- Department of Integrated Traditional Chinese and Western Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Rhabdomyolysis syndrome refers to the breakdown and necrosis of muscle tissue due to various reasons and caused by the release of intracellular contents into the blood stream, which can lead to acute renal failure or even death. In this article, we describe for the first time a case report of severe rhabdomyolysis induced by etoposide-nedaplatin chemotherapy in a small cell lung cancer (SCLC IIIb) patient. The patient developed progressive general muscle pain and weakness after the first cycle of chemotherapy, accompanied by elevated creatine kinase (CK), myoglobin (Mb), alanine aminotransferase (ALT), spartate aminotransferase (AST), and lactate dehydrogenase (LDH). Examination of and inquiry regarding the medical history were used to exclude various factors of rhabdomyolysis caused by trauma, strenuous activities, infections, drugs, hyperthermia, and immunity; the patient was diagnosed with severe rhabdomyolysis induced by chemotherapy. After treatment with intravenous fluids and methylprednisolone, the patient’s symptoms were relieved and laboratory results were significantly improved. An unexpected situation arose, in that the lung CT scan showed that the lung mass was significantly smaller than that before chemotherapy; the reason for this is not clear. Rhabdomyolysis induced by anti-cancer drugs, especially chemotherapy drugs, is rarely reported and easily overlooked. Therefore, physicians should be aware of this rare but potentially serious complication when using chemotherapy drugs.
Introduction
Rhabdomyolysis syndrome refers to the breakdown and necrosis of muscle tissue due various reasons and caused by the release of intracellular contents into the blood stream. It is characterized by muscle weakness, pain, and dark tea–colored urine (Cabral et al., 2020). Laboratory tests show that serum creatine kinase (CK) is elevated, which is an important basis for the diagnosis of rhabdomyolysis. Rhabdomyolysis is generally considered to be diagnosed when CK is at least five times the normal level or >1,000 U/L. Severe rhabdomyolysis can lead to acute renal failure or even death. It usually results from trauma, strenuous activities, infections, hyperthermia, inherited enzyme deficiencies, and myopathies. Medications such as lipid-reducers, antidiabetics, psychiatrics, bisphosphonates, or antimicrobials are important causes of rhabdomyolysis (Cetrone et al., 2014; Maqoud et al., 2021; Scala et al., 2022). However, rhabdomyolysis induced by anti-cancer drugs, especially chemotherapy drugs, is rarely reported. In this article, we describe for the first time a case report of severe rhabdomyolysis induced by etoposide-nedaplatin chemotherapy in an SCLC patient.
Drug-induced rhabdomyolysis is not a common problem of chemotherapy drugs; however, it is easily overlooked by physicians. Most cases can be improved after immediate treatment; nevertheless, symptoms of fatigue and pain can still remain, which seriously affect the quality of life. This reminds physicians to be aware of this rare but potentially serious complication when using chemotherapy drugs, although the cause of adverse reactions induced by anti-tumor drugs is still unclear.
Case report
A 71-year-old male was admitted to Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology on 9 April 2022, and complained of chest tightness, wheezing, and coughing for more than 3 months. A biopsy and brushing were performed at the upper left lobar bronchial opening by electronic bronchoscopy on 12 April 2022. The histopathological diagnosis was small cell lung carcinoma (SCLC IIIb). Immunohistochemistry showed PCK (weak+), CK8/18 (weak+), CD56 (+), Syn (partially weak+), CgA (partially weak+), INSM1 (partially+), NKX2.2 (partially weak+), SSTR2 (−), TTF-1 (−), RB1 (+), CD20 (−), CD20 (positive control+), CD3 (2V6) (−), LCA (−), P53 (diffuse strong+, suggesting mutation), and Ki-67 (LI approximately 90%).
After the patient provided written informed consent, he received the first cycle of EP (etoposide, 100 mg/m2, D1–D3 + nedaplatin, 80 mg/m2, D1) regimen chemotherapy starting on 20 April (as Day 1). On 22 April (Day 3), the patient developed chest tightness, weakness, wheezing, palpitation, increased blood pressure, and decreased oxygen saturation; therefore, chemotherapy on the third day was suspended. He underwent a lung enhanced CT examination that showed a mass shadow in the left upper lobe, a neoplastic lesion with involvement of the left pulmonary artery with atelectasis in the left upper lobe, micro-nodules in the upper lobe of the right lung and the lower lobe of both lungs, left pleural effusion, left lung insufficiency, aortic wall calcification, and gallstones. An electrocardiogram revealed sinus bradycardia and frequent ventricular premature beats. On 24 April (Day 5), color doppler echocardiography showed no abnormal heart structure or valve activity. The symptoms improved after treatment with oxygen inhalation and cardio-protective therapy; however, the patient refused to continue chemotherapy and was discharged on 27 April (Day 8).
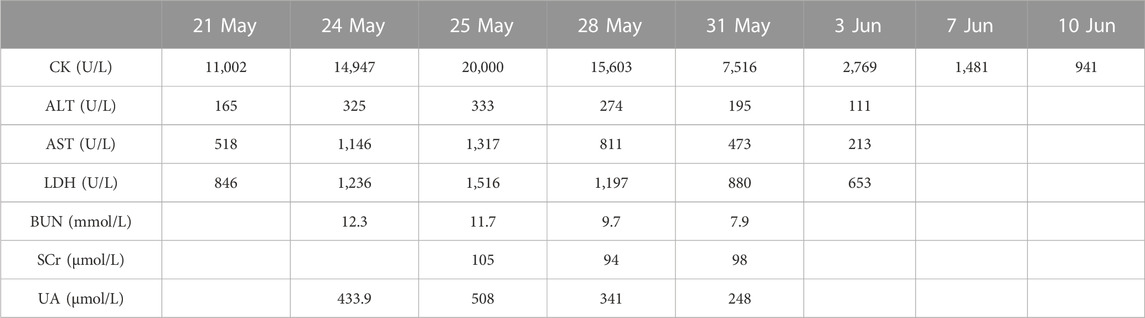
After discharge, the patient developed a general feeling of fatigue, which gradually worsened over the next week so that he could not stand up and walk on his own. This was accompanied by shoulder pain that gradually developed into a general feeling of muscle pain and tightness of the four limbs. On 21 May (Day 32), he was admitted to a local hospital for emergency treatment. Laboratory tests showed creatine kinase (CK) 11,002 U/L (normal range 59–248 U/L), myoglobin (Mb) > 2,000 ng/mL (normal range ≤154.9 ng/mL), alanine aminotransferase (ALT) 165 U/L (normal range ≤41 U/L), aspartate aminotransferase (AST) 518 U/L (normal range ≤40 U/L), and lactate dehydrogenase (LDH) 846 U/L (normal range 135–225 U/L). After rehydration and diuretic treatment, the levels of serum CK, Mb, ALT, AST, and LDH were markedly elevated to 14,947 U/L, >2,000 ng/mL, 325 U/L, 1,146 U/L, and 1,236 U/L, respectively, on 24 May (Day 35). Physicians at the local hospital recommended that the patient be transferred to the higher-level hospital for further diagnosis and treatment.
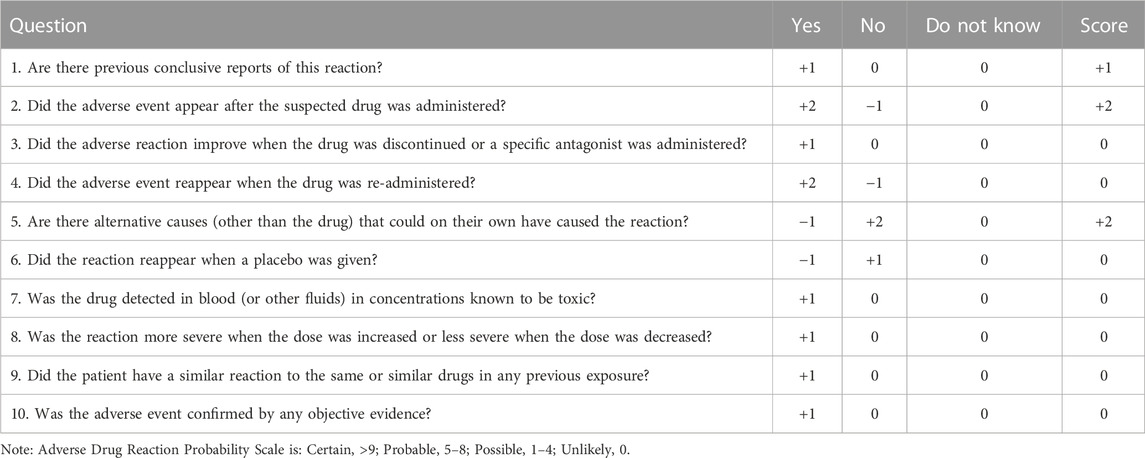
Therefore, the patient was admitted to our hospital on 24 May (Day 35) with complaints of progressed generalized muscle pain and weakness after chemotherapy for small cell lung cancer. The patient was a smoker but had no history of hypertension, diabetes mellitus, autoimmune disease, or renal disease and no relevant family history. He did not have any history of infection, trauma, hyperthermia, myopathies (myositis, dystrophy, or others) or taking statins, bisphosphonates, antidiabetics, or other drugs prior to admission. He began to have difficulty swallowing food or drinking water and his urine was dark yellow at admission. At that time, his height, weight, blood pressure, heart rate, temperature, respiratory rate, and peripheral capillary oxygen saturation (SpO2) were 180 cm, 73 kg, 135/75 mmHg, 71 beats/min, 36.1°C, 20 breaths/min, and 95% on room air, respectively. Laboratory tests on admission showed substantially increased serum CK, Mb, ALT, AST, and LDH (Table 1). In addition, the renal function and myocardial were abnormal: cardiac troponin I (cTnI) 46.5 pg/mL, blood urea nitrogen (BUN) 11.70 mmol/L, serum creatinine (Scr) 105 μmol/L, and uric acid (UA) 508.0 μmol/L. Autoimmune myositis–associated antibodies (anti-Ro-52, anti-Jo-1, anti-Mi-2, anti-Ku, anti-PM-Scl100, anti-PM-Scl75, anti-SRP, anti-PL-7, anti-PL-12, and anti-EJ), vasculitis-associated autoantibodies (anti-pANCA, anti-cANCA, anti-MPO, and anti-PR3), and rheumatism autoantibodies (anti-ANA, anti-dsDNA, anti-ENA, anti-RNP A, anti-RNP 68, anti-Sm/nRNP, anti-Sm, anti-SS-A, anti-SS-B, and anti-Scl-70) were negative and thyroid function tests (thyroid-stimulating hormone and free thyroxine) were normal. In order to exclude infection-induced rhabdomyolysis, IgM antibody tests for pathogens (mycoplasma pneumoniae, chlamydia pneumoniae, coxsackievirus, adenovirus, respiratory syncytial virus, and cytomegalovirus) were performed and were negative. Surprisingly, a lung CT scan showed that the lung mass was significantly smaller than that before chemotherapy (Figure 1). We recommended a muscle biopsy and electromyography to rule out myopathy; however, this was refused by the patient and his family. According to the Naranjo Scale of adverse drug reactions, the score was 5, which indicates that adverse events were probably adverse drug reactions (Table 2) (Khan et al., 2021). The patient was diagnosed with severe rhabdomyolysis induced by chemotherapy.
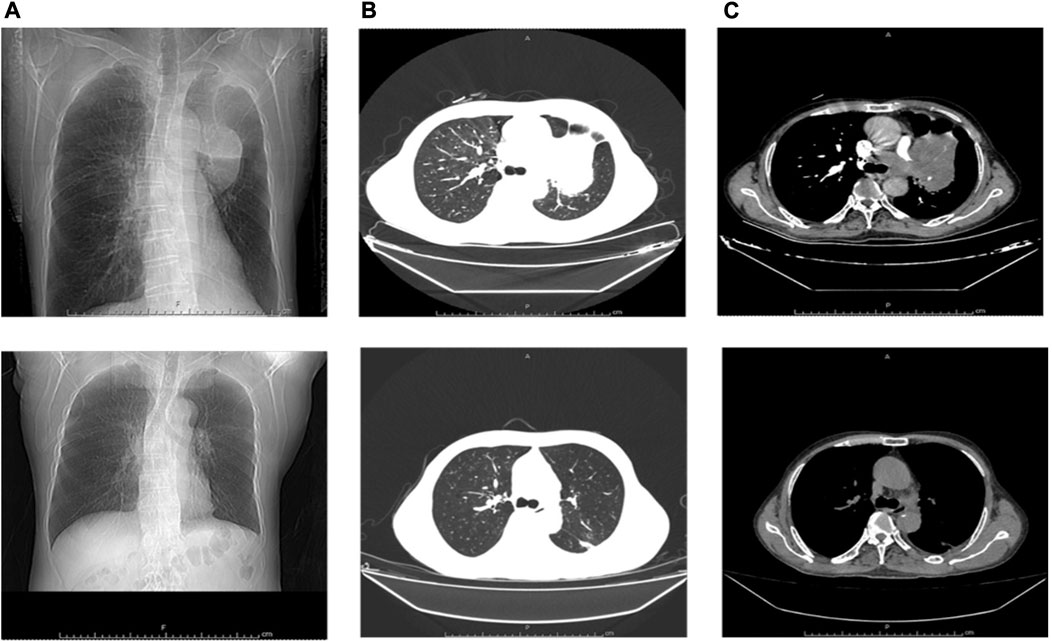
FIGURE 1. Comparison of lung CT scan before and after chemotherapy. (A–C) Before chemotherapy; (A–C) After chemotherapy; (A), (a) Frontal; (B), (b) Lung Window; (C), (c) Mediastinum Window.
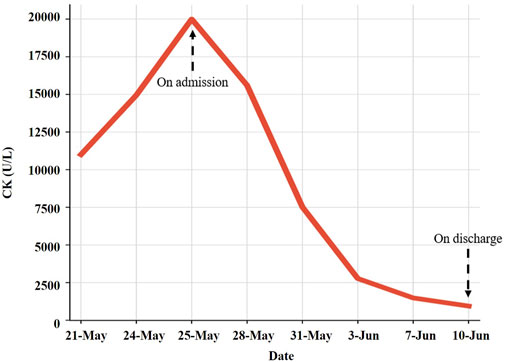
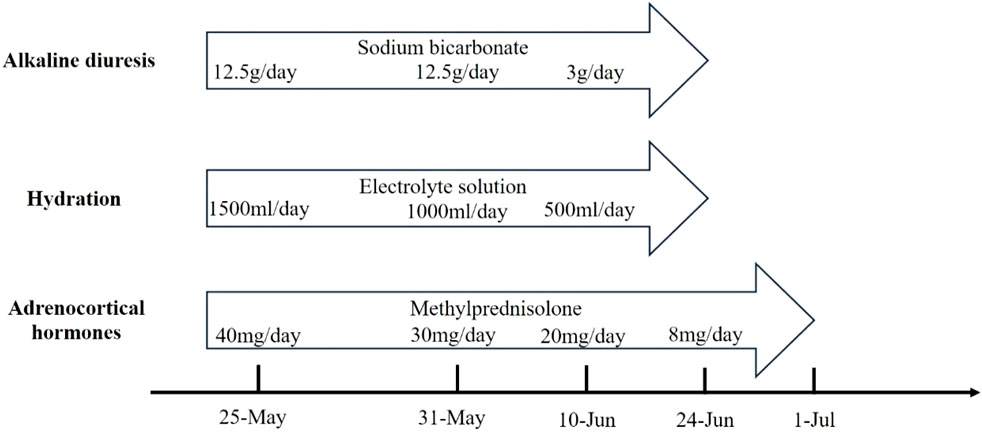
Large amounts of intravenous fluids were administered to prevent acute kidney injury and intravenous methylprednisolone (40 mg/day) was started immediately for rhabdomyolysis. Seven days later, intravenous methylprednisolone was changed to 30 mg/day. Serum CK, ALT, AST, and LDH gradually declined within 2 weeks (Table 1). The symptoms of fatigue and myalgia improved. After the 17th day of hospitalization, CK levels decreased to 941 U/L, and the patient requested discharge back to the local hospital for further rehabilitation (Figure 2). Methylprednisolone was continued during the hospitalization and upon discharge. On 10 June (Day 52), the patient was discharged from our hospital with an oral dose of methylprednisolone 20 mg/day, and the dosage was gradually reduced. Serum CK normalized 2 weeks after discharge with an oral dose of methylprednisolone 8 mg/day; however, the symptoms of fatigue and myalgia improved slowly. The treatment regime of this patient within 2 months is shown in Figure 3. After 6 months of follow-up, the patient’s fatigue symptoms still existed and he still refused to receive further anti-tumor drug treatment.
Discussion
The patient in this case had no previous history of myopathy or immune disease. Liver function, renal function, and electrolyte and urine routines were normal before chemotherapy. After intravenous infusion of etoposide-nedaplatin, the patient gradually developed a general feeling of fatigue, muscle pain, and dark yellow urine. Laboratory tests showed increased serum CK, Mb, ALT, AST, LDH, BUN, Scr, and UA. After excluding trauma, strenuous activity, infections, hyperthermia, and immune factors, the patient was diagnosed with severe rhabdomyolysis induced by chemotherapy. After treatment with intravenous fluids and methylprednisolone, the patient’s symptoms were relieved and laboratory results were significantly improved. Rhabdomyolysis induced by anti-cancer therapy, especially chemotherapy, is still rarely reported. To the best of our knowledge, this is the first case of etoposide-nedaplatin chemotherapy–induced rhabdomyolysis ever reported.
Etoposide, an inhibitor of topoisomerase-II, exerts an anti-cancer effect by inhibiting DNA repair and cell mitosis. It is widely used in treating lung cancer, germ cell tumors, and refractory lymphoma. The main adverse reactions of etoposide include myelosuppression, gastrointestinal symptoms, allergy, alopecia, neurotoxicity, fever, and phlebitis (Khan and Gurav, 2017). Nedaplatin is the second generation of platinum drugs, and is an analog of cisplatin. The anti-cancer mechanism is similar to cisplatin, which inhibits the growth of tumor cells by binding to DNA. It was approved in Japan for use in several cancers, including SCLC (Zhou et al., 2020). It has been allowed to be used in the treatment of esophageal cancer, lung cancer, gynecological cancer, and head and neck tumors in China based on the results of many clinical trials, which showed that nedaplatin has similar anti-tumor therapeutic effects, with a lower toxicity than cisplatin (Ge et al., 2018; Wang et al., 2016; Yang et al., 2012; Zhang et al., 2014). The common adverse effects of nedaplatin include bone marrow suppression, gastrointestinal symptoms, kidney dysfunction, ototoxicity, and alopecia, while allergic reaction and Adams–Stokes syndrome are rare complications (Kawarada et al., 2017).
Although cases of rhabdomyolysis related to etoposide and platinum drugs including carboplatin, cisplatin, and oxaliplatin were reported, no report related to nedaplatin was found. In 1999, Hoshi et al. (1999) reported that a 38-year-old man with choriocarcinoma in the left testis suffered from unconsciousness and hallucinations after receiving high-dose chemotherapy (ifosfamide, carboplatin, and etoposide), and died of respiratory failure. Matsuzaki et al. (2013) reported that a 15-year-old girl with alveolar rhabdomyosarcoma developed severe acute rhabdomyolysis shortly after the second multi-drug chemotherapy cycle, which consisted of etoposide, ifosfamide, actinomycin-D, and vincristine. After mechanical ventilation and fluid replacement, the patient’s condition slowly improved. Sokolova et al. (2017) reported that a 21-year-old man with relapsed non-seminomatous germ cell tumor, who received high-dose chemotherapy with carboplatin and etoposide, presented with bilateral leg pain, and finally improved after treatment. Etoposide combined with platinum drugs is the first-line chemotherapy for small cell lung cancer. To the best of our knowledge, this complication has not been reported with an etoposide-nedaplatin regimen. At present, it is generally believed that the core mechanism of rhabdomyolysis is the decline of intracellular ATP levels and the elevation of myoplasmic Ca2+ concentrations (Hohenegger, 2012). Although the mechanism of rhabdomyolysis caused by chemotherapy drugs is not clear, we can speculate on its possible mechanism; that is, chemotherapeutic drugs may damage the muscle membrane, leading to the leakage of cell contents and increased levels of Na+ entering the cell. Higher Na+ concentrations in cells will activate energy-dependent Na/K ATPase, thus depleting ATP in cells. With the increase of intracellular Na+, cells increase the activity of the 2Na+/Ca2+ exchanger, which removes excess intracellular Na+ from the cytoplasm by exchanging it with Ca2+, finally increasing the concentration of cytoplasmic calcium (Zhang, 2012). The increased Ca2+ concentration activates phospholipase A2 and various neutral proteases to degrade cellular phospholipid membranes and various intracellular organelles, which eventually leads to the destruction of muscle cells.
Another unexpected situation is that although the patient had rhabdomyolysis after the first cycle of chemotherapy, the lung mass was obviously reduced on the lung CT scan. One possible hypothesis is that the patient was too sensitive to etoposide or nedaplatin, which led to the simultaneous occurrence of tumor necrosis and muscle injury. However, whether rhabdomyolysis is associated with mass reduction remains uncertain; there is no evidence to support it at present. Certainly, we also consider the possibility of tumor lysis syndrome. Tumor lysis syndrome is characterized by a massive cytolysis, with the release of intracellular electrolytes, nucleic acids, and metabolites into the circulation, resulting in hyperuricemia, hyperkalemia, hyperphosphatemia, hypocalcemia, and even acute renal failure. However, the presented case developed chest tightness, weakness, asthma, and palpitation after Day-2 chemotherapy. Laboratory examination showed that serum UA 369.0 μmol/L, calcium (Ca) 2.22 mmol/L, potassium (K) 4.01 mmol/L, and phosphate (P) 1.24 mmol/L were within normal levels at that time. Serum UA (302.0 μmol/L), Ca (2.20 mmol/L), K (4.33 mmol/L), and P (0.90 mmol/L) were also within normal levels on the third day after chemotherapy. After discharge, the patient developed progressive generalized muscle pain and weakness. Serum CK, Mb, ALT, AST, and LDH levels were significantly increased, while serum UA (341.0 μmol/L), Ca (2.34 mmol/L), and K (4.31 mmol/L) were still within normal levels on this admission. Therefore, it is impossible to diagnose tumor lysis syndrome.
Conclusion
In conclusion, severe rhabdomyolysis can lead to acute renal failure or even death. Rhabdomyolysis induced by anti-cancer drugs, especially chemotherapy drugs, is rarely reported and easily overlooked. Here, we report the first case of etoposide-nedaplatin chemotherapy–induced severe rhabdomyolysis. It is tempting to speculate that the patient is too sensitive to etoposide or nedaplatin, which would lead to the simultaneous occurrence of tumor necrosis and muscle injury. However, the cause of etoposide-nedaplatin–induced severe rhabdomyolysis is still unclear and more cases need to be collected for analysis in the future. Therefore, we think that physicians should be aware of this rare but potentially serious complication when using chemotherapy drugs. When progressive fatigue and muscle pain occur in patients undergoing chemotherapy, the possibility of acute rhabdomyolysis should be considered. Once the diagnosis is confirmed, the medication should be discontinued immediately and treatment should be carried out, especially to maintain kidney function.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Clinical Trial Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
QW designed the idea. XX and XW collected the data and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the sixth Young Talent Lifting Project of China Association for Science and Technology (No. YESS20200255) and the Traditional Chinese Medicine Research Project of Hubei Provincial Administration of Traditional Chinese Medicine (No. ZY2023Q011).
Acknowledgments
The authors would like to thank the patient for the written informed consent provided to publish the report.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Cabral, B. M. I., Edding, S. N., Portocarrero, J. P., and Lerma, E. V. (2020). Rhabdomyolysis. Disease-a-Month 66, 101015. doi:10.1016/j.disamonth.2020.101015
Cetrone, M., Mele, A., and Tricarico, D. (2014). Effects of the antidiabetic drugs on the age-related atrophy and sarcopenia associated with diabetes type II. Curr. diabetes Rev. 10 (4), 231–237. doi:10.2174/1573399810666140918121022
Ge, L., Li, N., Yuan, G. W., Sun, Y. C., and Wu, L. Y. (2018). Nedaplatin and paclitaxel compared with carboplatin and paclitaxel for patients with platinum-sensitive recurrent ovarian cancer. Am. J. cancer Res. 8 (6), 33–34. doi:10.1016/j.ygyno.2018.04.077
Hohenegger, M. (2012). Drug induced rhabdomyolysis. Curr. Opin. Pharmacol. Jun 12 (3), 335–339. doi:10.1016/j.coph.2012.04.002
Hoshi, S., Itoh, A., Kato, S., Suzuki, K., Kawamura, S., and Orikasa, S. (1999). Severe rhabdomyolysis as a complication of high-dose chemotherapy in a patient with advanced testicular cancer. Int. J. Urol. 6, 56–58. doi:10.1046/j.1442-2042.1999.06130.x
Kawarada, Y., Miyazaki, M., Itoh, A., Araki, R., Iwamizu, H., Kataoka, T., et al. (2017). Incidence of and risk factors associated with nedaplatin-related hypersensitivity reactions. Int. J. Clin. Oncol. 22, 593–599. doi:10.1007/s10147-017-1091-4
Khan, T., and Gurav, P. (2017). PhytoNanotechnology: enhancing delivery of plant based anti-cancer drugs. Front. Pharmacol. 8, 1002. doi:10.3389/fphar.2017.01002
Khan, Z., Karatas, Y., and Kiroglu, O. (2021). Evaluation of adverse drug reactions in paediatric patients: a retrospective study in Turkish hospital. Front. Pharmacol. 12, 786182. doi:10.3389/fphar.2021.786182
Maqoud, F., Scala, R., Tragni, V., Pierri, C. L., Perrone, M. G., Scilimati, A., et al. (2021). Zoledronic acid as a novel dual blocker of kir6.1/2-SUR2 subunits of ATP-sensitive K+ channels: role in the adverse drug reactions. Pharmaceutics 13 (9), 1350. doi:10.3390/pharmaceutics13091350
Matsuzaki, H., Koga, Y., Suminoe, A., Oba, U., Takimoto, T., and Hara, T. (2013). Severe acute rhabdomyolysis induced by multi-agent chemotherapy for alveolar rhabdomyosarcoma in a 15-year-old female: a case report. Case Rep. Oncol. 6, 397–402. doi:10.1159/000354271
Scala, R., Maqoud, F., Antonacci, M., Dibenedetto, J. R., Perrone, M. G., Scilimati, A., et al. (2022). Bisphosphonates targeting ion channels and musculoskeletal effects. Front. Pharmacol. 13, 837534. doi:10.3389/fphar.2022.837534
Sokolova, A., Chan, O., Ullah, W., Hamdani, A. A., and Anwer, F. (2017). Delayed rhabdomyolysis with paclitaxel, ifosfamide, carboplatin, and etoposide regimen: a case report. J. Med. Case Rep. 11, 100. doi:10.1186/s13256-017-1272-9
Wang, H., Zhu, X., Huang, J., Chen, P., Han, S., and Yan, X. (2016). Nedaplatin sensitization of cisplatin-resistant human non-small cell lung cancer cells. Oncol. Lett. 11 (4), 2566–2572. doi:10.3892/ol.2016.4276
Yang, J. J., Zhou, Q., Liao, R. Q., Huang, Y. S., Xu, C. R., Wang, Z., et al. (2012). Nedaplatin/gemcitabine versus carboplatin/gemcitabine in treatment of advanced non-small cell lung cancer: a randomized clinical trial. Chin. J. cancer Res. 24 (2), 97–102. doi:10.1007/s11670-012-0097-8
Zhang, K., Qin, H., Pan, F., Liu, E., Liang, H., and Ruan, Z. (2014). Nedaplatin or oxaliplatin combined with paclitaxel and docetaxel as first-line treatment for patients with advanced non-small cell lung cancer. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 20, 2830–2836. doi:10.12659/MSM.891318
Zhang, M. H. (2012). Rhabdomyolosis and its pathogenesis. World J. Emerg. Med. 3 (1), 11–15. doi:10.5847/wjem.j.issn.1920-8642.2012.01.002
Keywords: rhabdomyolysis, etoposide, nedaplatin, adverse reactions, case report
Citation: Xu X, Wu X, Zhang M and Wang Q (2023) Case Report: Etoposide-nedaplatin induced rhabdomyolysis in a small cell lung cancer patient. Front. Pharmacol. 14:1214149. doi: 10.3389/fphar.2023.1214149
Received: 29 April 2023; Accepted: 02 August 2023;
Published: 22 August 2023.
Edited by:
Yu Yao, The First Affiliated Hospital of Xi’an Jiaotong University, ChinaReviewed by:
Zhifei Xu, Zhejiang University, ChinaYanzhen Wan, Qingdao Women and Children’s Hospital, China
Copyright © 2023 Xu, Wu, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi Wang, wangqixlp@163.com
 Xiaohu Xu
Xiaohu Xu Xiao Wu
Xiao Wu Mingmin Zhang
Mingmin Zhang Qi Wang
Qi Wang