- 1Department of Pharmacy, West China Hospital of Sichuan University, Chengdu, China
- 2Department of Pharmacy, Karamay Central Hospital, Karamay, China
- 3Department of Endocrinology and Metabolism, West China Hospital of Sichuan University, Chengdu, China
- 4West China School of Pharmacy, Sichuan University, Chengdu, China
Background: Sodium–glucose cotransporter-2 (SGLT2) inhibitors have proven to be effective in improving glycemic control in patients with type 2 diabetes mellitus (T2DM). However, the risk of diabetic ketoacidosis (DKA) in patients remains unclear. The purpose of this study is to conduct this systematic review and network meta-analysis for the risk of DKA of SGLT2 inhibitors in patients with T2DM.
Methods: We searched for randomized controlled trials (RCTs) concerning SGLT2 inhibitors in patients with T2DM in PubMed, EMBASE (Ovid SP), Cochrane Central Register of Controlled Trials (Ovid SP), and ClinicalTrials.gov from inception to January 2022. The primary outcomes were the risk of DKA. We assessed the sparse network with a fixed-effect model and consistency model in a frequentist framework with a graph-theoretical method by the netmeta package in R. We assessed the evidence quality of evidence of outcomes according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE).
Results: In total, 36 studies involving 52,264 patients were included. The network showed that there was no significant difference observed among SGLT2 inhibitors, other active antidiabetic drugs, and placebo in the risk of DKA. There was no significant difference in the DKA risk between different doses of SGLT2 inhibitors. The certainty of the evidence ranged from very low to moderate. The probabilities of rankings and P-score showed that compared to placebo, SGLT2 inhibitors might increase the risk of DKA (P-score = 0.5298). Canagliflozin might have a higher DKA risk than other SGLT2 inhibitors (P-score = 0.7388).
Conclusion: Neither SGLT2 inhibitors nor other active antidiabetic drugs were associated with an increased risk of DKA compared to placebo, and the risk of DKA with SGLT2 inhibitors was not found to be dose-dependent. In addition, the use of canagliflozin was less advisable than other SGLT2 inhibitors according to the rankings and P-score.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier PROSPERO, CRD42021297081.
1 Introduction
Type 2 diabetes mellitus (T2DM), the most common type of chronic disease characterized by hyperglycemic metabolism, has become a public health problem, prevalence and incidence of which have been increasing in recent years (Katsiki et al., 2020; Zhou et al., 2021). T2DM may cause irreversible damage to the heart and blood vessels, kidneys, and eyes (Chiang et al., 2021). At present, sodium-glucose cotransporter 2 (SGLT2) inhibitors are novel therapeutic targets for the treatment of T2DM through inhibiting glucose reabsorption in renal proximal convoluted tubules, reducing blood glucose fluctuations, promoting urinary glucose excretion, improving insulin sensitivity and ß-cell function in the liver and peripheral tissues, and further improving hepatic insulin resistance (Li et al., 2020; Scheen, 2020). In addition, SGLT2 inhibitors can exert the protective effect of the cardiovascular system and kidney, and delay the occurrence and development of T2DM complications by affecting blood lipid, weight loss, and blood pressure reduction (Häring et al., 2013; Li et al., 2021; Zheng et al., 2021; Zou et al., 2022).
Diabetic ketoacidosis (DKA) rarely occurs spontaneously in people with T2DM, but when appeared, it might be associated with the use of certain drugs (American Diabetes Association Professional Practice Committee, 2022). In May 2015, the Food and Drug Administration (FDA) issued a drug safety bulletin warning that canagliflozin, dapagliflozin, and empagliflozin could lead to hospitalization in patients with T2DM due to DKA (FDA, 2015a). However, according to a joint statement issued by the American Society of Clinical Endocrinologists (AACE) and the Endocrine Society (ACE), patients with T2DM treated with SGLT2 inhibitors have no higher risk of DKA than the general population, and there was no clear evidence that SGLT2 inhibitors are associated with DKA in T2DM (Handelsman et al., 2016). It remains unclear whether SGLT2 inhibitors increase the risk of DKA compared with other active antidiabetic drugs until now, and the risk of DKA among different doses of SGLT2 inhibitors also remains unknown. Therefore, we conducted this systematic review and network meta-analysis of the available evidence for the risk of DKA of SGLT2 inhibitors in patients with T2DM.
2 Methods
We conducted this systematic review and network meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA). This network meta-analysis was registered on the International Prospective Register of Systematic Review.
2.1 Literature search and eligible criteria
We comprehensively searched PubMed, EMBASE (Ovid SP), and Cochrane Central Register of Controlled Trials (Ovid SP) for studies published from the time when the databases were established to 26 January 2022. ClinicalTrial.gov was screened for unpublished studies. The reference lists of relevant published research studies investigating the risk of DKA of SGLT2 inhibitors in patients with T2DM were also screened for potentially relevant studies. The key terms searched in this study were based on the PICOS framework (Supplementary Table S1 and Supplementary Table S2). Duplicate records were removed with EndNote X9.
2.2 Study selection
We included studies meeting the following criteria: 1) participants: adults (>18 years) with a diagnosis of T2DM; 2) interventions/comparisons: SGLT2 inhibitors, active antidiabetic drugs (we defined active antidiabetic drugs as antidiabetic drugs other than SGLT2 inhibitors), or placebo; 3) outcomes: reporting the risk of DKA; and 4) study design: published or unpublished randomized controlled trials (RCTs) limited to the English language. The exclusion criteria were as follows: 1) including pregnant participants; 2) animal experiments; 3) studies published in a language other than English; 4) published as abstract only; 5) including patients with prediabetes; and 6) DKA caused by T2DM.
2.3 Screening process and data extraction
All retrieved literature studies were identified by two independent reviewers (YL and SY), and data were extracted by a predefined form. Any discrepancies were resolved by discussion with a third reviewer (NS), as required. We extracted the data including the first author’s name, publication year, sample size, follow-up length, intervention and comparison, outcomes, and the characteristics of participants.
SGLT2 inhibitors with diverse doses were separated to several trials. If a study contained more than one SGLT-2 inhibitor or more than one dose of SGLT-2 inhibitors, we defined them as a different comparison.
2.4 Quality assessment and the certainty of evidence
Four reviewers (N.S., P.F., Y.L., and S.Y.) conceived the study. Two independent reviewers (Y.L. and S.Y.) assessed the risk of bias of all included studies according to ROB 2, a revised Cochrane risk-of-bias tool for randomized trials (Sterne et al., 2019), and the discrepancies were resolved by consulting the third reviewer (N.S.). The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) was used to assess the certainty of the evidence for the outcome. Three reviewers (F.W., D.L., and S.Z.) conducted the analysis and interpreted the data. Two reviewers (Q.W. and H.Z.) checked the analysis data on the review. The members of the research team assessed the confidence rating for each comparison as high, moderate, low, or very low, based on the direct and indirect estimates. Discrepancies were resolved by discussions.
2.5 Treatment nodes
Treatment nodes were grouped by different kinds of active antidiabetic drugs and different doses of SGLT2 inhibitors. We drew network plots with the multinma package in R (version 4.1.3) (Multinma, 2020).
2.6 Statistical analysis
We conducted a network meta-analysis of randomized controlled trials that assessed the sparse network with a fixed-effect model (Efthimiou et al., 2019) and consistency model in the frequentist framework with a graph-theoretical method by the netmeta package in R (version 4.1.3) (Rücker et al., 2015). The effect size for assessing DKA safety was calculated as odds ratios (ORs) with accompanying 95% credible intervals (CIs). We calculated the consistency by node-splitting models (Van Valkenhoef et al., 2016). We calculated the P-score to rank treatments (Salanti et al., 2011). We assessed the global and local statistical heterogeneity with generalized Cochran’s Q. We estimated the variance in heterogeneity between studies using the DerSimonian–Laird random-effects model. We assessed transitivity using descriptive statistics from studies and population baselines (Cipriani et al., 2013).
We assessed publication bias using funnel plots and Egger’s test with the netmeta package in R. Multiple sensitivity analyses were carried out to assess the robustness of the final results, including the following: 1) this analysis was estimated in a Bayesian framework; 2) exclusion of studies with treatment duration <24 weeks; 3) exclusion of studies without a placebo control; 4) exclusion of the high risk of bias studies (exclusion of unblinded studies.); 5) exclusion of studies where the risk of DKA was 0 percent; and 6) exclusion of studies with fewer than 100 participants.
3 Results
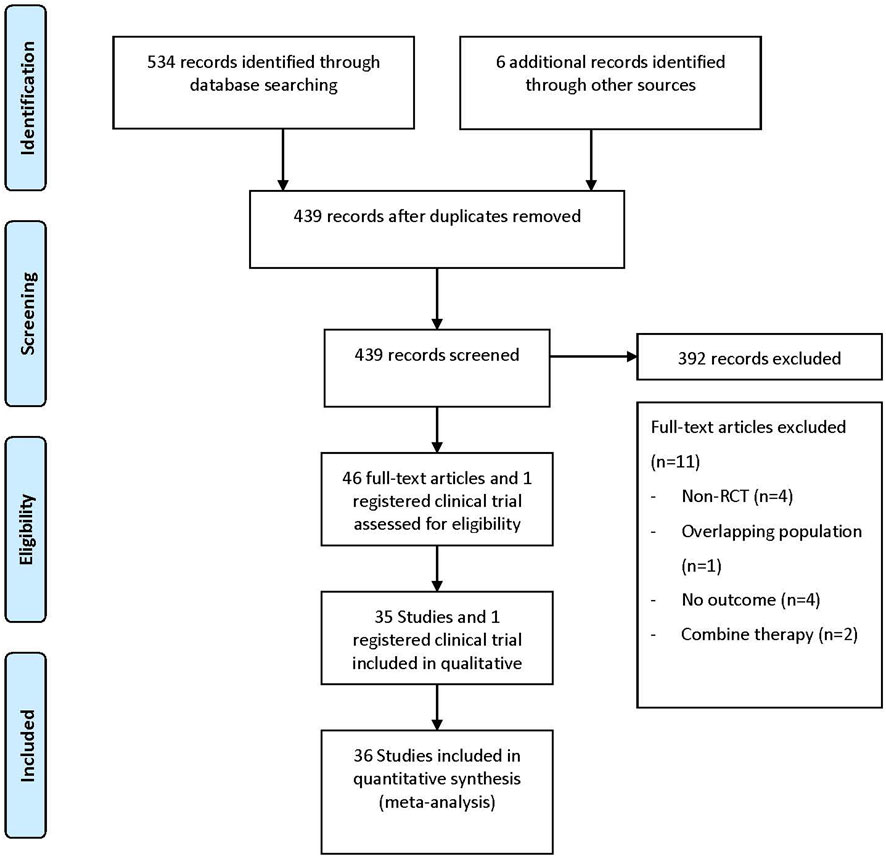
3.1 Characteristics of eligible studies
The literature search flow diagram is shown in Figure 1. After screening 534 articles and 6 registered clinical trials, a total of 36 studies between 2013 and 2021 were included in the meta-analysis according to predetermined criteria (35 articles and 1 registered clinical trial), involving 52,264 patients and 70 DKA events (Bode et al., 2013; Lavalle-González et al., 2013; NCT, 2013; Barnett et al., 2014; Rosenstock et al., 2014; Erondu et al., 2015; Haering et al., 2015; Roden et al., 2015; Rosenstock et al., 2015; Araki et al., 2016; Frías et al., 2016; Hadjadj et al., 2016; Mancia et al., 2016; Rodbard et al., 2016; Rosenstock et al., 2016; Ito et al., 2017; Neal et al., 2017; Søfteland et al., 2017; Aronson et al., 2018; Dagogo-Jack et al., 2018; Fioretto et al., 2018; Han et al., 2018; Hollander et al., 2018; Kawamori et al., 2018; Pratley et al., 2018; Ridderstrale et al., 2018; Rosenstock et al., 2018; Scott et al., 2018; Terauchi et al., 2018; Allegretti et al., 2019; Cho et al., 2019; Ji et al., 2019; Perkovic et al., 2019; Pollock et al., 2019; Cahn et al., 2020; Persson et al., 2021; Weng et al., 2021). Of the included studies, 21 were conducted in multinational country studies, and most studies were registered (35/36, 97%) and all published in English. The baseline characteristics of the included studies are presented in Table 1 and Supplementary Table S3, where 34 were two-arm studies and two were three-arm studies. Among the 36 studies (the retrieved SGLT2 inhibitors contain 1 study about bexagliflozin; 7 studies about canagliflozin; 8 studies about dapagliflozin; 10 studies about empagliflozin; 6 studies about ertugliflozin; 1 study about henagliflozin; 2 studies about ipragliflozin; and 1 study about tofogliflozin), 19 studies with different doses of SGLT2 inhibitors were compared; 27 studies compared SGLT2 inhibitors to placebo; 11 studies compared SGLT2 inhibitors to active antidiabetic drugs (active antidiabetic drugs retrieved include pioglitazone, exenatide, glimepiride, metformin, and sitagliptin); and two studies compared active drugs to placebo. The study population comprised 31,829 males (60.9%) and 20,435 females (39.1%), with the mean age being 59.3 years (ranging from 51.6 to 69.9 years); the mean HbA1c was 8.1% (ranging from 6.9% to 9.3%); the baseline mean BMI was 30.4 kg/m2 (ranging from 25.4 to 35.0 kg/m2); the mean disease duration was 9.8 years (ranging from 3.3 to 17.7 years); and the mean duration of treatment was 61.7 weeks (ranging from 12.0 to 271.0 weeks). In addition, most trials were funded by pharmaceutical companies (34/36, 94%).
3.2 Risk of bias of included studies
The overall risk of bias was low. The assessment of the risk of bias in the included studies is shown in Supplementary Table S4. Overall quality assessment indicated that more than half of the studies had a low risk of bias.
3.3 Results of network meta-analysis
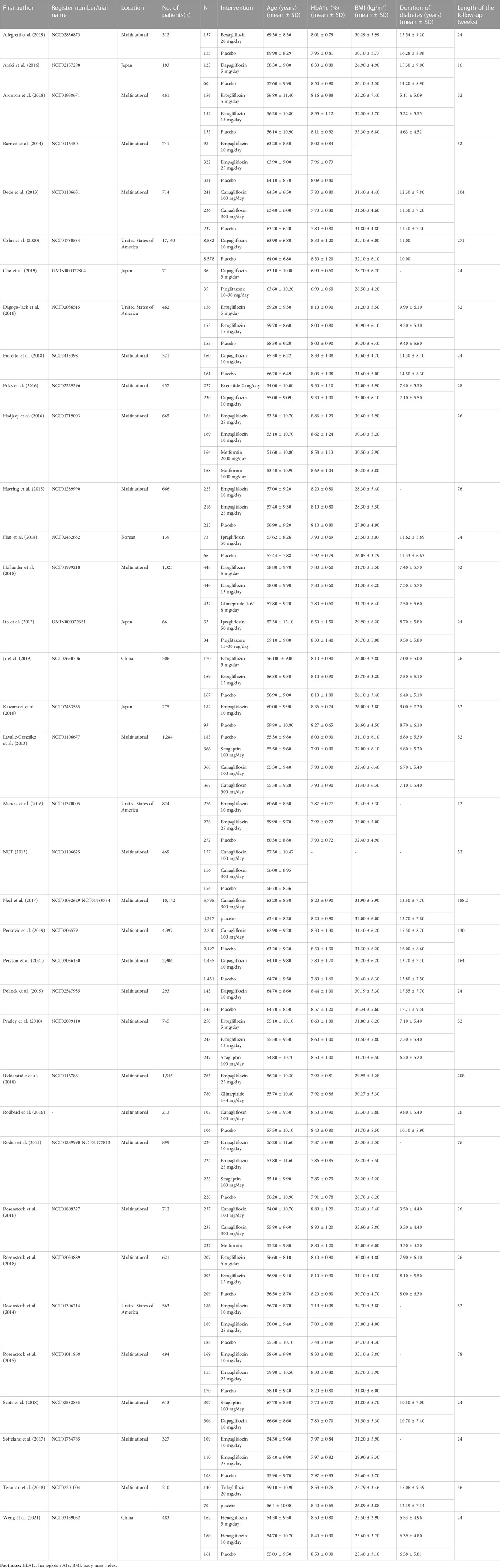
The network plots of each outcome are presented in Figure 2A and Figure 2B, presenting the results and quality of evidence for the different doses of SGLT2 inhibitors and the different active antidiabetic drugs. The inconsistency of the network meta-analysis is also evaluated in Supplementary Figure S1 and Supplementary Figure S2. Heterogeneity and intransitivity of the network meta-analysis were also evaluated (Supplementary Table S5–6, Supplementary Figures S3–S5).
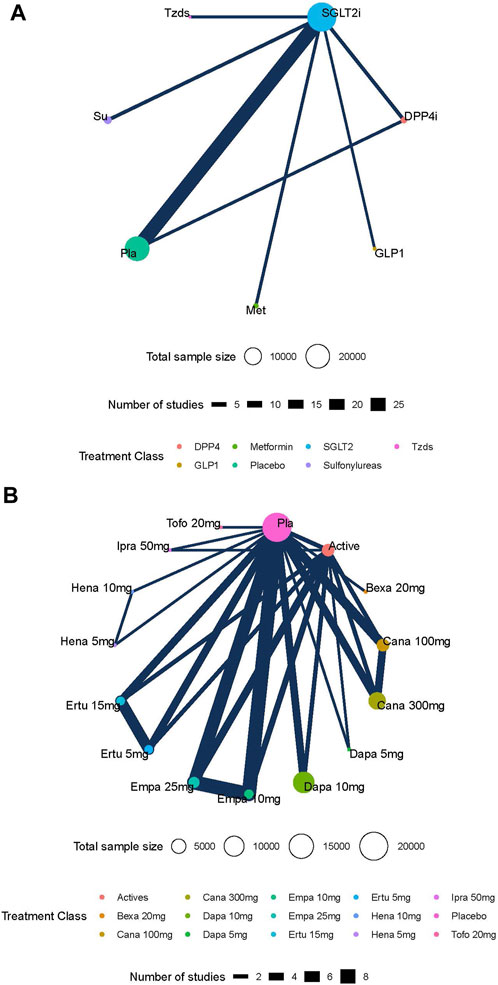
FIGURE 2. (A) Network plots of the risk of diabetic ketoacidosis (DKA) with different kinds of active antidiabetic drugs. Footnotes: nodes in different colors indicate different processing. The node size corresponds to the number of participants treated in the study. The thickness of the edge represents the number of tests. The lack of lines suggests that there have been no head-to-head trials of this outcome between the two treatments. (B) Network plots of the risk of diabetic ketoacidosis (DKA) with different kinds of active antidiabetic drugs. Footnotes: nodes in different colors indicate different processing. The node size corresponds to the number of participants treated in the study. The thickness of the edge represents the number of tests. The lack of lines suggests that there have been no head-to-head trials of this outcome between the two treatment procedures.
3.4 DKA
In total, 36 studies including 52,264 patients were reported on the risk of DKA, with a total of 70 DKA events occurring at a rate of 0.13%. Intervention nodes included in this network meta-analysis were different doses of SGLT2 inhibitors, metformin, sitagliptin, glimepiride, pioglitazone, and placebo. The SGLT2 inhibitor was not associated with a statistically significant increase in the risk of DKA (Figure 3A). There was also no difference in the risk of DKA between different doses of SGLT2 inhibitors (Figure 3B). The global I2 of pairwise was 0%, and the global I2 of the consistency model was 0%. The node split analysis showed that the results were consistent. The GRADE quality for the network meta-analysis is shown in Supplementary Table S7 and Supplementary Table S8.
FIGURE 3. (A) Network estimates (league tables) for different kinds of active antidiabetic drugs. Footnotes: outcome: the risk of DKA (odds ratio; 95% confidence interval). The league table presented the relative effects of different kinds of active antidiabetic drugs (the risk of DKA on the column to the risk of DKA of the row). DPP4i: dipeptidyl peptidase-4 inhibitor; SGLT2i: sodium–glucose cotransporter 2 inhibitors; SU: sulphonylurea; Tzds: thiazolidinediones; Met: metformin; Pla: placebo; DKA: diabetic ketoacidosis. (B) Network estimates (league tables) for different doses of SGLT-2i. Footnotes: outcome: the risk of DKA (odds ratio; 95% confidence interval). The league table presented the relative effects of different kinds of SGLT2 inhibitors (the risk of DKA on the column to the risk of DKA of the row). SGLT2i: sodium–glucose cotransporter 2 inhibitors; Cana: canagliflozin; Dapa: dapagliflozin; Ertu: ertugliflozin; Ipra: ipragliflozin; Hena: henagliflozin; Bexa: bexagliflozin; Empa: empagliflozin; Tofo: tofogliflozin; Pla: placebo; DKA: diabetic ketoacidosis.
3.5 Rankings and P-score
The P-score of DKA for different kinds of active antidiabetic drugs is illustrated in Supplementary Table S9, and the P-score of DKA for different doses of SGLT-2 inhibitors is illustrated in Supplementary Table S10. A higher P-score indicated a higher risk of DKA. There were no significant differences in network estimates between different hypoglycemic agents, but the P-score of DKA suggested that different kinds of active antidiabetic drugs ranked for the risk of DKA. Supplementary Table S9 shows that the highest P-score was of glucagon-like peptide-1 agonists (GLP1RAs) (P-score = 0.7361). The SGLT2 inhibitor ranked the third (P-score = 0.5298). Likewise, Supplementary Table S10 shows that canagliflozin (100 mg) had the highest P-score (P-score = 0.7388), and the lowest was tofogliflozin (20 mg) (P-score = 0.3491). The P-score was not found to be dose-dependent.
3.6 Funnel plot and sensitivity analysis
Egger’s test showed that there is no publication bias for different doses of SGLT2 inhibitors (p = 0.10, Supplementary Figure S6), but there is a publication bias for different kinds of active antidiabetic drugs (p < 0.01, Supplementary Figure S7). The sensitivity analyses are presented in Supplementary Table S11 and Supplementary Table S12. Studies with a duration of less than 24 weeks were excluded in the sensitivity analysis. The results showed that all sensitivity analyses demonstrated consistency with the primary results, regardless of the inclusion or exclusion of studies lasting less than 24 weeks.
4 Discussion
This network meta-analysis provides an overview of the evidence regarding the DKA safety of SGLT2 inhibitors and antidiabetic drugs in patients with T2DM. This result indicated that there was no significant difference in the risk of DKA between different kinds of antidiabetic drugs or different doses of SGLT2 inhibitors with very low-to-moderate certainty. There was no dose-dependent relation between SGLT2 inhibitors and the risk of DKA. Neither antidiabetic drugs nor SGLT2 inhibitors increased the risk of DKA compared with placebo in patients with T2DM. Nevertheless, although not statistically significant, our results indicate that the SGLT2 inhibitor ranked third in the risk of DKA, behind only the GLP-1RA and DPP4 inhibitor. According to the results of ranking and P-score, the risk of DKA slightly elevated among the different kinds of active antidiabetic drugs for glucagon-like peptide 1 receptor agonists (the type of GLP-1RAs included exenatide). Among the different doses of SGLT2 inhibitors, canagliflozin showed a slightly higher risk of DKA, with tofogliflozin being the lowest risk. According to the ranking and P-score, it is found that compared to the high dose, the incidence of DKA with low-dose canagliflozin is even lower.
There have been some DKA case reports linked to the use of canagliflozin, but the exact cause remains to be determined (Turner et al., 2016; Sloan et al., 2018). The potential causes of DKA in patients taking canagliflozin are believed to be four mechanisms: first, fluid loss; second, glucagon secretion increases; third, elevated glucagon–insulin ratio; and finally, acute prerenal azotemia (Chai et al., 2017).
The FDA issued a warning that the use of SGLT2 inhibitors in type 2 diabetes may cause euglycemia DKA (eDKA) in 2015 (FDA, 2015b). The American Diabetes Association also indicated that all patients on SGLT2 inhibitors were at risk for DKA but was rare in T2DM (Draznin et al., 2022). SGLT2 inhibitors may increase the risk of DKA through three possible mechanisms. First, SGLT2 inhibitors lower blood glucose concentrations and increase urinary glucose excretion, which reduces insulin production and promote glucagon production. The lack of insulin leads to the release of α-glycerol from liberals and amino acids from muscle decomposition, which promotes gluconeogenesis and leads to the increase of ketone bodies in the body. Decreasing insulin levels promote the process of lipolysis, which leads to the accumulation of ketones in the body. Second, SGLT2 inhibitors promote ketone body reabsorption by increasing the concentration of sodium ions in the renal tubules. Finally, SGLT2 inhibitors have a diuretic effect and reduce blood volume, thereby promoting the development of ketoacidosis (Cohen et al., 1956; Vallon et al., 2014; Yokono et al., 2014; Mudaliar et al., 2015; Fleming et al., 2020). However, not all patients on SGLT2 inhibitors are at a high risk of DKA. Other factors, such as infection, recent surgery, serious illness, insufficient insulin supply, low-carbohydrate diet, past pancreatitis, and dehydration, can interact with the use of SGLT2 inhibitors and amplify the risk (Bamgboye et al., 2021). Co-action of these risk factors with SGLT2 inhibitors ultimately leads to alter glucose production and increases the production of lipolysis and ketone bodies.
Regarding the use of SGLT2 inhibitors in T2DM, ketoacidosis has been a subject of debate. Different studies have reached inconsistent conclusions about the association between SGLT2 inhibitors and DKA risk. A systematic review including 10 RCTs with a total of 71,553 subjects showed that SGLT2 inhibitors led to increased risks of DKA, and the DKA was approximately three times higher with SGLT2 inhibitors (95%CI 1.36–3.63) (Lin et al., 2021). On the contrary, one unpublished registered clinical trial (NCT03764631) reported that there was no difference in the risk of DKA between empagliflozin and DPP4 inhibitors (Mansour, 2022).
Our analysis confirms the safety of SGLT2 inhibitors with regards to the risk of DKA, similar to that of placebo. In 2019, the United Kingdom Medicines and Health Products Regulatory Agency (MHRA) issued a warning that the use of GLP1RAs in combination with insulin may increase the risk of DKA (MHRA, 2019). This warning is consistent with the conclusions reached in our meta-analysis.
4.1 Strengths and limitations
Our study is the first frequentist network meta-analysis of SGLT2 inhibitors investigating the risk of DKA; in addition, we included one unpublished trial from the ClinicalTrials database that provided additional DKA data. Apart from that, we performed the quality assessment on all the included literature studies, ensuring that the literature studies were of high quality. Our systematic review and network meta-analysis included a large pool of trials and patients retrieved through a comprehensive literature search, and we used up-to-date and rigorous methodological tools to assess the risk of bias for the outcome. We included clinical trials from inception through 26 January 2022, with access to additional long-term trials (19 long-term follow-up of 52 weeks or more) and recent studies. A major strength of this network meta-analysis was to compare the risk of DKA among different active antidiabetic drugs. Furthermore, our study supports the evidence of the risk of DKA among different doses of SGLT2 inhibitors, which was the first time to be reported.
Several potential limitations should be acknowledged. First of all, the duration of treatment varied widely among the included studies, ranging from 12 weeks to 271 weeks. Second, because the incidence of DKA was low, the included trials reported a relatively small number of DKA, even reported no DKA event, which caused too much sparse data. We assessed the sparse network with a fixed-effect model and used the sensitivity analysis which excluded the sparse data’ studies for reducing the impact of sparse data. Third, all included studies did not distinguish eDKA and DKA. Therefore, our outcome was DKA not eDKA. Fourth, due to the influence of data collection, the results of our meta-analysis are more relevant to a population that is predominantly male, white, older, longer duration of diabetes, higher body mass index (BMI), and higher HbA1c levels. Moreover, we did not conduct a subgroup analysis to explore the correlation between the risk of SGLT2i-associated DKA and the duration of diabetes because of the range of data variations. There were few studies about the relationship between DKA and the duration of T2DM. An analysis of data on the incidence of DKA in SGLT2 inhibitors from the FDA Adverse Event Reporting System examined that the range of duration was from 1 day to >8 years, and there was no significance due to the limited availability of treatment duration information in some reports (Fadini et al., 2017).
5 Conclusion
This network meta-analysis suggested that neither SGLT2 inhibitors nor other active antidiabetic drugs increase the risk of DKA compared with placebo. At the same time, a consistent dose-effect gradient with increasing SGLT2 inhibitors doses was observed for treatment effect but not for the risk of DKA. However, given that the P-score of SGLT2 inhibitors was higher than placebo, SGLT2 inhibitors still needed to be cautiously used for patients with T2DM with a history of DKA. Furthermore, it is better to avoid the use of canagliflozin compared with other SGLT2 inhibitors based on the ranking and P-score. In the future, with the increasing use of SGLT-2 inhibitors, it is crucial to enhance and improve the safety studies of SGLT2 inhibitors by incorporating more clinical trials with large sample sizes and high quality.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
NS, PF, YL, and SY negotiated a plan for this study. SY and YL completed the retrieval and data extraction. NS is the primary monitor for this study. FW, DL, and SZ completed the analysis of the data together. QW and HZ mainly checked the final data on the review. SY and YL wrote this manuscript together. All authors contributed to the article and approved the submitted version.
Funding
NS was supported by grants from the Sichuan Province Science and Technology Support Program (Grant number: 2023JDR0243) and the Health Commission Program (Grant number: 2020-111). This research was supported by the National Key Clinical Specialties Construction Program.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1145587/full#supplementary-material
References
Allegretti, A. S., Zhang, W., Zhou, W., Thurber, T. K., Rigby, S. P., Bowman-Stroud, C., et al. (2019). Safety and effectiveness of bexagliflozin in patients with type 2 diabetes mellitus and stage 3a/3b CKD. Am. J. Kidney Dis. 74 (3), 328–337. doi:10.1053/j.ajkd.2019.03.417
American Diabetes Association Professional Practice Committee (2022). 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2022. Diabetes Care 45 (1), S17–s38. doi:10.2337/dc22-S002
Araki, E., Onishi, Y., Asano, M., Kim, H., and Yajima, T. (2016). Efficacy and safety of dapagliflozin over 1 year as add-on to insulin therapy in Japanese patients with type 2 diabetes: The DAISY (dapagliflozin added to patients under InSulin therapY) trial. Diabetes Obes. Metab. 19 (4), 562–570. doi:10.1111/dom.12853
Aronson, R., Frias, J., Goldman, A., Darekar, A., Lauring, B., and Terra, S. G. (2018). Long-term efficacy and safety of ertugliflozin monotherapy in patients with inadequately controlled T2DM despite diet and exercise: VERTIS MONO extension study. Diabetes Obes. Metab. 20 (6), 1453–1460. doi:10.1111/dom.13251
Bamgboye, A. O., Oni, I. O., and Collier, A. (2021). Predisposing factors for the development of diabetic ketoacidosis with lower than anticipated glucose levels in type 2 diabetes patients on SGLT2-inhibitors: A review. Eur. J. Clin. Pharmacol. 77 (5), 651–657. doi:10.1007/s00228-020-03051-3
Barnett, A. H., Mithal, A., Manassie, J., Jones, R., Rattunde, H., Woerle, H. J., et al. (2014). Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: A randomised, double-blind, placebo-controlled trial. Lancet Diabetes and Endocrinol. 2 (5), 369–384. doi:10.1016/S2213-8587(13)70208-0
Bode, B., Stenlof, K., Sullivan, D., Fung, A., and Usiskin, K. (2013). Efficacy and safety of canagliflozin treatment in older subjects with type 2 diabetes mellitus: A randomized trial. Hosp. Pract. 41(2), 72–84. doi:10.3810/hp.2013.04.1020
Cahn, A., Raz, I., Bonaca, M., Mosenzon, O., Murphy, S. A., Yanuv, I., et al. (2020). Safety of dapagliflozin in a broad population of patients with type 2 diabetes: Analyses from the DECLARE-TIMI 58 study. Diabetes Obes. Metab. 22 (8), 1357–1368. doi:10.1111/dom.14041
Chai, P. R., Bonney, C., Blohm, E., Boyer, E. W., and Babu, K. M. (2017). Canagliflozin-associated diabetic ketoacidosis: A case report. Toxicol. Commun. 1 (1), 2–5. doi:10.1080/24734306.2017.1331604
Chiang, C.-E., Ueng, K.-C., Chao, T.-H., Lin, T.-H., Wu, Y.-J., Wang, K.-L., et al. (2021). 2021 consensus pathway of the taiwan society of cardiology on novel therapy for type 2 diabetes. JACC Asia 1 (2), 129–146. doi:10.1016/j.jacasi.2021.08.003
Cho, K. Y., Nakamura, A., Omori, K., Takase, T., Miya, A., Manda, N., et al. (2019). Effect of switching from pioglitazone to the sodium glucose co-transporter-2 inhibitor dapagliflozin on body weight and metabolism-related factors in patients with type 2 diabetes mellitus: An open-label, prospective, randomized, parallel-group comparison trial. Diabetes Obes. Metab. 21 (3), 710–714. doi:10.1111/dom.13557
Cipriani, A., Higgins, J. P., Geddes, J. R., and Salanti, G. (2013). Conceptual and technical challenges in network meta-analysis. Ann. Intern. Med. 159 (2), 130–137. doi:10.7326/0003-4819-159-2-201307160-00008
Cohen, J. J., Berglund, F., and Lotspeich, W. D. (1956). Renal tubular reabsorption of acetoacetate, inorganic sulfate and inorganic phosphate in the dog as affected by glucose and phlorizin. Am. J. Physiol. 184 (1), 91–96. doi:10.1152/ajplegacy.1955.184.1.91
Dagogo-Jack, S., Liu, J., Eldor, R., Amorin, G., Johnson, J., Hille, D., et al. (2018). Efficacy and safety of the addition of ertugliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sitagliptin: The VERTIS SITA2 placebo-controlled randomized study. Diabetes Obes. Metab. 20 (3), 530–540. doi:10.1111/dom.13116
Draznin, B., Aroda, V. R., Bakris, G., Benson, G., Brown, F. M., Freeman, R., et al. (2022). 9. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes-2022. Diabetes Care 45 (1), S125–s143. doi:10.2337/dc22-S009
Efthimiou, O., Rücker, G., Schwarzer, G., Higgins, J. P. T., Egger, M., and Salanti, G. (2019). Network meta-analysis of rare events using the Mantel-Haenszel method. Stat. Med. 38 (16), 2992–3012. doi:10.1002/sim.8158
Erondu, N., Desai, M., Ways, K., and Meininger, G. (2015). Diabetic ketoacidosis and related events in the canagliflozin type 2 diabetes clinical Program. Diabetes Care 38 (9), 1680–1686. doi:10.2337/dc15-1251
Fadini, G. P., Bonora, B. M., and Avogaro, A. (2017). SGLT2 inhibitors and diabetic ketoacidosis: Data from the FDA Adverse event reporting System. Diabetologia 60 (8), 1385–1389. doi:10.1007/s00125-017-4301-8
FDA (2015a). FDA updates SGLT2 inhibitor labels for diabetes to include warnings about blood acid levels that are too high and serious urinary tract infections. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/la-fda-actualiza-las-etiquetas-de-los-inhibidores-del-sglt2-para-la-diabetes-fin-de-incluir (Accessed December 12, 2022).
FDA (2015b). SGLT2 inhibitor diabetes drugs may cause ketoacidosis. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/comunicado-de-la-fda-sobre-la-seguridad-de-los-medicamentos-la-fda-advierte-que-el-uso-de (Accessed December 12, 2022).
Fioretto, P., Del Prato, S., Buse, J. B., Goldenberg, R., Giorgino, F., Reyner, D., et al. (2018). Efficacy and safety of dapagliflozin in patients with type 2 diabetes and moderate renal impairment (chronic kidney disease stage 3A): The DERIVE Study. Diabetes Obes. Metab. 20 (11), 2532–2540. doi:10.1111/dom.13413
Fleming, N., Hamblin, P. S., Story, D., and Ekinci, E. I. (2020). Evolving evidence of diabetic ketoacidosis in patients taking sodium-glucose cotransporter 2 inhibitors. J. Clin. Endocrinol. Metab. 105 (8), dgaa200. doi:10.1210/clinem/dgaa200
Frías, J. P., Guja, C., Hardy, E., Ahmed, A., Dong, F., Öhman, P., et al. (2016). Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): A 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes and Endocrinol. 4 (12), 1004–1016. doi:10.1016/S2213-8587(16)30267-4
Hadjadj, S. R. J., Meinicke, T., Woerle, H. J., and Broedl, U. C. (2016). Initial combination of empagliflozin and metformin in patients with type 2 diabetes. Diabetes Care 39 (10), 1718–1728. doi:10.2337/dc16-0522
Haering, H. U., Merker, L., Christiansen, A. V., Roux, F., Salsali, A., Kim, G., et al. (2015). Empagliflozin as add-on to metformin plus sulphonylurea in patients with type 2 diabetes. Diabetes Res. Clin. Pract. 110 (1), 82–90. doi:10.1016/j.diabres.2015.05.044
Han, K. A., Chon, S., Chung, C. H., Lim, S., Lee, K. W., Baik, S., et al. (2018). Efficacy and safety of ipragliflozin as an add-on therapy to sitagliptin and metformin in Korean patients with inadequately controlled type 2 diabetes mellitus: A randomized controlled trial. Diabetes Obes. Metab. 20 (10), 2408–2415. doi:10.1111/dom.13394
Handelsman, Y., Henry, R. R., Bloomgarden, Z. T., Dagogo-Jack, S., DeFronzo, R. A., Einhorn, D., et al. (2016). American association of clinical Endocrinologists and American college of endocrinology position statement on the association of sglt-2 inhibitors and diabetic ketoacidosis. Endocr. Pract. 22 (6), 753–762. doi:10.4158/ep161292.ps
Häring, H. U., Merker, L., Seewaldt-Becker, E., Weimer, M., Meinicke, T., Woerle, H. J., et al. (2013). Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: A 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care 36 (11), 3396–3404. doi:10.2337/dc12-2673
Hollander, P., Liu, J., Hill, J., Johnson, J., Jiang, Z. W., Golm, G., et al. (2018). Ertugliflozin compared with glimepiride in patients with type 2 diabetes mellitus inadequately controlled on metformin: The VERTIS SU randomized study. Diabetes Ther. 9 (1), 193–207. doi:10.1007/s13300-017-0354-4
Ito, D. S. S., Inoue, K., Saito, D., Yanagisawa, M., Inukai, K., Akiyama, Y., et al. (2017). Comparison of ipragliflozin and pioglitazone effects on nonalcoholic fatty liver disease in patients with type 2 diabetes: A randomized, 24-week, open-label, active-controlled trial. Diabetes Care 40 (10), 1364–1372. doi:10.2337/dc17-0518
Ji, L., Liu, Y., Miao, H., Xie, Y., Yang, M., Wang, W., et al. (2019). Safety and efficacy of ertugliflozin in asian patients with type 2 diabetes mellitus inadequately controlled with metformin monotherapy: VERTIS asia. Diabetes Obes. Metab. 21 (6), 1474–1482. doi:10.1111/dom.13681
Katsiki, N., Ferrannini, E., and Mantzoros, C. (2020). New American Diabetes Association (ADA)/European Association for the Study of Diabetes (EASD) guidelines for the pharmacotherapy of type 2 diabetes: Placing them into a practicing physician's perspective. Metabolism 107, 154218. doi:10.1016/j.metabol.2020.154218
Kawamori, R., Haneda, M., Suzaki, K., Cheng, G., Shiki, K., Miyamoto, Y., et al. (2018). Empagliflozin as add-on to linagliptin in a fixed-dose combination in Japanese patients with type 2 diabetes: Glycaemic efficacy and safety profile in a 52-week, randomized, placebo-controlled trial. Diabetes Obes. Metab. 20 (9), 2200–2209. doi:10.1111/dom.13352
Lavalle-González, J. A., Davidson, J., Tong, C., Qiu, R., Canovatchel, W., Meininger, G., et al. (2013). Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: A randomised trial. Diabetologia 56 (12), 2582–2592. doi:10.1007/s00125-013-3039-1
Li, S., Nemeth, I., Donnelly, L., Hapca, S., Zhou, K., and Pearson, E. R. (2020). Visit-to-Visit HbA(1c) variability is associated with cardiovascular disease and microvascular complications in patients with newly diagnosed type 2 diabetes. Diabetes Care 43 (2), 426–432. doi:10.2337/dc19-0823
Li, S., Vandvik, P. O., Lytvyn, L., Guyatt, G. H., Palmer, S. C., Rodriguez-Gutierrez, R., et al. (2021). SGLT-2 inhibitors or GLP-1 receptor agonists for adults with type 2 diabetes: A clinical practice guideline. BMJ 373, n1091. doi:10.1136/bmj.n1091
Lin, D. S., Lee, J. K., and Chen, W. J. (2021). Clinical Adverse events associated with sodium-glucose cotransporter 2 inhibitors: A meta-analysis involving 10 randomized clinical trials and 71 553 individuals. J. Clin. Endocrinol. Metab. 106 (7), 2133–2145. doi:10.1210/clinem/dgab274
Mancia, C. C., Tikkanen, I., Zeller, C., Ley, L., Woerle, H. J., Broedl, U. C., et al. (2016). Impact of empagliflozin on blood pressure in patients with type 2 diabetes mellitus and hypertension by background antihypertensive medication. Hypertension 68 (6), 1355–1364. doi:10.1161/HYPERTENSIONAHA.116.07703
Mansour, A. (2022). Post-authorization safety study in type 2 diabetic patients in Saudi arabia treated with empagliflozin to assess the incidence of ketoacidosis, severe complications of urinary tract infection, volume depletion, and dehydration. Available at: https://clinicaltrials.gov/ct2/show/record/NCT03764631 (Accessed December 12, 2022).
MHRA (2019). GLP-1 receptor agonists: Reports of diabetic ketoacidosis when concomitant insulin was rapidly reduced or discontinued. Available at: https://www.gov.uk/drug-safety-update/glp-1-receptor-agonists-reports-of-diabetic-ketoacidosis-when-concomitant-insulin-was-rapidly-reduced-or-discontinued (Accessed December 12, 2022).
Mudaliar, S., Polidori, D., Zambrowicz, B., and Henry, R. R. (2015). Sodium-glucose cotransporter inhibitors: Effects on renal and intestinal glucose transport: From bench to bedside. Diabetes Care 38 (12), 2344–2353. doi:10.2337/dc15-0642
Multinma, S. (2020). Bayesian network meta-analysis of individual and aggregate data. R package version 0.1.3 version, Available at: https://zenodo.org/record/7033094#.Y8QAllFByUk (Accessed December 12, 2022).
NCT (2013). The CANTATA-MSU trial (CANagliflozin treatment and trial analysis - metformin and SUlphonylurea). Available at: https://clinicaltrials.gov/ct2/show/NCT01106625?term=NCT01106625&draw=2&rank=1 (Accessed December 12, 2022).
Neal, B., Perkovic, V., Mahaffey, K. W., de Zeeuw, D., Fulcher, G., Erondu, N., et al. (2017). Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 377 (7), 644–657. doi:10.1056/NEJMoa1611925
Perkovic, V., Jardine, M. J., Neal, B., Bompoint, S., Heerspink, H. J. L., Charytan, D. M., et al. (2019). Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N. Engl. J. Med. 380 (24), 2295–2306. doi:10.1056/NEJMoa1811744
Persson, R. P., Vart, P., Chertow, G. M., Hou, F. F., Jongs, N., McMurray, J. J. V., et al. (2021). Efficacy and safety of dapagliflozin by baseline glycemic status: A prespecified analysis from the DAPA-CKD trial committees and investigators. Diabetes Care 44(8), 1894–1897. doi:10.2337/dc21-0300
Pollock, C., Stefánsson, B., Reyner, D., Rossing, P., Sjöström, C. D., Wheeler, D. C., et al. (2019). Albuminuria-lowering effect of dapagliflozin alone and in combination with saxagliptin and effect of dapagliflozin and saxagliptin on glycaemic control in patients with type 2 diabetes and chronic kidney disease (DELIGHT): A randomised, double-blind, placebo-controlled trial. Lancet Diabetes and Endocrinol. 7 (6), 429–441. doi:10.1016/S2213-8587(19)30086-5
Pratley, R. E., Eldor, R., Raji, A., Golm, G., Huyck, S. B., Qiu, Y., et al. (2018). Ertugliflozin plus sitagliptin versus either individual agent over 52 weeks in patients with type 2 diabetes mellitus inadequately controlled with metformin: The VERTIS FACTORIAL randomized trial. Diabetes Obes. Metab. 20 (5), 1111–1120. doi:10.1111/dom.13194
Ridderstrale, M., Rosenstock, J., Andersen, K. R., Woerle, H. J., Salsali, A., and investigators, E.-R. H. H. S. (2018). Empagliflozin compared with glimepiride in metformin-treated patients with type 2 diabetes: 208-week data from a masked randomized controlled trial. Diabetes Obes. Metab. 20 (12), 2768–2777. doi:10.1111/dom.13457
Rodbard, H. W., Seufert, J., Aggarwal, N., Cao, A., Fung, A., Pfeifer, M., et al. (2016). Efficacy and safety of titrated canagliflozin in patients with type 2 diabetes mellitus inadequately controlled on metformin and sitagliptin. Diabetes Obes. Metab. 18 (8), 812–819. doi:10.1111/dom.12684
Roden, M., Merker, L., Christiansen, A. V., Roux, F., Salsali, A., Kim, G., et al. (2015). Safety, tolerability and effects on cardiometabolic risk factors of empagliflozin monotherapy in drug-naive patients with type 2 diabetes: A double-blind extension of a phase III randomized controlled trial. Cardiovasc Diabetol. 14, 154. doi:10.1186/s12933-015-0314-0
Rosenstock, J. A., Frappin, G., Salsali, A., Kim, G., Woerle, H. J., Broedl, U. C., et al. (2014). Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care 37 (7), 1815–1823. doi:10.2337/dc13-3055
Rosenstock, J. C. L., González-Ortiz, M., Merton, K., Craig, J., Capuano, G., Qiu, R., et al. (2016). Initial combination therapy with canagliflozin plus metformin versus each component as monotherapy for drug-naïve type 2 diabetes. Diabetes Care 39 (3), 353–362. doi:10.2337/dc15-1736
Rosenstock, J., Frias, J., Pall, D., Charbonnel, B., Pascu, R., Saur, D., et al. (2018). Effect of ertugliflozin on glucose control, body weight, blood pressure and bone density in type 2 diabetes mellitus inadequately controlled on metformin monotherapy (VERTIS MET). Diabetes Obes. Metab. 20 (3), 520–529. doi:10.1111/dom.13103
Rosenstock, J., Jelaska, A., Zeller, C., Kim, G., Broedl, U. C., Woerle, H. J., et al. (2015). Impact of empagliflozin added on to basal insulin in type 2 diabetes inadequately controlled on basal insulin: A 78-week randomized, double-blind, placebo-controlled trial. Diabetes Obes. Metab. 17 (10), 936–948. doi:10.1111/dom.12503
Rücker, G., Schwarzer, G., Krahn, U., and König, J. (2015). Network meta-analysis using frequentist methods. Available at: https://rdrr.io/cran/netmeta/ (Accessed December 12, 2022).
Salanti, G., Ades, A. E., and Ioannidis, J. P. (2011). Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. J. Clin. Epidemiol. 64 (2), 163–171. doi:10.1016/j.jclinepi.2010.03.016
Scheen, A. J. (2020). Pharmacokinetic/pharmacodynamic properties and clinical use of SGLT2 inhibitors in non-asian and asian patients with type 2 diabetes and chronic kidney disease. Clin. Pharmacokinet. 59 (8), 981–994. doi:10.1007/s40262-020-00885-z
Scott, R., Morgan, J., Zimmer, Z., Lam, R. L. H., O'Neill, E. A., Kaufman, K. D., et al. (2018). A randomized clinical trial of the efficacy and safety of sitagliptin compared with dapagliflozin in patients with type 2 diabetes mellitus and mild renal insufficiency: The CompoSIT-R study. Diabetes Obes. Metab. 20 (12), 2876–2884. doi:10.1111/dom.13473
Sloan, G., Kakoudaki, T., and Ranjan, N. (2018). Prolonged diabetic ketoacidosis associated with canagliflozin. Endocrinol. Diabetes Metab. Case Rep. 17, 0177. doi:10.1530/EDM-17-0177
Søfteland, E. M. J., Vangen, B., Toorawa, R., Maldonado-Lutomirsky, M., and Broedl, U. C. (2017). Empagliflozin as add-on therapy in patients with type 2 diabetes inadequately controlled with linagliptin and metformin: A 24-week randomized, double-blind, parallel-group trial. Diabetes Care 40 (2), 201–209. doi:10.2337/dc16-1347
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898. doi:10.1136/bmj.l4898
Terauchi, Y., Tamura, M., Senda, M., Gunji, R., and Kaku, K. (2018). Long-term safety and efficacy of tofogliflozin as add-on to insulin in patients with type 2 diabetes: Results from a 52-week, multicentre, randomized, double-blind, open-label extension, Phase 4 study in Japan (J-STEP/INS). Diabetes Obes. Metab. 20 (5), 1176–1185. doi:10.1111/dom.13213
Turner, J., Begum, T., and Smalligan, R. D. (2016). Canagliflozin-Induced diabetic ketoacidosis: Case report and review of the literature. J. Investig. Med. High. Impact Case Rep. 4 (3), 2324709616663231. doi:10.1177/2324709616663231
Vallon, V., Gerasimova, M., Rose, M. A., Masuda, T., Satriano, J., Mayoux, E., et al. (2014). SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am. J. Physiol. Ren. Physiol. 306 (2), F194–F204. doi:10.1152/ajprenal.00520.2013
Van Valkenhoef, G., Dias, S., Ades, A. E., and Welton, N. J. (2016). Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res. Synth. Methods. 7 (1), 80–93. doi:10.1002/jrsm.1167
Weng, J., Zeng, L., Zhang, Y., Qu, S., Wang, X., Li, P., et al. (2021). Henagliflozin as add-on therapy to metformin in patients with type 2 diabetes inadequately controlled with metformin: A multicentre, randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Obes. Metab. 23 (8), 1754–1764. doi:10.1111/dom.14389
Yokono, M., Takasu, T., Hayashizaki, Y., Mitsuoka, K., Kihara, R., Muramatsu, Y., et al. (2014). SGLT2 selective inhibitor ipragliflozin reduces body fat mass by increasing fatty acid oxidation in high-fat diet-induced obese rats. Eur. J. Pharmacol. 727, 66–74. doi:10.1016/j.ejphar.2014.01.040
Zheng, H., Liu, M., Li, S., Shi, Q., Zhang, S., Zhou, Y., et al. (2021). Sodium-glucose Co-Transporter-2 inhibitors in non-diabetic adults with overweight or obesity: A systematic review and meta-analysis. Front. Endocrinol. (Lausanne) 12, 706914. doi:10.3389/fendo.2021.706914
Zhou, Y. L., Zhang, Y. G., Zhang, R., Zhou, Y. L., Li, N., Wang, M. Y., et al. (2021). Population diversity of cardiovascular outcome trials and real-world patients with diabetes in a Chinese tertiary hospital. Chin. Med. J. (Engl.). 134 (11), 1317–1323. doi:10.1097/cm9.0000000000001407
Keywords: sodium–glucose cotransporter 2 inhibitors, type 2 diabetes mellitus, diabetic ketoacidosis, network meta-analysis, placebo
Citation: Yang S, Liu Y, Zhang S, Wu F, Liu D, Wu Q, Zheng H, Fan P and Su N (2023) Risk of diabetic ketoacidosis of SGLT2 inhibitors in patients with type 2 diabetes: a systematic review and network meta-analysis of randomized controlled trials. Front. Pharmacol. 14:1145587. doi: 10.3389/fphar.2023.1145587
Received: 16 January 2023; Accepted: 23 May 2023;
Published: 13 June 2023.
Edited by:
Tin Wui Wong, Universiti Teknologi MARA Puncak Alam, MalaysiaReviewed by:
Godfrey Mutashambara Rwegerera, University of Botswana, BotswanaSunita Nair, Consultant, Mumbai, India
Copyright © 2023 Yang, Liu, Zhang, Wu, Liu, Wu, Zheng, Fan and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Na Su, zoya159@163.com; Ping Fan, 825370320@qq.com
†These authors have contributed equally to this work and share first authorship
 Shiwen Yang1†
Shiwen Yang1† Shengzhao Zhang
Shengzhao Zhang Fengbo Wu
Fengbo Wu Hanrui Zheng
Hanrui Zheng Ping Fan
Ping Fan Na Su
Na Su