- 1Department of Studies and Research in Biochemistry, Jnana Kaveri Post Graduate Centre, Mangalore University, Kodagu, Karnataka, India
- 2Department of Studies and Research in Microbiology, Jnana Kaveri Campus, Mangalore University, Kodagu, Karnataka, India
- 3Department of Studies in Food Technology, Davangere University, Davangere, Karnataka, India
- 4Center for Biotechnology, Pravara Institute of Medical Sciences (Deemed to Be University), Ahmednagar, Maharashtra, India
- 5SDM Research Institute for Biomedical Sciences (SDMRIBS) Shree Dharmasthala Manjunatheshwara University, Dharwad, Karnataka, India
- 6School of Life Sciences, B.S. Abdur Rahman Crescent Institute of Science and Technology, Chennai, India
- 7Department of Biochemistry, Jnanasahyadri, Kuvempu University, Shivamogga, Karnataka, India
- 8Department of Biology, Faculty of Science, University of Tabuk, Tabuk, Saudi Arabia
- 9Genome and Biotechnology Unit, Faculty of Sciences, University of Tabuk, Tabuk, Saudi Arabia
- 10Department of Science and Technology, University College-Ranyah, Taif University, Taif, Saudi Arabia
- 11MS Swaminathan School of Agriculture, Shoolini University of Biotechnology and Management Sciences, Solan, Himachal Pradesh, India
Alzheimer’s disease (AD) is a type of neurodegenerative disease, associated with the hastening of ROS, acetylcholinesterase (AChE) activity, and amyloid β peptides plaques in the brain. The limitations and side effects of existing synthetic drugs incline toward natural sources. In the present communication active principles of methanolic extract of Olea dioica Roxb, leaves are explored as an antioxidant, AChE inhibitor, and anti-amyloidogenic. Furthermore, neuroprotection against the amyloid beta-peptide has been studied. The bioactive principles were identified by GC-MS and LC-MS and further subjected to antioxidant (DPPH and FRAP) and neuroprotection (AChE inhibition, ThT binding, and MTT assay, DCFH-DA and lipid peroxidation (LPO) assay using neuroblastoma (SHSY-5Y) cell lines) assays. Methanolic extract of O. dioica Roxb, leaves was found to contain polyphenols and flavonoids. In vitro assays exhibited potential antioxidant and anti-AChE (˃50%) activities. ThT binding assay indicated protection against amyloid-beta aggregation. MTT assay, Aβ1-40 (10 µM) with extract increase the cell viability (˃50%) and showed significant cytotoxicity to SHSY-5Y cells. ROS level (˃25%) significantly decreased in the Aβ1-40 (10 µM) + extract (15 and 20 μM/mL) and LPO assay (˃50%) suggesting prevention of cell damage. Results advocate that O. dioica leaves are a good source of antioxidants, anti-AChE, and anti-amyloidogenic compounds which may be further evaluated as a natural medicine for the treatment of AD.
Introduction
Among neurodegenerative diseases, common dementia or Alzheimer’s disease (AD) attributes to the loss of neurons in different parts of the brain. The build-ups of amyloid β peptide plaques are commonly seen in the brains of people with AD. Moreover enhanced oxidative stress in neural cells causes cell damage and cell death that results in progressive AD with mild to the severe cognitive deficit (Querfurth et al., 2010; Thompson et al., 2012). Low levels of acetylcholine (ACh), a neurotransmitter of the parasympathetic nervous system are another attribute of AD.
Acetylcholinesterase (AChE), is a serine hydrolase that performs ACh hydrolysis to acetate and choline resulting in the termination of impulse transmission at cholinergic synapses in the brain of AD patients (R. Wang & Xi, 2005). AChE stimulates the development of amyloid fibrils and produces AChE-Aβ complexes (Hardy & Selkoe, 2002). These AChE-Aβ complexes accelerate neurotoxicity leading to greater neuro-degeneration. Inhibition of AChE activity has been acknowledged to be a significant therapeutic approach to controlling AD progression. Synthetic anti-cholinesterases or AChE inhibitors such as galantamine, donepezil, memantine, rivastigmine, tacrine, and physostigmine are currently known drugs for the management of AD (Js et al., 2015). Although these anti-cholinesterases exert toxic effects while pharmacological manipulation of the AChE activity in AD patients. Toxic effects include vomiting, nausea, diarrhea, headache, anorexia, and abdominal pain.
Plants are a valuable resource in human medicine, especially for the manufacture of pharmaceutical and herbal drugs. These plant-based drugs are increasingly appreciated to treat many human ailments nowadays (Grossi et al., 2013). The identification of new pharmaceutical leads and the safe use of plant-based drugs are both aided by phytochemical and pharmacological characterizations. The increase in the rate of allopathic medicines, the reliable cure for many chronic diseases, the easy availability of drugs from natural sources, and the prohibitive cost of neuroprotective allopathic drugs with added side effects are some of the reasons for the popularity of plant-based medicines. Moreover, products made from plants are less expensive and have fewer adverse effects (Bhandary et al., 1995; Harsha et al., 2003) In this perspective, many phytochemicals from medicinal plants are investigated for anti-cholinesterases or anti-AChE activity and the most popular field of research in the management of AD as the prevalence of AD has increased (Adewusi & Steenkamp, 2011; Chapter 5 Purification and Characterisation, n.d.; Luccarini et al., 2015). Ethno-medicinal plants such as Bacopa monera, Mentha longifolia L.), Ocimum basilicum L., and others are used to treat neurological illnesses including AD recently (Pratap et al., 2017; Pratap & Shantaram, 2020a).
Olea dioica Roxb or Rose Sandal Wood is a medicinal angiosperm tree commonly found in the Western Ghats of the Indian subcontinent. It is an important ethnomedicinal tree and belongs to the family of Oleaceae. Earlier studies reported antibacterial, antioxidant, aphrodisiac, and cytotoxic properties from Olea dioica Roxb, (Rao & Naika, 2015). According to Siddha medicine, the roots of Olea dioica are effective in the treatment of cancer and snake bite (Ashwathanarayana & Naika, 2017). The fruits of Olea dioica are used to cure skin diseases (Yesodharan & Sujana, 2007). The plant extract of Olea dioica exhibits neuroprotective effects in Drosophila against acrylamide-induced neurotoxicity (Pratap et al., 2021). According to a literature survey, Olea dioica Roxb, is a very important ethnomedicinal plant owing several pharmacological activities and could be a source of anti-AChE and anti-amyloidogenic compounds which could be a promising candidate for creating new drug lead for the controlling progression of AD in humans. With this impetus, the current study was planned to isolate, identify and characterize different phytochemicals for anti-oxidative, anti-AChE, and anti-amyloidogenic properties.
Materials and methods
Chemicals
Dulbecco’s modified eagle’s medium and FBS were purchased from Gibco (United States), and SHSY5Y cells were from ATCC. 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB), 2′,7 di-chloro fluorescein di-acetate (DCFD), SDS, TBA, TLC plates, Aβ1-40 peptide, acetylcholine iodide (ACTI) and MTT were from (Merck-Sigma Aldrich) and other chemicals were purchased from Hi-media.
Plant extract preparation
O. dioica Roxb plant leaves were collected from Pilikula, Mangalore, Karnataka. After two rounds of distilled water rinsing, the leaves were left to dry for 23 days in the shade. The dried leaves were then pulverized into a fine powder using a mixer grinder. The powder (50 g) was Soxhlet-extracted with methanol for 12 hs, and it was kept at room temperature (RT) in brown bottles.
Screening for phytochemicals
The plant leaves extractwas then subjected to a screening of different phytochemicals as flavonoids, saponins, tri-terpenoids, alkaloids, phenolic compounds, reducing sugars, steroids, proteins, carbohydrates, etc. Using the procedure reported previously (Pratap and Shantaram, 2020a).
Spectral analysis
GC-MS analysis
Shimadzu 17A GC with Head Space Sampling System HSS-4A and an HP-5MS column (30 m 0.25 mm 0.1 m) were used to perform the analysis of the active fractions of the methanolic extract using gas chromatography-mass spectrometry (GCMS-S80) (Attimarad et al., 2011) (The sample was injected in split mode at 220°C, and the transfer line was set to 240°C. Helium was used as the carrier gas at a constant flow rate (1 mL/min). The temperature was ramped up from 60°C to 260°C at the rate of 3°C/min and then exposed at 260°C for 25 min in full scan mode, m/z (mass-to-charge ratio) 20–600. Electron impact ionisation was employed (70 eV), and the MS (mass spectrometry) ion source was maintained at 240°C. Compounds were identified by their gas chromatography retention times and mass spectra using standard compounds for comparison with methanolic fraction’s GC-MS graph (Pratap et al., 2020a; Pratap et al., 2020b).
LC-MS analysis
Instruments for LC-MS (Agilent 1100 LC, Bruker-make MS model Esquire 3000) having an ESI (electrospray ionisation), an APCI (atmospheric pressure chemical ionisation), and a PDA detection source with a mass range of 100 amu in quadrupole and 10,000 amu (Jain et al., 2016). The mass-charge ratio and molecular mass of the methanolic fractions were determined by using the spectrum database for organic compounds in MassBank, which was then utilised to identify the mass fragmentations (Pratap et al., 2020b; Gk et al., 2022).
FTIR analysis
By using FTIR analysis (Bruke), which scans spectra in the 4000–500 cm1 range, the functional groups on the methanolic fractions were identified. The sample was formed by uniformly dispersing the methanolic fractions within a matrix of KBr (Owokotomo et al., 2015). The intensity bands were compared to standard values in order to identify the functional groups (Pratap et al., 2020b; Gk et al., 2022).
Determination of total flavonoid content
The total flavonoids in the methanolic fractions were estimated using colorimetric techniques (Sugimoto et al., 2000; Adebiyi and Olayemi, 2017). A clean test tube with 2% AlCl3 solution (0.5 mL), 0.25 mL of distilled water, and 200 µL (1 mg/mL) of methanolic fraction was added. The absorbance at 510 nm was measured after 1 hour incubation period at room temperature. Using quercetin (1 mg/g), a positive control, the total flavonoid content was determined. The equation employed was y = 0.0255x, R2 = 0.9822, where x is the absorbance and y is the positive control of quercetin equivalent (mg/g), based on the calibration curve (Pratap et al., 2020a).
Antioxidant assay
DPPH assay
DPPH assay for antiradical activity was performed by the Brand-Williams method (Cuvelier & Berset, 1995). In order to prepare fresh DPPH, 7.8 mg of DPPH were dissolved in 20 mL of methanol and stored at −20°C. One millilitre of 0.1 mM solution of DPPH +100 μL of the samples at different concentrations (20–100 μg/mL) was taken. The mixture was incubated in the dark for 15 min and the absorbance was read at 517 nm. Quercetin was used as standard and % inhibitions were calculated using the given formula.
FRAP (ferric-reducing antioxidant power) assay
To assess antioxidant power of leaf extract of O. dioica, Benzie and Strain method (Benzie and Strain, 1996) with little modifications were used. The assay mixture was prepared by 150 mM of acetate buffer (pH 3.6), 20 mM ferric chloride hexa hydrate (FeCl3.6H2O) in the proportion of 10:1:1 and 10 mM of TPTZ in 40 mM hydrochloric acid at RT. Then 5 μL leaves extract (1mg/1 mL) was added to a freshly prepared working FRAP reagent (3.95 mL), mixed carefully, and incubated at RT for 30min. At low pH ferric ions were reduced to ferrous ion in the presence of leaves extract and blue coloured ferrous-tri-pyridyl tri-azine complex were formed. The absorbance values were measured at 593 nm against the blank.
AChE inhibition by bioautographic enzyme assay
According to the bioautographic approach (Devika & Koilpillai, 2014; Pratap et al., 2020a), the acetylcholinesterase inhibitory activity of the methanolic fractions was discovered using TLC. The methanolic fraction was spotted (10 g/mL) and developed with a standardising mobile-phase (CHCl3:MeOH, 9.5:0.5) before being dried at room temperature on a silica gel-coated TLC plate, 2.5 mm F-254, 10 10 cm (Merck, Germany). The plates were then sprayed with freshly made AChE (Electric eel AChE enzyme; Sigma-Aldrich) enzyme solution, and they were let to sit at room temperature for 3 min. They were then sprayed till they reached saturation level with 3 mM DTNB or Ellman’s reagent and 15 mM acetylcholine iodide (ATCI) solution in phosphate buffer (pH 7.2). In order to establish the presence of putative AChE inhibition zones, the plates were later dried at room temperature for 1 min, resulting in colourless or white specks on a yellow background. After measuring the Rf value of the plant bioactive components that were calculated for the separation of the molecule in preparative scale, the spots on the plates were confirmed for being possible AChE inhibitors and recognised as clear zones against a yellow background (Marston et al., 2002; Pratap et al., 2020a; Pratap et al., 2020b).
AChE inhibition assay by Ellman’s method
The Ellman spectrophotometric approach (Ellman et al., 1961) (Wal et al., 2011) was used to measure the AChE inhibition activity, albeit with a little modification. Briefly, 20 μL of AChE (3 U/mL) solution, 3 mL of 0.1 M Tris-HCl buffer (pH 8.0), 100 μL of methanolic fractions at various concentrations (20, 40, 60, 80, 100, and 120 g/mL), and incubated at RT for 15 min and later 50 μL of 3 mM DTNB was added. When a yellow colour develops, 50 μL of 15 mM AChI (acetylthiocholine iodide) was then added to start the reaction. The positive control (1 mg/mL) utilised was galantamine. With a UV-visible spectrophotometer, the mixture’s absorbance was determined at 412 nm (Beckman Coulter, United States). This assay was performed in triplicate. By comparing the enzyme activity to the negative control, the percentage inhibition was computed using the following formula:
ThT assay of disaggregation of preformed Aβ1-40 aggregates
Thioflavin T (ThT) fluorescence assay is frequently used to evaluate the fibrillogenesis (amyloids) rate. A ThT molecule produces fluorescence upon binding to amyloids and could be used to monitor in vitro amyloid fibril aggregation (Xue et al., 2017). The leaves extracts (10–100 μg/mL) were incubated with 10 µM of Aβ1-40 (40 µL) at 37°C for 1 day. Then, 100 µL of ThT (10 µM in PBS buffer, pH 7.4) was added. An FP-6200 spectrofluorometer (Shimadzu RF-5000) was used to assess the amounts of ThT fluorescence in the samples and the fluorescence was measured after 30 min at the wavelengths of excitation and emission Ex450nm and Ex483nm respectively along with the positive control (Galantamine + Aβ).
Cell culture
The neuroblastoma (SHSY-5Y) cells were cultured in a DMEM (Dulbecco’s Modified Eagle Medium) medium and supplemented with 10% FBS, antibiotics, and growth factor, at 37°C in a humid atmosphere with 5% CO2.
Preparation of amyloid-beta (Aβ 1–40)
Aβ 1–40 stock solution (0.1 mg/mL) was prepared in milli-Q. Before using, Aβ 1–40 solution was incubated at 37°C in a water bath for about 7–8 h with mixing (every 1 h) for aggregation and then diluted in the medium. After aggregation, the solution was divided into aliquots and kept at −20°C in sterile Eppendorf tubes.
MTT assay
SHSY-5Y cells (3 × 104cells/well) were seeded in 96 well plates. The medium was replaced every 24 h so that the cells become adherent. Further, the cells were added to a serum-free medium and treated with or without leaves extract + Aβ1-40 at different concentrations and then incubated for 24 h after which the cell viability was checked and measured by MTT (5 mg/mL) assay. The MTT assay (Dalberto et al., 2020) was performed by adding MTT solution to each well and incubating for 4 h at 37°C. After removing the MTT solution, 100 μL of DMSO (100%) was added to each well, and absorption was read at 570 nm using a microplate reader. Cell viability data were compared in two ways: treated cells versus untreated cells with Aβleaves extract, and leaves extract + Aβ. The concentration was selected based on the MTT results.
Dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay
Reactive Oxygen Species (ROS) levels were monitored using DCFH-DA assay (Aranda et al., 2013). SHSY-5Y cells (25 × 104) were seeded into 96 well plates and allowed to grow for 24 h. The cells were then washed with PBS, replaced with fresh medium (no FBS), and incubated for 24 h with 10 μM Aβ1-40 solution in the presence or absence of leaf extract at different concentrations. The cells were further incubated with DCFH-DA (5 μM) at 37°C for 30 min and washed with cold phosphate-buffered saline (PBS 7.4 pH). The fluorescence intensity was measured at wavelengths of 490 nm for excitation and 520 nm for emission in the microplate reader (Perkin Elmer LS 55 Luminescence Spectrometer). After completion of the process, the results were expressed as Relative Fluorescence Units (RFU).
Lipid peroxidation assay
Lipid peroxidation assay was performed using neuroblastoma cells (SHSY-5Y) by a method described earlier with slight modification (Sereia et al., 2019). The SH-SY5Y cells were seeded with the same density and same treatment as that of the ROS assay described above. The lipid peroxidation was evaluated to the extract during the exposure of SHSY-5Y cells at 20 μg/mL for 2 h. After that, it was combined with 10 μM of Aβ1-40 and incubated for another 24 h using the diphenyl-1-pyrenylphosphine (DPPP, 45 µM) probe for 15 min at 35°C and then the fluorescence was measured at λex355 nm and λem460 nm.
AChE activity
Ellman assay was used to measure the AChE activity in a 96-well microplate (Ellman et al., 1961). The SHSY-5Y cells were seeded at the same density and same treatment (ROS assay described above). After treatment, the cell culture media was collected and the cells were scraped in a cold phosphatebuffer. The reaction mixture containing 60 µL of cell scraped, 30 µL of DTNB (0.5 mM), and 30 µL of AChI (1 mM) was incubated for 5 min. Absorbance was measured at 412 nm for up to 10 min. The AChE activity was expressed as Unit/mg protein.
Statistical analysis
The differential significance of the results obtained was determined by one-way ANOVA followed by Bonferroni’s multiple comparisons test at the 0.05 level. All values are presented with Means ± SD, except where otherwise indicated. Statistical analysis was carried out using GraphPad Prism 8. Comparisons between two groups were performed using a one-way analysis of variance, whereas the comparisons among multiple groups were performed with one-way ANOVA. p < 0.05 was considered to indicate a statistically significant difference.
Results
Plants produce a different array of chemical compounds among them certain bioactive compounds like phenolics, anthocyanins, and flavonoids, etc. Have gained extensive approval for their physiological functions, which include free radical scavenging, anti-diabetic, and anti-cancer effects (Bhandary et al., 1995). The present study has revealed the bioactive constituents and neuroprotective potential of the leaf extract of Olea dioica. Earlier we have demonstrated in vitro anti-oxidant and anti-AChE potential of column-purified fractions of methanol extract of leaves (Pratap et al., 2020a). Here we specifically focusedon the detailed phytochemical analysis and characterization of the methanol extract of O. dioica Roxb leaves. We have also explored in vitro anti-AChE, anti-oxidant, anti-amyloids, and neuroprotective potential of O. dioica Roxb leaves extract using neuroblastoma (SHSY-5Y) cells.
Phytochemical analysis
During the primary phytochemical screening, methanolic extract of O. dioica Roxb leaves was found to contain saponins, tri-terpenoids, coumarins, flavonoids, and phenolic compounds. However, proteins, sugars, and steroids were absent (data is not represented). A literature survey illustrates positive correlations between antioxidant potential and phenolics and flavonoids contents (Yang and Gadi, 2008; Okpuzor et al., 2009). These natural compounds are found to prevent the development of AD pathology in vitro and in vivo experimental settings (Hamaguchi et al., 2009). Here, the total phenolic and flavonoid content of the methanolic leaf extract of O. dioica Roxb was determined from the calibration curve of standard Gallic acid and Quercetin which was then expressed as mg equivalent of Gallic acid and Quercetin per gram of dry extract respectively. The phenolic content was found to be 26 μg/mL (SD ± required) gallic acid equivalents/g, whereas the flavonoids were found to be 18.3 μg/mL (Mean ± SD) quercetin equivalent/g. Besides, phenolics and flavonoids the leaves extract were found to contain tannins, and saponins (data is not shown).
GC-MS and LC-MS analysis
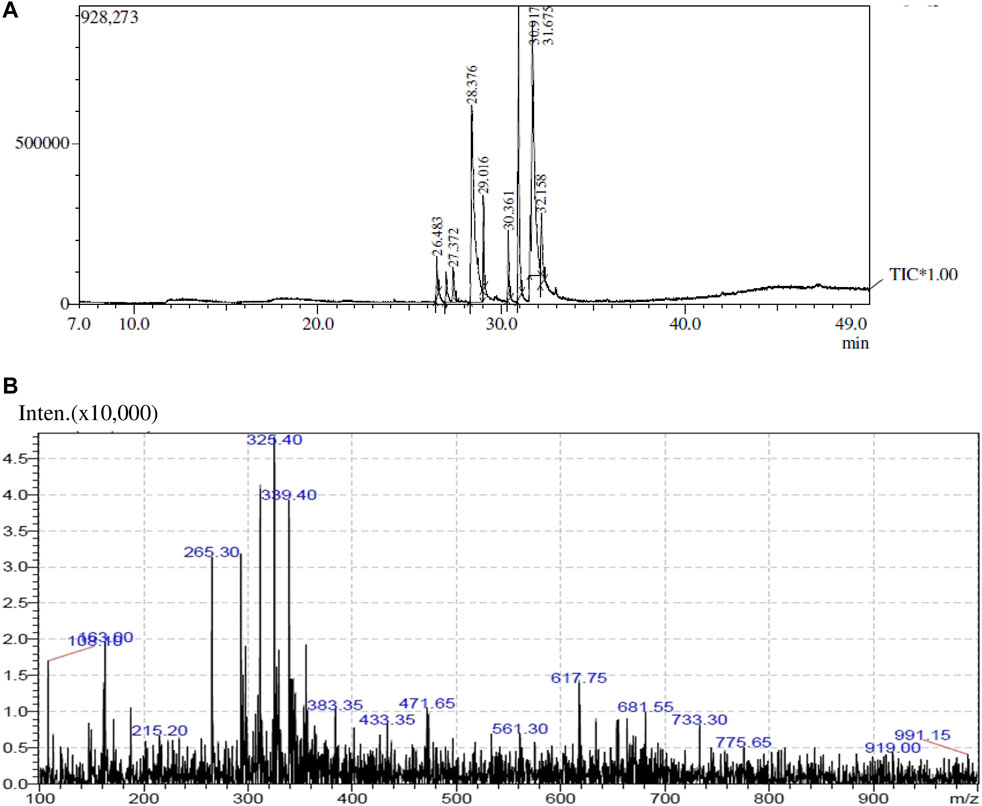
The GC-MS analysis spectra of the extract of O. dioicaRoxb leaves is shown in Figure 1 exhibited multiple peaks that represented the presence of distinct phytochemical components. A comparison of the MS constituents with the NIST library confirmed the identity of eight phytochemicals. The LC-MS spectra also showed different peaks and the masses compared with the mass bank library confirmed the identity of four compounds (Tables 1, 2).
TABLE 2. The identified components present in GC-MS and LC-MS analysis and biological activity of the components and the mass compared with the MassBank database.
FTIR analysis
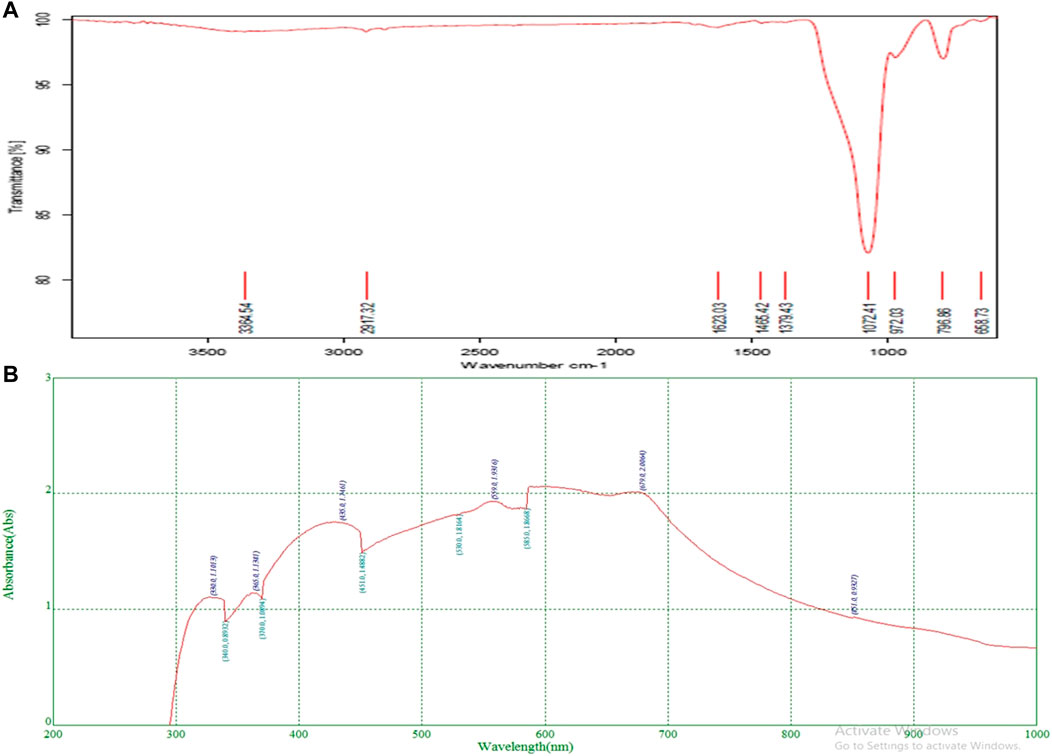
The functional groups and chemical components of structural components can be identified using the high-resolution analytical technique known as FTIR (Hussain et al., 2009). The functional groups of bioactive components in the leaf extract were essentially discovered by the FTIR measurement (Figure 2). FTIR results showed the band in the range of 3500–500 cm−1. The stretch and vibration of each band show the existence of functional groups such as O-H, which indicate alcohols and phenols. The FTIR analysis indicated the existence of the functional groups listed in Table 3. Specifically functional groups werealcohols, carboxylic acids, alkenes, amines, phenols, aldehydes, quinines, amides, anhydrides, and organic halogen compounds. All these compounds belong to the plant secondary metabolites.
UV-VIS analysis
The phytoconstituents present in methanolic leaves extract of Olea dioica Roxb., were identified by the UV-VIS spectra analysis, and were performed at the appropriate baseline, 200–1000 nm wavelength. (Figure 2). The flavonoids and their derivatives are represented by absorption peaks at 200–400 nm (Attimarad et al., 2011) The flavonoids have only two absorption maxima in the range of 230–285 nm and 300–350 nm. However, alkaloids, flavanols, flavonoids, and phenolic acid are known to have a presence range of 270–670 nm (Table 4). The crude extract included alkaloids, flavanols, flavonoids, and phenolic acid. Information on the secondary metabolites of the plants is provided by these intensity measurements (Domenici et al., 2014).

TABLE 4. Absorption peaks ranging from 200 to 300 nm and 400–700 nm indicating the presence of plant compounds.
The phytoconstituents present in methanolic leaves extract of Olea dioica Roxb., were identified by the UV-VIS spectra analysis performed at the appropriate baseline, 200–1000 nm wavelength.
Antioxidant activity
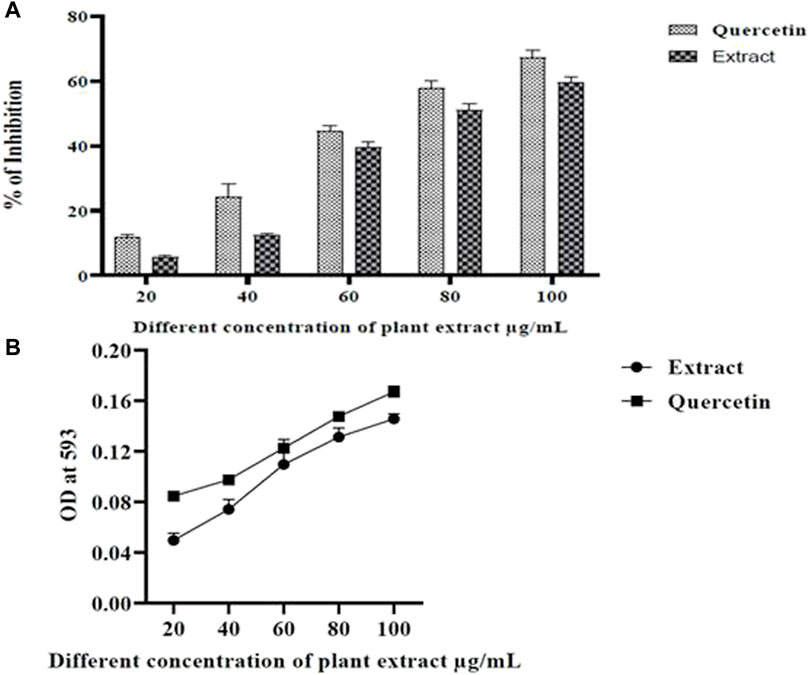
The oxidative stress markers have induced neurodegeneration in the brain and caused cell damage and cell death (Zhu et al., 2004; Head, 2009). The DPPH assay is stable with free radical absorption at 515 nm when using a colorimetric method while the FRAP assay is stable with free radical absorption at 593 nm when using a spectrophotometric approach. There is a distinct color shift from deep violet to pale yellow. A significant antioxidant activity of the plant extract was also evidenced by the decreased absorbance of the reaction mixture. Good scavenging activity was exhibited by the extract (Figure 3). The most significant scavenging activity was seen in Olea dioica Roxb., plant leaf extracts at 100 μg/mL comparable to that of quercetin (Figure 3). As the quantity of the Fe2+-TPTZ complex increased, as observed in the reference antioxidant, the absorbance of leaf extracts increased (Figure 3). Furthermore, the leaf extracts could well be able to donate electrons to free radicals.
Bioautography assay
Bioautographic enzyme assay is a simple and rapid screening of AChE inhibition by plant extracts (Marston et al., 2002; Pratap et al., 2020a; Pratap et al., 2020b). Our results with methanolic extract of Olea dioica showed AChE inhibition on the TLC plate displayingwhite spots or colourless regions of acetylcholinesterase inhibition over yellow background (Figure 4).
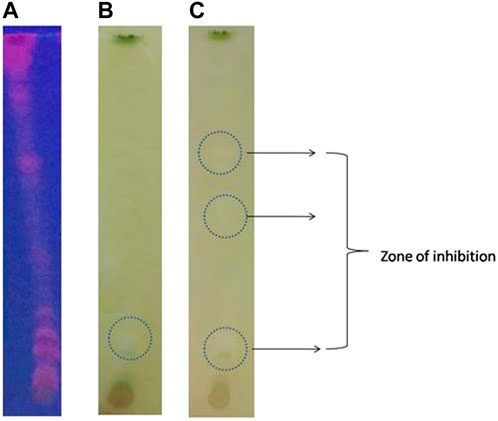
FIGURE 4. Bio-autographic assay of AChE inhibition activity in the TLC plates. (A) TLC plates at UV-light. (B) and (C) White spots over a yellow background develops as an indication of acetylcholinesterase inhibition zones at different time interval.
AChE inhibition and ThT binding potential
AChE enzyme inhibitory potential of the methanolic extract of Olea dioica Roxb. Is shown in Figure 5. The extracts demonstrated strong AChE inhibition, with values > 60% at various sample concentrations (10, 20, 40, 60, 80, and 100 μg/mL). The phenolic compounds are known to be neuroprotective exhibiting AChE inhibitory activity and could be one of the main approaches for the treatment of AD (Pratap and Shantaram, 2020b) (Pratap et al., 2021). The neuroprotective action of the methanolic extract of Olea dioica Roxb. Leaves were further assessed by ThT fluorescence assay (Figure 5). ThT binding to amyloid indicated that the methanolic extracts of Olea dioica Roxb. Leaves were capable of preventing amyloid-beta aggregation.
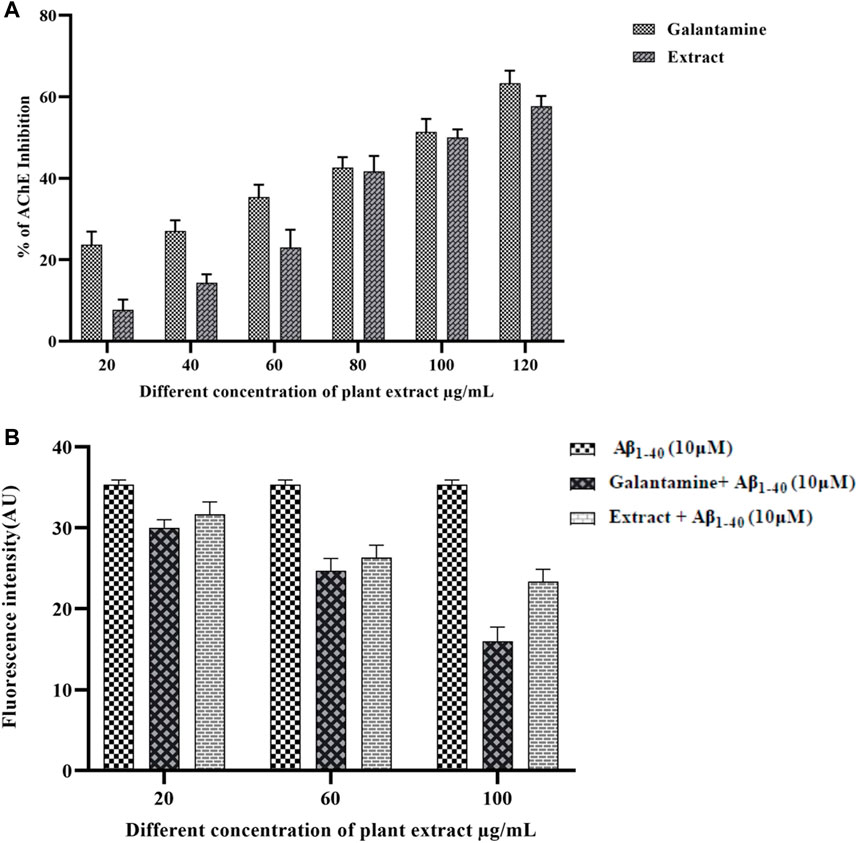
FIGURE 5. AChE inhibition by plant extracts positive control (A) and Thioflavin-T assay of plant extract and positive control (B).
MTT assay and cell viability
Treatment of Aβ1-40 alone at different concentrations (5, 10, and 20 µM) showed different level of cytotoxicity. Aβ1-40 concentration (5 and 10 µM) shows apparent cytotoxicity, but Aβ1-40 (60 μg/mL) shows a significant cytotoxicity effect on the SHSY-5Y cells. At 10 µMAβ1-40concentrations, changes in the cell morphology, cell loss, and cell shrinkage were observed and these changes were analyzed by microscopic examination (Figure 6).

FIGURE 6. Neuroprotective effect of plant extract against Aβ1-40 at different concentrations of inducing cell toxicity in SHSY-5Y cells. Gal (Galantamine) was used as a positive control.
SHSY-5Y cells were exposed to Aβ1-40 (10 µM) with different concentrations of plant extracts (5–20 μM/mL) for 24 h and the cell survival was assessed by MTT assay (Figure 7). Amyloid beta-induced groups have significantly decreased cell survivability with increasing toxicity in a dose-dependent manner. Different concentrations of plant extract along with Aβ1-40 (10 µM)treatment groups for 24 h incubation have significantly increased cell survivability compared to the treatment with Aβ1-40 (10 µM) alone and confirmed the neuroprotective role.
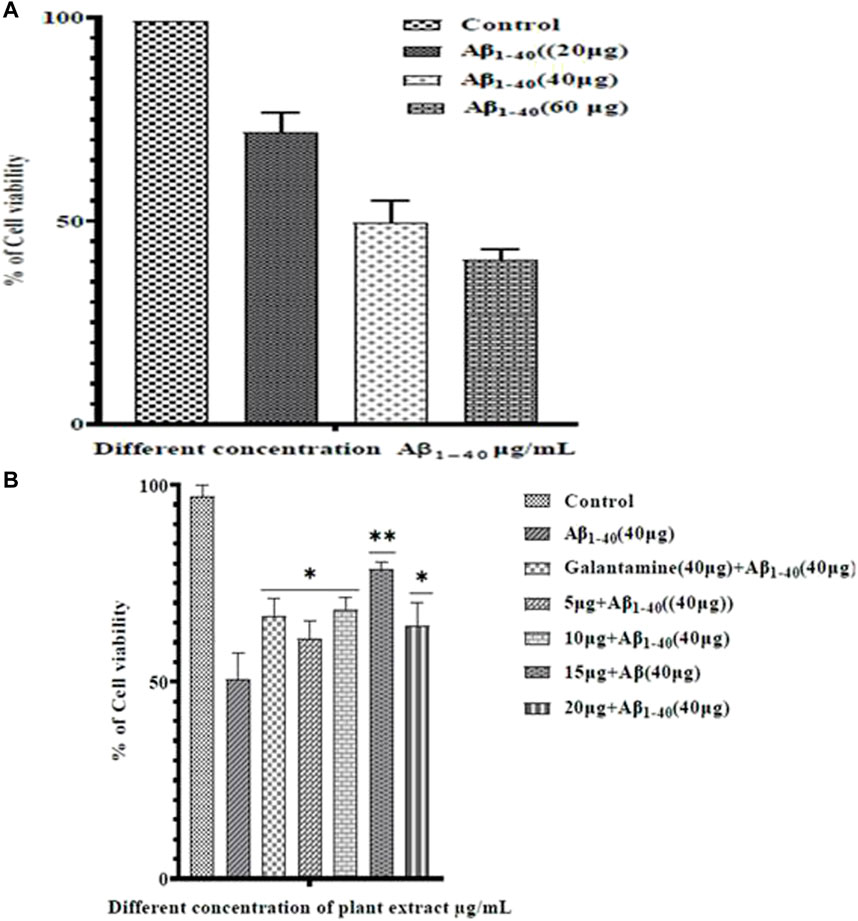
FIGURE 7. Cell viability in SHSY-5Y cells which were exposed to different concentrations of plant extract for 24 h. Cell viability was assessed by MTT assay.
ROS activity
Literature surveys were showcasing that amyloid-beta (Aβ) induced oxidative stress plays a significant role in the progression of AD (Butterfield et al., 2013). Oxidative stress stimulates amyloid-beta production (Adam Moser, Kevin Range and Manuscript, 2008) (H. Wang et al., 2009). The reaction of intracellular ROS with DCFH-DA through fluorescence noticed ROS production. SHSY-5Y cells were exposed to Aβ1-40 (10 µM) with extracts and without extracts for 24h, and the ROS level significantly decreased in the Aβ1-40 (10 µM) + extract (15 and 20 μM/mL) treatment (Figure 8). The extract at different concentrations significantly prevented Aβ1-40 induced ROS production, so the treated group showed a remarkable decrease in the ROS level (Figure 8). Using flow cytometry, the results were obtained. As cellular ROS generation increased, the intensity of the DCFH-DA fluorescence also increased. The extracts at different concentrations inhibited Aβ1-40 (10 µM) induced cell toxicity, observed by the shifting of fluorescent intensity, showing the implication of plant extract in preventing the production of ROS.
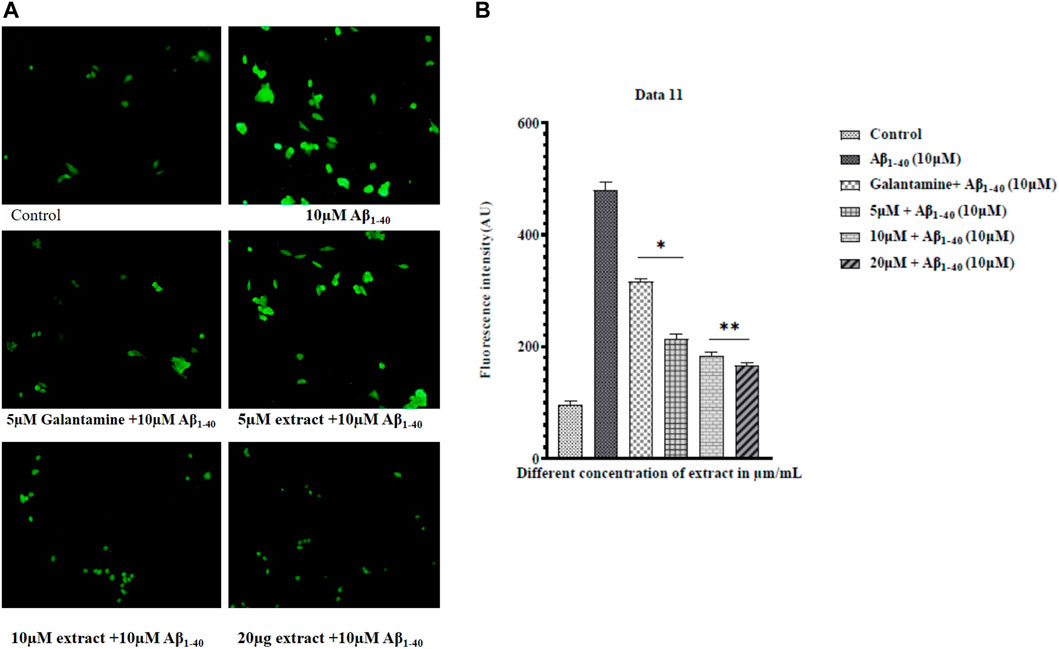
FIGURE 8. ROS measurement by fluorescence microscope (A) and ROS levels in control (Aβ1-40) and treated groups of extract + amyloid beta (B). The treated groups decrease the level of ROS in extract treated groups compared to control. The results were expressed in fluorescence intensity. Significance was checked by one-way ANOVA. The error bars represent the Mean ± SD. The p-value *p < 0.01 and **p < 0.001, were considered as significant while comparing Aβ1-40 treated group and Aβ1-40+ extract.
Lipid peroxidation assay (LPO)
LPO assay, a distinct indicator of cell death, further supported the protective role of extract. Moreover, the extract could effectively prevent cell injury which can be caused by the Aβ1-40 (Figure 9). These study results showed that the extract considerably protected SHSY-5Y cells from the cytotoxicity caused by Aβ1-40. Moreover, the LPO level was significantly reduced in the treated group compared to the control group.
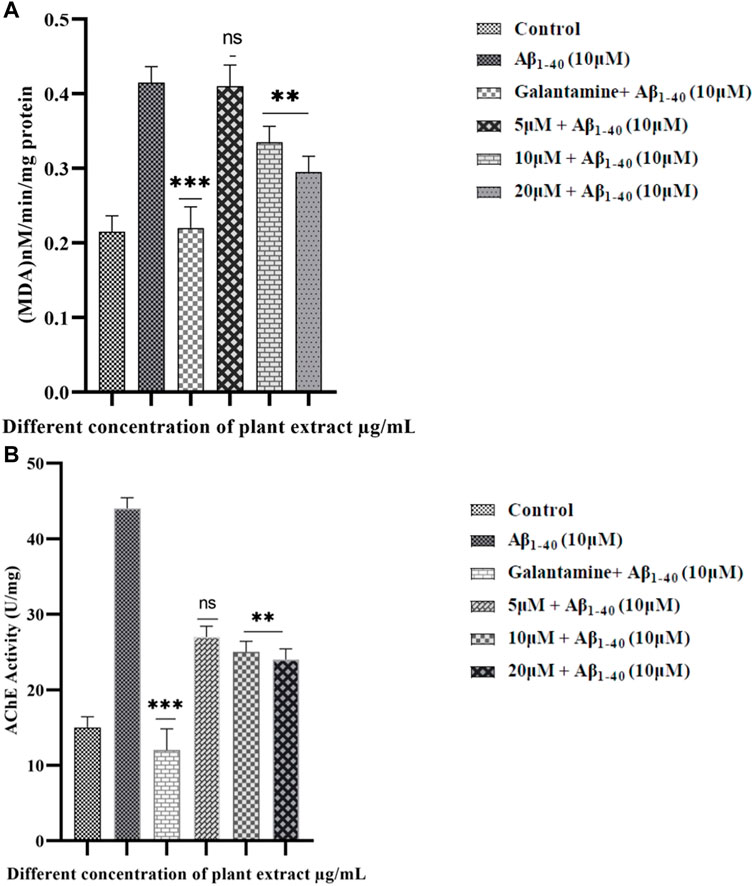
FIGURE 9. LPO in SHSY-5Y cells were treated with Aβ1-40 at different concentrations (5 and 10 µM) (A). The effect of extract on treated groups showed a decreasing LPO activity in Aβ1-40+ extract treated group. Quantitative evaluation of the extent of inhibition of acetylcholinesterase activity (AChE) by plant extract (5, 15, and 20 μM/mL) and the standard AChE inhibitor galantamine (5 μM/mL) (B). The data shown are with Mean ± SD. The p-value **p < 0.001, and ***p < 0.001 were considered as significant while comparing Aβ1-40 treated group and Aβ1-40+ extract.
Acetylcholinesterase activity
AChE has played an important role in the implication of the aging processes (Haddadi et al., 2014), where AChE enhances the formation of amyloid-β fibrils and forms the AChE-Aβ complexes which are very neurotoxic (Figure 9). The AChE activity level has increased in SHSY-5Y cells when exposed to Aβ1-40 (10 µM) for 24 h. However, the AChE activity level significantly decreased when exposed to Aβ1-40 (10 µM) along with the extract (5, 15, and 20 μM/mL) for 24 h.
Discussion
Bioactive substances found in medicinal plants included phenolics, anthocyanins, and flavonoids. According to reports, the phenolic and flavonoid compounds possess a variety of pharmacological properties, such as the ability to fight cancer, anti-diabetes, and scavenge free radicals. Antioxidants are extremely significant compounds that have the power to shield the body from harm brought on by oxidative stress brought on by free radicals. The phenol and flavonoid contents discovered in leafsample accord with the DPPH results obtained. By virtue of their hydroxyl groups’ ability to donate hydrogen, plant phenols and flavonoids have reducing and antioxidant properties.
The oxidative stress markers cause Parkinson’s disease, and Alzheimer’s disease, as well as neurodegeneration in the brain. According to earlier research on the chemical makeup of various natural compounds, curcumin, Gallic acid, Diterpene, Apigenin, Luteolin, and others are essential for cell division, anti-inflammatory activity, neuroprotection, and anti-amyloidogenic activity. They also act as pro-oxidants (Forni et al., 2019) (Pratap et al., 2021; Gk et al., 2022). According to Haddadi et al. (2014), AChE contributes significantly to the aging process by promoting the production of highly neurotoxic amyloid-b fibrils and AChE-Aβ complexes.
It has been suggested that AChE-Aβ complexes cause more neurodegenerationin the brain than does amyloid-β peptide. AChE is essential for the development of AD (Dhanasekaran et al., 2015). In recent years, the anti-AChE activity of a variety of plant extracts and chemicals produced from plants (Indole, Isoquinoline, Quinolizidine, andPiperidine) has been examined (Devi and Syad, 2014). AChE activity is a widespread biochemical indicator of AD. The current study’s findings revealed that the methanol extract of O. dioica contained sizable levels of phenolics and flavonoids. In some medicinal plants such as Curculigo orchioides (Pratap et al., 2020a) Tanacetum parthenium, Nigella sativa (Chen et al., 2021) Ginkgo biloba, Bacopa monniera L.) (Pratap et al., 2017), methanolic extract shows good antioxidant, anti-AChE activity, neuroprotection against amyloid beta toxicity The understudied herb may have a substantial impact on the treatment of human disorders because this leaf extract showed considerable anti-AChE, anti-oxidant, anti-Aβ, and neuroprotective effects.
Conclusion
As a result of their antioxidant, anti-AChE, and anti-inflammatory qualities, numerous herbal medicines, extracts, phytochemicals, and herbal formulations are said to have anti-Alzheimer’s effects. This results in the decrease of the amyloid beta-induced toxicity. Here our study reports antioxidant, AChE inihibitor, and anti-amyloidogenic activities from methanolic extract of Olea dioica Roxb. Leaves aids in neuroprotection. Further detailed investigation of bioactive principles from Olea dioica Roxb is needed to establish medicinal values in the control of AD. Based on these results, further studies on the identified bioactive compounds from these two plants are being planned.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding authors.
Author contributions
PK designed the study and wrote the protocol and the first draft of the manuscript. MS, YK, PV, RH, AK, AP, SJ, MA, AA, SS, and MM have conceived the study and contributed to the writing and editing of the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The authors are thankful to the Mangalore University, Department of Biochemistry and Food Technology, Davangere University and SDM Research Institute for Biomedical Sciences (SDMRIBS) for facilitating cell culture work. Authors thank Taif University Researches Supporting Project number (TURSP-2020/92), Taif University, Taif, Saudi Arabia for supporting this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adam Moser, Kevin Range and Manuscript (2008) Genetic alteration NIH public access. Bone 23 (1), 1–7. D. M. Y., and , A.
Adebiyi, O., and Olayemi, F. T. N.-H.-B.-S. U. (2017). In vitro antioxidant activity, total phenolic and flavonoid contents of ethanol extract of stem and leaf of Grewia carpinifolia. Elsevier. undefined. (n.d.). Available at: https://www.sciencedirect.com/science/article/pii/S2314853516301007. (Accessed October 7, 2019).
Adewusi, E. A., and Steenkamp, V. (2011). In vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from southern Africa. Asian Pac. J. Trop. Med. 4 (10), 829–835. doi:10.1016/S1995-7645(11)60203-4
Aranda, A., Sequedo, L., Tolosa, L., Quintas, G., Burello, E., Castell, J. V., et al. (2013). Dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay: A quantitative method for oxidative stress assessment of nanoparticle-treated cells. Toxicol. Vitro 27 (2), 954–963. doi:10.1016/j.tiv.2013.01.016
Ashwathanarayana, R., and Naika, R. (2017). Comparative study of qualitative phytochemical and quantitative GC-MS analysis of Olea dioica Roxb. from different forest types of Western Ghats, Karnataka, India. Trop. Plant Res. 4 (1), 134–144. doi:10.22271/tpr.2017.v4.i1.020
Attimarad, M., Mueen Ahmed, K. K., Aldhubaib, B. E., and Harsha, S. (2011). High-performance thin layer chromatography: A powerful analytical technique in pharmaceutical drug discovery. Pharm. Methods 2 (2), 71–75. doi:10.4103/2229-4708.84436
Benzie, I. F. F., and Strain, J. J. (1996). The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 239 (1), 70–76. doi:10.1006/abio.1996.0292
Bhandary, M. J., Chandrashekar, K. R., and Kaveriappa, K. M. (1995). Medical ethnobotany of the siddis of uttara Kannada district, Karnataka, India. J. Ethnopharmacol. 47 (3), 149–158. doi:10.1016/0378-8741(95)01274-H
Butterfield, D. A., Swomley, A. M., and Sultana, R. (2013). Amyloid β-Peptide (1-42)-induced oxidative stress in alzheimer disease: Importance in disease pathogenesis and progression. Antioxidants Redox Signal. 19 (8), 823–835. doi:10.1089/ars.2012.5027
Chen, X., Drew, J., Berney, W., and Lei, W. (2021). Neuroprotective natural products for alzheimer’s disease. Cells 10 (6), 1309–1338. doi:10.3390/cells10061309
Cuvelier, M. E., and Berset, C. (1995). 4A standard calibration techniques. Microflown E-Book 28, 25–30.
Dalberto, D., Nicolau, C. C., Garcia, A. L. H., Nordin, A. P., Grivicich, I., and da Silva, J. (2020). Cytotoxic and genotoxic evaluation of cotinine using human neuroblastoma cells (Sh-sy5y). Genet. Mol. Biol. 43 (2), e20190123–e20190127. doi:10.1590/1678-4685-GMB-2019-0123
Devi, P., and Syad, A. N. (2014). Botanics: A potential source of new therapies for Alzheimer;s disease? Botanics Targets Ther. 11, 11. doi:10.2147/btat.s33554
Devika, R., and Koilpillai, J. (2014). In vitro quantification study of flavonoids from Tagetes erecta. Asian J. Biotechnol. 6 (1), 21–24. doi:10.3923/ajbkr.2014.21.24
Dhanasekaran, S., Perumal, P., and Palayan, M. (2015). In-vitro Screening for acetylcholinesterase enzyme inhibition potential and antioxidant activity of extracts of Ipomoea aquatica Forsk: Therapeutic lead for Alzheimer’s disease. J. Appl. Pharm. Sci. 5 (2), 012–016. doi:10.7324/JAPS.2015.50203
Domenici, V., Ancora, D., Cifelli, M., Serani, A., Veracini, C. A., and Zandomeneghi, M. (2014). Extraction of pigment information from near-UV vis absorption spectra of extra virgin olive oils. doi:10.1021/jf503818k
Ellman, G. L., Courtney, K. D., Andres, V., and Featherstone, R. M. (1961). A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 7 (2), 88–95. doi:10.1016/0006-2952(61)90145-9
Forni, C., Facchiano, F., Bartoli, M., Pieretti, S., Facchiano, A., D'Arcangelo, D., et al. (2019). Beneficial role of phytochemicals on oxidative stress and age-related diseases. BioMed Res. Int. 2019, 8748253. 2019(Figure 1). doi:10.1155/2019/8748253
Gk, P., Nagaraju, P. G., Danagoudar, A., Joshi, C. G., Priyadarshini, P., Hussein, Y., et al. (2022). Neuroprotective, lifespan and memory enhancing potential, and molecular docking studies of natural compound from Curculigo orchioides: A study on Alzheimer's disease model of appl-GAL4 Drosophila melanogaster. South Afr. J. Bot. 149, 60–66. doi:10.1016/j.sajb.2022.05.047
Grossi, C., Rigacci, S., Ambrosini, S., Dami, T., Luccarini, I., Traini, C., et al. (2013). The polyphenol oleuropein aglycone protects TgCRND8 mice against Aß plaque pathology. PLoS ONE 8 (8), e71702. doi:10.1371/journal.pone.0071702
Haddadi, M., Jahromi, S. R., Sagar, B. K. C., Patil, R. K., Shivanandappa, T., and Ramesh, S. R. (2014). Brain aging, memory impairment and oxidative stress: A study in Drosophila melanogaster. Behav. Brain Res. 259, 60–69. doi:10.1016/j.bbr.2013.10.036
Hamaguchi, T., Ono, K., Murase, A., and Yamada, M. (2009). Phenolic compounds prevent Alzheimer’s pathology through different effects on the amyloid-β aggregation pathway. Am. J. Pathology 175 (6), 2557–2565. doi:10.2353/ajpath.2009.090417
Hardy, J., and Selkoe, D. J. (2002). The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 297 (5580), 353–356. doi:10.1126/science.1072994
Harsha, V. ., Hebbar, S. ., Shripathi, V., and Hegde, G. (2003). Ethnomedicobotany of uttara Kannada district in Karnataka, India—Plants in treatment of skin diseases. J. Ethnopharmacol. 84 (1), 37–40. doi:10.1016/S0378-8741(02)00261-1
Head, E. (2009). Oxidative damage and cognitive dysfunction: Antioxidant treatments to promote healthy brain aging. Neurochem. Res. 34 (4), 670–678. doi:10.1007/s11064-008-9808-4
Hussain, K., Ismail, Z., and Amirin Sadikun, P. I. (2009). Evaluation of metabolic changes in fruit of piper sarmentosum in various seasons by metabolomics using fourier transform infrared (FTIR) spectroscopy. Int. J. Pharm. Clin. Res. 1 (2), 68–71.
Jain, P. K., Soni, A., Jain, P., and Bhawsar, J. (2016). Phytochemical analysis of Mentha spicata plant extract using UV-VIS, FTIR and GC/MS technique. J. Chem. Pharm. Res. 8 (2), 1–6.
Js, B., J, G. E., Birks, J. S., and Evans, J. G. (2015). Rivastigmine for alzheimer ’ s disease (review) rivastigmine for alzheimer ’ s disease. Cochrane Database Syst. Rev. (Online) 4, 4–7.
Luccarini, I., Grossi, C., Rigacci, S., Coppi, E., Pugliese, A. M., Pantano, , et al. (2015). Oleuropein aglycone protects against pyroglutamylated-3 amyloid-ß toxicity: Biochemical, epigenetic and functional correlates. Neurobiol. Aging 36 (2), 648–663. doi:10.1016/j.neurobiolaging.2014.08.029
Marston, A., Kissling, J., and Hostettmann, K. (2002). A rapid TLC bioautographic method for the detection of acetylcholinesterase and butyrylcholinesterase inhibitors in plants. Phytochem. Anal. 13 (1), 51–54. doi:10.1002/pca.623
Okpuzor, J., Ogbunugafor, H., Kareem, G. K., and Igwo-Ezikpe, M. N. (2009). In vitro investigation of antioxidant phenolic compounds in extracts of Senna alata. Res. J. Phytochemistry 3 (4), 68–76. doi:10.3923/rjphyto.2009.68.76
Owokotomo, I. A., Ekundayo, O., Abayomi, T. G., and Chukwuka, A. V. (2015). In-vitro anti-cholinesterase activity of essential oil from four tropical medicinal plants. Toxicol. Rep. 2, 850–857. doi:10.1016/j.toxrep.2015.05.003
Pratap, G. K., Ananda, D., Joshi, C. G., and Shantaram, M. (2021). Ameliorative activity of medicinal plant fraction for neuroprotection against acrylamide-induced neurotoxicity in Drosophila melanogaster—A comparative study. J. Basic Appl. Zoology 82 (1), 43. doi:10.1186/s41936-021-00240-z
Pratap, G. K., Ashwini, S., and Shantaram, M. (2017). Alzheimer’s disease: A challenge inits management with certain medicinal plants-a review. Int. J. Pharm. Sci. Res. 8 (12), 4960–4972. doi:10.13040/IJPSR.0975-8232.8(12).4960-72
Pratap, G. K., Rather, S. A., and Shantaram, M. (2020b). Anticholinesterase Activity Mass Spectr. Analysis Olea dioica Roxb ., vitro Study 82, 601–611.
Pratap, G. K., Rather, S. A., and Shantaram, M. (2020a). In vitro anti-cholinesterase activity and mass spectrometric analysis of Curculigo orchioides gaertn., rhizome extract. Anal. Chem. Lett. 10 (4), 442–458. doi:10.1080/22297928.2020.1837669
Pratap, G. K., and Shantaram, M. (2020b). A kinetic study of acetylcholinesterase inhibition by fractions of oleo diox Roxb.leaf and Curculigo orchioides gaertn rhizome for the treatment of alzheimer’s disease. Eur. J. Med. Plants, 1–12. doi:10.9734/ejmp/2019/v30i430188
Pratap, G. K., and Shantaram, M. (2020a). An alternative approach for anti-alzheimer’s compounds from plant extracts. Tyler Fr. 1 (1), 15–42.
Querfurth, W., Henry, , and LaFerla, M. F. (2010). Alzheimer's disease. N. Engl. J. Med. 4 (362), 329–344. doi:10.1056/NEJMra0909142
Rao, A., and Naika, R. (2015). Preliminary phytochemical and antimicrobial properties of Olea dioica Roxb bark extract collected from Western Ghats, Karnataka, India Preliminary phytochemical and antimicrobial properties of Olea dioica Roxb bark extract collected from Western Ghats. December 2018.
Sereia, A. L., Oliveira, M. T. D., Baranoski, A., Larisa, L., Marques, M., Ribeiro, F. M., et al. (2019). In vitro evaluation of the protective effects of plant extracts against amyloid-beta peptide-induced toxicity in human neuroblastoma SH-. PLoS ONE 1–24.
Sugimoto, H., Yamanish, Y., Iimura, Y., and Kawakami, Y. (2000). Donepezil hydrochloride (E2020) and other acetylcholinesterase inhibitors. Curr. Med. Chem. 7 (3), 303–339. doi:10.2174/0929867003375191
Thompson, P. A., Wright, D. E., Counsell, C. E., and Zajicek, J. (2012). Statistical analysis, trial design and duration in alzheimer’s disease clinical trials: A review. Int. Psychogeriatrics 24 (5), 689–697. doi:10.1017/S1041610211001116
Wal, P., Wal, A., Sharma, G., and Rai, A. K. (2011). Biological activities of lupeol. Syst. Rev. Pharm. 2 (2), 96–103. doi:10.4103/0975-8453.86298
Wang, H., Xu, Y., Yan, J., Zhao, X., Sun, X., Zhang, , et al. (2009). Acteoside protects human neuroblastoma SH-SY5Y cells against β-amyloid-induced cell injury. Brain Res. 1283, 139–147. doi:10.1016/j.brainres.2009.05.101
Wang, R., and Xi, C. T. (2005). Neuroprotective effects of huperzine A: A natural cholinesterase inhibitor for the treatment of alzheimer’s disease. NeuroSignals 14 (1–2), 71–82. doi:10.1159/000085387
Xue, C., Lin, T. Y., Chang, D., and Guo, Z. (2017). Thioflavin T as an amyloid dye: Fibril quantification, optimal concentration and effect on aggregation. R. Soc. Open Sci. 4 (1), 160696. doi:10.1098/rsos.160696
Yang, J., and Gadi, R. L. (2008). Effects of steaming and dehydration on anthocyanins, antioxidant Activity,Total phenols and color characteristics of purple-fleshed sweet potatoes(Ipomoea batatas). Am. J. Food Technol. 3 (4), 224–234. doi:10.3923/ajft.2008.224.234
Yesodharan, K., and Sujana, K. A. (2007). Wild edible plants traditionally used by the tribes in the Parainbikulam Wildlife Sanctuary, Kerala, India. Nat. Product. Radiance 6 (1), 74–80.
Keywords: alzeheimer’s disease, olea dioica roxb, amyloid, beta, SHSY-5Y, GC-LCMS, acetylcholinesterase
Citation: Kenchappa PG, Karthik Y, Vijendra PD, Hallur RLS, Khandagale AS, Pandurangan AK, Jayanna SG, Alshehri MA, Alasmari A, Sayed S, Shantaram M and Mushtaq M (2023) In vitro evaluation of the neuroprotective potential of Olea dioica against Aβ peptide-induced toxicity in human neuroblastoma SH-SY5Y cells. Front. Pharmacol. 14:1139606. doi: 10.3389/fphar.2023.1139606
Received: 07 January 2023; Accepted: 14 April 2023;
Published: 10 May 2023.
Edited by:
Shoib Sarwar Siddiqui, University of Hertfordshire, United KingdomReviewed by:
Prabhat Upadhyay, Harvard Medical School, United StatesSyed Haris Omar, Charles Sturt University, Australia
Copyright © 2023 Kenchappa, Karthik, Vijendra, Hallur, Khandagale, Pandurangan, Jayanna, Alshehri, Alasmari, Sayed, Shantaram and Mushtaq. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Manjula Shantaram, manjula59@gmail.com; Muntazir Mushtaq, muntazirhuda@gmail.com
 Pratap G. Kenchappa
Pratap G. Kenchappa Yalpi Karthik2
Yalpi Karthik2 Raghavendra L. S. Hallur
Raghavendra L. S. Hallur Ajay S. Khandagale
Ajay S. Khandagale Ashok K. Pandurangan
Ashok K. Pandurangan Mohammed Ali Alshehri
Mohammed Ali Alshehri Samy Sayed
Samy Sayed Manjula Shantaram
Manjula Shantaram Muntazir Mushtaq
Muntazir Mushtaq