Inhibitor Treatment and the Risk of Diabetic Ketoacidosis in Denmark: A Retrospective Cohort Study of Five Years of Use. Curr. Drug Saf. 16 (1), 73–81. doi:10.2174/1574886315666200819114629
Lee, H. F., Chen, S. W., Liu, J. R., Li, P. R., Wu, L. S., Chang, S. H., et al. (2020). Major adverse cardiovascular and limb events in patients with diabetes and concomitant peripheral artery disease treated with sodium glucose cotransporter 2 inhibitor versus dipeptidyl peptidase-4 inhibitor. Cardiovasc Diabetol. 19 (1), 160. Epub 2020/10/02. doi:10.1186/s12933-020-01118-0
Li, C. X., Liang, S., Gao, L., and Liu, H. (2021). Cardiovascular outcomes associated with SGLT-2 inhibitors versus other glucose-lowering drugs in patients with type 2 diabetes: A real-world systematic review and meta-analysis. PLoS ONE 16 (2), e0244689. doi:10.1371/journal.pone.0244689
Liu, J., Li, L., Li, S., Wang, Y., Qin, X., Deng, K., et al. (2020). Sodium-glucose co-transporter-2 inhibitors and the risk of diabetic ketoacidosis in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 22 (9), 1619–1627. doi:10.1111/dom.14075
Mantovani, A., Grani, G., Chioma, L., Vancieri, G., Giordani, I., Rendina, R., et al. (2016). Severe hypoglycemia in patients with known diabetes requiring emergency department care: A report from an Italian multicenter study. J. Clin. Transl. Endocrinol. 5, 46–52. doi:10.1016/j.jcte.2016.08.004
Marilly, E., Cottin, J., Cabrera, N., Cornu, C., Boussageon, R., Moulin, P., et al. (2022). SGLT2 inhibitors in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials balancing their risks and benefits. Diabetologia 65 (12), 2000–2010. doi:10.1007/s00125-022-05773-8
Monami, M., Nreu, B., Zannoni, S., Lualdi, C., and Mannucci, E. (2017). Effects of SGLT-2 inhibitors on diabetic ketoacidosis: A meta-analysis of randomised controlled trials. Diabetes Res. Clin. Pract. 130, 53–60. doi:10.1016/j.diabres.2017.04.017
Neal, B., Perkovic, V., Mahaffey, K. W., de Zeeuw, D., Fulcher, G., Erondu, N., et al. (2017). Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 377 (7), 644–657. doi:10.1056/NEJMoa1611925
Nicolle, L. E., Capuano, G., Fung, A., and Usiskin, K. (2014). Urinary tract infection in randomized phase III studies of canagliflozin, a sodium glucose co-transporter 2 inhibitor. Postgrad. Med. 126 (1), 7–17. doi:10.3810/pgm.2014.01.2720
Nitzan, O., Elias, M., Chazan, B., and Saliba, W. (2015). Urinary tract infections in patients with type 2 diabetes mellitus: review of prevalence, diagnosis, and management. Diabetes, metabolic syndrome Obes. targets Ther. 8, 129–136. doi:10.2147/dmso.s51792
Norhammar, A., Bodegård, J., Nyström, T., Thuresson, M., Nathanson, D., and Eriksson, J. W. (2019). Dapagliflozin and cardiovascular mortality and disease outcomes in a population with type 2 diabetes similar to that of the DECLARE-TIMI 58 trial: A nationwide observational study. Diabetes Obes. Metab. 21 (5), 1136–1145. doi:10.1111/dom.13627
Nyström, T., Bodegard, J., Nathanson, D., Thuresson, M., Norhammar, A., and Eriksson, J. W. (2017). Novel oral glucose-lowering drugs are associated with lower risk of all-cause mortality, cardiovascular events and severe hypoglycaemia compared with insulin in patients with type 2 diabetes. Diabetes Obes. Metab. 19 (6), 831–841. doi:10.1111/dom.12889
Pasternak, B., Ueda, P., Eliasson, B., Svensson, A. M., Franzén, S., Gudbjörnsdottir, S., et al. (2019). Use of sodium glucose cotransporter 2 inhibitors and risk of major cardiovascular events and heart failure: Scandinavian register based cohort study. Bmj 366, l4772. doi:10.1136/bmj.l4772
Patorno, E., Pawar, A., Bessette, L. G., Kim, D. H., Dave, C., Glynn, R. J., et al. (2021). Comparative Effectiveness and Safety of Sodium-Glucose Cotransporter 2 Inhibitors Versus Glucagon-Like Peptide 1 Receptor Agonists in Older Adults. Diabetes Care 44 (3), 826–835. doi:10.2337/dc20-1464
Patorno, E., Pawar, A., Wexler, D. J., Glynn, R. J., Bessette, L. G., Paik, J. M., et al. (2022). Effectiveness and safety of empagliflozin in routine care patients: Results from the EMPagliflozin compaRative effectIveness and SafEty (EMPRISE) study. Diabetes Obes. Metab. 24 (3), 442–454. doi:10.1111/dom.14593
Paul, S. K., Bhatt, D. L., and Montvida, O. (2021). The association of amputations and peripheral artery disease in patients with type 2 diabetes mellitus receiving sodium-glucose cotransporter type-2 inhibitors: real-world study. Eur. Heart J. 42 (18), 1728–1738. doi:10.1093/eurheartj/ehaa956
Persson, F., Nyström, T., Jørgensen, M. E., Carstensen, B., Gulseth, H. L., Thuresson, M., et al. (2018). Dapagliflozin is associated with lower risk of cardiovascular events and all-cause mortality in people with type 2 diabetes (CVD-REAL Nordic) when compared with dipeptidyl peptidase-4 inhibitor therapy: A multinational observational study. Diabetes Obes. Metab. 20 (2), 344–351. doi:10.1111/dom.13077
Peters, J. L., Sutton, A. J., Jones, D. R., Abrams, K. R., and Rushton, L. (2008). Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J. Clin. Epidemiol. 61 (10), 991–996. doi:10.1016/j.jclinepi.2007.11.010
Puckrin, R., Saltiel, M. P., Reynier, P., Azoulay, L., Yu, O. H. Y., and Filion, K. B. (2018). SGLT-2 inhibitors and the risk of infections: a systematic review and meta-analysis of randomized controlled trials. Acta Diabetol. 55 (5), 503–514. doi:10.1007/s00592-018-1116-0
Rådholm, K., Wu, J. H., Wong, M. G., Foote, C., Fulcher, G., Mahaffey, K. W., et al. (2018). Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular disease, death and safety outcomes in type 2 diabetes - A systematic review. Diabetes Res. Clin. Pract. 140, 118–128. doi:10.1016/j.diabres.2018.03.027
Ryan, P. B., Buse, J. B., Schuemie, M. J., DeFalco, F., Yuan, Z., Stang, P. E., et al. (2018). Comparative effectiveness of canagliflozin, SGLT2 inhibitors and non-SGLT2 inhibitors on the risk of hospitalization for heart failure and amputation in patients with type 2 diabetes mellitus: A real-world meta-analysis of 4 observational databases (OBSERVE-4D). Diabetes Obes. Metab. 20 (11), 2585–2597. doi:10.1111/dom.13424
Salah, H. M., Al'Aref, S. J., Khan, M. S., Al-Hawwas, M., Vallurupalli, S., Mehta, J. L., et al. (2021). Effect of sodium-glucose cotransporter 2 inhibitors on cardiovascular and kidney outcomes-Systematic review and meta-analysis of randomized placebo-controlled trials. Am. Heart J. 232, 10–22. doi:10.1016/j.ahj.2020.10.064
Storgaard, H., Gluud, L. L., Bennett, C., Grøndahl, M. F., Christensen, M. B., Knop, F. K., et al. (2016). Benefits and Harms of Sodium-Glucose Co-Transporter 2 Inhibitors in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. PLoS One 11 (11), e0166125. Epub 2016/11/12. doi:10.1371/journal.pone.0166125
Tang, H., Cui, W., Li, D., Wang, T., Zhang, J., Zhai, S., et al. (2017). Sodium-glucose co-transporter 2 inhibitors in addition to insulin therapy for management of type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 19 (1), 142–147. doi:10.1111/dom.12785
Tang, H., Li, D., Wang, T., Zhai, S., and Song, Y. (2016). Effect of Sodium-Glucose Cotransporter 2 Inhibitors on Diabetic Ketoacidosis Among Patients With Type 2 Diabetes: A Meta-analysis of Randomized Controlled Trials. Diabetes Care 39 (8), e123–e124. doi:10.2337/dc16-0885
Toulis, K. A., Bilezikian, J. P., Thomas, G. N., Hanif, W., Kotsa, K., Thayakaran, R., et al. (2018). Initiation of dapagliflozin and treatment-emergent fractures. Diabetes, Obes. Metabolism 20 (4), 1070–1074. doi:10.1111/dom.13176
Udell, J. A., Yuan, Z., Rush, T., Sicignano, N. M., Galitz, M., and Rosenthal, N. (2018). Cardiovascular Outcomes and Risks After Initiation of a Sodium Glucose Cotransporter 2 Inhibitor: Results From the EASEL Population-Based Cohort Study (Evidence for Cardiovascular Outcomes With Sodium Glucose Cotransporter 2 Inhibitors in the Real World). Circulation 137 (14), 1450–1459. doi:10.1161/circulationaha.117.031227
Udell, J. A., Yuan, Z., Ryan, P., Rush, T., Sicignano, N. M., Galitz, M., et al. (2020). Cardiovascular outcomes and mortality after initiation of canagliflozin: Analyses from the EASEL Study. Endocrinol. Diabetes Metab. 3 (1), e00096. Epub 2020/01/11. doi:10.1002/edm2.96
Ueda, P., Svanström, H., Melbye, M., Eliasson, B., Svensson, A. M., Franzén, S., et al. (2018). Sodium glucose cotransporter 2 inhibitors and risk of serious adverse events: Nationwide register based cohort study. BMJ (Online) 363, k4365. doi:10.1136/bmj.k4365
van Dalem, J., Werkman, N. C. C., van den Bergh, J. P., Rossi, B., Viggers, R., Eastell, R., et al. (2022). Use of sodium-glucose co-transporter 2 inhibitors, changes in body mass index and risk of fracture: A population-based cohort study. Diabetes Res. Clin. Pract. 190, 109993. doi:10.1016/j.diabres.2022.109993
Wang, L., Voss, E. A., Weaver, J., Hester, L., Yuan, Z., DeFalco, F., et al. (2019). Diabetic ketoacidosis in patients with type 2 diabetes treated with sodium glucose co-transporter 2 inhibitors versus other antihyperglycemic agents: An observational study of four US administrative claims databases. Pharmacoepidemiol. Drug Saf. 28 (12), 1620–1628. doi:10.1002/pds.4887
Wang, Y., Desai, M., Ryan, P. B., DeFalco, F. J., Schuemie, M. J., Stang, P. E., et al. (2017). Incidence of diabetic ketoacidosis among patients with type 2 diabetes mellitus treated with SGLT2 inhibitors and other antihyperglycemic agents. Diabetes Res. Clin. Pract. 128, 83–90. doi:10.1016/j.diabres.2017.04.004
Werkman, N. C. C., Nielen, J. T. H., van den Bergh Jpw, , Ejskjaer, N., Røikjer, J., Schaper, N. C., et al. (2021). Use of Sodium-Glucose Co-Transporter-2-Inhibitors (SGLT2-Is) and Risk of Lower Limb Amputation. Curr. Drug Saf. 16 (1), 62–72. doi:10.2174/1574886315666200805103053
Wu, J. H., Foote, C., Blomster, J., Toyama, T., Perkovic, V., Sundström, J., et al. (2016). Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 4 (5), 411–419. doi:10.1016/s2213-8587(16)00052-8
Yang, J. Y., Wang, T., Pate, V., Gower, E. W., Crowley, M. J., Buse, J. B., et al. (2019). Sodium-glucose co-transporter-2 inhibitor use and risk of lower-extremity amputation: Evolving questions, evolving answers. Diabetes Obes. Metab. 21 (5), 1223–1236. doi:10.1111/dom.13647
Yu, O. H. Y., Dell'Aniello, S., Shah, B. R., Brunetti, V. C., Daigle, J. M., Fralick, M., et al. (2020). Sodium-Glucose Cotransporter 2 Inhibitors and the Risk of Below-Knee Amputation: A Multicenter Observational Study. Diabetes Care 43 (10), 2444–2452. doi:10.2337/dc20-0267
Yu, X., Zhang, S., and Zhang, L. (2018). Newer perspectives of mechanisms for euglycemic diabetic ketoacidosis. Int. J. Endocrinol. 2018, 7074868. doi:10.1155/2018/7074868
Yuan, Z., DeFalco, F. J., Ryan, P. B., Schuemie, M. J., Stang, P. E., Berlin, J. A., et al. (2018). Risk of lower extremity amputations in people with type 2 diabetes mellitus treated with sodium-glucose co-transporter-2 inhibitors in the USA: A retrospective cohort study. Diabetes Obes. Metab. 20 (3), 582–589. doi:10.1111/dom.13115
Zaccardi, F., Webb, D. R., Htike, Z. Z., Youssef, D., Khunti, K., and Davies, M. J. (2016). Efficacy and safety of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes mellitus: systematic review and network meta-analysis. Diabetes Obes. Metab. 18 (8), 783–794. doi:10.1111/dom.12670
Zerovnik, S., Kos, M., and Locatelli, I. (2022). Risk of lower extremity amputations in patients with type 2 diabetes using sodium-glucose co-transporter 2 inhibitors. Acta Diabetol. 59 (2), 233–241. doi:10.1007/s00592-021-01805-8
Zhang, L., Zhang, M., Lv, Q., and Tong, N. (2018a). Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in patients with type 2 diabetes and moderate renal function impairment: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 140, 295–303. doi:10.1016/j.diabres.2018.03.047
Zhang, X. L., Zhu, Q. Q., Chen, Y. H., Li, X. L., Chen, F., Huang, J. A., et al. (2018). Cardiovascular Safety, Long-Term Noncardiovascular Safety, and Efficacy of Sodium-Glucose Cotransporter 2 Inhibitors in Patients With Type 2 Diabetes Mellitus: A Systemic Review and Meta-Analysis With Trial Sequential Analysis. J. Am. Heart Assoc. 7 (2), e007165. doi:10.1161/jaha.117.007165
Keywords: sodium-glucose transporter 2 inhibitors, safety, diabetic ketoacidosis, lower limb amputation, urinary tract infections, genital tract infections, meta-analysis
Citation: Li CX, Liu TT, Zhang Q, Xie Q, Geng XH, Man CX, Li JY, Mao XY, Qiao Y and Liu H (2023) Safety of sodium-glucose transporter 2 (SGLT-2) inhibitors in patients with type 2 diabetes: a meta-analysis of cohort studies. Front. Pharmacol. 14:1275060. doi: 10.3389/fphar.2023.1275060
Received: 09 August 2023; Accepted: 21 September 2023;
Published: 13 October 2023.
Edited by:
Trond Geir Jenssen, Oslo University Hospital, NorwayReviewed by:
Aiko P. J. De Vries, Leiden University Medical Center (LUMC), NetherlandsAikaterini Andreadi, University of Rome Tor Vergata, Italy
Copyright © 2023 Li, Liu, Zhang, Xie, Geng, Man, Li, Mao, Qiao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua Liu, lcyx721@126.com
†These authors have contributed equally to this work and share first authorship
 Chun Xing Li
Chun Xing Li Tian Tian Liu1†
Tian Tian Liu1† Hua Liu
Hua Liu