- 1Department of Clinical Pharmacology Lab, Nanjing First Hospital, Nanjing Medical University, Nanjing, China
- 2Nanjing Hongqiao Pharmaceutical Technology Research Institute Co Ltd, Nanjing, China
Background: Salvianolic acid B (Sal B) is one of the main active ingredients of Salvia miltiorrhiza Bunge. In China, many traditional Chinese medicines have been modified into injections for higher bioavailability and better efficacy. Salvianolic acid injection has been widely used in the clinic.
Objective: This phase 1, randomized, double-blind, placebo-controlled, single-center study aimed to evaluate the safety, tolerance, and pharmacokinetics of Sal B injection in healthy Chinese volunteers.
Methods: For the single-ascending-dose study, forty-seven healthy volunteers were randomly divided into 25, 75, 150, 200, 250, and 300 mg groups. For the multiple-ascending-dose study, sixteen healthy volunteers were randomly divided into 150 and 300 mg groups. In each group, volunteers were treated with Sal B or placebo randomly. Their safety was evaluated by a skin test, physical examination, vital sign, laboratory examination, 12-lead electrocardiogram, Holter, and clinical symptoms and signs. Blood samples were collected in 75, 150, and 300 mg single-ascending-dose study groups and 150 mg multiple-ascending-dose study groups to determine the concentration of salvianolic acid B.
Results: In single-ascending-dose study groups, there were 41 adverse events in 24 cases (51.1%, 24/47). In multiple-ascending-dose study groups, there were 13 adverse events in eight cases (50.0%, 8/16). Sixty-six volunteers received the skin test, and three of them were excluded because of the positive result. Adverse events related to the treatment included increased alanine aminotransferase (4.0%), increased bilirubin (2.0%), increased creatinine kinase-MB (2.0%), increased brain natriuretic peptide (8.0%), increased urine N-acetyl-β-D-glucosidase (4.0%), dizziness (2.0%), and chest discomfort (2.0%). No serious adverse events occurred. No volunteers withdrew from the trial. Peak plasma concentration and the area under the plasma concentration–time curve of salvianolic acid B progressively increased in a dose-dependent manner in 75, 150, and 300 mg single-ascending-dose study groups. There was no accumulation after 5 consecutive days of administration of 150 mg salvianolic acid B.
Conclusion: Salvianolic acid B injections administered up to 300 mg in a single dose and 250 mg for 5 consecutive days showed excellent safety and tolerability in healthy Chinese volunteers.
Clinical Trial Registration: www.chinadrugtrials.org.cn, identifier CTR20192236
1 Introduction
Salvia miltiorrhiza (SM) refers to the dried root and rhizome of Salvia miltiorrhiza Bunge. SMis a kind of very popular traditional Chinese medicines (TCMs) that has been extensively applied for thousands of years in East Asia (Su et al., 2015). It has been used to treat cardiovascular diseases (Wu et al., 2020), cerebrovascular diseases (Feng et al., 2019), neurodegenerative diseases (Zhao et al., 2019), diabetes (Jia et al., 2019), etc. Moreover, it was widely used in China due to its safety and efficacy in treating cardiovascular and cerebrovascular diseases (Xu et al., 2018; Feng et al., 2019). The pharmacological properties include anti-inflammatory, anti-oxidant, anti-coagulatory, hypolipidemic, anti-apoptotic, vasodilatory, and angiogenesis-promoting actions (Li et al., 2018b). SM could react directly to the Western cardiovascular drug targets relevant to antithrombotic pathways (i.e., thrombin, coagulation factor Xa, and cyclooxygenase-1) (Zuo et al., 2020). Therefore, it could be exploited as an important complementary medicine preparation for pharmacotherapy of cardiovascular and cerebrovascular diseases.
The active ingredients of SM are mainly water-soluble salvianolic acids, which promote blood flow and improve perfusion (Zhang et al., 2018). Sal B is the most active salvianolic acid with the highest content in water-soluble substances (Bi et al., 2022). Since the 1980s, Chinese scientists have researched the water-soluble components of SM, which was used as a decoction. At least 15 water-soluble chemical components had been isolated named in sequences such as Sal A, B, C, and D (Du et al., 2020). In China, at least three kinds of SM polyphenolic acid injections were used in the clinic, which had effects against oxidative stress, platelet aggregation, coagulation, thrombosis, endothelial dysfunction, and inflammation targeting multiple vascular cell types. Salvianolic B (Sal B) is one of the main active ingredients of SM and scavenges different types of free radicals (Wu et al., 2020). Until now, there is no salvianolic acid B injection on the market.
The limited absorption in the gastrointestinal tract has a detrimental effect on the clinical application of SM; injections can increase the concentration of effective substances in vivo and increase the bioavailability (Bi et al., 2016). With the development and widespread use of TCM injections, adverse drug reactions (ADRs) have gradually become a public concern (Guo et al., 2015; Li et al., 2018a; Huang et al., 2021). The extraction progresses from the TCMs and the purity of the injections had been considered as the most important factors for severe ADRs (Ji et al., 2009). The Chinese Pharmacopoeia specifies the content index of Sal B and its preparations. In this study, the research institute developed a new preparation technology to extract Sal B with the purity of 96% on a large scale. We hope to provide a new promising Sal B for the clinical use with higher bioavailability and less ADRs. We completed the evaluation of safety, tolerance, and pharmacokinetics of Sal B injections in healthy volunteers from 2019 to 2021.
2 Methods
2.1 Drugs and preparations
The dried root and rhizome of S. miltiorrhiza Bunge have been identified by Dr. Shengjin Liu (the School of Pharmacy, Nanjing University of Chinese Medicine). A voucher specimen (No. nzy-ds-180423) was deposited at the Chinese Medicine Herbarium of Nanjing University of Chinese Medicine. The extraction of S. miltiorrhiza is concentrated and freeze-dried after refining (Patent No: ZL02160771.0). The content of salvianolic acid B in the refined stock solution is higher than 96%. A quality inspection report of salvianolic acid B is available on request to Junlin Cheng.
Sal B was made into freeze-dried powder. The powder was dissolved into 250 mL of 0.9% sodium chloride injection. A placebo was used as a control to eliminate the influence of intravenous infusion and menstruum on subjects. The placebo was 250 mL of 0.9% sodium chloride injection from the market, which was the same batch as the menstruum of the Sal B injection. They were infused intravenously within 60 ± 5 min, and the dripping speed was accurately controlled using the infusion pump.
2.2 Study design
This was a single-center, double-blind, randomized placebo-controlled study conducted at the Department of Clinical Pharmacology Lab, Nanjing First Hospital, Nanjing Medical University, Nanjing, China. The study is registered at http://www.chinadrugtrials.org.cn with the identifier CTR20192236, and the study protocol is included therein. According to the animal long-term toxicity test and drug specification of the salvianolic acid B injection, the initial dose is proposed to be 25 mg. From the phase 1 clinical trial of other salvianolic acid salt injection, the maximum tolerance equivalent of salvianolic acid B was 300 mg. The MAD dosage was referred to as the commonly used dose equivalent and the second highest tolerated dose of clinical salvianolic acid salt injection. That was between 150 and 250 mg.
2.3 Participants
Healthy Chinese volunteers aged 18–50 years were recruited, whose body mass index was between 19.0 and 26.0 kg/m2 and body weight was no less than 50 kg. Key exclusion criteria included any medicine administration in 2 weeks, adoption in another clinical trial during 3 months, donating blood during 3 months prior to the trial, history or clinical evidence of chronic diseases, abnormal results with clinical significance in vital signs, 12-lead electrocardiography, and laboratory examinations of allergic constitution. Volunteers signed the informed consent form before screening.
2.4 Randomization and masking
Suitable volunteers enrolled in the study according to the aforementioned criteria. Two volunteers received 25 mg of Sal B, which is the lowest dose in the study. Volunteers were randomized at a ratio of 1:3 in the 75, 200, and 250 mg groups and at a ratio of 1:4 in the 150 and 300 mg groups to receive either a placebo or increasing doses of Sal B in the single-ascending-dose (SAD) study. Other volunteers were randomized at a ratio of 1:3 to receive either a placebo or Sal B in the multiple-ascending-dose (MAD) study. The blind lists were kept under control and would only be uncovered in case of emergency.
2.5 Procedures
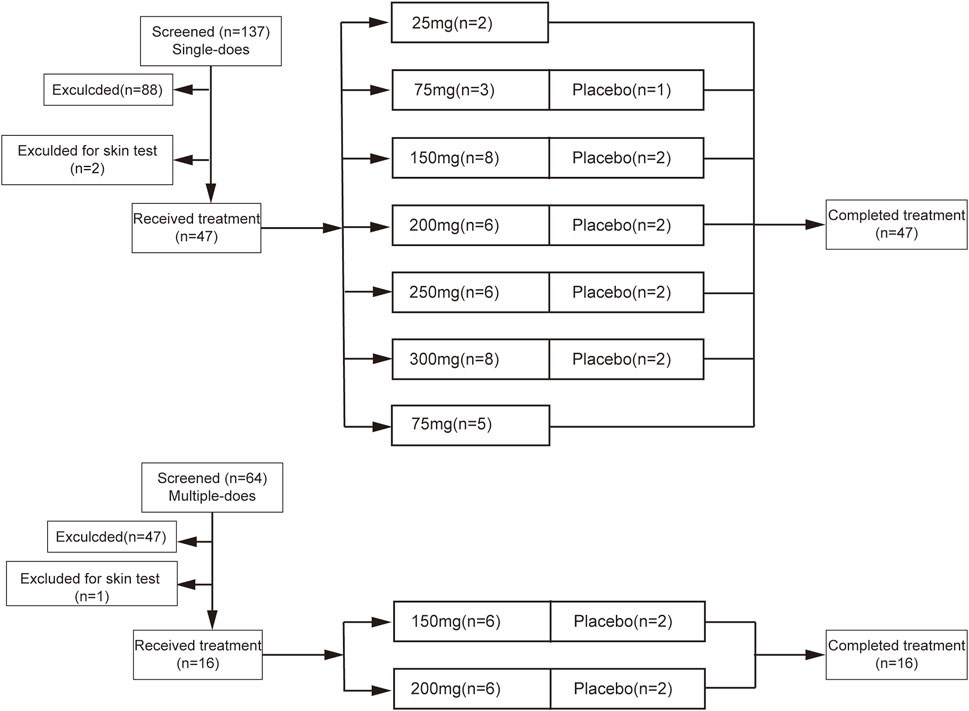
The study included the SAD study and MAD study (Figure 1). In the SAD study, two eligible volunteers included in the first 25 mg group were treated with Sal B one by one. After the safety was evaluated, the second volunteer was treated with Sal B. Eligible volunteers were randomized to receive the placebo or escalating doses of Sal B (75, 150, 200, 250, and 300 mg) successively. Electrocardiography was taken prior to administration and lasted for 24 h by Holter. The same procedure was conducted in the MAD study. Eligible volunteers were randomized to placebo or Sal B (150, 250 mg) groups for five consecutive days. All the volunteers were hospitalized throughout the trial and came back for a visit on Day 9 ± 1 of the last administration of Sal B. ECG was taken on the 1st and 5th administration days and lasted for 24 h by Holter.
2.6 Pharmacokinetics
In the 75, 150, and 300 mg SAD study, blood samples (4 mL each) were taken at 0, 0.25, 0.5, 0.75, and 1 h after the intravenous dose. After the termination of infusion, additional blood samples were taken at 0.17, 0.33, 0.67, 1, 1.5, 2, 3, 4, 5, 6, 7, and 8 h. In the 150 mg MAD study, blood samples were taken at 0, 0.25, 0.5, 0.75, and 1 h after the first dose, 0.17, 0.33, 0.67, 1, 1.5, 2, 3, 4, 5, 6, 7, and 8 h after the termination of the first dose, pre-dose of third and fourth dosing, 0, 0.25, 0.5, 0.75, and 1 h after the last dose, and 0.17, 0.33, 0.67, 1, 1.5, 2, 3, 4, 5, 6, 7, 8, and 24 h after the termination of the last dose. Blood samples were collected in tubes containing ethylenediaminetetraacetic acid dipotassium and centrifuged at 4°C within 1 h after sampling for plasma preparation. Sal B was detected using a liquid chromatography–tandem mass spectrometry method (HPLC 30-AD, SHIMADZU, Japan. AB SCIEX API 4000, Applied Biosystems, United States). The method was validated as per the Chinese Pharmacopoeia.
2.7 Safety assessments
Safety and tolerance were determined by clinical evaluation. Volunteers were monitored in hospital from the day before the first administration, and adverse events (AEs) were recorded. All AEs were evaluated in terms of severity (mild, moderate, or severe), duration, measures taken, outcomes, and relationship to treatment. Serious adverse events (SAEs) include death, life-threatening, permanent, or severe disability or functional loss, hospitalization or prolonged hospitalization, and congenital abnormality or birth defect.
2.8 Statistical analysis
SAS9.4 software was used for safety analysis. The pharmacokinetic parameters were estimated and analyzed using WinNonlin software (version 8.0) using the non-compartment model. Cmax, Tmax, T1/2, AUC0–24h, AUC0-∞, Vd, and CL/F were calculated in the SAD study, and Cmax, Tmax, AUC0–24h, Cav(ss), Cmax(ss), Cmin(ss), DF, and Rac were calculated in the MAD study.
3 Results
3.1 Study population
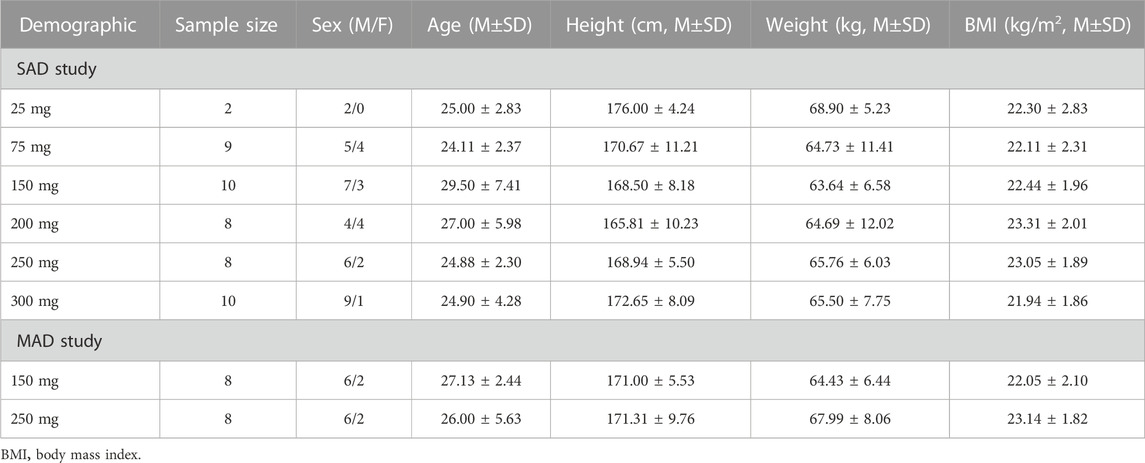
In the SAD study, 47 eligible volunteers were selected from 137 volunteers and assigned to six groups. In the MAD study, 16 eligible volunteers were selected from 64 volunteers and assigned to two groups. All the included volunteers completed the study as planned. The demographics of 63 volunteers are illustrated in Table 1. No significant difference was observed in the age, height, weight, and BMI of all groups.
3.2 Skin test and allergic reaction
In the study, 66 volunteers received the Sal B injection skin test. The concentration of Sal B in the skin test solution was 1/10 that of Sal B in the corresponding group. There were three volunteers who failed the skin test for enlarged and elevated erythema, and the positive rate was 4.4%. According to the protocol, these three volunteers were excluded from the trial. No skin and systemic allergic reactions were observed in the following procedure in the other 63 volunteers with negative skin tests.
3.3 Safety data
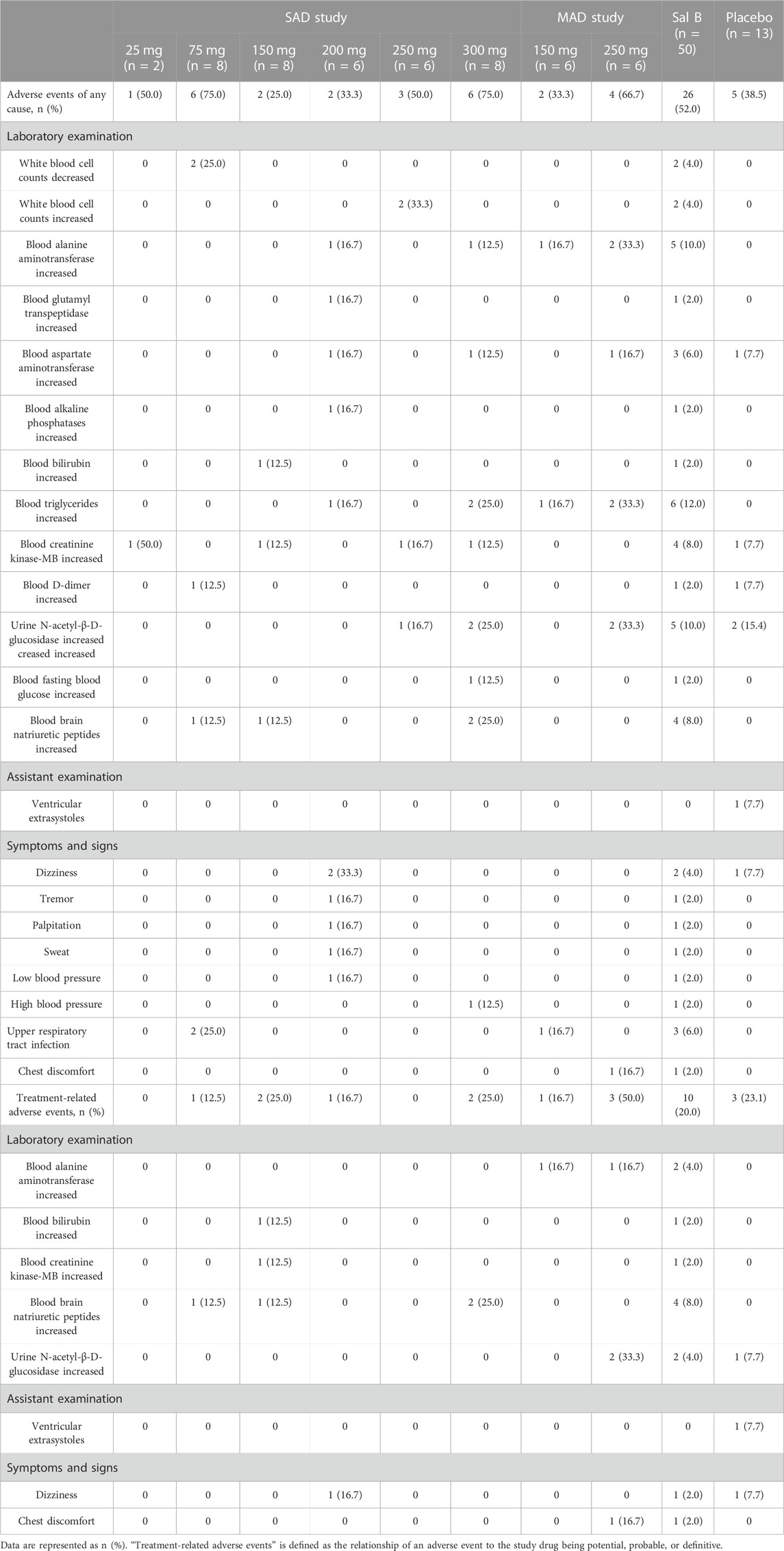
In the SAD study, there were 41 AEs in 24 cases with an incidence of 51.1% (24/47). Moreover, 10 treatment-related AEs occurred in nine cases with an incidence of 19.1% (9/47). In the MAD study, there were 13 AEs in eight cases with an incidence of 50.0% (8/16). Furthermore, five treatment-related AEs occurred in four cases with an incidence of 25% (4/16).
Changes in white blood cells occurred in two volunteers who received 75 mg and 250 mg of Sal B. Changes in liver function indicators occurred in 11 volunteers who received 150 mg or more of Sal B. Blood creatinine kinase-MB increased in four volunteers who received 25, 150, 250, and 300 mg of Sal B. Urine N-acetyl-β-D-glucosidase increased in five volunteers, who received 250 mg and 300 mg of Sal B. Blood brain natriuretic peptides increased in four volunteers who received 75, 150, and 300 mg of Sal B. One volunteer who received 200 mg of Sal B felt dizziness, tremor, palpitation, sweat, and low blood pressure but recovered in 40 min. Chest discomfort occurred in one volunteer who received 250 mg of Sal B for 5 consecutive days. Increased triglyceride is the most common adverse event (six cases), followed by increased alanine aminotransferase (five cases), increased NAG (five cases), increased creatinine kinase-MB (four cases), and increased BNP (four cases).
No clinically significant findings regarding physical examination, vital sign, or ECG were observed in volunteers receiving Sal B. No SAEs occurred, and no volunteers withdrew from the trial due to AEs. All observed AEs are shown in Table 2.
3.4 Pharmacokinetics
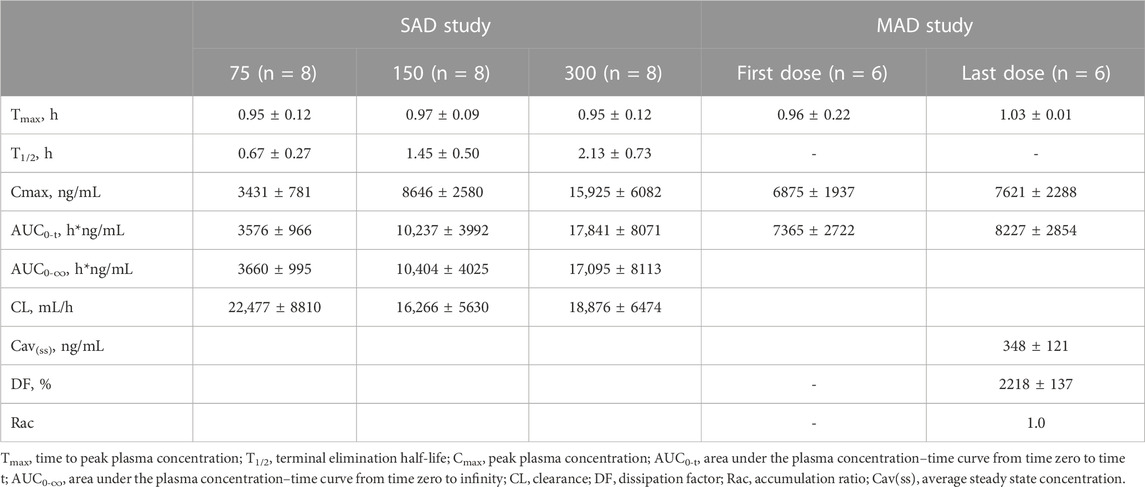
After a single administration of Sal B injection, the plasma exposure of Sal B increased in proportion to the dose, approximately. The mean Cmax was 3431, 8646, and 15,925 ng/mL, and the mean AUC0-t was 3576, 10,237, and 17,841 h*ng/mL in the 75, 150, and 300 mg groups. The plasma concentration–time profiles of Sal B are shown in Figure 2.
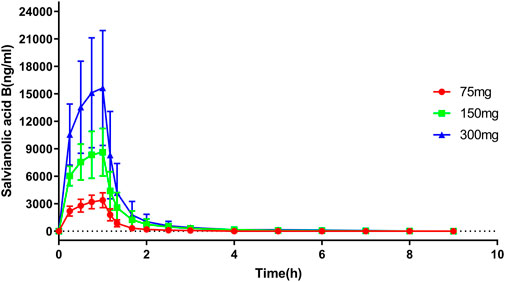
FIGURE 2. Plasma concentration–time profile of salvianolic B in the SAD study. n = 8. Data are presented as the mean ± SD.
After 5-consecutive-day administration of Sal B, Cav(ss) ranged from 227 ng/mL to 348 ng/mL, and Cmax(ss) ranged from 5010 to 10,700 ng/mL. The mean Cmax was 6875 and 7621 ng/mL, and the mean AUC0-t was 7365 and 8227 h*ng/mL in the first and last doses of Sal B. The accumulation coefficient (Rac) is 1.0. The plasma concentration–time profiles and pharmacokinetic parameter data are shown in Figure 3 and Table 3.
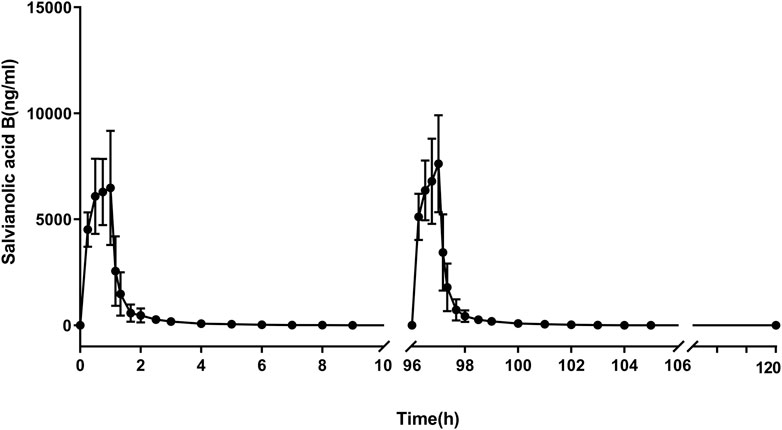
FIGURE 3. Plasma concentration–time profile of salvianolic B in the MAD study. n = 6. Data are presented as the mean ± SD.
4 Discussion
The purpose of this phase 1 clinical trial was to evaluate the safety, tolerability, and pharmacokinetics of Sal B injection in healthy volunteers. Overall, Sal B injection was well tolerated in healthy Chinese volunteers (25–300 mg), and the pharmacokinetic data exhibited dose-proportional increase in the 75, 150, and 300 mg groups. The MAD study revealed no accumulation. All the AEs were mild and resolved without any medical treatment, and continuous Sal B injection administration was possible. The physiological conditions of the volunteers were stable throughout the trial.
TCM injections in clinical application were usually the extracted mixture of TCMs, which were prone to cause allergic reaction because of their low purity (Xu and Dou, 2015). On the market, about 80% of the extracted mixture of salvianolic acid injection is salvianolic acid, while in this study the extract contains Sal B up to 96%. Only three cases of erythema were observed in the skin test, and no allergic reaction was observed in the following trial, which is probably due to the small amount of impurities.
Sal B is methylated by the liver to produce active metabolites, which may cause potential hepatotoxicity. Therefore, serum biochemical indicators should be concerned (Jia et al., 2010; Cheng et al., 2020). Blood alanine aminotransferase, glutamyl transpeptidase, aspartate aminotransferase, alkaline phosphatase, and/or bilirubin increased in 11 volunteers treated with Sal B, while only aspartate aminotransferase increased in the placebo group. The indicators increased within twice of the upper limit and recovered to the baseline in several days, indicating slight damage to the liver. A real-world study in SM depside salt revealed the same mild liver dysfunction in patients (Yan et al., 2017).
No significant damage of Sal B on renal function was found (Du et al., 2020). As a sensitive indicator of acute tubular injury, NAG increased in five volunteers with Sal B administration and once in the placebo group, which recovered in less than 10 days. Therefore, the damage of Sal B on the kidney seems to be slight and transient.
The elevated BNP is a marker of heart failure, and it is also a protective hormone to decrease blood pressure. We found the elevated BNP in four volunteers treated by Sal B, which was consistent with preclinical studies in healthy rats (Meng et al., 2018). Several TCMs showed dual-directional regulation in vivo, so we believe that Sal B has the same mechanism in healthy volunteers (Huo et al., 2000). In this study, the recipients enrolled were healthy volunteers with an average age of 26.2 years; further studies of the Sal B injection should be conducted in patients with cardiovascular disease.
Dizziness was the most common symptom among AEs (two cases, 4.0%) in Sal B groups, which is consistent with other reports (Ling et al., 2012; Yan et al., 2017). Two cases were mild and occurred at about 2 and 7 h after dosing and were experienced for 3 and 1.5 h, respectively, in the 200 mg group. Tremor, palpitation, sweat, and low blood pressure (SBP88mmHg/DBP49 mmHg) occurred in one volunteer during the same period, which were possibly complicated symptoms caused by dizziness. Chest discomfort (one cases, 2.0%) and increased creatinine kinase-MB (four cases, 8.0%) may be mediated by cardiovascular system damage, which were also found in other studies (Cong et al., 2022). Consistent with other studies (Ling et al., 2012), no clinical abnormalities were recorded in 12-lead ECG and Holter. All the clinical symptoms and signs were mild. Sal B showed a little effect on the coagulation system (Yan et al., 2017; Cong et al., 2022).
Cmax and AUC of Sal B proportionally increased with the dose in the 75, 150, and 300 SAD study. Cmax of the first (d1) and last doses (d5) in the 150-mg MAD study and the 150-mg SAD group were basically the same (p = 0.56, 0.46, and 0.19). The AUC0–t showed no significant difference between the first (d1) and last doses (d5) in the MAD phase and the 150-mg SAD group (p = 0.55, 0.31, and 0.16). The accumulation index was 1.0, which revealed no Sal B accumulated in vivo.
In conclusion, the current study showed that SAD at 25–300 mg and MAD up to 250 mg of Sal B were well tolerated with no SAEs observed. The accumulated safety and PK data in healthy volunteers support further evaluation in patient’s clinical trials for the treatment of Sal B.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee at Nanjing First Hospital Nanjing Medical University (YW20190926-02). The patients/participants provided their written informed consent to participate in this study.
Author contributions
HF and ZZ designed the study. JC wrote the article. JC, JZ, YH, JL, RQ, and HF performed the clinical trial. JL performed preclinical studies. All authors read and approved the final version of the manuscript for publication.
Funding
This study received funding from Nanjing Hongqiao Pharmaceutical Technology Research Institute Co., Ltd., and the Ministry of Science and Technology of the People’s Republic of China (Project No. 2017ZX09309022).
Acknowledgments
The authors thank all the volunteers in this trial, all investigators and site personnel, and company. Value pharmaceutical services Co., Ltd., was responsible for LC-MS analysis of salvianolic acid B, and Beijing Yeedozencom Science & Technology Co., Ltd., was responsible for the data analysis.
Conflict of interest
Authors JL, LH, and ZZ were employed by the company Nanjing Hongqiao Pharmaceutical Technology Research Institute Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bi, S. J., Dong, X. Y., Wang, Z. Y., Fu, S. J., Li, C. L., Wang, Z. Y., et al. (2022). Salvianolic acid B alleviates neurological injury by upregulating stanniocalcin 1 expression. Ann. Transl. Med. 10, 739. doi:10.21037/atm-21-4779
Bi, X., Liu, X., Di, L., and Zu, Q. (2016). Improved oral bioavailability using a solid self-microemulsifying drug delivery system containing a multicomponent mixture extracted from Salvia miltiorrhiza. Molecules 21, 456. doi:10.3390/molecules21040456
Cheng, J. Q., Shi, Q. P., Ding, F., Kong, L. T., Yu, M. L., and Wang, C. (2020). Liver function monitoring: A prospective nested case-control study of Salvia miltiorrhiza polyphenol injection. Sci. Rep. 10, 3538. doi:10.1038/s41598-020-60608-z
Cong, S., Dong, C., Hu, Y., Wang, C., Zhang, B., and Li, N. (2022). Effect of Salvia miltiorrhiza polyphenolic acid injection on improving limb use and cognitive impairment in patients with acute stroke. Comput. Math. Methods Med. 2022, 1481294. doi:10.1155/2022/1481294
Du, G., Song, J., Du, L., Zhang, L., Qiang, G., Wang, S., et al. (2020). Chemical and pharmacological research on the polyphenol acids isolated from danshen: A review of salvianolic acids. Adv. Pharmacol. 87, 1–41. doi:10.1016/bs.apha.2019.12.004
Feng, X., Li, Y., Wang, Y., Li, L., Little, P. J., Xu, S. W., et al. (2019). Danhong injection in cardiovascular and cerebrovascular diseases: Pharmacological actions, molecular mechanisms, and therapeutic potential. Pharmacol. Res. 139, 62–75. doi:10.1016/j.phrs.2018.11.006
Guo, Y. J., Wang, D. W., Meng, L., and Wang, Y. Q. (2015). Analysis of anaphylactic shock caused by 17 types of traditional Chinese medicine injections used to treat cardiovascular and cerebrovascular diseases. Biomed. Res. Int. 2015, 420607. doi:10.1155/2015/420607
Huang, R., Cai, Y., Yang, L., Shangguan, X., Ghose, B., and Tang, S. (2021). Safety of traditional Chinese medicine injection based on spontaneous reporting system from 2014 to 2019 in Hubei Province, China. Sci. Rep. 11, 8875. doi:10.1038/s41598-021-88339-9
Huo, H. R., Tan, Y. Q., Zhou, A. X., Li, X. Q., Guo, S. Y., Sun, Y. R., et al. (2000). Effect and mechanism of active fraction A guizhi decoction on dual-directional thermoregulation: Effect on heat shock protein in hypothalamus of rats. Zhongguo Zhong Yao Za Zhi 25, 619–621.
Ji, K., Chen, J., Li, M., Liu, Z., Xia, L., Wang, C., et al. (2009). Comments on serious anaphylaxis caused by nine Chinese herbal injections used to treat common colds and upper respiratory tract infections. Regul. Toxicol. Pharmacol. 55, 134–138. doi:10.1016/j.yrtph.2009.06.008
Jia, J. Y., Lu, Y. L., Li, X. C., Liu, G. Y., Li, S. J., Liu, Y., et al. (2010). Pharmacokinetics of depside salts from Salvia miltiorrhiza in healthy Chinese volunteers: A randomized, open-label, single-dose study. Curr. Ther. Res. Clin. Exp. 71, 260–271. doi:10.1016/j.curtheres.2010.08.004
Jia, Q., Zhu, R., Tian, Y., Chen, B., Li, R., Li, L., et al. (2019). Salvia miltiorrhiza in diabetes: A review of its pharmacology, phytochemistry, and safety. Phytomedicine 58, 152871. doi:10.1016/j.phymed.2019.152871
Li, Z. M., Thai, S., Lu, W., Sun, S., Tang, H., Zhai, S., et al. (2018a). Traditional Chinese medicine and drug-induced anaphylaxis: Data from the beijing pharmacovigilance database. Int. J. Clin. Pharm. 40, 921–927. doi:10.1007/s11096-018-0699-4
Li, Z. M., Xu, S. W., and Liu, P. Q. (2018b). Salvia miltiorrhizaBurge (danshen): A golden herbal medicine in cardiovascular therapeutics. Acta Pharmacol. Sin. 39, 802–824. doi:10.1038/aps.2017.193
Ling, S., Luo, R. Z., Nheu, L., Guo, Z. X., Sun, H., and Komesaroff, P. A. (2012). A phase I dose-escalation study to evaluate tolerability in a Western population to T89, a modern cardiovascular herbal medicine. J. Cardiovasc Pharmacol. 60, 513–519. doi:10.1097/FJC.0b013e31826f6179
Meng, Z. J., Wang, C., Meng, L. T., Bao, B. H., Wu, J. H., and Hu, Y. Q. (2018). Sodium tanshinone IIA sulfonate attenuates cardiac dysfunction and improves survival of rats with cecal ligation and puncture-induced sepsis. Chin. J. Nat. Med. 16, 846–855. doi:10.1016/S1875-5364(18)30126-2
Su, C. Y., Ming, Q. L., Rahman, K., Han, T., and Qin, L. P. (2015). Salvia miltiorrhiza: Traditional medicinal uses, chemistry, and pharmacology. Chin. J. Nat. Med. 13, 163–182. doi:10.1016/S1875-5364(15)30002-9
Wu, Y., Xu, S., and Tian, X. Y. (2020). The effect of salvianolic acid on vascular protection and possible mechanisms. Oxid. Med. Cell Longev. 2020, 5472096. doi:10.1155/2020/5472096
Xu, J., Wei, K., Zhang, G., Lei, L., Yang, D., Wang, W., et al. (2018). Ethnopharmacology, phytochemistry, and pharmacology of Chinese Salvia species: A review. J. Ethnopharmacol. 225, 18–30. doi:10.1016/j.jep.2018.06.029
Xu, Y. B., and Dou, D. Q. (2015). Advance and prospect in studies on anaphylactoid reaction of traditional Chinese medicine injections. Zhongguo Zhong Yao Za Zhi 40, 2765–2773.
Yan, Y. Y., Yang, Y. H., Wang, W. W., Pan, Y. T., Zhan, S. Y., Sun, M. Y., et al. (2017). Post-marketing safety surveillance of the Salvia miltiorrhiza depside salt for infusion: A real world study. PLoS One 12, e0170182. doi:10.1371/journal.pone.0170182
Zhang, J., Zhang, X., Zhang, J., Li, M., Chen, D., and Wu, T. (2018). Minor compounds of the high purity salvianolic acid B freeze-dried powder from Salvia miltiorrhiza and antibacterial activity assessment. Nat. Prod. Res. 32, 1198–1202. doi:10.1080/14786419.2017.1323212
Zhao, R., Liu, X., Zhang, L., Yang, H., and Zhang, Q. (2019). Current progress of research on neurodegenerative diseases of salvianolic acid B. Oxid. Med. Cell Longev. 2019, 3281260. doi:10.1155/2019/3281260
Keywords: salvianolic acid B, safety, tolerance, pharmacokinetics, traditional Chinese medicine injection
Citation: Cheng J, Long J, Zhang J, Han L, Hu Y, Liu J, Qiu R, Zhu Z and Fan H (2023) Safety, tolerance, and pharmacokinetics of salvianolic acid B in healthy Chinese volunteers: A randomized, double-blind, placebo-controlled phase 1 clinical trial. Front. Pharmacol. 14:1146309. doi: 10.3389/fphar.2023.1146309
Received: 17 January 2023; Accepted: 29 March 2023;
Published: 13 April 2023.
Edited by:
Alessandro Venditti, Sapienza University of Rome, ItalyReviewed by:
Chen Zhao, Institute of Basic Research in Clinical Medicine (CACMS), ChinaYi Li, China Pharmaceutical University, China
Copyright © 2023 Cheng, Long, Zhang, Han, Hu, Liu, Qiu, Zhu and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongwei Fan, fanhongwei178@sina.com; Zhibin Zhu, zzb5504@163.com
 Junlin Cheng
Junlin Cheng Jun Long2
Jun Long2 Runze Qiu
Runze Qiu Hongwei Fan
Hongwei Fan