- 1Department of Orthopedics, Orthopedic Research Institute, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Scientific Research and Experiment Management, West China Hospital, Sichuan University, Chengdu, China
- 3Department of Ultrasound, West China Hospital, Sichuan University, Chengdu, China
- 4West China Biobanks and National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University, Chengdu, China
- 5West China School of Nursing, Sichuan University/Department of Orthopedics, West China Hospital, Sichuan University, Chengdu, China
With the increase in human lifespan and the aggravation of global aging, the incidence of osteoarthritis (OA) is increasing annually. To better manage and control the progression of OA, prompt diagnosis and treatment for early-stage OA are important. However, a sensitive diagnostic modality and therapy for early OA have not been well developed. The exosome is a class of extracellular vesicles containing bioactive substances, that can be delivered directly from original cells to neighboring cells to modulate cellular activities through intercellular communication. In recent years, exosomes have been considered important in the early diagnosis and treatment of OA. Synovial fluid exosome and its encapsulated substances, e.g., microRNA, lncRNA, and proteins, can not only distinguish OA stages but also prevent the progression of OA by directly targeting cartilage or indirectly modulating the immune microenvironment in the joints. In this mini-review, we include recent studies on the diagnostic and therapeutic modalities of exosomes and hope to provide a new direction for the early diagnosis and treatment of OA disease in the future.
1 Introduction
Osteoarthritis (OA) is a prevalent degenerative joint disease that affects articular cartilage, subchondral bone, and even the entire joints, e.g., knee and hip. This complex disease is characterized by cartilage degeneration, subchondral ossification, and synovitis (Walsh et al., 2023). According to a recent longitudinal study, there were 5.7% of men and 10.3% of women over the age of 60 had symptomatic knee OA (Tang et al., 2016), and there are approximately 15 million newly diagnosed OA patients in the world (Jacob and Kostev, 2021). The pain, stiffness, and disability caused by cartilage damage in joints severely affect the quality of the patient’s life. Although total joint replacement is recognized as the most effective treatment for reducing joint pain and improving joint mobility for OA patients but it is applicable only for end-stage OA patients and has serious complications, e.g., periprosthetic joint infection, pulmonary embolism, deep venous thrombosis (Neuprez et al., 2020). The key to managing and controlling the progression of OA is the proper timing, i.e., early diagnosis and treatment. Unfortunately, few diagnostic modalities and effective treatments exist for the pathological alternations of the articular cartilage on early-stage OA. At present, the treatment of OA patients aims to temporarily alleviate the pain and inflammation by using glucosamine, non-steroidal anti-inflammatory drugs (NSAIDs), physical therapy, or intraarticular injection (Schnitzer et al., 2019), which are symptomatic treatments but do not prevent or reverse the underlying pathological alternations. Therefore, a sensitive diagnostic modality for early-stage OA and an effective treatment to counteract the progression of early-stage OA are major concerns for patients and health insurance systems. In recent decades, a variety of biological therapy has been proposed to treat early-stage OA, e.g., stem cell therapy (McGonagle et al., 2017; Murphy et al., 2020), platelet-rich plasma (Raeissadat et al., 2021), functional biomaterials (Chuang et al., 2023), and tissue engineering technology (Chuang et al., 2023; Duan et al., 2023). Although the above treatments exhibited great therapeutic potential, they also have inevitable limitations respectively. Notably, exosome-based therapy is an emerging treatment and has recently been recognized as a promising way for the early diagnosis and treatment of various diseases, including but not limited to OA.
In this review, we will introduce the advantages of using exosomes for early-stage OA diagnosis and the current applications of exosomes for OA treatment, and point out the critical directions for studies and clinical applications in the future.
2 Synovial fluid exosomes serve as the biomarkers for the diagnosis of early-stage OA
The lack of early diagnostic modalities delay the management and prevention of early-stage OA, thus indirectly exacerbating the progression of OA. Clinically, OA is diagnosed by imaging and physical examination, which are relatively inaccurate assessments for early-stage OA as the mild symptoms and unobvious radiographic evidence, e.g., joint space narrowing and the loss of cartilage thickness (Crossley et al., 2008). Considering early-stage OA is basically limited to the joint, the cytokines alternation either lacks specificity or is barely altered in blood (Zhao and Xu, 2018). To figure out a sensitive target for early diagnosis, synovial fluid has attracted attention. Synovial fluid is a type of viscous body fluid that is secreted by the inner layer of synovial membranes and stored in the joint cavity, the earliest pathophysiological hints of OA should appear in the synovial fluid, i.e., secreted exosomes (Skriner et al., 2006). Therefore, synovial fluid exosome is one of the best diagnostic targets for early-stage OA. Molecules and proteins which are distinctively expressed in different stages of diseases thus are considered the essential markers for diagnosis. Considering that few detectable alternations exist in the early-stage OA, the phenotype and components of enriched synovial fluid exosome provide a promising marker for early diagnosis. The function of wrapped substances in the exosome, e.g., messenger RNA, microRNAs (miRNAs), DNA, and proteins, are generally affected by the type and status of the original cell, and subsequently involved in the intercellular communications. Therefore, the above merits endow synovial fluid-derived exosomes with the diagnostic and therapeutic potential for early-stage OA.
In recent years, the diagnostic value of exosomes was intensively explored. For instance, the blood-derived exosomes with a high level of CD47+ were recognized as a screening indicator for the breast cancer (Lian et al., 2019; Chen et al., 2022). Similarly, the urinary exosomes can function as a diagnostic index for specific renal diseases (Lee et al., 2023a). Therefore, harvesting the exosomes in the synovial fluid in a minimally invasive way, then analyzing the markers of exosomes may provide a novel and sensitive diagnostic modality for early-stage OA. The Homeobox (Hox) gene, involves the encoding of a transcription factor that aims to modulate limb morphogenesis and bone formation, while the dysregulation of Hox is associated with the initiation and further progression of early-stage OA (Pelttari et al., 2015). As a result, the gene expression of Hox in chondrocyte-derived exosomes in the synovial fluid could be a potential diagnostic modality. In addition, exosome-expressed miRNA is also a specific marker for early-stage OA. It was reported that differentiated hUC-MSCs (human umbilical cord mesenchymal stromal cells) have a high expression of miRNA-140-5 P, which is a downstream effector of SOX9 and involves in the regeneration of the extracellular matrix of cartilage (Geng et al., 2020). The defective interaction between miRNA-140 and Let-7 has been found to cause significant growth defects of chondrocytes in OA disease (Li et al., 2018). Besides, miRNA-145 was reported to be highly expressed in OA patients, it targets Notch signaling and to involve in the apoptosis of chondrocytes (Wang et al., 2020). Interestingly, database analysis identified synovial fluid-derived exosomal miRNA expressed in OA patients in a gender-dependent manner (Kolhe et al., 2020), the differently expressed exosome-derived markers verified the fact that more female OA patients were found than male OA patients. Circular RNA (circRNA) is one of the non-coding RNAs which has a stable closed-loop structure in exosomes (Hsiao et al., 2017). Similar to miRNA, the expression of certain circRNA can also indicate the progression of OA (Wu and Zou, 2021), e.g., OA patients have higher expression of circRUNX2 (Wang et al., 2021) and circCDH13, which can modulate both miRNA-127-5p and MMP13 to aggravate the OA (Li et al., 2019). CircRNA_0032131 was reported to correlate with OA progression by regulating miR-502-5p and PRDX3 (Xu and Ma, 2021). Likewise, circRNA_0005105 involves in the degradation of extracellular matrix in cartilages (Wu et al., 2017), and circ_0008365 is highly expressed in serum-derived exosomes from OA patients and thus showed a diagnostic value for OA patients (Shuai et al., 2022). In addition, exosomal lncRNA PCGEM1 was also highly expressed in OA patients, and it was different in different stages of OA, which suggests that exosomal lncRNA PCGEM1 from the synovial fluid not only served as a marker to diagnose OA but also distinguish the stages of OA (Zhao and Xu, 2018). Furthermore, synovial-derived exosomes can also indicate the inflammatory status of the OA joint in its early stage. Theoretically, various inflammatory cytokines are expressed in synovial fluid, but they are difficult to be directly detected due to the low concentration at the early stage of inflammation (Wang and He, 2018). However, synovial fluid exosomes provide an approach to indirectly indicate the inflammation and identify the OA progression. For instance, IL1R+ synoviocyte can be activated after the binding of IL1R and IL-1 from the synovial fluid, and it subsequently secrete specific exosomes (MMP-13high and ADAM5high) (Mehta et al., 2019), therefore, the content of exosomes (MMP-13high and ADAM5high) may indirectly indicate the IL-1 levels in the synovial cavity. In summary, the phenotypes and wrapped molecules of synovial fluid exosomes can function as the accurate and effective biomarkers for the diagnosis of early-stage OA.
3 The exosome-based treatment has multidimensionally therapeutic effects on OA
Exosome also has multidimensionally therapeutic effects on the progression of OA, as they envelop various bioactive substances, e.g., cytokines, growth factors, and RNA, which can be directly transferred in neighboring cells through membrane fusion and then modulate the signal transduction to promote the cell proliferation, differentiation, and matrix formation (Lee et al., 2023b). Thus, the exosome-based treatment can function as an effective therapy to modulate the pathological damage of cartilage for OA patients (Xian Bo et al., 2022).
The therapeutic effects of exosome on OA is basically related to its original cells, e.g., bone mesenchymal stem cells (BMSCs), adipose tissue mesenchymal stem cells (AMSCs), synovial mesenchymal stem cells (SMSCs), and embryonic stem cells (ESCs), because these cells exhibit different cellular activities and biological responses to the different stages of OA (Asghar et al., 2020; Fan et al., 2022). Specific exosomes in the synovial fluid are involved in the expression of collagen type II alpha 1 (Col2A1), and aggrecan (ACAN) in articular cartilage thus alleviating the development of OA (Kato et al., 2014). Modification of bone targeting exosomes with siShn3 to silence Shn3 in osteoblasts enhances new bone formation and inhibits osteoclasts formation by downregulated RANKL and TRAP (Cui et al., 2022). MSC-derived exosome has similar therapeutic effects with its original cells on the treatment of OA, i.e., increasing the expression of chondrogenesis (Col2A1 and ACAN) and inhibiting catabolic enzyme (MMP-13 and ADAMTS5) (Cosenza et al., 2017). Granulocytic-myeloid-derived suppressor cells (GMDSCs)-derived exosomal miRNAs miRNA-29A-3 P and miRNA-93-5 P can effectively reduce arthritis index, leukocyte infiltration, and cartilage destruction in an OA mouse model by inhibiting inflammatory responses of T helper (Th1) cells, i.e., Th1 cells, and Th17 cells (Zhu et al., 2019). Exosomes with a high expression of miRNA-26a-5p can alleviate the injury of synovial fibroblasts induced by prostaglandin-endoperoxide synthase (Rizzi et al., 2022). Exosomal miRNA-100-5 P showed inhibitory effects on the mammalian target of rapamycin (mTOR) and inflammation thus having therapeutic effects on OA (Luo et al., 2019). Likewise, exosomal miR-92a-3p can inhibit WNT family member 5 A (WNT5a) to relieve the cartilage damage caused by OA (Yan et al., 2021). In conclusion, the above studies demonstrated that various exosome-encapsulated miRNAs exhibited multidimensionally therapeutic effects on OA, the application of exosomes can not only directly promote the proliferation, differentiation of chondrocytes, increase the extracellular matrix formation and the regeneration of cartilage, but also indirectly prevent the progression of OA through interacting with surrounding immune cells. Nevertheless, the exploration and selection of an appropriate target gene or molecule still require more systematic and rigorous research in the future.
4 Perspectives
The characteristics of exosomes provide us with a novel, sensitive, and effective diagnostic modality and, treatment for early-stage OA (illustrative scheme) (Figure 1). Nevertheless, there still exist many challenges and a long way to the wide clinical utilization, e.g., 1) the short circulating half-life of exosomes limits the systemic application; 2) the targeting ability of exosome-based therapy could be further improved to increase its efficiency and safety. For instance, superparamagnetic iron oxide nanoparticles (SPION) have been well developed to assist in the targeting of exosomes (Zhuo et al., 2021); 3) the efficiency of production, purification, and storage are also key factors that affect its applications; 4) the pathology of OA is complicated that can be affected by various factors, e.g., environment, genic factors, metabolism, or mechanical damage, thus personalized exosomes-based diagnostic modality and therapy should be encouraged. Overall, the advantages of applying exosomes in the early diagnostics and treatment of OA far outweigh its weakness. With the above weakness overcome in the future, exosome-based technology shall be the next-generation of diagnosis modality and treatment for OA.
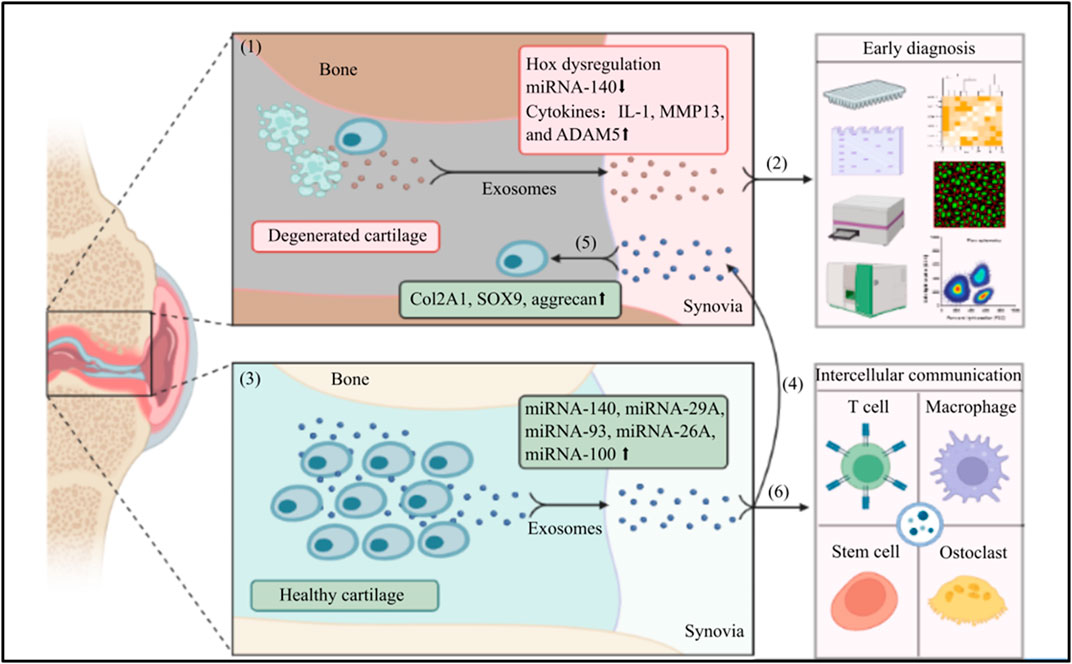
FIGURE 1. The application of exosomes in the early diagnosis and treatment for OA: (1) In an OA joint, chondrocytes undergo apoptosis, the structure of cartilage is damaged. Chondrocytes and mesenchymal stem cells in the OA environment intend to secrete exosomes with Hox gene dysregulation, low expression of miRNA-140, and high levels of IL-1, MMP13 and ADAM5; (2) The exosomes are secreted into synovial fluid that can be harvested in a minimally invasive way, the specific phenotype or enveloped component of exosomes are sensitive markers to early diagnose OA and differentiate the pathological stages; (3) In contrast, chondrocytes and mesenchymal stem cells in a healthy joint can secrete exosomes with high expression of various miRNA; (4) The exosomes with specific miRNAs are secreted into the synovial fluid; (5) The synovial fluid derived-exosomes can be harvested and injected in to a OA joint, the wrapped functional miRNAs can increase the levels of Col2A1, SOX9, and aggrecan, to promote the regeneration of cartilage and reverse the progression of OA; (6) Meanwhile, the synovial fluid derived-exosomes can also involve in a intercellular communication with many neighboring cells, e.g., T cell, macrophage, osteoclast, and stem cell to multidimensionally protect the joint harmed by OA. (The graphic components of this illustrative scheme were provided by BioRender.com).
Author contributions
Conceptualization, AC and YC; investigation, AC and YC; resources, YC; curation, YC; writing—original draft preparation, AC and YC; writing—review and editing, all authors; supervision, WZ and ZZ.
Funding
This work was supported by research grants from the National Natural Science Foundation of China (No. 82172394 and No. U22A20280 awarded to ZZ; No. 82272483 awarded to WZ), the Provincial Natural Science Foundation of Sichuan, (No. 2022NSFSC1445 awarded to WZ), Regional Innovation & Cooperation program of Science & Technology Department of Sichuan Province (No. 2021YFQ0028 awarded to ZZ), the Natural Science Foundation of Chongqing (cstc2020jcyjmsxmX0178 awarded to WZ), the Basic Research and Frontier Exploration of Yuzhong District (No. 20190109 awarded to WZ), West China Nursing Discipline Development Special Fund Project (No. HXHL20003 awarded to JC).
Acknowledgments
We would like to thank Wesley Huang, Sujan Shakya, and Jiali Chen for their constructive suggestions and excellent assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Asghar, S., Litherland, G. J., Lockhart, J. C., Goodyear, C. S., and Crilly, A. (2020). Exosomes in intercellular communication and implications for osteoarthritis. Rheumatol. Oxf. 59, 57–68. doi:10.1093/rheumatology/kez462
Chen, C., Wang, R., Chen, X., Hou, Y., and Jiang, J. (2022). Targeting CD47 as a novel immunotherapy for breast cancer. Front. Oncol. 12, 924740. doi:10.3389/fonc.2022.924740
Chuang, C. H., Kuo, C. C., Chiang, Y. F., Lee, P. Y., Wang, F. H., Hsieh, C. Y., et al. (2023). Enriched peripheral blood-derived mononuclear cells for treating knee osteoarthritis. Cell Transpl. 32, 9636897221149445. doi:10.1177/09636897221149445
Cosenza, S., Ruiz, M., Toupet, K., Jorgensen, C., and Noel, D. (2017). Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci. Rep. 7, 16214. doi:10.1038/s41598-017-15376-8
Crossley, K. M., Vicenzino, B., Pandy, M. G., Schache, A. G., and Hinman, R. S. (2008). Targeted physiotherapy for patellofemoral joint osteoarthritis: A protocol for a randomised, single-blind controlled trial. BMC Musculoskelet. Disord. 9, 122. doi:10.1186/1471-2474-9-122
Cui, Y., Guo, Y., Kong, L., Shi, J., Liu, P., Li, R., et al. (2022). A bone-targeted engineered exosome platform delivering siRNA to treat osteoporosis. Bioact. Mater 10, 207–221. doi:10.1016/j.bioactmat.2021.09.015
Duan, W. L., Zhang, L. N., Bohara, R., Martin-Saldana, S., Yang, F., Zhao, Y. Y., et al. (2023). Adhesive hydrogels in osteoarthritis: From design to application. Mil. Med. Res. 10, 4. doi:10.1186/s40779-022-00439-3
Fan, W. J., Liu, D., Pan, L. Y., Wang, W. Y., Ding, Y. L., Zhang, Y. Y., et al. (2022). Exosomes in osteoarthritis: Updated insights on pathogenesis, diagnosis, and treatment. Front. Cell Dev. Biol. 10, 949690. doi:10.3389/fcell.2022.949690
Geng, Y., Chen, J., Alahdal, M., Chang, C., Duan, L., Zhu, W., et al. (2020). Intra-articular injection of hUC-MSCs expressing miR-140-5p induces cartilage self-repairing in the rat osteoarthritis. J. Bone Min. Metab. 38, 277–288. doi:10.1007/s00774-019-01055-3
Hsiao, K. Y., Sun, H. S., and Tsai, S. J. (2017). Circular RNA - new member of noncoding RNA with novel functions. Exp. Biol. Med. (Maywood, N.J.) 242, 1136–1141. doi:10.1177/1535370217708978
Jacob, L., and Kostev, K. (2021). Osteoarthritis and the incidence of fracture in the United Kingdom: A retrospective cohort study of 258,696 patients. Osteoarthr. Cartil. 29, 215–221. doi:10.1016/j.joca.2020.12.006
Kato, T., Miyaki, S., Ishitobi, H., Nakamura, Y., Nakasa, T., Lotz, M. K., et al. (2014). Exosomes from IL-1β stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis Res. Ther. 16, R163. doi:10.1186/ar4679
Kolhe, R., Owens, V., Sharma, A., Lee, T. J., Zhi, W., Ghilzai, U., et al. (2020). Sex-specific differences in extracellular vesicle protein cargo in synovial fluid of patients with osteoarthritis. Life (Basel) 10, 337. doi:10.3390/life10120337
Lee, A. H., Ghosh, D., Koh, I. L., and Dawson, M. R. (2023). Senescence-associated exosomes transfer miRNA-induced fibrosis to neighboring cells. Aging (Albany NY) 15, 1237–1256. doi:10.18632/aging.204539
Lee, C. C., Chen, C. C., Hsu, C. K., Chen, Y. T., Chen, C. Y., Yang, K. J., et al. (2023). Urinary microRNA in diabetic kidney disease: A literature review. Med. Kaunas. 59, 354. doi:10.3390/medicina59020354
Li, Q., Huang, Q. P., Wang, Y. L., and Huang, Q. S. (2018). Extracellular vesicle-mediated bone metabolism in the bone microenvironment. J. Bone Min. Metab. 36, 1–11. doi:10.1007/s00774-017-0860-5
Li, Z., Yuan, B., Pei, Z., Zhang, K., Ding, Z., Zhu, S., et al. (2019). Circ_0136474 and MMP-13 suppressed cell proliferation by competitive binding to miR-127-5p in osteoarthritis. J. Cell Mol. Med. 23, 6554–6564. doi:10.1111/jcmm.14400
Lian, S., Xie, X., Lu, Y., and Jia, L. (2019). Checkpoint CD47 function on tumor metastasis and immune therapy. OncoTargets Ther. 12, 9105–9114. doi:10.2147/OTT.S220196
Luo, P., Jiang, C., Ji, P., Wang, M., and Xu, J. (2019). Exosomes of stem cells from human exfoliated deciduous teeth as an anti-inflammatory agent in temporomandibular joint chondrocytes via miR-100-5p/mTOR. Stem Cell Res. Ther. 10, 216. doi:10.1186/s13287-019-1341-7
McGonagle, D., Baboolal, T. G., and Jones, E. (2017). Native joint-resident mesenchymal stem cells for cartilage repair in osteoarthritis. Nat. Rev. Rheumatol. 13, 719–730. doi:10.1038/nrrheum.2017.182
Mehta, S., Akhtar, S., Porter, R. M., Onnerfjord, P., and Bajpayee, A. G. (2019). Interleukin-1 receptor antagonist (IL-1Ra) is more effective in suppressing cytokine-induced catabolism in cartilage-synovium co-culture than in cartilage monoculture. Arthritis Res. Ther. 21, 238. doi:10.1186/s13075-019-2003-y
Murphy, M. P., Koepke, L. S., Lopez, M. T., Tong, X., Ambrosi, T. H., Gulati, G. S., et al. (2020). Articular cartilage regeneration by activated skeletal stem cells. Nat. Med. 26, 1583–1592. doi:10.1038/s41591-020-1013-2
Neuprez, A., Neuprez, A. H., Kaux, J. F., Kurth, W., Daniel, C., Thirion, T., et al. (2020). Total joint replacement improves pain, functional quality of life, and health utilities in patients with late-stage knee and hip osteoarthritis for up to 5 years. Clin. Rheumatol. 39, 861–871. doi:10.1007/s10067-019-04811-y
Pelttari, K., Barbero, A., and Martin, I. (2015). A potential role of homeobox transcription factors in osteoarthritis. Ann. Transl. Med. 3, 254. doi:10.3978/j.issn.2305-5839.2015.09.44
Raeissadat, S. A., Ghazi Hosseini, P., Bahrami, M. H., Salman Roghani, R., Fathi, M., Gharooee Ahangar, A., et al. (2021). The comparison effects of intra-articular injection of Platelet Rich Plasma (PRP), Plasma Rich in Growth Factor (PRGF), Hyaluronic Acid (HA), and ozone in knee osteoarthritis; a one year randomized clinical trial. BMC Musculoskelet. Disord. 22, 134. doi:10.1186/s12891-021-04017-x
Rizzi, L., Turati, M., Bresciani, E., Anghilieri, F. M., Meanti, R., Molteni, L., et al. (2022). Characterization of microRNA levels in synovial fluid from knee osteoarthritis and anterior cruciate ligament tears. Biomedicines 10, 2909. doi:10.3390/biomedicines10112909
Schnitzer, T. J., Easton, R., Pang, S., Levinson, D. J., Pixton, G., Viktrup, L., et al. (2019). Effect of tanezumab on joint pain, physical function, and patient global assessment of osteoarthritis among patients with osteoarthritis of the hip or knee: A randomized clinical trial. Jama 322, 37–48. doi:10.1001/jama.2019.8044
Shuai, S., Cai, Q., and Ou, Y. (2022). Circular RNA circ_0008365 regulates SOX9 by targeting miR-338-3p to inhibit IL-1β-induced chondrocyte apoptosis and extracellular matrix degradation. J. Orthop. Surg. Res. 17, 452. doi:10.1186/s13018-022-03240-z
Skriner, K., Adolph, K., Jungblut, P. R., and Burmester, G. R. (2006). Association of citrullinated proteins with synovial exosomes. Arthritis Rheum. 54, 3809–3814. doi:10.1002/art.22276
Tang, X., Wang, S., Zhan, S., Niu, J., Tao, K., Zhang, Y., et al. (2016). The prevalence of symptomatic knee osteoarthritis in China: Results from the China health and retirement longitudinal study. Arthritis Rheumatol. 68, 648–653. doi:10.1002/art.39465
Walsh, D. A., Sofat, N., Guermazi, A., and Hunter, D. J. (2023). Osteoarthritis bone marrow lesions. Osteoarthr. Cartil. 31, 11–17. doi:10.1016/j.joca.2022.09.007
Wang, C., Li, N., Liu, Q., Su, L., Wang, S., Chen, Y., et al. (2021). The role of circRNA derived from RUNX2 in the serum of osteoarthritis and its clinical value. J. Clin. Lab. Anal. 35, e23858. doi:10.1002/jcla.23858
Wang, T., and He, C. (2018). Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 44, 38–50. doi:10.1016/j.cytogfr.2018.10.002
Wang, W. F., Liu, S. Y., Qi, Z. F., Lv, Z. H., Ding, H. R., and Zhou, W. J. (2020). MiR-145 targeting BNIP3 reduces apoptosis of chondrocytes in osteoarthritis through Notch signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 24, 8263–8272. doi:10.26355/eurrev_202008_22622
Wu, W., and Zou, J. (2021). Studies on the role of circRNAs in osteoarthritis. Biomed. Res. Int. 2021, 1–10. doi:10.1155/2021/8231414
Wu, Y., Zhang, Y., Zhang, Y., and Wang, J. J. (2017). CircRNA hsa_circ_0005105 upregulates NAMPT expression and promotes chondrocyte extracellular matrix degradation by sponging miR-26a. Cell Biol. Int. 41, 1283–1289. doi:10.1002/cbin.10761
Xian Bo, S., Chen, W., Chang, L., Hao Ran, Y., Hui Hui, G., Ya Kun, Z., et al. (2022). The research progress of exosomes in osteoarthritis, with particular emphasis on the therapeutic effect. Front. Pharmacol. 13, 731756. doi:10.3389/fphar.2022.731756
Xu, J., and Ma, X. (2021). Hsa_circ_0032131 knockdown inhibits osteoarthritis progression via the miR-502-5p/PRDX3 axis. Aging (Albany NY) 13, 15100–15113. doi:10.18632/aging.203073
Yan, L., Liu, G., and Wu, X. (2021). The umbilical cord mesenchymal stem cell-derived exosomal lncRNA H19 improves osteochondral activity through miR-29b-3p/FoxO3 axis. Clin. Transl. Med. 11, e255. doi:10.1002/ctm2.255
Zhao, Y., and Xu, J. (2018). Synovial fluid-derived exosomal lncRNA PCGEM1 as biomarker for the different stages of osteoarthritis. Int. Orthop. 42, 2865–2872. doi:10.1007/s00264-018-4093-6
Zhu, D., Tian, J., Wu, X., Li, M., Tang, X., Rui, K., et al. (2019). G-MDSC-derived exosomes attenuate collagen-induced arthritis by impairing Th1 and Th17 cell responses. Biochim. Biophys. Acta Mol. Basis Dis. 1865, 165540. doi:10.1016/j.bbadis.2019.165540
Keywords: exosome, mesenchymal stem cell, osteoarthritis, inflammation, immune activation, bone
Citation: Chen A, Chen Y, Rong X, You X, Wu D, Zhou X, Zeng W and Zhou Z (2023) The application of exosomes in the early diagnosis and treatment of osteoarthritis. Front. Pharmacol. 14:1154135. doi: 10.3389/fphar.2023.1154135
Received: 30 January 2023; Accepted: 11 April 2023;
Published: 28 April 2023.
Edited by:
Chen-he Zhou, Zhejiang University, ChinaReviewed by:
Subhash Chand, University of Nebraska Medical Center, United StatesCopyright © 2023 Chen, Chen, Rong, You, Wu, Zhou, Zeng and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weinan Zeng, weinanzeng@163.com; Zongke Zhou, zhouzongke@scu.edu.cn
†These authors have contributed equally to this work
 Anjing Chen
Anjing Chen Yangmengfan Chen1
Yangmengfan Chen1 Xiao Rong
Xiao Rong Weinan Zeng
Weinan Zeng Zongke Zhou
Zongke Zhou